Abstract
Previous studies indicate that the ability of Arabidopsis seedlings to recover normal growth following an ethylene treatment involves histidine kinase activity of the ethylene receptors. As histidine kinases can function as inputs for a two‐component signaling system, we examined loss‐of‐function mutants involving two‐component signaling elements. We find that mutants of phosphotransfer proteins and type‐B response regulators exhibit a defect in their ethylene growth recovery response similar to that found with the loss‐of‐function ethylene receptor mutant etr1‐7. The ability of two‐component signaling elements to regulate the growth recovery response to ethylene functions independently from their well‐characterized role in cytokinin signaling, based on the analysis of cytokinin receptor mutants as well as following chemical inhibition of cytokinin biosynthesis. Histidine kinase activity of the receptor ETR1 also facilitates growth recovery in the ethylene hypersensitive response, which is characterized by a transient decrease in growth rate when seedlings are treated continuously with a low dose of ethylene; however, this response was found to operate independently of the type‐B response regulators. These results indicate that histidine kinase activity of the ethylene receptor ETR1 performs two independent functions: (a) regulating the growth recovery to ethylene through a two‐component signaling system involving phosphotransfer proteins and type‐B response regulators and (b) regulating the hypersensitive response to ethylene in a type‐B response regulator independent manner.
Keywords: Arabidopsis, ethylene, histidine kinase, phosphotransfer protein, receptor, response regulator
1. INTRODUCTION
Two‐component signaling systems involve histidine kinases, response regulators, and sometimes histidine‐containing phosphotransfer proteins (Mizuno, 2005; Schaller, Shiu, & Armitage, 2011). Two‐component signaling systems are found in bacteria, archaea, fungi, slime molds, and plants (Mizuno, 2005; Schaller et al., 2011). The two‐component signaling system is so named because, in its simplest form, it incorporates a receptor histidine kinase and a response regulator (Gao & Stock, 2009; Stock, Robinson, & Goudreau, 2000). The receptor histidine kinase autophosphorylates on a conserved histidine residue in response to an environmental stimulus, and the phosphate is then transferred to a conserved aspartic acid residue within the receiver domain of a response regulator. Response regulators frequently serve as transcription factors, with phosphorylation modulating their ability to control gene expression. Plants make use of a permutation of the two‐component system known as the multistep phosphorelay (Schaller, Kieber, & Shiu, 2008). As found in plants, the multistep phosphorelay typically incorporates three components: (a) a hybrid receptor kinase that contains both histidine kinase and receiver domains in one protein, (b) a histidine‐containing phosphotransfer protein, and (c) a separate response regulator. In Arabidopsis, these are referred to as ARABIDOPSIS HISTIDINE KINASEs (AHKs), ARABIDOPSIS HISTIDINE‐CONTAINING PHOSPHOTRANSMITTERs (AHPs), and ARABIDOPSIS RESPONSE REGULATORs (ARRs). The two‐component signaling system has an established role in mediating cytokinin signal transduction in plants (Gruhn & Heyl, 2013; Hwang, Sheen, & Müller, 2012; Kieber & Schaller, 2014; To & Kieber, 2008; Werner & Schmülling, 2009), but a potential role in mediating ethylene signal transduction is still unclear despite the fact that several ethylene receptors have histidine kinase activity (Gamble, Coonfield, & Schaller, 1998; Moussatche & Klee, 2004).
The ethylene receptor family of plants is related to histidine kinases, containing sensor domains near their N‐termini and histidine kinase‐like domains in the C‐terminal halves. In Arabidopsis, the ethylene receptor family consists of five members that divide into two subfamilies based on phylogenetic analysis and some shared structural features. Subfamily 1 is composed of ETHYLENE RESPONSE1 (ETR1) and ETHYLENE RESPONSE SENSOR1 (ERS1), and subfamily 2 is composed of ETR2, ERS2, and ETHYLENE INSENSITIVE4 (EIN4) (Chang & Stadler, 2001; Chen, Etheridge, & Schaller, 2005; Schaller & Kieber, 2002). The subfamily‐1 receptors have canonical histidine kinase domains and exhibit histidine kinase activity based on in vitro analysis (Gamble et al., 1998; Moussatche & Klee, 2004), whereas the subfamily‐2 receptors contain diverged histidine kinase‐like domains and exhibit serine/threonine kinase activity based on in vitro analysis (Chen et al., 2009; Moussatche & Klee, 2004). Three of the five receptors are hybrid receptors (ETR1, ETR2, and EIN4) that contain receiver domains with all the conserved residues required for functioning in a multistep phosphorelay. In spite of these conserved features, no substantive role has been identified for the multistep phosphorelay in ethylene signaling (Binder, O'Malley, et al., 2004; Hall et al., 2012; Hass et al., 2004; Mason et al., 2005; Qu & Schaller, 2004). Instead, genetic analysis indicates that the primary elements functioning downstream of the ethylene receptors are the Raf‐like kinase CONSTITUTIVE TRIPLE RESPONSE1 (CTR1), the transmembrane protein EIN2, and the EIN3 family of transcription factors (Alonso, Hirayama, Roman, Nourizadeh, & Ecker, 1999; Chao et al., 1997; Huang, Li, Hutchison, Laskey, & Kieber, 2003; Ju et al., 2012; Kieber, Rothenberg, Roman, Feldman, & Ecker, 1993; Qiao et al., 2012; Solano, Stepanova, Chao, & Ecker, 1998). However, it has recently been shown that ETR1 and ETR2 also signal independently of CTR1, supporting the possibility for other, noncanonical signaling pathways (Bakshi et al., 2018).
If not involved in the primary signaling pathway, what role does the histidine kinase activity of the ethylene receptors play in signal transduction? Several studies suggest that the enzymatic activity of ETR1 modulates ethylene signal output (Binder, O'Malley, et al., 2004; Hall et al., 2012; Qu & Schaller, 2004; Street et al., 2015). This might seem to imply that a multistep phosphorelay operates downstream of the receptors; however, the histidine kinase and receiver domains of the receptors physically interact with CTR1 and EIN2 and autophosphorylation may also modulate interactions with the established ethylene signaling pathway (Binder, Chang, & Schaller, 2012; Bisson & Groth, 2010; Clark, Larsen, Wang, & Chang, 1998; Gao et al., 2003). It is thus necessary to specifically evaluate the role of two‐component signaling elements in ethylene‐mediated responses to resolve their level of contribution.
