Abstract
G-protein–coupled receptors (GPCRs) are the largest family of transmembrane signaling proteins. In the gastrointestinal tract, GPCRs expressed by epithelial cells sense contents of the lumen, and GPCRs expressed by epithelial cells, myocytes, neurons, and immune cells participate in communication among cells. GPCRs control digestion, mediate digestive diseases, and coordinate repair and growth. GPCRs are the target of more than one third of therapeutic drugs, including many drugs used to treat digestive diseases. Recent advances in structural, chemical, and cell biology research have shown that GPCRs are not static binary switches that operate from the plasma membrane to control a defined set of intracellular signals. Rather, GPCRs are dynamic signaling proteins that adopt distinct conformations and subcellular distributions when associated with different ligands and intracellular effectors. An understanding of the dynamic nature of GPCRs has provided insights into the mechanism of activation and signaling of GPCRs and has shown opportunities for drug discovery. We review the allosteric modulation, biased agonism, oligomerization, and compartmentalized signaling of GPCRs that control digestion and digestive diseases. We highlight the implications of these concepts for the development of selective and effective drugs to treat diseases of the gastrointestinal tract.
Keywords: Receptors, Signal Transduction, Trafficking, G Proteins, Drug Discovery
G-protein–coupled receptors (GPCRs) are the largest family of transmembrane signaling proteins, with approximately 800 members in the human genome. GPCRs transmit information about the external environment to the interior of the cell and thereby control most physiologic and pathologic processes. Approximately half the GPCRs have a sensory function and mediate olfaction, taste, perception of light, and pheromone signaling. Other GPCRs detect hormones, neurotransmitters, and paracrine factors and mediate communication among cells. GPCRs are the target of more than one third of therapeutic drugs, which illustrates their importance in disease and therapy.1
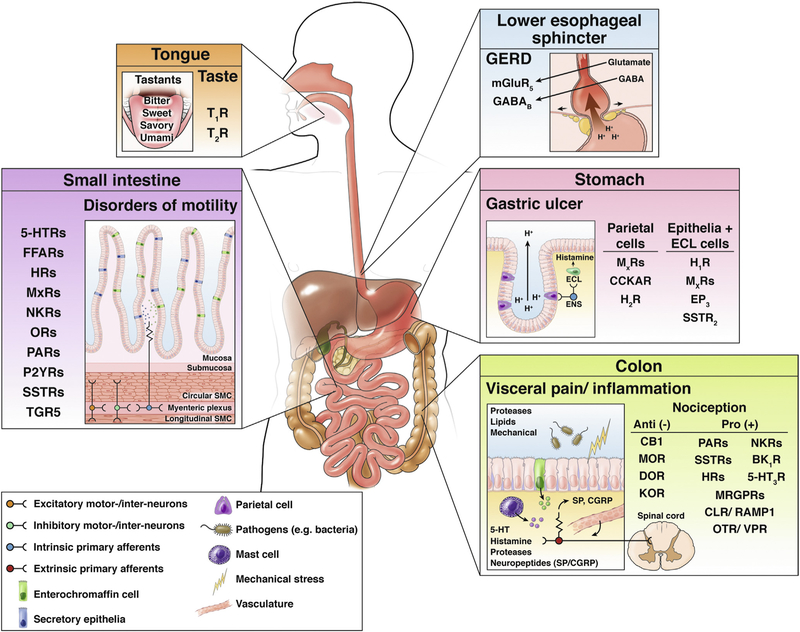
The importance, diversity, and complexity of GPCRs are illustrated by their role in digestion and as targets for digestive disease (Figure 1). GPCRs with sensory functions in the digestive tract include receptors of taste buds for sweet, bitter, and savory tastes,2 receptors of enteroendocrine cells for amino acids and proteins,3 and receptors of colonocytes for luminal proteases.4 GPCRs also sense the products of the microbiome. For instance, secondary bile acids, which are synthesized by bacteria in the colon, activate Takeda GPCR5 on enterochromaffin cells and enteric neurons to evoke peristalsis.5 Takeda GPCR5 expressed by cutaneous sensory nerves has been implicated in cholestatic pruritus.6,7 GPCRs of epithelial cells, myocytes, enteric neurons, and immune cells participate in cell-to-cell communication in the digestive system. They include receptors for structurally diverse ligands, including biogenic amines (catecholamines, histamine, serotonin), eicosanoids, amino acid transmitters, purine nucleotides, and neuropeptides, peptide hormones, and proteins. Thus, GPCRs orchestrate digestion (secretion, motility, transport), control disease processes (diseases of motility, secretion, inflammation, pain), and regulate growth and repair. Drugs that activate or inhibit GPCRs are effective therapies for digestive diseases (Figure 1).
Figure 1.
GPCRs and their ligands in digestion and digestive disease. GPCRs are expressed throughout the digestive tract. Expression of some functionally and clinically important GPCRs in specific cell types in the tongue, lower esophageal sphincter, stomach, small intestine, and colon are depicted. GPCRs control multiple processes in the gut and are targets for common diseases (eg, GERD, gastric ulcer disease, disorders of intestinal motility, colonic pain, and inflammation). 5HTxR, serotonin receptor; CLR, calcitonin receptor-like receptor; EP3, prostaglandin receptor 3; FFARs, free fatty acid receptors; GABABR, GABA B receptor; HxR, histamine receptor; MxR, muscarinic acetylcholine receptor; NKR, neurokinin receptor; OTR, oxytocin receptor; P2YR, purinergic 2Y receptor; RAMP1, receptor activity modifying protein 1; TGR5, Takeda GPCR 5 bile-acid receptor; TxR, taste receptor; VPR, vasopressin receptor.
Although the endogenous ligands of many GPCRs are known, there remain approximately 100 GPCRs with unidentified natural ligands. Some of these orphan GPCRs have roles in the digestive system. For example, the Mas-related GPCR (MRGPR) family is composed of approximately 40 orphan receptors expressed by primary sensory neurons and mast cells.8 MrgprX2 (human) or MrgprB2 (murine homologue) is expressed by mast cells and mediates antibody-independent responses to basic secretagogues, including drugs and peptides associated with pseudoallergic reactions.9 Substance P (SP), a gut neuropeptide, can activate MrgprX2. Mast cells are in proximity to sensory nerves containing SP and calcitonin gene-related peptide in the intestine.10 Therefore, neuropeptides and MrgprX2 might mediate the communication between sensory nerves and mast cells. Communication between sensory neurons and mast cells has been implicated in irritable bowel syndrome (IBS).11
GPCRs share a conserved structure with 7 transmembrane domains, 3 extracellular and 3 intracellular loops, and extracellular (N-terminal) and intracellular (C-terminal) tails of varying sizes. GPCRs are grouped into 5 families based on structural and functional similarities. The rhodopsin family (class A) includes receptors for neurotransmitters, peptides, visual pigments, odorants, tastants, and pheromones. The secretin family (class B) is composed of receptors for polypeptide gut hormones, including glucagon, glucagon-like peptides, glucose-dependent insulinotropic polypeptide, secretin, vasoactive intestinal peptide, pituitary adenylate cyclase-activating polypeptide, and growth hormone-releasing hormone. The glutamate family (class C) includes metabotropic glutamate receptors, a calcium-sensing receptor, and γ-aminobutyric acid (GABA) B receptors. Adhesion family GPCRs possess a large extra-cellular N-terminus that is cleaved during activation. The frizzled family, which includes frizzled and smoothened proteins, is activated by lipo-glycoproteins of the Wnt family (frizzled) and hedgehog family (smoothened). All GPCR families are represented in the digestive system.
This review highlights how recent advances in structural, chemical, and cellular biology research have provided an understanding of the mechanism of action of GPCRs. The traditional view that GPCRs are simple on and off switches that operate at the surface of cells to control a defined set of intracellular signals has been superseded by the realization that GPCRs are dynamic signaling proteins that can adopt different conformations and subcellular distributions, depending on the mechanisms of their activation.12
One aspect of the dynamic nature of GPCRs was exposed using x-ray crystallography and cryo-electron microscopy to probe GPCR structures. These approaches provided information about the organization of transmembrane, loop, and tail domains and their association with agonists, antagonists, G proteins, β-arrestins (ARRBs), and other signaling effectors.13–17 Limitations of structural studies of GPCRs include a requirement to stabilize receptors and signaling complexes by mutation, fusion to stabilizing proteins, or with single-domain antibodies (nano-bodies). Moreover, structural studies only provide snapshots of receptors frozen in time. However, structural analyses have shown that GPCRs adopt distinct conformations when bound to different agonists, antagonists, and intracellular effector and regulators. Two pharmacologic paradigms have emerged from an appreciation of the structural dynamism of GPCRs: allosteric modulation18 and biased agonism.19 Structural studies also have provided evidence that certain GPCRs exist as oligomers rather than as monomers.20,21
A second component of the dynamic nature of GPCRs was discovered using biosensors, biophysical approaches, and advanced imaging to study the trafficking and signaling of GPCRs in subcellular micro-domains. These studies showed that GPCRs are motile signaling proteins that, at activation, can traffic from the cell surface to endosomes by dynamin- and clathrin-mediated endocytosis. GPCRs in endosomes can generate sustained signals in subcellular compartments (ie, compartmentalized signaling) that control physiologic and pathologic processes.22–27 Thus, GPCRs in endosomes, rather than at the plasma membrane, might be a target for therapy.28
We discuss allosteric modulation, biased agonism, oligomerization, and compartmentalized signaling of GPCRs that control digestion and digestive diseases and consider the implications of these concepts for the development of drugs to treat gastrointestinal diseases.
Allosteric Modulators of GPCRs: Signaling Rheostats
Concept of Allosteric Modulation of GPCRs
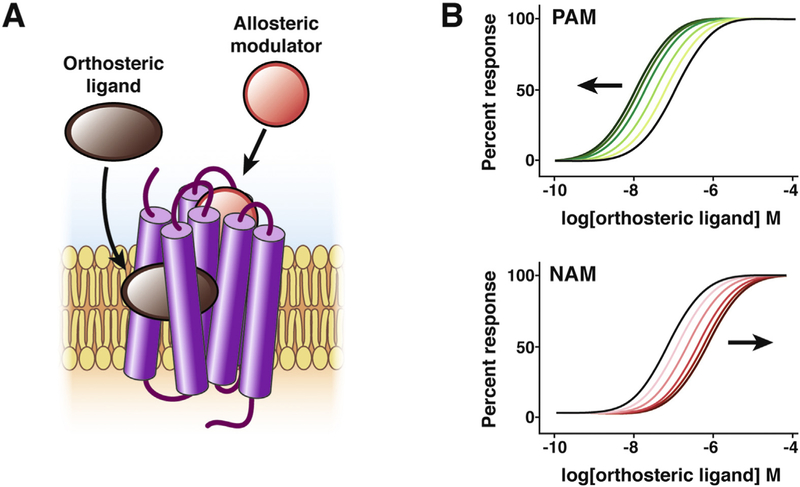
Allosteric modulators are drugs or endogenous molecules that fine-tune the ability of agonists to activate GPCRs. The challenge of developing drugs that are selective for a particular GPCR subtype illustrates the potential of allosteric modulation for drug discovery. A single endogenous ligand can activate several GPCRs (eg, acetylcholine activates 5 muscarinic receptors, M1–5Rs). These GPCR subtypes regulate processes within the digestive system and elsewhere. For example, studies in receptor knockout mice indicate that M1R and M3R regulate salivary secretion,29 whereas M2R and M3R control intestinal smooth muscle contraction.30 M1R, M4R, and M5R function in the central nervous system.31 Because the binding sites for endogenous ligands (orthosteric sites; “right” or “proper” in Greek) are conserved between GPCR subtypes, it is challenging to identify subtype-selective drugs that occupy the same site as the natural ligand. An alternative approach to attain subtype selectivity is to develop drugs that bind to a different site (allosteric site; “other” in Greek).32,33 Ligands that interact with allosteric sites can induce changes in GPCR conformation that potentiate (positive allosteric modulators [PAMs]) or inhibit (negative allosteric modulators [NAMs]) endogenous agonists (Figure 2). Intracellular effectors, including G proteins and ARRBs, are physiologic allosteric modulators, because interaction with GPCRs induces changes in conformation that alter agonist affinity.34,35
Figure 2.
