Abstract
Nicotine is naturally synthesized in tobacco roots and accumulates in leaves as a defense compound against herbivory attack. Nicotine biosynthesis pathway has been extensively studied with major genes and enzymes being isolated and functionally characterized. However, the molecular regulation of nicotine synthesis has not been fully understood. The phytohormone jasmonic acid (JA) mediates many aspects of plant defense responses including nicotine biosynthesis. In this study, five key genes (AtLOX2, AtAOS, AtAOC2, AtOPR3, AtJAR1) involved in JA biosynthesis from Arabidopsis were individually overexpressed, and a JA‐Ile hydrolysis‐related gene, NtJIH1, was suppressed by RNAi approach, to understand their effects on nicotine accumulation in tobacco. Interestingly, while transgene expression was high, levels of JA‐Ile (the biologically active form of JA) were often significantly reduced. Meanwhile, nicotine content in these transgenic plants did not increase. The research revealed a tightly controlled JA signaling pathway and a complicated regulatory network for nicotine biosynthesis by JA signaling.
Keywords: jasmonic acid biosynthesis, jasmonic acid signaling, nicotine synthesis regulation, tobacco, transgene
1. INTRODUCTION
Tobacco (Nicotiana tabacum L.) is an important nonfood crop widely grown across the world due to its great economic value brought by the widespread usage of tobacco products (Davis & Nielsen, 1999). Nicotine is specifically synthesized in tobacco roots and accumulated in leaves as a defensive compound against herbivores because it causes a continual excitation of neurons and even paralysis or death of insects (Baldwin, Halitschke, Kessler, & Schittko, 2001). Thus, nicotine is used as an insecticide in agriculture practice (Davis & Nielsen, 1999). In medical applications, nicotine can be used to make smoking cessation devices (Wang et al., 2015) and is applied to treating Parkinson's disease and alleviating inflammatory bowel syndrome (Polosa, Rodu, Caponnetto, Maglia, & Raciti, 2013; Quik, O'Leary, & Tanner, 2008). Nicotine is a major type of alkaloids in tobacco plants, accounting for 90% of the total alkaloids. The rest 10% are mainly composed of anabasine, anatabine, and nornicotine (Saitoh, Nona, & Kawashima, 1985).
Nicotine is composed of a pyridine ring and a pyrrolidine ring, synthesized from two separate branches as demonstrated in Figure S1. The pyrrolidine ring is originated from arginine or ornithine while the pyridine ring is formed from quinolinic acid. Early studies reported that the putrescine methyltransferase (PMT) and quinolinic acid phosphoribosyltransferase 2 (QPT2) are the rate‐limiting enzymes in the pyrrolidine branch and pyridine branch, respectively, because they had much lower enzyme activities than other enzymes in the two branches (Feth, Wagner, & Wagner, 1986; Saunders & Bush, 1979; Wagner & Wagner, 1985). The isoflavone reductase‐like enzyme A622 and a berberine bridgelike (BBL) enzyme are proposed to be involved in the condensation step of the pyridine and pyrrolidine rings to form nicotine (DeBoer, Lye, Aitken, Su, & Hamill, 2009; Kajikawa, Hirai, & Hashimoto, 2009). The synthesis and accumulation of the major and minor alkaloids is closely related and dynamically regulated (Chintapakorn & Hamill, 2003; Hung et al., 2013; Kajikawa et al., 2009; Lewis et al., 2015).
Previous research indicates that many factors affect nicotine biosynthesis, including mechanical wounding, topping (decapitation of the apical meristem), plant hormones, transcription factors, and negative feedback by pathway products (Baldwin, Schmelz, & Ohnmeiss, 1994; Elliot, 1966; Wasternack & Hause, 2013). Topping and wounding induce nicotine biosynthesis through mediating phytohormones, mainly jasmonate (JA) and auxin. The transcription factor NtMYC2a is a master positive regulator for nicotine biosynthesis (Wang et al., 2015). Overexpression of NtMYC2a in tobacco plants increased nicotine content by approximately 1–1.5‐fold. RNAi‐induced knockdown of NtMYC2 decreased the nicotine level by approximately fivefold (Wang, 2011). The high nicotine phenotype in NtMYC2a overexpression lines was consistent from T0 to T3 generations in field tests (Wang et al., 2015). It was shown that NtMYC2 upregulates nicotine biosynthesis by binding to the cis elements of an NtPMT and the NtQPT2 promoter regions and activating the expression of these two genes (Zhang, Bokowiec, Rushton, Han, & Timko, 2012). Interestingly, previous research in our laboratory showed that overexpression of NtPMT1a and/or NtQPT2 by a strong root‐specific promoter (NtQPT2 gene promoter) did not change the nicotine content in a field‐grown commercial cultivar while the transcripts of these two genes increased, indicating a possible layer of regulation at post‐transcriptional levels (Wang, 2011). The speculation was recently confirmed by a miRNA–mimicry regulatory system on QPT2 gene expression, and nicotine synthesis and accumulation (Li et al., 2015).
Jasmonic acid treatment induced expression of many genes involved in nicotine biosynthesis pathway and nicotine transportation (Baldwin et al., 1994; Goossens et al., 2003; Shoji & Hashimoto, 2011). Jasmonic acid regulates nicotine biosynthetic gene expression through the MYC2 and the jasmonate ZIM‐domain (JAZ) repressors system. In the absence of JA, the JAZs bind to MYC2 and form a repression complex, blocking MYC2 from activating nicotine biosynthetic genes. In the presence of JA, (+)‐7‐iso‐JA‐Ile forms a complex with COI1 and JAZs, releasing MYC2 transcription factor and activating nicotine biosynthesis (Kazan & Manners, 2013; Pauwels & Goossens, 2011; Wasternack & Hause, 2013). In addition, some ethylene responsive factors (ERFs) also play positive roles in nicotine biosynthesis (De Boer et al., 2011; Shoji & Hashimoto, 2011; Shoji, Kajikawa, & Hashimoto, 2010). Moreover, nicotine accumulation is regulated by a negative feedback loop as well: Nicotine itself is cellular toxic to tobacco root growth and negatively regulates its own biosynthesis (Shoji et al., 2009; Wang et al., 2015). Wang et al. (2015) demonstrated that transcript levels of all major nicotine synthesis genes in tobacco seedlings were reduced by about 50% 2 hr after 0.4 mM nicotine treatment, indicating a negative feedback pathway.
The JA biosynthesis pathway has been well established with major pathway components being functionally characterized, as shown in Figure S2 (Goossens, Fernández‐Calvo, Schweizer, & Goossens, 2016; Wasternack, 2007; Zhang, 2016). JA biosynthesis starts from α‐linolenic acid (C18:3), which is released from glycolipids of chloroplast membrane. The α‐linolenic acid is oxidized by lipoxygenases (LOXs) to form 13 (S)—hydroperoxy‐octadecatrienoic acid (13‐HPOT), which is then converted to 12,13 (S)‐epoxy‐octadecatrienoic acid [12,13 (S)‐EOT] by allene oxide synthase (AOS). The unstable allene oxide of 12,13 (S)‐EOT is immediately cyclized by allene oxide cyclase (AOC) to produce cis‐(+)‐12‐oxophytodienoic acid (OPDA). Thereafter, OPDA is translocated from the chloroplasts into the peroxisomes where the peroxisomal OPDA reductase (OPR) reduces OPDA. The reduction product goes through three rounds of beta‐oxidative side‐chain shortening to yield jasmonoyl‐CoA, which is then converted to (+)‐7‐iso‐JA. (+)‐7‐iso‐JA equilibrates to the more stable (−)‐JA, the predominant form of JA in plants. JA is released into the cytosol where it is metabolized to various derivatives, including JA‐Ile, the most active form of jasmonates. JA‐Ile formation is catalyzed by the JA amino acid synthetase (JAR1; Suza & Staswick, 2008).
