Abstract
We aimed to determine long-term outcomes for eculizumab-treated, positive crossmatch (+XM) kidney transplant recipients compared with +XM and age-matched negative crossmatch (−XM) controls. We performed an observational retrospective study and examined allograft survival, histologic findings, long-term B-cell flow cytometric XM (BFXM), and allograft-loss–associated factors. The mean (SD) posttransplant follow-up was 6.3 (2.5) years in the eculizumab group; 7.6 (3.5), +XM control group; 7.9 (2.5), −XM control group. Overall and death-censored allograft survival were similar in +XM groups (P=.73, P=.48) but reduced compared with −XM control patients (P<.001, P<.001). In the eculizumab-treated group, 57.9% (11/19) of allografts had chronic antibody-mediated rejection, but death-censored allograft survival was 76.6%, 5 years; 75.4%, 7 years. Baseline IgG3 positivity and BFXM ≥300 were associated with allograft loss. C1q positivity was also associated with allograft loss but did not reach statistical significance. Donor-specific antibodies appeared to decrease in eculizumab-treated patients. After excluding patients with posttransplant plasmapheresis, 42.3% (9/21) had negative BFXMs; 31.8% (7/22), completely negative single-antigen beads 1 year posttransplant. Eculizumab-treated +XM patients had reduced allograft survival compared with −XM controls but similar survival to +XM controls. BFXM and complement-activating DSA (by IgG3 and C1q testing) may be used for risk stratification in +XM transplantation.
Introduction
Many highly sensitized kidney transplant candidates have a prolonged wait time for transplant despite implementation of kidney-paired donation and priority on deceased donor waiting lists as part of the new kidney allocation system (1–3). Early impact analysis of the new kidney allocation system has shown that transplant candidates with the highest calculated panel-reactive antibodies (cPRA) (>99.95%) continue to receive transplants at disproportionately low rates (1). Additionally, a group of highly sensitized individuals are unlikely to receive a human leukocyte antigen (HLA)–compatible transplant under any circumstance. In a match-run simulation, 26% of waitlisted patients with a 100% cPRA finding were incompatible on virtual crossmatch with every deceased donor organ in the system (3). Thus, “desensitization” and a kidney transplant despite a high level of donor-specific antibody (DSA) may be an important option for select, highly sensitized kidney transplant candidates, especially if they have a living donor. Previous studies have already shown that HLA-incompatible transplantation is associated with improved patient survival compared with ongoing dialysis treatment in the United States (4, 5).
Unfortunately, a kidney transplant in patients with pre-existing DSA and a positive crossmatch (+XM) comes with risk. The incidence of clinical early active antibody-mediated rejection (ABMR) in the first month after transplant can be up to 50% in these recipients (6). This has major implications because it can lead to early graft loss (6–8). Even when clinical early active ABMR is prevented or successfully managed, eventual chronic active antibody-mediated rejection (CAMR) and premature graft loss are common (9–14). Understanding the factors associated with CAMR and early allograft loss is a major unmet need to inform transplant decisions for highly sensitized candidates with limited transplant options.
We have previously shown that eculizumab (an anti-C5 antibody that blocks terminal complement) after +XM kidney transplants is associated with reduced clinical early active ABMR, yet the incidence of CAMR and death-censored allograft survival was similar to that of historical controls after 2 years of follow-up (15). Eculizumab has also been shown to decrease the incidence of ABMR within 3 months post transplant in +XM kidney transplant recipients whose results for DSA were C1q positive (16). The long-term, death-censored allograft survival of patients treated with eculizumab remains unknown. Studying this eculizumab-treated, +XM cohort is important because most of these patients did not receive treatments to remove antibody after transplant (ie, rituximab, intravenous immunoglobulin [IgG], or plasmapheresis), and their outcomes can provide insights into the natural history of DSA after transplantation.
Therefore, we aimed to 1) determine allograft survival and histologic characterization for eculizumab-treated, +XM kidney transplant recipients followed up for 6.5 to 9.5 years after transplant; 2) assess DSA longitudinally in a +XM cohort who largely did not receive treatments to reduce DSA after transplant; and 3) evaluate whether there were pretransplant characteristics associated with early death-censored allograft loss in eculizumab-treated, +XM recipients (IgG3 subclass, C1q binding, and DSA class).
Materials and Methods
Patients and Treatment Protocols
This study was approved by the Mayo Clinic Institutional Review Board (Protocol No. 15–007703), and informed consent was waived for those who provided research authorization. We performed a retrospective observational study of recipients of a +XM kidney transplant. We extended the follow-up of a +XM cohort who received eculizumab to prevent early active ABMR (n=30 ABO-compatible, living-donor transplant recipients who received a transplant from June 2008 through October 2011) and a historical +XM group (n=48 ABO-compatible, living-donor transplant recipients who received a transplant from January 2005 through September 2007) (17). We also compared patient survival, allograft survival, and allograft function of these +XM kidney transplant recipients with an age-matched negative crossmatch (−XM) control group (n=78 ABO-compatible, living-donor kidney transplant recipients who received a transplant from October 2007 through October 2011).
As previously reported, the eculizumab-treated +XM group and historical control group consisted of a cohort of +XM kidney transplant recipients with a living donor and a B-cell flow cytometric crossmatch (BFXM) channel shift ≥200 (17). Our protocol was that patients with a BFXM channel shift ≥300 underwent pretransplant plasmapheresis to achieve BFXM channel shifts of <300 on the day of transplant (18). However, 1 patient in the eculizumab-treated group had a pretransplant BFXM of 420 despite multiple plasmapheresis treatments. Immunosuppression consisted of antithymocyte globulin induction and maintenance with tacrolimus, mycophenolate mofetil, and prednisone.
The eculizumab dosing regimen was as previously reported (15). Briefly, each patient received 1,200 mg immediately before transplant, 600 mg on postoperative day 1, and 600 mg weekly for the next 4 weeks. The BFXM was repeated at postoperative weeks 4, 9, 26, and 39 to determine further eculizumab dosing. If the BFXM was <200, eculizumab was discontinued. In patients whose BFXM remained ≥200, eculizumab treatment was continued (1,200 mg at week 5 and then every 2 weeks).
BFXM and DSA Testing
A 3-color cytometric XM was used for BFXM testing. Specifically, fluorescein isothiocyanate-conjugated F(ab)′2 goat antihuman IgG was used to assess alloantibody binding with indirect immunofluorescence and 2 other fluorescence parameters (CD3 PerCP and CD19PE) to distinguish T and B cells. Donor cells were treated with pronase (1 μg/2 mL) for 15 minutes, and then aliquots (300,000) were mixed with 20 μL of control or recipient serum. The mixture was incubated for 30 minutes and washed. Fluorescent antibodies were added, incubated for 15 minutes, washed, and aliquots acquired on FACSCalibur (BD Biosciences) using a 1024 scale. The fluorescence intensity of the donor B lymphocytes mixed with recipient serum was compared to the fluorescent intensity of donor cells mixed with negative control serum. The interpretation was based on the right “shift” of the mean channel fluorescence obtained from the mixture of donor lymphocytes and negative control serum. For the BFXM, a mean channel shift ≥106 was considered a positive finding.
Single antigen bead (SAB) assays (LABScreen, One Lambda, Inc) were performed to determine alloantibody specificities and levels (mean fluorescence intensity [MFI]) at baseline (before any desensitization procedures but <30 days before transplant), at regular intervals during the first year after transplant, and yearly thereafter. Specifically, in the eculizumab-treated +XM group, SAB was tested posttransplant at day 7, 14, 28, 90, 180, and 365. In the +XM control group, SAB was checked retrospectively on banked serum from baseline. Dilution analysis to identify prozone or serum interference, or both, was not systemically performed, and the sera was not pretreated.
