Abstract
Cadherin-mediated cell-cell adhesion is a dynamic process that is regulated during embryonic development, cell migration, and differentiation. Different cadherins are expressed in specific tissues consistent with their roles in cell type recognition. In this study, we examine the formation of N-cadherin–dependent cell-cell contacts in fibroblasts and myoblasts. In contrast to E-cadherin, both endogenous and ectopically expressed N-cadherin shuttles between an intracellular and a plasma membrane pool. Initial formation of N-cadherin–dependent cell-cell contacts results from the recruitment of the intracellular pool of N-cadherin to the plasma membrane. N-cadherin also localizes to the Golgi apparatus and both secretory and endocytotic vesicles. We demonstrate that the intracellular pool of N-cadherin is tightly associated with the microtubule (MT) network and that junction formation requires MTs. In addition, localization of N-cadherin to the cortex is dependent on an intact F-actin cytoskeleton. We show that N-cadherin transport requires the MT network as well as the activity of the MT-associated motor kinesin. In conclusion, we propose that N-cadherin distribution is a regulated process promoted by cell-cell contact formation, which controls the biogenesis and turnover of the junctions through the MT network.
INTRODUCTION
Cell-cell adhesion molecules are essential for the organization of cells into tissues during embryonic development. They are also involved in cell growth, migration, and differentiation (Takeichi, 1991; Gumbiner, 1996). Cell-cell adhesion is often modified in cancer cells and during cell invasion (Takeichi, 1993). Cadherins found in adherens junctions constitute the major family of transmembrane glycoproteins that mediate cell-cell adhesion by virtue of their ability to self-associate in a Ca2+-dependent manner. This homophilic binding is mediated by the N-terminal extracellular domain, which consists of five 110 amino acid repeats (EC1-EC5). Cadherins provide sites of attachment to the actin cytoskeleton through the binding of cytoplasmic proteins, called catenins (Kemler, 1993). β-Catenin and γ-catenin (plakoglobin) bind directly to the distal region of the cadherin cytoplasmic tail and interact with α-catenin, which associates with actin filaments (Jou et al., 1995). A fourth catenin, the phosphoprotein p120 interacts with the juxtamembrane region of cadherins, thereby modulating their dimerization and adhesive function (Yap et al., 1998; Ohkubo and Ozawa, 1999). Changes in the composition or phosphorylation of cadherin/catenin complexes or in the interaction with the actin cytoskeleton have all been suggested to play a role in regulation of adhesion (Gumbiner, 2000).
Adherens junctions are highly dynamic structures that turnover rapidly. During embryonic development and tumor progression, changes in cadherin function and availability at cell-cell contacts have been reported. For example, in epithelium-mesenchyme transitions, which occur during specific stages of embryonic development but also under pathological conditions, intercellular adhesions are disrupted through down-regulation of E-cadherin activity (Takeichi, 1991). Similarly, during migration of neural crest cells regulation of the localization and function of N-cadherin has been reported (Akitaya and Bronner-Fraser, 1992; Monier-Gavelle and Duband, 1995). One possible mechanism for modulation of adhesive function could occur through the turnover of cadherins at the cell surface. The availability of cadherins might be modulated through changes in either secretory or endocytotic pathways. The observation that a recycling E-cadherin pool increases in the absence of stable cell-cell contacts supports this hypothesis (Le et al., 1999).
To learn about the biogenesis of cell-cell junctions and the mechanisms regulating trafficking toward the junctions, we have examined the dynamics of cell-cell contact formation by using a fully functional fusion protein of N-cadherin and green fluorescent protein (Ncad/GFP). We described Ncad/GFP distribution in living cells and demonstrated that Ncad/GFP-containing vesicular structures associate with and move along MTs in a kinesin-dependent way. Our data revealed that cell-cell contact formation can control intracellular Ncad/GFP localization and transport. We also conclude that Ncad/GFP-regulated transport participates in the biogenesis of N-cadherin–dependent cell-cell junctions.
MATERIALS AND METHODS
DNA Constructs
The complete murine N-cadherin coding sequence (nucleotides 220-3050 of the cDNA, accession number AB008811) was cloned into the pEGFP-N1 vector (CLONTECH, Palo Alto, CA). The cyan fluorescent protein (CFP)-Ncad was obtained by replacing the GFP cassette with the CFP coding sequence. The vesicular stomatitis virus-G protein (VSVG)/yellow fluorescent protein (YFP) was obtained from J. White (White et al., 1999).
Cells Culture, Transfection, and Microinjection
Rat embryo (REF-52) fibroblasts or mouse C2 myoblasts were cultured at 37°C in the presence of 5% CO2 in DMEM supplemented with 10% fetal calf serum. Cells were plated on 18-mm-diameter glass coverslips 16–24 h before transfection (Meriane et al., 2000). Cells were transfected with plasmids encoding Ncad/GFP or empty vector (pEGFPN1) as described previously (Gauthier et al., 1998). Four hours after transfection with Ncad/CFP and VSVG/YFP, cells were incubated at 40°C for 15 h then shifted to 32°C and fixed at different times thereafter. To disrupt MTs, cells were incubated with 1 μM nocodazole (Nz) for 15–60 min. EGTA was used at 0.1 mM for 30–60 min. Cycloheximide was used at 10 μg/ml for 8–10 h.
Ncad/GFP-expressing cells were microinjected with H2 anti-kinesin or control mouse IgG antibodies (1 mg/ml in 100 mM HEPES pH 7.2, mixed 1:1 with rhodamine-labeled dextran [0.5 mg/ml-1 in 100 mM potassium glutamate, 39 mM potassium citrate]). After microinjection, time-lapse sequences were recorded or cells were fixed and processed for immunocytochemistry.
Western Blot Analysis of GFP-tagged Cadherin Contructs
REF-52 cells transfected with either empty pEGFPN1 vector (MOCK) or Ncad/GFP were lysed in 2% boiling SDS, 10 mM Tris-HCl pH 7.4. Samples (50 μg of protein) were loaded on an 8% polyacrylamide gel and transferred onto nitrocellulose. Membranes were saturated in 8% milk in Tris-HCl pH 7.5 containing 1% Tween 20 (TBST) for 1 h and incubated with a monoclonal antibody directed against the GFP tag (dilution 1:1000 in 4% nonfat milk-TBST; Zymed Laboratories, South San Francisco, CA) followed by a peroxidase-conjugated anti-mouse antibody (Amersham Biosciences, Piscataway, NJ). After washing, membranes were incubated with chemiluminescence reagents (enhanced chemiluminescence; PerkinElmer Life Sciences, Boston, MA). Protein quantification (bicinchoninic acid; Sigma, Saint Quentin Fallavier, France) was performed on the recovered lysates. Autoradiographs were scanned and images obtained in Adobe Photoshop were assembled in Adobe Illustrator (Adobe Systems, Mountain View, CA).
Immunoprecipitation
REF-52 cells transfected with either empty pEGFPN1 vector (MOCK) or Ncad/GFP were lysed for 20 min in ice-cold modified RIPA buffer (1% Triton X-100, 10 mM NaPPi, 0.1% SDS, 1% sodium deoxycholate, 10% glycerol, 20 mM Tris-HCl pH 7.4, 150 mM NaCl, 2.5 mM EDTA) supplemented with 20 mM β-glycerophosphate, 1 mM phenylmethylsulfonyl fluoride, 20 mM NaF, 100 μM Na3VO4. Extracts were immunoprecipitated using an anti-GFP antibody (1/100 dilution), separated on a 10% polyacrylamide gel, and then transferred onto nitrocellulose. Membranes were probed with β-catenin, γ-catenin, and p120 antibodies (Transduction Laboratories, Lexington, KY) followed by peroxidase-conjugated anti-mouse antibody (Amersham Biosciences). After washing, membranes were incubated with chemiluminescence reagent (enhanced chemiluminescence; PerkinElmer Life Sciences) and analyzed with a PhosphorImager (Molecular Dynamics, Sunnyvale, CA).
