Abstract
Plants encode at least two ancient and divergent classes of actin, reproductive and vegetative, and each class produces several subclasses of actin isovariants. To gain insight into the functional significance of the actin isovariants, we generated transgenic Arabidopsis lines that expressed a reproductive actin, ACT1, under the control of the regulatory sequences of a vegetative actin gene, ACT2. In the wild-type plants, ACT1 is predominantly expressed in the mature pollen, growing pollen tubes, and ovules, whereas ACT2 is constitutively and strongly expressed in all vegetative tissues and organs, but not in pollen. Misexpression of ACT1 in vegetative tissues causes dwarfing of plants and altered morphology of most organs, and the effects are in direct proportion to protein expression levels. Similar overexpression of ACT2 has little effect. Immunolocalization of actin in leaf cells from transgenic plants with highest levels of ACT1 protein revealed massive polymerization, bundling, and reorganization of actin filaments. This phenomenon suggests that misexpression of ACT1 isovariant in vegetative tissues affects the dynamics of actin and actin-associated proteins, in turn disrupting the organization of actin cytoskeleton and normal development of plants.
INTRODUCTION
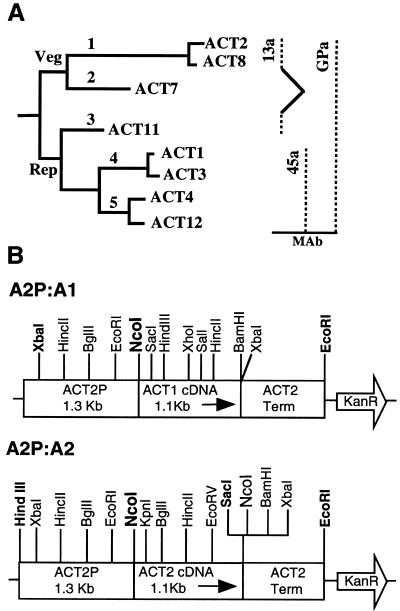
Actin is a multifunctional protein that mediates a number of important cellular processes in plants, including programming cell polarity, tip growth, cell shape and division plane determination, cell elongation, cell differentiation, cytoplasmic streaming, organelle movement and positioning, and cellular responses to external stimuli such as pathogen attack and incompatible growth of pollen in the pistil (Kost et al., 1999; Nick 1999; Meagher et al., 2000; Staiger, 2000; Wasteneys, 2000). The functional diversity of actins is reflected in the diversity within plant actin gene families, which often contain dozens of actin sequences (Meagher and Williamson, 1994). In the model plant Arabidopsis, there are eight actin genes that are expressed in a tissue-specific manner. Based on their phylogeny and expression pattern, those eight genes can be grouped into two major classes: vegetative and reproductive. The vegetative class is comprised of two subclasses of genes (Figure 1A), and both of them are expressed in nearly all the vegetative organs (An et al., 1996b; McDowell et al., 1996a; Meagher et al., 1999b). The reproductive class comprises three subclasses (Figure 1A), all of which are strongly expressed in the mature pollen and growing pollen tubes (An et al., 1996a; Huang et al., 1996, 1997). The reproductive genes ACT1, ACT3, and ACT11 are also expressed in ovules and to a certain extent in embryos and young meristematic tissue (Meagher et al., 1999b). The vegetative actins differ from the reproductive actins by only 4–7% at the amino acid level, yet they show distinct expression patterns and have not shared a common ancestor for at least 350 million years. Moreover, the closely related pair of genes within a subclass (e.g., ACT1 and 3 and ACT2 and 8; Figure 1A) differs from each other by only a single amino acid, but they have been maintained in the genome for 30–60 million years (McDowell et al., 1996b). These data strongly suggest that there are functional constraints preserving actin isovariant multiplicity, but it is still formally possible that the protein sequence differences are due to neutral drift, and natural selection has been acting primarily to preserve the divergent regulatory patterns of plant actins.
Figure 1.
Arabidopsis actin family and the specificity of anti-actin antibodies. (A) Actin tree showing two major classes, reproductive (Rep) and vegetative (Veg), and five subclasses of actin isovariants that are encoded by eight expressed genes. Specificity of antibodies is shown to the right. (B) Actin misexpression (A2P:A1) and overexpression (A2P:A2) constructs used in the present study.
We previously characterized the regulation of all eight genes with measurements of RNA levels and expression of reporter gene fusions (Meagher et al., 1999b). We are further unraveling the functional importance of the actin isovariants themselves by studying the protein expression patterns in different tissues and organs by using isovariant-specific antibodies (Kandasamy et al., 1999, 2001) and by characterizing T-DNA insertion mutants that are defective in the expression of different actin isovariants (McKinney et al., 1995; Gilliland et al., 1998). Mutations in most of the actin genes analyzed so far produced only mild morphological phenotypes, when plants were grown on soil under normal growth conditions (Gilliland et al., 1998). However, it still seemed possible that ectopic expression of actins in transgenic plants would produce strong phenotypes and provide valuable information regarding the physiological roles of the actin subclasses. Ectopic expression has been widely used to analyze the role of a variety of novel gene products, including that of floral homeotic or organ identity genes in plants (Mizukami and Ma, 1992; Uberlacker et al., 1996; Jack et al., 1997; Kirk et al., 1998; Jang et al., 1999; Kater et al., 2000). However, this type of misexpression study has not been used to dissect the function of isovariants encoded by plant cytoskeletal gene family members, although this method has been successfully used to analyze the role of fly and animal cytoskeletal gene products (Hutchens et al., 1997; Kumar et al., 1997; Fyrberg et al., 1998). Herein, we examine the effects on plant growth and development of ectopic expression of a reproductive actin (ACT1) under the control of a vegetative actin gene (ACT2) promoter. We chose ACT1 and ACT2 because they are the two most divergent and strongly expressed reproductive and vegetative actins, respectively, in Arabidopsis. Therefore, the broad aim of our investigation is to test the hypothesis that plant actin gene families contain ancient and divergent isovariant subclasses that have specialized protein functions linked to their specific expression patterns.
