Abstract
Background:
Empirical data on the link between stress and cardiovascular (CVD) risk among African-American women is limited. We examined associations of stressful life events and social strain with incident CVD among African-American women and tested for effect modification by resilience.
Methods and Results:
Our analysis included 10,785 African-American women enrolled in the Women’s Health Initiative Observational Study and Clinical Trials cohort. Participants were followed for CVD for up to 23 years (mean, 12.5). Multivariable Cox regression was used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for associations between stress-related exposures and incident CVD. We included interactions between follow-up time (age) and stressful life events due to evidence of non-proportional hazards. Effect modification by resilience was examined in the sub-cohort of 2,765 women with resilience and stressful life events measures. Higher stressful life events was associated with incident CVD at ages 55 (HR for highest vs lowest quartile=1.80, 95% CI=1.27–2.54) and 65 (HR for highest vs lowest quartile=1.40, 95% CI=1.16–1.68), but not at older ages. Adjustment for CVD risk factors attenuated these associations. Similar associations were observed for social strain. In the sub-cohort of women with updated stressful life events and resilience measures, higher stressful life events was associated with incident CVD in multivariable-adjusted models (HR=1.61, 95% CI=1.04–2.51). Resilience did not modify this association nor was resilience independently associated with incident CVD.
Conclusions:
In this cohort of older African-American women, recent reports of stressful life events were related to incident CVD. Resilience was unrelated to incident CVD.
Clinical Trials Registration Information:
URL: www.clinicaltrials.gov. Unique identifier: NCT00000611.
Keywords: Cardiovascular Disease, race/ethnicity, stress, resilience, effect modification, Race and Ethnicity, Risk Factors, Women
Introduction
Cardiovascular disease (CVD) mortality has declined dramatically in the U.S. This decline has paralleled advances in primary prevention, including improved hypertension treatment and control, smoking reductions, and the prevalent use of statins, as well as the use of early interventions in those with atherosclerotic events 1, 2. Age-adjusted annual heart disease mortality fell by 56% between 1950 and 1996, while age-adjusted annual stroke rates fell by 70% during the same period 3. However, these reductions have not been equitably experienced across the U.S. population. In particular, African-American women continue to experience a higher burden of CVD compared to their White counterparts 3–5. These differences appear to persist, even after accounting for traditional CVD risk factors. As such, the continued disparate health outcomes experienced by African-American women mandate additional research into the etiology and preventive strategies for CVD among African-American women.
Stress is hypothesized to contribute to CVD through inflammation, endothelial dysfunction, and atherosclerosis 6. In an analysis of the Women’s Health Initiative (WHI) Observational Study (OS), 7 higher social strain and stressful life events were associated with increased risk of coronary heart disease and stroke in models adjusted for sociodemographic characteristics and depressive symptoms; however, subsequent inclusion of potential mediating factors, including alcohol use, cigarette smoking, hypertension, waist circumference, high cholesterol, diabetes, physical activity, and dietary quality attenuated these relationships. These associations were not modified by race, suggesting similar relationships among White and African-American women.
Stress may be particularly relevant for African-American women, given the discriminatory environment in which these women may live. However, research on the relationship between stress and CVD among African-American women is sparse. Furthermore, resilience, or the ability to bounce back from adversity, may mitigate some of the harmful effects of stress and/or CVD risk factors. Emerging data suggest that at-risk populations, including individuals stigmatized because of their race/ethnicity, have developed culturally-specific mechanisms that help them not only survive, but also thrive 8. While the potential moderating effect of resilience has not been specifically explored in the context of stress and CVD among African-American women, resilience has been identified as a protective factor in the relationship between substance abuse, violence, and HIV/AIDS with depressive symptoms among African-American women 9.
We sought to build on the prior WHI analysis by examining the association between stress and incident CVD in a larger population of African-American women and evaluating whether resilience modifies this relationship. We hypothesized that higher self-reports of stressful life events and social strain are associated with increased incident CVD among African-American women. Moreover, we postulated that high levels of resilience may attenuate the harmful effects of stress on CVD development among African-American women.
Methods
Study population
Because of the sensitive nature of the data collected for this study, requests to access the dataset from qualified researchers trained in human subject confidentiality protocols may be sent to the Women’s Health Initiative at www.whi.org. Full details of the WHI have been described previously 10–12. Briefly, between 1993 and 1998, postmenopausal women between the ages of 50 and 79 years were recruited from 40 clinical sites across the U.S. into one or more randomized clinical trials (WHI-CT n=68,132) or an observational study (WHI-OS n=93,676). Women in the WHI-OS were either unwilling or ineligible to be included in the WHI CT 13. Moreover, African-American women were oversampled in the WHI-OS. The WHI-CT and WHI-OS were closed in 2004–2005, and participants were invited to continue follow-up in the WHI Extension Study 1 (2005–2010), Extension Study 2 (2010–2015), and Extension Study 3 (2015–2020). Written informed consent was obtained from all study participants. Ethics approval was obtained from institutional review boards at all participating institutions. A standardized written protocol, centralized training of staff, and quality assurance visits by the Clinical Coordinating Center were used to ensure uniform data collection 13.
Our study sample was drawn from the 161,808 women participating in either the WHI-CT or WHI-OS. Of these, we excluded non-African-American women (n=147,190), women missing baseline stressful life events or social strain measures (n=1,322), women with a history of CVD at baseline (n=2,435), and women who were missing follow-up time (n=76), leaving 10,785 women in our analytic sample.
Exposure variables
At baseline, women completed questionnaires on stressful life events and social strain. The stressful life events questionnaire was adapted from the Beta-Blocker Heart Attack Trial and modified for women of older age 14. Participants indicated whether any of 11 life changes had occurred over the past year: spouse died, spouse had serious illness, close friend died, had major problems with money, experienced a divorce or break up, close friend divorced, major conflict with children or grandchildren, lost job, physically abused, verbally abused, and pet died. Additionally, women were asked to appraise each of the 11 life events that occurred based on the degree of upset that it caused on a scale of 1 (did not upset me) to 3 (upset me very much), generating a scale ranging from 0 to 33, with higher scores indicating greater stressful events.
Social strain was derived from four items from a previously validated measure of negative aspects of social relationships 15. Participants were asked how many of the people who were important to them got on their nerves, asked too much of them, did not include them, or tried to get them to do things they do not want to do. Responses to each item ranged from 1 (none) to 5 (all), yielding a social strain score ranging from 4 to 20, with higher scores indicating greater social strain.
Resilience, or the ability to bounce back from stressful situations, was assessed during the WHI Extension Study 2 and quantified with the Modified Brief Resilience Scale (BRS) 16. Of the six items included in the original BRS, scoring of three items was available in the WHI study. Participants are asked to rate the following statements on a scale of 1 to 6: “I tend to bounce back quickly after hard times; It does not take me long to recover from a stressful event; I have a hard time making it through stressful events.” Scores range from 3–18, with higher scores indicating higher resilience.
Other covariates
At baseline, participants completed self-administered questionnaires detailing demographic characteristics, medical and reproductive history, previous use of postmenopausal hormone therapy, physical activity, smoking history, alcohol use, diet, and other risk factors. Physical activity was quantified with questions on frequency, duration, and intensity of participation in different forms of physical activity. Weekly recreational physical activity was calculated by multiplying an assigned energy expenditure level for each category of activity by the hours exercised per week to calculate total metabolic equivalents per week (METs per week). Participants also underwent a clinic visit where trained staff measured each participant’s height and weight using a standardized protocol. Body mass index (BMI) was calculated based on these height and weight measurements.
Outcome variables
The primary outcome of our analysis was time to any CVD event, including coronary heart disease (angina, myocardial infarction), revascularization procedure (coronary revascularization, percutaneous transluminal coronary angioplasty, carotid revascularization, coronary artery bypass graft), carotid artery disease, peripheral artery disease, stroke/transient ischemic attack, heart failure, and CVD-related death. These events were centrally adjudicated using standardized case definitions and clinical criteria and updated annually through December 31, 2015 (end of Extension Study 2). Death certificate and medical record reviews were used to determine cause of death. A 94% rate of agreement between local and central clinical adjudicators for cause of death in WHI has been previously reported 17.
