Abstract
Background:
Treatment decisions for early-stage breast cancer patients often involve discussions with multiple oncology providers. However, the extent to which primary care providers (PCPs) are involved in initial treatment decisions remains unknown.
Methods:
A stratified random sample of PCPs identified by newly diagnosed early-stage breast cancer patients from the Georgia and Los Angeles SEER registries were surveyed (N=517, 61% response rate). PCPs were asked how frequently they discussed surgery, radiation and chemotherapy options with patients, how comfortable they were with these discussions, whether they had the necessary knowledge to participate in decision-making, and their confidence in their ability to help (5-item Likert-type scales). Multivariable logistic regression was used to identify PCP-reported attitudes associated with more PCP participation in each treatment decision.
Results:
In this sample, 34% of PCPs reported that they discussed surgery, 23% discussed radiation, and 22% discussed chemotherapy options with their patients. Of those who reported more involvement in surgical decisions, 22% reported they were not comfortable having a discussion, and 17% did not feel they had the necessary knowledge to participate in treatment decision-making. PCPs who positively appraised their ability to participate were more likely to participate in all three decisions (Surgery OR: 6.01, 95%CI: 4.16–8.68; Radiation OR: 8.37, 95%CI: 5.16–13.58; Chemotherapy OR: 6.56, 95%CI: 4.23–10.17).
Conclusions:
A third of PCPs reported participating in breast cancer treatment decisions, yet gaps in knowledge about decision making and confidence in their ability to help exist. Efforts to increase PCP knowledge about breast cancer treatment options may be warranted.
Keywords: primary care provider, cancer care, cancer treatment decisions, treatment decision making, breast cancer
Precis:
The extent to which primary care providers are involved in their patients’ multimodal breast cancer treatment decisions across surgery, radiation, and chemotherapy options is assessed in this study. While a third reported participating in these decisions, a notable proportion acknowledged gaps in knowledge of treatment options and lack of confidence in their ability to help with patients’ decision making.
INTRODUCTION
Women diagnosed with early-stage breast cancer are faced with multiple complex treatment decisions. During this treatment decision-making process, they often discuss their treatment options with a number of cancer doctors, as well as with family and friends1–8. Patients may also increasingly consult their PCPs about treatment decisions, particularly as the cancer population ages9 and collaborative care models10, 11 are adopted in practice. Prior studies suggest PCP involvement in collaborative cancer care often begins as early as diagnosis, and supports that many PCPs remain engaged during the acute treatment phase.12, 13 In addition, population-based surveys of early stage cancer patients suggest that patients perceive their PCPs to be participating in their treatment decisions, with approximately one-third of breast cancer patients reporting that their PCP participated in their treatment decisions.12 Yet, we know little about the extent to which PCPs perceive they are participating in multimodal treatment decisions, and how they appraise their ability to effectively participate in shared decision-making in this context remains unclear.
Therefore, the goals of this study were to evaluate to what extent PCPs report that they participate in surgical, radiation and chemotherapy decisions for early-stage breast cancer, and to characterize PCPs’ perspectives on their ability to participate in these decisions. We also examined if PCPs’ appraisal of their ability to participate in these decisions was associated with their participation in treatment-decision making. Finally, we explored whether PCP-reported level of involvement in the three treatment decisions was concordant with patient-report of their PCPs’ involvement in their treatment decisions
METHODS
Study Population
The Individualized Cancer Care (iCanCare) Study is a large, population-based survey study of women with early-stage breast cancer and their providers, which has been described previously.14–16 We identified and accrued women, ages 20 to 79 years, with newly diagnosed, early-stage breast cancer (stages I and II) as reported to the SEER registries of Georgia and Los Angeles County, California, in 2013 to 2015 (N=5080, 70% response rate).
