Abstract
We report here that the anterograde transport from the endoplasmic reticulum (ER) to the Golgi was markedly suppressed by diacylglycerol kinase δ (DGKδ) that uniquely possesses a pleckstrin homology (PH) and a sterile α motif (SAM) domain. A low-level expression of DGKδ in NIH3T3 cells caused redistribution into the ER of the marker proteins of the Golgi membranes and the vesicular-tubular clusters (VTCs). In this case DGKδ delayed the ER-to-Golgi traffic of vesicular stomatitis virus glycoprotein (VSV G) and also the reassembly of the Golgi apparatus after brefeldin A (BFA) treatment and washout. DGKδ was demonstrated to associate with the ER through its C-terminal SAM domain acting as an ER-targeting motif. Both of the SAM domain and the N-terminal PH domain of DGKδ were needed to exert its effects on ER-to-Golgi traffic. Kinase-dead mutants of DGKδ were also effective as the wild-type enzyme, suggesting that the catalytic activity of DGK was not involved in the present observation. Remarkably, the expression of DGKδ abrogated formation of COPII-coated structures labeled with Sec13p without affecting COPI structures. These findings indicate that DGKδ negatively regulates ER-to-Golgi traffic by selectively inhibiting the formation of ER export sites without significantly affecting retrograde transport.
INTRODUCTION
Diacylglycerol kinase (DGK) phosphorylates diacylglycerol to yield phosphatidic acid and is known in higher eukaryotes to be composed of a family of nine related genes (Topham and Prescott, 1999; van Blitterswijk and Houssa, 2000). All of the family members show unique structural features, suggesting different mechanisms of the enzyme regulation and distinct functions of DGK isozymes. DGKδ uniquely contains a set of folds consisting of a pleckstrin homology (PH) domain at the N-terminus and a sterile α motif (SAM) domain at the C terminus (see Figure 1; Sakane et al., 1996). The functional significance of these domains remains unknown and needs to be defined in order to address the specific function of this DGK species. SAM domain has been detected in a wide range of proteins involved in developmental regulation and signal transduction (Schultz et al., 1997). Initially, SAM domain was reported to serve as the site of homotypic oligomerization (Stapleton et al., 1999; Thanos et al., 1999), although several SAM-containing proteins were later found to be monomeric (Chi et al., 1999; Smalla et al., 1999; Wang et al., 2001). In view of the EphB2 binding to the SH2 domain upon tyrosine phosphorylation (Stein et al., 1996), the function of SAM domains appears to be variable in different proteins. The PH domain is another structural module of a wide occurrence and has been found in more than a hundred different proteins involved in intracellular signaling, cytoskeletal organization, vesicular transport, and lipid metabolism (Rebecchi and Scarlata, 1998; Lemmon and Ferguson, 2000). This domain generally binds phosphoinositides, albeit with extremely varying affinities depending on the structural classification (Rebecchi and Scarlata, 1998; Lemmon and Ferguson, 2000). In the case of DGKδ, its PH domain showed a weak and promiscuous binding affinity to phosphoinositides in in vitro binding experiments (Kavran et al., 1998), the physiological significance of which remains unknown.
Figure 1.
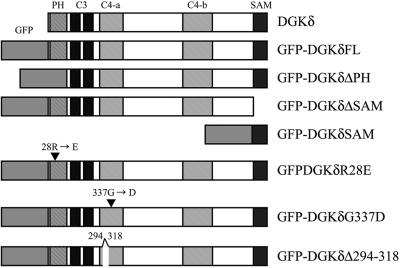
The schematic structures of DGKδ and its GFP-tagged mutants. The structure of the full-length DGKδ (DGKδFL) is shown as previously designated (Sakane et al., 1996). PH, Pleckstrin homology domain; C3, zinc finger structures; C4-a and C4-b, catalytic regions separated by an insertion of 317 residues; SAM, sterile α motif. GFP-DGKδ FL corresponds to the full-length DGKδ fused to GFP. Truncation mutants of DGKδ fused to GFP were, residues 101-1170(GFP-DGKδΔPH), residues 1–1096(GFP-DGKδΔSAM) and residues 1098–1170(GFP-DGKδSAM), respectively. In the case of GFP-DGKδR28E and GFP-DGKδG337D, Arg-28 and Gly-337 were changed to Glu and Asp, respectively. In GFP-DGKδΔ(294–318), a region of residues 294–318 was deleted from the full-length DGKδ. In some experiments (given in Figures 5–7), DGKδ were fused to the cyan variant, CFP, instead of GFP.
In the early secretory pathway, the membrane components are continuously recycled among the constituent compartments (Cole et al., 1998; Storrie et al., 1998), resulting in the constitutive transport of cargo molecules to the downstream secretory pathway. The dynamics of anterograde and retrograde transports is tightly linked to attachment to the membranes of the two types of proteinaceous coat complexes, COPI and COPII (Kirchhausen, 2001). Recent studies have revealed that coat formations are strictly controlled processes in which multiple proteins are sequentially involved (Kirchhausen, 2001). Attachment of COPI to the donor membranes is triggered by recruitment of ARF1-GTP to membranes (Donaldson et al., 1992; Helms and Rothman, 1992). The membrane association of ARF1 coupled to its activation is regulated positively by guanine nucleotide exchange factors (GEFs) and negatively by GTPase-activating proteins (GAPs; Puertollano et al., 2001). On the other hand, the attachment of COPII coat, which occurs only in the ER membranes, is caused by recruitment of GTP-Sar1p (Kuge et al., 1994). The activated Sar1p recruits the Sec23/24 complex, and budding of cargo-containing vesicles requires additional protein complex of Sec13/31 (Matsuoka et al., 1998). The experiments using permeabilized cells in the presence and absence of cytosol have shown that GTP-Sar1p alone can determine the ER export sites and that the cytosol contains suppressive factors interfering with the formation of COPII coats (Aridor et al., 2001). Only limited information of the cytosolic negative regulators has been available except for the GAP activity of Sec23p itself (Saito-Nakano and Nakano, 2000).
In the present work, we describe unexpected findings on the negative regulation by DGKδ of the COPII coat formation at the ER exit sites where anterograde transport is initiated. It is known that inhibition of each step of membrane transport in the early secretory pathway often results in redistribution of membrane proteins (e.g., Storrie et al., 1998). We therefore monitored in single cells the disturbances of membrane protein dynamics caused by DGKδ at the early stage of its expression. We also examined the effects of DGKδ on the two types of anterograde transport: reassembly of Golgi membrane proteins from brefeldin A (BFA)-induced fusion with the ER and the transport of temperature-sensitive folding mutant (ts045) of vesicular stomatitis virus glycoprotein (VSV G). We found that DGKδ inhibited ER-to-Golgi traffic through interfering with the formation of COPII-coated structures. Interestingly, the blockage of anterograde transport occurred only when DGKδ equipped with an intact PH domain was targeted to the ER membranes via its SAM domain.
MATERIALS AND METHODS
Plasmid Construction
To construct expression vectors coding for the full-length DGKδ fused at its N-terminus to green fluorescent protein (GFP-DGKδFL), SalI and XbaI sites were created by PCR at nucleotides 75 and 3594 of the DGK δ cDNA (Sakane et al., 1996). The restricted fragment was ligated in-frame into the corresponding sites of pEGFP-C3 (Clontech, Tokyo, Japan). Similarly, cDNAs encoding several deletion mutants of DGKδ, such as those lacking SAM domain (DGKδΔSAM), PH domain (DGKδΔPH), or residues 294–318 in the catalytic region (DGKδΔ294–318), were amplified from pSRE-DGKδ (Sakane et al., 1996) using the specific primers with SalI and XbaI sites at the ends. The amplified fragments were digested and subsequently subcloned into the XhoI-XbaI site of pEGFP-C3 to construct GFP-tagged chimera. The expression plasmids encoding cyan fluorescent protein (CFP)-fused DGKδ were also prepared using pECFP-C1 (Clontech). Point mutations were introduced into the PH domain (DGKδR28E) and the catalytic site (DGKδG337D) as described in the QuickChange protocol (Stratagene, La Jolla, CA) using the sets of complementary oligonucleotides (DGKδR28E: 5′-CATTCCAGCGATCAAAAGAGAGATACTTTAAGCTTC-3′ and 5′-GAAGCTTAAAGT-ATCTCTCTTTTGATCGCTGGAATG-3′; DGKδG337D: 5′-GTGGCGG-GGATGACAGTGTTGGCTGGG-3′ and 5′-CCCAGCCAACACTGTCA-TCCCCGCCAC-3′). The SAM domain of DGKδ (3292–3532 base pairs) was also in-frame fused to pEGFP-C3 or to a protein A fusion vector, pRIT2 (Amersham Pharmacia Biotech, Tokyo, Japan). The constructs of DGKδ used in the present work are summarized in Figure 1.
To construct an expression vector for yellow fluorescent protein (YFP)-tagged KDEL receptor, the cDNA encoding ELP1, a human homolog of Erd2 (Hsu et al., 1992), was obtained from the human liver QUICK-Clone cDNA (Clontech), and SalI and ApaI sites were created by PCR at the ends of the cDNA. The fragment was digested with SalI and ApaI and ligated in-frame into the corresponding sites of pEYFP-N1 (Clontech). Vectors for YFP-GT1–81 (GT1–81; the amino-terminal 81 amino acids of human β-1,4-galactosyltransferase) and CFP-GT1–81 (pEYFP-Golgi and pECFP-Golgi, respectively) were purchased from Clontech. An expression vector for YFP-ERGIC53 (Itin et al., 1996) was constructed as follows. The ERGIC53 cDNA was first obtained by reverse-transcribing HepG2 cell mRNA. BsrGI sites were then created at both ends by PCR, followed by BsrGI restriction, and ligated to pEYFP-ER (Clontech). An expression vector for hSec13-YFP was constructed by subcloning its cDNA, isolated from HepG2 cells, in-frame into pEYFP-N1 (Clontech) as described previously (Hammond and Glick, 2000). The authenticity of all cDNA constructs was verified by DNA sequencing.