Meanwhile, JA signaling could be attenuated by hydrolysis of JA‐Ile by jasmonoyl‐L‐isoleucine hydrolase 1 (JIH1; Woldemariam, Onkokesung, Baldwin, & Galis, 2012) and hydroxylation of JA‐Ile by p450 enzymes CYP94B3 and CYP94C1 (Miersch, Neumerkel, Dippe, Stenzel, & Wasternack, 2008). Although addition of methyl JA (MeJA) to the culture medium of tobacco BY‐2 cells has been shown to enhance alkaloids accumulation in the cells (Goossens et al., 2003), few reports studied effects on alkaloid levels by altering gene expression of JA metabolism pathways. Laudert, Schaller, and Weiler (2000) overexpressed AtAOS in tobacco but did not report its effects on nicotine accumulation. In another report, constitutive overexpression of the Hyoscyamus niger L. AOC gene in tobacco reportedly led to a notable fourfold to eightfold increase in NtPMT transcripts, a slight increase in NtQPT2 transcripts, and a 4.8‐fold increase in nicotine content as compared to WT (wild type, Jiang et al., 2009).
In this study, we intended to gain more insights on JA regulated nicotine synthesis in tobacco by modifying expression of the JA biosynthetic genes. As we do not know which enzyme controls the rate‐limiting step of JA biosynthesis, we investigated all five major genes in the pathway. However, as none of the JA biosynthesis genes were cloned and functionally characterized in tobacco and the gene sequences were not available in the database at the time when we started this project, cDNAs of these genes from Arabidopsis, LOX2, AOS, AOC2, OPR3, and JAR1, were individually introduced into tobacco and constitutively overexpressed under the CaMV 35S promoter. In addition, RNAi‐mediated gene silencing was used to knock down NtJIH1 (Woldemariam et al., 2012), which hydrolyzes JA‐Ile, in an attempt to enhance JA‐Ile level. The transgenic tobacco plants were developed with these individual gene constructs and analyzed.
2. MATERIALS AND METHODS
2.1. Generation of overexpression constructs
Vector pBI121 was used as the vector backbone and digested at BamHI and EcoRI restriction sites. Arabidopsis AOS (AY128733), AOC2 (AY054131), OPR3 (AY097367), and JAR1 (AY15043) cDNAs were acquired from Arabidopsis Biological Resource Center (ABRC, Ohio State University, Columbus, OH) while LOX2 cDNA (L23968) was kindly provided by Dr. Carmen Castresana (Spain National Research Council, Madrid, Spain). The coding sequences of these genes were amplified by HiFi PCR premix (Clontech, Mountain View, CA), and the amplified sequences were verified by sequencing analysis. The amplified genes were inserted into the pBI121 to replace the GUS gene individually by homologous recombination using In‐Fusion HD cloning system (Clontech). Primers used for PCR are listed in Table S1.
2.2. Generation of NtJIH1 RNAi construct
A Gateway binary RNAi vector pB7GWIWG2(II) (http://www.psb.ugent.be/gateway/) was used to generate inverted repeats and to express NtJIH1 dsRNA in tobacco plants (McGinnis, 2010). Two copies of NtJIH1 gene were found in tobacco genome database. A conserved 453‐bp region (Figure S3) was targeted by RNAi to knockdown both family members. The target region was amplified by HiFi PCR premix (Clontech) using sequence‐specific primers flanked by gateway attL sites (primers are shown in Table S1). PCR product was purified by ZymoClean gel DNA recovery kit (Zymo Research, Irvine, CA) and validated by sequencing analysis. An LR reaction was carried out to assemble the amplified fragment into pB7GWIWG2 (II) gateway vector using Gateway LR Clonase II Enzyme mix (Invitrogen, Carlsbad, CA).
2.3. Generation of transgenic plants
Transgenic tobacco plants were generated by Agrobacterium‐mediated leaf disk transformation as described (Horsch et al., 1989) with LBA4404 Agrobacterium strain harboring an individual gene construct. For overexpression constructs, the selection medium contained 100 mg/L kanamycin for NPTII gene selection. For the RNAi construct, the selection medium included 3 mg/L phosphinothricin for bar gene selection. After root formation, the plantlets were transplanted to greenhouse approximately 3 weeks later. PCR was performed on T0 plants to confirm the existence of the transgene(s) (primers used are listed in Table S1). PCR‐positive plants were grown to the preflowering stage and topped. Before topping, the third fully expanded leaves from the top were sampled for transgene expression analysis. Seven days after topping, the top 12 leaves from each plant were collected, dried, and mixed, and the total alkaloids levels were determined as an indicator of nicotine level. After sampling, flowers from suckers (branches developed from lateral buds after topping) of the T0 transgenic plants were self‐pollinated to produce T1 seeds.
2.4. Sampling of T1 plants for gene expression and alkaloids analysis
Two T0 plants per construct with higher transgene expression level and total alkaloids content were self‐pollinated to produce T1 generation plants. PCR was performed to identify transgenic plants in the T1 segregation population. Root tissues were pooled at preflowering stage from three plants to assess expression levels of nicotine synthesis‐related genes. Top 12 leaves of three T1 plants were collected 1 week after flowering in order to determine nicotine, nornicotine, anabasine, and anatabine contents.
2.5. Quantitative reverse transcription PCR (qRT‐PCR)
Leaf samples of T0 plants were collected for transgene expression. Root samples of T1 plants were collected for testing nicotine biosynthetic gene expression. Tissue samples were immediately frozen in liquid nitrogen after collection. Total RNA was isolated using the TRIzol Reagent (Invitrogen). RNA was treated with RQ1 DNase (Promega, Madison, WI) to remove genomic DNA. The cDNA was synthesized with iScript™ cDNA Synthesis Kit (Bio‐Rad, Hercules, CA). Real‐time qRT‐PCR was performed using the Stratagene Mx3005P system (Agilent Technologies, Santa Clara, CA) with iTaq™ Universal SYBR® Green Supermix (Bio‐Rad). The thermal cycling program was set at 95°C for 30 s, followed by 40 cycles of 95°C for 5 s, and then 57°C for 30 s. Three technical repeats were performed for each assay. A tobacco actin gene (GenBank: U60490.1) was used as a reference gene for normalization. The gene‐specific primers are listed in Table S1.
2.6. Quantification of total alkaloids in T0 plants
Total alkaloids of T0 transgenic plants were determined by the segmented‐flow colorimetric method in the Tobacco Analytical Chemistry Laboratory at NCSU as previously described (Collins, Sarji, & Williams, 1969; Davis, 1976). In brief, the top 12 fully expanded leaves were collected from each tobacco plant 7 days after topping. Leaves were oven‐dried for 72 hr at 65°C and ground. Twenty‐five milliliter distilled water was added to 250 mg leaf sample. After 30 min of shaking, the aqueous extract was filtered through a Whatman filter paper. The collected aqueous extract was reacted with the sulphanilic acid buffer and cyanogen chloride buffer. The developed color was measured at 460 nm by colorimetry.
