Abstract
Alfalfa, like other legumes, establishes a symbiotic relationship with the soil bacteria, Sinorhizobium meliloti, which results in the formation of the root nodules. Nodules contain the bacteria enclosed in a membrane‐bound vesicle, the symbiosome where it fixes atmospheric N2 and converts it into ammonia using the bacterial enzyme, nitrogenase. The ammonia released into the cytoplasm from the symbiosome is assimilated into glutamine (Gln) using carbon skeletons produced by the metabolism of sucrose (Suc), which is imported into the nodules from the leaves. The key enzyme involved in the synthesis of Suc in the leaves is sucrose phosphate synthase (SPS) and glutamine synthetase (GS) is the enzyme with a role in ammonia assimilation in the root nodules. Alfalfa plants, overexpressing SPS or GS, or both showed increased growth and an increase in nodule function. The endogenous genes for the key enzymes in C/N metabolism showed increased expression in the nodules of both sets of transformants. Furthermore, the endogenous SPS and GS genes were also induced in the leaves and nodules of the transformants, irrespective of the transgene, suggesting that the two classes of plants share a common signaling pathway regulating C/N metabolism in the nodules. This study reaffirms the utility of the nodulated legume plant to study C/N interaction and the cross talk between the source and sink for C and N.
Keywords: forage quality, malate dehydrogenase, nitrogenase activity, phosphoenolpyruvate carboxylase, SPS and GS activity, sucrose synthase
1. INTRODUCTION
Carbon (C) and nitrogen (N) assimilations are closely interconnected and plant growth is dependent on this interaction. Plants possess intricate regulatory machineries that coordinate N assimilation with C metabolism and meet the demands created by plant growth and development (Ljung, Nemhauser, & Prata, 2015). The control of the C–N interaction requires a complex network involving signals emanating from both C and N metabolisms (Amiour et al., 2012; Coruzzi & Zhou, 2001; Miller, Fan, Shen, & Smith, 2008; Sang, Sun, & Yang, 2012). These metabolic signals interact with hormones that respond to the N supply and regulate metabolism and development (Argueso, Ferreira, & Kieber, 2009; Coruzzi & Zhou, 2001; Ljung et al., 2015; Sakakibara, Takei, & Hirose, 2006).
Photosynthesis drives C and N assimilation by providing assimilatory power (reduced ferredoxin, NADPH, ATP) and the C skeletons required for the assimilation of N. With regard to the origin of C skeletons required for glutamate/glutamine (Glu/Gln) synthesis in illuminated leaves, the 2‐oxoglutarate molecules could be newly synthesized via photosynthesis or derived from metabolites in the night. Based on isotopic tracing experiments on illuminated leaves of Brassica napus, Gauthier et al. (2010) showed that night stored carbon, such as organic acids, played a significant role in nitrogen assimilation and Glu synthesis. Similar study by Abadie, Lothier, Boex‐Fontvieille, Carroll, and Tcherkez (2017), using NMR‐based metabolic flux analysis in illuminated leaves of Helianthus annuus, has shown that the C for Glu/Gln synthesis depends on previous C stored in the vacuoles and that it is citrate and other organic acids and not sucrose (Suc). However, such experiments have not been done for heterotrophic tissues.
Sucrose is the main photoassimilate that is exported from the leaves to supply the rest of the plant with reducing power and C skeletons needed for growth and for the synthesis of storage reserves (Lunn & Furbank, 1999), thus playing a role in both metabolism and development (Eveland & Jackson, 2012; Lunn & MacRae, 2003). Suc also acts as a signaling molecule for regulation of gene expression (Loreti, Bellis, Alp, & Perata, 2001; Ruan, 2012; Smeekens, 2000; Smeekens & Hellmann, 2014; Tognetti, Pontis, & Martinez‐Noel, 2013; Weise, Elzinga, Wobbes, & Smeekens, 2004; Wind, Smeekens, & Hanson, 2010).
Because large amounts of N are invested in the photosynthetic machinery, optimal CO2 assimilation through photosynthesis requires an adequate N supply. Plants acquire N from two principal sources: (a) the soil, as nitrate through commercial fertilizer or mineralization of indigenous organic matter and (b) in the case of legumes, the atmosphere, through symbiotic N2 fixation. Nitrate is converted into NH4 + by nitrate reductase (NR) and nitrite reductase (NiR) while atmospheric N2 is reduced to NH4 + by the bacterial enzyme, nitrogenase. Significant amounts of NH4 + are also derived from photorespiration and phenylpropanoid biosynthesis (Sengupta‐Gopalan & Ortega, 2015). The primary assimilation of ammonia into amino acids involves the ATP‐dependent amination of Glu to Gln by the enzyme glutamine synthetase (GS). This reaction is followed by the reductive transfer of the amide‐amino group of Gln to α‐ketoglutarate (α‐KG) to yield two Glu, with the reaction catalyzed by ferredoxin (Fd−) or NADH‐glutamate synthase (GOGAT).
Alfalfa, like all other legumes, establishes a symbiotic relationship with the soil bacteria, Sinorhizobium meliloti, which results in the formation of a novel organ, the root nodule. The root nodule contains the bacteria enclosed in a membrane‐bound vesicle, the symbiosome (Clarke, Loughlin, Day, & Smith, 2014), where it fixes atmospheric N and converts it into ammonia using the bacterial enzyme, nitrogenase. The ammonia released into the cytoplasm from the symbiosome is assimilated into Gln using C skeletons produced by the metabolism of Suc, which is imported into the nodules from the leaves. A product of Suc metabolism, malate, moves into the symbiosome and acts as a source of energy for nitrogenase activity (Oldroyd & Downie, 2008). While the key enzyme in Suc synthesis is sucrose phosphate synthase (SPS), the enzyme with the key role in ammonia assimilation is GS.
Sucrose phosphate synthase is regulated by a hierarchy of several interacting mechanisms, which includes transcriptional regulation, covalent modification via reversible phosphorylation (Huber & Huber, 1996; Toroser & Huber, 1997), and allosteric regulation via metabolites, Glc‐6‐P and phosphate (Lunn, Gillespie, & Furbank, 2003). Even though the major role of SPS is in photosynthetic tissues, SPS is also found in heterotrophic tissues like the roots, stem, and nodules (Aleman et al., 2010; Haigler et al., 2007). SPS is encoded by a small gene family. There are two families (A and B) present in the genomes of alfalfa, Medicago truncatula and pea (Aleman et al., 2010). While SPSB exhibits leaf‐specific expression, SPSA is expressed in the N2‐fixing zone of the nodules and in the vasculature of the nodules, roots, stem, and leaves (Aleman et al., 2010; Grimes, 2013). It is not known what the function of SPS is in the nodules but one could envision it having a role along with sucrose synthase (SucS) and invertase, in maintaining optimum levels of Suc to provide substrates for starch and cellulose synthesis, ammonia assimilation, and nitrogenase activity, and for functioning as a signaling molecule for gene expression in the nodules.
Glutamine synthetase is the key enzyme in the conversion of inorganic N to an organic form and there are two major isoforms of GS: cytosolic GS (GS1) occurring in the cytosol and chloroplastic GS (GS2), the latter, though nuclear encoded, is located in the chloroplasts/plastids. In the leaves, GS2 assimilates ammonia from nitrate reduction and reassimilates ammonia released during photorespiration (Oliveira, Brears, Knight, Clark, & Coruzzi, 2002). GS1 is the predominant isoform found in non‐photosynthetic tissues and its role is more complex due to its numerous isoforms (Bernard & Habash, 2009; Lea & Miflin, 2011). In roots, GS1 assimilates ammonia derived from NO3 − reduction or from the soil (Bernard & Habash, 2009; Lothier et al., 2010; White, Prell, James, & Poole, 2007), and in the leaves and stem, GS1 is located in the vasculature and plays a role in N translocation. In root nodules, the primary function of GS1 is the rapid assimilation of ammonia excreted into the plant cytosol by N2‐fixing bacteroids (Oldroyd & Downie, 2008). There are two GS 1 gene members in alfalfa, although constitutively expressed in all organs, the expression of both isoforms, specifically GS1a, is the highest in the nodules. The regulation of GS 1 gene expression is not limited to transcription (Sengupta‐Gopalan & Ortega, 2015). It has been shown that GS 1 is regulated at the level of transcript stability, mediated by its 3′UTR (Ortega et al., 2006; Simon & Sengupta‐Gopalan, 2010) and at the translational level by the 5′UTR (Ortega, Wilson, & Sengupta‐Gopalan, 2012). Furthermore, GS is subject to extensive posttranslational modification like phosphorylation (Finnemann & Schjoerring, 2000; Lima, Seabra, Melo, Cullimore, & Carvalho, 2006), ubiquitination, and binding with other proteins (Seabra, Silva, & Carvalho, 2013). GS is also subject to regulation at the level of holoenzyme turnover (Ortega, Roche, & Sengupta‐Gopalan, 1999).
Since SPS and GS play key roles in primary metabolism, efforts have been made to modulate the expression of these genes using transgenic approaches. The outcomes of overexpression of SPS have been quite varied, but in general, increased SPS activity is associated with the production of new sinks and increased sink strength (Baxter, Foyer, Turner, Rolfe, & Quick, 2003; Gebril et al., 2015; Haigler et al., 2007; Ishimaru et al., 2008; Laporte et al., 2001; Micallef et al., 1995; Nguyen‐Quoc, N'Tchobo, Foyer, & Yelle, 1999; Park, Canam, Kang, Ellis, & Mansfield, 2008; Park, Canam, Kang, Unda, & Mansfield, 2009; Seger, Gebril, Tabilona, Peel, & Sengupta‐Gopalan, 2015). Similarly, there have been several attempts to modulate the levels of GS1 enzyme in different plants using genetic engineering tools with the goal of improving the plant performance. However, no overriding picture has emerged from all these studies (Bao et al., 2014; Carvalho, Lopes‐Cardoso, Lima, Melo, & Cullimore, 2003; Fuentes, Allen, Ortiz‐Lopez, & Hernández, 2001; Harrison, de Crescenzo, Sené, & Hirel, 2003; Kirby, Gallardo, Man, & El‐Khatib, 2006; Oliveira et al., 2002; Ortega, Temple, Bagga, Ghoshroy, & Sengupta‐Gopalan, 2004; Seger, Ortega, Bagga, & Sengupta‐Gopalan, 2009; Seger et al., 2015; Temple, Knight, Unkefer, & Sengupta‐Gopalan, 1993; Temple, Bagga, & Sengupta‐Gopalan, 1994, 1998; Thomsen, Eriksson, Møller, & Schjoerring, 2014).
Legumes are unique in that they have nodules which function as a C sink and N source while the leaves function as a N sink and C source, thus making it an ideal system for studying C/N interaction and the cross talk between the source and sink for C and N. To determine if there is any inter/codependence between C and N metabolic pathways, this study was undertaken to compare GS 1 overexpressing alfalfa plants (35S‐GS) with the 35S‐SPS transformants. Our hypothesis is that increased SPS expression in the leaves would lead to an increase in Suc transport to the nodules while upregulating GS1 would increase the nitrogen assimilation in the nodules. The most intriguing outcome of this study was that both classes of transformants, irrespective of the transgene, showed a very similar response at different levels.
2. MATERIALS AND METHODS
2.1. Gene manipulations
The CaMV 35S‐SPS (ZmSPS) and the CaMV 35S‐GS (Gmglnb 1) constructs are as described by Seger et al. (2015). Plasmid pCAMBIA 2300 was used as the control vector for transformation.
2.2. Plant transformation and growth conditions
Agrobacterium‐mediated plant transformations were carried out as described by Gebril et al. (2015). DNA was isolated from the leaves of alfalfa using a DNeasy Mini Plant Kit (Qiagen). The DNA was subjected to PCR using the NPTII primer set. The randomly selected PCR‐positive transformants from tissue culture, three with Cambia 2300 (control), three with 35S‐SPS and three with 35S‐GS gene constructs, were transferred into the greenhouse. Plants were inoculated with S. meliloti 2011 to initiate symbiotic N2 fixation, and then fed N‐free Hoagland's nutrient solution, weekly. Since alfalfa is self‐incompatible and seeds cannot be obtained by selfing, plants were clonally propagated to make biological replicates. This was done by cutting the stem with apical meristem, dipping the cut end in a rooting hormone, then placing them in wet vermiculite in pots. Cuttings were also inoculated after acclimation and fed N‐free Hoagland, weekly. For each control plant and individual transformants, three to five clones were analyzed as biological replicates and averaged or pooled for experiments. The plants were grown in the greenhouse with full sunlight during the day along with supplemental LED grow lights (LIFTED, Rio Rancho, NM) for an extended light period during the winter.
2.3. RNA isolation and RT‐PCR
Total RNA was isolated from alfalfa tissues by the LiCl precipitation method (Ortega et al., 2006). A quantity of 2 μg of DNase‐treated RNA was used to prepare cDNA using a Superscript III first strand synthesis RT‐PCR kit (Invitrogen). The standard synthesis profile and reaction conditions were used as described in the Invitrogen protocol.
To perform semiquantitative RT‐PCR, the cDNA for each sample was subjected to PCR amplification using the primer sets for the different genes: ZmSPS1, Gmglnb1, and endogenous genes SPSA, SPSB, GS1a, GS1b, and Actin (Table 1). The primers were designed using IDT primer quest tool software and PCR was carried out using a OneTaq Hot Start PCR Kit (New England Biolabs). The amount of cDNA used and the number of cycles in the thermocycler were varied depending on the expression level of the endogenous genes in the leaves and nodules of alfalfa plants. The number of cycles was fine tuned to ensure that the reaction was terminated during the linear phase of amplification. Actin primers were used as an internal control. The products, following PCR, were subjected to electrophoresis and the band intensity for all the amplicons was measured and the values were adjusted based on the band intensity of the amplicon for Actin.
Table 1.
Primers used to monitor the expression of the GS and SPS genes in the alfalfa transformants
| Gene | Primer direction | Sequence |
|---|---|---|
| Medicago sativa Actin | Forward | 5′‐ACTTAACCCAAAGGCCAATAGA‐3′ |
| Reverse | 5′‐TGCTCATACGGTCAGCAATAC‐3′ | |
| M. sativa SPSA | Forward | 5′‐CCACTCACTTGGTCGAGATAAG‐3′ |
| Reverse | 5′‐TATGCCACTTGCCCATACAG‐3′ | |
| M. sativa SPSB5 | Forward | 5′‐CTCTTCACGCGCCAAATATCATCTC‐3′ |
| Reverse | 5′‐AGCTCTTCCGCTTCTATCCTTCTC‐3′ | |
| M. sativa GS1a | Forward | 5′‐CACAAATCAAGCTCCAGGACAAGATAG‐3′ |
| Reverse | 5′‐CATCTCCAGCAGAAATACCAACAGAAG‐3′ | |
| M. sativa GS1b | Forward | 5′‐TGGTCCCTCAGTTGGTATCT‐3′ |
| Reverse | 5′‐TGTGTGTGTGGTGGCTTATG‐3′ | |
| Zea mays SPS1 | Forward | 5′‐CCCGAAGAAGAACATCACTACC‐3′ |
| Reverse | 5′‐CGTCGATGTCATCTCTGTTACC‐3′ |
2.4. Protein isolation and analysis
Leaf and nodule tissues from biological replicates (three to five clones) were harvested for each independent transformant, and three independent transformants for each class were immediately placed in liquid N, and stored at −80°C until experiments were done. Tissues were ground in liquid N and homogenized with 6 volumes of extraction buffer. The SPS extraction buffer consisted of 50 mM HEPES, pH 7.5, 20% (v/v) glycerol, 5% (v/v) ethylene glycol, 5 mM EDTA, 5 mM magnesium acetate, 0.5% Triton X‐100, 10 mM DTT, and a protease inhibitors cocktail (Thermo Fisher). The GS extraction buffer consisted of 50 mM Tris‐HCl pH 8.0, 20% glycerol (v/v), 5% ethylene glycol (v/v), 1 mM MgCl2, 1 mM DTT, 1 mM EDTA, and a protease inhibitors cocktail. For SPS activity assays, the total protein extract was desalted in Sephadex G25 columns with desalting buffer (25 mM HEPES pH 7.5, 2.5 mM magnesium acetate, 20% glycerol (v/v), 5% ethylene glycol (v/v), and 1 mM EDTA). Protein concentration was measured using the Bradford protein assay (BioRad, Hercules, CA) with bovine serum albumin for protein standard concentrations.
2.5. Enzyme activity
SPS activity was assayed by quantifying the fructosyl moiety of Suc using the anthrone test (Seger et al., 2015) and the activity is expressed as nmol Suc‐P mg−1 protein min−1. Total GS activity was measured using the transferase assay as described by Seger et al. (2015). Transferase units were calculated from a standard curve of γ‐glutamyl hydroxamate. The activity is reported as μmole of γ‐glutamyl hydroxamate produced per mg of protein at 30°C.
2.6. Western blotting
The protein extracts used for enzyme activities were subjected to SDS–PAGE followed by western blotting. The percentage of these gels varied from 7% to 12% depending on molecular weight of all different proteins. The amount of protein loaded on the gels for each enzyme was adjusted to ensure that the bands were not saturated. The fractionated protein from these gels was electroblotted on the Immobilon‐P, PVDF membrane (Milipore, Bedford, MA). The detection of polypeptides was performed using polyclonal antibodies raised against soybean GS1 (Ortega et al., 2006), SPS (Agrisera, Sweden), SucS (provided by Dr. Raymond Chollet, University of Nebraska, Lincoln), PEPC, AS, NADH‐GOGAT, and nodule‐enhanced malate dehydrogenase (neMDH) (provided by Dr. Carol Vance, University of Minnesota, MN). The immunoreactive bands were visualized with alkaline phosphatase linked secondary antibody using nitroblue tetrazolium (NBT) and 5‐bromo‐4‐chloro‐3‐indoyl‐phosphate (BCIP) as substrates. The immunoreactive bands were quantified using the KODAK 1D image analysis system (CARESTREAM). Experiments were performed at least three to four times and only representative results were presented.
