Abstract
Background:
Influenza-like illness and inflammation are known risk factors for venous thromboembolism (VTE), which includes deep vein thrombosis (DVT) and pulmonary embolism (PE). However, few studies have characterized the risk of VTE following influenza vaccination. We examined VTE risk after vaccination in adults 50 years old and older within the Vaccine Safety Datalink (VSD).
Methods:
We used the self-controlled case series method to determine the risk of VTE among age-eligible adults who received influenza vaccine (with or without pandemic H1N1) and experienced a VTE during the months of September through December in 2007 through 2012. Presumptive VTE cases were identified among VSD participants using diagnostic codes, diagnostic tests, and oral anticoagulant prescription. Potential cases were validated by medical record review. The VTE incidence rate ratio was calculated among confirmed cases for the risk window 1 to 10 days after vaccination relative to all other person-time from September through December.
Results:
Of the 1,488 presumptive cases identified, 508 were reviewed, of which 492 (97%) were confirmed cases of VTE. The analysis included 396 incident, confirmed cases. Overall, there was no increased risk of VTE in the 1 to 10 days after influenza vaccination (IRR = 0.89, 95% CI 0.69–1.17) compared to the control period. Results were similar when all person-time was censored before vaccination. A post hoc analysis showed an increased risk among current tobacco smokers (IRR = 2.57, 95% CI 1.06–6.23). No clustering of VTE was observed in the 1–42 days after vaccination.
Discussion:
Overall, there was no evidence that inactivated influenza vaccine was associated with VTE in adults ≥50 years old. An increased risk was found among current smokers in a post hoc analysis. These findings are consistent with previous research and support the safety of annual vaccination in this population.
1. Background
The risk of venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE) increases markedly after age 50 years [1,2]. Known risk factors for VTE include cancer, tissue trauma and surgery, immobilization, hypertension, metabolic disorders, hormone therapy, smoking, and prior thrombosis [3,4]. Research has indicated that there is also a higher risk of VTE following influenza-like illness [5,6]. Investigators postulated that the infection-associated risk could be due to endothelial inflammation [7,8]. Influenza vaccination has been shown to cause a transient increase in pro-inflammatory cytokine production [9,10]. Despite this biological plausibility, there have been few studies examining the risk of VTE following influenza vaccination.
Annual influenza vaccination has been recommended for adults ≥50 years old since 2000 [11]. As of 2014, vaccine coverage was 48% in adults 50–64 years of age and 72% in adults 65 and older [12]. It is important to characterize the vaccine’s safety in this population which is at higher risk for comorbid conditions. The aim of our study was to evaluate the risk of VTE following influenza vaccination in adults 50 years old and older in the Vaccine Safety Datalink (VSD). The secondary aim was to characterize this risk for DVT and PE separately.
2. Methods
2.1. Study population
We used an exposed-only, case-only approach, where the patient population included those ≥50 years old who had experienced a VTE and received influenza vaccine in 2007 through 2012 during the months of September through December. Patients meeting these criteria were identified through the VSD, a collaborative effort funded by the Centers for Disease Control and Prevention to study the risk of adverse events following vaccination. The VSD utilizes data from several integrated healthcare delivery systems across the United States [13]. Each VSD site provides patient demographics, vaccination history, and other healthcare information on an annual basis from their electronic medical records and health insurance databases. This study was conducted with data from five participating sites (Kaiser Permanente Colorado, Kaiser Permanente Northwest, Kaiser Permanente Northern California, Kaiser Permanente Southern California, and Marshfield Clinic).
2.2. Case ascertainment and data sources
Only patients enrolled in the participating VSD site’s health plan for a year before and after VTE diagnosis (or with less follow up if patient died within 1 year after the diagnosis) were included. Patients were excluded if they had a history of any of the following conditions that are known to change the risk of VTE: hospital stay longer than 3 days from admission date; trauma, or selected surgical procedures within 4 weeks of the VTE diagnosis; any cancer treatment occurring August through December 2007 through 2012; or diagnosis with atrial fibrillation or long term oral anticoagulant therapy at any time prior to the study period. Exclusion codes are listed in the supplemental table.
