Abstract
In the kidney, final urinary acidification is achieved by V-ATPases expressed in type A intercalated cells. The B1 subunit of the V-ATPase is required for maximal urinary acidification, while the role of the homologous B2 subunit is less clear. Here we examined the effect of acute acid/alkali loading in humans on B1 and B2 subunit abundance in urinary exosomes in normal individuals and of acid loading in patients with distal renal tubular acidosis (dRTA). Specificities of B1 and B2 subunit antibodies were verified by yeast heterologously expressing human B1 and B2 subunits, and murine wild-type and B1-deleted kidney lysates. Acute ammonium chloride loading elicited systemic acidemia, a drop in urinary pH, and increased urinary ammonium excretion. Nadir urinary pH was achieved at four to five hours, and exosomal B1 abundance was significantly increased at two through six hours after ammonium chloride loading. After acute equimolar sodium bicarbonate loading, blood and urinary pH rose rapidly, with a concomitant reduction of exosomal B1 abundance within two hours, which remained lower throughout the test. In contrast, no change in exosomal B2 abundance was found following acid or alkali loading. In patients with inherited or acquired distal RTA, the urinary B1 subunit was extremely low or undetectable and did not respond to acid loading in urine, whereas no change in B2 subunit was found. Thus, both B1 and B2 subunits of the V-ATPase are detectable in human urinary exosomes, and acid and alkali loading or distal RTA cause changes in the B1 but not B2 subunit abundance in urinary exosomes.
Keywords: distal tubule, renal tubular acidosis, urinary exosome
The kidneys play a paramount role in the maintenance of acid-base homeostasis by reabsorbing filtered bicarbonate and excreting nonvolatile acid equivalents to regenerate decomposed bicarbonate. In the distal nephron, type A intercalated cells secrete H+ in the urine via an apical vacuolar-type H+-adenosine triphosphatase (V-ATPase) and bicarbonate into the blood via the basolateral anion exchanger (AE1 or SLC4A1). In contrast, type B intercalated cells secrete bicarbonate into the urine by the apical chloride/bicarbonate exchanger pendrin (SLC26A4), which is distinct from basolateral chloride/bicarbonate exchanger SLC4A1 in type A intercalated cells. The plasticity of intercalated cells as well as a the complex regulation of the acid-base transporters expressed in intercalated cells all contribute to the fine tuning of acid-base homeostasis by the kidney.1–4
The V-ATPase is an ATP-driven multi-subunit enzyme highly conserved in evolution and is involved in acidification of intracellular organelles and extracellular fluids in multicellular organisms.5 The B subunit is part of the peripheral domain V1 and 2 isoforms of this subunit, B1 and B2, exist. B1 expression is restricted to the kidney, epididymis, vas deferens, ciliary body of the eye, and inner ear.6,7 In the kidney, the B1 subunit in the apical membrane of type A intercalated cells has been proven to be critical for urinary acidification in humans and rodents.8,9 In contrast, the B2 subunit is ubiquitously expressed but is predominantly localized to intracellular compartments.10 However, B2 is not entirely intracellular as it can be detected at the plasma membrane of different cells, including renal tubular epithelial cells.10 In a mouse model of genetic B1 deficiency, B2 expression in renal medullary intercalated cells is up-regulated and was proposed to be a compensatory mechanism to attenuate the phenotype of B1 deletion, indicating that there is functional redundancy of B subunit isoforms, at least in mice.11 Autosomal-recessive mutations in the ATP6V1B1 gene encoding for the B1 subunit result in distal renal tubular acidosis (dRTA). The disease is characterized by hyperchloremic normal anion gap metabolic acidosis, alkalinuria, hypocitraturia, and reduction in renal net acid excretion.9,12 A recent report describes a mild form of urinary acidification deficit in individuals who are heterozygous carriers of B1 with the inactivating allele p.E161K.13 In mice, genetic deletion of B1 leads to impaired urinary acidification, but the phenotype is much milder than in humans with the ATP6V1B1 mutation, possibly due to partial compensation by the B2 subunit.8 While the concordance of dRTA with naturally occurring mutations in the B1 subunit unequivocally testifies to the relevance of the V-ATPase in renal acid-base homeostasis in humans, little data exist on the physiological regulation of the V-ATPase and its subunits in the human kidney.9,13
The detection of B1 and B2 subunits along with other subunits of V-ATPase by liquid chromatography–tandem mass spectroscopy in human urinary exosomes demonstrates that intercalated cells “secrete” apical membrane proteins as do other types of epithelial cells lining the renal tubule.14 Many elegant morphological studies in rodents revealed that acid-base alterations induce significant changes in the apical V-ATPase surface abundance in A-type intercalated cells.15–17 With these observations in mind, we hypothesized that acute systemic acid-base alterations in humans affect B1 and B2 abundance in urinary exosomes.
RESULTS
Antibody specificity for B subunit isoforms and comparison of 2 methods to harvest human urinary exosomes
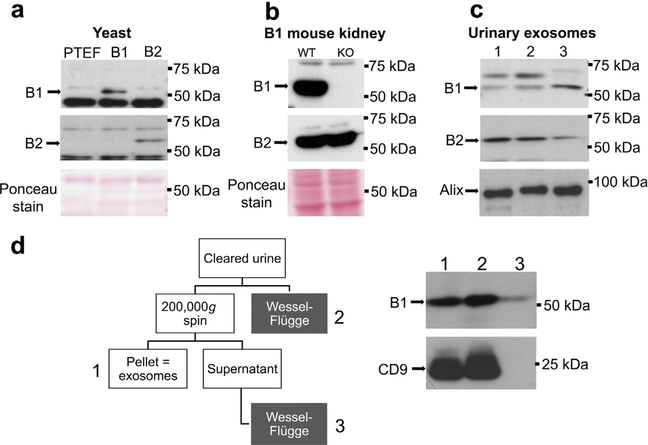
It is imperative that we determine the specificity and sensitivity of the new B1 and B2 subunit antibodies used in this study toward the human B subunit isoforms. An S. cerevisiae strain lacking the endogenous B subunit (ΔVMA2) was transformed with the empty vector or constructs containing the human B1 or B2 subunit.18 As shown in Figure 1a, both antibodies detected the appropriate B subunit isoform and showed no cross-reactivity. Next, we tested the specificity of the 2 antibodies using B1 subunit-deficient mice. The B1 antibody detected a ~55 kDa band only in wild-type kidney lysate while the B2 antibody detected ~55 kDa bands of equal intensity in both wild-type and B1−/− kidney lysates (Figure 1b). To detect B1 and B2 subunits in human urine, we collected second morning spot urine samples from 3 healthy subjects and isolated urinary exosomes. As shown in Figure 1c, both antibodies detected bands at the expected mobility for B subunits. Alix, an exosomal housekeeping protein, was used as loading control.
Figure 1 |. Specificity of the antibodies against the B1 and B2 subunit isoforms, detection of both subunit isoforms in urinary exosomes, and comparison of the centrifugation versus precipitation methods.