The data presented herein show that misexpression of ACT1 in vegetative tissues is very toxic, when it makes up significantly high level of the total actin pool, inducing alterations in the organization of actin filaments and causing severe structural and developmental perturbations in the transgenic plants. The ACT1 misexpression-induced dwarfism in plants, in addition to delayed flowering, significantly reduced organ size and altered branching pattern of the leaf trichomes and inflorescence stem. On the other hand, control plants overexpressing ACT2 isovariant to similar levels did not reveal any obvious phenotype. Our studies suggest that even though ACT1 and ACT2 are structurally similar, having ∼94% amino acid identity, they must have different functional capabilities. Isovariant specialization and interaction with the actin-associated proteins control F-actin assembly and organization and thereby plant growth and morphogenesis.
MATERIALS AND METHODS
Plant Material
Arabidopsis thaliana (ecotypes RLD and Columbia) plants were grown on germination medium (Murashige and Skoog salts [Life Technologies, Rockville, MD] and vitamins [Sigma, St. Louis, MO] supplemented with 1% sucrose and 0.8% Phytagar [Life Technologies]) or on soil and maintained in growth chambers at 22°C with a 16-h photoperiod. Phenotypic assessments of wild-type and kanamycin-resistant transgenic plants were made at different stages of development. For leaf length measurement, we used the two largest rosette leaves from 15 plants each of dwarf, medium, and normal transgenic plants at the time of bolting. To determine silique size, we measured the length of two mature siliques from 10 plants belonging to each category.
Construction of Binary Vectors and Plant Transformation
Two constructs were made for the present ectopic expression study: 1) A2P-A1, a misexpression construct that contains the 1.1-kb full-length ACT1 cDNA inserted between a 1.3-kb promoter and the terminator region of ACT2 (Figure 1B); 2) A2P-A2, a control construct in which the ACT1 cDNA was replaced with a 1.1-kb full-length ACT2 cDNA (Figure 1B). The ACT2-promoter was polymerase chain reaction (PCR) amplified from a genomic subclone pACT2-H, and the ACT1 and ACT2 cDNAs were PCR amplified from a mature flower library in the plasmid vector pCDNAII (Invitrogen, Carlsbad, CA) and ACT2 pCDNAII clone 3A1, respectively. The expression plasmids were mobilized into the Agrobacterium tumefaciens strain C58C1 and transformed into wild-type Arabidopsis plants by vacuum infiltration. Transformants were selected by plating the seeds on medium containing 35 mg/l kanamycin and examined for alterations in morphology.
Antibodies
We used the following three monoclonal antibodies to detect actin either by Western blot analysis or by fluorescence microscopy: 1) MAbGPa, a general plant-actin–specific antibody that detects all eight expressed Arabidopsis actins (Kandasamy et al., 1999); 2) MAb45a, a reproductive actin-specific antibody that reacts with actin subclasses 4 and 5 representing ACT1, ACT3, ACT4, and ACT12 (Kandasamy et al., 1999); and 3) MAb13a, an actin subclass-specific antibody that reacts with actin subclasses 1 and 3 representing the two major vegetative actins ACT2 and ACT8 and the closely related reproductive actin, ACT11 (Kandasamy et al., 2001; Figure 1A). Moreover, we used an anti-Hibiscus tubulin polyclonal antibody (courtesy of Dr. Richard Cyr, Pennsylvania State University, Pennsylvania) to detect microtubules at the cellular level. An anti-PEP carboxylase polyclonal antibody (Rockland, Gilbertsville, PA) was used as control to monitor variations in protein loading or transfer during electroblotting.
Western Blot Analysis
Protein samples from wild-type and transgenic Arabidopsis plants were prepared and analyzed by Western blotting as described previously (Kandasamy et al., 1999). Equal loading and uniform transfer of proteins to polyvinylidene difluoride membrane were monitored by Coomassie brilliant blue staining of gels and probing of identical blots or strips from the actin blots (>80-kDa region) with a control anti-PEP carboxylase antibody, respectively. Quantification of the actin bands, which were detected by using the ECL kit (Amersham Biosciences, Piscataway, NJ), was done by scanning the film in a densitometer loaded with the ImageQuant software (Molecular Dynamics, Sunnyvale, CA). Bands on exposed films that were used for quantification were shown to be in the linear range, compared with purified actin proteins run at known concentrations.
Light and Scanning Electron Microscopy
Hand sections from the inflorescence stem (basal node) of mature wild-type and transgenic plants were stained with phloroglucinol (1% in 6 N HCl), a lignin stain, and photographed using a Leica dissection microscope fitted with a color chilled 3 charge-coupled device camera (Hamamatsu, Tokyo, Japan). Scanning electron microscopic observations of leaf samples from 3- to 4-wk-old seedlings were performed following a previously described protocol (Kandasamy et al., 1994).
Immunofluorescence Labeling and Confocal Microscopy
Leaves from young seedlings were cryofixed by rapidly freezing them in liquid propane held at −180°C and then freeze substituted in acetone at −80°C for 48–72 h. The samples were gradually brought to room temperature over an 8-h period and rehydrated through a graded acetone series. After washing in PME [50 mM piperazine-N,N′-bis(2-ethanesulfonic acid) pH 7.0, 5 mM EGTA, 1 mM MgSO4, 0.5% casein], the leaves were permeabilized by treating with 1% Cellulysin (Calbiochem, San Diego, CA) and 0.1% Pectolyase (Sigma, St. Louis, MO) in PME containing protease inhibitors (complete mini EDTA-free tablets; Roche Molecular Biochemicals, Mannheim, Germany) for 15–20 min. The leaf samples were washed one time (5 min) in PME and two times (5 min each) in phosphate-buffered saline (PBS), and squashed onto chrom-alum and gelatin-coated slides to disperse the cells as described in Liu and Palevitz (1992). The leaf cells were further permeabilized in 0.5% Triton X-100 in PBS for 20 min and −20°C methanol for 10 min. After rinsing in PBS, the slides were blocked for 1 h in TBST-BSA-GS (10 mM Tris-HCl pH 7.5, 150 mM NaCl, 0.05% Tween 20, 5% bovine serum albumin [BSA], and 10% goat serum) and then incubated in the primary antibody diluted (5 μg/ml) in TBST-BSA-GS. Tubulin labeling was performed with an anti-Hibiscus tubulin polyclonal antibody. After overnight incubation, the slides were rinsed with PBS and then labeled for 2–3 h with fluorescein isothiocyanate-conjugated anti-mouse or anti-rabbit secondary antibody (Sigma) at 1:100 dilution. The slides were rinsed in PBS (3× 10 min) and mounted with 80% glycerol in PBS containing 1 mg/ml p-phenylenediamine (Sigma). The actin microfilaments and microtubules in the labeled cells were visualized with a Bio-Rad (Hercules, CA) MRC-600 confocal laser-scanning microscope. The images were further processed using Adobe Photoshop software (Adobe Systems, Mountain View, CA).
