Abstract
Purpose of Review
Patients with autoimmune rheumatic disease are at increased risk of infection after surgery. The goal of this manuscript is to review current evidence on important contributors to infection risk in these patients and the optimal management of immunosuppression in the perioperative setting.
Recent Findings
Recent studies have confirmed that patients with autoimmune rheumatic disease, including rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE), are at increased risk of infection after surgery, with most evidence coming from studies of joint replacement surgery. Immunosuppression, disease activity, comorbidities, demographics, and surgeon and hospital volume are all important contributors to post-operative infection risk. Recently published guidelines regarding immunosuppression management before joint replacement recommend continuing the conventional disease-modifying drugs used to treat RA (e.g., methotrexate) without interruption, holding more potent conventional therapies for 1 week unless the underlying disease is severe, and holding biologic therapies for one dosing interval before surgery. Recent observational data suggests that holding biologics may not have a substantial impact on infection risk. These data also implicate glucocorticoids as a major contributor to post-operative infection risk.
Summary
Observational data supports recent recommendations to continue many therapies in the perioperative period with only short interruptions of biologics and other potent immunosuppression. Even brief interruptions may not significantly lower risk, although the field continues to evolve. Clinicians should also consider other risk factors and should focus on minimizing glucocorticoids before surgery when possible to limit the risk of post-operative infection.
Keywords: Autoimmune disease, Surgery, Immunosuppression, Disease modifying anti-rheumatic drugs, Infection, Perioperative management
Introduction
Patients with autoimmune rheumatic diseases such as rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), spondyloarthritis, and other less common autoimmune diseases are frequently managed with immunosuppressing medications to achieve disease control. While immunosuppression can improve outcomes and quality of life, many of these medications also carry a risk of infection.
Infection is of particular concern in patients who are undergoing surgery, because the perioperative period is a high-risk time for infections. Clinicians are frequently asked to provide recommendations for perioperative management to minimize the risk of post-operative infection in these patients. In this review, we will discuss the risk of post-operative infection in patients with autoimmune disease and important contributors to post-operative infection risk, recognizing that immunosuppression is not the only, and perhaps not even the most important, contributor to infection risk. Finally, we will review the current evidence and recommendations for the management of immunosuppression in the perioperative period, including recently published guidelines.
Risk of Infection in the Perioperative Period
Rheumatoid arthritis is the most common autoimmune rheumatic disease, affecting approximately 1% of the general population. Even with improvements in treatments over the past two decades, orthopedic surgery remains particularly common in RA because of the potential for joint destruction [1•, 2]. In fact, 3–5% of all joint replacements may occur in patients with RA [3••]. Consequently, much of the data regarding infection risk in the perioperative period in patients with autoimmune rheumatic disease comes from studies of joint arthroplasty in patients with RA, with results extrapolated to other populations and procedures.
While arthroplasty has been studied in the greatest detail, patients with autoimmune diseases undergo a variety of elective and non-elective surgeries that have varying rates of complications. It is important to consider the risk of the procedure when considering perioperative management. In low-risk procedures such as cataract surgery, infection is not of major concern [4]. In contrast, infection after abdominal surgery may occur in up to 10% of patients, most commonly wound infections, pneumonia, and urinary infections [5]. The risk of infection after joint replacement surgery is approximately 3–5%, but the high morbidity of deep prosthetic infections, occurring in 0.5 to 1% of patients, adds to the concern with these procedures [6, 7].
A robust literature has shown that patients with RA are at increased risk of post-operative infection, at least after orthopedic surgery [7, 8••, 9–11, 12•, 13, 14, 15•]. A recent study, for example, taking advantage of linked Danish patient data, arthroplasty registers, and the DANBIO biologic registry, evaluated 3913 patients with RA and 120,499 patients with OA undergoing hip or knee arthroplasty. Rates of prosthetic joint infection were substantially higher in patients with RA compared to those with OA [1.7 vs. 1.1 per 100 person-years, HR 1.46 (95% CI 1.13–1.88)] [8••].
Although there are less data available regarding post-operative outcomes in patients with SLE, data from smaller studies suggests that these patients may also be at greater risk of post-operative complications after arthroplasty [15•, 16–18]. A study of 58 patients with SLE matched to patients with RA and OA from a single center found that patients with SLE had the longest length of stay and highest rates of complications after total hip arthroplasty [16]. Similarly, Schnaser et al. used the Nationwide Inpatient Sample to evaluate inpatient complications after hip arthroplasty and found that patients with RA and SLE had more post-operative complications than patients with OA, although infections and other complications after discharge could not be assessed [15•]. Patients with SLE may also be at increased risk of infections and other complications after non-orthopedic major surgery [19•, 20••, 21, 22]. One large study evaluating 4321 patients with SLE using administrative inpatient data in Taiwan found increased rates of 30-day mortality (OR 1.71 (95% CI 1.09–2.67)), pneumonia (OR 1.31 (1.01–1.70)), and septicemia (OR 1.75 (1.38–2.21)) after major surgery in patients with SLE compared to controls [20••].
Despite the greater risk of complications, these surgeries can provide tremendous benefit. A study by Shah et al. of 99 patients with SLE undergoing total hip or knee arthroplasty (primarily for avascular necrosis or osteoarthritis) found that long-term functional outcomes were similar to a population of patients with osteoarthritis alone, despite the fact that SLE patients had substantially greater pre-operative pain [23]. In studies by Goodman et al., patients with RA similarly had substantial improvements in pain and function after total knee replacement and total hip replacement, with 2-year outcomes similar to patients with OA at least for knee replacement [24, 25].
