Abstract
G-protein-coupled receptors (GPCRs) are conventionally considered to function at the plasma membrane, where they detect extracellular ligands and activate heterotrimeric G proteins that transmit intracellular signals. Consequently, drug discovery efforts have focused on identification of agonists and antagonists of cell surface GPCRs. However, β-arrestin (ARR)-dependent desensitization and endocytosis rapidly terminate G protein signaling at the plasma membrane. Emerging evidence indicates that GPCRs can continue to signal from endosomes by G-protein- and βARR-dependent processes. By regulating the duration and location of intracellular signaling events, GPCRs in endosomes control critically important processes, including gene transcription and ion channel activity. Thus, GPCRs in endosomes, in addition to at the cell surface, have emerged as important therapeutic targets.
Revision of the Plasma-Membrane-Centric View of GPCR Signaling
With almost 1000 members in the human genome, G-protein-coupled receptors (GPCRs, see Glossary) are the largest family of transmembrane signaling proteins [1]. Photons, odorants, tastants, and many hormones and neurotransmitters activate GPCRs, which control most physiological and pathological processes. Over one-third of marketed drugs target GPCRs [2].
GPCRs are conventionally considered to function primarily at the plasma membrane, and drug discovery efforts target cell surface receptors. GPCRs at the plasma membrane interact with extracellular ligands and undergo a conformational change that promotes association with intracellular heterotrimeric G proteins (Gαβγ), which induces guanine nucleotide exchange, G protein activation, and dissociation of Gα and Gβγ subunits [1]. G protein subunits in turn regulate downstream enzymes and effectors that control many cellular processes [1]. GPCR signaling at the plasma membrane is tightly regulated and often transient. GPCR kinases (GRKs) phosphorylate activated GPCRs, thereby enhancing their affinity for β-arrestins (βARRs) [3,4]. βARRs bind phosphorylated GPCRs in two stages: first to phosphorylated residues within the receptor C-terminal tail, and second within the transmembrane core [5]. Since the binding site of βARRs in the receptor core overlaps with the G-protein-binding site, βARR recruitment sterically hinders further G protein activation, which desensitizes G protein signaling [5–7]. βARRs also couple GPCRs to clathrin and adaptor protein-2 (AP2), which mediate endocytosis of GPCRs, removing them from the site of activation [4]. The extent of GRK-mediated phosphorylation of GPCRs is a major determinant of the stability of GPCR/βARR complexes, which largely governs the fate of internalized GPCRs. GPCRs with few GRK phosphorylation sites (Class A GPCRs) interact with βARR in endosomes with low affinity and transiently, and can rapidly recycle back to the plasma membrane. In contrast, GPCRs with many, often clustered, GRK phosphorylation sites (Class B GPCRs) exhibit sustained high-affinity interactions with βARR in endosomes, and either slowly recycle or traffic to lysosomes for degradation [8,9]. Ligand stability in endosomes also determines the fate of endosomal GPCRs. By degrading neuropeptide ligands in acidified endosomes, endothelin-converting enzyme-1 can destabilize neuropeptide/GPCR/ARR complexes and promote receptor recycling [10,11].
This plasma-membrane-centric view of GPCRs is incomplete since many activated GPCRs traffic, with their ligands, to endosomes, where they can continue to signal by βARR- and G-protein-dependent mechanisms [12–14]. The concept that GPCRs can signal from endosomes raises important questions. If βARRs uncouple GPCRs from G proteins at the plasma membrane, how do GPCRs continue to activate G proteins in endosomes? What is the contribution of GPCR signaling at the plasma membrane and in endosomes to complex pathophysiological processes? How is endosomal signaling of GPCRs regulated and can this be exploited for therapy? Are GPCRs in endosomes, rather than at the cell surface, the key therapeutic target? This article reviews the latest developments in endosomal GPCR signaling, and discusses how this information can be used to design more selective and potentially effective drugs.
Paradox of G Protein- and βARR-Dependent Signaling of GPCRs in Endosomes
By recruiting GPCRs and signaling partners, including components of mitogen-activated protein kinase cascades, to endosomes, βARRs are key mediators of endosomal signaling of GPCRs [4]. However, G proteins, in addition to βARRs, also mediate signaling of GPCRs in endosomes. Signaling of G proteins in endosomes was first discovered for the yeast G protein, Gpa1 [15]. A growing number of GPCRs have been shown to activate Gαs, Gαi/o, and Gαq in endosomes of mammalian cells, including the parathyroid hormone receptor (PTHR), vasopressin type 2 receptor (V2R), thyroid stimulating hormone receptor (TSHR), luteinizing hormone receptor (LHR), sphingosine-1-phosphate 1 receptor (S1PR1), neurokinin type 1 receptor (NK1R), calcitonin receptor-like receptor (CLR), and C-C chemokine receptor-1 (CCR1) [12,14,16–22]. However, the concept of endosomal G protein signaling was met with skepticism because βARRs compete with G proteins for GPCR binding sites and, once recruited, βARRs would be expected to terminate G protein signaling [4,7]. High-resolution structural studies have confirmed overlapping binding sites for visual arrestin and transducin (Gt protein) at rhodopsin [6,7,23]. Thus, it would seem impossible that GPCR/βARR complexes could stimulate G protein signaling from endosomes.
Despite this paradox, class B GPCRs (e.g., PTHR, V2R, and TSHR), which form stable endosomal complexes with βARRs through phosphorylation site clusters within the receptor C-terminal tail, stimulate G protein signaling that, unlike Class A GPCRs, is insensitive to agonist wash-out or cell impermeable antagonists [16–19,21,24–26]. In addition, fluorescence microscopy has demonstrated that these internalized and βARR-associated GPCRs colocalize with G proteins in endosomes [12,17,24,25]. Disrupting the Class B GPCR C-terminal tail-βARR interaction markedly diminishes the ability of these GPCRs to stimulate endosomal G protein signaling, which suggests a role for βARRs in directing these signals. For example, deletion of NK1R C terminus or antagonism of NK1R/βARR interactions prevents βARR recruitment and internalization, as well as endosomal signaling outputs [12]. The correlation between a strong βARR/GPCR interaction and endosomal G protein signaling was confirmed by exchanging the C-terminal tail of the Class A β2-adrenergic receptor (β2AR) with the C-tail of the Class B V2R, which enhanced interactions of the chimera β2V2R with βARRs and magnified G protein signaling from endosomes [24,27]. Moreover, coexpression of constitutively active βARR1 enhances sustained endosomal G protein signaling of the PTHR and V2R, whereas the siRNA knockdown of βARR1/2 has the opposite effect [18,25,26]. Thus, not only do βARRs fail to desensitize G protein signaling from endosomal GPCRs, they can also enhance endosomal G protein signaling. How is this possible given the conventional view that βARRs uncouple GPCRs and G proteins?
