Abstract
This study aims to investigate anthropometric measures and their effectiveness as screening method for diagnosing obstructive sleep apnea (OSA) in each gender. We also evaluated which measures were associated with OSA in the adult population of a large metropolitan city, Sao Paulo, Brazil. 552 women and 450 men were submitted to polysomnography (PSG), and the anthropometric measurements as body mass index (BMI), waist-to-height ratio, neck and waist circumference were collected. The measurements were then compared with the OSA classification established by the PSG. In women, waist circumference and waist-to-height ratio were found to be the best predictor, while in men, the factors with great potential for identification varied according to severity of the disease, highlighting waist-to-height ratio, neck circumference and BMI had strongest association. The accuracy of the classification in relation to mild-to-severe OSA based on cut-off values of 92.5cm for waist circumference was greater than 72.9% in men, and 78.9% in women based on cut off values of 95cm. Regarding severe OSA, cut-off values of 116.1cm were greater than 91.3% accurate in the male population, and 95.1% in the female population with a cut-off value of 126.5cm. The study found waist circumference and waist-to-height ratio to be the best measure to assess sleep-disordered breathing in women. Waist-to-height ratio and neck circumferences were the best measures in men with mild OSA, but BMI was more closely associated with severe OSA. The present study identified the anthropometric variables with the highest risk for OSA and their respective cutoff value, according to gender.
Keywords: Sleep, Abdominal Obesity, Body Mass Index, Waist Circumference, Waist-Height Ratio
INTRODUCTION
Obstructive sleep apnea (OSA) is globally the most commonly diagnosed sleep disorder1. The disease causes changes in respiratory patterns leading to intermittent hypoxia, hypercapnia and increased frequency of awakenings2. OSA is mainly characterized by a set of symptoms resulting from apnea events that have negative outcomes on health, such as excessive daytime sleepiness3, cardiovascular impairment4 and increased morbidity and mortality5. Epidemiological studies indicate that the prevalence of OSA can reach up to 32.9%1. OSA has major economic costs for health systems6. Direct and indirect costs of moderate-severe OSA in the USA are estimated to be between US$65 billion and US$165 billion/year7.
Given large extent of the problem, it is important to develop simple, reliable, cost-effective methods to predict or diagnose OSA. One option is to use anthropometric variables relating to being overweight. The amount of an individual’s adipose tissue has been correlated with irregular respiratory patterns which result in obstructive respiratory events8. Obesity is a worldwide public health problem, resulting from an excessive intake of calories and sedentary behaviors9. Sedentary behaviors can result in an energy imbalance and lead to weight gain as well10. In 2014, global estimates indicate that more than 1.9 billion adults were overweight. Of these, over 600 million were obese9. Obesity increases probability of an individual developing a range of conditions including cardiovascular diseases, diabetes, musculoskeletal disorders and even some types of cancer (e.g., endometrial, pancreas, and colon)11,12.
Anthropometric measures are essential tools used for health evaluation, especially in relation to obesity. Body mass index (BMI), waist-to-height ratio, neck and waist circumferences are the most widely used measures. The use of anthropometric measures is simple, cheap, accessible and with high clinical correlation. The effectiveness of these measures in general assessing health has been previously demonstrated13, and they have a strong correlation with sleep apnea.
Neck circumference is a variable often considered in sleep medicine due to its capability to predict sleep apnea events14. It has been shown that fat accumulation in cervical region considerably reduces the diameter of the upper airway. In general, increased anthropometric measurements are directly related to the accumulation of visceral fat, which brings with it a higher risk for insulin resistance, type-2 diabetes, metabolic syndrome and cardiovascular disorders15,16. BMI is the anthropometric measure most widely used, and is considered the main classifier for overweight/obesity17. According to the World Health Organization definition, an individual with an index equal or higher than to 25 is considered to be overweight and with an index equal or higher than 30 to be obese9.
Because of importance of these measurements as outlined above, and their frequent use for clinical evaluation of obesity, the objective of this study is to investigate anthropometric measures and their effectiveness as screening method for diagnosing OSA in each gender.
METHODS
We used data from the Sao Paulo Epidemiologic Sleep study (EPISONO), which was designed to evaluate parameters related to overall sleep disorders in Sao Paulo, Brazil1. São Paulo is the biggest city of Brazil and their residents are highly representative of the Brazilian population. In this study, volunteers were randomly selected to represent the population of Sao Paulo according to gender, age (20-80 years) and socioeconomic status. In the first stage, to assure the representativeness of different levels of wealth, 96 districts (from the 1500 districts in which the city was divided for census purposes) were proportionally selected from the 4 homogenous socioeconomic macro-regions of Sao Paulo. Addresses with difficult accessibility were excluded. Households were selected if they were permanently occupied private homes, so clinics, schools, and other commercial and non-commercial establishments were excluded. In the second stage, the selection of a given household was made by randomly picking the first home and subsequently skipping a specified number, calculated by dividing the total number of homes by a fixed number, to select 11 households in each sector. Each apartment in a building was considered a home, and counting was carried out from the upper floor to the lower floor. Finally, in the third stage of sampling, all eligible residents of each chosen home were ranged from the youngest to the oldest, and the participant was selected by means of 96 pre-established random tables, which designated the rank number to be chosen for each of the 11 households, from the 96 selected districts.