Statistical Analysis
Summary statistics of baseline characteristics according to stressful life events and social strain were evaluated with chi-square tests or t-tests, respectively. Stressful life events and social strain were investigated in four categories (based on quartiles) and as continuous variables. We estimated hazard ratios (HRs) and 95% confidence intervals (CIs) for the association between stressful life events, social strain, and incident CVD using the Cox proportional hazards regression model. Age at study randomization/enrollment was used as the underlying time scale and women who died from non-CVD events were censored. We tested the proportional hazards assumption by examining the Wald test for the multiplicative interaction between our main exposure variables, stressful life events and social strain, and follow-up time (natural log scale). Due to evidence of non-proportional hazards for baseline stressful life events, but not social strain, we included a multiplicative interaction between baseline stressful life events and age to allow for non-proportional hazards. We present age-specific HRs and 95% CIs to illustrate the time-varying relationship of baseline stress on incident CVD as women age. All models were adjusted for known CVD risk factors, including diabetes status (no, yes), BMI (<25 kg/m2, 25–30 kg/m2, ≥30 kg/m2), physical activity (>0–3.75 MET-hours/week, 3.75–8.75 MET-hours/week, 8.75–17.5 MET-hours/week, >=17.5 MET-hours/week), hypertension history (none, treated hypertension, or untreated hypertension), use of anti-hyperlipidemia drugs (no, yes), smoking status (never, past, current), and education (less than High school or GED, High School diploma or GED, some college, college graduate or higher).
We also examined whether resilience modified the effect of stressful life events and incident CVD using the likelihood-ratio procedure, comparing models with and without an interaction term between resilience and stressful life events. This analysis included the subset of African-American women who responded to the Extension Study 2 questionnaire. We did not examine interactions with social strain, as this was not included in the Extension Study 2 questionnaire. Analyses were conducted using STATA software (version 11, STATA Corp., Texas, USA). All P values were two-sided with the probability of a Type I error set at <5%.
Results
Study population
Compared to African-American women in the lowest quartile of stressful life events, women in the highest quartile of stressful life events were younger at baseline, had lower educational attainment, were less physically active, and had a higher proportion of current smoking, obesity, oral contraceptive use, and diabetes mellitus (Table 1). African-American women in the highest quartile of social strain were more commonly never users of menopausal hormone therapy compared to those in the lowest quartile of social strain (Table 2).
Table 1.
Baseline Characteristics of Study Participants by Stressful Life Events, Women's Health Initiative Observational and Clinical Trials, n=10,785
| Stressful life events | ||||
|---|---|---|---|---|
| Q1 (0 – 1) | Q2 (2 – 3) | Q3 (4 – 6) | Q4 (7 – 30) | |
| (n=2,495) | (n=2,917) | (n=2,966) | (n=2,407) | |
| Age at WHI entry Mean (SD) | 61.7 (7) | 61.4 (7.1) | 60.8 (7) | 60.0 (6.8) |
| Education | ||||
| Missing | 22 (1%) | 31 (1%) | 31 (1%) | 42 (2%) |
| Less than High school diploma or GED | 226 (9%) | 256 (9%) | 291 (10%) | 317 (13%) |
| High school diploma or GED | 342 (14%) | 397 (14%) | 395 (13%) | 323 (13%) |
| Some college | 897 (36%) | 1066 (37%) | 1170 (39%) | 1014 (42%) |
| College graduate or higher | 1008 (40%) | 1167 (40%) | 1079 (36%) | 711 (30%) |
| Smoking status | ||||
| Missing | 46 (2%) | 35 (1%) | 45 (2%) | 35 (1%) |
| Never smoked | 1286 (52%) | 1444 (50%) | 1425 (48%) | 1133 (47%) |
| Past smoker | 933 (37%) | 1142 (39%) | 1122 (38%) | 932 (39%) |
| Current smoker | 230 (9%) | 296 (10%) | 374 (13%) | 307 (13%) |
| BMI | ||||
| Missing | 25 (1%) | 28 (1%) | 25 (1%) | 23 (1%) |
| <25 kg/m2 | 443 (18%) | 506 (17%) | 464 (16%) | 329 (14%) |
| 25–29 kg/m2 | 886 (36%) | 984 (34%) | 991 (33%) | 712 (30%) |
| ≥30 kg/m2 | 1141 (46%) | 1399 (48%) | 1486 (50%) | 1343 (56%) |
| Hormone use | ||||
| Missing | 4 (<1%) | 2 (<1%) | 4 (<1%) | 6 (<1%) |
| Never | 1446 (58%) | 1701 (58%) | 1727 (58%) | 1456 (60%) |
| Past user | 383 (15%) | 420 (14%) | 443 (15%) | 352 (15%) |
| Current user <5 years | 267 (11%) | 291 (10%) | 313 (11%) | 263 (11%) |
| Current user 5 to <10 years | 141 (6%) | 195 (7%) | 187 (6%) | 126 (5%) |
| Current user ≥10 years | 254 (10%) | 308 (11%) | 292 (10%) | 204 (8%) |
| Type of hormone use | ||||
| Missing | 4 (<1%) | 2 (<1%) | 4 (<1%) | 6 (<1%) |
| Never user | 1446 (58%) | 1701 (58%) | 1727 (58%) | 1456 (60%) |
| Past user of either E alone or E+P | 383 (15%) | 420 (14%) | 443 (15%) | 352 (15%) |
| E-alone | 498 (20%) | 599 (21%) | 574 (19%) | 458 (19%) |
| E+P | 164 (7%) | 195 (7%) | 218 (7%) | 135 (6%) |
| Oral contraceptive use | ||||
| Missing | 0 | 0 | 0 | 1 (<1%) |
| Yes | 905 (36%) | 1142 (39%) | 1216 (41%) | 1039 (43%) |
| Diabetes status | ||||
| Missing | 6 (<1%) | 7 (<1%) | 5 (<1%) | 2 (<1%) |
| Yes | 222 (9%) | 279 (10%) | 301 (10%) | 287 (12%) |
| Use of anti-hyperlipidemia drugs | ||||
| Missing | 125 (5%) | 186 (6%) | 188 (6%) | 173 (7%) |
| Yes | 328 (13%) | 391 (13%) | 359 (12%) | 303 (13%) |
| Hypertension history | ||||
| Missing | 112 (4%) | 164 (6%) | 169 (6%) | 145 (6%) |
| Never hypertensive | 1187 (48%) | 1333 (46%) | 1335 (45%) | 1050 (44%) |
| Treated hypertensive | 993 (40%) | 1191 (41%) | 1200 (40%) | 973 (40%) |
| Untreated hypertensive | 203 (8%) | 229 (8%) | 262 (9%) | 239 (10%) |
| Physical activity | ||||
| Missing | 77 (3%) | 125 (4%) | 135 (5%) | 108 (4%) |
| None | 513 (21%) | 639 (22%) | 641 (22%) | 565 (23%) |
| >0–3.75 MET-hours/week | 436 (17%) | 478 (16%) | 540 (18%) | 461 (19%) |
| 3.75–8.75 MET-hours/week | 515 (21%) | 584 (20%) | 614 (21%) | 473 (20%) |
| 8.75–17.5 MET-hours/week | 463 (19%) | 513 (18%) | 502 (17%) | 404 (17%) |
| >=17.5 MET-hours/week | 491 (20%) | 578 (20%) | 534 (18%) | 396 (16%) |
| WHI Trial membership | ||||
| OS | 1340 (54%) | 1495 (51%) | 1444 (49%) | 1180 (49%) |
| E-only | 158 (6%) | 177 (6%) | 224 (8%) | 187 (8%) |
| E+P | 108 (4%) | 154 (5%) | 158 (5%) | 126 (5%) |
| DM | 741 (30%) | 878 (30%) | 904 (30%) | 704 (29%) |
| E+P and DM | 148 (6%) | 213 (7%) | 236 (8%) | 210 (9%) |
GED: General equivalency diploma; MET: metabolic equivalent; OS: Observational study; E+P: Estrogen plus progestin; DM: Dietary modification
Table 2.