(a) Lysates of yeast transformed with empty vector (lane 1) or expression vectors containing the human B1 (lane 2) or B2 subunit (lane 3). Upper and middle panels display signals obtained with B1- and B2-specific antibodies, respectively. Lower panel shows corresponding Ponceau staining. (b) Kidney lysates of wild-type (WT) (lane 1) and B1 knockout (KO) mice (lane 2). Upper and middle panels display signals obtained with B1- and B2-specific antibodies, respectively. Lower panel shows corresponding Ponceau staining. (c) Urinary exosomes isolated from 3 healthy subjects, blotted with B1 (upper panel) or B2 (middle panel) antibody or an antibody for the housekeeping exosomal marker alix (lower panel). (d) After clearing (17,000 g for 15 minutes at 24 °C), the same urine sample was subjected to parallel differential centrifugation and total protein precipitation using a modified method of Wessel-Flügge. The resultant proteins were loaded equally and immunoblotted for B1 and the housekeeping exosomal marker CD9. PTEF, emtpy yeast vector p426TEF. To optimize viewing of this image, please see the online version of this article at www.kidney-international.org.
Comparison of methods
In addition to the standard differential centrifugation method,19 the Wessel-Flügge precipitation method20 was tested because it captures all urinary B1 antigen, regardless of whether it is associated with the lipid bilayer, and it also has a slightly simpler preparative protocol. The 2 methods were compared using the same urine sample (Figure 1d). In addition to urinary exosomes, B1 antigen can be detected and quantified in whole urinary protein precipitates from cleared urine (Figure 1d, right panel lane 2). Importantly, this method also detects residual B1 antigen in the supernatant after ultracentrifugation of exosomes at 200,000g; a fraction that likely represents soluble B1 that are not associated with lipid membranes (i.e., free B1 or free V1 prior to assembly into V1V0 holoenzyme). These unassembled B1 subunits are of undetermined functional significance. No residual antigen is detectable in the supernatant for the exosomal marker CD9 (Figure 1d, right panel lane 3). Importantly, when compared with total urinary B1 antigen obtained by the Wessel-Flügge method, quantification of exosomal B1 antigen shows less intra- and interindividual variability with the differential centrifugation method and better reflects physiological regulation (data not shown).
Effect of NH4Cl loading on B subunits in urinary exosomes
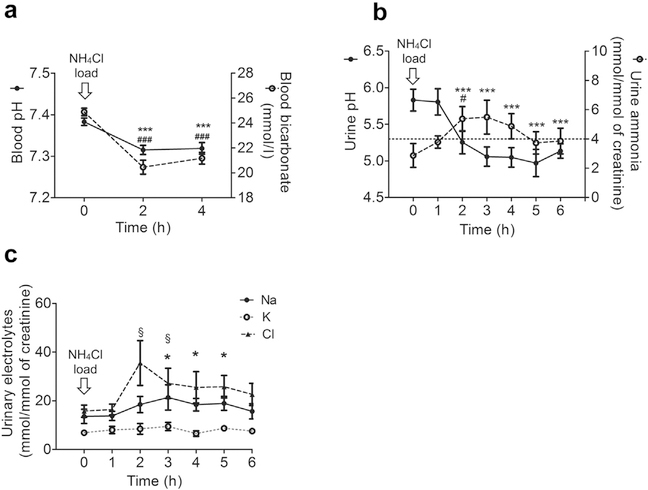
Results of acute NH4Cl loading are depicted in Figure 2. All participants acidified their urine to a pH <5.3 (Figure 2b), which is considered normal.21 Nadir urinary pH was achieved at 4 to 5 hours, which is comparable to previous studies.13,22–24 Blood pH and bicarbonate, measured at baseline, 2 hours, and 4 hours, revealed systemic metabolic acidosis at 2 hours with slight improvement at 4 hours (Figure 2a). Urinary ammonium excretion increased slightly, but only reached statistical significance at 2 hours (Figure 2b). As expected, urinary chloride excretion rose after NH4Cl ingestion (Figure 2c). Urinary creatinine concentration and urinary osmolality did not change significantly throughout the experiment (Supplementary Figure S1).
Figure 2 |. Blood and urinary parameters during NH4Cl loading.
Time 0 represents baseline (prior to NH4Cl ingestion). Arithmetic mean ± SEM of different parameters (n = 8). (a) Blood pH and bicarbonate. The * and # symbols indicate comparison of pH and bicarbonate, respectively, with reference to baseline; the number of these symbols indicates significance. (b) Urine pH and ammonia excretion. The * and # symbols indicate comparison of pH and ammonia, respectively, with reference to baseline; the number of these symbols indicates significance. (c) Urinary Na, K, and Cl excretion. The * and § symbols indicate comparison of Na and Cl excretion, respectively, with reference to baseline; the number of these symbols indicates significance. Urinary K excretion was not found to be significantly different during the course of experiment. Data are mean ± SEM; n = 8. */#/§P < 0.05; ***/###P < 0.001 compared with baseline.
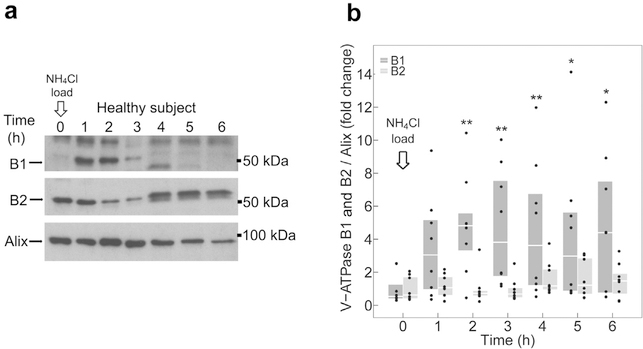
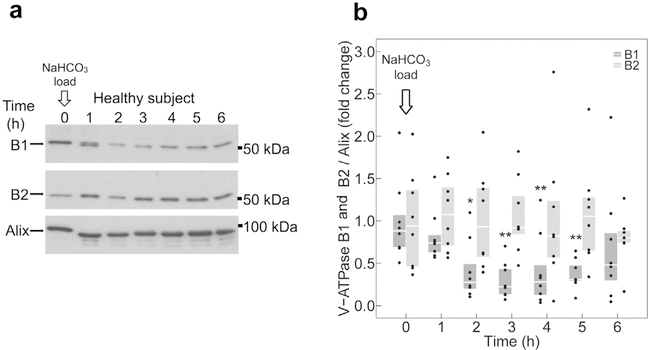
Figure 3a shows a representative immunoblot of exosomes isolated from a NH4Cl-loaded participant, probed for B1 and B2 subunits protein abundance, and normalized to the exosomal housekeeping protein alix, and compared with baseline. Pooled analysis of 8 individuals tested independently revealed an acute and sustained increase of B1 subunit abundance in urinary exosomes on acid loading (Supplementary Figure S2). At 2 hours, B1 abundance was significantly higher compared with baseline and continued to be higher throughout the experiment (Figure 3b). In contrast, exosomal B2 subunit abundance was not altered during the entire course of the experiment (Figure 3b).