Recent biophysical approaches have provided a mechanistic understanding of endosomal G protein signaling (Figure 1). Investigation of the structure of a β2V2R/βARR1 complex using negative stain electron microscopy revealed that the β2V2R/βARR1 complex adopts at least two conformations: (i) a tail conformation, where βARR1 binds β2V2R through the phosphorylated receptor C tail; and (ii) a core conformation, where βARR1, in addition to binding to the C tail, also binds to the β2V2R transmembrane core through a flexible region within βARR1 (fingerloop region, FLR), which sterically blocks the G-protein-binding site [5]. This core conformation is analogous to high-resolution structures of the rhodopsin-visual arrestin complex [6,7], When complexed in the core conformation, βARR1 desensitizes G protein signaling in the expected manner, but when complexed in the tail conformation, G protein signaling could proceed normally [27].
Figure 1. Conformations of GPCR/βARR Complex.
(A) GPCRs interact with G proteins through their transmem-brane core region to stimulate G protein signaling. (B, C) Upon phosphorylation of the receptor C-terminal tail, GPCRs form complexes with βARR in two different conformations: one in which the βARR is bound only to the phosphorylated receptor C-terminal tail and appears to hang from the receptor (tail conformation, B); and a second more fully engaged conformation where, in addition to the tail interaction, a flexible loop in βARR, termed the fingerloop, inserts into the transmembrane core of the receptor (core conformation, C). Since the insertion of the βARR fingerloop into the receptor in the core conformation sterically blocks the G-protein-binding site, G protein activation is terminated. However, since βARR does not occupy the receptor G-protein-binding site in the tail conformation, GPCRs can internalize in complex with βARR and still stimulate G protein signaling from internalized compartments such as endosomes. Abbreviations: βARR, β-arrestin; GPCR, G-protein-coupied receptor.
The unexpected finding that βARRs can interact with GPCRs via the phosphorylated C tail only, as well as in the core conformation, has been further investigated using a βARR1 mutant, βARR1 (∆FLR), which lacks the FLR that normally interacts with the receptor core. The β2V2R/ βARRI(∆FLR) complex exclusively adopts the tail conformation, and removal of the FLR abolishes its function to desensitize G protein signaling. However, the mutant maintains its ability to promote receptor internalization and βARR-mediated signaling [27]. Other studies have confirmed the role of the βARR tail conformation in GPCR internalization and signaling [28–31]. Notably, only Class B GPCRs with phosphorylation site clusters in their C tail form stable tail conformation complexes with βARR(∆FLR), suggesting that Class A GPCR are dependent on the core interaction [27].
These findings can explain the paradoxical role of βARRs as negative regulators of G protein signaling at the plasma membrane, and positive regulators of G protein signaling in endosomes: when βARRs binds GPCRs only through the C tail to promote internalization, the entire receptor core region is exposed, and thus βARRs do not block the G-protein-binding site. Therefore, the receptor maintains its ability to couple to G proteins in endosomes [5,27–29]. In support of this concept, Class B GPCRs including PTHR, V2R, CCR1, TSHR, and Y1R promote close proximity between G proteins and βARR upon activation [18,19,22,25,30]. Furthermore, Class B GPCRs, which form GPCR/βARR tail conformation complexes, have been directly shown to interact simultaneously with both G proteins and βARRs to form GPCR/G protein/βARR ‘megaplexes’ [18,24,27,30]. The structural architecture of a megaplex consisting of β2V2R/ Gs/βARR1 has been investigated by electron microscopy, and shows that the GPCR within megaplexes couple with G proteins at the receptor core while interacting with βARRs through the C tail [24]. The existence of megaplexes can explain how Class B GPCRs can stimulate G protein signaling while being internalized to endosomes by βARR [24].
Although endosomal signaling has been mostly described for Class B GPCRs, class A GPCRs can also signal from endosomes, albeit by distinct mechanisms. The recruitment of βARRs to Class A GPCRs, such as the β2AR, terminates G protein signaling by occluding the G protein-binding site [20,27]. However, following βARR-mediated receptor internalization, Class A GPCRs can activate a second wave of G protein signaling from endosomes, which appears to proceed after βARR dissociates from the receptor [20]. In contrast to the large and sustained G protein signals that emanate from Class B GPCRs in endosomes, endosomal G protein signaling of Class A GPCRs is modest and transient.
The assembly of a GPCR/βARR complex, which is stabilized by interaction of βARR with the phosphorylated C tail of the receptor, is generally considered to be necessary for endosomal signaling of GPCRs. This long-held view has been challenged by the discovery of a mechanism of βARR activation that does not require a stable GPCR/βARR complex or the receptor C tail. Atomic level simulations of ARR/GPCR interactions indicate that the transmembrane core and C tail of the receptor, which bind to distinct surfaces of ARR, can independently activate ARR [32]. In the case of the β1AR and β2AR, the transient engagement of βARR with the receptor core can promote the accumulation of βARR within clathrin-coated endocytic structures after dissociation from the receptor [33]. The sequestration of βARR in these structures is associated with βARR-mediated activation of extracellular signal-regulated kinase (ERK), without the engagement with an activated receptor.
GPCRs in Endosomes Control Signaling in Time and Space
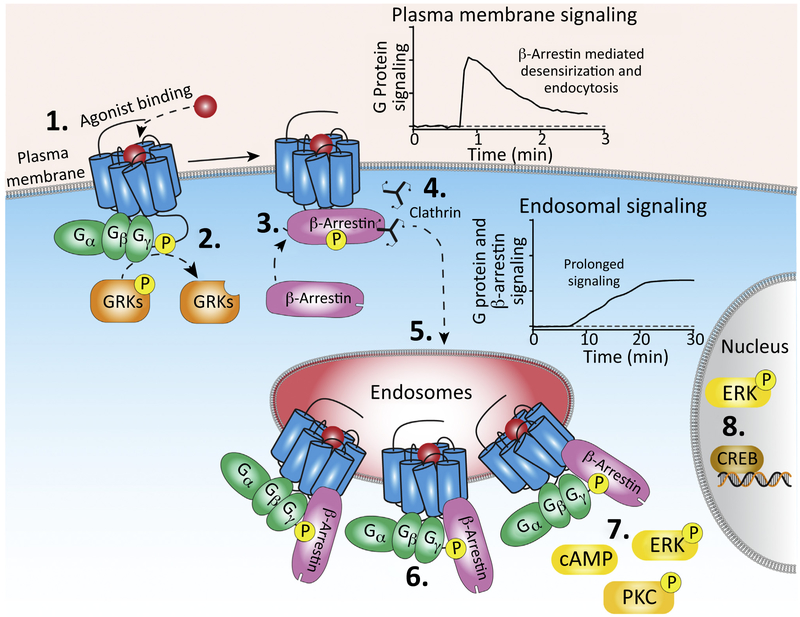
βARR-dependent desensitization and endocytosis of GPCRs ensure that signaling at the plasma membrane is fleeting and largely confined to the regulation of plasma-membrane-delimited events (e.g., activation of membrane-associated enzymes, growth factor receptors, and ion channels) (Figure 2, Key Figure). In contrast, GPCR signaling in endosomes can be sustained by βARR- and G-protein-dependent processes. Given the dynamic nature of the tubulovesicular endosomal network, which ramifies throughout the cytosol, GPCRs in endosomes can generate signals in various subcellular compartments. Compartmentalized signaling may explain how GPCRs, which often activate a common set of G proteins and downstream effectors, can selectively regulate cellular responses.
