Abstract
Longitudinal studies of chronic wasting disease (CWD) in the native host have provided considerable understanding of how this prion disease continues to efficiently spread among cervid species. These studies entail great cost in animal, time and financial support. A variety of methods have emerged including transgenic mouse bioassay, western blot, enzyme-linked immunoassay (ELISA), immunohistochemistry (IHC), serial protein misfolding cyclic amplification (sPMCA) and real time quaking-induced conversion (RT-QuIC), that deepen our understanding of this and other protein misfolding disorders. To further characterize an inoculum source used for ongoing CWD studies and to determine how the readouts from each of these assays compare, we assayed a CWD-positive brain pool homogenate (CBP6) and a mouse dilutional bioassay of this homogenate using the above detection methods. We demonstrate that: (i) amplification assays enhanced detection of amyloid seeding activity in the CWD+ cervid brain pool to levels beyond mouse LD50, (ii) conventional detection methods (IHC and western blot) performed well in identifying the presence of PrPSc in terminal brain tissue yet lack sufficient detection sensitivity to identify all CWD-infected mice, and (iii) the incorporation of amplification assays enhanced detection of CWD-infected mice near the LD50. This cross-platform analysis provides a basis to calibrate the relative sensitivities of CWD detection assays.
Introduction
Chronic wasting disease (CWD) is unique among the prion diseases due to its capacity to infect a wildlife population and for its unparalleled transmission efficiency. Infectious particles shed in several bodily fluids and excretions from infected, yet asymptomatic cervids likely contribute to this efficient transmissibility [1–6]. Additional concern is warranted for cervids and species sympatric with cervid populations as the geographical distribution, host range [7, 8, 9] and strain identification [10–12] of CWD continues to expand, undoubtedly resulting in increased environmental contamination. Although no cases of CWD transmission to humans have been reported, its zoonotic potential remains a concern [10, 13] due to the prevalence of asymptomatic CWD in deer and elk [14, 15] (commonly consumed by humans) and the precedent of BSE transmission to humans causing vCJD [16]. This growing zoonotic concern, in concert with increased import in early detection for all protein misfolding disorders, has led to enhanced efforts to develop novel tools to detect and monitor prion infections earlier in the disease course.
Diagnoses of prion diseases have historically relied upon detection of the biological marker associated with disease (PrPSc) [17] in terminal brain and lymphoid tissues using western blot (WB), immunohistochemistry (IHC) and enzyme linked immunoassay (ELISA) [18]. Although these methods efficiently diagnose prion infections postmortem, they lack sufficient sensitivity for consistent acute phase detection [18]. Enhanced early detection could estimate prion burdens in tissue and biological fluids, and provide new insights into peripheral prion trafficking, disease pathogenesis, and transmission dynamics to help guide the development of non-invasive diagnostics, therapeutic strategies and management practices.
The major challenge to early diagnosis of prion diseases has been the very low prion concentrations and/or inhibitors that are present in accessible biological samples [19–21]. In addition, prion infectivity is comprised of variably protease sensitive particles [22], yet conventional prion detection methods require the use of protease digestion, which ablates sensitive forms of prion infectivity (PrPSen) and reduces sensitivity. Thus, while bioassay in native and transgenic hosts remains the gold standard for assessment of prion infectivity in biological samples, it remains burdensome due to animals, time required and cost.
The emergence of in vitro prion amplification detection methods, including serial protein misfolding cyclic amplification (sPMCA) and real-time quaking induced conversion (RT-QuIC), have provided rapid and highly sensitive means for prion detection. Unlike IHC, WB and ELISA, sPMCA and RT-QuIC are seeding assays that rely upon the conversion of the native prion protein (PrPC) into PrPSc. sPMCA [23] uses brain homogenate from transgenic mice overexpressing PrPC as substrate, alternating sonication and incubation to amplify PrPSc [24]. This method has been successful in detecting misfolded prion protein in a variety of tissues and bodily fluids from prion-infected hosts [18, 25]. RT-QuIC is a second in vitro amplification assay [26] that has been widely used to detect protein misfolding disorders, including prions [6, 27–32]. RT-QuIC employs recombinant normal prion protein (rPrPC) and intermittent shaking and incubation to initiate the templated conversion of PrP to a misfolded amyloid PrPSc-like form [26], detected by the intercalation of thioflavin T(ThT) into growing amyloid fibrils. RT-QuIC has been shown to detect amyloid seeding activity in a variety of biological tissue and fluid samples [6, 33–40]. Importantly, both sPMCA and RT-QuIC are capable of detecting prions during the early asymptomatic phase of disease [40–45].
We have conducted several past and ongoing analyses, including longitudinal studies in the native host, utilizing a single CWD+ brain homogenate pool (CBP6) to garner better understanding of CWD pathogenesis and transmission dynamics. A direct analysis of this pool by the above in vitro assays and bioassay has not been conducted. To directly address this gap in characterization and to gain a better understanding of how readouts from these methods compare across a single inoculum, we have assessed: (i) CWD+ cervid brain homogenate pool CBP6, and (ii) brain tissue from a transgenic mouse end-point dilutional bioassay of the same homogenate [36] by in vitro assays: WB, ELISA, IHC, sPMCA, RT-QuIC and a combination of sPMCA + RT-QuIC.
Results
Prion detection in CWD+ deer brain homogenate (CBP6)
Detection of PrPSc by conventional western blot (WB) and BioRad enzme-linked immunosorbent assay (ELISA)
Previous studies have demonstrated that conventional methods are adequate to detect prions in tissues harvested postmortem [46–49]. As expected, we were able to demonstrate detection of PrPSc by WB in the 10−1 dilution of unamplified CWD+ cervid brain homogenate following proteinase K (PK) digestion. No amyloid was detected in further dilutions of CWD+ cervid brain CBP6 (10−2 to 10−7), nor in age-matched naïve deer brain (Fig 1, Table 1). BioRad ELISA detected PrPSc in 10−1 and 10−2 dilutions (OD readings of 3.999 and 0.259 respectively) of unamplified CWD+ cervid brain pool CBP6. No amyloid was detected in further dilutions (10−3–10−9), nor in age-matched naïve deer brain (Table 1).
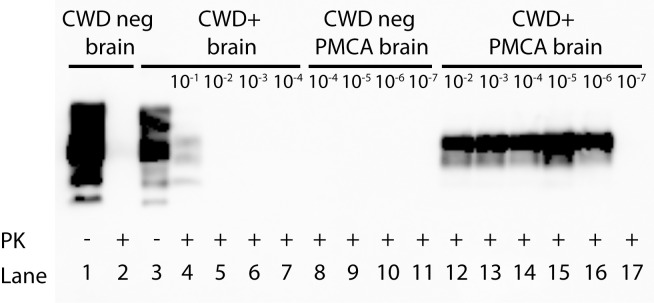
Fig 1. Western blot comparison of prion detection in a dilutional series of unamplified and sPMCA amplified CWD+ cervid brain.
Detection of PrPSc is shown in the 10−1 dilution of unamplified CWD+ cervid brain homogenate CBP6 (500 μg; lane 4). No PrPSc was detected in further dilutions of unamplified CBP6 (lanes 5–7). Detection of PrPSc is shown in the 10−2–10−6 dilutions of sPMCA amplified CBP6 (5 rounds PMCA; initiating seed = 20 μg to 2 ng; lanes 12–16), but not in the 10−7 dilution (200 pg). Complete PK digestion of PrPC is shown in unamplified (lane 2) and sPMCA amplified naïve deer brain (5 rounds; lanes 8–11).
Table 1. Comparison of prion detection in a dilutional series of CWD+ cervid brain pool.
| Cervid brain pool homogenate dilution | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Assay | 10−1 | 10−2 | 10−3 | 10−4 | 10−5 | 10−6 | 10−7 | 10−8 | 10−9 | |
| Western Blot | ||||||||||
| BioRad ELISA | ||||||||||
| Mouse Bioassay | ND | *LD50 | ND | ND | ||||||
| sPMCA/WB | ND | ND | ||||||||
| RT-QuIC | NT | NA | ||||||||
| sPMCA/RT-QuIC | NT | |||||||||
CWD+ cervid brain pool homogenate (CBP6) was serially diluted and assayed for prion endpoint dilution. Conventional WB detected prions at 10−1 (unamplified; 500 μg). BioRad ELISA detected prions at 10−1 and 10−2 dilutions (unamplified; 3 mg-300 μg. Tg(CerPrP) 5037 mouse bioassay demonstrates prion infectivity within CBP6 dilutions 10−2–10−6 (300 μg-30 ng). sPMCA/WB detects amyloid seeding activity from 10−1–10−6 (20 μg-2 ng; 10−8–10−9 not done (ND)). Amyloid seeding activity is detected by RT-QuIC from 10−3–10−7 (2 μg-200 pg). RT-QuIC demonstrates no amplification (NA) at dilution 10−2 (20 μg). sPMCA/RT-QuIC detects amyloid seeding activity from 10−2–10−8 (20 μg-20 pg). No assay detected prions at CBP6 dilution 10−9. Bioassay not done (ND) at 10−1 or 10−8–10−9. 10−1 dilutions were not tested (NT) for RT-QuIC and sPMCA/RT-QuIC due to required dilution into PrPC substrate. Shaded squares indicate prion positivity. *LD50 = 105.5.
