Abstract
Background.
Posterior fossa ependymoma (PFE) comprises two groups, PFA and PFB with stark differences in outcome. However, the long-term outcomes of PFA ependymoma have not been fully described. We aimed to identify predictors of survival and neurocognitive outcome in a large consecutive cohort of subgrouped PFE over three decades.
Methods.
Demographic, survival and neurocognitive data was collected from consecutive patients diagnosed with PFE from 1985–2014 at the Hospital for Sick Children. Subgroup was assigned using genome wide methylation array and/or immunoreactivity to H3K27me3.
Results.
Seventy-two PFE were identified, of which 89% were PFA. There were no relapses amongst PFB. Ten-year PFS of all PFA was poor at 37.1% (95% CI, 25.9 to 53.1%). Analysis of consecutive 10-year epochs revealed significant improvement in PFS/OS over time. This pertains to the GTR rate increasing from 35% to 77% and use of upfront radiation increasing from 65% to 96% over the observed period and confirmed in a multivariable model. Analysis of longitudinal neuropsychological outcomes restricted to PFA patients treated with focal irradiation, applying a mixed linear model shows significant continuous declines in full scale IQ over time with upfront conformal radiation even when correcting for hydrocephalus, number of surgeries and age at diagnosis (−1.33 ± 0.42 points/year, p=0.0042).
Conclusions.
Data from a molecularly informed large cohort of PFE clearly indicate improved survival over time, related to more aggressive surgery and upfront radiation. However, for the first time in a subgrouped cohort, we show that this approach results in reduced neurocognitive outcomes over time.
Keywords: Ependymoma, molecular subgroup, survival, neurocognitive outcome
Precis:
In a subgroup specific manner, we show that survival of posterior fossa ependymoma has improved over time, and this is clearly related to a change in practice to pursue aggressive surgical resections with upfront postoperative radiation in all patients. This significant improvement highlighting an urgent need for implementation of early intervention and neuroprotective strategies.
Introduction
Ependymoma is the third most common malignancy of the posterior fossa and can arise at any age, albeit with a bimodal age distribution with peaks in infancy and mid 30’s.1 Current therapies are essentially based on surgery and external beam irradiation in children over the age of 1, resulting in 10-year survival rates between 50–60%2, 3. Indeed, this represents a major shift over the past decade, where previously most children under 3 were treated with radiation-sparing approaches and only recently has conformal radiation to the tumor bed become the standard of care in North America.3 Recently it has been shown that childhood posterior fossa ependymoma is not a single entity rather is comprised of at least two distinct molecular variants, Group A (PFA) and Group B (PFB) with distinct epigenetics, demographics and outcomes.4 Whereby PFA is strongly enriched in infants, PFB is prevalent in adults and with a near equal distribution in adolescence.5–9 A recent retrospective study across 820 posterior fossa ependymomas has shown that molecular subgroup is the most powerful predictor of outcome, and even within PFA, upfront postsurgical external beam irradiation and a complete resection are strong predictors of good outcome.2 Although radiation was uniform in the majority of cases in this study, chemotherapy regimens were not, including infants treated with delayed radiation prior to progression.
Several questions persist with respect to optimal treatment of PFE of childhood. First, the impact of molecular subgroup has not been evaluated in a prospectively-followed, molecularly profiled cohort. Indeed, in Europe, chemotherapy remains a mainstay in the treatment for posterior fossa ependymoma.10, 11 Although the on-going studies from SIOPe and COG are evaluating upfront radiation with maintenance chemotherapy closely following recently closed studies from the Children’s Oncology Group (ACNS0121), there is still a reluctance to radiate children under the age of 3. Although previously suggested that upfront irradiation across ependymomas of all locations does not significantly impact 5-year neurocognitive outcomes, long-term neurocognitive outcomes in the context of molecular subgroup are lacking.12, 13 As such, knowledge of the long-term functional outcomes in PFA ependymoma would significantly improve our ability to rationally risk-stratify patients, but also prioritize children for early intervention and neuroprotective strategies.
Our centre is the only primary tertiary care referral centre for children with brain tumours under the age of 18 in Southern Ontario representing a population of approximately eight million. As such we sought to determine the subgroup specific survival and neurocognitive outcome across a cohort of consecutive patients with posterior fossa ependymoma.
Methods
Patient cohort
After obtaining approval from the Hospital for Sick Children Research Ethics Board, seventy-two consecutive patients with posterior fossa ependymoma diagnosed and treated at the Hospital for Sick Children between 1985 and 2014 were included in this study. Both frozen and formalin fixed paraffin embedded (FFPE) samples were collected with informed consent from the parents/guardians or a waiver of consent where applicable under approval of the Hospital for Sick Children Research Ethics Board. Criteria for inclusion were a histological diagnosis of Grade II–III ependymoma, and location within the posterior fossa. Subtotal resection was defined as more than 5mm postoperative residual on a post-operative MRI or post-operative contrast enhanced CT scan as per the guidelines of the Children’s Oncology Group. Clinical variables pertaining to treatment and survival were collected blinded to molecular subgroup. After 2004, all patients over age 1 were treated as per ACNS0121 (NCT00027846) or after 2010 ACNS0831 (NCT01096368). ACNS0121 administered upfront radiation to all completely resected patients, 54 to those under 18 months and 59.4Gy to those above 18 months, and 2 cycles of chemotherapy prior to radiation for incompletely resected patients. ACNS0831 is the ongoing Children’s Oncology Group study randomizing maintenance chemotherapy after upfront radiation. Prior to 2004, patients over age 3 were treated using 3D conformal radiation to the tumor bed and those under age 3 were treated using chemotherapy only protocols, most commonly BabyPOG.14, 15 Only one patient was treated with proton beam irradiation.