IgG3 subclass testing was performed for patients in the eculizumab group who had banked serum. Testing was performed with Luminex (Luminex Corp), as described elsewhere (19). Briefly, the LABScreen assay was performed according to manufacturer instructions, except for replacement of phycoerythrin-conjugated secondary mouse monoclonal antihuman IgG (One Lambda, Inc). For IgG3 subclass testing, antihuman IgG specific to IgG subclass hinge regions was used (IgG3: HP6050, SouthernBiotech). Trimmed MFI values were normalized against the negative control (bead #1) and with phosphate-buffered saline (negative control sample). The subclass secondary antibody sensitivity and cross-reactivity were verified with control beads coated with an IgG3 subclass protein (EMD Chemicals) at the Terasaki Research Institute, Los Angeles, California. The secondary antibody used for subclass testing was found to be sensitive at detection of IgG3 subclass proteins and not cross-reactive with other IgG subclasses at a dilution of 1:20. Bead #2 of the LABScreen assay was the positive control bead.
For the C1q assay, testing was done by using heat-inactivated serum (56°C for 30 minutes) spiked with 150 mg/mL of purified human C1q in HEPES buffer (One Lambda, Inc) to obtain equivalent functional quantities of C1q per sample. LABScreen SABs were added, incubated for 20 minutes at room temperature, and then phycoerythrin-conjugated antihuman C1q added. Beads were washed twice and analyzed on a LABScan 200 flow analyzer (Luminex Corp). Total IgG, IgG3 subclass, and C1q positivity were defined as having a normalized MFI >1,000.
Biopsy Assessment
Biopsy scores were compared between eculizumab-treated and +XM control patients at the 5-year biopsy time point. Results from −XM patients have been previously published by our group and were not repeated (20, 21). Kidney biopsy tissue from the eculizumab-treated patients was processed for light microscopy and stained by immunofluorescence for C4d (AbD Serotec).
The updated Banff 2017 classification was used for ABMR (22). The classification includes 1) DSA detected in the serum; 2) histologic evidence of tissue injury (glomerulitis [g] score >0 and/or peritubular capillaritis [ptc] score >0); acute thrombotic microangiopathy; or acute tubular injury; and 3) evidence of antibody interaction with the vascular endothelium indicated by linear peritubular capillarity C4d staining (C4d2 or C4d3 by immunofluorescence on frozen section) or moderate microvascular inflammation (ptc + g score >2). CAMR was determined by the presence of active ABMR findings with concomitant transplant glomerulopathy (Banff chronic glomerulopathy >0 lesion) (22). C4d positivity was defined as at least focal (≥10%) peritubular capillary staining (Banff C4d score >1) with immunofluorescence on frozen section.
Statistical Analysis
Statistical analysis was done with JMPv13 (SAS Institute, Inc). Means or medians with standard deviation and interquartile range (IQR) were used to express the results, depending on whether data were normally distributed. t tests and analysis of variance were used to compare the quantitative values with a normal distribution, and Wilcoxon or Kruskal-Wallis tests were used for variables without normal distribution. χ2 or Fisher exact tests, or both, were used to evaluate categorical variables. Categorical variables were compared using χ2 or 2-tailed Fisher exact tests. The Kaplan-Meier method was used to determine patient and allograft survival, and the log-rank test was used to compare data. Matched-pairs analysis was done to compare the change in BFXM or SAB results from baseline to 1 and 5 years. Statistical significance was determined by a 2-tailed P value of <.05.
Results
Patient Demographics
From June 2008 through October 2011, 30 patients had a +XM transplant and received eculizumab, as previously described (15, 17). Forty-eight historical +XM control patients received transplants from January 2005 through September 2007. All +XM patients from the 2 groups received a living-donor transplant. Both +XM groups were similar in age, sex, race, cause of end-stage renal disease (ESRD), mean HLA mismatch, and history of kidney transplant (Table 1). The mean (SD) age of all +XM patients was 47.9 (10.0) years. Most patients were white (93.6% [73/78]) women (76.9% [60/78]), and the main cause of ESRD was glomerulonephritis (37.2% [29/78]). The eculizumab-treated and +XM control patients only differed in type of living-donor transplant. In the eculizumab group, 26.7% (8/30) received a living-related donor kidney transplant, whereas 66.7% (32/48) of patients in the +XM control group received a living-related donor kidney transplant (P=.001).
Table 1.
Baseline Demographic Characteristics
| Characteristics | Eculizumab-Treated +XM Patients (n=30) | +XM Control Patients (n=48) | All +XM Patients (n=78) | −XM Control Patients (Age-Matched) (n=78) | P Value Eculizumab-Treated +XM vs +XM Control Patients | P Value −XM Control (Age-Matched) vs All +XM Patients |
|---|---|---|---|---|---|---|
| Age at transplant, mean (SD), y | 47.8 (12.7) | 47.9 (11.0) | 47.9 (10.0) | 48.4 (12.3) | .91 | .70 |
| Male sex, No. (%) | 8 (26.7) | 10 (20.8) | 18 (23.1) | 50 (64.1) | .36 | .001 |
| Race, No. (%) | .25 | .18 | ||||
| White | 29 (96.7) | 44 (91.7) | 73 (93.6) | 74 (94.9) | ||
| African American | 0 (0) | 3 (6.3) | 3 (3.8) | 2 (2.6) | ||
| Hispanic | 0 (0) | 1 (2.1) | 1 (1.3) | 0 (0) | ||
| Asian | 1 (3.3) | 0 (0) | 1 (1.3) | 1 (1.3) | ||
| American Indian | 0 (0) | 0 (0) | 0 (0) | 1 (1.3) | ||
| Cause of end-stage renal disease, No. (%) | .16 | .03 | ||||
| Glomerulonephritis | 13 (43.3) | 16 (33.3) | 29 (37.2) | 37 (47.4) | ||
| Polycystic kidney disease | 4 (13.3) | 7 (14.6) | 11 (14.1) | 14 (17.9) | ||
| Diabetes mellitus | 1 (3.3) | 8 (16.7) | 9 (11.5) | 14 (17.9) | ||
| Hypertension | 2 (6.7) | 0 (0) | 2 (2.6) | 5 (6.4) | ||
| Other/unknown | 10 (33.3) | 17 (35.4) | 27 (34.6) | 8 (10.2) | ||
| Type of donor, No. (%) | ||||||
| Living-related | 8 (26.7) | 32 (66.7) | 40 (51.3) | 49 (62.8) | .001 | .20 |
| Living-unrelated | 22 (73.3) | 16 (33.3) | 38 (48.7) | 29 (37.2) | ||
| HLA mismatch, mean (SD) | 3.9 (1.3) | 3.3 (1.4) | 3.5 (1.4) | 3.2 (1.8) | .34 | .26 |
| History of transplant, No. (%) | 14 (46.7) | 20 (41.7) | 34 (43.6) | 10 (12.8) | .52 | <.001 |
| Prior dialysis, No. (%) | 17 (56.7) | 36 (75.0) | 53 (67.9) | 45 (57.7) | .25 | .13 |
| Posttransplant allograft follow-up, mean (SD), y | 6.3 (2.5) | 7.6 (3.5) | 7.09 (3.2) | 7.9 (2.5) | .03 | .03 |
| Patient survival, mean (SD), y | 6.8 (2.2) | 8.7 (3.2) | 8.0 (3.0) | 8.3 (2.3) | .005 | .45 |
Abbreviations: HLA, human leukocyte antigen; −XM, negative crossmatch; +XM, positive crossmatch.
Compared with the +XM groups, the −XM control group was age matched but comprised more men (64.1% [50/78] vs 23.1% [18/78]; P=.001) and fewer prior kidney transplant recipients (12.8% [10/78] vs 43.6% [34/78]; P<.001).