Subcellular Fractionation
Isolated, confluent, and EGTA-treated confluent C2 myoblasts were lysed in cold hypotonic buffer containing 10 mM Tris-HCl pH 7.5, 5 mM MgCl2, 1 mM dithiothreitol, and 1 mM phenylmethylsulfonyl fluoride as described (Gauthier et al., 1998). Cell extracts were centrifuged (600 × g for 5 min at 4°C) to pellet nuclei and nuclei-associated structures, including the Golgi and endoplasmic reticulum membranes (P1). Supernatants were ultracentrifugated (100,000 × g for 45 min) to separate cytoplasmic membranes (P100) and cytosolic proteins (S100). Samples were fractionated on a 12.5% SDS-polyacrylamide gel and transferred to nitrocellulose membranes. Membranes were blotted with an anti-N-cadherin (Transduction Laboratories) and anti-α-tubulin antibodies.
Purification of MTs and Associated Proteins
Isolated, confluent, and nocodazole-treated confluent Ncad/GFP-transfected REF-52 cells were trypsinized and sedimented at 2000 rpm for 5 min. The cell pellet was resuspended in 3 volumes of PEM [100 mM piperazine-N,N′-bis(2-ethanesulfonic acid) pH 6.9, 5 mM EGTA, 1 mM MgSO4, 100 mM dithiothreitol] containing 900 mM glycerol and the complete protease inhibitor cocktail (Roche Molecular Biochemicals, Mannheim, Germany). After sonication, cells extracts were centrifugated at 100,000 × g for 60 min at 4°C. The pellet (C2), which contains nuclei and associated internal membranes (Golgi, endoplasmic reticulum), was resuspended in PEM/glycerol and later analyzed by immunoblotting. The supernatant was incubated with 10 μM taxol and 0.5 mM GTP for 30 min, layered on a 10% sucrose cushion in PEM, and centrifugated at 40,000 × g for 30 min. Pellet (C3; microtubule-associated protein [MAP]-containing microtubules) and supernatant (S3; MAP depleted supernatant) were analyzed by SDS-PAGE and Western blotting for Ncad/GFP, α-tubulin, and a known MT-associated protein TOGp (Charrasse et al., 1998).
Immunocytochemistry
Eighteen hours after transfection, cells were fixed for 5 min in 3.7% formalin (in PBS) followed by a 2-min permeabilization in 0.1% Triton X-100 (in PBS) and incubation in PBS containing 0.1% bovine serum albumin (BSA). Alternatively, cells were fixed for 20 min in a microtubule-stabilizing buffer containing 3% formalin, 0.05% glutaraldehyde, 0.05% Triton X-100 in 60 mM piperazine-N,N′-bis(2-ethanesulfonic acid), 25 mM HEPES pH 6.9, 10 mM EGTA, and 10 mM MgCl2 followed by 2-min permeabilization with 0.2% Triton X-100 in 50 mM Tris-HCl pH 7.5 and 150 mM NaCl. Cells were stained for F-actin by using tetramethylrhodamine B isothiocyanate (TRITC) or coumarin isothiocyanate (CPITC)-conjugated phalloidin (Sigma). Cells were stained for vimentin by using a mouse monoclonal anti-vimentin antibody (Sigma) and for microtubules by using an anti-α-tubulin antibody (P. Mangeat, Centre National de la Recherche Scientifique, Montpellier, France). cis-, medial-, and trans-compartments of the Golgi apparatus were stained using mouse antibodies against p115 (Transduction Laboratories), CTR433 (1 10; M. Bornens, Curie Institute, Paris, France) and TGN38 (Transduction Laboratories). Anti-β-catenin and anti-N-cadherin antibodies are from Transduction Laboratories. All these mouse antibodies were revealed with an affinity-purified tetramethylrhodamine B isothiocyanate-conjugated goat anti-mouse antibody (Cappel Laboratories, Durham, NC).
For the localization of endocytotic compartments, 20 h after transfection, cells were incubated 45 min at 37°C with rhodamin-labeled human transferrin (20 μg/ml, rhod-Tf; Molecular Probes, Eugene, OR). Cells were rinsed twice with DMEM containing 1% BSA, fixed with 3.7% paraformaldehyde containing 30 mM sucrose for 15 min at room temperature, and incubated 10 min with 30 mM NH4Cl before permeabilization in 0.2% BSA, 0.05% saponin in PBS for 15 min.
Cells were prepared and observed as described (Gauthier et al., 1998). Images were captured with a MicroMax 1300 charge-coupled device camera (Princeton Instruments, Trenton, NJ) driven by MetaMorph (version 4.11; Universal Imaging, Westchester, PA) software. Images were processed using Adobe Photoshop and Adobe Illustrator.
Confocal Laser Scanning Microscopy
Dual-channel confocal laser scanning microscopy was performed using the Bio-Rad (Hercules, CA) MRC 1024 confocal laser scanning microscope equipped with an argon/krypton ion laser. For all experiments, at least 50 transfected cells were examined. Images were collected sequentially to avoid cross-contamination between the fluorochromes. For colocalization analysis, individual confocal optical sections were processed using the colocalization analysis Bio-Rad Lasersharp software.
Deconvolution and Colocalization
Stacks of 16-bit files (Z step 0.1 μm) were captured as described above and epifluorescence images were first restored with Huygens (Scientific Volume Imaging b.v, Hilversum, The Netherlands). Huygens is an iterative program that can reassign light, after encoding as gray level, to its sources in the stack with a very high probability by using a point spread function. This results in removing the fuzziness contained in the stack, while keeping the three-dimensional information. In the present study, the maximum likelihood estimation algorithm was used throughout. Restored stacks were then further processed with Imaris (Bitplane, Zurich, Switzerland), for visualization and volume rendering. Respective colocalization of Ncad/GFP, tubulin, vimentin, and F-actin fluorescence were analyzed with the Imaris Colocalization module.
Time-Lapse Imaging
Time-lapse epifluorescence microscopy was performed on a Leica DM IRBE (Leica, Wetzlar, Germany) inverted microscope equipped with an automatic shutter and GFP filter sets, a 63× oil immersion objective (numerical aperture 1.3; Leica), sample heater (37°C), and a home-made CO2 incubation chamber. To minimize bleaching and phototoxicity, fluorescence illumination was supplied by a halogen bulb (100 W). Images were captured with a MicroMax 1300 charge-coupled device camera (Princeton Instruments) driven by MetaMorph (version 4.11; Universal Imaging) imaging software, converted to TIF files that were edited with NIH Image and compiled into QuickTime movies. The exposure time was fixed to 500 ms.
RESULTS
Expression of Ncad/GFP Fusion Protein
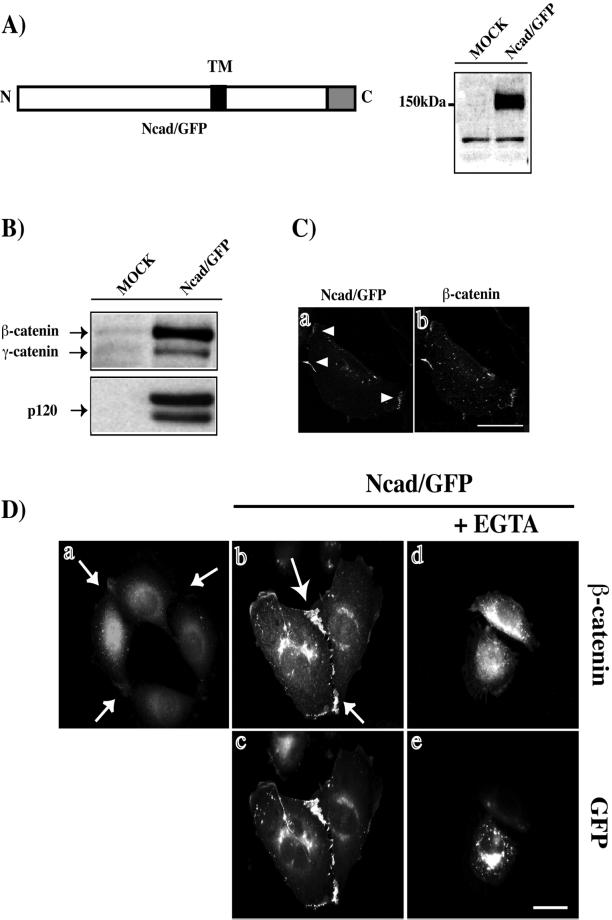
To better understand the biogenesis of adherens junctions, we examined the transport of N-cadherin to the plasma membrane in living cells, by using a mouse N-cadherin tagged with GFP at the C terminus (Figure 1A). Before analyzing the transport of the fusion protein by video microscopy, we ensured that Ncad/GFP was expressed as a full-length protein, correctly localized, and possessed adhesive properties similar to those of endogenous cadherins. Ncad/GFP expressed in REF-52 cells displayed the expected apparent molecular mass (∼150 kDa) consistent with the combined molecular masses of N-cadherin and GFP (Figure 1A). We next examined the ability of catenins to bind to the Ncad/GFP fusion protein. As expected, β-, γ-, and p120 catenins coimmunoprecipitated with Ncad/GFP (Figure 1B).
