Abstract
Sexual dimorphism includes the physical and reproductive differences between the sexes, including differences that are conserved across species, ranging from the common fruit fly, Drosophila melanogaster, to humans. Sex-dependent variations in adaptive homeostasis, and adaptive stress responses may offer insight into the underlying mechanisms for male and female survival differences and into differences in chronic disease incidence and severity in humans. Earlier work showed sex-specific differences in adaptive responses to oxidative stressors in hybrid laboratory strains of D. melanogaster. The present study explored whether this phenomenon is also observed in wild-type D. melanogaster strains Oregon-R (Or-R) and Canton-S (Ca-S), as well as the common mutant reference strain w[1118], in order to better understand whether such findings are descriptive of D. melanogaster in general. Flies of each strain were pretreated with non-damaging, adaptive concentrations of hydrogen peroxide (H2O2) or of different redox cycling agents (paraquat, DMNQ, or menadione). Adaptive homeostasis, and changes in the expression of the proteasome and overall cellular proteasomal proteolytic capacity were assessed. Redox cycling agents exhibited a male-specific adaptive response, whereas H2O2 exposure provoked female-specific adaptation. These findings demonstrate that different oxidants can elicit sexually dimorphic adaptive homeostatic responses in multiple fly strains. These results (and those contained in a parallel study [1]) highlight the need to address sex as a biological variable in both fundamental science, clinical research, and toxicology.
Keywords: proteasome, proteostasis, adaptive homeostasis, oxidative stress, hydrogen peroxide, redox cycling
INTRODUCTION
Across several species, females consistently outlive males [2–7]. In humans the robustness of this trend begins as early as childhood (birth to age 5), wherein females uniformly show greater survival. This trend is continued into later life (age 50), with women comprising 90% of supercentenarians [8]. Women have a lower mortality risk from certain chronic diseases [9], and the majority of current longevity interventions favor females [10]. Some findings point to genetic factors [11–14], hormonal exposure [15, 16], and mating [17, 18] as the major causes for sex differences [19]. Nor are cellular differences between the sexes entirely attributable to hormonal exposure [20], as genetic differences result in varying embryonic growth rates [21, 22], and higher sensitivity in male hippocampal neuronal cells following peroxynitrite exposure compared to female neuronal cells [23]. Additionally, hormonal ablation does not completely eliminate sex-specific responses in tumor progression [24], or differences in sensitivity to carcinogens [25], nor in survival differences in pediatric stroke studies [26], suggesting that additional mechanisms may be involved.
Key signaling pathways, such as the nutrient sensing, insulin/insulin-like growth factor (IGF-1) pathway, show a female-specific benefit. Female mice heterozygous for IGF-1 mutation survive longer and are more stress-resistant compared to their wild-type counterparts, whereas heterozygous males show no difference [27, 28]. Tissue-specific differences also arise in response to changes in nutrient signaling, as short-term caloric restriction triggers increased oxidative phosphorylation to a greater extent in females than in males [29]. Similarly, male D. melanogaster show reduced heat tolerance following subsequent removal of the insulin-like receptor homolog, InR [30], whereas females exhibit increased lifespan. The impact of the insulin-sensing pathway also appears to be sex-specific in humans, as females show greater sensitivity to insulin, as evidenced by differences in glucose utilization in both muscle and liver tissues [31].
Sex-bias in the cellular response to acute oxidative stress has been reported for humans and mice, where cells from females generally show greater stress resistance than do cells from males [32]. Furthermore, sex-dependent difference are also evident in adaptation, or adaptive homeostasis [33], albeit less-explored. The adaptive homeostatic response is a signaling cascade induced in cells or organisms by a non-toxic amount of an oxidant or compound that protects against future, more damaging, insults. Early cell-culture studies demonstrated the adaptive increase in multiple stress responsive enzymes, including the 20S proteasome [34–36] and the Lon protease [37, 38] in response to adaptive doses of H2O2. Replication of the adaptive methodology in model organisms, most notably D. melanogaster [39–41] and C. elegans [42, 43] demonstrated that adaptation to H2O2 is also observed in these multicellular animals.
The model organism, D. melanogaster is an excellent system in which to study sex differences in life span regulation, due to the short life span and the presence of both sexes. Comparisons between males and females for several Drosophila genotypes [17, 44, 45], indicates that females, on average, are longer-lived than their male counterparts. Unique physiological characteristics of females, including larger body size [46] and greater rate of regeneration of the gut epithelium [47], are interesting and testable possible explanations for differences in the stress response between the sexes, including differences in the adaptive stress response.
The purpose of the present study was to assess the sex-specific differences in the adaptive stress response in wild-type and w[1118] D. melanogsster strains. The sex-dependent response of the superoxide-producing redox cycling agent, paraquat was compared to the short-term oxidant, hydrogen peroxide. 3 day old flies of the three strains: Canton-S (Ca-S), Oregon-R (Or-R), and w[1118] were pretreated with micromolar, ‘adaptive’ amounts of each oxidant. Following pretreatment, flies were subjected to semi-lethal concentrations of either paraquat or hydrogen peroxide to measure changes in survival. The 20S proteasome expression and activity were measured following pretreatment. Additionally, other redox cycling agents, 2,3-Dimethoxy-1,4-naphthoquinone (DMNQ), and menadione, were tested to measure sex-specific adaptive differences. These experiments (and those contained in an additional study [1]) reveal that the redox-cyclers consistently trigger a male-specific adaptive response, whereas H2O2 caused a female-specific adaptive response. Overall this work improves our understanding of the sex-specific differences in adaptive responses to oxidative stress. More importantly, it emphasizes the need to address sex as a vital biological variable in aging, and in determining disease susceptibility and severity.
MATERIALS AND METHODS
D. melanogaster culture
Three common strains of D. melanogaster were utilized: Canton-S, Oregon-R, and w[1118]. These strains were selected due to their high usage as standard control strains in D. melanogaster genetics, with each strain having been utilized in at least 2000 publications (PubMed). These strains have also been extensively utilized in lifespan and stress-resistance studies and provide the genetic background for genetically modified strains. For further explanation of these standard strains, please see the following [1]. All strains were cultured at 25°C, with 12 hour light/dark cycles, on a standard agar/dextrose/corn meal/yeast media [48]. Flies were collected over 48 hours from pre-cleared bottles prior to treatments.
D. melanogaster challenge assay
Ten flies were transferred into vials with a Kimwipe soaked in 1mL of 5% sucrose for 24 hours. Upon treatment initiation, flies were transferred to vials with 1mL of 5% sucrose and increasing concentrations of H2O2 [0M-8M] or paraquat [0mM-40mM] as indicated. Survival was scored every 8 hours, until all flies were dead.
D. melanogaster adaptation
24 hours prior to treatment, 10 flies were transferred to vials containing 1mL of 5% sucrose. Upon treatment, flies were transferred to vial with 1mL of 5% sucrose and low amounts of H2O2 [0μM-100μM] or paraquat [0μM-10μM], as indicated, for 8 hours, and subsequently transferred to vials containing only 1mL of 5% sucrose for an additional 16 hours. Afterwards, flies were transferred to vials containing a toxic dose of H2O2 [4.4M] or paraquat [30mM]. Survival was scored every 8 hours, until all flies were dead.
Pretreatment with various oxidants
Ten flies were transferred to vials containing 1mL of 5% sucrose. Upon treatment initiation, flies were transferred to vials containing menadione [0μM-1mM], DMNQ [0μM-10μM], paraquat [0μM-10μM] or H2O2 [0μM-100μM] for 8 hours before being placed back onto vials with only 1mL of 5% sucrose for an additional 16 hours. Afterwards flies were frozen for down-stream processing.
Preparation of D. melanogaster
Flies were homogenized in 200μL proteolysis buffer (50mM Tris/HCl, 20mM KCl, 5mM MgAc, 1mM DTT, pH 7.5) using an electric pestle. Further lysis was conducted by three ‘freeze-thaw’ cycles, consisting of 5-minute intervals on dry ice, followed by incubation in water, and vortexed. Samples were centrifuged for 10,000g for 10min at 4°C to remove cuticle fragments. Protein concentration was measured using the Bicinchoninic acid assay (BCA) reducing agent compatible kit (no. 23252, ThermoFisher Scientific).
Fluoropeptide Proteolytic Activity Assays
5μg of whole fly lysate was aliquoted, in triplicate, to 96-well plates. Proteasome’s chymotrypsin-like activity was measured by adding 2μM of the proteasomal β5-specific fluorogenic substrate, Suc-LLVY-AMC (no. 539141, Calbiochem). Lysate was incubated at 37°C, and fluorescence readings were recorded every 10 minutes for 4h using an excitation/emission of 355nm/444nm. Fluorescence units were converted to moles of free 7-amino-4-methylcuomarin (AMC), using an AMC standard curve (no. 164545, Merck), with background subtracted. To measure proteolytic inhibition, 20μM of the proteasome inhibitor, lactacystin (no. 80052–806, VWR) was added directly to lysate, and incubated on plate shaker for 30min at 300rpm, after which, substrate was added.
Western Blots
10μg of whole fly lysate was run on a 4–15% gradient SDS-PAGE gel (no. 4568084, Bio-Rad) for 1 hour at 100V before being transferred at 4°C to a PVDF membrane (no. 1620177XTU, Bio-Rad). The goat polyclonal anti-Actin-HRP antibody, conjugated to horseradish peroxidase (1:1000 dilution, no sc-1616, Santa Cruz Biotechnology) was used as the protein loading control. Monoclonal antibody against the α-subunit of the 20S core proteasome of D. melanogaster was used (1:100 dilution, no. sc-65755, Santa Cruz Biotechnology).
