Abstract
Cancer is considered a fetal disease caused by uncontrolled proliferation and progression of abnormal cells. The most efficient cancer therapies suppress tumor growth, prevent progression and metastasis, and are minimally toxic to normal cells. Natural compounds have shown a variety of chemo-protective effects alone or in combination with standard cancer therapies. Along with better understanding of the dynamic interactions between our immune system and cancer development, nutritional immunology—the use of natural compounds as immunomodulators in cancer patients—has begun to emerge.
Cancer cells evolve strategies that target many aspects of the immune system to escape or even edit immune surveillance. Therefore, the immunesuppressive tumor microenvironment is a major obstacle in the development of cancer therapies. Because interaction between the tumor microenvironment and the immune system is a complex topic, this review focuses mainly on human clinical trials and animal studies, and it highlights specific immune cells and their cytokines that have been modulated by natural compounds, including carotenoids, curcumin, resveratrol, EGCG, and β-glucans. These natural compounds have shown promising immune-modulating effects, such as inhibiting myeloid-derived suppressor cells and enhancing natural killer and cytolytic T cells, in tumor-bearing animal models, but their efficacy in cancer patients remains to be determined.
Keywords: cancer immunology, carotenoids, curcumin, resveratrol, EGCG, β-glucans
Introduction
Cancer is caused by uncontrolled proliferation and progression of abnormal cells, which could eventually lead to mortality. The most efficient cancer therapies suppress tumor growth, and prevent progression and metastasis. Advanced medical technologies and modern cancer therapies, including chemotherapy, radiotherapy, and immunotherapy, have achieved successful clinical outcomes in certain types of cancer. In addition, early detection programs, such as mammography, prostate cancer screening, and colonoscopy, lead to precise diagnosis of precancerous lesions. All of these advances improve overall survival of cancer patients. However, we now appreciate the existence of heterogeneity among cancer types and even among individual patients. Therefore, targeted therapies based on molecular signatures of cancer cells can significantly enhance therapeutic responses in selected subpopulations. Two well-documented examples are ErbB2-targeted therapies for ErbB2-positive breast cancer (Shepard et al. 1991) and estrogen antagonists for estrogen receptor-positive breast cancer (Heiser et al. 2012).
The host’s immune system, which continuously monitors the body for pathogens and invading antigens, also plays an important role in the tumor microenvironment and contributes to cancer heterogeneity. The immune editing that permits tumor development and progression consists of three phases: elimination, equilibrium, and escape. First, cancer cells prevent the immune system from eliminating new cancer cells; then they achieve an equilibrium with immune cells; and last, they escape immune surveillance. Efficient immune-therapies should be able to rescue the dysregulated immune cells at all three stages and enhance the potency of cancer-combatting cells. For example, T cells, which normally recognize and kill cancer cells, can become exhausted and dysfunctional, as evidenced by the upregulation of a programmed cell death marker (PD-1), on their surface. Blocking PD-1, or its ligand PD-L1, which is expressed on cancer cells, has restored T cell function against many types of cancer (Thommen and Schumacher 2018).
An efficient cancer therapy regimen also needs to minimize toxicity to normal cells, as many cancer patients respond to cytotoxic treatments but succumb to side effects such as cachexia, weight loss, fatigue, and cytokine storm. Therefore, there is growing interest in supplementing standard cancer therapies with natural compounds and dietary components to improve quality of life during chemotherapy (Bjorklund et al. 2018, Serna-Thome et al. 2018). Importantly, the potential capabilities of these natural compounds and dietary components to inhibit tumor growth have begun to be explored. Accumulating evidence is demonstrating that natural compounds can have a wide variety of effects against cancer, such as anti-proliferation, anti-angiogenesis, anti-migration, and pro-apoptosis (Hussain, Kumar, and Ghosh 2016, Zang et al. 2014, Ferraz da Costa, Fialho, and Silva 2017, Rather and Bhagat 2018, Ko et al. 2017, Lee 2017, Rauf et al. 2017).
Nutritional immunology is also drawing more attention, given the recent success with immunotherapy. Natural compounds and dietary components have been shown to influence immune cells in vitro and to enhance immune responses against multiple conditions, such as inflammatory diseases (viral infection, obesity, and diabetes) and cancer (Sultan et al. 2014, Kim et al. 2015, Chirumbolo 2012, Ferguson and Philpott 2007, del Corno et al. 2016, Baraya, Wong, and Yaacob 2017, Janakiram et al. 2016, Burkard et al. 2017, Zheng et al. 2012, Ghiringhelli et al. 2012, Casey et al. 2015, Mohamed, Jantan, and Haque 2017). For instance, our group demonstrated that berries, which contain multiple chemopreventive compounds, enhanced the function of natural killer (NK) cells and decreased the infiltration of neutrophils in animal models and human patients with colorectal cancer (Pan, Kang, et al. 2017, Pan, C, et al. 2017, Pan et al. 2015). Because of the complexity of the tumor microenvironment and immune system, this review will focus on human clinical studies (Table 1 and Figure 1) and tumor-bearing animal studies (Table 2 and Figure 2). In addition, it will highlight specific immune cells and their cytokines in tumors that have been shown to be modulated by natural compounds.
Table 1.
Immune-modulating effects of natural compounds in human studies
| Compound | Doses | Human participants | Effects | Refs |
|---|---|---|---|---|
| β-carotene | 50 mg on alternate days for 10–12 years or placebo | 38 middle-aged men 51–64 y, 21 elderly men 65–86 y | Increased NK cell activities in elderly men to levels similar to those in younger men | Santos, 1996 |
| β-carotene | 15 mg daily for 26 days or placebo | 25 healthy nonsmoking men | Increased the percentages of monocytes expressing antigen-presenting molecules | Hughes, 2000 |
| β-carotene | 90 mg daily for 21 days or placebo | 25 healthy elderly women 60–80 y | No differences in the profiles of lymphocyte subsets (CD3+, CD4+, CD8+ T cells, and CD19+ B cells) | Santos, 1997 |
| 50 mg on alternate days for 10–12 years or placebo | 50 healthy elderly men 50–86 y | |||
| Lycopene | 13.3 mg daily for 12 weeks or placebo | 58 healthy volunteers >65 years | No differences in T cell subsets and their expression of functional surface molecules | Corridan, 2001 |
| β-carotene | 8.2 mg daily for 12 weeks or placebo | |||
| Curcumin | 3 g daily for 2 weeks | 30 non-small-cell lung cancer patients | Increased Th1 cells, decreased Treg cells in peripheral blood | Zou, 2018 |
| Curcumin | 3 g daily for 1 month | 40 advanced colon cancer patients | Increased Th1 cells, decreased Treg cells in peripheral blood | Xu, 2017 |
| Resveratrol | 1 g daily for 28 days | 9 healthy volunteers | Increased the number of circulating Treg cells (CD3+CD4+CD25+CD127-) and CD3+NKG2D+ T cells | Espinoza, 2017 |
| Decreased levels of proinflammatory cytokines in plasma, such as TNF-α and MCP-1 | ||||
| Polyphenon E | 400–2000 mg daily for up to 6 months | 33 Rai stage 0-II CLL patients | Decreased absolute lymphocyte count (ALC) | Shanafelt, 2009 |
| Polyphenon E | 2000 mg daily for up to 6 months | 42 Rai stage 0-II CLL patients | Decreased absolute lymphocyte count (ALC) | Shanafelt, 2013 |
| Green tea extract | 4 capsules daily for 1 month + 6 capsules for 5 months | 12 Rai stage 0 CLL patients | Decreased lymphocytosis and circulating Treg cells | D’Arena, 2013 |
| 6 capsules contain 4602 mg of green tea leaves, 189 mg of EGCG, and 97.5 mg of caffeine | Decreased IL-10 and TGF-β serum levels | |||
| Agaricus blazei Murill Kyowa (ABMK) | 9 packs daily for 9 weeks | 100 gynecological cancer patients | Increased NK cell activity | Ahn, 2004 |
| Ganoderma lucidum polysaccharide (GLPS) | 1800 mg daily for 12 weeks | 34 advanced-stage cancer patients | Increased the absolute number of CD56+ NK cells | Gao, 2003 |
| Increased levels of IL-2, IL-6, and IFN-γ in plasma | ||||
| Decreased levels of IL-1 and TNF-α in plasma | ||||
| Ganoderma lucidum polysaccharide extract | 1800 mg daily for 12 weeks | 47 advanced colorectal cancer patients | Increased the number of CD3+, CD4+, CD8+, and CD56+ lymphocytes | Chen, 2006 |
| Increased NK cell activities | ||||
| Increased levels of IL-2, IL6, and IFN-γ in plasma | ||||
| Decreased levels of IL-1 and TNF-α in plasma | ||||
| Lentinula edodes mycelia extract (LEM) | 1800 mg daily for 3 weeks | 10 breast cancer patients with nodal metastases | Prevented chemotherapy-induced decline in cytotoxic activities of NK and LAK cells | Nagashima, 2013 |
| Prevented chemotherapy-induced decline in the proportion of activated NK and NK T cells in lymphocytes | ||||
| Lentinula edodes mycelia extract (LEM) | 1800 mg daily for 4 weeks | 1 gastric and 7 colorectal cancer patients | Increased IFN-γ production by CD4+ T, CD8+ T, and CD56+ NK/NKT cells | Okuno, 2011 |
| Protein-bound polysaccharide K (PSK) | 3 g daily for 2 years | 139 stage III colorectal cancer patients | Increased the number of NK cells | Ohwada, 2006 |
| Sizofiran (SPG) | 20 mg injected intramuscularly at 5 and 2 days before surgery | 40 stage III–IV head and neck cancer patients | Increased cytotoxic activities of NK cells and LAK cells | Kano, 1996 |
| Increased CD4+ T cells in lymph nodes | ||||
| Increased IL-2 production | ||||
| Sizofiran (SPG) | 0.2 mg or 20 mg injected intramuscularly 7 and 3 days before radiation therapy | 45 stage II–III invasive cervical cancer patients | Increased tumor-infiltrating T cells | Nakano, 1996 |
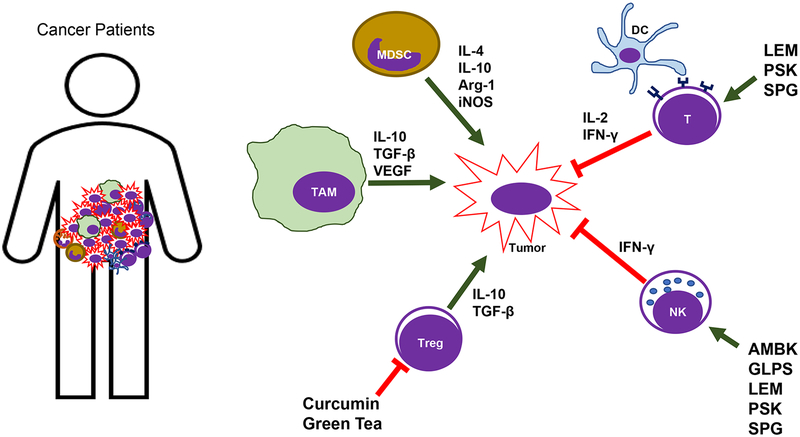
Figure 1: The schematic summary of immune-modulating effects of natural compounds in humans.
Curcumin and green tea have shown to suppress Treg cell function, and mushroom extracts (LEM, PSK, SPG, AMBK, and GLPS) have shown to enhance T and NK cell function in humans.
Table 2.