Cell Culture, Transfection, and Time-lapse Analysis
NIH3T3, COS7, or normal rat kidney (NRK) cells (HSRRB, Osaka, Japan) were cultured in Dulbecco's modified minimum essential medium (DMEM) supplemented with 10% fetal calf serum at 37°C under 5% CO2. In the experiments given in Figures 3, 8, and 9, plasmids were transfected into cells using LipofectAMINE PLUS (Life Technologies-BRL, Gaithersburg, MD) according to the manufacturer's instructions. VSV G (ts045) cDNA was kindly donated by Dr. T. Nakada (Tokyo University) and subcloned into pECFP-N1 (Clontech) essentially as described previously (Presley et al., 1997). To achieve synchronized transport of VSV G (ts045)-CFP, NIH3T3 cells were incubated at 39.5°C for 24 h after introduction of expression vectors, and the anterograde transport of VSV G was then initiated in the medium prewarmed to 32°C. For detecting disturbance of membrane protein dynamics in single cells (see Figures 5–7 and 10), expression plasmids were introduced mechanically into the cells using siliconized glass microbeads. The original method using the beads (McNeil and Warder, 1987) was to deliver small molecules but was unsuitable for introducing large molecules such as plasmid DNA. However, we found that plasmid DNA could be efficiently introduced to cells when the beads were siliconized. This method was adopted because of its synchronized and rapid protein expression (see RESULTS), thus enabling us to achieve time-lapse analysis of cells from 30 min to 6 h of post–plasmid loading. Briefly, acid-washed beads (φ < 106 μm; Sigma-Aldrich, Tokyo, Japan) were siliconized in a closed chamber by dimethyldichlorosilane (Sigma-Aldrich) for 1 h at room temperature and then heated to 180°C for 3 h. Before plasmid loading, the medium was removed, and 1–2 μl of DNA (3 μg/ml DMEM) was placed onto the cells cultured on glass-based dishes (ATG, Tokyo, Japan). The cells were then covered with ca. 100 μl of dry glass beads, tapped vertically three times, and immediately rinsed with DMEM to remove the beads. The cells were further cultured in Phenol Red–free, CO2-independent MEM (Life Technologies-BRL) supplemented with 10% (wt/vol) fetal bovine serum on the stage of an inverted confocal laser microscope (LSM 510; Zeiss, Thornwood, NY) with a 100× oil planapochromat lens (NA 1.4). The temperature on the microscope stage was maintained using an objective lens heater (Bioptechs, Butler, PA) in combination with a stage heater (Kitazato Supply, Fujinomiya, Japan). Images were captured and processed using Photoshop 5.0 (Adobe Systems, San Jose, CA). IPLab (Scanalytics, Fairfax, VA) was used to quantitate the signal.
Figure 3.
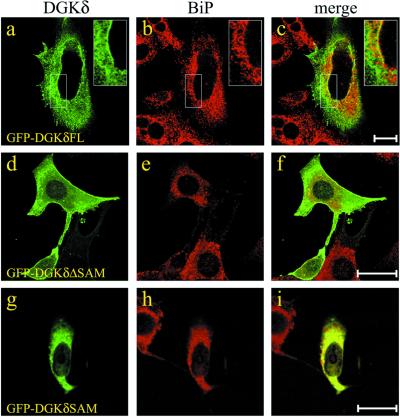
The SAM domain of DGKδ serves as an ER-targeting signal. NIH3T3 cells were transfected with the expression plasmids for GFP-DGKδFL (a–c), GFP-DGKδΔSAM (d–f), or GFP-DGKδSAM (g–i). At 24 h after transfection, the cells were fixed with methanol at −20°C and immunostained with anti-BiP antibody (b, e, and h). Note that GFP-DGKδFL (a) was partially colocalized with BiP (b), particularly at the nuclear rim (insets). In contrast, DGKδΔSAM (d) showed little overlap with BiP (e). Distribution of GFP-DGKδSAM (g) was nearly identical to BiP (h). Bars, 10 μm. DGKδFL, the full-length DGKδ; DGKδΔSAM, DGKδ lacking SAM domain; DGKδSAM, the SAM domain at the C terminus of DGKδ.
Figure 8.
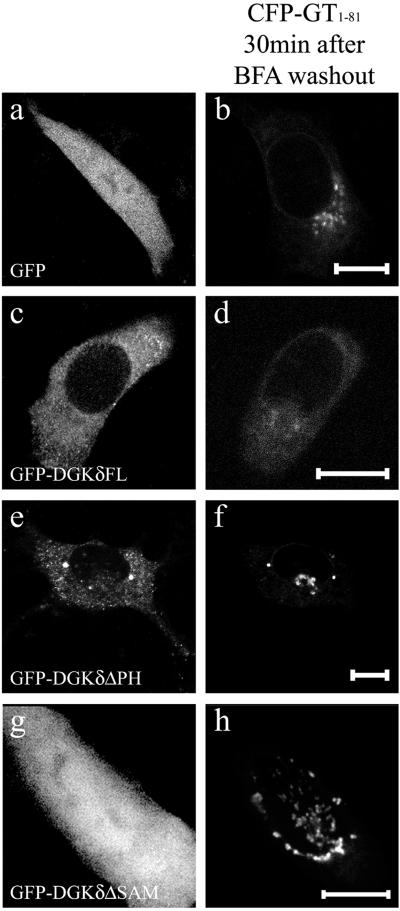
DGKδ inhibited anterograde transport through its SAM and PH domains. The ER-to-Golgi anterograde transport to reassemble Golgi apparatus was observed after washing-out NIH3T3 cells pretreated with BFA. The cells were transfected using LipofectAMINE PLUS with expression vectors coding for CFP-GT1–81 and GFP (a and b), CFP-GT1–81 and GFP-DGKδFL (c and d), or CFP-GT1–81 and GFP-DGKδΔSAM (e and f), or CFP-GT1–81 and GFP-DGKδΔPH (g and h). At 24 h posttransfection, cells were treated with BFA (10 μg/ml) in DMEM containing 10% fetal calf serum for 60 min at 37°C. The cells were then washed twice with the BFA-free medium. The cells were further incubated with BFA-free medium for 30 min at 37°C. The confocal images of single cells coexpressing CFP-GT1–81 (b, d, f, and g) and various DGKδ constructs (a, c, e, and g) are shown. Bars, 10 μm.
Figure 9.
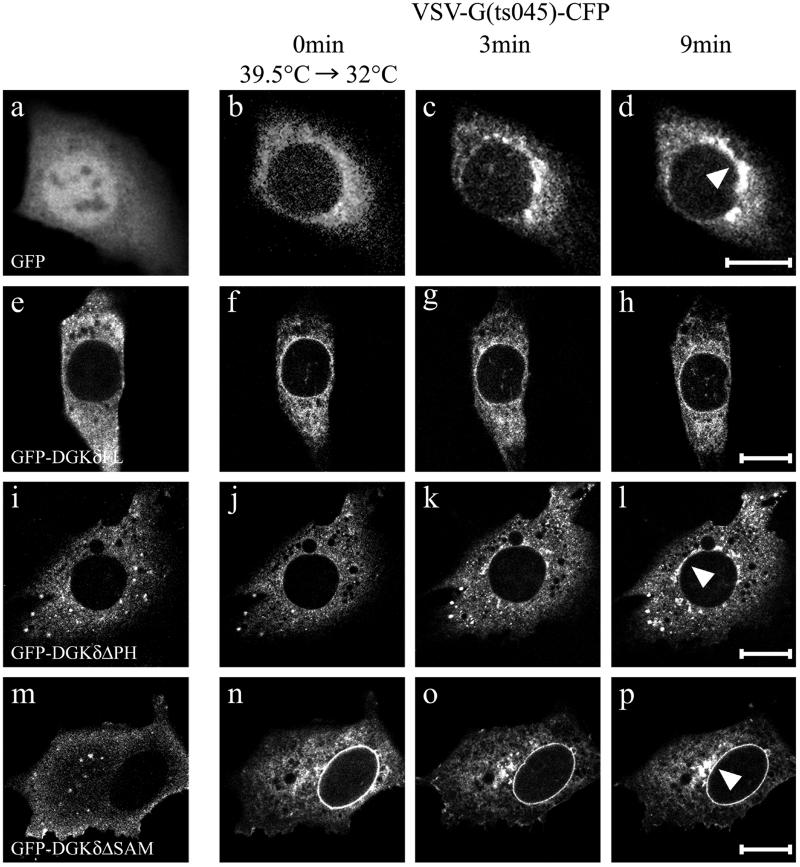
DGKδ slowed the ER-to-Golgi transport of VSV G(ts045). Kinetics of ER-to-Golgi transport was measured in NIH3T3 cells expressing temperature-sensitive folding mutant, VSV G(ts045) fused to CFP (b–d, f–h, j–l, and n–p). The cells coexpressed GFP (a–d), GFP-DGKδFL (e–h), GFP-DGKδΔPH (i–l), and GFP-DGKδΔSAM (m–p). Cells transfected with the expression vectors were cultured first at nonpermissive temperature (39.5°C) for 24 h (=0 min; a, b, e, f, i, j, m, and n), and then the incubation temperature was shifted to permissive temperature (32°C). Thereafter, the confocal images were recorded at 1-min intervals. Images taken at 0 (a, b, e, f, i, j, m, and n), 3 (c, g, k, and o), and 9 min (d, h, l, and p) are shown. Arrowheads indicate the Golgi areas. Bars, 10 μm.