Presumptive cases of VTE were identified in the VSD datasets by meeting all of the following criteria (1) an inpatient or outpatient visit during the study period of with an assignment of International Classification of Disease, 9th revision (ICD-9) code for VTE (diagnosis of VTE), (2) No other assignments of an ICD-9 code for VTE in the year prior to the study period, (3) a diagnostic test for VTE within 7 days of the VTE code assignment, and (4) a prescription for oral anticoagulant medication within 2 days of the VTE code assignment. ICD-9 codes for VTE included acute venous embolism and thrombosis of deep vessels of lower extremity (453.4X) and/or pulmonary embolism and infarction (415.1X). Diagnostic tests included ultrasound, chest/thorax CT scan, chest MRI, thoracic MRI, venography, plethysmography, ventilation/perfusion scan, and pulmonary angiography (see supplemental table for CPT codes). Qualifying oral anticoagulant medications included apixaban, dabigatran etexilate mesylate, rivaroxaban and warfarin. Diagnoses of comorbidities (assignment of ICD-9 codes for obesity, diabetes, asthma, emphysema, COPD, or abnormal coagulation profile- see supplemental table for ICD-9 codes) and vaccination history were collected from the VSD datafiles one year before through one year after the VTE symptom onset date. Influenza vaccines included the seasonal vaccine containing pre-2009 H1N1, monovalent pandemic vaccine, or post-pandemic seasonal vaccine containing the H1N1pdm09 antigen. Only non-adjuvant influenza vaccines were licensed for use in the United States during the study period. Any co-administered vaccines within 1 day of influenza vaccination that contained any antigens of tetanus, diphtheria, pertussis, zoster, pneumococcal, meningococcal, or hepatitis A or B were also included.
Manual medical record abstraction was performed for a sample of the patients who met the above criteria and also received influenza vaccine during the same year. Each site reviewed either a random sample of 140 presumptive cases or all cases if fewer than 140 presumptive cases were identified. Reported symptoms, date of symptom onset, results of diagnostic testing, and risk factors for VTE (including family history of VTE, hormonal medication use, and smoking history) were manually abstracted. Reviewed cases were classified into definite, probable, possible, or non-case categories of certainty according to the criteria used by Spencer, et al. [14]. Cases that were not definite for either type of VTE were adjudicated by two physicians and case certainty assigned by consensus.
We included only incident cases, where symptom onset was less than 32 days before the occurrence of the VTE code in the medical record. Cases where onset was outside of the study period or where the VTE diagnosis was made ≥32 days after symptom onset were excluded from the final sample. Any cases where onset occurred on the date of vaccination were excluded to avoid situations where the patient was prompted to receive care due to the VTE and the provider took the opportunity to administer the influenza vaccine.
2.3. Statistical analysis
The primary analysis was performed with all confirmed, incident cases of definite, possible, and probable VTE. We plotted VTE onset and dates of first influenza vaccination by month and year to qualitatively visualize incidence of VTE related to the exposure. The risk of VTE onset following influenza vaccination was calculated using the self-controlled case series (SCCS) method [15]. The risk window was defined as 1–10 days after any influenza vaccination. We chose this window because research indicates that peak pro-inflammatory cytokine production occurs shortly after vaccination, within 7 days [9,10]; three extra days were included in this period to be certain that the risk window captured the increased inflammation. The date of vaccination was excluded from the person-time in the analysis.
If records indicated that a second vaccination was received with the same formulation as the first, person-time was censored after the second vaccination as this record was likely to a clerical error. The control window included all other person-time between September 1st through December 31st in the year that both influenza vaccination and VTE occurred.
The Incident Rate Ratio (IRR) of VTE onset in the risk window compared to the control window was calculated using Poisson regression which was conditioned on each patient. In addition to the primary analysis, we also calculated the ratio among those with DVT only, PE only, and both diagnoses.
