INTRODUCTION
Early T-cell precursor acute lymphoblastic leukemia (ETP-ALL) is a recently recognized, high-risk subgroup of ALL characterized by early differentiation arrest and distinct genetic and transcriptional features. ETP-ALL represents 10% to 30% of T-cell ALL (T-ALL) cases in adults and 10% to 15% of cases in children, exhibits inferior response rates to chemotherapy, and is characterized by high relapse rates with dismal outcomes in the relapsed/refractory setting.1 Recent insights into the biology of ETP-ALL using BH3 profiling have revealed BCL-2 dependence2 with exquisite in vitro sensitivity to the BCL-2–selective antagonist venetoclax.3 Herein, we report a patient with refractory ETP-ALL and another with T-ALL and some features suggestive of the ETP subtype who had excellent responses to venetoclax given in combination with cytotoxic chemotherapy. Both patients received venetoclax off-label and provided written informed consent after receiving counseling about the unapproved nature of this treatment, the rationale for its use, and possible adverse effects from the medication.
PATIENT 1
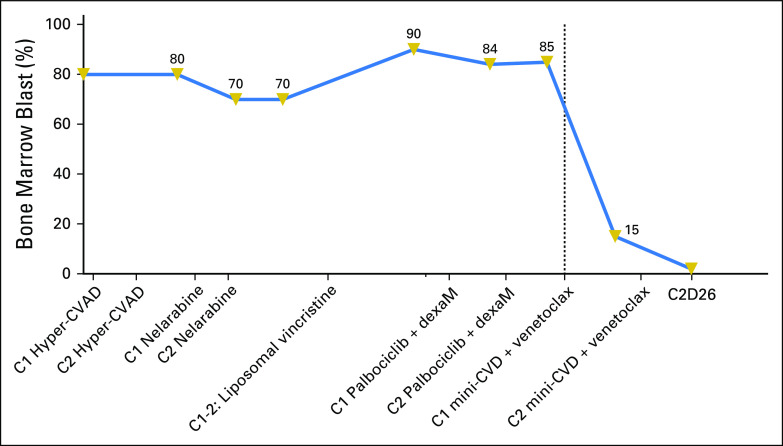
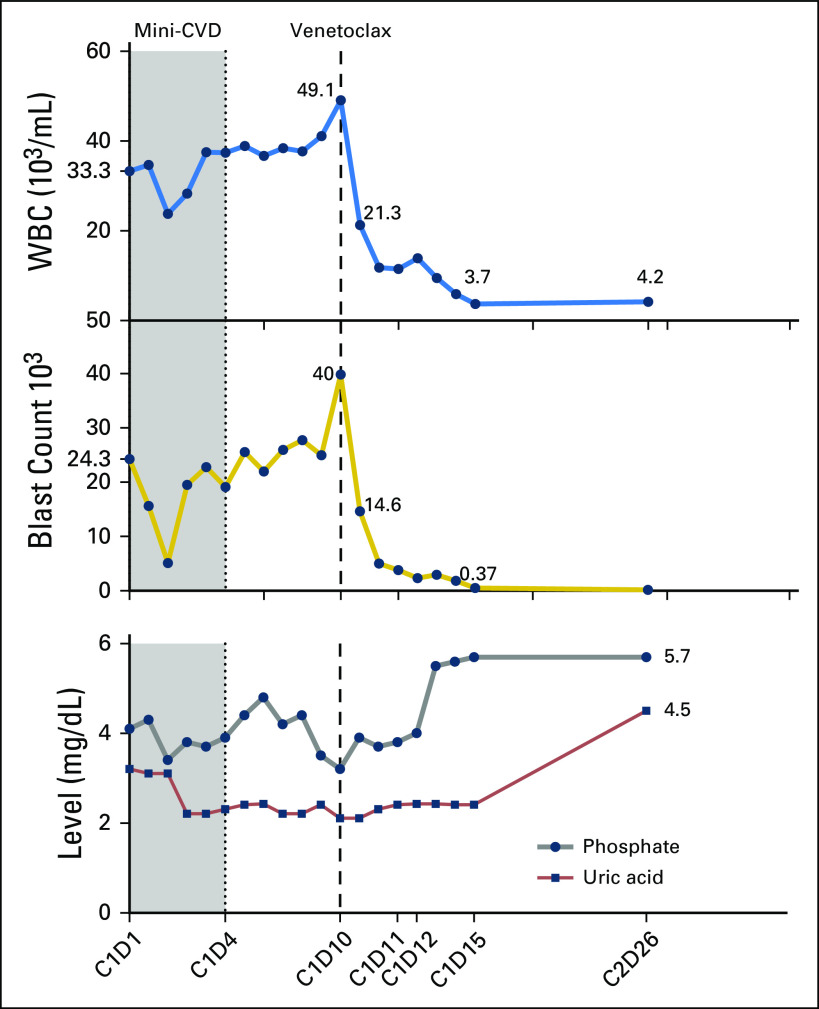
A 71-year-old woman was diagnosed with ETP-ALL in April 2017 with 80% bone marrow blasts. She received two cycles of hyperfractionated cyclophosphamide, vincristine, doxorubicin and dexamethasone (hyper-CVAD) alternating with high-dose methotrexate and cytarabine and was refractory to treatment. Subsequently, she experienced treatment failure during second-line therapy with nelarabine and third-line therapy with liposomal vincristine (Fig 1). At presentation to our center, she had 90% bone marrow blasts. Flow cytometry was positive for cytoplasmic CD3, CD4 (partial), CD5 (dim, small subset, 6.8%), CD7 (bright), CD10 (dim, small subset, 2%), CD13, CD33, CD34, CD38, CD45 (dim), CD56 (dim, small subset, 9%), CD117, CD123 (partial), and HLA-DR. Negative markers included CD1a, CD2, surface CD3, CD8, CD14, CD15, CD19, CD22, CD25, CD36, CD41, myeloperoxidase, and