Figure 5.
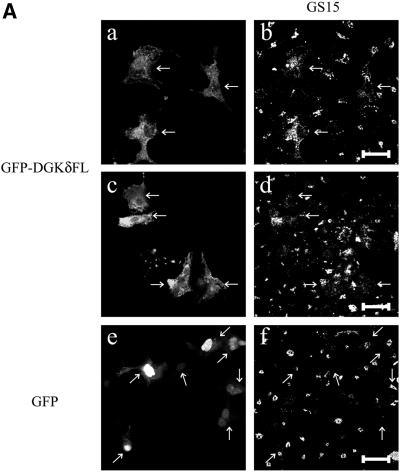
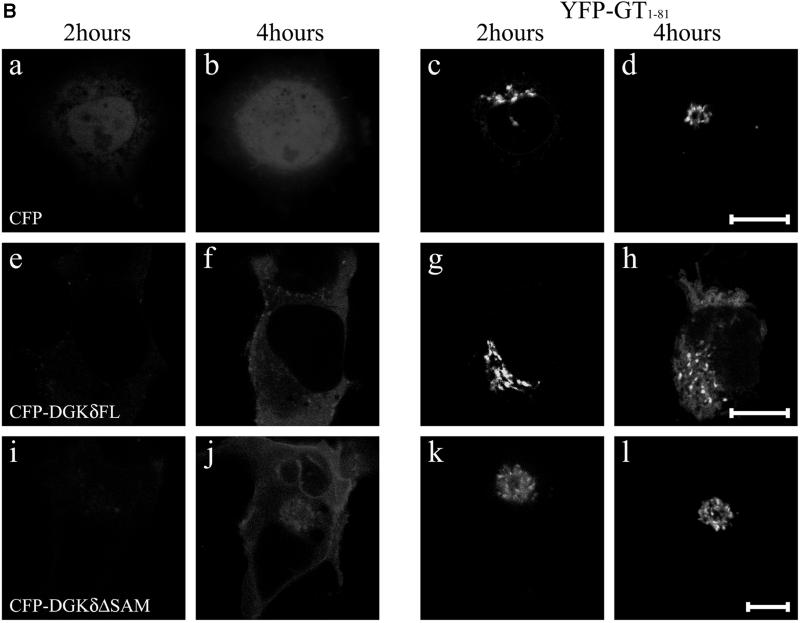
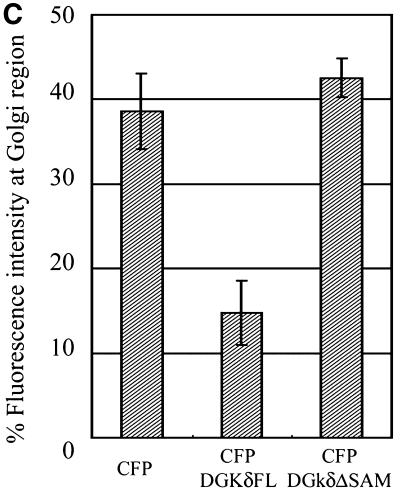
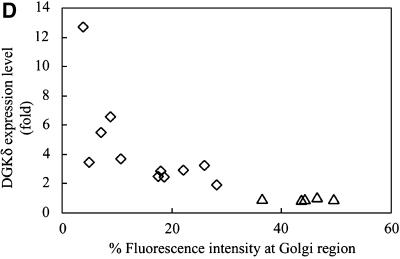
Redistribution of Golgi membrane proteins caused by DGKδ expression. (A) COS7 cells were transfected with the expression plasmids for GFP-DGKδFL (a–d) or GFP (e and f) using Fugene 6. At 36 h after transfection, the cells were fixed with paraformaldehyde and immunostained with anti-GS15 antibody (Transduction Laboratories, Lexington, KY) and Alexa 594–conjugated anti-mouse antibody (b, d, and f). Arrows show cells expressing GFP-DGKδFL or GFP. Bars, 50 μm. (B) pEYFP-GT1–81 and expression vectors for CFP (a–d), CFP-DGKδFL (e–h), or CFP-DGKδΔSAM (i–l) were introduced into COS7 cells using siliconized glass microbeads as described in MATERIALS AND METHODS. Confocal images were taken after 1 h at 30-min intervals. Shown are the images at 2 h (a, c, e, g, i, and k) and 4 h (b, d, f, h, j, and l) of plasmid loading. Bars, 10 μm. (C) On the basis of the assumption that the juxtanuclear aggregates with intense signal of YFP-GT1–81 represented the Golgi region, we selected such areas in the LSM images by setting threshold fluorescence levels using IPLab software (Scanalytics, VA). Distribution of YFP-GT1–81 was calculated by dividing fluorescent intensities at the Golgi by those of whole cell signal at 4 h after plasmid loading. Mean values ± SEM of four cells are presented. (D) Expression levels of DGKδ and distribution of YFP-GT1–81. Either of the vectors for CFP (open triangles) or CFP-DGKδFL (open rhombuses) was cointroduced into COS7 cells with the vector for YFP-GT1–81 using microbeads. At 4 h after loading, the cells were fixed with methanol and immunostained with anti-DGKδ antibody or preimmune IgG followed by secondary anti-rabbit antibody conjugated to Alexa 594 (Molecular Probes, Eugene, OR). The YFP and Alexa594 signals of the LSM images were quantitated by IPLab. In quantitating the Alexa594 signal, the average signal obtained by the preimmune IgG incubation was set to zero. Distribution of YFP-GT1–81 was calculated as in C. The relative expression levels of DGKδ were obtained by dividing the whole-cell Alexa 594 signal in CFP-DGKδFL–expressing cells by the averaged endogenous DGK signal in control CFP-expressing cells and were compared with distribution of YFP-GT1–81 in the same cell.
Figure 7.
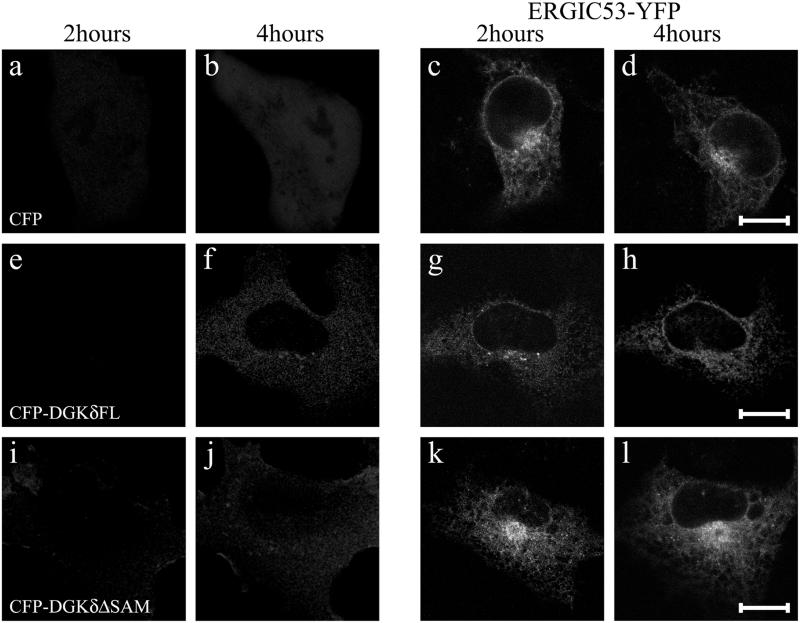
Redistribution of YFP-ERGIC53 caused by DGKδ expression. The expression vector coding for YFP-ERGIC53 was introduced into COS7 cells using microbeads together with those encoding CFP (a–d), CFP-DGKδFL (e–h), or CFP-DGKδΔSAM (i–l). Confocal images were taken as in Figure 5. Bars, 10 μm.
Figure 10.
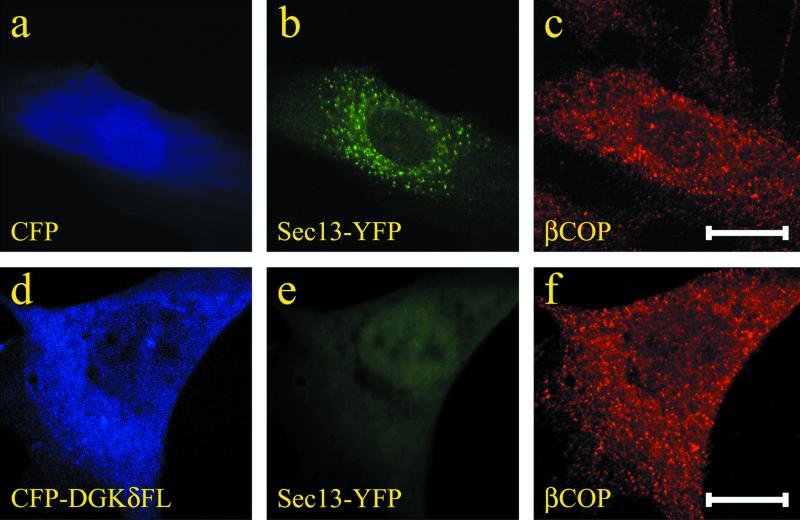
Expression of DGKδ inhibited formation of COPII-coated structures in the ER network whereas COPI-coated structures remained unchanged. Expression vectors coding for Sec13-YFP plus CFP (a–c) or Sec13-YFP plus CFP-DGKδFL (d–e) were transfected into NRK cells cultured on collagen-coated glass dishes. The confocal images of Sec13p were taken at 12 h posttransfection (b and e). To visualize COPI-coated vesicles, cells (c and f) were fixed with paraformaldehyde and immunostained with anti-βCOP antibody. Bars, 10 μm.