The mean BFXM and DSA class present pretransplant in the eculizumab and +XM control groups were similar (Table 2). The mean (SD) BFXM in the eculizumab group was 306 (92) compared with 323 (78) in the +XM control group (P=.35). Class I DSA only, class II DSA only, and class I and II DSA were present in 36.7%, 30.0%, and 33.3% of eculizumab-treated patients and 39.5%, 25.0%, and 35.4% of +XM control patients, respectively (P=.89).
Table 2.
Baseline DSA and Crossmatch Testing
| Variable | Eculizumab-Treated +XM Patients (n=30) | +XM Control Patients (n=48) | P Value Eculizumab vs +XM Control Patients |
|---|---|---|---|
| Baseline B-cell flow cytometric crossmatch, mean (SD) | 306 (92) | 323 (78) | .35 |
| Class I DSA only, No. (%) | 11 (36.7) | 19 (39.5) | |
| Class II DSA only, No. (%) | 9 (30.0) | 12 (25.0) | |
| Both class I + II DSA, No. (%) | 10 (33.3) | 17 (35.4) | .89 |
| Anti-A specificity, No. (%) | 17 (56.7) | 24 (50.0) | .57 |
| Anti-B specificity, No. (%) | 17 (56.7) | 20 (41.7) | .20 |
| Anti-DR specificity, No. (%) | 13 (43.3) | 18 (37.5) | .61 |
| Anti-DQ specificity, No. (%) | 12 (40.0) | 20 (41.7) | .88 |
| Sum class I DSA MFI, mean (SD) | 4,193.3 (4,889.0) | 4,556.68 (5,083.0) | .76 |
| Sum class II DSA MFI, mean (SD) | 4,037.07 (5,183.3) | 3,128 (4,141.2) | .40 |
| Sum of class I and class II DSA MFI, mean (SD) | 11,905.0 (8,985.3) | 9,592.5 (7,806.2) | .24 |
| Number of DSA per patient, mean (SD) | 2.2 (1.0) | 2.6 (1.4) | 0.12 |
| Pretransplant plasmapheresis, No. (%) | 17 (56.7) | 32 (66.7) | .52 |
| Number of pretransplant plasmapheresis treatments, mean (SD) | 4.6 (1.3) | 4.4 (1.4) | .78 |
| IgG3+ (n=15), No. (%) | 8 (53.3) | NA | |
| C1q+ (n=18), No. (%) | 14 (77.8) | NA |
Abbreviations: DSA, donor-specific antibody; IgG, immunoglobulin G; MFI, mean fluorescence intensity; NA, not applicable; −XM, negative crossmatch; +XM, positive crossmatch.
Patient and Allograft Survival
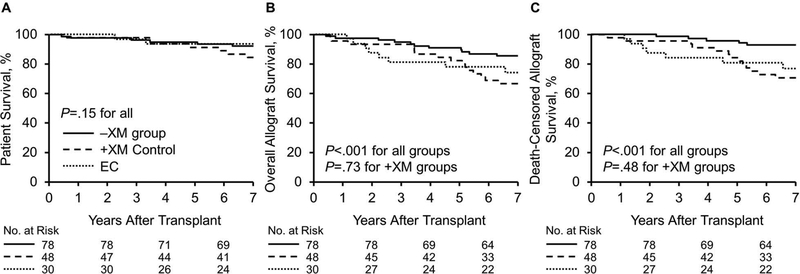
Patient survival was similar among all +XM and −XM kidney transplant recipients over a mean (SD) follow-up of 6.8 (2.2) years in the eculizumab group; 8.7 (3.2) years, +XM control group; and 8.3 (2.3) years, age-matched −XM control group (P=.15) (Figure 1A). The mean (SD) posttransplant allograft follow-up was 6.3 (2.5) years for the eculizumab group; 7.6 (3.5) years, +XM control group; and 7.9 (2.5) years, −XM control group. Overall allograft survival and death-censored allograft survival were similar in the +XM groups (P=.73, P=.48, respectively), but both were reduced compared with the −XM control group, (P<.001, P<.001, respectively) (Figure 1B and 1C).
Figure 1.
Patient and Allograft Survival. A, Patient survival was similar among all groups over mean (SD) posttransplant patient follow-up of 6.8 (2.2) years in the eculizumab group, 8.7 (3.2) years in the +XM control group, and 8.3 (2.3) years in the age-matched −XM group. B and C, Overall and death-censored allograft survival was similar in the +XM groups, but both were reduced as compared with the −XM group over mean (SD) posttransplant follow-up of 6.3 (2.5) years, 7.6 (3.5), and 7.9 (2.5) years in the eculizumab, +XM control, and −XM control groups, respectively. EC indicates eculizumab; −XM, negative crossmatch; +XM, positive crossmatch.
Specifically, the overall 5- and 7-year posttransplant survival rates were 81.3% and 74.1% in the eculizumab group, 82.2% and 66.7% in the +XM control group, and 92.1% and 85.3% in the −XM group. Death-censored allograft survival rates at 5 and 7 years were 80.9% and 76.8% in the eculizumab group, 84.3% and 70.5% in the +XM control group, and 95.9% and 91.6% in the −XM control group.
Thirty-seven patients studied had death-censored allograft failure during follow-up. The cause of failure was identified by allograft biopsy in 94.6% (35/37) of cases (Table 3). Most death-censored allograft failures resulted from CAMR in the +XM groups, whereas causes of allograft failure in the −XM control group varied. CAMR caused graft loss in only 12.5% (1/8) of the −XM control patients.
Table 3.
Causes of Death-Censored Allograft Failure
| No. (%) | |||
|---|---|---|---|
| Cause of Allograft Failure | Eculizumab-Treated +XM Patients (n=8) | +XM Control Patients (n=21) | −XM Control Patients (n=8) |
| Chronic antibody-mediated rejection | 8 (100) | 18 (85.7) | 1 (12.5) |
| Nonadherence/acute rejection | 0 (0) | 0 (0) | 3 (37.5) |
| Chronic volume depletion | 0 (0) | 0 (0) | 1 (12.5) |
| Recurrent IgA nephropathy | 0 (0) | 1 (4.8) | 1 (12.5) |
| Focal segmental glomerulosclerosis | 0 (0) | 1 (4.8) | 0 (0) |
| Infection | 0 (0) | 0 (0) | 1 (12.5) |
| Unknown | 0 (0) | 1 (4.8) | 1 (12.5) |
Abbreviations: IgA, immunoglobulin A; −XM, negative crossmatch; +XM, positive crossmatch.
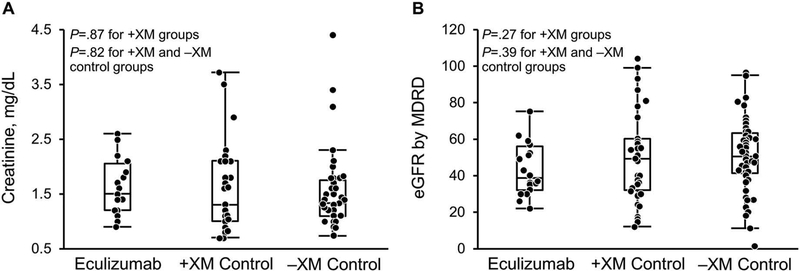
Allograft Function
Among patients with functioning allografts at 5 years after transplant, allograft function was similar in the +XM and −XM groups (Figure 2). The median (IQR) creatinine (mg/dL) in the eculizumab, +XM control, and −XM groups was 1.5 (1.2–2.1), 1.3 (1.0–2.1), and 1.4 (1.1–1.8), respectively, at 5 years (P=.87, comparing eculizumab and +XM control group; P=.82, comparing all +XM patients with −XM control patients). The median (IQR) estimated glomerular filtration rate using the Modification of Diet in Renal Disease equation was 38.5 (32.0–55.8) in the eculizumab group; 49 (32–60), +XM control group; and 49.8 (40.7–63.4), −XM control group (P=.27, comparing eculizumab and the +XM control group; P=.39, comparing all +XM patients with −XM control patients).