One situation in which histidine kinase activity of the ethylene receptors appears to play a modulating role is in the ability of seedlings to recover normal growth following cessation of ethylene treatment (Binder, O'Malley, et al., 2004). Treatment of wild‐type etiolated seedlings with ethylene inhibits their growth rate; upon removal of ethylene, the seedlings return to their basal growth rate within two hours. Loss‐of‐function receptor mutants for the family members with receiver domains (ETR1, ETR2, and EIN4) all result in a slower recovery to normal growth rate following removal of ethylene, the slow growth recovery phenotype being particularly strong in the etr1‐7 mutant. In addition, the double mutant etr1‐7;ers1‐2 is delayed in its ability to recover normal growth rate, and this can be rescued by introducing a wild‐type version of ETR1 but not by a kinase‐inactive version of ETR1 (Binder, O'Malley, et al., 2004).
These results suggest that histidine kinase activity and, by extension, the multistep phosphorelay may mediate the ability of seedlings to recover normal growth following cessation of ethylene treatment. We tested this hypothesis by performing a kinetic analysis of the ethylene growth response and recovery in mutants of Arabidopsis two‐component signaling elements. Results from this study indicate that histidine kinase activity of the ethylene receptor ETR1 performs two independent functions. First, it regulates the growth recovery to ethylene through a two‐component signaling system involving AHPs and type‐B ARRs. Second, it also plays a role in regulating growth recovery during a “hypersensitive” response to ethylene, which is characterized by a transient decrease in growth rate when seedlings are treated with a continuous very low dose of ethylene, but does so in a type‐B ARR‐independent manner. The potential mechanisms underlying these differences in histidine kinase‐mediated regulation are discussed.
2. MATERIALS AND METHODS
2.1. Plant materials
Loss‐of‐function mutants analyzed were the etr1‐7 ethylene receptor mutant (Hua & Meyerowitz, 1998), AHK double mutants constructed from published T‐DNA insertion lines (Argueso et al., 2012), AHP mutants ahp5‐2, ahp2 ahp3, and ahp2 ahp3 ahp5‐2 (Hutchison et al., 2006), type‐B ARR mutants arr2‐4, arr10‐2 arr12‐1, and arr2‐2 arr10‐2 arr12‐1 (Mason et al., 2005), and are all of the Arabidopsis ecotype Col‐0. Construction of the transgenic etr1‐7 ers1‐2 lines containing wild‐type ETR1 (gETR1) or kinase‐inactivated ETR1 (getr1‐HGG) was as described (Wang, Hall, O'Malley, & Bleecker, 2003).
2.2. Kinetic analysis of hypocotyl growth rate
Measurements were performed using two‐day‐old etiolated Arabidopsis seedlings as described previously (Binder, O'Malley, et al., 2004). To examine the growth response and recovery kinetics to ethylene, 10 μl/L ethylene was introduced 1 hr after measurements were initiated and then removed 2 hr later. To examine the hypersensitive growth response to ethylene, a concentration of 8.7 nl/L ethylene was used. All data represent the mean of at least three seedlings for the recovery analysis and of at least four seedlings for the hypersensitive response analysis. The Kolmogorov–Smirnov test, performed with JMP 10.0, was used to assess the significance of differences in growth curves. For kinetic recovery analysis, the 3‐5 hr time points were compared for significance; for the hypersensitive response, the 1.5‐3.5 hr time points were compared for significance.
2.3. Hypocotyl growth response to cytokinin
Dose–response for the effect of the cytokinin trans‐zeatin on hypocotyl elongation of 4‐d‐old dark‐grown seedlings was performed as described (Argyros et al., 2008). Seedlings were grown on vertical plates on MS media with 1% (w/v) sucrose, along with the indicated concentrations of cytokinin, scanned, and lengths measured using ImageJ software. All data represent the mean of at least nine seedlings.
2.4. Accession numbers
Sequence data from this article can be found in the EMBL/GenBank data libraries under accession number(s): AHK2 (AT5G35750), AHK3 (AT1G27320), AHK4 (AT2G01830), AHP2 (AT3G29350), AHP3 (AT5G39340), AHP5 (AT1G03430), ARR2 (AT4G16110), ARR10 (AT4G31920), ARR12 (AT2G25180), ERS1 (AT2G40940), ETR1 (AT1G66340).
3. RESULTS
3.1. Two‐component signaling elements regulate the growth recovery to ethylene
We examined short‐term changes in seedling growth response and recovery to ethylene using two‐day‐old etiolated seedlings. Under these growth conditions, wild‐type seedlings exhibit a growth rate of approximately 0.4 mm/hr in the absence of ethylene (Figure 1). Treatment with 10 μl/L exogenous ethylene results in a rapid decrease in the growth rate, initiated within 15 min of the treatment and reaching a new steady‐state growth rate approximately 75 min after ethylene addition. As has been observed previously (Binder, Mortimore, Stepanova, Ecker, & Bleecker, 2004; Binder, O'Malley, et al., 2004), the growth response has two kinetic phases: a rapid inhibition response lasting for approximately 15 min, followed by a slower inhibition response that lasts for approximately 60 min. Removal of ethylene results in a recovery of seedling growth rate, with the initial growth rate being attained approximately 90 min after ethylene removal.
Figure 1.