2.7. Carbohydrate analysis
Carbohydrate analysis was done using the anthrone method essentially as described in our earlier papers (Aleman et al., 2010; Gebril et al., 2015). For Suc and starch analysis, plants (35–40 days post‐inoculation) were kept under supplemental light for 48 hr, and leaves and nodules were harvested at 3:00 p.m.
2.8. Leaf gas exchange measurement
Gas exchange or net photosynthetic rates (P net) was measured with a Li‐Cor 6400‐05 conifer chamber with an external light source attached to an infrared gas analyzer‐based photosynthesis system (Li‐Cor Inc., Lincoln, Nebraska). Measurements were performed on mature trifoliate leaves between 10:00 a.m. and 12:00 p.m. after plants had been under supplemental light for 2 hours. Net photosynthetic rates were measured at a CO2 flow rate of 400 μmol/s and an internal CO2 of 400 μmol CO2 mol−1. Trifoliate leaf area was measured using a Li‐Cor LI3000 portable area meter (Li‐Cor Inc., Lincoln, Nebraska). Two measurements were done on each plant, consisting of three biological replicates for each of the three independent transformants and the average of the three values for each transformant was calculated and presented.
2.9. Nitrogenase activity
Nitrogenase activity of nodules was measured using an acetylene reduction assay (ARA) method described by Hardy et al. (1968). Alfalfa plants were removed from pots and excess vermiculite was shaken off the roots. These roots were placed in mason jars having Teflon lined septa on the top. The reaction was started by replacing 10% of internal air with acetylene (C2H2), which was generated from calcium carbide (CaC2) and water. The contents in the jar were shaken after every half an hour and 3 ml of acetylene/ethylene mixture was taken out at 0, 30, 60, and 90 min using a syringe. The mixture was kept in vacuum tubes until analysis. Empty jars with 10% acetylene was used as a blank to determine any background level. The amount of ethylene production was measured using gas chromatography (GC). Using a tight syringe, 1.0 ml from each vacuum tube containing acetylene/ethylene mixture was taken and injected into a GC. A standard curve with known concentrations of pure ethylene was generated and nitrogenase activity was measured as nmol ethylene per plant.
2.10. Biomass measurements
The primary transformants were moved from tissue culture containers to pots with vermiculite and grown in the greenhouse. The plants were inoculated with S. meliloti and grown for 2–3 months to produce the “mother plants.” Cuttings of the same size from these plants were made and planted in pots (vermiculite), and were inoculated. The plants were allowed to grow until the onset of flowering and then cut down and allowed to grow until the onset of flowering. At this time, the aerial parts of the plants were harvested weighed and then put in bags for drying. The fresh and dry biomass were measured. Since the control plants flowered later than the other two classes of transformants, they were allowed to grow for another 20 days before being cut.
2.11. Forage quality
Three sets of each control, 35S‐SPS, and 35S‐GS alfalfa transformants grown under symbiotic conditions were used to determine forage quality. These plants were treated the same way as for biomass measurement. About 10–12 shoots from each class of plants were harvested, pooled, and fresh samples were sent to SDK laboratories (Hutchinson, KS) for complete N profile and fiber analysis.
2.12. Statistical analysis
The data were analyzed with ANOVA comparison test using SAS software (v9.4). Each bar on the graphs is the average of three biological replicates and bars represent the value of standard deviation. Significant differences were evaluated using ANOVA planned contrast and shown by asterisks. Single asterisk (*) indicates 0.01 < p < 0.05 and double asterisk (**) indicates 0.001 < p < 0.01.
3. RESULTS
3.1. The 35S‐SPS and the 35S‐GS transformants showed the accumulation of the transgene product corresponding to the gene construct in both the leaves and nodules
DNA isolated from three independent transformants representing the three sets of transformants: CAMBIA2300 (control), the CaMV 35S promoter driving the maize SPS gene (35S‐SPS), and the CaMV35S promoter driving a soybean GS 1 gene (35S‐GS) were subjected to PCR amplification using NPTII gene‐specific primers followed by electrophoresis. NPTII is the selectable marker in the tDNA conferring resistance to the antibiotic kanamycin. As seen in Figure 1, all the putative transformants selected for this study showed an amplicon of the expected size (800 bp), confirming that the putative transformants were indeed transformed. These plants were selected for further analysis. The three independent transformants representing the three classes were propagated vegetatively and the cuttings were inoculated with S. meliloti.
Figure 1.
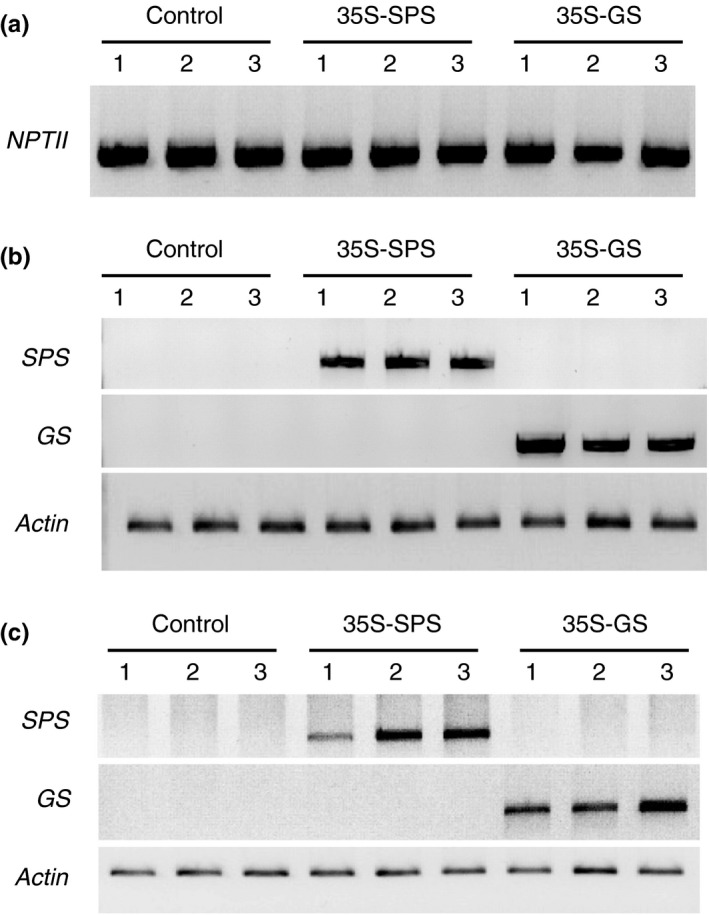
Analysis of the three classes of transformants: control (Cambia 2300), 35S‐SPS, and 35S‐GS plants for the integration and functionality of the gene constructs. (a) DNA isolated from three independent transformants for each class was isolated and subjected to genomic PCR using a NPTII specific primer set. The products were then fractionated on agarose gels. (b) RNA isolated from the leaves of the same plants used in panel A were subjected to RT‐PCR using primer sets specific for the SPS transgene, the GS transgene, and Actin and the products were electrophoresed. (c) RNA isolated from the nodules of the same set of plants used in panels A and B were subjected to RT‐PCR using primer sets specific for SPS transgene, GS transgene, and Actin and the products were subjected to electrophoresis
To check if the gene constructs are functional and that the promoter CaMV35S was functioning in a constitutive manner, we examined the plants for the expression of the transgenes in the leaves and nodules. RNA isolated from the leaves and nodules of the three classes of plants (control, 35S‐SPS, and 35S‐GS) was subjected to RT‐PCR using the two transgene specific primer sets along with the Actin primer set that was used as an internal control for RNA loads. The products along with known molecular weight DNA markers were electrophoresed on agarose gel. The 35S‐SPS transformants showed an amplicon of the expected size (800 bp) and the 35S‐GS transformants showed a 750‐bp amplicon in both the leaves and nodules. The control plants did not show an amplicon with either set of primers (Figure 1). These results confirm that the transgene was being transcribed in both the leaves and nodules.
3.2. The 35S‐GS transformants along with the 35S‐SPS transformants showed an increase in SPS level in their leaves
Western blot analysis was performed on total protein extracted from the leaves of three clonally propagated plants for each of the three independent transformants representing the three classes of plants using SPS antibodies. A representative blot with samples from one of the replicates for each individual transformant is shown in Figure 2a. The intensity of the immunoreactive bands from this blot was measured and plotted as bar graphs (Figure 2b). An immunoreactive band (~140 kD) was seen in all the samples, but the band intensity in the samples from both the 35S‐SPS and 35S‐GS transformants, in general, was higher when compared to controls. While the increase in SPS level in the 35S‐SPS transformants over the control plants can probably be an attribute of the expression of the SPS transgene, the increase in SPS levels in the 35S‐GS transformants over the control plants can only be ascribed to an increase in the endogenous SPS level.
Figure 2.
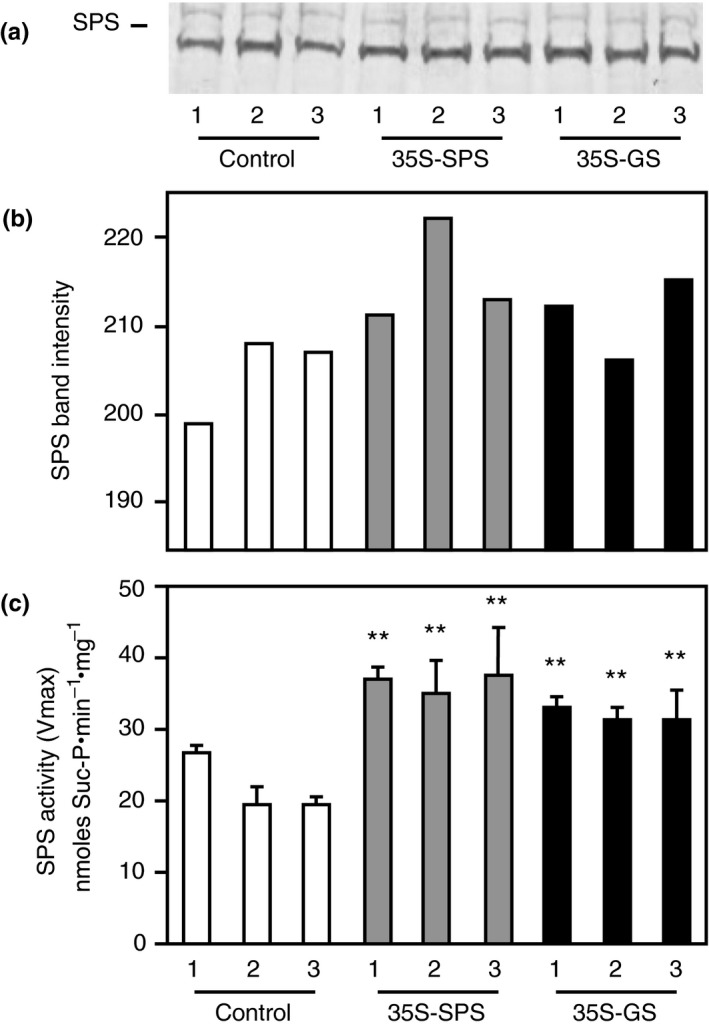
Analysis of SPS protein and SPS enzyme activity in the leaves of control, 35S‐SPS, and 35S‐GS transformants. (a) A quantity of 70 μg of total protein extracted from the leaves of three clonally propagated plants for each independent transformant was subjected to SDS–PAGE (7.5% acrylamide) followed by western blot analysis using SPS antibodies. A representative blot is shown here. The size of the immunoreactive band was determined based on the migration of proteins of known molecular weight. (b) The immunoreactive bands from the western blot were quantified using the KODAK image analysis software and plotted as band intensity. (c) The same leaf extracts used for western blot analysis were used for enzyme activity measurement by quantifying the synthesis of Suc‐6P from UDP‐Glc and Fru‐6P. SPS enzyme activity values are plotted as nmol Sucrose‐P mg−1 protein min−1. Values are the means ± SD of samples from three different replicates for each independent transformant. Significant differences from the average value obtained for the control plants were evaluated by ANOVA contrast test and shown by asterisks (*p < 0.05 or **<0.01)
Total soluble protein extracted from the leaves of the same plants as used for western blot analysis was subjected to the SPS enzyme activity assay. The enzyme activity was measured by quantifying the synthesis of Suc‐6P from UDP‐Glc and Fru‐6P, and SPS enzyme activity values were plotted as nmol Suc‐P mg−1 protein min−1. The bars represent the average of the activities of the three replicates for each of the three independent transformants representing each of the three classes of plants (Figure 2c). SPS activity was significantly higher in the leaves of both the 35S‐SPS and 35S‐GS transformants when compared to the samples from the control plants with the levels being higher in the 35S‐SPS transformants. These results indicate that the SPS activity in the leaves match the accumulation pattern of the corresponding protein.
3.3. The 35S‐GS transformants showed accumulation of the GS1 transgene product in the leaves
Western blot analysis using the GS antibodies was performed on total protein extracted from the leaves of the same three clonally propagated plants for each of the three independent transformants representing each of the three classes, as used for the analysis of SPS protein (Figure 2). A representative blot with samples from a replicate of each individual transformant is shown in Figure 3a. The intensity of the immunoreactive bands from this blot was measured and plotted as bar graphs (Figure 3b). An immunoreactive band corresponding to GS2 was seen in all the lanes and the intensity of the band was the same across all the lanes. A major immunoreactive band, migrating faster than the GS2 protein band was seen only in the lanes with leaf protein from the 35S‐GS transformants, most likely representing the transgene product. All the lanes, besides showing the major GS2 band also showed an immunoreactive band migrating slightly slower than the band corresponding to the soybean GS1 (transgene) protein, though the band in the 35S‐GS transformants appeared to be masked by the stronger transgene protein band. This band probably represents the endogenous GS1 protein.
Figure 3.
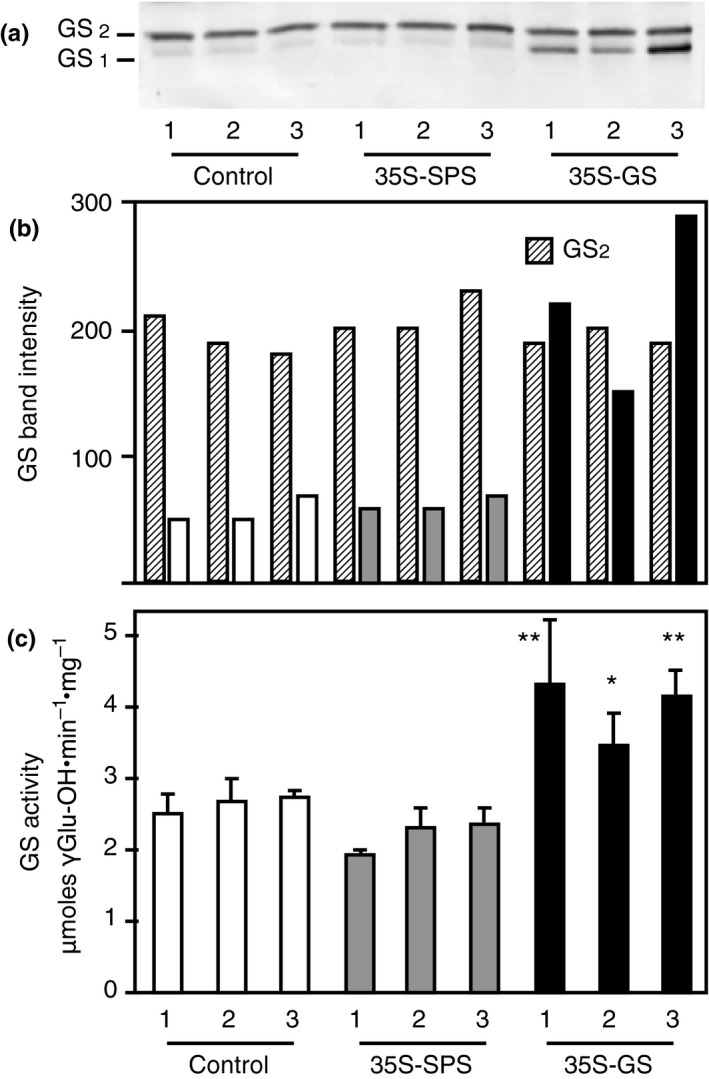
Analysis of GS protein and GS enzyme activity in the leaves of control, 35S‐SPS, and 35S‐GS transformants. (a) A quantity of 10 μg of total protein extracted from the leaves of three clonally propagated plants for each independent transformant was subjected to SDS–PAGE (10% acrylamide) followed by western blot analysis using GS antibodies. A representative blot is shown here. The size of the immunoreactive band was determined based on the migration of proteins of known molecular weight. (b) The immunoreactive bands from the western blot were quantified using the KODAK image analysis software and plotted as band intensity. (c) GS transferase activity was measured in the leaf extracts used for GS western blot analysis and GS activity values were plotted as μmol γ‐glutamyl hydroxamate produced per minute per mg of protein at 30°C. Values are the means ± SD of samples from three different replicates for each independent transformant. Significant differences from the average value obtained for the control plants were evaluated by ANOVA contrast test and shown by asterisks (*p < 0.05 or **<0.01)
The same samples used for western blotting were subjected to GS activity measurement using the transferase assay. The enzyme activity for the three replicates for each transformant was averaged and plotted as μmol γ‐glutamyl hydroxamate produced per minute per mg of protein. (Figure 3c). GS activity was significantly higher in the 35S‐GS transformants compared to the activities in the 35S‐SPS transformants and the control plants. One of the three 35S‐SPS transformants had lower GS activities than the control plants. The overall means of control plants and 35S‐SPS transformants were not different, and both means were highly statistically different from the 35S‐GS transformants. These results indicate that the GS activity was higher in the 35S‐GS transformants compared to the activity in the 35S‐SPS transformants and control plants, which can be attributed to the accumulation of the transgene polypeptide.