Women who participated in the iCanCare Study were asked to identify via survey their attending physicians, including their PCPs. Participants identified 2,946 unique PCPs. PCPs were considered to be ineligible if they were of a different specialty, were unable to be located, retired, or deceased (n=150). A stratified sample of eligible PCPs was then surveyed about their experiences caring for breast cancer patients. High-volume PCPs (identified by >1 patient in the iCanCare Study, n=618) were first selected for inclusion, and then a 10% random sample of low-volume PCPs (identified by only 1 patient in iCanCare, n=234) were selected for inclusion. Survey packets contained the PCP survey, a $40 incentive, study brochure, introductory letter, a pre-stamped return envelope, and informed consent information (physicians were not required to sign and return it with the completed survey). To encourage response, we provided a $40 cash incentive and again used a modified Dillman approach, including reminders to non-respondents. Of the 852 eligible PCPs who were mailed surveys, 518 completed them, resulting in a 60.8% response rate. Included in this analysis are 517 PCPs who were linked to 1077 eligible patients in the iCanCare patient sample. This study was approved by the University of Michigan Institutional Review Board and the state and institution institutional review boards of the SEER registries.
MEASURES
Questionnaire content was developed based upon our prior work,12, 16 literature review, and a conceptual framework hypothesizing that PCP involvement leads to improved primary care quality. We used standard techniques to assess content validity, including systematic review by design experts, cognitive pretesting with clinicians, and pilot studies in selected clinician populations.
PCP-reported level of involvement in treatment decision-making:
PCP-reported level of involvement in treatment decision-making was ascertained by asking the respondent PCPs “How often do you discuss: (1) which type of surgery a patient should have? (2) whether or not the patient should have radiation therapy? and (3) whether or not the patient should have chemotherapy? Response categories included never-always (5-pt. Likert-type scale), which were categorized into more involvement (sometimes/often/always) vs. less involvement (rarely/never) for each of the three treatment modalities (surgery, radiation, chemotherapy).
PCP-reported ability to participate in treatment decision making:
PCPs’ ability to participate in treatment decision-making was then ascertained by asking respondent PCPs how comfortable they were in discussing specific breast cancer treatments (surgery, radiation, chemotherapy) with response options not at all comfortable--extremely comfortable (5-point Likert-type scales). We also asked participants whether they had the knowledge necessary to participate in treatment decision-making, and whether they were confident in their ability to help patients’ with treatment-related decision-making, both with response options strongly disagree—strongly agree on 5-point Likert-type scales. An overall score of PCPs’ ability to participate was then created using the mean responses to the three items, with higher scores reflecting a more positive appraisal for each specific treatment decision (surgery, radiation, chemotherapy).
PCP-reported characteristics:
The PCP-reported covariates in this analysis included demographic and practice factors. Demographic characteristics collected via survey included: age at survey (in 10 year increments), gender (male/female) and race (white, black, Hispanic, Asian, or other/unknown). Practice characteristics included specialty (General/Internal Medicine, Family Medicine, OB/GYN or other), breast cancer volume (patients/year), and practice type (Physician practice vs. other practice type).
STATISTICAL ANALYSES
We first evaluated the overall proportion of PCPs who reported being more vs. less involved in surgical, radiation and chemotherapy decisions, among the respondent PCPs. The bivariate associations of PCP-reported characteristics with PCP-reported involvement in surgery, radiation and chemotherapy decisions were then evaluated in the PCP sample using two-sided t-tests and chi-square tests where appropriate. The bivariate association between the PCP-reported ability to participate in treatment decision making scores and PCP-reported involvement in surgical, radiation and chemotherapy decisions were examined using two-sided t-tests. Multivariable logistic regression was then used to characterize the association between PCP-reported ability to participate in treatment-decision making and PCP involvement in in the three treatment decisions using three separate models, adjusting for age, race, breast cancer patients/year, practice type, and SEER site. All multivariable analyses incorporated weights to account for differential probabilities of survey response across characteristics of these PCPs.
We then explored whether PCP-reported level of involvement in the three treatment decisions was associated with patient-report of their PCP involvement in their treatment decisions using the linked PCP-patient dataset (N=517 PCPs linked to 1077 patients). All analyses were performed using SAS 9.4 (SAS Institute, Cary, NC), and P < .05 was considered statistically significant.