Immunoprecipitation (IP)
200 flies were homogenized in 400μL proteolysis buffer (50mM Tris/HCl, 20mM KCl, 5mM MgAc, 1mM DTT, pH 7.5) using an electric pestle. Further lysis was conducted by three ‘freeze-thaw’ cycles, consisting of 5-minute intervals on dry ice, followed by incubation in water, and vortexed. Samples were centrifuged for 10,000g for 10min at 4°C to remove cuticle fragments. Protein concentration was measured using the Bicinchoninic acid assay (BCA) reducing agent compatible kit (no. 23252, ThermoFisher Scientific). Each IP sample was normalized to have 400μg protein in a final volume of 300μL proteolysis buffer. Next, 20μL of washed protein G Sepharose 4B beads (no. 10–1242, ThermoFisher Scientific) were added to each sample and placed on an end-over-end shaker for 30min at 4°C to pre-clear the lysate. (Antibody binding beads were washed twice by first adding 500μL 1X PBS, inverting the sample twice to mix, and centrifuging at 2000rpm for 1min at 4°C to pellet the beads, at which point the supernatant was removed). Afterwards, samples were centrifuged at 2000rpm for 1min at 4°C to pellet the beads and the lysate was transferred to fresh tubes, at which point 20μL of the monoclonal antibody against the α-subunit of the 20S core proteasome of D. melanogaster (no. sc-65755, Santa Cruz Biotechnology) was added and samples were rotated at 4°C, overnight. Next, 40μL of washed antibody binding beads were added to each IP and rotated at 4°C, overnight. Samples were centrifuged at 2000 rpm for 2min at 4°C. Afterwards, the ‘Flow-through’ (supernatant) was transferred to fresh tubes for down-stream analysis. Beads were washed once with 1x mPER buffer (no. 78501, ThermoFisher Scientific) and three times with ice-cold 1x PBS solution. Samples were rotated for 5min between washes. Beads were eluted by adding 50μL of sample buffer containing 5% SDS and heated at 95°C for 5min prior to loading on an 10% SDS-PAGE gel.
RESULTS
Sex differences in resistance to hydrogen peroxide toxicity
In a previous study, sex-specific differences were identified in the adaptation to hydrogen peroxide (H2O2) in several laboratory strains of flies [39–41, 43]. To determine if the sex-specific differences in H2O2 adaptation are observed in wild-type D. melanogaster strains, two common wild-type strains were analyzed, Canton-S (Ca-S) and Oregon-R (Or-R), along with the common reference strain w[1118]. Three-day old progeny from each strain were fed various concentrations of H2O2 [0M-8M], and survival was scored every 8 hours. In all three strains, males showed similar sensitivity to challenge doses greater than 2M (Figure 1A,C,E), with males of the w[1118] strain showing the greatest resistance at 1M, matching earlier findings [43]. Females showed similar sensitivity to increasing concentrations of H2O2, with greatest resistance observed for w[1118] (Figure 1B,D,F).
Figure 1. Sex-specific sensitivity to hydrogen peroxide (H2O2).

(A-F) Male and female progeny were collected and placed onto 5% sucrose for 24 hours prior to hydrogen peroxide challenge [0M-8M]. (A,B) Ca-S male and female survival curves. (C,D) Or-R male and female survival curves. (E,F) w[1118] male and female survival curves.
Hydrogen peroxide pretreatment results in female-specific adaptation
Adaptation, or adaptive homeostasis [33], occurs for a broad spectrum of oxidative (and many other) stressors [33, 49, 50]. Exposure to adaptive doses (micromolar amounts) of H2O2 have been previously shown to upregulate cytoprotective genes, which protect against future, potentially more damaging, oxidative insults. Prior studies in cell culture [34, 36], and the model organisms C. elegans [42, 43] and Drosophila melanogaster [39, 40, 43] have shown that H2O2 pretreatment increases survival from a subsequent toxic H2O2 insult, and in D. melanogaster this H2O2 adaptation was shown to be female-specific [39, 40, 43]
Here, we sought to determine if H2O2 pretreatment in common laboratory strains also caused a female-specific adaptive response [39, 40, 43]. To do so, flies of the three strains were collected and placed, 24 hours prior to pretreatment, upon 5% sucrose. Afterwards, flies received either no H2O2 or adaptive concentrations of H2O2 [10μM or 100μM] for 8 hours, before being placed back onto 5% sucrose for an additional 16-hour recovery. Flies were challenged with 4.4M H2O2 and survival was scored every 8 hours. In all three strains, males, irrespective of pretreatment, showed no difference in survival (Figure 2A–C). The lack of adaptation in males was not due to insufficient pretreatment concentrations, as higher doses were detrimental to survival [43]. In contrast, females of all three strains showed longer survival times compared to females that did not receive the pretreatment (Figure 2D–F). Pretreatment with adaptive concentrations of H2O2 [10μM, 100μM, or 1000μM] also resulted in females (Figure 2G,I,K), but not males (Figure 2H,J,L), exhibiting higher levels of the 20S proteasome, as measured by 20S proteasome α subunit protein levels.
Figure 2. Pretreatment with hydrogen peroxide results in female-specific adaptation.
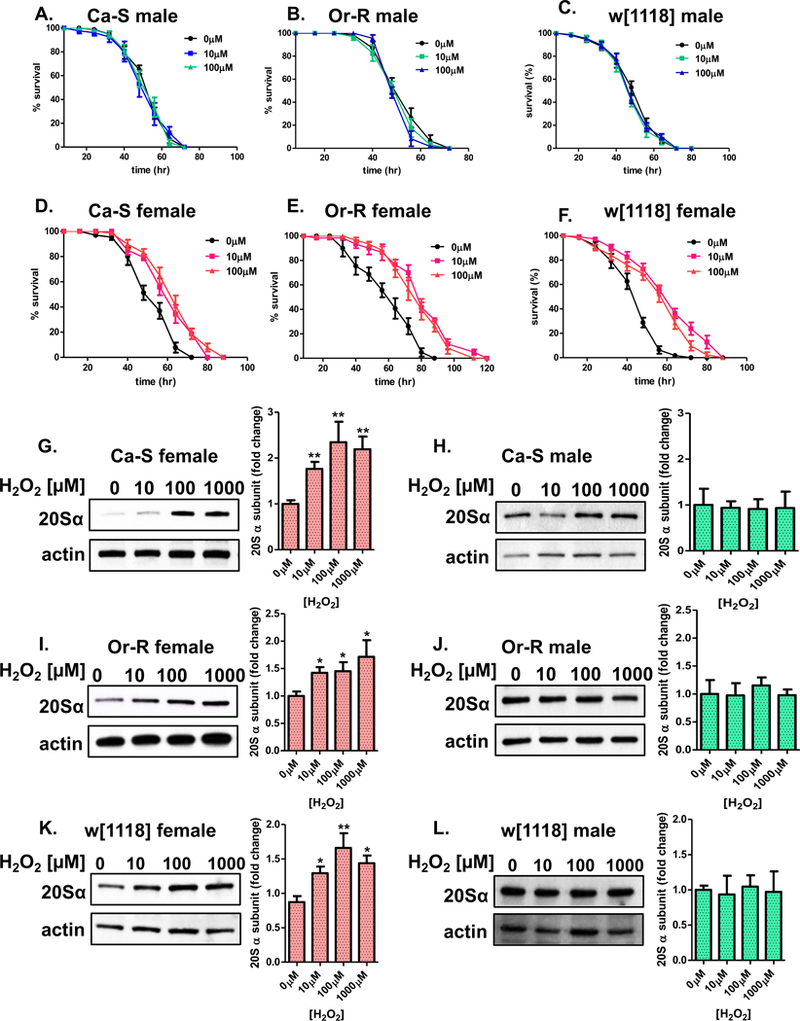
(A-F) Male and female progeny were collected and placed onto 5% sucrose for 24 hours prior to H2O2 pretreatment [0μM-100μM] for 8 hours. After a 16-hour recovery, flies were placed upon H2O2 challenge dose [4.4M]. (A-C) Males, irrespective pretreatment concentration, show no difference in survival compared to the controls. (D-F) Pretreated females showed longer survival times compared to controls upon administration of the challenge dose. Statistical difference in survival was calculated using the Log-Rank test. Statistical summary is located in Table 1 [1]. (G-K) The higher amounts of the 20S proteasome α subunits was measured between males and females of the three strains. Females and males were pretreated with adaptive doses of H2O2 [0μM-100μM] for 8 hours, before allowed a 16-hour recovery before collection. (G,I,K) Pretreated females show increased proteasome expression. (H,J,L) Males, regardless pretreatment, show no adaptive change in the amount of proteasome. Samples were done in triplicate, protein loading was normalized to Actin-HRP, and quantified using ImageJ. Error bars denote the standard error of the mean (S.E.M) values. * P < 0.05 and ** P < 0.01, relative to the control using one-way ANOVA.
Hydrogen peroxide adaptation involves a female-specific increase in proteasomal proteolytic capacity
Next, we measured proteasome function to determine if there was an adaptive change in overall proteolytic capacity. Progeny were subjected to adaptive doses of H2O2 [0μM-1000μM] for 8 hours before a 16-hour recovery and subsequent collection. Proteasome function was assessed in whole fly lysates by the degradation of the fluogenic substrate Suc-LLVY-AMC. Similar to the inability to adapt in Fig 2, males showed no change in proteolytic activity upon H2O2 pretreatment (Figure 3A,C,F). In contrast, pretreated females showed higher amounts of proteolytic capacity (Figure 3B,D,E). To confirm that the higher levels of proteolytic activity was due to the proteasome, the proteasome inhibitor Lactacystin was added to the lysate from pretreated females and, as expected, proteolytic activity decreased (see Figure 1A in [1]). Lysate from pretreated males also showed decreased basal activity upon addition of the proteasome inhibitor (see Figure 1B in [1]).
Figure 3. Hydrogen peroxide adaptation involves female-specific increases in proteasomal proteolytic capacity.