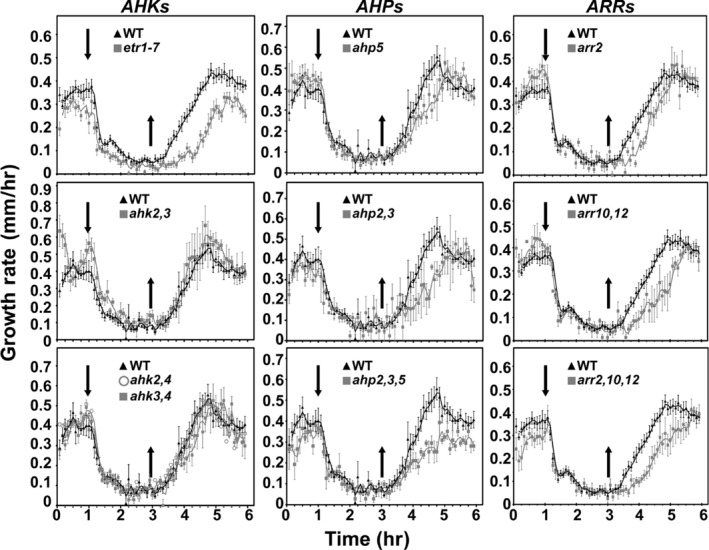
Growth kinetics of two‐day‐old etiolated Arabidopsis hypocotyls containing mutations in two‐component signaling elements. Short‐term growth kinetic analysis in response to 10 μl/L ethylene was performed on mutants involving receptors (column 1), AHPs (column 2), and type‐B ARRs (column 3) and compared to the wild type. Ethylene was introduced one hour after measurements were initiated (down arrow) and then removed two hours later (up arrow). For receptor mutants, we examined the etr1‐7 mutant as well as ahk double mutants. For ahp mutants, we examined ahp5‐2, ahp2 ahp3, and ahp2 ahp3 ahp5‐2. For type‐B arr mutants, we examined arr2‐4, arr10‐2 arr12‐1, and arr2‐2 arr10‐2 arr12‐1. Error bars indicate SE (n ≥ 3)
Loss‐of‐function mutations in the ethylene receptors ETR1, ETR2, or EIN4 affect these growth recovery kinetics to ethylene (Binder, O'Malley, et al., 2004). As shown in Figure 1, the loss‐of‐function etr1‐7 mutant exhibits a normal growth response to ethylene, but is significantly delayed in its recovery kinetics compared to wild type (p < 0.0001), taking approximately 30 min longer than wild type to attain its initial growth rate. We examined loss‐of‐function mutants involving the histidine kinase‐linked cytokinin receptors AHK2, AHK3, and AHK4 to determine whether the slow growth recovery phenotype was unique to ethylene receptor mutants (Figure 1). Because there is functional redundancy in the cytokinin receptor family, we examined the double mutants ahk2 ahk3, ahk2 ahk4, and ahk3 ahk4, all of which have demonstrated effects on shoot and root growth (Argueso et al., 2012; Higuchi et al., 2004; Nishimura et al., 2004; Riefler, Novak, Strnad, & Schmülling, 2006). All three ahk double mutant combinations exhibited ethylene growth response and recovery kinetics similar to those observed in wild type (Figure 1), indicating that ethylene receptors play a role in mediating these growth responses that does not require activity of the cytokinin receptors.
Arabidopsis contains five genes encoding phosphotransfer proteins (AHP1, AHP2, AHP3, AHP4, and AHP5) that are predicted to contain the conserved histidine for phosphorylation (Schaller et al., 2008). Single loss‐of‐function mutants do not display significant effects on cytokinin signaling, but higher order mutants involving ahp1, ahp2, ahp3, and ahp5 result in decreased cytokinin sensitivity (Hutchison et al., 2006). We analyzed mutants involving ahp2, ahp3, and ahp5 for their ethylene growth response and recovery kinetics (Figure 1). All the mutants exhibited an ethylene response similar to wild type, but the ahp5, ahp2 ahp3, and ahp2 ahp3 ahp5 mutants exhibited a significantly slower growth recovery phenotype (p < 0.0001).
Arabidopsis contains 11 type‐B response regulators (type‐B ARRs), which serve as transcription factors to mediate the final step in the multistep phosphorelay (Schaller et al., 2008). ARR1, ARR2, ARR10, and ARR12 are broadly expressed and based on genetic analysis contribute the most to cytokinin signal transduction (Argyros et al., 2008; Ishida, Yamashino, Yokoyama, & Mizuno, 2008; Mason, Li, Mathews, Kieber, & Schaller, 2004; Mason et al., 2005). ARR2 has been implicated in ethylene signaling (Hass et al., 2004), and we therefore focused our analysis on arr2 mutants (Figure 1). As with the other mutants examined, all the type‐B arr mutants exhibited an ethylene response similar to wild type, but the arr2, arr10 arr12, and arr2 arr10 arr12 mutants exhibited a significantly slower growth recovery phenotype (p < 0.0001).
3.2. Two‐component signaling elements regulate ethylene growth recovery independently from their role in cytokinin signaling
We performed several controls to confirm that the two‐component signaling elements regulate the growth recovery to ethylene independently from their role in cytokinin signaling. First, as described earlier, double mutants of the cytokinin receptors do not exhibit the same ethylene recovery phenotype as mutants involving the downstream AHPs and ARRs, which is not what would be predicted if cytokinin played a role in the ethylene response (Figure 1). We note that we could not test the ahk triple mutant because it is infertile and needs to be propagated in a segregating population, and the homozygous triple mutant cannot be differentiated phenotypically from other genotypes in the etiolated seedlings used for the kinetic analysis. To confirm the lack of correlation between the effects of the mutants on the cytokinin and ethylene responses, we specifically examined how mutations in the cytokinin receptors (AHKs), AHPs, and type‐B ARRs affect the hypocotyl growth response to cytokinin (Figure 2), doing so because our kinetic results are based on a hypocotyl growth response to ethylene. We observed that the ahk double mutants ahk2 ahk4 and ahk3 ahk4 were less responsive to cytokinin than any of the ahp and type‐B arr mutant combinations we tested, even though the ahp and arr mutants exhibit a stronger effect on ethylene growth recovery kinetics.
Figure 2.

Hypocotyl growth response of two‐component mutants to cytokinin. Dose–response for the effect of the cytokinin t‐zeatin on hypocotyl elongation of four‐day‐old etiolated seedlings, performed as described (Argyros et al., 2008). The hypocotyl length of each line in the presence of cytokinin is expressed as a percentage of the control. Error bars indicate SE (n ≥ 9); error bars not shown if smaller than symbol
As an alternative approach to assess a potential role for cytokinin in the ethylene growth response, we performed kinetic analysis in the presence of the cytokinin biosynthesis inhibitor lovastatin (Crowell & Salaz, 1992). Lovastatin is an inhibitor of the cytosolic pathway for isoprenoid biosynthesis, one of the two pathways that generate the isopentenyl groups used in the biosynthesis of cytokinins. Treatment of four‐day‐old dark‐grown seedlings with 1 μM lovastatin inhibited hypocotyl growth, reducing hypocotyl length from 9.87 mm (±0.15 SE) for the untreated control to 7.10 mm (±0.07 SE) for the lovastatin‐treated seedlings (i.e., a 28% reduction in growth). When examined by kinetic analysis, the control seedlings exhibiting a growth rate of 0.33 mm/hr (± 0.01 SE) compared to 0.24 mm/hr (± 0.01 SE) for the lovastatin‐treated seedlings (i.e., a 23% reduction in growth rate). This lovastatin‐induced reduction in hypocotyl growth is consistent with that previously observed (Nagata, Suzuki, Yoshida, & Muranaka, 2002), is indicative of the efficacy of the inhibitor, and also still allowed sufficient growth to perform kinetic analysis (Figure 3). We found that the ethylene growth response and recovery kinetics are not affected by lovastatin, consistent with these growth responses being independent of cytokinin (Figure 3). Thus, the effects we observe for the mutants and lovastatin on the ethylene response do not correlate with their effects on cytokinin signaling responses.
Figure 3.