Allosteric modulation of GPCRs. The orthosteric site of a GPCR is the site where the endogenous ligand (brown) binds. Sites that are topographically distinct from the orthosteric site are known as allosteric sites. Ligands that bind to allosteric sites (red) can potentiate (PAMs) or depress (NAMs) orthosteric ligand affinity and efficacy. The simulated concentration response curves show the effect of increasing concentrations of PAMs (green lines) or NAMs (red lines) on the response to a GPCR agonist (black line).
There are advantages to drugs that interact with allosteric rather than orthosteric sites. First, allosteric modulators might provide subtype selectivity, because the allosteric site is likely to be less conserved than the orthosteric site, which evolved to bind the same endogenous transmitter. Second, allosteric ligands modulate the activity of GPCRs that are bound to endogenous ligands, providing an opportunity to fine-tune physiologic responses. Third, because the magnitude of an allosteric effect is limited by cooperativity between orthosteric and allosteric sites, allosteric ligands have a ceiling level beyond which no further modulation occurs, with decreased propensity for overdose and toxicity. These advantages have led to drug discovery efforts focused on the identification of allosteric modulators of GPCRs,18 some of which have progressed to clinical trials.1 However, there are only 2 approved allosteric modulators of GPCRs: maraviroc, a chemokine receptor 5 NAM that inhibits human immuno-deficiency virus entry,36 and cinacalcet, a calcium-sensing receptor PAM used to treat hyperparathyroidism.37 These drugs were found to be allosteric modulators after regulatory approval.
Translational and Clinical Impact of Allosteric Modulators for Digestive Diseases
Consideration of the clinical utility of allosteric modulators of GPCRs raises 2 questions: are allosteric modulators a potential treatment for digestive diseases and will gastrointestinal-related adverse events prohibit use of PAMs and NAMs for non-gastrointestinal disorders? PAMs and NAMs have been developed for several GPCRs found in the gastrointestinal tract; some have progressed to clinical trials (Table 1).
Table 1.
Clinical Trials of Allosteric Modulators, Biased Agonists, and Bivalent Ligands of GPCRs for Treatment of Disorders of the GI Tract or With Side Effects in the GI Tract
| Drug | Mechanism of action | Clinical indication | Potential GI effect | Outcome of trial | ClinicalTrials.gov identifier |
|---|---|---|---|---|---|
| Allosteric modulators | |||||
| MK-7622 | M1R PAM | Improved cognition in Alzheimer disease | Diarrhea | Trial stopped for futility; diarrhea was most common side effect | NCT01852110 |
| ADX10059 | MGLUR5 NAM | GERD | Decreased reflux | Further testing stopped due to increased hepatic transaminases | NCT00820079 |
| Biased agonists | |||||
| TRV130 | MOR agonist | Pain | Decreased nausea and vomiting; constipation not measured in trials | Analgesia comparable to or better than morphine | NCT02335294, NCT02083315 |
| ADL5859 | DOR agonist | Pain | Possible decreased effect on GI motility vs MOR agonist; however, not measured in trial | No analgesia | NCT00993863, NCT00626275, NCT00603265, NCT00979953 |
| ADL5747 | DOR agonist | Pain | Possible decreased effect on GI motility vs MOR agonist; however, not measured in trial | Not effective for analgesia | NCT00979953, NCT01058642 |
| Oligomer targets | |||||
| Eluxadoline | MOR agonist and DOR antagonist | IBS-D abdominal pain | Analgesia for abdominal pain | Approved for clinical use | NCT01553747, NCT01553591 |
| Eluxadoline | MOR agonist and DOR antagonist | IBS-D with bile acid Malabsorption | Improved stool consistency | Recruiting | NCT03441581 |
| Eluxadoline | MOR agonist and DOR antagonist | Diarrhea-associated fecal incontinence | Fewer days with fecal incontinence | Recruitment pending | NCT03489265 |
GI, gastrointestinal.
PAMs and NAMs have been identified for M1–5R.38 Allosteric targeting of M1R, M4R, and M5R is an attractive treatment for disorders of the central nervous system, including schizophrenia, in which subtype-specificity would limit off-target effects on peripheral M2R and M3R, which are expressed in the digestive tract.39 The M1R PAM benzyl quinolone carboxylic acid alleviates cognitive deficits but induces diarrhea in mice.40,41 Compounds with differential positive cooperativity across subtypes could improve cognition with a lower risk of gastrointestinal side effects.42 MK-7622, an M1R PAM, sensitizes M1R to acetylcholine in the nanomolar range with no effect on M2R, M3R, or M4R up to 100 µmol/L.43 MK-7622 improved cognitive testing in preclinical models. Two phase I trials tested MK-7622. MK-7622 produced an increase on the sigma band awake electroencephalogram, which indicated alertness. It also reversed the negative cognitive effects induced by scopolamine, a muscarinic receptor antagonist.43 Based on these results, a phase IIa and IIb, multicenter, randomized, double-blind, placebo-controlled, parallel group trial was undertaken to evaluate the efficacy and safety of MK-7622 as an adjunctive therapy to acetylcholinesterase inhibitors for Alzheimer disease (ClinicalTrials.gov, identifier NCT01852110). The trial was stopped because MK-7622 failed to improve cognition. Diarrhea, which is induced by acetylcholine, was the most common adverse event. Given the prominent role of M2R and M3R in regulating gastrointestinal smooth muscle, peripherally restricted allosteric modulators that fine-tune the actions of acetylcholine might offer a potential therapy for motility and secretory disturbances and visceral pain of IBS.44
Opioids and associated µ-, δ-, and k-opioid receptors (µ-opioid receptor [MOR], δ-opioid receptor [DOR], and k-opioid receptor, respectively) are expressed throughout the gut. In addition to their analgesic properties, which are mediated by opioid receptors (ORs) expressed by primary sensory neurons and second-order spinal neurons, opioids inhibit intestinal motility and electrolyte and fluid secretion by activating ORs on enteric neurons. Orthosteric agonists of MOR are used to treat pain (eg, morphine, fentanyl) and diarrhea (eg, loperamide). However, their usefulness is limited by respiratory depression, constipation, and addiction. Morphine-induced analgesia is limited by tolerance (ie, decreased effectiveness with sustained use). MOR PAMs could provide effective therapy without adverse effects by amplifying the actions of endogenous opioids or by allowing a decrease of the dose of synthetic opioids. BMS-986122 is a MOR PAM that potentiates opioids and morphine.45,46 However, because respiratory depression and constipation are mediated by MOR, PAMs would be expected to potentiate these side effects. Although MOR is the prominent target of opioid analgesics, DOR also controls intestinal contractility.47 DOR is a target for diarrhea-predominant IBS (IBS-D),48 and enhancement of enkephalinergic signaling attenuates secretory diarrhea.49 BMS-986187 is a DOR PAM that amplifies the actions of DOR agonists.50 By modulating endogenous opioids, DOR PAMs have the potential to inhibit motility without causing constipation. Despite the promise of the MOR PAM (BMS-986122) and the DOR PAM (BMS-986187), the therapeutic potential of these drugs is yet to be assessed and they have not been tested in clinical trials.
Allosteric modulators of gut GPCRs have been described for the treatment of other digestive disorders. Glutamate, a transmitter of visceral and somatic pain, can activate ionotropic receptors (ion channels) and metabotropic GPCRs (MGLUR1–8). MGLUR5, which is expressed by vagal afferent endings of the gastroesophageal sphincter, regulates sphincter tone, providing a basis for the development of allosteric modulators of MGLUR5 for gastroesophageal reflux disease (GERD). ADX10059 is a MGLUR5 NAM. A randomized, patient-blind, placebo-controlled trial demonstrated that ADX10059 decreased GERD-related symptoms.51 Dizziness developed in 75% of participants. Then, ADX10059 was tested, at a lower dose, in a double-blind, placebo-controlled, multicenter trial in participants with proton pump inhibitor–responsive GERD. At this lower dose, ADX10059 increased symptom- and heartburn-free days and decreased regurgitation and sleep disturbance. Mild to moderate dizziness and vertigo were experienced by only 16% and 12% of patients, respectively52 (ClinicalTrials.gov, identifier NCT00820079). Testing was stopped because long-term administration of ADX10059 in a trial for the prevention of migraine increased hepatic transaminases (ClinicalTrials.gov, identifier NCT00820105). Liver enzyme increase resulted from metabolism of ADX10059 rather than MGLUR5 inhibition; therefore, negative allosteric modulation of MGLUR5 remains a viable approach for GERD.