Figure 1.
Ncad/GFP has properties similar to those of endogenous N-cadherin. (A) GFP was fused to the C terminus of murine N-cadherin (Ncad/GFP). TM, transmembrane region; Greybox, GFP. Cell lysates of Ncad/GFP or mock-transfected REF-52 fibroblasts were analyzed by SDS-PAGE and immunoblotting with an anti-GFP antibody. (B) Cell lysates of Ncad/GFP or mock-transfected REF-52 fibroblasts were immunoprecipitated using an anti-GFP antibody and immunoblotted for the presence of β, γ-catenins and p120. (C) Ncad/GFP-expressing REF-52 fibroblasts were monitored for GFP fluorescence (a) and β-catenin localization (b) by using confocal microscopy. (D) Parental (a) or L cells transfected with plasmids encoding Ncad/GFP (b–e) were stained for β-catenin distribution (a, b, and d). In d and e, cells were treated for 60 min with EGTA. Cells shown are representative of more than 100 observed cells. Bar, 10 μm.
We then examined the colocalization of Ncad/GFP and β-catenin by confocal microscopy (Figure 1C). Ncad/GFP (Figure 1C, a) and β-catenin (Figure 1C, b) were closely associated at cell-cell junctions. Similar Ncad/GFP and β-catenin association was observed in C2 myoblasts (our unpublished results). Mouse L cells do not express cadherin and show no recruitment of β-catenin to cell-cell contact sites (Figure 1D, a). In these cells, expression of Ncad/GFP resulted in the formation of cell-cell attachments rich in β-catenin (Figure 1D, b and c), which were lost in the absence of extracellular Ca2+ (Figure 1D, d and e). Similarly, expression of Ncad/GFP in L cells resulted in the formation of Ca2+-dependent cell aggregates in suspension culture (our unpublished results). Taken together, these data show that Ncad/GFP has properties similar to those of endogenous cadherins and constitutes a suitable tool to study the transport of N-cadherin to the plasma membrane.
Redistribution of Ncad/GFP upon Cell-Cell Contact Formation
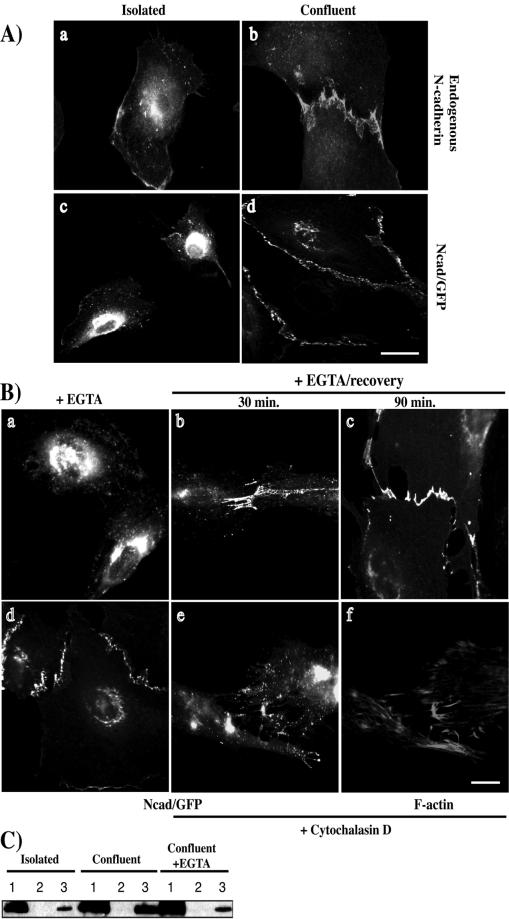
In neural crest cells, an intracellular pool of N-cadherin has been described, which might be recruited to the plasma membrane when stable cell-cell contacts are formed (Monier-Gavelle and Duband, 1997). We thus decided to study both endogenous N-cadherin and ectopically expressed Ncad/GFP in cells grown at different densities (Figure 2A). Whereas N-cadherin was cytoplasmic and perinuclear in isolated C2 myoblasts (Figure 2A, a), cell-cell contact formation was accompanied by N-cadherin accumulation at the plasma membrane (Figure 2A, b). Ncad/GFP localization in REF-52 cells exhibited the same distribution as endogenous N-cadherin in C2 cells. In isolated cells, the Ncad/GFP fusion protein exhibited a punctate distribution throughout the cytoplasm as well as a marked concentration in the perinuclear region (Figure 2A, c). However, when Ncad/GFP-expressing cells established cell-cell contacts we observed a decrease of the perinuclear and cytoplasmic fluorescence and an accumulation of Ncad/GFP at the plasma membrane (Figure 2A, d). When an Ncad/GFP-expressing cell was surrounded by nonexpressing cells, the distribution of Ncad/GFP was similar to that observed in isolated Ncad/GFP-expressing cells (our unpublished results). GFP protein was localized diffusely throughout the cytoplasm, independent of cell confluence (our unpublished results). These data show that both endogenous N-cadherin and Ncad/GFP shuttle between an intracellular pool and the plasma membrane depending on cell confluence and on the propensity for establishment of homophilic interactions.
Figure 2.
N-cadherin localization is dependent on cell confluence. (A) Isolated (a) and confluent C2 myoblasts (b) were stained for endogenous N-cadherin. Alternatively, exponentially growing REF-52 fibroblasts were transfected with a plasmid encoding Ncad/GFP. Cells were fixed 20 h after transfection and monitored for GFP fluorescence in either isolated (c) or confluent cells (d). (B) Ncad/GFP-expressing cells were treated with EGTA for 30 min (a) followed by a 30- or a 90-min rinse in complete medium (b and c, respectively). Ncad/GFP-expressing cells were treated with cytochalasin D for 15 min (e and f). Control untreated Ncad/GFP-expressing cells are shown in d. After fixation cells were monitored for GFP fluorescence (a–e) or stained with rhodamine-labeled phalloidin (f). Cells shown are representative of >100 observed cells. Bar, 10 μm. (C) Isolated, confluent, or EGTA-treated confluent C2 were lysed in the absence of detergent. Nuclei and associated membranes were pelleted by low-speed centrifugation (lane 1). The supernatant was then subjected to ultracentrifugation, resulting in the isolation of cytosolic extracts (lane 2) and plasma membranes (lane 3). Equal concentrations of proteins from each fraction were analyzed by Western blotting with an anti-N-cadherin antibody.
To further investigate the importance of cell-cell contacts on N-cadherin distribution, we treated cells with EGTA to chelate extracellular Ca2+ (Figure 2B). Within 10–15 min Ncad/GFP accumulated at the perinuclear region and was excluded from the junctions (Figure 2B, a). Restoration of extracellular Ca2+ rapidly resumed Ncad/GFP at cell-cell contact sites and reduced the intracellular fluorescence (Figure 2B, b). After 90 min, Ncad/GFP distribution was comparable to untreated confluent cells (compare Figure 2B, c to A, d). These data show that the function of the N-cadherin extracellular domain is required for plasma membrane localization, preventing cadherin redistribution to perinuclear and cytoplasmic compartments. Because cadherin-mediated cell-cell adhesion has been reported to depend on the ability of cadherin to bind the F-actin cytoskeleton (Matsuzaki et al., 1990), we examined whether Ncad/GFP distribution would be affected by F-actin cytoskeleton disorganization. In cells treated with cytochalasin D (Figure 2B, f), Ncad/GFP was barely detectable at the junctions (compare Figure 2B, e with untreated Ncad/GFP-expressing cells in Figure 2B, d). Its distribution resembled what we observed after EGTA treatment. This demonstrates that the integrity of the F-actin cytoskeleton is required to maintain N-cadherin at the plasma membrane.