Several sub-analyses were performed in order to confirm robustness of the SCCS analysis results. We repeated the analysis excluding person-time in the two weeks prior to vaccination and all person-time prior to vaccination to determine if the main results were subject to the healthy-vaccinee effect [19]. We also repeated the analysis using a stricter definition for incident cases with symptom onset less than 8 days prior to diagnosis of VTE, compared to the definition of less than 32 days used in the main analysis. A temporal scan statistic [16] was also performed to identify if VTE events were clustered within the 1–42 days after vaccination, in order to check appropriateness of the 10 day risk window and to determine if there were other appropriate risk windows. Exploratory sub-analyses were performed to examine risk of VTE in different patient groups based on presence of different underlying risk factors for VTE, such as age, sex, diagnosis with comorbidities, smoking status, vaccine composition with respect to the A(H1N1)pdm09 antigen, and presence of co-administered vaccines.
Statistical analysis was performed using SAS 9.4. Institutional Review Boards at each VSD site approved this study with a waiver of informed consent.
3. Results
3.1. Case Identification
During the study period, 3774 patients aged ≥50 years in the VSD received both an ICD-9 code for VTE and an influenza vaccine during the same year (Fig. 1). Of these, 39% (1488) met the diagnostic testing and prescription criteria for the presumptive case definition. Of the 508 cases chart reviewed, 97% (492) were confirmed cases of VTE. Most of the non-cases were excluded because they were suspected to have VTE but the diagnosis was ultimately ruled out by diagnostic testing.
Fig. 1.
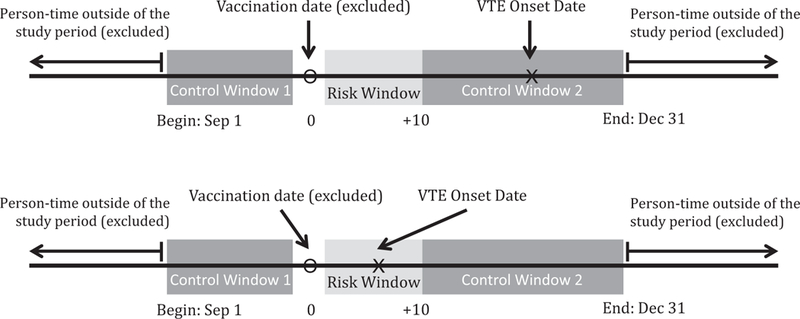
Self-controlled case series design for determining the risk of venous thromboembolism (VTE) following influenza vaccination.
Of the 492 confirmed cases, 72 (15%) non-incident cases and 20 (4%) cases with onset outside of the study period were excluded from the final analysis. In order to use the SCCS method, four cases were excluded because vaccination occurred on December 31st (there was no risk period) or on the same day as VTE onset (censored onset date). The final analysis sample included 396 confirmed, incident cases. The majority (n = 382) were definite cases, with relatively few probable and possible cases of VTE (n = 4 and n = 10, respectively) (Fig. 2).
Fig. 2.
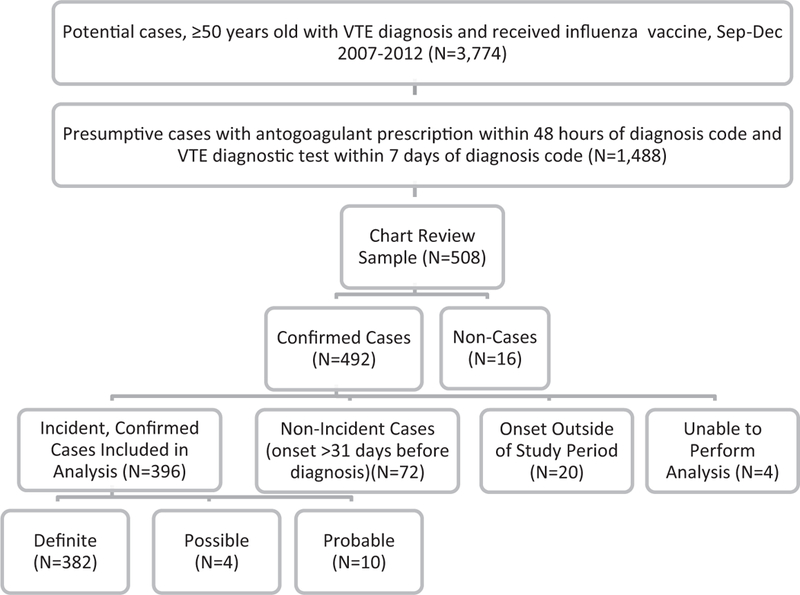
Identification of chart-confirmed, incident cases of venous thromboembolism (VTE).