Biased-Agonism of GPCRs: Shapeshifting Receptors and Pathway-Selective Drugs
Concept of Biased Agonism of GPCRs
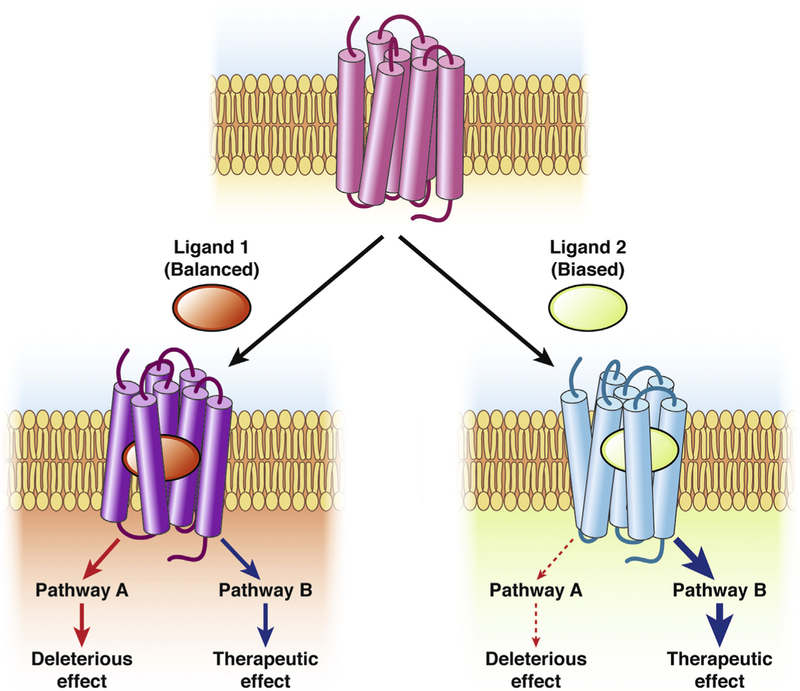
Biased agonism describes the phenomenon in which the binding of different ligands, including endogenous ligands or drugs, to the same receptor in an identical cellular background results in differential activation of signaling pathways19 (Figure 3). Although this is the definition of ligand-biased agonism, other descriptions include differential localization of activated GPCRs (location bias) or differential signaling between various cell types (system bias). Biased agonism provides an avenue for pathway-selective drug discovery (ie, the development of drugs that modulate the beneficial pathways rather than those that give rise to adverse effects). Ligand bias can be attributed to different agonists stabilizing distinct conformations of GPCRs that couple to particular signaling effectors. Studies of serotonin receptors bound to the ARRB-biased agonists ergotamine and lysergic acid diethylamide support this concept.53 However, robust structural evidence for this mechanism of biased agonism is lacking and will require studies of GPCRs in multiple activation states. The realization that GPCRs can be differentially activated within intracellular compartments (see Compartmentalized Signaling) has sparked interest in location bias as a therapeutic avenue.54 System bias, which can be attributed to differences in the stoichiometric ratios of signaling effectors between cells, also offers a strategy for the design of effective therapies. However, these endeavors require an understanding of the signaling pathways in functionally relevant cells and of how they can be altered during disease, which, in most cases, is still lacking. Biased agonism of GPCRs has implications for physiologic control and drug discovery.
Figure 3.
The therapeutic potential of biased agonists of GPCRs. Biased agonism describes the phenomenon in which different ligands binding to the same GPCR in an identical cellular background elicit distinct signaling outcomes (path-ways A and B). Balanced agonists (ligand 1) are those that activate all signaling pathways to the same extent, leading to therapeutic effects but also to deleterious effects. When there is a distinction between the signaling pathways that drive a therapeutic response and those that mediate the adverse effects of a drug, biased agonists provide a novel avenue for pathway-directed therapeutics. In such a case, the drug would only trigger the desired response and spare the unwanted, deleterious effects (ligand 2).
The mechanisms by which serine and cysteine proteases activate protease-activated receptor-2 (PAR2) illustrate the relevance of biased agonism of a GPCR that controls gut functions. PAR2 is expressed throughout the digestive system, where it regulates inflammation, pain, motility, and secretion, and is a therapeutic target for inflammatory and functional disorders.55 During disease, proteases become activated and trigger PAR2 by distinct mechanisms.56 Trypsin, from pancreatic secretions and colonocytes, and mast cell tryptase cleave within the extracellular N-terminus of PAR2 at the R36↓S37 to expose a new N-terminal tethered ligand domain (S37LIGKV). This domain then binds to extracellular loops of cleaved PAR2, which couples to Gaq, Gas, and ARRBs. PAR2 internalizes and can continue to signal from endosomes (see Compartmentalized Signaling).25,57 This canonical mechanism, which operates in model cell lines and primary sensory neurons, was once considered the only way proteases could activate PAR2. However, cathepsin-S from macrophages and neutrophil elastase cleave PAR2 at different sites from trypsin and tryptase and activate PAR2 by biased mechanisms.58,59 Cathepsin-S cleaves at E56↓T57 to show a distinct tethered ligand (T57VFSVDEFSA), which binds to PAR2 and induces coupling to Gas.58 Elastase cleaves PAR2 at S67↓V68, close to the first transmembrane domain, and activates the receptor by a mechanism that likely involves a conformational change rather than exposure of a tethered ligand and induces PAR2 coupling to Gas and Ga12,13.59 After cleavage by cathepsin-S and elastase, PAR2 couples to neither Gaq nor ARRBs and does not internalize. An understanding of these mechanisms provides insights into how these proteases signal PAR2-dependent pain, including inflammatory pain in the colon.25 Trypsin evokes hyperexcitability of primary sensory neurons by mechanisms that depend on protein kinase C and extracellular signal regulated kinase, which are downstream from Gaq.25 Cathepsin-S and elastase evoke hyperexcitability of neurons by adenylyl cyclase- and protein kinase A– mediated pathways, downstream from Gas.25,58,59 The mechanisms by which proteases of different selectivity can activate PAR2 represents biased signaling, in which the receptor couples to different G proteins depending on the site of cleavage. Other GPCRs that control gut functions also might be activated by biased mechanisms, although this has not been studied. Biased agonism is likely to be pertinent for GPCRs for neuropeptides, which often exist in multiple forms that might interact with receptors in different ways.
In addition to its physiologic relevance, biased agonism of GPCRs has implications for drug discovery. A limitation of most agonist drugs is that the same receptor mediates the beneficial and detrimental effects (ie, on-target side effects). For example, MOR mediates morphine-induced analgesia, but also causes constipation and respiratory depression. If the signaling pathways that are responsible for the beneficial and detrimental actions of agonists are known and are different, then it might be possible to develop drugs that activate only the beneficial signaling events, thereby minimizing on-target side effects. Such drugs would be not only receptor specific but also pathway specific, offering selectivity (Figure 3). Although this concept is attractive, the development of pathway-selective biased agonists is challenging.60 The signaling pathways that underlie the beneficial and detrimental actions of agonists in vivo are not always known because of the difficulty of studying signaling in primary cells and intact animals.
Despite these challenges, there has been interest in developing pathway-selective biased agonists of ORs that would treat pain without on-target side effects. Interest in this area was sparked by the observation that mice lacking β-arrestin2 (ARRB2) displayed altered responses to morphine.61,62 ARRB2 deletion enhanced and prolonged morphine-induced analgesia, which is attributable to decreased MOR desensitization. In contrast, ARRB2 deletion attenuated morphine-induced tolerance, respiratory depression, and constipation, which suggests that ARRB2 mediates the signaling that underlies these effects.61–63 Observations with loperamide, a peripherally restricted MOR agonist, confirmed that ARRB2 mediates opioid-induced constipation.64,65 However, ARRB2 plays a role in the digestive tract, where it mediates the development of tolerance to morphine in the colon but not in the ileum.66–68 The observation that ARRB2 plays distinct roles in regulating MOR signaling that underlies analgesia vs respiratory suppression and constipation prompted efforts to identify biased agonists of MOR that activate G proteins but not ARRBs. Potentially, G-protein–biased agonists would induce analgesia without on-target side effects. Several candidates have emerged.
Translational and Clinical Impact of Biased Agonists for Digestive Diseases
TRV130 (Oliceridine, Olinvo) is a weak G-protein–biased agonist of MOR.69 Consistent with its lesser ability to recruit ARRB2, TRV130 stimulates minimal MOR phosphorylation or internalization compared with other opioids.70 TRV130 retains analgesic activity in rodents, with decreased adverse effects of gastrointestinal function and respiration.70 ClinicalTrials.gov lists 10 trials related to TRV130 (Table 1). A double-blind, patient-controlled analgesia phase IIb study was designed to investigate the efficacy, safety, and tolerability of TRV130 compared with morphine and placebo in patients with moderate to severe pain after abdominoplasty (ClinicalTrials.gov, identifier NCT02335294). Although the analgesic efficacy of TRV130 was similar to that of morphine, TRV130 produced less nausea and vomiting.71 In healthy men, TRV130 produced greater analgesia than morphine, with a smaller decrease in respiratory function and less nausea and vomiting (ClinicalTrials.gov, identifier NCT02083315).72 These clinical trials do not report whether the incidence of constipation after administration of TRV130 is lower compared with morphine. Oliceridine was granted novel drug application status by the US Food and Drug Administration in 2017, but this application was rejected for safety issues and dosing concerns.
Structure-based drug design has been used to develop G-protein–biased agonists of ORs. PZM21 is a G-protein– biased MOR agonist derived from structure-based drug design efforts facilitated by the resolution of the crystal structure of all OR subtypes.73 Together with PZM-21, multiple G-protein–biased MOR agonists have been identified that provide analgesia with fewer on-target side effects.74 However, recent studies suggest that TRV130 and PZM21 retain their undesirable side effects with repeated use despite being G protein biased.75,76 Further studies are required to ascertain the therapeutic utility of G-protein– biased agonists of MOR.
Biased agonists of DOR have been tested for analgesic efficacy.77 The attractiveness of DOR agonists for clinical use is their decreased propensity to inhibit gastrointestinal motility and cause constipation compared with MOR agonists.78,79 The DOR agonists SNC80 and ARM390 produce comparable analgesia but show biased effects at the cellular and behavioral levels.80 SNC80 causes endocytosis of DOR, whereas ARM390 does not. Repeated injection of SNC80 produces analgesic, locomotor, and anxiolytic tolerance and receptor down-regulation. Repeated administration of ARM390 produces analgesic tolerance, but not locomotor or anxiolytic tolerance. Dorsal root ganglia from the mice treated with these agonists demonstrated intact DOR expression, although DOR coupling to calcium channels was lost. ADL5859 and ADL5747 are DOR agonists that, similar to ARM390, produce biased effects in preclinical studies.81,82 ADL5747 and ADL5859 produce antinociception in inflammatory and neuropathic pain models, do not activate locomotion, and do not induce DOR internalization. ADL5859 has been tested in clinical trials for analgesic efficacy after molar removal (ClinicalTrials.gov, identifier NCT009938363), rheumatoid arthritis (ClinicalTrials.gov, identifier NCT00626275), diabetes-induced peripheral neuropathy (ClinicalTrials.gov, identifier NCT00603265), and osteoarthritis (ClinicalTrials.gov, identifier NCT00979953). ADL5859 did not demonstrate analgesic efficacy in these trials. Preclinical testing showed ADL5747 to have greater analgesic potency than ADL5859 in a model of inflammatory pain in rats. However, it failed to show analgesic efficacy for osteoarthritic pain (ClinicalTrials.gov, identifier NCT00979953) and post-herpetic neuralgia (ClinicalTrials.gov, identifier NCT01058642).