Subgroup assignment
Subgroup was assigned using a combination of genome-wide methylation arrays and H3K27me3 immunoreactivity. 31 samples profiled by methylation were analysed on the Illumina Infinium HumanMethylation450 BeadChip at the Princess Margaret Cancer Centre-Ontario Institute for Cancer Research Translational Genomics Laboratory and, subgroups were assigned using the Molecular Neuropathology 2.0 algorithm.6, 16 HK27me3 immunostaining was performed in 54 cases with limited tissue availability as previously described (07–449, Millipore, 0.1ug/mL).17 In 13 overlapping cases between H3K27me3 and methylation profiling, no discrepancies were observed. Three cases could not be subgrouped due to weak staining, and a lack of sufficient tissue for methylation profiling.
Neuropsychological Testing
Neuropsychological testing was performed at various longitudinal timepoints. Our strategy to the testing is to perform the first test 9 months from radiation therapy and subsequently every two years, with the exception of young children where testing was delayed until they were capable of performing the preschool version of the test. There is variability in both the number of times patients in our sample were assessed and the number of years over which they were assessed. Due to the longitudinal nature of this study, several different Wechsler Intelligence test versions were used. Test versions used included the: Wechsler Intelligence Scale for Children: WISC-III, WISC-IV, WISC-V, Wechsler Preschool and Primary Scale of Intelligence: (WPPSI-R, WPPSI-III, WPPSI-IV), and Wechsler Adult Intelligence Scale: (WAIS-III, WAIS-IV). The Full Scale Intelligence Quotient (FSIQ) is a reliable measure of overall cognitive functioning, the Verbal Comprehension Index (VCI) measures verbal reasoning, the Perceptual Reasoning Index (PRI) evaluates interpretation/organization of visual/non-verbal information, Working Memory Index (WMI) measures attentional and concentration abilities, the Processing Speed Index (PSI) evaluates the speed of graphomotor and mental processing. Only patients who received focal radiation were included in this analysis, patients who received craniospinal irradiation either upfront or at recurrence were excluded.
Statistical analysis
Progression-free survival (PFS) and overall survival (OS) were analysed by the Kaplan-Meier method and p-values reported using the log-rank test. Administrative censoring was employed due to the longer follow-up times of older patients. Survival data are presented as survival estimates including 95% confidence intervals. Associations between covariates and risk groups were tested by the Fisher’s exact test. Univariable and multivariable Cox proportional hazard regression was used to estimate hazard ratios including 95% confidence intervals. All p-values reported are two-sided. All statistical analyses were performed in the R statistical software (v3.4.2), using R packages survival (v2.40–1), rms (5.1–0), Coxphf (v1.12) and ggplot2 (v2.2.1).
Linear mixed models were used to evaluate change in intellectual outcome from time since diagnosis. First, an uncorrected model was conducted with time since diagnosis as a fixed effect, and patient and number of assessments as random effects. Models were generated for FSIQ, VCI, PRI, PSI and WMI. The intercept produced by the model estimates group functioning at the beginning of the modelled time period, which is shortly after surgery in our cohort. Several risk factors for reduced neurocognitive functional are known in children undergoing radiation, including hydrocephalus, age at diagnosis, the number of surgical resections, and mutism.18 Hence, we conducted a second set of linear mixed models where we included presence of hydrocephalus, age at diagnosis, and number of surgeries as fixed effects in addition to time since diagnosis to control for these variables. Although mutism is known to have an impact on outcomes, it was observed for only 13% of patients in our sample, with only two patients having multiple assessments. As such, we elected not to include this variable in our corrected models. All analyses were performed in the R statistical software (v3.4.2).
Results
Demographics and Patient Cohort
Between 1985 and 2014, 72 children under the age of 17 with newly diagnosed posterior fossa ependymomas were identified as being treated at the Hospital for Sick Children. In 69 of these patients, sufficient material was available for molecular subgrouping into PFA and PFB using either genome wide methylation arrays, or in those cases with limited material H3K27me3 immunoreactivity. The concordance between both methods was 100% as previously described. The overall composition of the cohort is described in Table 1. The distribution of molecular subgroup, age and gender were consistent with previous reports. Specifically, PFB ependymoma comprised 9 of 69 known subgrouped cases, and were primarily older cases. In 3 cases, IHC was equivocal with insufficient tissue for methylation array testing.