3.4. The expression of SPSB gene was upregulated in the leaves of both the 35S‐SPS and 35S‐GS transformants
To check if an increase in the accumulation of the SPS proteins in the leaves of the 35S‐GS transformants is due to the induction of endogenous SPS genes, semiquantitative RT‐PCR was conducted on the RNA isolated from the leaves of the same plants that were used for western blot analysis (Figures 2 and 3) using primer sets for SPSA and SPSB. The number of cycles was fine tuned to ensure that the reaction was terminated during the log phase of amplification. Actin primers were also used to amplify Actin transcripts, used as an internal control for RNA concentration. The PCR products were subjected to electrophoresis and the band intensity for all the amplicons was measured and the values were adjusted based on the band intensity of the amplicon for Actin. All the three independent transformants for 35S‐SPS and the 35S‐GS classes, showed an increase in the amplicon intensity for SPSB over control. The value for each class of transformants (control, 35S‐SPS, and 35S‐GS) was then calculated as the average of the values for the three independent transformants (Figure 4). A significant increase in the level of the amplicon for SPSB was seen in the leaves of both the 35S‐SPS and 35S‐GS transformants when compared to the control plants. No difference in the amplicon levels for SPSA was seen among the three classes of transformants. This analysis was done on RNA from different replicates with the same overall outcome; both the 35S‐SPS and 35S‐GS transformants showed an increase in the SPSB transcript level when compared to the control plants.
Figure 4.
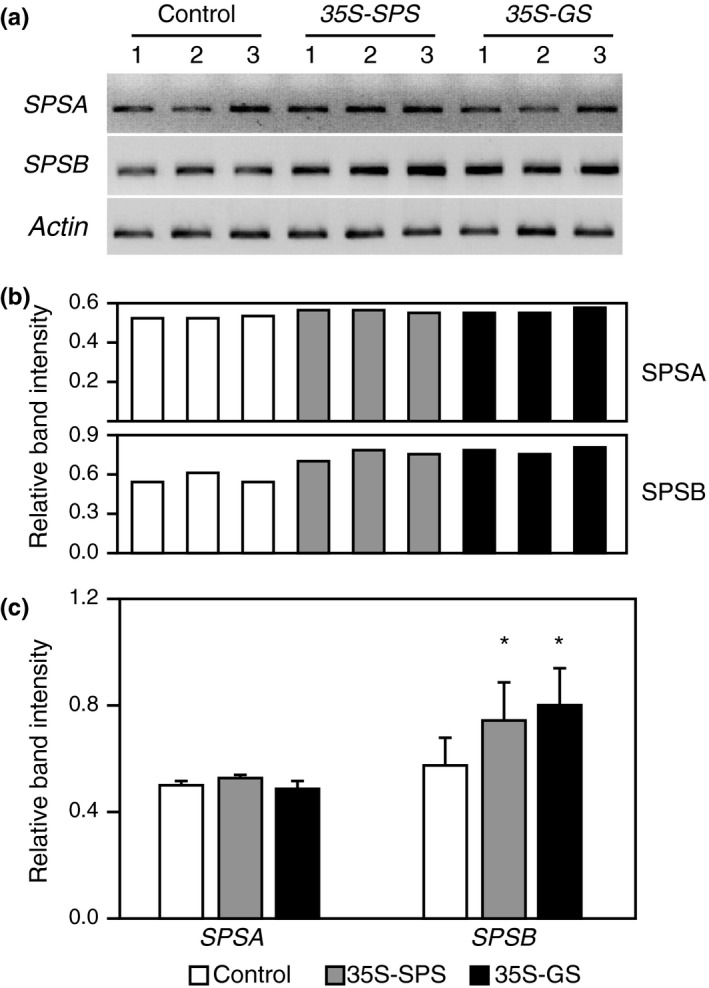
Analysis of transcript accumulation for the endogenous SPS genes in the leaves of control, 35S‐SPS, and 35S‐GS transformants. (a) A quantity of 2 μg of total RNA isolated from the leaves of the same set of plants used for protein analysis was subjected to semiquantitive RT‐PCR using primer sets specific for SPSA,SPSB, and Actin (internal control for RNA concentration). Following amplification, the products were subjected to electrophoresis. Data from a representative experiment is shown here. (b) The band intensities were measured using the KODAK image software. The ratio of the band intensity obtained for SPSA and SPSB genes relative to the band intensity obtained with the Actin primer set was calculated for each transformant. The values obtained for the three independent transformants representing the 35S‐SPS and 35S‐GS classes were then compared with the average value obtained for the three control plants and plotted as bar graphs. Significant differences from the control plants were evaluated by ANOVA contrast test and shown by asterisks (*p < 0.05 or **<0.01)
3.5. The 35S‐SPS and 35S‐GS transformants showed higher Suc and starch content in their leaves compared to the control plants
With the rationale that an increase in SPS activity could translate into higher rates of Suc synthesis, Suc content was measured in the leaves of all the three classes of transformants. Total carbohydrates were extracted from the leaves of three clonally propagated plants for each transformed line and the average of these was then calculated (Table 2A). The average Suc content in the leaves of the 35S‐GS transformants and 35S‐SPS transformants were compared with the average Suc concentration for all the three control plants. All three individuals representing the two classes of transformants showed a statistically significant (50%–70%) increase in the Suc content in their leaf tissue compared to the leaves of control plants. These results indicate that the increase in SPS activity in the leaves of both the 35S‐SPS and 35S‐GS transformants is accompanied by an increase in Suc level.
Table 2.
Analysis of forage quality of control, 35S‐SPS, and 35S‐GS transformants
| (A) | |||
|---|---|---|---|
| Genotype | Avg. lignin % dry basis | Avg. ADF % dry basis | Avg. NDF % dry basis |
| Control 1 | 4.82 ± 0.07 | 23.6 ± 5.07 | 29.21 ± 2.24 |
| Control 2 | 5.24 ± 0.14 | 26.56 ± 0.3 | 31.95 ± 2.58 |
| Control 3 | 4.82 ± 0.035 | 27.29 ± 0.05 | 31.44 ± 0.65 |
| 35S‐SPS 1 | 4.35 ± 0.03* | 22.9 ± 0.2* | 25.32 ± 0.93** |
| 35S‐SPS 2 | 4.21 ± 0.11* | 21.94 ± 0.411* | 25.97 ± 0.13** |
| 35S‐SPS 3 | 4.16 ± 0.12* | 22.47 ± 0.96* | 26.04 ± 0.16** |
| 35S‐GS 1 | 4.50 ± 0.127* | 23.91 ± 0.07 | 29.62 ± 0.02 |
| 35S‐GS 2 | 4.70 ± 0.06* | 22.01 ± 1.97** | 25.51 ± 1.41** |
| 35S‐GS 3 | 4.22 ± 0.12* | 22.67 ± 0.30* | 24.50 ± 1.30** |
| (B) | |||
|---|---|---|---|
| Genotype |
Avg. CP % dry basis |
Avg. RFQ | Avg. RFV |
| Control 1 | 20.16 ± 0.64 | 204.66 ± 4.5 | 197.33 ± 2.51 |
| Control 2 | 20.77 ± 0.20 | 210 ± 11.7 | 200.33 ± 15.5 |
| Control 3 | 19.89 ± 0.78 | 229.5 ± 4.5 | 198 ± 6 |
| 35S‐SPS 1 | 24.24 ± 0.7** | 253.66 ± 4.7** | 253.33 ± 1.52** |
| 35S‐SPS 2 | 23.84 ± 0.17** | 248 ± 2.0** | 251.33 ± 0.57** |
| 35S‐SPS 3 | 24.84 ± 0.23** | 259 ± 2.0** | 258.66 ± 1.1.52** |
| 35S‐GS 1 | 22.01 ± 0.70** | 213.33 ± 4.16 | 225.5 ± 4.5** |
| 35S‐GS 2 | 22.47 ± 0.06** | 238.33 ± 3.51** | 245.16 ± 1.7** |
| 35S‐GS 3 | 22.08 ± 0.69** | 252 ± 3.0** | 257 ± 2.0** |
The clonally propagated plants was grown 50 days post‐inoculation at which time they were all cut down to the base and grown till the onset of flowering. The shoots were then cut at the base and sent out to the SDK labs for forage quality analysis. The control plants were cut ~2 weeks later since they flowered late compared to the other two classes of transformants. (A) The lignin content, acid detergent fiber (ADF), and neutral detergent fiber (NDF) were measured. Values for three different replicates for each independent transformant were measured, and the mean value ± SD was calculated for each plant. Significant differences between the 35S‐SPS and 35S‐GS from the average value obtained from the control plants were evaluated by ANOVA contrast test and shown by asterisks (* p < 0.05 or **<0.01). (B) Relative forage quality (RFQ), relative feed value (RFV), and crude protein (CP) content were measured. The RFQ and RFV were calculated based on fiber and protein content. Values for three different replicates for each independent transformant representing each class were measured, and the mean value ± SD was calculated for each plant. Significant differences between the 35S‐SPS and 35S‐GS from the average values obtained for the control plants were evaluated by ANOVA contrast test and shown by asterisks (* p < 0.05 or **<0.01).
Starch was isolated from the same samples as used for Suc extraction as described in Materials and Methods. To measure starch content, it was digested with α‐amyloglucosidase to release the Glc units and the Glc content was measured spectrophotometrically. The starch content like the Suc content was measured in the leaves of the three clonally propagated plants for each transformed line and the average of these values was then calculated. The average starch content in each independent transformant for the two classes, 35S‐SPS and 35S‐GS, was then compared with the average value for the three independent control plants. The starch content in both classes, 35S‐SPS and 35S‐GS transformants, showed significantly higher level compared to the control plants with the level being higher in the 35S‐SPS transformants (Figure 8b). The results suggest that there is higher conversion of Suc to starch in the leaves of the 35S‐SPS transformants compared to the leaves in the 35S‐GS transformants.
3.6. Both the 35S‐SPS and 35S‐GS transformants showed higher accumulation of SPS protein and enzyme activity in their nodules compared to the control plants
To check how constitutive expression of SPS and GS 1 transgenes affects the accumulation of the SPS proteins in the nodules, total protein extracted from the nodules of three clonally propagated plants for each of the three independent transformants for each of the three classes of plants was subjected to western blot analysis using the SPS antibodies. A representative blot with samples from one replicate for each transformant is shown in Figure 5a. The intensity of the immunoreactive bands was measured in each case and plotted as bar graphs (Figure 5b). An immunoreactive band (~140 kD) was seen in all the samples, but the band intensity in all the samples from both the 35S‐SPS and 35S‐GS transformants, was higher when compared to controls (Figure 5a,b). While the increase in SPS level in the 35S‐SPS transformants over the control plants can probably be an attribute to the expression of the SPS transgene, the increase in SPS levels in the 35S‐GS transformants over the control plants can only be ascribed to an increase in the endogenous SPS level.
Figure 5.
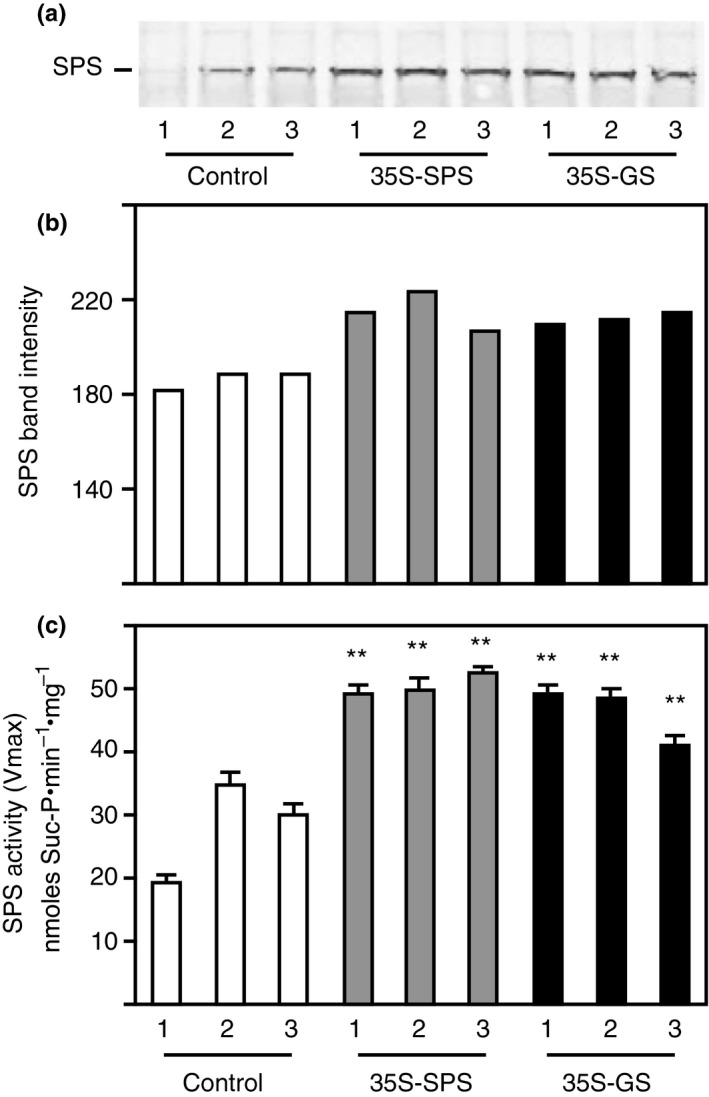
Analysis of SPS protein and SPS enzyme activity in the nodules of control, 35S‐SPS and 35S‐GS transformants. (a) A quantity of 50 μg of total protein extracted from the nodules of three clonally propagated plants for each independent transformant was subjected to SDS–PAGE (7.5% acrylamide) followed by western blot analysis using SPS antibodies. A representative blot is shown here. The size of the immunoreactive band was determined based on the migration of proteins of known molecular weight. (b) The immunoreactive bands from the western blot were quantified using the KODAK image analysis software and plotted as band intensity. (c) The same nodule extracts used for western blot analysis were used for enzyme activity measurement by quantifying the synthesis of Suc‐6P from UDP‐Glc and Fru‐6P. SPS enzyme activity values are plotted as nmol Suc‐P mg−1 protein min−1. Values are the means ± SD of samples from three different replicates for each independent transformant. Significant differences from the average value obtained for the control plants were evaluated by ANOVA contrast test and shown by asterisks (*p < 0.05 or **<0.01)
The samples used for western blot analysis were used for measuring SPS enzyme activity by quantifying the synthesis of Suc‐6P from UDP‐Glc and Fru‐6P and SPS enzyme activity values was plotted as nmol Suc‐P mg−1 protein min−1. The bars represent the average of the activities of the three replicates for each of the three independent transformants for each of the three classes of plants (Figure 5c). SPS activity was significantly higher in the nodules of both the 35S‐SPS and 35S‐GS transformants when compared to the samples from control plants. The results on enzyme activity closely follows SPS protein accumulation pattern, suggesting that the increase in SPS levels and SPS enzyme activity in the nodules of the 35S‐GS transformants is probably due to the activation of endogenous SPS gene/s.
3.7. Both the 35S‐SPS and 35S‐GS transformants showed higher accumulation of GS protein and GS enzyme activity in their nodules compared to the control plants
Western blot analysis using the GS antibodies was performed on total protein extracted from the nodules of the same three clonally propagated plants for each of the three independent transformants, as used for analysis of SPS protein (Figure 5). A representative blot with samples from one replicate for each transformant is shown in Figure 6a. The intensity of the immunoreactive bands was measured in each case and plotted as bar graphs (Figure 6b). As expected, all the samples showed an immunoreactive band of 39 kD, with the GS antibodies; however, both the 35S‐SPS and 35S‐GS transformants showed higher accumulation of the immunoreactive band when compared to the control plants with the levels being higher in the 35S‐SPS transformants (Figure 6a,b).
Figure 6.