GPCR Oligomerization: It Takes 2 to Tango
Concept of Oligomerization of GPCRs
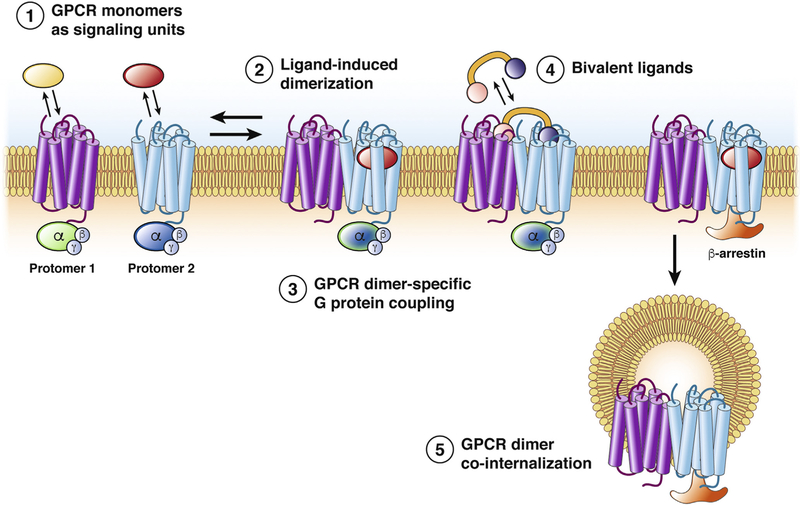
Although receptor tyrosine kinases and ion channels can assemble into multimeric functional units, the oligomerization of GPCRs is controversial. In light of this controversy, the International Union of Basic and Clinical Pharmacology developed criteria for the acceptance of GPCR oligomers.83 Criteria include evidence of physical association of GPCRs in native tissues and cells, rather than in transfected cells; evidence of a new or different pharmacologic property of the oligomer in native systems; and the observation of functional changes when one of the protomers is deleted in animals. Despite this controversy, the development of drugs that target components of a GPCR oligomer offers the possibility of selectivity and efficacy (Figure 4).
Figure 4.
Potential roles of GPCR dimerization. GPCRs have been shown to function as monomers (1) and dimers (2). (3) The formation of GPCR dimers can be triggered by agonist activation and change the specificity of G-protein coupling. (4) Such differences in effector coupling elicited by dimerization have prompted the development of bivalent drugs, which specifically target the 2 protomers within a dimer. (5) Dimerization also can provide an alternative mechanism of receptor trafficking, in which ligands can promote the co-internalization of the 2 receptors after the stimulation of only 1 protomer. Alternatively, the presence of a protomer that is resistant to agonist-promoted endocytosis, within a heterodimer, can inhibit the internalization of the complex.
Oligomerization of Class C GPCRs
The strongest evidence for the existence of dimers comes from class C GPCRs (glutamate, GABA, calcium). Dimerization of some class C GPCRs is necessary for function, in which the association of 2 identical or distinct subunits forms a functional receptor. In contrast to other families, the ligand binding site of these GPCRs is not located within the heptahelical domain, but rather within a large extracellular Venus flytrap domain. Class C GPCR dimers are stabilized by a disulfide covalent linkage between the 2 subunits. Dimerization of these receptors is essential for allosteric coupling between the Venus flytrap domain and the heptahelical domain and thus between sites for ligand binding and G-protein activation. Heterodimerization of the GABAB1 and GABAB2 receptors is required to mask an endoplasmic reticulum retention sequence, allowing trans-location of receptors to the plasma membrane.84–86 Agonist binding to GABAB1 allosterically activates GABAB2 to initiate intracellular signal transduction. Although this heteromerization was first described in the brain,85 it has been postulated to occur in the digestive tract87 and is supported by the colocalization of the 2 subunits in the upper gut.88 GABAA and GABAB receptors are expressed throughout the gut and can regulate relaxation of the lower esophageal sphincter, gastric and intestinal motility, and colonic pain.89 GABAB agonists have been proposed as a treatment for GERD but the incidence of centrally mediated side effects has limited therapeutic applicability.90
Oligomerization of Class A GPCRs
The dimerization of class A GPCRs, although more controversial than for class C GPCRs, illustrates the dynamism of this receptor family, because the assembly of class A oligomers has been proposed to be ligand dependent and to modulate GPCR biogenesis and endocytosis91,92 (Figure 4). Dimerization of ORs has attracted attention. Studies of purified receptors reconstituted into a phospholipid bilayer indicate that monomeric MOR can bind agonists and antagonists and is the minimal functional unit necessary for G-protein activation.93 However, structural and functional observations suggest that ORs can dimerize. Antagonist-bound MOR crystalized as a symmetrical dimer with the interfaces within transmembrane helices 5 and 6,20 although these interfaces were not observed in the agonist-bound structure.94 MOR homodimers have been detected in heterologous expression systems and in vivo.95
MOR can dimerize with DOR, because in recombinant systems a MOR-DOR heterodimer displays binding and functional properties that can be observed in native membranes of wild-type but not of knockout mice.96 However, these data have been debated. In transgenic mice expressing DOR fused to green fluorescent protein (GFP), there is little overlap between DOR-GFP and immunoreactive MOR in primary sensory and spinal neurons,97 although DOR-GFP and MOR-mCherry are coexpressed in limited neuronal populations.98 Within pain pathways, DOR-MOR coexpression is limited to excitatory interneurons and projection neurons in the dorsal horn of the spinal cord and to neurons in parabrachial, amygdala, and cortical regions of the brain.99 In these neurons, DOR and MOR traffic and function independently. Despite this controversy, the MOR-DOR heterodimer has been suggested as a therapeutic target that could provide analgesia with decreased tolerance.100,101 Bifunctional ligands, composed of a MOR agonist and a DOR antagonist, have been generated with the rationale that DOR antagonists could enhance MOR responses.
Although functional coexpression of MOR and DOR by the same neuron was first demonstrated using electrophysiologic recordings from enteric neurons,102 the definitive demonstration of MOR-DOR heteromers in enteric neurons is lacking. DOR-GFP is coexpressed in a subpopulation of myenteric neurons with immunoreactive MOR.103 However, whether they form heteromers or functionally interact through other mechanisms has not been determined. Electrophysiologic and molecular studies show that MOR and DOR are coexpressed by afferent neurons innervating the mouse colon, where receptors might suppress neuronal excitability during inflammation.104
Translational and Clinical Impact of GPCR Oligomers for Digestive Diseases
The utility of bivalent drugs that recognize the 2 components of a GPCR dimer is illustrated by finding that a molecule with MOR agonist and DOR antagonist activity (Eluxadoline) acts through the MOR-DOR heteromer105 (Table 1 and Figure 4). Eluxadoline relieves abdominal pain in patients with IBS-D (ClinicalTrials.gov, identifier NCT01553747; NCT01553591).48,106 Despite the MOR activity, the drug showed no evidence of abuse potential in phase II and III clinical studies.107 A clinical trial is open to test whether Eluxadoline is effective for the management of IBS-D in patients with bile acid malabsorption (ClinicalTrials.gov, identifier NCT03441581). Eluxadoline will be tested for the management of diarrhea-associated fecal incontinence (ClinicalTrials.gov, identifier NCT03489265).
Compartmentalized Signaling: Adding Texture to GPCR Responses
Concept of Compartmentalized Signaling of GPCRs
Although alterations in the conformation of GPCRs might account for allosteric modulation and biased agonism and could explain the altered functions of GPCR oligomers, GPCRs also undergo positional changes during their activation–deactivation cycle, exemplified by agonist-induced endocytosis. Agonist-induced endocytosis in vivo has been demonstrated for the neurokinin-1 receptor (NK1R) and DOR, because of the availability of selective NK1R antibodies and transgenic mice expressing DOR-GFP. Physiologic stimuli evoke NK1R endocytosis in endothelial cells of post-capillary venules at sites of neurogenic inflammation,108 in enteric neurons during inflammation,109 and in second-order spinal neurons after painful stimuli.24,110,111 Exogenous and endogenously released opioids induce endocytosis of DOR in myenteric neurons.47,103 These studies led to the appreciation that GPCRs can signal from endosomes and the plasma membrane, with implications for physiologic control and drug discovery.23,26,28 GPCRs in endosomes can generate sustained signals in subcellular compartments (ie, compartmentalized signaling) that contribute to important pathophysiologic processes, and endosomal GPCRs could be an important target for therapy.
Control of Plasma Membrane Signaling of GPCRs
Plasma membrane signaling is regulated by ligand degradation and reuptake and by receptor desensitization and endocytosis and is often transient (Figure 5). Cell-surface peptidases degrade neuropeptides and terminate their biological effects. Neprilysin degrades and inactivates SP and bradykinin and attenuates their proinflammatory actions.112–114 Neprilysin deletion causes NK1R-dependent plasma extravasation in the digestive tract115 and exacerbates inflammation of the intestine by impaired degradation of SP.114 Enkephalin-degrading enzymes regulate activation of ORs, and inhibitors of these enzymes suppress diarrhea by enhancing the antisecretory actions of endogenous opioids.49
Figure 5.
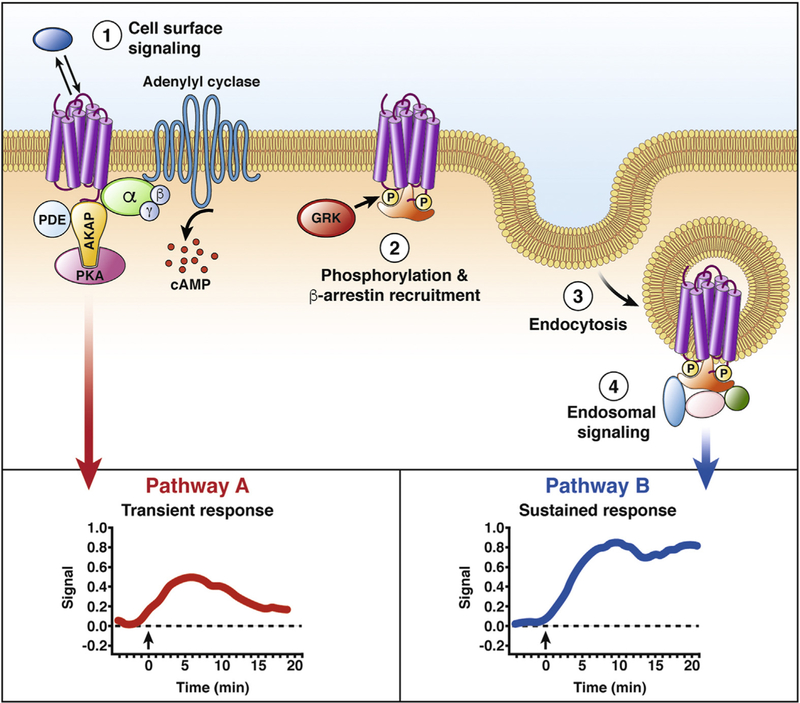
GPCR trafficking and compartmentalized signaling. The formation of GPCR-mediated signaling platforms provides a mechanism to sculpt specific cellular responses. (1) GPCRs at the plasma membrane form multiprotein complexes that participate in the regulation of a specific signaling pathway (pathway A). For example, AKAP interactions with GPCRs can scaffold the formation of complexes that regulate cAMP signaling by bringing in close proximity enzymes that degrade cAMP (PDEs) and kinases that are activated by this second messenger (PKA). (2) With prolonged agonist stimulation, GPCRs are phosphorylated by GRKs. The phosphorylated receptor has higher affinity for the cytosolic protein ARRB. (3) ARRBs are adaptors that promote clathrin-and dynamin-mediated endocytosis of GPCRs. (4) ARRBs scaffold the formation of multi-protein complexes that result in a second wave of intracellular signaling (pathway B). Genetically encoded biosensors have shown differences in the spatial and temporal profile of GPCR signaling from different subcellular locations (insets). AKAP, A-kinase anchor protein; GRK, G-protein receptor kinase; PDE, phosphodiesterase; PKA, protein kinase A.