Contributors to Infection Risk and Management of Risk Factors
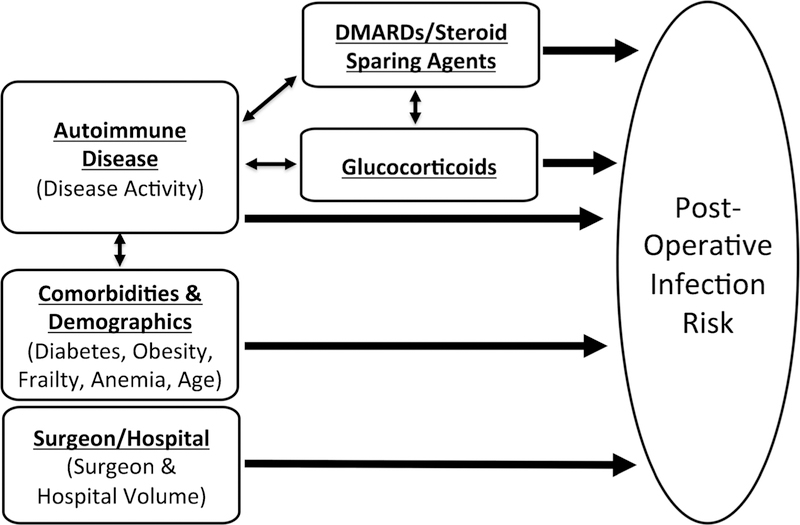
While immunosuppression management is important and often a focus of the perioperative assessment, it is also important to consider other risk factors, which may be at least as important for post-operative outcomes and in some cases are modifiable (Fig. 1). Many of these risk factors are not specific to patients with autoimmune disease. Comorbidities, obesity, age, and a history of previous infections are important contributors to post-operative infection [10, 12•, 26••, 27, 28]. Frailty and poor physical functioning, commonly observed in patients with autoimmune disease, are important predictors of post-operative complications, longer hospital stay, and greater post-operative mortality [29–32]. Control of diabetes is important, as hemoglobin A1c and pre-operative hyperglycemia have been associated with outcomes after joint replacement [33•, 34, 35]. Pre-operative anemia is also associated with both transfusion requirements and adverse post-operative outcomes [19•, 28, 36•]. While optimal pre-operative management of anemia is uncertain, it may be helpful to at least identify easily correctable causes such as iron deficiency.
Fig. 1.
Contributors to post-operative infection risk in patients with autoimmune rheumatic disease. Both the disease activity and the immunosuppression used to treat the disease (conventional therapies, biologic/targeted therapies, and especially glucocorticoids) are contributors to post-operative infection risk and are interconnected. Comorbidities (some of which can be affected by the underlying autoimmune disease), demographics, and surgeon and hospital volume are also important contributors to post-operative infection risk and are important to consider when evaluating a patient planning surgery. DMARDs, disease-modifying antirheumatic drugs
Disease activity itself may also be an important contributor to post-operative infection risk. A notable study by Cordtz et al. including almost 4000 patients with RA undergoing hip or knee arthroplasty found that disease activity as measured by the DAS28 was strongly associated with the risk of prosthetic joint infection (HR 2.00 (1.28–3.13) per 1 unit increase in DAS28) [8••]. Additionally, Lin et al. observed a higher risk of infection and mortality after major surgery in patients with SLE and found that this risk was especially elevated in patients who had received recent inpatient care for their SLE. This finding presumably reflects an increased risk in patients with more severe and active disease [20••]. Thus, there is observational evidence to suggest that optimization of disease control prior to surgery might improve post-operative outcomes, an important consideration when balancing the risks and benefits of immunosuppression (Fig. 1).
In addition to considering modifiable patient risk factors for infection, guiding patients to high-volume surgeons at high-volume centers may also improve outcomes. Both surgeon and hospital volume are strongly associated with patient outcomes after hip and knee replacement [6, 37•]. It may also be important for patients with autoimmune disease to be treated by surgeons who have experience treating similar patients [3••, 26••]. Ravi et al. evaluated a cohort of 4762 patients with RA undergoing total knee or hip arthroplasty and found that greater surgeon volume treating patients with RA, but not overall surgeon volume, was associated with lower rates of complications, including infection [3••]. Thus, it is important to encourage patients to have surgery performed by a high-volume surgeon in a high-volume center and, especially for joint replacement, with a surgeon experienced in treating patients with autoimmune rheumatic disease. The specific surgical techniques, materials, and processes associated with outcomes are beyond the scope of this review and will be determined by the treating surgeon.
Perioperative Management of Immunosuppression
Determining the appropriate management of immunosuppressive therapies in the perioperative period is of intense interest, given their potential contribution to post-operative infection. Unfortunately, there are no adequately powered randomized controlled trials that compare strategies of interrupting vs. continuing immunosuppression before surgery. It is unlikely that such studies, which would require several thousand patients, will be available in the near future. A growing body of knowledge from observational studies, however, can help guide perioperative management of immunosuppression.
A major advance in the perioperative management of patients with immunosuppression has been the publication of guidelines from the American College of Rheumatology (ACR) in conjunction with the American Association of Hip and Knee Surgeons (AAHKS), which provide specific recommendations for the management of immunosuppression in patients with RA and SLE undergoing elective hip and knee arthroplasty [38••]. In the following sections, we will review studies informing perioperative management of immunosuppression, including more recent data published after the ACR/AAHKS guidelines, and review guideline recommendations (Table 1).
Table 1.
