Abstract
Patient‐centered drug development (PCDD) is a shift in the way that drugs are developed, systematically incorporating patient participation in all stages of medicines development. The more the research sector understands the needs and values of patients, the more effective and efficient it can be in bringing meaningful drugs and evidence to patients and providers. In this paper, we describe PCDD, provide examples of PCDD work across the phases of drug development, and discuss the challenges to making PCDD systematic. We describe how the developing Learning Health System will enable PCCD: we believe that the Learning Health System will address PCDD barriers by connecting stakeholders, enabling the more efficient flow of data, information, and evidence in the health ecosystem, and by providing governance for the connected ecosystem.
Keywords: clinical research, drug approval, drug development, Learning Health Network, Learning Health System, medicines development, patient engagement, patient participation, patient centered, patient‐centered drug development, patient centric, patient focused
1. INTRODUCTION
Patient‐centered drug development (PCDD) has been defined as an approach “in which developers and regulators systematically consider patient perspectives in the design, conduct, and reporting of research.”1 Patient‐centered drug development work has been fragmentary at best2—not systematically and holistically planned across development phases. Roles, methods, and best practices for PCDD are being formed by entities such as the Patient‐Centered Outcomes Research Institute,3 the INVOLVE project,4 and the Clinical Trials Transformation Initiative.5 As medicines development shifts to more systematic PCDD, the global Learning Health System (LHS) is also evolving. The LHS is defined as a system “in which progress in science, informatics, and care culture align to generate new knowledge as an ongoing, natural by‐product of the care experience, and seamlessly refine and deliver best practices for continuous improvement in health and health care.”6 The LHS will enable PCDD by providing the technical architecture for more efficient flow of data, information, and evidence in the health ecosystem and defining policy and governance rules of the road for the health ecosystem. This paper will describe PCDD and provide examples of Lilly progress to date on PCDD; we will describe the challenges to PCDD and elucidate how the developing LHS will facilitate PCDD.
2. ABOUT PCDD
The traditional drug development model is intended to discover, develop, and market medications to satisfy regulatory requirements. Patient‐centered drug development shifts this focus by putting the patient at the center of drug development phases, actively involving patients in all phases of development. The PCDD approach arose from two key FDA initiatives: FDA Safety and Innovation Act and Prescription Drug User Fee Act reauthorization.7 FDA described patient‐focused drug development as intending
more systematic and expansive approach to obtaining the patient perspective on disease severity or the unmet medical need in a therapeutic area to benefit the drug review process. In other words, the patient perspective will provide context in which regulatory decision‐making is made, specifically the analysis of the severity of the condition treatment and the current state of the treatment armamentarium for a given disease.8
A 2015 conference on patient‐focused drug development proposed this definition:
Patient‐focused drug development is a formal process by which drug developers and regulators form a partnership with the patient to enhance drug development, research, regulatory, and reimbursement processes with the patient voice. This partnership engages patients to obtain as critical input their views, experiences, and preferences throughout a product's lifecycle.9
Box 1: Characteristics of patient‐centered drug development
Patients are valued co‐researchers, informing decisions about unmet need, trial endpoints, device design, trial design and execution, and evidence translation and dissemination
Developers systematically seek, well understand, and incorporate patients' views of value, benefit, and risk into all phases of development
The research process is more transparent and well understood by patients
Clinical research participation is more convenient for patients and is seen as a care option
Development includes caregivers' perspectives
These definitions get us closer to aligning on PCDD as a concept. There is, though, no agreed upon framework for PCDD—no clear consensus on roles and rules. A 2014 systematic review of the literature on PCDD concluded that
Patient engagement in healthcare research is likely feasible in many settings. However, this engagement comes at a cost and can become tokenistic. Research dedicated to identifying the best methods to achieve engagement is lacking and clearly needed.10
A draft Patient‐Focused Development Plan has been created by FDA11; this conceptual framework shows how patient and caregiver involvement can be incorporated. Perfetto and Oehrlein adapted the FDA Plan and created a patient‐focused drug development plan, shown in Figure 1.