terminal deoxynucleotidyl transferase (TdT). Immunohistochemical staining revealed uniformly strong BCL-2 expression on the blasts (Fig 2D). Targeted next-generation sequencing (NGS) revealed mutations in ASXL1 and PTPN11. Cytogenetics revealed add(1)(p13) and t(7;13)(q36;q12). She was enrolled in a phase I clinical trial of palbociclib and dexamethasone and received two cycles with no response. The patient was then started on attenuated doses of hyper-CVAD (minus doxorubicin) alternating with methotrexate and cytarabine (mini-CVD)4 plus venetoclax. Venetoclax was started on cycle 1 day 10 at a dose of 100 mg and rapidly dose escalated to 400 mg by day 15 (Fig 2). A dramatic decrease in peripheral blood leukocyte and blast count after initiation of venetoclax was observed (Fig 3). The patient had hyperphosphatemia without evidence of frank tumor lysis, which was easily managed with oral phosphate binders (Fig 3). Bone marrow examination on cycle 1 day 24 showed a hypocellular marrow with 15.6% blasts by flow cytometry (down from 85% on the aspirate differential pretreatment). Venetoclax was continued throughout the first cycle. A change in antifungal prophylaxis from caspofungin to voriconazole necessitated lowering the dose of venetoclax to 100 mg once per day beginning with cycle 2. The second cycle of chemotherapy plus venetoclax was then initiated, and a repeat bone marrow examination performed on day 26 showed 40% cellularity with 2% blasts. Flow cytometry revealed positive minimal residual disease (MRD; 0.16%). Karyotype was diploid. A third cycle of mini-CVD was then administered, but venetoclax was held on day 31 of cycle 3 because of profound neutropenia and thrombocytopenia. At the time of writing, she remains in morphologic remission with progressively declining levels of MRD in the bone marrow (0.02% on cycle 3 day 44; 0.01% on cycle 3 day 78). There have been no significant changes in immunophenotype. Filgrastim was initiated on cycle 3 day 84 enabling resumption of venetoclax (without chemotherapy) on cycle 3 day 93.