Summary of American College of Rheumatology (ACR)/American Association of Hip and Knee Surgeons (AAHKS) recommendations for the perioperative management of immunosuppression in patients undergoing total hip and knee arthroplasty, with comments on current evidence
| ACR/AAHKS recommendations | Current evidence | |
|---|---|---|
| Conventional DMARDs | ||
| Hydroxychloroquine, sulfasalazine, methotrexate, leflunomide | Continue without interruption | No or low serious infection risk in non-surgical studies; continuing not associated with increased risk in small randomized trials |
| Other conventional immunosuppressive drugs | ||
| Azathioprine, cyclosporine mycophenolate mofetil, tacrolimus | Stop for 7 days before surgery in non-severe SLE | Limited direct evidence |
| Continue without interruption in severe SLE | ||
| Biologic/targeted therapies | ||
| TNF inhibitors, abatacept, rituximab, tocilizumab, secukinumab, ustekinumab, belimumab, anakinra | Stop for 1 dosing interval before surgery | Risk of serious infection from non-surgical studies; holding therapy not associated with large benefits in observational studies of infliximab and abatacept |
| Tofacitinib | Stop for 7 days before surgery | No direct studies of timing; similar serious infection risk to biologic therapies |
| Glucocorticoids | ||
| Prednisone, prednisolone, methylprednisolone, dexamethasone, etc. | Taper to < 20 mg/day (prednisone equivalent) if possible | Increased risk at doses ≥ 10 mg/day in observational studies with possible risk even at lower doses |
ACR/AAHKS guidelines recommend waiting ≥ 14 days after surgery to restart therapy in cases in which treatment is interrupted before surgery, making sure the wound is healing well and there are no signs of local or systemic infection before restarting
DMARDs disease-modifying antirheumatic drugs
Conventional Synthetic Disease-Modifying Antirheumatic Drugs
Two older, small randomized controlled trials of 64 and 160 patients evaluated the impact of holding low-dose methotrexate on infection risk after orthopedic surgery in patients with RA [39, 40]. While these studies were underpowered and patients were treated with lower doses of methotrexate, one showed no increase in the risk of infections or wound healing, while the other found that the group that stopped methotrexate both had more flares (8% vs. 0%) and more complications (15% vs. 2%) after surgery. This information is further bolstered by data from the non-surgical setting showing that use of methotrexate is not associated with a substantial increase in serious infections [41]. Similar results from a small trial of interrupting leflunomide in patients undergoing arthroplasty [42] and safety data regarding hydroxychloroquine and sulfasalazine [43] have led to recommendations in ACR/AAHKS guidelines to continue these conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) without interruption in the perioperative period [38••].
Other conventional therapies such as azathioprine, mycophenolate, cyclosporine, and tacrolimus may be of greater concern for their immunosuppressive effects, but there is limited data about how these therapies affect post-operative infection risk. A Medicare study that included 3339 solid organ transplant patients undergoing arthroplasty (often treated with a combination of these therapies) found that organ transplant patients had a significantly greater risk of pneumonia, sepsis, and periprosthetic infection compared to controls (2.4% vs. 1%), although this risk is almost certainly not due solely to immunosuppression use [44]. The risk of these therapies during surgery when used for the treatment of autoimmune disease remains uncertain. Reflecting this uncertainty, ACR/AAHKS guidelines suggest continuing therapy in patients with severe SLE (in whom consequences of a disease flare would be greater) but holding therapy for 1 week before arthroplasty in patients with non-severe SLE [38••]. Cyclophosphamide therapy was not addressed in the guidelines, but this highly immunosuppressing and short-term treatment is used to treat severe disease and presumably elective surgery would be strongly discouraged during cyclophosphamide treatment.
Biologics and Targeted Small Molecules
Tumor necrosis factors inhibitors (TNFi) are known to be associated with an increase in the risk of serious infections in the non-operative setting and carry a black box warning to this effect [45]. Risk overall appears similar with non-TNF biologic therapies with small differences (e.g., lower risk with abatacept or higher risk with tocilizumab) suggested by some observational studies [46, 47]. Consequently, biologic therapies have been of particular concern in patients undergoing surgery.
A number of studies, including recent meta-analyses, have shown that patients with RA treated with biologics are more likely to have a post-operative infection compared to patients treated with conventional therapies [8••, 48, 49]. It is not clear, however, to what degree this increase in infection risk is due to biologic treatment versus differences in the severity of the underlying disease. In the recent study by Cordtz et al., for example, patients with RA treated with a biologic had higher rates of prosthetic joint infection, but also had greater disease activity and were more likely to receive glucocorticoids [8••]. These inherent differences between biologic-treated patients and those treated with conventional therapy are a major challenge to overcome for any observational study seeking to inform how to manage biologics in the perioperative period.
Some studies have addressed this inherent challenge by comparing patients who stopped biologic therapy before surgery to those who continued treatment, more directly addressing the question of how to manage biologic therapy in the perioperative setting. Zahr et al. studied more than 6000 patients with RA undergoing major surgery, including almost 900 patients treated with a biologic (mostly TNF inhibitors), using Veteran’s Administration databases [50•]. In this study, patients who stopped a biologic before surgery did not have lower rates of post-operative infection than those who continued treatment, although the exact timing of treatment in the perioperative period was uncertain, particularly for non-infusion therapies.
Two of our studies using administrative claims data required that patients be treated with an infusion therapy, because the date of each infusion is accurately recorded in this data. In a study of more than 4000 infliximab-treated patients undergoing hip or knee arthroplasty, we found that the timing of infliximab was not associated with rates of hospitalized infection within 30 days of surgery or prosthetic joint infection within 1 year [26••]. Rates of these outcomes were similar in patients who received infliximab within 4 weeks of surgery compared to those who received infliximab 8–12 weeks (at least one dosing interval) prior to surgery (serious infection OR 0.90 (0.60 to 1.34) and prosthetic joint infection HR 0.98 (0.52, 1.87)). A follow-up study evaluating 1939 patients with RA treated with intravenous abatacept showed similar results, with no significant difference in the rate of hospitalized infection or prosthetic joint infection in patients receiving abatacept < 4 weeks before surgery compared to those receiving abatacept 4–8 weeks (at least one dosing interval) before surgery OR 0.93 (0.65–1.34) and HR 1.29 (0.62–2.69) [51•]. A study from a French abatacept registry also found that abatacept timing was not associated with post-operative infection among the 263 patients who underwent surgery [52•].