Immune-modulating effects of natural compounds in tumor-bearing mice
| Compound | Doses | Animal models | Effects | Refs |
|---|---|---|---|---|
| All-trans retinoic acid (ATRA) | Subcutaneous implantation of 5 mg ATRA + PML-RARFrC DNA vaccine | Acute promyelocytic leukemia (APL) | Increased the production of IFN-γ | Furugaki, 2010 |
| Enhanced cytototoxic T cells (CD4+ and CD8+) | ||||
| All-trans retinoic acid (ATRA) | Subcutaneous implantation of 5 mg ATRA (21-day release) + HA-specific vaccine/C3 peptide-specific vaccine | 7,12-dimethylbenz(a)anthracene-induced mammalian adenocarcinoma (DA3-HA cells injected to BALB/c mice) | Decreased immature myeloid cells (ImCs) | Kusmartsev, 2003 |
| Methylcholanthrene-induced sarcoma (MethA cells injected into BALB/c mice) | ||||
| Fibrosarcoma (C3 cells injected into C57BL/6 mice) | ||||
| Curcumin | Oral 50 mg/kg on alternate days for 14 days | Ehrlich’s ascites carcinoma (EAC) cells injected into Swiss albino mice | Curumin | Bhattacharyya, 2010 |
| Diminished the Treg cell population (CD4+CD25+Foxp3+) | ||||
| Increased IFN-γ secretion, decreased production of IL-4, TGF-β, and IL-10. Reversed Th2-type immune bias | ||||
| Curcumin | Oral 50 mg/kg every day for 20–25 days | Lewis lung carcinoma (LLC) cells injected into C57BL/6 mice | Reduced the accumulation of MDSCs in spleen and tumors | Liu, 2016 |
| Promoted the maturation and differentiation of MDSCs | ||||
| Inhibited the expression of Arg-1 and ROS in purified MDSCs | ||||
| Decreased the level of IL-6 in tumor tissue and serum | ||||
| TriCurin | 1.28 mM+ TriCurin (1.28 mM curcumin + 0.32 mM epicatechin gallate + 4 mM resveratrol) | HPV+ mouse lung cancer (TC-1 cells injected into C57BL/6 mice) | Increased tumor-infiltrating NK cells and CD8+ cytolytic T cells | Mukherjee, 2018 |
| Head and neck squamous cell carcinoma (UMSCC47 cells injected into athymic nude/nude mice) | Repolarized M2-like TAMs (Arg-1highIL-10highIL-12low) to M1-like TAMs (iNOShighIL-10lowIL-12high) in the tumors | |||
| Curcumin | 0.1% in AIN-76A diet till 110 days of age | Familial adenomatous polyposis (ApcMin/+ mice) | Increased mucosal CD4+ T cells and B cells | Churchill, 2000 |
| Bisdemethoxycurcumin (BDMC) | 3 mg/kg every 3 days for 2–3 weeks, i.v. + 200 μg α-PD-L1 antibody for 2–4 weeks, i.p. | Mouse bladder carcinoma (MB49 cells injected into C57BL/6 mice) | Increased intratumoral CD8+ T cell infiltration | Shao, 2017 |
| Increased the level of IFN-γ | ||||
| Decreased the number of intratumoral myeloid-derived suppressor cells | ||||
| α-PD-L1 facilitated the secretion of IFN-γ, granzyme B, and perforin through these CD8+ T-cells | ||||
| Curcumin | Oral 0.8 or 2 g/kg curcumin daily + Listeriaat-Mage-b vaccine | Triple-negative breast cancer (4T1 cells injected into BALB/c mice) | Decreased levels of IL-6 and increased IL-12 production by MDSCs | Singh, 2013 |
| Improved CD4 and CD8 T cell responses | ||||
| TriCurin lipososme (TrLp) or curcumin liposome (CLp) | TrLp (1.28 mM+) or CLp (5 mM) daily for 5 days or every 72 h for 60 days | Mouse glioblastoma (GL261 cells injected into C57BL/6 mice) | Increased activated NK cells in tumors | Mukherjee, 2018 |
| TAMs repolarized from M2-like (Arg-1high) to M1-like (iNOShigh). | ||||
| TrLp more effective than CLp | ||||
| Curcumin–polyethylene glycol conjugate (CUR–PEG) | 40 mg/kg CUR-PET every other day, i.v. + TRP2 peptide vaccine | Melanoma (B16F10 cells injected into C57BL/6 mice) | Increased cytotoxic T lymphocyte response and IFN-γ production | Lu, 2016 |
| Decreased levels of tumor-infiltrating MDSCs and Treg cells | ||||
| Decreased levels of IL-6 | ||||
| Resveratrol | 20 mg or 50 mg/mouse on alternate days for 19 days, i.p. | Triple-negative breast cancer (4T1 cells injected into BALB/c mice) | Decreased B regulatory cells (Breg, CD25+CD81high cells within CD19+ B cells) and Foxp3+ Treg cells | Lee-Chang, 2013 |
| 50 mg or 500 mg/mouse on alternate days for 14 days, i.p. | Melanoma (B16F10 cells injected into C57BL/6 mice) | Activated CD8+ cytolytic T cells | ||
| Resveratrol | 1, 2.5, and 5 mg/kg daily for 22 days, i.p. | Renal cancer (Renca cells injected into BALB/c mice) | Increased the number and cytotoxicity of CD8+ T cells | Chen, 2015 |
| Switched from expression of Th2 cytokines (IL-6 and IL-10) to Th1 cytokines (IFN-γ) | ||||
| Resveratrol | Oral 12.5, 25, 50 mg/kg daily for 3 weeks | Mouse lymphocytic leukemia (L1210 cells injected into BALB/c mice) | Increased NK cell activity | Li, 2007 |
| Grape seed proanthocyanidins | Oral 200 mg/kg proanthocyanidins daily + 2 mg/kg doxorubicin on alternate days for 9 doses, i.p. | Mouse sarcoma (S180 cells injected into BALB/c mice) | Increased NK cell cytotoxicity and percentages of CD4+ T helper cells and CD8+ cytolytic T cells | Zhang, 2005 |
| Reversed doxorubicin-induced side effects, including lower levels of IFN-γ, IL-2 expression and NK cell function | ||||
| Polyphenon E | 0.3% in the drinking water | Mouse neuroblastomas (Transgenic TH-MYCN mice; Human SHSY5Y cells injected into NOD/SCID mice; Syngeneic Neuro 2A cells injected into A/J mice) | Shifted the balance from monocytic MDSCs (CD11b+Ly6G-Ly6high) toward granulocytic MDSCs (CD11b+Ly6G+Ly6low) | Santilli, 2013 |
| Rescued MDSC-suppressed production of IFN-γ in T cells | ||||
| Increased tumor-infiltrating CD8+ cytolytic T cells | ||||
| EGCG | 0.5 mg/mL in drinking water for 78 days + 2 μg pcDNA3-Sig/E7/LAMP-1 vaccine | E7-expressing tumors (TC-1 cells injected into C57BL/6 mice) | Increased the E7-specific CD8+ T-cell immune response (IFN-γ secretion) | Kang, 2007 |
| EGCG | Oral 5, 20, and 40 mg/kg for two weeks | Mouse leukemia (WEHI-3 cells injected into BALB/c mice) | Increased the percentages of CD3+ T cells, CD19+ B cells, and macrophages | Huang, 2013 |
| Increased NK cell activity | ||||
| Reduced the percentage of CD11b+ monocyte cells | ||||
| EGCG | Oral 2 mg | Mouse bladder cancer (MBT-2 cells injected into C3H/He mice) | Increased NK cell cytotoxicity | Hsieh, 2011 |
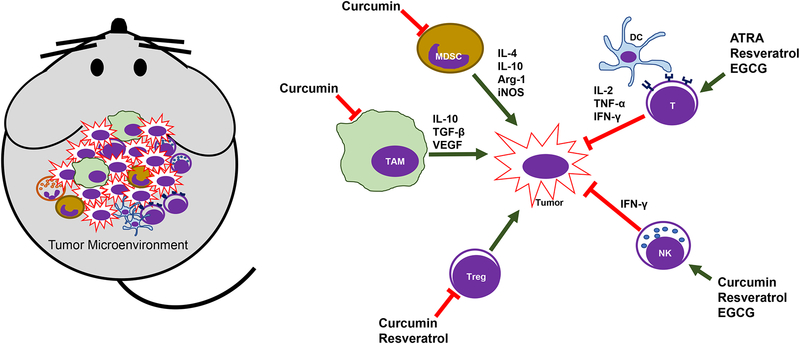
Figure 2: The schematic summary of immune-modulating effects of natural compounds in tumor-bearing mice.
Curcumin, all-trans retinoic acid (ATRA), resveratrol, and EGCG have shown to suppress MDSCs, TAMs and Treg cells, as well as enhance T and NK cell function.
We performed a PubMed search using the following key words: cancer immunity, natural compounds, diet, polyphenols, and human clinical trials. Research articles, clinical trials, and reviews that investigated and compared the immune-modulating effects of natural compounds against cancer were first screened according to abstracts and titles. Full texts of selected articles were then assessed. Reference lists from studies selected by the electronic search were manually searched to identify further relevant reports and to select the best-studied natural compounds. Therefore, this review summarizes the immune-modulating effects of carotenoids and retinoic acid (RA), curcumin, resveratrol, (−)-epigallocatechin-3-gallate (EGCG), and β-glucans.
The cancer-immunity cycle
The lymphoid compartment of the immune system includes NK cells, NKT cells, CD4+ T cells, CD8+ T cells, and B cells. It can exert antigen-specific cytotoxicity, mainly with T cells, or non-specific cytotoxicity, mainly with NK cells, against cancer cells. CD8+ T cells are the major anti-cancer effector cells. They can differentiate into cytotoxic T lymphocytes in lymphoid organs upon encountering antigen-presenting cells (APCs). Dendritic cells (DCs) are the major APCs that present tumor-specific peptides to CD8+ T cells (Palucka and Coussens 2016). T cells can also secrete different types of cytokines to promote their functions, and they can interact with other immune cells. For example, Th1-polarized CD4+ T cells secret interleukin-2 (IL-2), tumor necrosis factor alpha (TNF-α), and interferon gamma (IFN-γ), which can enhance the cytotoxic activities of macrophages and the antigen-presenting functions of DCs (Palucka and Coussens 2016). In the immunesuppressive tumor microenvironment, however, chronic inflammation can promote cancer cell growth and survival, and switch the Th1-like immune response to the Th2-like response. Th2-type cytokines, such as IL-4, IL-10, and transforming growth factor beta (TGF-β), are dominant, and they can recruit Foxp3+ T regulatory (Treg) cells, M2-polarized macrophages and monocytes, as well as B regulatory (Breg) cells. These cells further promote cancer angiogenesis, migration, and metastasis (Chen and Mellman 2013, Thommen and Schumacher 2018, Ribas 2015, Godfrey et al. 2018). In addition, NK cells are the major components of the innate immune response. They recognize pathogens, harmful agents, and cancer cells by “missing-self” signals (Malmberg et al. 2017). Thus, NK cells can select and lyse “non-self” cells that fail to express major histocompatibility complex (MHC) class I molecules on their surface. The cytotoxicity of NK cells is also controlled by activation receptors, such as natural cytotoxicity receptors (NCRs), and by inhibitory receptors, such as killer cell inhibitory immunoglobulin-like receptors (KIRs). These receptors recognize stress signals and self-MHC class I ligands, respectively, to determine NK cell functions (Woo, Corrales, and Gajewski 2015, Iannello et al. 2016, Marcus et al. 2014). However, the functions of NK cells can be suppressed by Treg cells and myeloid-derived suppressor cells (MDSCs), as well as by their cytokines, including IL-10 and TGF-β, in the immunesuppressive tumor microenvironment (Malmberg et al. 2017).
The myeloid compartment of the immune system includes macrophages, DCs, granulocytes, and monocytes. The most important feature of these cells is their functional plasticity in the microenvironment. DCs can capture and display cancer-derived antigens to allow recognition by T lymphocytes. Therefore, DCs are key initiators of the immune response against cancer cells. However, Treg cells and high levels of IL-10 can suppress DC maturation so those cells remain immature and become subgroups of MDSCs (Veglia and Gabrilovich 2017). Macrophages are differentiated cells of the mononuclear phagocytic lineage. “Activated” macrophages, or M1-like macrophages, are involved in the Th1-type response to pathogens. Upon activation by IFN-γ and Toll-like receptors (TLRs), M1-like macrophages secrete IL-12, TNF-α, reactive oxygen species (ROS), and nitric oxide (NO) to kill pathogens and abnormal cells. “Alternatively activated” macrophages, or M2-like or tumor-associated macrophages (TAMs), have been found in the tumor microenvironment. They secrete vascular endothelial growth factors (VEGFs), IL-10, and TGF-β to recruit Treg cells, as well as to suppress CD8+ T cells and NK cells (Ruffell and Coussens 2015, Noy and Pollard 2014, Ostuni et al. 2015, Qian and Pollard 2010). Neutrophils, the first defense against bacteria and virus, are responsible for acute inflammation and wound healing. Like TAMs, tumor-associated neutrophils can generate VEGFs, IL-10, arginase 1 (Arg-1), and matrix metalloproteinase (MMP) to promote angiogenesis, migration, and metastasis of cancer cells (Powell and Huttenlocher 2016, Sionov, Fridlender, and Granot 2015). MDSCs are regarded as the most potent immunesuppressive cells in the tumor microenvironment. The two main types are polymorphonuclear MDSCs (PMN-MDSCs), which are morphologically and phenotypically close to neutrophils, and monocytic MDSCs (M-MDSCs), which resemble monocytes (Kumar et al. 2016, Marvel and Gabrilovich 2015). These cells are characterized by their functions: suppressing T cells and NK cells and secreting cytokines such as Arg-1, inducible NOS (iNOS), TGF-β, and IL-10 (Tcyganov et al. 2018, Gabrilovich 2017).
Carotenoids and Retinoic acid (RA)
Carotenoids are liposoluble pigments belonging to the tetraterpene family (C40-based isoprenoids), and they impart yellow, orange, or red colors to fruits, leaves, and flowers (Milani et al. 2017). More than 600 carotenoids with natural structural variants have been identified. The major carotenoids in the diet and human body include β-carotene, α-carotene, lycopene, lutein, and cryptoxanthin. Lycopene is the red pigment in ripe tomatoes, and β-carotene is the orange pigment in carrots (Milani et al. 2017).
Vitamin A (retinol) and its bioactive metabolite, retinoic acid (RA), share a polyene backbone structure with β-carotene. During the development of our immune system, RAs are criticial checkpoint immune-modulators for maintaining the immune system (Carratu et al. 2012, Brown and Noelle 2015). RA-induced Foxp3+ Treg cells maintain the stable immune tolerance against innocuous antigens, thus preventing excessive, self-destructive immune responses that could lead to the development of inflammatory bowel disease and autoimmune disease. RAs have also been shown to regulate the differentiation of CD4+ T cells, Th1 lineage stability, and the balance of the Th1/Th17 versus the Th2 response (Brown and Noelle 2015).