Fig 1.
Timeline of treatment and bone marrow blast percentages for patient 1. C, cycle; DexaM, dexamethasone; hyper-CVAD, hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone alternating with methotrexate and cytarabine; mini-CVD, dose-attenuated cyclophosphamide, vincristine, and dexamethasone alternating with methotrexate and cytarabine.
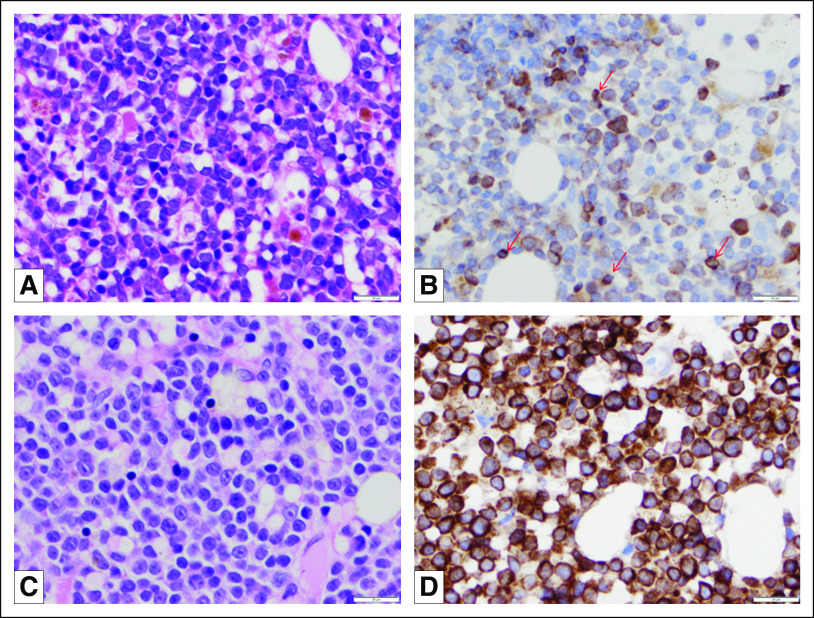
Fig 2.
Routinely stained sections of bone marrow core biopsy specimens obtained from patients with (A) T-cell acute lymphoblastic leukemia and (C) early T-cell precursor acute lymphoblastic leukemia demonstrate sheets of medium-sized blasts. Immunohistochemical studies performed with anti-BCL-2 antibody (clone 100/D5, Leica/Novocastra, Buffalo Grove, IL) on formalin-fixed, paraffin-embedded tissue of bone marrow core biopsy specimen of (B) the patient with T-cell acute lymphoblastic leukemia demonstrate that blasts show variable BCL-2 expression. The BCL-2 expression of blasts was weaker than the BCL-2 expression on intermixed small mature lymphocytes (arrows). In contrast, (D) the blasts of the patient with early T-cell precursor lymphoblastic leukemia demonstrated uniformly strong BCL-2 expression. Magnification, ×500.
Fig 3.
Trajectory of circulating leukocyte count (103/μL) and blast percentage from start of treatment for patient 1 with corresponding serum phosphate (mg/dL) and plasma uric acid levels (mg/dL), and absolute blast count (103/μL). A dramatic decrease in peripheral blood leukocyte and blast count was observed after initiation of venetoclax as shown by the dashed vertical line. C, cycle; D, day; mini-CVD, dose-attenuated cyclophosphamide, vincristine, and dexamethasone alternating with methotrexate and cytarabine; WBC, white blood cell count.