Detection of seeding activity by new generation assays: sPMCA, RT-QuIC or sPMCA/RT-QuIC
We and others [50, 51] have demonstrated that tissues harvested from infected hosts, especially those in the asymptomatic phase of disease, may contain low levels or variably PK resistant forms of the misfolded protein. The presence of very low concentrations of prion deposition diminishes ability to rely solely upon IHC, ELISA or WB for the detection of PrPSc deposition associated with prion disease. Thus, we assessed and compared endpoint dilution of the same CWD+ cervid brain (CBP6) dilutional series using these assays—sPMCA, RT-QuIC and RT-QuIC readout of sPMCA 5th round product (sPMCA/RT-QuIC).
The conventional readout for prion seeding activity generated by sPMCA is WB (sPMCA/WB). We subjected a dilutional series of naive and CBP6 CWD+ cervid brain homogenate (10−2 to 10−7) to 5 rounds of sPMCA. When assessed by WB, we revealed the presence of amyloid seeding activity to an endpoint dilution of 10−6 (Fig 1, Table 1). Upon assessment of the same CWD+ cervid brain dilutional series by RT-QuIC we determined the endpoint for amyloid seeding activity to 10−7 (Fig 2, Table 1). We have recently used RT-QuIC as a readout for sPMCA product [52] (sPMCA/RT-QuIC). Incorporation of RT-QuIC as the readout for sPMCA 5th round product improved detection of amyloid seeding activity in CWD+ cervid brain homogenate to 10−8 (Fig 2, Table 1). No amyloid seeding activity was detected at any dilution of the naïve brain homogenate post sPMCA/WB, RT-QuIC or sPMCA/RT-QuIC amplification.
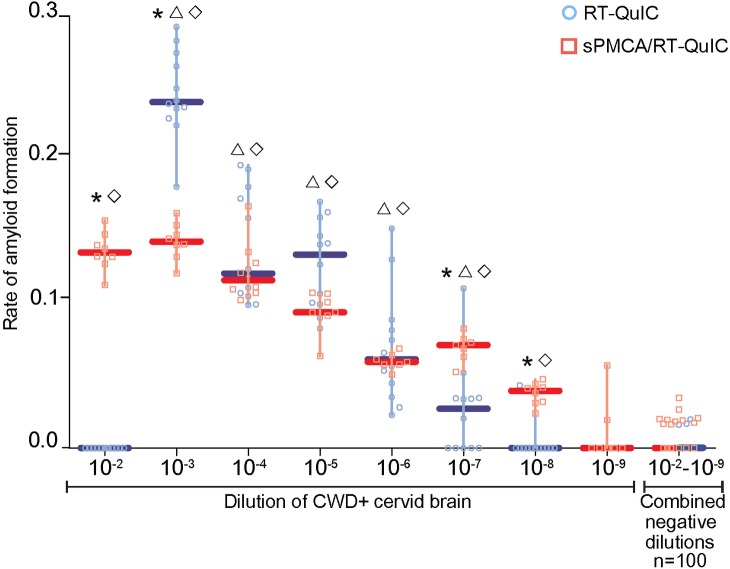
Fig 2. Comparison of amyloid seeding activity in a dilutional series of CWD+ cervid brain by RT-QuIC and sPMCA/RT-QuIC.
Amyloid seeding activity was detected by RT-QuIC in the 10−3–10−7 dilutions of CWD+ cervid brain pool CBP6 (2 μg-200 pg seed material; blue circles and blue median lines). sPMCA/RT-QuIC assay (round 5 sPMCA product diluted 1:100) detected amyloid seeding activity in the 10−2–10−8 dilutions of CBP6 (20 μg-20 pg seed material; red squares and red median lines). Neither assay detected significant amyloid seeding activity in the 10−9 dilution of CBP6 (2 pg seed material) or in naïve cervid brain homogenate dilutions (100 replicatesϒ). 7–12 replicates are shown per assay dilution. Statistical significance between assays is indicated with an asterisk (≤ 0.0017). Statistical significance between samples and same dilution negative controls is indicated with Δ RT-QuIC and ◊ sPMCA/RT-QuIC (≤ 0.0001). ϒnegative controls from all dilutions were combined as no dilutional effect was noted.
sPMCA/RT-QuIC optimum seed input
RT-QuIC amyloid seeding activity can be inhibited at high seed concentrations and rescued by dilution [5, 53, 54]. To establish the optimal seed input for maximal amyloid seeding detection by sPMCA/RT-QuIC we assessed a dilutional series of CBP6 5th round sPMCA product by RT-QuIC (Fig 3). We demonstrate that RT-QuIC amyloid seeding activity is rescued when 5th round sPMCA CBP6 is diluted beyond a 1:10 dilution (Fig 3). We also reveal maximal readout for the combined assay when CBP6 sPMCA 5th round product (initiated by 1:1000 dilution) is spiked at 1:100 dilution into RT-QUIC (Fig 3, asterisk). This combination of dilutions is used for sPMCA/RT-QuIC throughout the remainder of this publication. Negative controls run with each dilution remained below the threshold and were combined for ease of graphics (Fig 3).
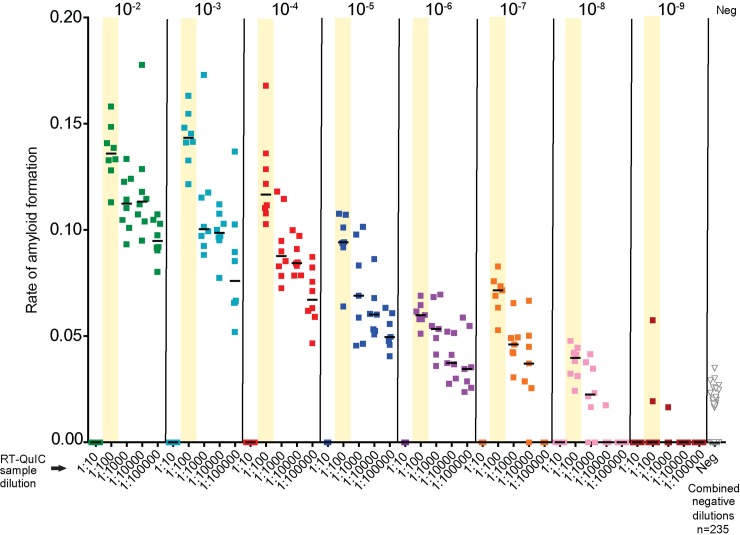
Fig 3. Detection of amyloid seeding activity by sPMCA/RT-QuIC in a CWD+ cervid brain dilutional series.
Amyloid seeding activity was detected in 10−2–10−8 dilutions of CWD+ cervid brain (CBP6) (20ug-20pg) using 1:100–1:100000 dilutions of sPMCA 5th round product. Amyloid seeding activity was inhibited at the 1:10 dilution. Optimal prion detection was demonstrated at a 1:100 dilution (yellow bars) of sPMCA 5th round product. No significant amyloid seeding activity was initiated by 10−2–10−9 dilutions of naïve cervid brain using 1:100–1:100000 dilutions of sPMCA 5th round product. n = 7–8 RT-QuIC replicates/sample. n = 235 negative control dilution replicates.
Prion detection in brain tissue harvested from CWD+ CBP6 mouse bioassay
The use of conventional and amplification assays has contributed much to our current understanding of amyloid disorders, including prions. Yet, a limitation of these assays is their inability to directly measure infectivity. Bioassay remains the gold standard to assess infectivity. To determine the role in vitro analysis may have in providing insight to prion infectivity we compared results from five of these methods. To this end, brain tissue harvested from a dilutional mouse bioassay of CBP6 were assessed by conventional (IHC, WB) and amplification (sPMCA, RT-QuIC and sPMCA/RT-QuIC) assays. Insufficient quantities of mouse brain tissue negated inclusion of these samples for BioRad ELISA analysis.
Survival and detection of PrPSc by immunohistochemistry or western blot
A dilutional series mouse bioassay was conducted using CBP6 [36]. Six mice were intracranially-inoculated for each dilution (10−2–10−7). Two mice (one each from the 10−5 and 10−6 cohorts) were removed from the study due to death from causes other than prion infection. We found that all mice (11/11; 5-6/cohort) that were inoculated with 10−2 or 10−3 CWD+ cervid brain CBP6 succumbed to disease after an average of 203 (range 160 to 255) or 239 (184 to 257) days post inoculation (DPI), respectively (Table 2). PrPSc deposition was confirmed in all eleven mice by WB and IHC (Fig 4, S1 and S2 Figs). Five of six (5/6) mice inoculated with 10−4 CWD+ cervid brain CBP6 (average clinical disease 232 DPI, range 181 to 257), three of five (3/5) mice inoculated with 10−5 (average clinical disease 332 DPI, range 231 to 502) and 2/5 mice inoculated with 10−6 (average clinical disease 426 DPI, range 286 to 502) CWD+ cervid brain CBP6 showed signs consistent with prion disease and demonstrated PrPSc deposition by IHC and WB (Fig 4, Table 2, S1 and S2 Figs). Neither WB nor IHC identified PrPSc deposition in brain tissue of the six mice (0/6) inoculated with 10−7 CWD+ cervid brain CBP6 (502 DPI), nor mice (0/12) receiving naïve deer brain inoculum (502 DPI) (Fig 4, Table 2, S1 and S2 Figs).