RESULTS
Overall, 34% of the respondent PCPs reported more (somewhat/often/always) involvement in their breast cancer patients’ surgical treatment decisions vs. less involvement (never/rarely). About one quarter reported more involvement in radiation decisions (23%) and chemotherapy decisions (22%), respectively. (Figure 1)
Figure 1:
Distribution (%) of more vs. less PCP-reported involvement in surgery, radiation and chemotherapy decisions. (N=517)
*PCP-reported involvement was defined as more involvement (sometimes/often/always) vs. less involvement (rarely/never) for each of the three treatment modalities (surgery, radiation, chemotherapy) by asking respondents how often did you discuss: (1) which type of surgery a patient should have? (2) whether or not the patient should have radiation therapy? and (3) whether or not the patient should have chemotherapy? Response categories included never-always (5-pt. Likert-type scale).
Table 1 displays the distribution of more PCP involvement in surgery, radiation and chemotherapy decisions by levels of PCP-reported demographic and practice characteristics. The median age at survey was greater among PCPs who reported more involvement in the treatment decisions across all three treatment types (all p<0.01). PCP-reported race was significantly associated with PCP-reported involvement in surgery and radiation decisions, with non-white PCPs comprising a greater proportion of those who reported more involvement vs. less involvement in surgery (39.6% vs. 32.7%, p<0.01), radiation (45.1% vs. 32.3%, p=0.02) and chemotherapy decisions (44.3% vs. 32.7%, p=0.03). PCPs in private practice (vs. other practice types) comprised a greater proportion of those who reported more involvement in surgery (75.0% vs. 63.8%, p=0.01), radiation (78.0 vs. 64.5%, p<0.01) and chemotherapy decisions (75.5% vs. 64.3%, p=0.05) when compared to those who reported less involvement in these decisions. A higher breast cancer volume was also significantly associated with more PCP involvement in radiation and chemotherapy decisions (both p=0.02). Medical specialty and PCP gender were not found to be associated with the extent of PCP involvement in any of the three treatment decisions. (Table 1)
Table 1:
Distribution of PCP-reported characteristics by PCP-reported involvement in breast cancer surgery, radiation, and chemotherapy treatment decisions. (N=517)
| Surgery decision | Radiation decision | Chemotherapy decision | |||||||
|---|---|---|---|---|---|---|---|---|---|
| PCP characteristics | Less involvement N (%) | More involvement N (%) | p-value | Less involvement N (%) | More involvement N (%) | p-value | Less involvement N (%) | More involvement N (%) | p-value |
| PCP Age (mean, SE) | 52.7 (0.5) | 55.9 (0.8) | <0.01 | 53.0 (0.5) | 56.6 (1.0) | <0.01 | 53.1 (0.5) | 56.2 (1.0) | <0.01 |
| PCP Gender | 0.19 | 0.85 | 0.54 | ||||||
| Male | 181 (57.3) | 83 (50.9) | 205 (55.3) | 58 (54.2) | 203 (54.3) | 60 (57.7) | |||
| Female | 135 (42.7) | 80 (49.1) | 166 (44.7) | 49 (45.8) | 171 (45.7) | 44 (42.3) | |||
| PCP race/ethnicity | 0.14 | 0.02 | 0.03 | ||||||
| White | 202 (67.3) | 93 (60.4) | 239 (67.7) | 56 (54.9) | 241 (67.3) | 54 (55.7) | |||
| Non-white | 98 (32.7) | 61 (39.6) | 114 (32.3) | 46 (45.1) | 117 (32.7) | 43 (44.3) | |||
| Breast cancer volume/yr | 0.08 | 0.02 | 0.02 | ||||||
| <=10 | 280 (89.5) | 134 (83.8) | 330 (89.4) | 84 (80.8) | 335 (89.3) | 79 (80.6) | |||
| 11 or more | 33 (10.5) | 26 (16.2) | 39 (10.6) | 20 (19.2) | 40 (10.7) | 19 (19.4) | |||
| Practice type | 0.01 | <0.01 | 0.05 | ||||||
| Physician practice | 203 (63.8) | 123 (75.0) | 240 (64.5) | 85 (78.0) | 245 (64.3) | 80 (75.5) | |||