GPCR desensitization also regulates signaling at the plasma membrane. ARRBs uncouple GPCRs from G proteins and couple GPCRs to the clathrin-mediated endocytic machinery.116 Desensitization of MOR and analgesic tolerance to opioids are associated with a decrease of MOR at the plasma membrane.117 However, tolerance to morphine develops for pain and for motility of the upper gut but not the colon, leading to constipation with escalating doses of opioids that are required to control pain.68 Differential functions of ARRBs could account for these differences in tolerance.
Intracellular Signaling of GPCRs
Although endosomes were considered a conduit for receptor trafficking to recycling or degradation pathways, endosomes currently are considered a major site of continued signaling by GPCRs.22–27,118–121 GPCRs in endosomes can assemble signaling complexes (signalosomes) in subcellular compartments. The spatial and temporal characteristics of these signals can provide a mechanism underlying specific cellular responses (Figure 5).
The idea of compartmentalized signaling, although initially proposed for cyclic adenosine monophosphate (cAMP),122 was first demonstrated for calcium signaling owing to the availability of fluorescent indicators that allowed observations of calcium sparks, puffs, and blinks within living cells.123 The use of genetically encoded Förster resonance energy transfer biosensors that are targeted to particular subcellular domains has shown that most signals are compartmentalized.124 Signal compartmentalization can be achieved by the formation of signaling micro-domains, such as those described for receptors that stimulate the formation of cAMP. Here, local second-messenger concentrations are controlled by the proximity of adenylyl cyclase (generates cAMP), phosphodiesterases (degrade cAMP), and cAMP-activated protein kinase A.125 Scaffolding proteins that lack enzymatic activity but participate in the organization of signaling effectors can mediate signal compartmentalization. A-kinase anchoring proteins are recognized for their roles in the formation of multi-protein complexes that modulate spatial and temporal cAMP signaling.125 ARRBs serve as molecular scaffolds that recruit GPCRs, including PAR2 and NK1R, and components of the mitogenactivated protein kinase cascade to endosomes for the activation of extracellular signal regulated kinase in subcellular compartments.57,126 Although most descriptions of compartmentalized GPCR signaling in physiologic settings have been focused on the heart and brain, signal compart-mentalization in the gastrointestinal tract has been reported for cAMP.127
Control of the Endosomal Signaling of GPCRs
The trafficking of GPCRs through the endosomal system, which depends in part on the stability of agonist-GPCR-ARRB complexes, governs the speed of receptor recycling and re-sensitization and the duration of endosomal signals. Initially, GPCRs that exhibited sustained interactions with ARRBs were designated class B GPCRs (eg, NK1R, PAR2)128,129 and those that exhibited low affinity and transient interactions with ARRBs were termed class A GPCRs (eg, NK3R, MOR).130 Although this initial classification has been linked to the dynamics of receptor internalization and recycling, it has become apparent that not all GPCRs fall in these 2 categories. Despite this, the differential affinity for ARRBs can affect signaling of receptors that are coexpressed in enteric neurons, where the activated NK1R sequesters ARRBs and thereby inhibits ARRB-dependent desensitization and endocytosis of the NK3R.130 This process could provide a mechanism for sustained signaling by tachykinins through the NK3R even after the NK1R is desensitized and internalized.
For neuropeptide receptors, degradation of ligands by endosomal peptidases also determines the stability of agonist-GPCR-ARRB complexes and controls GPCR trafficking and signaling. Endothelin-converting enzyme 1 (ECE1) is a transmembrane peptidase found in early endosomes of many cells, including enteric neurons and endothelial cells.131–134 By degrading SP and calcitonin gene-related peptide in acidic endosomes, ECE1 destabilizes the agonist-GPCR-ARRB complex, which terminates endosomal signaling and promotes receptor recycling and re-sensitization. This mechanism controls the proinflammatory and neurotoxic actions of SP and NK1R.135 The susceptibility of endogenous peptides and peptide drugs to degradation by endosomal ECE1 has implications for physiologic control and therapy. Somatostatin (SST) isoforms exist with 14 or 28 amino acids. The 2 isoforms of SST evoke endocytosis of the SST receptor 2 (SSTR2), which is expressed throughout the enteric nervous system. After activation by SST14, SSTR2 recycles, whereas after activation by SST28, SSTR2 remains in endosomes, from where it can continue to signal.136 This difference is attributable to differential susceptibility of the SST isoforms to degradation by ECE1. ECE1 degrades SST14 in endosomes, which destabilizes the SST14-SSTR2-ARRB complex, allowing the receptor to recycle.136,137 Because ECE1 does not degrade SST28, SSTR2 remains in endosomes. Although metabolically stable SST analogues (eg, octreotide) are effective treatments for several disorders,138 they have side effects in the gastrointestinal tract (constipation, cramps, nausea). Stable SST analogues that are resistant to ECE1 evoke prolonged sequestration of SSTR2 in enteric neurons, which could generate long-lasting signals that underlie beneficial and detrimental actions.136
Mechanisms of Endosomal GPCR Signaling
The concept that endosomes are a major site for sustained GPCR signaling was suggested by observations that ARRBs serve as molecular scaffolds that recruit GPCRs and components of the mitogen-activated protein kinase cascades to endosomes.57,126 It is apparent that GPCRs in endosomes can signal by ARRB- and G-protein–mediated mechanisms, and that endosomal signaling activates kinases and generates cAMP in defined subcellular compartments22–27,118–121 (Figure 5). How is it possible that GPCRs can signal from endosomes by ARRB- and G-protein–mediated mechanisms, when ARRBs uncouple GPCRs from G proteins at the plasma membrane? Structural studies of the β2-adrenergic receptor have identified receptor-G protein-ARRB mega-complexes and shown that conformations of GPCR-ARRB complexes retain the capacity to couple to Gα subunits.139,140
Translational and Clinical Impact of GPCR Compartmentalized Signaling for Digestive Diseases
The therapeutic relevance of endosomal GPCR signaling is evident.28 Although GPCR signaling at the plasma membrane is transient, endosomal signaling by the same receptor can be sustained and regulate events in the cell, including gene transcription in the case of the β2-adrenergic receptor and NK1R.24,121 Endosomal signaling by GPCRs in the pain pathway, including the SP NK1R and the calcitonin gene-related peptide calcitonin receptor-like receptor in second-order spinal neurons,24,27 and PAR2 in primary spinal afferent neurons,25 is critical for the sustained activation and hyperexcitability of neurons that is a hallmark of chronic pain. Indeed, receptor endocytosis is required for these receptors to exhibit the full repertoire of signaling responses. Inhibitors of clathrin and dynamin and lipid-conjugated antagonists that target NK1R, calcitonin receptor-like receptor, and PAR2 in endosomes block signaling derived from endosomal receptors. Such inhibitors provide relief from pain in preclinical models of somatic and colonic pain,24,25,27 illustrating the pathophysiologic relevance of endosomal GPCR signaling. Endosomal-targeted antagonists of PAR2 could be effective treatments for IBS pain, in which colonic proteases and PAR2 are strongly implicated.25,141,142 Endosomal-targeted agonists and antagonists of GPCRs could provide options for therapy in which this has proved clinically ineffective.28
Future Directions
GPCRs control digestion and digestive diseases and are a target for therapy. GPCRs sense the contents of the lumen, mediate the actions of gut hormones, neurotransmitters, and paracrine agents, and control inflammation and pain. Drugs that activate or inhibit these receptors have been a mainstay for the treatment of digestive disorders (eg, histamine H2 receptor antagonists for peptic ulcer disease143).
However, we have but a superficial understanding of this large and complex family of receptors in digestion and digestive diseases. The functions and roles in the gut of orphan GPCRs, such as MRGPRs, leucine-rich GPCRs, and frizzled and adhesion receptors, are still unknown. The concepts of allosteric modulation, biased agonism, oligomerization, and compartmentalized signaling offer new opportunities for therapy. The successful exploitation of these concepts for the development of superior therapies requires a complete understanding of receptor expression, signaling, and trafficking in important cell types in health and diseased states, which is lacking.
Progress in structural, chemical, and cell biology and genetics will advance the understanding of the function of GPCRs and the development of GPCR-directed therapies. Conventional drug discovery involves screens of libraries of millions of drug-like molecules. Although this approach has yielded success, some GPCRs have been found to be undruggable. An understanding of the structural basis of GPCR activation and signaling, coupled with advances in molecular modeling, has enabled screening of virtual libraries in silico, allowing rational structure-based drug design, even for orphan GPCRs.144 Cryo-electron microscopy13,14 and proximity ligation techniques coupled to mass spectrometry and proteomics145 have provided fresh insights into the formation and structure of GPCR-signaling platforms. The realization that GPCRs can signal in defined subcellular compartments to control pathophysiologically important processes, such as pain, has led to the development of compartment-selective agonists and antagonists.28 Analysis of compartmentalized signaling using genetically encoded biosensors has shown that some drugs can activate GPCRs in unexpected intracellular locations. Opioid peptides can activate MOR at the plasma membrane and then in endosomes, secondary to receptor endocytosis, whereas morphine also can activate MOR in the Golgi apparatus because of of its ability to penetrate membranes.54 In this context, developments such as organoids, which replicate the complex organization of organs in tissue culture, and advanced genome editing using CRISPR Cas 9 hold remarkable potential in basic and translational GPCR research.146 The development of designer receptors exclusively activated by designer drugs and opto-genetics have provided important insights into GPCR signaling pathways that underlie important physiologic processes in vivo. Designer receptors exclusively activated by designer drugs are engineered to respond to inert drugs, but not to endogenous ligands. By using transgenic and viral-delivery approaches, it is possible to express designer receptors exclusively activated by designer drugs in particular cell types and then examine the consequences of GPCR activation in defined cell types.147,148 Chemo-genetic approaches have been used to control the activity of enteric glial cells to investigate their roles in intestinal motility149 and secretomotor function.150
Much of the focus of these new technologies has been to define the function of GPCRs in the central nervous system and to develop more effective GPCR-directed therapies for neurologic diseases. In light of the undoubted importance of GPCRs in the digestive system, the application of similar technologies to analysis of gut function could lead to advances in understanding digestive diseases.
Acknowledgments
Funding
This work is supported by the National Institutes of Health (grants NS102722, DE026806, and DK118971), the Department of Defense (grant W81XWH1810431 to Nigel W. Bunnett and Brian L. Schmidt), and the National Health and Medical Research Council of Australia (grant 1121029 to Meritxell Canals and Daniel P. Poole).