Table 2. Prion detection in tissue harvested from a mouse dilutional bioassay of CWD+ cervid brain.
| Mouse cohort (average dpi to terminal disease ± SD) n+/total n |
||||||||
|---|---|---|---|---|---|---|---|---|
| Tissue tested | Assay | 10−2 CWD (203±36) | 10−3 CWD (239±46) | 10−4 CWD (232±54) | 10−5 CWD (372±133) | 10−6 CWD (423±108) | 10−7 CWD (>450) | Neg (NA) |
| Brain | WB | 6/6 | 6/6 | 5/6 | 2/5 | 2/5 | 0/6 | 0/12 |
| IHC | 6/6 | 6/6 | 5/6 | 3/5 | 2/5 | 0/6 | 0/12 | |
|
sPMCA/ WB |
5/5 | 6/6 | 5/6 | 2/5 | 2/5 | 0/6 | 0/12 | |
| RT-QuIC | 5/5 | 6/6 | 5/6 | 3/5 | 2/5 | 0/6 | 0/12 | |
|
sPMCA/ RT-QuIC |
5/5 | 6/6 | 6/6 | 3/5 | 2/5 | 0/6 | 0/12 | |
| Spleen | RT-QuIC | 5/5 | 6/6 | 5/6 | 3/5 | 2/5 | 0/6 | 0/12 |
Comparison of in vitro conventional (WB, IHC) and amplification (RT-QuIC, sPMCA/WB and sPMCA/RT-QuIC) assays. Prions were detected in the brains of mice inoculated with CWD+ cervid brain (CBP6) dilutions 10−2–10−6 (300 μg-30 ng) by all assays. sPMCA/RT-QuIC detected prions in brain tissue of one additional mouse in the 10−4 cohort (3 μg seed material). In the 10−5 cohort, IHC, RT-QuIC, sPMCA/RT-QuIC and RT-QuIC of spleen tissue revealed one additional prion+ mouse than WB and sPMCA/WB. Prions were not detected in mice inoculated with the 10−7 CBP6 dilution (3 ng) nor in n = 12 negative control mice inoculated with naïve cervid brain homogenate.
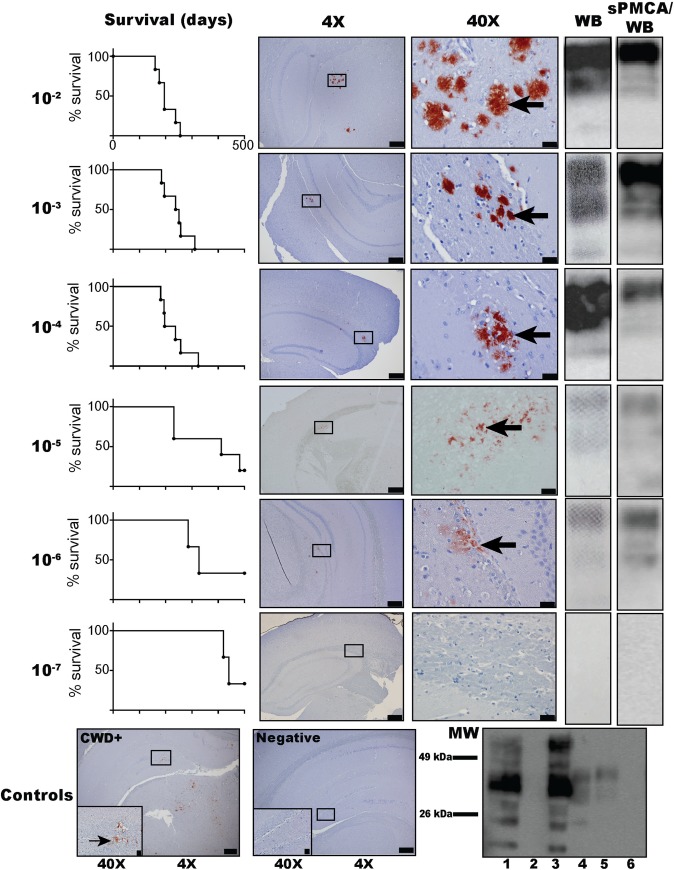
Fig 4. Dilutional mouse bioassay of CWD+ cervid brain into Tg(CerPrP) 5037 mice: survival curves, IHC, WB, and sPMCA/WB prion detection.
PrPSc is shown in the brains of mice inoculated with dilutions 10−2–10−6 of CBP6 (300 μg-30 ng) using IHC (red deposits indicated with arrows), WB, and sPMCA/WB (5th round product) (S1–S3 Figs). PrPSc was not detected in mice inoculated with the 10−7 CBP6 dilution (3 ng) nor in n = 12 negative control mice inoculated with naïve cervid brain. Bottom panels (left to right) show IHC PrPSc deposition in the positive control mouse brain (IC-inoculated 30 μl 10% CWD+ cervid brain homogenate) and no deposition in the negative control mouse (IC-inoculated 30 μl 10% naïve cervid brain homogenate). Control western blot (bottom right) shows complete PK digestion (50 μg/ml) of PrPc in the unamplified (lane 2; without PK in lane 1) and amplified (lane 6) negative control mouse (IC-inoculated 30 μl 10% naïve cervid brain homogenate). PrPSc is revealed after PK digestion (50 μg/ml) in unamplified and sPMCA amplified CWD+ mouse brain (IC-inoculated with 30 μl 10% CWD+ cervid brain homogenate) (lanes 4 and 5; unamplified sample without PK in lane 3). IHC image objectives are 4X (scale bar = 200 μm) and 40X (scale bar = 20 μm).
Detection of amyloid seeding activity by RT-QuIC, sPMCA/WB, sPMCA/RT-QuIC
To further this cross-platform analysis brain tissue harvested from mice in the above dilutional bioassay were assessed by RT-QuIC and sPMCA. RT-QuIC, sPMCA/WB and sPMCA/RT-QuIC equally demonstrate amyloid seeding activity in the brain tissue of all eleven (11/11) mice receiving the highest concentration of CWD+ cervid brain (5-6/cohort; 10−2 and 10−3) (Fig 5, Table 2, S3 Fig). Insufficient tissue from one mouse in the 10−2 cohort precluded analysis for seeding activity by sPMCA and RT-QuIC. Upon analysis of brain tissue from mice receiving an intermediate dose of CWD+ cervid brain (10−4 cohort), both RT-QuIC and sPMCA/WB demonstrate amyloid seeding activity in 5/6 mice (Fig 5, Table 2, S3 Fig). Employing sPMCA/RT-QuIC we revealed amyloid seeding activity in all six (6/6) mice in the intermediate dose cohort (10−4) (Fig 5, Table 2). RT-QuIC analysis of brain tissue from bioassay mice receiving even lower doses of CWD+ cervid brain resulted in the detection of prion seeding activity in 3/5 mice (10−5 cohort), 2/5 mice (10−6 cohort) and 0/5 mice (10−7 cohort) (Fig 5, Table 2). sPMCA/RT-QuIC improved the amyloid conversion rates but did not increase the overall number of prion positive mice in cohorts 10−5–10−7 (Fig 5, Table 2). Negative assay controls and naïve mouse brain remained negative (Fig 5, Table 2).
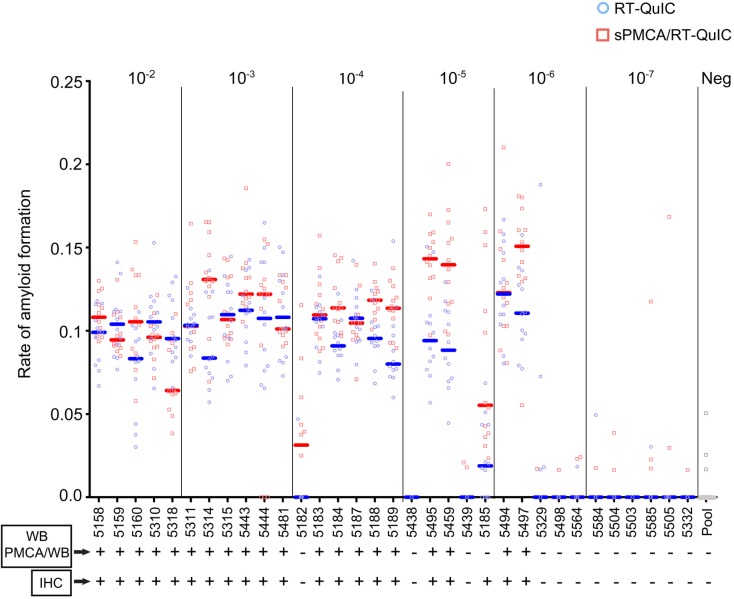
Fig 5. Prion detection in brain tissue harvested from a mouse dilutional bioassay of CWD+ cervid brain pool.