Lovastatin does not affect growth kinetics of two‐day‐old etiolated Arabidopsis hypocotyls. Short‐term growth kinetic analysis in response to 10 μl/L ethylene was performed on wild‐type seedlings grown in the absence (DMSO control) or the presence of 1 μM lovastatin. Data were normalized to growth rate in air prior to treatment with ethylene to facilitate the comparison. Unnormalized growth rates in air were 0.33 ± 0.01 mm/hr for the control and 0.24 ± 0.01 mm/hr for the lovastatin‐treated seedlings. Error bars indicate SE (n ≥ 3)
3.3. Histidine kinase activity of ETR1 regulates the hypersensitive response to ethylene in a type‐B ARR‐independent manner
The first phase for ethylene growth inhibition, characterized by a rapid decrease in seedling growth, can be differentiated from the second slower response phase based on its hypersensitivity to ethylene (Binder, Mortimore, et al., 2004). As shown in Figure 4, wild‐type seedlings treated with 8.7 nl/L ethylene (an approximately 1000‐fold lower dose than that used in Figure 1) exhibit a rapid but transient decrease in their growth rate, recovering to their pretreatment growth rate in approximately 2 hr, even though the seedlings are still in the presence of ethylene. We examined the receptor mutant etr1‐7 to determine whether ETR1 plays a role in the hypersensitive ethylene response. The etr1‐7 mutant responded similarly to wild type, but its reduced growth rate was significantly prolonged compared to wild type (p < 0.0001), requiring approximately 1 hr longer to return to the initial growth rate (Figure 4). To determine whether type‐B ARR transcription factors might play a role in the hypersensitive response, we examined the ethylene response of arr2 arr10 arr12 but observed similar growth kinetics to wild type (Figure 4). To determine whether the histidine kinase activity of ETR1 plays a role in the hypersensitive response, we made use of the subfamily‐1 loss‐of‐function mutant etr1‐7 ers1‐2 transformed with wild‐type ETR1 (gETR1) or a kinase‐inactive ETR1 (getr1‐HGG) (Binder, O'Malley, et al., 2004). The gETR1 line exhibited a hypersensitive response similar to wild type, but the getr1‐HGG line exhibited a significantly prolonged hypersensitive response (p < 0.0001), suggesting that histidine kinase activity plays a role in the response.
Figure 4.
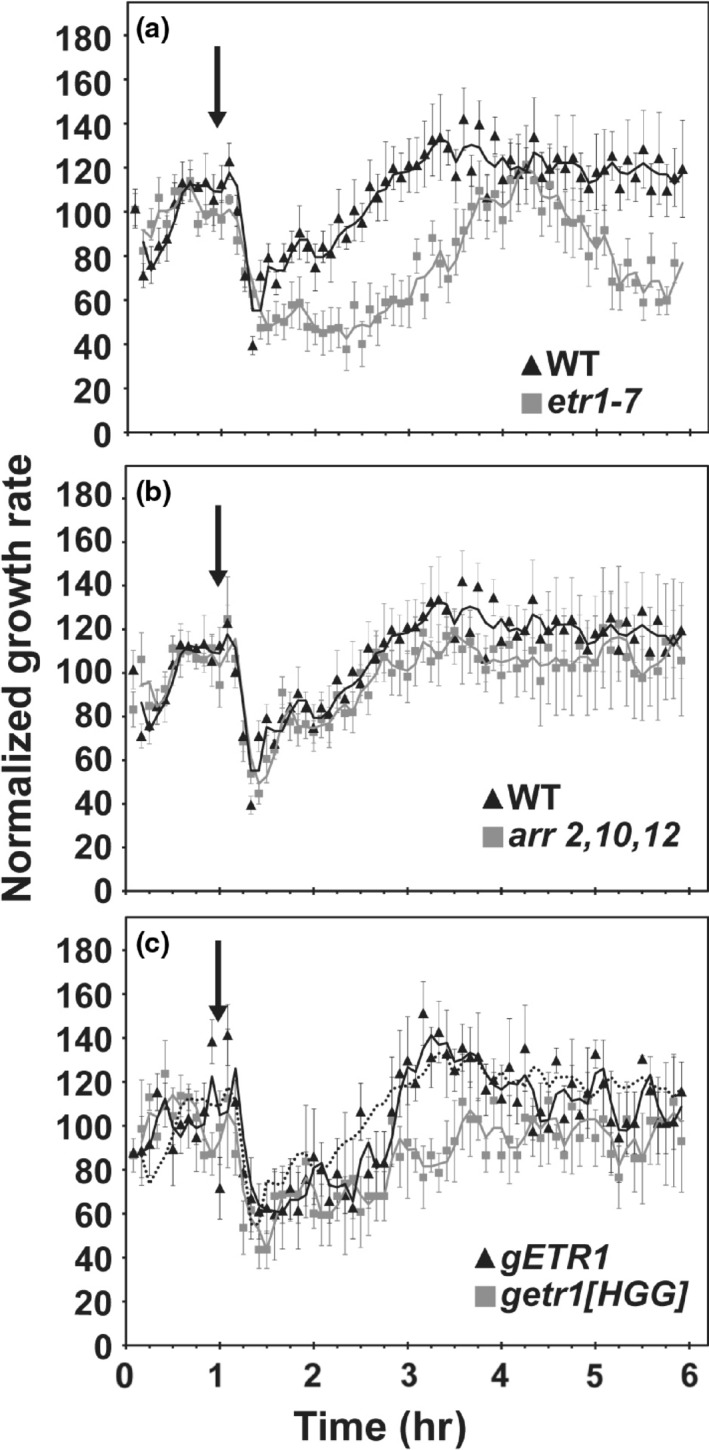
Kinetic analysis of the hypersensitive response to ethylene. Growth kinetic analysis was performed with 8.7 nl/L ethylene introduced 1 hr after measurements were initiated (down arrow). The hypersensitive response of etr1‐7 (a), arr2‐2 arr10‐2 arr12‐1 (b), and transgenic etr1‐7 ers1‐2 lines containing wild‐type ETR1 (gETR1), or kinase‐inactive ETR1 (getr1‐HGG) (c) are all compared to wild type. The dotted line in panel C shows data for wild type for comparison. Data were normalized to growth rate in air prior to treatment with ethylene to facilitate comparisons. Error bars indicate SE (n ≥ 4)
4. DISCUSSION
These results indicate that histidine kinase activity of the ethylene receptor ETR1 performs two independent functions: (a) regulating the growth recovery to ethylene through a two‐component signaling system involving AHPs and type‐B ARRs and (b) regulating the hypersensitive response to ethylene in a type‐B ARR‐independent manner. Several points can be made about the role of the two‐component system in ethylene signaling. First, loss‐of‐function mutants in ethylene receptors ETR1, ETR2, and EIN4 (Binder, O'Malley, et al., 2004), the AHPs, and the type‐B ARRs, although having no effect on the initial ethylene response, all affect ethylene growth recovery kinetics in a similar manner, consistent with their functioning in the same regulatory pathway (Figure 5). The 45‐min delay in recovery time observed in some of the mutants is substantive when one considers that growth recovery usually occurs in less than 2 hr, and indicates that the two‐component signaling pathway plays a significant role in mediating the plant's responsiveness to changes in its phytohormone environment. Second, there is functional overlap within members of each two‐component family for mediating the ethylene recovery response, similar to what has been found in the cytokinin signaling pathway (Schaller et al., 2008; To & Kieber, 2008; Werner & Schmülling, 2009), a finding consistent with physical interactions being detected for multiple AHPs with ETR1 and the type‐B ARRs (Dortay et al., 2008; Scharein, Voet‐van‐Vormizeele, Harter, & Groth, 2008; Urao, Miyata, Yamaguchi‐Shinozaki, & Shinozaki, 2000). Third, given the role of type‐B ARRs as transcription factors, the effect of the two‐component signaling pathway is likely to be on transcriptional output from ethylene signaling, potentially regulating the expression of genes involved in the growth response (Hass et al., 2004). Fourth, the AHPs and type‐B ARRs that function in ethylene signaling also play roles in cytokinin signaling (Kieber & Schaller, 2014; To & Kieber, 2008; Werner & Schmülling, 2009), raising the possibility of cross talk between these phytohormone signaling pathways as well as the question as to whether and how specificity is obtained when utilizing the same signaling elements.