Figure 3 |. Effect of NH4Cl loading on B subunit abundance in urinary exosomes.
(a) Representative immunoblots of urinary exosomes isolated from a single participant, probed with B1 (upper panel), B2 (middle panel), or alix (lower panel) antibody. (b) Quantification of immunoblots from 8 participants. B1 (dark gray boxplots) and B2 (light gray boxplots) subunit abundance were normalized to alix. All data were normalized to the respective baseline. Boxplots represent medians and interquartile ranges. Data points corresponding to the boxplots are presented. B1 and B2 at each time point are compared with baseline (time 0 hours) by Wilcoxon signed-rank test. *P < 0.05 and **P < 0.01, compared with baseline. To optimize viewing of this image, please see the online version of this article at www.kidney-international.org.
Effect of NaHCO3 loading on B subunit isoforms in urinary exosomes
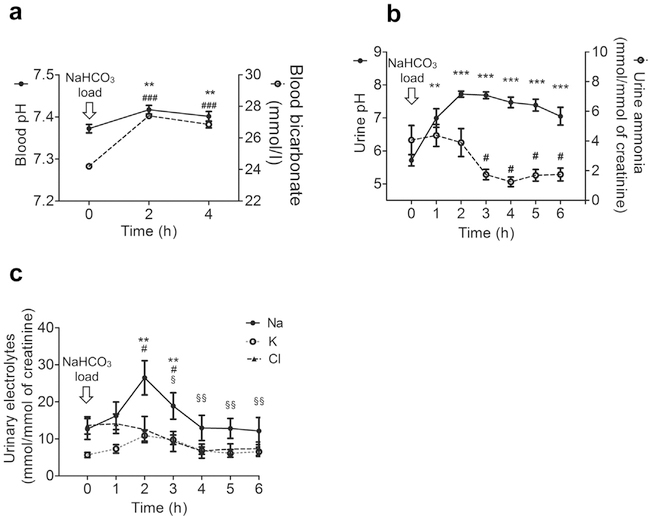
In the next step, an equimolar alkali challenge in the form of NaHCO3 was administered to all participants. After NaHCO3 loading, blood pH and bicarbonate (Figure 4a) and urinary pH (Figure 4b) rose rapidly, indicating the presence of a transient systemic metabolic alkalosis and appropriate bicarbonaturia. Urinary ammonium excretion decreased compared with baseline and remained significantly lower at 3 hours (Figure 4b). Urinary sodium and potassium excretion rose after NaHCO3 administration, while chloride excretion dropped (Figure 4c). Urinary creatinine concentration and osmolality remained unaltered throughout the experiment (Supplementary Figure S3). Urinary exosomes were immunoblotted for B1, B2, and alix. Figure 5a shows representative immunoblots of a participant subjected to acute alkali loading. Pooled analysis of 8 participants demonstrated rapid and sustained down-regulation of B1 abundance in urinary exosomes after NaHCO3 loading (Figure 5b and Supplementary Figure S4) with lowest level at 3 hours. In contrast, exosomal B2 subunit abundance did not change significantly throughout the test period (Figure 5b and Supplementary Figure S4).
Figure 4 |. Blood and urinary parameters during NaHCO3 loading.
Time 0 represents baseline (prior to NaHCO3 ingestion). Arithmetic mean ± SEM of different parameters (n = 8). (a) Blood pH and bicarbonate. The * and # symbols indicate comparison of pH and bicarbonate, respectively, with reference to baseline; the number of these symbols indicates significance. (b) Urine pH and ammonia excretion. The * and # symbols indicate comparison of pH and ammonia, respectively, with reference to baseline; the number of these symbols indicates significance. (c) Urinary Na, K, and Cl excretion. The *, #, and § symbols indicate comparison of Na, K, and Cl excretion, respectively, with reference to baseline; the number of these symbols indicates significance. Data are mean ± SEM; n = 8. #P < 0.05, **/§§P < 0.01, ***P < 0.001 compared with baseline.
Figure 5 |. Effect of NaHCO3 on B subunit abundance in urinary exosomes.
(a) Representative immunoblots of urinary exosomes isolated from a single participant, probed with B1 (upper panel), B2 (middle panel), or alix (lower panel) antibody. (b) Quantification of immunoblots from 8 participants. B1 (dark gray boxplots) and B2 (light gray boxplots) subunit abundance were normalized to alix. All data were normalized to the respective baseline. Boxplots represent medians and interquartile ranges. Data points corresponding to each boxplot are presented. B1 and B2 at each time point are compared with baseline (time 0 hours) by Wilcoxon signed-rank test. *P < 0.05 and **P < 0.01 compared with baseline. To optimize viewing of this image, please see the online version of this article at www.kidney-international.org.
Exosomal B subunit isoforms in patients with B1 subunit mutations
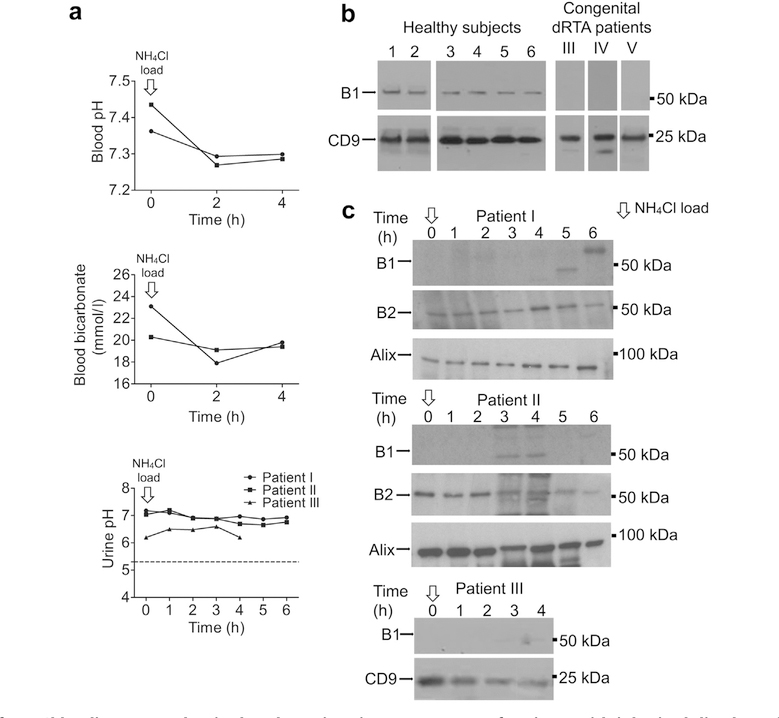
Three patients with inherited dRTA provided urinary exosomes before and during NH4Cl loading test. All patients have homozygous mutations in the gene coding for the B1 subunit ATP6V1B1. Genotype and phenotype of patients I and II were published previously.12 The genetic information of the patients is summarized in Table 1. Figure 6a shows blood pH and bicarbonate levels (patients I and II) and urinary pH (patients I, II, and III) after acid loading. Figure 6b shows exosomes blotted for B1 of patient III and of 2 additional patients with congenital dRTA (patient IV, patient V) along with normal subjects. Patients IV and V presented with classical dRTA with a positive family history but did not provide DNA for genotyping.