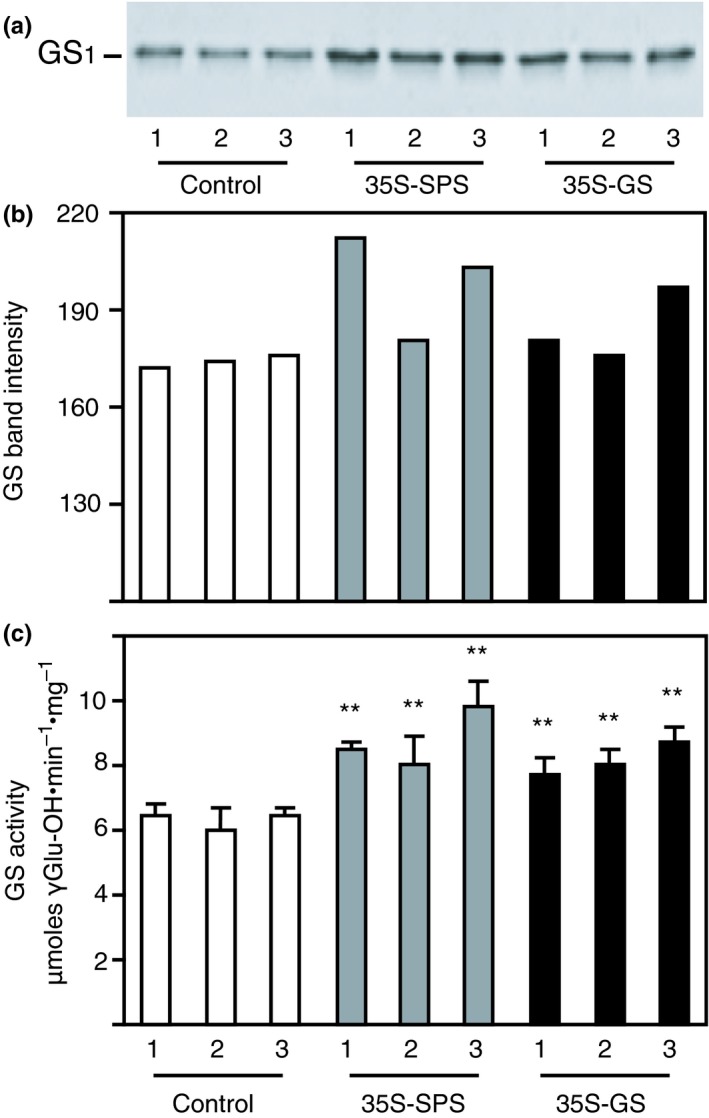
Analysis of GS protein and GS enzyme activity in the nodules of control, 35S‐SPS, and 35S‐GS transformants. (a) A quantity of 1 μg of total protein extracted from the leaves of three clonally propagated plants for each independent transformant was subjected to SDS–PAGE (10% acrylamide) followed by western blot analysis using GS antibodies. A representative blot is shown here. The size of the immunoreactive band was determined based on the migration of proteins of known molecular weight. (b) The immunoreactive bands from the western blot were quantified using the KODAK image analysis software and plotted as band intensity. (c) GS transferase activity was measured in the nodule extracts used for GS western blot analysis and GS activity values were plotted as μmol γ‐glutamyl hydroxamate produced per minute per mg of protein at 30°C. Values are the means ± SD of samples from three different replicates for each independent transformant. Significant differences from the average value obtained for the control plants were evaluated by ANOVA contrast test and shown by asterisks (*p < 0.05 or **<0.01)
The same samples used for western blotting were subjected to GS enzyme activity measurement using the transferase assay. The enzyme activity for the three replicates for each individual transformant was averaged and plotted as μmol γ‐glutamyl hydroxamate produced per minute per mg of protein. (Figure 6c). GS activity was significantly higher in both the 35S‐SPS and 35S‐GS transformants compared to the activities in the control plants. Taken together, it appears that the GS enzyme activity level is consistent with GS protein accumulation in the nodules. What is most intriguing is that both GS protein accumulation and GS enzyme activity was higher in the nodules of the 35S‐SPS transformants compared to the 35S‐GS transformants, implying that there is an induction of GS 1 gene(s) in the nodules of the 35S‐SPS transformants.
3.8. The expression of the endogenous SPS and GS 1 gene members were upregulated in the nodules of both the 35S‐SPS and 35S‐GS transformants
An increase in the accumulation of SPS proteins in the nodules of the 35S‐GS transformants and that of GS in the nodules of the 35S‐SPS transformants prompted us to check the expression level of the endogenous SPS and GS 1 genes. Semiquantitative RT‐PCR was performed on the RNA isolated from the nodules of the same plants used for western blot analysis using primer sets for GS1a, GS1b, SPSA, and SPSB. The number of cycles was fine tuned to ensure that the reaction was terminated during the log phase of amplification. Actin primers were also used to amplify the Actin transcript as an internal control for RNA concentration. The products, following PCR, were subjected to electrophoresis and the band intensity for all the amplicons was measured and the values were adjusted based on the band intensity of the amplicon for Actin. This analysis was done on RNA isolated from multiple replicates and the results of a representative experiment are shown. The bar graph shows the intensity of the bands for the amplification products (Figure 7b). No amplicon was obtained with the SPSB primer set consistent with the fact that SPSB is not expressed in the nodules (Aleman et al., 2010). All the three independent transformants for both the 35S‐SPS and 35S‐GS classes showed an increase in the amplicon level for SPSA and GS1a in their nodules when compared to the control. The value for each class of transformants (control, 35S‐SPS, and 35S‐GS) was then calculated as the average of the values for the three independent transformants. A significant increase in the amplicon level for SPSA and GS1a was seen in both the 35S‐SPS and 35S‐GS transformants when compared to the control plants with the levels in the 35S‐SPS transformants superseding the levels in the 35S‐GS transformants (Figure 7). No difference in the amplicon level for GS1b was seen across all the samples. The results indicate that the expression of the gene members SPSA and GS1a are upregulated in the nodules of both the 35S‐SPS and 35S‐GS transformants, more so in the nodules of the 35S‐SPS transformants.
Figure 7.
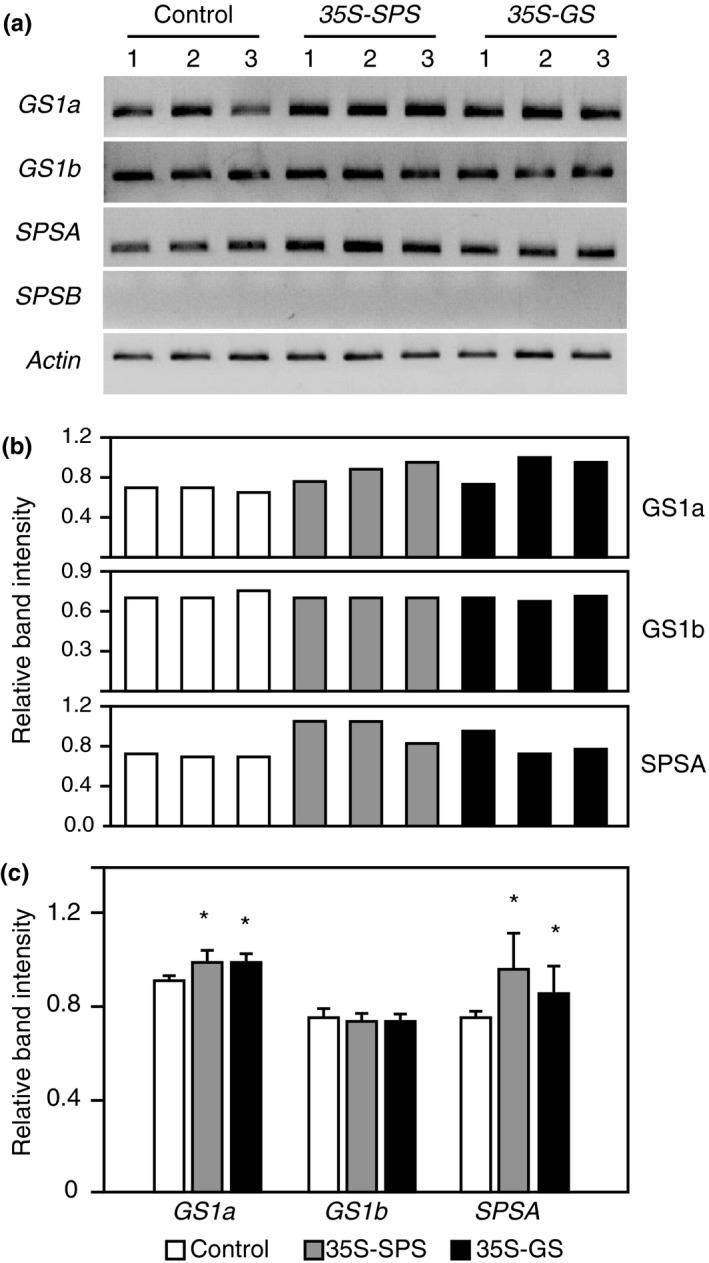
Analysis of transcript accumulation for the endogenous SPS genes in the nodules of Control, 35S‐SPS, and 35S‐GS transformants. (a) A quantity of 2 μg of total RNA isolated from the nodules of the same set of plants used for protein analysis was subjected to semiquantitive RT‐PCR using primer sets specific for GS1a,GS1b,SPSA,SPSB, and Actin (internal control for RNA concentration). Following amplification, the products were subjected to electrophoresis. Data from a representative experiment are shown here. (b) The band intensities were measured using the KODAK image software. (c) The ratio of the band intensity obtained for GS1a,GS1b,SPSA,SPSB genes relative to the band intensity obtained with the Actin primer set was calculated for each transformant. The values obtained for the three independent transformants representing the 35S‐SPS and 35S‐GS classes were then compared with the average value obtained for the three control plants and plotted as bar graphs. Significant differences from the control plants were evaluated by ANOVA contrast test and shown by asterisks (*p < 0.05 or **<0.01)
3.9. The 35S‐SPS and 35S‐GS transformants showed higher Suc and starch content in their nodules compared to the control plants
The Suc content was measured in the nodules, the same way as for leaves. The Suc content in the nodules of three replicates for each independent transformant was measured and the average value for each of the three independent transformants for each of the three classes was tabulated as average Suc content (Figure 8c).
Figure 8.
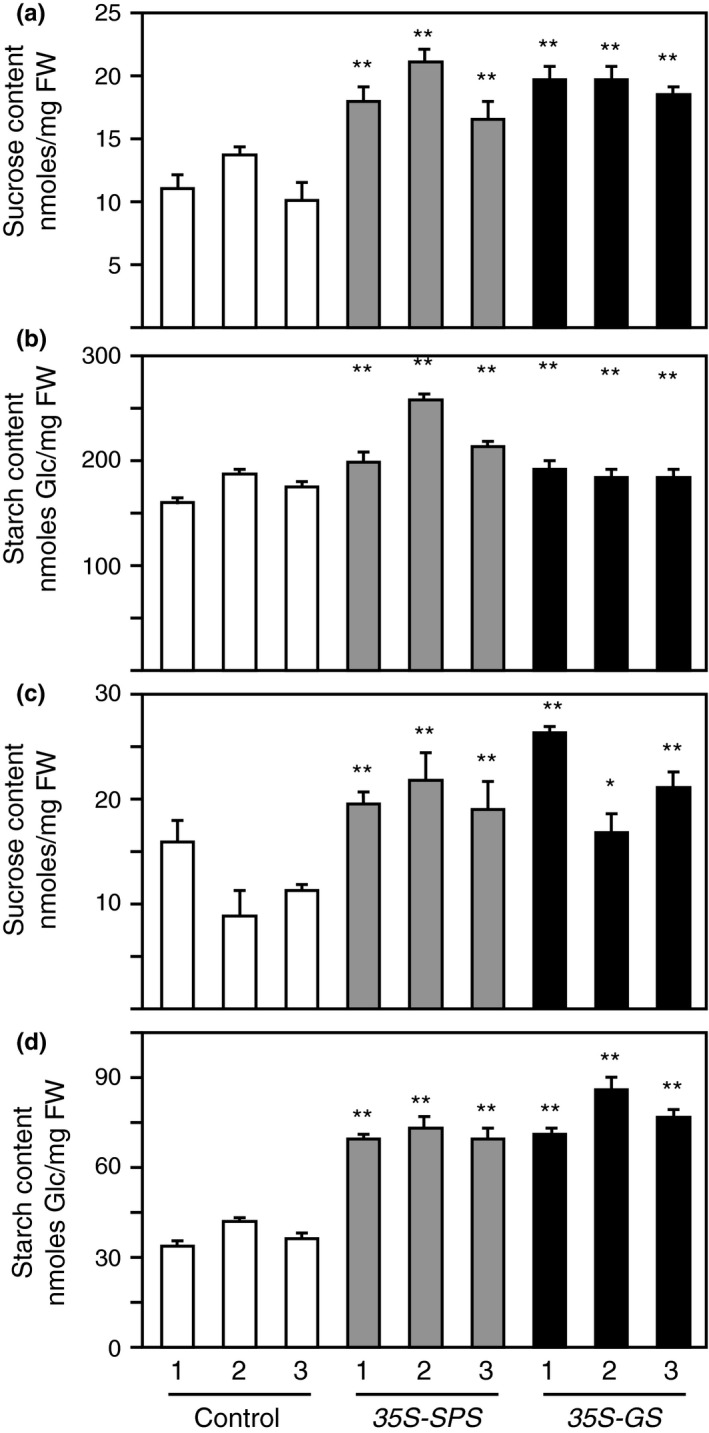
Sucrose and starch content in the leaves and nodules of control, 35S‐SPS, and 35S‐GS transformants. Sucrose and starch content were measured in the leaves (a, b) and nodules (c, d) as described in Section 2. Sucrose content was plotted as nmoles Suc mg−1 fresh weight (fwt) and starch content was plotted as nmoles Gluc mg−1 fresh weight (fwt). Values for three replicates for each of the three independent transformants representing each class were measured, and the mean value ± SD was calculated for each transformant. Significant differences between the 35S‐SPS and 35S‐GS transformants from the average value obtained from the three control plants were evaluated by ANOVA contrast test and shown by asterisks (*p < 0.05 or **<0.01)
Starch was isolated from the same samples as used for Suc extraction. The starch content like the Suc content was measured in the nodules of the three clonally propagated plants for each independent transformant representing the three classes of plants. The starch content in the nodules of three replicates for each independent transformant was measured and the average value for each of the three independent transformants representing each of the three classes was plotted as average starch content (Figure 8d).
The average values for Suc and starch for each of the three independent transformants representing each of the 35S‐SPS and 35S‐GS classes were compared with the average Suc and starch content in the three independent control plants. Both the Suc and starch concentration in all the three independent transformants representing the 35S‐SPS and 35S‐GS classes showed a significant increase of ~60%–70% in Suc content and up to 100% in starch content (Figure 8c,d). The results would indicate that there is more Suc transported into the nodules of the transformants and a high proportion is converted into starch. The higher levels of SPS activity in the nodules may play a role in the synthesis of starch by increasing Suc cycling.
3.10. The 35S‐SPS and 35S‐GS transformants showed higher level of the key enzymes involved in ammonia assimilation in their nodules compared to the control plants
Sucrose imported from the leaves to the nodules is acted upon by SucS to produce hexoses and hexose phosphates which are metabolized through the glycolytic pathway to produce phosphoenolpyruvate (PEP). PEP carboxylase (PEPC) and malate dehydrogenase (MDH) activities divert C flux from glycolysis to form malate which is the primary source of C transported into the symbiosomes that houses the bacteroids (White et al., 2007). The pyruvate derived from PEP enters the Krebs cycle to produce α‐KG. GOGAT in the GS‐GOGAT cycle combines Gln and α‐KG to produce Glu which then provides the C skeletons for loading up with ammonia, the reaction being catalyzed by GS. Glutamine can then be converted into asparagine (Asn); one of the enzymes in this conversion is asparagine synthetase (AS).
An increase in the amount of Suc imported in the nodules of the 35S‐SPS and 35S‐GS transformants would imply that there is an increase in the availability of substrates that is needed to support an increase in both N2 fixation and ammonia assimilation. One would then conjecture that an increase in these metabolic pathways would require increased enzyme level and or/enzyme activity for the different reactions in the pathways. To check for the levels of the different enzymes, western blot analysis was performed on nodule proteins from the three classes of plants. Protein extracted from the nodules of three independent transformants from each class was subjected to immunoblot analysis using antibodies for the different enzymes as shown in Figure 9a. As an internal control for protein loads, a panel from a gel with fractionated proteins stained with Coomassie blue is shown. The amount of protein loaded on the gels for each enzyme was adjusted to ensure that the band intensities were not saturated. Immunoreactive bands were seen with all the antibodies used in all the lanes (Figure 9a). The intensity of the immunoreactive bands was quantified and plotted. As seen in Figure 9b, in all cases, each of the three 35S‐SPS and 35S‐GS transformants showed higher level of band intensities when compared to the control plants. The average band intensity for each class of transformants was then calculated and subjected to statistical analysis. The values obtained for the 35S‐SPS and 35S‐GS transformants were compared with the average value obtained for the control plants.
Figure 9.
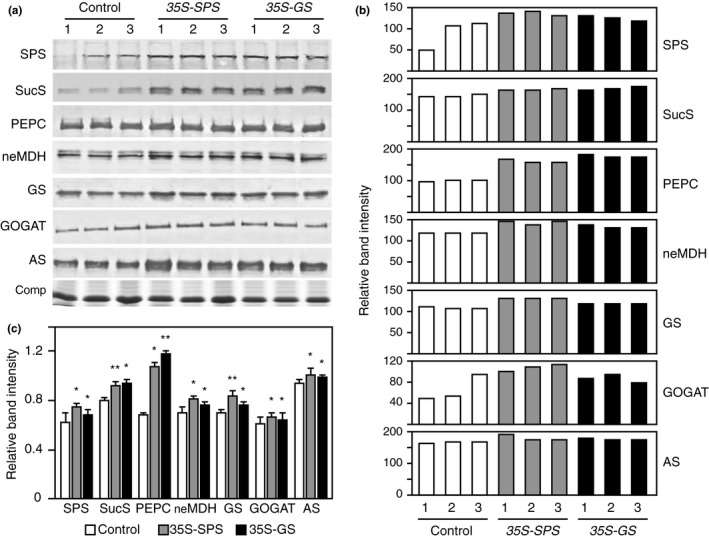
Examination of the steady‐state levels of key enzymes in C and N metabolic pathways in the nodules of control, 35S‐SPS, and 35S‐GS transformants. (a) Protein extracts from the nodules were subjected to SDS–PAGE followed by western blot analysis using the different antibodies (as indicated). A Coomassie blue stained (Comp) region of the protein gel is shown here as a reference for protein loads. The MW in kD indicated for each panel were based on the migration of known molecular weight markers. (b) The immunoreactive bands were quantified using the KODAK image analysis software, and the band intensity was normalized to the Coomassie stained endogenous protein band (Comp). The normalized band intensity was the plotted for each individual transformant representing the three classes. (c) The average of the values obtained for the three independent transformants representing the 35S‐SPS and 35S‐GS classes were then compared with the average value obtained for the three control plants and plotted as bar graphs. The values are the means ± SE of samples from three different independent transformants (Panel A) (n = 3) for each class of transformants. Significant differences from the control plants were evaluated by ANOVA contrast test and shown by asterisks (*p < 0.05 or **<0.01)
Sucrose synthase, neMDH, and AS appear to be in the form of a doublet indicating that in each case there are two gene members that are expressed in the nodules. In each case, however, only one of the bands in the doublet showed an increase in the 35S‐SPS and 35S‐GS transformants. Both the 35S‐SPS and 35S‐GS transformants showed a significant increase in the levels of SPS, SucS, PEPC, neMDH, NADH‐GOGAT, GS1 and AS, when compared to the control plants. An increase in the SucS level would imply increased sink strength or enhanced Suc import from the leaves while an increase in the levels of the other enzymes would mean a boost in N2 fixation and the assimilation of ammonia.