Figure 1.
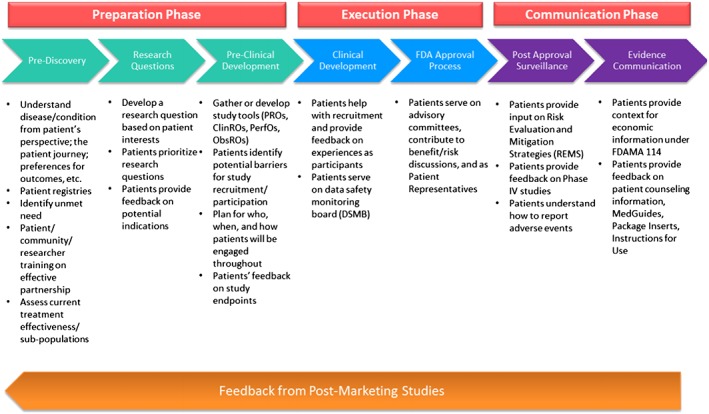
Perfetto et al. A Proposed conceptual framework for Patient‐Focused Drug Development. Reproduced with permission of the authors9
Categorized per the framework in Figure 1, we present case examples of adapting drug development to make it more patient‐centered. These examples are not meant to be exhaustive but illustrative of the experience at one pharmaceutical company.
| Preparation phase: This is the development phase where we explore unmet needs, generate hypotheses, create research plans, and design trials. In this phase, patients can advocate for new treatments and advise on clinical study design. The PCDD approach to planning includes consideration of the humanistic attributes of people; this systematic approach to listening and understanding informs the type of data the study will capture, e.g., quality of life metrics, physical functioning, social participation or productivity (absenteeism and presenteeism), or other aspects of patient experiences. Examples of PCDD work in the preparation phase include the following. | |
|---|---|
| Experiment | Learnings |
| Using clinical outcome assessments (COAs) (including patient‐reported outcomes—PRO instruments) to learn about patient values and needs. The COA data collection can be built into clinical trial plans and used as endpoints in research studies, including clinical trials. | The COA data collection can be built into clinical trial plans and used as endpoints in research studies, including clinical trials. |
| Using real world data to enhance our understanding of patient lives: gathering de‐identified real world data and analyzing it to complement qualitative stories of patient experiences. | Real world data can be analyzed to increase our understanding of combined qualitative and quantitative patient experiences; this understanding can be used to inform all aspects of the planning phase. |
| Execution phase: This is the phase where we recruit patients for trials, conduct studies, and seek regulatory approval and guidance. In this phase, patients can help recruit the right patients to trials, advise on making the enrollment process more accessible and participation in trials less burdensome, and provide input to regulators. Examples of PCDD experiments in the execution phase include the following. | |
|---|---|
| Experiment | Learnings |
| Educating and being transparent about how trials work: Lilly TrialGuide (https://www.lillytrialguide.com/en‐US) is Lilly's customer facing, persistent digital site that provides educational information about clinical research, along with easy to find listings of Lilly's clinical trials. Clinical trials are described in patient‐friendly language and iconography, with clear ways to contact investigator sites in order to learn more about participating in clinical research. | We have discovered/confirmed that patients are most interested in content that showcases the authentic experiences of real patients. |
| Making trial data collection more convenient: exploration to enable controlled, regulatory‐quality data capture through a variety of wearables to reduce the burden of participation in clinical trials while increasing the types, volume, and accuracy of data captured. Current trial design plans include use of smartphones and wearables to measure movement, ambulatory blood pressure, and sleep latency. | We are wrapping up two studies that measured itching and movement as clinical endpoints—two historically difficult things to measure with great reliability and convenience. |
| Making trial site visits more convenient: Lilly is partnering with leaders in the field of connected clinical trials to bring mobile technology and high‐touch personal support to provide clinical trial participants choices in how they experience trials. We are working to provide options for in‐home clinical trial visits supported by device capture of data, networks of mobile nurses, and varying other support options. In addition, we are identifying blended models that enable clinical trial visits to occur at any doctor's office, clinics, or local pharmacies. | We will be ready to execute this model on an actual study in the second half of 2017 and will have more data to share in 2018 regarding improved patient access and satisfaction in clinical research. |
| Making study protocols meaningful to patients: Lilly CoLAB is a process Lilly uses with patients and investigative site personnel to assure that clinical trial protocols are designed for participation by key stakeholders. CoLAB participants work face‐to‐face with study teams to identify and address operational issues with the protocols, using simulation as a means to gather insight and recommendations. | To date, we have executed dozens of CoLABs. We have also engaged many patients for their input in a number of studies, and we are confident that our protocols are becoming easier to execute and participate in. |