A number of studies have examined the effects of β-carotene and other carotenoids on immune function in healthy volunteers (Hughes 2001). They have used β-carotene doses ranging from levels achievable from the diet (15 mg/day) to pharmacologic doses (180 mg/day) for periods of 14 to 365 days. Many immune-modulating effects have been observed, such as increases in the number of CD4+ T cells and the ratio of CD4+/CD8+ cells, as well as expression of activation markers, including IL-2 receptors and transferrin receptors on lymphocytes (Bryant and Prasad 1991, Watson et al. 1991, Murata et al. 1994). In addition, Santos et al observed that supplementation with β-carotene (50 mg on alternate days) significantly increased NK cell activity compared with that in the placebo group. Interestingly, after β-carotene supplementation for 10–12 years, elderly male participants (65–86 years old) had similar levels of NK cell activity as younger male subjects (51–64 years old), suggesting that β-carotene might be able to maintain a strong immune system in older people (Santos et al. 1996). Another interesting study compared the effects of different dietary carotenoids on the expression of antigen-presenting molecules (the MHC class II molecule (HLA-DR), intercellular adhesion molecule-I, and leukocyte function-associated antigen-3) on the surface of monocytes (Hughes et al. 2000). Hughes et al found that lycopein (found in tomatoes) and lutein (found in peas, watercress, and other vegetables) were less able than β-carotene to regulate the expression of antigen-presenting molecules on the surface of moncytes (Hughes et al. 2000), which is not surprising, given their low plasma levels after supplemention with 15 mg of lycopene or lutein daily. However, lycopene was found to reach higher concentrations in the prostate than in serum (Gerster 1997), which could contribute to the reduced risk of prostate caner associated with the consumption of tomato-based foods (Clinton et al. 1996). Outcomes of human studies can also be influenced by many other confounding factors, such as age, gender, race, and baseline levels of carotenoids in the participants’ plasma. Santos et al also conducted two studies in elderly subjects: a short-term, high-dose study (90 mg/day for 21 days) in women and a longer-term, lower-dose trial (50 mg on alternate days for 10 to 12 years) in men. Neither study showed signficiant changes in the functions or numbers of lymphocyte subsets (CD3+, CD4+, CD8+ T cells, and CD19+ B cells) (Santos et al. 1997). Another intervention study, in Ireland, gave elderly volunteers (>65 years old) either placebo, 8.2 mg of β-carotene, or 13.3 mg of lycopene daily for 12 weeks. This study found no changes in T cell subsets or their expression of functional surface molecules despite significant increases in plasma levels of these carotenoids, suggesting that supplementing with relatively low levels of carotenoids in a well-nourished population may not substantially affect the immune system (Corridan et al. 2001). This may also explain inconsistent observations between epidemiologic studies (Ziegler, Mayne, and Swanson 1996, Sommer and Vyas 2012) and intervention trials (Alpha-Tocopherol 1994, Hennekens et al. 1996, Omenn et al. 1996, Watkins et al. 2000) regarding the effects of β-carotene intake on lung cancer risk.
In light of the essential role of RAs in the immune system, all-trans RAs have been used to treat cutaneous T cell lyphoma (Zhang and Duvic 2003, 2006, Huen and Kim 2015) and hematological malignancies (Montrone et al. 2009), including acute promyelocytic leukemia (APL) (Castaigne et al. 1990, Tallman et al. 1997, Tallman et al. 2002). Human clinical trials have shown that supplementation with all-trans RAs significantly improve disease-free and overal survival of patients compared to chemotherapy alone. The mechanisms could involve promoting differentiation and maturation of leukemic promyelocytes (Castaigne et al. 1990, Tallman et al. 1997, Tallman et al. 2002). In addition, in a mouse model of APL, all-trans RAs in combination with DNA vaccination effectively boosted the production of IFN-γ and increased the number of cytototoxic T cells (CD4+ and CD8+) (Furugaki et al. 2010).
Furthermore, several studies, including animal studies (Kusmartsev et al. 2003, Song et al. 2009) and human clinical trials (Recchia et al. 2005, Recchia et al. 2001, Recchia, Cesta, and Rea 2003, Recchia et al. 2006, Mirza et al. 2006, Haque, Banik, and Ray 2007), have reported that RA exerts certain immune-modulating effects on solid tumors. For example, 13-cis RA combined with IL-2 significantly prolonged overal survival when 44 patients with advanced ovarian cancer were compared with 82 well-matched patients receiving standard therapies (102 versus 29 months). IL-2/RA treatment strongly increased the number of NK cells and the CD4+/CD8+ ratio after 1 and 2 years of treatment (Recchia et al. 2005). Similar results were observed in two other clinical trials. One involved patients with metastastic solid tumors who had undergone agressive surgery and chemotherapy (Recchia et al. 2001, Recchia, Cesta, and Rea 2003), and the other was a multicenter phase II study of patients with non-small-cell lung cancer (Recchia et al. 2006). RAs also dramatically suppressed the number of immature myeloid suppressor cells (ImC) (Lin−HLA-DR−CD33+) and significantly increased the tetanus-toxoid-specific T cell response in patients with metastatic renal cell carcinoma. However, these RA-mediated effects were observed only in patients with high plasma concentrations of RAs (>150 ng/ml) but not in patients with lower concentrations (<135 ng/ml) (Mirza et al. 2006). In addition, a combination of RAs and IFN-γ showed promise as chemoimmunotherapy for patients with glioblastoma (Haque, Banik, and Ray 2007).
In summary, baseline levels of carotenoids in plasma should be taken into account when designing clinical trials, giving that this effect has contributed to discrepancy of study outcomes. Encouragingly, the combination of RA with IL-2 demonstrated promising anti-cancer and immune-modulating effects in patients with solid tumors, making RA a potential adjuvent agent.
Curcumin
Curcumin (diferuloylmethane), also a carotenoid, is the primary active polyphenolic component of turmeric, a yellowish powder derived from Curcuma longa of the Zingiberacea (ginger) plant family (Bose et al. 2015, Jagetia and Aggarwal 2007, Gautam, Gao, and Dulchavsky 2007, Srivastava et al. 2011). Curcumin has been widely used in traditional Indian and Chinese medicine for centuries, mainly to treat inflammatory diseases including viral infection, arthritis, colitis and hepatitis (Bose et al. 2015, Jagetia and Aggarwal 2007, Gautam, Gao, and Dulchavsky 2007, Srivastava et al. 2011, Mollazadeh et al. 2017). Given its anti-inflammatory effects, curcumin has also been intensively studied as an anti-cancer agent in the past several decades, and promising anti-tumorigenic potential has been reported. Importantly, many animal studies have revealed that curcumin can modulate cancer immunology by enhancing the cytolytic function of immune cells and inhibiting the immunesuppressive cancer microenvironment (Bhattacharyya et al. 2010, Shao et al. 2017, Singh et al. 2013, Lu et al. 2016, Liu et al. 2016, Churchill et al. 2000, Luo et al. 2011, Chang et al. 2012, Bhattacharyya et al. 2007, Mukherjee, Hussaini, et al. 2018, Mukherjee, Baidoo, et al. 2018, Pal et al. 2005, Shiri et al. 2015, Bose et al. 2015).
One group gave daily curcumin to Swiss albino mice who bore Ehrlich’s ascites carcinoma cells. They observed significantly less tumor growth and enhanced survival rates. Curcumin prevented the loss of CD4+ T helper cells and CD8+ cytolytic T cells and diminished the Treg cell population (CD4+CD25+Foxp3+) in circulating blood, lymph nodes, and tumors. Curcumin also switched the Th2-type response to the Th1-type response by increasing IFN-γ secretion and decreasing the production of IL-4, TGF-β, and IL-10 (Bhattacharyya et al. 2010). In another study, C57BL/6 mice bearing Lewis lung carcinoma (LLC) received 50 mg/kg curcumin, which restored levels of CD4+ and CD8+ T cells and inhibited the production of Arg-1, ROS, and IL-6 by MDSCs (Liu et al. 2016). Also, a cocktail of natural compounds—1.28 mM+ TriCurin (1.28 mM curcumin:0.32 mM epicatechin gallate:4 mM resveratrol)—increased levels of tumor-infiltrating NK cells and CD8+ cytolytic T cells in C57BL/6 mice bearing HPV+ mouse lung cancer (TC-1 cells). In addition, TriCurin repolarized M2-like TAMs (Arg-1highIL-10highIL-12low) to M1-like TAMs (iNOShighIL-10lowIL-12high) in the tumors (Mukherjee, Hussaini, et al. 2018).
Many other studies involving either genetic models (Churchill et al. 2000) or xenograft models of other types of cancer (Luo et al. 2011, Chang et al. 2012, Bhattacharyya et al. 2007, Pal et al. 2005, Shiri et al. 2015) have demonstrated that curcumin can reverse the tumor-favoring microenvironment, leading to further investigations of curcumin combined with standard cancer therapies. One study combined the anti-PD-L1 antibodies with bisdemethoxycurcumin (BDMC), a natural dimethoxy derivative of curcumin, in C57BL/6 mice with metastasized bladder cancer (MB49 cells) (Shao et al. 2017). They observed that BDMC alone increased levels of tumor-infiltrating CD8+ T cells, IFN-γ secretion in the blood, and decreased the number of tumor-infiltrating MDSCs. Importantly, the combination of anti-PD-L1 antibodies with curcumin further enhanced the secretion of IFN-γ, granzyme B, and perforin from CD8+ T cells. Furthermore, curcumin has been combined with vaccines against tumor development. For instance, one group applied two vaccination strategies to BALB/c mice bearing triple-negative breast tumors (4T1 cells) (Singh et al. 2013). One strategy gave the mice curcumin and all the vaccinations after tumors had developed. The other treated the mice with curcumin before they were inoculated with tumor cells and then vaccinated the animals after tumors developed. For the vaccine, they used attenuated Listeria monocytogenes encoding tumor-associated antigen against metastasis (Listeriaat-Mage-b). Interestingly, the second strategy was more effective against metastasis than the first, as it decreased the production of MDSCs and IL-6, and increased levels of IL-12 and IFN-γ.
Despite the accumulating evidence that curcumin is an anti-cancer agent, research in this area is still challenged by one main obstacle: curcumin’s low bioavailability (Panda et al. 2017). To overcome this problem, curcumin molecules have been modified. For example, curcumin liposomes were prepared and given to C57BL/6 mice bearing glioblastoma (GL261 cells) (Mukherjee, Baidoo, et al. 2018). A larger number of activated NK cells were recruited into the tumors, and TAMs were repolarized from M2-like (Arg-1high) to M1-like (iNOShigh). However, this study found that TriCurin produced stronger immune-modulating effects than curcumin liposomes alone. Another study investigated the anti-tumor effects of a curcumin–polyethylene glycol conjugate (CUR-PEG, an amphiphilic CUR-based micelle) combined with a lipid-based Trp2 peptide vaccine in melanoma (B16F10 cells)-bearing C57BL/6 mice (Lu et al. 2016). The resulting synergistic effect associated with increased CD8+ cytolytic T cells, TNF-α secretion, and IFN-γ production along with decreased levels of tumor-infiltrating MDSCs and Treg cells.
In summary, curcumin is one of the most widely studied natural anti-cancer compounds. Strong evidence from in vitro and in vivo studies have demonstrated its promising chemoprotective effects. Its potential to modulate cancer immunology has been proposed as one of the mechanisms. However, successful transition from the lab to the clinic will need a solution to the issue of its low bioavailability.
Several human clinical trials have used new drug delivery systems to optimize plasma levels of curcumin when investigating the potential effects of curcumin in cancer patients. Curcumin was found to improve quality of life and reduce the systemic inflammation induced by chemotherapy (Kanai et al. 2013, Hejazi et al. 2016, Pan, Skaer, et al. 2017, Dhillon et al. 2008, Epelbaum et al. 2010, Parsons et al. 2016, Mahammedi et al. 2016, Panahi et al. 2014). Only a few studies have examined the potential immune-modulating effects of curcumin in humans, however. For example, 2 weeks of curcumin treatment increased the number of Th1 cells but decreased the number of Treg cells in peripheral blood of lung cancer patients (Zou et al. 2018). Similar results were observed in colorectal cancer patients (Xu, Yu, and Zhao 2017). Thus, further investigations of combining curcumin with standard cancer therapies could be a new field of cancer research.
Resveratrol
Resveratrol (3,4′,5-trihydroxy-trans stilbene) is a non-flavonoid polyphenolic antioxidant found predominantly in grapes (Vitis vinifera) but also in many other natural sources, including peanuts, soy, and berries (Varoni et al. 2016, Udenigwe et al. 2008). The suggestion that the “French paradox”—the interesting observation that the mortality rate from coronary heart disease is much lower in France than in other European countries and the USA—could be attributed to the high consumption of red wine in that country drew close attention to resveratrol, red wine’s primary active ingredient. Since then, many potential benefits of resveratrol, such as antioxidation, anti-inflammation, neuroprotection, anti-proliferation, and immune-modulation, have been discovered (Katiyar 2008, Leischner et al. 2016, Soto et al. 2011, Tong et al. 2011).