Figure 5.
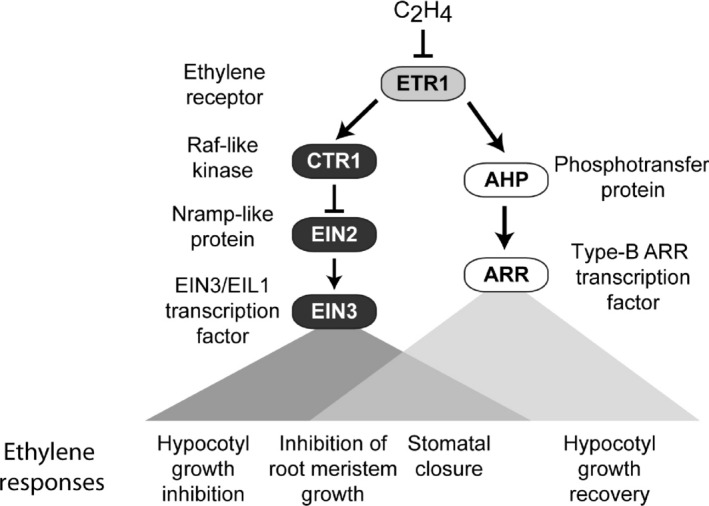
Model for ethylene signal transduction. Signal output from the ethylene receptors diverges to regulate the CTR1/EIN2/EIN3 and AHP/ARR pathways. ETR1 is shown here as a representative ethylene receptor that has histidine kinase activity. Ethylene responses may be dependent on an individual pathway or have varying contributions from both pathways
Our results complement prior studies that have implicated two‐component signaling elements in the regulation of ethylene responses, including effects on hypocotyl growth, stomatal closure, and root meristem development (Figure 5). The type‐B response regulator ARR2 was previously implicated in ethylene responses based on an arr2 mutant exhibiting a hyposensitive ethylene response for the inhibition of hypocotyl elongation, and the ARR2 protein being phosphorylated in an ETR1‐dependent manner and binding to a promoter element for the ethylene‐induced gene ERF1 (Hass et al., 2004). In contrast to this study by Hass et al. (2004), we do not observe an effect of arr2 mutants in the hypocotyl growth response assay, either as a long‐term effect (Mason et al., 2005) or when assessing the short‐term kinetic response as described here in our current study; however, our results do support a role for ARR2 in the hypocotyl based on the ethylene growth recovery kinetics of arr2. Differences observed between the two studies on arr2 mutant phenotypes may relate to growth conditions used for studying the effects of ethylene on hypocotyl growth. In addition to potentially mediating ethylene effects on hypocotyl growth, ARR2 as well as three AHPs (AHP1, AHP2, and AHP3) have been implicated in mediating the stimulatory effects of ethylene on stomatal closure (Desikan et al., 2006; Mira‐Rodado et al., 2012). Furthermore, the type‐B response regulator ARR1, but not ARR2, ARR10, or ARR12, contributes to ethylene‐mediated inhibition of cell proliferation at the primary root meristem; kinase‐inactive versions of ETR1 similarly reduce this ethylene response, consistent with it being mediated through a phosphorelay initiated at the ethylene receptors (Street et al., 2015).
Our study supports the existence of two independent ethylene signaling pathways initiated at the receptors: the well‐documented CTR1/EIN2/EIN3‐dependent pathway as well as an AHP/ARR‐dependent pathway (Figure 5). The degree to which these pathways contribute to various ethylene responses varies depends on the response assayed. For example, both pathways contribute to a similar extent in ethylene‐mediated regulation of stomatal closure, but the CTR1/EIN2/EIN3 pathway is predominant in the regulation of cell proliferation at the root meristem (Desikan et al., 2006; Mira‐Rodado et al., 2012; Street et al., 2015). Although genetic analysis reveals a role for the AHP/ARR pathway in mediating the growth recovery response to ethylene, whether the CTR1/EIN2/EIN3 pathway also plays a role cannot be readily assessed because this pathway mediates the growth inhibition response (i.e., a growth recovery response cannot be assessed unless there is an initial growth inhibition response) (Binder, Mortimore, et al., 2004). As noted above, both pathways contribute to varying extents in the control of different ethylene responses. A potential mechanism that would facilitate such control is combinatorial regulation between the EIN3/EIL and type‐B ARR transcription factors, EIN3 and ARR having each individually been found to coregulate gene expression in conjunction with other transcription factors (Feng et al., 2017; Liu et al., 2017; Marin‐de la Rosa et al., 2015).