3.2. Characteristics of incident, confirmed cases
By VTE sub-type, 251 (63%) cases were diagnosed with DVT only, 117 (30%) with PE only, and 28 (7%) with both types of VTE (Table 1). The final analysis sample was 57% male and the majority (58%) had at least one possible risk factor for VTE. Many were diagnosed with at least one comorbid condition (51%) and many were current or former smokers (49%). However, hormonal medication use and documented family history of VTE occurred infrequently (6% or less) among the final study population. Patients with PE or both conditions were more likely to be hospitalized than patients with DVT only (74% and 79% of cases vs 10%). Only one patient died within 31 days of diagnosis, from an unrelated event.
Table 1.
Patient characteristics and clinical characteristics of incident, confirmed cases of venous thromboembolism (VTE).
| Characteristic (N,%) | VTE Overall(N = 396) | DVT (N = 251) | PE (N = 117) | Both (N = 28) |
|---|---|---|---|---|
| Demographics | ||||
| Sex | ||||
| Female | 169 (43%) | 109 (43%) | 50 (43%) | 10 (36%) |
| Age group | ||||
| 50–59 | 87 (22%) | 51 (20%) | 26 (22%) | 10 (36%) |
| 60–69 | 121 (31%) | 74 (29%) | 38 (32%) | 9 (32%) |
| 70–79 | 111 (28%) | 71 (28%) | 35 (30%) | 5 (18%) |
| 80–89 | 67 (17%) | 47 (19%) | 16 (14%) | 4 (14%) |
| 90+ | 10 (3%) | 8 (3%) | 2 (2%) | 0 (0%) |
| Risk factors | ||||
| Diagnosis with any comorbid conditiona | 203 (51%) | 121 (48%) | 63 (54%) | 19 (68%) |
| Smoking statusb | ||||
| Current | 32 (8%) | 19 (8%) | 9 (8%) | 4 (14%) |
| Former/Ever | 162 (41%) | 98 (40%) | 55 (47%) | 9 (32%) |
| Never | 197 (50%) | 130 (53%) | 52 (45%) | 115 (54%) |
| Hormonal Contraceptive or Replacement Therapy Use | 24 (6%) | 11 (4%) | 11 (9%) | 2 (7%) |
| Family history of VTE | 13 (3%) | 10 (4%) | 3 (3%) | 0 (0%) |
| Vaccine containing A(H1N1)pdm09 antigen | 236 (60%) | 146 (59%) | 73 (62%) | 17 (61%) |
| Co-administered vaccine c | 26 (7%) | 13 (5%) | 10 (9%) | 3 (11%) |
DVT = Deep Vein Thrombosis PE = Pulmonary Embolism.
Obesity, diabetes, asthma, emphysema, COPD,or abnormal coagulation profile.
5 patients (1%) did not have a smoking status in their medical record.
Co-administered vaccine = any tetanus, diphtheria, pertussis, zoster, pneumococcal, meningococcal, or hepatitis A or B within 1 day of influenza vaccine.
According to vaccination records, only seven patients received both the seasonal and monovalent H1N1 vaccines in 2009. Of the incident VTE cases, 236 (60%) received a vaccine that contained the H1N1pdm09 antigen (monovalent or post-pandemic seasonal vaccines). Five patients had vaccination records indicating that they had received two vaccines of the same formulation.