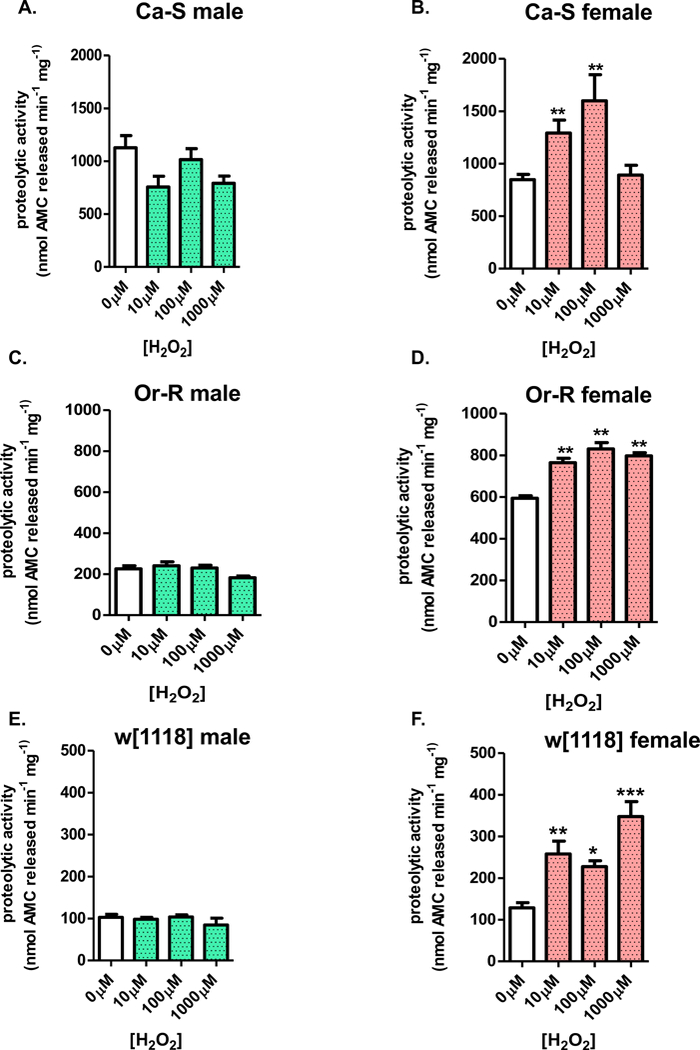
(A-F) Male and female progeny were collected and placed onto 5% sucrose for 24 hours prior to H2O2 pretreatment [0μM-1000μM] for 8 hours, at which point, flies were transferred to 5% sucrose for a 16-hour recovery before collection. Proteolytic capacity was assessed in whole fly lysate by degradation of the fluorogenic peptide, Suc-LLVY-AMC. (A,C,E) In all three strains, males show no change in proteolytic capacity, irrespective pretreatment. (B,D,F) Females from all three strains show higher levels of proteolysis upon H2O2 pretreatment. Error bars denote the standard error of the mean (S.E.M) values. * P <0.05, ** P < 0.01, and *** P < 0.001, relative to control using one-way ANOVA.
Sex differences in resistance to paraquat toxicity
The redox cycling agent, paraquat (methyl viologen dichloride) accepts electrons from intracellular one-electron donors (such as mitochondrial complexes I and III and cytochrome P450 reductase) to generate the paraquat radical, and then donates those single electrons to molecular O2 resulting in superoxide (O2•® ) generation [51]. Since this process regenerates oxidized paraquat, it can be continuously repeated to generate a constant stream of O2•® in a process called redox cycling. Paraquat (PQ) has been used extensively as an oxidant in D. melanogaster studies, and resistance to paraquat often correlates with strain life-span [52]. To determine if paraquat toxicity is similar among the three D. melanogaster strains, 3-day old progeny were subjected to increasing concentrations of paraquat [0mM-40mM] to assess survival. Males of the Ca-S strain showed greater sensitivity to paraquat toxicity compared to males of the Or-R or w[1118] strains (Figure 4A,C,E). Similarly, females of the Ca-S strain showed the greatest sensitivity to paraquat toxicity, whereas Or-R and w[1118] females had greater resistance at the same paraquat concentrations (Figure 4B,D,F).
Figure 4. Sex-specific sensitivity to paraquat (PQ).

(A-F) Male and female progeny were collected and placed onto 5% sucrose for 24 hours prior to paraquat challenge [0mM-40mM]. (A,B) Ca-S male and female survival curves. (C,D) Or-R male and female survival curves. (E,F) w[1118] male and female survival curves.
Paraquat pretreatment results in male-specific adaptation
Next, the potential ability to adapt following adaptive exposure to paraquat was tested. We have recently reported that males, but not females adapt to paraquat pretreatment using limited studies of common hybrid laboratory strains [39]. To determine whether adaptation is observed in males from wild-type and w[1118] strains, progeny were either placed on 5% sucrose or were pretreated with adaptive doses of paraquat [1μM or 10μM] before being subjected to a challenge dose of paraquat [30mM]. Pretreated males of all three strains showed longer survival times compared to those that did not receive the pretreatment (Figure 5A–C, see Table 2 [1]). In contrast, females of all three strains, regardless of pretreatment, showed no change in survival (Figure 5D–F, see Table 2 [1]).
Figure 5. Pretreatment with paraquat results in male specific adaptation.
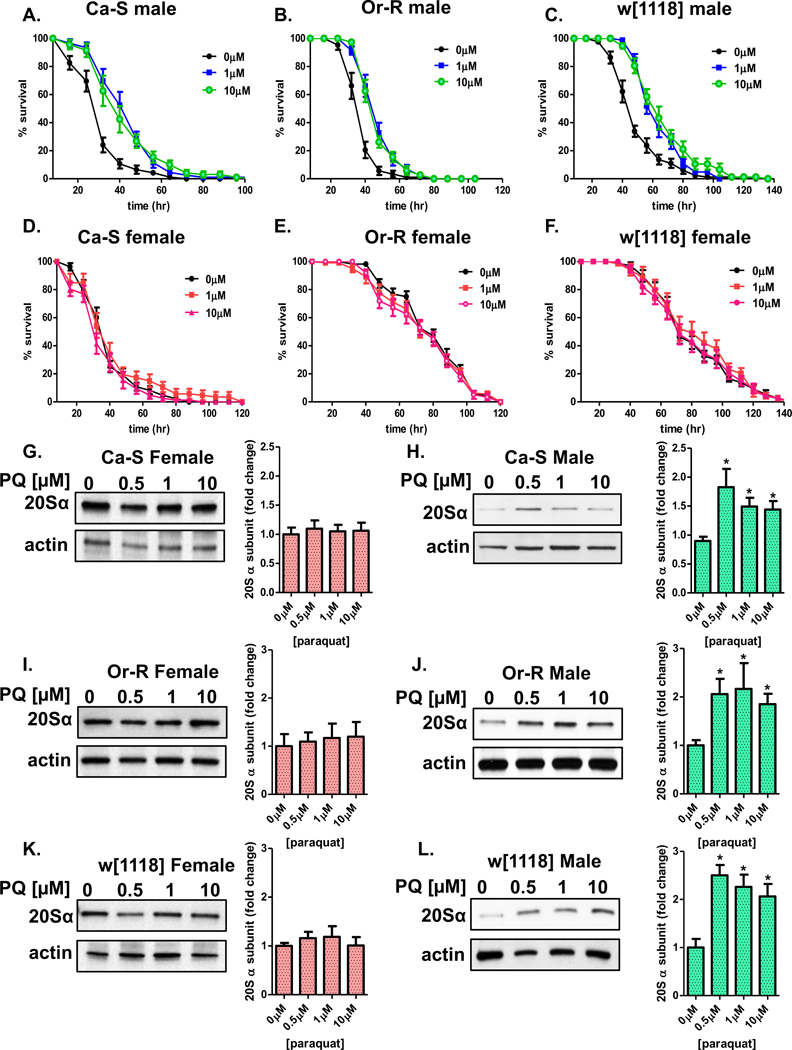
(A-F) Male and female progeny were collected and placed onto 5% sucrose for 24 hours prior to paraquat pretreatment [0μM-10μM] for 8 hours. After a 16-hour recovery, flies were placed upon paraquat challenge dose [30mM]. (A-C) Upon pretreatment, males survive longer compared to males receiving only the challenge dose. (D-F) Females, irrespective pretreatment, show no difference in survival upon introduction of the challenge dose. Statistical difference in survival was calculated using the Log-Rank test. Statistical summary is located in Table 2 [1]. (G-K) The adaptive change of 20S proteasome α subunit expression was measured between males and females of the three wild-type strains. Females and males were pretreated with adaptive doses of paraquat [0μM-10μM] for 8 hours, before allowed a 16-hour recovery before collection. (G,I,K) Females show no change in proteasome expression following paraquat pretreatment. (H,J,L) Paraquat pretreated males show increased proteasome expression compared to controls. Samples were done in triplicate, protein loading was normalized to Actin-HRP, and quantified using ImageJ. Error bars denote the standard error of the mean (S.E.M) values. * P < 0.05 and ** P < 0.01, relative to the control using one-way ANOVA.
To determine if the adaptive increase in survival was associated with an increase in proteasome expression, western blots were performed to measure proteasome levels. Upon paraquat pretreatment, females showed no change in proteasome levels (Figure 5G,I,K). In contrast, males showed higher protein levels of the proteasome (Figure 5H,J,L).