Preliminary results of our study comparing the risk of different biologics in the perioperative setting suggest that post-operative risk is similar in patients treated with different biologics [53], but data regarding the optimal timing of other biologics before surgery is limited. Studies of tocilizumab-and rituximab-treated patients undergoing surgery, for example, are too small to fully evaluate the impacts of timing on outcomes [54, 55]. Data to guide perioperative management in non-orthopedic surgery is also limited. One study of 311 patients with inflammatory bowel disease treated with infliximab found that infliximab level ≥ 3 μg/mL was associated with greater risk of infection after abdominal surgery, although the timing of infliximab was not reported [56•]. A separate study of 193 patients with inflammatory bowel disease receiving a biologic (mostly infliximab) undergoing abdominal surgery found no association between biologic timing and post-operative infection [57].
ACR/AAHKS guidelines currently recommend that biologics be held for one dosing interval and that the targeted small molecule tofacitinib be held for 1 week prior to joint replacement, and that these therapies be resumed 14 days after surgery as long as the site is healing well and there are no signs of infection [38••]. Although evidence is lacking that holding biologics or targeted small molecules has a substantial impact on reducing the risk of post-operative infection, the guideline panel (including patient representatives) recognized the known risk of these therapies in the non-operative setting and prioritized the prevention of infection over the risk of disease flare given the high morbidity with infection, especially prosthetic infection after joint replacement [38••, 58].
Glucocorticoids
Glucocorticoids have consistently been found to be associated with the risk of post-operative infection in patients with RA [8••, 12•, 26••, 59–61], SLE [19•, 20••], and inflammatory bowel disease undergoing surgery [62, 63]. Our study of infliximab-treated patients undergoing arthroplasty and preliminary results from a study of more than 7000 patients with RA receiving a biologic before hip or knee arthroplasty showed that patients receiving > 10 mg/day of prednisone in the 3 months before surgery had approximately twice the risk of post-operative infection as patients not receiving glucocorticoids [26••, 53].
A strong direct effect of glucocorticoids on post-operative infection risk is supported by data from the non-operative setting; in studies of RA, chronic glucocorticoid use at doses > 10 mg/day is associated with a doubling in the risk of serious infection [43, 64, 65]. While patients receiving glucocorticoids may have more severe or active disease, the consistency and strength of the association suggests a causal relationship as opposed to confounding by indication. This risk with glucocorticoids, notably, is substantially higher than the risk associated with biologic therapies.
ACR/AAHKS guidelines recommend avoiding elective surgery in patients receiving > 20 mg/day of prednisone [38••]. As noted above, however, infection risk may be substantially increased at doses of 10–20 mg and perhaps even at lower doses.
Summary for Management of Immunosuppression in the Perioperative Period
Managing perioperative immunosuppression is inherently a balance between the potential benefits of treatment interruptions and the risk of disease flares. As the ACR/AAHKS guidelines emphasize, prevention of infection is of the utmost importance, particularly in high-risk surgeries and in surgeries involving prosthetic material. Disease flares themselves, however, may contribute to adverse post-operative outcomes, and the glucocorticoids often used to treat disease flares may be the strongest contributors to post-operative infection risk.
With these principles in mind, methotrexate, leflunomide, hydroxychloroquine, and sulfasalazine should be continued without interruption, as they do not have a substantial impact on post-operative infection risk and their interruption can lead to flares. More potent immunosuppressants such as azathioprine, mycophenolate mofetil, cyclosporine, and tacrolimus can be held for 1 week before surgeries carrying significant infection risk if the underlying disease is not severe (meaning the risk of a severe flare with treatment interruption is low) but should be continued in patients in whom interruption poses a more severe risk.
It seems unlikely that interruption of biologic therapy has a strong impact on post-operative infection risk, but the available data is all observational. ACR/AAHKS recommendations to stop for one dosing interval and restart 14 days after surgery are reasonable for many patients undergoing major surgery given this uncertainty. Fortunately, treatment interruptions are relatively short with this approach. In surgeries with a low risk of serious infections, however, or in patients considered to be at higher risk of flares with treatment interruption (especially if glucocorticoids will be required), continuing treatment in the perioperative period may be preferred.
We believe that limiting glucocorticoid exposure in the months before elective surgery, where possible, is likely to have a stronger impact on outcomes than interruptions of other immunosuppression. Since glucocorticoids cannot be abruptly stopped, reductions in use need to be anticipated and planned over the months leading up to elective surgery.
In patients who require urgent or emergent surgery, optimization may not be possible; these patients should be carefully monitored post-operatively, recognizing that they are at increased risk of post-operative infections and that decisions need to be made regarding when to restart therapies (particularly biologics).
Conclusion
In patients with autoimmune rheumatic disease planning elective surgery, the treating physician plays an important role in minimizing post-operative risk of infection. Preventing infections in these patients requires not only considering the patient’s immunosuppressive therapy (especially glucocorticoids), but also optimizing disease control, managing comorbidities, and encouraging patients to see a surgeon that performs a high volume of the procedure at a high-volume center (Fig. 1).
Acknowledgments
Funding Michael George is supported by the Rheumatology Research Foundation Scientist Development Award and the National Institute of Arthritis and Musculoskeletal and Skin Diseases K23-AR073931–01. Joshua Baker has been supported by a VA Clinical Science Research & Development Career Development Award (IK2 CX000955) and Merit Award (I01 CX001703). The contents of this work do not represent the views of the Department of the Veterans Affairs or the US Government.