3.11. Compared to the control plants, the 35S‐SPS and 35S‐GS transformants showed increased photosynthetic rates
Tobacco plants overexpressing cytosolic GS have been shown to have higher photosynthetic rates (Fuentes et al., 2001; Seger et al., 2015), and similarly, overexpression of SPS has also been shown to be accompanied by an increase in photosynthetic rates (Baxter et al., 2003; Seger et al., 2015). To check if alfalfa plants overexpressing SPS and GS 1 transgene show the same trend with regard to photosynthesis, we measured net photosynthetic rates (P net) under ambient (400 mmol mol−1) CO2 concentration in the control, 35S‐SPS, and 35S‐GS transformants. Three clonally propagated plants representing each independent transformant were used in these measurements, and the average of the values obtained from the three replicates was plotted (Figure 10a). The P net values for each of the three transformants for the 35S‐SPS and 35S‐GS classes were compared with the average of P net values for all the three control plants. The P net rates were significantly higher in all the transformants belonging to both the 35S‐SPS and 35S‐GS classes.
Figure 10.
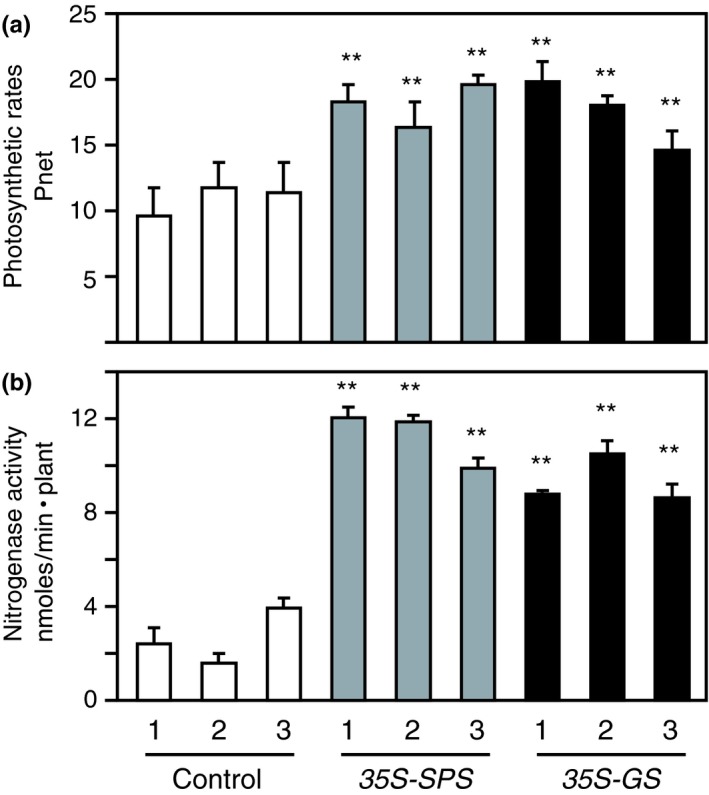
Analysis of (a) photosynthetic rates in the leaves and (b) nitrogenase activity in the nodules of control, 35S‐SPS and 35S‐GS transformants. (a) Clonally propagated plants were inoculated with Sinorhizobium meliloti and 30 days post‐inoculation, the photosynthetic rates were measured in mature trifoliate leaves from three replicates for each independent transformant. Photosynthetic rates (P net), measured as mmole CO 2 m−2 s−1, were determined using a conifer chamber attached to a Li‐Cor 6400 photosynthesis system. Values for three different replicates for each independent transformants were calculated as mean value ± SD. Significant differences between each independent transformant belonging to the 35S‐SPS and 35S‐GS classes, and the average value obtained for the three control plants were evaluated by ANOVA contrast test and shown by asterisks (*p < 0.05 or **<0.01). (b) Established cuttings were inoculated with S. meliloti and allowed to grow for a period of 30 days. The plants were then uprooted, and the roots of the plants were placed individually in mason jars and nitrogenase activity was measured as nmol ethylene per plant using the acetylene reduction assay as described in Section 2. Values for three to five different replicates for each independent transformants representing each class were measured, and the mean value ± SD was calculated for each individual transformant. Significant differences between the 35S‐SPS and 35S‐GS transformants from the average value obtained for the control plants were evaluated by ANOVA contrast test and shown by asterisks (*p < 0.05 or **<0.01)
3.12. Compared to the control plants, the 35S‐SPS and 35S‐GS transformants showed elevated nitrogenase activity
An increase in the MDH level in the nodules of the 35S‐SPS and 35S‐GS transformants could be an indicator of increased nitrogenase activity since malate is the form of organic acid used by the bacteroids (White et al., 2007). The root system of three replicates for each independent transformant representing all three classes of plants was assayed for nitrogenase activity using the ARA method as described in Section 2. The activity was measured as the amount of ethylene produced per plant and the values were plotted as nmoles min−1 plant−1. The average values for nitrogenase activity for each of the three independent transformants representing each of the three classes of plants were based on the values obtained from three to five replicates. Nitrogenase activity for the individual transformants belonging to the two classes, 35S‐SPS and 35S‐GS, was compared to the average values obtained for all the control plants. As seen in Figure 10b, all the transformants from each class showed significantly higher nitrogenase activity, with the level being higher in the 35S‐SPS transformants.
3.13. Compared to the control plants, the 35S‐SPS and 35S‐GS transformants showed increased growth, biomass, and nodule number
Cuttings of the same size from the primary transformants were made and planted in pots (vermiculite), and were then inoculated with S. meliloti. Thirty days following inoculation, a set of cuttings were uprooted, and the vermiculite was washed off from the roots, and the whole plant with the root system was photographed (Figure 11a). The 35S‐SPS transformants grew the fastest and had the most robust root system compared to the 35S‐GS transformants, which in turn performed better than the control plants. The nodules were harvested, counted, and weighed. The number and weight of the nodules for each plant (three replicates) were analyzed and the average value for the three independent transformants representing each class of transformants was plotted as average nodule number and nodule mass per plant (Figure 12). The nodule number and weight followed the same trend as the shoot and root growth pattern. The 35S‐SPS transformants showed the highest number of nodules and the nodules had a higher mass.
Figure 11.
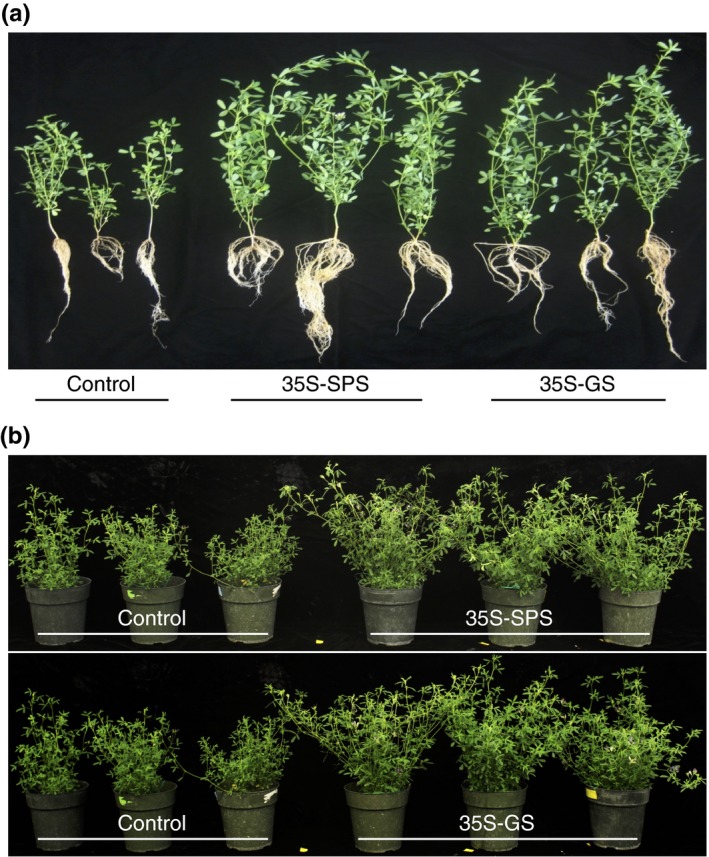
Visualization of growth in the control, 35S‐SPS, and 35S‐GS transformants at different stages. (a) Established transformants were used to obtain shoots for propagation. The cut shoots were planted on vermiculite and once established (~10 days) after the start day, the cuttings were inoculated with Sinorhizobium meliloti, and allowed to grow for a period of 30 days. The plants were uprooted and visualized. A plant representing each of the three independent transformant for each class were photographed. (b) Clonal replicates (2 per pot) were used for each individual transformant for each of the three classes of plants. The plants were grown till the onset of flowering and then cut down to the base. This process was repeated, and then, the plants were grown till the onset of flowering in the 35S‐SPS and 35S‐GS transformants, at which time they were photographed. The plants were arranged as: control and 35S‐SPS (top panel), and control and 35S‐GS transformants (bottom panel)
Figure 12.
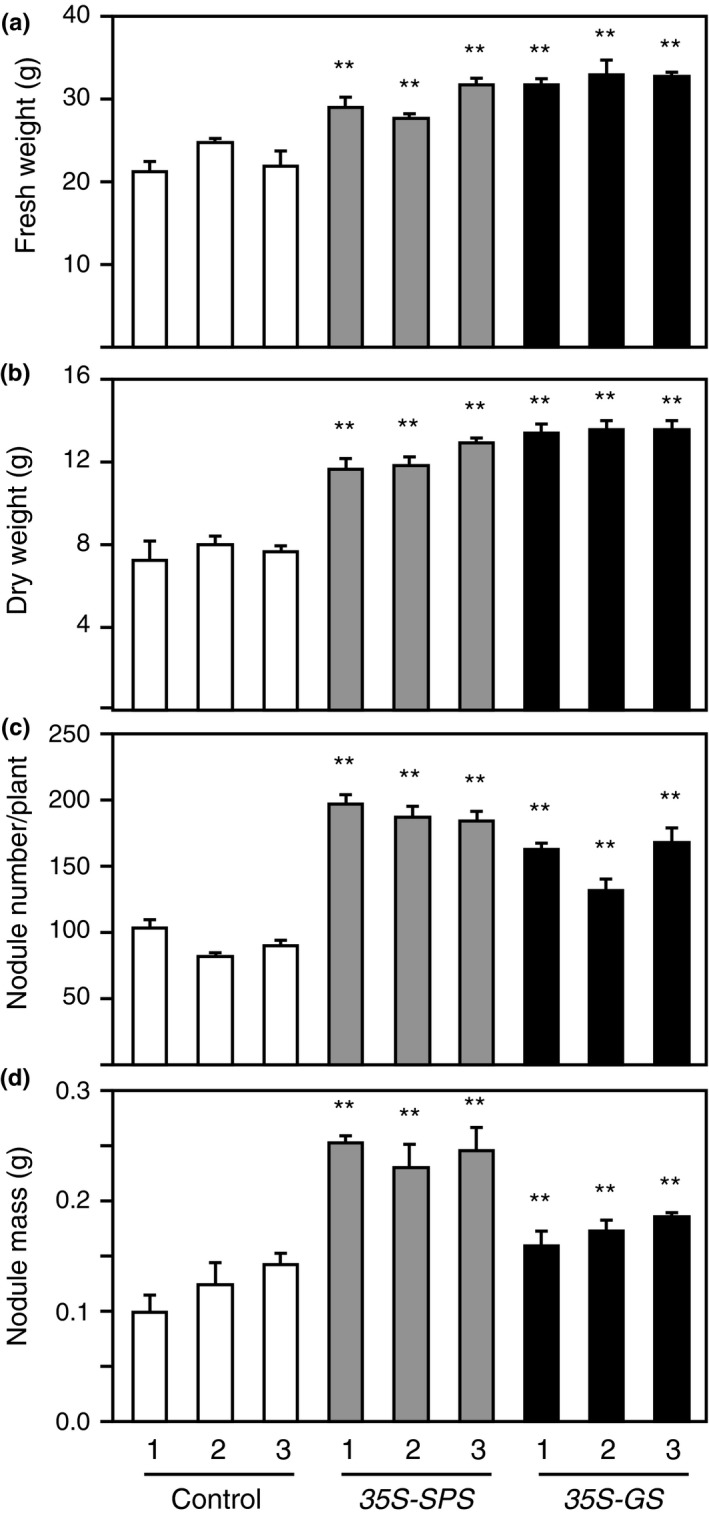
Analysis of fresh weight and dry weight of shoots and nodule number and mass of control, 35S‐SPS, and 35S‐GS transformants. (a, b) The plants from Figure 11, after being photographed, were cut down and allowed to grow back to just before the onset of flowering and were then cut at the base and weighed for fresh weight. For dry weight, the tissue was kept in paper bags for 2 weeks and then weighed. The control plants were cut ~2 weeks later since they flowered late compared to the other two classes of transformants. Values in grams for three different replicates for each independent transformants representing each class were measured, and the mean value ± SD was calculated for each individual transformant. Significant differences between the 35S‐SPS and 35S‐GS transformants from the average value obtained for the control plants were evaluated by ANOVA contrast test and shown by asterisks (*p < 0.05 or **<0.01). (c, d) Established cuttings were inoculated with Sinorhizobium meliloti and allowed to grow for a period of 30 days. The plants were uprooted and the nodules were harvested. The nodules per plant were counted and weighed. Values in numbers/grams for three different replicates for each independent transformant representing each class were measured, and the mean value ± SD was calculated for each individual transformant. Significant differences between the 35S‐SPS and 35S‐GS transformants from the average value obtained for the control plants were evaluated by ANOVA contrast test and shown by asterisks (*p < 0.05 or **<0.01)
Another set of cuttings were grown for a longer period of time to check for the timing of flowering and measurement of fresh and dry weight of the shoots. The 35S‐SPS transformants started flowering around 30 days post‐inoculation, the 35S‐GS transformants started flowering about a week after the 35S‐SPS plants while flowering in the control plants happened around 50 days post‐inoculation, about 2 weeks later than the 35S‐SPS transformants. The plants were cut down to the base after 50 days of growth following inoculation, and allowed to regrow for 45 days and then photographed (Figure 11b). Both the 35S‐SPS and 35S‐GS plants outgrew the control plants and the flowering in the control plants was delayed by about 2 weeks. At this stage, both the 35S‐SPS and 35S‐GS transformants showed no difference in growth. After being photographed, the plants were cut down at the time of onset of flowering, as such the control plants were allowed to grow for another 15 days before being cut. The cut material was then weighed and put in bags for drying. The fresh and dry biomass were measured when the plants were mature (at the onset of flowering), and as seen in Figure 12c,d, both the fresh weight and dry weight of the 35S‐SPS and 35S‐GS plants were significantly higher than the control plants.
3.14. The 35S‐SPS and 35S‐GS transformants exhibited improved forage quality when compared to the control plants
Forage quality is defined as a measure of the potential of forage to produce a desired animal response. Forage quality is positively associated with protein content and negatively with fiber content. Laboratory analyses can be used to determine the nutritive value. In general, forage quality is calculated based on the protein and fiber content. The fiber content is classified into two categories: acid detergent fiber (ADF) and neutral detergent fiber (NDF). ADF includes cellulose and lignin while NDF is made up of the structural components including the cell walls. The lignin content was also measured by itself. The material from the same batch as used for biomass measurement was sent away to the SDK lab for analysis of forage quality (Table 2). The average values for lignin content, ADF, NDF, CP, RFQ, and RFV were calculated from the replicates (clonally propagated plants) for each individually transformed lines. The values for these parameters for the individual transformant belonging to the 35S‐SPS and 35S‐GS classes were compared to the average of the values obtained for all the three control plants.
Both the 35S‐SPS plants and 35S‐GS plants showed lower NDF and ADF content when compared to the control plants. While the 35S‐SPS transformants showed a 19% drop in ADF and 14% in NDF, the 35S‐GS transformants showed a drop of 15% in ADF and 10% in NDF, when compared to control. The insoluble lignin content in the 35S‐SPS and 35S‐GS transformants showed a 13% and 6% drop, respectively, when compared to the control plants. The crude protein content showed a ~20% increase in the 35S‐SPS transformants and ~10% increase in the 35S‐GS transformants. The relative forage quality (RFQ) in the 35S‐SPS plants was 21% higher than the control plants, whereas the 35S‐GS plants showed a 12% increase in forage quality. Moreover, the relative feed value (RFV) in the 35S‐SPS and 35S‐GS transformants was 20% and 10% higher compared to the control plants, respectively.
4. DISCUSSION
In this study, we have shown that the constitutive overexpression of GS 1 improves plant growth and performance in alfalfa just as has been reported for alfalfa plants overexpressing SPS in a constitutive manner (Gebril et al., 2015). The question that we have raised in this study is why an increase in the expression of these two genes with completely different functions but with key roles in primary metabolism have the same outcome in nodulated alfalfa plants—increased nodule number, growth rates, and biomass. To address this, our experimental approach was to compare the two classes of alfalfa plants, 35S‐SPS and 35S‐GS transformants, at the molecular, biochemical, and physiological levels under N2‐fixing conditions. This would allow us to check for any commonalities or differences in how they might promote growth. Based on our previous study with the 35S‐SPS alfalfa transformants, we had surmised that the increased growth of those transformants was due to an increase in Suc transport to the nodules (Gebril et al., 2015), resulting in enhanced nodule function, a consequence of an increase in the availability of both energy and C skeletons. The subsequent outcome was an increase in the amount of N transported into the aerial parts, which then contributed to enhanced growth. Based on the literature, the underlying belief with regard to improved performance of plants overexpressing GS 1 in a constitutive manner is that the transgene product participates in the reassimilation of photorespiratory ammonia and/or ammonia from nitrate reduction in the leaves and/or the recycling of ammonia produced by the turnover of amino acids (Sengupta‐Gopalan & Ortega, 2015). There is also a study where increased growth of GS1 overexpressing poplar trees has been proposed to be due to the role of Gln in the synthesis of indole acetic acid (IAA). A transfer of the amino group from Gln to chorismate produces anthranilate in a reaction catalyzed by anthranilate synthase and anthranilate is involved in the synthesis of IAA (Man, Boriel, El‐Khatib, & Kirby, 2005). While there are a few reports of legume plants transformed with gene constructs to overexpress/downregulate GS 1 in either a constitutive or an organ‐specific manner, none of these studies have evoked nodule function to be responsible for increased plant growth (Carvalho et al., 2003; Harrison et al., 2003; Ortega et al., 2004).