Pregnant and lactating women, people with physical or mental impairments that prevent self-care, individuals below 20 or over 80 years old and people who work every night were not included in the selection from the household. Substitutes were chosen from the next home, following the same random selection criteria described above. In addition, individuals were not included in the following instances: 3 unsuccessful attempts to contact the target individual, total refusal to participate, obstruction by a family member, or inability to participate for a specified reason. These individuals were replaced using the same method described above18.
Participants
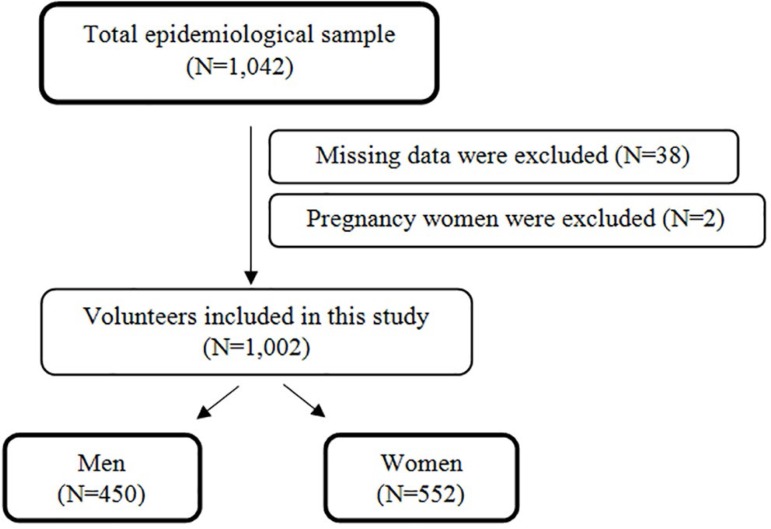
A total of 1,042 volunteers underwent a complete full-night polysomnography (PSG). Individuals who had missing values in the variables analyzed (N=38) and pregnant women (N=2) were excluded from the study. At the time of selection, all the volunteers read and signed an informed consent form, which authorized us to collect and use the data for future research. The methodology of recruiting volunteers in this study was similar to the conceptual framework used in the North American National Health Surveys19. The study was approved by the Ethics Committee of the Universidade Federal de Sao Paulo (CEP #593/2006 and #258917/2014) and registered with ClinicalTrials.gov (NCT00596713). Informed consent was obtained from all individual participants included in the study. Figure 1 shows the flow diagram of study identification, selection and exclusion due to missing data.
Figure 1.
Flow sheet regarding the criteria for selecting patients for the study.
Polysomnography
A full-night PSG was performed using a digital system (EMBLA® S7000, Embla Systems, Inc., Broomfield, CO, USA) at the sleep laboratory during the subject’s habitual sleep time which had previously been established with the subject1. The following physiological variables were monitored simultaneously: electroencephalogram; electrooculogram; electrocardiogram; airflow; surface electromyogram (submental, temporal, masseter and tibialis anterior muscles); respiratory effort of the thorax and abdomen using inductance plethysmography; snoring; body position; saturation of peripheral oxygen (SpO2) and pulse rate.
The criteria for OSA was diagnosed in conformity with the International Classification of Sleep Disorders20. OSA classification was defined according to the Apnea-Hypopnea Index (AHI)2. Subjects were diagnosed with OSA if they presented an AHI≥5 and at least one of the following complaints: loud snoring, daytime sleepiness, fatigue and breathing interruptions during sleep. Subjects with an AHI≥15 were diagnosed with OSA, regardless of whether they had any additional complaints. Subjects were distributed into 4 groups based on OSA severity: control group (<5 events/h), mild OSA (5-15 events/h), moderate OSA (15-30 events/h) and severe OSA (>30 events/h). Four trained technicians visually scored all PSG for investigating sleep disorders21. Hypopneas were defined using the alternative rule22. Regarding PSG, the random re-scoring of 4% of all recordings showed an agreement rate of 93.3±5.1%, and a Kappa 0.91±0.03, which guaranteed the scoring reliability of the polysomnographies.
Anthropometric measurements
General physical measurements were taken by trained technicians immediately before the subjects were prepared for the PSG hook-up, following recommended procedures and using precise instruments. Waist-to-height ratio (waist/height), BMI (weight/height2), and waist and neck circumferences (cm) were included in this study.
Statistical analysis
All the data which did not meet the assumptions of normality and homogeneity were z-score transformed for suitable parametric evaluation. The statistical analysis for description of the sample group was carried out using a General Linear Model through one-way ANOVA.χ2 test was performed to examine the association between categorical variables. To evaluate the relationship between AHI and the anthropometric variables used in this study, a Pearson’s correlation test was performed. To determine the possible associated factors with OSA, the variables were included in a binary logistic regression. The identification of crude odds ratio (cOR) was performed based on the Enter method, which evaluates the odds ratio of each single factor. The adjusted odds ratio (aOR) was based on the Backward Wald method. In this logistic regression the factors were withdrawn, one by one, based on the P value non-significant, until remained only the significant variables.