Through analysis of the hypersensitive response to ethylene, our results also indicate that the histidine kinase activity of ETR1 may play a transcriptionally independent role in ethylene signaling. Previous genetic analysis demonstrated that the hypersensitive response of Arabidopsis to ethylene is independent of the transcription factors EIN3 and EIL1 (Binder, Mortimore, et al., 2004), our results here demonstrating that this response is also independent of the type‐B ARR transcription factors ARR2, ARR10, and ARR12. Nevertheless, histidine kinase activity of ETR1 appears to play a role in the hypersensitive response, because loss of ETR1 or transformation of Arabidopsis with a kinase‐inactivated ETR1 results in a prolonged response. Because the ethylene receptors are components within larger signaling complexes (Chen et al., 2010), phosphorylation could potentially modulate the interactions and activity of proteins physically associated with ETR1 such as CTR1, EIN2, and/or other ethylene receptors (Clark et al., 1998; Gao et al., 2003, 2008; Grefen et al., 2008). However, the output in such a case would not be through an established transcriptionally based pathway but potentially through a phosphorylation/dephosphorylation cascade, which often mediates rapid eukaryotic signaling responses.
AUTHOR CONTRIBUTIONS
BMB, JJK, and GES conceived and supervised the study. BMB and HJK designed and performed experiments. DEM and CEH generated mutant lines used in the study. BMB, HJK, and GES analyzed data. GES wrote the manuscript with contributions from all other authors.
Supporting information
ACKNOWLEDGMENTS
We thank Tobias Zutz for technical assistance in the kinetic analysis, and Brad Taylor for assistance in the statistical analysis. This work was supported by the NSF grants #IOS‐1456487 to GES, #MCB‐1716279 to BMB, and #IOS‐1022053 to JJK, DEM, and GES, and the Human Frontier Science Program grant #LT000757/2009‐L and Institute for Basic Science # IBS‐R013‐D1 to HJK.
Binder BM, Kim HJ, Mathews DE, Hutchison CE, Kieber JJ, Eric Schaller G. A role for two‐component signaling elements in the Arabidopsis growth recovery response to ethylene. Plant Direct. 2018;2:1–9. 10.1002/pld3.58
Contributor Information
Brad M. Binder, Email: bbinder@utk.edu
G. Eric Schaller, Email: george.e.schaller@dartmouth.edu.
REFERENCES
- Alonso, J. M. , Hirayama, T. , Roman, G. , Nourizadeh, S. , & Ecker, J. R. (1999). EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science, 284, 2148–2152. 10.1126/science.284.5423.2148 [DOI] [PubMed] [Google Scholar]
- Argueso, C. T. , Ferreira, F. J. , Epple, P. , To, J. P. , Hutchison, C. E. , Schaller, G. E. , … Kieber, J. J. (2012). Two‐component elements mediate interactions between cytokinin and salicylic acid in plant immunity. PLoS Genetics, 8, e1002448 10.1371/journal.pgen.1002448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argyros, R. D. , Mathews, D. E. , Chiang, Y.‐H. , Palmer, C. M. , Thibault, D. M. , Etheridge, N. , … Schaller, G. E. (2008). Type B response regulators of Arabidopsis play key roles in cytokinin signaling and plant development. Plant Cell, 20, 2102–2116. 10.1105/tpc.108.059584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakshi, A. , Piya, S. , Fernandez, J. C. , Chervin, C. , Hewezi, T. , & Binder, B. M. (2018). Ethylene receptors signal via a noncanonical pathway to regulate abscisic acid responses. Plant Physiology, 176, 910–929. 10.1104/pp.17.01321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder, B. M. , Chang, C. , & Schaller, G. E. (2012). Perception of ethylene by plants: Ethylene receptors In McManus M. T. (Ed.), Annual plant reviews: The plant hormone ethylene, Vol 44 (pp. 117–145). Hoboken, NJ: Wiley‐Blackwell; 10.1002/9781118223086.ch5 [DOI] [Google Scholar]
- Binder, B. M. , Mortimore, L. A. , Stepanova, A. N. , Ecker, J. R. , & Bleecker, A. B. (2004). Short‐term growth responses to ethylene in Arabidopsis seedlings are EIN3/EIL1 independent. Plant Physiology, 136, 2921–2927. 10.1104/pp.104.050393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder, B. M. , O'Malley, R. C. , Wang, W. , Moore, J. M. , Parks, B. M. , Spalding, E. P. , & Bleecker, A. B. (2004). Arabidopsis seedling growth response and recovery to ethylene. A kinetic analysis. Plant Physiology, 136, 2913–2920. 10.1104/pp.104.050369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisson, M. M. , & Groth, G. (2010). New insight in ethylene signaling: Autokinase activity of ETR1 modulates the interaction of receptors and EIN2. Molecular Plant, 3, 882–889. 10.1093/mp/ssq036 [DOI] [PubMed] [Google Scholar]
- Chang, C. , & Stadler, R. (2001). Ethylene hormone receptor action in Arabidopsis. BioEssays, 23, 619–627. 10.1002/(ISSN)1521-1878 [DOI] [PubMed] [Google Scholar]
- Chao, Q. , Rothenberg, M. , Solano, R. , Roman, G. , Terzaghi, W. , & Ecker, J. R. (1997). Activation of the ethylene gas response pathway in Arabidopsis by the nuclear protein ETHYLENE‐INSENSITIVE3 and related proteins. Cell, 89, 1133–1144. 10.1016/S0092-8674(00)80300-1 [DOI] [PubMed] [Google Scholar]
- Chen, Y. F. , Etheridge, N. , & Schaller, G. E. (2005). Ethylene signal transduction. Annals of Botany, 95, 901–915. 10.1093/aob/mci100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y. F. , Gao, Z. , Kerris, R. J. 3rd , Wang, W. , Binder, B. M. , & Schaller, G. E. (2010). Ethylene receptors function as components of high‐molecular‐mass protein complexes in Arabidopsis. PLoS ONE, 5, e8640 10.1371/journal.pone.0008640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, T. , Liu, J. , Lei, G. , Liu, Y. F. , Li, Z. G. , Tao, J. J. , … Zhang, J. S. (2009). Effects of tobacco ethylene receptor mutations on receptor kinase activity, plant growth and stress responses. Plant and Cell Physiology, 50, 1636–1650. 10.1093/pcp/pcp107 [DOI] [PubMed] [Google Scholar]