Paraquat, adaptation involves a male-specific increase in proteasomal proteolytic capacity
Because paraquat pretreatment resulted in increased expression of the proteasome and of survival in males, we sought to determine if proteasome capacity was also increased. Progeny were pretreated with either no paraquat or low adaptive doses [1μM-100μM] for 8 hours before an additional 16-hour recovery prior to collection. Whole fly lysate was assessed for proteasome activity by the addition of the fluorogenic peptide, Suc-LLVY-AMC. Males of each strain showed higher amounts of proteolytic capacity compared to controls (Figure 6A,C,E). In contrast, no female flies of any strain exhibited higher proteasomal proteolytic capacity following paraquat treatment; in fact, Or-R females showed a significant decrease at the highest paraquat dose (Fif. 6B,D,F). Importantly, the higher levels of proteolysis in pre-treated males was shown to be dependent upon the proteasome. Lysates from males and females either pre-treated or not with paraquat were also incubated with the proteasome inhibitor, lactacystin. No change in proteolytic capacity was measured in paraquat pre-treated females incubated with lactacystin (see Figure 1C in [1]), but lactacystin inhibition blocked the adaptive rise in paraquat pretreated males (see Figure 1D in [1]).
Figure 6. Paraquat adaptation involves male-specific increases in proteasomal proteolytic capacity.
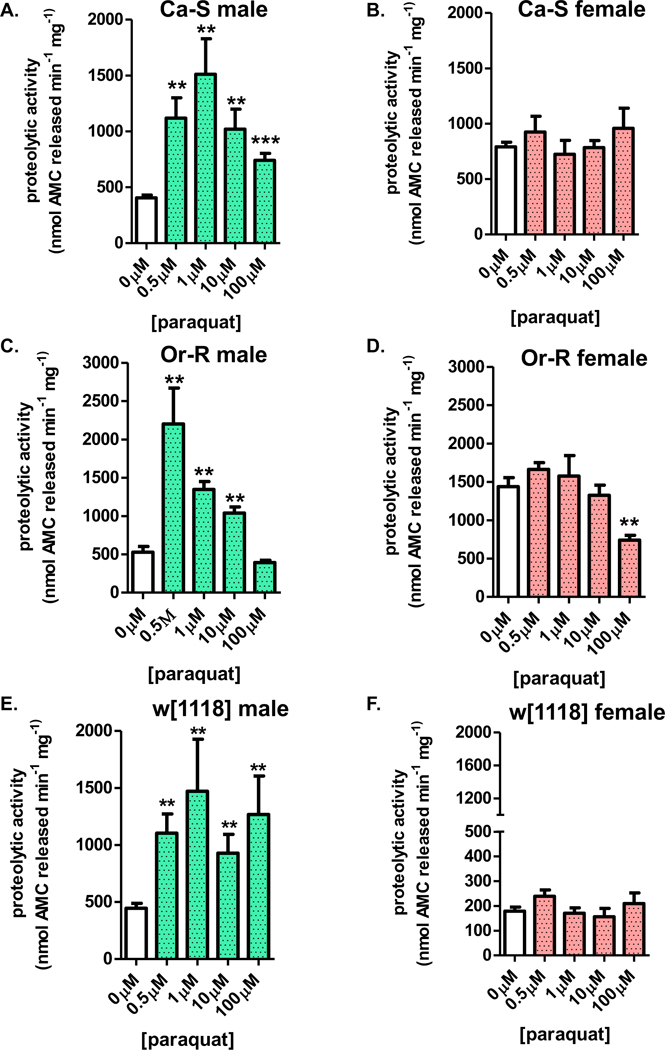
(A-F) Male and female progeny were collected and placed onto 5% sucrose for 24 hours prior to paraquat pretreatment [0μM-100μM] for 8 hours, at which point, flies were transferred to 5% sucrose for a 16-hour recovery before collection. Proteasomal proteolytic capacity was assessed in whole fly lysate by degradation of the fluorogenic peptide, Suc-LLVY-AMC. (A,C,E) Males from all three strains show higher levels of proteolysis upon paraquat pretreatment. (B,D,F) All three strains, females show no change in proteolytic capacity, irrespective pretreatment. Error bars denote the standard error of the mean (S.E.M) values. * P <0.05, ** P < 0.01, and *** P < 0.001, relative to control using one-way ANOVA.
Pretreatment with multiple redox-cyclers results in a male-specific rise in proteolysis
Because paraquat showed a male-specific proteasome increase (in contrast with H2O2), we sought to address whether this phenomenon is a feature of redox cycling agents in general. To test this hypothesis, 2,3-Dimethoxy-1,4-naphthoquinone (DMNQ) and menadione (an analog of 1,4 naphthoquinone with vitamin K activity) were studied. DMNQ is a redox cycler, and during its one electron reduction, and subsequent oxidation by molecular O2, acts as a continual generator of superoxide [53]. DMNQ was tested to determine if it would cause an adaptive rise in proteasomal proteolytic capacity and if the response would be male-specific. Progeny from the three strains were pretreated with increasing concentrations of DMNQ [500nM-10μM]. Following pretreatment, whole fly lysate was assayed for proteolytic capacity of the proteasome following the addition of the flurogenic peptide, Suc-LLVY-AMC. Upon pretreatment, males of all three strains showed higher proteolysis levels, measured by increased fluorescence (Figure 7A,C,E). Strikingly, females pretreated with DMNQ, showed no change in proteolytic capacity (Figure 7B,D,F). Together, suggesting DMNQ may play a similar role in triggering a male-specific response, as previously shown with paraquat (see above) [39].
Figure 7. Pretreatment with DMNQ results in male specific increases in proteasomal proteolytic capacity.

(A-F) Male and female progeny of the Ca-S, Or-R, and w[1118] strains were collected and placed onto 5% sucrose for 24 hours prior to DMNQ pretreatment [0μM-10μM] for 8 hours, at which point, flies were transferred to 5% sucrose for a 16-hour recovery before collection. Proteasomal proteolytic capacity was assessed in whole fly lysate by degradation of the fluorogenic peptide, Suc-LLVY-AMC. (A,C,E) Males from all three strains showed higher levels of proteolysis upon DMNQ pretreatment. (B,D,F) All three strains, females show no change in proteolytic capacity, irrespective pretreatment. Error bars denote the standard error of the mean (S.E.M) values. * P <0.05, ** P < 0.01, and *** P < 0.001, relative to control using one-way ANOVA.
Next, menadione, commonly used as a nutritional supplement due to its Vitamin K activity, was explored. Menadione acts as both a redox cycler and arylating quinone, and is reported to increase the intracellular concentration of superoxide [54]. Earlier studies found pretreatment with a low concentration of menadione was able to protect S. cerevisiae from future insults [55], suggesting menadione may act as an inducer of an adaptive stress response. Here, low concentrations of menadione [500nM-1mM] were fed to progeny of the three D. melanogaster strains. Following pretreatment, whole fly lysate was assayed for changes in the proteasome proteolytic capacity. Males pretreated with menadione exhibited an elevated capacity to degrade the small fluorgenic peptide Suc-LLVY-AMC (Figure 8A,C,E), whereas females pretreated with menadione showed no change in proteolysis (Figure 8B,D,F). Thus several redox cycling agents are capable of inducing not only the stress response, but also a male-specific adaptive response.
Figure 8. Pretreatment with menadione results in male specific increases in proteasomal proteolytic capacity.
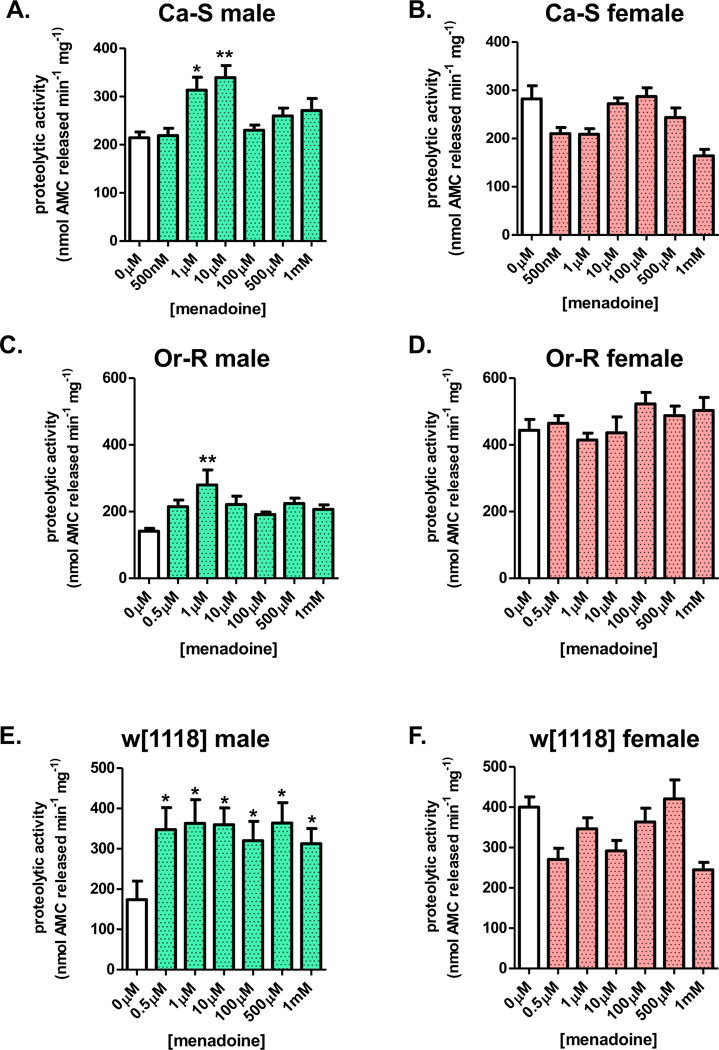
(A-F) Male and female progeny of the Ca-S, Or-R, and w[1118] strains were collected and placed onto 5% sucrose for 24 hours prior to menadione pretreatment [0μM-10μM] for 8 hours, at which point, flies were transferred to 5% sucrose for a 16-hour recovery before collection. Proteasomal proteolytic capacity was assessed in whole fly lysate by degradation of the fluorogenic peptide, Suc-LLVY-AMC. (A,C,E) Males from all three strains show increased proteolysis upon menadione pretreatment. (B,D,F) All three strains, females show no change in proteolytic capacity, irrespective pretreatment. Error bars denote the standard error of the mean (S.E.M) values. * P <0.05, ** P < 0.01, and *** P < 0.001, relative to control using one-way ANOVA.