Abbreviations used in this paper:
- ARRB
β-arrestin
- ARRB2
β-arrestin2
- cAMP
cyclic adenosine monophosphate
- DOR
δ-opioid receptor
- ECE1
endothelin converting enzyme 1
- GABA
γ-aminobutyric acid
- GERD
gastroesophageal reflux disease
- GFP
green fluorescent protein
- GPCR
G-protein–coupled receptor
- GRK
G-protein–coupled receptor kinase
- IBS
irritable bowel syndrome
- IBS-D
diarrhea-predominant irritable bowel syndrome
- MGLUR
metabotropic glutamate receptor
- MOR
µ-opioid receptor
- MRGPR
Mas-related GPCR
- NAM
negative allosteric modulator
- NK1R
neurokinin-1 receptor
- OR
opioid receptor
- PAM
positive allosteric modulator
- PAR
protease-activated receptor
- SP
substance P
- SST
somatostatin
- SSTR
somatostatin receptor
Footnotes
Conflicts of interest
Nigel W. Bunnett is a founding scientist of Endosome Therapeutics Inc. Research in the laboratories of Nigel W. Bunnett, Daniel P. Poole, and Nicholas A. Veldhuis is funded in part by Takeda Pharmaceuticals Inc.
References
- 1.Hauser AS, Attwood MM, Rask-Andersen M, et al. Trends in GPCR drug discovery: new agents, targets and indications. Nat Rev Drug Discov 2017;16:829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chaudhari N, Roper SD. The cell biology of taste. J Cell Biol 2010;190:285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reimann F, Tolhurst G, Gribble Fiona M. G-protein–coupled receptors in intestinal chemosensation. Cell Metab 2012;15:421–431. [DOI] [PubMed] [Google Scholar]
- 4.Kong W, McConalogue K, Khitin LM, et al. Luminal trypsin may regulate enterocytes through proteinase-activated receptor 2. Proc Natl Acad Sci U S A 1997; 94:8884–8889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alemi F, Poole DP, Chiu J, et al. The receptor TGR5 mediates the prokinetic actions of intestinal bile acids and is required for normal defecation in mice. Gastroenterology 2013;144:145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abu-Hayyeh S, Ovadia C, Lieu T, et al. Prognostic and mechanistic potential of progesterone sulfates in intrahepatic cholestasis of pregnancy and pruritus gravidarum. Hepatology 2016;63:1287–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alemi F, Kwon E, Poole DP, et al. The TGR5 receptor mediates bile acid-induced itch and analgesia. J Clin Invest 2013;123:1513–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Solinski HJ, Gudermann T, Breit A. Pharmacology and signaling of MAS-related G protein-coupled receptors. Pharmacol Rev 2014;66:570–597. [DOI] [PubMed] [Google Scholar]
- 9.McNeil BD, Pundir P, Meeker S, et al. Identification of a mast-cell–specific receptor crucial for pseudo-allergic drug reactions. Nature 2015;519:237–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stead RH, Tomioka M, Quinonez G, et al. Intestinal mucosal mast cells in normal and nematode-infected rat intestines are in intimate contact with peptidergic nerves. Proc Natl Acad Sci U S A 1987;84:2975–2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barbara G, Stanghellini V, De Giorgio R, et al. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology 2004;126:693–702. [DOI] [PubMed] [Google Scholar]
- 12.Geppetti P, Veldhuis NA, Lieu T, et al. G protein-coupled receptors: dynamic machines for signaling pain and itch. Neuron 2015;88:635–649. [DOI] [PubMed] [Google Scholar]
- 13.Liang YL, Khoshouei M, Deganutti G, et al. Cryo-EM structure of the active, Gs-protein complexed, human CGRP receptor. Nature 2018;561:492–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liang YL, Khoshouei M, Glukhova A, et al. Phase-plate cryo-EM structure of a biased agonist-bound human GLP-1 receptor-Gs complex. Nature 2018;555:121–125. [DOI] [PubMed] [Google Scholar]
- 15.Rasmussen SG, Choi HJ, Fung JJ, et al. Structure of a nanobody-stabilized active state of the beta(2) adrenoceptor. Nature 2011;469:175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rasmussen SG, DeVree BT, Zou Y, et al. Crystal structure of the beta2 adrenergic receptor-Gs protein complex. Nature 2011;477:549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shukla AK, Westfield GH, Xiao K, et al. Visualization of arrestin recruitment by a G-protein–coupled receptor. Nature 2014;512:218–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Christopoulos A Advances in G protein-coupled receptor allostery: from function to structure. Mol Pharmacol 2014;86:463–478. [DOI] [PubMed] [Google Scholar]
- 19.Kenakin T Functional selectivity and biased receptor signaling. J Pharmacol Exp Ther 2011;336:296–302. [DOI] [PubMed] [Google Scholar]
- 20.Manglik A, Kruse AC, Kobilka TS, et al. Crystal structure of the micro-opioid receptor bound to a morphinan antagonist. Nature 2012;485:321–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu H, Wacker D, Mileni M, et al. Structure of the human kappa-opioid receptor in complex with JDTic. Nature 2012;485:327–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Irannejad R, Tomshine JC, Tomshine JR, et al. Conformational biosensors reveal GPCR signalling from endosomes. Nature 2013;495:534–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Irannejad R, Tsvetanova NG, Lobingier BT, et al. Effects of endocytosis on receptor-mediated signaling. Curr Opin Cell Biol 2015;35:137–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jensen DD, Lieu T, Halls ML, et al. Neurokinin 1 receptor signaling in endosomes mediates sustained nociception and is a viable therapeutic target for prolonged pain relief. Sci Transl Med 2017;9(392). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jimenez-Vargas NN, Pattison LA, Zhao P, et al. Protease-activated receptor-2 in endosomes signals persistent pain of irritable bowel syndrome. Proc Natl Acad Sci U S A 2018;115:E7438–E7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murphy JE, Padilla BE, Hasdemir B, et al. Endosomes: a legitimate platform for the signaling train. Proc Natl Acad Sci U S A 2009;106:17615–17622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yarwood RE, Imlach WL, Lieu T, et al. Endosomal signaling of the receptor for calcitonin gene-related peptide mediates pain transmission. Proc Natl Acad Sci U S A 2017;114:12309–12314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thomsen ARB, Jensen DD, Hicks GA, et al. Therapeutic targeting of endosomal G-protein–coupled receptors. Trends Pharmacol Sci 2018;39:879–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gautam D, Heard TS, Cui Y, et al. Cholinergic stimulation of salivary secretion studied with M1 and M3 muscarinic receptor single- and double-knockout mice. Mol Pharmacol 2004;66:260–267. [DOI] [PubMed] [Google Scholar]
- 30.Matsui M, Motomura D, Fujikawa T, et al. Mice lacking M2 and M3 muscarinic acetylcholine receptors are devoid of cholinergic smooth muscle contractions but still viable. J Neurosci 2002;22:10627–10632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thomsen M, Sorensen G, Dencker D. Physiological roles of CNS muscarinic receptors gained from knockout mice. Neuropharmacology 2018;136:411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Christopoulos A, Kenakin T. G protein-coupled receptor allosterism and complexing. Pharmacol Rev 2002; 54:323–374. [DOI] [PubMed] [Google Scholar]
- 33.Monod J, Changeux JP, Jacob F. Allosteric proteins and cellular control systems. J Mol Biol 1963;6:306–329. [DOI] [PubMed] [Google Scholar]
- 34.De Lean A, Stadel JM, Lefkowitz RJ. A ternary complex model explains the agonist-specific binding properties of the adenylate cyclase-coupled beta-adrenergic receptor. J Biol Chem 1980;255:7108–7117. [PubMed] [Google Scholar]
- 35.Gurevich VV, Pals-Rylaarsdam R, Benovic JL, et al. Agonist-receptor-arrestin, an alternative ternary complex with high agonist affinity. J Biol Chem 1997;272:28849–28852. [DOI] [PubMed] [Google Scholar]
- 36.Dorr P, Westby M, Dobbs S, et al. Maraviroc (UK-427, 857), a potent, orally bioavailable, and selective small-molecule inhibitor of chemokine receptor CCR5 with broad-spectrum anti-human immunodeficiency virus type 1 activity. Antimicrob Agents Chemother 2005; 49:4721–4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Block GA, Martin KJ, de Francisco AL, et al. Cinacalcet for secondary hyperparathyroidism in patients receiving hemodialysis. N Engl J Med 2004;350:1516–1525. [DOI] [PubMed] [Google Scholar]
- 38.Gentry PR, Sexton PM, Christopoulos A. Novel allosteric modulators of G protein-coupled receptors. J Biol Chem 2015;290:19478–19488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Conn PJ, Christopoulos A, Lindsley CW. Allosteric modulators of GPCRs: a novel approach for the treatment of CNS disorders. Nat Rev Drug Discov 2009;8:41–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kurimoto E, Matsuda S, Shimizu Y, et al. An approach to discovering novel muscarinic M1 receptor positive allosteric modulators with potent cognitive improvement and minimized gastrointestinal dysfunction. J Pharmacol Exp Ther 2018;364:28–37. [DOI] [PubMed] [Google Scholar]
- 41.Thomsen M, Lindsley CW, Conn PJ, et al. Contribution of both M1 and M4 receptors to muscarinic agonistmediated attenuation of the cocaine discriminative stimulus in mice. Psychopharmacology (Berl) 2012; 220:673–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sako Y, Kurimoto E, Mandai T, et al. TAK-071, a novel M1 positive allosteric modulator with low cooperativity, improves cognitive function in rodents with few cholinergic side effects. Neuropsychopharmacology 2019;44:950–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Uslaner JM, Kuduk SD, Wittmann M, et al. Preclinical to human translational pharmacology of the novel M1 positive allosteric modulator MK-7622. J Pharmacol Exp Ther 2018;365:556–566. [DOI] [PubMed] [Google Scholar]
- 44.Wess J, Eglen RM, Gautam D. Muscarinic acetylcholine receptors: mutant mice provide new insights for drug development. Nat Rev Drug Discov 2007;6:721–733. [DOI] [PubMed] [Google Scholar]
- 45.Burford NT, Clark MJ, Wehrman TS, et al. Discovery of positive allosteric modulators and silent allosteric modulators of the mu-opioid receptor. Proc Natl Acad Sci U S A 2013;110:10830–10835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Livingston KE, Traynor JR. Disruption of the Na+ ion binding site as a mechanism for positive allosteric modulation of the mu-opioid receptor. Proc Natl Acad Sci U S A 2014;111:18369–18374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.DiCello JJ, Saito A, Rajasekhar P, et al. Inflammation-associated changes in DOR expression and function in the mouse colon. Am J Physiol Gastrointest Liver Physiol 2018;315:G544–G559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lembo AJ, Lacy BE, Zuckerman MJ, et al. Eluxadoline for Irritable Bowel Syndrome with Diarrhea. N Engl J Med 2016;374:242–253. [DOI] [PubMed] [Google Scholar]
- 49.Turck D, Berard H, Fretault N, et al. Comparison of racecadotril and loperamide in children with acute diarrhoea. Aliment Pharmacol Ther 1999;13(Suppl 6):27–32. [DOI] [PubMed] [Google Scholar]