Figure 2. Compartmentalized Signaling GPCRs in Endosomes.
1. Agonist binding at the plasma membrane stabilizes GPCRs in an active conformation. GPCRs interact with and activate heterotrimeric G proteins, which induce signaling. 2. GPCR kinases phosphorylate C-terminal domains of GPCRs. 3. Recruitment of βARRs to the phosphorylated GPCR. In addition to the C-terminal tail, βARRs also bind the GPCR core, which sterically hinders G protein binding and thereby terminates G protein signaling. 4. βARRs scaffold clathrin and AP2 to mediate GPCR endocytosis. 5. GPCRs continue to signal in endosomes. 6. GPCRs that promote prolonged endosomal G protein signaling can interact with βARRs in the tail conformation. Since βARR does not occupy the G-protein-binding site when in complex with a GPCR in the tail conformation, the receptor can form GPCR/G protein/βARR complex that continues to stimulate G protein signaling. 7. G-protein- and βARR-dependent signaling from endosomes leads to generation of second messengers such as cAMP, and phosphorylation of signaling proteins including PKC and ERK1/2, which can regulate nuclear events (8). Abbreviations: AP2, adaptor protein-2; βARR, β-arrestin; CREB, cAMP response element binding protein; ERK, extracellular signal-regulated kinase; GPCR, G-protein-coupled receptor; GRK, G-protein-coupled receptor kinase; PKC, protein kinase C.
The use of inhibitors of endocytosis, coupled with biophysical approaches to study GPCR trafficking and signaling with high spatial and temporal resolution, have provided insights into the link between trafficking and signaling of GPCRs. Disruption of clathrin or dynamin inhibits substance P (SP)-evoked endocytosis of the NK1R, and suppress activation of cytosolic cAMP and protein kinase C (PKC) and nuclear ERK [12]. In contrast, these treatments do not affect SP stimulation of cAMP at the plasma membrane or ERK in the cytosol, and enhance PKC activation at the plasma membrane. Thus, the NK1R in early endosomes activates cAMP and PKC in the cytosol and ERK in the nucleus, which proceed by βARR- and G-protein-dependent mechanisms. The receptor for calcitonin gene-related peptide (CGRP), CLR, signals from endosomes in a similar manner [14]. Barbadin, an inhibitor of βARR/AP2 interactions, blocks agonist-induced endocytosis of the β2AR and V2R, prevents V2R-dependent ERK activation, and inhibits β2AR- and V2R-mediated cAMP production [34]. Endosomal signaling of PTHR, TSHR, and β2AR also drives cytosolic cAMP production [35]. Signaling of GPCRs in endosomes may underlie long-term effects, since the β2AR and NK1R in endosomes regulate cAMP- and ERK1/2-dependent regulation of transcription [12,35]. Disruption of the endosomal transmembrane peptidase endothelin-converting enzyme-1, which degrades SP in endosomes and destabilizes the NK1 R/βARR complex [11], leads to enhanced activation of nuclear ERK [36].
Studies of endosomal signaling of receptor tyrosine kinases (RTKs), notably the epidermal growth factor receptor (EGFR), have also provided insights into the mechanisms by which different GPCRs can selectively regulate cellular responses. RTKs, like GPCRs, can internalize by clathrin- and dynamin-mediated mechanisms, and can continue to signal from endosomes. The answer to the quandary of how different growth factors can elicit divergent cellular responses by engaging the same signaling machinery may lie in the mechanisms by which the EGFR signals from endosomes [37]. The use of quantitative high-resolution microscopy has revealed that the phosphorylated EGFR is packaged into discrete amounts or quanta in endosomes; cells respond to increasing amounts of EGF by increasing the number of endosomes, each with a constant amount of phosphorylated EGFR. By altering the number of endosomes containing phosphorylated EGFR, different growth factors can differentially control the cellular response. Thus, the packaging of phosphorylated EGFR in endosomes ensures the fidelity of signaling [37]. This quantal packaging of receptors has not been shown with GPCRs but does provide a possible framework to understand how increased internalization and endosomal signaling of GPCRs can regulate cellular responses.
Given the heterogeneous nature of the endosomal network, it is not surprising that certain subcompartments are particularly important sites of GPCR signaling. For example, very early endosomes (VEEs), which lack markers for early endosomes (e.g., Rab5a, phosphatidylino-sitol-3), are sites of sustained LHR-dependent ERK signaling. The adaptor protein containing pleckstrin homology domain, phosphotyrosine-binding domain, and leucine zipper motif 1 (APPL1) regulates LHR sorting and signaling within VEEs, since APPL1 knockdown causes LHR retention in VEEs and enhanced cAMP responses [38]. Thus, APPL1 mediates rapid LHR recycling from VEEs to the plasma membrane, and APPL1 is a negative regulator of LHR-mediated cAMP production from VEEs.
GPCRs can translocate from endosomes to other organelles, where they can continue to signal. The S1PR1 and TSHR receptor retrogradely traffic from endosomes to the trans-Golgi network (TGN). S1PR1 generates sustained Gαi-dependent signals from the TGN [16] and, once trafficked to the TGN and positioned next to the nucleus, TSHR activates Gαs, resulting in cAMP accumulation and the activation of the cAMP response element binding protein (CREB) within the nucleus [39]. TSHR in the TGN also generates a delayed yet sustained PKA response. Inhibition of TSHR endocytosis or disruption of the TGN abrogates cAMP and PKA responses. Thus, GPCRs shuttle between the plasma membrane, endosomes and TGN, and each of these subcellular domains provides a platform to modify and fine-tune cellular responses to extracellular stimuli.