Table 1:
Demographics of the entire cohort
| Variable | All (n=72) | PFA (n=60) | PFB (n=9) | P value PFA vs PFB |
|||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Age, mean | 4.94 | 3.62 | 12.44 | <0.0001 | |||
| Age, range | 0.43–17.68 | 0.43–12.88 | 4.39–17.68 | ||||
| Sex, M | 37 | 51.4 | 31 | 51.7 | 4 | 44.4 | 0.73 |
| Hydrocephalus at Dx | 59 | 81.9 | 50 | 83.3 | 7 | 77.8 | 1 |
| Hydrocephalus, VPS | 18 | 25 | 15 | 25 | 2 | 22.2 | 1 |
| GTR | 41 | 56.9 | 35 | 58.3 | 5 | 55.6 | 1 |
| Upfront M+ disease | 6 | 8.3 | 6 | 10 | 0 | 1 | |
| M1 | 4 | 5.6 | 4 | 6.7 | |||
| M2/3 | 2 | 2.8 | 2 | 3.3 | |||
| Upfront RADS | 59 | 81.9 | 48 | 80 | 8 | 88.9 | 1 |
| Focal 59–59.4 Gy | 26 | 36.1 | 19 | 31.7 | 6 | 66.7 | |
| Focal 54–55.8Gy | 20 | 27.8 | 18 | 30 | 0 | 0 | |
| Focal 50Gy | 2 | 2.8 | 2 | 3.3 | 0 | 0 | |
| CSI (23.4–36Gy) | 11 | 15.3 | 9 | 15 | 2 | 22.2 | |
| No Rads | 13 | 18.1 | 12 | 20 | 2 | 22.2 | |
| Upfront Chemotherapy + Radiation | 28 | 38.9 | 24 | 40 | 2 | 22.2 | 0.47 |
| ≥2 Surgeries at Dx | 12 | 16.7 | 11 | 18.3 | 1 | 11.1 | 1 |
| Recurrence | 38 | 52.8 | 36 | 60 | 0 | 0 | 0.0102 |
| Deceased | 25 | 34.7 | 23 | 38.3 | 0 | 0 | 0.0826 |
Legend: VPS - ventriculoperitoneal shunt, CSI – Craniospinal irradiation, all were prior to 1990, GTR – gross total resection, Dx - diagnosis
Long-term survival of posterior fossa ependymoma
The median follow-up time of our cohort was 5.54 years (95% CI 1.38–16.83). Five and ten-year progression-free survival and overall survival across the entire cohort was 48.7% (95% CI, 38.1 to 62.1%) and 42.1% (95% CI, 30.7 to 57.9%) for PFS and 72.4% (95% CI, 62.5 to 83.9%) and 58.1% (95% CI, 46.1 to 73.3%) for OS. When stratifying progression-free survival in a subgroup specific manner survival specifically for PFA was 42.9% (95% CI, 31.9 to 57.6%) at 5 years and 37.1% (95% CI, 25.9 to 53.1%) at 10 years. No failures were observed amongst any PFB patients (Supplemental Figure 1). Five-year overall survival for PFA only was 67.6% (95% CI 56.5–80.8%) and ten-year survival was 57.7% (95% CI 45.4–73.2%).
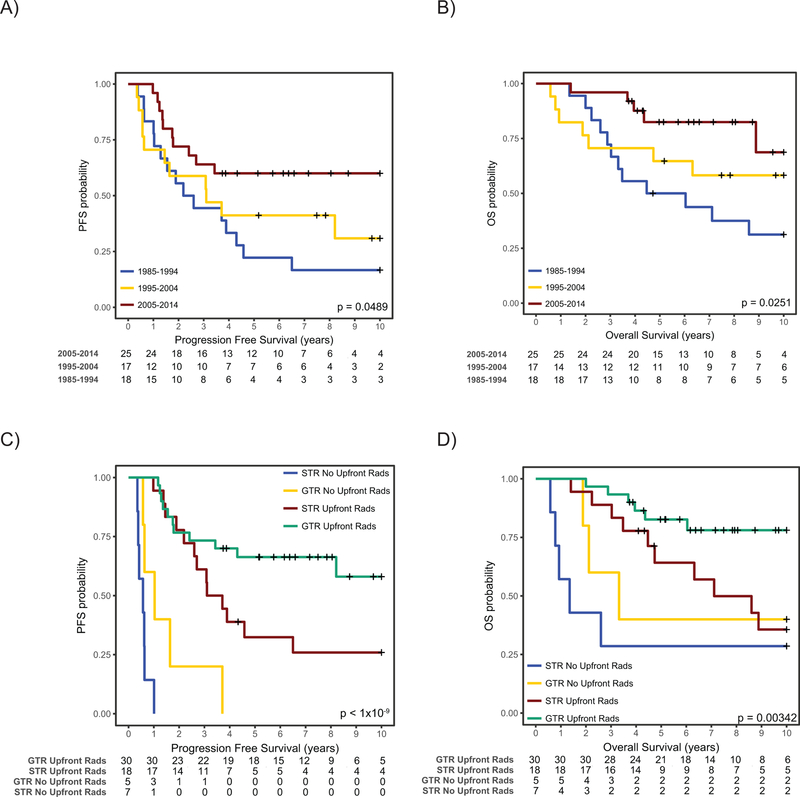
In order to discern the pattern of survival over time of posterior fossa ependymoma, we then divided our cohort into three 10-year epochs, specifically 1985–1994, 1995–2004 and 2005–2014. Strikingly, both progression free survival and overall survival were significantly better at the third epoch of 2005–2015 compared to patients treated previously. In particular, 10-year PFS was 22.5% (95% CI, 9.3 to 54.1%) for epoch 1985–1994, 30.8% (95% CI, 14.2 to 66.6%) for 1995–2004 and 67.7 (95% CI, 53.1 to 86.4%) for 2005–2014 (p= 0.021). Similarly, 10-year OS was 34.9% (95% CI, 18.1 to 67.3) for epoch 1985–1994, 53.1% (95% CI, 34.5 to 81.6%) for 1995–2004 and 85.1% (95% CI, 72.5 to 99.9%) for 2005–2014 (p= 0.009). Similar patterns were observed when restricting the analysis only to PFA patients suggestive that even when correcting for molecular subgroup, survival is clearly improving over time (Figure 1A, 1B).