Logistic regression models were performed to identify anthropometric factors associated with OSA in 3 logistic models, separately, for each gender. The first logistic model was performed to identify the main associated factors related to OSA, regardless of its severity. The second logistic model was performed to evaluate the associated factors for moderate to severe OSA. The third logistic model aimed to analyze the predictive factors for severe OSA.
Based on a judgment of accuracy provided by the anthropometric variables, the optimal cut-off point for each parameter as classifiers of OSA or non-OSA was determined. The accuracy of cut-off points was determined by the sum of all true-positive and true-negative results divided by the number of participants. Data are reported as mean±standard error of the mean or frequency (percentage). The significance level was set at p<0.05.
RESULTS
From the 1,042 volunteers enrolled in the study, the following parameters were missing for 40 individuals: waist circumference (N=34); neck circumference (N=3), BMI (N=1) and pregnancy (N=2). Of the remaining 1,002 participants, 450 were men (44.9%) and 552 were women. Descriptive parameters in Table 1 show that the following anthropometric parameters were affected by gender: age (F1,1000=9.97; p<0.01); waist circumference (F1,1000=72.03; p<0.001) and neck circumference (F1,1000=582.32; p<0.001). The PSG parameters resulting from OSA such as AHI (F1,1000=29.85; p<0.001), basal SpO2 (F1,1000=40.78; p<0.001), medium SpO2 (F1,1000=25.94; p<0.001) and minimum SpO2 (F1,1000=15.98; p<0.001) were significantly different between genders. In a comparison between genders, the frequency of patients with OSA was significantly higher in the male sample, especially among moderate and severe cases (χ2=36.44; df=3; p<0.001).
Table 1.
Descriptive parameters of the epidemiological sample according to gender.
| Variable | Men (n = 450) | Women (n = 552) | p value |
|---|---|---|---|
| Age (y) | 40.9±0.7 | 43.7±0.6 ** | 0.002 |
| Body mass index (kg/m2) | 26.6±0.2 | 27.0±0.2 | 0.225 |
| Waist circumference (cm) | 90.1±0.7 *** | 82.8±0.6 | <0.001 |
| Neck circumference (cm) | 39.2±0.1 *** | 33.7±0.2 | <0.001 |
| Waist-to-height ratio | 0.525±0.004 | 0.525±0.004 | 0.937 |
| High risk (>0.5) | 285/63.3% | 334/60.5% | 0.360 |
| Apnea-hypopnea index (events/h) | 10.6±0.7 *** | 6.1±0.5 | <0.001 |
| Basal oxygen saturation (%) | 95.6±0.1 *** | 96.2±0.1 | <0.001 |
| Medium oxygen saturation (%) | 94.8±0.1 *** | 95.4±0.1 | <0.001 |
| Minimum oxygen saturation (%) | 87.6±0.3 *** | 89.0±0.2 | <0.001 |
| Obstructive sleep apnea (n/%) | <0.001 | ||
| Normal | 260/57.8% | 412/74.6% *** | |
| Mild | 82/18.2% | 77/13.9% | |
| Moderate | 64/14.2% *** | 36/6.5% | |
| Severe | 44/9.8% *** | 27/4.9% |
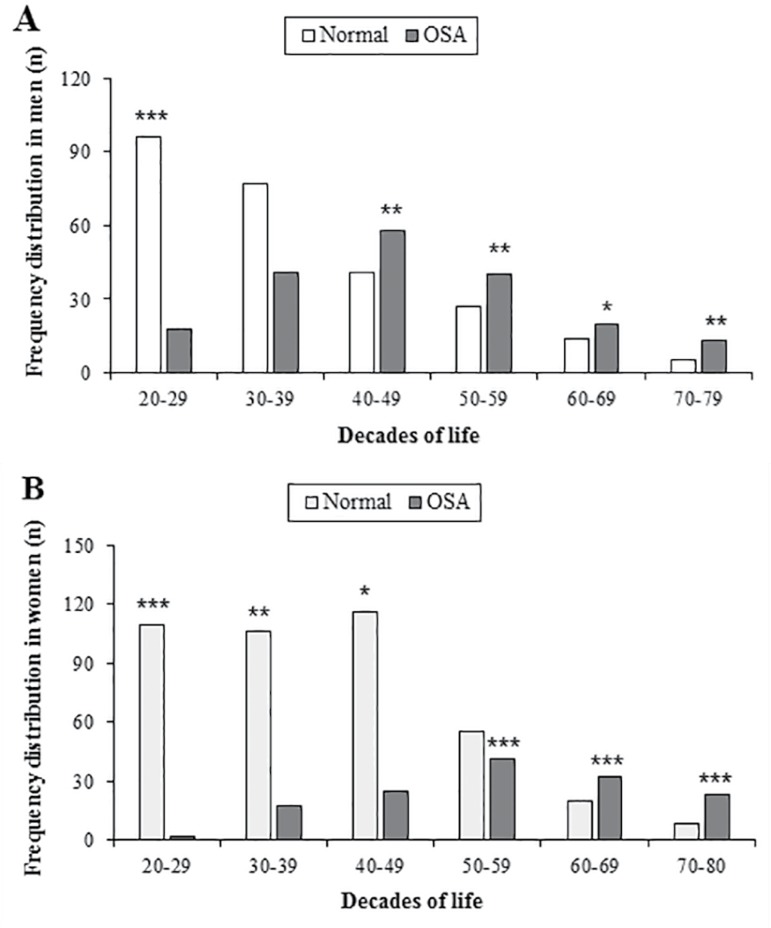
The study identified that genders were related to anthropometric differences, as well as prevalence of sleep apnea in several age groups. Figure 2 shows the distribution of OSA among decades of life from 20 to 80 years. The male sample presented significant results (χ 2=65.09; df=5; p<0.001), especially a higher frequency of men aged 20-29 years without OSA (p<0.001) (Figure 2A). On the other hand, diagnosis of OSA increased significantly only during the following decades of life: 40-49 years (p<0.01), 50-59 years (p<0.01), 60-69 years (p<0.05) and 70-80 years (p<0.01). In Figure 2B, the results obtained from the female sample (χ2=135.13; df=5; p<0.001) indicate that up to the fifth decade of life there is a higher frequency of women without sleep-disordered breathing: 20-29 years (p<0.001), 30-39 years (p<0.01) and 40-49 years (p<0.05). After the sixth decade of life there was a sudden rise in frequency of women diagnosed with OSA: 50-59 years (p<0.001), 60-69 years (p<0.001) and 70-80 years (p<0.001).