Indirect Immunofluorescence
Cells cultured on coverslips were fixed for 10 min at room temperature with 4% paraformaldehyde in phosphate-buffered saline (PBS) and permeabilized with 0.2% Triton X-100 for 1 min on ice. Alternatively, cells were fixed with methanol for 4 min at −20°C. The fixed and permeabilized cells were blocked in 0.1% bovine serum albumin (BSA) in PBS for 30 min and then incubated with the antibody solution in the blocking buffer for 45 min at 37°C. Cells were washed three times with PBS (for 5 min each) and then incubated with Alexa 594–conjugated anti-mouse IgG antibody (Molecular Probes, Eugene, OR) for 30 min at room temperature. The cells were again washed three times with PBS (5 min each), and the coverslips were mounted onto glass-slides using VECTASHIELD (Vector Laboratories, Burlingame, CA). Images were taken with an inverted confocal laser scanning microscopy (Zeiss LSM 510) with a 40× oil plan-neofluar (NA1.3; Figure 5A) or a 100× oil objective lens (other figures) and processed by Adobe Photoshop version 5.0.
Preparation of Protein A-DGKδSAM Fusion Protein
Escherichia coli N4830–1 was transformed with pRIT2 vector (Amersham Pharmacia) encoding protein A-DGKδSAM fusion protein. Protein expression was induced by incubating cells at 42°C for 6 h. The harvested cells were then washed twice with PBS, suspended in 0.1% Tween 20 in PBS, and lysed by sonication. The lysate was centrifuged at 12,000 × g for 20 min to remove cell debris, and the protein A fusion protein was purified using IgG-Sepharose (Amersham Pharmacia).
Preparation of ER-enriched Microsomes
ER-enriched microsomes were prepared essentially as described previously (Kappeler et al., 1997). Briefly, mouse liver was homogenized with a Dounce homogenizer in 5 ml of homogenizing buffer/g tissues (10 mM Bes-KOH, pH 7.2/120 mM NaCl/5 mM KCl) supplemented with protease inhibitor cocktail (Roche, Tokyo, Japan). The homogenate was centrifuged at 10,000 × g for 10 min. The supernatant was then centrifuged at 300,000 × g for 30 min. The 300,000 × g pellet (microsomes) was suspended in the homogenizing buffer containing 35% (wt/vol) Nycodenz (Nycomed Pharma, Oslo, Norway) and transferred to centrifuge tubes. A Nycodenz gradient of 29–13% in the homogenizing buffer was made on top of the microsomal suspension and centrifuged at 36,000 rpm at 4°C for 3 h in a Hitachi (Tokyo, Japan) RPS-40T rotor. Ten 1-ml fractions were collected from the bottom, and each fraction was subjected to Western blot analysis using antibodies to GM130 (Transduction laboratories, Lexington, KY), ERGIC53 (a gift of Dr. F. Tokunaga at Himeji Institute of Technology, Himeji, Japan), and calnexin (StressGen Biotech, Sidney, British Columbia, Canada). Fractions enriched with calnexin were diluted with the homogenizing buffer and centrifuged at 300,000 × g for 30 min. The pellet was then resuspended with homogenization buffer. The ER-enriched microsomes thus prepared were further incubated with 1 M KCl in the buffer for 10 min on ice and then recovered by centrifuging at 300,000 × g for 30 min. The salt-washed microsomes were dialyzed against the binding buffer as described below and stored at −80°C.
Binding Assay
The salt-washed microsomes (100 μg of protein) were first preincubated at 4°C for 10 min with BSA (10 mg/ml) in the binding buffer containing 10 mM Bes-KOH (pH 7.2), 120 mM potassium acetate, 2 mM magnesium acetate, and 5 mM sodium acetate to block nonspecific binding. After preincubation, the microsomes were further incubated with protein A-DGKδSAM fusion protein in the same buffer for 10 min at 4°C. The microsomes were then recovered by centrifugation at 300,000 × g for 30 min and washed once with the binding buffer. The pellets were analyzed for the bound protein A fusion protein by Western blotting using horse radish peroxidase–conjugated IgG, and the signals were detected by SuperSignal (Pierce, Rockford, IL). In some experiments, the microsomes (100 μg of protein) were treated with trypsin (11 μg/ml; Sigma-Aldrich) for 5 min at 37°C before the binding studies. The mixture was added with aprotinin (44 μg/ml; Sigma-Aldrich) and was subjected to the binding assays.
RESULTS
ER Localization of DGKδ
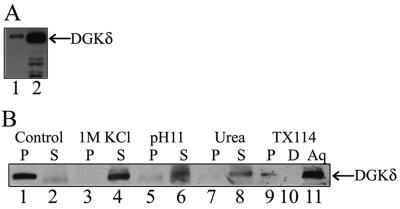
Previously, we reported that most of DGKδ expressed in COS7 cells was recovered in the total particulate fraction (Sakane et al., 1996) despite the apparent lack of membrane-binding sequence motifs. We therefore attempted first to define the intracellular distribution of DGKδ and also to characterize the mechanisms underlying its membrane association. The analysis of cellular DGKδ protein in the subcellular fractions of NIH3T3 cells showed that DGKδ was markedly enriched in the microsomal fraction (Figure 2A) compared with the cytosol. To examine the nature of the enzyme interactions with microsomes, we next treated the membranes with chemicals that selectively disrupt interactions on the surface without extracting proteins from phospholipid bilayers. Treatments with alkaline buffer and high salt concentration, as well as incubation with 6 M urea, all abolished the membrane association of DGKδ (Figure 2B, lanes 3–8). This suggests that DGKδ associates with the microsomal surface through ionic protein/protein interactions. Consistent with this notion, the microsomal DGKδ partitioned into the aqueous phase in the phase separation experiments using Triton X-114 (Bordier, 1981), although a significant fraction was not solubilized (Figure 2B, lane 9). We also noted that a part of the microsomal DGKδ remained insoluble when treated with Triton X-100. These data indicate that a considerable part of the cellular DGKδ is associated with the microsomal surface, although a minor portion exists in the cytosol and in the detergent-insoluble cytoskeletal elements.
Figure 2.
Association of endogenous DGKδ with microsomes. (A) Homogenates of NIH3T3 cells were subjected to subcellular fractionation as described in MATERIALS AND METHODS. Ten micrograms of proteins of the cytosolic (lane 1) and microsomal (lane 2) fractions was analyzed by SDS-PAGE (10%) followed by Western blot analysis using anti-DGKδ antibody (Sakane et al., 1996). (B) Microsomes (10 μg) were incubated for 30 min on ice with homogenization buffer alone (lanes 1 and 2) or the buffer containing 1 M KCl (lanes 3 and 4), or 6 M urea (lanes 7 and 8). The microsomes were also treated with the buffer added with 0.2 M sodium carbonate (pH 11.0; lanes 5 and 6). After treatments, the microsomes were separated into supernatant (S) and pellet (P) fractions by centrifuging at 300,000 × g for 30 min. Microsomes (10 μg) were also subjected to Triton X-114 phase separation, and the insoluble aggregates (P, lane 9), aqueous phase (Aq, lane 11), and detergent phase (D, lane 10) were obtained. Samples were separated by SDS-PAGE (7.5%) and analyzed by Western blotting using anti-DGKδ antibody.
We next expressed in NIH3T3 cells GFP-tagged DGKδ in order to further characterize its intracellular localization in intact cells. In the experiments given in Figure 3, the cells at 24 h posttransfection were immunostained with antibody against BiP, an ER marker. Although the localization profiles of the full-length DGKδ were rather heterogenous, a portion of the GFP-DGKδ signal was consistently overlapped with BiP. Because of the cytoplasmic signal, the structure of the ER as detected by the BiP staining (Figure 3b) was not clearly demarcated by GFP-DGKδ, except for the nuclear rim (Figure 3a, inset). The N-terminus tagging of DGKδ with GFP had little influence on the enzyme localization because immunostaining of nontagged DGKδ expressed in NIH3T3 cells showed a staining pattern indistinguishable from that given in Figure 3a. The heterogenous distribution of DGKδ indicates two possibilities; 1) the enzyme contains multiple localization signals resulting in its association with different intracellular sites, or 2) DGKδ has no particular localization signal. To distinguish between these possibilities, we made a series of truncated DGKδ mutants fused N-terminally to GFP (Figure 1). When their distributions were examined in transfected cells, we found that deletion of the C-terminal SAM domain abrogated its signal at the ER (Figure 3, d–f). Other deletion mutants with intact SAM domain showed cellular localizations indistinguishable from those of the full-length enzyme. If the SAM domain possesses an ER-targeting signal, then GFP fused to the SAM domain alone should localize in the ER. We thus expressed the GFP-tagged SAM domain and found that the localization pattern was nearly identical to that of BiP (Figure 3, g–i). These data suggest that the SAM domain is responsible for targeting DGKδ to the ER.