Figure 2.
Renal Allograft Function 5 Years After Transplant. Allograft function in surviving allografts was similar for the +XM and −XM groups. eGFR indicates estimated glomerular filtration rate; MDRD, Modification of Diet in Renal Disease; −XM, negative crossmatch; +XM, positive crossmatch.
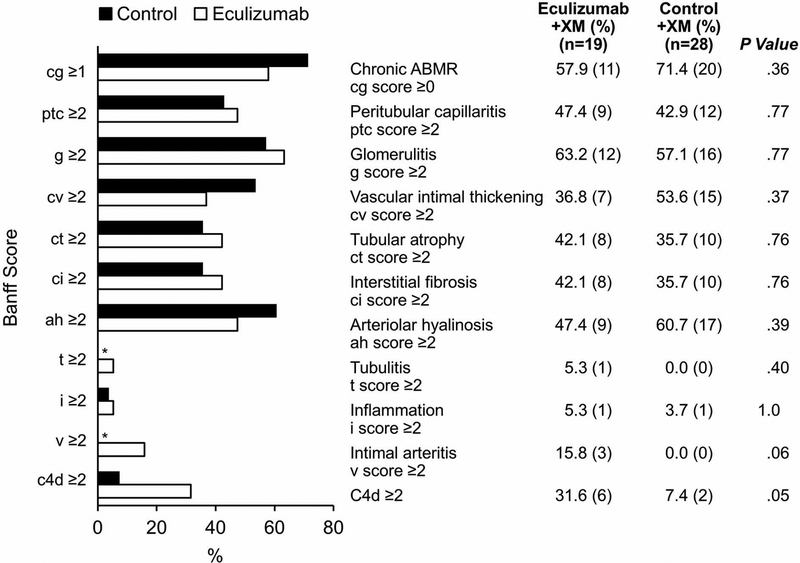
Histologic Findings for Renal Allografts 5 Years After Transplant
Of the eculizumab cohort, 79.2% (19/24) of surviving patients with a functioning allograft underwent a 5-year protocol biopsy (Figure 3). Of the +XM control group, 68.3% (28/41) underwent a 5-year protocol biopsy. The biopsy findings were similar between the 2 groups. In the eculizumab group, 57.9% (11/19) had CAMR (Banff cg score ≥0) compared with 71.4% (20/28) of the +XM control group (P=.36). The prevalence of peritubular capillaritis was 47.4% (9/19) in the eculizumab group and 42.9% (12/28) in the historical +XM control group (P=.77). Glomerulitis was present in 63.2% (12/19) of the eculizumab-treated patients and 57.1% (16/28) of the +XM control patients (P=.77). Among the eculizumab-treated patients, 31.6% (6/19) were positive for C4d, whereas only 7.1% (2/28) of the +XM control patients had C4d positivity (P=.05).
Figure 3.
5-Year Protocol Biopsy Findings for +XM Recipients. Findings were similar in the eculizumab and +XM control groups across all Banff scores. C4d staining was performed using immunofluorescence. ABMR indicates antibody-mediated rejection; g, glomerulitis; cg, chronic glomerulopathy; +XM, positive crossmatch. *No patients in this group had these biopsy findings.
Longitudinal Assessment of DSA After Transplantation in Eculizumab-Treated Patients
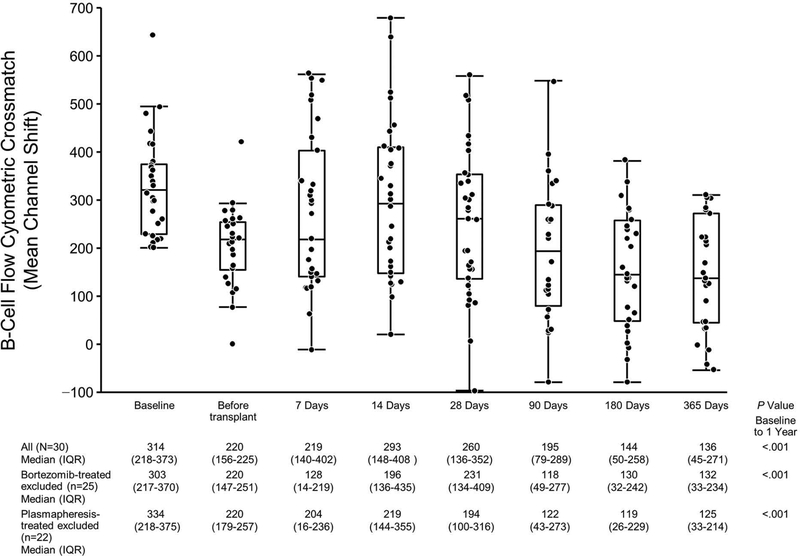
Most patients (66.7%, 20/30) in the eculizumab +XM cohort did not receive any antibody-depleting therapy post transplant, but bortezomib was given to 16.7% (5/30), and plasmapheresis therapy was used for 26.7% (8/30) of patients. However, both the BFXM channel shift and sum MFI from the SAB assay decreased over the first year regardless of therapy in most patients (Figures 4 and 5).
Figure 4.
BFXM the First Year Post Transplant in Eculizumab-Treated Patients. The scatterplot shows the BFXM for all tested patients at the time points indicated. BFXM was expressed as mean channel shift (≥106 was considered positive). Matched-pairs analysis was performed to compare BFXM at baseline to that at 1 year. BFXM indicates B-cell flow cytometric crossmatch; IQR, interquartile range.
Figure 5.