The adaptive proteolytic response is dependent upon the proteasome
Finally, we sought to determine if the adaptive increase in proteolysis was primarily dependent upon the proteasome. To address this question, flies of the Ca-S strain were pretreated with or without an adaptive dose of H2O2. Following pretreatment, fly lysates were immunoprecipitated using the D. melanogaster specific monoclonal antibody against the 20S proteasome α subunit, to remove the proteasome from the lysate. Following removal of proteasomes from the flow-through, proteolytic activity in the flow-through and input lysate was assessed. Additionally, efficiency of proteasome removal was confirmed by western blot (see Figure 2 in [1]). The fluorogenic peptide, Suc-LLVY-AMC was used to assay changes in proteasome activity, with increased fluorescence indicative of higher proteasome degradation capacity. Higher proteolytic activity was evident in the input lysate from pretreated females (Figure 9A). Lysate from the female flow-through showed no change in proteolytic activity, showing that the higher proteolytic activity is primarily dependent upon the proteasome. In males, although the input lysate showed no adaptive change in proteolytic activity following H2O2 pretreatment, there was a significant decrease in the basal proteasome activity in the flow-through lysate (Figure 9B).
Figure 9. Adaptive increases in proteolytic capacity are dependent upon the proteasome.
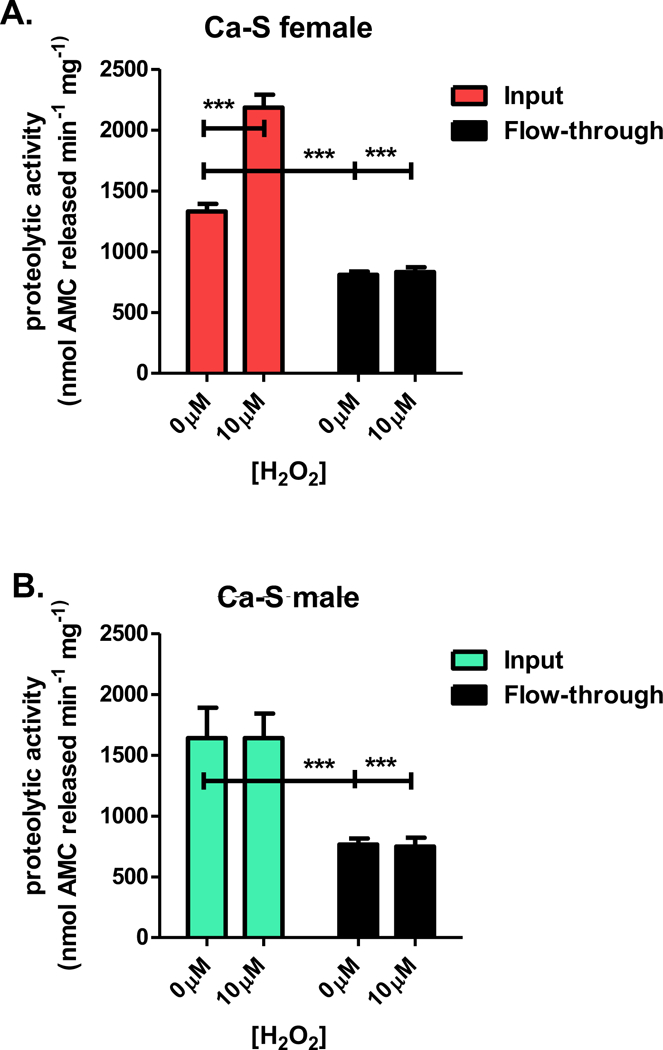
Flies of the Ca-S strain were pretreated with either no H2O2 or 10μM H2O2. Afterwards, whole fly lysate underwent immunoprecipitation (IP) against the 20S proteasome α subunit D. melanogaster specific monoclonal antibody. Proteolytic capacity was compared between lysate treated with or without H2O2 pretreatment and lysate from IP flow-through (FT). (A) Lysate from flow-through show no change in proteolytic capacity in pretreated females. (B) Male lysate from input show no change in proteolysis, irrespective pretreatment, with flow-through showing decreased basal 20S proteolysis. Error bars denote the standard error of the mean (S.E.M) values. * P <0.05, ** P < 0.01, and *** P < 0.001, relative to input control using one-way ANOVA.
DISCUSSION
Redox cycling agents and hydrogen peroxide are extensively used as oxidizing agents in redox biology and free radical research. Yet, only recently has the relationship between sex-specific adaptive responses and the type of oxidant employed begun to be explored [39]. Our previous studies with laboratory strains of flies showed that females, but not males adapt to H2O2 stress [39–41, 43] and that males, but not females, adapt to paraquat stress [39]. The present study demonstrates that additional redox cycling agents (DMNQ and menadione) induce a male-favored adaptive response similar to paraquat, whereas females show adaptation only upon hydrogen peroxide pretreatment. These results demonstrate the sex-specific adaptive response is observed in wild-type D. melanogaster strains, including the Oregon-R strain and the relatively short-lived, highly oxidant-sensitive Canton-S (Ca-S) strain [56, 57], along with the common w[1118] reference strain. These results further establish Drosophila melanogaster as a tractable invertebrate model system to study sex-specific adaptive responses, which may help elucidate the underlying mechanistic sex differences evident in higher organisms.
Adaptation, or ‘adaptive homeostasis’ is a highly conserved process, wherein cells, tissues, and whole organisms transiently activate various signaling pathways in response to short-term mild perturbations, triggering transcriptional and translational modifications. These adjustments protect against future, potentially more-damaging insults [33]. We feel it is important to differentiate between the physiological concept of adaptive homeostasis [33] which posits that discrete signal transduction pathways (such as Nrf2) mediate transient adaptation, and the more toxicological concept of hormesis [33, 49, 50] in which actual damage or toxicity itself is proposed to be the mechanistic ‘signal’ for adaptation. We have begun to address this question in previous publications. Thus, in studies using mouse embryonic fibroblasts (MEF) [32], we have shown that low concentrations of H2O2 (e.g. 10.0μM) which cause no cellular damage whatever, produce increases in Nrf2 levels and increased Nrf2 translocation to the nucleus, along with induction of increased Proteasome and Lon protease synthesis and increased stress resistance (after a suitable period of adaptation). In contrast, higher (but non-lethal) concentrations of H2O2 (e.g. 100.0μM) cause discernable cellular damage and slow growth, but still increase Nrf2 levels, Nrf2 nuclear translocation, and increased Proteasome and Lon protease synthesis albeit at lower levels, and still increase stress resistance but to a lesser extent than do low H2O2 concentrations. Similar results were obtained with low (non-damaging) versus higher (mildly damaging) levels of peroxynitrite, paraquat, and menadione. Importantly, all adaptive responses were transient, and all were blocked by Nrf2 inhibitors and/or knock-down. From these studies one may conclude that cells in culture use the Nrf2 signal transduction pathway to adapt very well to low signaling levels of agents such as H2O2, peroxynitrite, paraquat, and menadione. In contrast, cultured cells are less able to undergo adaptive homeostasis when damaging levels of the same agents are employed.
The above studies provide important basic information for cells in culture, but do not tell us if a whole organism or animal would respond similarly. Therefore, we initiated studies in C. elegans worms and D. melanogaster fruit flies. Our results [37, 38] all reveal a consistent trend; that low, non-damaging, or signaling levels of various oxidants and redox cycling agents effectively induce transient adaptive homeostasis responses including increased Proteasome and Lon and increased stress resistance. These adaptive responses were all dependent on Nrf2 orthologues: SKN-1 in worms and Cnc-C in flies. In contrast, as with our mammalian cell culture studies above, higher levels of oxidants or redox cycling agents caused measurable damage and were less effective in engendering adaptive homeostasis.
Taken together, our results with mammalian cells in culture, worms, and flies [37, 38] are all consistent with the proposal that adaptive homeostasis operates via signal transduction, not because of damage per se. Adaptation can still occur in the presence of damage, but it may be less efficient or effective. Our results suggest the importance of keeping real-life exposures to potential toxicants at low levels, in order to obtain maximum adaptive homeostasis benefits. We are now perusing more detailed studies to try to define the mechanistic relationships between the concentration of a signaling agent, the degree and type(s) of toxicity that may be caused, and the ability of pathways like Nrf2 to effectuate adaptation.
Mounting evidence indicates that the adaptive stress response is not uniform between the sexes. Indeed, early studies assessing the adaptive response in mammalian cell culture almost all involve female-derived cell lines [20]. In these studies pretreatment with hydrogen peroxide showed rapid and robust activation of the stress-responsive transcriptional activator, Nrf2 [58–61], resulting in upregulation of stress inducible enzymes, including the 20S proteasome [35, 36, 62]. In addition, our previous studies of the adaptive response in the model organism, D. melanogaster, uncovered a female-specific adaptive response to hydrogen peroxide [39–41, 43], resulting in the upregulation of the 20S proteasome [41, 43], and a male-specific adaptation to paraquat. The present findings further demonstrate the induction of the 20S proteasome is sex and oxidant-dependent, as blockage of the 20S proteasome prevents the adaptive increase.