Figure 1:
Survival of PFA ependymoma over Time. A) Progression-Free and B) Overall Survival of all PFA ependymomas stratified by epochs. C) Progression-Free and D) Overall survival of all PFA ependymoma stratified by extent of surgical resection and administration of upfront radiation. P-values determined using the log-rank test.
In 2004, a more aggressive surgical approach was adopted at both diagnosis and recurrence, along with more frequent upfront radiation, and re-irradiation. The rate of GTR increased over three epochs from 36% through 52% to 77% in 2004–2015. Similarly, rate of upfront radiation increased from 65% through 76% to 96% during three subsequent 10-year epochs. To determine if more aggressive surgery and radiation accounted for this increased survival, we performed a multivariable Cox regression analysis integrating extent of surgical resection, epoch of therapy, grade, molecular subgroup, upfront radiation and age at diagnosis. When applying this model, omission of upfront radiation was the strongest predictor of poor outcome followed by extent of resection (Figure 1C, 1D; Supplemental Figure 2, Table 2, Upfront XRT: HR 28.33 95% CI 8.4258–95.240; Subtotal Resection: HR 3.8 95% CI 1.6465–8.771). PFA ependymomas treated with upfront radiation-sparing approaches all progressed rapidly with a median time to progression of 0.62 years (IQR: 0.52–1.02). Histological grade, epoch of treatment and age at diagnosis were not significant in this multivariable model when accounting for molecular subgroup (Table 2, Supplemental Figure 2). When restricting the analysis to PFA tumors only, an identical pattern emerges, with upfront radiation being a very strong predictor of improved outcome (Table 2). Overall, eleven patients received upfront craniospinal irradiation at doses between 23.4 and 36Gy, the remainder of patients received focal irradiation to the tumour bed. The patients who received upfront craniospinal irradiation were all treated prior to 1995 and only 2 had upfront metastatic disease. The rationale behind administering craniospinal irradiation in the remainder is unclear.
Table 2:
Multivariable Analysis of 10 year progression free and overall survival across PFA ependymoma (n=60)
| Progression Free Survival | ||||
|---|---|---|---|---|
| p-value | HR | Lower CI 95% | Upper CI 95% | |
| Age | 0.19 | 1.101 | 0.9534 | 1.27 |
| Incomplete Resection | 0.00599 ** | 3.216 | 1.398 | 7.398 |
| 1995–2004 vs 2005–2014 | 0.31 | 0.576 | 0.1988 | 1.67 |
| 1985–1994 vs 2005–2014 | 0.9435 | 1.038 | 0.3665 | 2.942 |
| Grade 3 vs 2 Histology | 0.67 | 1.188 | 0.537 | 2.628 |
| Upfront Radiation | 4.2e-08 *** | 30.72 | 9.029 | 104.5 |
| Female Gender | 0.538 | 1.264 | 0.599 | 2.665 |
| Overall Survival | ||||
| p-value | HR | Lower CI 95% | Upper CI 95% | |
| Age | 0.51 | 0.94 | 0.7826 | 1.129 |
| Incomplete Resection | 0.273 | 1.665 | 0.6688 | 4.147 |
| 1995–2004 vs 2005–2014 | 0.2524 | 2.204 | 0.4536 | 8.535 |
| 1985–1994 vs 2005–2014 | 0.0493* | 3.774 | 1.004 | 14.191 |
| Grade 3 vs 2 Histology | 0.198 | 1.909 | 0.713 | 5.116 |
| Upfront Radiation | 0.192 | 1.956 | 0.714 | 5.356 |
| Female Gender | 0.914 | 1.049 | 0.443 | 2.485 |
Growth Hormone Deficiency
In light of previous reports that growth hormone deficiency after conformal radiation is highly dependent on the dose to the pituitary, we evaluated the effect to the pituitary gland. Surprisingly, we observe that in patients who are progression free at two years and only receive focal irradiation, 39% had growth hormone deficiency. There was a clear trend to younger age being a risk factor for growth hormone deficiency after focal irradiation (p=0.024, Supplemental Figure 3).