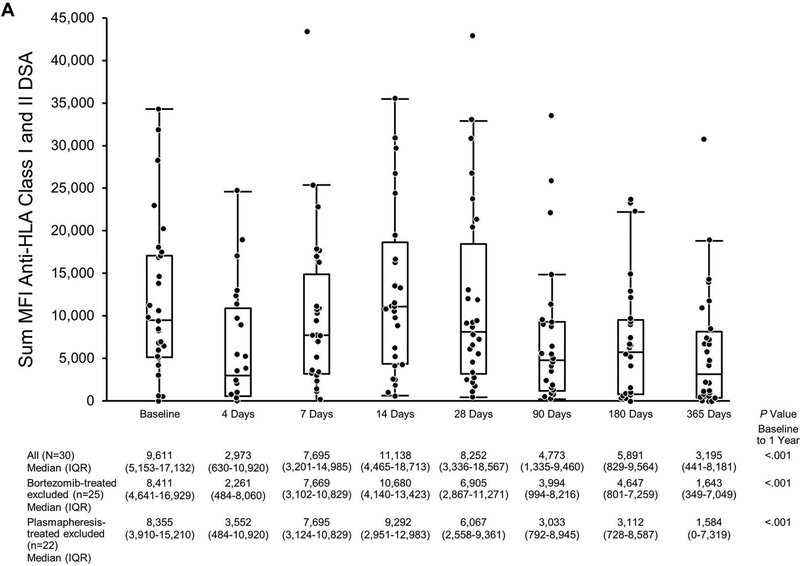
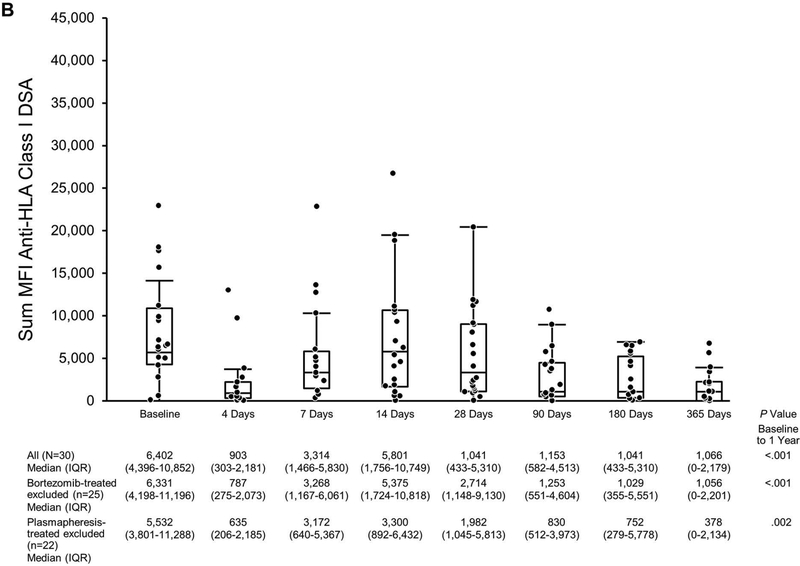
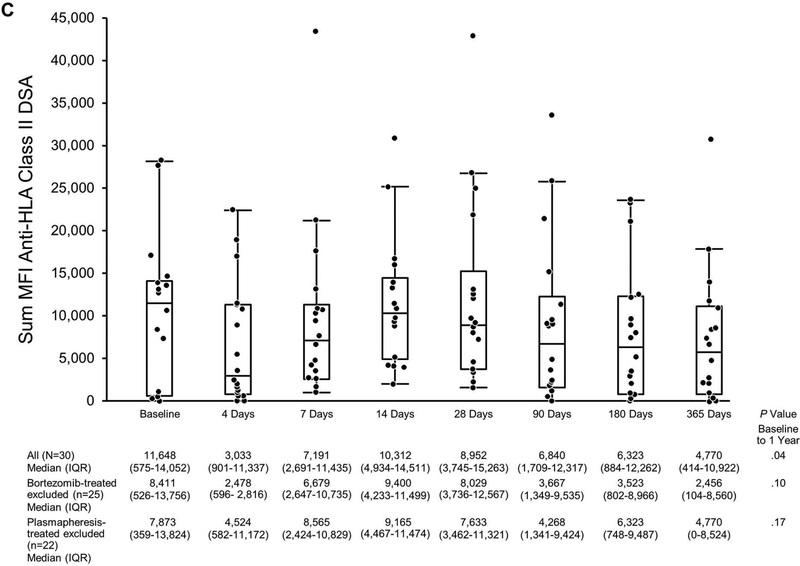
Donor-Specific Antibody the First Year Post Transplant in Eculizumab-Treated Patients Based on MFI. The scatterplot shows sum MFIs: A, Anti-HLA class I and class II DSA; B, Anti-HLA class I DSA only; C, Anti-HLA class II DSA only. Matched pairs analysis was performed to compare sum MFI at baseline to that at 1 year. DSA indicates donor-specific antibody; HLA, human leukocyte antigen; MFI, mean fluorescence intensity.
At 1 year post transplant, 33.3% (9/27) of patients had a negative BFXM. The median (IQR) BFXM channel shift at baseline was 314 (218–373). Within 1 year, the channel shift decreased to a median (IQR) of 136 (45–271) (P<.001, matched-pairs analysis) (Figure 4). At 1 year post transplant, 23.3% (7/30) of eculizumab +XM patients also did not have DSA based on the SAB assay (MFI was below the level of detection).
The median (IQR) sum MFI anti-HLA class I + class II DSA at baseline was 9,611 (5,153–17,132) and 3,195 (441–8,181) at 1 year (P<.001, matched-pairs analysis) (Figure 5A).
Class I and class II anti-HLA DSA MFI were analyzed separately as shown in Figures 5B and 5C. Class I median (IQR) sum MFI decreased from 6,402 (4,396–10,852) at baseline to 1,066 (0–2,179) at 1 year (P<.001, matched-pairs analysis). Class II median (IQR) sum MFI was 11,648 (575–14,052) at baseline; 4,770 (414–10,922) at 1 year (P=.04, matched-pairs analysis).
Even when patients treated with bortezomib or plasmapheresis were excluded, several BFXM and SAB tests became negative (Figures 4 and 5). In the eculizumab-treated patients who did not receive bortezomib, 40.9% (9/22) had a negative BFXM result at 1 year, and 28.0% (7/25) had negative SAB tests (MFI below the level of detection). When patients who received plasmapheresis were excluded, 42.3% (9/21) had a negative BFXM result, and 31.8% (7/22) had completely negative SAB tests for DSA at 1 year post transplant.
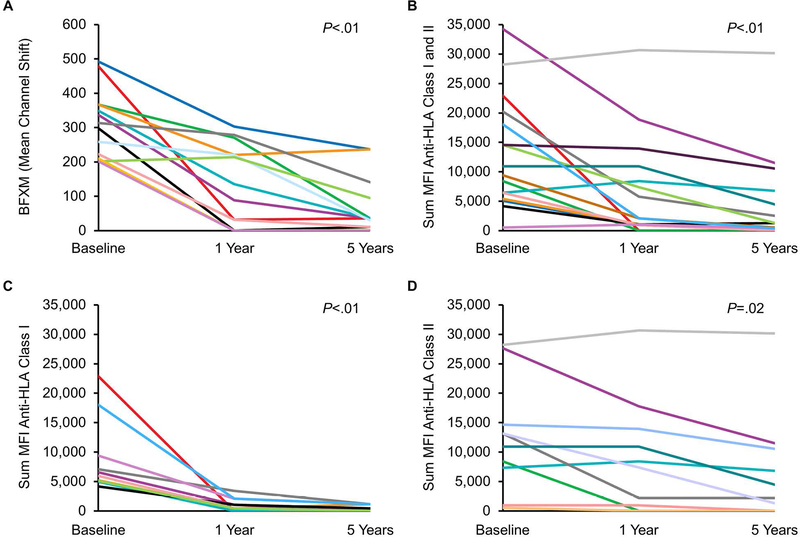
In the subset of 13 patients who had a BFXM at 5 years, the median (IQR) BFXM mean channel shift decreased from 306 (215–363) to 37 (13–118) (P<.01, matched-pairs analysis) (Figure 6A). Specifically, 76.9% (10/13) of the tested patients had a negative BFXM by 5 years. In the subset of patients who had an SAB test at 5 years, the median (IQR) of sum MFI decreased from 9,999 (5,377–19,681) at baseline to 1,006 (0–5,059) at 5 years (P<.01, matched-pairs analysis) (Figure 6B). This decrease was found for both anti-HLA class I and anti-HLA class II (Figures 6C and 6D). At 5 years, 25.0% (4/16) of patients had completely negative SAB tests for DSA (MFI below the level of detection). Only 50% (8/16) had SAB tests that were positive for DSA with MFI >1,000.
Figure 6.
Longitudinal Assessment of Donor-Specific Antibody the First 5 Years Post Transplant in Eculizumab-Treated Patients. The line plots shows results from baseline to 5 years in subsets of patients with testing: A, BFXM; B, Sum MFI anti-HLA class I and II; C, Sum MFI anti-HLA class I; D, Sum MFI anti-HLA class II. BFXM was expressed as mean channel shift (≥106 was considered positive). Matched-pairs analysis was performed to compare results from baseline to 5 years. BFXM indicates B-cell flow cytometric crossmatch; HLA, human leukocyte antigen; MFI, mean fluorescence intensity.