Unique to this study is the testing of the effects of additional redox cycling agents, specifically menadione (Vitamin K-3, 2-methyl-1,4-napthoquinone) and DMNQ (2,3-Dimethoxy-1,4-napthoquinone) on adaptive differences between the sexes. These quinones can continuously generate superoxide (and via dismutation, hydrogen peroxide) by accepting electrons from intracellular sources such as mitochondrial complexes I and III [37, 38] and cytochrome P450 reductase. In addition to redox cycling, menadione, which is a potent electrophile, also reacts with reduced glutathione (GSH) or the cysteine residues of proteins, to form arylation products, causing cellular damage [54]. In contrast, DMNQ continuously cycles between its reduced one-electron semiquinone state back to its oxidized state, generating the superoxide radical, which is subsequently dismutated to hydrogen peroxide by cellular superoxide dismutase enzymes [63]. A key difference between menadione and DMNQ, is the absence of arylation sites on DMNQ, resulting in an underlying difference in cytotoxicity, as first identified in male-derived hepatocytes [64]. Interestingly, the findings from the present study show that in multiple fly strains pretreatment with non-damaging amounts of DMNQ and menadione both trigger a male-specific adaptive response, as measured by increased resistance to the quinones, increased cellular content of proteasomal protein, and increased proteasomal proteolytic capacity.
The sex-specific differences in adaptive response are potentially linked to the mode of action, and/or the delivery methods, of the differing oxidants: redox cyclers versus hydrogen peroxide. Unlike introduction of bolus amounts of hydrogen peroxide via the digestive tract (through ad libitum feeding), redox cyclers work via the continuous generation of the superoxide radical and its dismutation into hydrogen peroxide. Though additional work is necessary to fully understand the mode of action, different pathways have been proposed for these two classes of oxidants. Specifically, non-damaging amounts of hydrogen peroxide are viewed as crucial mediators of cellular signaling. Indeed, at low concentrations, H2O2 has been implicated to act as an insulin mimetic [65], induce cell proliferation [66, 67], and activate various transcription factors involved in the adaptive stress response [68]. Indeed, ablation of the Drosophila insulin-like peptide producing neuronal cells caused increased oxidative stress resistance in females [69]. Moreover, a localized increase in H2O2 concentration created by membrane-associated NADPH oxidase and extracellular SOD enhances insulin-stimulated signaling through the AKT pathway [70–72], and H2O2 is reported to increase transcriptional binding by Nrf2 by disrupting the interaction between Nrf2 and its inhibitory factor Keap1 [73].
H2O2 signaling is not limited to transcription factors. This is most notable in the dynamic fluctuation in the cytosolic proteasomal pool. Under homeostatic conditions the proteasome exists in multiple forms in the cell cytoplasm, nucleus, and endoplasmic reticulum. These include the 26S proteasome, the 20S proteasome (± 11S or PA28 regulators), and hybrid proteasomes. The 26S proteasome form, which consists of the 20S catalytic core and two 19S regulatory caps, is responsible for degrading ubiquitin-tagged proteins in an ATP-dependent manner. Adaptive concentrations of H2O2 trigger the sequestering of the 19S regulatory caps by HSP70, as demonstrated in the female K562 lymphoma cells and the immortalized mouse hippocampal HT22 neuronal cells (sex unknown) [74, 75]. Additionally, ECM 29, a regulatory protein involved in the assembly of the proteasome core and regulatory subunits [76], was identified, using S. cerevisiae, as a novel partner in 19S separation from the 20S catalytic core, under periods of oxidative stress [77]. Together, these interactions facilitate an immediate pool of available 20S proteasome for turnover of oxidized proteins during stress conditions. To further supplement the available pool of 20S proteasome, H2O2 stimulation also activates de novo transcriptional upregulation of the 20S proteasome subunits, providing additional protection against future oxidative insults [59]. Here, we observed that low, signaling amounts of H2O2, stimulated the synthesis of increased cellular content and activity of the 20S proteasome subunits [1], and also improved survival in female D. melanogaster, consistent with a conserved signaling role for H2O2.
In contrast, paraquat, a very strong redox cycling agent, gains its toxicity from its redox cycling capacity to continually generate superoxide through its reduction by mitochondria and cytochrome P450 reductase [78–80]. In turn, the redox cycling ability of paraquat to continually generate superoxide, has been shown to increase the expression of key antioxidant enzymes, including superoxide dismutase and catalase, both necessary in the breakdown of superoxide [81, 82]. In vitro cell culture studies showed that paraquat toxicity triggered Nrf2 activation in male-derived cell lines (not tested in female cell lines), including the rat adrenal medulla (PC12) cells [83], human bronchial epithelial cells [84], and mouse Type II alveolar epithelial cells [85]. Due to the redox cycling capacity of paraquat, it can quickly deplete cellular stores of NADPH [80]. Moreover, due to the necessity of NADPH for paraquat cycling, it has been shown to induce metabolic remodeling. Specifically, as one of the primary sources of intracellular NADPH is the pentose phosphate pathway, it is not surprising that this pathway is found to be elevated upon paraquat exposure [86]. Additionally, D. melanogaster studies assessing the neurological targets of paraquat, especially as a model for spontaneous Parkinson’s disease, identified DJ-1, an oxidant sensitive chaperone protein, which when removed, increased male paraquat sensitivity [87].
However, paraquat’s role as a signaling molecule is less well-understood. Earlier work found the activation of a group of mitogen-activated protein kinases (MAPKs), specifically the c-Jun N-terminal kinase (JNKs) [88], were activated in cell culture studies in response to extracellular stress, including heat shock (as evident in the ovarian-derived hamster cell line, CHO-K1), UV radiation (as shown in the male-derived rat adrenal medulla PC-12 cell line), and during oxidative stress, including paraquat (as demonstrated in rat dopaminergic neural N27 cells) [88–90]. The JNK pathway has been found to be activated as a means of cellular protection [91], yet under prolonged stress, may promote apoptosis [92]. Moreover, studies conducted in D. melanogaster larvae, found a series of genes that were regulated by the JNK pathway [93]. Follow-up studies found that paraquat exposure resulted in the activation of the JNK signaling pathway, tested by measuring changes in four JNK-dependent genes (hsp68, gstD1, fer1HCH, and mtnA) [94]. In addition, overexpression of JNK signaling, limited to neurons, was capable of improving male survival in D. melanogaster [94]. The present study and the one completed in parallel [1], show that not only paraquat, but additional redox cycling agents, including DMNQ and menadione, are capable of inducing cellular signaling responses, measured through increased proteasomal proteolytic capacity.
Overall, the sex-dependent differences in response to the type of oxidant observed in Drosophila may be related to underlying differences in the adaptive stress response in mammalian models. Indeed, studies using rat striatal astrocytes found male-derived cells to show higher levels of oxidative stress and toxicity following hydrogen peroxide and DMNQ exposure, compared to levels in female-derived cells [95]. Furthermore, many diseases, including coronary heart disease (male-favored) [96], certain cancers (breast, thyroid, anal, female-favored; tongue, pharynx, esophagus, stomach, and liver, male-favored) [97], and Parkinson’s (male-favored) [98], demonstrate an underlying elevation in reactive oxygen species in a sex-specific manner. Additionally, sex disparities in the response to oxidative stress even appear to arise at an early age, with young healthy men showing higher basal levels of oxidative stress markers compared to healthy age-matched premenopausal women [99]. Moreover, the higher rate of Parkinson’s disease prevalence in men may be related to the ability of male-derived neuronal cells to have higher paraquat uptake, as it has been previously shown to be incorporated via the dopamine transporter [100]. Conversely, females may be better able to cope against oxidative stress, as findings suggest higher basal expression of antioxidant and stress-responsive cellular defenses [101, 102].
HIGHLIGHTS.
Sex-specific differences in the adaptive homeostatic response are oxidant-dependent
Redox cycling agents induce a male-specific adaptive response
Hydrogen peroxide induces a female-specific adaptive response
Sex-specific differences are consistent across wild-type D. melanogaster strains
FUNDING
This work was supported by NSF grant [DGE-1418060] to LCDP, NIH/NIA grants [AG011833 and R56AG049629] to JT, and NIH/NIA grant [AG052374] and NIH/NIEHS grant [ES003598] to KJAD.