RESULTS
Misexpression of Reproductive Actin Isovariant in Vegetative Tissues Produces Severe Morphological Defects
To express ACT1 in vegetative tissues, a 1.1-kb, full-length coding sequence of the ACT1 cDNA was fused in frame to the initiation codon, downstream from a 1.3-kb ACT2 promoter fragment and upstream from the ACT2 3′ untranslated region containing multiple poly(A) addition sites. With the resulting construct, A2P:A1 (Figure 1B), we generated >100 independent kanamycin-resistant plants, of which 30% exhibited a strong dwarf (D) morphology, reaching less than one-third of wild-type plant height at maturity (Figure 2). Forty percent of the mature A2P:A1 plants showed ∼50% reduction in height (M), whereas the remaining 30% of the plants were almost normal (N) in stature with <20% decrease in height (Table 1). Thus, based on their size at maturity, we placed these transgenic plants into three categories: dwarf, medium, and normal. As a control to distinguish the effect of overexpression of an actin isovariant that is already active in the vegetative tissue from ACT1 isovariant-specific effects, a 1.1-kb ACT2 cDNA sequence was expressed under the control of the same ACT2 promoter and terminator sequence (A2P:A2; Figure 1B) in a parallel experiment. With this construct, we generated 30 independent AP2:A2 transgenic plants, of which 33% were classified as medium in stature (M), and the rest were normal sized (N; Table 1). None of the AP2:A2 plants exhibited any significant morphological aberration.
Figure 2.
Dwarf phenotype of mature ACT1-misexpressing plants. Seedlings were grown in Petri plates for 3 wk, transferred to soil, and photographed after 6 wk. WT, wild-type plant. A2P:A1, two independent transgenic dwarf plants misexpressing ACT1.
Table 1.
Effect of ectopic expression of actins on plant morphology Normal, <20% reduction in plant size compared with wild-type; medium, 30–40% reduction in plant size; dwarf, >60% reduction in plant size. Mature plants were used for plant size measurements.
| Construct | No. of transgenic lines produced | Dwarf (%) | Medium (%) | Normal (%) |
|---|---|---|---|---|
| A2P:A1 | 100 | 30 | 40 | 30 |
| A2P:A2 | 30 | 33 | 67 |
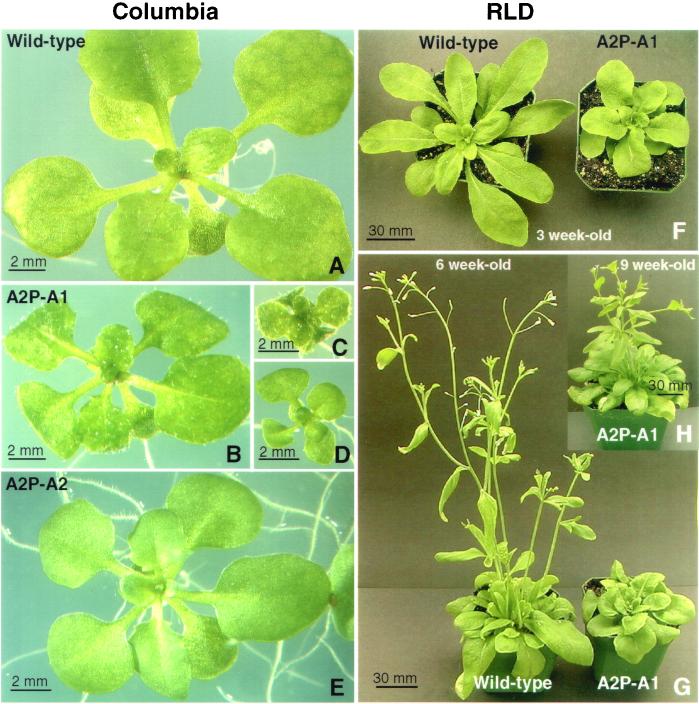
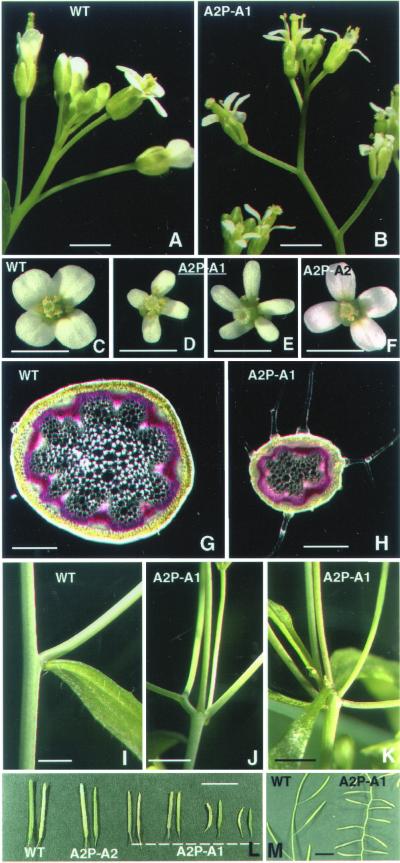
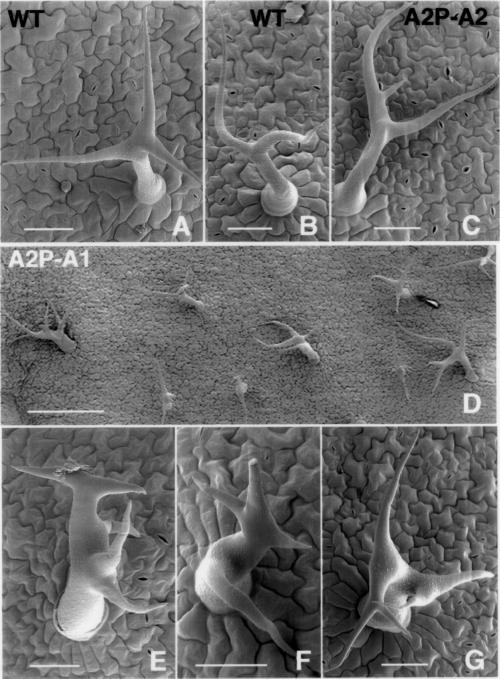
Analysis of the ACT1-misexpressing Arabidopsis cv. Columbia lines revealed clear morphological defects even in 2- to 3-wk-old seedlings, which showed varying levels of reduction in plant size compared with wild-type, and curling of leaves toward the lower side (Figure 3, A–D). A2P:A2 control seedlings, on the other hand, looked almost the same as wild-type plants (Figure 3E). Similar results were obtained when we introduced the ACT2:A1 and ACT2:A2 constructs into Arabidopsis cv. RLD (Figure 3, F and G). The severely dwarfed plants showed delayed bolting and flowering (Figure 3, F–H). The dwarf transformants eventually produced smaller flowers with sepals and petals having half normal width (compare Figure 4, A and C with B and D). A significant number of the dwarf lines (∼20%) showed flowers with smaller anthers and five petals, as depicted in Figure 4E, and produced very few seeds. However, the flowers on the medium- and normal-sized A2P:A1 plants and all A2P:A2 transformants (Figure 4F) were very similar to those