3.3. Risk of VTE following influenza vaccination
Peak vaccination occurred in October of each study year, while VTE onset was fairly consistent throughout the study months (Fig. 3). Onset occurred prior to first vaccination for 48% of patients with VTE. Findings from the SCCS analysis indicated that there was no overall increased risk of any VTE in the 1–10 days following an influenza vaccination compared to the control period (IRR = 0.89, 95% CI 0.68–1.17) (Table 2). Similar results were observed when we restricted the analysis to those who had been diagnosed with DVT only (IRR = 0.79, 95% CI 0.56–1.12), PE only (IRR = 1.12, 95% CI 0.68–1.85), or were diagnosed with both DVT and PE (IRR = 0.84, 95% 0.29–2.42).
Fig. 3.
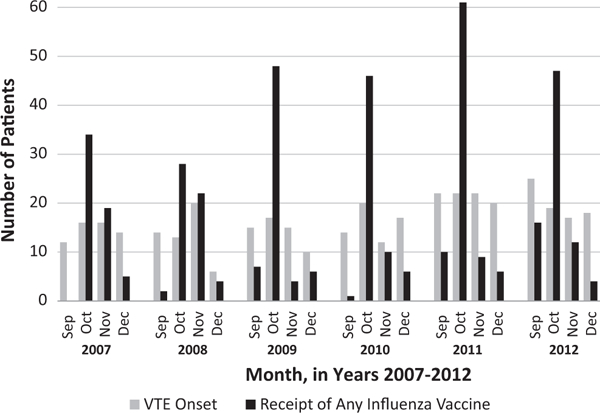
Month of venous thromboembolism (VTE) onset and month of influenza vaccination among adults ≥50years old, 2007–2012.
Table 2.
Incidence Rate Ratio (IRR) of Venous Thromboembolism (VTE) with study design modifications and exploratory analysis among certain risk groups.
| Patient groupa,b | Cases | IRR of VTE Overall (95% CI) |
|---|---|---|
| All patients | 396 | 0.89 (0.68, 1.17) |
| Sex | ||
| Female | 169 | 0.82 (0.54, 1.25) |
| Male | 227 | 0.94 (0.67, 1.35) |
| Age group | ||
| 50–64 | 152 | 0.76 (0.48, 1.19) |
| 65–79 years | 167 | 1.01 (0.66, 1.53) |
| ≥80 years | 77 | 0.90 (0.48–1.68) |
| Diagnosed comorbid conditionc | ||
| 1 or more | 203 | 0.93 (0.64–1.37) |
| None | 0.84 (0.57, 1.25) | |
| Smoking status | ||
| Current smokers | 32 | 2.57 (1.06–6.23) |
| Former smokers | 161 | 0.96 (0.62–1.47) |
| Never smokers | 197 | 0.64 (0.43–0.96) |
| Exposure to A(H1N1)pdm09 antigen | ||
| Pre-pandemic seasonal vaccine only | 158 | 0.89 (0.58–1.38) |
| Monovalent A(H1N1)pdm09 (with or without seasonal) or post-pandemic seasonal vaccine | 236 | 0.84 (0.59–1.20) |
| Hormonal therapy | 21 | 0.56 (0.16–1.98) |
All persons with two vaccinations in one season were excluded.
26 Persons had co-administered vaccines, none had VTE occurring 1 to 10 days following an influenza vaccination.
Obesity, diabetes, asthma, emphysema, COPD, or abnormal coagulation profile.
The temporal scan statistic indicated that there was no temporal clustering of onset of this outcome related to the vaccination date in the 1–42 days post-vaccination. Results from sensitivity analyses excluding person-time in the 2 weeks prior to vaccination (IRR= 1.02, 95% 0.68–1.17), excluding all pre-vaccination person-time (IRR= 1.10, 95% 0.77–1.57), and defining incident cases as those with symptom onset less than 8 days prior to VTE diagnosis (IRR = 0.91, 95% 0.67–1.25) also indicated no increased risk of VTE following vaccination.