Comparison of in vitro conventional (WB, IHC) and amplification (RT-QuIC, sPMCA/WB and sPMCA/RT-QuIC) assays. Readout for WB and PMCA/WB were the same for all mice (S2 and S3 Figs). IHC (S1 Fig), RT-QuIC and sPMCA/RT-QuIC detected prions in one additional mouse at 10−5 dilution of CBP6 (300ng seed material) than WB or PMCA/WB. RT-QuIC and sPMCA/RT-QuIC similarly detected prions in mice inoculated with 10−2, 10−3, 10−5 and 10−6 dilutions of CBP6 (300 μg, 30 μg, 300ng and 30 ng seed material; blue circles and median lines and red squares and median lines respectively). sPMCA/RT-QuIC detected prions in one additional mouse at 10−4 dilution of CBP6 (3 μg seed material). No assay detected prions in mice inoculated with the 10−7 dilution of CBP6 (3 ng seed material) or in negative control mice inoculated with naïve cervid brain (n = 12). Amplification assays represent 12 replicates/mouse/assay.
Detection of additional prion-infected mice when assessed by RT-QuIC and sPMCA/RT-QuIC
Amyloid seeding activity was detected in the brain tissue of additional mice (5182, 5185) when assessed by RT-QuIC and sPMCA/RT-QuIC, but not by sPMCA/WB. Both of these mice had received a CWD+ cervid brain CBP6 dose in the LD50 range of the bioassay (10−5.5) (Fig 5, Table 2). To determine if the 11 CWD-inoculated yet brain negative mice, 5/11 in cohorts 10−5 and 10−6, the LD50 range, (5329, 5438, 5439, 5498 and 5564) and 6/11 in cohort 10−7 dilution cohort (5584, 5504, 5503, 5585, 5505, 5332) were not subclinical carriers we assessed spleen tissue by RT-QuIC (Fig 5, Table 2). Amyloid seeding activity was not detected in the spleen of any (0/11) of these mice (Table 2).
Discussion
RT-QuIC and sPMCA/QuIC amplification assays detect amyloid conversion beyond mouse bioassay LD50
The incorporation of amplification assays, sPMCA and RT-QuIC, enhanced the detection of prions in a CWD+ cervid brain pool CBP6 to levels beyond the mouse bioassay LD50 (Fig 4, Table 1). This may be due to a threshold requirement (minimum infectious dose: MID) to initiate infectivity in vivo. The existence of a MID threshold for viral and bacterial infectious agents has been observed [55]. While a small dose of viral or bacterial antigen may initiate a vaccinating immune response (live attenuated vaccine strategies), to initiate infection and disease progression a larger dose of pathogen is required. For prions this may be reflected by the initial success within the endoplasmic reticulum (ER) to activate the unfolded protein response (UPR) in an attempt to restore normal protein folding and ER function [56, 57], resulting in prolonged development of sufficient accumulation of amyloid required to initiate prion disease. Alternatively, these findings may suggest that a fraction of conversion competent amyloid, as detected by in vitro amplification assays, is noninfectious. Previous studies have demonstrated that both infectious and noninfectious amyloid particles exist in prion inoculums [58]. It is also possible that this attests to the in vitro amplification process being more efficient at initiating amyloid conversion events than its in vivo counterpart.
sPMCA and RT-QuIC amyloid seeding activity is inhibited at high seed concentrations and rescued by dilution
We confirm earlier reports [36, 54, 59] that sPMCA and RT-QuIC amyloid seeding activity remains undetectable in tissue and biological fluid samples when spiked into either assay at high concentrations (10−1–10−2 dilution) (Figs 2 and 3). Here, employing the CWD+ cervid brain pool CBP6, we demonstrate that amyloid detection in sPMCA product by RT-QuIC is rescued and provides peak detection when dilutions for both sPMCA and RT-QuIC are optimized (Fig 2). Observed inhibition may be related to components or matrix effects that occur in less dilute samples. Optimization may be required when assessing seeding activity by single or combined use of amplification assays to account for variation in amyloid conversion rates dependent upon tissue/fluid type, host species, prion strain or PRNP genotype.
Amplification assays detect prions in terminal brain tissue with similar efficacy as conventional IHC
We found that conventional IHC detected prions (PrPSc deposition) in mouse bioassay terminal brain tissue with similar efficacy as the new generation of amplification assays (prion seeding activity) (Figs 4 and 5, S1–S3 Figs, and Table 2). These findings affirm the robust nature of conventional test assays and supports their continued use in the application of testing tissues harvested from terminal, end stage disease cases, when PrPSc levels are high. The detection of high concentrations of prions by both conventional and amplification assays has been previously substantiated in tissues harvested during the late and terminal phases of prion disease [60–64]. Of increasing interest is the ability to detect very low concentrations of prions associated with early infection and silent carriers of disease. Conventional assays have been used to detect prions in lymphoid biopsies harvested from hosts lacking signs/symptoms of disease [65–67]. Yet, these methods present concerns regarding diagnostic accuracy due to limitations associated with the amount of tissue that can be analyzed, the low detection capacity inherent to these methods, and by the presence of protease sensitive prion isoforms that may be abolished by PK digestion [68].
New generation in vitro amplification assays provide improved detection rigor
We demonstrate that amplification assays can improve detection rigor as prion burden decreases (Figs 3 and 5). The ability to assess 8–16 replicates from a single sample improves readout reliability, and thus provides a screening mechanism for the detection of very low concentrations of prions present shortly after prion exposure, or for the assessment of nonclassical and asymptomatic disease states. These findings are supported by previous studies that have employed these techniques to demonstrate prions in samples harvested at very early time points post experimental infection or during the asymptomatic phase of disease [6, 18, 38, 40, 43, 44, 50, 69–72].
In vitro amplification assays provide enhanced detection of prion infection at the mouse bioassay LD50
As mentioned above, our study finds that in vitro amplification by RT-QuIC and sPMCA/RT-QuIC demonstrate enhanced detection of seeding activity in a CWD+ cervid brain pool homogenate than that reached by conventional test results from mouse bioassay of the same homogenate. Further analysis of terminal mouse brain tissue from the dilutional bioassay by RT-QuIC and sPMCA revealed 2 additional prion-infected mice at the mouse bioassay LD50 (Fig 5). These findings further corroborate the published LD50 established for this CWD+ brain pool [36]. Interestingly, use of sPMCA/RT-QuIC revealed an increase in amyloid seeding rates for most of the bioassay mice (Fig 5). Both findings may have impact upon earlier detection and diagnosis of disease status, and prion detection in samples containing minute quantities of amyloid seeding activity. Our findings are isolated to the detection of prions in brain tissues and a single CWD+ brain pool, but are supported by a recent report [52] where the use of sPMCA/RT-QuIC demonstrate enhanced detection of seeding activity in biological fluids containing very low prion burdens. These findings may be especially important for antemortem prion diagnosis. Bioassay remains the gold standard and definitive readout for the detection of prion infectivity. Its use has provided tremendous insight to our understanding of prion pathogenesis and transmission dynamics [3, 71, 73–78]. Yet, it is costly in money, time and animal use. Bioassay of prion concentrations near the LD50 requires the use of 9–12 animals/cohort and between 400–500 days to reach a study endpoint. Conservatively, adding in animal costs, per diem and assessment of tissue, this equates to thousands of dollars and 1–2 years.
Future use for new generation amplification assays
This new generation of in vitro amplification assays have been reported to obtain sensitivity levels rivaling that of bioassay [27, 79]. Our results confirm that sPMCA and RT-QuIC detect prions to bioassay endpoint (Fig 5, Table 2). We also show that RT-QuIC can detect additional seeding activity not demonstrated when sPMCA is analyzed by WB. Prion infectivity is known to consist of variably protease sensitive/resistant isoforms [80]. RT-QuIC avoids the use of proteinase K, and thus, may enhance detection of protease sensitive isoforms. While more testing is required, we propose that RT-QuIC and sPMCA/RT-QuIC may be useful to assess prion infectivity instead of bioassay.
Recent evidence suggests that other neurodegenerative diseases such as Parkinson’s Disease (PD), Alzheimer’s Disease (AD), and Huntington’s disease produce prion-like aggregates [81]. Studies have utilized sPMCA [82–84] and RT-QuIC [30, 84] to detect α-synuclein proteins and β-amyloid oligomers in cerebral spinal fluid of AD and PD patients, respectively. Modified PMCA is able to detect polymers with high sensitivity and specificity, and has the capacity to distinguish these neurodegenerative diseases from others [82]. Similar to prion diseases, PD, AD and other neurodegenerative diseases have a long latency period during which time detection is limited. Current diagnostic methods for these human protein misfolding disorders rely upon imaging techniques, clinical examination and screening for potential biomarkers, however, a definitive diagnosis can only be made from post-mortem histology [85]. Earlier diagnosis will be key to continued development of therapeutics for all neurodegenerative diseases.