Both the 35S‐SPS and the 35S‐GS transformants showed an increase in the SPS protein level in the leaves and the nodules. While the increase in SPS levels in the 35S‐SPS transformants is likely an attribute of the expression of the SPS transgene, the increased SPS levels in the leaves and nodules of the 35S‐GS transformants can only be ascribed to the expression of the endogenous SPS genes. Similarly, both classes of transformants showed an increase in the level of GS1 protein in the nodules, which in the case of the 35S‐GS transformants is probably due to the expression of the GS 1 transgene. In the case of the 35S‐SPS transformants, however, the increase in GS1 level can only be attributed to the induction of endogenous GS 1 genes. Transcript levels for the endogenous SPSB gene in the leaves and SPSA and GS1a genes in the nodules showed a significant increase when compared to the leaves and nodules from control plants. In the leaves, the 35S‐GS transformants showed the transgene product and there was no increase in the transcript level for either of the endogenous GS 1 gene members in both classes of transformants. What is, however, intriguing is that both classes of transformants had the same response with regard to the induction of the endogenous SPS and GS 1 genes, irrespective of the transgene.
The 35S‐SPS transformants and the 35S‐GS transformants showed a 60% and 40% increase in SPS activity in the nodules, respectively. Both sets of transformants also showed an increase in SucS levels in the nodules. We can postulate that the increase in the levels of SPS and SucS enhances the cycle of breakdown and synthesis of Suc (Nguyen‐Quoc & Foyer, 2001), allowing for an increase in the allotment of substrates for N metabolism, the synthesis of starch, cellulose, and signaling molecules. The starch level in the nodules of the two classes of transformants was ~80% higher than in the nodules of control plants. Based on western blot analysis, some of the key enzymes in C and N metabolism were also found to be present in significantly higher levels in the nodules of the 35S‐SPS transformants and the 35S‐GS transformants, compared to control, probably an attribute of the increased activation of these genes by a signaling molecule/s.
Both classes of transformants showed an increase in photosynthetic rates as has been reported for other plants overexpressing either SPS or GS 1 genes (Baxter et al., 2003; Fuentes et al., 2001; Oliveira et al., 2002; Seger et al., 2015). An increase in photosynthetic rates in the 35S‐SPS transformants can be attributed to an increase in Suc level resulting from the constitutive overexpression of SPS, as has been proposed by Baxter et al. (2003). A direct proof for proposing that Suc is the activator for photosynthetic rates was demonstrated by Furbank, Pritchard, and Jenkins (1997) where they showed that photosynthetic rates went up in tobacco leaves when they were fed Suc. Oliveira et al. (2002) attributed the increase in photorespiratory and photosynthetic rates in tobacco plants overexpressing GS1 in a constitutive manner to improve reassimilation of photorespiratory ammonia in the mesophyll cells. Thus, in both cases, the end result of increased photosynthetic rates is an increase in Suc levels. We could thus propose that Suc may act as the signal for the induction of SPSB in the leaves of both the 35S‐SPS and 35S‐GS transformants.
An increase in the level of SPSA and GS1a transcripts in the nodules of both classes of transformants would imply that the signal regulating SPSA and GS1a is common between the two sets of plants. It has been suggested that the internal amino acid pools function as a signal to regulate N uptake and assimilation (Miller et al., 2008), and both Gln and Glu are considered important signaling molecules (Forde & Lea, 2007). Carvalho et al. (2003) showed a drop in GS1a transcript level in nodules of M. truncatula plants grown in the presence of phosphinothricin (PPT; which irreversibly inhibits GS activity) leading to a drop in Gln synthesis. A direct correlation between inhibition of GS activity and GS1a transcript level suggests that Gln might have a role in the transcriptional activation of GS1a. Both the 35S‐GS and 35S‐SPS transformants showed higher levels of GS1a transcript, GS1 protein, and GS enzyme activity in their nodules compared to controls, thus lending credence to the possibility of Gln being the signal for induction of the endogenous GS1a gene. There are, however, no reports of Gln/Glu inducing SPS genes.
Besides the induction of GS1a and SPSA in the nodules of the 35S‐SPS and 35S‐GS transformants, the nodules also showed an increase in the levels of AS, neMDH, PEPC, NADH‐GOGAT, and SucS (Figure 9). Carvalho et al. (2003), however, had demonstrated that the genes for enzymes mentioned above were not induced in the nodules of M. truncatula plants overexpressing GS 1 in a nodule‐specific manner and exhibiting a two‐ to threefold increase in GS activity. This would imply that the inducing signal for the aforementioned genes along with GS1a and SPSA in the nodules is probably not Gln but some other signaling molecule that is transported from the leaves. These two classes of plants showed a significant increase in Suc concentration in both the leaves and nodules raising the possibility that Suc might be the signal for the induction of SPSA and GS1a along with the other genes involved in C/N metabolism in the nodules. There are several reports suggesting that besides having a metabolic role, Suc also functions as an effector of gene expression (Ruan, 2012; Smeekens & Hellmann, 2014; Tognetti et al., 2013; Wind et al., 2010). There is evidence in the literature suggesting that Suc plays a role in inducing GS genes. Oliveira and Coruzzi (1999) reported that Arabidopsis plants treated with Suc showed an induction of all three of its GS 1 genes. Arabidopsis plants expressing antisense gene construct for chloroplastic Fru‐1,6‐bisphosphatase showed not only an increase in Suc content but also an increase in GS1 activity, implying that Suc may be inducing GS 1 expression (Sahrawy, Avila, Chueca, Cánovas, & Lopez‐Gorgé, 2004). However, there are not too many reports on metabolite‐mediated regulation of SPS genes. In some recent work from our laboratory, we have shown that overexpression of SPS in a leaf‐specific manner in alfalfa results in an increase in Suc level in both the leaves and nodules and the induction of SPS activity in the nodules (Padhi, 2017). Verma, Upadhyay, Verma, Solomom, and Singh (2011) have demonstrated a positive correlation between Suc levels and SPS activity in sugarcane.
Glutamine synthetase protein accumulation in the nodules of the 35S‐SPS transformants exceeded that in the 35S‐GS transformants. While in the 35S‐GS plants, the GS1 protein accumulation in the nodules is accounted for by both the endogenous GS1 proteins and transgene product (soybean GS1), the 35S‐SPS transformants, have GS1 protein encoded only by the endogenous GS1a and GS1b genes in their nodules. Since there was no difference in the transcript level for GS1a in the nodules between the two sets of plants the question arises as to why the GS1 protein level is higher in the nodules of the 35S‐SPS transformants. Besides regulation at the transcriptional and posttranscriptional levels, GS is also subject to regulation by protein turnover (Ortega et al., 1999; Seabra et al., 2013; Seger et al., 2015; Temple et al., 1994). We could invoke posttranslational regulation to account for this difference in protein levels in the nodules between the two classes of transformants.
Neither one of the two GS 1 genes was induced in the leaves of the two classes of transformants. We have proposed that GS1a gene is induced by Suc in the nodules of the two classes of transformants. However, GS1a was not induced in the leaves even though the Suc concentration was higher in both classes of plants when compared to the control plants. Of the two GS 1 genes in alfalfa, the major isoform that is expressed in the nodules is GS1a and its expression is minimal in the leaves (Seabra et al., 2013). It is likely that expression of GS1a in the nodules requires both nodule factors and Suc imported from the leaves. The increase in the levels of some of the nodule‐enhanced isoforms of key enzymes in C and N metabolism, like neMDH, AS, and SucS, in the nodules of both sets of transformants would imply that the genes encoding these enzymes are also probably regulated by both Suc and nodule factors.
There is evidence that downstream products of N assimilation such as Glu or Gln might serve as the signals of organic N status (Coruzzi & Bush, 2001). Plants have complex regulatory machinery that coordinates the N and C metabolism with nutrient availability, environmental factors, and the demands for growth and development (Nunes‐Nesi, Fernie, & Stitt, 2010). It has been shown that C and N metabolism is coordinated via a mechanism that involves sensing the cellular C and N balance and regulating the transcription of genes involved in several processes such as photosynthesis, respiration, and N assimilation (Gutiérrez et al., 2008; Palenchar, Kouranov, Lejay, & Coruzzi, 2004; Sang et al., 2012. The sensory systems appear to monitor levels of different metabolites, which includes Suc and Gln (Foyer & Noctor, 2006).
While we have tried to implicate Gln or Suc or their derivatives, functioning as signaling molecules to regulate the various genes with a role in C/N metabolism in the nodules, the possibility still exists that both Gln and Suc interact with each other in order to regulate the expression of these genes. Based on feeding cotyledons of Brassica juncea, with Suc alone or Suc with a N source (KNO3 or NH4NO3), Goel and Singh (2015) showed that several genes including a gene for cytosolic GS were induced when both C and N were present. Since Gln is the primary product of N assimilation from inorganic N, we could assume that in some cases it is not the inorganic N, rather Gln or Glu, in combination with Suc that act as the signal. Several DNA microarray studies have shown that more than half of the transcriptome is regulated by C, N, and C–N combination (Zheng, 2009). Many enzymes are regulated by both C and N signals (Sang et al., 2012).
An ideal signaling metabolite to coordinate the regulation of N and C metabolism should be in the intersection between these two metabolic pathways. It has long been recognized that the TCA cycle intermediate α‐KG could, in theory, fulfill such a regulatory role and also function as the major C skeleton in N‐assimilatory reactions (Araújo, Martins, Fernie, & Tohge, 2014; Commichau, Forchhammer, & Stulke, 2006). PII proteins are able to sense and integrate signals from central metabolism, in particular α‐KG, an indicator of C/N balance as well as the energy status. PII in bacteria, interacts with other regulatory proteins to modulate GS at the transcriptional and posttranslational level in response to the C/N ratio (Jiang, Mayo, & Ninfa, 2007). It has been speculated that PII in plants could have a role in regulating some steps in C and N metabolism (Chellamuthu et al., 2014; Ferrario‐Méry, Besin, Pichon, Meyer, & Hodges, 2006; Ferrario‐Méry et al., 2005; Forchhammer & Luddecke, 2016; Hsieh, Lam, van de Loo, & Coruzzi, 1998). Besides PII, plants have several known N and C metabolism regulatory pathways like the TOR pathway, Glu receptor, SNF1/AMP‐dependent kinase, and trehalose‐6‐phosphate (Tre‐6P) (Nunes‐Nesi et al., 2010). It is known that the Arabidopsis TOR homologs play roles in nutrient signaling regulating metabolism, growth, and life span (Anderson & Hanson, 2005; Mahfouz, Kim, Delauney, & Verma, 2006; Ren et al., 2012) .
While there are no reports of coordination between PS and GS 1 expression and of Suc or Gln acting as signaling molecules for their induction, there is evidence in the literature that shows a link between organic acid metabolism and GS activity. Arabidopsis plants expressing a Dof1 gene from maize showed improved N assimilation and growth under low N conditions. Maize Dof1 is a member of the Dof transcription factors that activates expression of multiple genes associated with organic acid metabolism. Dof1 thus could be a key factor in coordinating gene expression involved in C skeleton production for N assimilation (Yanagisawa, Akiyama, Kisaka, Uchimiya, & Miwa, 2004). The overexpression of Dof1 increases GS activity and promotes plant growth (Yanagisawa et al., 2004). There is also a report suggesting that a Dof1 transcription factor in pine could have a role in the synthesis of Gln (Rueda‐López, Crespillo, Cánovas, & Ávila, 2008). It would be justifiable to check if Dof1 transcription factor induces the SPS genes.
As in the case of the 35S‐SPS transformants, the 35S‐GS transformants also exhibited early flowering, increased growth, and biomass, higher photosynthetic rates and increased nodule numbers. Both Suc and Gln have been implicated to play a role in growth and development (Kamada‐Nobusada, Makita, Kojima, & Sakakibara, 2013; Lastdrager, Hanson, & Smeekens, 2014). Gln has been shown to induce the expression of transcription factor genes that are involved in the induction of genes with a role in plant growth (Kan, Chung, Juo, & Hsieh, 2015). It has also been shown that Gln has a role in the synthesis of IAA (Man et al., 2005) and in the regulation of N and cytokinin biosynthesis. Cytokinin regulates a variety of processes in plant growth which includes both root and shoot growth (Matsumoto‐Kitano et al., 2008). Suc is a dominant regulator of growth processes in plants (Lastdrager et al., 2014) and the derivative, Tre‐6P, has been shown to have a role in plant growth (Yadav et al., 2014). Suc is known to induce auxin levels (Lilley, Gee, Sairanen, Ljung, & Nembhauser, 2012; Sairanen et al., 2012), and also induces auxin transport and signal transduction in Arabidopsis (Stokes, Chattopadhyay, Wilkins, Nambara, & Campbell, 2013). In more recent studies, it has been shown that Suc rather than auxin is possibly responsible for apical dominance (Mason, Ross, Babst, Wienclaw, & Beveridge, 2014). Early flowering in both sets of plants, as proposed by Gebril et al. (2015), could be in response to increased Suc in the phloem (Corbesier, Bernier, & Périlleux, 2002).
In this study, it appears that the 35S‐SPS transformants exhibit higher growth rates than the 35S‐GS transformants during the early stages following inoculation, which could be attributed to increased nodule number in the 35S‐SPS transformants compared to the 35S‐GS transformants and control. That raises the question of why the 35S‐SPS transformants have more nodules than the 35S‐GS transformants and control plants. The regulatory pathway that controls the number of nodules in the roots has been shown to be mediated by signaling molecules and phytohormones (Ferguson et al., 2010). The same signaling molecules also interact or crosstalk with sugar pathways in the regulation of many other pathways (Engels, Kirby, & White, 2011; Hammond & White, 2008). Nodule initiation may be influenced by the Suc level and it is likely that the 35S‐SPS transformants, due to the constitutive expression of the SPS transgene export more Suc to the site of nodule initiation via their root system when compared to the 35S‐GS transformants and the control plants. The overexpression of GmNMHC5, a MADS box containing transcription factor, in transgenic soybean, significantly promoted lateral root development and nodule building. GmNMHC5 is upregulated by exogenous Suc. These results indicate that GmNMHC5 can sense the Suc signal and plays significant roles in lateral root development and nodule building (Liu et al., 2015).
The phenomenon of endogenous GS 1 and SPS genes being upregulated in plants overexpressing either SPS or GS appears to be unique for alfalfa but then there are no reports of this kind of analysis in any other leguminous plant. We have shown that tobacco transformants containing the 35S‐GS gene construct, while showing an increase in GS1 level, did not show an increase in SPS level, SPS enzyme activity or Suc content in the leaves when compared to the control plants. Similarly, the 35S‐SPS tobacco transformants did not show an increase in GS protein levels or GS enzyme activity (Seger et al., 2015). Leguminous plants could be unique in that they have nodules, the site of N2‐fixation and ammonia assimilation. The root nodules act both as the C sink and as the N source and the leaves act as the C source and N sink requiring that there be close cross talk between C and N metabolic pathways and between the nodules and leaves. This would require fine‐tuning in the expression of the endogenous GS and SPS genes.
One of the hallmarks of good forage quality is low fiber content and this has been accomplished in alfalfa by genetic manipulation of the lignin pathway (Guo et al., 2001; Shadle et al., 2007). It is, however, important to note that improvement in forage quality cannot be at the cost of biomass. Gallego‐Giraldo et al. (2016) have shown that significant increase in forage quality and biomass can be increased by downregulating the expression of a family of WRKY transcription factors that act as the repressor of secondary cell wall formation. We have shown here that the 35S‐SPS and 35S‐GS plants, besides showing increased growth and biomass, also have superior forage quality—relatively higher protein content and/or lower fiber content. The increase in protein content in the aerial parts of the plant can be attributed to an increase in N assimilation in the nodules resulting in increased availability of amino acids for protein synthesis in the aerial parts of the plant. The only explanation that we have to offer for a decrease in fiber content is that secondary cell wall synthesis may not be able to keep up with the faster growth rates in these two classes of transformants. A point to note here is that the 35S‐SPS plants have 10% lower fiber content compared to the 35S‐GS transformants. This could be accounted for by the expression of the GS 1 transgene in the vasculature of the 35S‐GS transformants (Seger et al., 2009), which is the major site for lignin synthesis. GS1 plays a role in the reassimilation of ammonia released from phenylalanine ammonia lyase during lignin production and allows the maintenance of high rates of lignification without affecting the N economy (Cánovas, Avila, Canton, Canas, & de la Torre, 2007). The higher lignin content in the 35S‐GS transformants could account for the higher biomass in this class of transformants compared to the 35S‐SPS transformants. Future studies will focus on performing detailed analysis of the sugars, polysaccharides and lignin in these two classes of transformants.