| Other practice type | 115 (36.2) | 41 (25.0) | 132 (35.5) | 24 (22.0) | 130 (34.7) | 26 (24.5) | |||
| Specialty | 0.51 | 0.14 | 0.36 | ||||||
| General internal med | 148 (46.7) | 79 (47.9) | 168 (45.2) | 58 (53.2) | 170 (45.3) | 56 (52.8) | |||
| Family medicine | 142 (44.8) | 66 (40.0) | 170 (45.7) | 38 (34.9) | 169 (45.1) | 39 (36.8) | |||
| Obstetrics/gynecology | 17 (5.4) | 14 (8.5) | 24 (6.4) | 7 (6.4) | 25 (6.7) | 6 (5.7) | |||
| Other | 10 (3.2) | 6 (3.6) | 10 (2.7) | 6 (5.5) | 11 (2.9) | 5 (4.7) | |||
| Site | <0.01 | 0.32 | 0.34 | ||||||
| Georgia | 197 (59.9) | 77 (46.1) | 218 (56.6) | (51.4) | 220 (56.6) | 55 (51.4) | |||
| California | 132 (40.1) | 90 (53.9) | 167 (43.4) | (48.6) | 169 (43.4) | 52 (48.6) | |||
Figure 2 displays the mean score for PCP-reported ability to participate in decision-making across levels of PCP-reported involvement in each of the three treatment decisions. PCP-reported ability to participate was positively associated with their level of participation in all three treatment decisions. PCPs with higher mean ability to participate scores were more likely to report more involvement (vs. less) in surgical decisions (mean 3.6 vs. 2.3), radiation (mean 3.8 vs. 2.3) and chemotherapy decisions (mean 3.7 vs. 2.3) (all p<0.001).
Figure 2:
Mean PCPs’ ability to participate scores (95% confidence intervals) of their ability to participate in surgery, radiation, and chemotherapy decisions by levels of PCP-reported involvement in each decision*
*all p-values for associations between PCP-reported ability to participate scores and PCP-reported involvement in treatment decisions <0.001.
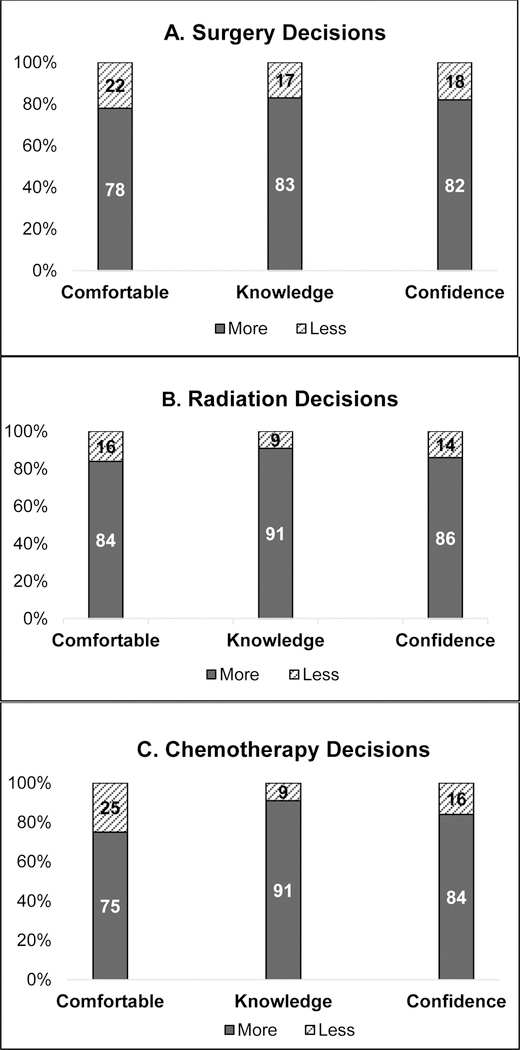
We also assessed the distribution of the individual components of the PCP-reported ability to participate scores among those who reported they were involved in the treatment decisions. Of the 34% of PCPs who reported more involvement in surgery decisions, 22% reported that they were not comfortable having these discussions and 17% reported they did not have necessary knowledge to participate in treatment decision-making. Of the 23% who reported more involvement in radiation decisions, 16% reported they were not comfortable having these discussions and 14% reported they lacked the confidence to help with these decisions. Similar gaps in comfort, knowledge and confidence were seen among those who reported they were more involved in chemotherapy decisions also. (Figure 3)
Figure 3:
Distribution (%) of the components of the PCPs’ ability to participate scores, among PCPs who reported they were more involved in treatment decisions. (N=167 surgery, N=107 radiation, N=111 chemotherapy)
Figure 4A–C display the multivariable-adjusted associations between PCP-reported ability to participate in treatment decision-making and their level of involvement in surgery (Figure 4A), radiation (Figure 4B) and chemotherapy decisions (Figure 4C). The odds of PCPs reporting more involvement in surgical treatment decisions increased greater than 6 fold for each unit increase in their ability to participate scores (OR: 6.01, 95%CI: 4.16–8.68). (Figure 4A) Similar statistically significant and positive associations were seen for the radiation and chemotherapy decisions (Radiation OR: 8.37, 95%CI: 5.16–13.58; Chemotherapy OR: 6.56, 95%CI: 4.23–10.17). (Figure 4B–C) PCP age was positively associated with more PCP-reported involvement in surgery and radiation decisions (Surgery OR: 1.41, 95%CI: 1.04–1.91; Radiation OR: 1.58, 95%CI: 1.09–2.28), but not chemotherapy decisions (OR: 1.38, 95%CI: 0.98–1.95). (Figure 4. A–C).
Figure 4:
Multivariate-adjusted odds ratios and 95% confidence intervals estimating the odds of more PCP involvement in surgery, radiation, and chemotherapy treatment decisions.
We also examined the validity of PCP report of their participation in the treatment decisions by comparing it against patient-report of their PCP’s participation in their breast cancer treatment decisions (517 PCPs linked to 1077 patients). There was a clear pattern of concordance for all three decisions, which are displayed in Online Table 1. PCP-report of their participation in surgery and radiation decisions was concordant with patient-report of the PCP’s involvement in their treatment decisions (both p<0.01).
DISCUSSION
Our findings from this sample of PCPs who treat women with early-stage breast cancer in general practice settings suggest that up to a third of PCPs report that they are involved in multimodal breast cancer treatment decisions, including surgical, radiation and chemotherapy decisions. In addition, older PCPs and those who more positively appraised their ability to participate in these decisions were more likely to be involved in these decisions. However, little variation was seen in PCP-reported involvement in these decisions across PCP-reported practice factors. To our knowledge, this is one of the first studies to assess the extent to which PCPs are involved in these complex treatment decisions and examine factors associated with their level of involvement.
Our results suggest that up to a third of PCPs participate in breast cancer surgery decisions, but participate less often in radiation and chemotherapy decisions. Prior literature suggests that PCPs may be increasingly called upon to engage in cancer treatment decision-making.12, 13 Our prior work in the iCanCare Study patient participants found that more than one-third of women reported that their PCP was involved in their breast cancer treatment decisions, and this participation was greatest among minority women, those with less education, and those with more comorbidities.12 In addition, prior research suggests that over one-third of men with prostate cancer report that their PCP helped them with their treatment decision.13 Our findings expand upon this prior work by investigating PCPs’ report of involvement in different cancer treatment decisions. As initial treatment options for early-stage breast cancer increasingly become more nuanced, PCPs may be more often consulted by their patients for their input. As such, efforts to educate PCPs about the specifics of the various treatment options so that they can effectively support patients in making high-quality decisions appears warranted.
Our findings also suggest that PCP involvement in these treatment decisions is more common among PCPs who positively appraise their ability to participate in these decisions. While the majority of PCPs in this sample positively appraised their ability to participate in these decisions, a notable minority of PCPs reported gaps in their ability to participate despite also reporting that they were involved in these treatment decisions. This included PCPs reporting they were not comfortable participating in these decisions, did not have the knowledge necessary to do so and were less confident in their ability to help with these decisions. We also assessed whether PCP appraisal of their ability to participate varied across patient demographic characteristics such as age, race, education and insurance status and found very little variation (data not shown). Thus, our findings highlight that opportunities exist to improve PCP knowledge about breast cancer treatment options, as well as their confidence in their ability to participate in cancer treatment decision-making more broadly.