PATIENT 2
A 75-year-old man was referred to our center with an outside diagnosis of refractory secondary acute myeloid leukemia (AML). The patient had initially been diagnosed with myelodysplastic syndrome (MDS) in June 2016 and treated with azacitidine for six cycles before it was discontinued, reportedly because of pancytopenia. His MDS then apparently progressed to AML in August 2017, for which he received 7+3 induction. A day-28 bone marrow examination showed persistent disease and he was referred to us for salvage treatment. Initial bone marrow examination at our center in October 2017 showed 85% blasts with a phenotype consistent with T-ALL (positive for cytoplasmic CD3, CD5, CD7, CD10 (partial), CD13, CD34, CD38 (dim), CD56 (dim), CD117 (dim), and HLA-DR and negative for CD1a, CD2, surface CD3, CD4, CD8, CD14, CD15, CD19, CD22, CD25, CD33, CD36, CD41, CD64, CD123, myeloperoxidase, and TdT). The blasts lacked CD1a and CD8 expression, while co-expressing the myeloid markers CD13, CD117 (dim), and CD34, consistent with ETP-ALL; however, CD5 expression was strong, which argued against ETP-ALL. The blasts showed variable BCL-2 expression, significantly lower than that of intermixed small mature lymphocytes (Fig 2B). Cytogenetics revealed der(1;15), der(1;22), trisomy 1, and monosomy 7. Targeted NGS revealed mutations in NRAS, SRSF2, NOTCH1, and variants of uncertain origin in TET2. We reviewed his previous bone marrow slides performed at the outside facility (August 2017), which showed an acute leukemia (70% blasts) without good evidence for a myeloid immunophenotype and dysplastic granulocytes. Flow cytometry performed at the outside facility had shown expression of CD5 (moderate), CD7 (bright), CD10 (partial), CD11b (partial), CD13 (variable), CD33 (variable), CD34 (moderate), CD38 (moderate), CD45 (dim), CD56 (partial), CD117 (variable), CD123 (partial), and HLA-DR (partial), and karyotyping had revealed monosomy 7, trisomy 1, and der(1;22)(q10;q10). His original diagnosis was confirmed at our institution as MDS with 70% ring sideroblasts and a diploid karyotype, with no evidence of T-ALL. The reported immunophenotype of the blasts (7%) was positive for CD34, CD117, HLA-DR, CD13, CD33 (partial), CD38, CD7, myeloperoxidase, and cytoplasmic CD3 (subset), and negative for CD2, CD3, CD4, CD10, CD11b, CD14, CD15, CD16, CD19, CD56, CD64, CD123, and TdT. Given his borderline performance status and active coronary artery disease, the patient was treated with mini-CVD plus venetoclax. A remarkable response was noted in the peripheral blood leukocyte and blast counts. Bone marrow examination after cycle 1 showed an approximate 50% reduction in bone marrow blasts, and subsequent bone marrow evaluation after cycle 2 revealed morphologic remission (3% blasts) with positive MRD at 0.2% by flow cytometry. Dysgranulopoiesis was present. Monosomy 7 was undetectable by karyotyping or fluorescent in situ hybridization, although a new del(20q) was detectable by both techniques. Mutational analysis disclosed the known SRSF2 mutation and TET2 variants, and a new MPL variant of uncertain origin. The response, however, was transient, with a repeat bone marrow examination 4 weeks later showing persistent T-ALL with 10% blasts, background MDS, and del(20q) on karyotyping. The patient then received a third cycle of mini-CVD with venetoclax, and a follow-up bone marrow examination 4 weeks later was markedly hypocellular with no morphologic evidence of acute leukemia, but flow cytometry showed persistent MRD (T-ALL) at 1.2%. Unfortunately, approximately 3 weeks afterward, he was found to have relapsed with 62% bone marrow blasts; flow cytometry revealed 18.8% aberrant T lymphoblasts with no appreciable change in immunophenotype, whereas karyotyping and fluorescent in situ hybridization again showed del(20q) and monosomy 7. Serial NGS was not performed.