Table 1 |.
Characteristics of congenital dRTA patients
| Patient | Sex | Age (yrs) | V-ATPase mutation | Plasma Na (mmol/l) | Plasma K (mmol/l) | Plasma Cl (mmol/l) | Plasma HCO3 (mmol/l) |
|---|---|---|---|---|---|---|---|
| I | Female | 30 | ATP6V1B1, homozygous (p.Leu81Pro) | 141 | 3.4 | 110 | 23.1 |
| II | Female | 26 | ATP6V1B1, compound heterozygous, (p.Leu81Pro and p.Pro346Arg) | 137 | 3.3 | 112 | 20.3 |
| III | Female | 34 | ATP6V1B1, compound heterozygous, (p.Thr30Ile and p. Ala272Val) | 135 | 3.2 | 111 | 20.5 |
| IV | Male | 28 | Positive family history but did not provide DNA | 140 | 3.8 | 110 | 19.5 |
| V | Female | 32 | Positive family history but did not provide DNA | 138 | 3.5 | 107 | 21.0 |
dRTA, distal renal tubular acidosis; V-ATPase, vacuolar-type H+-adenosine triphosphatase.
Figure 6 |. Effect of NH4Cl loading on B subunit abundance in urinary exosomes of patients with inherited distal renal tubular acidosis (dRTA) due to B1 subunit mutations.
(a) Blood and urine parameters for patients I, II, and III after NH4Cl loading. (b) Immunoblots of urinary exosomes from 3 patients (III, IV, and V) with congenital dRTA. (c) Urinary exosome from patients I, II, and III during NH4Cl loading probed with B1 (upper panel), B2 (middle panel), and alix (lower panel) antibodies (patients I and II), and for B1 and CD9 (patient III). To optimize viewing of this image, please see the online version of this article at www.kidney-international.org.
Figure 6a shows that 2 subjects had frankly alkaline urine (patients I and II) and their urinary pH was unresponsive to acute acid loading. Additional urinary parameters of patients I and II are shown in Supplementary Figure S5. Patient III had lower starting urine pH but it did not fall in response to acid loading (Figure 6a, lower panel). When urinary exosomes were immunoblotted, the B2 subunit isoform was present throughout the test in all samples from patients I and II, the B1 subunit isoform was not detectable either at baseline or during NH4Cl loading in all 3 dRTA patients (Figure 6c).
NH4Cl loading in patients with acquired complete dRTA
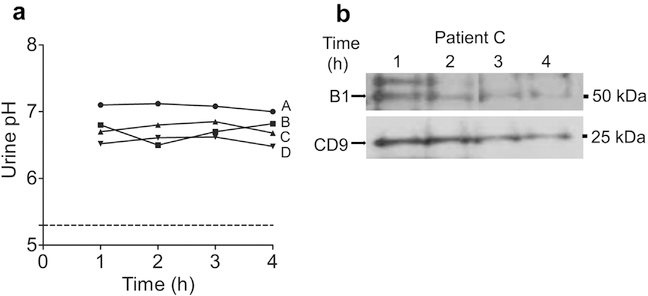
We next investigated 4 calcium phosphate stone-formers without nephrocalcinosis who were diagnosed of complete dRTA based on nonanion gap metabolic acidosis, alkalinuria, and hypocitraturia (Table 2). None of the patients had a positive family history, their genotype did not harbor any nonsynonymous mutations in V-ATPase subunits B1 and a4 (not shown). Two of the patients had Sjögren’s syndrome (patients A and C), 1 patient (patient B) had lupus nephritis with normal serum creatinine, with the remaining 1 (patient D) designated as having “idiopathic dRTA.” None of the patients with acquired dRTA showed a fall in urine pH on challenge with NH4Cl (Figure 7a). Three of the 4 subjects had undetectable B1 in urinary exosomes at baseline and throughout NH4Cl loading (not shown). One subject with detectable B1 levels is shown in Figure 7b. B1 abundance in this subject did not show changes with NH4Cl loading. Thus, lack or low B1 and flat time profile with acid loading are not limited to congenital dRTA with B1 mutations but they also seem to be a hallmark of acquired forms of dRTA.
Table 2 |.
Characteristics of acquired dRTA patients
| Patient | Sex | Age (yr) | Clinical diagnosis | Plasma Na (mmol/l) | Plasma K (mmol/l) | Plasma Cl (mmol/l) | Plasma HCO3 (mmol/l) |
|---|---|---|---|---|---|---|---|
| A | Female | 29 | Sjögren’s syndrome | 140 | 3.3 | 106 | 20 |
| B | Female | 24 | Lupus nephritis | 137 | 3.6 | 108 | 19 |
| C | Female | 42 | Sjögren’s syndrome | 138 | 3.4 | 106 | 18 |
| D | Male | 36 | Idiopathic | 136 | 4.0 | 105 | 20 |
dRTA, distal renal tubular acidosis.
Figure 7 |. Effect of NH4Cl loading on B subunit abundance in urinary exosomes in patients with acquired distal renal tubular acidosis.
Four patients (A, B, C, and D) with acquired distal renal tubular acidosis were studied with NH4Cl loading. (a) Urinary pH profile. (b) Subject C had enough B1 in the exosome preparation to provide a quantifiable signal. To optimize viewing of this image, please see the online version of this article at www.kidney-international.org.
DISCUSSION
The purpose of this study was to test whether one can visualize the regulation of B1 and B2 V-ATPase subunit isoforms in human urinary exosomes. Our data indicate that acute systemic acid-base changes exert rapid and significant changes in the abundance of the B1 but not the B2 subunit in human urine from healthy individuals. In addition, B1 subunit is not detectable in urinary exosomes of patients with ATP6V1B1 missense mutations and generally is very low or undetectable in patients with acquired dRTA and not responsive to acute acid challenge.
The rationale behind analyzing urinary exosomes deserves a comment. There has been no study on the renal expression patterns of V-ATPase isoforms after acid-base changes in humans. Characterization of V-ATPase subunits in human tissue after acid or alkali loading is not possible, so we chose to study the regulation of V-ATPase B1 and B2 subunits in humans by employing the surrogate urinary exosomes, which contain membrane proteins of nephron-lining epithelial cells.19,25 The results obtained with B1-deleted mouse kidney lysates and yeast heterologously expressing human B1 and B2 subunits confirmed the specificity of our B1 and B2 antibodies.