Baseline Characteristics of Study Participants by Social Strain, Women's Health Initiative Observational and Clinical Trials, n=10,785
| Social Strain | ||||
|---|---|---|---|---|
| Q1 (4) | Q2 (5 – 6) | Q3 (7 – 9) | Q4 (10 –20) | |
| (n=2,649) | (n=3,220) | (n=2,477) | (n=2,439) | |
| Age at WHI entry Mean (SD) | 61.3 (7) | 60.8 (7) | 60.3 (6.8) | 61.8 (7.2) |
| Education | ||||
| Missing | 22 (1%) | 40 (1%) | 39 (2%) | 25 (1%) |
| Less than High school diploma or GED | 207 (8%) | 288 (9%) | 376 (15%) | 219 (9%) |
| High school diploma or GED | 308 (12%) | 459 (14%) | 368 (15%) | 322 (13%) |
| Some college | 1011 (38%) | 1289 (40%) | 1009 (41%) | 838 (34%) |
| College graduate or higher | 1101 (42%) | 1144 (36%) | 685 (28%) | 1035 (42%) |
| Smoking status | ||||
| Missing | 52 (2%) | 43 (1%) | 36 (1%) | 30 (1%) |
| Never smoked | 1297 (49%) | 1593 (49%) | 1173 (47%) | 1225 (50%) |
| Past smoker | 1016 (38%) | 1220 (38%) | 936 (38%) | 957 (39%) |
| Current smoker | 284 (11%) | 364 (11%) | 332 (13%) | 227 (9%) |
| BMI | ||||
| Missing | 27 (1%) | 34 (1%) | 17 (1%) | 23 (1%) |
| <25 kg/m2 | 503 (19%) | 524 (16%) | 298 (12%) | 417 (17%) |
| 25–29 kg/m2 | 895 (34%) | 1052 (33%) | 738 (30%) | 888 (36%) |
| ≥30 kg/m2 | 1224 (46%) | 1610 (50%) | 1424 (57%) | 1111 (46%) |
| Hormone use | ||||
| Missing | 6 (<1%) | 4 (<1%) | 3 (<1%) | 3 (<1%) |
| Never | 1456 (55%) | 1919 (60%) | 1483 (60%) | 1472 (60%) |
| Past user | 452 (17%) | 450 (14%) | 351 (14%) | 345 (14%) |
| Current user <5 years | 275 (10%) | 340 (11%) | 259 (10%) | 260 (11%) |
| Current user 5 to <10 years | 180 (7%) | 190 (6%) | 157 (6%) | 122 (5%) |
| Current user ≥10 years | 280 (11%) | 317 (10%) | 224 (9%) | 237 (10%) |
| Type of hormone use | ||||
| Missing | 6 (<1%) | 4 (<1%) | 3 (<1%) | 3 (<1%) |
| Never user | 1456 (55%) | 1919 (60%) | 1483 (60%) | 1472 (60%) |
| Past user of either E alone or E+P | 452 (17%) | 450 (14%) | 351 (14%) | 345 (14%) |
| E-alone | 561 (21%) | 618 (19%) | 489 (20%) | 461 (19%) |
| E+P | 174 (7%) | 229 (7%) | 151 (6%) | 158 (6%) |
| Oral contraceptive use | ||||
| Missing | 1 (<1%) | 0 | 0 | 0 |
| Yes | 1026 (39%) | 1343 (42%) | 1025 (41%) | 908 (37%) |
| Diabetes status | ||||
| Missing | 6 (<1%) | 4 (<1%) | 5 (<1%) | 5 (<1%) |
| Yes | 235 (9%) | 311 (10%) | 306 (12%) | 237 (10%) |
| Use of anti-hyperlipidemia drugs | ||||
| Missing | 178 (7%) | 212 (7%) | 163 (7%) | 119 (5%) |
| Yes | 342 (13%) | 404 (13%) | 346 (14%) | 289 (12%) |
| Hypertension history | ||||
| Missing | 157 (6%) | 192 (6%) | 132 (5%) | 109 (4%) |
| Never hypertensive | 1229 (46%) | 1445 (45%) | 1060 (43%) | 1171 (48%) |
| Treated hypertensive | 1037 (39%) | 1314 (41%) | 1028 (42%) | 978 (40%) |
| Untreated hypertensive | 226 (9%) | 269 (8%) | 257 (10%) | 181 (7%) |
| Physical activity | ||||
| Missing | 128 (5%) | 147 (5%) | 97 (4%) | 73 (3%) |
| None | 539 (20%) | 718 (22%) | 589 (24%) | 512 (21%) |
| >0–3.75 MET-hours/week | 472 (18%) | 580 (18%) | 456 (18%) | 407 (17%) |
| 3.75–8.75 MET-hours/week | 520 (20%) | 656 (20%) | 497 (20%) | 513 (21%) |
| 8.75–17.5 MET-hours/week | 490 (18%) | 531 (16%) | 426 (17%) | 435 (18%) |
| >=17.5 MET-hours/week | 500 (19%) | 588 (18%) | 412 (17%) | 499 (20%) |
| WHI Trial membership | ||||
| OS | 1304 (49%) | 1579 (49%) | 1236 (50%) | 1340 (55%) |
| E-only | 178 (7%) | 239 (7%) | 188 (8%) | 141 (6%) |
| E+P | 141 (5%) | 171 (5%) | 113 (5%) | 121 (5%) |
| DM | 827 (31%) | 978 (30%) | 739 (30%) | 683 (28%) |
| E+P and DM | 199 (8%) | 253 (8%) | 201 (8%) | 154 (6%) |
GED: General equivalency diploma; MET: metabolic equivalent; OS: Observational study; E+P: Estrogen plus progestin; DM: Dietary modification
Baseline stressful life events, social strain, and incident CVD
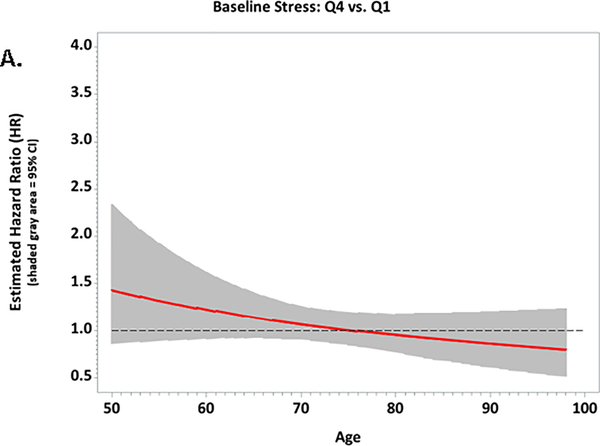
During a mean 12.5 years of follow-up, 1,863 women (17%) experienced a CVD event, at a mean age of 72.6 years. Angina was the most common CVD event (n=636; 34%), followed by stroke (n=348; 19%), coronary heart disease (n=308; 17%), and congestive heart failure (n=302, 16%). In univariable models, higher baseline reports of stressful life events were associated with higher incident CVD at ages 55 (HR for highest vs. lowest quartile=1.80, 95% CI=1.27–2.54) and 65 (HR for highest vs. lowest quartile =1.40, 95% CI=1.16–1.68). In multivariable models that adjusted for established CVD risk factors, the association between stressful life events and incident CVD was no longer significant (Table 3 and Figure 1). Similarly, we observed a univariable association between higher baseline reports of social strain and incident CVD (HR for highest vs. lowest quartile =1.30, 95% CI=1.13–1.49) that was attenuated in the multivariable models (HR=1.13, 95% CI=0.97–1.31). Associations between established CVD risk factors and CVD events were in the expected directions in the multivariable-adjusted model. For example, diabetes (HR=2.25, 95% CI=1.98–2.56), obesity HR=1.18, 95% CI=1.06–1.32), physical inactivity (HR=1.27, 95% CI=1.09, 1.49), and current smoking (HR=2.05, 95% CI=1.76–2.38) were all related to higher CVD event risk.
Table 3.