Footnotes
This article is part of the Topical Collection on Infection and Arthritis
Compliance with Ethical Standards
Conflict of Interest Dr. Michael George reports consultancy for AbbVie and grants from Bristol-Myers Squibb. Dr. Joshua Baker reports consultancy for Bristol-Myers Squibb and expert testimony for Burns White LLC.
Michael George has received a research grant from Bristol-Myers Squibb and consulting fees < $10,000 from AbbVie. Joshua Baker has received consulting fees < $10,000 from Bristol-Myers Squibb.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
Publisher's Disclaimer: Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.•.Cordtz RL, Hawley S, Prieto-Alhambra D, Højgaard P, Zobbe K, Overgaard S, et al. Incidence of hip and knee replacement in patients with rheumatoid arthritis following the introduction of biological DMARDs: an interrupted time-series analysis using nationwide Danish healthcare registers. Ann Rheum Dis 2018;77:684–9Interrupted time-series using Danish registries showing that rates of total knee and hip replacement have decreased in RA in the era of biologic therapy but still remain substantially higher than for the general population.
- 2.Shourt CA, Crowson CS, Gabriel SE, Matteson EL. Orthopedic surgery among patients with rheumatoid arthritis 1980–2007: a population-based study focused on surgery rates, sex, and mortality. J Rheumatol 2012;39:481–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.••.Ravi B, Croxford R, Austin PC, Hollands S, Paterson JM, Bogoch E, et al. Increased surgeon experience with rheumatoid arthritis reduces the risk of complications following total joint arthroplasty: surgical experience performing TJA in RA is protective. Arthritis Rheumatol 2014;66:488–96Canadian administrative database study which found that surgeon experience with arthroplasty in RA patients (surgeon RA joint replacement volume) was more important than overall surgeon volume in predicting complications.
- 4.Creuzot-Garcher CP, Mariet AS, Benzenine E, Daien V, Korobelnik J-F, Bron AM, et al. Is combined cataract surgery associated with acute postoperative endophthalmitis? A nationwide study from 2005 to 2014. Br J Ophthalmol 2018:bjophthalmol-2018–312171. [DOI] [PubMed]
- 5.Van Arendonk KJ, Tymitz KM, Gearhart SL, Stem M, Lidor AO. Outcomes and costs of elective surgery for diverticular disease: a comparison with other diseases requiring colectomy. JAMA Surg 2013;148:316–21. [DOI] [PubMed] [Google Scholar]
- 6.Katz JN, Barrett J, Mahomed NN, Baron JA, Wright RJ, Losina E. Association between hospital and surgeon procedure volume and the outcomes of total knee replacement. J Bone Joint Surg Am 2004;86-A:1909–16. [DOI] [PubMed] [Google Scholar]
- 7.Stundner O, Danninger T, Chiu Y-L, Sun X, Goodman SM, Russell LA, et al. Rheumatoid arthritis vs osteoarthritis in patients receiving total knee arthroplasty: perioperative outcomes. J Arthroplast 2014;29:308–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.••.Cordtz RL, Zobbe K, Højgaard P, Kristensen LE, Overgaard S, Odgaard A, et al. Predictors of revision, prosthetic joint infection and mortality following total hip or total knee arthroplasty in patients with rheumatoid arthritis: a nationwide cohort study using Danish healthcare registers. Ann Rheum Dis 2018;77:281–8Study of joint replacement using linked Danish registries found greater risk of prosthetic joint infection in patients with RA vs. osteoarthritis; biologic use in RA was associated with a non-significant increase in risk, while disease activity and glucocorticoid use were strongly associated with infection risk.
- 9.Ravi B, Croxford R, Hollands S, Paterson JM, Bogoch E, Kreder H, et al. Increased risk of complications following total joint arthroplasty in patients with rheumatoid arthritis: increased risk of specific complications in TJA recipients with RA. Arthritis Rheumatol 2014;66:254–63. [DOI] [PubMed] [Google Scholar]
- 10.Bongartz T, Halligan CS, Osmon DR, Reinalda MS, Bamlet WR, Crowson CS, et al. Incidence and risk factors of prosthetic joint infection after total hip or knee replacement in patients with rheumatoid arthritis. Arthritis Rheum 2008;59:1713–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ravi B, Escott B, Shah PS, Jenkinson R, Chahal J, Bogoch E, et al. A systematic review and meta-analysis comparing complications following total joint arthroplasty for rheumatoid arthritis versus for osteoarthritis. Arthritis Rheum 2012;64:3839–49. [DOI] [PubMed] [Google Scholar]
- 12.•.Salt E, Wiggins AT, Rayens MK, Morris BJ, Mannino D, Hoellein A, et al. Moderating effects of immunosuppressive medications and risk factors for post-operative joint infection following total joint arthroplasty in patients with rheumatoid arthritis or osteoarthritis. Semin Arthritis Rheum 2017;46:423–9Administrative data study identified RA, male gender, obesity, and prednisone use as risk factors for infection after arthroplasty.
- 13.Kurdi AJ, Voss BA, Tzeng TH, Scaife SL, El-Othmani MM, Saleh KJ. Rheumatoid arthritis vs osteoarthritis: comparison of demographics and trends of joint replacement data from the nationwide inpatient sample. Am J Orthop 2018;47. [DOI] [PubMed] [Google Scholar]
- 14.Horowitz JA, Puvanesarajah V, Jain A, Li XJ, Shimer AL, Shen FH, et al. Rheumatoid arthritis is associated with an increased risk of postoperative infection and revision surgery in elderly patients undergoing anterior cervical fusion. Spine 2018;43:E1040–4. [DOI] [PubMed] [Google Scholar]
- 15.•.Schnaser EA, Browne JA, Padgett DE, Figgie MP, D’Apuzzo MR. Perioperative complications in patients with inflammatory arthropathy undergoing total hip arthroplasty. J Arthroplast 2016;31: 2286–90Study using the Nationwide Inpatient Sample found increased rates of immediate complications after hip arthroplasty in patients with RA and SLE, with increased mortality in patients with SLE.