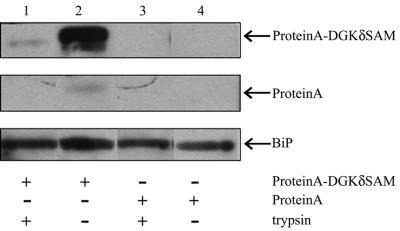
To confirm that the DGKδ SAM domain acts as an ER-anchoring sequence, we intended to see whether the SAM domain could bind to the ER in vitro. For this purpose, we expressed and purified DGKδSAM fused to protein A at its N-terminus. As the source of the ER membranes, we used mouse liver microsomes highly enriched with the ER on a Nycodenz gradient. The ER preparation was further washed with 1 M KCl to remove endogenous DGKδ. The ER fraction thus prepared was incubated with protein A fused to DGKδ SAM, and then the ER membranes were recovered through a sucrose cushion. As shown in lanes 2 and 4 of Figure 4, DGKδSAM fused to Protein A, but not unfused Protein A, was bound to the recovered ER membranes. Because SAM domains are generally known to interact with other proteins (Schultz et al., 1997), we tested whether the binding of DGKδSAM to the ER depended on protein–protein interactions. As seen in lane 1 of Figure 4, the binding of DGKδSAM to the ER was markedly reduced when the membranes were pretreated with trypsin. Taken together with the effects of the SAM domain on the intracellular localization (Figure 3), it becomes now clear that DGKδ binds to the ER membrane protein(s) through its SAM domain acting as an ER-targeting signal.
Figure 4.
Binding in vitro of DGKδSAM to ER-enriched microsomes. ER-enriched microsomes purified by Nycodenz gradients were further treated with 1 M KCl to remove endogenous DGKδ. The salt-washed microsomes were preincubated with (lanes 1 and 3) or without trypsin (lanes 2 and 4) for 30 min on ice, and then aprotinin was added to stop the digestion. The pretreated microsomes were next incubated with protein A-DGKδSAM (lanes 1 and 2) or protein A (lanes 3 and 4) in the binding buffer containing BSA (10 mg/ml) for 10 min on ice. Microsomes were then recovered by centrifugation and analyzed by SDS-PAGE (12.5%) followed by Western blotting using horseradish peroxidase–conjugated rabbit IgG. The blots were stripped and reprobed with anti-BiP antibody to confirm an equal loading of the microsomal fractions.
Redistribution of Golgi Markers Caused by DGKδ Expression
We next examined the possibility that the ER-resident DGKδ might be involved in the regulation of vesicular traffics. In the early secretory pathway, membrane components are rapidly exchanged among the organelle compartments, resulting in the achievements of their specific distributions (Cole et al., 1998; Storrie et al., 1998). Alteration of each process of the pathway should cause a shift in the apparent distribution of the membrane components at a given time. Hence, we monitored disturbance of membrane protein distribution in a single cell upon expression of DGKδ. Initially, we examined the effects of DGKδ overexpression on an endogenous Golgi membrane protein, GS15. After expressing DGKδ fused to GFP for 36 h using Fugene 6, the cells were fixed, and the distribution of GS15 was observed at a low magnification. As shown in Figure 5A, whereas GS15 signals in control cells were confined to the ribbon-like juxtanuclear structures of Golgi apparatus, the Golgi protein in all cells expressing DGKδ-GFP was redistributed to the ER-like perinuclear structures. We next attempted to keep the expression level of the various constructs as low as possible to minimize the spurious effects of overexpression. At the same time, we attempted to evaluate the effects of DGKδ at a very early phase of its expression so that we could minimize various adaptive responses of the cells. For this purpose, we directly introduced into COS7 cells expression plasmids using siliconized glass microbeads. Throughout these experiments, we usually obtained detectable protein expression as early as 1 h of bead loading.
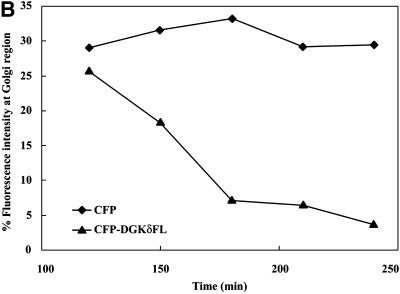
To monitor possible perturbation of membrane dynamics, cells were introduced with expression plasmids of specific membrane markers tagged with YFP. In the initial experiments (Figure 5B), we introduced plasmids of CFP-DGKδ and of YFP fused to the N-terminal 81 amino acids of galactosyltransferase (YFP-GT1–81), thus engineered to possess medial-trans Golgi localization signal (Yamaguchi and Fukuda, 1995). YFP-GT1–81 usually became detectable as early as 1 h postloading, whereas CFP-DGKδ was barely detected during the first 2 h (Figure 5B, e). At 2 h postloading, YFP-GT1–81 (Figure 5B, g) was mostly detected at the juxtanuclear region, and little ER network was labeled. However, CFP-DGKδ became detectable after 4 h of incubation (Figure 5B, f), when the YFP signal at the juxtanuclear Golgi region in the same cell was diminished and the ER network structure was highlighted (Figure 5B, h). In contrast, when a plasmid for CFP was loaded (Figure 5B, a–d), no redistribution of YFP-GT1–81 to the ER was observed (Figure 5B, d). These findings demonstrated that DGKδ caused redistribution of the Golgi proteins into the ER at a very early phase of its expression. In these experiments, we confirmed that redistribution of YFP-GT1–81 occurred similarly when nontagged full-length DGKδ was expressed instead of CFP-fusion protein. We also confirmed that the expression of the full-length DGKγ (Kai et al., 1994) fused N-terminally to GFP failed to affect the distribution of the Golgi marker during the experimental periods. If the observed effects of DGKδ were caused by its association with the ER membranes via the SAM domain as shown in Figures 3 and 4, deletion of this domain should abolish the redistribution of Golgi markers. Hence the cells were coloaded with plasmids coding for CFP-DGKδΔSAM and YFP-GT1–81 (Figure 5B, i–l) and cultured on a microscope stage for up to 4 h. As expected, the intense YFP signal at the Golgi region was unchanged (Figure 5B, l) even after expression of CFP-DGKδΔSAM (Figure 5B, j), and little signal was detected at the ER network (Figure 5B, l). Quantitation of the YFP signal intensity at the Golgi region revealed that nearly 60% of Golgi membrane protein was redistributed to the reticular network within 4 h of DGKδ expression, whereas CFP or CFP-DGKδΔSAM caused little change in its distribution (Figure 5C). To assess how many fold overexpression is required for the phenotype, we measured the levels of DGKδ by immunostaining using anti DGKδ antibody. As shown in Figure 5D, increased ER localization of YFP-GT1–81 was detectable at a few-fold enhancement of DGKδ over the endogenous level. It becomes thus clear that relatively low-level expression of DGKδ is sufficient to cause apparent redistribution of the Golgi proteins (Figure 5D).
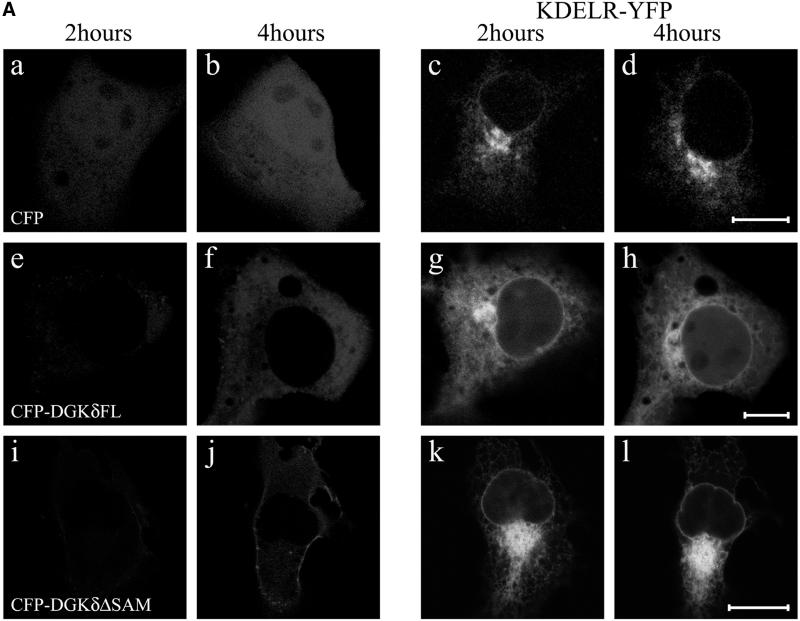
Considering the constitutive recycling of Golgi membrane components to the ER (Cole et al., 1998; Storrie et al., 1998), the above data indicate that DGKδ may affect membrane dynamics in the early secretory pathway. Because the kinetics of recycling of Golgi membrane proteins was generally known to be slow (Cole et al., 1998; Storrie et al., 1998; Zaal et al., 1999), we used in the next experiments the two membrane proteins that rapidly recycle via COPI mediated retrograde transport: KDEL receptor (Hsu et al., 1992) and ERGIC53 (Hauri et al., 2000). To visualize the KDEL receptor, which is involved in the retrieval of ER-resident proteins from the cis-Golgi network to the ER, YFP was fused to the C terminus of the KDEL receptor. When KDEL receptor-YFP was expressed, intense labeling of the Golgi region was observed with a faint ER signal (Figure 6A, c and d). In the cells coexpressing CFP and KDEL receptor, the ratio of the signals between the ER and Golgi remained unchanged throughout the experiments (Figure 6A, c and d, and 6B). In contrast, even at 2 h when CFP-DGKδ was expressed to a very limited extent, the signal of KDEL receptor-YFP at the Golgi area was significantly reduced with its redistribution to the reticular network of the ER (Figure 6A, g). More redistribution was observed at 4 h (Figure 6A, h, and 6B). This phenotype was not observed when DGKδ lacking SAM domain was introduced (Figure 6A, k and l). We also used another well-characterized recycling protein, ERGIC53 (Hauri et al., 2000), a marker of vesicular-tubular clusters (VTCs). Because this type I membrane protein contains a cytoplasmic tail that interacts with COPI (Tisdale et al., 1997) and COPII coats (Kappeler et al., 1997), we inserted YFP immediately after the signal sequence cleavage site so that the cytoplasmic tail was unchanged. YFP-tagged ERGIC53 showed a distribution pattern identical to that of untagged ERGIC53 when expressed in NIH3T3 cells. Different from the localization profile of the KDEL receptor (Figure 6A), a portion of ERGIC53 was found in punctate structures adjacent to the ER network (Figure 7, c and d). However, upon expression of DGKδ, such clusters were nearly abolished and the ER network became highlighted (Figure 7, g and h). Consistent with the observations described above, a DGKδ mutant lacking the C-terminal SAM domain had little influence on the dynamics of YFP-ERGIC53 (Figure 7, k and l).