5. CONCLUSIONS
By comparing the two classes of transformants, 35S‐SPS and 35S‐GS, we have shown that the biochemical basis for improved growth is the same, even though SPS and GS are key enzymes in C and N metabolism, respectively. Some of the growth attributes in the 35S‐GS transformants could be a consequence of improved reassimilation of photorespiratory ammonia. In both sets of plants, improvement in plant performance can be attributed to enhanced sink strength of the nodules, which could be the outcome of increased synthesis of Suc in the source leaves (Kaschuk, Hungria, Leffelaar, Giller, & Kuyper, 2010). It would thus appear that a common signaling mechanism exists in both sets of plants that regulate the expression of key genes with a role in nodule function. We could also entertain the possibility that Suc activates a transcription factor/s which in turn regulates all the key genes needed for nodule function. It is important to point out that even though the two genes, SPS and GS 1, used for overexpression in this study, target two different metabolic pathways, the two sets of plants undergo molecular changes such that they end up being similar with regard to the levels of SPS and GS1 proteins in the nodules.
While both Suc and Gln (or their derivatives) have been implicated to function as signaling molecules that induce gene expression, the data presented here is more supportive of Suc being the signaling molecule. Taken together, the results demonstrate the close interaction and interdependence between C and N metabolism. Furthermore, this study reaffirms the utility of the nodulated legume plant to study C/N interaction and the cross talk between the source and sink for C and N. Experiments are in progress to manipulate expression of SPS and GS in an organ‐specific manner.
CONFLICTS OF INTEREST
The authors declare no conflict of interest associated with the work described in this manuscript.
AUTHOR CONTRIBUTIONS
C.S.G. and J.L.O. supervised the experiments; H.K. and A.P. performed the experiments; K.A. and S.G. provided technical assistance; C.S.G. conceived the project and wrote the article with contributions of H.K, A.P., and J.L.O.
Supporting information
ACKNOWLEDGMENTS
This work was funded by grants from Arrowhead Innovation Fund, New Mexico State University and Agriculture Experiment Station, New Mexico State University. The support from LIFTED, LED grown lights (Rio Rancho, NM) is greatly appreciated.
Kaur H, Peel A, Acosta K, Gebril S, Ortega JL, Sengupta‐Gopalan C. Comparison of alfalfa plants overexpressing glutamine synthetase with those overexpressing sucrose phosphate synthase demonstrates a signaling mechanism integrating carbon and nitrogen metabolism between the leaves and nodules. Plant Direct. 2019;3:1–23. 10.1002/pld3.115
REFERENCES
- Abadie, C. , Lothier, J. , Boex‐Fontvieille, E. , Carroll, A. , & Tcherkez, G. (2017). Direct assessment of the metabolic origin of carbon atoms in glutamate from illuminated leaves using 13C‐NMR. New Phytologist, 216, 1079–1089. 10.1111/nph.14719 [DOI] [PubMed] [Google Scholar]
- Aleman, L. , Ortega, J. L. , Martinez‐Grimes, M. , Seger, M. , Holguin, F. O. , Uribe, D. J. , & Sengupta‐Gopalan, C. (2010). Nodule‐enhanced expression of a sucrose phosphate synthase gene member (MsSPSA) has a role in carbon and nitrogen metabolism in the nodules of alfalfa (Medicago sativa L.). Planta, 231, 233–244. 10.1007/s00425-009-1043-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiour, N. , Imbaud, S. , Clément, G. , Agier, N. , Zivy, M. , Valot, B. , & Tercet‐Laforgue, T. (2012). The use of metabolomics integrated with transcriptomic and proteomic studies for identifying key steps involved in the control of nitrogen metabolism in crops such as maize. Journal of Experimental Botany, 63, 5017–5033. 10.1093/jxb/ers186 [DOI] [PubMed] [Google Scholar]
- Anderson, G. H. , & Hanson, M. R. (2005). The Arabidopsis Mei2 homologue AML1 binds AtRaptor1B, the plant homologue of a major regulator of eukaryotic cell growth. BMC Plant Biology, 5, 2 10.1186/1471-2229-5-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araújo, W. L. , Martins, A. O. , Fernie, A. R. , & Tohge, T. (2014). 2‐Oxoglutarate: Linking TCA cycle function with amino acid, glucosinolate, flavonoid, alkaloid, and gibberellin biosynthesis. Frontiers in Plant Science, 5, 552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argueso, C. T. , Ferreira, F. J. , & Kieber, J. J. (2009). Environmental perception avenues: The interaction of cytokinin and environmental response pathways. Plant, Cell and Environment, 32, 1147–1160. 10.1111/j.1365-3040.2009.01940.x [DOI] [PubMed] [Google Scholar]
- Bao, A. , Zhao, Z. , Ding, G. , Shi, L. , Xu, F. , & Cai, H. (2014). Accumulated expression level of cytosolic glutamine synthetase 1 gene (OsGS1; 1 or OsGS1; 2) alter plant development and the carbon‐nitrogen metabolic status in rice. PLoS ONE, 9, e95581 10.1371/journal.pone.0095581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter, C. J. , Foyer, C. H. , Turner, J. , Rolfe, S. A. , & Quick, W. P. (2003). Elevated sucrose phosphate synthase activity in transgenic tobacco sustains photosynthesis in older leaves and alters development. Journal of Experimental Botany, 54, 1813–1820. 10.1093/jxb/erg196 [DOI] [PubMed] [Google Scholar]
- Bernard, S. M. , & Habash, D. Z. (2009). The importance of cytosolic glutamine synthetase in nitrogen assimilation and recycling. New Phytologist, 182, 608–620. 10.1111/j.1469-8137.2009.02823.x [DOI] [PubMed] [Google Scholar]
- Cánovas, F. M. , Avila, C. , Canton, F. R. , Canas, R. A. , & de la Torre, F. (2007). Ammonium assimilation and amino acid metabolism in conifers. Journal of Experimental Botany, 58, 2307–2318. 10.1093/jxb/erm051 [DOI] [PubMed] [Google Scholar]
- Carvalho, H. G. , Lopes‐Cardoso, I. A. , Lima, L. M. , Melo, P. M. , & Cullimore, J. V. (2003). Nodule‐specific modulation of glutamine synthetase in transgenic Medicago truncatula leads to inverse alterations in asparagine synthetase expression. Plant Physiology, 133, 243–252. 10.1104/pp.102.017830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chellamuthu, V.‐R. , Emilova, E. , Lapina, T. , Luddecke, J. , Minaeva, E. , Hermann, C. , … Forchhammer, K. (2014). A widespread glutamine sensing‐mechanism in the plant kingdom. Cell, 159, 1188–1199. 10.1016/j.cell.2014.10.015 [DOI] [PubMed] [Google Scholar]
- Clarke, V. C. , Loughlin, P. C. , Day, D. A. , & Smith, P. M. C. (2014). Transport processes of legume symbiosome. Frontiers in Plant Science, 5, 699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commichau, F. M. , Forchhammer, K. , & Stulke, J. (2006). Regulatory links between carbon and nitrogen metabolism. Current Opinion in Microbiology, 9, 167–172. 10.1016/j.mib.2006.01.001 [DOI] [PubMed] [Google Scholar]
- Corbesier, L. , Bernier, G. , & Périlleux, C. (2002). C:N ratio increases in the phloem sap during floral transition of the long‐day plants Sinapis alba and Arabidopsis thaliana . Plant and Cell Physiology, 43, 684–688. 10.1093/pcp/pcf071 [DOI] [PubMed] [Google Scholar]
- Coruzzi, G. , & Bush, D. R. (2001). Nitrogen and carbon nutrient and metabolite signaling in plants. Plant Physiology, 125, 61–64. 10.1104/pp.125.1.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coruzzi, G. M. , & Zhou, L. (2001). Carbon and nitrogen sensing and signaling in plants: Emerging ‘matrix effects’. Current Opinion in Plant Biology, 4, 247–253. 10.1016/S1369-5266(00)00168-0 [DOI] [PubMed] [Google Scholar]
- Engels, C. , Kirby, E. , & White, P. (2011). Mineral nutrition, yield and source‐sink relationships In Marschner P. (Ed.), Marschner's mineral nutrition of higher plants (pp. 85–135). London, UK: Academic Press (Elsevier). [Google Scholar]
- Eveland, A. L. , & Jackson, D. P. (2012). Sugars, signaling, and plant development. Journal of Experimental Botany, 63, 3367–3377. 10.1093/jxb/err379 [DOI] [PubMed] [Google Scholar]
- Ferguson, B. J. , Indrasumunar, A. , Hayashi, S. , Lin, M.‐H. , Lin, Y.‐H. , Reid, D. E. , & Gresshoff, P. M. (2010). Molecular analysis of legume nodule development and autoregulation. Journal of Integrative Plant Biology, 52, 61–76. 10.1111/j.1744-7909.2010.00899.x [DOI] [PubMed] [Google Scholar]
- Ferrario‐Méry, S. , Besin, E. , Pichon, O. , Meyer, C. , & Hodges, M. (2006). The regulatory PII protein controls arginine biosynthesis in Arabidopsis. FEBS Letters, 580, 2015–2020. 10.1016/j.febslet.2006.02.075 [DOI] [PubMed] [Google Scholar]
- Ferrario‐Méry, S. , Bouvet, M. , Leleu, O. , Savino, G. , Hodges, M. , & Meyer, C. (2005). Physiological characterisation of Arabidopsis mutants affected in the expression of the putative regulatory protein PII. Planta, 223, 28–39. 10.1007/s00425-005-0063-5 [DOI] [PubMed] [Google Scholar]
- Finnemann, J. , & Schjoerring, J. K. (2000). Post‐translational regulation of cytosolic glutamine synthetase by reversible phosphorylation and 14‐3‐3 protein interaction. Plant Journal, 24, 171–181. 10.1046/j.1365-313x.2000.00863.x [DOI] [PubMed] [Google Scholar]
- Forchhammer, K. , & Luddecke, J. (2016). Sensory properties of the PII signaling protein family. FEBS Journal, 283, 425–437. 10.1111/febs.13584 [DOI] [PubMed] [Google Scholar]
- Forde, B. G. , & Lea, P. J. (2007). Glutamate in plants: Metabolism, regulation, and signaling. Journal of Experimental Botany, 58, 2339–2358. 10.1093/jxb/erm121 [DOI] [PubMed] [Google Scholar]
- Foyer C. H., & Noctor G. (Eds.) (2006). Photosynthetic nitrogen assimilation and associated carbon and respiratory metabolism,, Vol. 12 Berlin, Germany: Springer Science & Business Media. [Google Scholar]
- Fuentes, S. I. , Allen, D. J. , Ortiz‐Lopez, A. , & Hernández, G. (2001). Over‐expression of cytosolic glutamine synthetase increases photosynthesis and growth at low nitrogen concentrations. Journal of Experimental Botany, 52, 1071–1081. 10.1093/jexbot/52.358.1071 [DOI] [PubMed] [Google Scholar]
- Furbank, R. T. , Pritchard, J. , & Jenkins, C. L. D. (1997). Effects of exogenous sucrose feeding on photosynthesis in the C3 plant tobacco and the C4 plant Flaveria bidentis . Australian Journal of Plant Physiology, 24, 291–299. [Google Scholar]
- Gallego‐Giraldo, L. , Shadle, G. , Shen, H. , Barros‐Rios, J. , Corrales, S. F. , Wang, H. , & Dixon, R. A. (2016). Combining enhanced biomass density with reduced lignin level for improved forage quality. Plant Biotechnology Journal, 14, 895–904. 10.1111/pbi.12439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier, P. P. G. , Bligny, R. , Gout, E. , Mahé, A. , Nogués, S. , Hodges, M. , & Tcherkez, G. (2010). In folio isotopic tracing demonstrates that nitrogen assimilation into glutamate is mostly independent from current CO2 assimilation in illuminated leaves of Brassica napus . New Phytologist, 185, 988–999. 10.1111/j.1469-8137.2009.03130.x [DOI] [PubMed] [Google Scholar]
- Gebril, S. , Seger, M. , Villanueva, F. M. , Ortega, J. L. , Bagga, S. , & Sengupta‐Gopalan, C. (2015). Transgenic alfalfa (Medicago sativa) with increased sucrose phosphate synthase activity shows enhanced growth when grown under N2‐fixing conditions. Planta, 242, 1009–1024. 10.1007/s00425-015-2342-0 [DOI] [PubMed] [Google Scholar]
- Goel, P. , & Singh, A. K. (2015). Abiotic stresses downregulate key genes involved in nitrogen uptake and assimilation in Brassica juncea L. PLoS ONE, 10, e0143645 10.1371/journal.pone.0143645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes, M. M. (2013). The role of Sucrose Phosphate Synthase (SPS) in the root nodules of alfalfa (Medicago sativa). Ph. D. dissertation, New Mexico State University.