In exploratory analyses restricted to risk groups, most groups showed the same results (Table 2). There were 26 patients co-administered other vaccinations and none had VTE occurring 1–10 days following an influenza vaccination. However, there was an increased risk of VTE in the ten days following influenza vaccination among current smokers (IRR = 2.57, 95% CI 1.06–6.23) and decreased risk among never smokers (IRR = 0.64, 95% CI 0.43–0.96). We repeated the analysis for current and never smokers with different censoring criteria to test the robustness of the result. The risk was still significantly greater in the 1–10 days post-vaccination for current smokers when the 2 weeks prior to any vaccination were excluded (IRR = 2.77, 95% CI 1.07–7.18) and all person-time prior to vaccination was excluded (IRR = 3.13, 95% C11.01–9.75). In contrast, the decreased risk for never smokers was no longer statistically significant following both changes in excluded time prior to vaccination (IRR = 0.73 95% C1 0.47–1.13 and IRR = 0.70 95% CI 0.42–1.18, respectively).
4. Discussion
Older adults are at higher risk for VTE [1,2] and they are also recommended for influenza vaccination because those 65 and older are at higher risk for complications from influenza infection [17]. We were able to examine the risk of VTE overall and for PE and DVT separately; there was no evidence of increased risk of VTE following influenza vaccination, for either distinct PE or DVT diagnosis, or for those with both diagnoses at the same time. Results were similar when the analysis was stratified by age group, presence of at least one comorbid condition, and by receipt of vaccine containing H1N1pdm009 antigen. This study provides additional evidence supporting the safety of annual vaccination of adults ≥50 years of age.
These findings are consistent with a previous study that characterized the risk of VTE following influenza vaccination [18]. The prior study was a case-control study which found no increased risk of VTE among vaccinated patients aged 52 years and older compared to non-vaccinated patients. However, vaccination status was reported by patients and therefore results may have been subject to recall bias. While there were differences between cases and controls in the proportions with VTE risk factors, these results were adjusted in a multivariate analysis for history of surgery, recent hospitalization, and oral contraceptive use. However, the case-control design could not fully account for any residual confounding due to a relationship between patient health and their decision to vaccinate.
Our study had several strengths and improvements on previous work in this topic. In our study, the electronic case-finding algorithm facilitated efficient detection of incident VTE cases and nearly all cases that met the electronic criteria were confirmed by medical record review. Recall bias was avoided through use of electronic immunization records.
By using the SCCS method, we were able to account for unmeasured confounders, such as underlying genetic risk factors, as long as they were constant throughout the observation period because the sample included only vaccinated (exposed) cases that served as their own controls. Presumably, potential VTE risk factors like effects from smoking, certain chronic medical conditions, and use of hormonal medications would not change considerably for any given case in the four-month study period. We did exclude cases with acute health events including trauma, hospitalization, and cancer treatment that are associated with increased VTE risk.
In an exploratory analysis among patient groups with different underlying risk of VTE, we observed an elevated VTE risk after influenza vaccination in current smokers and a reduced risk in never smokers. Smoking has been identified as a risk factor for VTE [3,4]; therefore, we examined the risk among patient groups stratified by smoking status to determine if there was effect modification. The elevated risk in the 1–10 days after vaccination for current smokers was more robust than the reduced risk for never smokers, as the significance held when we changed the person-time exclusion criteria. Current smokers may be at a higher risk of VTE directly after influenza vaccination, but additional research is needed to confirm this finding. Previous research did not examine the effect of smoking status in adjusted models characterizing the risk of VTE related to vaccination.
We were able to confirm the robustness of our main results by varying the parameters of analysis and checking our risk window with the temporal scan. 1n the case of the healthy-vaccinee effect, the background rate of illness drops just prior to vaccination because those who are ill do not receive vaccinations; therefore, the increase of the rate back to baseline levels immediately after vaccination can manifest as a false-positive increased risk. Excluding the person-time immediately prior to vaccination tests whether results are subject to this effect. We observed no change in risk associated with vaccination indicating that our results are not likely affected by a healthy-vaccinee effect. While our incident case definition requiring diagnosis within one month of onset of symptoms of VTE was broad, results remained the same when we used a seven day incident case definition. Our choice of risk window was based on only two studies measuring cytokine production after influenza-vaccination [9,10]; however, we were able to check the appropriateness of the window using the temporal scan statistic. Since we identified no clustering of the VTE outcome in the 1 through 42 days post vaccination, we conclude that the risk window we chose was valid.