on untransformed wild-type plants (Figure 4C). A few A2P:A1 plants (<10%) showed partial pollen sterility, but this did not correlate with ACT1 protein expression levels. All other plants produced pollen with no obvious phenotype. Transverse sections of the floral stem of dwarf plants revealed fewer vascular bundles and smaller cells (Figure 4H) compared with wild-type (Figure 4G) or ACT2-overexpressing plants. A scanning electron microscopy (SEM) observation of leaves from the dwarf plants also revealed smaller epidermal cells compared with the wild plants (our unpublished observations). The other morphological defects observed in the A2P:A1-dwarf lines include abnormal branching of the inflorescence stem (Figure 4, I–K) and trichomes (Figure 5, D–G), and strikingly smaller leaves (Figure 6B) and siliques (Figures 4, L and M; 6C). SEM studies of wild-type and ACT2-overexpressing plants reveal that leaf trichomes in these plants are stellate and erect with two to three mostly even branches (Figure 5, A–C), and those on the inflorescence stem and leaf petiole are seldom branched. On the other hand, A2P:A1-dwarf plants have leaf trichomes with two to five uneven and distorted branches (Figure 5, D–G). The inflorescence stem of these plants also produced branched trichomes (Figure 4H). In the mature dwarf plants, the angle at which the siliques were attached to the inflorescence stem was strikingly different compared with wild-type plants (Figure 4M). The roots of A2P:A1 dwarf plants showed ∼15 to 20% reduction in growth compared with the wild-type plants, whereas the roots of medium and normal-sized plants of both ACT1-misexpressing and ACT2-overexpressing lines showed normal growth.
Figure 3.
Effects of ACT1 misexpression on plant morphology and flowering in Arabidopsis. (A–E) Arabidopsis thaliana cv. Columbia. (A) Three-week-old wild-type seedling. (B–D) Three-week-old seedlings misexpressing ACT1. (E) Three-week-old seedling overexpressing ACT2. (F–H) Arabidopsis thaliana cv. RLD. Three- (F) and 6-wk-old (G) wild-type and A2P:A1-transgenic seedlings. (H) Nine-week-old same A2P:A1-transgenic plant. Note the delayed bolting of A2P:A1 transformant.
Figure 4.
Effects of ACT1 misexpression on different organs of Arabidopsis. (A and B) Inflorescence of a wild-type plant (A) and an A2P:A1-dwarf transgenic plant (B). (C–F) Flowers from wild-type (C) and ACT1-misexpressing (D and E) or ACT2-overexpressing (F) transgenic plants. (G and H) Cross section of inflorescence stem of wild-type (G) and ACT1-misexpressing dwarf (H) plants. (I–K) Nodal regions of inflorescence stem of wild-type (I) and transgenic dwarf plants (J and K). Note the multiple branches arising from the nodal region of the A2P-A1 transformants. (L and M) Siliques of wild-type and transgenic plants misexpressing ACT1 or overexpressing ACT2. WT, wild-type. A2P-A1, ACT1-misexpressing plant. A2P-A2, ACT2-overexpressing plant. Bar, 1 mm (A–F), 0.5 mm (G and H), 2 mm (I–K), and 10 mm (L and M).
Figure 5.
SEM analysis of effect of ectopic actin expression on leaf trichome morphology. (A and B) Wild-type leaf trichomes. (C) Leaf trichomes from a medium plant overexpressing ACT2. (D–G) Leaf trichomes from dwarf plants misexpressing ACT1. (D) Overview. All samples are from Arabidopsis cv. Columbia. Bar, 50 μm (A–C and E–G) and 250 μm (D).
Figure 6.