Age-specific hazard ratios (HRs) and 95% confidence intervals (CIs) for associations of stressful life events, social strain and CVD risk among African-American women in the Women's Health Initiative, n=10,785
| Univariable Model | Multivariable Model* | |||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Stressful life events (at age 55) | ||||
| Q4 (7 – 30) vs. Q1 (0 – 1) | 1.80 (1.27, 2.54) | <0.001 | 1.32 (0.91, 1.92) | 0.15 |
| Q3 (4 – 6) vs. Q1 (0 – 1) | 1.25 (0.88, 1.77) | 0.21 | 1.13 (0.78, 1.63) | 0.51 |
| Q2 (2 – 3) vs. Q1 (0 – 1) | 1.03 (0.72, 1.46) | 0.89 | 0.84 (0.57, 1.23) | 0.36 |
| Stressful life events (at age 65) | ||||
| Q4 (7 – 30) vs. Q1 (0 – 1) | 1.40 (1.16, 1.68) | <0.001 | 1.14 (0.94, 1.40) | 0.19 |
| Q3 (4 – 6) vs. Q1 (0 – 1) | 1.13 (0.94, 1.36) | 0.19 | 1.05 (0.86, 1.28) | 0.62 |
| Q2 (2 – 3) vs. Q1 (0 – 1) | 1.04 (0.86, 1.26) | 0.68 | 0.94 (0.77, 1.15) | 0.55 |
| Stressful life events (at age 75) | ||||
| Q4 (7 – 30) vs. Q1 (0 – 1) | 1.13 (0.97, 1.31) | 0.11 | 1.01 (0.86, 1.19) | 0.88 |
| Q3 (4 – 6) vs. Q1 (0 – 1) | 1.04 (0.9, 1.19) | 0.61 | 0.99 (0.85, 1.14) | 0.85 |
| Q2 (2 – 3) vs. Q1 (0 – 1) | 1.05 (0.92, 1.2) | 0.45 | 1.04 (0.90, 1.20) | 0.60 |
| Stressful life events (at age 85) | ||||
| Q4 (7 – 30) vs. Q1 (0 – 1) | 0.94 (0.73, 1.19) | 0.60 | 0.91 (0.70, 1.18) | 0.48 |
| Q3 (4 – 6) vs. Q1 (0 – 1) | 0.96 (0.77, 1.20) | 0.73 | 0.93 (0.73, 1.18) | 0.57 |
| Q2 (2 – 3) vs. Q1 (0 – 1) | 1.06 (0.86, 1.32) | 0.57 | 1.13 (0.90, 1.43) | 0.29 |
| Stressful life events (at age 95) | ||||
| Q4 (7 – 30) vs. Q1 (0 – 1) | 0.79 (0.55, 1.13) | 0.20 | 0.83 (0.56, 1.22) | 0.34 |
| Q3 (4 – 6) vs. Q1 (0 – 1) | 0.90 (0.64, 1.25) | 0.53 | 0.89 (0.62, 1.27) | 0.52 |
| Q2 (2 – 3) vs. Q1 (0 – 1) | 1.08 (0.77, 1.49) | 0.67 | 1.22 (0.86, 1.74) | 0.26 |
| Social strain | 0.001 | 0.39 | ||
| Q4 (10 – 20) vs. Q1 (4) | 1.30 (1.13, 1.49) | 1.13 (0.97, 1.31) | ||
| Q3 (7 – 9) vs. Q1 (4) | 1.24 (1.09, 1.42) | 1.09 (0.95, 1.25) | ||
| Q2 (5 – 6) vs. Q1 (4) | 1.14 (1.01, 1.30) | 1.04 (0.90, 1.21) | ||
| Diabetes status | ||||
| Yes vs. No | 2.61 (2.32, 2.93) | <0.001 | 2.25 (1.98, 2.56) | <0.001 |
| BMI | <0.001 | <0.001 | ||
| <25 vs. 25–29 kg/m2 | 0.79 (0.68, 0.93) | 0.79 (0.67, 0.93) | ||
| ≥30 vs. 25–29 kg/m2 | 1.39 (1.25, 1.54) | 1.18 (1.06, 1.32) | ||
| Physical activity | <0.001 | 0.01 | ||
| None | 1.44 (1.24, 1.68) | 1.27 (1.09, 1.49) | ||
| >0–3.75 vs. ≥17.5 MET-hours/week | 1.47 (1.26, 1.72) | 1.28 (1.09, 1.51) | ||
| 3.75–8.75 vs. ≥17.5 MET-hours/week | 1.26 (1.08, 1.47) | 1.16 (0.99, 1.37) | ||
| 8.75–17.5 vs. ≥17.5 MET-hours/week | 1.16 (0.98, 1.36) | 1.10 (0.93, 1.30) | ||
| Hypertension history | <0.001 | <0.001 | ||
| Treated hypertensive vs. Never hypertensive | 1.71 (1.55, 1.9) | 1.46 (1.31, 1.63) | ||
| Untreated hypertensive vs. Never hypertensive | 1.44 (1.22, 1.72) | 1.31 (1.09, 1.56) | ||
| Use of anti-hyperlipidemia drugs | ||||
| Yes vs. No | 1.42 (1.25, 1.6) | <0.001 | 1.24 (1.09, 1.41) | <0.001 |
| Smoking status | <0.001 | <0.001 | ||
| Past smoker vs. Never smoked | 1.21 (1.09, 1.33) | 1.24 (1.12, 1.38) | ||
| Current smoker vs. Never smoked | 1.86 (1.62, 2.13) | 2.05 (1.76, 2.38) | ||
| Education | <0.001 | <0.001 | ||
| Less than High school diploma/GED vs. College graduate or higher | 1.99 (1.72, 2.31) | 1.61 (1.37, 1.90) | ||
| High school diploma/GED vs. College graduate or higher | 1.38 (1.19, 1.59) | 1.14 (0.98, 1.34) | ||
| Some college vs. College graduate or higher | 1.27 (1.14, 1.41) | 1.19 (1.06, 1.34) | ||
Adjusted for stressful life events, social strain, diabetes status, BMI, physical activity, hypertension history, use of anti-hyperlipidemia drugs, smoking status, and education
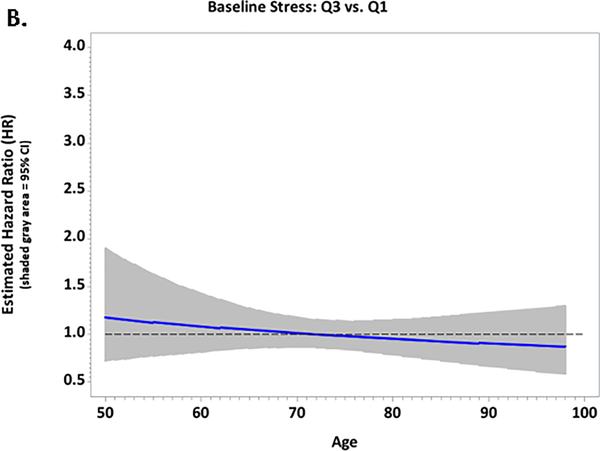
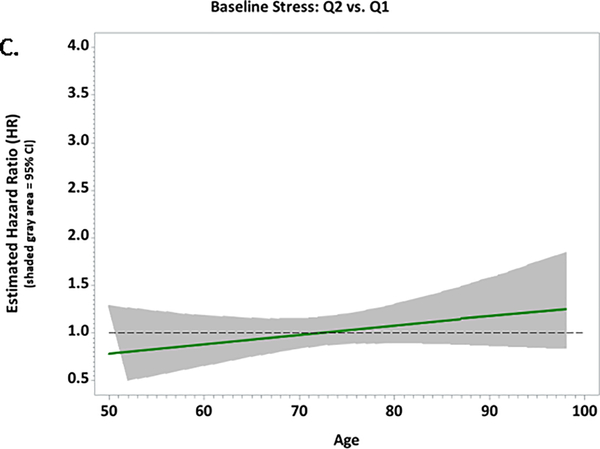
Figure 1.