Resveratrol has been shown to enhance cytolytic immune responses and reverse immune suppression in the tumor microenvironment (Lee-Chang et al. 2013, Chen et al. 2015, Yang et al. 2008, Pan, Shen, et al. 2017, Zhang et al. 2005, Chang et al. 2016, Li et al. 2007, Guan et al. 2012). For example, one group investigated the immune-modulating effects of resveratrol in the triple-negative breast cancer (4T1 cells)-bearing BALB/c mice and in the melanoma (B16F10 cells)-bearing C57BL/6 mice (Lee-Chang et al. 2013). Resveratrol significantly reduced levels of Breg cells (CD25+CD81high cells within CD19+ B cells) and Foxp3+ Treg cells, though it did not affect MDSCs. In addition, resveratrol activated CD8+ cytolytic T cells. In renal cancer (Renca cells)-bearing BALB/c mice, resveratrol significantly increased the number and activity of CD8+ cytolytic T cells, but not NK cells, as well as decreased the percentage of Treg cells, but not MDSCs, in tumors. Resveratrol also switched the Th2-like immune response (IL-6 and IL-10 secretion) to favor the Th1-like response (IFN-γ secretion) in the tumor microenvironment (Chen et al. 2015). Moreover, enhanced NK cell cytotoxicity was observed during resveratrol treatment in skin lymphocytic leukemia (L1210 cells)-bearing BALB/c mice (Li et al. 2007). Furthermore, grape seed proanthocyanidins have been reported to significantly increase NK cell cytotoxicity, as well as the percentages of CD4+ T helper cells and CD8+ cytolytic T cells in sarcoma (S180 cells)-bearing BALB/c mice (Zhang et al. 2005). Interestingly, that study observed that grape seed proanthocyanidins enhanced the anti-tumor effects of doxorubicin. They completely reversed the side effects induced by doxorubicin treatment, which included reduced levels of IFN-γ and IL-2 and suppressed NK cell function (Zhang et al. 2005).
Clinical studies with healthy volunteers have demonstrated that resveratrol can be safely administered (Brown et al. 2010, Chow et al. 2010, Boocock et al. 2007, Espinoza et al. 2017). Importantly, a recent study that examined the effects of resveratrol on circulating T cell subsets in healthy volunteers found that it significantly increased the number of circulating Treg cells (CD3+CD4+CD25+CD127−) and CD3+NKG2D+ T cells while decreasing plasma levels of proinflammatory cytokines such as TNF-α and monocyte chemoattractant protein (MCP-1) (Espinoza et al. 2017). Though several clinical trials have given resveratrol to cancer patients (van Die et al. 2017, Paller et al. 2015, Howells et al. 2011, Patel et al. 2010), the results are not yet available.
In summary, the benefits of red wine are widely recognized, but most studies of resveratrol have focused on cardiovascular diseases and diabetes. Therefore, more studies are needed to evaluate the effects of resveratrol, alone or in combination with standard therapies, in cancer patients.
(−)-Epigallocatechin-3-gallate (EGCG)
Tea plants (Camellia sinensis) have been grown in Asia for thousands of years, and tea consumption has become the second-most common beverage worldwide. This high popularity can be attributed to tea’s charming smell and flavor as well as to its multiple health benefits, including antioxidative, anti-inflammatory, antimicrobial, anti-carcinogenic, anti-hypertensive, neuroprotective, cholesterol-lowering, and thermogenic properties (Hayat et al. 2015, Crespy and Williamson 2004, Katiyar, Elmets, and Katiyar 2007). Moreover, many epidemiologic studies have suggested that green tea consumption associates with chemoprotective effects against cancer (Shirakami, Shimizu, and Moriwaki 2012). The primary active ingredients in green tea are epigallocatechin-3-gallate (EGCG), epicatechin, epicatechin-3-gallate, and epigallocatechin (Butt and Sultan 2009). Multiple anti-cancer mechanisms have been reported, such as induction of apoptosis, promotion of cell growth arrest, alteration of cell cycle regulatory proteins, activation of killer caspases, and suppression of the nuclear factor kappa-B (NF-κB) pathway (Butt and Sultan 2009, Shirakami, Shimizu, and Moriwaki 2012). Several animal studies have shown that EGCG modulates the immune system in tumor-bearing mice (Santilli et al. 2013, Kang et al. 2007, Huang et al. 2013, Mantena, Roy, and Katiyar 2005, Hsieh et al. 2011).
One group used two xenograft models, SHSY5Y tumor-bearing NOD/SCID mice and neuro 2A tumor-bearing A/J mice, to investigate the immune-modulating effects of Polyphenon E, a clinical-grade mixture of green tea catechins, against neuroblastoma (Santilli et al. 2013). Polyphenon E shifted the balance from monocytic MDSCs (CD11b+Ly6G-Ly6high) toward granulocytic MDSCs (CD11b+Ly6G+Ly6low) by enhancing myeloid cell maturation and upregulating the secretion of granulocyte colony stimulating factor (G-CSF) in monocytic MDSCs. These effects rescued the MDSC-suppressed production of IFN-γ in T cells and increased the number of tumor-infiltrating CD8+ cytolytic T cells (Santilli et al. 2013). In a model of mice bearing E7-expressing tumors (TC-1 cells), EGCG significantly increased the E7-specific (antigen-specific) CD8+ T-cell immune response (IFN-γ secretion) in tumors (Kang et al. 2007). E7 is an oncoprotein in the human papilloma virus. It induces and maintains cancer cell transformation, making it a perfect candidate for a targeted vaccine. Moreover, EGCG has been reported to increase NK cell cytotoxicity in leukemia (WEHI-3 cells)-bearing BALB/c mice (Huang et al. 2013) and in bladder cancer (MBT-2 cells)-bearing C3H/He mice (Hsieh et al. 2011).
In several human clinical trials, evidence for EGCG-mediated benefits against solid tumors was largely lacking (Lazzeroni et al. 2017, Gee et al. 2017, Zhao et al. 2016, Zhao et al. 2014, Crew et al. 2012). In contrast, one group evaluated the safety and clinical efficacy of Polyphenon E in patients with early stage chronic lymphocytic leukemia (CLL) (Shanafelt et al. 2009, Shanafelt et al. 2013). Polyphenon E was well tolerated, and reduction in absolute lymphocyte count and/or lymphadenopathy was observed in the majority of patients. Similarly, another group found that green tea extract reduced or stabilized lymphocytosis and lowered the number of circulating Treg cells in 90% of CLL subjects. In addition, both IL-10 and TGF-β levels in the serum decreased during the period of green tea consumption (D’Arena et al. 2013). These studies highlight the possibility that green tea could become a chemopreventive agent against hematological malignancies. However, the effects of green tea on solid tumors are yet to be determined.
β-Glucans
β-glucans are a group of polysaccharides found in the cell walls of bacteria, fungi (including mushrooms), and cereals such as barley and oats. All β-glucans are glucose polymers that share a β1–3 linear glycosidic chain core but differ in length and branching structures. Two main types of branching are β1–4 glycosidic chains in β-glucans from bacteria and β1–6 glycosidic chains in β-glucans from fungi (Aleem 2013). Epidemiological studies show that diets containing β-glucans may prevent or slow the development of cancer, including gastric cancer and breast cancer. However, β-glucans are administered mostly to improve quality of life and overall survival in patients undergoing chemotherapy or radiation treatment (Aleem 2013).
Various β-glucan extracts have been investigated in clinical studies, including Lentinan from shiitake mushrooms (Lentinus edodes), Schizophyllan (SPG) from Schizophyllum commune, Krestin (PSK and PSP) from Trametes (Coriolus or Polyporus) versicolor, Maitake from Grifola frondosa, Agaricus sylvaticus, and A. blazei. These fungal extracts have had beneficial effects on patients with cancers, including gastric cancer, colorectal cancer, esophageal cancer, hepatocellular carcinoma, pancreatic cancer, breast cancer, lung cancer, stomach cancer, and leukemia (Aleem 2013, Roudi et al. 2017, Ramberg, Nelson, and Sinnott 2010, Chan, Chan, and Sze 2009, Murphy, Davis, and Carmichael 2010, Jin et al. 2012, Roupas et al. 2012, Standish et al. 2008). For example, enhanced number and/or activity of NK cells were reported in studies of an extract from Agaricales mushrooms—Agaricus blazei Murill Kyowa (ABMK)—in patients with different types of cancer, including cervical, ovarian, endometrial (Ahn et al. 2004), and breast cancer (Novaes et al. 2011). Similar results were obtained with Ganopoly, a polysaccharide extract of Ganoderma lucidum (Lingzhi), in advanced-stage cancer patients (Gao et al. 2003, Chen et al. 2006); with Lentinula edodes mycelia (LEM) extract in breast cancer patients (Nagashima et al. 2013) and gastrointestinal cancer patients (Okuno and Uno 2011, Yamaguchi, Miyahara, and Hihara 2011, Ina, Kataoka, and Ando 2013); with PSK in stage II–III colorectal cancer patients (Ohwada et al. 2006, Ohwada et al. 2004); and with SPG in stage III–IV head and neck cancer patients (Kano, Kakuta, and Hashimoto 1996). Moreover, evidence of glucan-mediated effects on T cells and their cytokine secretions have also been observed. Intratumoral SPG administration augmented tumor-infiltrating T cells in patients with stage II–III invasive cervical cancers (Nakano et al. 1996). SPG also increased CD4+ T helper cell and IL-2 production in regional lymph nodes of patients with stage III–IV head and neck cancer (Kano, Kakuta, and Hashimoto 1996). Ganopoly (Chen et al. 2006) and LEM (Okuno and Uno 2011) increased the number of CD3+, CD4+, and CD8+ T cells, as well as plasma levels of IL-2, IL-6, and IFN-γ, in advanced-stage cancer patients.
A two-stage model has been proposed to explain the mechanism of immune modulation by β-glucans (Murphy, Davis, and Carmichael 2010, Albeituni and Yan 2013, Wasser 2017). The first stage is initiated when β-glucans bind to dectin-1 and TLRs that are expressed on macrophages and DCs. β-glucans are then taken up by macrophages into endosomes, where they are fragmented and transported to the spleen, lymph nodes, and bone marrow for release. The second stage is achieved by NK cells or granulocytes that express complement receptor 3 (CR3) on their surface. The β-glucan fragments released by macrophages are recognized by CR3, which triggers signaling pathways that eventually release cytokines and stimulate adaptive immune responses, such as CR3-dependent cellular cytotoxicity (CR3-DCC).
In summary, glucans, with largely diverse structures, are becoming promising adjuvant anti-cancer agents. Some have been standardized and clinically approved, such as Lentinan, which has been used clinically against several types of cancer in Japan since the early 1980s. However, most of the abovementioned mushrooms grow in Asia, where most of the clinical trials were conducted. This could limit their use in other regions of the world.
Summary
The current review summarizes some immune-modulating effects of natural compounds as determined in human clinical trials and tumor-bearing animal models. Promising results have been observed mainly in animal studies, and mostly in xenograft mouse models where the tumor microenvironment could be significantly different from that in cancer patients and genetically modified models (Olive et al. 2009). Therefore, further investigations need to consider the similarity of the tumor microenvironment in cancer patients and proposed animal models. Ultimately, efficacy in cancer patients needs to be fully examined.
Large epidemiologic studies have suggested that diet could influence the risk of at least certain types of cancer. For example, a higher intake of fruits and vegetables might associate with a lower risk of colorectal cancer (Pan, Yu, and Wang 2018). Indeed, fruits and vegetables are enriched in many well-studied chemopreventive agents, such as curcumin and resveratrol. However, the relationship between diets that might lower cancer risk and their effects on the immune system remains largely unknown. It certainly would be valuable to investigate whether natural compounds and dietary components could strengthen the immune system in populations at high risk for cancer.
Acknowledgments
This work was partially supported by NIH grants 5 R01 CA148818 (to L.-S. Wang) and 5 R01 CA185301, AI129582 and NS106170 (to J.Y.), as well as American Cancer Society Research Scholar Grant RSG-13138-01-CNE (to L.-S. Wang) and RSG-14-243-01-LIB (to J.Y.) We apologize to the researchers whose studies could not be cited due to space limitation.