2.7. Quantification of individual alkaloids in T1 plants
At preflowering stage, the top 12 fully expanded leaves were collected and oven‐dried at 65°C for 72 hr and ground into fine powder. 0.2000 ± 0.0010 g of the leaf powder was weighed for total alkaloid extraction. Two milliliter 2 N NaOH solution was added to each sample. After incubation for 15 min, 10.0 ml quinoline working solution (0.4 g quinoline/ml methyl‐tert‐butyl ether) was added. After 2.5 hr of shaking, the mixture was incubated overnight for separation. Approximately 1.0 ml of the top methyl‐tert‐butyl ether (MTBE) layer was transferred into GC vials. Quantification was performed on an Agilent HP 6890 Gas Chromatograph (Agilent). One microliter sample from the GC vial was injected. The carrier gas helium was set at an average velocity of approximately 38 cm/s. The injector and detector were both set at 250°C. The oven temperature program was set as 110°C held for 1 min, 200°C at a rate of 10°C/min, 300°C at 25°C/min and held for 10 min. Data were collected and analyzed using Agilent Chemstation software. Pure nicotine (Sigma Aldrich, St. Louis, MO), anabasine (Alfa Aesar, Haverhill, MA), and nornicotine and anatabine (Toronto Research Chemicals, Toronto, Canada) were purchased and used as internal standards to make a calibration table.
2.8. Determination of JA‐Ile content in T1 lines
T1 lines were analyzed for JA‐Ile content, the biologically active form of JA. A 38‐mm‐diameter leaf disk was collected at the third fully expanded leaf from the top by a paper punch before flowering without topping/wounding treatment. The samples were immediately frozen in liquid nitrogen and stored in −80°C freezer until used. The frozen tissue was ground into fine powder, and 100 mg powder was quickly weighed before it thaws. One milliliter cold extraction buffer I (80% methanol spiked with 10 μl lidocaine as an internal control) was added. After well mixing, the mixture was vortexed at 4°C for 15 min, followed by sonication for another 15 min in ice water. After centrifugation at 15,700 g at 4°C for 15 min, the supernatant was transferred into a glass tube on ice. The extraction steps were repeated once more with 1 ml of cold extraction buffer II (acetonitrile: isopropanol: H2O = 3:3:2) and then with cold extraction buffer III (acetonitrile: H2O = 1:1). The supernatants from three extractions were combined in a glass tube. The total extracts were dried by a rotary evaporator at 30–40°C for approximately 15 min. The dry extracts were stored at −80°C until used. The residue was dissolved in 100 μl of sterile distilled water and centrifuged at 15,700 g at 4°C for 15 min, and the cleared supernatant was transferred to glass vials. HPLC‐MRM‐MS was performed using an Agilent 1100 HPLC (Agilent) coupled with an AB Sciex 4000 QTRAP™ (AB Sciex, Framingham, MA). Optimized detection conditions including precursor ion, product ion, declustering potential (DP), collision energy (EP), and cell exit potential (CXP) were established for quantification of JA, MeJA, JA‐Ile, OPDA. A reverse‐phase C18 column (Agilent, Eclipse XDB‐C18, 4.6 × 250 mm, 5 μm) was used for metabolite separation with 0.1% formic acid in water as solvent A and 0.1% formic acid in acetonitrile as solvent B. The LC gradient was as follows: 1% solvent B for 5 min; a linear gradient from 1% B to 99.5% B over 41.5 min; 99.5% B for 4.5 min; and return to 1% B. The flow rate was 0.5 ml/min, and the total analysis time was 1 hr. The mass spectrometer conditions were as follows: 30 psi curtain gas, 50 psi GS1, 55 psi GS2, ion source voltage at ±4,500 V, with the Turbo ElectroSpray Ionization (ESI) interface temperature at 350°C. A multiple period (segment) method was followed as previously described (Geng et al., 2016).
2.9. Statistical analysis
In nicotine content analysis of T1 lines, one‐way ANOVA and Dunnett comparison (α = .05) was used. For JA‐Ile content analysis, considering the relative heterogeneity within each genotype sample, a set of 1‐df contrasts was created in order to test the null hypothesis of transgenic JA‐Ile content is equal to control VC content, against the alternative hypothesis of VC having greater JA‐Ile content, at a significance level of .05. Each contrast analyzed the difference in JA‐Ile content between a particular genotype and VC, and the bootstrap stepdown method, for a one‐sided t test, was used within the SAS procedure MULTTEST (Satterthwaite analysis, SAS Institute Inc. 2015) to determine overall significance for this set of contrasts (Westfall & Young, 1993).
To improve the resolution of the statistical analysis, we conducted a follow‐up randomized complete block (RCB) design experiment in the greenhouse using two T1 lines with higher nicotine levels. PCR‐positive T1 plants of lines AOC2‐49, JAR1‐20, and WT were randomly assigned to three blocks with four plants in each plot. Plants were topped at preflowering stage, and leaves were collected 7 days after topping for nicotine analysis. Leaves from the same plot were pooled for nicotine analysis. The ANOVA and Tukey's studentized range test was used to determine difference significance and to make multiple comparisons.
3. RESULTS
Six to 11 T0‐independent transgenic plants were obtained from each gene construct. Plants transformed with the empty pBI121 vector (VC) or WT were used as a control. In T0 plants from two transgene constructs (AtAOS and NtJIH1 RNAi), the transgene expression varied a great deal among T0 plants within a construct whereas the total alkaloids levels were comparable to, or even slightly lower than, VC (Figures S4 and S5), indicating very little effect of transgene expression on alkaloids synthesis. Thus, plants from these two constructs were not pursued further. However, some plants from the other four transgene constructs (AtLOX2, AtAOC2, AtOPR3, and AtJAR1) showed relatively higher total alkaloids contents at T0 generation. To further characterize these plants, two lines from each of these constructs were studied at T1 generation for expression of the five key nicotine synthesis‐related genes (NtPMT1, NtQPT2, NtMYC2, NtBBLa, and Nt622; Wang et al., 2015), and the contents of nicotine and the other three minor alkaloids (nornicotine, anabasine, and anatabine). Very small differences on the minor alkaloids were observed between transgenic plants and VC (Table S2). Results of gene expression and contents of total alkaloids or nicotine of these transgenic plants are reported below.
3.1. Overexpression of AtAOS or knockdown of NtJIH1
13‐AOS is the first dedicated enzyme in the JA biosynthesis pathway and catalyzes formation of unstable allene oxide (Wasternack, 2007). Arabidopsis has only one AOS gene in its genome (Kubigsteltig, Laudert, & Weiler, 1999). Six AtAOS‐overexpression T0 plants were generated. Various AtAOS transcript levels were detected in transgenic plants by qRT‐PCR (Figure S4a). The nicotine content of transgenic plants was similar to, or even slightly lower than, that of WT (Figure S4b), suggesting overexpression of AOS had little impact on nicotine biosynthesis.
Jasmonoyl‐L‐isoleucine hydrolase 1 (JIH1) catalyzes hydrolysis of jasmonoyl‐Ile, the active form of JA, so to regulate JA signaling in plants (Woldemariam et al., 2012). Seven NtJIH1 RNAi plants were generated. Nicotine levels and JIH1 transcript levels of these plants were determined. JIH1 transcript levels were reduced in transgenic lines to only 8%–37% of that in WT (Figure S5a). However, nicotine content of transgenic plants was very similar to, or slightly lower than, WT (Figure S5b). These results suggest that JIH1 downregulation does not affect nicotine synthesis.
3.2. Overexpression of AtLOX2
13‐Lipoxygenase (13‐LOX) catalyzes oxygenation of α‐linolenic acid at an early step of JA biosynthesis. Among four 13‐LOX genes in Arabidopsis, LOX2 is responsible for the bulk JA formation at early wounding, and generation of oxylipins (Wasternack & Hause, 2013). Seven independent transgenic plants were obtained. The qRT‐PCR results indicate that all the transgenic plants had detectable AtLOX2 transcript levels (Figure 1a). The total alkaloid increase ranges from 8% to 47% (Figure 1b). Two LOX2 overexpression plants (LOX2‐20 and LOX2‐33) were chosen for further analysis at the T1 generation. NtPMT1, NtQPT2, NtMYC2, NtBBLa, and NtA622 transcript levels in LOX2‐20 T1 line were comparable to, or slightly lower than, those in VC. In LOX2‐33 line, however, the NtPMT1, NtQPT2, and NtMYC2 transcripts were doubled as compared to VC (Figure 1c). The means of nicotine content of the two transgenic lines were slightly higher than those of VC (Figure 1d); however, the difference was not statistically significant.