- 16.Merayo-Chalico J, Gónzalez-Contreras M, Ortíz-Hernández R, Alcocer-Varela J, Marcial D, Gómez-Martín D. Total hip arthroplasty outcomes: an 18-year experience in a single center: is systemic lupus erythematosus a potential risk factor for adverse outcomes? J Arthroplast 2017;32:3462–7. [DOI] [PubMed] [Google Scholar]
- 17.Fein AW, Figgie CA, Dodds TR, Wright-Chisem J, Parks ML, Mandl LA, et al. Systemic lupus erythematosus does not increase risk of adverse events in the first 6 months after total knee arthroplasty. J Clin Rheumatol 2016;22:355–9. [DOI] [PubMed] [Google Scholar]
- 18.Roberts JE, Mandl LA, Su EP, Mayman DJ, Figgie MP, Fein AW, et al. Patients with systemic lupus erythematosus have increased risk of short-term adverse events after total hip arthroplasty. J Rheumatol 2016;43:1498–502. [DOI] [PubMed] [Google Scholar]
- 19.•.Quintanilla-González L, Torres-Villalobos G, Hinojosa-Azaola A. Risk factors for development of early infectious and noninfectious complications in systemic lupus erythematosus patients undergoing major surgery. Lupus 2018;27:1960–72Retrospective single center study including 191 patients with SLE undergoing major surgery found increased post-operative complications in patients with SLE, with risk factors for infection including prednisone use, anemia, hypoalbuminemia, and lymphopenia.
- 20.••.Lin J-A, Liao C-C, Lee Y-J, Wu C-H, Huang W-Q, Chen T-L. Adverse outcomes after major surgery in patients with systemic lupus erythematosus: a nationwide population-based study. Ann Rheum Dis 2014;73:1646–51Taiwanese study including 4321 patients with SLE undergoing major surgery demonstrated increased pneumonia, septicemia, and mortality in patients with SLE, with greater risk in patients with recent inpatient care for SLE or recent glucocorticoid injections.
- 21.Babazade R, Yilmaz HO, Leung SM, Zimmerman NM, Turan A. Systemic lupus erythematosus is associated with increased adverse postoperative renal outcomes and mortality: a historical cohort study using administrative health data. Anesth Analg 2017;124: 1118–26. [DOI] [PubMed] [Google Scholar]
- 22.Domsic RT, Lingala B, Krishnan E. Systemic lupus erythematosus, rheumatoid arthritis, and postarthroplasty mortality: a cross-sectional analysis from the nationwide inpatient sample. J Rheumatol 2010;37:1467–72. [DOI] [PubMed] [Google Scholar]
- 23.Shah UH, Mandl LA, Mertelsmann-Voss C, Lee YY, Alexiades MM, Figgie MP, et al. Systemic lupus erythematosus is not a risk factor for poor outcomes after total hip and total knee arthroplasty. Lupus 2015;24:900–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goodman SM, Johnson B, Zhang M, Huang W-T, Zhu R, Figgie M, et al. Patients with rheumatoid arthritis have similar excellent outcomes after total knee replacement compared with patients with osteoarthritis. J Rheumatol 2016;43:46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goodman SM, Ramsden-Stein DN, Huang W-T, Zhu R, Figgie MP, Alexiades MM, et al. Patients with rheumatoid arthritis are more likely to have pain and poor function after total hip replacements than patients with osteoarthritis. J Rheumatol 2014;41:1774–80. [DOI] [PubMed] [Google Scholar]
- 26.••.George MD, Baker JF, Hsu JY, Wu Q, Xie F, Chen L, et al. Perioperative timing of infliximab and the risk of serious infection after elective hip and knee arthroplasty. Arthritis Care Res (Hoboken) 2017;69:1845–54Administrative claims study using Medicare data including 4288 patients receiving infliximab prior to total knee or hip arthroplasty found no significant difference in the risk of hospitalized infection or prosthetic joint infection in patients who continued infliximab vs. patients in whom infliximab was stopped before surgery; average glucocorticoid dose > 10 mg/day was associated with increased infection risk.
- 27.Mahomed NN, Barrett JA, Katz JN, Phillips CB, Losina E, Lew RA, et al. Rates and outcomes of primary and revision total hip replacement in the United States Medicare population. J Bone Joint Surg Am 2003;85-A:27–32. [DOI] [PubMed] [Google Scholar]
- 28.Bozic KJ, Lau E, Kurtz S, Ong K, Rubash H, Vail TP, et al. Patient-related risk factors for periprosthetic joint infection and postoperative mortality following total hip arthroplasty in Medicare patients. The Journal of Bone and Joint Surgery (American) 2012. [cited 2015 Mar 6];94 Available from: 10.2106/JBJS.K.00072 [DOI] [PubMed] [Google Scholar]
- 29.Lin H-S, Watts JN, Peel NM, Hubbard RE. Frailty and post-operative outcomes in older surgical patients: a systematic review. BMC Geriatr 2016;16:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Partridge JSL, Fuller M, Harari D, Taylor PR, Martin FC, Dhesi JK. Frailty and poor functional status are common in arterial vascular surgical patients and affect postoperative outcomes. Int J Surg 2015;18:57–63. [DOI] [PubMed] [Google Scholar]
- 31.Baker JF, Giles JT, Weber D, Leonard MB, Zemel BS, Long J, et al. Assessment of muscle mass relative to fat mass and associations with physical functioning in rheumatoid arthritis. Rheumatology (Oxford) 2017;56:981–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andrews JS, Trupin L, Yelin EH, Hough CL, Covinsky KE, Katz PP. Frailty and reduced physical function go hand in hand in adults with rheumatoid arthritis: a US observational cohort study. Clin Rheumatol 2017;36:1031–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.•.Tarabichi M, Shohat N, Kheir MM, Adelani M, Brigati D, Kearns SM, et al. Determining the threshold for HbA1c as a predictor for adverse outcomes after total joint arthroplasty: a multicenter, retrospective study. J Arthroplast 2017;32:S263–S267.e1Retrospective study of 1645 patients with diabetes undergoing arthroplasty found substantially higher rates of prosthetic joint infection in patients with higher hemoglobin A1c values.