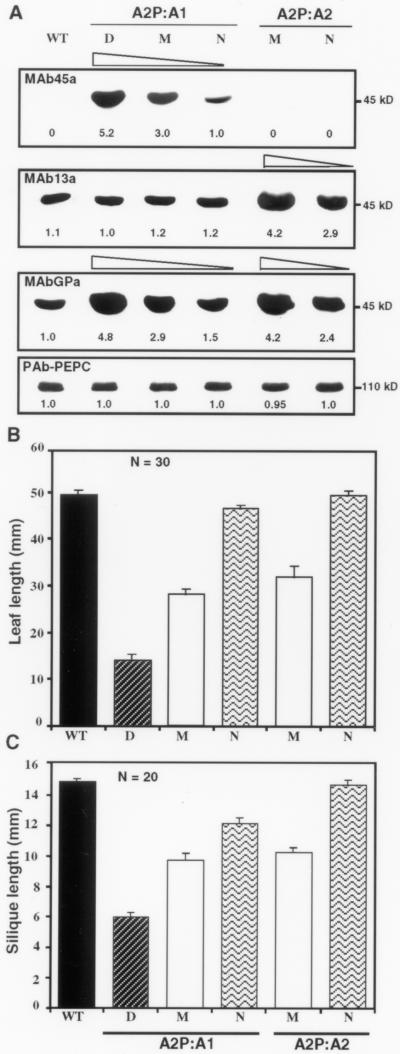
Correlation between actin protein levels and morphological phenotypes. (A) Western blot analysis of actin protein expression in ACT1-misexpressing (A2P-A1) and ACT2-overexpressing (A2P-A2) plants. Twenty-five micrograms of total protein from leaf samples was loaded per each lane. Identical blots were probed with anti-actin monoclonal antibodies MAb45a, MAb13a, and MAbGPa, and an anti-PEP carboxylase polyclonal antibody (PAb-PEPC, con trol). Relative intensity of protein bands is indicated at the bottom of the blots. Similar intensities of PEP carboxylase bands reveal equal loading of total proteins in all the lanes. WT, wild-type control; D, dwarf; M, medium; N, normal. (B) Leaf length of wild-type plants (WT) and transformants misexpressing ACT1 (A2P:A1) or overexpressing ACT2 (A2P:A2). (C) Silique length in wild-type and transgenic plants misexpressing ACT1 or overexpressing ACT2. The bars show the SE of mean values. N = 30 in (B) and N = 20 in (C) indicate sample size for each category and wild-type.
Level of ACT1 Protein Expression Correlates Well with Morphological Phenotypes
The targeted expression of ACT1 in the vegetative organs was examined by protein blot analysis with MAb45a, an antibody specific for the late pollen-specific reproductive actins, including ACT1 (Figure 1A). The reproductive actins are not present in detectable levels in the wild-type leaf (Figure 6A). Therefore, this antibody was used to analyze the expression of ACT1 protein in the leaves of dwarf, medium, and normal A2P:A1-transformants. There was an excellent correlation between the level of ectopic ACT1 expression and the severity of the morphological phenotype (Figure 6A, top). Quantification of the protein levels indicate that the dwarf (D) and medium (M) plants contained about five- and threefold higher amounts of ACT1 protein, respectively, compared with the normal (N) transgenic plants (Figure 6A, top). Interestingly, the misexpression of the A2P:A1 construct in the leaves did not repress expression of vegetative actins ACT2 and ACT8 or reproductive actin ACT11, as revealed by MAb13a (Figure 6A, middle). This antibody specifically reacts with these three actins comprising subclasses 1 and 3 (Figure 1A). The general antibody, MAbGPa, which reacts equally with all Arabidopsis actins, showed a gradual (about fourfold) increase in the level of total actin from the normal to dwarf ectopic plants, compared with the wild-type control (Figure 6A, middle). An identical blot probed with an anti-PEP carboxylase antibody confirmed that all the lanes contained equal amounts of total protein (Figure 6A, bottom). Protein blot analysis of ACT2-overexpressing plants (A2P:A2) with MAb13a revealed about four- and threefold increases in the levels of ACT2 proteins in the medium and normal transgenic lines, respectively, compared with the wild-type or ACT1-misexpressing plants (Figure 6A, middle). In spite of high levels of ACT2 in these plants, they were only slightly smaller than the wild-type plants (Table 1) and did not show any of the morphological phenotypes exhibited by A2P:A1 plants.
ACT1-Misexpressing Dwarf Plants Exhibit Aberrant Organization of Actin Filaments
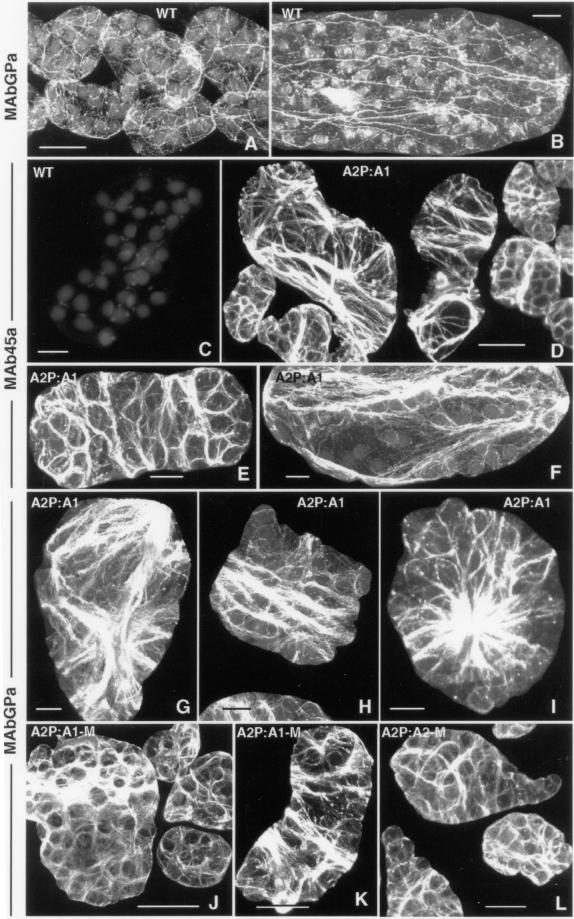
The assembly of G-actin into F-actin polymers is central to the function of actin filaments in directing the plane of cell division, cell expansion, cell differentiation, and ultimately the morphology of the plant. It is controlled by the direct interaction of actin with dozens of actin-binding proteins (ABPs). Therefore, the dwarf phenotype induced by forced misexpression of the ACT1 isovariant in vegetative tissues might be due to the misassembly of actin filaments. Hence, we examined the F-actin organization in the severely affected dwarf transgenic plants and compared it with that of wild-type and A2P:A2 transformants with the highest ACT2 expression. Figure 7, A and B, reveals the typical arrangement of the actin filaments in wild-type leaf cells with longitudinal arrays of actin bundles (Figure 7B) and a network of actin filaments surrounding the chloroplasts (Figure 7, A and B). These actin bundles and thin filaments are composed of vegetative actins, because MAb45a is unable to stain any actin filament in the wild-type leaf cells (Figure 7C). However, in the ACT1-misexpressing dwarf plants, MAb45a showed dense staining of transversely and longitudinally oriented thin actin filaments and sheets of actin bundles (Figure 4, D–F). Staining with MAbGPa revealed transverse, radial, or longitudinal sheets or ribbons of dense actin bundles (Figure 7, G–I). We found that the effect of ACT1 misexpression on the organization of actin bundles is less severe in medium-sized transgenic plants compared with the dwarf plants. However, many leaf cells in the medium plants still contained unevenly distributed transverse sheets of actin bundles (Figure 7, J and K). In normal-sized transgenic plants that showed very low levels of ACT1 protein expression, the arrangement of actin filaments and bundles, as revealed by MAbGPa labeling, was very similar to wild-type plants. The reproductive actin-specific MAb45a also detected actin cables in the leaf cells of normal-sized plants, but the intensity of staining was low (our unpublished observations). A drastic alteration in the orientation or organization of actin bundles was never observed in A2P:A2-transgenic leaf cells, although those cells stained strongly due to enhanced expression of ACT2 proteins (Figure 7L) compared with wild-type cells (Figure 7, A and B). Hence, it might be argued that the polarity of actin deposition was mostly affected in A2P:A1-dwarf plants.