Figure 1.
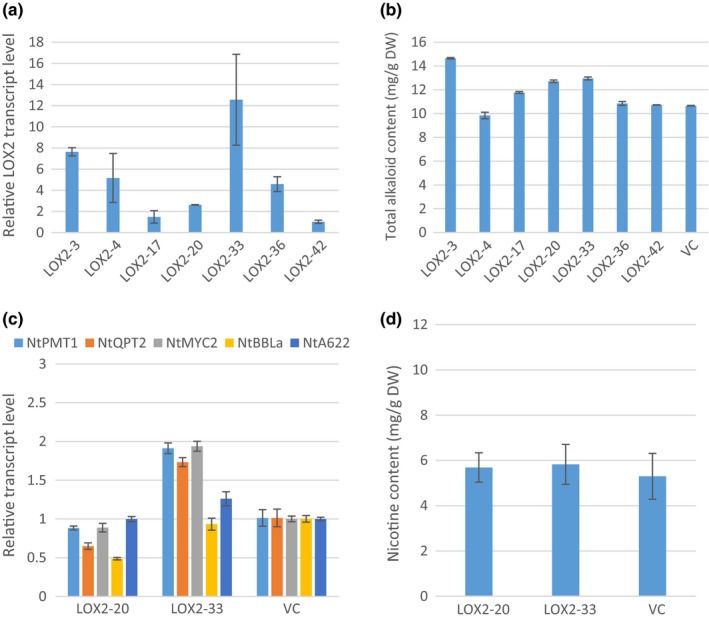
Overexpression of AtLOX2 in tobacco. (a) Relative AtLOX2 transcript levels in T0 plants RNA were isolated from leaf tissues collected before topping. Transcript levels were determined by qRT‐PCR. Values are means from three technical replicates. AtLOX2 transcript levels are normalized to actin and presented as fold to the AtLOX2 level in LOX2‐42 plant which is arbitrarily set at 1. (b) Total alkaloid levels of T0 plants. Total alkaloids (mg/g dry weight) were extracted and quantified from leaves 7 days after topping. The values shown are the means of total alkaloids (mg) per gram leaf dry weight from three technical replicates. (c) Transcript levels of five nicotine synthesis‐related genes in roots of two T1 lines. Roots from three plants were pooled at preflowering stage. Transcript levels were determined by qRT‐PCR with three technical replicates. Transcript levels were normalized to actin and presented as fold to the vector control (VC) which was arbitrarily set at 1. (d) Nicotine content of two T1 lines. Nicotine was extracted and quantified from leaves at preflowering stage. The values shown are the means of nicotine (mg) per gram of leaf dry weight from three biological replicates. VC, vector control. No significant difference was observed
3.3. Overexpression of AtAOC2
There are four allene oxide cyclase (AOC) isoforms with partial functional redundancy in Arabidopsis, among which function of AOC2 is better understood (Wasternack & Hause, 2013). Among 11 AtAOC2 overexpression T0 plants, the transgene expression varied by hundreds of fold (Figure 2a). However, total alkaloids contents varied only from 26% reduction up to 19% increase as compared to VC (Figure 2b). Lines AOC2‐49 and AOC2‐50 were chosen for further analysis at T1 generation. The basal levels of NtPMT1, NtQPT2, NtMYC2, NtBBLa, and NtA622 transcripts in these transgenic plants were similar to VC (Figure 2c). The means of nicotine content in the AOC2‐49 and AOC2‐50 transgenic plants were 10%–20% higher than VC (Figure 2d). However, the differences were not statistically significant.
Figure 2.
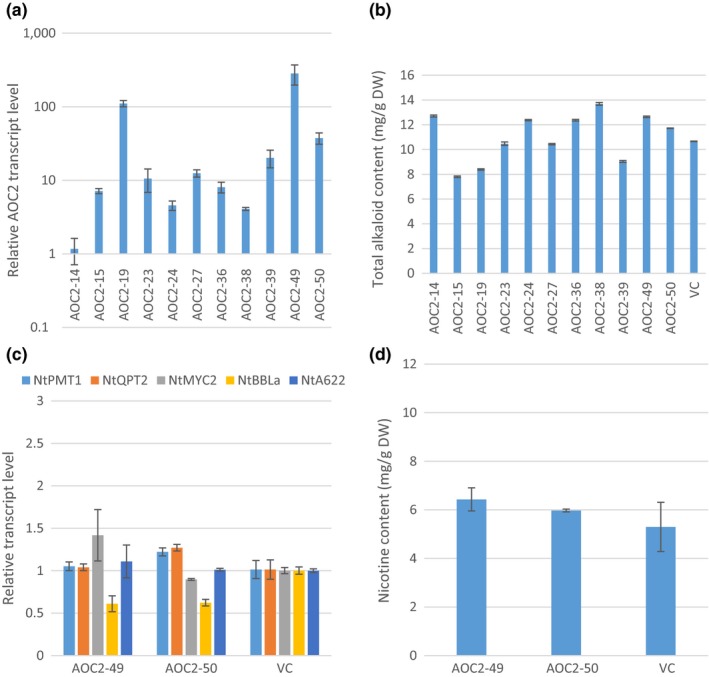
Overexpression of AtAOC2 in tobacco. (a) Relative AtAOC2 transcript levels in T0 plants. RNA was isolated from leaf tissues collected before topping. Transcript levels were determined by qRT‐PCR. Values are means from three technical replicates. AtAOC2 transcript levels were normalized to actin and presented as fold to the AtAOC2 level in AOC2‐14 plant which is arbitrarily set at 1. (b) Total alkaloid levels of AOC2 T0 plants. Total alkaloids were extracted and quantified from leaves 7 days after topping. The values shown are the means of total alkaloids (mg) per gram leaf dry weight from three technical replicates. (c) Transcript levels of five nicotine synthesis‐related genes in roots of two AOC2 T1 lines. Roots from three plants were pooled at preflowering stage. Transcript levels were determined by qRT‐PCR with three technical replicates. Transcript levels were normalized to actin and presented as fold to the vector control (VC) which was arbitrarily set at 1. (d) Nicotine content of two AOC2 T1 lines. Nicotine was extracted and quantified from leaves at preflowering stage. The values shown are the means of nicotine (mg) per gram of leaf dry weight from three biological replicates. VC, vector control. No significant difference was observed
3.4. Overexpression of AtOPR3
Among six OPDA reductases (OPR) in Arabidopsis, only OPR3 is involved in JA biosynthesis (Wasternack & Hause, 2013). Nine transgenic plants overexpressing AtOPR3 were generated. The transgene expression varied by more than 1,000‐fold (Figure 3a), and four T0 plants had total alkaloid content increase, ranging from 14% to 43% as compared to VC (Figure 3b). Two transgenic lines, OPR3‐21 and OPR3‐40, were chosen to be further studied at T1 generation.
Figure 3.