Contribution of Endosomal Signaling of GPCRs to Pathophysiological Control
Recent observations suggest that endosomal signaling of GPCRs regulates important physiological and pathophysiological processes in intact tissues and animals. The NK1R mediates SP-dependent pain transmission by second-order neurons in the dorsal horn of the spinal cord. Painful stimuli of peripheral tissues, for example, intraplantar injection (injection into the foot) of capsaicin, formalin, or complete Freund’s adjuvant, trigger clathrin- and dynamin-dependent endocytosis of the NK1R in spinal neurons, which is attributable to the release of SP from the central projections of nociceptors in the dorsal horn [12]. NK1R endocytosis correlates with activation of ERK½ in spinal neurons [12]. The intrathecal injection of pharmacological inhibitors of dynamin and clathrin, or of dynamin-1 of βARR siRNA, inhibits NK1R endocytosis and ERK1/2 activation in spinal neurons, and suppresses mechanical nociception in response to intraplantar injection of capsaicin, formalin or complete Freund’s adjuvant. Although disruption of dynamin and clathrin can affect the trafficking of many receptors and channels, selective inhibitors of NK1R/βARR interactions also prevent NK1R endocytosis and inhibit nociception in mice. Electrophysiological studies in slice preparations of rat spinal cord provide insights into the link between NK1R endocytosis and pain transmission. Transient stimulation with SP induces a rapid-onset firing of action potentials that persists after washout and is accompanied by NK1R endocytosis. Inhibitors of dynamin and of signals that derive from the NK1R in endosomes (PKC and ERK) prevent sustained SP-induced excitation of spinal neurons, which requires persistent signaling of NK1R from endosomes [12]. The CGRP receptor, CLR, is coexpressed on spinal neurons with the NK1R, and also undergoes clathrin- and dynamin-dependent endocytosis [14]. Dynamin inhibitors, and inhibitors of ERK and PKC, similarly prevent the sustained actions of CGRP in excitation of spinal neurons. Studies with NK1R and CLR antagonists that are targeted to endosomes, which is described in ‘Therapeutic Targeting of GPCRs in Subcellular Compartments’ section, reinforce the concept that endosomes are platforms for sustained SP- and CGRP-mediated pain transmission.
Pituitary adenylate cyclase 1 receptor (PAC1) provides another example of the importance of endosomal signaling in regulating neuronal excitability. PAC1 signaling from endosomes is necessary for MEK/ERK activation in cardiac ganglia neurons [40] and inhibitors of endocytosis block pituitary adenylate cyclase-activating peptide (PACAP)-induced increases in neuronal excitability [41]. In the amygdala, endosomal signaling of PAC1 is necessary for neuronal activation and nociceptive responses [42].
Endosomal signaling of GPCRs may contribute to human disease [43]. Irritable bowel syndrome (IBS) is characterized by abdominal pain and disrupted bowel habits (constipation and diarrhea). Proteases derived from colonocytes and immune cells that infiltrate the colon wall (e.g., mast cells, macrophages, and neutrophils), and protease-activated receptor-2 (PAR2), a GPCR that is expressed by primary sensory neurons or nociceptors, are strongly implicated in IBS pain. Proteases released from biopsies of colon from IBS patients cause a sustained hyperexcitability of nociceptors, a hallmark of chronic pain, and inhibitors of clathrin- and dynamin-dependent endocytosis, and an antagonist of endosomal PAR2 (described in the section ‘Therapeutic Targeting of GPCRs in Subcellular Compartments’) prevent excitability of nociceptors, and may represent a novel therapy for IBS pain [43]. However, different proteases can activate PAR2 and cause pain by distinct signaling mechanisms. Proteases such as trypsin and mast cells tryptase cleave and activate PAR2 by canonical mechanisms, which include coupling to Gαq, recruitment of βARRs, and receptor endocytosis. Inhibitors of dynamin and clathrin-dependent endocytosis and of ERK activation prevent sustained trypsin-induced hyperexcitability of nociceptors and pain, which presumably require PAR2 endocytosis and endosomal signaling to activate ERK [43]. ERK in turn then regulates ion channels that mediate neuronal hyperexcitability. In contrast, neutrophil elastase and macrophage cathepsin S cleave PAR2 at different sites and activate the receptor by biased mechanisms, which include coupling to Gαs, but not βARRs nor endocytosis. Inhibitors of dynamin and clathrin do not affect sustained elastase- and cathepsin-S-evoked nociceptor hyperexcitability or pain, which instead depend on PAR2 signaling from the plasma membrane, activation of adenylyl cyclase, and cAMP-mediated activation of protein kinase A [43]. These studies provide insights into the relative contributions of signals that emanate from GPCRs at the plasma membrane and in endosomes to pain transmission. They show that the same GPCR can signal by divergent pathways from distinct subcellular domains (plasma membrane or endosomes) to induce a physiological response.
Thus, GPCRs in endosomes signal by mechanisms that are distinct from those that originate from GPCRs at plasma membrane, and can control unique physiological and pathological processes. A better understanding of the signaling events that are regulated by GPCRs at plasma and endosomal membranes, and of the contribution of these signals to the control of physiological and pathophysiological responses, may enable the design of more selective and effective therapies for GPCR-regulated diseases.
Therapeutic Targeting of GPCRs in Subcellular Compartments
The discovery that GPCRs in endosomes can generate sustained signals in subcellular compartments that underlie important pathophysiological processes has implications for therapy with antagonists and agonists. In situations where endosomal signaling mediates disease-relevant processes, for example, chronic pain, antagonists that target GPCRs in endosomes may be a superior therapeutic approach compared with agents that act primarily at the plasma membrane. Conversely, the therapeutic efficacy of agonists of GPCRs could be enhanced by improving their capacity to generate sustained signals from endosomes.
Endosomally Biased GPCR Agonists
Endogenous agonists that stimulate G protein signaling from endosomes tend to bind GPCRs tightly. These agonists include peptides or small proteins that remain associated with GPCRs even in the acidified endosomal lumen. The tightagonist/GPCR association maintains the receptorinan active state within endosomes, and thereby enables continued G protein signaling.
The correlation between tight agonist/GPCR association and sustained endosomal signaling is illustrated by the PTHR, for which there are several peptide agonists. PTH(1–34) displays an exceptional ability to stabilize an active state and remain associated with PTHR for prolonged periods, whereas PTH-related protein [PTHrP(1–36)] rapidly dissociates, especially when PTHR is not complexed with G proteins [17,44]. PTH(1–34) remains associated with PTHR in endosomes, leading to sustained endosomal signaling. In contrast, PTHrP(1–36) dissociates from PTHR soon after internalization; in consequence, there is minimal endosomal signaling before the PTHR recycles. The ability of parathyroid hormone (PTH), but not PTHrP, to promote sustained endosomal signaling may account for enhanced renal production of 1,25- dihydroxy-vitamin D3 and resulting increases in serum calcium [45,46].
The V2R provides another example of the correlation between the stability of agonist/GPCR interaction with the balance between plasma membrane and endosomal signaling. Arginine vasopressin binds tightly to the V2R, which results in prolonged internalization and endosomal G protein signaling, whereas oxytocin binds to V2R with lower affinity and dissociates from the receptor soon after internalization, which results in predominant plasma membrane G protein signaling [18,47]. V2R-stimulated Gαs/cAMP signaling promotes translocation of aquaporin water channels and epithelial sodium channels (ENaCs) from intracellular vesicles to the apical membrane of collecting duct cells, which increases renal water and sodium reabsorption. The sustained endosomal Gαs/cAMP response to arginine vasopressin explains why it has superior antidiuretic and antinatriuretic effects over oxytocin [18].