- Clark, K. L. , Larsen, P. B. , Wang, X. , & Chang, C. (1998). Association of the Arabidopsis CTR1 Raf‐like kinase with the ETR1 and ERS1 ethylene receptors. Proceedings of the National Academy of Sciences of the United States of America, 95, 5401–5406. 10.1073/pnas.95.9.5401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowell, D. N. , & Salaz, M. S. (1992). Inhibition of growth of cultured tobacco cells at low concentrations of lovastatin is reversed by cytokinin. Plant Physiology, 100, 2090–2095. 10.1104/pp.100.4.2090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan, R. , Last, K. , Harrett‐Williams, R. , Tagliavia, C. , Harter, K. , Hooley, R. , … Neill, S. J. (2006). Ethylene‐induced stomatal closure in Arabidopsis occurs via AtrbohF‐mediated hydrogen peroxide synthesis. The Plant Journal, 47, 907–916. 10.1111/j.1365-313X.2006.02842.x [DOI] [PubMed] [Google Scholar]
- Dortay, H. , Gruhn, N. , Pfeifer, A. , Schwerdtner, M. , Schmülling, T. , & Heyl, A. (2008). Toward an interaction map of the two‐component signaling pathway of Arabidopsis thaliana . Journal of Proteome Research, 7, 3649–3660. 10.1021/pr0703831 [DOI] [PubMed] [Google Scholar]
- Feng, Y. , Xu, P. , Li, B. , Li, P. , Wen, X. , An, F. , … Guo, H. (2017). Ethylene promotes root hair growth through coordinated EIN3/EIL1 and RHD6/RSL1 activity in Arabidopsis. Proceedings of the National Academy of Sciences, 114, 13834–13839. 10.1073/pnas.1711723115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble, R. L. , Coonfield, M. L. , & Schaller, G. E. (1998). Histidine kinase activity of the ETR1 ethylene receptor from Arabidopsis. Proceedings of the National Academy of Sciences of the United States of America, 95, 7825–7829. 10.1073/pnas.95.13.7825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, Z. , Chen, Y. F. , Randlett, M. D. , Zhao, X. C. , Findell, J. L. , Kieber, J. J. , & Schaller, G. E. (2003). Localization of the Raf‐like kinase CTR1 to the endoplasmic reticulum of Arabidopsis through participation in ethylene receptor signaling complexes. Journal of Biological Chemistry, 278, 34725–34732. 10.1074/jbc.M305548200 [DOI] [PubMed] [Google Scholar]
- Gao, R. , & Stock, A. M. (2009). Biological insights from structures of two‐component proteins. Annual Review of Microbiology, 63, 133–154. 10.1146/annurev.micro.091208.073214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, Z. , Wen, C.‐K. , Binder, B. M. , Chen, Y.‐F. , Chang, J. , Chiang, Y.‐H. , … Schaller, G. E. (2008). Heteromeric interactions among ethylene receptors mediate signaling in Arabidopsis. Journal of Biological Chemistry, 283, 23081–23810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grefen, C. , Städele, K. , Růžička, K. , Obrdlik, P. , Harter, K. , & Horák, J. (2008). Subcellular localization and in vivo interaction of the Arabidopsis thaliana ethylene receptor family members. Molecular Plant, 1, 308–320. 10.1093/mp/ssm015 [DOI] [PubMed] [Google Scholar]
- Gruhn, N. , & Heyl, A. (2013). Updates on the model and the evolution of cytokinin signaling. Current Opinion in Plant Biology, 16, 569–574. 10.1016/j.pbi.2013.09.001 [DOI] [PubMed] [Google Scholar]
- Hall, B. P. , Shakeel, S. N. , Amir, M. , Haq, N. U. , Qu, X. , & Schaller, G. E. (2012). Histidine kinase activity of the ethylene receptor ETR1 facilitates the ethylene response in Arabidopsis. Plant Physiology, 159, 682–695. 10.1104/pp.112.196790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hass, C. , Lohrmann, J. , Albrecht, V. , Sweere, U. , Hummel, F. , Yoo, S. D. , … Harter, K. (2004). The response regulator 2 mediates ethylene signalling and hormone signal integration in Arabidopsis. EMBO Journal, 23, 3290–3302. 10.1038/sj.emboj.7600337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi, M. , Pischke, M. S. , Mahonen, A. P. , Miyawaki, K. , Hashimoto, Y. , Seki, M. , … Kakimoto, T. (2004). In planta functions of the Arabidopsis cytokinin receptor family. Proceedings of the National Academy of Sciences, 101, 8821–8826. 10.1073/pnas.0402887101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua, J. , & Meyerowitz, E. M. (1998). Ethylene responses are negatively regulated by a receptor gene family in Arabidopsis thaliana . Cell, 94, 261–271. 10.1016/S0092-8674(00)81425-7 [DOI] [PubMed] [Google Scholar]
- Huang, Y. , Li, H. , Hutchison, C. E. , Laskey, J. , & Kieber, J. J. (2003). Biochemical and functional analysis of CTR1, a protein kinase that negatively regulates ethylene signaling in Arabidopsis. The Plant Journal, 33, 221–233. 10.1046/j.1365-313X.2003.01620.x [DOI] [PubMed] [Google Scholar]
- Hutchison, C. E. , Li, J. , Argueso, C. , Gonzalez, M. , Lee, E. , Lewis, M. W. , … Kieber, J. J. (2006). The Arabidopsis histidine phosphotransfer proteins are redundant positive regulators of cytokinin signaling. Plant Cell, 18, 3073–3087. 10.1105/tpc.106.045674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang, I. , Sheen, J. , & Müller, B. (2012). Cytokinin signaling networks. Annual Review of Plant Biology, 63, 353–380. 10.1146/annurev-arplant-042811-105503 [DOI] [PubMed] [Google Scholar]
- Ishida, K. , Yamashino, T. , Yokoyama, A. , & Mizuno, T. (2008). Three type‐B response regulators, ARR1, ARR10, and ARR12, play essential but redundant roles in cytokinin signal transduction throughout the life cycle of Arabidopsis thaliana . Plant and Cell Physiology, 49, 47–57. 10.1093/pcp/pcm165 [DOI] [PubMed] [Google Scholar]
- Ju, C. , Yoon, G. M. , Shemansky, J. M. , Lin, D. Y. , Ying, Z. I. , Chang, J. , … Chang, C. (2012). CTR1 phosphorylates the central regulator EIN2 to control ethylene hormone signaling from the ER membrane to the nucleus in Arabidopsis. Proceedings of the National Academy of Sciences, 109, 19486–19491. 10.1073/pnas.1214848109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieber, J. J. , Rothenberg, M. , Roman, G. , Feldman, K. A. , & Ecker, J. R. (1993). CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the Raf family of protein kinases. Cell, 72, 427–441. 10.1016/0092-8674(93)90119-B [DOI] [PubMed] [Google Scholar]