Clonal selection and survival advantage mediated by antiapoptotic BCL-2 family proteins have a central role in lymphoblast biology.5,6 ABT-263 (navitoclax), the clinical equivalent of the prototypical BH3-mimetic ABT-737,7 targeted both BCL-XL and BCL-2; however, severe thrombocytopenia as a result of BCL-XL inhibition in megakaryocytes limited its application in the clinic.8-10 Venetoclax (formerly ABT-199) is a BCL-2–selective BH3-mimetic that spares platelets while inducing apoptosis of tumor cells.6
By using BH3 profiling in patient-derived tumor xenograft models, Chonghaile et al3 demonstrated that T-ALL cells are, in general, dependent on BCL-XL for survival. They further demonstrated differential dependence on antiapoptotic proteins depending on the stage of maturation, with the more immature ETP-ALL demonstrating strong selective dependence on BCL-2. Other investigators have reported similar findings and shown synergism between venetoclax and cytotoxic chemotherapy against T-ALL cell lines and primary samples.11,12
To our knowledge, we report the first clinical response of ETP-ALL (case 1), which has been durable thus far, to venetoclax administered in combination with low-intensity chemotherapy. The other patient’s T-ALL response was also complete but transient, although ETP-ALL was less likely in that case. Furthermore, BCL-2 expression was uniformly strong in the first patient’s blasts, whereas it was variable in the blasts from the second patient and considerably weaker than the level of expression in mature lymphocytes from the same patient. This is in line with the preclinical data showing particular susceptibility of the ETP subtype to BCL-2 inhibition, with greater dependence on BCL-XL exhibited by other, more mature subtypes of T-ALL. Given these preclinical data and such anecdotal clinical responses, as well as preclinical evidence of efficacy of venetoclax against mixed lineage leukemia–rearranged B-cell ALL (B-ALL),13 a phase IB clinical trial (ClinicalTrials.gov identifier: NCT03319901) testing the combination of venetoclax and mini-CVD in previously untreated adults with ALL (both B- and T-ALL, including ETP-ALL) has been initiated at Dana-Farber Cancer Institute and MD Anderson Cancer Center. Patients with Philadelphia chromosome–positive ALL, Burkitt’s lymphoma/leukemia, or lymphoblastic lymphoma are excluded. Venetoclax is administered on days 1 to 21 of a 28-day cycle. Changes in expression of both pro- and antiapoptotic proteins of the BCL-2 family are monitored throughout.
AUTHOR CONTRIBUTIONS
Conception and design: Yazan Numan, Mansour Alfayez, Abhishek Maiti, Yesid Alvarado, Elias J. Jabbour, Alessandra Ferrajoli, Hagop Kantarjian, Prithviraj Bose
Provision of study materials or patients: Yesid Alvarado, Elias J. Jabbour, Alessandra Ferrajoli, Sergej N. Konoplev, Prithviraj Bose
Collection and assembly of data: Yazan Numan, Mansour Alfayez, Abhishek Maiti, Sergej N. Konoplev, Prithviraj Bose
Data analysis and interpretation: Yazan Numan, Mansour Alfayez, Abhishek Maiti, Yesid Alvarado, Elias J. Jabbour, Alessandra Ferrajoli, Sergej N. Konoplev, Prithviraj Bose
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Yazan Numan
No relationship to disclose
Mansour Alfayez
No relationship to disclose
Abhishek Maiti
Research Funding: Celgene (Inst)
Yesid Alvarado
No relationship to disclose
Elias J. Jabbour
Consulting or Advisory Role: Pfizer
Research Funding: Pfizer
Alessandra Ferrajoli
Research Funding: Celgene, Genentech, Acerta Pharma
Sergej N. Konoplev
No relationship to disclose
Hagop M. Kantarjian
Honoraria: AbbVie, Amgen, ARIAD Pharmaceuticals, Bristol-Myers Squibb, Immunogen, Orsenix, Pfizer
Research Funding: Pfizer (Inst), Amgen (Inst), Bristol-Myers Squibb (Inst), Novartis (Inst), ARIAD Pharmaceuticals (Inst), Astex Pharmaceuticals (Inst)