Several factors are likely to determine the stability and agonist/GPCR interactions in endosomes and thus the duration of endosomal signals. The charge and conformation of agonists and their receptors, which depend on the local pH and association of the receptor with signaling and regulatory partners, can affect the affinity and duration of agonist/GPCR association in acidic endosomes. The stability of agonists can also determine the half-life of agonist/ GPCR complexes in endosomes. By degrading neuropeptides (SP, CGRP, and somatostatin-14) in acidified early endosomes, endothelin-converting enzyme-1 triggers disassembly of agonist/GPCR/βARR complexes, promotes receptor recycling, and terminates endosomal ERK signaling [10,11,36]. Analogs that are resistant to this peptidase, including somatostatin-28 and octreotide, induce remarkably sustained sequestration of somatostatin receptors in endosomes, which may explain their therapeutically beneficial actions [48].
An understanding of how the agonist/GPCR residence time affects the subcellular location and endpoints of receptor activation can inform the development of therapeutic agonists with unique and sustained actions. Modified versions of PTH have been made to promote prolonged endosomal PTHR-mediated responses [49,50]. Long-acting PTH (LA-PTH), which is a hybrid between modified PTH(1–14) and PTHrP(15–36), binds PTHR much tighter than PTH(1–34) does and promotes remarkably sustained endosomal G protein signaling after agonist washout [50]. A single intravenous injection of LA-PTH promotes hypercalcemia and hypophosphatemia that persists for >48 h, despite clearance from the circulation within 1 h. LA-PTH effectively corrects calcium and phosphorus homeostasis in animals with hypoparathyroidism [50,51].
Thus, the design of GPCR agonists that tightly bind to receptors in endosomes, in a manner that is insensitive to low endosomal pH and to endosomal enzymatic activity, may be an effective strategy to develop drugs with superior and long-lasting efficacy.
Endosomally Biased GPCR Antagonists
The appreciation that GPCR signaling in endosomes can underlie pathology of some chronic diseases suggests that the targeting of endosomal GPCRs with antagonists would offer an improved therapeutic approach. This concept has been explored by conjugating peptidic antagonists of the NK1R (Spantide) and CLR (CGRP8–37) to polyethylene glycol (PEG) and the transmembrane lipid cholestanol [12,14]. Cholestanol-conjugated probes rapidly incorporate into the plasma membrane and then become concentrated and retained in early endosomes containing the NK1R and CLR. Once accumulated in endosomes, spantide–PEG–cholestanol and CGRP8–37–PEG–cholestanol selectively inhibit signals that emanate from endosomal NK1R and CLR (e.g., nuclear ERK), without affecting signals from plasma membrane receptors (e.g., cytosolic ERK). Spantide–PEG–cholestanol and CGRP8–37–PEG–cholestanol respectively inhibit sustained SP- and CGRP-induced excitation of spinal neurons in slice preparations, whereas unconjugated antagonists are less effective. When injected intrathecally, spantide–PEG–cholestanol and CGRP8–37–PEG–cholestanol cause a remarkably sustained inhibition of mechanical nociceptive responses to intraplantar injection of capsaicin, formalin, or complete freund’s adjuvant, compared to unconjugated antagonists [12,14]. In a similar manner, a cholestanol-conjugated antagonist of PAR2 prevents the capacity of proteases that are released from biopsies of colonic mucosa obtained from patients with IBS to cause sustained hyperexcitability of nociceptors [43]. Such endosomally targeted antagonists of PAR2 could represent a treatment for the common problem of IBS pain.
These studies reveal that endosomally targeted GPCR antagonists not only inhibit sustained endosomal signaling, but more effectively inhibit pain than conventional antagonists in preclinical models. The modification of existing antagonists to target endosomes or to promote their sustained interactions with GPCRs in endosomes may enhance their therapeutic efficacy. The results raise the possibility that the inability of antagonists to engage GPCRs in endosomes explains the limited efficacy of some of these agents in chronic diseases, where GPCRs could be largely internalized due to the continuous release of endogenous ligands.
Concluding Remarks and Future Perspectives
The traditional views that GPCR signaling emanates principally from the plasma membrane and that cell surface GPCRs are the optimal target for therapeutically beneficial drugs are incomplete. Upon activation at the cell surface, many GPCRs and agonists traffic to endosomes, where they can continue to signal by G protein- and βARR-dependent mechanisms. In contrast to the signals that arise from GPCRs at the plasma membrane, which are rapidly terminated by βARR-mediated receptor desensitization and endocytosis, signals from GPCRs in endosomes are often sustained. As GPCRs traffic through the endosomal network, they can generate signals in subcellular microdomains that control important cellular responses, including gene transcription in the nucleus and channel activity at the plasma membrane. Thus, endosomes are not merely a conduit for GPCR trafficking to degradatory or recycling pathways, but are a vital site of intracellular GPCR signaling.
Our knowledge of GPCR signaling from endosomes is incomplete (see Outstanding Questions). Structural studies have provided major insights into the molecular mechanisms by which GPCRs can engage G proteins and βARRs. However, these studies mostly focus on plasma membrane signaling events, rely on the use of GPCRs that are stabilized by extensive mutations or crosslinking, usually require antibodies or nanobodies to stabilize signaling complexes, and offer a fleeting snapshot of the structural underpinnings of signal transduction. New technologies are required to reveal the molecular details of signaling by unmodified GPCRs and their spectrum of signaling and regulatory partners in subcellular microdomains of functionally relevant cells and in real time. Approaches such as cryoelectron tomography, which can provide structural information about protein signaling complexes in intact cells, may be useful in this regard [52]. GPCRs in endosomes can engage G proteins (Gs, Gi/o, and Gq) and βARRs, which in turn control formation of second messengers (e.g., cAMP) and activation of kinases (e. g., PKC and ERK). However, the ability of GPCRs in endosomes to control activation of the full spectrum of signaling pathways awaits further investigation. Unbiased proteomic approaches, including GPCR-APEX, can be used to identify components of GPCR signaling complexes in living cells, and may provide information about the full spectrum of signaling events that emanate from GPCRs in endosomes [53]. Most information about endosomal signaling derives from observations in model cells; cell lines that are used to express and study particular protein-protein interactions and signaling cascades. Unfortunately, the relative contributions of plasma membrane and endosomal signaling to the control of complex pathophysiological processes in intact tissues and animals is poorly understood. The mechanisms that terminate plasma membrane GPCR signaling have been intensively studied, whereas the control of endosomal signaling remains to be fully defined.