Plots of the multivariable-adjusted hazard ratios and 95% confidence intervals for associations between baseline stressful life events and CVD risk according to age among African-American women in the Women’s Health Initiative; A. Quartile 4 vs. Quartile 1; B. Quartile 3 vs. Quartile 1; C. Quartile 2 vs. Quartile 1
The modifying role of resilience
Among the 10,785 African-American women included in the baseline analyses, 2,765 (25.6%) had data on resilience and stressful life events from the Extension Study 2 questionnaire. Associations between resilience, stressful life events, and baseline factors are shown in Table 4. African-American women in the highest quartile of resilience reported lower stress, had higher educational attainment, were less likely to be obese, more commonly used oral contraceptives, less commonly used anti-hyperlipidemia drugs, and were more physically active compared with African-American women in the lower quartile of resilience.
Table4.
Characteristics of Study Participants according to Resilience, Women's Health Initiative Observational and Clinical Trials, n=2,765*
| Resilience | ||||
|---|---|---|---|---|
| Q1 (3 – 12) | Q2 (13 – 15) | Q3 (16 – 17) | Q4 (18) | |
| (n=604) | (n=766) | (n=500) | (n=895) | |
| Age at WHI entry Mean (SD) | 59.5 (6.2) | 59.2 (6.1) | 58.7 (6.1) | 58.8 (6) |
| Age at Extension 2 questionnaire Mean (SD) | 75.0 (6.1) | 74.6 (6) | 74.2 (6) | 74.2 (5.9) |
| Stressful life events: | ||||
| Q4 (5 – 22) | 232 (38%) | 226 (30%) | 131 (26%) | 143 (16%) |
| Q3 (3 – 4) | 143 (24%) | 191 (25%) | 156 (31%) | 232 (26%) |
| Q2 (1 –2) | 126 (21%) | 192 (25%) | 122 (24%) | 272 (30%) |
| Q1 (0) | 103 (17%) | 157 (20%) | 91 (18%) | 248 (28%) |
| Education | ||||
| Missing | 6 (1%) | 5 (1%) | 5 (1%) | 6 (1%) |
| Less than High school diploma or GED | 31 (5%) | 25 (3%) | 15 (3%) | 26 (3%) |
| High school diploma or GED | 83 (14%) | 97 (13%) | 46 (9%) | 83 (9%) |
| Some college | 252 (42%) | 296 (39%) | 169 (34%) | 321 (36%) |
| College graduate or higher | 232 (38%) | 343 (45%) | 265 (53%) | 459 (51%) |
| Smoking status: | ||||
| Missing | 7 (1%) | 12 (2%) | 4 (1%) | 9 (1%) |
| Never smoked | 287 (48%) | 373 (49%) | 234 (47%) | 449 (50%) |
| Past smoker | 254 (42%) | 299 (39%) | 205 (41%) | 372 (42%) |
| Current smoker | 56 (9%) | 82 (11%) | 57 (11%) | 65 (7%) |
| BMI: | ||||
| Missing | 11 (2%) | 5 (1%) | 5 (1%) | 9 (1%) |
| <25 kg/m2 | 94 (16%) | 140 (18%) | 99 (20%) | 177 (20%) |
| 25–29 kg/m2 | 216 (36%) | 270 (35%) | 177 (35%) | 342 (38%) |
| ≥30 kg/m2 | 283 (47%) | 351 (46%) | 219 (44%) | 367 (41%) |
| Hormone use: | ||||
| Never | 330 (55%) | 422 (55%) | 267 (53%) | 496 (55%) |
| Past user | 90 (15%) | 115 (15%) | 73 (15%) | 118 (13%) |
| Current user <5 years | 76 (13%) | 85 (11%) | 75 (15%) | 94 (11%) |
| Current user 5 to <10 years | 49 (8%) | 61 (8%) | 38 (8%) | 87 (10%) |
| Current user ≥10 years | 59 (10%) | 83 (11%) | 47 (9%) | 100 (11%) |
| Type of hormone use: | ||||
| Never user | 330 (55%) | 422 (55%) | 267 (53%) | 496 (55%) |
| Past user of either E alone or E+P | 90 (15%) | 115 (15%) | 73 (15%) | 118 (13%) |
| E-alone | 134 (22%) | 172 (22%) | 106 (21%) | 194 (22%) |
| E+P | 50 (8%) | 57 (7%) | 54 (11%) | 87 (10%) |
| Oral contraceptive use | 263 (44%) | 352 (46%) | 258 (52%) | 449 (50%) |
| Diabetes status: | ||||
| Missing | 2 (<1%) | 0 | 0 | 1 (<1%) |
| Yes | 35 (6%) | 40 (5%) | 33 (7%) | 45 (5%) |
| Use of anti-hyperlipidemia drugs: | ||||
| Missing | 48 (8%) | 38 (5%) | 37 (7%) | 37 (4%) |
| Yes | 71 (12%) | 93 (12%) | 44 (9%) | 74 (8%) |
| Hypertension history: | ||||
| Missing | 39 (6%) | 31 (4%) | 36 (7%) | 35 (4%) |
| Never hypertensive | 286 (47%) | 395 (52%) | 252 (50%) | 487 (54%) |
| Treated hypertensive | 217 (36%) | 288 (38%) | 175 (35%) | 299 (33%) |
| Untreated hypertensive | 62 (10%) | 52 (7%) | 37 (7%) | 74 (8%) |
| Physical activity: | ||||
| Missing | 33 (5%) | 27 (4%) | 33 (7%) | 30 (3%) |
| None | 122 (20%) | 158 (21%) | 93 (19%) | 191 (21%) |
| >0–3.75 MET-hours/week | 95 (16%) | 122 (16%) | 71 (14%) | 113 (13%) |
| 3.75–8.75 MET-hours/week | 132 (22%) | 142 (19%) | 95 (19%) | 178 (20%) |
| 8.75–17.5 MET-hours/week | 119 (20%) | 153 (20%) | 95 (19%) | 175 (20%) |
| ≥17.5 MET-hours/week | 103 (17%) | 164 (21%) | 113 (23%) | 208 (23%) |
| WHI Trial membership: | ||||
| OS | 248 (41%) | 342 (45%) | 228 (46%) | 425 (47%) |
| E-only | 37 (6%) | 44 (6%) | 30 (6%) | 55 (6%) |
| E+P | 27 (4%) | 33 (4%) | 28 (6%) | 50 (6%) |
| DM | 236 (39%) | 268 (35%) | 174 (35%) | 293 (33%) |
| E+P and DM | 56 (9%) | 79 (10%) | 40 (8%) | 72 (8%) |
OS: Observational study; E+P: Estrogen plus progestin; DM: Dietary modification
Demographics are restricted to the 2,765 participants with available resilience information from Extension Study 2.
Of the Extension Study 2 cohort, 202 (7.3%) experienced a CVD event, with a mean age at first CVD event of 79.5 years (SD=6.6 years; range: 64.6 – 96.3 years). We observed no interaction between resilience and stressful life events in either a univariable (p=0.74) or multivariable Cox proportional hazards model for incident CVD (p=0.48). Therefore, we present the multivariable-adjusted main effects of stressful life events and resilience in relation to incident CVD in Table 5. Similar to estimates in the overall cohort, higher stressful life events was positively associated with incident CVD (HR for highest vs. lowest quartile =1.61, 95% CI=1.04–2.51; Q2 vs Q1 HR=1.83, 95% CI=1.19–2.82) even after accounting for CVD risk factors. However, resilience was not associated with incident CVD (HR for lowest vs. highest quartile=0.95, 95% CI=0.63–1.42).
Table 5.