Figure 2.
A. Frequency distribution of patients with OSA by decades of life in men. Legend: Significant difference *(p<0.05), **(p<0.01) and ***(p<0.001) B. Frequency distribution of patients with OSA by decades of life in women. Legend: Significant difference *(p<0.05), **(p<0.01) and ***(p<0.001).
Analysis of Pearson’s correlation coefficients between the evaluated anthropometric parameters and AHI were considered significant (p<0.001). With regard to men, variables were decreasingly ordered by the “r” values: body mass index (r=0.467), waist-to-height ratio (r=0.464), waist circumference (r=0.447) and neck circumference (r=0.401). In women, the sequence of the variables was different: waist circumference (r=0.411), waist-to-height ratio (r=0.411), body mass index (r=0.353) and neck circumference (r=0.300).
The cut-off values for each parameter and the respective accuracy obtained between genders in all anthropometric variables analyzed were presented in the Table 2. In the male population, a waist circumference of 92.5cm corresponds to 72.9% accuracy in rating mild-moderate-severe OSA. In women, a waist circumference of 95.0 corresponds to 78.9% accuracy in rating OSA, regardless of severity. In men, when considering only moderate-severe OSA, a 0.61 waist-to-height ratio represented a higher accuracy (78.4%). Meanwhile, in women, a 0.70 waist-to-height ratio resulted in 88.2% accuracy in the diagnosis of moderate-severe OSA. Lastly, an analysis of individuals with severe OSA indicated that a waist circumference of 116.1cm resulted in a 91.3% accuracy of diagnosis in the male population, while a waist circumference of 126.5cm resulted in 95.1% accuracy in the identification of the respiratory sleep disorder in women.
Table 2.
Anthropometric indices and their cutoff values according to obstructive sleep apnea severity in each gender.
| Body Mass Index | Waist Circumference | Neck Circumference | Waist-to-height ratio | |||||
|---|---|---|---|---|---|---|---|---|
| Cut-off (kg/m2) | Accuracy (%) | Cut-off (cm) | Accuracy (%) | Cut-off (cm) | Accuracy (%) | Cut-off | Accuracy (%) | |
| Mild to moderate to severe OSA (model 1) | ||||||||
| Men (n=190/450) | 27.2 | 71.6±0.02 | 92.5* | 72.9±0.02 | 40.2 | 72.0±0.02 | 0.56 | 71.8±0.02 |
| Women (n=140/552) | 33.0 | 76.7±0.02 | 95.0* | 78.9±0.02 | 36.2 | 76.0±0.02 | 0.58 | 77.9±0.02 |
| Moderate to severe OSA (model 2) | ||||||||
| Men (n=108/450) | 32.0 | 77.8±0.02 | 96.0 | 74.9±0.02 | 42.3 | 78.0±0.02 | 0.61* | 78.4±0.02 |
| Women (n=63/552) | 36.1 | 86.3±0.01 | 101.0 | 87.4±0.01 | 37.9 | 86.3±0.01 | 0.70* | 88.2±0.01 |
| Severe OSA (model 3) | ||||||||
| Men (n=44/450) | 34.1 | 91.1±0.01 | 116.1* | 91.3±0.01 | 46.4 | 90.2±0.01 | 0.67 | 91.1±0.01 |
| Women (n=27/552) | 42.2 | 94.8±0.01 | 126.5* | 95.1±0.01 | 40.5 | 94.6±0.01 | 0.72 | 94.0±0.01 |
Legend: OSA (obstructive sleep apnea).
Higher accuracy value between the anthropometric variables evaluated in this study.