Prithviraj Bose
Honoraria: Incyte
Consulting or Advisory Role: Celgene
Research Funding: Celgene (Inst), CTI BioPharma (Inst), Incyte (Inst), Blueprint Medicines (Inst), NS Pharma
(Inst), Gilead Sciences (Inst), Promedior (Inst)
Travel, Accommodations, Expenses: Incyte, Celgene
REFERENCES
- 1.Jain N, Lamb AV, O’Brien S, et al. Early T-cell precursor acute lymphoblastic leukemia/lymphoma (ETP-ALL/LBL) in adolescents and adults: A high-risk subtype. Blood. 2016;127:1863–1869. doi: 10.1182/blood-2015-08-661702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Del Gaizo Moore V, Schlis KD, Sallan SE, et al. BCL-2 dependence and ABT-737 sensitivity in acute lymphoblastic leukemia. Blood. 2008;111:2300–2309. doi: 10.1182/blood-2007-06-098012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chonghaile TN, Roderick JE, Glenfield C, et al. Maturation stage of T-cell acute lymphoblastic leukemia determines BCL-2 versus BCL-XL dependence and sensitivity to ABT-199. Cancer Discov. 2014;4:1074–1087. doi: 10.1158/2159-8290.CD-14-0353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kantarjian H, Ravandi F, Short NJ, et al. Inotuzumab ozogamicin in combination with low-intensity chemotherapy for older patients with Philadelphia chromosome-negative acute lymphoblastic leukaemia: A single-arm, phase 2 study. Lancet Oncol. 2018;19:240–248. doi: 10.1016/S1470-2045(18)30011-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Merino R, Ding L, Veis DJ, et al. Developmental regulation of the Bcl-2 protein and susceptibility to cell death in B lymphocytes. EMBO J. 1994;13:683–691. doi: 10.1002/j.1460-2075.1994.tb06307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Souers AJ, Leverson JD, Boghaert ER, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med. 2013;19:202–208. doi: 10.1038/nm.3048. [DOI] [PubMed] [Google Scholar]
- 7.Oltersdorf T, Elmore SW, Shoemaker AR, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 8.Roberts AW, Advani RH, Kahl BS, et al. Phase 1 study of the safety, pharmacokinetics, and antitumour activity of the BCL2 inhibitor navitoclax in combination with rituximab in patients with relapsed or refractory CD20+ lymphoid malignancies. Br J Haematol. 2015;170:669–678. doi: 10.1111/bjh.13487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roberts AW, Seymour JF, Brown JR, et al. Substantial susceptibility of chronic lymphocytic leukemia to BCL2 inhibition: Results of a phase I study of navitoclax in patients with relapsed or refractory disease. J Clin Oncol. 2012;30:488–496. doi: 10.1200/JCO.2011.34.7898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson WH, O’Connor OA, Czuczman MS, et al. Navitoclax, a targeted high-affinity inhibitor of BCL-2, in lymphoid malignancies: A phase 1 dose-escalation study of safety, pharmacokinetics, pharmacodynamics, and antitumour activity. Lancet Oncol. 2010;11:1149–1159. doi: 10.1016/S1470-2045(10)70261-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson NM, Harrold I, Mansour MR, et al. BCL2-specific inhibitor ABT-199 synergizes strongly with cytarabine against the early immature LOUCY cell line but not more-differentiated T-ALL cell lines. Leukemia. 2014;28:1145–1148. doi: 10.1038/leu.2013.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peirs S, Matthijssens F, Goossens S, et al. ABT-199 mediated inhibition of BCL-2 as a novel therapeutic strategy in T-cell acute lymphoblastic leukemia. Blood. 2014;124:3738–3747. doi: 10.1182/blood-2014-05-574566. [DOI] [PubMed] [Google Scholar]
- 13.Khaw SL, Suryani S, Evans K, et al. Venetoclax responses of pediatric ALL xenografts reveal sensitivity of MLL-rearranged leukemia. Blood. 2016;128:1382–1395. doi: 10.1182/blood-2016-03-707414. [DOI] [PMC free article] [PubMed] [Google Scholar]