Long-term neurocognitive outcomes in PFA ependymoma
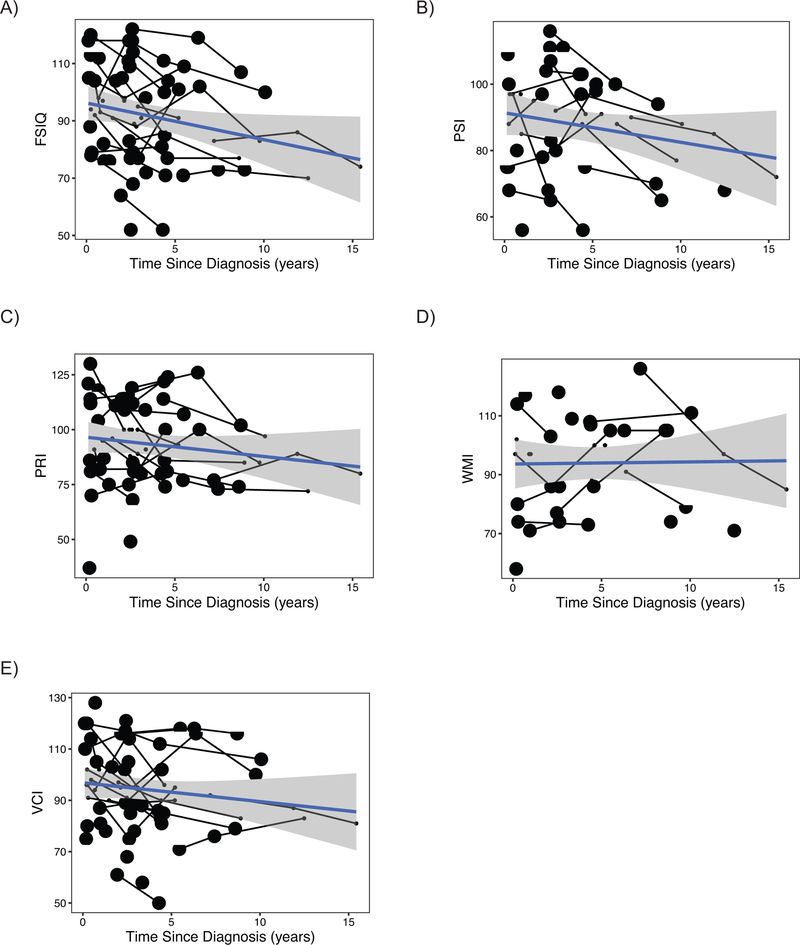
In order to discern the impact of radiation on neurocognitive outcomes in PFA ependymoma, we first calculated the decline in intellectual function with respect to time since diagnosis. Only patients with PFA ependymoma treated with conformal radiation upfront were included in these analyses, and in those patients who relapsed, time points after salvage craniospinal re-irradiation were excluded. Strikingly, before correcting for potential confounding variables, the FSIQ slope showed significant decline over time, with average declines of 1.33 (±0.42) IQ points per year (p=0.004; Table 3). Individual patient trajectories show that the majority of patients displayed declines in FSIQ over time. (Figure 2). Four patients with PFB ependymoma had neurocognitive testing, of which only two had multiple assessments, at intervals of 1 and 2.4 years. Neither of the two PFB patients had any decline in FSIQ.
Table 3:
Estimated Intercepts and Slopes for change in IQ indices (uncorrected model including time since diagnosis as a fixed effect and patient and number of assessments as random effects).
| Intercept | Slope | ||||||
|---|---|---|---|---|---|---|---|
| IQ Indices | No. Patients | Estimate | SE | Estimate | SE | t-value | p-value |
| Full-scale IQ | 34 | 94.88 | 2.97 | −1.33 | 0.42 | −3.17 | 0.004** |
| Perceptual reasoning | 33 | 94.36 | 3.63 | −0.73 | 0.55 | −1.31 | 0.20 |
| Processing speed | 26 | 93.28 | 3.48 | −1.34 | 0.58 | −2.29 | 0.03* |
| Verbal comprehension | 34 | 94.47 | 3.02 | −0.69 | 0.45 | −1.53 | 0.13 |
| Working memory | 24 | 96.60 | 4.31 | −0.56 | 0.68 | −0.82 | 0.43 |
Figure 2:
Declines in neurocognitive status over time in PFA ependymoma. Estimated declines in (A) Full Scale Intelligence Quotient (IQ) score over time for PFA ependymoma patients receiving focal radiation (B) Processing Speed Index, (C) Perceptual Reasoning/Organization Index, (D) Working Memory/Freedom From Distractibility Index, and (E) Verbal Comprehension in linear-term models corrected for hydrocephalus, number of surgeries and age at diagnosis. Lines represent patients seen for longitudinal intellectual assessments; each square represents patient seen once.