- 34.Cancienne JM, Werner BC, Browne JA. Is there a threshold value of hemoglobin A1c that predicts risk of infection following primary total hip arthroplasty? J Arthroplast 2017;32:S236–40. [DOI] [PubMed] [Google Scholar]
- 35.Chrastil J, Anderson MB, Stevens V, Anand R, Peters CL, Pelt CE. Is hemoglobin A1c or perioperative hyperglycemia predictive of periprosthetic joint infection or death following primary total joint arthroplasty? J Arthroplast 2015;30:1197–202. [DOI] [PubMed] [Google Scholar]
- 36.•.Salt E, Wiggins AT, Rayens MK, Brown K, Eckmann K, Johannemann A, et al. Risk factors for transfusions following total joint arthroplasty in patients with rheumatoid arthritis. J Clin Rheumatol 2018;24:422–6Administrative data study found that patients with RA with a history of anemia were substantially more likely to receive a transfusion after total joint arthroplasty.
- 37.•.Katz JN, Losina E, Barrett J, Phillips CB, Mahomed NN, Lew RA, et al. Association between hospital and surgeon procedure volume and outcomes of total hip replacement in the United States Medicare population. J Bone Joint Surg Am 2001;83-A:1622–9Key older study using Medicare data showing the association between higher surgeon and hospital volume and improved outcomes after joint replacement.
- 38.••.Goodman SM, Springer B, Guyatt G, Abdel MP, Dasa V, George M, et al. 2017 American College of Rheumatology/American Association of Hip and Knee Surgeons Guideline for the perioperative management of antirheumatic medication in patients with rheumatic diseases undergoing elective total hip or total knee arthroplasty. Arthritis Rheumatol (Hoboken, NJ) 2017;69:1538–51Guidelines from the American College of Rheumatology and American Association of Hip and Knee Surgeons providing recommendations for the management of immunosuppression in patients with RA and SLE undergoing total joint arthroplasty.
- 39.Sany J, Anaya JM, Canovas F, Combe B, Jorgensen C, Saker S, et al. Influence of methotrexate on the frequency of postoperative infectious complications in patients with rheumatoid arthritis. J Rheumatol 1993;20:1129–32. [PubMed] [Google Scholar]
- 40.Grennan DM, Gray J, Loudon J, Fear S. Methotrexate and early postoperative complications in patients with rheumatoid arthritis undergoing elective orthopaedic surgery. Ann Rheum Dis 2001;60:214–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lopez-Olivo MA, Siddhanamatha HR, Shea B, Tugwell P, Wells GA, Suarez-Almazor ME. Methotrexate for treating rheumatoid arthritis. Cochrane Database Syst Rev 2014;CD000957. [DOI] [PMC free article] [PubMed]
- 42.Tanaka N, Sakahashi H, Sato E, Hirose K, Ishima T, Ishii S. Examination of the risk of continuous leflunomide treatment on the incidence of infectious complications after joint arthroplasty in patients with rheumatoid arthritis. J Clin Rheumatol 2003;9: 115–8. [DOI] [PubMed] [Google Scholar]
- 43.Wolfe F, Caplan L, Michaud K. Treatment for rheumatoid arthritis and the risk of hospitalization for pneumonia: associations with prednisone, disease-modifying antirheumatic drugs, and anti-tumor necrosis factor therapy. Arthritis Rheum 2006;54:628–34. [DOI] [PubMed] [Google Scholar]
- 44.Klement MR, Penrose CT, Bala A, Wellman SS, Bolognesi MP, Seyler TM. How do previous solid organ transplant recipients fare after primary total knee arthroplasty? J Arthroplast 2016;31:609–615.e1. [DOI] [PubMed] [Google Scholar]
- 45.Singh JA, Cameron C, Noorbaloochi S, Cullis T, Tucker M, Christensen R, et al. Risk of serious infection in biological treatment of patients with rheumatoid arthritis: a systematic review and meta-analysis. Lancet 2015;386:258–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yun H, Xie F, Delzell E, Levitan EB, Chen L, Lewis JD, et al. Comparative risk of hospitalized infection associated with biologic agents in rheumatoid arthritis patients enrolled in Medicare. Arthritis Rheumatol (Hoboken NJ) 2016;68:56–66. [DOI] [PubMed] [Google Scholar]
- 47.Rutherford AI, Subesinghe S, Hyrich KL, Galloway JB. Serious infection across biologic-treated patients with rheumatoid arthritis: results from the British Society for Rheumatolgy Biologics Register for Rheumatoid Arthritis. Ann Rheum Dis 2018:annrheumdis 2017–212825. [DOI] [PubMed]
- 48.Goodman SM, Menon I, Christos PJ, Smethurst R, Bykerk VP. Management of perioperative tumour necrosis factor α inhibitors in rheumatoid arthritis patients undergoing arthroplasty: a systematic review and meta-analysis. Rheumatology (Oxford) 2016;55: 573–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mabille C, Degboe Y, Constantin A, Barnetche T, Cantagrel A, Ruyssen-Witrand A. Infectious risk associated to orthopaedic surgery for rheumatoid arthritis patients treated by anti-TNFalpha. Joint Bone Spine 2017;84:441–5. [DOI] [PubMed] [Google Scholar]
- 50.•.Abou Zahr Z, Spiegelman A, Cantu M, Ng B. Perioperative use of anti-rheumatic agents does not increase early postoperative infection risks: a Veteran Affairs’ administrative database study. Rheumatol Int 2015;35:265–72Study using Veteran’s Administration data found that neither interruption of conventional DMARDs nor interruption of biologic therapy was associated with a decreased risk of post-operative infection.