In summary, we provide evidence that the use of ‘new generation’ amplification assays enhance prion detection sensitivity and improve test validity and rigor for the analysis of biological samples containing very low concentrations of prions. Methods employing in vitro detection will never replace bioassay, but they can augment findings and become tools for the early diagnosis of human and animal protein misfolding disorders.
Materials and methods
Ethics statement
All animals were handled in strict accordance with guidelines for animal care and use provided by the United States Department of Agriculture (USDA), National Institutes of Health (NIH) and the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC), and all animal work was approved by Colorado State University Institutional Animal Care and Use Committee (IACUC protocol numbers 10-2189A, 12-3773A, and 13-4482A).
Cervids handling
Naïve deer brain homogenate: Naïve brain homogenate derived from pooling the brain tissue harvested from two naïve and IHC confirmed CWD-free white-tailed deer served as a negative control. Hereafter referred to as naïve cervid brain.
CWD-infected deer brain homogenates (CBP6): CWD positive inoculum was derived from pooling the brain tissue harvested from six terminal CWD-infected and IHC-confirmed positive white-tailed deer (CBP6) generated from previous experimental studies conducted at CSU [86, 87]. The inoculum is hereafter referred to as CWD+ cervid brain CBP6. The deer were acquired from Warnell School of Forestry, University of Georgia-Athens. They were housed socially in 6×12 meter suites with sand and epoxy mixture sealed floors to afford normal wear on hooves. The sealed floors were covered in a layer of aspen chip bedding. A natural light equivalent was mimicked, humidity was maintained at 25–40%, and temperature ranged from 16–26 degrees Celsius. Enrichment consisted of cardboard boxes, ropes and plastic toys, and daily interaction with caretaker personnel. Deer received 50 g complete pelleted feed per kg/day along with hay forage and water ad libitum. For sample collection (including prior to euthanasia), deer were anesthetized with IM injections of Ketamine (2–8 mg/kg) and Medetomidine (0.1–0.2 mg/kg). Euthanasia was performed by IV injection of pentobarbital sodium with phenytoin (1 ml per 4.5 kg).
Mice handling
Naïve mouse brain homogenate: Brain homogenate from transgenic mice overexpressing the elk prion protein, Tg(CerPrP) 5037, [88] inoculated with naïve cervid brain served as a negative control for all assays.
Bioassay mouse brain tissues: Tg(CerPrP) 5037 mice were used to establish an LD50 of CBP6 CWD+ cervid brain [36]. Mice were housed socially in a commercial caging system in cages filled with aspen chips, provided nesting materials, checked daily, and provided commercial irradiated rodent chow and water ad libitum. Prior to inoculation, mice are given a 2 mg/kg dose of meloxicam analgesic. Briefly, mouse cohorts (n = 6/cohort) were inoculated intracranially in the right parietal lobe of the cerebral cortex with 30 μl of 10−1–10−7 dilutions of CBP6 and were observed for signs of prion disease. Mice were terminated by inhalation of CO2 upon development of prion disease or 500 days post infection (dpi). Brain tissue harvested from each inoculated mouse was divided in half with one half frozen at -80°C and one half fixed in periodate-lysine-paraformaldehyde (PLP) fixative for a minimum of 48 h. After a minimum of 48 h fixed tissues were transferred to 1xPBS at room temperature until further analysis.
Western blot (WB)
Brain tissue from Tg(CerPrP) 5037-inoculated mice was prepared as a 10% (w/v) homogenate in 1xPBS and stored at 4°C until further analysis. Homogenates were mixed with proteinase K (PK; Invitrogen) at 50 μg/mL, incubated at 37°C for 30 min followed by 45°C for 10 min. Samples were mixed with reducing agent (10x)—lithium dodecyl sulfate (LDS) sample buffer (4x) (Invitrogen) at a concentration of 1x, heated at 95°C for 5 min and separated on NuPAGE 12% Bis-Tris gel at 125V for 1.5 h. Protein was transferred to a polyvinylidene fluoride (PVDF) membrane at 80 V for 1 h in transfer buffer (0.025 M Trizma base, 0.2 M glycine, 20% methanol, pH 8.3). The membrane was then incubated with 5% nonfat milk in 1xTris-buffered saline (TBS) with 0.1% Tween 20 (TBST) for 3 min and then for 12 min with BAR224-HRP (0.2 μg/ml final concentration; Cayman Chemical) diluted in TBST, followed by a 30 min wash with TBST. The membrane was then developed with ECL Plus Western blotting detection reagents (Pierce) and viewed on a Luminescent Image Analyzer LAS-4000 (Fujifilm).
BioRad enzyme-linked immunosorbant assay (ELISA)
Brain tissue dilutional series (10−1–10−9) of naïve and CWD+ cervid brain homogenate (CBP6) were performed at the Colorado State University Diagnostic Laboratory as per BioRad instructions. The read-out criteria used were Suspect (OD ≥ 0.116), Questionable (OD 0.094–0.115) or Not Detected (< 0.093).
Immunohistochemistry (IHC)
PLP fixed mouse brains were paraffin-embedded and 5 μm tissue sections were cut and mounted on glass positive charge slides. Tissues were deparaffinized in a 65°C oven followed by successive xylene immersions (100%), rehydrated through graded ethanol washes (100% x 2, 95% x 2 and 70% at 5 min per wash), and 88% formic acid treated for 60 min. Tissues were then subjected to hydrated autoclaving using an automated antigen-retrieval system 2100-Retriever (Prestige Medical) and a citrate buffer (0.01M sodium citrate, 0.05% tween 20, pH 6) for 30 min. Samples were then blocked with 3% H2O2 (30 min) followed by a proprietary protein block (TNB, PerkinElmer Life and Analytical Sciences) (30 min) and stained with unconjugated BAR-224 at 2 μg/ml (Cayman Chemical) (overnight at 4°C). Detection was completed using HRP-conjugated anti-mouse secondary antibody (Envision+, Dako) (30 min) and AEC substrate chromogen (Dako) (1 min). Tissues were then counterstained with Meyer’s hematoxylin (Dako) (2 min), followed by 0.1% calcium bicarbonate bluing reagent (5 min) and coverslipped with aqueous mounting media (Dako).
Serial protein misfolding cyclic amplification (sPMCA)
Tg(CerPrP) 5037normal brain homogenate (NBH): NBH, the PrPC substrate used for sPMCA prion conversion, was prepared as follows: naïve Tg(CerPrP) 5037 mice <4 months of age were euthanized by CO2 inhalation and perfused with 35 mL of 5 mM ethylenediaminetetraacetic acid tetrasodium salt (EDTA) in PBS via intracardiac catheterization. The brain was removed and flash frozen using liquid nitrogen. Brain homogenate was then prepared at a 10% (w/v) solution in sPMCA buffer (1% Triton-X 100 [v/v], 5 mM EDTA, and 150 mM NaCl) with the addition of Complete Protease Inhibitors (Roche Pharmaceuticals, Indianapolis, IN) in a homogenizer (Omni Bead Ruptor). Homogenates were then centrifuged for 1 min at 3000 rpm to remove bulk brain material, and the supernatant frozen in single-experiment aliquots at -80°C in a prion-free room until use in sPMCA.
sPMCA: Thirty microliters (30 μl) of each sample (3 replicates for each sample, 2 investigators/sample; investigators were blinded to sample identity) was spiked into 50 μl of NBH and sonicated for 10 sec every 5 min for each 24 h round. After each 24 h round 30 μl of sonicated material was transferred into 50 μl of fresh NBH and subjected to another 24 h round for a total of 5 rounds. After 5 rounds material was analyzed by WB as described previously [71]. Naïve and CWD+ cervid brain inoculum (CBP6) served as positive and negative sPMCA controls.
Real-time quaking induced conversion assay (RT-QuIC)
Substrate for seeded RT-QuIC reactions was prepared by adding truncated Syrian Hamster recombinant protein encoding residues 90–231 (SHrPrP) prepared as previously reported [38] to RT-QuIC buffer (320 mM NaCl, 1.0 mM EDTA, 10 μM Thioflavin T [ThT, Sigma]) at a final concentration of 0.1 mg/ml. Ninety-eight microliters (98 μl) RT-QuIC substrate was added to optical bottom black 96-well plates (Nunc). Each mouse brain homogenate from the mouse CBP6 bioassay was diluted 10−2 to 10−7, and 2 μl of each was run in quadruplicate on 2–3 plates by 2–3 investigators for a total of 7–12 replicates/sample. Positive and negative controls were included on all plates. Prepared plates were placed in a BMG Labtech Polarstar fluorometer and subjected to 700 rpm double-orbital shaking for 1 min ever other min for 15 min for 250 cycles. After each cycle ThT fluorescence was read at an excitation of 450 nm and emission of 480 nm. Gain was set at 1700 and read using orbital averaging with 20 flashes per well with the 4 mm setting. Fluorescent readings were recorded for all sample reactions for a total time of 62 h at a temperature of 42°C. Samples were considered positive if they crossed a threshold (5 SD above the mean of the initial 5 readings). The inverse of the time when the reaction reached the threshold (1/time to threshold) was then used to determine the amyloid formation rate. Statistical analyses were run in Prism v6, GraphPad Software, La Jolla, CA. A Mann-Whitney test was used to generate (p-values less than 0.05 were considered significant) by comparing the sample rates to the rates of known negative control tissues. A Reed-Muench calculation was used to calculate the LD50 for the titrated mouse bioassay as previously described [36].