Abbreviations:
- ABMK
Agaricus blazei Murill Kyowa
- APCs
antigen-presenting cells
- APL
acute promyelocytic leukemia
- Arg-1
arginase 1
- BDMC
bisdemethoxycurcumin
- Breg
B regulatory
- CLL
chronic lymphocytic leukemia
- CR3
complement receptor 3
- CR3-DCC
CR3-dependent cellular cytotoxicity
- CUR-PEG
curcumin–polyethylene glycol conjugate
- DCs
dendritic cells
- EGCG
(−)-epigallocatechin-3-gallate
- G-CSF
granulocyte colony stimulating factor
- IFN-γ
interferon gamma
- IL-2
interleukin-2
- ImC
immature myeloid suppressor cells
- iNOS
inducible NOS
- KIRs
killer cell inhibitory immunoglobulin-like receptors
- LEM
Lentinula edodes mycelia
- LLC
Lewis lung carcinoma
- MCP-1
monocyte chemoattractant protein
- MDSCs
myeloid-derived suppressor cells
- MHC
major histocompatibility complex
- M-MDSCs
monocytic MDSCs
- MMP
matrix metalloproteinase
- NCRs
natural cytotoxicity receptors
- NF-κB
nuclear factor kappa-B
- NK
natural killer
- NO
nitric oxide
- PD-1
programmed cell death marker
- PMN-MDSCs
polymorphonuclear MDSCs
- RA
retinoic acid
- ROS
reactive oxygen species
- TAMs
tumor-associated macrophages
- TLRs
Toll-like receptors
- TNF-α
tumor necrosis factor alpha
- Treg
T regulatory
- VEGFs
vascular endothelial growth factors
References
- Ahn WS, Kim DJ, Chae GT, Lee JM, Bae SM, Sin JI, Kim YW, Namkoong SE, and Lee IP. 2004. Natural killer cell activity and quality of life were improved by consumption of a mushroom extract, Agaricus blazei Murill Kyowa, in gynecological cancer patients undergoing chemotherapy. Int J Gynecol Cancer 14 (4):589–94. doi: 10.1111/j.1048-891X.2004.14403.x. [DOI] [PubMed] [Google Scholar]
- Albeituni SH, and Yan J. 2013. The effects of beta-glucans on dendritic cells and implications for cancer therapy. Anticancer Agents Med Chem 13 (5):689–98. [DOI] [PubMed] [Google Scholar]
- Aleem E 2013. beta-Glucans and their applications in cancer therapy: focus on human studies. Anticancer Agents Med Chem 13 (5):709–19. [DOI] [PubMed] [Google Scholar]
- Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. 1994. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N Engl J Med 330 (15):1029–35. doi: 10.1056/NEJM199404143301501. [DOI] [PubMed] [Google Scholar]
- Baraya YS, Wong KK, and Yaacob NS. 2017. The Immunomodulatory Potential of Selected Bioactive Plant-Based Compounds in Breast Cancer: A Review. Anticancer Agents Med Chem 17 (6):770–783. doi: 10.2174/1871520616666160817111242. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Mandal D, Saha B, Sen GS, Das T, and Sa G. 2007. Curcumin prevents tumor-induced T cell apoptosis through Stat-5a-mediated Bcl-2 induction. J Biol Chem 282 (22):15954–64. doi: 10.1074/jbc.M608189200. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Md Sakib Hossain D, Mohanty S, Sankar Sen G, Chattopadhyay S, Banerjee S, Chakraborty J, Das K, Sarkar D, Das T, and Sa G. 2010. Curcumin reverses T cell-mediated adaptive immune dysfunctions in tumor-bearing hosts. Cell Mol Immunol 7 (4):306–15. doi: 10.1038/cmi.2010.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorklund G, Dadar M, Aaseth J, Chirumbolo S, and Pen JJ. 2018. Cancer-associated cachexia, reactive oxygen species, and nutrition therapy. Curr Med Chem. doi: 10.2174/0929867325666180629123817. [DOI] [PubMed] [Google Scholar]
- Boocock DJ, Faust GE, Patel KR, Schinas AM, Brown VA, Ducharme MP, Booth TD, Crowell JA, Perloff M, Gescher AJ, Steward WP, and Brenner DE. 2007. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol Biomarkers Prev 16 (6):1246–52. doi: 10.1158/1055-9965.EPI-07-0022. [DOI] [PubMed] [Google Scholar]
- Bose S, Panda AK, Mukherjee S, and Sa G. 2015. Curcumin and tumor immune-editing: resurrecting the immune system. Cell Div 10:6. doi: 10.1186/s13008-015-0012-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CC, and Noelle RJ. 2015. Seeing through the dark: New insights into the immune regulatory functions of vitamin A. Eur J Immunol 45 (5):1287–95. doi: 10.1002/eji.201344398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown VA, Patel KR, Viskaduraki M, Crowell JA, Perloff M, Booth TD, Vasilinin G, Sen A, Schinas AM, Piccirilli G, Brown K, Steward WP, Gescher AJ, and Brenner DE. 2010. Repeat dose study of the cancer chemopreventive agent resveratrol in healthy volunteers: safety, pharmacokinetics, and effect on the insulin-like growth factor axis. Cancer Res 70 (22):9003–11. doi: 10.1158/0008-5472.CAN-10-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant DG, and Prasad C. 1991. Effect of beta-carotene on lymphocyte subpopulations in elderly humans. Am J Clin Nutr 54 (2):432–3. [DOI] [PubMed] [Google Scholar]
- Burkard M, Leischner C, Lauer UM, Busch C, Venturelli S, and Frank J. 2017. Dietary flavonoids and modulation of natural killer cells: implications in malignant and viral diseases. J Nutr Biochem 46:1–12. doi: 10.1016/j.jnutbio.2017.01.006. [DOI] [PubMed] [Google Scholar]
- Butt MS, and Sultan MT. 2009. Green tea: nature’s defense against malignancies. Crit Rev Food Sci Nutr 49 (5):463–73. doi: 10.1080/10408390802145310. [DOI] [PubMed] [Google Scholar]
- Carratu MR, Marasco C, Mangialardi G, and Vacca A. 2012. Retinoids: novel immunomodulators and tumour-suppressive agents? Br J Pharmacol 167 (3):483–92. doi: 10.1111/j.1476-5381.2012.02031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey SC, Amedei A, Aquilano K, Azmi AS, Benencia F, Bhakta D, Bilsland AE, Boosani CS, Chen S, Ciriolo MR, Crawford S, Fujii H, Georgakilas AG, Guha G, Halicka D, Helferich WG, Heneberg P, Honoki K, Keith WN, Kerkar SP, Mohammed SI, Niccolai E, Nowsheen S, Vasantha Rupasinghe HP, Samadi A, Singh N, Talib WH, Venkateswaran V, Whelan RL, Yang X, and Felsher DW. 2015. Cancer prevention and therapy through the modulation of the tumor microenvironment. Semin Cancer Biol 35 Suppl:S199–S223. doi: 10.1016/j.semcancer.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castaigne S, Chomienne C, Daniel MT, Ballerini P, Berger R, Fenaux P, and Degos L. 1990. All-trans retinoic acid as a differentiation therapy for acute promyelocytic leukemia. I. Clinical results. Blood 76 (9):1704–9. [PubMed] [Google Scholar]
- Chan GC, Chan WK, and Sze DM. 2009. The effects of beta-glucan on human immune and cancer cells. J Hematol Oncol 2:25. doi: 10.1186/1756-8722-2-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang WA, Hung JY, Jian SF, Lin YS, Wu CY, Hsu YL, and Kuo PL. 2016. Laricitrin ameliorates lung cancer-mediated dendritic cell suppression by inhibiting signal transducer and activator of transcription 3. Oncotarget 7 (51):85220–85234. doi: 10.18632/oncotarget.13240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YF, Chuang HY, Hsu CH, Liu RS, Gambhir SS, and Hwang JJ. 2012. Immunomodulation of curcumin on adoptive therapy with T cell functional imaging in mice. Cancer Prev Res (Phila) 5 (3):444–52. doi: 10.1158/1940-6207.CAPR-11-0308. [DOI] [PubMed] [Google Scholar]
- Chen DS, and Mellman I. 2013. Oncology meets immunology: the cancer-immunity cycle. Immunity 39 (1):1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- Chen L, Yang S, Liao W, and Xiong Y. 2015. Modification of Antitumor Immunity and Tumor Microenvironment by Resveratrol in Mouse Renal Tumor Model. Cell Biochem Biophys 72 (2):617–25. doi: 10.1007/s12013-015-0513-z. [DOI] [PubMed] [Google Scholar]
- Chen X, Hu ZP, Yang XX, Huang M, Gao Y, Tang W, Chan SY, Dai X, Ye J, Ho PC, Duan W, Yang HY, Zhu YZ, and Zhou SF. 2006. Monitoring of immune responses to a herbal immuno-modulator in patients with advanced colorectal cancer. Int Immunopharmacol 6 (3):499–508. doi: 10.1016/j.intimp.2005.08.026. [DOI] [PubMed] [Google Scholar]
- Chirumbolo S 2012. Plant phytochemicals as new potential drugs for immune disorders and cancer therapy: really a promising path? J Sci Food Agric 92 (8):1573–7. doi: 10.1002/jsfa.5670. [DOI] [PubMed] [Google Scholar]
- Chow HH, Garland LL, Hsu CH, Vining DR, Chew WM, Miller JA, Perloff M, Crowell JA, and Alberts DS. 2010. Resveratrol modulates drug- and carcinogen-metabolizing enzymes in a healthy volunteer study. Cancer Prev Res (Phila) 3 (9):1168–75. doi: 10.1158/1940-6207.CAPR-09-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill M, Chadburn A, Bilinski RT, and Bertagnolli MM. 2000. Inhibition of intestinal tumors by curcumin is associated with changes in the intestinal immune cell profile. J Surg Res 89 (2):169–75. doi: 10.1006/jsre.2000.5826. [DOI] [PubMed] [Google Scholar]
- Clinton SK, Emenhiser C, Schwartz SJ, Bostwick DG, Williams AW, Moore BJ, and Erdman JW Jr. 1996. cis-trans lycopene isomers, carotenoids, and retinol in the human prostate. Cancer Epidemiol Biomarkers Prev 5 (10):823–33. [PubMed] [Google Scholar]
- Corridan BM, O’Donoghue M, Hughes DA, and Morrissey PA. 2001. Low-dose supplementation with lycopene or beta-carotene does not enhance cell-mediated immunity in healthy free-living elderly humans. Eur J Clin Nutr 55 (8):627–35. doi: 10.1038/sj.ejcn.1601187. [DOI] [PubMed] [Google Scholar]
- Crespy V, and Williamson G. 2004. A review of the health effects of green tea catechins in in vivo animal models. J Nutr 134 (12 Suppl):3431S–3440S. [DOI] [PubMed] [Google Scholar]
- Crew KD, Brown P, Greenlee H, Bevers TB, Arun B, Hudis C, McArthur HL, Chang J, Rimawi M, Vornik L, Cornelison TL, Wang A, Hibshoosh H, Ahmed A, Terry MB, Santella RM, Lippman SM, and Hershman DL. 2012. Phase IB randomized, double-blinded, placebo-controlled, dose escalation study of polyphenon E in women with hormone receptor-negative breast cancer. Cancer Prev Res (Phila) 5 (9):1144–54. doi: 10.1158/1940-6207.CAPR-12-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Arena G, Simeon V, De Martino L, Statuto T, D’Auria F, Volpe S, Deaglio S, Maidecchi A, Mattoli L, Mercati V, Musto P, and De Feo V. 2013. Regulatory T-cell modulation by green tea in chronic lymphocytic leukemia. Int J Immunopathol Pharmacol 26 (1):117–25. doi: 10.1177/039463201302600111. [DOI] [PubMed] [Google Scholar]
- del Corno M, Scazzocchio B, Masella R, and Gessani S. 2016. Regulation of Dendritic Cell Function by Dietary Polyphenols. Crit Rev Food Sci Nutr 56 (5):737–47. doi: 10.1080/10408398.2012.713046. [DOI] [PubMed] [Google Scholar]
- Dhillon N, Aggarwal BB, Newman RA, Wolff RA, Kunnumakkara AB, Abbruzzese JL, Ng CS, Badmaev V, and Kurzrock R. 2008. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin Cancer Res 14 (14):4491–9. doi: 10.1158/1078-0432.CCR-08-0024. [DOI] [PubMed] [Google Scholar]
- Epelbaum R, Schaffer M, Vizel B, Badmaev V, and Bar-Sela G. 2010. Curcumin and gemcitabine in patients with advanced pancreatic cancer. Nutr Cancer 62 (8):1137–41. doi: 10.1080/01635581.2010.513802. [DOI] [PubMed] [Google Scholar]
- Espinoza JL, Trung LQ, Inaoka PT, Yamada K, An DT, Mizuno S, Nakao S, and Takami A. 2017. The Repeated Administration of Resveratrol Has Measurable Effects on Circulating T-Cell Subsets in Humans. Oxid Med Cell Longev 2017:6781872. doi: 10.1155/2017/6781872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson LR, and Philpott M. 2007. Cancer prevention by dietary bioactive components that target the immune response. Curr Cancer Drug Targets 7 (5):459–64. [DOI] [PubMed] [Google Scholar]
- Ferraz da Costa DC, Fialho E, and Silva JL. 2017. Cancer Chemoprevention by Resveratrol: The p53 Tumor Suppressor Protein as a Promising Molecular Target. Molecules 22 (6). doi: 10.3390/molecules22061014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furugaki K, Pokorna K, Le Pogam C, Aoki M, Reboul M, Bajzik V, Krief P, Janin A, Noguera ME, West R, Charron D, Chomienne C, Pla M, Moins-Teisserenc H, and Padua RA. 2010. DNA vaccination with all-trans retinoic acid treatment induces long-term survival and elicits specific immune responses requiring CD4+ and CD8+ T-cell activation in an acute promyelocytic leukemia mouse model. Blood 115 (3):653–6. doi: 10.1182/blood-2007-08-109009. [DOI] [PubMed] [Google Scholar]