Outstanding Questions.
How do βARRs positively modulate endosomal G protein signaling of GPCRs?
Do βARRs simply increase the endo-somal to plasma membrane GPCR ratio to enhance G protein signaling from endosomes?
Do βARRs in the GPC/G protein/βARR megaplex maintain close proximity between GPCRs and G proteins to enhance the activation rate of G proteins?
How is endosomal GPCR signaling regulated?
What scaffolding proteins maintain the endosomal GPCR signaling complex?
What is the termination signal – peptidases that degrade peptide agonists, proteases that cleave GPCRs, phosphatases that dephosphorylate GPCRs?
Although agonists promote GPCR endocytosis, what are the factors that control GPCR trafficking within the cell, from endosomes and the TGN?
Are there specific molecular determinants located within GPCRs, which controls this mode of trafficking?
What regulatory proteins are involved in this mode of trafficking?
What is the relative contribution of plasma membrane and endosomal signaling of GPCRs to physiological control and mechanisms of disease in intact animals?
Are endosomally biased antagonists the optimal therapy?
Does this happen in all cell types or is it regulated differently from one cell/tissue type to another?
The concept that GPCRs in endosomes control important pathophysiological processes has implications for drug discovery and development. Although GPCRs are the single largest target of therapeutic drugs, drug discovery efforts have focused on the identification of agonists or antagonists of GPCRs at the plasma membrane. Whether drugs that preferentially target GPCRs in endosomes might show superior efficacy and selectivity remains to be determined. Agonists of GPCRs are likely to activate GPCRs at the plasma membrane and in endosomes, but whether their therapeutically beneficial effects derive from GPCRs in a defined subcellular region remains to be determined. It may be possible to engineer endosomally biased agonists that preferentially interact with GPCRs in an acidic environment, which could be therapeutically useful. The μ-opioid receptor is a GPCR present on sensory neurons that, when activated, has an analgesic effect. Unfortunately, μ-opioid receptor agonists also give rise to many adverse side effects like constipation and respiratory depression. An analog of fentanyl that preferentially interacts with the μ-opioid receptor in the slightly acidic conditions of injured and diseased tissue is efficacious for the treatment of inflammatory pain but largely devoid of the detrimental side effects of respiratory depression, sedation, and constipation, by virtue of its inability to interact with the receptor at normal extracellular pH [54]. It remains to be determined whether the therapeutically beneficial effects of this analog are due to activation of the μ-opioid receptor in acidified endosomes. Another approach to deliver GPCR ligands to endosomes could be encapsulation into nanoparticles, which enter cells by clathrin-dependent endocytosis and have been used to deliver chemotherapeutic agents to tumor cells [55]. The ability of agonists to activate GPCRs in subcellular compartments could also relate to their distribution throughout the cell. The use of a genetically encoded biosensor, a fluorescent protein that can sense and report changes in cellular responses, to detect activated conformations of opioid receptors in specific cellular domains revealed that whereas peptide agonists activate receptors first at the plasma membrane and then in endosomes, non-peptide drugs such as morphine can also activate receptors in the Golgi apparatus [56]. Whether abnormal activation of GPCRs in subcellular domains contributes to adverse effects of certain agonists deserves further study. During chronic disease, it is likely that many GPCRs redistribute from the plasma membrane to endosomes due to the continued generation of endogenous agonists. Under these circumstances, GPCRs in endosomes are likely to be a valid therapeutic target. However, whether drugs can penetrate the plasma and endosomal membranes and effectively engage GPCR conformations in an acidified endosomal environment in which receptors are likely complexed with multiple signaling and regulatory partners is uncertain. Compartment-specific GPCR ligands, designed to produce beneficial effects by targeting pathological GPCR signaling but without affecting signaling from microdomains that may provoke unwanted side effects represents a novel approach to improve upon current therapies for chronic diseases.
Highlights.
G protein signaling by some GPCRs is not only stimulated at the plasma membrane but also from endosomes after βARR-dependent internalization.
Some GPCRs interact with βARR in a specific conformation, the ‘tail’ conformation, where βARR only is bound to the phosphorylated C-terminal tail and does not block the G-protein-binding site at the intracellular receptor core.
This tail conformation allows GPCRs to interact with G protein and βARR simultaneously to form GPCR–G protein– βARR supercomplexes, which provides a mechanistic basis for endosomal G protein signaling by internalized GPCRs.
Endosomal GPCR signaling plays important cell biological and physiological roles. It was recently found that endosomal GPCR signaling plays a central role in regulating nociception.
Agonists and antagonists can be developed to specifically target endosomal GPCRs to achieve more specific modulation of physiological responses.
Acknowledgments
Supported by grants from the National Institutes of Health (NS102722, DE026806, DK118971), and Department of DefenseW81XWH1810431 (NWB).
Glossary
- Adaptor protein-2 (AP2)
binds active βARR and is directly involved in clathrin-mediated receptor endocytosis.
- β-Arrestin (βARR)
interacts with agonist-occupied, phosphorylated GPCRs and mediate GPCR desensitization, endocytosis, and signal transduction.
- β2-Adrenergic receptor (β2AR)
GPCR for epinephrine that regulates cardiovascular functions.
- cAMP
secondary messenger produced by activation of adenylyl cyclase.
- C-C chemokine receptor-1 (CCR1)
GPCR for chemokines that regulates migration of immune cells.
- Clathrin
protein that interacts with βARRs and adaptins to mediate endocytosis of GPCRs and many other cell surface proteins.
- Calcitonin receptor-like receptor (CLR)
receptor for CGRP that is expressed in the cardiovascular and nervous systems where it controls vascular tone and nociception. A new target for migraine pain.
- cAMP response element binding protein (CREB)
cAMP-responsive transcription factor.
- Cryoelectron tomography
form of electron microscopy that can be used to provide high-resolution images of macromolecules in intact cells.
- ∆FLR
βARR mutant without the FLR.
- Extracellular signal-regulated kinase (ERK)
essential component of the mitogen-activated protein kinase signaling pathway.
- Fingerloop region (FLR)
βARR region that inserts into the receptor core and blocks GPCR-G protein interactions.
- Gαβγ - heterotrimeric G protein
consists of three subunits Gα, Gbβ and Gγ, and is activated by GPCRs to generate signaling outputs throughout the cell.
- G-protein-coupled receptor (GPCR)
integral membrane protein with seven transmembrane domains. GPCRs are the largest class of receptors, serve to communicate extracellular signals to the inside of the cell, control almost all physiological and pathological processes, and are the target of >30% of clinically used drugs.
- GPCR-APEX (GPCR ascorbate peroxidase)
a proximity labeling approach that can be used for the identification of proteins that are closely associated with GPCRs by mass spectrometry.