The decline in FSIQ with time since diagnosis remained significant in the model controlling for the other medical/treatment variables (p=0.004; Supplemental Table 1): Notably, more than 1 surgery also predicted poorer FSIQ in this model (p=0.04; Supplemental Table 1). However, only three patients in our cohort with greater than 1 upfront surgery had multiple neurocognitive assessments - limiting the interpretation of this finding. Persistent hydrocephalus requiring CSF diversion was present in 25% of cases but was not associated with outcome in FSIQ (p=0.59, Supplemental Table 1). Neither was age at diagnosis associated with FSIQ patient outcomes (p=0.59, Supplemental Table 1).
The Processing Speed Index (PSI) also declined over time since diagnosis, with a slope of −1.34 points per year (p=0.03, Table 3, Figure 2). This decline remained significant in the model controlling for presence of hydrocephalus, age at diagnosis, or number of surgeries (−1.73 points per year, p=0.009). None of these other variables predicted outcome in PSI, (p>0.10, Table 3).
Analysis Perceptual Reasoning/Organization Index (PRI), Verbal Comprehension Index (VCI) and Working Memory/Freedom From Distractibility Index (WMI) did not show significant changes over time from diagnosis in either the uncorrected or corrected models (p-values>0.10, Table 3, Supplemental Table 1): Slopes trended downward for PRI and VCI, however (Figure 2, Supplemental Table 1). Finally, younger age at diagnosis was associated with poorer WMI outcome, (−1.81 points per year, SE=0.7, p=0.02, Supplemental Table 1).
Discussion
We have defined the long-term survival and functional outcomes in posterior fossa ependymoma, in a large unselected, consecutive, molecularly-characterized cohort. To our knowledge this is the first report of functional outcomes in posterior fossa ependymoma in a subgroup specific manner. Our study highlights the necessity of post-operative adjuvant radiation for PFA ependymoma, while demonstrating for the first time the significant risk of long-term neurocognitive sequelae associated with long-term survival using this approach.
We have previously shown in a very large retrospective cohort of 820 posterior fossa ependymoma that the three strongest predictors of outcome were PFA subgroup, incomplete surgical resection and post-surgical upfront radiation.2 This study was confounded by its retrospective design, however we find in our consecutive institutional cohort that post-operative upfront radiation is necessary for cure in PFA ependymoma. PFA ependymoma patients treated with chemotherapy alone had a very poor outcome across 3 independent non-overlapping cohorts in that study, and our results here confirm this finding. Although it is possible that small subsets of PFA ependymoma could potentially be treated with surgery alone; it is clear that this would represent a very small proportion of patients at best. Currently no clinical or biological factor has been able to identify this subgroup, and as such the majority of PFA patients remain high-risk. As such, upfront external beam irradiation will remain the standard of care for the time being.
We have previously evaluated the impact of molecular subgroup on long-term neurocognitive outcomes in medulloblastoma, and have shown that there are clear differences across the four principal subgroups of medulloblastoma.19 This suggests a strong benefit to interpretation of neurocognitive outcomes in the context of biologically homogeneous groups; particularly when biological factors can influence confounders such as hydrocephalus, tumor location and the emergence of cerebellar mutism.20 Although we did not have sufficient PFB ependymomas with multiple longitudinal testing for comparison, by constraining the analysis to PFA ependymoma, we potentially account for unobserved variables inherent to the biological subgroup. Incorporation of prospective neuropsychological assessments, growth parameters and precise dosimetry with molecular subgroup, including the newly described 9 subtypes of PFA into upfront clinical trials will be required to provide a more granular assessment of long-term trajectories.21
Although previously it has been suggested that at 5 years, there is minimal impact to neurocognitive outcomes in ependymoma patients treated with conformal radiation; these studies did not provide longer-term trajectories limiting interpretation.22–25 Furthermore, these studies were limited to knowledge of genomics at the time they were conducted, where they combined biologically heterogeneous entities. Our analysis shows a similar trajectory at 5 years, however, our results suggest that neurocognitive declines continue through late childhood and adolescence placing these children at high-risk for learning difficulties as they advance through school. This is similar to medulloblastoma, where although the decline in FSIQ with time is more profound, the decline continues through childhood highlighting the importance of testing beyond 5 years post radiation.18, 19 Previous studies have not specifically evaluated the rate of growth hormone deficiency after focal irradiation for PFA ependymoma.26 Our finding that growth hormone deficiency was common after focal irradiation, further supports early monitoring from a young age, although precise dosimetry collected in cooperative group studies will be required to further discern this observation. Studies of proton radiotherapy have been promising, but are severely limited by short follow-up times, and urgent long-term endocrinological and neuropsychological studies in population based cohorts are required to establish if this is a strategy which may improve long-term outcomes.27
Unfortunately, our data are in keeping with previous studies that radiation-sparing approaches in PFA ependymoma result in dismal outcomes and are unlikely to be dismissed from upfront therapy anytime in the near future. As most PFA ependymoma patients are diagnosed prior to school-age, our studies highlight the importance of rapid implementation of early interventional strategies in all patients. The emergence of more conformal radiation technologies through advances in proton beam radiotherapy may help reduce the neurocognitive impact. However, it is unclear if it is the combination of direct radiation injury to the cerebellum and aggressive surgery, rather than radiation scatter, that underlies the decline in FSIQ. This requires further investigation as part of multicentre cooperative group trials. With the emerging field of neuroprotection, with interventions such as exercise, and cognitive training showing promise28–33, our results clearly indicate that cooperative groups need to prioritize children with PFA ependymoma for detailed neuropsychological evaluation, and incorporate early intervention and trials involving neuroprotective strategies.