REFERENCES
- 1.Pomatto LC, et al. , Data on sex-specific adaptive homeostasis in D. melanogaster depends on increased proteolysis by the 20S Proteasome. Data in Brief. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maklakov AA and Lummaa V, Evolution of sex differences in lifespan and aging: causes and constraints. BioEssays, 2013. 35(8): p. 717–724. [DOI] [PubMed] [Google Scholar]
- 3.Zhu F, et al. , Chronic lithium treatment diminishes the female advantage in lifespan in Drosophila melanogaster. Clinical and Experimental Pharmacology and Physiology, 2015. 42(6): p. 617–621. [DOI] [PubMed] [Google Scholar]
- 4.Holzenberger M, et al. , IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature, 2003. 421(6919): p. 182–187. [DOI] [PubMed] [Google Scholar]
- 5.Barford A, et al. , Life expectancy: women now on top everywhere. Bmj, 2006. 332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gems D, Evolution of sexually dimorphic longevity in humans. Aging (Albany NY), 2014. 6(2): p. 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tower J, Sex-specific regulation of aging and apoptosis. Mechanisms of Ageing and Development, 2006. 127(9): p. 705–718. [DOI] [PubMed] [Google Scholar]
- 8.Austad SN and Bartke A, Sex differences in longevity and in responses to anti-aging interventions: a mini-review. Gerontology, 2015. 62(1): p. 40–46. [DOI] [PubMed] [Google Scholar]
- 9.Austad SN and Fischer KE, Sex Differences in Lifespan. Cell Metabolism, 2016. 23(6): p. 1022–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shen J, Landis GN, and Tower J, Multiple Metazoan Life-span Interventions Exhibit a Sex-specific Strehler–Mildvan Inverse Relationship Between Initial Mortality Rate and Age-dependent Mortality Rate Acceleration. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barrett EL and Richardson DS, Sex differences in telomeres and lifespan. Aging cell, 2011. 10(6): p. 913–921. [DOI] [PubMed] [Google Scholar]
- 12.Du S, et al. , XY sex chromosome complement, compared with XX, in the CNS confers greater neurodegeneration during experimental autoimmune encephalomyelitis. Proceedings of the National Academy of Sciences, 2014. 111(7): p. 2806–2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen X, et al. , The number of X chromosomes causes sex differences in adiposity in mice. PLoS Genet, 2012. 8(5): p. e1002709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang J, Bingaman S, and Huxley VH, Intrinsic sex-specific differences in microvascular. Am J Physiol Heart Circ Physiol, 2010. 298: p. H1146–H1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ross JL and Howlett SE, Age and ovariectomy abolish beneficial effects of female sex on rat ventricular myocytes exposed to simulated ischemia and reperfusion. PLoS One, 2012. 7(6): p. e38425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yuan Y, et al. , Steroid receptor coactivator-3 is required for inhibition of neointima formation by estrogen. Circulation, 2002. 105(22): p. 2653–2659. [DOI] [PubMed] [Google Scholar]
- 17.Landis GN, et al. , The progesterone antagonist mifepristone/RU486 blocks the negative effect on life span caused by mating in female Drosophila. Aging (Albany NY), 2015. 7(1): p. 53–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi C and Murphy CT, Mating induces shrinking and death in Caenorhabditis mothers. Science, 2014. 343(6170): p. 536–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arya GH, et al. , Natural variation, functional pleiotropy and transcriptional contexts of odorant binding protein genes in Drosophila melanogaster. Genetics, 2010. 186(4): p. 1475–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pomatto LC, Tower J, and Davies KJ, Sexual Dimorphism and Aging Differentially Regulate Adaptive Homeostasis. The Journals of Gerontology: Series A, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pergament E, et al. , Fertilization and early embryology: Sexual differentiation and preimplantation cell growth. Human reproduction, 1994. 9(9): p. 1730–1732. [DOI] [PubMed] [Google Scholar]
- 22.Xu K, et al. , Sex‐related differences in developmental rates of bovine embryos produced and cultured in vitro. Molecular reproduction and development, 1992. 31(4): p. 249–252. [DOI] [PubMed] [Google Scholar]
- 23.Du L, et al. , Innate gender-based proclivity in response to cytotoxicity and programmed cell death pathway. Journal of Biological Chemistry, 2004. 279(37): p. 38563–38570. [DOI] [PubMed] [Google Scholar]
- 24.Ceribelli A, Pino MS, and Cecere FL, Gender differences: implications for clinical trials and practice. Journal of Thoracic Oncology, 2007. 2(5): p. S15–S18. [DOI] [PubMed] [Google Scholar]
- 25.Mollerup S, et al. , Sex differences in lung CYP1A1 expression and DNA adduct levels among lung cancer patients. Cancer Research, 1999. 59(14): p. 3317–3320. [PubMed] [Google Scholar]
- 26.Johnston MV and Hagberg H, Sex and the pathogenesis of cerebral palsy. Developmental Medicine & Child Neurology, 2007. 49(1): p. 74–78. [DOI] [PubMed] [Google Scholar]
- 27.Holzenberger M, et al. , IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature, 2003. 421(6919): p. 182–187. [DOI] [PubMed] [Google Scholar]
- 28.Selman C, et al. , Evidence for lifespan extension and delayed age-related biomarkers in insulin receptor substrate 1 null mice. The FASEB Journal, 2008. 22(3): p. 807–818. [DOI] [PubMed] [Google Scholar]
- 29.Valle A, et al. , Sexual dimorphism in liver mitochondrial oxidative capacity is conserved under caloric restriction conditions. American Journal of Physiology-Cell Physiology, 2007. 293(4): p. C1302–C1308. [DOI] [PubMed] [Google Scholar]
- 30.Gruntenko NE, et al. , Probable mechanism of sexual dimorphism in insulin control of Drosophila heat stress resistance. Physiological Entomology, 2016. 41(1): p. 59–66. [Google Scholar]
- 31.Magkos F, Wang X, and Mittendorfer B, Metabolic actions of insulin in men and women. Nutrition, 2010. 26(7): p. 686–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang F, et al. , Female adult mouse cardiomyocytes are protected against oxidative stress. Hypertension, 2010. 55(5): p. 1172–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davies KJ, Adaptive homeostasis. Molecular aspects of medicine, 2016. 49: p. 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pickering AM, et al. , The immunoproteasome, the 20S proteasome and the PA28αβ proteasome regulator are oxidative-stress-adaptive proteolytic complexes. Biochemical Journal, 2010. 432(3): p. 585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grune T, et al. , HSP70 mediates dissociation and reassociation of the 26S proteasome during adaptation to oxidative stress. Free Radical Biology and Medicine, 2011. 51(7): p. 1355–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pickering AM and Davies KJ, Differential roles of proteasome and immunoproteasome regulators Pa28αβ, Pa28γ and Pa200 in the degradation of oxidized proteins. Archives of biochemistry and biophysics, 2012. 523(2): p. 181–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ngo JK and Davies KJ, Mitochondrial Lon protease is a human stress protein. Free Radical Biology and Medicine, 2009. 46(8): p. 1042–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ngo JK, et al. , Impairment of lon-induced protection against the accumulation of oxidized proteins in senescent wi-38 fibroblasts. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 2011. 66(11): p. 1178–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pomatto LC, et al. , The Mitochondrial Lon Protease Is Required for Age-Specific and Sex-Specific Adaptation to Oxidative Stress. Current Biology, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pickering AM, et al. , Oxidative stress adaptation with acute, chronic, and repeated stress. Free Radical Biology and Medicine, 2013. 55: p. 109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pomatto L, et al. , The age-and sex-specific decline of the 20s proteasome and the Nrf2/CncC signal transduction pathway in adaption and resistance to oxidative stress in Drosophila melanogaster. Aging, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raynes R, et al. , Aging and SKN-1-dependent Loss of 20S Proteasome Adaptation to Oxidative Stress in C. elegans. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 2016: p. glw093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pickering AM, et al. , A conserved role for the 20S proteasome and Nrf2 transcription factor in oxidative stress adaptation in mammals, Caenorhabditis elegans and Drosophila melanogaster. Journal of Experimental Biology, 2013. 216(4): p. 543–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Malick LE and Kidwell J, The effect of mating status, sex and genotype on longevity in Drosophila melanogaster. Genetics, 1966. 54(1): p. 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shen J, Landis GN, and Tower J, Multiple metazoan life-span interventions exhibit a sex-specific strehler–mildvan inverse relationship between initial mortality rate and age-dependent mortality rate acceleration. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 2016: p. glw005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rideout EJ, Narsaiya MS, and Grewal SS, The sex determination gene transformer regulates male-female differences in Drosophila body size. PLoS Genet, 2015. 11(12): p. e1005683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hudry B, Khadayate S, and Miguel-Aliaga I, The sexual identity of adult intestinal stem cells controls organ size and plasticity. Nature, 2016. 530(7590): p. 344–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ren C, Finkel SE, and Tower J, Conditional inhibition of autophagy genes in adult Drosophila impairs immunity without compromising longevity. Experimental gerontology, 2009. 44(3): p. 228–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ceci M, Ross J, and Condorelli G, Molecular determinants of the physiological adaptation to stress in the cardiomyocyte: a focus on AKT. Journal of molecular and cellular cardiology, 2004. 37(5): p. 905–912. [DOI] [PubMed] [Google Scholar]
- 50.Demirovic D and Rattan SI, Establishing cellular stress response profiles as biomarkers of homeodynamics, health and hormesis. Experimental gerontology, 2013. 48(1): p. 94–98. [DOI] [PubMed] [Google Scholar]
- 51.Hassan HM, Exacerbation of superoxide radical formation by Paraquat. Methods in enzymology, 1984. 105: p. 523–532. [DOI] [PubMed] [Google Scholar]
- 52.Arking R, et al. , Elevated paraquat resistance can be used as a bioassay for longevity in a genetically based long‐lived strain of Drosophila. Developmental genetics, 1991. 12(5): p. 362–370. [DOI] [PubMed] [Google Scholar]