| Engaging with and learning from ePatient advisors: Lilly has sought an active partnership with ePatient leaders to better understand the experiences of their everyday lives, how disease management fits into their lives, and the implications of participation in clinical trials. Recognizing that clinical trial participation is just one part of a complicated journey, people who are living with a disease or new diagnosis are expected to become aware of relevant clinical trials, figure out how to navigate them, and then alter their lives to participate in them. The ePatient advisor role is an effort in working collaboratively to design solutions to improve clinical trials and clinical trial participation. | The ePatient advisor role has brought insights on how increased focus on patient‐centeredness and real world evidence is changing the health care ecosystem and how these trends may affect drug development and has helped in keeping the patient voice front and center in clinical trial innovation. Through further collaboration with patients, we developed a tiered‐consent form for clinical trial enrollment, affording patients access to the level of information they desire while meeting the institutional review boards requirements for informed trial participation. |
| Showing gratitude to patients: one way that Lilly shows gratitude to participants in clinical trials is by honoring them through The Hero's Journey Art project (https://www.lillytrialguide.com/en‐US/heros‐journey‐art). Clinical trial participants and the clinical trial community express their thoughts and feelings about clinical trials, decorating wooden bricks that are incorporated into 3 large crowdsourced sculptures honoring clinical trial participants. | We recently unveiled the first sculpture at the LiveStrong headquarters in Austin, Texas, in March of 2017. There is a strong, positive sentiment around this activity which can be seen by searching on the tag #herosjourneyart on social media. |
| Communication phase: This is the phase where we share information on the research process, learnings from our clinical trials, and study what happens in the real world (i.e., in naturalistic settings) with our medications. Examples of PCDD experiments in the communication phase include the following. | |
|---|---|
| Experiment | Learnings |
| Engaging in social media dialogue with patients: Lilly engages in social media focused on clinical trial innovation through the @LillyTrials Twitter handle and the LillyTrials blog. Topics of social discussion include how to improve clinical research as well as a variety of ways to raise awareness of clinical research. The LillyTrials blog is a home for regular patient guest bloggers to share their perspective on clinical trials, their importance, and how they can be improved. @LillyTrials is an eager participant in the global #WhyWeDoResearch social media campaign, encouraging patients, research staff, and the public to lend their voices toward raising awareness of clinical research and research opportunities. | We have doubled our growth rate of followers and continue to push the bounds of Lilly's use in social media. Our most recent example is our Instagram Story series on the unveiling of Hero's Journey Art at LiveStrong. |
| Using health literacy principles and best practices in the development of documents such as medication labels, trial enrollment forms, potential risks and side effects information and for translating our scientific evidence into nonbranded health education materials that are clear and meaningful to patients; testing these documents and improving them with patient input. | It is our responsibility to make our communications clear for patients. Using health literacy principles and processes improves patient understanding; patient understanding is essential for successful outcomes. |
| Gathering and applying patient advice and input on the design and function of medications and devices: to design convenient and successful drug devices, we look for insights into the daily lives of patients to help us understand preferences, limitations, and needs. | This approach can impact everything from medication color, shape, and size to the design of the container that holds the medication; we foresee improved patient outcomes and increased adherence due to greater convenience for patients. |
3. CHANGES NEEDED FOR MORE SYSTEMATIC PCDD
As described above, we have learned from work to date in PCDD. These case experiments and those of others in industry indicate the changes needed for the maturation and evolution of PCDD.