Hazard ratios (HRs) and 95% confidence intervals (CIs) for associations of stressful life events, established CVD risk factors, and CVD risk among African-American women in the Women's Health Initiative, n=2,765*
| events/N | Total person-years | Multivariable Model HR (95% CI)† | p | |
|---|---|---|---|---|
| Stressful life events | 0.02 | |||
| Q4 (5 – 22) | 65/732 | 2832.8 | 1.61 (1.04, 2.51) | |
| Q3 (3 – 4) | 49/722 | 2838.2 | 1.20 (0.75, 1.89) | |
| Q2 (1 –2) | 66/712 | 2733.9 | 1.83 (1.19, 2.82) | |
| Q1 (0) | 35/599 | 2358.8 | 1.00 | |
| Resilience | 0.66 | |||
| Q4 (18) | 60/895 | 3536.3 | 1.00 | |
| Q3 (16 – 17) | 36/500 | 2015.9 | 1.04 (0.72, 1.51) | |
| Q2 (13 – 15) | 61/766 | 2962.8 | 1.04 (0.72, 1.51) | |
| Q1 (3 – 12) | 58/604 | 2248.7 | 0.95 (0.63, 1.42) | |
| Diabetes status | 0.07 | |||
| No | 194/2609 | 10217.9 | 1.00 | |
| Yes | 21/153 | 534.0 | 1.59 (0.97, 2.62) | |
| BMI | 0.09 | |||
| <25 kg/m2 | 35/510 | 2044.3 | 1.02 (0.66, 1.57) | |
| 25–29 kg/m2 | 66/1005 | 3990.3 | 1.00 | |
| ≥30 kg/m2 | 111/1220 | 4614.6 | 1.40 (1.01, 1.95) | |
| Physical activity | 0.20 | |||
| None | 32/564 | 2210.4 | 0.70 (0.44, 1.13) | |
| >0–3.75 MET-hours/week | 35/401 | 1516.8 | 0.92 (0.57, 1.49) | |
| 3.75–8.75 MET-hours/week | 47/547 | 2102.9 | 1.18 (0.77, 1.82) | |
| 8.75–17.5 MET-hours/week | 44/542 | 2160.4 | 1.14 (0.74, 1.77) | |
| ≥17.5 MET-hours/week | 45/588 | 2301.9 | 1.00 | |
| Hypertension history | 0.99 | |||
| Never hypertensive | 93/1420 | 5630.8 | 1.00 | |
| Treated hypertensive | 20/225 | 861.8 | 1.01 (0.74, 1.38) | |
| Untreated hypertensive | 89/979 | 3723.3 | 0.99 (0.59, 1.66) | |
| Use of anti-hyperlipidemia drugs | 0.60 | |||
| No | 176/2323 | 9072.2 | 1.00 | |
| Yes | 25/282 | 1070.8 | 0.89 (0.57, 1.39) | |
| Smoking status | <0.001 | |||
| Never smoked | 82/1343 | 5314.0 | 1.00 | |
| Past smoker | 102/1130 | 4338.4 | 1.48 (1.08, 2.02) | |
| Current smoker | 27/260 | 991.2 | 2.53 (1.58, 4.04) | |
| Education | 0.01 | |||
| Less than High school diploma or GED | 10/97 | 359.7 | 1.64 (0.83, 3.24) | |
| High school diploma or GED | 22/309 | 1183.3 | 1.02 (0.61, 1.71) | |
| Some college | 101/1038 | 3991.5 | 1.63 (1.19, 2.25) | |
| College graduate or higher | 81/1299 | 5149.5 | 1.00 | |
Analysis is restricted to the 2,765 participants with available resilience information from Extension Study 2.
Adjusted for stressful life events, diabetes status, BMI, physical activity, hypertension history, use of anti-hyperlipidemia drugs, smoking status, and education.
Discussion
In this large study of older African-American women, we observed associations between baseline reports of higher stressful life events and increased incident CVD that diminished with age. Moreover, the age-specific associations were subsequently attenuated when conventional CVD risk factors were accounted. Likewise, higher social strain was related to incident CVD, but associations were attenuated with CVD risk factor adjustment. In the sub-cohort of women with updated information on stressful life events, we observed a significant association with CVD that was independent of CVD risk factors. Yet, contrary to our hypothesis, the relationship between stressful life events and incident CVD was not modified by resilience, nor was resilience independently related to incident CVD in the sub-cohort.
Epidemiological research conducted among male and female study populations support a link between psychosocial factors and CVD 18, 19. For example, a London-based study of psychological distress in 4,374 men and 1,895 women reported increased risk of coronary heart disease associated with higher baseline levels of psychological distress; associations among women were of a smaller magnitude compared to men 20. In the large INTERHEART study, general stress, adverse life events, and financial stress were consistently associated with myocardial infarction risk, independent of smoking behavior and socioeconomic status for both men and women 21. Moreover, adverse childhood events, depression, and anger were more strongly related to ischemic heart disease risk than traditional risk factors in a study including 9,367 women and 7,970 men 22. However, it is widely known that sex is an important biological variable. In the female-only Nurses’ Health Study II, trauma and posttraumatic stress disorder were associated with elevated incident CVD 23. Inclusion of race along with female sex is a focal point in understanding the impact of psychosocial factors and incident CVD.
Few studies have investigated stress and CVD in study populations with large numbers of African-American women, which is unfortunate given that African-American women are disproportionately affected by various psychosocial challenges, including more limited access to healthcare through insurance 24, generally lower median household income 25, less access to healthy food options 26, and higher exposure to crime 27 when compared with White women. Moreover, there is evidence to suggest that race-related stress is associated with cardiovascular health 28. These factors may act independently and interactively to uniquely increase CVD risk among African-American women.
In the Jackson Heart Study cohort, a cross-sectional analysis found that higher levels of stress were positively associated with CVD risk factors, including hypertension, diabetes, and obesity – findings consistent with our own data 29. In a prior WHI analysis including women of any race, baseline reports of higher stressful life events and social strain were associated with CVD; however, associations were attenuated with adjustment for traditional risk factors 7. Further, effect modification by race was not observed in the prior WHI study, suggesting that associations were similar among White and African-American women. In the current WHI analysis of African-American women, we observed an association between baseline reports of stressful life events and incident CVD that was also attenuated in multivariable models. However, in the sub-cohort of women with updated assessments of stressful life events, the association of higher stressful life events and incident CVD was independent of traditional risk factors. Of note, women in quartiles two and four of stressful life events had significantly higher CVD risk than women in quartile one. The lack of a clear dose-response relationship might suggest that the scale used to measure stressful life events is inadequate in capturing the full multidimensional nature of stress. In addition, the WHI study assessed an acute measure of stressful life events, i.e. events occurring in the preceding year. As such, we hypothesize that the age-related waning effect of stressful life events on incident CVD reflects a distance from events that no longer impact cardiovascular health. The observation that an updated assessment of stressful life events (evaluated during Extension Study 2) was independently associated with CVD reinforces this concept. Additional studies with assessment of cumulative or chronic stress are needed to understand pathways that lend to modification and mediation of stress and disease.
The association between stress and disease was first introduced by Selye 30 and later adapted within the context of specific diseases, including CVD. Stress is hypothesized to contribute to CVD through direct and indirect routes. The biological response to stress includes raised blood pressure, reduced insulin sensitivity, increased hemostasis, and endothelial dysfunction, all of which could conceivably contribute to CVD 31. Moreover, repeated exposure to stress over time causes “wear and tear” on the body that initiates additional cascades of stress and disease 31, 32. Indirectly, stress might influence CVD-related risk factors as observed in this study and by others 29, 33. Evidence suggesting that stress (e.g., discrimination) is linked to disease occurrence and is perhaps intergenerational, places greater stakes on eliminating psychosocial disparities 34, 35.
Missing from the literature is an evaluation of resilience, or the ability to bounce back or recover from stress in the context of African-Americans women’s cardiovascular health. Resilience is operationalized as the ability to adapt to experiences of stress or adversity and maintain a stable trajectory of healthy psychosocial and physical functioning 36. Some have demonstrated that higher levels of resilience are linked with longitudinal declines in depressive symptoms among individuals with long-term physical disabilities 37 and overall longevity 38 in study populations inclusive of men and women. In another study, resilience was shown to significantly buffer the association between the co-occurrence of substance abuse, violence, and HIV/AIDS and depressive symptoms among African-American women 9. In the current study, we did not observe a direct relationship between resilience and incident CVD, nor did resilience modify the association between stressful life events and incident CVD. We used a shortened version of the Brief Resilience Scale, administered to women during the WHI Extension Study 2. Of note, the women who participated in the WHI Extension Study 2 sub-cohort were women who were alive and motivated to participate, likely culminating in a healthier and non-representative study population. As such, selection bias may underlie the null findings we observed here. In addition, the Brief Resilience Scale is limited to the individual context. Other dimensions, as described by the multisystemic social-ecological theory of resilience, including the quality of the environment 39 and resilience resources 40 might be important considerations for African-American women. Despite our null findings, future studies should explore these features.