In rodents consuming regular chow, type A intercalated cells display some V-ATPase in luminal membrane, but most of the protein is located in a specialized intracellular tubulovesicular system in the apical pole of the cell.26–29 In rats subjected to chronic acid load, the V-ATPase undergoes a profound redistribution.28,30 After 14 days, type A intercalated cells have most of the V-ATPase on the plasma membrane and exhibit a profound loss of intracellular tubulovesicles from the apical cytoplasm.31 In contrast, in alkali-loaded rats, the V-ATPase staining of cytoplasmic vesicles was greatly enhanced while the plasma membrane labeling disappeared. Interestingly, there was no significant change in V-ATPase total protein or mRNA levels in either cortex or medulla of acid- or alkali-treated rats.31 Similar results have been obtained in mice, rats, and rabbits.6,15–17,32 Together, these data support a model where the regulation of the V-ATPase in type A intercalated cells occurs mostly at a posttranslational level via shuttling between subapical vesicles and the plasma membrane in response to acid-base stimuli.17,33,34
We speculate that shuttling analogous to the rodent V-ATPase17,33,34 also occurs in the human kidney, which causes alterations of the B1 protein abundance in urinary exosomes, thus providing a window of detection. True urinary exosomes are released into the urine by fusion of the outer membrane of the specialized multivesicular bodies with the apical plasma membrane during vesicular trafficking.19,35 In support of our hypothesis, such subapical to apical trafficking and concomitant alterations in the exosomal content has been described for several other apical membrane proteins, including the sodium chloride cotransporter36 or aquaporin 2.37,38 The major drawback of this study or any study using conventional “exosomal preparations” is the inability to distinguish between exosomes derived from multivesicular bodies from ectosomes, which originates from constitutive shedding rather than membrane trafficking. There is also the lack of experimental proof for the hypothesis that exosomal B1 is directly proportional to apical membrane B1. Additional studies comparing exosomal B subunit abundance with quantification of plasma membrane or subcellular V-ATPase expression from renal tissue or both are needed to unequivocally substantiate our claim.
In contrast to the extensive immunohistologic studies in rodents, human data is sparse. In patients with congenital dRTA or certain forms of acquired dRTA, V-ATPase expression is very low or absent,39–42 but the staining in dRTA from transplant rejection43 and lupus44 were more equivocal and failed to establish concordance between clinical chemistry data and immunohistochemistry. We previously found the distribution of B1 has a largely intracellular localization in a patient with a B1 mutation that disrupts pump assembly.18
In this study, we employed the short “1-day” ammonium chloride loading test as originally described by Wrong and Davies in 1959.21 One advantage of this classical test is the isolated assessment of urinary acidification. Once ammonia production increases (at ~12–24 hours of acid loading), urinary pH is not only determined by distal H+–pumping but also by the increased availability of the urinary buffer ammonia. However, there are other ways to assess urinary acidification, such as the 3-day ammonium chloride loading test45 or the furosemide or the furosemide and fludrocortisone tests.22,46 It will be interesting to assess in future studies the regulation of exosomal B1 abundance in individuals subjected to more chronic acid loads (e.g., the 3-day ammonium chloride loading test) or subjected to tests that use acid-independent mechanisms of urinary acidification (e.g., furosemide or furosemide and fludrocortisone tests).
We also analyzed urinary exosomes of dRTA patients with homozygous and compound heterozygous ATP6V1B1 mutations. Our data indicate that the diagnosis of dRTA from B1 subunit mutations or nongenetic causes can potentially be confirmed by urinary exosome analysis. Whether this is also the case for patients with ATP6V1B1 mutations other than the ones examined remains to be tested. In addition, we do not know whether other subunits, such as a4 mutations, lead to reduced or absent B1 in urinary exosomes. If there are differences between subjects with a4 versus B1 mutations, exosome analysis may be an easy and fast way to discriminate patients with ATP6V1B1 mutations from patients with congenital dRTA due to mutations in other known genes (ATP6V0A4 or SLC4A1). Our data indicate that the exosomal B2 subunit abundance in these patients is unaffected, supporting the critical and perhaps exclusive role of the B1 subunit for maximal urinary acidification in humans.
In summary, both B1 and B2 V-ATPase subunits can be specifically detected in human urinary exosomes by immunoblotting. Our results furthermore suggest that the B1 but not B2 subunit abundance in urinary exosomes is altered within hours by acute acid or alkali loading, probably via a mechanism involving translocation of this apical protein.
MATERIALS AND METHODS
Study participants and test procedures
All study participants (men, aged 25–45 years) gave written informed consent, and the study protocol was approved by the ethics commission of the canton of Bern, or the Institutional Review Board of the University of Texas Southwestern Medical Center, Dallas, TX. Participants underwent the classical “short” NH4Cl loading test21,23,24 and equimolar NaHCO3 loading on separate days after overnight fasting and with at least 3 weeks between tests. All tests started at 8:00 AM, venous blood samples were obtained for chemistry, pH and blood gases at 8:00 AM, 10:00 AM, 12:00 PM. Urine was collected hourly from 8:00 AM to 2:00 PM.
Venous blood gas and electrolyte analysis was performed immediately after collection on a ABL800FLEX blood gas analyzer (Radiometer, Thalwil, Switzerland) or a Radiometer ABL5 (Radiometer America, Westlake, OH). Urine pH was measured by a S20 SevenEasy pH meter (Mettler Toledo, Greifensee, Switzerland) immediately after collection. For the NH4Cl loading test, oral NH4Cl at a dose of 1.87 mmol/kg (100 mg/kg) body weight was given. For NaHCO3 loading, oral NaHCO3 at a dose of 1.87 mmol/kg (157 mg/ kg) was given. In both NH4Cl and NaHCO3 loading, study participants were recommended to drink 200 ml of water hourly. Protease inhibitors cocktail tablets (Roche, Mannheim, Germany) were added immediately after urine collection. Samples were stored at −80 °C until use.
Measurement of urinary parameters
Urine samples were aliquoted and sent to the Central Laboratory of the University Hospital of Bern, Switzerland, for determination of urinary electrolytes (Na, K, Cl) and creatinine. Urinary ammonium was measured using the Berthelot method.47 Urinary osmolality was measured on a Vapro 5600 (Wescor, Logan, UT) vapor pressure osmometer. For the experiments done at UT Southwestern, blood and urine samples were transferred from the outpatient research facility immediately to the Mineral Metabolism Clinical Laboratory. A systemic multichannel analysis measured creatinine, sodium, potassium, chloride, total carbon dioxide, glucose, and uric acid (Beckman CX9ALX; Beckman Coulter, Fullerton, CA). Urine ammonium was measured by the glutamate dehydrogenase method while citrate was assessed enzymatically with reagents from Boehringer-Mannheim Biochemicals (Indianapolis, IN).
Urinary exosomes
Twenty milliliters of collected urine were centrifuged at 17,000g for 15 minutes at 24 °C in Ultra Centrifuge (TFT70.38 rotor, Beckman Coulter). The supernatant was removed and incubated at room temperature for 25 minutes. The pellet was resuspended in 200 μl isolation solution (250 mmol/l sucrose and 10 mmol/l triethanolamine-HCl, pH 7.6) and 50 μl 3.24 mol/l dithiothreitol, and subsequently centrifuged at 17,000g for 15 minutes at 24 °C. This supernatant was collected and combined with supernatant obtained from the previous step and centrifuged at 200,000g for 2 hours at 24 °C. The exosome pellet was dissolved in 50 μl of Laemmli buffer (0.6% wt/vol sodium dodecylsulfate [SDS], 3% vol/ vol glycerol, 18 mmol/l tris-HCl pH 6.8 and 0.003% wt/vol bromophenol blue), and stored at −20 °C for further use.