- G-protein-coupled receptor kinases (GRKs)
family of kinases that phosphorylate active GPCRs, which triggers βARR recruitment to phosphorylated receptors.
- Gt protein - transducin
heterotrimeric G protein activated by rhodopsin.
- Irritable bowel syndrome (IBS)
disorder in humans that is characterized by abdominal pain and altered bowel habits (constipation and diarrhea). Although the cause is unknown, IBS often occurs after intestinal inflammation and infection.
- Long-acting parathyroid hormone (LA-PTH)
modified version of PTH that stimulates PTHR signaling for prolonged periods of time.
- Luteinizing hormone receptor (LHR)
GPCR for the reproductive LH. LHR is predominantly expressed in testis and ovaries.
- Nociceptor
a sensory nerve, with cell bodies in dorsal root and trigeminal ganglia, that is specialized for the detection of painful stimuli in peripheral tissues and for the transmission of painful signals to the central nervous system.
- Neurokinin type 1 receptor (NK1R)
GPCR for tachykinin peptides, notably SP. NK1R is expressed in central and peripheral nervous systems and is involved in nociception, neurogenic inflammation, and smooth muscle cell contraction.
- Pituitary adenylate cyclase 1 receptor (PAC1)
GPCR for PACAP. PAC1 is expressed in the peripheral and central nervous systems, is involved in neural protection, and is highly expressed in neuroendocrine tumors.
- Pituitary adenylate cyclaseactivating polypeptide (PACAP)
member of the vasoactive intestinal polypeptide family that activates PAC1.
- Protease-activated receptor 2 (PAR2)
GPCR for serine (e.g., trypsin, tryptase, and elastase) and cysteine (cathepsin S) proteases. PAR2 that is activated by proteolytic cleavage within the extracellular C terminus.
- Polyethylene glycol (PEG)
synthetic polymer used for formulation and delivery of drugs.
- Protein kinase C (PKC)
family of kinases that are activated by diacyl glycerol and intracellular calcium ions, and which control activity of signaling proteins through phosphorylation.
- Parathyroid hormone (PTH)
protein hormone secreted by the parathyroid glands that regulates mineral homeostasis.
- Parathyroid hormone receptor (PTHR)
GPCR for PTH that maintains mineral homeostasis.
- Parathyroid hormone-related protein (PTHrP–)
protein secreted from cancer cells that activates the PTHR.
- Receptor tyrosine kinase (RTK)
family of cell surface receptors that are activated by growth factors, cytokines, and hormones.
- Sphingosine-1-phosphate 1 receptor (S1P1R)
GPCR involved in endothelial cell and lymphocyte regulation.
- Substance P (SP)
tachykinin family neuropeptide that activates neurokinin receptors.
- Trans-Golgi network (TGN)
secretory pathway sorting station that directs proteins to different subcellular destinations.
- Thyroid stimulating hormone receptor (TSHR)
GPCR expressed in the thyroid gland that is activated by TSH to produce thyroxine and triiodothyronine hormones.
- Very early endosome (VEE)
endosomal compartment physically and biochemically distinct from early endosomes.
- Vasopressin type 2 receptor (V2R)
GPCR for arginine vasopressin that regulates water homeostasis in the kidney.
Footnotes
Disclaimer Statement
N.B. and G.H. are founding scientists of Endosome Therapeutics Inc.
References
- 1.Hanlon CD and Andrew DJ (2015) Outside-in signaling – a brief review of GPCR signaling with a focus on the Drosophila GPCR family. J. Cell Sci 128, 3533–3542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hauser AS et al. (2017) Trends in GPCR drug discovery: new agents,targetsand indications. Nat. Rev. DrugDiscov 16,829–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Komolov KE and Benovic JL (2018) G protein-coupled receptor kinases: past, present and future. Cell Signal. 41, 17–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peterson YK and Luttrell LM (2017) The diverse roles of arrestin scaffolds in G protein-coupled receptor signaling. Pharmacol. Rev 69, 256–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shukla AK et al. (2014) Visualization of arrestin recruitment by a G-protein-coupled receptor. Nature 512, 218–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kang Y et al. (2015) Crystal structure of rhodopsin bound to arrestin by femtosecond X-ray laser. Nature 523, 561–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szczepek M et al. (2014) Crystal structure of a common GPCR-binding interface for G protein and arrestin. Nat. Commun 5, 4801–4809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oakley RH et al. (2000) Differential affinities of visual arrestin, beta arrestin1, and beta arrestin2 for G protein-coupled receptors delineate two major classes of receptors. J. Biol. Chem 275, 17201–17210 [DOI] [PubMed] [Google Scholar]
- 9.Oakley RH et al. (1999) Association of beta-arrestin with G protein-coupled receptors during clathrin-mediated endocytosis dictates the profile of receptor resensitization. J. Biol. Chem 274, 32248–32257 [DOI] [PubMed] [Google Scholar]
- 10.Padilla BE et al. (2007) Endothelin-converting enzyme-1 regulates endosomal sorting of calcitonin receptor-like receptor and beta-arrestins. J. Cell Biol 179, 981–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roosterman D et al. (2007) Endothelin-converting enzyme 1 degrades neuropeptides in endosomes to control receptor recycling. Proc. Natl. Acad. Sci. U. S. A. 104, 11838–11843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jensen DD et al. (2017) Neurokinin 1 receptor signaling in endosomes mediates sustained nociception and is a viable therapeutic target for prolonged pain relief. Sci. Transl. Med. 9, eaal3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vilardaga JP et al. (2014) Endosomal generation of cAMP in GPCR signaling. Nat. Chem. Biol 10, 700–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yarwood RE et al. (2017) Endosomal signaling of the receptor for calcitonin gene-related peptide mediates pain transmission. Proc. Natl. Acad. Sci. U. S. A. 114, 12309–12314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Slessareva JE et al. (2006)Activation of the phosphatidylinositol 3-kinase Vps34 by a G protein alpha subunit at the endosome. Cell 126, 191–203 [DOI] [PubMed] [Google Scholar]
- 16.Mullershausen F et al. (2009) Persistent signaling induced by FTY720-phosphate is mediated by internalized S1P1 receptors. Nat. Chem. Biol 5, 428–434 [DOI] [PubMed] [Google Scholar]
- 17.Ferrandon S et al. (2009) Sustained cyclic AMP production by parathyroid hormone receptor endocytosis. Nat. Chem. Biol 5, 734–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feinstein TN et al. (2013) Noncanonical control of vasopressin receptor type 2 signaling by retromer and arrestin. J. Biol. Chem 288, 27849–27860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calebiro D et al. (2009) Persistent cAMP-signals triggered by internalized G-protein-coupled receptors. PLoS Biol 7, e1000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Irannejad R et al. (2013) Conformational biosensors reveal GPCR signalling from endosomes. Nature 495, 534–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lyga S et al. (2016) Persistent cAMP signaling by internalized LH receptors in ovarian follicles. Endocrinology 157, 1613–1621 [DOI] [PubMed] [Google Scholar]