- Gabrilovich DI 2017. Myeloid-Derived Suppressor Cells. Cancer Immunol Res 5 (1):3–8. doi: 10.1158/2326-6066.CIR-16-0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Zhou S, Jiang W, Huang M, and Dai X. 2003. Effects of ganopoly (a Ganoderma lucidum polysaccharide extract) on the immune functions in advanced-stage cancer patients. Immunol Invest 32 (3):201–15. [DOI] [PubMed] [Google Scholar]
- Gautam SC, Gao X, and Dulchavsky S. 2007. Immunomodulation by curcumin. Adv Exp Med Biol 595:321–41. doi: 10.1007/978-0-387-46401-5_14. [DOI] [PubMed] [Google Scholar]
- Gee JR, Saltzstein DR, Kim K, Kolesar J, Huang W, Havighurst TC, Wollmer BW, Stublaski J, Downs T, Mukhtar H, House MG, Parnes HL, and Bailey HH. 2017. A Phase II Randomized, Double-blind, Presurgical Trial of Polyphenon E in Bladder Cancer Patients to Evaluate Pharmacodynamics and Bladder Tissue Biomarkers. Cancer Prev Res (Phila) 10 (5):298–307. doi: 10.1158/1940-6207.CAPR-16-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerster H 1997. The potential role of lycopene for human health. J Am Coll Nutr 16 (2):109–26. [DOI] [PubMed] [Google Scholar]
- Ghiringhelli F, Rebe C, Hichami A, and Delmas D. 2012. Immunomodulation and anti-inflammatory roles of polyphenols as anticancer agents. Anticancer Agents Med Chem 12 (8):852–73. [DOI] [PubMed] [Google Scholar]
- Godfrey DI, Le Nours J, Andrews DM, Uldrich AP, and Rossjohn J. 2018. Unconventional T Cell Targets for Cancer Immunotherapy. Immunity 48 (3):453–473. doi: 10.1016/j.immuni.2018.03.009. [DOI] [PubMed] [Google Scholar]
- Guan H, Singh NP, Singh UP, Nagarkatti PS, and Nagarkatti M. 2012. Resveratrol prevents endothelial cells injury in high-dose interleukin-2 therapy against melanoma. PLoS One 7 (4):e35650. doi: 10.1371/journal.pone.0035650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque A, Banik NL, and Ray SK. 2007. Emerging role of combination of all-trans retinoic acid and interferon-gamma as chemoimmunotherapy in the management of human glioblastoma. Neurochem Res 32 (12):2203–9. doi: 10.1007/s11064-007-9420-z. [DOI] [PubMed] [Google Scholar]
- Hayat K, Iqbal H, Malik U, Bilal U, and Mushtaq S. 2015. Tea and its consumption: benefits and risks. Crit Rev Food Sci Nutr 55 (7):939–54. doi: 10.1080/10408398.2012.678949. [DOI] [PubMed] [Google Scholar]
- Heiser LM, Sadanandam A, Kuo WL, Benz SC, Goldstein TC, Ng S, Gibb WJ, Wang NJ, Ziyad S, Tong F, Bayani N, Hu Z, Billig JI, Dueregger A, Lewis S, Jakkula L, Korkola JE, Durinck S, Pepin F, Guan Y, Purdom E, Neuvial P, Bengtsson H, Wood KW, Smith PG, Vassilev LT, Hennessy BT, Greshock J, Bachman KE, Hardwicke MA, Park JW, Marton LJ, Wolf DM, Collisson EA, Neve RM, Mills GB, Speed TP, Feiler HS, Wooster RF, Haussler D, Stuart JM, Gray JW, and Spellman PT. 2012. Subtype and pathway specific responses to anticancer compounds in breast cancer. Proc Natl Acad Sci U S A 109 (8):2724–9. doi: 10.1073/pnas.1018854108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hejazi J, Rastmanesh R, Taleban FA, Molana SH, Hejazi E, Ehtejab G, and Hara N. 2016. Effect of Curcumin Supplementation During Radiotherapy on Oxidative Status of Patients with Prostate Cancer: A Double Blinded, Randomized, Placebo-Controlled Study. Nutr Cancer 68 (1):77–85. doi: 10.1080/01635581.2016.1115527. [DOI] [PubMed] [Google Scholar]
- Hennekens CH, Buring JE, Manson JE, Stampfer M, Rosner B, Cook NR, Belanger C, LaMotte F, Gaziano JM, Ridker PM, Willett W, and Peto R. 1996. Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. N Engl J Med 334 (18):1145–9. doi: 10.1056/NEJM199605023341801. [DOI] [PubMed] [Google Scholar]
- Howells LM, Berry DP, Elliott PJ, Jacobson EW, Hoffmann E, Hegarty B, Brown K, Steward WP, and Gescher AJ. 2011. Phase I randomized, double-blind pilot study of micronized resveratrol (SRT501) in patients with hepatic metastases--safety, pharmacokinetics, and pharmacodynamics. Cancer Prev Res (Phila) 4 (9):1419–25. doi: 10.1158/1940-6207.CAPR-11-0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh DS, Wang H, Tan SW, Huang YH, Tsai CY, Yeh MK, and Wu CJ. 2011. The treatment of bladder cancer in a mouse model by epigallocatechin-3-gallate-gold nanoparticles. Biomaterials 32 (30):7633–40. doi: 10.1016/j.biomaterials.2011.06.073. [DOI] [PubMed] [Google Scholar]
- Huang AC, Cheng HY, Lin TS, Chen WH, Lin JH, Lin JJ, Lu CC, Chiang JH, Hsu SC, Wu PP, Huang YP, and Chung JG. 2013. Epigallocatechin gallate (EGCG), influences a murine WEHI-3 leukemia model in vivo through enhancing phagocytosis of macrophages and populations of T- and B-cells. In Vivo 27 (5):627–34. [PubMed] [Google Scholar]
- Huen AO, and Kim EJ. 2015. The Role of Systemic Retinoids in the Treatment of Cutaneous T-Cell Lymphoma. Dermatol Clin 33 (4):715–29. doi: 10.1016/j.det.2015.05.007. [DOI] [PubMed] [Google Scholar]
- Hughes DA 2001. Dietary carotenoids and human immune function. Nutrition 17 (10):823–7. [DOI] [PubMed] [Google Scholar]
- Hughes DA, Wright AJ, Finglas PM, Polley AC, Bailey AL, Astley SB, and Southon S. 2000. Effects of lycopene and lutein supplementation on the expression of functionally associated surface molecules on blood monocytes from healthy male nonsmokers. J Infect Dis 182 Suppl 1:S11–5. doi: 10.1086/315910. [DOI] [PubMed] [Google Scholar]
- Hussain SS, Kumar AP, and Ghosh R. 2016. Food-based natural products for cancer management: Is the whole greater than the sum of the parts? Semin Cancer Biol 40–41:233–246. doi: 10.1016/j.semcancer.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannello A, Thompson TW, Ardolino M, Marcus A, and Raulet DH. 2016. Immunosurveillance and immunotherapy of tumors by innate immune cells. Curr Opin Immunol 38:52–8. doi: 10.1016/j.coi.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ina K, Kataoka T, and Ando T. 2013. The use of lentinan for treating gastric cancer. Anticancer Agents Med Chem 13 (5):681–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagetia GC, and Aggarwal BB. 2007. “Spicing up” of the immune system by curcumin. J Clin Immunol 27 (1):19–35. doi: 10.1007/s10875-006-9066-7. [DOI] [PubMed] [Google Scholar]
- Janakiram NB, Mohammed A, Madka V, Kumar G, and Rao CV. 2016. Prevention and treatment of cancers by immune modulating nutrients. Mol Nutr Food Res 60 (6):1275–94. doi: 10.1002/mnfr.201500884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X, Ruiz Beguerie J, Sze DM, and Chan GC. 2012. Ganoderma lucidum (Reishi mushroom) for cancer treatment. Cochrane Database Syst Rev (6):Cd007731. doi: 10.1002/14651858.CD007731.pub2. [DOI] [PubMed] [Google Scholar]
- Kanai M, Otsuka Y, Otsuka K, Sato M, Nishimura T, Mori Y, Kawaguchi M, Hatano E, Kodama Y, Matsumoto S, Murakami Y, Imaizumi A, Chiba T, Nishihira J, and Shibata H. 2013. A phase I study investigating the safety and pharmacokinetics of highly bioavailable curcumin (Theracurmin) in cancer patients. Cancer Chemother Pharmacol 71 (6):1521–30. doi: 10.1007/s00280-013-2151-8. [DOI] [PubMed] [Google Scholar]
- Kang TH, Lee JH, Song CK, Han HD, Shin BC, Pai SI, Hung CF, Trimble C, Lim JS, Kim TW, and Wu TC. 2007. Epigallocatechin-3-gallate enhances CD8+ T cell-mediated antitumor immunity induced by DNA vaccination. Cancer Res 67 (2):802–11. doi: 10.1158/0008-5472.CAN-06-2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano Y, Kakuta H, and Hashimoto J. 1996. Effect of sizofiran on regional lymph nodes in patients with head and neck cancer. Biotherapy 9 (4):257–62. [DOI] [PubMed] [Google Scholar]
- Katiyar S, Elmets CA, and Katiyar SK. 2007. Green tea and skin cancer: photoimmunology, angiogenesis and DNA repair. J Nutr Biochem 18 (5):287–96. doi: 10.1016/j.jnutbio.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Katiyar SK 2008. Grape seed proanthocyanidines and skin cancer prevention: inhibition of oxidative stress and protection of immune system. Mol Nutr Food Res 52 Suppl 1:S71–6. doi: 10.1002/mnfr.200700198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YS, Sayers TJ, Colburn NH, Milner JA, and Young HA. 2015. Impact of dietary components on NK and Treg cell function for cancer prevention. Mol Carcinog 54 (9):669–78. doi: 10.1002/mc.22301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko JH, Sethi G, Um JY, Shanmugam MK, Arfuso F, Kumar AP, Bishayee A, and Ahn KS. 2017. The Role of Resveratrol in Cancer Therapy. Int J Mol Sci 18 (12). doi: 10.3390/ijms18122589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V, Patel S, Tcyganov E, and Gabrilovich DI. 2016. The Nature of Myeloid-Derived Suppressor Cells in the Tumor Microenvironment. Trends Immunol 37 (3):208–220. doi: 10.1016/j.it.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusmartsev S, Cheng F, Yu B, Nefedova Y, Sotomayor E, Lush R, and Gabrilovich D. 2003. All-trans-retinoic acid eliminates immature myeloid cells from tumor-bearing mice and improves the effect of vaccination. Cancer Res 63 (15):4441–9. [PubMed] [Google Scholar]
- Lazzeroni M, Guerrieri-Gonzaga A, Gandini S, Johansson H, Serrano D, Cazzaniga M, Aristarco V, Macis D, Mora S, Caldarella P, Pagani G, Pruneri G, Riva A, Petrangolini G, Morazzoni P, DeCensi A, and Bonanni B. 2017. A Presurgical Study of Lecithin Formulation of Green Tea Extract in Women with Early Breast Cancer. Cancer Prev Res (Phila) 10 (6):363–370. doi: 10.1158/1940-6207.CAPR-16-0298. [DOI] [PubMed] [Google Scholar]
- Lee-Chang C, Bodogai M, Martin-Montalvo A, Wejksza K, Sanghvi M, Moaddel R, de Cabo R, and Biragyn A. 2013. Inhibition of breast cancer metastasis by resveratrol-mediated inactivation of tumor-evoked regulatory B cells. J Immunol 191 (8):4141–51. doi: 10.4049/jimmunol.1300606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y 2017. Cancer Chemopreventive Potential of Procyanidin. Toxicol Res 33 (4):273–282. doi: 10.5487/tr.2017.33.4.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leischner C, Burkard M, Pfeiffer MM, Lauer UM, Busch C, and Venturelli S. 2016. Nutritional immunology: function of natural killer cells and their modulation by resveratrol for cancer prevention and treatment. Nutr J 15 (1):47. doi: 10.1186/s12937-016-0167-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Fan GX, Wang W, Li T, and Yuan YK. 2007. Resveratrol induces apoptosis, influences IL-6 and exerts immunomodulatory effect on mouse lymphocytic leukemia both in vitro and in vivo. Int Immunopharmacol 7 (9):1221–31. doi: 10.1016/j.intimp.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Liu D, You M, Xu Y, Li F, Zhang D, Li X, and Hou Y. 2016. Inhibition of curcumin on myeloid-derived suppressor cells is requisite for controlling lung cancer. Int Immunopharmacol 39:265–272. doi: 10.1016/j.intimp.2016.07.035. [DOI] [PubMed] [Google Scholar]
- Lu Y, Miao L, Wang Y, Xu Z, Zhao Y, Shen Y, Xiang G, and Huang L. 2016. Curcumin Micelles Remodel Tumor Microenvironment and Enhance Vaccine Activity in an Advanced Melanoma Model. Mol Ther 24 (2):364–374. doi: 10.1038/mt.2015.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo F, Song X, Zhang Y, and Chu Y. 2011. Low-dose curcumin leads to the inhibition of tumor growth via enhancing CTL-mediated antitumor immunity. Int Immunopharmacol 11 (9):1234–40. doi: 10.1016/j.intimp.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Mahammedi H, Planchat E, Pouget M, Durando X, Cure H, Guy L, Van-Praagh I, Savareux L, Atger M, Bayet-Robert M, Gadea E, Abrial C, Thivat E, Chollet P, and Eymard JC. 2016. The New Combination Docetaxel, Prednisone and Curcumin in Patients with Castration-Resistant Prostate Cancer: A Pilot Phase II Study. Oncology 90 (2):69–78. doi: 10.1159/000441148. [DOI] [PubMed] [Google Scholar]
- Malmberg KJ, Carlsten M, Bjorklund A, Sohlberg E, Bryceson YT, and Ljunggren HG. 2017. Natural killer cell-mediated immunosurveillance of human cancer. Semin Immunol 31:20–29. doi: 10.1016/j.smim.2017.08.002. [DOI] [PubMed] [Google Scholar]
- Mantena SK, Roy AM, and Katiyar SK. 2005. Epigallocatechin-3-gallate inhibits photocarcinogenesis through inhibition of angiogenic factors and activation of CD8+ T cells in tumors. Photochem Photobiol 81 (5):1174–9. doi: 10.1562/2005-04-11-RA-487. [DOI] [PubMed] [Google Scholar]