Quantitative Western blot analysis of the subcellular distribution of endogenous N-cadherin in isolated versus confluent C2 myoblasts (Figure 2C) revealed that in isolated cells, N-cadherin predominantly associated with nuclear-bound membranes (lane 1), and only a minor amount was recovered in the plasma membrane fraction (lane 3). In contrast, in confluent cells a much greater proportion of N-cadherin was present in the plasma membrane-containing fraction (lane 3). On EGTA treatment little N-cadherin was found in the plasma membrane-containing fraction (lane 3). No protein could be detected in the cytosolic fraction (lanes 2). The same protein concentration was used in all three conditions as monitored by α-tubulin quantification (our unpublished results). Similar results were obtained for Ncad/GFP expressed in REF-52 cells (our unpublished results). These results confirm that endogenous N-cadherin redistributes from an intracellular pool to cell-cell contact sites and that this is dependent on cell confluence and the functionality of the N-cadherin extracellular domain.
In summary, these results suggest that cell-cell contact is one of the regulatory mechanisms that controls the assembly of N-cadherin–based junctions.
Intracellular Ncad/GFP Localizes to Golgi Apparatus and into Endocytotic and Secretory Vesicles
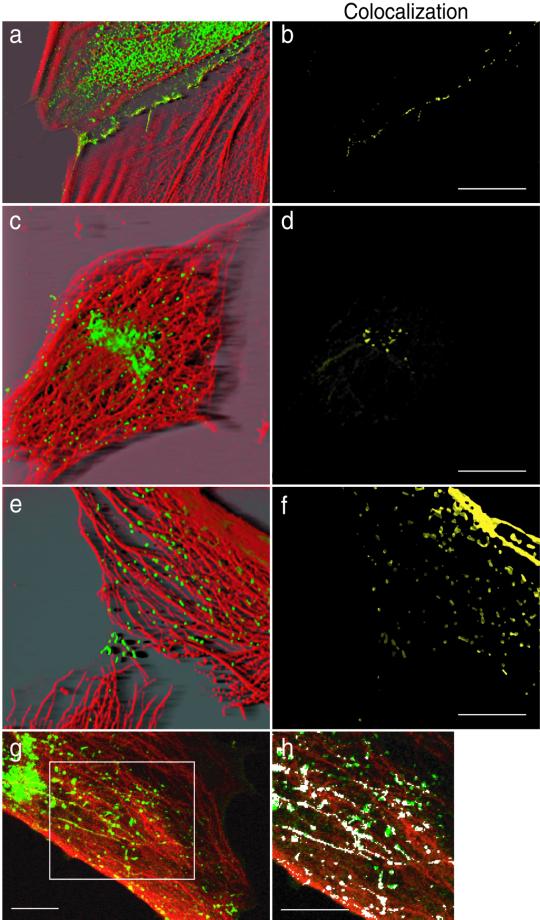
To analyze the subcellular localization of Ncad/GFP, REF-52 fibroblasts were transfected with Ncad/GFP and fixed and stained with p115, CTR433, and TGN38 antibodies recognizing the cis-, medial-, and trans-compartments of the Golgi apparatus, respectively. Confocal analysis revealed that perinuclear Ncad/GFP corresponded to the three Golgi stacks (Figure 3A, a–c). Colocalizing red and green pixels in a single confocal plan are pseudocolored in white, and the Golgi staining alone is shown in each inset. Similar results were obtained with endogenous N-cadherin in C2 cells (our unpublished results).
Figure 3.
Intracellular localization of Ncad/GFP. (A) Ncad/GFP-expressing cells were fixed and stained with antibodies against the cis- (p115) (a, inset), medial- (CTR433) (b, inset), and trans-compartments (TGN38) (c, inset) of the Golgi apparatus. Cells were monitored for GFP fluorescence (a–c, green). Shown are confocal images, colocalized red and green pixels appear in white. Cells shown are representative of >100 observed cells. Bar, 10 μm. (B) Precontacting or fully contacting Ncad/GFP-expressing cells were incubated with rhod-Tf for 45 min. After fixation, cells were monitored for GFP (green) and rhodamine fluorescence (red and a and b, insets). Confocal sections revealing the colocalization of Ncad/GFP and rhod-Tf (a and b, white) are shown. (C) Ncad/CFP and VSVG/YFP-expressing cells were monitored for CFP and YFP (a). Colocalization analysis was performed using the Imaris Colocalization module (b).
We next wanted to determine whether the punctate vesicular pattern of Ncad/GFP might be attributed to endocytotic vesicles as described for E-cadherin (Le et al., 1999). Ncad/GFP-expressing cells were incubated with rhod-Tf for 45 min to visualize the entire endocytotic pathway. Confocal analysis showed a partial colocalization (shown in white) of Ncad/GFP and rhod-Tf in isolated or precontacting cells (Figure 3B, a). The percentage of colocalization decreased when cell-cell contacts were formed (Figure 3B, b). Quantitative analysis performed on numerous cells showed that in isolated or precontacting cells ∼30% of the Ncad/GFP signal colocalized with the rhod-Tf signal, whereas this value dropped to ∼10% in contacting cells. We have also found colocalization of N-cadherin–containing vesicles with both Rab5 and EEA1 (our unpublished results), providing strong evidence of N-cadherin recycling through the early endosomal compartment.
Finally, to determine whether N-cadherin was also associated with secretory vesicles, we performed colocalization analysis of N-cadherin fused to CFP (Ncad/CFP) and the vesicular stomatitis virus ts045 G protein fused to YFP (VSVG/YFP). This thermoreversible VSVG folding mutant has been widely used to study membrane transport in living cells. The misfolded protein is retained in the endoplasmic reticulum at 40°C and moves to the Golgi and the plasma membrane after a temperature shift to 32°C (Figure 3C, a) (Hirschberg et al., 1998). Colocalization analysis with N-cadherin by using the Imaris Colocalization module was performed on fixed cells 60 min after the shift from 40 to 32°C to maximize the amount of VSVG/YFP between the Golgi and the plasma membrane. Ncad/CFP and VSVG/YFP colocalization was observed in all expressing cells (Figure 3C, b).
To further confirm the presence of N-cadherin in the secretory pathway, live REF-52 cells expressing Ncad/GFP were analyzed by time-lapse microscopy. Figure 4A (video) shows a video sequence of Ncad/GFP-containing vesicles and tubules emerging from the Golgi region (arrows) and moving toward the edge of the cell. These tubules sometimes divided into smaller structures (asterisk), which followed different tracks and displayed elastic properties (extension and retraction during movement) (black arrow). Both tubules and vesicles moved at rates of 0.35 ± 0.1 μm/s. Similar structures have been described in VSVG-GFP and neurotrophin receptor p75-GFP post-Golgi traffic (Kreitzer et al., 2000). Fusions of these vesicles with the plasma membrane were visible especially at the junctions (Figure 4B). The black arrow shows a vesicle moving toward the cell edge close to a cell-cell contact (J), which disperses into the plasma membrane after a short resting phase. The white arrow indicates a vesicle that did not fuse with the plasma membrane which remains visible over this period. Thus, our data demonstrate that N-cadherin localizes to the Golgi apparatus, and endocytotic and secretory vesicles in addition to its plasma membrane localization.
Figure 4.
Post-Golgi transport and fusion at the plasma membrane of Ncad/GFP-containing vesicles. (A) Images of post-Golgi carriers of Ncad/GFP-expressing cells were captured every 3 s. Inverted contrast images are displayed at the indicated time intervals. The arrows indicate different sites where vesicles exit the Golgi. Asterisks indicate bifurcating transport carriers. Bar, 10 μM. (B) Inverted images of Ncad/GFP-expressing cells collected every 3 s. The sequence shows fusion of an Ncad/GFP-containing vesicle with the plasma membrane (black arrow). A different vesicle does not fuse or leaves the plane of focus during this time period (white arrow). Bar, 10 μM.
Transport of Ncad/GFP-containing Vesicular Structures Is Dependent on Cell-Cell Contacts
To investigate the influence of cell-cell contacts on intracellular Ncad/GFP transport, isolated, contacting, and confluent REF-52 cells expressing Ncad/GFP were analyzed by time-lapse microscopy. As shown in Figure 5A (video), massive centrifugal and centripetal movement was observed in this isolated cell between the Golgi region and the plasma membrane. When two cells were in contact (Figure 5B and video), centrifugal movement of the Ncad/GFP-containing vesicular structures still occurred, whereas centripetal motion was diminished. Brightest point projections of all video frames in a stack are shown in Figure 5, A and B. In this representation, moving structures appear as a linear series of dots. Quantitative analysis of these movements demonstrated that in early contacting cells the proportion of centrifugal flow was decreased, whereas the centripetal flow was increased compared with isolated cells (Table 1). Interestingly, the proportion of resting phases descreased with contact formation (23% in isolated cells vs. 8.7% in contacting cells). Additionally, the velocity of Ncad/GFP-containing vesicles in contacting cells was slightly increased by contact formation. When a similar recording of Ncad/GFP fluorescence was performed in fully contacting cells (Figure 5C and video), almost no Ncad/GFP-containing vesicular structures could be detected between the Golgi and the cell-cell junction. The projection of the video frames confirmed the absence of moving Ncad/GFP-containing vesicular structures. Together, these data suggest that the dynamics of N-cadherin transport is regulated by cell contact formation.