The differential centrifugation method described was compared with a precipitation method modified from Wessel and Flügge20 that captures total urinary protein (including non-exosome-associated proteins). Briefly, 16 ml of urine was processed as described for exosomes but stopping before the 200,000g centrifugation. Each volume (V) of processed urine was combined sequentially with 4V of methanol, 1V of chloroform, and 3V of ultrapure water, with vigorous vortexing between additions. The resulting solution was centrifuged (5500g for 20 minutes at 24 °C), followed by careful discard of the upper phase without disturbing the protein precipitate at the interface. Another 3V of methanol were added, with vigorous vortexing, followed by centrifugation (5500g for 20 minutes at 24 °C). The resulting pellet was dissolved in 150 μl radioimmunoprecipitation assay buffer and 2.5-fold concentrated Laemmli buffer (1:1 by volume) and stored at −20 °C until further use.
Yeast experiments
Generation and expression of human B1 and B2 subunit constructs in the p426TEF yeast expression vectors were described previously.18 Briefly, constructs were transformed into S. cerevisiae (genotype: MATa/MATalpha his3Δ1/his3Δ1 leu2Δ0/leu2Δ0 lys2Δ0/+ met15Δ0/ + ura3Δ0/ura3Δ0 ΔVMA2/ΔVMA2) and grown at 30 °C in the synthetic minimal uracil (SD-Ura) dropout medium (DO-Ura supplement 0.77 g/l, yeast nitrogen base 6.7 g/l, 20 g/l D-glucose, 100 mg/l adenine hemisulfate, 50 mmol/l methanesulfonic acid, pH 5.5). All yeast media contained 200 μg/l geneticin. Transformation of yeast was performed as described by Schiestl and Gietz.48 For immunoblotting, 10 optical density units of yeast grown in SD-Ura medium were washed once with 1mmol/l ethylenediamine tetraacetic acid and H2O and lysed in 2 mol/l NaOH (10 minutes at 4 °C). Yeast protein was isolated by sequential trichloroacetic acid or acetone precipitation and resuspended in SDS sample buffer (25 mmol/l tris-Cl, pH 6.8, 9 mol/l urea, 1 mmol/l ethylenediamine tetraacetic acid, 1% (wt/vol) SDS, 0.7 mol/l β-mercaptoethanol, 10% (vol/vol) glycerol). After incubation (37 °C for 15 minutes), equal portions of the lysates were separated by SDS-polyacrylamide gel electrophoresis, transferred to polyvinylidene diflouride membranes, and analyzed by immunoblotting.
Preparation of mouse kidney lysates
Animal experiments were approved by the local Veterinary Authority (Kantonales Veterinäramt Zürich) and conducted in accordance with Swiss Animal Welfare Laws. Generation and breeding of V-ATPase B1 subunit-deficient mice (B1−/−) was described previously.8 Wildtype and B1−/− mice were anesthetized, and kidneys were removed and immediately shock-frozen in liquid nitrogen. Renal tissue was homogenized with an electric homogenizer at 4 °C in lysis buffer (54.6 mmol/l N-2-hydroxyethylpiperazine-N′−2-ethanesulfonic acid; 2.69 mmol/l Na4 P2 O7; 360 mmol/l NaCl; 10% [vol/vol] glycerol; 1% [vol/vol] NP40) containing protease inhibitors (Roche, Mannheim, Germany). Homogenates were clarified by centrifugation at 20,000g for 20 minutes and subsequently used for SDS–polyacrylamide gel electrophoresis and immunoblotting.
Immunoblotting and antibodies
Urinary exosomal pellets resuspended in Laemmli buffer were incubated at 60 °C for 15 minutes. The volume of urinary exosomes suspension loaded per lane for gel electrophoresis was adjusted according to the urinary creatinine concentration. After separation by SDS-PAGE, proteins were transferred to polyvinylidene difluoride membranes (Immobilon-P, Millipore Corporation, Bedford, MA) and then used for immunoblotting. The following primary antibodies were used: rabbit polyclonal anti-B1 and anti-B2 at 1:2000 dilutions, mouse polyclonal anti-alix (Bern subjects, 1:500 dilution; ab88743, Abcam, Cambridge, UK), and anti-CD9 at 1:400 dilution (Dallas subjects, Santa Cruz Biotechnology, Dallas, TX). Blots were either probed with alix or CD9 antibody as both exosomal housekeeping protein have virtually equal normalizing qualities.49 The anti-B1 serum was produced in rabbits using a C-terminal peptide of the murine sequence that had a N-terminally introduced cysteine for linkage to keyhole limpet hemocyanin (KLH, NH2-CQQDPASDTAL-COOH, Pineda antibody service, Berlin, Germany).6 Alternatively, a previously well-characterized rabbit anti-B1 polyclonal was also used.50 Similarly, a rabbit anti-B2 serum was produced against the last 10 amino acids of the B2 subunit and completely conserved between humans and mice (EFYPRDSAKH, Pineda antibody service, Berlin, Germany) as described.10 Secondary antibodies used were horseradish peroxidase–conjugated anti-rabbit (1:20,000 dilution; Sigma-Aldrich, St. Louis, MO), and anti-mouse (1:3000 dilution; Sigma-Aldrich). Image quantification was performed by the ImageJ software (National Institutes of Health, Bethesda, MD).
Statistical analysis
Data are expressed as mean ± SEM. Comparisons were made between groups using either Student t test or 1-way analysis of variance with post hoc Tukey analysis, as appropriate. Effect of NH4Cl and NaHCO3 on B subunit abundance in urinary exosomes was visualized by boxplots and data points within the median ± 3 × interquartile range were shown for each B subunit and each time point. All statistical tests were 2-sided. A P value of <0.05 was considered statistically significant. Data were analyzed using Prism 5 software (GraphPad Software, La Jolla, CA) and the R software version 3.2.2 (R Foundation for Statistical Computing, Vienna, Austria).
Supplementary Material
Figure S1. Urinary creatinine concentration and osmolality during NH4Cl loading. Time 0 represents baseline (prior to treatment). Data are mean ± SEM; n = 8. No significant differences compared with baseline were observed.
Figure S2. Effect of NH4Cl loading on B subunit abundance in urinary exosomes. Immunoblots of urinary exosomes isolated from 7 different participants, probed with B1 (upper panel), B2 (middle panel), and alix (lower panel) antibodies.
Figure S3. Urinary creatinine concentration and osmolality during NaHCO3 loading. Time 0 represents baseline (prior to treatment). Data are mean ± SEM; n = 8. No significant differences compared with baseline were observed.
Figure S4. Effect of NaHCO3 loading on B subunit abundance in urinary exosomes. Immunoblots of urinary exosomes isolated from 7 different participants, probed with B1 (upper panel), B2 (middle panel) and alix (lower panel) antibodies.