Logistic regression analysis between anthropometric measures and OSA severity was performed for both genders. Table 3 shows factors associated with OSA severity in the male sample. The factors significantly associated with the diagnosis of OSA, regardless of severity, were: neck circumference and waist-to-height ratio. Interpreting this finding, an increase in neck circumferences and waist-to-height ratio of 1-unit causes an increase of 15% and 13% in risk for OSA, respectively. In analysis of subjects with moderate-severe OSA, waist-to-height ratio was considered the only significantly associated factor. In the logistic regression, a 1-unit increase in the waist-to-height ratio led to an increase of 14% in the risk for OSA. In the last analysis in Table 3, considering only severe classification of OSA, BMI remained the only factor associated with sleep disorder.
Table 3.
Binary logistic regression models for calculation of crude odds ratio and adjusted odds ratio for obstructive sleep apnea in the male sample.
| cOR | 95% CI for cOR | p value | aOR | 95% CI for aOR | p value | |
|---|---|---|---|---|---|---|
| Associated factors to mild-moderate-severe OSA (model 1) | ||||||
| Constant | - | - | - | <0.001 | - | <0.001 |
| Body mass index | 1.25 | 1.19-1.33 | <0.001 | - | - | - |
| Waist-to-height ratio | 1.18 | 1.13-1.22 | <0.001 | 1.13 | 1.08-1.18 | <0.001 |
| Neck circumference | 1.38 | 1.28-1.50 | <0.001 | 1.15 | 1.05-1.27 | 0.004 |
| Waist circumference | 1.10 | 1.07-1.12 | <0.001 | - | - | - |
| Associated factors to moderate-severe OSA (model 2) | ||||||
| Constant | - | - | - | <0.001 | - | <0.001 |
| Body mass index | 1.21 | 1.15-1.28 | <0.001 | - | - | - |
| Waist-to-height ratio | 1.14 | 1.10-1.18 | <0.001 | 1.14 | 1.10-1.18 | <0.001 |
| Neck circumference | 1.26 | 1.16-1.35 | <0.001 | - | - | - |
| Waist circumference | 1.07 | 1.05-1.09 | <0.001 | - | - | - |
| Associated factors to severe OSA (model 3) | ||||||
| Constant | - | - | - | <0.001 | - | <0.001 |
| Body mass index | 1.23 | 1.15-1.31 | <0.001 | 1.23 | 1.15-1.31 | <0.001 |
| Waist-to-height ratio | 1.14 | 1.09-1.19 | <0.001 | - | - | - |
| Neck circumference | 1.18 | 1.08-1.29 | <0.001 | - | - | - |
| Waist circumference | 1.08 | 1.05-1.11 | <0.001 | - | - | - |
Legend: aOR (adjusted odds ratio), CI (confidence interval), cOR (crude odds ratio), OSA (obstructive sleep apnea).
Table 4 shows the factors associated with OSA severity in women. Regarding the presence of OSA, regardless of its severity, an increase of 1-unit in waist-to-height ratio increased by 14% in the risk for presenting the sleep disorder. Women with moderate-severe OSA had a 7% higher risk of having sleep-disordered breathing for each increase of 1cm in length of the waist circumference. In the third stage, women with severe OSA had 8% higher risk for each increase of 1 cm in length of the waist circumference.
Table 4.
Binary logistic regression models for calculation of crude odds ratio and adjusted odds ratio for obstructive sleep apnea in the female sample.
| cOR | 95% CI for cOR | p value | aOR | 95% CI for aOR | p value | |
|---|---|---|---|---|---|---|
| Associated factors to mild-moderate-severe OSA (model 1) | ||||||
| Constant | - | - | - | <0.001 | - | <0.001 |
| Body mass index | 1.25 | 1.19-1.33 | <0.001 | - | - | - |
| Waist-to-height ratio | 1.14 | 1.11-1.17 | <0.001 | 1.14 | 1.11-1.17 | <0.001 |
| Neck circumference | 1.38 | 1.28-1.50 | <0.001 | - | - | - |
| Waist circumference | 1.10 | 1.07-1.12 | <0.001 | - | - | - |
| Associated factors to moderate-severe OSA (model 2) | ||||||
| Constant | - | - | - | 0.001 | - | <0.001 |
| Body mass index | 1.13 | 1.09-1.18 | <0.001 | - | - | - |
| Waist-to-height ratio | 1.11 | 1.08-1.15 | <0.001 | - | - | - |
| Neck circumference | 1.25 | 1.14-1.38 | <0.001 | - | - | - |
| Waist circumference | 1.07 | 1.05-1.10 | <0.001 | 1.07 | 1.05-1.10 | <0.001 |
| Associated factors to severe OSA (model 3) | ||||||
| Constant | - | - | - | <0.001 | - | <0.001 |
| Body mass index | 1.15 | 1.09-1.22 | <0.001 | - | - | - |
| Waist-to-height ratio | 1.13 | 1.08-1.18 | <0.001 | - | - | - |
| Neck circumference | 1.25 | 1.11-1.41 | <0.001 | - | - | - |
| Waist circumference | 1.08 | 1.05-1.11 | <0.001 | 1.08 | 1.05-1.11 | <0.001 |
Legend: aOR (adjusted odds ratio), CI (confidence interval), cOR (crude odds ratio), OSA (obstructive sleep apnea).