Figure 6.
Redistribution of KDEL receptor-YFP caused by DGKδ expression. (A) The expression vector coding for KDEL receptor (KDELR)-YFP was coloaded to COS7 cells using microbeads with those encoding CFP (a–d), CFP-DGKδFL (e–h), or CFP-DGKδΔSAM (i–l). LSM images were recorded as in Figure 5. Bars, 10 μm. (B) The intense signal of KDELR-YFP at the Golgi areas was quantitated as in Figure 5C.
Inhibition of ER-to-Golgi Traffic by DGKδ
The results described so far suggested that the ER-resident DGKδ disturbed membrane dynamics of the early secretory pathway by affecting the rate-limiting steps operating on the ER membranes. To determine if the ER exit was regulated by DGKδ, we next studied its effects on the two types of anterograde transport: reformation of the Golgi after BFA treatment and transport of VSV G. A fungus metabolite, BFA, causes rapid redistribution of Golgi membrane proteins to the ER and blocks the ER-to-Golgi transport (Fujiwara et al., 1988). Removal of BFA reinitiates rapid anterograde membrane transport from the ER, resulting in the reformation of Golgi apparatus (Fujiwara et al., 1988). We treated NIH3T3 cells with BFA and observed the time-dependent reassembly of CFP-GT1–81 upon washout of BFA. As shown in Figure 8b, the Golgi apparatus was completely reformed within 30 min after removal of BFA from the cells. However, the expression of DGKδ markedly retarded the reformation of the Golgi apparatus in all cells examined. This retardation was not seen when the SAM domain was omitted (Figure 8h), in accordance with the results obtained for Golgi marker redistribution (Figure 5). Furthermore, we found that in addition to the SAM domain, the N-terminal PH domain was also required for the effects of DGKδ as shown for the PH domain–deleted mutant (Figure 8, e and f).
In the next experiments analyzing the constitutive transport from the ER to the Golgi, we used a temperature-sensitive folding mutant of VSV G, ts045, tagged to CFP. This type I membrane protein is the most extensively studied cargo protein, and it has been shown that the cytoplasmic tail directly interacts with Sar1p to trigger the COPII coat assembly (Aridor et al., 2001). At the restrictive temperature, VSV G-CFP was mostly arrested in the ER (Figure 9, b, f, j, and n), and a rapid transport to the juxtanuclear region occurred maximally at 9 min after a temperature shift to 32°C (Figure 9, c and d). When GFP-DGKδ was coexpressed, the transport of VSV G-CFP to the Golgi region was barely detected at 9 min (Figure 9h). In contrast, the expression of the C terminus truncation mutant of DGKδ, GFP-DGKδΔSAM, failed to significantly affect the exit from the ER (Figure 9p). We found that deletion of the N-terminal 100 amino acids corresponding to the PH domain also caused an apparent lack of the phenotype for both types of anterograde transports. Interestingly, the expression of GFP-DGKδΔPH had little effect, if any, on the kinetics of the ER-to-Golgi transport of CFP-GT1–81 (Figure 8f) and VSV G-CFP (Figure 9l). In a separate experiment, we confirmed that the deletion of PH domain did not significantly affect the ER-localization of the enzyme molecule. The results therefore showed that the PH domain was also required for the inhibition of both types of anterograde transports in addition to targeting DGKδ to the ER via its SAM domain.
Membrane dynamics in the early secretory pathway is controlled by the formation and detachment of COPI and COPII coats on the membranes (Kirchhausen, 2001). The presence of several regulating factors (Kirchhausen, 2001) has been suggested in the process of coated vesicle formation. It now seems possible that DGKδ is one of the negative regulators of anterograde transport. To analyze the membrane dynamics at the early stage of anterograde transport, we tagged with YFP Sec13p, a component of the COPII coat (Tang et al., 1997). When expressed in NRK cells, punctate structures of Sec13p were clearly observed adjacent to the ER network (Figure 10b). Previously, Sec13p-tagged with GFP was used to record movement of COPII vesicles (Hammond and Glick, 2000). We confirmed that the punctate structure disappeared when treated with H89, a protein kinase inhibitor, in accord with previous report (Lee and Linstedt, 2000). It is therefore clear that these Sec13p structures represent VTCs. Remarkably, the expression of DGKδ almost completely abolished the VTC structures of Sec13p and instead Sec13p was evenly distributed in the cytoplasm (Figure 10e). In contrast, the profiles of COPI-coated vesicles, as revealed by anti-βCOP antibody staining, were unchanged upon expression of CFP-DGKδ (Figure 10f). This finding indicated that retrograde transport was not affected by DGKδ, consistent with the notion that DGKδ selectively inhibited anterograde transport. From these results, we concluded that DGKδ could regulate negatively anterograde transport by preventing formation of COPII-coated transitional ER structures.
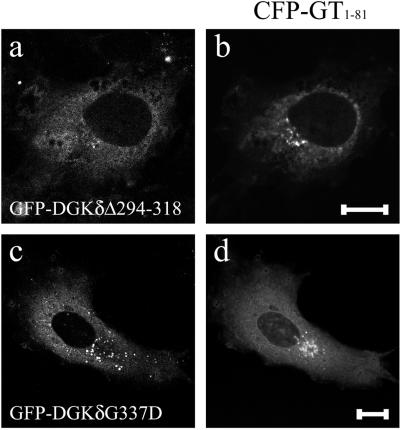
In the final experiments, we introduced into NIH3T3 cells two types of DGKδ constructs mutated at the catalytic domain, i.e., a kinase-dead mutant, DGKδG337D, and that deleted with 25 amino acids at 294–318, which corresponded to the internal truncation detected previously in a catalytically inactive form of DGKγ (Kai et al., 1994). These mutants expressed as GFP-fusions caused redistribution of CFP-GT1–81 to the ER similarly as did the full-length DGKδ (Figure 11). We also noted that addition into the culture media of phosphatidic acid or diacylglycerol did not affect the vesicular transport in NIH3T3 cells. We considered from these results that the catalytic activity of DGKδ was not significantly involved in the negative control of ER-to-Golgi traffic.
Figure 11.
Kinase-dead mutants of DGKδ also induced redistribution of CFP-GT1–81. Expression vectors for GFP-DGKδΔ294–318 (a and b) or GFP-DGKδG337D (c and d) were coloaded into COS7 cells with a vector coding for CFP-GT1–81 using microbeads. Shown are the GFP (a and c) or CFP images (b and d) of cells at 4 h postloading. Bars, 10 μm.
DISCUSSION
In the present work, we have demonstrated that DGKδ can be involved in the negative regulation of the early secretory pathway by inhibiting ER exit. This conclusion is based on three lines of observations obtained from single cells expressing DGKδ: 1) redistribution of Golgi-related membrane proteins, 2) reduced anterograde transport rate, and 3) inhibition of transitional ER structure formation. The negative effects of DGKδ were selectively observed in the COPII-dependent pathway, and no effect was discernible in COPI-dependent transport from the Golgi to the ER or clathrin-mediated endocytosis (our unpublished data). Furthermore, we found that both of the two domains uniquely present in the DGKδ were essential for this function. The SAM domain was shown to act as an ER-targeting motif, whereas the targeted molecule further required the PH domain for expressing the function. Such a selective mode of inhibitory effects and the requirements of definite structural motifs strongly suggest that DGKδ constitutes one of the negative regulators of the COPII-dependent pathway.
Interestingly, the effects of DGKδ closely resemble those recently described for H89, an isoquinolinesulfonamide protein kinase inhibitor (Aridor and Balch, 2000; Lee and Linstedt, 2000), which was described to cause loss of transitional ER structures labeled with Sec13p without affecting constitutive Golgi-to-ER retrograde transport (Lee and Linstedt, 2000). Balch and colleagues later showed that the H89-sensitive step in the COPII-dependent pathway is the recruitment of Sar1p rather than the late assembly step (Aridor and Balch, 2000). Based on analysis using a panel of protein kinase inhibitors, both reports concluded that the target molecule of H89 in COPII coat recruitment was neither protein kinases A nor D but probably unidentified serine-threonine kinase. Considering the consequences of DGKδ expression, we think it likely that DGKδ may regulate an unknown machinery including the putative protein kinase required for COPII coat recruitment. Classical members of the DGK family phosphorylate diacylglycerol to produce phosphatidic acid so that the action of protein kinase C, which requires diacylglycerol for activation, is attenuated (Sakane and Kanoh, 1997). Recently, phospholipid metabolism has been implicated in the control of membrane traffic particularly at the distal stage of secretory pathway (De Camilli et al., 1996; Siddhanta and Shields, 1998; Roth, 1999). Phosphatidic acid generated by the action of phospholipase D has been shown to regulate protein traffic from the Golgi complex, although the significance of lipids in the control of ER-to-Golgi traffic remains largely unknown. Initially, we assumed that the effects of the expressed DGKδ were mediated by the formation of phosphatic acid in restricted areas at the ER surface. However, the results of expression of DGKδ mutated at its catalytic region have led us to consider at the present stage of investigation that the alteration of lipid metabolism at the ER is not involved in the observed effects of DGKδ. Furthermore, neither the PH domain nor SAM domain appears to be directly involved in the DGK catalytic action (Sakane et al., 1996). We also analyzed cellular lipids in COS7 cells overexpressing DGKδ for 3 d and failed to detect significant increase of phosphatidic acid. DGKδ that contains a large insert in the catalytic domain exhibits a very low molecular activity compared with the other isozymes (Sakane et al., 1996), and the present work revealed unexpectedly a novel function specifically ascribed to this DGK isozyme. This may be consistent with the reports that suggest an involvement of a novel protein kinase sensitive to H89, rather than diacylglycerol-dependent protein kinase C or D for the transitional ER formation (Lee and Linstedt, 2000; Aridor et al., 2001).