Figure S5. Urinary Na (A), K (B), Cl (C), NH4 (D), creatinine (E), and osmolality (F) during NH4Cl loading in distal renal tubular acidosis patients. Time 0 represents baseline (prior to treatment).
ACKNOWLDGMENTS
GP was supported by the Marie Curie Actions International Fellowship Program. DF was supported by the Swiss National Centre of Competence in Research TransCure, the Swiss National Science Foundation (grants 31003A_152829 and 33IC30_166785/1), and by a Medical Research Position Award of the Foundation Prof. Dr. Max Cloëtta. CAW is supported by the National Centre of Competence in Research Kidney. CH and the Swiss National Science Foundation grant (31003A_155959). OWM was supported by the National Institutes of Health (R01-DK081423, R01-DK091392) and the O’Brien Kidney Research Center P30 DK-079328). IAB was supported by the National Institute of Health (K01-DK090282), the Charles and Jane Pak Center of Mineral Metabolism Seed Grants, and the Department of Internal Medicine Chairman’s Fund in UT Southwestern.
Footnotes
DISCLOSURE
All the authors declared no competing interests.
Supplementary material is linked to the online version of the paper at www.kidney-international.org.
REFERENCES
- 1.Al-Awqati Q Plasticity in epithelial polarity of renal intercalated cells: targeting of the H(+)-ATPase and band 3. Am J Physiol. 1996;270: C1571–C1580. [DOI] [PubMed] [Google Scholar]
- 2.Brown D, Hirsch S, Gluck S. An H+-ATPase in opposite plasma membrane domains in kidney epithelial cell subpopulations. Nature. 1988;331:622–624. [DOI] [PubMed] [Google Scholar]
- 3.Christensen EI, Wagner CA, Kaissling B. Uriniferous tubule: structural and functional organization. Compr Physiol. 2012;2:805–861. [DOI] [PubMed] [Google Scholar]
- 4.Wagner CA, Devuyst O, Bourgeois S, et al. Regulated acid-base transport in the collecting duct. Pflugers Arch. 2009;458:137–156. [DOI] [PubMed] [Google Scholar]
- 5.Stevens TH, Forgac M. Structure, function and regulation of the vacuolar (H+)-ATPase. Annu Rev Cell Dev Biol. 1997;13:779–808. [DOI] [PubMed] [Google Scholar]
- 6.Finberg KE, Wagner CA, Stehberger PA, et al. Molecular cloning and characterization of Atp6v1b1, the murine vacuolar H+ -ATPase B1-subunit. Gene. 2003;318:25–34. [DOI] [PubMed] [Google Scholar]
- 7.Breton S, Smith PJ, Lui B, et al. Acidification of the male reproductive tract by a proton pumping (H+)-ATPase. Nat Med. 1996;2:470–472. [DOI] [PubMed] [Google Scholar]
- 8.Finberg KE, Wagner CA, Bailey MA, et al. The B1-subunit of the H(+) ATPase is required for maximal urinary acidification. Proc Natl Acad Sci U S A. 2005;102:13616–13621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karet FE, Finberg KE, Nelson RD, et al. Mutations in the gene encoding B1 subunit of H+-ATPase cause renal tubular acidosis with sensorineural deafness. Nat Genet. 1999;21:84–90. [DOI] [PubMed] [Google Scholar]
- 10.Paunescu TG, Da Silva N, Marshansky V, et al. Expression of the 56-kDa B2 subunit isoform of the vacuolar H(+)-ATPase in proton-secreting cells of the kidney and epididymis. Am J Physiol Cell Physiol. 2004;287:C149–C162. [DOI] [PubMed] [Google Scholar]
- 11.Paunescu TG, Russo LM, Da Silva N, et al. Compensatory membrane expression of the V-ATPase B2 subunit isoform in renal medullary intercalated cells of B1-deficient mice. Am J Physiol Renal Physiol. 2007;293:F1915–F1926. [DOI] [PubMed] [Google Scholar]
- 12.Mohebbi N, Vargas-Poussou R, Hegemann SC, et al. Homozygous and compound heterozygous mutations in the ATP6V1B1 gene in patients with renal tubular acidosis and sensorineural hearing loss. Clin Genet. 2013;83:274–278. [DOI] [PubMed] [Google Scholar]
- 13.Dhayat NA, Schaller A, Albano G, et al. The vacuolar H+-ATPase B1 subunit polymorphism p.E161K associates with impaired urinary acidification in recurrent stone formers. J Am Soc Nephrol. 2016;27: 1544–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzales PA, Pisitkun T, Hoffert JD, et al. Large-scale proteomics and phosphoproteomics of urinary exosomes. J Am Soc Nephrol. 2009;20: 363–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Madsen KM, Verlander JW, Kim J, et al. Morphological adaptation of the collecting duct to acid-base disturbances. Kidney Int Suppl. 1991;33: S57–S63. [PubMed] [Google Scholar]
- 16.Verlander JW, Madsen KM, Cannon JK, et al. Activation of acid-secreting intercalated cells in rabbit collecting duct with ammonium chloride loading. Am J Physiol. 1994;266:F633–F645. [DOI] [PubMed] [Google Scholar]
- 17.Verlander JW, Madsen KM, Tisher CC. Effect of acute respiratory acidosis on two populations of intercalated cells in rat cortical collecting duct. Am J Physiol. 1987;253:F1142–F1156. [DOI] [PubMed] [Google Scholar]
- 18.Fuster DG, Zhang J, Xie XS, et al. The vacuolar-ATPase B1 subunit in distal tubular acidosis: novel mutations and mechanisms for dysfunction. Kidney Int. 2008;73:1151–1158. [DOI] [PubMed] [Google Scholar]
- 19.Pisitkun T, Shen RF, Knepper MA. Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci U S A. 2004;101: 13368–13373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wessel D, Flugge UI. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal Biochem. 1984;138:141–143. [DOI] [PubMed] [Google Scholar]
- 21.Wrong O, Davies HE. The excretion of acid in renal disease. Q J Med. 1959;28:259–313. [PubMed] [Google Scholar]
- 22.Walsh SB, Shirley DG, Wrong OM, et al. Urinary acidification assessed by simultaneous furosemide and fludrocortisone treatment: an alternative to ammonium chloride. Kidney Int. 2007;71:1310–1316. [DOI] [PubMed] [Google Scholar]
- 23.Pathare G, Dhayat N, Mohebbi N, et al. Acute regulated expression of pendrin in human urinary exosomes [e-pub ahead of print]. Pflugers Arch. 10.1007/s00424-017-2049-0. Accessed. [DOI] [PubMed] [Google Scholar]
- 24.Dhayat NA, Gradwell MW, Pathare G, et al. Furosemide/fludrocortisone test and clinical parameters to diagnose incomplete distal renal tubular acidosis in kidney stone formers. Clin J Am Soc Nephrol. 2017;12: 1507–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pathare G, Tutakhel OA, van der Wel MC, et al. Hydrochlorothiazide treatment increases the abundance of the NaCl cotransporter in urinary extracellular vesicles of essential hypertensive patients. Am J Physiol Renal Physiol. 2017;312:F1063–F1072. [DOI] [PubMed] [Google Scholar]