RT-QuIC readout of sPMCA material (sPMCA/RT-QuIC)
sPMCA 5th round product was diluted 1:100 or 1:1000 in 0.1% SDS/PBS (see figure legends for sample dilution). Two microliters (2 μl) of each sample was analyzed by RT-QuIC as described above. The rates were calculated and graphed as previously described [52].
Supporting information
(XLSX)
(XLSX)
(XLSX)
(TIF)
(TIF)
(TIF)
Acknowledgments
We sincerely thank Joseph Westrich for manuscript editing and Craig Ramsey for discussion. Funding was provided by NIH-NIAID R01AI112956 and R01AI093634.
Data Availability
All relevant data are within the manuscript and Supporting Information files.
Funding Statement
This work was supported by the National Institutes of Health R01AI112956 (CKM), National Institutes of Health R01I093634 (CKM), Department of Microbiology, Immunology and Pathology, and the College of Veterinary Medicine and Biomedical Sciences, Colorado State University. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No conflicts of interest are associated with this manuscript.
References
- 1.Miller MW, Williams ES, Hobbs NT, Wolfe LL. Environmental sources of prion transmission in mule deer. Emerging infectious diseases. 2004;10(6):1003–6. 10.3201/eid1006.040010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mathiason CK, Hays SA, Powers J, Hayes-Klug J, Langenberg J, Dahmes SJ, et al. Infectious prions in pre-clinical deer and transmission of chronic wasting disease solely by environmental exposure. PloS one. 2009;4(6):e5916 10.1371/journal.pone.0005916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mathiason CK, Powers JG, Dahmes SJ, Osborn DA, Miller KV, Warren RJ, et al. Infectious prions in the saliva and blood of deer with chronic wasting disease. Science. 2006;314(5796):133–6. 10.1126/science.1132661 [DOI] [PubMed] [Google Scholar]
- 4.Safar JG, Lessard P, Tamguney G, Freyman Y, Deering C, Letessier F, et al. Transmission and detection of prions in feces. The Journal of infectious diseases. 2008;198(1):81–9. 10.1086/588193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haley NJ, Mathiason CK, Zabel MD, Telling GC, Hoover EA. Detection of sub-clinical CWD infection in conventional test-negative deer long after oral exposure to urine and feces from CWD+ deer. PLoS One. 2009;4(11):e7990 10.1371/journal.pone.0007990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nalls AV, McNulty E, Hoover CE, Pulscher LA, Hoover EA, Mathiason CK. Infectious Prions in the Pregnancy Microenvironment of CWD-infected Reeves' Muntjac Deer. Journal of virology. 2017;pii: JVI.00501-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.USGS-NWHC. USGS CWD Map October 2018 [Available from: https://www.usgs.gov/media/images/distribution-chronic-wasting-disease-north-america-0.
- 8.Sohn HJ, Kim JH, Choi KS, Nah JJ, Joo YS, Jean YH, et al. A case of chronic wasting disease in an elk imported to Korea from Canada. The Journal of veterinary medical science. 2002;64(9):855–8. [DOI] [PubMed] [Google Scholar]
- 9.Benestad SL, Mitchell G, Simmons M, Ytrehus B, Vikoren T. First case of chronic wasting disease in Europe in a Norwegian free-ranging reindeer. Veterinary research. 2016;47(1):88 10.1186/s13567-016-0375-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benestad SL, Telling GC. Chronic wasting disease: an evolving prion disease of cervids. Handbook of clinical neurology. 2018;153:135–51. 10.1016/B978-0-444-63945-5.00008-8 [DOI] [PubMed] [Google Scholar]
- 11.Perrott MR, Sigurdson CJ, Mason GL, Hoover EA. Evidence for distinct chronic wasting disease (CWD) strains in experimental CWD in ferrets. The Journal of general virology. 2012;93(Pt 1):212–21. 10.1099/vir.0.035006-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Angers RC, Kang HE, Napier D, Browning S, Seward T, Mathiason C, et al. Prion strain mutation determined by prion protein conformational compatibility and primary structure. Science. 2010;328(5982):1154–8. 10.1126/science.1187107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Requena JR, Kristensson K, Korth C, Zurzolo C, Simmons M, Aguilar-Calvo P, et al. The Priority position paper: Protecting Europe's food chain from prions. Prion. 2016;10(3):165–81. 10.1080/19336896.2016.1175801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Monello RJ, Galloway NL, Powers JG, Madsen-Bouterse SA, Edwards WH, Wood ME, et al. Pathogen-mediated selection in free-ranging elk populations infected by chronic wasting disease. Proceedings of the National Academy of Sciences of the United States of America. 2017;114(46):12208–12. 10.1073/pnas.1707807114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson C, Johnson J, Vanderloo JP, Keane D, Aiken JM, McKenzie D. Prion protein polymorphisms in white-tailed deer influence susceptibility to chronic wasting disease. The Journal of general virology. 2006;87(Pt 7):2109–14. 10.1099/vir.0.81615-0 [DOI] [PubMed] [Google Scholar]
- 16.Bruce ME, Will RG, Ironside JW, McConnell I, Drummond D, Suttie A, et al. Transmissions to mice indicate that 'new variant' CJD is caused by the BSE agent. Nature. 1997;389(6650):498–501. 10.1038/39057 [DOI] [PubMed] [Google Scholar]
- 17.Bolton DC, McKinley MP, Prusiner SB. Identification of a protein that purifies with the scrapie prion. Science. 1982;218(4579):1309–11. [DOI] [PubMed] [Google Scholar]
- 18.Haley NJ, Richt JA. Evolution of Diagnostic Tests for Chronic Wasting Disease, a Naturally Occurring Prion Disease of Cervids. Pathogens. 2017;6(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orru CD, Wilham JM, Raymond LD, Kuhn F, Schroeder B, Raeber AJ, et al. Prion disease blood test using immunoprecipitation and improved quaking-induced conversion. mBio. 2011;2(3):e00078–11. 10.1128/mBio.00078-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elder AM, Henderson DM, Nalls AV, Wilham JM, Caughey BW, Hoover EA, et al. In vitro detection of prionemia in TSE-infected cervids and hamsters. PloS one. 2013;8(11):e80203 10.1371/journal.pone.0080203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng YC, Hannaoui S, John TR, Dudas S, Czub S, Gilch S. Real-time Quaking-induced Conversion Assay for Detection of CWD Prions in Fecal Material. Journal of visualized experiments: JoVE. 2017(127). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sajnani G, Requena JR. Prions, proteinase K and infectivity. Prion. 2012;6(5):430–2. 10.4161/pri.22309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saborio GP, Permanne B, Soto C. Sensitive detection of pathological prion protein by cyclic amplification of protein misfolding. Nature. 2001;411(6839):810–3. 10.1038/35081095 [DOI] [PubMed] [Google Scholar]
- 24.Colby DW, Zhang Q, Wang S, Groth D, Legname G, Riesner D, et al. Prion detection by an amyloid seeding assay. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(52):20914–9. 10.1073/pnas.0710152105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moda F. Protein Misfolding Cyclic Amplification of Infectious Prions. Progress in molecular biology and translational science. 2017;150:361–74. 10.1016/bs.pmbts.2017.06.016 [DOI] [PubMed] [Google Scholar]
- 26.Atarashi R, Moore RA, Sim VL, Hughson AG, Dorward DW, Onwubiko HA, et al. Ultrasensitive detection of scrapie prion protein using seeded conversion of recombinant prion protein. Nature methods. 2007;4(8):645–50. 10.1038/nmeth1066 [DOI] [PubMed] [Google Scholar]