Factors Associated With Death-Censored Allograft Survival in +XM Groups
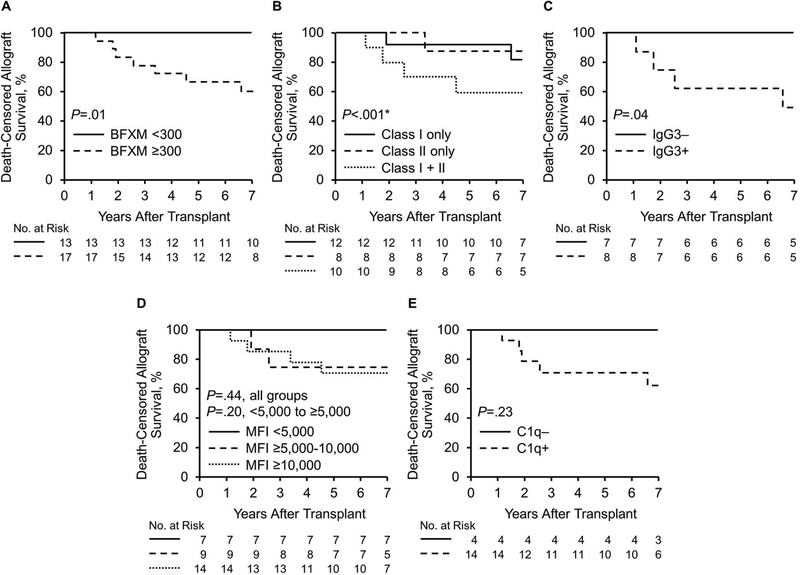
The main pretransplant factors associated with death-censored allograft loss in the eculizumab-treated +XM cohort included having a BFXM ≥300, both class I and class II DSA, and IgG3+ DSA (Figure 7). During mean (SD) follow-up of 6.3 (2.5) years, 44.4% (8/18) of allografts failed in patients with a baseline BFXM of ≥300, whereas 0% (0/13) of allografts failed in patients with a BFXM <300 (P=.01). The 5- and 7-year actuarial death-censored allograft survival was 66.7% and 60.0% for patients with a BFXM ≥300 (P=.01) (Figure 7A).
Figure 7.
Factors Associated With Death-Censored Allograft Loss in the Eculizumab-Treated +XM Cohort. A, BFXM; B and C, Class I + II donor-specific antibodies and IgG3 positivity were associated with death-censored allograft loss in eculizumab-treated patients. D, Death-censored allograft loss based on sum MFI DSA; E, Death-censored allograft loss in C1q negative and positive patients. BFXM indicates B-cell flow cytometric crossmatch; IgG, immunoglobulin; MFI, mean fluorescence intensity. *Hypothesis testing was between the class I + class II DSA group and the class I only and class II only groups combined.
Patients with class I + II DSA in the eculizumab group had a death-censored allograft failure rate of 54.5% (6/11) during follow-up, whereas patients with only class I DSA had a failure rate of 15.4% (2/13) and only class II DSA, 12.5% (1/8) (P<.001, class I + II vs class I or II only). The 5- and 7-year actuarial death-censored allograft survival rates in patients with class I + II DSA were 60.0% (Figure 7B). Half (4/8) of the patients who had IgG3+ DSA at baseline had allograft failure during follow-up, whereas 0% (0/8) of patients who had negative findings for IgG3 had death-censored allograft failure. The 5- and 7-year actuarial survival rates of patients with IgG3+ DSA were 62.5% and 50.0% (Figure 7C).
When the sum MFI was <5,000, the death-censored allograft loss was numerically lower than that in patients with an MFI of ≥5,000, but this did not reach statistical significance (comparing MFI <5,000, ≥5,000 and <10,000, and ≥10,000 [P=.44] and comparing MFI <5,000 and ≥5,000 [P=.20]) (Figure 7D). Additionally, patients who were C1q positive at baseline had a numerically higher rate of death-censored allograft loss, but this finding also did not reach statistical significance (0% [0/4] vs 35.7% [5/14] (P=.23) (Figure 7E).
Relationships existed among BFXM, IgG3, and class I + II DSA. Both the presence of class I + II DSA (P=.03) and IgG3 positivity (P=.03) were associated with having a BFXM ≥300. All 8 patients with IgG3+ DSA at baseline had a BFXM ≥300, and 72.7% (8/11) of all patients with a BFXM ≥300 were IgG3+. Of the 11 patients with class I + II DSA, 81.8% (9/11) had a BFXM ≥300. In contrast, the proportion of patients with C1q positivity was similar in both BFXM groups (P=.87). In the group with BFXM ≥300, 78.8% (11/14) were positive for C1q; in the BFXM <300 group, 75.0% (3/4) were positive for C1q.
In the +XM control group, patients with a BFXM <300 had a numerically higher 5- and 7-year actuarial allograft survival rate than those with a BFXM ≥300, but this did not reach statistical significance (P=.12). The 5- and 7-year actuarial survival rates in the BFXM <300 group were 94.1% and 81.6% compared with 80.0% and 66.5% in the BFXM ≥300 group (Figure S1A). The sum of the DSA MFI correlated with death-censored allograft survival in this group. The 5- and 7- year death-censored allograft survival rates in patients with sum MFI of DSA of <5,000 was 93.7% compared with 80.7% and 60.9% in the group with sum MFI of ≥5,000 (Figure S1B and S1C).
Unlike in the eculizumab cohort, death-censored allograft survival was similar in patients with only 1 class of DSA as compared with those with class I + II DSA (P=.31). The 5- and 7-year death-censored allograft survival rates in patients with class I + II were 75.0% and 68.8% in the +XM control group (Figure S1D).
Discussion
Reduction in the rate of clinical early active ABMR in eculizumab-treated recipients with a positive BFXM result did not translate into a reduced rate of chronic CAMR or improve death-censored allograft survival over a mean (SD) follow-up of 6.8 (2.2) years. However, 5- and 7-year death-censored allograft survival appears acceptable for subgroups of +XM kidney transplant recipients, particularly those with a low BFXM (<300) or negative IgG3 findings at baseline. No death-censored allograft loss during follow-up was observed in eculizumab-treated +XM recipients with a BFXM <300; and in the +XM control group with BFXM <300, the 5- and 7-year death-censored allograft survival rates were 94.1% and 81.6%. Thus, a +XM kidney transplant may be a reasonable option for select, highly sensitized kidney transplant candidates with an approved living donor, limited transplant options, and the known high mortality rate on hemodialysis.
Given the similar allograft survival rates between the +XM groups, we do not see the benefit of using eculizumab preemptively for all recipients of a +XM kidney transplant. However, eculizumab may be beneficial in certain patient subgroups or when used for a prolonged period. The benefits of using eculizumab for the treatment of early acute ABMR (rather than prevention) have also not been thoroughly investigated.
We also validated previous observations that anti-HLA DSA strength appears to decrease long-term even without antibody-reducing therapies posttransplant (23, 24). We found that in the subset of eculizumab-treated patients who did not receive antibody-depleting therapy (n=25), 40.1% had a negative BFXM, and nearly 30% had negative SAB testing 1 year after transplant. Our observation was distinctive because of the long follow-up and lack of antibody-depleting therapy after transplant in most patients. These data underscore the importance of randomized controlled trials when testing potential therapies intended to reduce DSA in the posttransplant period. The data also suggest that therapies other than DSA reduction are likely needed to prevent or treat CAMR, or both, because natural DSA reduction in most of our patients was not associated with a low CAMR rate.
Our results are consistent with what has been previously published (9, 24, 25). The 5-year allograft survival of eculizumab-treated, +XM patients was similar to that of other +XM cohorts (26). We have also confirmed the predictive value of pretransplant IgG3 DSA in patients with alloantibodies (27, 28). In a cohort of mainly deceased-donor kidney transplant recipients with pre-existing DSA, the presence of IgG3+ DSA was associated with reduced death-censored allograft survival 4 years after transplant (61.4% in IgG3+ patients compared with 92.8% in IgG3– patients) (27). Unlike other studies (29, 30), our study did not show that C1q was associated with inferior outcomes (31), probably because of the high incidence of C1q positivity in the eculizumab cohort—nearly 75% of all tested patients were C1q+ and the small sample size. The prognostic value of C1q positivity is likely increased in patients with a lower level of pre-existing DSA.