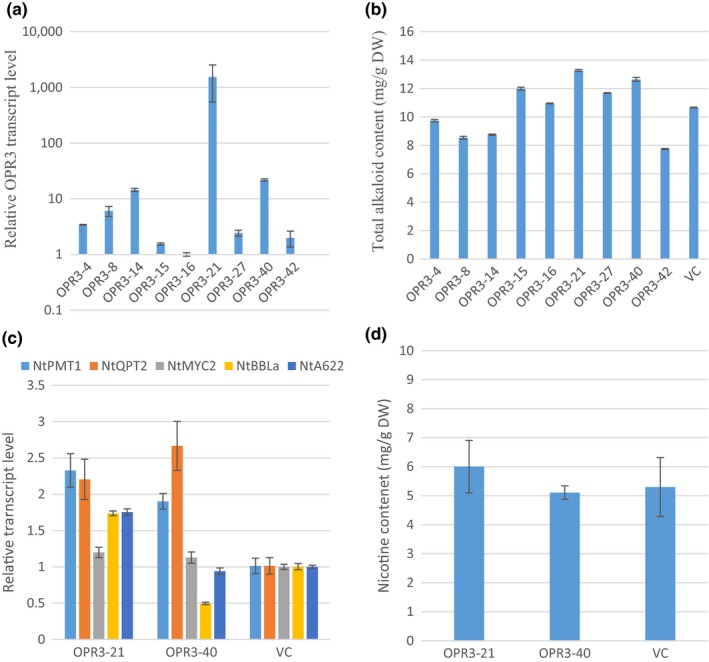
Overexpression of AtOPR3 in tobacco. (a) Relative AtOPR3 transcript levels in OPR3 T0 transgenic plants. RNA was isolated from leaf tissues collected before topping. Transcript levels were determined by qRT‐PCR. Values are means from three technical replicates. AtOPR3 transcript levels were normalized to actin and presented as fold to the AtOPR3 level in OPR3‐16 plant which is arbitrarily set at 1. (b) Total alkaloid levels of OPR3 T0 plants. Total alkaloids were extracted and quantified from leaves 7 days after topping. The values shown are the means of total alkaloids (mg) per gram leaf dry weight from three technical replicates. (c) Transcript levels of five nicotine synthesis‐related genes in roots of two OPR3 T1 lines. Roots from three plants were pooled at preflowering stage. Transcript levels were determined by qRT‐PCR with three technical replicates. Transcript levels were normalized to actin and presented as fold to the vector control (VC) which was arbitrarily set at 1. (d) Nicotine content of two OPR3 T1 lines. Nicotine was extracted and quantified from leaves at preflowering stage. The values shown are the means of nicotine (mg) per gram of leaf dry weight from three biological replicates. VC, vector control. No significant difference was observed
In T1 generation of both lines, NtPMT1 and NtQPT2 transcript levels were doubled of those in VC (Figure 3c). In OPR3‐21 line, NtBBLa and NtA622 transcript levels had more than 50% increase as compared to VC. However, the means of nicotine content in transgenic plants are not significantly different from VC (Figure 3d). Based on these data, we conclude that overexpression of OPR3 somewhat enhanced nicotine pathway gene transcript levels, but had little effect on nicotine accumulation.
3.5. Overexpression of AtJAR1
Six AtJAR1 transgenic plants were generated. More than 30‐fold difference of AtJAR1 transcript level was detected among the six plants (Figure 4a). Five of them had increased total alkaloid content as compared to VC (Figure 4b). For example, in JAR1‐20 and JAR1‐60 plants, total alkaloid increased by 50% and 11.7%, respectively. When the two lines were further analyzed at T1 generation, compared to VC, the basal levels of NtPMT1, NtQPT2, NtBBLa, and NtA622 transcript did not change, but MYC2 was nearly doubled in JAR1‐20 and almost unchanged in JAR1‐60 (Figure 4c). However, no significant changes in the nicotine levels were detected, as shown in Figure 4d.
Figure 4.
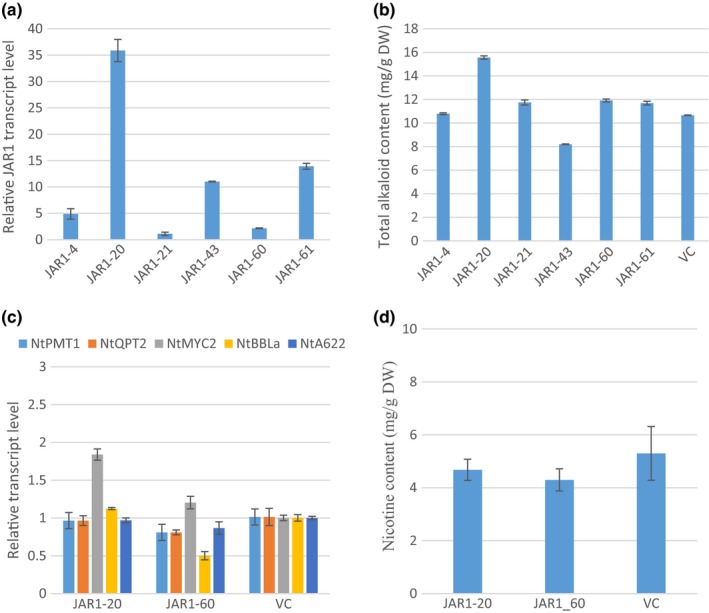
Overexpression of AtJAR1 in tobacco. (a) Relative AtJAR1 transcript levels in JAR1 T0 transgenic plants. RNA was isolated from leaf tissues collected before topping. Transcript levels were determined by qRT‐PCR. Values are means from three technical replicates. AtJAR1 transcript levels were normalized to actin and presented as fold to the AtJAR1 level in JAR1‐21 plant which is arbitrarily set at 1. (b) Total alkaloid levels of JAR1 T0 plants. Total alkaloids were extracted and quantified from leaves 7 days after topping. The values shown are the means of total alkaloids (mg) per gram leaf dry weight from three technical replicates. (c) Transcript levels of five nicotine synthesis‐related genes in roots of two JAR1 T1 lines. Roots from three plants were pooled at pre‐flowering stage. Transcript levels were determined by qRT‐PCR with three technical replicates. Transcript levels were normalized to actin and presented as fold to the vector control (VC) which was arbitrarily set at 1. (d) Nicotine content of two JAR1 T1 lines. Nicotine was extracted and quantified from leaves at pre‐flowering stage. The values shown are the means of nicotine (mg) per gram of leaf dry weight from three biological replicates. VC, vector control. No significant difference was observed
3.6. An effort to improve statistical analysis
Due to elevated total alkaloid content in the T0 generation (Figures 2 and 4), some lines, such as AOC2‐49 and JAR1‐20, looked more likely to have elevated nicotine content (Saitoh et al., 1985), and the sensitivity of the statistical analysis in the T1 experiments might have been suffered by the limited sample size. To improve the resolution of the statistical analysis, we conducted a follow‐up randomized complete block (RCB) design experiment in the greenhouse. Such design could help control location effect and reduce variance among the replicates. However, the calculated minimum significant difference is 3.7454, indicating that the nicotine levels of lines AOC2‐49 and JAR1‐20 were not significantly different from WT (Table 1).
Table 1.