Figure 5.
Ncad/GFP transport is dependent on cell-cell contacts. (A–C) Images of Ncad/GFP-expressing cells were captured every 5 s for 5 min. Projections of all video frames in a movie sequence are shown with the contrast inverted to better visualize the transport paths. Distinct trafficking behaviors are observed in isolated (A), recently (B) or fully contacting cells (C). Circles indicate vesicles with random Brownian-like motion. Inset in B illustrates two centrifugal (1 and 2) and one centripetal (3) pathway. Bar, 10 μM.
Table 1.
Distinct behavior for the traffic of the Ncad/GFP-containing vesicles in isolated or early contacting cells This table displays data summarizing frequency of centrifugal versus centripetal motion events of Ncad/GFP-containing vesicles. Results are means (±SEM) of several experimental performed in either isolated or early contacting Ncad/GFP-expressing cells (r = 9–10 cells). MetaMorph software has been used to measure the movement of the Ncad/GFP structures (tubules and individual vesicles) between frames from a continuously acquired live image (stack). We have tracked the positions with respect to a defined origin. Data regarding the X and Y coordinates and displacement of the vesicles were logged to disk and treated with Excel software.
| Features of Ncad/GFP vesicles in | Isolated cells | Early contacting cells |
|---|---|---|
| % of centripetal flow | 29 | 12 |
| % of centrifugal flow | 47 | 79 |
| % stop phases | 23 | 8.7 |
| Velocities (μm/s) | 0.18 ± 0.06 | 0.35 ± 0.1 |
Ncad/GFP-containing Vesicles Associate with MTs
The observed movement of vesicular structures en route to the plasma membrane and of the centripetal flow along linear tracks prompted us to analyze the cytoskeletal structures involved in this process. Ncad/GFP-expressing cells were stained for F-actin, vimentin IF, or MTs. Image stacks were deconvolved using the Huygens System image restoration software as described in MATERIALS AND METHODS. Respective colocalization of Ncad/GFP, F-actin, vimentin, and tubulin fluorescence was studied with the Imaris Colocalization module. As shown in Figure 6, F-actin staining (Figure 6a, red) of Ncad/GFP-expressing cells (Figure 6a, green) revealed that Ncad/GFP colocalized with F-actin at the cell-cell junctions, whereas cytoplasmic vesicular structures showed no obvious association with the F-actin cytoskeleton (Figure 6b, colocalization in yellow). Staining of vimentin IF (Figure 6c, red) of Ncad/GFP-expressing cells (Figure 6c, green) revealed an almost complete lack of colocalization of Ncad/GFP and vimentin both in the cytoplasm (Figure 6d) and at the junctions (our unpublished results). In contrast, MT staining (Figure 6e, red) of Ncad/GFP-expressing cells (Figure 6e, green) revealed that all Ncad/GFP-containing vesicular structures were detected along MTs (Figure 6f, colocalization). Confocal imaging confirmed this proximity. A confocal optical section of an Ncad/GFP-expressing cell (Figure 6 g, green) stained for microtubules (Figure 6g, red) was analyzed using the colocalization analysis Lasersharp software. Figure 6h shows colocalized red and green pixels in white for the selected area in Figure 6g.
Figure 6.
Ncad/GFP-containing vesicular structures associate with MTs. Ncad/GFP-expressing cells were fixed and processed for immunofluorescence by using rhodamine-labeled phalloidin and anti-vimentin or anti-α-tubulin antibodies followed by rhodamin-labeled secondary antibodies. Stacks of images were acquired using a wide-field microscope (0.1 μm Z step) and deconvolved using the Huygens System image restoration software. Deconvolved stacks were visualized using the Iview command of the Imaris software (a, c, and e). a, c, and e show the Ncad/GFP (in green), F-actin (a, red), vimentin IF (c, red), and α-tubulin staining (e, red). Respective voxel colocalizations of Ncad/GFP, with F-actin, vimentin, and tubulin obtained using the Imaris Colocalization module are shown in b, d, and f. A single confocal section of Ncad/GFP-expressing cells (green) stained with an anti-α-tubulin antibody (red) is shown in g. h illustrates in white the colocalized objects in the selected area in g. Bar, 10 μm.
To examine whether Ncad/GFP-containing vesicles bind to MTs, we purified the MAP fraction from Ncad/GFP-expressing cells in the presence of GTP and taxol (Figure 7A). The microsomal fraction (C2), which contains nuclei and associated internal membranes (Golgi, endoplasmic reticulum), MAP fraction (C3), and MAP-depleted supernatant (S3), was analyzed by Western blotting for the presence of Ncad/GFP and α-tubulin. As expected, Ncad/GFP was present in the microsomal fraction, which contained most of the cellular membranes (C2) (Figure 7B). In addition, some Ncad/GFP was present in the MAP fraction (C3) of isolated cells. Interestingly, in contacting cells or in Nz-treated isolated cells, we could no longer detect Ncad/GFP in the MAP fraction (C3). Ponceau staining of the membranes and immunodetection of α-tubulin revealed that tubulin was highly enriched in the C3 fraction. As a positive control we used TOGp, a previously described MAP (Charrasse et al., 1998), that was also enriched in the MAP fraction. Thus, N-cadherin is found in a complex with F-actin at the cell-cell contact sites and localizes to vesicles associated with MTs.
Figure 7.
Ncad/GFP-containing vesicles cofractionate with microsomes and MTs. (A) Schematic protocol of the different purification steps. (B) SDS-PAGE and immunoblot analysis of Ncad/GFP in the microsomal fraction (C2), MAP-depleted supernatant (S3), and MT/MAP fraction (C3) prepared from isolated, contacting, or Nz-treated isolated Ncad/GFP-expressing cells. Immunoblot detection of a known MAP protein, TOGp, is shown as a control. The amount of tubulin in the fractions is monitored by ponceau red staining of the membranes (middle panels) and immunoblot analysis by using an anti-α-tubulin antibody (bottom panels).
N-Cadherin Transport and Junction Formation Are MT Dependent
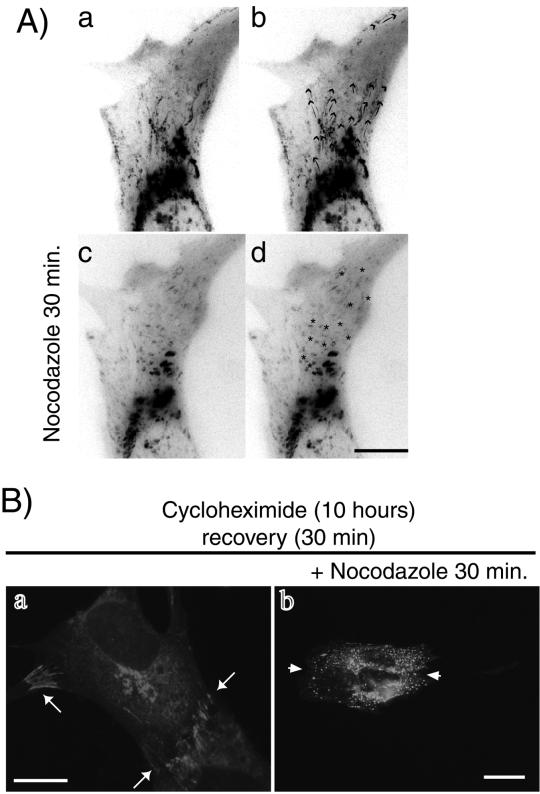
First, we assessed whether MT disruption would affect the motion of Ncad/GFP-containing vesicular structures. Ncad/GFP-expressing cells treated with 1 μM Nz were analyzed by video microscopy. Efficient MT depolymerization was verified by immunofluorescence with an anti-α-tubulin antibody (our unpublished results). As shown in Figure 8A and the accompanying video, 30 min after Nz addition, the motion of Ncad/GFP-containing vesicular structures was dramatically reduced; neither trans-Gogi network-to-plasma membrane nor centripetal movements were detectable (Figure 8A, c and d). In contrast, we could clearly observe long-range movements before Nz treatment (Figure 8A, a and b). In addition, we could not detect any linear tracks, indicating vesicle movement in a projection of the whole stack after Nz treatment (Figure 8A, d, asterisks). This demonstrates that the absence of MTs prevents both centrifugal and centripetal transport of Ncad/GFP-containing vesicular structures.