As previous work from our group has suggested, BFXM may be useful to stratify patients with a high level of pretransplant DSA (25, 26). Patients with a BFXM <300 had numerically higher 5- and 7-year allograft survivals in both +XM cohorts. This only reached statistical significance in the eculizumab cohort, however. Given that this was a nonrandomized study and the control +XM group had transplants during a different era, further study is needed to determine whether the use of eculizumab actually modified outcomes in the subset of patients with a lower BFXM of 200–300.
Although the BFXM has particular advantages in the field of +XM transplantation, careful interpretation of MFI DSA can also be used to risk stratify patients if the BFXM is not available, as has been previously shown (14, 32–34). The results from SAB assays can be unreliable because of prozone and bead saturation in highly sensitized patients, which often necessitates serial dilution and identification of antibody titer. Identifying the DSA titer is labor intensive and expensive. The BFXM is also a means of assessing the strength of multiple DSA specificities simultaneously.
The significant overlap between DSA MFI, IgG3 subclass, C1q, and BFXM must be recognized. Our study was not designed to determine the superiority of 1 test over the other but to illustrate the potential importance of these tests individually for risk stratification before transplant in the setting of known DSA.
We acknowledge that most of the +XM recipients from the eculizumab and control +XM groups developed CAMR and had reduced death-censored allograft survival. However, over 75% of allografts in the +XM groups (eculizumab or +XM control) were functioning 5 years after transplant; allograft function in the surviving allografts was similar to that of a matched −XM cohort at that time point; and +XM recipients had similar survival rates. Early data analysis from the new kidney transplant allocation system showed that many sensitized patients will continue to have prolonged waits for a compatible deceased-donor kidney. Considering this, our encouraging results support HLA-incompatible transplantation in select settings, particularly for ultrasensitized patients with a cPRA >99.5% (1). Large observational trials have shown that the patient survival benefit with incompatible transplantation is the same if not better than that of a patient who continues dialysis (5, 35).
The strength of our study is long-term, detailed follow-up of a unique +XM group of patients who received eculizumab after transplant. We also had long follow-up of a large, well-characterized +XM cohort in the modern era of SAB and BFXM testing. Our study is limited by the small number of patients in both +XM groups, lack of randomization, and use of a historical +XM control group. Furthermore, our results may not be generalized to the deceased donor population because all of the +XM recipients had living donors. Our ability to detect histologic changes between the 2 +XM groups was limited because not all patients had 5-year allograft biopsies per protocol. A major limitation in studying IgG3 and C1q positivity was our limited data for the eculizumab cohort. It is also possible that we did not detect all patients with IgG3 present because of the reduced sensitivity of subclass-specific reagents. We chose to study IgG3 and C1q rather than IgG subclasses 1, 2, and 4. Although some studies have suggested an important role of DSA characteristics other than IgG3 and C1q (36), the largest cohort of patients with pre-existing DSA (n=110) and comprehensive follow-up showed that only IgG3 and C1q were predictive of allograft loss (28).
In conclusion, +XM kidney transplant recipients who received eculizumab after transplant remained at high risk for CAMR and had similar long-term outcomes as historical +XM control patients. The death-censored allograft survival in +XM kidney transplant recipients was less than that of a matched −XM cohort but reasonable at 5 and 7 years after transplant, considering that some highly sensitized kidney transplant patients may not otherwise receive a transplant. Additionally, natural DSA reduction in eculizumab-treated patients was not associated with a reduced rate of CAMR; thus, therapies to reduce DSA alone may be inadequate to prevent or treat CAMR. Tools such as BFXM and IgG3 positivity may be useful for risk stratification to assist with donor selection in +XM transplantation.
Supplementary Material
Acknowledgment
This publication was made possible by CTSA Grant Number KL2 TR002379 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Abbreviations
- ABMR
antibody-mediated rejection
- BFXM
B-cell flow cytometric crossmatch
- CAMR
chronic active antibody-mediated rejection
- cPRA
calculated panel-reactive antibodies
- DSA
donor-specific antibody
- ESRD
end-stage renal disease
- g
glomerulitis
- HLA
human leukocyte antigen
- IgG
immunoglobulin G
- IQR
interquartile range
- MFI
mean fluorescent intensity
- −XM
negative crossmatch
- +XM
positive crossmatch
- ptc
peritubular capillaritis
- SAB
single antigen bead
Footnotes
Disclosure
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. The authors (C.A.S. and M.D.S.) previously received research funding from Alexion Pharmaceuticals. The other authors have no conflicts of interest to disclose.
Supporting Information
Additional Supporting Information may be found online in the supporting informationtab for this article.
References
- 1.Stewart DE, Kucheryavaya AY, Klassen DK, Turgeon NA, Formica RN, Aeder MI. Changes in Deceased Donor Kidney Transplantation One Year After KAS Implementation. Am J Transplant 2016;16(6):1834–1847. [DOI] [PubMed] [Google Scholar]
- 2.Hahn AB, Mackey M, Constantino D, Ata A, Chandolias N, Lopez-Soler R et al. The new kidney allocation system does not equally advantage all very high cPRA candidates - A single center analysis. Hum Immunol 2017;78(1):37–40. [DOI] [PubMed] [Google Scholar]
- 3.Gebel HM, Kasiske BL, Gustafson SK, Pyke J, Shteyn E, Israni AK et al. Allocating Deceased Donor Kidneys to Candidates with High Panel-Reactive Antibodies. Clin J Am Soc Nephrol 2016;11(3):505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montgomery RA, Lonze BE, King KE, Kraus ES, Kucirka LM, Locke JE et al. Desensitization in HLA-incompatible kidney recipients and survival. N Engl J Med 2011;365(4):318–326. [DOI] [PubMed] [Google Scholar]
- 5.Orandi BJ, Luo X, Massie AB, Garonzik-Wang JM, Lonze BE, Ahmed R et al. Survival Benefit with Kidney Transplants from HLA-Incompatible Live Donors. N Engl J Med 2016;374(10):940–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gloor JM, Winters JL, Cornell LD, Fix LA, DeGoey SR, Knauer RM et al. Baseline donor-specific antibody levels and outcomes in positive crossmatch kidney transplantation. Am J Transplant 2010;10(3):582–589. [DOI] [PubMed] [Google Scholar]
- 7.Locke JE, Zachary AA, Haas M, Melancon JK, Warren DS, Simpkins CE et al. The utility of splenectomy as rescue treatment for severe acute antibody mediated rejection. Am J Transplant 2007;7(4):842–846. [DOI] [PubMed] [Google Scholar]
- 8.Kaplan B, Gangemi A, Thielke J, Oberholzer J, Sankary H, Benedetti E. Successful rescue of refractory, severe antibody mediated rejection with splenectomy. Transplantation 2007;83(1):99–100. [DOI] [PubMed] [Google Scholar]