- 27.Wilham JM, Orru CD, Bessen RA, Atarashi R, Sano K, Race B, et al. Rapid end-point quantitation of prion seeding activity with sensitivity comparable to bioassays. PLoS pathogens. 2010;6(12):e1001217 10.1371/journal.ppat.1001217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Franceschini A, Baiardi S, Hughson AG, McKenzie N, Moda F, Rossi M, et al. High diagnostic value of second generation CSF RT-QuIC across the wide spectrum of CJD prions. Scientific reports. 2017;7(1):10655 10.1038/s41598-017-10922-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoover CE, Davenport KA, Henderson DM, Denkers ND, Mathiason CK, Soto C, et al. Pathways of Prion Spread during Early Chronic Wasting Disease in Deer. Journal of virology. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Groveman BR, Orru CD, Hughson AG, Raymond LD, Zanusso G, Ghetti B, et al. Rapid and ultra-sensitive quantitation of disease-associated alpha-synuclein seeds in brain and cerebrospinal fluid by alphaSyn RT-QuIC. Acta neuropathologica communications. 2018;6(1):7 10.1186/s40478-018-0508-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Foutz A, Appleby BS, Hamlin C, Liu X, Yang S, Cohen Y, et al. Diagnostic and prognostic value of human prion detection in cerebrospinal fluid. Annals of neurology. 2017;81(1):79–92. 10.1002/ana.24833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Race B, Williams K, Hughson AG, Jansen C, Parchi P, Rozemuller AJM, et al. Familial human prion diseases associated with prion protein mutations Y226X and G131V are transmissible to transgenic mice expressing human prion protein. Acta neuropathologica communications. 2018;6(1):13 10.1186/s40478-018-0516-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yao Y, Dong X, Guan H, Lu Q. Cerebrospinal fluid real-time quaking-induced conversion test for sporadic Creutzfeldt-Jakob disease in an 18-year-old woman: A case report. Medicine. 2017;96(48):e8699 10.1097/MD.0000000000008699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Orru CD, Yuan J, Appleby BS, Li B, Li Y, Winner D, et al. Prion seeding activity and infectivity in skin samples from patients with sporadic Creutzfeldt-Jakob disease. Science translational medicine. 2017;9(417). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Denkers ND, Henderson DM, Mathiason CK, Hoover EA. Enhanced prion detection in biological samples by magnetic particle extraction and real-time quaking-induced conversion. The Journal of general virology. 2016;97(8):2023–9. 10.1099/jgv.0.000515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoover CE, Davenport KA, Henderson DM, Pulscher LA, Mathiason CK, Zabel MD, et al. Detection and Quantification of CWD Prions in Fixed Paraffin Embedded Tissues by Real-Time Quaking-Induced Conversion. Scientific reports. 2016;6:25098 10.1038/srep25098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Orru CD, Groveman BR, Hughson AG, Zanusso G, Coulthart MB, Caughey B. Rapid and sensitive RT-QuIC detection of human Creutzfeldt-Jakob disease using cerebrospinal fluid. mBio. 2015;6(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Henderson DM, Manca M, Haley NJ, Denkers ND, Nalls AV, Mathiason CK, et al. Rapid antemortem detection of CWD prions in deer saliva. PloS one. 2013;8(9):e74377 10.1371/journal.pone.0074377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.John TR, Schatzl HM, Gilch S. Early detection of chronic wasting disease prions in urine of pre-symptomatic deer by real-time quaking-induced conversion assay. Prion. 2013;7(3):253–8. 10.4161/pri.24430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elder AM, Henderson DM, Nalls AV, Hoover EA, Kincaid AE, Bartz JC, et al. Immediate and Ongoing Detection of Prions in the Blood of Hamsters and Deer following Oral, Nasal, or Blood Inoculations. Journal of virology. 2015;89(14):7421–4. 10.1128/JVI.00760-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Henderson DM, Davenport KA, Haley NJ, Denkers ND, Mathiason CK, Hoover EA. Quantitative assessment of prion infectivity in tissues and body fluids by real-time quaking-induced conversion. The Journal of general virology. 2015;96(Pt 1):210–9. 10.1099/vir.0.069906-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hwang S, West Greenlee MH, Balkema-Buschmann A, Groschup MH, Nicholson EM, Greenlee JJ. Real-Time Quaking-Induced Conversion Detection of Bovine Spongiform Encephalopathy Prions in a Subclinical Steer. Frontiers in veterinary science. 2017;4:242 10.3389/fvets.2017.00242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kramm C, Pritzkow S, Lyon A, Nichols T, Morales R, Soto C. Detection of Prions in Blood of Cervids at the Asymptomatic Stage of Chronic Wasting Disease. Scientific reports. 2017;7(1):17241 10.1038/s41598-017-17090-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Douet JY, Lacroux C, Aron N, Head MW, Lugan S, Tillier C, et al. Distribution and Quantitative Estimates of Variant Creutzfeldt-Jakob Disease Prions in Tissues of Clinical and Asymptomatic Patients. Emerging infectious diseases. 2017;23(6):946–56. 10.3201/eid2306.161734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bougard D, Brandel JP, Belondrade M, Beringue V, Segarra C, Fleury H, et al. Detection of prions in the plasma of presymptomatic and symptomatic patients with variant Creutzfeldt-Jakob disease. Science translational medicine. 2016;8(370):370ra182 10.1126/scitranslmed.aag1257 [DOI] [PubMed] [Google Scholar]
- 46.Demart S, Fournier JG, Creminon C, Frobert Y, Lamoury F, Marce D, et al. New insight into abnormal prion protein using monoclonal antibodies. Biochemical and biophysical research communications. 1999;265(3):652–7. 10.1006/bbrc.1999.1730 [DOI] [PubMed] [Google Scholar]
- 47.Stack MJ, Balachandran A, Chaplin M, Davis L, Czub S, Miller B. The first Canadian indigenous case of bovine spongiform encephalopathy (BSE) has molecular characteristics for prion protein that are similar to those of BSE in the United Kingdom but differ from those of chronic wasting disease in captive elk and deer. The Canadian veterinary journal = La revue veterinaire canadienne. 2004;45(10):825–30. [PMC free article] [PubMed] [Google Scholar]
- 48.Spraker TR, Zink RR, Cummings BA, Sigurdson CJ, Miller MW, O'Rourke KI. Distribution of protease-resistant prion protein and spongiform encephalopathy in free-ranging mule deer (Odocoileus hemionus) with chronic wasting disease. Veterinary pathology. 2002;39(5):546–56. 10.1354/vp.39-5-546 [DOI] [PubMed] [Google Scholar]
- 49.Miller JM, Jenny AL, Taylor WD, Marsh RF, Rubenstein R, Race RE. Immunohistochemical detection of prion protein in sheep with scrapie. Journal of veterinary diagnostic investigation: official publication of the American Association of Veterinary Laboratory Diagnosticians, Inc. 1993;5(3):309–16. [DOI] [PubMed] [Google Scholar]
- 50.Manne S, Kondru N, Nichols T, Lehmkuhl A, Thomsen B, Main R, et al. Ante-mortem detection of chronic wasting disease in recto-anal mucosa-associated lymphoid tissues from elk (Cervus elaphus nelsoni) using real-time quaking-induced conversion (RT-QuIC) assay: A blinded collaborative study. Prion. 2017;11(6):415–30. 10.1080/19336896.2017.1368936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Haley NJ, Seelig DM, Zabel MD, Telling GC, Hoover EA. Detection of CWD prions in urine and saliva of deer by transgenic mouse bioassay. PloS one. 2009;4(3):e4848 10.1371/journal.pone.0004848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Davenport KA, Hoover CE, Denkers ND, Mathiason CK, Hoover EA. Modified Protein Misfolding Cyclic Amplification Overcomes Real-Time Quaking-Induced Conversion Assay Inhibitors in Deer Saliva To Detect Chronic Wasting Disease Prions. Journal of clinical microbiology. 2018;56(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hoover CE, Davenport KA, Henderson DM, Pulscher LA, Mathiason CK, Zabel MD, et al. Detection and Quantification of CWD Prions in Fixed Paraffin Embedded Tissues by Real-Time Quaking-Induced Conversion. Scientific Reports. 2016;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Orru CD, Wilham JM, Vascellari S, Hughson AG, Caughey B. New generation QuIC assays for prion seeding activity. Prion. 2012;6(2):147–52. 10.4161/pri.19430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schmid-Hempel P, Frank SA. Pathogenesis, virulence, and infective dose. PLoS pathogens. 2007;3(10):1372–3. 10.1371/journal.ppat.0030147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shah SZA, Zhao D, Hussain T, Yang L. The Role of Unfolded Protein Response and Mitogen-Activated Protein Kinase Signaling in Neurodegenerative Diseases with Special Focus on Prion Diseases. Frontiers in aging neuroscience. 2017;9:120 10.3389/fnagi.2017.00120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xiang C, Wang Y, Zhang H, Han F. The role of endoplasmic reticulum stress in neurodegenerative disease. Apoptosis: an international journal on programmed cell death. 2017;22(1):1–26. [DOI] [PubMed] [Google Scholar]
- 58.Silveira JR, Raymond GJ, Hughson AG, Race RE, Sim VL, Hayes SF, et al. The most infectious prion protein particles. Nature. 2005;437(7056):257–61. 10.1038/nature03989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Haley NJ, Van de Motter A, Carver S, Henderson D, Davenport K, Seelig DM, et al. Prion-seeding activity in cerebrospinal fluid of deer with chronic wasting disease. PloS one. 2013;8(11):e81488 10.1371/journal.pone.0081488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Spraker TR, Miller MW, Williams ES, Getzy DM, Adrian WJ, Schoonveld GG, et al. Spongiform encephalopathy in free-ranging mule deer (Odocoileus hemionus), white-tailed deer (Odocoileus virginianus) and Rocky Mountain elk (Cervus elaphus nelsoni) in northcentral Colorado. Journal of wildlife diseases. 1997;33(1):1–6. 10.7589/0090-3558-33.1.1 [DOI] [PubMed] [Google Scholar]