Figure 8.
MT disruption affects the movement of Ncad/GFP-containing vesicular structures and junction formation. (A) Images of untreated or Nz-treated recently contacting Ncad/GFP-expressing cells were captured every 5 s for 10 min. Projections of all video frames in a movie sequence from control cells (a and b) or Nz-treated cells (c and d) are shown. Arrows in b show the routes delineated by the Ncad/GFP-containing structures. Asterisks in d indicate structures with random Brownian-like movements. Bar, 10 μM. (B) Ncad/GFP-expressing cells were treated with cycloheximide for 10 h. Cells were rinsed for 30 min (a). Alternatively, cells were incubated with the MT disrupting agent Nz (1 μM) for 30 min and then rinsed in the presence of Nz (b). After fixation, cells were monitored for GFP fluorescence. Bar, 10 μM.
Second, we examined whether the centrifugal transport of N-cadherin along MTs is responsible for junction formation. For this purpose, cells were incubated in medium containing cycloheximide for 10 h to inhibit Ncad/GFP translation. Then the cells were rinsed with fresh medium with or without the MT-disrupting agent Nz for 30 min (Figure 8B). After 10 h in cycloheximide we could not detect any Ncad/GFP (our unpublished results). After 30 min of recovery, Ncad/GFP was highly expressed and cell-cell contacts were reformed (Figure 8B, a, arrows). In contrast, in cells treated with Nz, Ncad/GFP remained undetectable at the cell-cell contacts (Figure 8B, b, arrowheads) and redistributed to perinuclear and cytoplasmic compartments. These data indicate that MTs play an essential role in the transport of Ncad/GFP-containing vesicular structures and subsequent adherens junction formation.
N-Cadherin Transport Is Kinesin Dependent
We finally examined whether the MT motor protein of the kinesin family is responsible for the motion of Ncad/GFP-containing vesicular structures. Anti-kinesin H2 antibody, previously shown to inhibit both retrograde and anterograde fast axonal transport (Brady et al., 1990), was microinjected into contacting Ncad/GFP-expressing cells. Thirty to 60 min after anti-kinesin H2 antibody microinjection, injected cells identified by coinjected rhodamine-labeled dextran were analyzed by video microscopy (Figure 9A, a; and video 9A). The projection of a whole time-lapse stack recorded 60 min after anti-kinesin H2 microinjection is shown in Figure 9A, b. Injection of the anti-kinesin H2 antibody resulted in a complete loss of directed movement of Ncad/GFP-containing vesicular structures; only Brownian motion of these vesicular structures was observed. Quantitative analysis of six cells injected with the anti-kinesin H2 antibody is shown in Figure 9B. The same inhibition was observed in isolated cells (our unpublished results). Injection of inert mouse Ig together with rhodamine-labeled dextran had no effect on motion of Ncad/GFP-containing vesicular structures (Figure 9B). In addition, the speed of movement of Ncad/GFP-containing vesicular structures (see above) is consistent with previous analysis of motor driven exocytotic transport (Kreitzer et al., 2000). These data argue for an essential role of the MT motor kinesin in the transport of Ncad/GFP-containing vesicular structures.
Figure 9.
Anti-kinesin antibody affects the movement of Ncad/GFP-containing vesicular structures. (A) Ncad/GFP-expressing cells were microinjected with anti-kinesin H2 antibody and rhodamine-labeled dextran. Sixty minutes after microinjection, images were captured every 5 s for 10 min. Panel a shows the microinjected cells; b is a brightest point projection of all video frames in the stack. Bar, 10 μM. (B) Stacks were analyzed to quantify the movement of Ncad/GFP-containing vesicles. For each condition (noninjected cells, control inert Ig-injected cells, and anti-kinesin H2 antibody-injected cells), six cells were examined.
DISCUSSION
Although adherens junctions are complex multimolecular structures, they are highly dynamic and have a fast rate of turnover. The regulated delivery of cadherins to the plasma membrane is one mechanism that might affect adherens junction assembly. We used a fully functional Ncad/GFP fusion protein to show that Ncad/GFP localizes to the Golgi, and endocytotic and secretory vesicles, and the plasma membrane. We further demonstrate that MT-dependent kinesin-driven transport of Ncad/GFP-containing vesicular structures is required for cell-cell contact formation and that contact formation stimulates the translocation of Ncad/GFP-containing vesicular structures from an intracellular pool to the cell periphery.
Microtubules as Structural and Functional Tracks for Ncad/GFP-containing Vesicles
Our results provide the first evidence that the biogenesis of cell-cell contacts requires MT-dependent transport of N-cadherin–containing vesicles (Figure 10A). MT-based transport has been best characterized in neuronal cells for proteins such as CAM and neurotransmitters (Hirokawa, 1996; Seiler et al., 1997). In these cells, the delivery of post-Golgi organelles to the cell surface occurs over a long distance. However, until very recently, it has been assumed that MTs might not play a major role in Golgi-to-plasma membrane transport in fibroblasts. Indeed, secretion was still observed after Nz treatment (Hirschberg et al., 1998). Under these high Nz concentration conditions, extensive Golgi fragmentation occurred and Golgi ministacks redistributed near the endoplasmic reticulum and the plasma membrane (Cole et al., 1996; Storrie et al., 1998). In contrast, in our low Nz concentration conditions, although MTs were disrupted, the central localization characteristic of the Golgi was preserved, demonstrating that in fibroblasts MTs are required for the short distance transport of N-cadherin between the Golgi and the plasma membrane. Recent studies reached a similar conclusion for the MT-dependent biosynthetic transport of the mannose-6-phosphate receptor (Nakagawa et al., 2000). Thus, the MT-dependent secretory pathway in mammalian cells might be an efficient and controlled mechanism to deliver molecules to specific sites at the plasma membrane.
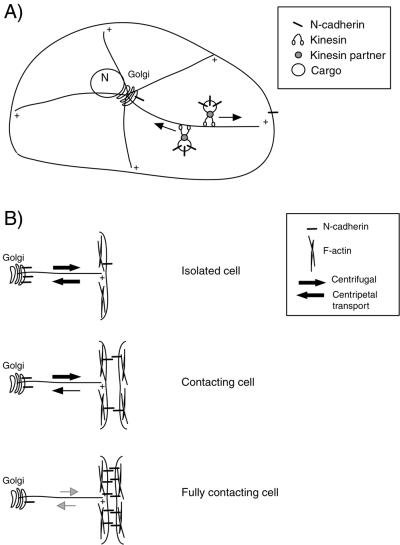
Figure 10.
Model for the regulation of N-cadherin traffic by cell-cell contact formation. (A) N-cadherin is found associated with the Golgi, both secretory and endocytotic vesicles, and the plasma membrane. Both N-cadherin–containing secretory and endocytotic vesicles are moved along MTs by kinesin motor proteins. (B) N-cadherin localization and transport are regulated by cell-cell contact formation. In isolated cells, both centrifugal and centripetal flow of N-cadherin–containing vesicles is observed. In contacting cells, the centripetal transport is diminished, whereas the centrifugal transport is maintained. In fully contacted cells, both centrifugal and centripetal N-cadherin transport is below the detectable level. Once delivered to the plasma membrane, N-cadherin associates, through binding to catenins, with the F-actin cytoskeleton.
In addition, the high viscosity of the cytoplasm is not consistent with a random diffusion process of secretory vesicles. Thus, is it likely that cytoplasmic motors are essential for secretion. Motor proteins of the kinesin family are involved in post-Golgi transport of at least two glycoproteins, VSVG and the low-affinity neurotrophin receptor p75 (Kreitzer et al., 2000). When we microinjected a function-blocking anti-kinesin antibody, formation and motion of post-Golgi Ncad/GFP-containing carriers was impaired demonstrating that kinesin is also required for N-cadherin transport. Identification of the motor proteins and their associated components will be required for a better understanding of the regulation of N-cadherin membrane traffic. MT disruption by Nz treatment or microinjection of the anti-kinesin antibody also completely inhibited the endocytotic pathway. Thus, because the inhibition of kinesin affects both the secretion and endocytosis of Ncad/GFP-containing vesicles it will be interesting to determine whether kinesin associates with different proteins in each of these processes (Klopfenstein et al., 2000).