- Guo, D. , Chen, F. , Wheeler, J. , Winder, J. , Selman, S. , Peterson, M. , & Dixon, R. A. (2001). Improvement of in‐rumen digestibility of alfalfa forage by genetic manipulation of lignin O‐methyltransferases. Transgenic Research, 10, 457–464. 10.1023/A:1012278106147 [DOI] [PubMed] [Google Scholar]
- Gutiérrez, R. A. , Stokes, T. , Thum, K. , Xu, X. , Obertello, M. , Katari, M. S. , & Coruzzi, G. M. (2008). Systems approach identifies an organic nitrogen‐responsive gene network that is regulated by the master clock control gene CCA1 . Proceedings of the National Academy of Sciences of the United States of America, 105, 4939–4944. 10.1073/pnas.0800211105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigler, C. H. , Singh, B. , Zhang, D. , Hwang, S. , Wu, C. , Cai, W. , & Hequent, E. F. (2007). Transgenic cotton over‐producing spinach SPS showed enhanced leaf sucrose synthesis and improved fiber quality under controlled environmental conditions. Plant Molecular Biology, 63, 815–832. 10.1007/s11103-006-9127-6 [DOI] [PubMed] [Google Scholar]
- Hammond, J. P. , & White, P. J. (2008). Sucrose transport in the phloem: Integrating root responses to phosphorus starvation. Journal of Experimental Botany, 59, 93–109. [DOI] [PubMed] [Google Scholar]
- Hardy, R. W. F. , Holsten, R. D. , Jackson, E. K. , & Burnes, R. C. (1968). The acetylene‐ethylene assay for N2 fixation: laboratory and field evaluation. Plant Physiology, 43, 1185‐1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison, J. , de Crescenzo, M. A. P. , Sené, O. , & Hirel, B. (2003). Does lowering glutamine synthetase activity in nodules modify nitrogen metabolism and growth of Lotus japonicus? Plant Physiology, 133, 253–262. 10.1104/pp.102.016766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh, M.‐H. , Lam, H.‐M. , van de Loo, F. J. , & Coruzzi, G. (1998). A PII‐like protein in Arabidopsis: Putative role in nitrogen sensing. Proceedings of the National Academy of Sciences of the United States of America, 95, 13965–13970. 10.1073/pnas.95.23.13965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber, S. C. , & Huber, J. L. (1996). Role and regulation of sucrose‐phosphate synthase in higher plants. Annual Review of Plant Biology, 47, 431–444. 10.1146/annurev.arplant.47.1.431 [DOI] [PubMed] [Google Scholar]
- Ishimaru, K. , Hirotsu, N. , Kashiwagi, T. , Madoka, Y. , Nagasuga, K. , Ono, K. , & Ohsugi, R. (2008). Overexpression of a maize SPS gene improves yield characters of potato under field conditions. Plant Production Science, 11, 104–107. 10.1626/pps.11.104 [DOI] [Google Scholar]
- Jiang, P. , Mayo, A. E. , & Ninfa, A. J. (2007). Escherichia coli glutamine synthetase adenylyltransferase (ATase, EC 2.7. 7.49): Kinetic characterization of regulation by PII, PII‐UMP, glutamine, and α‐ketoglutarate. Biochemistry, 46, 4133–4146. 10.1021/bi0620510 [DOI] [PubMed] [Google Scholar]
- Kamada‐Nobusada, T. , Makita, N. , Kojima, M. , & Sakakibara, H. (2013). Nitrogen‐dependent regulation of de novo cytokinin biosynthesis in rice: The role of glutamine metabolism as an additional signal. Plant and Cell Physiology, 54, 1881–1893. 10.1093/pcp/pct127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan, C. C. , Chung, T. Y. , Juo, Y. A. , & Hsieh, M. H. (2015). Glutamine rapidly induces the expression of key transcription factor genes involved in nitrogen and stress responses in rice roots. BMC Genomics, 16, 731 10.1186/s12864-015-1892-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaschuk, G. , Hungria, M. , Leffelaar, P. A. , Giller, K. E. , & Kuyper, T. W. (2010). Differences in photosynthetic behavior and leaf senescence of soybean (Glycine max L.) dependent on N2 fixation or nitrate supply. Plant Biology, 12, 60–69. 10.1111/(ISSN)1438-8677 [DOI] [PubMed] [Google Scholar]
- Kirby, E. G. , Gallardo, F. , Man, H. , & El‐Khatib, R. (2006). The overexpression of glutamine synthetase in transgenic poplar: A review. Silvae Genetica, 55, 278–284. 10.1515/sg-2006-0036 [DOI] [Google Scholar]
- Laporte, M. M. , Galagan, J. A. , Prasch, A. L. , Vanderveer, P. J. , Hanson, D. T. , Shewmaker, C. K. , & Sharkey, T. D. (2001). Promoter strength and tissue specificity effects on growth of tomato plants transformed with maize sucrose‐phosphate synthase. Planta, 212, 817–822. 10.1007/s004250000433 [DOI] [PubMed] [Google Scholar]
- Lastdrager, J. , Hanson, J. , & Smeekens, S. (2014). Sugar signals and the control of plant growth and development. Journal of Experimental Botany, 65, 799–807. 10.1093/jxb/ert474 [DOI] [PubMed] [Google Scholar]
- Lea, P. , & Miflin, B. (2011). Nitrogen assimilation and its relevance for crop improvement In Foyer C., & Zang H. (Eds.), Nitrogen metabolism in the post‐genomic era, Plant Annual Reviews, Vol. 42 (pp. 1–40). Hoboken, NJ: Wiley‐Blackwell. [Google Scholar]
- Lilley, J. L. , Gee, C. W. , Sairanen, I. , Ljung, K. , & Nembhauser, J. L. (2012). An endogenous carbon‐sensing pathway triggers increased auxin flux and hypocotyl elongation. Plant Physiology, 160, 2261–2270. 10.1104/pp.112.205575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima, L. , Seabra, A. , Melo, P. , Cullimore, J. , & Carvalho, H. (2006). Phosphorylation and subsequent interaction with 14‐3‐3 proteins regulate plastid glutamine synthetase in Medicago truncatula . Planta, 223, 558–567. 10.1007/s00425-005-0097-8 [DOI] [PubMed] [Google Scholar]
- Liu, W. , Han, X. , Zhan, G. , Zhao, Z. , Feng, Y. , & Wu, C. (2015). A novel sucrose‐regulatory MADS‐Box transcription factor GmNMHC5 promotes root development and nodulation in soybean (Glycine max [L.] Merr.). International Journal of Molecular Sciences, 16, 20657–20673. 10.3390/ijms160920657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljung, K. , Nemhauser, J. L. , & Prata, P. (2015). New mechanistic links between sugar and hormone signaling. Current Opinion in Plant Biology, 25, 130–137. 10.1016/j.pbi.2015.05.022 [DOI] [PubMed] [Google Scholar]
- Loreti, E. , Bellis, L. D. , Alp, A. , & Perata, P. (2001). Why and how do plant cells sense sugars? Annals of Botany, 88, 803–812. 10.1006/anbo.2001.1526 [DOI] [Google Scholar]
- Lothier, J. , Gaufichon, L. , Sormani, R. , Lemaître, T. , Azzopardi, M. , Morin, H. , & Masclaux‐Daubresse, C. (2010). The cytosolic glutamine synthetase GLN1; 2 plays a role in the control of plant growth and ammonium homeostasis in Arabidopsis rosettes when nitrate supply is not limiting. Journal of Experimental Botany, 62, 1375–1390. [DOI] [PubMed] [Google Scholar]
- Lunn, J. E. , & Furbank, R. T. (1999). Tansley Review No. 105. Sucrose biosynthesis in C 4 plants. New Phytologist, 143, 221–237. 10.1046/j.1469-8137.1999.00450.x [DOI] [Google Scholar]
- Lunn, J. E. , Gillespie, J. , & Furbank, R. T. (2003). Expression of a cyanobacterial sucrose phosphate synthase from Synechocystis sp. PCC6803 in transgenic plants. Journal of Experimental Botany, 54, 223–237. 10.1093/jxb/erg023 [DOI] [PubMed] [Google Scholar]
- Lunn, J. E. , & MacRae, E. (2003). New complexities in the synthesis of sucrose. Current Opinion in Plant Biology, 6, 208–214. 10.1016/S1369-5266(03)00033-5 [DOI] [PubMed] [Google Scholar]
- Mahfouz, M. M. , Kim, S. , Delauney, A. J. , & Verma, D. P. S. (2006). Arabidopsis TARGET OF RAPAMYCIN interacts with RAPTOR, which regulates the activity of S6 kinase in response to osmotic stress signals. Plant Cell, 18, 477–490. 10.1105/tpc.105.035931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man, H. M. , Boriel, R. , El‐Khatib, R. , & Kirby, E. G. (2005). Characterization of transgenic poplar with ectopic expression of pine cytosolic glutamine synthetase under conditions of varying nitrogen availability. New Phytologist, 167, 31–39. 10.1111/j.1469-8137.2005.01461.x [DOI] [PubMed] [Google Scholar]
- Mason, M. G. , Ross, J. J. , Babst, B. A. , Wienclaw, B. N. , & Beveridge, C. A. (2014). Sugar demand, not auxin, is the initial regulator of apical dominance. Proceedings of the National Academy of Sciences of the United States of America, 111, 6092–6097. 10.1073/pnas.1322045111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto‐Kitano, M. , Kusumoto, T. , Tarkowski, P. , Kinoshita‐Tsujimura, K. , Václavíková, K. , Miyawaki, K. , & Kakimoto, T. (2008). Cytokinins are central regulators of cambial activity. Proceedings of the National Academy of Sciences of the United States of America, 105, 20027–20031. 10.1073/pnas.0805619105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micallef, B. J. , Haskins, K. A. , Vanderveer, P. J. , Roh, K. S. , Shewmaker, C. K. , & Sharkey, T. D. (1995). Altered photosynthesis, flowering, and fruiting in transgenic tomato plants that have an increased capacity for sucrose synthesis. Planta, 196, 327–334. [Google Scholar]
- Miller, A. J. , Fan, X. , Shen, Q. , & Smith, S. J. (2008). Amino acids and nitrate as signals for the regulation of nitrogen acquisition. Journal of Experimental Botany, 59, 111–119. 10.1093/jxb/erm208 [DOI] [PubMed] [Google Scholar]
- Nguyen‐Quoc, B. , & Foyer, C. H. (2001). A role for ‘futile cycles’ involving invertase and sucrose synthase in sucrose metabolism of tomato fruit. Journal of Experimental Botany, 52, 881–889. 10.1093/jexbot/52.358.881 [DOI] [PubMed] [Google Scholar]
- Nguyen‐Quoc, B. , N'Tchobo, H. , Foyer, C. H. , & Yelle, S. (1999). Overexpression of sucrose phosphate synthase increases sucrose unloading in transformed tomato fruit. Journal of Experimental Botany, 50, 785–791. 10.1093/jxb/50.335.785 [DOI] [Google Scholar]
- Nunes‐Nesi, A. , Fernie, A. R. , & Stitt, M. (2010). Metabolic and signaling aspects underpinning the regulation of plant carbon nitrogen interactions. Molecular Plant, 3, 973–996. 10.1093/mp/ssq049 [DOI] [PubMed] [Google Scholar]
- Oldroyd, G. E. , & Downie, J. A. (2008). Coordinating nodule morphogenesis with rhizobial infection in legumes. Annual Review of Plant Biology, 59, 519–546. 10.1146/annurev.arplant.59.032607.092839 [DOI] [PubMed] [Google Scholar]
- Oliveira, I. C. , Brears, T. , Knight, T. J. , Clark, A. , & Coruzzi, G. M. (2002). Overexpression of cytosolic glutamine synthetase. Relation to nitrogen, light, and photorespiration. Plant Physiology, 129, 1170–1180. 10.1104/pp.020013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira, I. C. , & Coruzzi, G. M. (1999). Carbon and amino acids reciprocally modulate the expression of glutamine synthetase in Arabidopsis. Plant Physiology, 121, 301–310. 10.1104/pp.121.1.301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega, J. , Moguel‐Esponda, S. , Potenza, C. , Conklin, C. F. , Quintana, A. , & Sengupta‐Gopalan, C. (2006). The 3′ untranslated region of a soybean cytosolic glutamine synthetase (GS1) affects transcript stability and protein accumulation in transgenic alfalfa. Plant Journal, 45, 832–846. 10.1111/j.1365-313X.2005.02644.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega, J. L. , Roche, D. , & Sengupta‐Gopalan, C. (1999). Oxidative turnover of soybean root glutamine synthetase. In vitro and in vivo studies. Plant Physiology, 119, 1483–1496. 10.1104/pp.119.4.1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega, J. L. , Temple, S. J. , Bagga, S. , Ghoshroy, S. , & Sengupta‐Gopalan, C. (2004). Biochemical and molecular characterization of transgenic Lotus japonicus plants constitutively over‐expressing a cytosolic glutamine synthetase gene. Planta, 219, 807–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega, J. L. , Wilson, O. L. , & Sengupta‐Gopalan, C. (2012). The 5′ untranslated region of the soybean cytosolic glutamine synthetase β1 gene contains prokaryotic translation initiation signals and acts as a translational enhancer in plants. Molecular Genetics and Genomics, 287, 881–893. 10.1007/s00438-012-0724-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padhi, S. (2017). Role of sucrose phosphate synthase (SPS) isoforms in the growth and development of nodulated alfalfa (Medicago Sativa L.) plants. Master thesis, New Mexico State University.
- Palenchar, P. M. , Kouranov, A. , Lejay, L. V. , & Coruzzi, G. M. (2004). Genome‐wide patterns of carbon and nitrogen regulation of gene expression validates the carbon and nitrogen (CN)‐signaling hypothesis in plants. Genome Biology, 5, R91 10.1186/gb-2004-5-11-r91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, J. Y. , Canam, T. , Kang, K. Y. , Ellis, D. D. , & Mansfield, S. D. (2008). Over‐expression of an Arabidopsis family A sucrose phosphate synthase (SPS) gene alters plant growth and fibre development. Transgenic Research, 17, 181–192. 10.1007/s11248-007-9090-2 [DOI] [PubMed] [Google Scholar]
- Park, J. Y. , Canam, T. , Kang, K. Y. , Unda, F. , & Mansfield, S. D. (2009). Sucrose phosphate synthase expression influences poplar phenology. Tree Physiology, 29, 937–946. 10.1093/treephys/tpp028 [DOI] [PubMed] [Google Scholar]
- Ren, M. , Venglat, P. , Qui, S. , Feng, L. , Zao, Y. , Wang, E. , … Datlaa, R. (2012). Target of Rapamycin signaling regulates metabolism, growth and life span in Arabidopsis . Plant Cell, 24, 4850–4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan, Y. L. (2012). Signaling role of sucrose metabolism in development. Molecular Plant, 5, 763–765. 10.1093/mp/sss046 [DOI] [PubMed] [Google Scholar]
- Rueda‐López, M. , Crespillo, R. , Cánovas, F. M. , & Ávila, C. (2008). Differential regulation of two glutamine synthetase genes by a single Dof transcription factor. Plant Journal, 56, 73–85. 10.1111/j.1365-313X.2008.03573.x [DOI] [PubMed] [Google Scholar]
- Sahrawy, M. , Avila, C. , Chueca, A. , Cánovas, F. M. , & Lopez‐Gorgé, J. (2004). Increased sucrose level and altered nitrogen metabolism in Arabidopsis thaliana transgenic plants expressing antisense chloroplastic fructose‐1, 6‐bis phosphatase. Journal of Experimental Botany, 55, 2495–2503. 10.1093/jxb/erh257 [DOI] [PubMed] [Google Scholar]
- Sairanen, I. , Novák, O. , Pěnčík, A. , Ikeda, Y. , Jones, B. , Sandberg, G. , & Ljung, K. (2012). Soluble carbohydrates regulate auxin biosynthesis via PIF proteins in Arabidopsis. Plant Cell, 24, 4907–4916. 10.1105/tpc.112.104794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakibara, H. , Takei, K. , & Hirose, N. (2006). Interactions between nitrogen and cytokinin in the regulation of metabolism and development. Trends in Plant Science, 11, 440–448. 10.1016/j.tplants.2006.07.004 [DOI] [PubMed] [Google Scholar]
- Sang, Y. , Sun, W. , & Yang, Z. (2012). Signalling mechanisms integrating carbon and nitrogen utilization. Frontiers in Biology, 7, 548–556. 10.1007/s11515-012-1249-4 [DOI] [Google Scholar]
- Seabra, A. R. , Silva, L. S. , & Carvalho, H. G. (2013). Novel aspects of glutamine synthetase (GS) regulation revealed by a detailed expression analysis of the entire GS gene family of Medicago truncatula under different physiological conditions. BMC Plant Biology, 13, 137 10.1186/1471-2229-13-137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seger, M. , Gebril, S. , Tabilona, J. , Peel, A. , & Sengupta‐Gopalan, C. (2015). Impact of concurrent overexpression of cytosolic glutamine synthetase (GS1) and sucrose phosphate synthase (SPS) on growth and development in transgenic tobacco. Planta, 241, 69–81. 10.1007/s00425-014-2165-4 [DOI] [PubMed] [Google Scholar]
- Seger, M. , Ortega, J. L. , Bagga, S. , & Sengupta‐Gopalan, C. (2009). Repercussion of mesophyll‐specific overexpression of a soybean cytosolic glutamine synthetase gene in alfalfa (Medicago sativa L.) and tobacco (Nicotiana tabaccum L.). Plant Science, 176, 119–129. 10.1016/j.plantsci.2008.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta‐Gopalan, C. , & Ortega, J. L. (2015). An insight into the role and regulation of glutamine synthetase in plants In D'Mello J. P. F. (Ed.), Amino acids in higher plants (pp. 82–99). Wallingford, UK: CABI. [Google Scholar]
- Shadle, G. , Chen, F. , Srinivasa Reddy, M. S. , Jackson, L. , Nakashima, J. , & Dixon, R. A. (2007). Down‐regulation of hydroxycinnamoyl CoA shikimatehydroxycinnamoyl transferase in transgenic alfalfa affects lignification, development and forage quality. Phytochemistry, 68, 1521–1529. 10.1016/j.phytochem.2007.03.022 [DOI] [PubMed] [Google Scholar]
- Simon, B. , & Sengupta‐Gopalan, C. (2010). The 3′ untranslated region of the two cytosolic glutamine synthetase (GS1) genes in alfalfa (Medicago sativa) regulates transcript stability in response to glutamine. Planta, 232, 1151–1162. 10.1007/s00425-010-1247-1 [DOI] [PubMed] [Google Scholar]
- Smeekens, S. (2000). Sugar‐induced signal transduction in plants. Annual Review of Plant Physiology and Plant Molecular Biology, 51, 49–81. 10.1146/annurev.arplant.51.1.49 [DOI] [PubMed] [Google Scholar]
- Smeekens, S. , & Hellmann, H. A. (2014). Sugar sensing and signaling in plant. Frontiers in Plant Science, 5, 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes, M. E. , Chattopadhyay, A. , Wilkins, O. , Nambara, E. , & Campbell, M. M. (2013). Interplay between sucrose and folate modulates auxin signalling in Arabidopsis. Plant Physiology, 162, 1552–1565. 10.1104/pp.113.215095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple, S. J. , Bagga, S. , & Sengupta‐Gopalan, C. (1994). Can glutamine synthetase activity levels be modulated in transgenic plants by the use of recombinant DNA technology? Biochemical Society Transactions, 22, 915–920. 10.1042/bst0220915 [DOI] [PubMed] [Google Scholar]
- Temple, S. J. , Bagga, S. , & Sengupta‐Gopalan, C. (1998). Down‐regulation of specific members of the glutamine synthetase gene family in alfalfa by antisense RNA technology. Plant Molecular Biology, 37, 535–547. 10.1023/A:1006099512706 [DOI] [PubMed] [Google Scholar]
- Temple, S. J. , Knight, T. J. , Unkefer, P. J. , & Sengupta‐Gopalan, C. (1993). Modulation of glutamine synthetase gene expression in tobacco by the introduction of an alfalfa glutamine synthetase gene in sense and antisense orientation: Molecular and biochemical analysis. Molecular and General Genetics, 236, 315–325. 10.1007/BF00277128 [DOI] [PubMed] [Google Scholar]
- Thomsen, H. C. , Eriksson, D. , Møller, I. S. , & Schjoerring, J. K. (2014). Cytosolic glutamine synthetase: A target for improvement of crop nitrogen use efficiency? Trends in Plant Science, 19, 656–663. 10.1016/j.tplants.2014.06.002 [DOI] [PubMed] [Google Scholar]
- Tognetti, J. A. , Pontis, H. G. , & Martinez‐Noel, G. M. A. (2013). Sucrose signaling in plants. Plant Signaling & Behavior, 8, e23316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toroser, D. , & Huber, S. C. (1997). Protein phosphorylation as a mechanism for osmotic‐stress activation of sucrose‐phosphate synthase in spinach leaves. Plant Physiology, 114, 947–955. 10.1104/pp.114.3.947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma, A. K. , Upadhyay, S. K. , Verma, P. C. , Solomom, S. , & Singh, S. B. (2011). Functional analysis of sucrose phosphate synthase (SPS) and sucrose synthase (SS) in sugarcane (Saccharum) cultivars. Plant Biology, 13, 325–332. 10.1111/j.1438-8677.2010.00379.x [DOI] [PubMed] [Google Scholar]
- Weise, A. , Elzinga, N. , Wobbes, B. , & Smeekens, S. (2004). A conserved upstream open reading frame mediates sucrose‐induced repression of translation. Plant Cell, 16, 1717–1729. 10.1105/tpc.019349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White, J. , Prell, J. , James, E. K. , & Poole, P. (2007). Nutrient sharing between symbionts. Plant Physiology, 144, 604–614. 10.1104/pp.107.097741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wind, J. , Smeekens, S. , & Hanson, J. (2010). Sucrose: Metabolite and signaling molecule. Phytochemistry, 71, 1610–1614. 10.1016/j.phytochem.2010.07.007 [DOI] [PubMed] [Google Scholar]
- Yadav, U. P. , Ivakov, A. , Feil, R. , Duan, G. Y. , Walther, D. , Giavalisco, P. , … Lunn, J. E. (2014). The sucrose‐trehalose 6‐phosphate (Tre6P) nexus: Specificity and mechanisms of sucrose signaling by Tre6P. Journal of Experimental Botany, 65, 1051–1068. 10.1093/jxb/ert457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa, S. , Akiyama, A. , Kisaka, H. , Uchimiya, H. , & Miwa, T. (2004). Metabolic engineering with Dof1 transcription factor in plants: Improved nitrogen assimilation and growth under low‐nitrogen conditions. Proceedings of the National Academy of Sciences of the United States of America, 101, 7833–7838. 10.1073/pnas.0402267101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, Z. (2009). Carbon and nitrogen nutrient balance signaling in plants. Plant Signaling & Behavior, 4, 584–591. 10.4161/psb.4.7.8540 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