DISCUSSION
The present findings indicate that anthropometric variables are gender specific and potentially indicate the severity of OSA. Complexity of the sleep disorders requires an objective analysis23. PSG is considered the gold standard method for diagnosis of sleep disorders, especially OSA.
Regarding gender in the sample composition, 190 men were diagnosed with mild, moderate and severe sleep apnea, respectively 82, 64 and 44 volunteers. Thus, sleep apnea had 42.2% prevalence in the men. In the female sample, that prevalence of the sleep disorder was approximately 25.4%, being 77 women diagnosed with mild apnea, 36 with moderate apnea and 27 with severe apnea. Directly or indirectly, the waist measurement (either by waist circumference or waist-to-height ratio) was associated to mild-moderate-severe and moderate-severe models in men, and in women the measure was related to all logistic models. These results clearly demonstrate that measurement of waist was significantly relevant in models adjusted for the risk of sleep apnea in both genders.
The results from the male sample identify an increase in frequency of men with OSA from beginning of the fifth decade of life (40-49 years) (Figure 2A). Gradual weight gain occurs primarily through to aging, unhealthy eating habits and lifestyle24,25. This study allowed us to identify possible cut-off points for anthropometric measures strongly associated with OSA, both men and women. Among men, smaller increases in specific measurements were associated with OSA, while in women the increase required was proportionally greater. Among women, waist circumference was the parameter with the highest accuracy as a classifier of OSA. The cut-off values for waist circumference, as well as BMI, were greater in the female sample compared to the male sample. In women, relations between visceral adipose tissue and risk factors were consistently stronger than in men15,26.
In this study, a sudden rise in the frequency of OSA is observed. The change in pattern occurs between the fifth and sixth decades of life, which coincides with the menopausal transition. Previous studies have indicated that progesterone increases the ventilatory drive and activity of the dilator muscles of the upper airway27. Postmenopausal women have an increased incidence of sleep-disordered breathing28. Some hypotheses which could explain the relationship between obesity and sleep apnea include: a reduction in intrathoracic volume, resulting in a lower current tidal volume29; a reduction of the size of the pharyngeal lumen caused by the accumulation of adipose tissue on the side walls of the upper airway30; decreased muscle strength resulting in reduced airway obstruction protection due to deposits of intramuscularfat31 and a reduction in the size of the upper airway, as a secondary result of central obesity, chest wall and tracheal traction32.
In previous studies, waist circumference measurements were found to be a better predictor of comorbidities than BMI16. The importance of waist circumference in sleep-disordered breathing has been previously demonstrated28,33. Waist circumference is the anthropometric measure that was mostly associated with obesity, as well as, the worsening of sleep-disordered breathing and cardiovascular diseases15,34. Previous studies reported that a waist circumference greater than 85 cm in men and 90 cm in women are suggestive of obesity due their approximates to this visceral fat mass35. In this sense, high levels of visceral fat have been associated with OSA risk36. The facts mentioned above highlight a strong relationship between abdominal fat and obesity. Some previous studies, contrary to this one, found that OSA was more closely associated to abdominal obesity in men than in women37-39. Among the main possible hypotheses to explain these previous findings are small sample sizes and a lack of epidemiological representativeness.
Some specific differences between genders have been highlighted. In women, we suggest waist-to-height ratio and waist circumference as the best screening methods for sleep apnea. In men, factors with great potential for identification varied according to severity of the disease, highlighting waist-to-height ratio and neck circumference for mild-moderate-severe OSA, waist-to-height ratio for moderate-severe OSA and BMI for severe OSA. The main novelty of this study is identification of obesity-related cut-off values of anthropometric measures and their respective accuracy as predictors of OSA in both genders. Considering clinically harmful models of the sleep disorder (moderate and severe) as well as obesity-related anthropometric measures, the findings of this study indicate the impact of fat distribution on OSA. In women, accumulation of central fat increases the risk of sleep apnea and in men, the risk is more related to overall distribution of fat in the body. Despite small difference of accuracy between anthropometric measures, the result refers to a representative epidemiological sample and associates values of each parameter to different severity groups of the sleep disorder.
It is worth mentioning that the findings come from a sample with different ethnic background, due to formation of the Brazilian population. Furthermore, a recent study has shown that 76% of population presented at least 1 sleep related problem and number of sleep complaints was higher in women and increased with age40. The main complaints were light and insufficient sleep, snoring and insomnia.
Some limitations of this study should be considered. The study considered the analysis of gender, as a whole, regardless of age range. The change of hormone levels, typical of the late reproductive period in women, can interfere with fat distribution, body shape and increased incidence of OSA. Moreover, it is noteworthy that the presence of severe comorbidities and/or advanced disease stages should be minimally considered in this study, due to voluntary recruitment of all participants and to epidemiological approach of the study. Nevertheless, the results emphasize the role of BMI and waist circumference in OSA in both men and women. Further investigations could evaluate pattern of oxygen desaturation with obesity-related cut-off values of anthropometric measures, and also applied to randomized controlled studies, follow-up studies, as well as in others population worldwide.