Nicotine levels of T1 plants of lines AOC2‐49 and JAR1‐20 after topping in a randomized complete block design experiment. (A) Analysis of variance of the RCB experiment. PCR‐positive T1 plants of lines AOC2‐49 and JAR1‐20, and WT were randomly assigned to three blocks in the greenhouse. Each plot contained four plants of a line. The third leaves from the top of each plot were pooled 7 days after topping, and nicotine levels were determined by GC‐MS. (B) Tukey's studentized range test of nicotine level in the RCB experiment shows no significant differences between transgenic and WT lines
| A | |||||
|---|---|---|---|---|---|
| Source of variation | df | Sum of squares | Mean square | F Value | Pr > F |
| Model | 5 | 60.28889672 | 12.05777934 | 2.27 | .1275 |
| Error | 6 | 26.59059696 | 4.43176616 | ||
| Location | 3 | 32.12215774 | 10.70738591 | 2.42 | .1648 |
| Treatment effect | 2 | 28.16673898 | 14.08336949 | 3.18 | .1145 |
| Corrected total | 11 | 86.87949368 | |||
| B | |||
|---|---|---|---|
| Tukey grouping | Mean (mg/g DW) | N | Treatment |
| A | 9.676 | 4 | AOC2_49 |
| A | 6.564 | 4 | JAR1_20 |
| A | 6.303 | 4 | WT |
3.7. Overexpressing JA synthesis pathway genes did not increase JA‐Ile content
Among JA and its derivatives in plants, JA‐Ile is the bioactive form functioning in JA signaling and regulation of downstream genes. To further understand how overexpression of JA synthesis pathway genes affects nicotine biosynthesis, JA‐Ile contents of six T1 lines with various transgene constructs were analyzed. As shown in Figure 5, four lines (AOC2‐49, JAR1‐60, LOX2‐20, and LOX2‐33) of six had significant JA‐Ile reduction (p < .05) when their means were compared with VC. The other two lines (AOC2‐50 and OPR3‐40) also had a great reduction in JA‐Ile. Obviously, the JA‐Ile contents in transgenic plants were substantially reduced by overexpression of the transgenes from the JA synthesis pathway.
Figure 5.
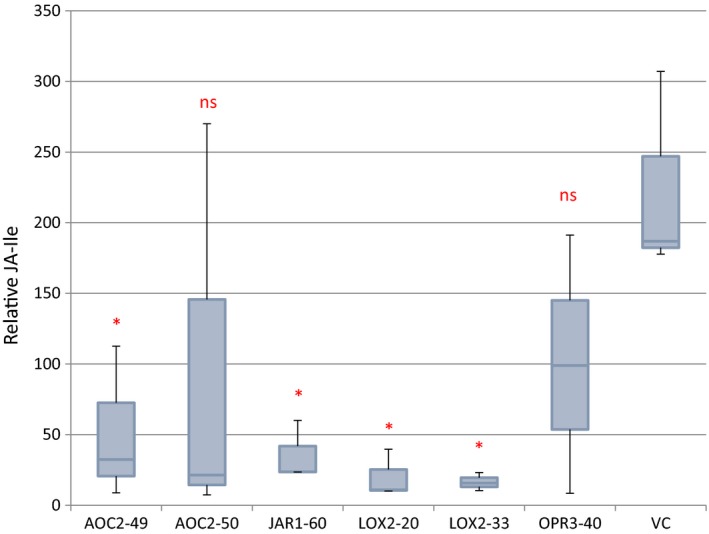
JA‐Ile contents in non‐topped transgenic T1 lines, and VC. 0.1 g of the third fully expanded leaves from top was collected from PCR positive transgenic plants (n = 3) of T1 lines prior to flowering, and the JA‐Ile peak area was measured. Bootstrap stepdown method is used to test the null hypothesis that genotype does not differ significantly from the control VC, with a one‐sided t test and the SAS procedure MULTTEST (Satterthwaite analysis) to determine significance. The x‐axis value (×104) represents JA‐Ile peak area per gram tissue normalized to internal control on the HPLC graph. *Significant difference at α = .05. ns, not significant
4. DISCUSSION
In this research, five Arabidopsis JA synthetic genes, LOX2, AOS, AOC2, OPR3, and JAR1, were individually introduced into tobacco genome and constitutively overexpressed. The NtJIH1 gene in tobacco, which hydrolyzes JA‐Ile, was knocked down by RNAi‐mediated gene silencing with an intention to reduce the degradation of JA‐Ile. The most striking phenomenon we observed in this experiment is that overexpression of these JA biosynthesis genes did not increase, and often reduced, JA‐Ile contents in transgenic plants. Laudert et al. (2000) recorded similar results: When AtAOS was overexpressed in tobacco, the basal JA level was reduced by nearly 10‐fold. The authors argued that the supply of the upstream substrate of JA synthesis pathway, α‐linolenic acid, is limited without wounding (Laudert et al., 2000). Although it could explain no increase in JA‐Ile, it cannot explain substantial reduction in JA‐Ile in the transgenic plants (their result and our Figure 5). Our data suggest that regulation of JA signaling is complicatedly regulated and tightly controlled at the various steps of JA biosynthesis. Because defense signaling is resource‐costly, plant fine‐tunes its responses to attenuate JA signaling at various levels. For instance, it is well documented that JA signaling negatively affects root cell division and thus root length (Wasternack & Hause, 2013). To tightly regulate JA signaling, plant developed fine tuning and JA attenuation mechanisms at various levels. For example, when studying the crystal structure of tomato OPR3, the authors unexpectedly observed a self‐inhibited dimer structure (Breithaupt et al., 2006), suggesting a strong and reversible dimerization in vivo involving phosphorylation of OPR3 as a regulatory step in JA biosynthesis. In another case, it was observed that any two of the four functional AOC polypeptides, AOC1, 2, 3, and 4, were able to interact to each other in BiFC experiments, prompting the authors to propose another layer of regulatory mechanism in JA biosynthesis through various combinations of heterodimerization of the four AOCs (Stenzel et al. 2012).
The second striking phenomenon we observed is that no matter how expression of nicotine synthesis‐related genes changed (or unchanged), the nicotine contents roughly stayed the same as in VC (Figures 1, 2, 3, 4c,d). The situation is similar to a previous observation in our laboratory: Overexpression of NtPMT1a and/or NtQPT2 under the NtQPT2 promoter did increase the transcript levels of both genes, but did not alter the nicotine content in the field test (Wang, 2011). However, knocking down expression of these genes by antisense or co‐suppression did reduce nicotine contents (Chintapakorn & Hamill, 2003; Wang, 2011; Xie et al., 2004), implicating that these key nicotine synthesis pathway genes are necessary but not sufficient in nicotine synthesis and accumulation. Based on these observations, we propose that nicotine biosynthesis in tobacco is also tightly regulated at multiple levels. One such a regulatory step is at transcriptional level. As shown previously, overexpression of transcription factors NtMYC2a or NtERF189 and alike could enhance expression of nicotine synthesis pathway genes leading to higher nicotine content in tobacco plants (Wang et al., 2015) or cultured cells (De Boer et al., 2011; Shoji & Hashimoto, 2011; Shoji et al., 2010). In addition, a negative feedback regulation was reported at the transcriptional level of AtMYC2, which is a “master” TF at the center of JA signaling (Kazan & Manners, 2013) and positively regulates pathway gene expression of nicotine synthesis (Wang et al., 2015). It was shown that AtMYC2 promoter has an MYC2 binding site, and thus, MYC2 was able to directly, negatively regulate its own transcript level (Dombrecht et al. 2007).
Furthermore, post‐transcriptional regulation is also shown to play an important role in nicotine biosynthesis. One example is the recently revealed mimicry and microRNA system tobacco uses to regulate the transcript level of NtQPT2 gene and thus the nicotine biosynthesis (Li et al., 2015). The nta‐eTMX27, the mimicry of NtQPT2 transcript, is upregulated after topping while the corresponding microRNA, nta‐miRX27, is downregulated, and the nicotine content increases. Moreover, phosphorylation of an MYC2 (NbbHLH1) and/or an ERF by an MAPKK (NtJAM1) further enhances the activities of these TFs and nicotine accumulation (De Boer et al., 2011), indicating a regulation step at post‐translational level. All these suggest that, in future experiments to study nicotine synthesis regulation, the enzymatic activities of NtPMT and NtQPT2, rather than their mRNA levels, need to be measured in both transgenic plants and control plants. Additionally, nicotine itself has a negative feedback loop on expression of its synthesis pathway genes (Wang et al., 2015) although the detailed mechanism(s) still needs to be elucidated.