- 51.•.George M, Baker J, Winthrop K, Alemao E, Chen L, Connolly S, et al. Timing of abatacept before elective arthroplasty and risk of post-operative outcomes. Arthritis Care Res Accepted for publication.Administrative claims study including 1939 surgeries found that patients who received intravenous abatacept within 4 weeks of surgery had similar outcomes to patients in which abatacept was held for 4–8 weeks or more than 8 weeks before surgery.
- 52.•.Latourte A, Gottenberg J-E, Luxembourger C, Pane I, Claudepierre P, Richette P, et al. Safety of surgery in patients with rheumatoid arthritis treated by abatacept: data from the French Orencia in Rheumatoid Arthritis Registry. Rheumatology (Oxford) 2017;56: 629–37French registry study showing no association between abatacept timing and post-operative complications among 263 patients undergoing surgery.
- 53.George M, Baker J, Winthrop K, Alemao E, Chen L, Connolly S, et al. Comparative risk of biologic therapies and risk of glucocorticoids in patients with rheumatoid arthritis undergoing elective arthroplasty. Presented at the European League Against Rheumatism annual meeting, June 2018. Ann Rheum Dis 2018;77:163. [Google Scholar]
- 54.Momohara S, Hashimoto J, Tsuboi H, Miyahara H, Nakagawa N, Kaneko A, et al. Analysis of perioperative clinical features and complications after orthopaedic surgery in rheumatoid arthritis patients treated with tocilizumab in a real-world setting: results from the multicentre TOcilizumab in Perioperative Period (TOPP) study. Mod Rheumatol 2013;23:440–9. [DOI] [PubMed] [Google Scholar]
- 55.Godot S, Gottenberg J-E, Paternotte S, Pane I, Combe B, Sibilia J, et al. Safety of surgery after rituximab therapy in 133 patients with rheumatoid arthritis: data from the autoimmunity and rituximab registry. Arthritis Care Res (Hoboken) 2013;65:1874–9. [DOI] [PubMed] [Google Scholar]
- 56.•.Lau C, Dubinsky M, Melmed G, Vasiliauskas E, Berel D, McGovern D, et al. The impact of preoperative serum anti-TNFα therapy levels on early postoperative outcomes in inflammatory bowel disease surgery. Ann Surg 2015;261:487–96Study of patients with inflammatory bowel disease undergoing abdominal surgery found that infliximab levels ≥ 3 μg/mL were associated with increased post-operative infection risk in patients with Crohn’s disease but not in patients with ulcerative colitis; timing of infliximab was not available.
- 57.Waterman M, Xu W, Dinani A, Steinhart AH, Croitoru K, Nguyen GC, et al. Preoperative biological therapy and short-term outcomes of abdominal surgery in patients with inflammatory bowel disease. Gut 2013;62:387–94. [DOI] [PubMed] [Google Scholar]
- 58.Goodman SM, Miller AS, Turgunbaev M, Guyatt G, Yates A, Springer B, et al. Clinical practice guidelines: incorporating input from a patient panel. Arthritis Care Res (Hoboken) 2017;69:1125–30. [DOI] [PubMed] [Google Scholar]
- 59.Somayaji R, Barnabe C, Martin L. Risk factors for infection following total joint arthroplasty in rheumatoid arthritis. Open Rheumatol J 2013;7:119–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Michaud K, Fehringer EV, Garvin K, O’Dell JR, Mikuls TR. Rheumatoid arthritis patients are not at increased risk for 30-day cardiovascular events, infections, or mortality after total joint arthroplasty. Arthritis Res Ther 2013;15:R195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gilson M, Gossec L, Mariette X, Gherissi D, Guyot M-H, Berthelot J-M, et al. Risk factors for total joint arthroplasty infection in patients receiving tumor necrosis factor α-blockers: a case-control study. Arthritis Res Ther 2010;12:R145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aberra FN, Lewis JD, Hass D, Rombeau JL, Osborne B, Lichtenstein GR. Corticosteroids and immunomodulators: postoperative infectious complication risk in inflammatory bowel disease patients. Gastroenterology 2003;125:320–7. [DOI] [PubMed] [Google Scholar]
- 63.Ferrante M, D’Hoore A, Vermeire S, Declerck S, Noman M, Van Assche G, et al. Corticosteroids but not infliximab increase short-term postoperative infectious complications in patients with ulcerative colitis. Inflamm Bowel Dis 2009;15:1062–70. [DOI] [PubMed] [Google Scholar]
- 64.Grijalva CG, Chen L, Delzell E, Baddley JW, Beukelman T, Winthrop KL, et al. Initiation of tumor necrosis factor-α antagonists and the risk of hospitalization for infection in patients with autoimmune diseases. JAMA 2011;306:2331–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dixon WG, Suissa S, Hudson M. The association between systemic glucocorticoid therapy and the risk of infection in patients with rheumatoid arthritis: systematic review and meta-analyses. Arthritis Res Ther 2011;13:R139. [DOI] [PMC free article] [PubMed] [Google Scholar]