First and foremost, we need culture change: a shift to valuing and incorporating—systematically and in all phases of drug development—the preferences, values, needs, and experiences of patients. We believe the pharma industry and indeed the entire research sector need to make cultural changes that will resolutely embrace patient needs as the north star of the drug development lifecycle. Patients need to be seen and recognized as co‐researchers in all development phases—planning, execution, and evidence dissemination. Putting the patient at the core of all we do will improve trust in the research sector, trust in the research process, and trust in the pharma industry. This culture shift requires a new interdisciplinary mindset: “The success of clinical epidemiology has taken the evidence‐based medicine movement to a stage where many of the unanswered research questions are no longer epidemiological but humanistic, social, and political.”12 The shift to PCDD also requires a commitment to learning from each patient and adding these learnings to the body of scientific evidence. We need to commit to translating our research findings—both successes and failures—in ways that are meaningful, clear, and accessible to patients and valued by them. To accomplish the above, departments within medicines development companies need to align internally on a unified PCDD vision.
Second, we need clear and modern governance and policies that enable patients and research to work together across the development lifecycle. Many of these are in process, for example, FDA has undertaken patient‐focused drug development meetings with individual patients and groups of patients and committed to holding more of these meetings.13 We need policies that guide on the following specific topics in order to enable more systematic PCDD:
The collection of COAs in drug development,14 eg, PROs in labels. Although the PRO Guidance provides some guidance on the development and validation of COA instruments, inclusion of COA data in drug labels has been limited.
The use of new technologies in research
The effective use of real world evidence
New, flexible, and secure privacy and trust models to enable appropriate access to data for research
Third, we need technical infrastructure—the connectivity and the standards—for data, information, and evidence to flow. We cannot shift to systematic PCDD without a connected system: our current fragmented health ecosystem does not enable reliable and efficient access to and use of health data, information, and evidence. We need to connect the fragmented nodes of the health ecosystem so that what happens in the delivery of care informs and guides research, relevant and current research insights are more available at the point of care for shared decision making, and so that patient experiences outside the delivery and research sectors are woven into decision‐making and enable new insights and learning.
Finally, we need clear methods and best practices in systematically and effectively including patients in all aspects of drug development. We need to find ways of incorporating the diverse perspectives of patients—patient perspectives are not monolithic—by studying patients in naturalistic settings and by listening more than asking. Patients are sharing their real world experiences living with and managing a variety of conditions within the context of their everyday lives. “The illness as lived will differ from the disease or risk state in the evidence‐based guideline, and may well be at odds with the outcomes (whether ‘patient reported’ or not) measured in the research trial.”13 It is important to incorporate a wide variety of these lived experiences to gain a comprehensive understanding of how we can most effectively partner to design solutions that will have the greatest impact in clinical trial design and drug development.
Clear and consistent stakeholder roles, approved processes, and best practices for PCDD are sorely needed and are now being defined.9, 15, 16 Specifically, we need to answer questions asked by Domecq et al:
What are the best methods to identify patients for engagement?
What are the best methods to engage patients?
What are the observed benefits of patient engagement?
What are the harms and barriers of patient engagement?10
4. HOW THE LHS WILL FACILITATE PCDD
The LHS will be a global network of networks, connecting the fragmented health ecosystem—health care delivery, health research, and the real world experiences of persons—so that data, information, and evidence flow more efficiently. The LHS will be person‐centered; the word “person” is deliberately chosen to indicate the whole person (i.e., not just when a person is a patient). The connected LHS will allow the system to learn from each person's experiences and will speed learning in all parts of the system: the healthcare delivery system and the health research sector will be more connected to each other, and both will be more connected to the real world experiences of persons. When research and care are better connected and both understand the real world health behaviors of persons, as a system, we can learn more rapidly, improve health outcomes, reduce errors, and ultimately reduce costs by providing more effective care.