In terms of limitations, it is possible that some true cases of VTE were not detected through the use of the electronic algorithm to identify diagnosis codes, diagnostic tests, and oral anticoagulant prescriptions. We would not have captured patients with severe VTE who died before they were able to receive a diagnosis. Additionally, 61% of potential cases that were identified by ICD-9 code alone did not meet the additional diagnostic test and anticoagulant prescription criteria; we were not able to characterize the population that did not meet these additional criterion. Our results may not be generalizable to patients who may be at highest risk of VTE due to recent surgery, trauma, or malignancy.
Due to the influenza vaccines in use during the study period, we were unable to assess other specific influenza vaccine formulations such as the high dose, recombinant, and adjuvant vaccines. We gathered risk information about smoking status and family history of VTE manually from the medical record, but this information is reported by patients; therefore, some patients may be inaccurately classified into different risk groups due to recall and reporting bias. The SCCS method may be subject to confounding if the risk of the outcome fluctuates during the study period; we restricted the timeframe of the study to the four-month period to reduce the likelihood of this confounding, but there may be residual confounding if the risk of VTE fluctuated during the 4-month study period. Despite these limitations, our study further strengthens the body of evidence indicating no association between influenza vaccine and thromboembolism.
5. Conclusion
Overall, there was no evidence of increased risk of VTE in the 1–10 days following influenza vaccination in adults ≥50 years old in 2007–2012. In post hoc analysis, an increased risk was found among current tobacco smokers.
Supplementary Material
Acknowledgements
The authors thank Melissa Simpson, PhD, Brian Chow, MD, and Huong McLean, PhD for their contributions to this study.
Funding statement
This work was supported by the Centers for Disease Control and Prevention [contract number 200–2012–53587].
Footnotes
Conflicts of Interest
ALN receives research support from Pfizer, Merck, and MedImmune (now AstraZeneca) for unrelated studies. NPK receives research support from GlaxoSmithKline, Sanofi Pasteur, Protein Science, Pfizer, Merck, and MedImmune (now AstraZeneca) for unrelated studies. The remaining authors report no conflicts of interest.
Appendix A. Supplementary material
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.vaccine.2017.08.086.
References
- [1].Cushman M, Tsai AW, White RH, Heckbert SR, Rosamond WD, Enright P, Folsom AR. Deep vein thrombosis and pulmonary embolism in two cohorts: the longitudinal investigation of thromboembolism etiology. Am J Med 2004. July;1(117):19–25. 10.1016/j.amjmed.2004.01.018. [DOI] [PubMed] [Google Scholar]
- [2].Fowkes FJ, Price JF, Fowkes FG. Incidence of diagnosed deep vein thrombosis in the general population: systematic review. Eur J Vasc Endovasc Surg 2003. January;25:1–5. 10.1053/ejvs.2002.1778. [DOI] [PubMed] [Google Scholar]
- [3].Goldhaber SZ, Bounameaux H. Pulmonary embolism and deep vein thrombosis. Lancet 2012. May 12;379(9828):1835–46. 10.1016/S0140-6736(11)61904-1. [DOI] [PubMed] [Google Scholar]
- [4].Goldhaber SZ. Venous thromboembolism: epidemiology and magnitude of the problem. Best Pract Res Clin Haematol 2012. September;25(3):235–42. 10.1016/j.beha.2012.06.007. [DOI] [PubMed] [Google Scholar]