Intracellular distribution of tagged DGKδ was highly heterogenous, but a portion of the molecules was consistently associated with the ER. Given that the SAM domain brings the molecule to the ER surface as revealed in the present work, why did a considerable part of DGKδ still associate with non-ER compartments? Deleterious effect of the enzyme overexpression seems unlikely, because we made time-lapse analysis of DGKδ at its very early stage of expression. Two explanations are possible. One is that the SAM domain in DGKδ may be covalently modified or sterically hindered in order to prevent its ER association. For example, the SAM domain of EphB1 was tyrosine phosphorylated so that the interaction of EphB1 with a low-molecular-weight phosphotyrosine phosphatase was regulated by the tyrosine phosphorylation cascade (Stein et al., 1996). The tyrosine residue phosphorylated in EphB1 is conserved at Tyr-1118 of the DGKδ SAM domain; thus it may be phosphorylated and prevented from association with the ER, although tyrosine phosphorylation of DGKδ is the subject of future investigation. The second possibility is the presence of another localization signal(s) in the DGKδ molecule. The PH domain at the N-terminus is a likely candidate. The PH domain was previously shown to bind to a number of polyphosphoinositides and phosphatidylserine with relatively low affinity (Kavran et al., 1998). The DGKδ PH domain appears to attribute to the cell surface association because deletion of this domain abolished the signal at the plasma membrane (see Figures 8e and 9i). The deletion of the SAM domain, on the other hand, always enhanced the enzyme localization at the plasma membranes, presumably representing binding through the PH domain (see, e.g., Figures 3d and 5B, j). Thus we suggest that DGKδ localization may be dynamically determined by a balance between actions of the two domains. We are currently trying to find the regulatory mechanisms of the membrane association of DGKδ.
In addition to the SAM domain, the presence of a PH domain distinguishes this isoform from the other members of DGK. The present work demonstrated that in addition to being targeted to the ER, the PH domain was needed for DGKδ to exert its negative effects upon the formation of the ER exit sites. Thus it is conceivable that the effects of DGKδ may be exerted through its PH domain sequestrating the target lipid(s) involved in the control of ER-to-Golgi transport, because the presence of phosphatidylinositol(3,4)P2 in the membranes is required for binding the COPII coat protein complex (Yoshihisa et al., 1993) and because association of an isoform of phosphatidylinositol-4-kinase with the ER is reported (Wong et al., 1997). A similar case has been reported for the GFP-fused PH domain of phospholipase D1, which was recruited to the cell surface and inhibited exocytosis in chromaffin cells (Holz et al., 2000). However, the possibility of DGKδ PH domain interacting with key phospholipids needs to be further explored because DGKδ with a mutation of Arg-28 to Glu in the PH domain, which is supposedly required for water-mediated contact with the 1-phosphate of phosphoinositides (Lietzke et al., 2000), inhibited anterograde transport of CFP-GT1–81 upon BFA washout as effectively as wild-type enzyme. Apparently further work is needed to characterize the role of DGKδPH domain acting in the COPII coat assembly. Previously, ionic interactions of PH domains with other proteins, presumably via a positively charged binding pocket, have been reported (Pitcher et al., 1996; Burks et al., 1998). It seems, therefore, possible that the DGKδ PH domain interacts with regulatory protein(s) rather than phospholipids required for COPII coat attachment.
ACKNOWLEDGMENTS
The authors thank Dr. Hiroshi Kimura (Oxford University, UK) for his suggestion on the bead-loading method. This study was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology, Japan (to I.W. and H.K.).
Abbreviations used:
- BFA
brefeldin A
- BSA
bovine serum albumin
- CFP
cyan fluorescent protein
- DGK
diacylglycerol kinase
- DMEM
Dulbecco's modified minimum essential medium
- ER
endoplasmic reticulum
- GFP
green fluorescent protein
- GT
galactosyltransferase
- PBS
phosphate-buffered saline
- PH
pleckstrin homology
- SAM
sterile α motif
- VSV G
vesicular stomatitis virus glycoprotein
- VTC
vesicular-tubular cluster
- YFP
yellow fluorescent protein
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.01–05-0255. Articel and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.01–05-0255.
REFERENCES
- Aridor M, Balch WE. Kinase signaling initiates coat complex II (COPII) recruitment and export from the mammalian endoplasmic reticulum. J Biol Chem. 2000;275:35673–35676. doi: 10.1074/jbc.C000449200. [DOI] [PubMed] [Google Scholar]
- Aridor M, Fish KN, Bannykh S, Weissman J, Roberts TH, Lippincott-Schwartz J, Balch WE. The Sar1 GTPase coordinates biosynthetic cargo selection with endoplasmic reticulum export site assembly. J Cell Biol. 2001;152:213–229. doi: 10.1083/jcb.152.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981;256:1604–1607. [PubMed] [Google Scholar]
- Burks DJ, Wang J, Towery H, Ishibashi O, Lowe D, Riedel H, White MF. IRS pleckstrin homology domains bind to acidic motifs in proteins. J Biol Chem. 1998;273:31061–31067. doi: 10.1074/jbc.273.47.31061. [DOI] [PubMed] [Google Scholar]
- Chi SW, Ayed A, Arrowsmith CH. Solution structure of a conserved C-terminal domain of p73 with structural homology to the SAM domain. EMBO J. 1999;18:4438–4445. doi: 10.1093/emboj/18.16.4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole NB, Ellenberg J, Song J, DiEuliis D, Lippincott-Schwartz J. Retrograde transport of Golgi-localized proteins to the ER. J Cell Biol. 1998;140:1–15. doi: 10.1083/jcb.140.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Camilli P, Emr SD, McPherson PS, Novick P. Phosphoinositides as regulators in membrane traffic. Science. 1996;271:1533–1539. doi: 10.1126/science.271.5255.1533. [DOI] [PubMed] [Google Scholar]
- Donaldson JG, Finazzi D, Klausner RD. Brefeldin A inhibits Golgi membrane-catalyzed exchange of guanine nucleotide onto ARF protein. Nature. 1992;360:350–352. doi: 10.1038/360350a0. [DOI] [PubMed] [Google Scholar]
- Fujiwara T, Oda K, Yokota S, Takatsuki A, Ikehara Y. Brefeldin A causes disassembly of the Golgi complex and accumulation of secretory proteins in the endoplasmic reticulum. J Biol Chem. 1988;263:18545–18552. [PubMed] [Google Scholar]
- Hammond AT, Glick BS. Dynamics of transitional endoplasmic reticulum sites in vertebrate cells. Mol Biol Cell. 2000;11:3013–3030. doi: 10.1091/mbc.11.9.3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauri HP, Kappeler F, Andersson H, Appenzeller C. ERGIC-53 and traffic in the secretory pathway. J Cell Sci. 2000;113:587–596. doi: 10.1242/jcs.113.4.587. [DOI] [PubMed] [Google Scholar]
- Helms JB, Rothman JE. Inhibition by brefeldin A of a Golgi membrane enzyme that catalyzes exchange of guanine nucleotide bound to ARF. Nature. 1992;360:352–354. doi: 10.1038/360352a0. [DOI] [PubMed] [Google Scholar]
- Holz RW, Hlubek MD, Sorensen SD, Fisher SK, Balla T, Ozaki S, Prestwich GD, Stuenkel EL, Bittner MA. A. pleckstrin homology domain specific for phosphatidylinositol 4, 5-bisphosphate (PtdIns-4,5-P2) and fused to green fluorescent protein identifies plasma membrane PtdIns-4,5-P2 as being important in exocytosis. J Biol Chem. 2000;275:17878–17885. doi: 10.1074/jbc.M000925200. [DOI] [PubMed] [Google Scholar]
- Hsu VW, Shah N, Klausner RD. A brefeldin A-like phenotype is induced by the overexpression of a human ERD-2-like protein, ELP-1. Cell. 1992;69:625–635. doi: 10.1016/0092-8674(92)90226-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itin C, Roche AC, Monsigny M, Hauri HP. ERGIC-53 is a functional mannose-selective and calcium-dependent human homologue of leguminous lectins. Mol Biol Cell. 1996;7:483–493. doi: 10.1091/mbc.7.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kai M, Sakane F, Imai S, Wada I, Kanoh H. Molecular cloning of a diacylglycerol kinase isozyme predominantly expressed in human retina with a truncated and inactive enzyme expression in most other human cells. J Biol Chem. 1994;269:18492–18498. [PubMed] [Google Scholar]