Supplementary Material
Supplemental Figure 1: Survival of PFA versus PFB. A) Progression-Free and B) Overall Survival. P-values determined using the log-rank test.
Supplemental Figure 3: Age at diagnosis stratified by growth hormone deficiency. P-value determined using a t-test assuming unequal variances.
Supplemental Figure 2: Progression-Free and Overall Survival of PFA ependymoma stratified by extent of surgical resection (A,B), upfront radiation (C,D) and histological grading (E,F). P-values determined using the log-rank test.
Acknowledgments
Funding:
MZ was supported by a Meagan’s Walk Fellowship in Pediatric Neuro-Oncology and fellowships from the Garron Family Cancer Center and Restracomp. MDT is supported by operating funds from the National Institutes of Health (R01CA148699 and R01CA159859) and the Pediatric Brain Tumor Foundation. DJM is supported by the Canadian Institutes for Health Research. EB is supported by the Garron Family Chair in Childhood Cancer Research of the Hospital for Sick Children and University of Toronto. VR is supported by the Collaborative Ependymoma Research Network, the Brain Tumour Foundation of Canada, the C.R. Younger Foundation, American Brain Tumor Association and the Canadian Institutes for Health Research.
Footnotes
Conflict of interest: Authors disclose no conflict of interest.
References
- 1.Khatua S, Ramaswamy V, Bouffet E. Current therapy and the evolving molecular landscape of paediatric ependymoma. Eur J Cancer 2017;70: 34–41. [DOI] [PubMed] [Google Scholar]
- 2.Ramaswamy V, Hielscher T, Mack SC, et al. Therapeutic Impact of Cytoreductive Surgery and Irradiation of Posterior Fossa Ependymoma in the Molecular Era: A Retrospective Multicohort Analysis. J Clin Oncol 2016. [DOI] [PMC free article] [PubMed]
- 3.Merchant TE, Li C, Xiong X, Kun LE, Boop FA, Sanford RA. Conformal radiotherapy after surgery for paediatric ependymoma: a prospective study. Lancet Oncology 2009;10: 258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cavalli FMG, Hubner JM, Sharma T, et al. Heterogeneity within the PF-EPN-B ependymoma subgroup. Acta Neuropathol 2018;136: 227–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pajtler KW, Mack SC, Ramaswamy V, et al. The current consensus on the clinical management of intracranial ependymoma and its distinct molecular variants. Acta Neuropathol 2017;133: 5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pajtler KW, Witt H, Sill M, et al. Molecular Classification of Ependymal Tumors across All CNS Compartments, Histopathological Grades, and Age Groups. Cancer Cell 2015;27: 728–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Witt H, Mack SC, Ryzhova M, et al. Delineation of two clinically and molecularly distinct subgroups of posterior fossa ependymoma. Cancer Cell 2011;20: 143–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mack SC, Witt H, Piro RM, et al. Epigenomic alterations define lethal CIMP-positive ependymomas of infancy. Nature 2014;506: 445–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zapotocky M, Ramaswamy V, Lassaletta A, Bouffet E. Adolescents and young adults with brain tumors in the context of molecular advances in neuro-oncology. Pediatr Blood Cancer 2018;65. [DOI] [PubMed] [Google Scholar]
- 10.Grundy RG, Wilne SH, Robinson KJ, et al. Primary postoperative chemotherapy without radiotherapy for treatment of brain tumours other than ependymoma in children under 3 years: Results of the first UKCCSG/SIOP CNS 9204 trial. European Journal of Cancer 2010;46: 120–133. [DOI] [PubMed] [Google Scholar]
- 11.Grundy RG, Wilne SA, Weston CL, et al. Primary postoperative chemotherapy without radiotherapy for intracranial ependymoma in children: the UKCCSG/SIOP prospective study. Lancet Oncol 2007;8: 696–705. [DOI] [PubMed] [Google Scholar]
- 12.Mulhern RK, Merchant TE, Gajjar A, Reddick WE, Kun LE. Late neurocognitive sequelae in survivors of brain tumours in childhood. Lancet Oncol 2004;5: 399–408. [DOI] [PubMed] [Google Scholar]
- 13.Merchant TE, Mulhern RK, Krasin MJ, et al. Preliminary results from a phase II trial of conformal radiation therapy and evaluation of radiation-related CNS effects for pediatric patients with localized ependymoma. J Clin Oncol 2004;22: 3156–3162. [DOI] [PubMed] [Google Scholar]
- 14.Strother DR, Lafay-Cousin L, Boyett JM, et al. Benefit from prolonged dose-intensive chemotherapy for infants with malignant brain tumors is restricted to patients with ependymoma: a report of the Pediatric Oncology Group randomized controlled trial 9233/34. Neuro Oncol 2014;16: 457–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duffner PK, Horowitz ME, Krischer JP, et al. Postoperative chemotherapy and delayed radiation in children less than three years of age with malignant brain tumors. N Engl J Med 1993;328: 1725–1731. [DOI] [PubMed] [Google Scholar]