- Marcus A, Gowen BG, Thompson TW, Iannello A, Ardolino M, Deng W, Wang L, Shifrin N, and Raulet DH. 2014. Recognition of tumors by the innate immune system and natural killer cells. Adv Immunol 122:91–128. doi: 10.1016/B978-0-12-800267-4.00003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvel D, and Gabrilovich DI. 2015. Myeloid-derived suppressor cells in the tumor microenvironment: expect the unexpected. J Clin Invest 125 (9):3356–64. doi: 10.1172/JCI80005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milani A, Basirnejad M, Shahbazi S, and Bolhassani A. 2017. Carotenoids: biochemistry, pharmacology and treatment. Br J Pharmacol 174 (11):1290–1324. doi: 10.1111/bph.13625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirza N, Fishman M, Fricke I, Dunn M, Neuger AM, Frost TJ, Lush RM, Antonia S, and Gabrilovich DI. 2006. All-trans-retinoic acid improves differentiation of myeloid cells and immune response in cancer patients. Cancer Res 66 (18):9299–307. doi: 10.1158/0008-5472.CAN-06-1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed SIA, Jantan I, and Haque MA. 2017. Naturally occurring immunomodulators with antitumor activity: An insight on their mechanisms of action. Int Immunopharmacol 50:291–304. doi: 10.1016/j.intimp.2017.07.010. [DOI] [PubMed] [Google Scholar]
- Mollazadeh H, Cicero AFG, Blesso CN, Pirro M, Majeed M, and Sahebkar A. 2017. Immune modulation by curcumin: The role of interleukin-10. Crit Rev Food Sci Nutr:1–13. doi: 10.1080/10408398.2017.1358139. [DOI] [PubMed] [Google Scholar]
- Montrone M, Martorelli D, Rosato A, and Dolcetti R. 2009. Retinoids as critical modulators of immune functions: new therapeutic perspectives for old compounds. Endocr Metab Immune Disord Drug Targets 9 (2):113–31. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Baidoo JNE, Sampat S, Mancuso A, David L, Cohen LS, Zhou S, and Banerjee P. 2018. Liposomal TriCurin, A Synergistic Combination of Curcumin, Epicatechin Gallate and Resveratrol, Repolarizes Tumor-Associated Microglia/Macrophages, and Eliminates Glioblastoma (GBM) and GBM Stem Cells. Molecules 23 (1). doi: 10.3390/molecules23010201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S, Hussaini R, White R, Atwi D, Fried A, Sampat S, Piao L, Pan Q, and Banerjee P. 2018. TriCurin, a synergistic formulation of curcumin, resveratrol, and epicatechin gallate, repolarizes tumor-associated macrophages and triggers an immune response to cause suppression of HPV+ tumors. Cancer Immunol Immunother. doi: 10.1007/s00262-018-2130-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata T, Tamai H, Morinobu T, Manago M, Takenaka H, Hayashi K, and Mino M. 1994. Effect of long-term administration of beta-carotene on lymphocyte subsets in humans. Am J Clin Nutr 60 (4):597–602. [DOI] [PubMed] [Google Scholar]
- Murphy EA, Davis JM, and Carmichael MD. 2010. Immune modulating effects of beta-glucan. Curr Opin Clin Nutr Metab Care 13 (6):656–61. doi: 10.1097/MCO.0b013e32833f1afb. [DOI] [PubMed] [Google Scholar]
- Nagashima Y, Maeda N, Yamamoto S, Yoshino S, and Oka M. 2013. Evaluation of host quality of life and immune function in breast cancer patients treated with combination of adjuvant chemotherapy and oral administration of Lentinula edodes mycelia extract. Onco Targets Ther 6:853–9. doi: 10.2147/OTT.S44169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano T, Oka K, Hanba K, and Morita S. 1996. Intratumoral administration of sizofiran activates Langerhans cell and T-cell infiltration in cervical cancer. Clin Immunol Immunopathol 79 (1):79–86. [DOI] [PubMed] [Google Scholar]
- Novaes MR, Valadares F, Reis MC, Goncalves DR, and Menezes Mda C. 2011. The effects of dietary supplementation with Agaricales mushrooms and other medicinal fungi on breast cancer: evidence-based medicine. Clinics (Sao Paulo) 66 (12):2133–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noy R, and Pollard JW. 2014. Tumor-associated macrophages: from mechanisms to therapy. Immunity 41 (1):49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohwada S, Ikeya T, Yokomori T, Kusaba T, Roppongi T, Takahashi T, Nakamura S, Kakinuma S, Iwazaki S, Ishikawa H, Kawate S, Nakajima T, and Morishita Y. 2004. Adjuvant immunochemotherapy with oral Tegafur/Uracil plus PSK in patients with stage II or III colorectal cancer: a randomised controlled study. Br J Cancer 90 (5):1003–10. doi: 10.1038/sj.bjc.6601619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohwada S, Ogawa T, Makita F, Tanahashi Y, Ohya T, Tomizawa N, Satoh Y, Kobayashi I, Izumi M, Takeyoshi I, Hamada K, Minaguchi S, Togo Y, Toshihiko T, Koyama T, and Kamio M. 2006. Beneficial effects of protein-bound polysaccharide K plus tegafur/uracil in patients with stage II or III colorectal cancer: analysis of immunological parameters. Oncol Rep 15 (4):861–8. [PubMed] [Google Scholar]
- Okuno K, and Uno K. 2011. Efficacy of orally administered Lentinula edodes mycelia extract for advanced gastrointestinal cancer patients undergoing cancer chemotherapy: a pilot study. Asian Pac J Cancer Prev 12 (7):1671–4. [PubMed] [Google Scholar]
- Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA, Caldwell ME, Allard D, Frese KK, Denicola G, Feig C, Combs C, Winter SP, Ireland-Zecchini H, Reichelt S, Howat WJ, Chang A, Dhara M, Wang L, Ruckert F, Grutzmann R, Pilarsky C, Izeradjene K, Hingorani SR, Huang P, Davies SE, Plunkett W, Egorin M, Hruban RH, Whitebread N, McGovern K, Adams J, Iacobuzio-Donahue C, Griffiths J, and Tuveson DA. 2009. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 324 (5933):1457–61. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, Keogh JP, Meyskens FL, Valanis B, Williams JH, Barnhart S, and Hammar S. 1996. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med 334 (18):1150–5. doi: 10.1056/NEJM199605023341802. [DOI] [PubMed] [Google Scholar]
- Ostuni R, Kratochvill F, Murray PJ, and Natoli G. 2015. Macrophages and cancer: from mechanisms to therapeutic implications. Trends Immunol 36 (4):229–39. doi: 10.1016/j.it.2015.02.004. [DOI] [PubMed] [Google Scholar]
- Pal S, Bhattacharyya S, Choudhuri T, Datta GK, Das T, and Sa G. 2005. Amelioration of immune cell number depletion and potentiation of depressed detoxification system of tumor-bearing mice by curcumin. Cancer Detect Prev 29 (5):470–8. doi: 10.1016/j.cdp.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Paller CJ, Rudek MA, Zhou XC, Wagner WD, Hudson TS, Anders N, Hammers HJ, Dowling D, King S, Antonarakis ES, Drake CG, Eisenberger MA, Denmeade SR, Rosner GL, and Carducci MA. 2015. A phase I study of muscadine grape skin extract in men with biochemically recurrent prostate cancer: Safety, tolerability, and dose determination. Prostate 75 (14):1518–25. doi: 10.1002/pros.23024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palucka AK, and Coussens LM. 2016. The Basis of Oncoimmunology. Cell 164 (6):1233–1247. doi: 10.1016/j.cell.2016.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J, Shen J, Si W, Du C, Chen D, Xu L, Yao M, Fu P, and Fan W. 2017. Resveratrol promotes MICA/B expression and natural killer cell lysis of breast cancer cells by suppressing c-Myc/miR-17 pathway. Oncotarget 8 (39):65743–65758. doi: 10.18632/oncotarget.19445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan P, Skaer CW, Wang HT, Oshima K, Huang YW, Yu J, Zhang J, Yearsley MM, Agle KA, Drobyski WR, Chen X, and Wang LS. 2017. Loss of free fatty acid receptor 2 enhances colonic adenoma development and reduces the chemopreventive effects of black raspberries in ApcMin/+ mice. Carcinogenesis 38 (1):86–93. doi: 10.1093/carcin/bgw122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan P, Kang S, Wang Y, Liu K, Oshima K, Huang YW, Zhang J, Yearsley M, Yu J, and Wang LS. 2017. Black Raspberries Enhance Natural Killer Cell Infiltration into the Colon and Suppress the Progression of Colorectal Cancer. Front Immunol 8:997. doi: 10.3389/fimmu.2017.00997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan P, Skaer CW, Stirdivant SM, Young MR, Stoner GD, Lechner JF, Huang YW, and Wang LS. 2015. Beneficial Regulation of Metabolic Profiles by Black Raspberries in Human Colorectal Cancer Patients. Cancer Prev Res (Phila) 8 (8):743–50. doi: 10.1158/1940-6207.CAPR-15-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan P, Skaer C, Yu J, Zhao H, Ren H, Oshima K, and Wang LS. 2017. Berries and other natural products in the pancreatic cancer chemoprevention in human clinical trials. J Berry Res 7 (3):147–161. doi: 10.3233/JBR-170159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan P, Yu J, and Wang LS. 2018. Colon Cancer: What We Eat. Surg Oncol Clin N Am 27 (2):243–267. doi: 10.1016/j.soc.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panahi Y, Saadat A, Beiraghdar F, and Sahebkar A. 2014. Adjuvant therapy with bioavailability-boosted curcuminoids suppresses systemic inflammation and improves quality of life in patients with solid tumors: a randomized double-blind placebo-controlled trial. Phytother Res 28 (10):1461–7. doi: 10.1002/ptr.5149. [DOI] [PubMed] [Google Scholar]
- Panda AK, Chakraborty D, Sarkar I, Khan T, and Sa G. 2017. New insights into therapeutic activity and anticancer properties of curcumin. J Exp Pharmacol 9:31–45. doi: 10.2147/JEP.S70568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons HA, Baracos VE, Hong DS, Abbruzzese J, Bruera E, and Kurzrock R. 2016. The effects of curcumin (diferuloylmethane) on body composition of patients with advanced pancreatic cancer. Oncotarget 7 (15):20293–304. doi: 10.18632/oncotarget.7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel KR, Brown VA, Jones DJ, Britton RG, Hemingway D, Miller AS, West KP, Booth TD, Perloff M, Crowell JA, Brenner DE, Steward WP, Gescher AJ, and Brown K. 2010. Clinical pharmacology of resveratrol and its metabolites in colorectal cancer patients. Cancer Res 70 (19):7392–9. doi: 10.1158/0008-5472.CAN-10-2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell DR, and Huttenlocher A. 2016. Neutrophils in the Tumor Microenvironment. Trends Immunol 37 (1):41–52. doi: 10.1016/j.it.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian BZ, and Pollard JW. 2010. Macrophage diversity enhances tumor progression and metastasis. Cell 141 (1):39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramberg JE, Nelson ED, and Sinnott RA. 2010. Immunomodulatory dietary polysaccharides: a systematic review of the literature. Nutr J 9:54. doi: 10.1186/1475-2891-9-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rather RA, and Bhagat M. 2018. Cancer Chemoprevention and Piperine: Molecular Mechanisms and Therapeutic Opportunities. Front Cell Dev Biol 6:10. doi: 10.3389/fcell.2018.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauf A, Imran M, Suleria HAR, Ahmad B, Peters DG, and Mubarak MS. 2017. A comprehensive review of the health perspectives of resveratrol. Food Funct 8 (12):4284–4305. doi: 10.1039/c7fo01300k. [DOI] [PubMed] [Google Scholar]
- Recchia F, Cesta A, and Rea S. 2003. Low dose interleukin-2 and 13-cis-retinoic acid as maintenance therapy in patients with solid tumors responsive to chemotherapy. J Exp Clin Cancer Res 22 (4 Suppl):135–43. [PubMed] [Google Scholar]
- Recchia F, De Filippis S, Rosselli M, Saggio G, Cesta A, Fumagalli L, and Rea S. 2001. Phase 1B study of subcutaneously administered interleukin 2 in combination with 13-cis retinoic acid as maintenance therapy in advanced cancer. Clin Cancer Res 7 (5):1251–7. [PubMed] [Google Scholar]
- Recchia F, Saggio G, Cesta A, Candeloro G, Nuzzo A, Lombardo M, Carta G, and Rea S. 2005. Interleukin-2 and 13-cis retinoic acid as maintenance therapy in advanced ovarian cancer. Int J Oncol 27 (4):1039–46. [PubMed] [Google Scholar]
- Recchia F, Saggio G, Nuzzo A, Biondi E, Di Blasio A, Cesta A, Candeloro G, Alesse E, and Rea S. 2006. Multicenter phase 2 study of interleukin-2 and 13-cis retinoic acid as maintenance therapy in advanced non-small-cell lung cancer. J Immunother 29 (1):87–94. [DOI] [PubMed] [Google Scholar]