After years of research, the planning and building of the LHS are now underway, coordinated by the Learning Health Community (LHC), a group of 109+ diverse health organizations.17 In the United States, an operational LHS is the pinnacle goal of the interoperability initiatives of the Office of the National Coordinator for Health Information Technology.18 The vision and practical initiatives of the LHS will enable PCDD by addressing the barriers and needed changes discussed previously: shifting culture, setting governance for the health ecosystem, and crafting technical infrastructure that enables interoperability. None of this will be easy and none of this will be quick: successfully connecting, maintaining, governing, and evolving the ultra‐large scale LHS is a daunting and audacious undertaking that will require the continuous cooperation and collective determination of stakeholders from all sectors of health.
4.1. Culture change
The LHS vision is person‐centered—that is, it aims to
protect and improve the health of individuals by informing choices about health and healthcare. The LHS will do this by enabling strategies that engage individuals, families, groups, communities, and the general population, as well as the United States healthcare system as a whole.19
This shift to patient centricity enables and reinforces the shift in drug development to systematically putting the patient at the center of development work; when the health ecosystem that PCDD works within is person‐centric, drug development can more effectively adapt and align to be systematic in its patient centricity.
The LHS is building toward a culture of rapid and continuous learning: using the more efficient flow of data, information, and knowledge to enable faster health insights for individuals and populations. The LHS vision is about learning from each person—when each person's experiences and characteristics are available, we can learn from each person and add that learning to the body of scientific evidence. This learning in turn forms a “virtuous cycle” where improvement and evolution is faster and continuous. Systematic PCDD within medicines development companies will be more enabled when the larger health ecosystem—the LHS—is focused on and designed for rapid learning from all persons.
4.2. Policy and governance
In 2014, the LHC Policy and Governance Initiative began the work of crafting a policy and governance model for the LHS; this model will define how the LHS will be governed and how decisions will be made. The LHC Policy and Governance Initiative is planning for managing the LHS in a way that supports its operations, to build and maintain trust on the part of all stakeholders, and to stimulate ongoing innovation.
In this model, a trust fabric will help all stakeholders understand how to ethically engage in the LHS, accessing and sharing data, information, and evidence. This in turn will help research engage in more systematic PCDD by having established trust, common goals, and rules of the road for communicating. New models for privacy and sharing will be established to enable stakeholders to connect more effectively, for example, informing how individuals communicate unmet needs to research and how the research sector communicates with patients (e.g., study recruitment, presenting clinical trial data and learnings back to study participants). In the operational LHS, we will have transparent policies and governance over how research undertakes appropriate secondary or co‐use and shares real world insights.
4.3. Technical infrastructure
Technical infrastructure for enabling interoperability is essential for the flow of data information and evidence. The LHS has a standards initiative underway to plan, architect, and build this infrastructure; this initiative is called Essential Standards to Enable Learning—ESTEL. The ESTEL initiative is working to identify and establish the technical rules of the road: how will we all speak the same language so that information, data, and evidence flow efficiently? The LHS standards foundation will enable research and delivery to connect to each other more efficiently by defining agreed upon ways to represent and transmit data, information, and evidence. The technical infrastructure of the LHS will enable the exchange, availability, and use of electronic health information; this will enable PCDD in the following ways:
Faster cures: we can speed innovation when we can practice PCDD in a connected ecosystem: the LHS will enable us to connect to and learn from patients to understand unmet need and inform hypothesis generation, recruit the right patients for trials more quickly, execute trials more efficiently and faster (e.g., using real world data and real world evidence to inform and speed pragmatic and adaptive trials), improve the convenience and relevance of clinical studies, collect data in trials more efficiently and more conveniently, provide study participants their data in context of patients like them, and connect providers and patients to research studies as care options.
More efficient research access to real world data: the LHS will provide the technical and behavioral rules of the road for appropriate research access to real world data.
More effective research evidence dissemination: the LHS can enable the flow of innovation evidence to delivery and help shorten the knowledge translation rate from the appalling average 17 years it still takes for a medical intervention to be used regularly in healthcare.20
The LHS can help us get closer to precision medicine by helping researchers understand patient experiences, values, and preferences and the phenotypic characteristics of patient groups.