Traditional health behavioral interventions for ideal cardiovascular health include smoking cessation, healthy diet, and physical activity; greatest CVD risk reduction is achieved when such interventions are concurrent 41. On the other hand, the extent to which interventions to reduce stress result in lower incidence of CVD events is questionable based on limited success when population-wide strategies are implemented 42. However, intervening on intermediary factors downstream of stress (e.g. physical activity) that are also implicated in CVD etiology may be a positive strategy to simultaneously reduce stress and CVD risk. Future studies among African-American women are needed to test these mediation hypotheses, and to inform future intervention studies.
Strengths of our study include the large sample size of African-American women, prospective design, use of previously validated stressful life events and social strain measures, and adjudication of clinical endpoints for all women included in this study. Further, this is the first study to examine the direct and moderating effects of resilience in relation to CVD among African-American women, which represents an important extension of the current literature.
Limitations of our study center on the scales used to characterize stress and resilience. Specifically, the stressful life events scale queries respondents on the occurrence of certain events during the one-year interval prior to questionnaire completion. This one year duration likely marks an acute as opposed to chronic or cumulative measure of stress. As such, we cannot make conclusions regarding chronic stress, which has been linked with higher CVD 43. Moreover, we used the stressful life events scale as a composite; however, not all of the individual stressors queried therein are equal. It is possible that some of the stressors may be more impactful and detrimental to CV health (e.g. financial stressors) than others, even if the respondent does not endorse worrying about it a great deal. Further, the stressful life events scale does not include discrimination or perceived racism domains, which might be more relevant for African-American women. We also lacked multiple repeat measurements for our main exposures, preventing an assessment how stress, strain, and resilience change over time and the potential impact on CVD risk. As part of our analytic approach, we chose to categorize stress and strain into quartiles. However, due to the skewed distributions of both stress and strain, the highest quartiles contained a wide range of responses, particularly for stress (range for the highest quartile: 7–30). Our analyses assumed that the relationship between stress/strain and incident CVD for participants within each quartile was consistent; unfortunately, with so few participants with higher stress and/or strain levels (for example, 95% of the participants had a composite stress sum less than or equal to 11), we were not able to verify this assumption.
The Brief Resilience Scale, a validated instrument for resilience, is a six-item scale 16. In WHI, a modified version consisting of three items was available. It is possible that the modified version does not recapitulate the validated scale, potentially contributing to null findings regarding resilience and CVD risk. Despite this, our study is the first to examine this novel effect modification hypothesis in a large prospective cohort of African-American women. Finally, our results are of limited generalizability given the older age distribution and high educational attainment.
In conclusion, we observed age-related associations between stressful life events reported within the previous year and risk of developing CVD among African-American women. In the overall cohort of women, these associations were attenuated when conventional CVD risk factors were accounted. However, in the sub-cohort of women with updated measures, persistent relationships were observed. Additional studies among African-American women with diverse education and income levels across a range of ages are needed. In addition, the context of resilience should be further explored as resilience and resilience resources represent novel and potentially more malleable CVD intervention targets.
Supplementary Material
What is Known
African-American women have a higher burden of CVD compared to their White counterparts.
Stress is modestly associated with higher risk of cardiovascular disease in mostly White study populations.
What the Study Adds
Higher baseline stressful life events and social strain were associated with higher CVD risk among African-American women; however, adjustment for traditional CVD risk factors attenuated these associations.
In a sub-cohort of women with updated stressful life events and resilience measures, higher stressful life events was independently associated with higher incident CVD.
Resilience was not independently associated with CVD risk nor did it modify the association between stressful life events and CVD risk.
Acknowledgements
The authors would like acknowledge the following WHI Principal Investigators and academic medical centers. Program office: Jacques Rossouw, Shari Ludlam, Dale Burwen, Joan McGowan, Leslie Ford, and Nancy Geller (National Heart, Lung, and Blood Institute, Bethesda, Maryland); Clinical Coordinating Center: Garnet Anderson, Ross Prentice, Andrea LaCroix, and Charles Kooperberg (Fred Hutchinson Cancer Research Center, Seattle, WA); Investigators and Academic Centers: JoAnn E. Manson (Brigham and Women’s Hospital, Harvard Medical School, Boston, MA); Barbara V. Howard (MedStar Health Research Institute/Howard University, Washington, DC); Marcia L. Stefanick (Stanford Prevention Research Center, Stanford, CA); Rebecca Jackson (The Ohio State University, Columbus, OH); Cynthia A. Thomson (University of Arizona, Tucson/Phoenix, AZ); Jean Wactawski-Wende (University at Buffalo, Buffalo, NY); Marian Limacher (University of Florida, Gainesville/Jacksonville, FL); Robert Wallace (University of Iowa, Iowa City/Davenport, IA); Lewis Kuller (University of Pittsburgh, Pittsburgh, PA); Sally Shumaker (Wake Forest University School of Medicine, Winston-Salem, NC).
Sources of Funding
This work was supported by the National Cancer Institute (K01CA21845701A1 to ASF and K12CA133250 to DA), the National Heart, Lung, and Blood Institute (K01HL142848 to KB), University of Arizona Health Sciences, Strategic Priorities Faculty Initiative Grant (KB), and University of Arizona Sarver Heart Center (KB). The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201600018C, HHSN268201600001C, HHSN268201600002C, HHSN268201600003C, and HHSN268201600004C.
Footnotes
Disclosures
None
REFERENCES
- 1.Mensah GA, Wei GS, Sorlie PD, Fine LJ, Rosenberg Y, Kaufmann PG, Mussolino ME, Hsu LL, Addou E, Engelgau MM and Gordon D. Decline in Cardiovascular Mortality: Possible Causes and Implications. Circulation research. 2017;120:366–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ford ES, Ajani UA, Croft JB, Critchley JA, Labarthe DR, Kottke TE, Giles WH and Capewell S. Explaining the decrease in U.S. deaths from coronary disease, 1980–2000. N Engl J Med. 2007;356:2388–98. [DOI] [PubMed] [Google Scholar]
- 3.Decline in deaths from heart disease and stroke--United States, 1900–1999. MMWR Morbidity and mortality weekly report. 1999;48:649–56. [PubMed] [Google Scholar]
- 4.Garcia M, Mulvagh SL, Merz CN, Buring JE and Manson JE. Cardiovascular Disease in Women: Clinical Perspectives. Circulation research. 2016;118:1273–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones KM, Carter MM and Schulkin J. Racial and Ethnic Disparities in Cardiovascular Disease: An Assessment of Obstetrician-Gynecologists’ Knowledge, Attitudes, and Practice Patterns. Journal of racial and ethnic health disparities. 2015;2:256–66. [DOI] [PubMed] [Google Scholar]
- 6.Steptoe A and Kivimäki M. Stress and cardiovascular disease. Nature Reviews Cardiology. 2012;9:360. [DOI] [PubMed] [Google Scholar]
- 7.Kershaw KN, Brenes GA, Charles LE, Coday M, Daviglus ML, Denburg NL, Kroenke CH, Safford MM, Savla T, Tindle HA, Tinker LF and Van Horn L. Associations of stressful life events and social strain with incident cardiovascular disease in the Women’s Health Initiative. Journal of the American Heart Association. 2014;3:e000687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ungar M. Researching and theorizing resilience across cultures and contexts. Preventive medicine. 2012;55:387–9. [DOI] [PubMed] [Google Scholar]