- 61.Porcario C, Hall SM, Martucci F, Corona C, Iulini B, Perazzini AZ, et al. Evaluation of two sets of immunohistochemical and Western blot confirmatory methods in the detection of typical and atypical BSE cases. BMC research notes. 2011;4:376 10.1186/1756-0500-4-376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wadsworth JD, Joiner S, Hill AF, Campbell TA, Desbruslais M, Luthert PJ, et al. Tissue distribution of protease resistant prion protein in variant Creutzfeldt-Jakob disease using a highly sensitive immunoblotting assay. Lancet. 2001;358(9277):171–80. [DOI] [PubMed] [Google Scholar]
- 63.Beekes M, Baldauf E, Cassens S, Diringer H, Keyes P, Scott AC, et al. Western blot mapping of disease-specific amyloid in various animal species and humans with transmissible spongiform encephalopathies using a high-yield purification method. The Journal of general virology. 1995;76 (Pt 10):2567–76. [DOI] [PubMed] [Google Scholar]
- 64.Rubenstein R, Kascsak RJ, Merz PA, Papini MC, Carp RI, Robakis NK, et al. Detection of scrapie-associated fibril (SAF) proteins using anti-SAF antibody in non-purified tissue preparations. The Journal of general virology. 1986;67 (Pt 4):671–81. [DOI] [PubMed] [Google Scholar]
- 65.Spraker TR, VerCauteren KC, Gidlewski T, Schneider DA, Munger R, Balachandran A, et al. Antemortem detection of PrPCWD in preclinical, ranch-raised Rocky Mountain elk (Cervus elaphus nelsoni) by biopsy of the rectal mucosa. Journal of veterinary diagnostic investigation: official publication of the American Association of Veterinary Laboratory Diagnosticians, Inc. 2009;21(1):15–24. [DOI] [PubMed] [Google Scholar]
- 66.Gill ON, Spencer Y, Richard-Loendt A, Kelly C, Dabaghian R, Boyes L, et al. Prevalent abnormal prion protein in human appendixes after bovine spongiform encephalopathy epizootic: large scale survey. BMJ. 2013;347:f5675 10.1136/bmj.f5675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ligios C, Cancedda MG, Madau L, Santucciu C, Maestrale C, Agrimi U, et al. PrP(Sc) deposition in nervous tissues without lymphoid tissue involvement is frequently found in ARQ/ARQ Sarda breed sheep preclinically affected with natural scrapie. Archives of virology. 2006;151(10):2007–20. 10.1007/s00705-006-0759-2 [DOI] [PubMed] [Google Scholar]
- 68.Fiorini M, Bongianni M, Monaco S, Zanusso G. Biochemical Characterization of Prions. Progress in molecular biology and translational science. 2017;150:389–407. 10.1016/bs.pmbts.2017.06.012 [DOI] [PubMed] [Google Scholar]
- 69.Orru CD, Hughson AG, Race B, Raymond GJ, Caughey B. Time course of prion seeding activity in cerebrospinal fluid of scrapie-infected hamsters after intratongue and intracerebral inoculations. Journal of clinical microbiology. 2012;50(4):1464–6. 10.1128/JCM.06099-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Aguilar-Calvo P, Garcia C, Espinosa JC, Andreoletti O, Torres JM. Prion and prion-like diseases in animals. Virus research. 2015;207:82–93. 10.1016/j.virusres.2014.11.026 [DOI] [PubMed] [Google Scholar]
- 71.Selariu A, Powers JG, Nalls A, Brandhuber M, Mayfield A, Fullaway S, et al. In utero transmission and tissue distribution of chronic wasting disease-associated prions in free-ranging Rocky Mountain elk. The Journal of general virology. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nalls AV, McNulty E, Powers J, Seelig DM, Hoover C, Haley NJ, et al. Mother to offspring transmission of chronic wasting disease in reeves' muntjac deer. PloS one. 2013;8(8):e71844 10.1371/journal.pone.0071844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ackermann I, Balkema-Buschmann A, Ulrich R, Tauscher K, Shawulu JC, Keller M, et al. Detection of PrP(BSE) and prion infectivity in the ileal Peyer's patch of young calves as early as 2 months after oral challenge with classical bovine spongiform encephalopathy. Veterinary research. 2017;48(1):88 10.1186/s13567-017-0495-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Moore SJ, West Greenlee MH, Kondru N, Manne S, Smith JD, Kunkle RA, et al. Experimental Transmission of the Chronic Wasting Disease Agent to Swine after Oral or Intracranial Inoculation. Journal of virology. 2017;91(19). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Marin-Moreno A, Espinosa JC, Fernandez-Borges N, Piquer J, Girones R, Andreoletti O, et al. An assessment of the long-term persistence of prion infectivity in aquatic environments. Environmental research. 2016;151:587–94. 10.1016/j.envres.2016.08.031 [DOI] [PubMed] [Google Scholar]
- 76.Konold T, Thorne L, Simmons HA, Hawkins SA, Simmons MM, Gonzalez L. Evidence of scrapie transmission to sheep via goat milk. BMC veterinary research. 2016;12:208 10.1186/s12917-016-0807-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Comoy EE, Mikol J, Luccantoni-Freire S, Correia E, Lescoutra-Etchegaray N, Durand V, et al. Transmission of scrapie prions to primate after an extended silent incubation period. Scientific reports. 2015;5:11573 10.1038/srep11573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hunter N, Foster J, Chong A, McCutcheon S, Parnham D, Eaton S, et al. Transmission of prion diseases by blood transfusion. The Journal of general virology. 2002;83(Pt 11):2897–905. 10.1099/0022-1317-83-11-2897 [DOI] [PubMed] [Google Scholar]
- 79.Saa P, Castilla J, Soto C. Ultra-efficient replication of infectious prions by automated protein misfolding cyclic amplification. The Journal of biological chemistry. 2006;281(46):35245–52. 10.1074/jbc.M603964200 [DOI] [PubMed] [Google Scholar]
- 80.Collinge J. Mammalian prions and their wider relevance in neurodegenerative diseases. Nature. 2016;539(7628):217–26. 10.1038/nature20415 [DOI] [PubMed] [Google Scholar]
- 81.Soto C, Pritzkow S. Protein misfolding, aggregation, and conformational strains in neurodegenerative diseases. Nature neuroscience. 2018;21(10):1332–40. 10.1038/s41593-018-0235-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Salvadores N, Shahnawaz M, Scarpini E, Tagliavini F, Soto C. Detection of misfolded Abeta oligomers for sensitive biochemical diagnosis of Alzheimer's disease. Cell reports. 2014;7(1):261–8. 10.1016/j.celrep.2014.02.031 [DOI] [PubMed] [Google Scholar]
- 83.Shahnawaz M, Tokuda T, Waragai M, Mendez N, Ishii R, Trenkwalder C, et al. Development of a Biochemical Diagnosis of Parkinson Disease by Detection of alpha-Synuclein Misfolded Aggregates in Cerebrospinal Fluid. JAMA neurology. 2017;74(2):163–72. 10.1001/jamaneurol.2016.4547 [DOI] [PubMed] [Google Scholar]
- 84.Redaelli V, Bistaffa E, Zanusso G, Salzano G, Sacchetto L, Rossi M, et al. Detection of prion seeding activity in the olfactory mucosa of patients with Fatal Familial Insomnia. Scientific reports. 2017;7:46269 10.1038/srep46269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Giacomelli C, Daniele S, Martini C. Potential biomarkers and novel pharmacological targets in protein aggregation-related neurodegenerative diseases. Biochemical pharmacology. 2017;131:1–15. 10.1016/j.bcp.2017.01.017 [DOI] [PubMed] [Google Scholar]
- 86.Denkers ND, Hayes-Klug J, Anderson KR, Seelig DM, Haley NJ, Dahmes SJ, et al. Aerosol transmission of chronic wasting disease in white-tailed deer. Journal of virology. 2013;87(3):1890–2. 10.1128/JVI.02852-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Goni F, Mathiason CK, Yim L, Wong K, Hayes-Klug J, Nalls A, et al. Mucosal immunization with an attenuated Salmonella vaccine partially protects white-tailed deer from chronic wasting disease. Vaccine. 2015;33(5):726–33. 10.1016/j.vaccine.2014.11.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Browning SR, Mason GL, Seward T, Green M, Eliason GA, Mathiason C, et al. Transmission of prions from mule deer and elk with chronic wasting disease to transgenic mice expressing cervid PrP. Journal of virology. 2004;78(23):13345–50. 10.1128/JVI.78.23.13345-13350.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(XLSX)
(XLSX)
(TIF)
(TIF)
(TIF)
Data Availability Statement
All relevant data are within the manuscript and Supporting Information files.