Figure 7.
Immunofluorescence staining of actin filaments in the leaf cells of Arabidopsis. (A and B) Wild-type cells labeled with the general antibody MAbGPa. (C) Wild-type cell labeled with MAb45a, a reproductive class-specific anti-actin antibody. (D–F) Leaf cells of dwarf plants labeled with MAb45a. (G–I) Dwarf plant leaf cells stained with MAbGPa. (J and K) Leaf cells from A2P:A1-medium–sized plants labeled with MAbGPa. (L) Leaf cells from a medium-sized A2P:A2 plant overexpressing ACT2. Bar, 25 μm (A, D, and J–L) and 10 μm (B, C, and E–I).
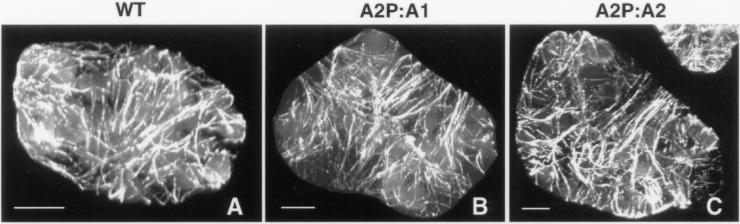
Organization of Tubulin Cytoskeleton Is Unaffected by Actin Misexpression
Because a high level of ACT1 isovariant misexpression severely altered the arrangement of actin bundles, we were interested in examining whether the organization of microtubules, the other major cytoskeletal component present in plant cells, was also affected in those leaf cells. It seems reasonable to expect cross communication between these two cytoskeletal systems (Brown, 1999). We stained the leaf cells from wild-type plants and plants expressing A2P:A1 and A2P:A2 constructs with an anti-tubulin polyclonal antibody. The staining pattern revealed no significant change in the density of microtubules in the A2P:A1-dwarf plants (Figure 8B), and their arrangement was indistinguishable from the microtubules of wild-type (Figure 8A) as well as ACT2-overexpressing plants (Figure 8C). As reported in our previous study (Kandasamy and Meagher, 1999), the microtubules exhibited mostly transverse orientation in the leaf mesophyll cells in the A2P:A1, A2P:A2, and wild-type plants analyzed.
Figure 8.
Immunofluorescence staining of microtubules in the leaf cells of Arabidopsis. (A–C) Organization of microtubules in a wild-type control (A), ACT1-misexpressing dwarf plant (B), and ACT2-overexpressing medium plant. Bar, 10 μm (A–C).
DISCUSSION
Arabidopsis contains two ancient classes of actin genes with different tissue-specific and developmental patterns of expression. Since their divergence from common ancestral sequences, the protein isovariants encoded by these genes may have evolved distinct reproductive and vegetative functions. We used ectopic expression to model the biological significance of two divergent classes of actin isovariants. This study demonstrates that misexpression of a pollen-specific reproductive actin isovariant in vegetative tissues drastically interferes with the growth of Arabidopsis plants. Ectopic expression of ACT1 alters actin polymerization, F-actin organization, and cyto-architecture of the cells, and thereby dramatically affects plant development and morphogenesis. Although the transgene-induced abnormalities in plant growth depend in part upon protein dosage, isovariant specificity plays the predominant role in inducing the dwarf or other phenotypes. For example, the overexpression of ACT2 in vegetative tissues has minimal effects on plant size and morphogenesis, whereas misexpression of ACT1 gave extreme phenotypes. Therefore, forcible alteration in actin isovariant composition appears to be the major cause for aberrant actin organization and plant development. This suggests that the two classes of actins, which differ by 4–7% at the amino acid level, have distinct biochemical characteristics. The isovariant-specific amino acid changes in the primary sequences of the two actin classes might favor entirely different interactions with distinct ABPs.
The actin system in eukaryotic cells is complex, consisting of >70 distinct classes of ABPs (Pollard, 2001). This is further complicated in higher plants because they express tissue-specific isoforms of ABPs such as profilins and actin-depolymerizing factors that control the polymerization and dynamics of the actin cytoskeleton (Staiger et al., 1997; Meagher et al., 1999a). The predominant expression of the highly divergent pollen-specific actins in the vegetative tissue of the dwarf transgenic plants might change actin isovariant dynamics and alter the balance for proper binding of different ABPs. Specifically, vegetative profilins might sequester ACT1 poorly, releasing too much actin for polymerization. This would lead to severe structural and functional perturbations that would cause altered cell structure and plant morphology. This suggestion is corroborated by the recent findings that the two classes of maize profilins have different binding properties to their ligands and different ability to disrupt actin architecture when injected into live cells (Kover et al., 2000). Thus, comparing the diverse responses of misexpression of ACT1 and overexpression of ACT2, we suggest that members of the plant actin gene family, which all exhibit distinct patterns of tissue-specific expression, might have isovariant-specific roles to play during plant growth and morphogenesis.