- 50.Burford NT, Livingston KE, Canals M, et al. Discovery, synthesis, and molecular pharmacology of selective positive allosteric modulators of the delta-opioid receptor. J Med Chem 2015;58:4220–4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Keywood C, Wakefield M, Tack J. A proof-of-concept study evaluating the effect of ADX10059, a metabotropic glutamate receptor-5 negative allosteric modulator, on acid exposure and symptoms in gastro-oesophageal reflux disease. Gut 2009;58:1192–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zerbib F, Bruley des Varannes S, Roman S, et al. Randomised clinical trial: effects of monotherapy with ADX10059, a mGluR5 inhibitor, on symptoms and reflux events in patients with gastro-oesophageal reflux disease. Aliment Pharmacol Ther 2011;33:911–921. [DOI] [PubMed] [Google Scholar]
- 53.Wacker D, Wang C, Katritch V, et al. Structural features for functional selectivity at serotonin receptors. Science 2013;340:615–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stoeber M, Jullie D, Lobingier BT, et al. A genetically encoded biosensor reveals location bias of opioid drug action. Neuron 2018;98:963–976 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ossovskaya VS, Bunnett NW. Protease-activated receptors: contribution to physiology and disease. Physiol Rev 2004;84:579–621. [DOI] [PubMed] [Google Scholar]
- 56.Edgington-Mitchell LE. Pathophysiological roles of proteases in gastrointestinal disease. Am J Physiol Gastrointest Liver Physiol 2016;310:G234–G239. [DOI] [PubMed] [Google Scholar]
- 57.DeFea KA, Zalevsky J, Thoma MS, et al. beta-arrestin–dependent endocytosis of proteinase-activated receptor 2 is required for intracellular targeting of activated ERK1/2. J Cell Biol 2000;148:1267–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhao P, Lieu T, Barlow N, et al. Cathepsin S causes inflammatory pain via biased agonism of PAR2 and TRPV4. J Biol Chem 2014;289:27215–27234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhao P, Lieu T, Barlow N, et al. Neutrophil elastase activates protease-activated receptor-2 (PAR2) and transient receptor potential vanilloid 4 (TRPV4) to cause inflammation and pain. J Biol Chem 2015;290:13875–13887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kenakin T Is the quest for signaling bias worth the effort? Mol Pharmacol 2018;93:266–269. [DOI] [PubMed] [Google Scholar]
- 61.Bohn LM, Gainetdinov RR, Lin FT, et al. Mu-opioid receptor desensitization by beta-arrestin-2 determines morphine tolerance but not dependence. Nature 2000; 408:720–723. [DOI] [PubMed] [Google Scholar]
- 62.Bohn LM, Lefkowitz RJ, Gainetdinov RR, et al. Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science 1999;286:2495–2498. [DOI] [PubMed] [Google Scholar]
- 63.Bohn LM, Raehal KM. Opioid receptor signaling: relevance for gastrointestinal therapy. Curr Opin Pharmacol 2006;6:559–563. [DOI] [PubMed] [Google Scholar]
- 64.Raehal KM, Schmid CL, Medvedev IO, et al. Morphine-induced physiological and behavioral responses in mice lacking G protein-coupled receptor kinase 6. Drug Alcohol Depend 2009;104:187–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Raehal KM, Walker JK, Bohn LM. Morphine side effects in beta-arrestin 2 knockout mice. J Pharmacol Exp Ther 2005;314:1195–1201. [DOI] [PubMed] [Google Scholar]
- 66.Akbarali HI, Inkisar A, Dewey WL. Site and mechanism of morphine tolerance in the gastrointestinal tract. Neurogastroenterol Motil 2014;26:1361–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kang M, Maguma HT, Smith TH, et al. The role of beta-arrestin2 in the mechanism of morphine tolerance in the mouse and guinea pig gastrointestinal tract. J Pharmacol Exp Ther 2012;340:567–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ross GR, Gabra BH, Dewey WL, et al. Morphine tolerance in the mouse ileum and colon. J Pharmacol Exp Ther 2008;327:561–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen X-T, Pitis P, Liu G, et al. Structure–activity relationships and discovery of a G protein biased µ opioid receptor ligand, [(3-methoxythiophen-2-yl)methyl]({2-[(9R)-9-(pyridin-2-yl)-6-oxaspiro-[4.5]decan-9-yl]ethyl}) amine (TRV130), for the treatment of acute severe pain. J Med Chem 2013;56:8019–8031. [DOI] [PubMed] [Google Scholar]
- 70.DeWire SM, Yamashita DS, Rominger DH, et al. A G protein-biased ligand at the mu-opioid receptor is potently analgesic with reduced gastrointestinal and respiratory dysfunction compared with morphine. J Pharmacol Exp Ther 2013;344:708–717. [DOI] [PubMed] [Google Scholar]
- 71.Singla N, Minkowitz HS, Soergel DG, et al. A randomized, Phase IIb study investigating oliceridine (TRV130), a novel micro-receptor G-protein pathway selective (mu-GPS) modulator, for the management of moderate to severe acute pain following abdominoplasty. J Pain Res 2017;10:2413–2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Soergel DG, Subach RA, Burnham N, et al. Biased agonism of the mu-opioid receptor by TRV130 increases analgesia and reduces on-target adverse effects versus morphine: a randomized, double-blind, placebo-controlled, crossover study in healthy volunteers. Pain 2014;155:1829–1835. [DOI] [PubMed] [Google Scholar]
- 73.Manglik A, Lin H, Aryal DK, et al. Structure-based discovery of opioid analgesics with reduced side effects. Nature 2016;537:185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schmid CL, Kennedy NM, Ross NC, et al. Bias factor and therapeutic window correlate to predict safer opioid analgesics. Cell 2017;171:1165–1175 e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Altarifi AA, David B, Muchhala KH, et al. Effects of acute and repeated treatment with the biased mu opioid receptor agonist TRV130 (oliceridine) on measures of antinociception, gastrointestinal function, and abuse liability in rodents. J Psychopharmacol 2017;31:730–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hill R, Disney A, Conibear A, et al. The novel mu-opioid receptor agonist PZM21 depresses respiration and induces tolerance to antinociception. Br J Pharmacol 2018;175:2653–2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Charfi I, Audet N, Bagheri Tudashki H, et al. Identifying ligand-specific signalling within biased responses: focus on delta opioid receptor ligands. Br J Pharmacol 2015; 172:435–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gallantine EL, Meert TF. A comparison of the antinociceptive and adverse effects of the mu-opioid agonist morphine and the delta-opioid agonist SNC80. Basic Clin Pharmacol Toxicol 2005;97:39–51. [DOI] [PubMed] [Google Scholar]
- 79.Eisenstein TK, Rahim RT, Feng P, et al. Effects of opioid tolerance and withdrawal on the immune system. J Neuroimmune Pharmacol 2006;1:237–249. [DOI] [PubMed] [Google Scholar]
- 80.Pradhan AA, Walwyn W, Nozaki C, et al. Ligand-directed trafficking of the delta-opioid receptor in vivo: two paths toward analgesic tolerance. J Neurosci 2010;30:16459–16468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Le Bourdonnec B, Windh RT, Ajello CW, et al. Potent, orally bioavailable delta opioid receptor agonists for the treatment of pain: discovery of N,N-diethyl-4-(5-hydroxyspiro[chromene-2,4ˊ-piperidine]-4-yl)benzamide (ADL5859). J Med Chem 2008;51:5893–5896. [DOI] [PubMed] [Google Scholar]
- 82.Le Bourdonnec B, Windh RT, Leister LK, et al. Spirocyclic delta opioid receptor agonists for the treatment of pain: discovery of N,N-diethyl-3-hydroxy-4-(spiro [chromene-2,4ˊ-piperidine]-4-yl) benzamide (ADL5747). J Med Chem 2009;52:5685–5702. [DOI] [PubMed] [Google Scholar]
- 83.Pin JP, Neubig R, Bouvier M, et al. International Union of Basic and Clinical Pharmacology. LXVII. Recommendations for the recognition and nomenclature of G protein-coupled receptor heteromultimers. Pharmacol Rev 2007; 59:5–13. [DOI] [PubMed] [Google Scholar]
- 84.Jones KA, Borowsky B, Tamm JA, et al. GABA(B) receptors function as a heteromeric assembly of the subunits GABA(B)R1 and GABA(B)R2. Nature 1998; 396:674–679. [DOI] [PubMed] [Google Scholar]
- 85.Kaupmann K, Malitschek B, Schuler V, et al. GABA(B)-receptor subtypes assemble into functional heteromeric complexes. Nature 1998;396:683–687. [DOI] [PubMed] [Google Scholar]
- 86.White JH, Wise A, Main MJ, et al. Heterodimerization is required for the formation of a functional GABA(B) receptor. Nature 1998;396:679–682. [DOI] [PubMed] [Google Scholar]
- 87.Kawakami S, Uezono Y, Makimoto N, et al. Characterization of GABA(B) receptors involved in inhibition of motility associated with acetylcholine release in the dog small intestine: possible existence of a heterodimer of GABA(B1) and GABA(B2) subunits. J Pharmacol Sci 2004;94:368–375. [DOI] [PubMed] [Google Scholar]
- 88.Torashima Y, Uezono Y, Kanaide M, et al. Presence of GABA(B) receptors forming heterodimers with GABA(B1) and GABA(B2) subunits in human lower esophageal sphincter. J Pharmacol Sci 2009;111:253–259. [DOI] [PubMed] [Google Scholar]
- 89.Hyland NP, Cryan JF. A gut feeling about GABA: focus on GABA(B) receptors. Front Pharmacol 2010;1:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lehmann A, Jensen JM, Boeckxstaens GE. GABAB receptor agonism as a novel therapeutic modality in the treatment of gastroesophageal reflux disease. Adv Pharmacol 2010;58:287–313. [DOI] [PubMed] [Google Scholar]
- 91.Bulenger S, Marullo S, Bouvier M. Emerging role of homo- and heterodimerization in G-protein–coupled receptor biosynthesis and maturation. Trends Pharmacol Sci 2005;26:131–137. [DOI] [PubMed] [Google Scholar]
- 92.Terrillon S, Bouvier M. Roles of G-protein–coupled receptor dimerization. EMBO Rep 2004;5:30–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kuszak AJ, Pitchiaya S, Anand JP, et al. Purification and functional reconstitution of monomeric mu-opioid receptors: allosteric modulation of agonist binding by Gi2. J Biol Chem 2009;284:26732–26741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Huang W, Manglik A, Venkatakrishnan AJ, et al. Structural insights into micro-opioid receptor activation. Nature 2015;524:315–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.He L, Fong J, von Zastrow M, et al. Regulation of opioid receptor trafficking and morphine tolerance by receptor oligomerization. Cell 2002;108:271–282. [DOI] [PubMed] [Google Scholar]
- 96.Gomes I, Gupta A, Filipovska J, et al. A role for heterodimerization of mu and delta opiate receptors in enhancing morphine analgesia. Proc Natl Acad Sci U S A 2004;101:5135–5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Scherrer G, Imamachi N, Cao YQ, et al. Dissociation of the opioid receptor mechanisms that control mechanical and heat pain. Cell 2009;137:1148–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Erbs E, Faget L, Scherrer G, et al. A mu-delta opioid receptor brain atlas reveals neuronal co-occurrence in subcortical networks. Brain Struct Funct 2015;220:677–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang D, Tawfik VL, Corder G, et al. Functional divergence of delta and mu opioid receptor organization in CNS pain circuits. Neuron 2018;98:90–108 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fujita W, Gomes I, Devi LA. Heteromers of mu-delta opioid receptors: new pharmacology and novel therapeutic possibilities. Br J Pharmacol 2015;172:375–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhu Y, King MA, Schuller AG, et al. Retention of supraspinal delta-like analgesia and loss of morphine tolerance in delta opioid receptor knockout mice. Neuron 1999;24:243–252. [DOI] [PubMed] [Google Scholar]