- 9.Thurston IB, Howell KH, Kamody RC, Maclin-Akinyemi C and Mandell J. Resilience as a moderator between syndemics and depression in mothers living with HIV. AIDS care. 2018:1–8. [DOI] [PubMed] [Google Scholar]
- 10.Anderson GL, Manson J, Wallace R, Lund B, Hall D, Davis S, Shumaker S, Wang CY, Stein E and Prentice RL. Implementation of the Women’s Health Initiative study design. Ann Epidemiol. 2003;13:S5–17. [DOI] [PubMed] [Google Scholar]
- 11.Hays J, Hunt JR, Hubbell FA, Anderson GL, Limacher M, Allen C and Rossouw JE. The Women’s Health Initiative recruitment methods and results. Ann Epidemiol. 2003;13:S18–77. [DOI] [PubMed] [Google Scholar]
- 12.Stefanick ML, Cochrane BB, Hsia J, Barad DH, Liu JH and Johnson SR. The Women’s Health Initiative postmenopausal hormone trials: overview and baseline characteristics of participants. Ann Epidemiol. 2003;13:S78–86. [DOI] [PubMed] [Google Scholar]
- 13.Design of the Women’s Health Initiative clinical trial and observational study. The Women’s Health Initiative Study Group. Controlled clinical trials. 1998;19:61–109. [DOI] [PubMed] [Google Scholar]
- 14.Ruberman W, Weinblatt E, Goldberg JD and Chaudhary BS. Psychosocial influences on mortality after myocardial infarction. N Engl J Med. 1984;311:552–9. [DOI] [PubMed] [Google Scholar]
- 15.Rook KS. The negative side of social interaction: impact on psychological well-being. Journal of personality and social psychology. 1984;46:1097–1108. [DOI] [PubMed] [Google Scholar]
- 16.Smith BW, Dalen J, Wiggins K, Tooley E, Christopher P and Bernard J. The brief resilience scale: assessing the ability to bounce back. International journal of behavioral medicine. 2008;15:194–200. [DOI] [PubMed] [Google Scholar]
- 17.Heckbert SR, Kooperberg C, Safford MM, Psaty BM, Hsia J, McTiernan A, Gaziano JM, Frishman WH and Curb JD. Comparison of self-report, hospital discharge codes, and adjudication of cardiovascular events in the Women’s Health Initiative. Am J Epidemiol. 2004;160:1152–8. [DOI] [PubMed] [Google Scholar]
- 18.Steptoe A and Kivimaki M. Stress and cardiovascular disease: an update on current knowledge. Annu Rev Public Health. 2013;34:337–54. [DOI] [PubMed] [Google Scholar]
- 19.Everson-Rose SA and Lewis TT. Psychosocial factors and cardiovascular diseases. Annu Rev Public Health. 2005;26:469–500. [DOI] [PubMed] [Google Scholar]
- 20.Stansfeld SA, Fuhrer R, Shipley MJ and Marmot MG. Psychological distress as a risk factor for coronary heart disease in the Whitehall II Study. International journal of epidemiology. 2002;31:248–55. [DOI] [PubMed] [Google Scholar]
- 21.Rosengren A, Hawken S, Ounpuu S, Sliwa K, Zubaid M, Almahmeed WA, Blackett KN, Sitthi-amorn C, Sato H and Yusuf S. Association of psychosocial risk factors with risk of acute myocardial infarction in 11119 cases and 13648 controls from 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:953–62. [DOI] [PubMed] [Google Scholar]
- 22.Dong M, Giles WH, Felitti VJ, Dube SR, Williams JE, Chapman DP and Anda RF. Insights into causal pathways for ischemic heart disease: adverse childhood experiences study. Circulation. 2004;110:1761–6. [DOI] [PubMed] [Google Scholar]
- 23.Sumner JA, Kubzansky LD, Elkind MS, Roberts AL, Agnew-Blais J, Chen Q, Cerda M, Rexrode KM, Rich-Edwards JW, Spiegelman D, Suglia SF, Rimm EB and Koenen KC. Trauma Exposure and Posttraumatic Stress Disorder Symptoms Predict Onset of Cardiovascular Events in Women. Circulation. 2015;132:251–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomasson MA. Racial Differences in Health Insurance Coverage and Medical Expenditures in the United States: A Historical Perspective. Social Science History. 2016;30:529–550. [Google Scholar]
- 25.Guzman GG. Household income: 2016. American community survey briefs, ACSBR/16–02, US Census Bureau. 2017.
- 26.Kirkpatrick SI, Dodd KW, Reedy J and Krebs-Smith SM. Income and race/ethnicity are associated with adherence to food-based dietary guidance among US adults and children. Journal of the Academy of Nutrition and Dietetics. 2012;112:624–635.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Unger E, Diez-Roux AV, Lloyd-Jones DM, Mujahid MS, Nettleton JA, Bertoni A, Badon SE, Ning H and Allen NB. Association of neighborhood characteristics with cardiovascular health in the multi-ethnic study of atherosclerosis. Circulation Cardiovascular quality and outcomes. 2014;7:524–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wyatt SB, Williams DR, Calvin R, Henderson FC, Walker ER and Winters K. Racism and cardiovascular disease in African Americans. The American journal of the medical sciences. 2003;325:315–31. [DOI] [PubMed] [Google Scholar]
- 29.Gebreab SY, Diez-Roux AV, Hickson DA, Boykin S, Sims M, Sarpong DF, Taylor HA and Wyatt SB. The contribution of stress to the social patterning of clinical and subclinical CVD risk factors in African Americans: the Jackson Heart Study. Social science & medicine (1982). 2012;75:1697–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Selye H. Stress and the general adaptation syndrome. British medical journal. 1950;1:1383–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peters A and McEwen BS. Stress habituation, body shape and cardiovascular mortality. Neuroscience and biobehavioral reviews. 2015;56:139–50. [DOI] [PubMed] [Google Scholar]
- 32.McEwen BS and Stellar E. Stress and the individual. Mechanisms leading to disease. Archives of internal medicine. 1993;153:2093–101. [PubMed] [Google Scholar]
- 33.Albert MA, Durazo EM, Slopen N, Zaslavsky AM, Buring JE, Silva T, Chasman D and Williams DR. Cumulative psychological stress and cardiovascular disease risk in middle aged and older women: Rationale, design, and baseline characteristics. American heart journal. 2017;192:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taylor JY, Wright ML, Crusto CA and Sun YV. The Intergenerational Impact of Genetic and Psychological Factors on Blood Pressure (InterGEN) Study: Design and Methods for Complex DNA Analysis. Biological research for nursing. 2016;18:521–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taylor JY, Sun YV, Barcelona de Mendoza V, Ifatunji M, Rafferty J, Fox ER, Musani SK, Sims M and Jackson JS. The combined effects of genetic risk and perceived discrimination on blood pressure among African Americans in the Jackson Heart Study. Medicine. 2017;96:e8369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bonanno GA, Westphal M and Mancini AD. Resilience to loss and potential trauma. Annual review of clinical psychology. 2011;7:511–35. [DOI] [PubMed] [Google Scholar]
- 37.Silverman AM, Molton IR, Alschuler KN, Ehde DM and Jensen MP. Resilience predicts functional outcomes in people aging with disability: a longitudinal investigation. Archives of physical medicine and rehabilitation. 2015;96:1262–8. [DOI] [PubMed] [Google Scholar]
- 38.Elliott AM, Burton CD and Hannaford PC. Resilience does matter: evidence from a 10-year cohort record linkage study. BMJ open. 2014;4:e003917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ungar M, Ghazinour M and Richter J. Annual Research Review: What is resilience within the social ecology of human development? Journal of child psychology and psychiatry, and allied disciplines. 2013;54:348–66. [DOI] [PubMed] [Google Scholar]
- 40.Connor KM and Davidson JR. Development of a new resilience scale: the Connor-Davidson Resilience Scale (CD-RISC). Depression and anxiety. 2003;18:76–82. [DOI] [PubMed] [Google Scholar]
- 41.Dong C, Rundek T, Wright CB, Anwar Z, Elkind MS and Sacco RL. Ideal cardiovascular health predicts lower risks of myocardial infarction, stroke, and vascular death across whites, blacks, and hispanics: the northern Manhattan study. Circulation. 2012;125:2975–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kivimäki M and Steptoe A. Effects of stress on the development and progression of cardiovascular disease. Nature Reviews Cardiology. 2017;15:215. [DOI] [PubMed] [Google Scholar]
- 43.Kershaw KN, Diez Roux AV, Bertoni A, Carnethon MR, Everson-Rose SA and Liu K. Associations of chronic individual-level and neighbourhood-level stressors with incident coronary heart disease: the Multi-Ethnic Study of Atherosclerosis. J Epidemiol Community Health. 2015;69:136–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.