Another interesting observation in our research is that reduction at JA‐Ile level did not affect nicotine contents (Figures 1, 2, 3, 4d), seemingly to suggest that the basal nicotine level (without wounding/topping) in tobacco be regulated by a pathway other than JA signaling. Alternatively, the endogenous JA‐Ile level may not correlate well with the expression of the JA signaling pathway genes and the accumulation of the nicotine content due to the complicated regulatory mechanisms in plant defense responses (De Vos et al., 2005).
In addition, we observed that total alkaloids contents measured in T0 generation often varied a great range, probably more sensitive to environmental factors, and did not appear to be reliable. Conversely, nicotine content in T1 generation looks more stable although we do not have a good explanation for the phenomenon.
Unlike the observations in our study, a previous research reported that constitutive overexpression of an AOC gene from a Solanaceae species, Hyoscyamus niger L., led to a 4.8‐fold increase in nicotine yield in tobacco plants (Jiang et al., 2009). The inconsistency may be caused by two possible reasons. First, the AOC from Hyoscyamus niger L. may have higher enzyme activity and thus stronger JA signaling effects. When the HnAOC (248 AAs) and AtAOC2 (253 AAs) proteins are analyzed, only 175 AAs can be aligned and 65% of those are identical. It is likely that the AOC enzyme activities from these two species are different. Second, Jiang et al. seem to have analyzed T0 transgenic plants only, and our data indicated that alkaloids data from T0 generation are not quite reliable (Figures 1, 2, 3, 4).
Taken together, overexpression of the key genes in JA biosynthesis pathway or RNAi approach of a catabolic gene is not an efficient way to alter nicotine biosynthesis, which is repeatedly shown in our experiments. The research revealed a tightly controlled JA signaling pathway and a very complicated regulatory network for nicotine biosynthesis.
AUTHOR CONTRIBUTIONS
HC performed most of the experiments and collected the data. HC, BW, and RQ designed the experiments, analyzed the data, and wrote the manuscript. SG and SC analyzed the content of JA‐Ile and discussed the data. CA and HC performed statistical analysis of the data. All authors read and approved the manuscript.
Supporting information
ACKNOWLEDGMENTS
We thank Tyler Steede and Karen Andres for their help in quantification of nicotine and alkaloids. We appreciate Dr. Carmen Castresana from Spain National Research Council, Madrid, Spain, for providing us AtLOX2 cDNA (L23968), and thank Dr. Deyu Xie for providing us the Gateway RNAi binary vector. We are grateful to the China Scholarship Council for supporting HC's graduate study. This study was also partially supported by grants from Yunnan Academy of Tobacco Agricultural Sciences to RQ (grants No. 110201202004, 2013YN04). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Chen H, Wang B, Geng S, Arellano C, Chen S, Qu R. Effects of overexpression of jasmonic acid biosynthesis genes on nicotine accumulation in tobacco. Plant Direct. 2018;2:1–11. 10.1002/pld3.36
REFERENCES
- Baldwin, I. T. , Halitschke, R. , Kessler, A. , & Schittko, U. (2001). Merging molecular and ecological approaches in plant–insect interactions. Current Opinion in Plant Biology, 4, 351–358. [DOI] [PubMed] [Google Scholar]
- Baldwin, I. T. , Schmelz, E. A. , & Ohnmeiss, T. E. (1994). Wound‐induced changes in root and shoot jasmonic acid pools correlate with induced nicotine synthesis in Nicotiana sylvestris spegazzini and comes. Journal of Chemical Ecology, 20, 2139–2157. [DOI] [PubMed] [Google Scholar]
- Breithaupt, C. , Kurzbauer, R. , Lilie, H. , Schaller, A. , Strassner, J. , Huber, R. , … Clausen, T. (2006). Crystal structure of 12‐oxophytodienoate reductase 3 from tomato: Self‐inhibition by dimerization. Proceedings of the National Academy of Sciences of the United States of America, 103, 14337–14342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chintapakorn, Y. , & Hamill, J. D. (2003). Antisense‐mediated down‐regulation of putrescine N‐methyltransferase activity in transgenic Nicotiana tabacum L. can lead to elevated levels of anatabine at the expense of nicotine. Plant Molecular Biology, 53, 87–105. [DOI] [PubMed] [Google Scholar]
- Collins, P. , Sarji, N. , & Williams, J. (1969). Determination of nicotine alkaloids in tobacco using the autoanalyzer. Tobacco Science, 13, 79–81. [Google Scholar]
- Davis, R. E. (1976). A combined automated procedure for the determination of reducing sugars and nicotine alkaloids in tobacco products using a new reducing sugar method. Tobacco Science, 20, 139–144. [Google Scholar]
- Davis, L. D. , & Nielsen, M. T. (1999). Tobacco: Production, chemistry and technology. Oxford, UK: Blackwell Science Ltd. [Google Scholar]
- De Boer, K. , Tilleman, S. , Pauwels, L. , Vanden Bossche, R. , De Sutter, V. , Vanderhaeghen, R. , … Goossens, A. (2011). APETALA2/ETHYLENE RESPONSE FACTOR and basic helix–loop–helix tobacco transcription factors cooperatively mediate jasmonate‐elicited nicotine biosynthesis. The Plant Journal, 66, 1053–1065. [DOI] [PubMed] [Google Scholar]
- De Vos, M. , Van Oosten, V. R. , Van Poecke, R. M. , Van Pelt, J. A. , Pozo, M. J. , Mueller, M. J. , … Pieterse, C. M. (2005). Signal signature and transcriptome changes of Arabidopsis during pathogen and insect attack. Molecular Plant‐Microbe Interactions, 18, 923–937. [DOI] [PubMed] [Google Scholar]
- DeBoer, K. D. , Lye, J. C. , Aitken, C. D. , Su, A. K. , & Hamill, J. D. (2009). The A622 gene in Nicotiana glauca (tree tobacco): Evidence for a functional role in pyridine alkaloid synthesis. Plant Molecular Biology, 69, 299–312. [DOI] [PubMed] [Google Scholar]
- Dombrecht, B. , Xue, G. P. , Sprague, S. J. , Kirkegaard, J. A. , Ross, J. J. , Reid, J. B. , … Kazan, K . (2007). MYC2 differentially modulates diverse jasmonate‐dependent functions in Arabidopsis. Plant Cell, 19, 2225–2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliot, J. (1966). Some effects of topping five flue‐cured tobacco varieties at three stages of floral development. Tobacco Science, 10, 100–104. [Google Scholar]
- Feth, F. , Wagner, R. , & Wagner, K. (1986). Regulation in tobacco callus of enzyme activities of the nicotine pathway. Planta, 168, 402–407. [DOI] [PubMed] [Google Scholar]
- Geng, S. , Misra, B. B. , de Armas, E. , Huhman, D. V. , Alborn, H. T. , Sumner, L. W. , & Chen, S. (2016). Jasmonate‐mediated stomatal closure under elevated CO2 revealed by time‐resolved metabolomics. The Plant Journal, 88(6), 947–962. [DOI] [PubMed] [Google Scholar]
- Goossens, J. , Fernández‐Calvo, P. , Schweizer, F. , & Goossens, A. (2016). Jasmonates: Signal transduction components and their roles in environmental stress responses. Plant Molecular Biology, 91, 673–689. [DOI] [PubMed] [Google Scholar]