CONCLUSIONS
This study revealed the importance of anthropometric measurements as parameters related to sleep apnea in men and women. The accuracy of variables and the best variables to predict OSA were different between genders. For men, waist-to-height ratio and neck circumferences were the most accurate variables when considering individuals with mild and moderate OSA. In respect of severe OSA, only BMI was strongly associated with the sleep-disordered breathing. For women, waist circumference showed the highest success rate when screening moderate-severe and severe OSA. The present study identified the anthropometric variables with the highest risk for OSA and their respective cutoff value, according to gender.
Acknowledgments
This study was funded by Associação Fundo de Incentivo à Pesquisa (AFIP), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Sao Paulo Research Foundation (FAPESP) (#2013/14945-7 to DNP). HH, LB, MLA and ST are recipients of CNPq fellowships. The funders had no role in study design, data collection and analysis, decision to publish, or manuscript preparation.
The authors thank all volunteers who took part in the study. The efforts of AFIP staff, in particular Roberta Siufi, are deeply appreciated.
REFERENCES
- 1.Tufik S, Santos-Silva R, Taddei JA, Bittencourt LR. Obstructive sleep apnea syndrome in the Sao Paulo Epidemiologic Sleep Study. Sleep Med. 2010;11(5):441–446. doi: 10.1016/j.sleep.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 2.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22(5):667–689. [PubMed] [Google Scholar]
- 3.Black J. Sleepiness and residual sleepiness in adults with obstructive sleep apnea. Respir Physiol Neurobiol. 2003;136(2-3):211–220. doi: 10.1016/s1569-9048(03)00083-1. [DOI] [PubMed] [Google Scholar]
- 4.Ljunggren M, Byberg L, Theorell-Haglöw J, Lindahl B, Michaëlsson K, Lindberg E. Increased risk of heart failure in women with symptoms of sleep-disordered breathing. Sleep Med. 2016;17:32–37. doi: 10.1016/j.sleep.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 5.Drager LF, Lorenzi-Filho G. CPAP for obstructive sleep apnea and the metabolic syndrome. N Engl J Med. 2012;366(10):964–964. doi: 10.1056/NEJMc1200497. [DOI] [PubMed] [Google Scholar]
- 6.Kapur V, Blough DK, Sandblom RE, Hert R, de Maine JB, Sullivan SD, et al. The medical cost of undiagnosed sleep apnea. Sleep. 1999;22(6):749–755. doi: 10.1093/sleep/22.6.749. [DOI] [PubMed] [Google Scholar]
- 7.Wittmann V, Rodenstein DO. Health care costs and the sleep apnea syndrome. Sleep Med Rev. 2004;8(4):269–279. doi: 10.1016/j.smrv.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Pillar G, Shehadeh N. Abdominal fat and sleep apnea: the chicken or the egg? Diabetes Care. 2008;31(Suppl 2):S303–S309. doi: 10.2337/dc08-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization . Obesity and overweight. Geneva: World Health Organization; 2016. [Google Scholar]
- 10.Hu FB, Li TY, Colditz GA, Willett WC, Manson JE. Television watching and other sedentary behaviors in relation to risk of obesity and type 2 diabetes mellitus in women. JAMA. 2003;289(14):1785–1791. doi: 10.1001/jama.289.14.1785. [DOI] [PubMed] [Google Scholar]
- 11.Wolk A, Gridley G, Svensson M, Nyrén O, McLaughlin JK, Fraumeni JF, et al. A prospective study of obesity and cancer risk (Sweden) Cancer Causes Control. 2001;12(1):13–21. doi: 10.1023/a:1008995217664. [DOI] [PubMed] [Google Scholar]
- 12.Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289(1):76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 13.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287(3):356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 14.Onat A, Hergenç G, Yüksel H, Can G, Ayhan E, Kaya Z, et al. Neck circumference as a measure of central obesity: associations with metabolic syndrome and obstructive sleep apnea syndrome beyond waist circumference. Clin Nutr. 2009;28(1):46–51. doi: 10.1016/j.clnu.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 15.Rexrode KM, Carey VJ, Hennekens CH, Walters EE, Colditz GA, Stampfer MJ, et al. Abdominal adiposity and coronary heart disease in women. JAMA. 1998;280(21):1843–1848. doi: 10.1001/jama.280.21.1843. [DOI] [PubMed] [Google Scholar]
- 16.Janssen I, Katzmarzyk PT, Ross R. Waist circumference and not body mass index explains obesity-related health risk. Am J Clin Nutr. 2004;79(3):379–384. doi: 10.1093/ajcn/79.3.379. [DOI] [PubMed] [Google Scholar]
- 17.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA. 2006;295(13):1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 18.Santos-Silva R, Tufik S, Conway SG, Taddei JA, Bittencourt LR. Sao Paulo Epidemiologic Sleep Study: rationale, design, sampling, and procedures. Sleep Med. 2009;10(6):679–685. doi: 10.1016/j.sleep.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Korn EL, Graubard BI. Analyses of health surveys. Hoboken: John Wiley & Sons; 1999. [Google Scholar]