This ectopic expression study also reveals that the actin cytoskeleton might be actively involved in several plant developmental events such as branching and bolting of the inflorescence stem, cell enlargement, and trichome morphogenesis in Arabidopsis. We found that elevated expression of ACT1 isovariant in leaves affected the normal branching and growth of trichomes, indicating a role for actin in trichome development. This observation is supported by the recent fluorescence microscopic and cytoskeletal inhibitor studies (Mathur et al., 1999; Szymanski et al., 1999) that indicate that actin is required for the coordination of cell growth at the later stages of trichome morphogenesis. In the ACT1-misexpressing dwarf plants, the initiation and distribution of trichomes on leaves and inflorescence stem are not affected, but the final morphology and branching pattern of the trichomes were altered, suggesting that actin may have a role in later, but not early, stages of trichome morphogenesis. The delayed bolting and flowering and altered branching of the inflorescence stem show that the misexpression of actin interferes with the meristematic activity and organization.
The plant cytoskeleton is involved in a number of critical cellular processes from cell division, organ initiation, and morphogenesis to responses to various environmental stimuli. Producing the correct class of isovariants, in the correct tissue and at the correct stage of development is therefore very important for normal growth and morphogenesis. For example, during microsporogenesis plants express only vegetative actins at the early stages, but switch to predominantly pollen-specific reproductive class of actins when the pollen matures (Kandasamy et al., 1999). We observed similar changes in isovariant expression with the actin monomer-sequestering protein profilin in Arabidopsis (Kandasamy and Meagher, unpublished data). This developmental switch occurs most likely to suit the needs of pollen tubes showing specialized tip growth. Also, in Drosophila when flies lacking an isoform of muscle actin were complemented with nonmuscle actin, they show severe defects in flight muscle structure and function (Fyrberg et al., 1998), demonstrating that the fly actin isoforms are not functionally equivalent. Similarly, even closely related α-tubulin isoforms in Drosophila have different functional capabilities (Hutchens et al., 1997). Collectively, these observations strongly indicate that individual actin isovariants, how similar as they may be, should not necessarily be functionally equivalent.
Our demonstration that misexpression of a highly divergent reproductive actin in vegetative tissues has a drastic effect on actin polymerization and plant development provides further evidence for the functional nonequivalency of the two classes of actin isovariants. This finding supports our hypothesis that the ancient classes of actin isovariants are preserved in the genome, because they show distinct patterns of tissue-specific and developmental regulation and perform isovariant class-specific functions. However, the possibility still remains that structurally very similar isovariants (e.g., ACT2 and ACT8) are functionally equivalent. To demonstrate that they are functionally specialized, we need to isolate isoform-specific null mutants that show clear morphological phenotypes, and that can be complemented with related isovariants to see whether the mutant phenotype can be completely restored. Ectopically expressing a vegetative actin in reproductive tissue and elucidating its effect on pollen tube growth and embryogenesis may also serve as an important area for future study.
ACKNOWLEDGMENTS
We thank Drs. Marcus Fechheimer, Kelly Dawe, and Rebecca Balish, and Gay Gragson for comments on the manuscript. We are grateful to Dr. Richard Cyr for the tubulin antibody and Beth Richardson for help with rapid freezing of samples. Confocal and scanning electron microscopy works were carried out at the Center for Advanced Ultrastructural Research at the University of Georgia. This work was supported by the National Institutes of Health (GM-36397).
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.01–7–0342. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.01–07-0342.
REFERENCES
- An Y-Q, Huang S, McDowell JM, McKinney EC, Meagher RB. Conserved expression of the Arabidopsis ACT1 and ACT3actin subclasses in organ primordia and mature pollen. Plant Cell. 1996a;8:15–30. doi: 10.1105/tpc.8.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An Y-Q, McDowell JM, Huang S, McKinney EC, Chambliss S, Meagher RB. Strong, constitutive expression of the Arabidopsis ACT2/ACT8actin subclass in vegetative tissues. Plant J. 1996b;10:107–121. doi: 10.1046/j.1365-313x.1996.10010107.x. [DOI] [PubMed] [Google Scholar]
- Brown SS. Cooperation between microtubule- and actin-based motor proteins. Annu Rev Cell Dev Biol. 1999;15:63–80. doi: 10.1146/annurev.cellbio.15.1.63. [DOI] [PubMed] [Google Scholar]
- Fyrberg EA, Fyrberg CC, Biggs JR, Saville D, Beall CJ, Ketchum A. Functional nonequivalence of Drosophilaactin isoforms. Biochem Genet. 1998;36:271–287. doi: 10.1023/a:1018785127079. [DOI] [PubMed] [Google Scholar]
- Gilliland LU, McKinney EC, Asmussen MA, Meagher RB. Detection of deleterious genotypes in multigenerational studies. 1. Disruptions in individual Arabidopsisactin genes. Genetics. 1998;149:717–725. doi: 10.1093/genetics/149.2.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, An Y-Q, McDowell JM, McKinney EC, Meagher RB. The Arabidopsis thaliana ACT4/ACT12actin gene subclass is strongly expressed throughout pollen development. Plant J. 1996;10:189–202. doi: 10.1046/j.1365-313x.1996.10020189.x. [DOI] [PubMed] [Google Scholar]
- Huang S, An Y-Q, McDowell JM, McKinney EC, Meagher RB. The Arabidopsis ACT11actin gene is strongly expressed in tissues of the emerging inflorescence, pollen, and developing ovules. Plant Mol Biol. 1997;33:125–139. doi: 10.1023/a:1005741514764. [DOI] [PubMed] [Google Scholar]