- [5].Clayton TC, Gaskin M, Meade TW. Recent respiratory infection and risk of venous thromboembolism: case-control study through a general practice database. Int J Epidemiol 2011. June;40(3):819–27. 10.1093/ije/dyr012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].van Wissen M, Keller TT, Ronkes B, Gerdes VE, Zaaijer HL, van Gorp EC, Brandjes DP, Levi M, Büller HR. Influenza infection and risk of acute pulmonary embolism. Thromb J 2007. October;16(5):16 10.1186/1477-9560-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Smeeth L, Cook C, Thomas S, Hall AJ, Hubbard R, Vallance P. Risk of deep vein thrombosis and pulmonary embolism after acute infection in a community setting. Lancet 2006. April 1;367(9516):1075–9. 10.1016/S0140-6736(06)68474-2. [DOI] [PubMed] [Google Scholar]
- [8].Schmidt M, Horvath-Puho E, Thomsen RW, Smeeth L, Sørensen HT. Acute infections and venous thromboembolism. J lntern Med 2012. June;271 (6):608–18. 10.1111/j.1365-2796.2011.02473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Christian LM, lams JD, Porter K, Karlsson E, Glasser R. Inflammatory responses to trivalent influenza virus vaccine among pregnant women. Vaccine 2011. November 8;29(48):8982–7. 10.1016/j.vaccine.2011.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Tsai MY, Hanson NQ, Straka RJ, Hoke TR, Ordovas JM, Peacock JM, Arends VL, Arnett DK. Effect of influenza vaccine on markers of inflammation and lipid profile. J Lab Clin Med 2005. June;145(6):323–7. 10.1016/j.lab.2005.03.009. [DOI] [PubMed] [Google Scholar]
- [11].Centers for Disease Control and Prevention. Notice to Readers: Delayed Supply of Influenza Vaccine and Adjunct ACIP Influenza Vaccine Recommendations for the 2000–01 Influenza Season. MMWR. Morbidity and Mortality Weekly Reports. Retrieved from https://www.cdc.gov/mmwr/preview/mmwrhtml/mm4927a4.htm 2010;24 [Accessed 10 January 2017]. [PubMed] [Google Scholar]
- [12].Centers for Disease Control and Prevention. Surveillance of Vaccination Coverage Among Adult Populations. Retrieved from 2016;5 [Accessed 22 November 2016]. [Google Scholar]
- [13].McNeil M, Gee J, Weintraub ES, Belongia EA, Lee GM, Glanz JM, Nordin JD, Klein NP, Baxter R, Naleway AL, Jackson LA, Omer SB, Jacobsen SJ, DeStefano F. The vaccine safety datalink: successes and challenges monitoring vaccine safety. Vaccine 2014;32(42):5390–8. 10.1016/j.vaccine.2014.07.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Spencer FA, Emery C, Lessard D, Anderson F, Emani S, Aragam J, Becker RC, Goldberg RJ. The worcester venous thromboembolism study: a population-based study of the clinical epidemiology of venous thromboembolism. J Gen Intern Med 2006;21(7):722–7. 10.1111/j.1525-1497.2006.00458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Whitaker HJ, Hocine MN, Farrington CP. The methodology of self-controlled case series studies. Stat Methods Med Res 2009;18(1):7–26. 10.1177/0962280208092342. [DOI] [PubMed] [Google Scholar]
- [16].McClure DL, Xu S, Weintraub E, Glanz JM. An efficient statistical algorithm for a temporal scan statistic applied to vaccine safety analyses. Vaccine 2012;30 (27):3986–91. 10.1016/j.vaccine.2012.04.040. [DOI] [PubMed] [Google Scholar]
- [17].Centers for Disease Control and Prevention. What you should know and do this flu season if you are 65 years and older. Retrieved from 2016;25 [Accessed 17 Jan 2017]. [Google Scholar]
- [18].Zhu T, Carcaillon L, Martinez l, Cambou JP, Kyndt X, Guillot K, Vergnes MC, Scarabin PY, Emmerich J. Association of influenza vaccination with reduced risk of venous thromboembolism. Thromb Haemost 2009;102(6):1259–64. 10.1160/TH09-04-0222. [DOI] [PubMed] [Google Scholar]
- [19].Hawken S, Potter BK, Little J, Benchimol El, Mahmud S, Ducharme R, Wilson K. The use of relative incidence ratios in self-controlled case series studies: an overview. BMC Med Res Methodol 2016;16(1):126 10.1186/s12874-016-0225-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