- Goossens, A. , Hakkinen, S. T. , Laakso, I. , Seppanen‐Laakso, T. , Biondi, S. , De Sutter, V. , … Oksman‐Caldentey, K. M. (2003). A functional genomics approach toward the understanding of secondary metabolism in plant cells. Proceedings of the National Academy of Sciences of the United States of America, 100, 8595–8600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsch, R. B. , Fry, J. , Hoffmann, N. , Neidermeyer, J. , Rogers, S. G. , & Fraley, R. T. (1989). Leaf disc transformation. Plant molecular biology manual (pp. 63–71). Dordrecht, The Netherlands and Boston, MA: Kluwer Academic. [Google Scholar]
- Hung, C. Y. , Fan, L. , Kittur, F. S. , Sun, K. , Qiu, J. , Tang, S. , … Conkling, M. A. (2013). Alteration of the alkaloid profile in genetically modified tobacco reveals a role of methylenetetrahydrofolate reductase in nicotine demethylation. Plant Physiology, 161, 1049–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, K. , Pi, Y. , Hou, R. , Jiang, L. , Sun, X. , & Tang, K. (2009). Promotion of nicotine biosynthesis in transgenic tobacco by overexpressing allene oxide cyclase from Hyoscyamus niger . Planta, 229, 1057–1063. [DOI] [PubMed] [Google Scholar]
- Kajikawa, M. , Hirai, N. , & Hashimoto, T. (2009). A PIP‐family protein is required for biosynthesis of tobacco alkaloids. Plant Molecular Biology, 69, 287–298. [DOI] [PubMed] [Google Scholar]
- Kazan, K. , & Manners, J. M. (2013). MYC2: The master in action. Molecular Plant, 6, 686–703. [DOI] [PubMed] [Google Scholar]
- Kubigsteltig, I. , Laudert, D. , & Weiler, E. (1999). Structure and regulation of the Arabidopsis thaliana allene oxide synthase gene. Planta, 208, 463–471. [DOI] [PubMed] [Google Scholar]
- Laudert, D. , Schaller, F. , & Weiler, E. W. (2000). Transgenic Nicotiana tabacum and Arabidopsis thaliana overexpressing allen oxide synthase. Planta, 211, 163–165. [DOI] [PubMed] [Google Scholar]
- Lewis, R. S. , Lopez, H. O. , Bowen, S. W. , Andres, K. R. , Steede, W. T. , & Dewey, R. E. (2015). Transgenic and mutation‐based suppression of a berberine bridge enzyme‐like (BBL) gene family reduces alkaloid content in field‐grown tobacco. PLoS One, 10, e0117273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, F. , Wang, W. , Zhao, N. , Xiao, B. , Cao, P. , Wu, X. , … Fan, L. (2015). Regulation of nicotine biosynthesis by an endogenous target mimicry of microRNA in tobacco. Plant Physiology, 169, 1062–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis, K. M. (2010). RNAi for functional genomics in plants. Briefings in Functional Genomics, 9, 111–117. [DOI] [PubMed] [Google Scholar]
- Miersch, O. , Neumerkel, J. , Dippe, M. , Stenzel, I. , & Wasternack, C. (2008). Hydroxylated jasmonates are commonly occurring metabolites of jasmonic acid and contribute to a partial switch‐off in jasmonate signaling. New Phytologist, 177, 114–127. [DOI] [PubMed] [Google Scholar]
- Pauwels, L. , & Goossens, A. (2011). The JAZ proteins: A crucial interface in the jasmonate signaling cascade. Plant Cell, 23, 3089–3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polosa, R. , Rodu, B. , Caponnetto, P. , Maglia, M. , & Raciti, C. (2013). A fresh look at tobacco harm reduction: The case for the electronic cigarette. Harm Reduction Journal, 10, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quik, M. , O'Leary, K. , & Tanner, C. M. (2008). Nicotine and Parkinson's disease: Implications for therapy. Movement Disorders, 23, 1641–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh, F. , Nona, M. , & Kawashima, N. (1985). The alkaloid contents of sixty Nicotiana species. Phytochemistry, 24, 477–480. [Google Scholar]
- SAS Institute Inc (2015). SAS/STAT® 14.1 user's guide. Cary, NC: SAS Institute Inc. [Google Scholar]
- Saunders, J. W. , & Bush, L. P. (1979). Nicotine biosynthetic enzyme activities in Nicotiana tabacum L. genotypes with different alkaloid levels. Plant Physiology, 64, 236–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji, T. , & Hashimoto, T. (2011). Tobacco MYC2 regulates jasmonate‐inducible nicotine biosynthesis genes directly and by way of the NIC2‐locus ERF genes. Plant and Cell Physiology, 52, 1117–1130. [DOI] [PubMed] [Google Scholar]
- Shoji, T. , Inai, K. , Yazaki, Y. , Sato, Y. , Takase, H. , Shitan, N. , … Hashimoto, T. (2009). Multidrug and toxic compound extrusion‐type transporters implicated in vacuolar sequestration of nicotine in tobacco roots. Plant Physiology, 149, 708–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji, T. , Kajikawa, M. , & Hashimoto, T. (2010). Clustered transcription factor genes regulate nicotine biosynthesis in tobacco. Plant Cell, 22, 3390–3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenzel, I. , Otto, M. , Delker, C. , Kirmse, N. , Schmidt, D. , Miersch, O. , … Wasternack, C. (2012). ALLENE OXIDE CYCLASE (AOC) gene family members of Arabidopsis thaliana: tissue‐ and organ‐specific promoter activities and in vivo heteromerization. Journal of Experimental Botany, 63, 6125–6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suza, W. P. , & Staswick, P. E. (2008). The role of JAR1 in jasmonoyl‐L‐isoleucine production during Arabidopsis wound response. Planta, 227, 1221–1232. [DOI] [PubMed] [Google Scholar]
- Wagner, R. , & Wagner, K. (1985). The pyridine‐nucleotide cycle in tobacco enzyme activities for the de‐novo synthesis of NAD. Planta, 165, 532–537. [DOI] [PubMed] [Google Scholar]
- Wang, B. (2011). Factors in nicotine biosynthesis in tobacco. PhD thesis, North Carolina State University, Raleigh, NC. [Google Scholar]
- Wang, B. , Lewis, R. S. , Shi, J. , Song, Z. , Gao, Y. , Li, W. , … Qu, R. (2015). Genetic factors for enhancement of nicotine levels in cultivated tobacco. Scientific Reports, 5, 17360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasternack, C. (2007). Jasmonates: An update on biosynthesis, signal transduction and action in plant stress response, growth and development. Annals of Botany, 100, 681–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasternack, C. , & Hause, B. (2013). Jasmonates: Biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Annals of Botany, 111, 1021–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westfall, P. , & Young, S. S. (1993). Resampling based multiple testing: Examples of p‐value adjustment. New York, NY: Wiley. [Google Scholar]
- Woldemariam, M. G. , Onkokesung, N. , Baldwin, I. T. , & Galis, I. (2012). Jasmonoyl‐l‐isoleucine hydrolase 1 (JIH1) regulates jasmonoyl‐l‐isoleucine levels and attenuates plant defenses against herbivores. The Plant Journal, 72, 758–767. [DOI] [PubMed] [Google Scholar]
- Xie, J. H. , Song, W. , Maksymowicz, W. , Jin, W. , Cheah, K. , Chen, W. X. , … Conkling, M. A. (2004). Biotechnology: A tool for reduced risk tobacco products ‐ the nicotine experience from test tube to cigarette pack. Reviews in Advanced Tobacco Science, 30, 17–37. [Google Scholar]
- Zhang, K. (2016). MYC transcription factors: masters in the regulation of jasmonate biosynthesis in Arabidopsis thaliana. PhD thesis, Leiden University, Leiden, The Netherlands. [Google Scholar]
- Zhang, H. B. , Bokowiec, M. T. , Rushton, P. J. , Han, S. C. , & Timko, M. P. (2012). Tobacco transcription factors NtMYC2a and NtMYC2b form nuclear complexes with the NtJAZ1 repressor and regulate multiple jasmonate‐inducible steps in nicotine biosynthesis. Molecular Plant, 5, 73–84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