- Hutchens JA, Hoyle HD, Turner FR, Raff EC. Structurally similar Drosophilaα-tubulins are functionally distinct in vivo. Mol Biol Cell. 1997;8:481–500. doi: 10.1091/mbc.8.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack T, Sieburth L, Meyerowitz E. Targeted misexpression of AGAMOUS in whorl 2 of Arabidopsisflowers. Plant J. 1997;11:825–839. doi: 10.1046/j.1365-313x.1997.11040825.x. [DOI] [PubMed] [Google Scholar]
- Jang S, Hong MY, Chung YY, An G. Ectopic expression of tobacco MADS genes modulates flowering time and plant architecture. Mol Cells. 1999;9:576–586. [PubMed] [Google Scholar]
- Kandasamy MK, Gilliland LU, McKinney EC, Meagher RB. One plant actin isovariant, ACT7, is induced by auxin and required for normal callus formation. Plant Cell. 2001;13:1541–1554. doi: 10.1105/TPC.010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandasamy MK, McKinney EC, Meagher RB. The late pollen-specific actins in angiosperms. Plant J. 1999;18:681–691. doi: 10.1046/j.1365-313x.1999.00487.x. [DOI] [PubMed] [Google Scholar]
- Kandasamy MK, Meagher RB. Actin-organelle interactions: association with chloroplast in Arabidopsisleaf mesophyll cells. Cell Motil Cytoskeleton. 1999;44:110–118. doi: 10.1002/(SICI)1097-0169(199910)44:2<110::AID-CM3>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Kandasamy MK, Nasrallah JB, Nasrallah ME. Pollen-pistil interactions and developmental regulation of pollen tube growth in Arabidopsis. Development. 1994;120:3405–3418. [Google Scholar]
- Kater MM, Franken J, van Aelst A, Angenent GC. Suppression of cell expansion by ectopic expression of the Arabidopsis SUPERMANgene in transgenic petunia and tobacco. Plant J. 2000;23:407–413. doi: 10.1046/j.1365-313x.2000.00785.x. [DOI] [PubMed] [Google Scholar]
- Kost B, Mathur J, Chau N-H. Cytoskeleton in plant development. Curr Opin Plant Biol. 1999;2:462–470. doi: 10.1016/s1369-5266(99)00024-2. [DOI] [PubMed] [Google Scholar]
- Kover DR, Drobak BK, Staiger CJ. Maize profilin isoforms are functionally distinct. Plant Cell. 2000;12:583–598. doi: 10.1105/tpc.12.4.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirik V, Kolle K, Wohlfarth T, Misera S, Baumlein H. Ectopic expression of a novel MYB gene modifies the architecture of the Arabidopsisinflorescence. Plant J. 1998;13:729–742. doi: 10.1046/j.1365-313x.1998.00072.x. [DOI] [PubMed] [Google Scholar]
- Kumar A, et al. Rescue of cardiac α-actin-deficient mice by enteric smooth muscle γ-actin. Proc Natl Acad Sci USA. 1997;94:4406–4411. doi: 10.1073/pnas.94.9.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Palevitz BA. Organization of cortical microfilaments in dividing root cells. Cell Motil Cytoskeleton. 1992;23:252–264. [Google Scholar]
- Mathur J, Spielhofer P, Kost B, Chau N-H. The actin cytoskeleton is required to elaborate and maintain spatial patterning during trichome cell morphogenesis in Arabidopsis thaliana. Development. 1999;126:5559–5568. doi: 10.1242/dev.126.24.5559. [DOI] [PubMed] [Google Scholar]
- McDowell JM, An Y-Q, Huang S, McKinney EC, Meagher RB. The Arabidopsis ACT7actin gene is expressed in rapidly developing tissues and responds to several external stimuli. Plant Physiol. 1996a;111:699–711. doi: 10.1104/pp.111.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell JM, Huang S, McKinney EC, An Y-Q, Meagher RB. Structure and evolution of the actin gene family in Arabidopsis thaliana. Genetics. 1996b;142:587–602. doi: 10.1093/genetics/142.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney EC, Ali N, Traut A, Feldmann KA, Beltostotsky DA, McDowell JM, Meagher RB. Sequence based identification of T-DNA insertion mutations in Arabidopsis: actin mutants act2-1 and act4-1. Plant J. 1995;8:613–622. doi: 10.1046/j.1365-313x.1995.8040613.x. [DOI] [PubMed] [Google Scholar]
- Meagher RB, McKinney EC, Kandasamy MK. Isovariant dynamics expands and buffers the responses of complex systems: the diverse plant actin gene family. Plant Cell. 1999a;11:995–1005. doi: 10.1105/tpc.11.6.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meagher RB, McKinney EC, Kandasamy MK. The significance of diversity in the plant actin gene family. In: Staiger CJ, Baluska F, Volkmann D, Barlow P, editors. Actin: A Dynamic Framework for Multiple Plant Cell Functions. Amsterdam, The Netherlands: Kluwer; 2000. pp. 3–27. [Google Scholar]
- Meagher RB, McKinney EC, Vitale AV. The evolution of new structures: clues from plant cytoskeletal genes. Trends Genet. 1999b;15:278–284. doi: 10.1016/s0168-9525(99)01759-x. [DOI] [PubMed] [Google Scholar]
- Meagher RB, Williamson RE. The plant cytoskeleton. In: Meyerowitz E, Somerville C, editors. Arabidopsis. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1994. pp. 1049–1084. [Google Scholar]
- Mizukami Y, Ma H. Ectopic expression of the floral homeotic gene AGAMOUS in transgenic Arabidopsisplants alters floral organ identity. Cell. 1992;71:119–131. doi: 10.1016/0092-8674(92)90271-d. [DOI] [PubMed] [Google Scholar]
- Nick P. Signals, motors, morphogenesis—the cytoskeleton in plant development. Plant Biol. 1999;1:169–179. [Google Scholar]
- Pollard TD. Genomics, the cytoskeleton and motility. Nature. 2001;409:842–843. doi: 10.1038/35057029. [DOI] [PubMed] [Google Scholar]
- Staiger CJ, Gibbon BC, Kovar DR, Zonia LE. Profilin and actin depolymerizing factor: modulators of actin organization in plants. Trends Plant Sci. 1997;2:275–281. [Google Scholar]
- Staiger CJ. Signaling to the actin cytoskeleton in plants. Annu Rev Plant Physiol Plant Mol Biol. 2000;51:257–288. doi: 10.1146/annurev.arplant.51.1.257. [DOI] [PubMed] [Google Scholar]
- Szymanski DB, Marks MD, Wick SM. Organized F-actin is essential for normal trichome morphogenesis in Arabidopsis. Plant Cell. 1999;11:2331–2347. doi: 10.1105/tpc.11.12.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uberlacker B, Klinge B, Werr W. Ectopic expression of the maize homeobox genes ZmHox1a or ZmHox1bcauses pleiotropic alterations in the vegetative and floral development of transgenic tobacco. Plant Cell. 1996;8:349–362. doi: 10.1105/tpc.8.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasteneys GO. The cytoskeleton and growth polarity. Curr Opin Plant Biol. 2000;3:503–511. doi: 10.1016/s1369-5266(00)00120-5. [DOI] [PubMed] [Google Scholar]