- Ribas A 2015. Adaptive Immune Resistance: How Cancer Protects from Immune Attack. Cancer Discov 5 (9):915–9. doi: 10.1158/2159-8290.CD-15-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roudi R, Mohammadi SR, Roudbary M, and Mohsenzadegan M. 2017. Lung cancer and beta-glucans: review of potential therapeutic applications. Invest New Drugs 35 (4):509–517. doi: 10.1007/s10637-017-0449-9. [DOI] [PubMed] [Google Scholar]
- Roupas Peter, Keogh Jennifer, Noakes Manny, Margetts Christine, and Taylor Pennie. 2012. The role of edible mushrooms in health: Evaluation of the evidence. Journal of Functional Foods 4 (4):687–709. doi: 10.1016/j.jff.2012.05.003. [DOI] [Google Scholar]
- Ruffell B, and Coussens LM. 2015. Macrophages and therapeutic resistance in cancer. Cancer Cell 27 (4):462–72. doi: 10.1016/j.ccell.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santilli G, Piotrowska I, Cantilena S, Chayka O, D’Alicarnasso M, Morgenstern DA, Himoudi N, Pearson K, Anderson J, Thrasher AJ, and Sala A. 2013. Polyphenon [corrected] E enhances the antitumor immune response in neuroblastoma by inactivating myeloid suppressor cells. Clin Cancer Res 19 (5):1116–25. doi: 10.1158/1078-0432.CCR-12-2528. [DOI] [PubMed] [Google Scholar]
- Santos MS, Leka LS, Ribaya-Mercado JD, Russell RM, Meydani M, Hennekens CH, Gaziano JM, and Meydani SN. 1997. Short- and long-term beta-carotene supplementation do not influence T cell-mediated immunity in healthy elderly persons. Am J Clin Nutr 66 (4):917–24. [DOI] [PubMed] [Google Scholar]
- Santos MS, Meydani SN, Leka L, Wu D, Fotouhi N, Meydani M, Hennekens CH, and Gaziano JM. 1996. Natural killer cell activity in elderly men is enhanced by beta-carotene supplementation. Am J Clin Nutr 64 (5):772–7. [DOI] [PubMed] [Google Scholar]
- Serna-Thome G, Castro-Eguiluz D, Fuchs-Tarlovsky V, Sanchez-Lopez M, Delgado-Olivares L, Coronel-Martinez J, Molina-Trinidad EM, de la Torre M, and Cetina-Perez L. 2018. Use of Functional Foods and Oral Supplements as Adjuvants in Cancer Treatment. Rev Invest Clin 70 (3):136–146. doi: 10.24875/ric.18002527. [DOI] [PubMed] [Google Scholar]
- Shanafelt TD, Call TG, Zent CS, LaPlant B, Bowen DA, Roos M, Secreto CR, Ghosh AK, Kabat BF, Lee MJ, Yang CS, Jelinek DF, Erlichman C, and Kay NE. 2009. Phase I trial of daily oral Polyphenon E in patients with asymptomatic Rai stage 0 to II chronic lymphocytic leukemia. J Clin Oncol 27 (23):3808–14. doi: 10.1200/JCO.2008.21.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanafelt TD, Call TG, Zent CS, Leis JF, LaPlant B, Bowen DA, Roos M, Laumann K, Ghosh AK, Lesnick C, Lee MJ, Yang CS, Jelinek DF, Erlichman C, and Kay NE. 2013. Phase 2 trial of daily, oral Polyphenon E in patients with asymptomatic, Rai stage 0 to II chronic lymphocytic leukemia. Cancer 119 (2):363–70. doi: 10.1002/cncr.27719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Y, Zhu W, Da J, Xu M, Wang Y, Zhou J, and Wang Z. 2017. Bisdemethoxycurcumin in combination with alpha-PD-L1 antibody boosts immune response against bladder cancer. Onco Targets Ther 10:2675–2683. doi: 10.2147/OTT.S130653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard HM, Lewis GD, Sarup JC, Fendly BM, Maneval D, Mordenti J, Figari I, Kotts CE, Palladino MA Jr., Ullrich A, and et al. 1991. Monoclonal antibody therapy of human cancer: taking the HER2 protooncogene to the clinic. J Clin Immunol 11 (3):117–27. [DOI] [PubMed] [Google Scholar]
- Shirakami Y, Shimizu M, and Moriwaki H. 2012. Cancer chemoprevention with green tea catechins: from bench to bed. Curr Drug Targets 13 (14):1842–57. [DOI] [PubMed] [Google Scholar]
- Shiri S, Alizadeh AM, Baradaran B, Farhanghi B, Shanehbandi D, Khodayari S, Khodayari H, and Tavassoli A. 2015. Dendrosomal curcumin suppresses metastatic breast cancer in mice by changing m1/m2 macrophage balance in the tumor microenvironment. Asian Pac J Cancer Prev 16 (9):3917–22. [DOI] [PubMed] [Google Scholar]
- Singh M, Ramos I, Asafu-Adjei D, Quispe-Tintaya W, Chandra D, Jahangir A, Zang X, Aggarwal BB, and Gravekamp C. 2013. Curcumin improves the therapeutic efficacy of Listeria(at)-Mage-b vaccine in correlation with improved T-cell responses in blood of a triple-negative breast cancer model 4T1. Cancer Med 2 (4):571–82. doi: 10.1002/cam4.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sionov RV, Fridlender ZG, and Granot Z. 2015. The Multifaceted Roles Neutrophils Play in the Tumor Microenvironment. Cancer Microenviron 8 (3):125–58. doi: 10.1007/s12307-014-0147-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer A, and Vyas KS. 2012. A global clinical view on vitamin A and carotenoids. Am J Clin Nutr 96 (5):1204S–6S. doi: 10.3945/ajcn.112.034868. [DOI] [PubMed] [Google Scholar]
- Song X, Ye D, Liu B, Cui J, Zhao X, Yi L, Liang J, Song J, Zhang Z, and Zhao Q. 2009. Combination of all-trans retinoic acid and a human papillomavirus therapeutic vaccine suppresses the number and function of immature myeloid cells and enhances antitumor immunity. Cancer Sci 100 (2):334–40. doi: 10.1111/j.1349-7006.2008.01037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto BL, Hank JA, Darjatmoko SR, Polans AS, Yanke EM, Rakhmilevich AL, Seo S, Kim K, Reisfeld RA, Gillies SD, and Sondel PM. 2011. Anti-tumor and immunomodulatory activity of resveratrol in vitro and its potential for combining with cancer immunotherapy. Int Immunopharmacol 11 (11):1877–86. doi: 10.1016/j.intimp.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava RM, Singh S, Dubey SK, Misra K, and Khar A. 2011. Immunomodulatory and therapeutic activity of curcumin. Int Immunopharmacol 11 (3):331–41. doi: 10.1016/j.intimp.2010.08.014. [DOI] [PubMed] [Google Scholar]
- Standish LJ, Wenner CA, Sweet ES, Bridge C, Nelson A, Martzen M, Novack J, and Torkelson C. 2008. Trametes versicolor mushroom immune therapy in breast cancer. J Soc Integr Oncol 6 (3):122–8. [PMC free article] [PubMed] [Google Scholar]
- Sultan MT, Butt MS, Qayyum MM, and Suleria HA. 2014. Immunity: plants as effective mediators. Crit Rev Food Sci Nutr 54 (10):1298–308. doi: 10.1080/10408398.2011.633249. [DOI] [PubMed] [Google Scholar]
- Tallman MS, Andersen JW, Schiffer CA, Appelbaum FR, Feusner JH, Ogden A, Shepherd L, Willman C, Bloomfield CD, Rowe JM, and Wiernik PH. 1997. All-trans-retinoic acid in acute promyelocytic leukemia. N Engl J Med 337 (15):1021–8. doi: 10.1056/NEJM199710093371501. [DOI] [PubMed] [Google Scholar]
- Tallman MS, Andersen JW, Schiffer CA, Appelbaum FR, Feusner JH, Woods WG, Ogden A, Weinstein H, Shepherd L, Willman C, Bloomfield CD, Rowe JM, and Wiernik PH. 2002. All-trans retinoic acid in acute promyelocytic leukemia: long-term outcome and prognostic factor analysis from the North American Intergroup protocol. Blood 100 (13):4298–302. doi: 10.1182/blood-2002-02-0632. [DOI] [PubMed] [Google Scholar]
- Tcyganov E, Mastio J, Chen E, and Gabrilovich DI. 2018. Plasticity of myeloid-derived suppressor cells in cancer. Curr Opin Immunol 51:76–82. doi: 10.1016/j.coi.2018.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thommen DS, and Schumacher TN. 2018. T Cell Dysfunction in Cancer. Cancer Cell 33 (4):547–562. doi: 10.1016/j.ccell.2018.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong H, Song X, Sun X, Sun G, and Du F. 2011. Immunomodulatory and antitumor activities of grape seed proanthocyanidins. J Agric Food Chem 59 (21):11543–7. doi: 10.1021/jf203170k. [DOI] [PubMed] [Google Scholar]
- Udenigwe CC, Ramprasath VR, Aluko RE, and Jones PJ. 2008. Potential of resveratrol in anticancer and anti-inflammatory therapy. Nutr Rev 66 (8):445–54. doi: 10.1111/j.1753-4887.2008.00076.x. [DOI] [PubMed] [Google Scholar]
- van Die MD, Williams SG, Emery J, Bone KM, Taylor JM, Lusk E, and Pirotta MV. 2017. A Placebo-Controlled Double-Blinded Randomized Pilot Study of Combination Phytotherapy in Biochemically Recurrent Prostate Cancer. Prostate 77 (7):765–775. doi: 10.1002/pros.23317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varoni EM, Lo Faro AF, Sharifi-Rad J, and Iriti M. 2016. Anticancer Molecular Mechanisms of Resveratrol. Front Nutr 3:8. doi: 10.3389/fnut.2016.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veglia F, and Gabrilovich DI. 2017. Dendritic cells in cancer: the role revisited. Curr Opin Immunol 45:43–51. doi: 10.1016/j.coi.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasser SP 2017. Medicinal Mushrooms in Human Clinical Studies. Part I. Anticancer, Oncoimmunological, and Immunomodulatory Activities: A Review. Int J Med Mushrooms 19 (4):279–317. doi: 10.1615/IntJMedMushrooms.v19.i4.10. [DOI] [PubMed] [Google Scholar]
- Watkins ML, Erickson JD, Thun MJ, Mulinare J, and Heath CW Jr. 2000. Multivitamin use and mortality in a large prospective study. Am J Epidemiol 152 (2):149–62. [DOI] [PubMed] [Google Scholar]
- Watson RR, Prabhala RH, Plezia PM, and Alberts DS. 1991. Effect of beta-carotene on lymphocyte subpopulations in elderly humans: evidence for a dose-response relationship. Am J Clin Nutr 53 (1):90–4. [DOI] [PubMed] [Google Scholar]
- Woo SR, Corrales L, and Gajewski TF. 2015. Innate immune recognition of cancer. Annu Rev Immunol 33:445–74. doi: 10.1146/annurev-immunol-032414-112043. [DOI] [PubMed] [Google Scholar]
- Xu B, Yu L, and Zhao LZ. 2017. Curcumin up regulates T helper 1 cells in patients with colon cancer. Am J Transl Res 9 (4):1866–1875. [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y, Miyahara E, and Hihara J. 2011. Efficacy and safety of orally administered Lentinula edodes mycelia extract for patients undergoing cancer chemotherapy: a pilot study. Am J Chin Med 39 (3):451–9. doi: 10.1142/S0192415X11008956. [DOI] [PubMed] [Google Scholar]
- Yang Y, Paik JH, Cho D, Cho JA, and Kim CW. 2008. Resveratrol induces the suppression of tumor-derived CD4+CD25+ regulatory T cells. Int Immunopharmacol 8 (4):542–7. doi: 10.1016/j.intimp.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Zang S, Liu T, Shi J, and Qiao L. 2014. Curcumin: a promising agent targeting cancer stem cells. Anticancer Agents Med Chem 14 (6):787–92. [DOI] [PubMed] [Google Scholar]
- Zhang C, and Duvic M. 2003. Retinoids: therapeutic applications and mechanisms of action in cutaneous T-cell lymphoma. Dermatol Ther 16 (4):322–30. [DOI] [PubMed] [Google Scholar]
- Zhang C, and Duvic M. 2006. Treatment of cutaneous T-cell lymphoma with retinoids. Dermatol Ther 19 (5):264–71. doi: 10.1111/j.1529-8019.2006.00083.x. [DOI] [PubMed] [Google Scholar]
- Zhang XY, Li WG, Wu YJ, and Gao MT. 2005. Amelioration of doxorubicin-induced myocardial oxidative stress and immunosuppression by grape seed proanthocyanidins in tumour-bearing mice. J Pharm Pharmacol 57 (8):1043–52. doi: 10.1211/0022357056523. [DOI] [PubMed] [Google Scholar]
- Zhao H, Zhu W, Jia L, Sun X, Chen G, Zhao X, Li X, Meng X, Kong L, Xing L, and Yu J. 2016. Phase I study of topical epigallocatechin-3-gallate (EGCG) in patients with breast cancer receiving adjuvant radiotherapy. Br J Radiol 89 (1058):20150665. doi: 10.1259/bjr.20150665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Zhu W, Xie P, Li H, Zhang X, Sun X, Yu J, and Xing L. 2014. A phase I study of concurrent chemotherapy and thoracic radiotherapy with oral epigallocatechin-3-gallate protection in patients with locally advanced stage III non-small-cell lung cancer. Radiother Oncol 110 (1):132–6. doi: 10.1016/j.radonc.2013.10.014. [DOI] [PubMed] [Google Scholar]
- Zheng YY, Viswanathan B, Kesarwani P, and Mehrotra S. 2012. Dietary agents in cancer prevention: an immunological perspective. Photochem Photobiol 88 (5):1083–98. doi: 10.1111/j.1751-1097.2012.01128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler RG, Mayne ST, and Swanson CA. 1996. Nutrition and lung cancer. Cancer Causes Control 7 (1):157–77. [DOI] [PubMed] [Google Scholar]
- Zou JY, Su CH, Luo HH, Lei YY, Zeng B, Zhu HS, and Chen ZG. 2018. Curcumin converts Foxp3+ regulatory T cells to T helper 1 cells in patients with lung cancer. J Cell Biochem 119 (2):1420–1428. doi: 10.1002/jcb.26302. [DOI] [PubMed] [Google Scholar]