As PCPs are typically the providers that manage patients’ other comorbidities and general medical care, they often have more established relationships with their patients. As a result, they may be more attuned to the preferences and values of their patients than cancer specialists whom patients have only recently met.17 Yet, discussions about how to best support PCP involvement in team-based cancer care often focus on their involvement after the initial treatment process is complete, and often do not encompass their involvement both before and during treatment. A growing body of evidence supports that PCPs want to be involved in the care of cancer patients throughout the continuum.18–21 If PCPs are engaged during the active treatment phase already, involving them in initial treatment decisions may provide an additional opportunity for shared decision-making.
Our prior work suggests that engaging other individuals who provide decision support and using online communication tools during the breast cancer decision process, led to more deliberative treatment decisions.1, 2 It is therefore plausible that additional provider engagement in the decision-making process may lead to a more deliberative and preference-sensitive decision. However, prior research suggests that receiving help from a PCP did not influence treatment patterns in men with localized prostate cancer.13 Therefore, the extent to which PCP-involvement in the treatment decision may influence patients’ appraisal of their decision-making process and their ultimate treatment decision itself remains poorly understood.
This study has a number of strengths, including the sampling of primary care doctors who care for patients with breast cancer, a high response rate for a primary care survey, and the validation of PCP report of engagement against patient experiences. However, there are limitations that merit comment. First, we do not have a direct measure of clinician involvement in decision-making. However, we did find that clinician and patient report were concordant. Second, the study was cross-sectional, and therefore our ability to make inferences about the temporality of these associations is limited. Third, we do not have information on the content of these conversations, nor do we know whether the patient or PCP initiated them. Fourth, as this was an observational study, we are unable to account for unmeasured confounders. Finally, our population included PCPs in Los Angeles County, California, and Georgia; thus, generalizability to other populations may be limited.
In conclusion, up to one-third of PCPs report participating in multimodal breast cancer treatment decisions. Yet, gaps exist in PCPs self-reported knowledge about these treatment options and their confidence in their ability to help patient with these decisions. Efforts to better incorporate and communicate with PCPs and educate them about the specifics of cancer treatments may be warranted to promote collaborative cancer care.
Supplementary Material
Acknowledgments
Funding: This work was funded by grant P01CA163233 to the University of Michigan from the National Cancer Institute and a research grant from the University of Michigan Rogel Cancer Center. Dr. Wallner’s time was also supported by K07 CA201052 from the National Cancer Institute.
Cancer incidence data collection was supported by the California Department of Public Health pursuant to California Health and Safety Code Section 103885; Centers for Disease Control and Prevention’s (CDC) National Program of Cancer Registries, under cooperative agreement 5NU58DP003862-04/DP003862; the NCI’s Surveillance, Epidemiology and End Results Program under contract HHSN261201000140C awarded to the Cancer Prevention Institute of California, contract HHSN261201000035C awarded to the University of Southern California (USC), and contract HHSN261201000034C awarded to the Public Health Institute.
Cancer incidence data collection in Georgia was supported by contract HHSN261201300015I, Task Order HHSN26100006 from the NCI and cooperative agreement 5NU58DP003875-04-00 from the CDC. The ideas and opinions expressed herein are those of the authors. The State of California, Department of Public Health, the NCI, and the CDC and their Contractors and Subcontractors had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Conflicts of Interest: Dr. Wallner reports grants from National Cancer Institute, grants from University of Michigan Rogel Cancer Center, during the conduct of the study.