- 16.Aarsen FK, Paquier PF, Arts WF, et al. Cognitive Deficits and Predictors 3 Years After Diagnosis of a Pilocytic Astrocytoma in Childhood. Journal of Clinical Oncology 2009;27: 3526–3532. [DOI] [PubMed] [Google Scholar]
- 17.Venneti S, Santi M, Felicella MM, et al. A sensitive and specific histopathologic prognostic marker for H3F3A K27M mutant pediatric glioblastomas. Acta Neuropathol 2014;128: 743–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moxon-Emre I, Bouffet E, Taylor MD, et al. Impact of Craniospinal Dose, Boost Volume, and Neurologic Complications on Intellectual Outcome in Patients With Medulloblastoma. J Clin Oncol 2014. [DOI] [PubMed]
- 19.Moxon-Emre I, Taylor MD, Bouffet E, et al. Intellectual Outcome in Molecular Subgroups of Medulloblastoma. J Clin Oncol 2016;34: 4161–4170. [DOI] [PubMed] [Google Scholar]
- 20.Lassaletta A, Bouffet E, Mabbott D, Kulkarni AV. Functional and neuropsychological late outcomes in posterior fossa tumors in children. Childs Nerv Syst 2015;31: 1877–1890. [DOI] [PubMed] [Google Scholar]
- 21.Pajtler KW, Wen J, Sill M, et al. Molecular heterogeneity and CXorf67 alterations in posterior fossa group A (PFA) ependymomas. Acta Neuropathol 2018;136: 211–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Merchant TE, Sharma S, Xiong X, Wu S, Conklin H. Effect of cerebellum radiation dosimetry on cognitive outcomes in children with infratentorial ependymoma. Int J Radiat Oncol Biol Phys 2014;90: 547–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Willard VW, Conklin HM, Boop FA, Wu S, Merchant TE. Emotional and behavioral functioning after conformal radiation therapy for pediatric ependymoma. Int J Radiat Oncol Biol Phys 2014;88: 814–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Netson KL, Conklin HM, Wu S, Xiong X, Merchant TE. A 5-year investigation of children’s adaptive functioning following conformal radiation therapy for localized ependymoma. Int J Radiat Oncol Biol Phys 2012;84: 217–223 e211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Conklin HM, Li C, Xiong X, Ogg RJ, Merchant TE. Predicting change in academic abilities after conformal radiation therapy for localized ependymoma. J Clin Oncol 2008;26: 3965–3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Merchant TE, Rose SR, Bosley C, Wu S, Xiong X, Lustig RH. Growth hormone secretion after conformal radiation therapy in pediatric patients with localized brain tumors. J Clin Oncol 2011;29: 4776–4780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Macdonald SM, Sethi R, Lavally B, et al. Proton radiotherapy for pediatric central nervous system ependymoma: clinical outcomes for 70 patients. Neuro Oncol 2013;15: 1552–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Conklin HM, Ashford JM, Clark KN, et al. Long-Term Efficacy of Computerized Cognitive Training Among Survivors of Childhood Cancer: A Single-Blind Randomized Controlled Trial. J Pediatr Psychol 2016. [DOI] [PMC free article] [PubMed]
- 29.Conklin HM, Ogg RJ, Ashford JM, et al. Computerized Cognitive Training for Amelioration of Cognitive Late Effects Among Childhood Cancer Survivors: A Randomized Controlled Trial. J Clin Oncol 2015;33: 3894–3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Piscione PJ, Bouffet E, Timmons B, et al. Exercise training improves physical function and fitness in long-term paediatric brain tumour survivors treated with cranial irradiation. Eur J Cancer 2017;80: 63–72. [DOI] [PubMed] [Google Scholar]
- 31.Ameis SH, Daskalakis ZJ, Blumberger DM, et al. Repetitive Transcranial Magnetic Stimulation for the Treatment of Executive Function Deficits in Autism Spectrum Disorder: Clinical Trial Approach. J Child Adolesc Psychopharmacol 2017;27: 413–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Riggs L, Piscione J, Laughlin S, et al. Exercise training for neural recovery in a restricted sample of pediatric brain tumor survivors: a controlled clinical trial with crossover of training versus no training. Neuro Oncol 2017;19: 440–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang J, Gallagher D, DeVito LM, et al. Metformin activates an atypical PKC-CBP pathway to promote neurogenesis and enhance spatial memory formation. Cell Stem Cell 2012;11: 23–35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Survival of PFA versus PFB. A) Progression-Free and B) Overall Survival. P-values determined using the log-rank test.
Supplemental Figure 3: Age at diagnosis stratified by growth hormone deficiency. P-value determined using a t-test assuming unequal variances.
Supplemental Figure 2: Progression-Free and Overall Survival of PFA ependymoma stratified by extent of surgical resection (A,B), upfront radiation (C,D) and histological grading (E,F). P-values determined using the log-rank test.