- Kappeler F, Klopfenstein DR, Foguet M, Paccaud JP, Hauri HP. The recycling of ERGIC-53 in the early secretory pathway. ERGIC-53 carries a cytosolic endoplasmic reticulum-exit determinant interacting with COPII. J Biol Chem. 1997;272:31801–31808. doi: 10.1074/jbc.272.50.31801. [DOI] [PubMed] [Google Scholar]
- Kavran JM, Klein DE, Lee A, Falasca M, Isakoff SJ, Skolnik EY, Lemmon MA. Specificity and promiscuity in phosphoinositide binding by pleckstrin homology domains. J Biol Chem. 1998;273:30497–30508. doi: 10.1074/jbc.273.46.30497. [DOI] [PubMed] [Google Scholar]
- Kirchhausen T. Three ways to make a vesicle. Nat Rev Mol Cell Biol. 2001;1:187–198. doi: 10.1038/35043117. [DOI] [PubMed] [Google Scholar]
- Kuge O, Dascher C, Orci L, Rowe T, Amherdt M, Plutner H, Ravazzola M, Tanigawa G, Rothman JE, Balch WE. Sar1 promotes vesicle budding from the endoplasmic reticulum but not Golgi compartments. J Cell Biol. 1994;125:51–65. doi: 10.1083/jcb.125.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TH, Linstedt AD. Potential role for protein kinases in regulation of bidirectional endoplasmic reticulum-to-Golgi transport revealed by protein kinase inhibitor H89. Mol Biol Cell. 2000;11:2577–2590. doi: 10.1091/mbc.11.8.2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmon MA, Ferguson KM. Signal-dependent membrane targeting by pleckstrin homology (PH) domains. Biochem J. 2000;350:1–18. [PMC free article] [PubMed] [Google Scholar]
- Lietzke SE, Bose S, Cronin T, Klarlund J, Chawla A, Czech MP, Lambright DG. Structural basis of 3-phosphoinositide recognition by pleckstrin homology domains. Mol Cell. 2000;6:385–394. doi: 10.1016/s1097-2765(00)00038-1. [DOI] [PubMed] [Google Scholar]
- Matsuoka K, Orci L, Amherdt M, Bednarek SY, Hamamoto S, Schekman R, Yeung T. COPII-coated vesicle formation reconstituted with purified coat proteins and chemically defined liposomes. Cell. 1998;93:263–275. doi: 10.1016/s0092-8674(00)81577-9. [DOI] [PubMed] [Google Scholar]
- McNeil PL, Warder E. Glass beads load macromolecules into living cells. J Cell Sci. 1987;88:669–678. doi: 10.1242/jcs.88.5.669. [DOI] [PubMed] [Google Scholar]
- Pitcher JA, Fredericks ZL, Stone WC, Premont RT, Stoffel RH, Koch WJ, Lefkowitz RJ. Phosphatidylinositol 4,5-bisphosphate (PIP2)-enhanced G protein-coupled receptor kinase (GRK) activity. Location, structure, and regulation of the PIP2 binding site distinguishes the GRK subfamilies. J Biol Chem. 1996;271:24907–24913. doi: 10.1074/jbc.271.40.24907. [DOI] [PubMed] [Google Scholar]
- Presley JF, Cole NB, Schroer TA, Hirschberg K, Zaal KJ, Lippincott-Schwartz J. ER-to-Golgi transport visualized in living cells. Nature. 1997;389:81–5. doi: 10.1038/38001. [DOI] [PubMed] [Google Scholar]
- Puertollano R, Randazzo PA, Presley JF, Hartnell LM, Bonifacino JS. The GGAs promotes arf-dependent recruitment of clathrin to the tgn. Cell. 2001;105:93–102. doi: 10.1016/s0092-8674(01)00299-9. [DOI] [PubMed] [Google Scholar]
- Rebecchi MJ, Scarlata S. Pleckstrin homology domains: a common fold with diverse functions. Annu Rev Biophys Biomol Struct. 1998;27:503–28. doi: 10.1146/annurev.biophys.27.1.503. [DOI] [PubMed] [Google Scholar]
- Roth MG. Lipid regulators of membrane traffic through the Golgi complex. Trends Cell Biol. 1999;9:174–179. doi: 10.1016/s0962-8924(99)01535-4. [DOI] [PubMed] [Google Scholar]
- Saito-Nakano Y, Nakano A. Sed4p functions as a positive regulator of Sar1p probably through inhibition of the GTPase activation by Sec23p. Genes Cells. 2000;5:1039–1048. doi: 10.1046/j.1365-2443.2000.00391.x. [DOI] [PubMed] [Google Scholar]
- Sakane F, Imai S, Kai M, Wada I, Kanoh H. Molecular cloning of a novel diacylglycerol kinase isozyme with a pleckstrin homology domain and a C-terminal tail similar to those of the EPH family of protein-tyrosine kinases. J Biol Chem. 1996;271:8394–8401. doi: 10.1074/jbc.271.14.8394. [DOI] [PubMed] [Google Scholar]
- Sakane F, Kanoh H. Molecules in focus: diacylglycerol kinase. Int J Biochem Cell Biol. 1997;29:1139–1143. doi: 10.1016/s1357-2725(97)00037-x. [DOI] [PubMed] [Google Scholar]
- Schultz J, Ponting CP, Hofmann K, Bork P. SAM as a protein interaction domain involved in developmental regulation. Protein Sci. 1997;6:249–253. doi: 10.1002/pro.5560060128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddhanta A, Shields D. Secretory vesicle budding from the trans-Golgi network is mediated by phosphatidic acid levels. J Biol Chem. 1998;273:17995–17998. doi: 10.1074/jbc.273.29.17995. [DOI] [PubMed] [Google Scholar]
- Smalla M, Schmieder P, Kelly M, Ter Laak A, Krause G, Ball L, Wahl M, Bork P, Oschkinat H. Solution structure of the receptor tyrosine kinase EphB2 SAM domain and identification of two distinct homotypic interaction sites. Protein Sci. 1999;8:1954–1961. doi: 10.1110/ps.8.10.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton D, Balan I, Pawson T, Sicheri F. The crystal structure of an Eph receptor SAM domain reveals a mechanism for modular dimerization. Nat Struct Biol. 1999;6:44–49. doi: 10.1038/4917. [DOI] [PubMed] [Google Scholar]
- Stein E, Cerretti DP, Daniel TO. Ligand activation of ELK receptor tyrosine kinase promotes its association with Grb10 and Grb2 in vascular endothelial cells. J Biol Chem. 1996;271:23588–235193. doi: 10.1074/jbc.271.38.23588. [DOI] [PubMed] [Google Scholar]
- Storrie B, White J, Rottger S, Stelzer EH, Suganuma T, Nilsson T. Recycling of golgi-resident glycosyltransferases through the ER reveals a novel pathway and provides an explanation for nocodazole-induced Golgi scattering. J Cell Biol. 1998;143:1505–1521. doi: 10.1083/jcb.143.6.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang BL, Peter F, Krijnse-Locker J, Low SH, Griffiths G, Hong W. The mammalian homolog of yeast Sec13p is enriched in the intermediate compartment and is essential for protein transport from the endoplasmic reticulum to the Golgi apparatus. Mol Cell Biol. 1997;17:256–266. doi: 10.1128/mcb.17.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanos CD, Goodwill KE, Bowie JU. Oligomeric structure of the human EphB2 receptor SAM domain. Science. 1999;283:833–836. doi: 10.1126/science.283.5403.833. [DOI] [PubMed] [Google Scholar]
- Tisdale EJ, Plutner H, Matteson J, Balch WE. p53/58 binds COPI and is required for selective transport through the early secretory pathway. J Cell Biol. 1997;137:581–593. doi: 10.1083/jcb.137.3.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topham MK, Prescott SM. Mammalian diacylglycerol kinases, a family of lipid kinases with signaling functions. J Biol Chem. 1999;274:11447–11450. doi: 10.1074/jbc.274.17.11447. [DOI] [PubMed] [Google Scholar]
- van Blitterswijk WJ, Houssa B. Properties and functions of diacylglycerol kinases. Cell Signal. 2000;12:595–605. doi: 10.1016/s0898-6568(00)00113-3. [DOI] [PubMed] [Google Scholar]
- Wang WK, Bycroft M, Foster NW, Buckle AM, Fersht AR, Chen YW. Structure of the C-terminal sterile alpha-motif (SAM) domain of human p73alpha. Acta Crystallogr D Biol Crystallogr. 2001;57:545–551. doi: 10.1107/s0907444901002529. [DOI] [PubMed] [Google Scholar]
- Wong K, Meyers R, Cantley LC. Subcellular locations of phosphatidylinositol 4-kinase isoforms. J Biol Chem. 1997;272:13236–13241. doi: 10.1074/jbc.272.20.13236. [DOI] [PubMed] [Google Scholar]
- Yamaguchi N, Fukuda MN. Golgi retention mechanism of beta-1,4-galactosyltransferase. Membrane-spanning domain-dependent homodimerization and association with alpha- and beta-tubulins. J Biol Chem. 1995;270:12170–12176. doi: 10.1074/jbc.270.20.12170. [DOI] [PubMed] [Google Scholar]
- Yoshihisa T, Barlowe C, Schekman R. Requirement for a GTPase-activating protein in vesicle budding from the endoplasmic reticulum. Science. 1993;259:1466–1468. doi: 10.1126/science.8451644. [DOI] [PubMed] [Google Scholar]
- Zaal KJ, Smith CL, Polishchuk RS, Altan N, Cole NB, Ellenberg J, Hirschberg K, Presley JF, Roberts TH, Siggia E, Phair RD, Lippencott-Schwartz J. Golgi membranes are absorbed into and reemerge from the ER during mitosis. Cell. 1999;99:589–601. doi: 10.1016/s0092-8674(00)81548-2. [DOI] [PubMed] [Google Scholar]