References
- 1.Wallner LP, Li Y, McLeod MC, et al. Decision-support networks of women newly diagnosed with breast cancer. Cancer 2017;123: 3895–3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wallner LP, Martinez KA, Li Y, et al. Use of Online Communication by Patients With Newly Diagnosed Breast Cancer During the Treatment Decision Process. JAMA Oncol 2016;2: 1654–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maly RC, Umezawa Y, Ratliff CT, Leake B. Racial/ethnic group differences in treatment decision-making and treatment received among older breast carcinoma patients. Cancer 2006;106: 957–965. [DOI] [PubMed] [Google Scholar]
- 4.Maly RC, Umezawa Y, Leake B, Silliman RA. Determinants of participation in treatment decision-making by older breast cancer patients. Breast Cancer Res Treat 2004;85: 201–209. [DOI] [PubMed] [Google Scholar]
- 5.Hawley ST, Griggs JJ, Hamilton AS, et al. Decision involvement and receipt of mastectomy among racially and ethnically diverse breast cancer patients. J Natl Cancer Inst 2009;101: 1337–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stiggelbout AM, Jansen SJ, Otten W, Baas-Thijssen MC, van Slooten H, van de Velde CJ. How important is the opinion of significant others to cancer patients’ adjuvant chemotherapy decision-making? Support Care Cancer 2007;15: 319–325. [DOI] [PubMed] [Google Scholar]
- 7.Ohlen J, Balneaves LG, Bottorff JL, Brazier AS. The influence of significant others in complementary and alternative medicine decisions by cancer patients. Soc Sci Med 2006;63: 1625–1636. [DOI] [PubMed] [Google Scholar]
- 8.Gilbar R, Gilbar O. The medical decision-making process and the family: the case of breast cancer patients and their husbands. Bioethics 2009;23: 183–192. [DOI] [PubMed] [Google Scholar]
- 9.American Cancer Society. Breast Cancer Facts and Figures 2016–2017 Atlanta, GA, 2017. [Google Scholar]
- 10.Institute of Medicine. Delivering High Quality Cancer Care: Charting a New Course for a System in Crisis Levit LA BE, Nass SJ, and Ganz PA, editor, 2013. [PubMed]
- 11.Cohen HJ. A model for the shared care of elderly patients with cancer. J Am Geriatr Soc 2009;57 Suppl 2: S300–302. [DOI] [PubMed] [Google Scholar]
- 12.Wallner LP, Abrahamse P, Uppal JK, et al. Involvement of Primary Care Physicians in the Decision Making and Care of Patients With Breast Cancer. J Clin Oncol 2016;34: 3969–3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Radhakrishnan A, Grande D, Ross M, et al. When Primary Care Providers (PCPs) Help Patients Choose Prostate Cancer Treatment. J Am Board Fam Med 2017;30: 298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hawley ST, Janz NK, Griffith KA, et al. Recurrence risk perception and quality of life following treatment of breast cancer. Breast Cancer Res Treat 2017;161: 557–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jagsi R, Abrahamse PH, Lee KL, et al. Treatment decisions and employment of breast cancer patients: Results of a population-based survey. Cancer 2017. [DOI] [PMC free article] [PubMed]
- 16.Wallner LP, Li Y, Furgal AKC, et al. Patient Preferences for Primary Care Provider Roles in Breast Cancer Survivorship Care. J Clin Oncol 2017;35: 2942–2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roorda C, de Bock GH, Scholing C, et al. Patients’ preferences for post-treatment breast cancer follow-up in primary care vs. secondary care: a qualitative study. Health Expect 2014. [DOI] [PMC free article] [PubMed]
- 18.Del Giudice ME, Grunfeld E, Harvey BJ, Piliotis E, Verma S. Primary care physicians’ views of routine follow-up care of cancer survivors. J Clin Oncol 2009;27: 3338–3345. [DOI] [PubMed] [Google Scholar]
- 19.Lawrence RA, McLoone JK, Wakefield CE, Cohn RJ. Primary Care Physicians’ Perspectives of Their Role in Cancer Care: A Systematic Review. J Gen Intern Med 2016;31: 1222–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Potosky AL, Han PK, Rowland J, et al. Differences between primary care physicians’ and oncologists’ knowledge, attitudes and practices regarding the care of cancer survivors. J Gen Intern Med 2011;26: 1403–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith SL, Wai ES, Alexander C, Singh-Carlson S. Caring for survivors of breast cancer: perspective of the primary care physician. Curr Oncol 2011;18: e218–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.