- 53.Ishihara Y, Shiba D, and Shimamoto N, Enhancement of DMNQ-induced hepatocyte toxicity by cytochrome P450 inhibition. Toxicology and applied pharmacology, 2006. 214(2): p. 109–117. [DOI] [PubMed] [Google Scholar]
- 54.Shi M, et al. , Extracellular glutathione and γ-glutamyl transpeptidase prevent H2O2-induced injury by 2, 3-dimethoxy-1, 4-naphthoquinone. Free Radical Biology and Medicine, 1993. 15(1): p. 57–67. [DOI] [PubMed] [Google Scholar]
- 55.Jamieson DJ, Saccharomyces cerevisiae has distinct adaptive responses to both hydrogen peroxide and menadione. Journal of Bacteriology, 1992. 174(20): p. 6678–6681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Linnen C, Tatar M, and Promislow D, Cultural artifacts: a comparison of senescence in natural, laboratory-adapted and artificially selected lines of Drosophila melanogaster. Evolutionary Ecology Research, 2001. 3(8): p. 877–888. [Google Scholar]
- 57.Tower J, Aging mechanisms in fruit flies. BioEssays, 1996. 18(10): p. 799–807. [DOI] [PubMed] [Google Scholar]
- 58.Chapple SJ, Siow RC, and Mann GE, Crosstalk between Nrf2 and the proteasome: therapeutic potential of Nrf2 inducers in vascular disease and aging. The international journal of biochemistry & cell biology, 2012. 44(8): p. 1315–1320. [DOI] [PubMed] [Google Scholar]
- 59.Pickering AM, et al. , Nrf2-dependent induction of proteasome and Pa28αβ regulator are required for adaptation to oxidative stress. Journal of Biological Chemistry, 2012. 287(13): p. 10021–10031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nopparat C, et al. , The anti-inflammatory effect of melatonin in SH-SY5Y neuroblastoma cells exposed to sublethal dose of hydrogen peroxide. Mechanisms of Ageing and Development. [DOI] [PubMed] [Google Scholar]
- 61.Covas G, et al. , Activation of Nrf2 by H2O2: de novo synthesis versus nuclear translocation. Methods Enzymol, 2013. 528: p. 157–171. [DOI] [PubMed] [Google Scholar]
- 62.Wiese AG, Pacifici RE, and Davies KJ, Transient adaptation to oxidative stress in mammalian cells. Archives of Biochemistry and Biophysics, 1995. 318(1): p. 231–240. [DOI] [PubMed] [Google Scholar]
- 63.Kappus H and Sies H, Toxic drug effects associated with oxygen metabolism: redox cycling and lipid peroxidation. Cellular and Molecular Life Sciences, 1981. 37(12): p. 1233–1241. [DOI] [PubMed] [Google Scholar]
- 64.Verrax J, et al. , Ascorbate potentiates the cytotoxicity of menadione leading to an oxidative stress that kills cancer cells by a non-apoptotic caspase-3 independent form of cell death. Apoptosis, 2004. 9(2): p. 223–233. [DOI] [PubMed] [Google Scholar]
- 65.Czech MP, Lawrence JC, and Lynn WS, Evidence for the involvement of sulfhydryl oxidation in the regulation of fat cell hexose transport by insulin. Proceedings of the National Academy of Sciences, 1974. 71(10): p. 4173–4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Christman MF, Storz G, and Ames BN, OxyR, a positive regulator of hydrogen peroxide-inducible genes in Escherichia coli and Salmonella typhimurium, is homologous to a family of bacterial regulatory proteins. Proceedings of the National Academy of Sciences, 1989. 86(10): p. 3484–3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Davies JM, Lowry CV, and Davies KJ, Transient adaptation to oxidative stress in yeast. Archives of Biochemistry and Biophysics, 1995. 317(1): p. 1–6. [DOI] [PubMed] [Google Scholar]
- 68.Schreck R, Rieber P, and Baeuerle PA, Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. The EMBO journal, 1991. 10(8): p. 2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Broughton SJ, et al. , Longer lifespan, altered metabolism, and stress resistance in Drosophila from ablation of cells making insulin-like ligands. Proceedings of the National Academy of Sciences of the United States of America, 2005. 102(8): p. 3105–3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Truong TH and Carroll KS, Redox regulation of protein kinases. Critical reviews in biochemistry and molecular biology, 2013. 48(4): p. 332–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tower J, Sex-Specific Gene Expression and Life Span Regulation. Trends in Endocrinology & Metabolism. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Iwakami S, et al. , Concentration-dependent dual effects of hydrogen peroxide on insulin signal transduction in H4IIEC hepatocytes. PloS one, 2011. 6(11): p. e27401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Taguchi K, Motohashi H, and Yamamoto M, Molecular mechanisms of the Keap1-Nrf2 pathway in stress response and cancer evolution. Genes Cells, 2011. 16(2): p. 123–40. [DOI] [PubMed] [Google Scholar]
- 74.Grune T, et al. , HSP70 mediates dissociation and reassociation of the 26S proteasome during adaptation to oxidative stress. Free Radical Biology and Medicine, 2011. 51(7): p. 1355–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reeg S, et al. , The molecular chaperone Hsp70 promotes the proteolytic removal of oxidatively damaged proteins by the proteasome. Free Radical Biology and Medicine, 2016. 99: p. 153–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gorbea C, et al. , Characterization of mammalian Ecm29, a 26 S proteasome-associated protein that localizes to the nucleus and membrane vesicles. Journal of Biological Chemistry, 2004. 279(52): p. 54849–54861. [DOI] [PubMed] [Google Scholar]
- 77.Lehmann A, et al. , Ecm29 fulfils quality control functions in proteasome assembly. Molecular cell, 2010. 38(6): p. 879–888. [DOI] [PubMed] [Google Scholar]
- 78.Iyanagi T, Makino N, and Mason H, Redox properties of the reduced nicotinamide adenine dinucleotide phosphate-cytochrome P-450 and reduced nicotinamide adenine dinucleotide-cytochrome b5 reductases. Biochemistry, 1974. 13(8): p. 1701–1710. [DOI] [PubMed] [Google Scholar]
- 79.Vermilion JL and Coon MJ, Identification of the high and low potential flavins of liver microsomal NADPH-cytochrome P-450 reductase. Journal of Biological Chemistry, 1978. 253(24): p. 8812–8819. [PubMed] [Google Scholar]
- 80.Forman H, Nelson J, and Fisher A, Rat alveolar macrophages require NADPH for superoxide production in the respiratory burst. Effect of NADPH depletion by paraquat. Journal of Biological Chemistry, 1980. 255(20): p. 9879–9883. [PubMed] [Google Scholar]
- 81.Abrashev R, et al. , Differential effect of paraquat and hydrogen peroxide on the oxidative stress response in Vibrio cholerae non O1 26/06. Biotechnology & Biotechnological Equipment, 2011. 25(sup1): p. 72–76. [Google Scholar]
- 82.Krůček T, et al. , Effect of low doses of herbicide paraquat on antioxidant defense in Drosophila. Archives of insect biochemistry and physiology, 2015. 88(4): p. 235–248. [DOI] [PubMed] [Google Scholar]
- 83.Izumi Y, et al. , Compensatory role of the Nrf2–ARE pathway against paraquat toxicity: Relevance of 26S proteasome activity. Journal of Pharmacological Sciences, 2015. 129(3): p. 150–159. [DOI] [PubMed] [Google Scholar]
- 84.Podder B, Song H-Y, and Kim Y-S, Naringenin exerts cytoprotective effect against paraquat-induced toxicity in human bronchial epithelial BEAS-2B cells through NRF2 activation. J Microbiol Biotechnol, 2014. 24(5): p. 605–613. [DOI] [PubMed] [Google Scholar]
- 85.Ding Y-W, et al. , SIRT1 exerts protective effects against paraquat-induced injury in mouse type II alveolar epithelial cells by deacetylating NRF2 in vitro. International journal of molecular medicine, 2016. 37(4): p. 1049–1058. [DOI] [PubMed] [Google Scholar]
- 86.Bassett D and Fisher A, Alterations of glucose metabolism during perfusion of rat lung with paraquat. American Journal of Physiology-Endocrinology And Metabolism, 1978. 234(6): p. E653. [DOI] [PubMed] [Google Scholar]
- 87.Meulener M, et al. , Drosophila DJ-1 Mutants Are Selectively Sensitive to Environmental Toxins Associated with Parkinson’s Disease. Current Biology, 2005. 15(17): p. 1572–1577. [DOI] [PubMed] [Google Scholar]
- 88.Johnson GL and Lapadat R, Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science, 2002. 298(5600): p. 1911–1912. [DOI] [PubMed] [Google Scholar]
- 89.Peng J, et al. , The Herbicide Paraquat Induces Dopaminergic Nigral Apoptosis through Sustained Activation of the JNK Pathway. Journal of Biological Chemistry, 2004. 279(31): p. 32626–32632. [DOI] [PubMed] [Google Scholar]
- 90.Kyriakis JM and Avruch J, Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiological reviews, 2001. 81(2): p. 807–869. [DOI] [PubMed] [Google Scholar]
- 91.Minamino T, et al. , MEKK1 suppresses oxidative stress-induced apoptosis of embryonic stem cell-derived cardiac myocytes. Proceedings of the National Academy of Sciences, 1999. 96(26): p. 15127–15132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tournier C, et al. , Requirement of JNK for stress-induced activation of the cytochrome c-mediated death pathway. Science, 2000. 288(5467): p. 870–874. [DOI] [PubMed] [Google Scholar]
- 93.Jasper H, et al. , The genomic response of the Drosophila embryo to JNK signaling. Developmental cell, 2001. 1(4): p. 579–586. [DOI] [PubMed] [Google Scholar]
- 94.Wang MC, Bohmann D, and Jasper H, JNK Signaling Confers Tolerance to Oxidative Stress and Extends Lifespan in Drosophila. Developmental Cell, 2003. 5(5): p. 811–816. [DOI] [PubMed] [Google Scholar]
- 95.Giordano G, et al. , GENDER DIFFERENCES IN BRAIN SUSCEPTIBILITY TO OXIDATIVE STRESS ARE MEDIATED BY LEVELS OF PARAOXONASE-2 (PON2) EXPRESSION. Free radical biology & medicine, 2013. 58: p. 98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jousilahti P, et al. , Sex, age, cardiovascular risk factors, and coronary heart disease. Circulation, 1999. 99(9): p. 1165–1172. [DOI] [PubMed] [Google Scholar]
- 97.Siegel RL, Miller KD, and Jemal A, Cancer statistics, 2016. CA: a cancer journal for clinicians, 2016. 66(1): p. 7–30. [DOI] [PubMed] [Google Scholar]
- 98.Wooten G, et al. , Are men at greater risk for Parkinson’s disease than women? Journal of Neurology, Neurosurgery & Psychiatry, 2004. 75(4): p. 637–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ide T, et al. , Greater Oxidative Stress in Healthy Young Men Compared With Premenopausal Women. Arteriosclerosis, Thrombosis, and Vascular Biology, 2002. 22(3): p. 438–442. [DOI] [PubMed] [Google Scholar]
- 100.Izumi Y, et al. , Endogenous dopamine is involved in the herbicide paraquat-induced dopaminergic cell death. Toxicological Sciences, 2014. 139(2): p. 466–478. [DOI] [PubMed] [Google Scholar]
- 101.Horvathova M, et al. , Sex differences in the blood antioxidant defense system in juvenile rats with various genetic predispositions to hypertension. Hypertension Research, 2016. 39(2): p. 64–69. [DOI] [PubMed] [Google Scholar]
- 102.Tsuber V, Kadamov Y, and Tarasenko L, Activation of antioxidant defenses in whole saliva by psychosocial stress is more manifested in young women than in young men. PloS one, 2014. 9(12): p. e115048. [DOI] [PMC free article] [PubMed] [Google Scholar]