- 9.Bentall A, Cornell LD, Gloor JM, Park WD, Gandhi MJ, Winters JL et al. Five-year outcomes in living donor kidney transplants with a positive crossmatch. Am J Transplant 2013;13(1):76–85. [DOI] [PubMed] [Google Scholar]
- 10.Gloor JM, Sethi S, Stegall MD, Park WD, Moore SB, DeGoey S et al. Transplant glomerulopathy: subclinical incidence and association with alloantibody. Am J Transplant 2007;7(9):2124–2132. [DOI] [PubMed] [Google Scholar]
- 11.Vo AA, Choi J, Cisneros K, Reinsmoen N, Haas M, Ge S et al. Benefits of rituximab combined with intravenous immunoglobulin for desensitization in kidney transplant recipients. Transplantation 2014;98(3):312–319. [DOI] [PubMed] [Google Scholar]
- 12.Mohan S, Palanisamy A, Tsapepas D, Tanriover B, Crew RJ, Dube G et al. Donor-specific antibodies adversely affect kidney allograft outcomes. J Am Soc Nephrol 2012;23(12):2061–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lefaucheur C, Suberbielle-Boissel C, Hill GS, Nochy D, Andrade J, Antoine C et al. Clinical relevance of preformed HLA donor-specific antibodies in kidney transplantation. Contrib Nephrol 2009;162:1–12. [DOI] [PubMed] [Google Scholar]
- 14.Lefaucheur C, Loupy A, Hill GS, Andrade J, Nochy D, Antoine C et al. Preexisting donor-specific HLA antibodies predict outcome in kidney transplantation. J Am Soc Nephrol 2010;21(8):1398–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stegall MD, Diwan T, Raghavaiah S, Cornell LD, Burns J, Dean PG et al. Terminal complement inhibition decreases antibody-mediated rejection in sensitized renal transplant recipients. Am J Transplant 2011;11(11):2405–2413. [DOI] [PubMed] [Google Scholar]
- 16.Lefaucheur C, Viglietti D, Hidalgo LG, Ratner LE, Bagnasco SM, Batal I et al. Complement-Activating Anti-HLA Antibodies in Kidney Transplantation: Allograft Gene Expression Profiling and Response to Treatment. J Am Soc Nephrol 2018;29(2):620–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cornell LD, Schinstock CA, Gandhi MJ, Kremers WK, Stegall MD. Positive crossmatch kidney transplant recipients treated with eculizumab: outcomes beyond 1 year. Am J Transplant 2015;15(5):1293–1302. [DOI] [PubMed] [Google Scholar]
- 18.Burns JM, Cornell LD, Perry DK, Pollinger HS, Gloor JM, Kremers WK et al. Alloantibody levels and acute humoral rejection early after positive crossmatch kidney transplantation. Am J Transplant 2008;8(12):2684–2694. [DOI] [PubMed] [Google Scholar]
- 19.Taniguchi M, Rebellato LM, Briley KP, Haisch CE, Bolin P, Banuelos N et al. Risk Stratification of Human Leukocyte Antigen Class II Donor Specific Antibody Positive Patients by Immunoglobulin G Subclasses. Clin Transpl 2015;31:293–301. [PubMed] [Google Scholar]
- 20.Stegall MD, Park WD, Larson TS, Gloor JM, Cornell LD, Sethi S et al. The histology of solitary renal allografts at 1 and 5 years after transplantation. Am J Transplant 2011;11(4):698–707. [DOI] [PubMed] [Google Scholar]
- 21.Bentall A, Herrera LP, Cornell LD, Gonzales MA, Dean PG, Park WD et al. Differences in chronic intragraft inflammation between positive crossmatch and ABO-incompatible kidney transplantation. Transplantation 2014;98(10):1089–1096. [DOI] [PubMed] [Google Scholar]
- 22.Haas M, Loupy A, Lefaucheur C, Roufosse C, Glotz D, Seron D et al. The Banff 2017 Kidney Meeting Report: Revised diagnostic criteria for chronic active T cell-mediated rejection, antibody-mediated rejection, and prospects for integrative endpoints for next-generation clinical trials. Am J Transplant 2018;18(2):293–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Higgins R, Lowe D, Hathaway M, Lam F, Kashi H, Tan LC et al. Rises and falls in donor-specific and third-party HLA antibody levels after antibody incompatible transplantation. Transplantation 2009;87(6):882–888. [DOI] [PubMed] [Google Scholar]
- 24.Amrouche L, Aubert O, Suberbielle C, Rabant M, Van Huyen JD, Martinez F et al. Long-term Outcomes of Kidney Transplantation in Patients With High Levels of Preformed DSA: The Necker High-Risk Transplant Program. Transplantation 2017;101(10):2440–2448. [DOI] [PubMed] [Google Scholar]
- 25.Schinstock CA, Gandhi M, Cheungpasitporn W, Mitema D, Prieto M, Dean P et al. Kidney Transplant With Low Levels of DSA or Low Positive B-Flow Crossmatch: An Underappreciated Option for Highly Sensitized Transplant Candidates. Transplantation 2017;101(10):2429–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orandi BJ, Garonzik-Wang JM, Massie AB, Zachary AA, Montgomery JR, Van Arendonk KJ et al. Quantifying the risk of incompatible kidney transplantation: a multicenter study. Am J Transplant 2014;14(7):1573–1580. [DOI] [PubMed] [Google Scholar]
- 27.Lefaucheur C, Viglietti D, Bentlejewski C, Duong van Huyen JP, Vernerey D, Aubert O et al. IgG Donor-Specific Anti-Human HLA Antibody Subclasses and Kidney Allograft Antibody-Mediated Injury. J Am Soc Nephrol 2016;27(1):293–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Viglietti D, Loupy A, Vernerey D, Bentlejewski C, Gosset C, Aubert O et al. Value of Donor-Specific Anti-HLA Antibody Monitoring and Characterization for Risk Stratification of Kidney Allograft Loss. J Am Soc Nephrol 2017;28(2):702–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loupy A, Lefaucheur C. Antibody-Mediated Rejection of Solid-Organ Allografts. N Engl J Med 2018;379(12):1150–1160. [DOI] [PubMed] [Google Scholar]
- 30.Viglietti D, Bouatou Y, Kheav VD, Aubert O, Suberbielle-Boissel C, Glotz D et al. Complement-binding anti-HLA antibodies are independent predictors of response to treatment in kidney recipients with antibody-mediated rejection. Kidney Int 2018;94(4):773–787. [DOI] [PubMed] [Google Scholar]
- 31.Loupy A, Lefaucheur C, Vernerey D, Prugger C, Duong van Huyen JP, Mooney N et al. Complement-binding anti-HLA antibodies and kidney-allograft survival. N Engl J Med 2013;369(13):1215–1226. [DOI] [PubMed] [Google Scholar]
- 32.Kannabhiran D, Lee J, Schwartz JE, Friedlander R, Aull M, Muthukumar T et al. Characteristics of Circulating Donor Human Leukocyte Antigen-specific Immunoglobulin G Antibodies Predictive of Acute Antibody-mediated Rejection and Kidney Allograft Failure. Transplantation 2015;99(6):1156–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schinstock CA, Cheungpasitporn W, Cosio F, Mitema D, Gandhi M, Prieto M et al. Kidney transplant with low level DSA and low positive B-flow crossmatch: an underappreciated option for highly sensitized transplant candidates. In.: Am J Transplant, 2016: 700–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fidler SJ, Irish AB, Lim W, Ferrari P, Witt CS, Christiansen FT. Pre-transplant donor specific anti-HLA antibody is associated with antibody-mediated rejection, progressive graft dysfunction and patient death. Transpl Immunol 2013;28(4):148–153. [DOI] [PubMed] [Google Scholar]
- 35.Manook M, Koeser L, Ahmed Z, Robb M, Johnson R, Shaw O et al. Post-listing survival for highly sensitised patients on the UK kidney transplant waiting list: a matched cohort analysis. Lancet 2017;389(10070):727–734. [DOI] [PubMed] [Google Scholar]
- 36.Khovanova N, Daga S, Shaikhina T, Krishnan N, Jones J, Zehnder D et al. Subclass analysis of donor HLA-specific IgG in antibody-incompatible renal transplantation reveals a significant association of IgG4 with rejection and graft failure. Transpl Int 2015;28(12):1405–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.