- 26.Hamm LL, Hering-Smith KS. Acid-base transport in the collecting duct. Semin Nephrol. 1993;13:246–255. [PubMed] [Google Scholar]
- 27.Preisig PA, Alpern RJ. Pathways for apical and basolateral membrane NH3 and NH4+ movement in rat proximal tubule. Am J Physiol. 1990;259: F587–F593. [DOI] [PubMed] [Google Scholar]
- 28.Tisher CC, Madsen KM, Verlander JW. Structural adaptation of the collecting duct to acid-base disturbances. Contrib Nephrol. 1991;95:168–177. [DOI] [PubMed] [Google Scholar]
- 29.Zimolo Z, Montrose MH, Murer H. H+ extrusion by an apical vacuolar-type H(+)-ATPase in rat renal proximal tubules. J Membr Biol. 1992;126: 19–26. [DOI] [PubMed] [Google Scholar]
- 30.Kuwahara M, Sasaki S, Marumo F. Mineralocorticoids and acidosis regulate H+/HCO3- transport of intercalated cells. J Clin Invest. 1992;89: 1388–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bastani B, Purcell H, Hemken P, et al. Expression and distribution of renal vacuolar proton-translocating adenosine triphosphatase in response to chronic acid and alkali loads in the rat. J Clin Invest. 1991;88:126–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Daryadel A, Bourgeois S, Figueiredo MF, et al. Colocalization of the (Pro) renin receptor/Atp6ap2 with H+-ATPases in mouse kidney but prorenin does not acutely regulate intercalated cell H+-ATPase activity. PLoS One. 2016;11:e0147831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brown D Membrane recycling and epithelial cell function. Am J Physiol. 1989;256:F1–F12. [DOI] [PubMed] [Google Scholar]
- 34.Madsen KM, Tisher CC. Structure-function relationships in H+-secreting epithelia. Fed Proc. 1985;44:2704–2709. [PubMed] [Google Scholar]
- 35.Gonzales P, Pisitkun T, Knepper MA. Urinary exosomes: is there a future? Nephrol Dial Transplant. 2008;23:1799–1801. [DOI] [PubMed] [Google Scholar]
- 36.van der Lubbe N, Jansen PM, Salih M, et al. The phosphorylated sodium chloride cotransporter in urinary exosomes is superior to prostasin as a marker for aldosteronism. Hypertension. 2012;60:741–748. [DOI] [PubMed] [Google Scholar]
- 37.Higashijima Y, Sonoda H, Takahashi S, et al. Excretion of urinary exosomal AQP2 in rats is regulated by vasopressin and urinary pH. Am J Physiol Renal Physiol. 2013;305:F1412–F1421. [DOI] [PubMed] [Google Scholar]
- 38.Takata K, Matsuzaki T, Tajika Y, et al. Localization and trafficking of aquaporin 2 in the kidney. Histochem Cell Biol. 2008;130:197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cohen EP, Bastani B, Cohen MR, et al. Absence of H(+)-ATPase in cortical collecting tubules of a patient with Sjogren’s syndrome and distal renal tubular acidosis. J Am Soc Nephrol. 1992;3:264–271. [DOI] [PubMed] [Google Scholar]
- 40.Walsh S, Turner CM, Toye A, et al. Immunohistochemical comparison of a case of inherited distal renal tubular acidosis (with a unique AE1 mutation) with an acquired case secondary to autoimmune disease. Nephrol Dial Transplant. 2007;22:807–812. [DOI] [PubMed] [Google Scholar]
- 41.Bastani B, Haragsim L, Gluck S, et al. Lack of H-ATPase in distal nephron causing hypokalaemic distal RTA in a patient with Sjogren’s syndrome. Nephrol Dial Transplant. 1995;10:908–909. [PubMed] [Google Scholar]
- 42.Han JS, Kim GH, Kim J, et al. Secretory-defect distal renal tubular acidosis is associated with transporter defect in H(+)-ATPase and anion exchanger-1. J Am Soc Nephrol. 2002;13:1425–1432. [DOI] [PubMed] [Google Scholar]
- 43.Jordan M, Cohen EP, Roza A, et al. An immunocytochemical study of H+ ATPase in kidney transplant rejection. J Lab Clin Med. 1996;127: 310–314. [DOI] [PubMed] [Google Scholar]
- 44.Bastani B, Underhill D, Chu N, et al. Preservation of intercalated cell H(+)-ATPase in two patients with lupus nephritis and hyperkalemic distal renal tubular acidosis. J Am Soc Nephrol. 1997;8:1109–1117. [DOI] [PubMed] [Google Scholar]
- 45.Batlle D, Haque SK. Genetic causes and mechanisms of distal renal tubular acidosis. Nephrol Dial Transplant. 2012;27:3691–3704. [DOI] [PubMed] [Google Scholar]
- 46.Batlle DC. Segmental characterization of defects in collecting tubule acidification. Kidney Int. 1986;30:546–554. [DOI] [PubMed] [Google Scholar]
- 47.Berthelot M. Violet d’aniline. Rep Chim App. 1859;1:688. [Google Scholar]
- 48.Schiestl RH, Gietz RD. High efficiency transformation of intact yeast cells using single stranded nucleic acids as a carrier. Curr Genet. 1989;16: 339–346. [DOI] [PubMed] [Google Scholar]
- 49.Fernandez-Llama P, Khositseth S, Gonzales PA, et al. Tamm-Horsfall protein and urinary exosome isolation. Kidney Int. 2010;77:736–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peng SB. Nucleotide labeling and reconstitution of the recombinant 58-kDa subunit of the vacuolar proton-translocating ATPase. J Biol Chem. 1995;270:16926–16931. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Urinary creatinine concentration and osmolality during NH4Cl loading. Time 0 represents baseline (prior to treatment). Data are mean ± SEM; n = 8. No significant differences compared with baseline were observed.
Figure S2. Effect of NH4Cl loading on B subunit abundance in urinary exosomes. Immunoblots of urinary exosomes isolated from 7 different participants, probed with B1 (upper panel), B2 (middle panel), and alix (lower panel) antibodies.
Figure S3. Urinary creatinine concentration and osmolality during NaHCO3 loading. Time 0 represents baseline (prior to treatment). Data are mean ± SEM; n = 8. No significant differences compared with baseline were observed.
Figure S4. Effect of NaHCO3 loading on B subunit abundance in urinary exosomes. Immunoblots of urinary exosomes isolated from 7 different participants, probed with B1 (upper panel), B2 (middle panel) and alix (lower panel) antibodies.
Figure S5. Urinary Na (A), K (B), Cl (C), NH4 (D), creatinine (E), and osmolality (F) during NH4Cl loading in distal renal tubular acidosis patients. Time 0 represents baseline (prior to treatment).