Improved safety surveillance: the LHS can help us continue to improve active safety surveillance. By using the LHS infrastructure to constantly and more efficiently look for safety signals on health interventions, we can improve patient outcomes and reduce adverse events.
Enable distributed and real‐time real world analytics so that we can more efficiently access and analyze data for rapid learnings.
Easier to integrate/link/connect disparate data sets (e.g., a patient's genomic data to their electronic medical records, a patient's personal health tracker data to their personal health record) in a connected system via the LHS technical standards for data representation and transmission.
Connecting additional stakeholders: the LHS will connect groups that have not been well connected to date, for example, caregivers, community‐based services, long‐term providers, behavioral providers, and dental providers. Information from the groups can add to the understanding of the experiences of persons beyond the delivery and research sectors.
The LHS flow will help increase the transparency of cost data, and cost transparency will in turn help enable the ecosystem shift to value‐based care and decision making.
4.4. PCDD methods and best practices
The LHS will enable the more efficient creation and sharing of PCDD best practices:
In a connected system with infrastructure for flow and clear rules of the road, we can more efficiently conduct PCDD experiments.
The LHS will connect additional stakeholders and enable PCDD learnings from all (vs. learnings from a subset of health ecosystem stakeholders).
The connected LHS will enable more efficient access to up‐to‐date best practices for PCDD.
Once we can effectively share PCDD learnings in a connected system, we can save time to insights by reducing duplication of experiments.
The development of the LHS will enhance and speed the shift to systematic PCDD. The LHS moves us toward the needed culture changes for PCDD. The technical infrastructure of the LHS will enable the more efficient flow of data, information, and evidence—faster and to more players. The policy and governance rules of the LHS will continuously drive clearer, more up to date policies, and the LHS will enable the more efficient creation and dissemination of best practices for PCDD.
5. CONCLUSION
Patient‐centered drug development systematically and actively includes patient participation and patient‐focused evidence in all phases of drug development: hypothesis generation, study planning and execution, medication and device design, and evidence translation and dissemination. Work in PCDD to date has highlighted the challenges to shifting to systematic PCDD. The developing LHS will enable PCDD: the LHS will be an ultra‐large scale system of systems that will provide the technical and governance fabric of the larger, connected health ecosystem. Connecting the fragmented, disconnected health landscape of today will be extremely complex and will require the innovation, trust, and tenacity of all stakeholders. In this connected LHS, data, information, and evidence will flow more efficiently; this flow, the governance around it, and the resulting rapid learning will enable systematic PCDD and ultimately result in beneficial drugs and meaningful evidence for patients, providers, and payers.
DISCLOSURES
Laura Crawford is a member of the Learning Health Community Interim Steering Committee.
Erin Moore is an FDA Patient Representative; FDA Pediatric Advisory Committee, Office of Pediatric Therapeutics.
The opinions expressed in this article are those of the authors and do not necessarily reflect the views of their employers or organizations.
ACKNOWLEDGMENTS
The authors thank Joe Kim (Eli Lilly and Company) for his contributions to this article and Leigh Anne Naas and Brande Yaist (Eli Lilly and Company) for their thoughtful review of this article.
Crawford LS, Matczak GJ, Moore EM, Haydar RA, Coderre PT. Patient‐centered drug development and the Learning Health System. Learn Health Sys. 2017;1:e10027 10.1002/lrh2.10027
REFERENCES
- 1. Basch E. Toward patient‐centered drug development in oncology. N Engl J Med. 2013;369(5):397‐400. 10.1056/NEJMp1114649 [DOI] [PubMed] [Google Scholar]
- 2. Hoos A, Anderson J, Boutin M, et al. Partnering with patients in the development and lifecycle of medicines: a call to action. Therapeutic Innovation & Regulatory Science. 2015;49(6):929‐939. 10.1177/2168479015580384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Patient‐Centered Outcomes Research Institute . http://www.pcori.org/. Accessed 2017 January.