- 102.Egan TM, North RA. Both mu and delta opiate receptors exist on the same neuron. Science 1981;214:923–924. [DOI] [PubMed] [Google Scholar]
- 103.Poole DP, Pelayo JC, Scherrer G, et al. Localization and regulation of fluorescently labeled delta opioid receptor, expressed in enteric neurons of mice. Gastroenterology 2011;141:982–991 e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Guerrero-Alba R, Valdez-Morales EE, Jimenez-Vargas NN, et al. Co-expression of mu and delta opioid receptors by mouse colonic nociceptors. Br J Pharmacol 2018;175:2622–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fujita W, Gomes I, Dove LS, et al. Molecular characterization of eluxadoline as a potential ligand targeting mudelta opioid receptor heteromers. Biochem Pharmacol 2014;92:448–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dove LS, Lembo A, Randall CW, et al. Eluxadoline benefits patients with irritable bowel syndrome with diarrhea in a phase 2 study. Gastroenterology 2013; 145:329–338 e1. [DOI] [PubMed] [Google Scholar]
- 107.Fant RV, Henningfield JE, Cash BD, et al. Eluxadoline demonstrates a lack of abuse potential in phase 2 and 3 studies of patients with irritable bowel syndrome with diarrhea. Clin Gastroenterol Hepatol 2017;15:1021–1029 e6. [DOI] [PubMed] [Google Scholar]
- 108.Bowden JJ, Garland AM, Baluk P, et al. Direct observation of substance P-induced internalization of neurokinin 1 (NK1) receptors at sites of inflammation. Proc Natl Acad Sci U S A 1994;91:8964–8968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Poole DP, Lieu T, Pelayo JC, et al. Inflammation-induced abnormalities in the subcellular localization and trafficking of the neurokinin 1 receptor in the enteric nervous system. Am J Physiol Gastrointest Liver Physiol 2015; 309:G248–G259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mantyh PW, DeMaster E, Malhotra A, et al. Receptor endocytosis and dendrite reshaping in spinal neurons after somatosensory stimulation. Science 1995; 268:1629–1632. [DOI] [PubMed] [Google Scholar]
- 111.Steinhoff MS, von Mentzer B, Geppetti P, et al. Tachykinins and their receptors: contributions to physiological control and the mechanisms of disease. Physiol Rev 2014;94:265–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Deddish PA, Marcic BM, Tan F, et al. Neprilysin inhibitors potentiate effects of bradykinin on b2 receptor. Hypertension 2002;39:619–623. [DOI] [PubMed] [Google Scholar]
- 113.Okamoto A, Lovett M, Payan DG, et al. Interactions between neutral endopeptidase (EC 3.4.24.11) and the substance P (NK1) receptor expressed in mammalian cells. Biochem J 1994;299:683–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sturiale S, Barbara G, Qiu B, et al. Neutral endopeptidase (EC 3.4.24.11) terminates colitis by degrading substance P. Proc Natl Acad Sci U S A 1999;96:11653–11658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lu B, Figini M, Emanueli C, et al. The control of micro-vascular permeability and blood pressure by neutral endopeptidase. Nat Med 1997;3:904–907. [DOI] [PubMed] [Google Scholar]
- 116.Peterson YK, Luttrell LM. The diverse roles of arrestin scaffolds in G protein-coupled receptor signaling. Pharmacol Rev 2017;69:256–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Williams JT, Ingram SL, Henderson G, et al. Regulation of mu-opioid receptors: desensitization, phosphorylation, internalization, and tolerance. Pharmacol Rev 2013; 65:223–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Calebiro D, Nikolaev VO, Gagliani MC, et al. Persistent cAMP-signals triggered by internalized G-protein–coupled receptors. PLoS Biol 2009;7:e1000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ferrandon S, Feinstein TN, Castro M, et al. Sustained cyclic AMP production by parathyroid hormone receptor endocytosis. Nat Chem Biol 2009;5:734–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Irannejad R, von Zastrow M. GPCR signaling along the endocytic pathway. Curr Opin Cell Biol 2014;27:109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tsvetanova NG, von Zastrow M. Spatial encoding of cyclic AMP signaling specificity by GPCR endocytosis. Nat Chem Biol 2014;10:1061–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Buxton IL, Brunton LL. Compartments of cyclic AMP and protein kinase in mammalian cardiomyocytes. J Biol Chem 1983;258:10233–10239. [PubMed] [Google Scholar]
- 123.Berridge MJ. Calcium microdomains: organization and function. Cell Calcium 2006;40:405–412. [DOI] [PubMed] [Google Scholar]
- 124.Halls ML, Canals M. Genetically encoded FRET bio-sensors to illuminate compartmentalised GPCR signalling. Trends Pharmacol Sci 2018;39:148–157. [DOI] [PubMed] [Google Scholar]
- 125.Willoughby D, Halls ML, Everett KL, et al. A key phosphorylation site in AC8 mediates regulation of Ca(2+)-dependent cAMP dynamics by an AC8-AKAP79-PKA signalling complex. J Cell Sci 2012; 125:5850–5859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.DeFea KA, Vaughn ZD, O’Bryan EM, et al. The proliferative and antiapoptotic effects of substance P are facilitated by formation of a beta-arrestin–dependent scaffolding complex. Proc Natl Acad Sci U S A 2000; 97:11086–11091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Moon C, Zhang W, Ren A, et al. Compartmentalized accumulation of cAMP near complexes of multidrug resistance protein 4 (MRP4) and cystic fibrosis transmembrane conductance regulator (CFTR) contributes to drug-induced diarrhea. J Biol Chem 2015;290:11246–11257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Oakley RH, Laporte SA, Holt JA, et al. Association of beta-arrestin with G protein-coupled receptors during clathrin-mediated endocytosis dictates the profile of receptor resensitization. J Biol Chem 1999;274:32248–32257. [DOI] [PubMed] [Google Scholar]
- 129.Oakley RH, Laporte SA, Holt JA, et al. Differential affinities of visual arrestin, beta arrestin1, and beta arrestin2 for G protein-coupled receptors delineate two major classes of receptors. J Biol Chem 2000;275:17201–17210. [DOI] [PubMed] [Google Scholar]
- 130.Schmidlin F, Dery O, Bunnett NW, et al. Heterologous regulation of trafficking and signaling of G protein-coupled receptors: beta-arrestin–dependent interactions between neurokinin receptors. Proc Natl Acad Sci U S A 2002;99:3324–3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cattaruzza F, Cottrell GS, Vaksman N, et al. Endothelin-converting enzyme 1 promotes re-sensitization of neurokinin 1 receptor-dependent neurogenic inflammation. Br J Pharmacol 2009;156:730–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Padilla BE, Cottrell GS, Roosterman D, et al. Endothelin-converting enzyme-1 regulates endosomal sorting of calcitonin receptor-like receptor and beta-arrestins. J Cell Biol 2007;179:981–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pelayo JC, Poole DP, Steinhoff M, et al. Endothelin-converting enzyme-1 regulates trafficking and signalling of the neurokinin 1 receptor in endosomes of myenteric neurones. J Physiol 2011;589:5213–5230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Roosterman D, Cottrell GS, Padilla BE, et al. Endothelin-converting enzyme 1 degrades neuropeptides in endosomes to control receptor recycling. Proc Natl Acad Sci U S A 2007;104:11838–11843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jensen DD, Halls ML, Murphy JE, et al. Endothelin-converting enzyme 1 and beta-arrestins exert spatiotemporal control of substance P-induced inflammatory signals. J Biol Chem 2014;289:20283–20294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhao P, Canals M, Murphy JE, et al. Agonist-biased trafficking of somatostatin receptor 2A in enteric neurons. J Biol Chem 2013;288:25689–25700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Roosterman D, Kempkes C, Cottrell GS, et al. Endothelin-converting enzyme-1 degrades internalized somatostatin-14. Endocrinology 2008;149:2200–2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Oberg KE, Reubi JC, Kwekkeboom DJ, et al. Role of somatostatins in gastroenteropancreatic neuroendocrine tumor development and therapy. Gastroenterology 2010;139:742–753 e1. [DOI] [PubMed] [Google Scholar]
- 139.Cahill TJ III, Thomsen AR, Tarrasch JT, et al. Distinct conformations of GPCR-beta-arrestin complexes mediate desensitization, signaling, and endocytosis. Proc Natl Acad Sci U S A 2017;114:2562–2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Thomsen ARB, Plouffe B, Cahill TJ III, et al. GPCR-G protein-beta-arrestin super-complex mediates sustained g protein signaling. Cell 2016;166:907–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cenac N, Andrews CN, Holzhausen M, et al. Role for protease activity in visceral pain in irritable bowel syndrome. J Clin Invest 2007;117:636–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rolland-Fourcade C, Denadai-Souza A, Cirillo C, et al. Epithelial expression and function of trypsin-3 in irritable bowel syndrome. Gut 2017;66:1767–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Brimblecombe RW, Duncan WA, Durant GJ, et al. The pharmacology of cimetidine, a new histamine H2-receptor antagonist. Br J Pharmacol 1975;53:435P–436P. [PMC free article] [PubMed] [Google Scholar]
- 144.Wacker D, Stevens RC, Roth BL. How ligands illuminate GPCR molecular pharmacology. Cell 2017;170:414–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Paek J, Kalocsay M, Staus DP, et al. Multidimensional tracking of GPCR signaling via peroxidase-catalyzed proximity labeling. Cell 2017;169:338–349 e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Driehuis E, Clevers H. CRISPR/Cas 9 genome editing and its applications in organoids. Am J Physiol Gastrointest Liver Physiol 2017;312:G257–G265. [DOI] [PubMed] [Google Scholar]
- 147.Gulbransen BD. Emerging tools to study enteric neuro-muscular function. Am J Physiol Gastrointest Liver Physiol 2017;312:G420–G426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhu H, Roth BL. DREADD: a chemogenetic GPCR signaling platform. Int J Neuropsychopharmacol 2014; 18(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.McClain JL, Fried DE, Gulbransen BD. Agonist-evoked Ca(2 ) signaling in enteric glia drives neural programs that regulate intestinal motility in mice. Cell Mol Gastroenterol Hepatol 2015;1:631–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Grubisic V, Gulbransen BD. Enteric glial activity regulates secretomotor function in the mouse colon but does not acutely affect gut permeability. J Physiol 2017; 595:3409–3424. [DOI] [PMC free article] [PubMed] [Google Scholar]