Our data also demonstrate the importance of the MT network for the biogenesis of the N-cadherin cell-cell contacts, which is supported by recent studies from Lambert et al. (2000). In newt lung epithelial cells, cell-cell contacts are disrupted upon Nz treatment, again suggesting a key role for MTs in epithelial cell-cell adhesion (Waterman-Storer et al., 2000). N-cadherin belongs to a large family of glycoproteins that are expressed only in certain tissues and it will be of interest to study whether MT-based transport is also required for the delivery of other cadherin family members such as E-cadherin, to the plasma membrane.
Regulation of Cadherin Adhesive Activity through Turnover of Cell Junctions
Formation of adherens junctions is a complex, multistep process that has mainly been studied in epithelial Madin-Darby canine kidney cells (McNeill et al., 1993; Hinck et al., 1994; Adams et al., 1996). In these cells, E-cadherin/catenin complexes are almost immediately incorporated into adherens junctions and associate with the F-actin cytoskeleton when they arrive at the plasma membrane (Adams et al., 1996). In contrast, our data show that N-cadherin in C2 myoblasts or expressed in REF-52 fibroblasts is subject to continuous intracellular vesicular transport. This membrane traffic includes both export of N-cadherin from the Golgi apparatus to the plasma membrane as well as endocytotic mechanisms. We further propose that N-cadherin–mediated cell-cell interactions are not a constitutive process but that they are influenced by cell-cell contacts. These observations are consistent with studies in neural crest cells. In these cells, an intracellular pool of N-cadherin is present and formation of stable intercellular contacts results from the recruitment of this pool to the plasma membrane and to adherens junctions rather than from a redistribution of a pool of already surface-bounded N-cadherin molecules (Monier-Gavelle and Duband, 1995, 1997). We propose that uninterrupted N-cadherin vesicular trafficking is essential for many developmental cell migratory processes such as neural crest cell and myoblastic precursor migration, condensation, and tissue elongation in the Xenopus embryo, which require rapid and continuous regulation of contact formation and adhesivness (Bronner-Fraser, 1993; Hall and Miyake, 2000).
The differences between E- and N-cadherin in cell-cell contact formation might be due to the cadherin extracellular domain (ectodomain) that is specific for different cadherins. Also, such a difference might arise from the different cell types in which these cadherins are expressed. Nonmotile epithelial cells and motile neural crest cells, fibroblasts, or myoblasts display very different behaviors. Finally, such discrepancies might result from distinct cytoskeletal architecture and/or relationships between microtubules and F-actin. In particular, constitutive E-cadherin association with the plasma membrane according to the rubber band model results from the specific cortical F-actin organization in epithelial cells (Vasioukhin and Fuchs, 2001).
Cell-Cell Contacts Regulate Trafficking of N-Cadherin
Our results establish that a molecular pathway might be elicited by cadherin-mediated cell-cell contacts that directly affects the distribution of N-cadherin. Such a cytoplasmic signaling event could act on both secretion and endocytotic/recycling pathways. The model we propose in Figure 10B shows both centripetal and centrifugal flow of N-cadherin in an isolated cell and the decrease of centripetal flow upon contact formation that allows accumulation of N-cadherin at the plasma membrane. This observation raises the question of how N-cadherin traffic could be regulated by cell-cell contact formation. In addition to the modification of the centripetal and centrifugal flows, cell-cell contact decreases the frequency of phases in which Ncad/GFP vesicles do not move. Combined with our results demonstrating kinesin-driven N-cadherin movement along MTs, we think that cell-cell contact formation might somehow regulate kinesin motor protein function. This is consistent with previous reports pointing to a role for phosphorylation of kinesin in regulating its activity (Lindesmith et al., 1997; De Vos et al., 2000). Along this line, recent studies propose that cadherins initiate a signaling pathway that interferes with MT organization (Chausovsky et al., 2000) and dynamics (Waterman-Storer et al., 2000). However, elements of such a pathway have yet to be identified. Important components of the cadherin-mediated signaling pathway may be small GTPases shown to be regulated in epithelial cells after cell-cell contact formation (Kim et al., 2000; Pece et al., 1999). In particular Cdc42Hs, which controls both cell growth and the post-Golgi secretory pathway, is an interesting candidate for integrating regulatory pathways at the Golgi for membrane sorting and cell signaling (Kroschewski et al., 1999). Further studies will be required to determine the mechanisms that control surface distribution of N-cadherin.
In conclusion, the MT network appears to be a critical coordinator for N-cadherin–dependent cell-cell contact formation, therefore providing a novel level of regulation for assembly of adherens junctions. In transformed cells, the cytoskeletal organization is often drastically affected. Thus, N-cadherin transport to the plasma membrane might be impaired under such conditions.
Supplementary Material
ACKNOWLEDGMENTS
We thank Phillipe Fort, Emmanuel Vignal, and Pierre Roux for valuable discussions; René-Marc Mège for N-cadherin cDNA; and Torsten Wittman and Nathalie Morin for critical reading of the manuscript. Confocal microscopy was performed at the Center Regional d'Imagerie Cellulaire de Montpellier. This work was supported by institutional grants from the Center National de la Recherche Scientifique, Institut National de la Santé et de la Recherche Medicale, and contracts from the Association Francaise pour la Recherche contre le Cancer and the Ligue Nationale Contre le Cancer (équipe labelisée). During this work S.M. was supported by a fellowship from the Association Francaise pour la Recherche contre le Cancer.
Abbreviations used:
- GFP
green fluorescent protein
- Ncad/GFP
N-cadherin/GFP
- MT
microtubule
- Nz
nocodazole
- IF
intermediate filament
Footnotes
Online version of this article contains video material for Figures 4, 5, 8, and 9. Online version available at www.molbiolcell.org.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.01–07-0337. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.01–07-0337.