- 4. National Institute for Health Research . INVOLVE Project. http://www.invo.org.uk/. Accessed 2017 January.
- 5. Clinical Trials Transformation Initiative web site . https://www.ctti‐clinicaltrials.org/ Accessed 2017 January.
- 6. Institute of Medicine (IOM) . The learning healthcare system: workshop summary. Washington, DC: The National Academies Press. 2007. https://www.nap.edu/catalog/11903/the‐learning‐healthcare‐system‐workshop‐summary‐iom‐roundtable‐on‐evidence. Accessed 2017 January. [PubMed]
- 7. Perfetto EM, Burke L, Oehrlein EM, Epstein RS. Patient‐focused drug development: a new direction for collaboration. Med Care. 2015;53(1):9‐17. 10.1097/MLR.0000000000000273 [DOI] [PubMed] [Google Scholar]
- 8. Food and Drug Administration Safety and Innovation Act (FDASIA) . Fact sheet: increased patient participation in medical product regulation. 2012. http://www.fda.gov/RegulatoryInformation/Legislation/SignificantAmendmentstotheFDCAct/FDASIA/ucm311045.htm. Accessed 2017 January.
- 9. Perfetto EM, Oerhlein EM. Assessing meaningful patient engagement in drug development: a definition, framework, and rubric. 2015. http://www.pharmacy.umaryland.edu/media/SOP/wwwpharmacyumarylandedu/centers/cersievents/pfdd/mcersi‐pfdd‐framework‐rubric.pdf. Accessed 2017 January.
- 10. Domecq JP, Prutsky G, Elraiyah T, et al. Patient engagement in research: a systematic review. BMC Health Serv Res. 2014;14:89 10.1186/1472-6963-14-89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Food and Drug Administration . Roadmap to patient‐focused outcome measurement in clinical trials. 2015. http://www.fda.gov/drugs/developmentapprovalprocess/drugdevelopmenttoolsqualificationprogram/ucm370177.htm Accessed 2017 January.
- 12. Greenhalgh T, Snow R, Ryan S, Rees S, Salisbury H. Six ‘biases’ against patients and careers in evidence‐based medicine. BMC Med. 2015;13:200 10.1186/s12916-015-0437-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pharmaceutical Researchers and Manufacturers Association . Fact sheet: patient‐focused drug development. 2016. http://www.phrma.org/fact‐sheet/patient‐focused‐drug‐development. Accessed 2017 January.
- 14. Food and Drug Administration . Guidance for industry: patient‐reported outcome measures: use in medical product development to support labeling claims. 2009. http://www.fda.gov/downloads/drugs/guidances/ucm193282.pdf. Accessed 2017 January. [DOI] [PMC free article] [PubMed]
- 15. Clinical Trial Transformation Initiative . Clinical trial Transformation initiative recommendations: effective engagement with patient groups around clinical trials. 2015. https://www.ctti‐clinicaltrials.org/files/pgctrecs.pdf. Accessed 2017 January.
- 16. Parmenter L. Patient‐centered drug development and market access: nine steps to success. QuintlesIMS blog. 2015. http://www.quintiles.com/blog/patient‐centered‐drug‐development‐and‐market‐access‐nine‐steps‐to‐success. Accessed 2017 January.
- 17. Learning health community web site. Endorsers. http://www.learninghealth.org/endorsers/. Accessed 2017 January.
- 18. Office of the National Coordinator for Health Information Technology . Connecting health and care for the nation: a shared nationwide interoperability roadmap. 2015. https://www.healthit.gov/sites/default/files/hie‐interoperability/nationwide‐interoperability‐roadmap‐final‐version‐1.0.pdf Accessed 2017 January.
- 19. Learning Health Community . Learning Health System Core Values. 2012. http://www.learninghealth.org/about‐the‐community/ Accessed 2017 January.
- 20. Balas EA, Boren SA. Managing Clinical Knowledge for Health Care Improvement In: Bemmel J, McCray AT, eds. Yearbook of Medical Informatics: Patient‐Centered Systems; 2000. [PubMed] [Google Scholar]