- 22.Gilliland CT et al. (2013) The chemokine receptor CCR1 is constitutively active, which leads to G protein-independent, beta-arrestin-mediated internalization. J. Biol. Chem 288, 32194–32210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou XE et al. (2017) Identification of phosphorylation codesfor arrestin recruitment by G protein-coupled receptors. Cell 170, 457–469 e13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomsen ARB et al. (2016) GPCR-G Protein-beta-arrestin super-complex mediates sustained G protein signaling. Cell 166, 907–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wehbi VL et al. (2013) Noncanonical GPCR signaling arising from a PTH receptor-arrestin-Gbetagamma complex. Proc. Natl. Acad. Sci. U. S. A. 110, 1530–1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feinstein TN et al. (2011) Retromer terminates the generation of cAMP by internalized PTH receptors. Nat. Chem. Biol 7, 278–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cahill TJ 3rd et al. (2017)Distinct conformations of GPCR-beta-arrestin complexes mediate desensitization, signaling, and endocytosis. Proc. Natl. Acad. Sci. U. S. A. 114, 2562–2567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumari P et al. (2016) Functional competence of a partially engaged GPCR-beta-arrestin complex. Nat. Commun 7, 13416–13432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumari P et al. (2017) Core engagement with beta-arrestin is dispensable for agonist-induced vasopressin receptor endocyto-sis and ERK activation. Mol. Biol. Cell 28, 1003–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wanka L et al. (2018) Different mode of arrestin-3 binding at the human Y1 and Y2 receptor. Cell Signal. 50, 58–71 [DOI] [PubMed] [Google Scholar]
- 31.Toth AD et al. (2018) Heterologous phosphorylation-induced formation of a stability lock permits regulation of inactive receptors by beta-arrestins. J. Biol. Chem 293, 876–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Latorraca NR et al. (2018) Molecular mechanism of GPCR-mediated arrestin activation. Nature 557, 452–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eichel K et al. (2018) Catalytic activation of beta-arrestin by GPCRs. Nature 557, 381–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beautrait A et al. (2017) A new inhibitor of the beta-arrestin/AP2 endocytic complex reveals interplay between GPCR internalization and signalling. Nat. Commun 8, 15054–15070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsvetanova NG and von Zastrow M (2014) Spatial encoding of cyclic AMP signaling specificity by GPCR endocytosis. Nat. Chem. Biol 10, 1061–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cottrell GS et al. (2009) Endosomal endothelin-converting enzyme-1: a regulator of beta-arrestin-dependent ERK signaling. J. Biol. Chem 284, 22411–22425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Villasenor R et al. (2015) Regulation of EGFR signal transduction by analogue-to-digital conversion in endosomes. eLife 4, e06156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sposini S et al. (2017) Integration of GPCR signaling and sorting from very early endosomes via opposing APPL1 mechanisms. Cell Rep 21, 2855–2867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Godbole A et al. (2017) Internalized TSH receptors en route to the TGN induce local Gs-protein signaling and gene transcription. Nat. Commun 8, 443–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.May V and Parsons RL (2017) G protein-coupled receptor endosomal signaling and regulation of neuronal excitability and stress responses: signaling options and lessons from the PAC1 receptor. J. Cell Physiol 232, 698–706 [DOI] [PubMed] [Google Scholar]
- 41.Merriam LA et al. (2013) Pituitary adenylate cyclase 1 receptor internalization and endosomal signaling mediate the pituitary adenylate cyclase activating polypeptide-induced increase in guinea pig cardiac neuron excitability. J. Neurosci 33, 4614–4622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Missig G et al. (2017) Parabrachial pituitary adenylate cyclase activating polypeptide activation of amygdala endosomal extracellular signal-regulated kinase signaling regulates the emotional component of pain. Biol. Psychiatry 81, 671–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jimenez-Vargas NN et al. (2018) Protease-activated receptor-2 in endosomes signals persistent pain of irritable bowel syndrome. Proc. Natl. Acad. Sci 115, e7438–7447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dean T et al. (2008) Altered selectivity of parathyroid hormone (PTH) and PTH-related protein (PTHrP) for distinct conformations of the PTH/PTHrP receptor. Mol. Endocrinol 22, 156–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Horwitz MJ et al. (2003) Direct comparison of sustained infusion of human parathyroid hormone-related protein-(1-36) hPTHrP-(1-36)] versus hPTH-(1-34) on serum calcium, plasma 1,25-dihy-droxyvitamin D concentrations, and fractional calcium excretion in healthy human volunteers. J. Clin. Endocrinol. Metab 88,1603–1609 [DOI] [PubMed] [Google Scholar]
- 46.Horwitz MJ et al. (2005) Continuous PTH and PTHrP infusion causes suppression of bone formation and discordant effects on 1,25(OH)2 vitamin D. J. Bone Miner. Res 20, 1792–1803 [DOI] [PubMed] [Google Scholar]
- 47.Zalyapin EA et al. (2008) Effects of the renal medullary pH and ionic environment on vasopressin binding and signaling. Kidney Int 74, 1557–1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao P et al. (2013) Agonist-biased trafficking of somatostatin receptor 2A in enteric neurons. J. Biol. Chem 288, 25689–25700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Okazaki M et al. (2008) Prolonged signaling at the parathyroid hormone receptor by peptide ligands targeted to a specific receptor conformation. Proc. Natl. Acad. Sci. U. S. A. 105, 16525–16530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maeda A et al. (2013) Critical role of parathyroid hormone (PTH) receptor-1 phosphorylation in regulating acute responses to PTH. Proc. Natl. Acad. Sci. U. S. A. 110, 5864–5869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shimizu M et al. (2016) Pharmacodynamic actions of a long-acting PTH analog (LA-PTH) in thyroparathyroidectomized (TPTX) rats and normal monkeys. J. Bone Miner. Res 31, 1405–1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pfeffer S et al. (2017) Dissecting the molecular organization of the translocon-associated protein complex. Nat. Commun 8, 14516–14525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Paek J et al. (2017)Multidimensional tracking of GPCR signaling via peroxidase-catalyzed proximity labeling. Cell 169, 338–349. e11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spahn V et al. (2017) A nontoxic pain killer designed by modeling of pathological receptor conformations. Science 355, 966–969 [DOI] [PubMed] [Google Scholar]
- 55.Sun J et al. (2017) A distinct endocytic mechanism of function-alized-silica nanoparticles in breast cancer stem cells. Sci. Rep 7, 16236–16249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stoeber M et al. (2018)A genetically encoded biosensor reveals location bias of opioid drug action. Neuron 98, 963–976 e5 [DOI] [PMC free article] [PubMed] [Google Scholar]