REFERENCES
- Adams CL, Nelson WJ, Smith SJ. Quantitative analysis of cadherin-catenin-actin reorganization during development of cell-cell adhesion. J Cell Biol. 1996;135:1899–1911. doi: 10.1083/jcb.135.6.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akitaya T, Bronner-Fraser M. Expression of cell adhesion molecules during initiation and cessation of neural crest cell migration. Dev Dyn. 1992;194:12–20. doi: 10.1002/aja.1001940103. [DOI] [PubMed] [Google Scholar]
- Brady ST, Pfister KK, Bloom GS. A monoclonal antibody against kinesin inhibits both anterograde and retrograde fast axonal transport in squid axoplasm. Proc Natl Acad Sci USA. 1990;87:1061–1065. doi: 10.1073/pnas.87.3.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronner-Fraser M. Mechanisms of neural crest cell migration. Bioessays. 1993;15:221–230. doi: 10.1002/bies.950150402. [DOI] [PubMed] [Google Scholar]
- Charrasse S, Schroeder M, Gauthier-Rouviere C, Ango F, Cassimeris L, Gard DL, Larroque C. The TOGp protein is a new human microtubule-associated protein homologous to the XenopusXMAP215. J Cell Sci. 1998;111:1371–1383. doi: 10.1242/jcs.111.10.1371. [DOI] [PubMed] [Google Scholar]
- Chausovsky A, Bershadsky AD, Borisy GG. Cadherin-mediated regulation of microtubule dynamics. Nat Cell Biol. 2000;2:797–804. doi: 10.1038/35041037. [DOI] [PubMed] [Google Scholar]
- Cole NB, Sciaky N, Marotta A, Song J, Lippincott-Schwartz J. Golgi dispersal during microtubule disruption: regeneration of Golgi stacks at peripheral endoplasmic reticulum exit sites. Mol Biol Cell. 1996;7:631–650. doi: 10.1091/mbc.7.4.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vos K, Severin F, Van Herreweghe F, Vancompernolle K, Goossens V, Hyman A, Grooten J. Tumor necrosis factor induces hyperphosphorylation of kinesin light chain and inhibits kinesin-mediated transport of mitochondria. J Cell Biol. 2000;149:1207–1214. doi: 10.1083/jcb.149.6.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier RC, Vignal E, Meriane M, Roux P, Montcourier P, Fort P. RhoG GTPase controls a pathway that independently activates Rac1 and Cdc42Hs. Mol Biol Cell. 1998;9:1379–1394. doi: 10.1091/mbc.9.6.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner BM. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- Gumbiner BM. Regulation of cadherin adhesive activity. J Cell Biol. 2000;148:399–404. doi: 10.1083/jcb.148.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall BK, Miyake T. All for one and one for all: condensations and the initiation of skeletal development. Bioessays. 2000;22:138–147. doi: 10.1002/(SICI)1521-1878(200002)22:2<138::AID-BIES5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Hinck L, Nathke IS, Papkoff J, Nelson WJ. Dynamics of cadherin/catenin complex formation: novel protein interactions and pathways of complex assembly. J Cell Biol. 1994;125:1327–1340. doi: 10.1083/jcb.125.6.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N. The molecular mechanism of organelle transport along microtubules: the identification and characterization of KIFs (kinesin superfamily proteins) Cell Struct Funct. 1996;21:357–367. doi: 10.1247/csf.21.357. [DOI] [PubMed] [Google Scholar]
- Hirschberg K, Miller CM, Ellenberg J, Presley JF, Siggia ED, Phair RD, Lippincott-Schwartz J. Kinetic analysis of secretory protein traffic and characterization of Golgi to plasma membrane transport intermediates in living cells. J Cell Biol. 1998;143:1485–1503. doi: 10.1083/jcb.143.6.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jou TS, Stewart DB, Stappert J, Nelson WJ, Marrs JA. Genetic and biochemical dissection of protein linkages in the cadherin-catenin complex, Proc. Natl Acad Sci USA. 1995;92:5067–5071. doi: 10.1073/pnas.92.11.5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemler R. From cadherins to catenins: cytoplasmic protein interactions and regulation of cell adhesion. Trends Genet. 1993;9:317–321. doi: 10.1016/0168-9525(93)90250-l. [DOI] [PubMed] [Google Scholar]
- Kim SH, Li Z, Sacks DB. E-Cadherin-mediated cell-cell attachment activates Cdc42. J Biol Chem. 2000;275:36999–37005. doi: 10.1074/jbc.M003430200. [DOI] [PubMed] [Google Scholar]
- Klopfenstein DR, Vale RD, Rogers SL. Motor protein receptors. Moonlighting on other jobs [In Process Citation] Cell. 2000;103:537–540. doi: 10.1016/s0092-8674(00)00144-6. [DOI] [PubMed] [Google Scholar]
- Kreitzer G, Marmorstein A, Okamoto P, Vallee R, Rodriguez-Boulan E. Kinesin and dynamin are required for post-Golgi transport of a plasma-membrane protein. Nat Cell Biol. 2000;2:125–127. doi: 10.1038/35000081. [DOI] [PubMed] [Google Scholar]
- Kroschewski R, Hall A, Mellman I. Cdc42 controls secretory and endocytic transport to the basolateral plasma membrane of MDCK cells. Nat Cell Biol. 1999;1:8–13. doi: 10.1038/8977. [DOI] [PubMed] [Google Scholar]
- Lambert M, Padilla F, Mege RM. Immobilized dimers of N-cadherin-Fc chimera mimic cadherin-mediated cell contact formation: contribution of both outside-in and inside-out signals. J Cell Sci. 2000;113:2207–2219. doi: 10.1242/jcs.113.12.2207. [DOI] [PubMed] [Google Scholar]
- Le TL, Yap AS, Stow JL. Recycling of E-cadherin: a potential mechanism for regulating cadherin dynamics. J Cell Biol. 1999;146:219–232. [PMC free article] [PubMed] [Google Scholar]
- Lindesmith L, McIlvain JM, Jr, Argon Y, Sheetz MP. Phosphotransferases associated with the regulation of kinesin motor activity. J Biol Chem. 1997;272:22929–22933. doi: 10.1074/jbc.272.36.22929. [DOI] [PubMed] [Google Scholar]
- Matsuzaki F, Mege RM, Jaffe SH, Friedlander DR, Gallin WJ, Goldberg JI, Cunningham BA, Edelman GM. cDNAs of cell adhesion molecules of different specificity induce changes in cell shape and border formation in cultured S180 cells. J Cell Biol. 1990;110:1239–1252. doi: 10.1083/jcb.110.4.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeill H, Ryan TA, Smith SJ, Nelson WJ. Spatial and temporal dissection of immediate and early events following cadherin-mediated epithelial cell adhesion, J. Cell Biol. 1993;120:1217–1226. doi: 10.1083/jcb.120.5.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meriane M, Roux P, Primig M, Fort P, Gauthier-Rouviere C. Critical activities of Rac1 and Cdc42Hs in skeletal myogenesis: antagonistic effects of JNK and p38 pathways. Mol Biol Cell. 2000;11:2513–2528. doi: 10.1091/mbc.11.8.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monier-Gavelle F, Duband JL. Control of N-cadherin-mediated intercellular adhesion in migrating neural crest cells in vitro. J Cell Sci. 1995;108:3839–3853. doi: 10.1242/jcs.108.12.3839. [DOI] [PubMed] [Google Scholar]
- Monier-Gavelle F, Duband JL. Cross talk between adhesion molecules: control of N-cadherin activity by intracellular signals elicited by beta1 and beta3 integrins in migrating neural crest cells. J Cell Biol. 1997;137:1663–1681. doi: 10.1083/jcb.137.7.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Setou M, Seog D, Ogasawara K, Dohmae N, Takio K, Hirokawa N. A novel motor, KIF13A, transports mannose-6-phosphate receptor to plasma membrane through direct interaction with AP-1 complex [In Process Citation] Cell. 2000;103:569–581. doi: 10.1016/s0092-8674(00)00161-6. [DOI] [PubMed] [Google Scholar]
- Ohkubo T, Ozawa M. p120(ctn) binds to the membrane-proximal region of the E-cadherin cytoplasmic domain and is involved in modulation of adhesion activity. J Biol Chem. 1999;274:21409–21415. doi: 10.1074/jbc.274.30.21409. [DOI] [PubMed] [Google Scholar]
- Pece S, Chiariello M, Murga C, Gutkind JS. Activation of the protein kinase Akt/PKB by the formation of E-cadherin-mediated cell-cell junctions. Evidence for the association of phosphatidylinositol 3-kinase with the E-cadherin adhesion complex. J Biol Chem. 1999;274:19347–19351. doi: 10.1074/jbc.274.27.19347. [DOI] [PubMed] [Google Scholar]
- Seiler S, Nargang FE, Steinberg G, Schliwa M. Kinesin is essential for cell morphogenesis and polarized secretion in Neurospora crassa , EMBO J. 1997;16:3025–3034. doi: 10.1093/emboj/16.11.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storrie B, White J, Rottger S, Stelzer EH, Suganuma T, Nilsson T. Recycling of Golgi-resident glycosyltransferases through the ER reveals a novel pathway and provides an explanation for nocodazole-induced Golgi scattering. J Cell Biol. 1998;143:1505–1521. doi: 10.1083/jcb.143.6.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeichi M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science. 1991;251:1451–1455. doi: 10.1126/science.2006419. [DOI] [PubMed] [Google Scholar]
- Takeichi M. Cadherins in cancer: implications for invasion and metastasis. Curr Opin Cell Biol. 1993;5:806–811. doi: 10.1016/0955-0674(93)90029-p. [DOI] [PubMed] [Google Scholar]
- Vasioukhin V, Fuchs E. Actin dynamics and cell-cell adhesion in epithelia. Curr Opin Cell Biol. 2001;13:76–84. doi: 10.1016/s0955-0674(00)00177-0. [DOI] [PubMed] [Google Scholar]
- Waterman-Storer CM, Salmon WC, Salmon ED. Feedback interactions between cell-cell adherens junctions and cytoskeletal dynamics in newt lung epithelial cells. Mol Biol Cell. 2000;11:2471–2483. doi: 10.1091/mbc.11.7.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J, et al. Rab6 coordinates a novel Golgi to ER retrograde transport pathway in live cells. J Cell Biol. 1999;147:743–760. doi: 10.1083/jcb.147.4.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap AS, Niessen CM, Gumbiner BM. The juxtamembrane region of the cadherin cytoplasmic tail supports lateral clustering, adhesive strengthening, and interaction with p120ctn. J Cell Biol. 1998;141:779–789. doi: 10.1083/jcb.141.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.