- 20.International Classification of Sleep Disorders: Diagnostic and Coding Manual (ICSD-2) 2nd ed. Westchester: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 21.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Los Angeles: University of California Los Angeles Brain Information Service; 1968. [Google Scholar]
- 22.Iber C, Ancoli-Israel S, Cheeson A, Quan SF. The AASM manual for scoring of sleep associated events: rules, terminology and techical specifications. Wetchester: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 23.Polesel DN, Nozoe KT, Decleva DVL, Tufik S, Andersen ML. Obesity, dyslipidemia, and sleep disorders: complexity requires complementary analysis. Chest. 2013;143(4):1187–1188. doi: 10.1378/chest.12-2648. [DOI] [PubMed] [Google Scholar]
- 24.Kawaguchi Y, Fukumoto S, Inaba M, Koyama H, Shoji T, Shoji S, et al. Different impacts of neck circumference and visceral obesity on the severity of obstructive sleep apnea syndrome. Obesity (Silver Spring) 2011;19(2):276–282. doi: 10.1038/oby.2010.170. [DOI] [PubMed] [Google Scholar]
- 25.Polesel DN, Okazaki KM, Nozoe KT, Andersen ML, Tufik S. Obstructive sleep apnea as a potential confounding factor in atherosclerosis in the Asian population. J Neurol Sci. 2014;346(1-2):333–334. doi: 10.1016/j.jns.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 26.Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116(1):39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 27.Marcouiller F, Boukari R, Laouafa S, Lavoie R, Joseph V. The nuclear progesterone receptor reduces post-sigh apneas during sleep and increases the ventilatory response to hypercapnia in adult female mice. PLoS One. 2014;9(6):e100421. doi: 10.1371/journal.pone.0100421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Polesel DN, Hirotsu C, Nozoe KT, Boin AC, Bittencourt L, Tufik S, et al. Waist circumference and postmenopause stages as the main associated factors for sleep apnea in women: a cross-sectional population-based study. Menopause. 2015;22(8):835–844. doi: 10.1097/GME.0000000000000406. [DOI] [PubMed] [Google Scholar]
- 29.Naimark A, Cherniack R. Compliance of the respiratory system and its components in health and obesity. J Appl Physiol. 1960;15:377–382. doi: 10.1152/jappl.1960.15.3.377. [DOI] [PubMed] [Google Scholar]
- 30.Hoffstein V, Zamel N, Phillipson EA. Lung volume dependence of pharyngeal cross-sectional area in patients with obstructive sleep apnea. Am Rev Respir Dis. 1984;130(2):175–178. doi: 10.1164/arrd.1984.130.2.175. [DOI] [PubMed] [Google Scholar]
- 31.Whittle AT, Marshall I, Mortimore IL, Wraith PK, Sellar RJ, Douglas NJ. Neck soft tissue and fat distribution: comparison between normal men and women by magnetic resonance imaging. Thorax. 1999;54(4):323–328. doi: 10.1136/thx.54.4.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown LK. A waist is a terrible thing to mind: central obesity, the metabolic syndrome, and sleep apnea hypopnea syndrome. Chest. 2002;122(3):774–778. doi: 10.1378/chest.122.3.774. [DOI] [PubMed] [Google Scholar]
- 33.Davidson TM, Patel MR. Waist circumference and sleep disordered breathing. Laryngoscope. 2008;118(2):339–347. doi: 10.1097/MLG.0b013e3181587d7c. [DOI] [PubMed] [Google Scholar]
- 34.Song X, Jousilahti P, Stehouwer CD, Söderberg S, Onat A, Laatikainen T, et al. DECODE Study Group Cardiovascular and all-cause mortality in relation to various anthropometric measures of obesity in Europeans. Nutr Metab Cardiovasc Dis. 2015;25(3):295–304. doi: 10.1016/j.numecd.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 35.New criteria for 'obesity disease' in Japan. Circ J. 2002;66(11):987–992. doi: 10.1253/circj.66.987. [DOI] [PubMed] [Google Scholar]
- 36.Luchnikova E, Shchekotov V. 7a.05: Visceral fat level determined using the bioelectrical impedance as a method to assess obstructive sleep apnea risk. J Hypertens. 2015;33(Suppl 1):e90 [Google Scholar]
- 37.Simpson L, Mukherjee S, Cooper MN, Ward KL, Lee JD, Fedson AC, et al. Sex differences in the association of regional fat distribution with the severity of obstructive sleep apnea. Sleep. 2010;33(4):467–474. doi: 10.1093/sleep/33.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kritikou I, Basta M, Tappouni R, Pejovic S, Fernandez-Mendoza J, Nazir R, et al. Sleep apnoea and visceral adiposity in middle-aged male and female subjects. Eur Respir J. 2013;41(3):601–609. doi: 10.1183/09031936.00183411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mazzuca E, Battaglia S, Marrone O, Marotta AM, Castrogiovanni A, Esquinas C, et al. Gender-specific anthropometric markers of adiposity, metabolic syndrome and visceral adiposity index (VAI) in patients with obstructive sleep apnea. J Sleep Res. 2014;23(1):13–21. doi: 10.1111/jsr.12088. [DOI] [PubMed] [Google Scholar]
- 40.Hirotsu C, Bittencourt L, Garbuio S, Andersen ML, Tufik S. Sleep complaints in the Brazilian population: Impact of socioeconomic factors. Sleep Sci. 2014;7(3):135–142. doi: 10.1016/j.slsci.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]