- Kieber, J. J. , & Schaller, G. E. (2014). Cytokinins. The Arabidopsis Book, 12, e0168 10.1199/tab.0168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X. , Liu, R. , Li, Y. , Shen, X. , Zhong, S. , & Shi, H. (2017). EIN3 and PIF3 Form an Interdependent Module That Represses Chloroplast Development in Buried Seedlings. Plant Cell, 29, 3051–3067. 10.1105/tpc.17.00508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin‐de la Rosa, N. , Pfeiffer, A. , Hill, K. , Locascio, A. , Bhalerao, R. P. , Miskolczi, P. , … Alabadi, D. (2015). Genome wide binding site analysis reveals transcriptional coactivation of cytokinin‐responsive genes by DELLA proteins. PLoS Genetics, 11, e1005337 10.1371/journal.pgen.1005337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason, M. G. , Li, J. , Mathews, D. E. , Kieber, J. J. , & Schaller, G. E. (2004). Type‐B response regulators display overlapping expression patterns in Arabidopsis. Plant Physiology, 135, 927–937. 10.1104/pp.103.038109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason, M. G. , Mathews, D. E. , Argyros, D. A. , Maxwell, B. B. , Kieber, J. J. , Alonso, J. M. , … Schaller, G. E. (2005). Multiple type‐B response regulators mediate cytokinin signal transduction in Arabidopsis. Plant Cell, 17, 3007–3018. 10.1105/tpc.105.035451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mira‐Rodado, V. , Veerabagu, M. , Witthoft, J. , Teply, J. , Harter, K. , & Desikan, R. (2012). Identification of two‐component system elements downstream of AHK5 in the stomatal closure response of Arabidopsis thaliana. Plant Signaling & Behavior, 7, 1467–1476. 10.4161/psb.21898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno, T. (2005). Two‐component phosphorelay signal transduction systems in plants: From hormone responses to circadian rhythms. Bioscience, Biotechnology, and Biochemistry, 69, 2263–2276. 10.1271/bbb.69.2263 [DOI] [PubMed] [Google Scholar]
- Moussatche, P. , & Klee, H. J. (2004). Autophosphorylation activity of the Arabidopsis ethylene receptor multigene family. Journal of Biological Chemistry, 279, 48734–48741. 10.1074/jbc.M403100200 [DOI] [PubMed] [Google Scholar]
- Nagata, N. , Suzuki, M. , Yoshida, S. , & Muranaka, T. (2002). Mevalonic acid partially restores chloroplast and etioplast development in Arabidopsis lacking the non‐mevalonate pathway. Planta, 216, 345–350. 10.1007/s00425-002-0871-9 [DOI] [PubMed] [Google Scholar]
- Nishimura, C. , Ohashi, Y. , Sato, S. , Kato, T. , Tabata, S. , & Ueguchi, C. (2004). Histidine kinase homologs that act as cytokinin receptors possess overlapping functions in the regulation of shoot and root growth in Arabidopsis. Plant Cell, 16, 1365–1377. 10.1105/tpc.021477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao, H. , Shen, Z. , Huang, S. S. , Schmitz, R. J. , Urich, M. A. , Briggs, S. P. , & Ecker, J. R. (2012). Processing and subcellular trafficking of ER‐tethered EIN2 control response to ethylene gas. Science, 338, 390–393. 10.1126/science.1225974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu, X. , & Schaller, G. E. (2004). Requirement of the histidine kinase domain for signal transduction by the ethylene receptor ETR1. Plant Physiology, 136, 2961–2970. 10.1104/pp.104.047126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riefler, M. , Novak, O. , Strnad, M. , & Schmülling, T. (2006). Arabidopsis cytokinin receptor mutants reveal functions in shoot growth, leaf senescence, seed size, germination, root development, and cytokinin metabolism. Plant Cell, 18, 40–54. 10.1105/tpc.105.037796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller, G. E. , & Kieber, J. J. (2002). Ethylene In Somerville C., & Meyerowitz E. (Eds.), The Arabidopsis Book, Vol. 1 (p. e0071). Rockville, MD: American Society of Plant Biologists. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller, G. E. , Kieber, J. J. , & Shiu, S.‐H. (2008). Two‐component signaling elements and histidyl‐aspartyl phosphorelays. The Arabidopsis Book(C. Somerville, E. Meyerowitz, editors), 6, e0112 10.1199/tab.0112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller, G. E. , Shiu, S. H. , & Armitage, J. P. (2011). Two‐component systems and their co‐option for eukaryotic signal transduction. Current Biology, 21, R320–R330. 10.1016/j.cub.2011.02.045 [DOI] [PubMed] [Google Scholar]
- Scharein, B. , Voet‐van‐Vormizeele, J. , Harter, K. , & Groth, G. (2008). Ethylene signaling: Identification of a putative ETR1‐AHP1 phosphorelay complex by fluorescence spectroscopy. Analytical Biochemistry, 377, 72–76. 10.1016/j.ab.2008.03.015 [DOI] [PubMed] [Google Scholar]
- Solano, R. , Stepanova, A. , Chao, Q. , & Ecker, J. R. (1998). Nuclear events in ethylene signaling: A transcriptional cascade mediated by ETHYLENE‐INSENSITIVE3 and ETHYLENE‐RESPONSE‐FACTOR1. Genes & Development, 12, 3703–3714. 10.1101/gad.12.23.3703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock, A. M. , Robinson, V. L. , & Goudreau, P. N. (2000). Two‐component signal transduction. Annual Review of Biochemistry, 69, 183–215. 10.1146/annurev.biochem.69.1.183 [DOI] [PubMed] [Google Scholar]
- Street, I. H. , Aman, S. , Zubo, Y. , Ramzan, A. , Wang, X. , Shakeel, S. N. , … Schaller, G. E. (2015). Ethylene inhibits cell proliferation of the Arabidopsis root meristem. Plant Physiology, 169, 338–350. 10.1104/pp.15.00415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- To, J. P. C. , & Kieber, J. J. (2008). Cytokinin signaling: Two‐components and more. Trends in Plant Science, 13, 85–92. 10.1016/j.tplants.2007.11.005 [DOI] [PubMed] [Google Scholar]
- Urao, T. , Miyata, S. , Yamaguchi‐Shinozaki, K. , & Shinozaki, K. (2000). Possible His to Asp phosphorelay signaling in an Arabidopsis two‐component system. FEBS Letters, 478, 227–232. 10.1016/S0014-5793(00)01860-3 [DOI] [PubMed] [Google Scholar]
- Wang, W. , Hall, A. E. , O'Malley, R. , & Bleecker, A. B. (2003). Canonical histidine kinase activity of the transmitter domain of the ETR1 ethylene receptor from Arabidopsis is not required for signal transmission. Proceedings of the National Academy of Sciences, 100, 352–357. 10.1073/pnas.0237085100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner, T. , & Schmülling, T. (2009). Cytokinin action in plant development. Current Opinion in Plant Biology, 12, 527–538. 10.1016/j.pbi.2009.07.002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


