Abstract
Enumeration of circulating tumor cells (CTCs) can provide valuable prognostic information to guide cancer treatment as well as help monitor disease progression. Analysis of these rare malignant cells has the potential to further our understanding of cancer metastasis by gaining insights into CTC characteristics and properties. Microfluidics presents a unique platform to isolate and study CTCs. In this chapter, we describe the detailed procedures for the fabrication and use of a microfluidic device to detect CTCs from the blood of pancreatic cancer patients.
Keywords: Microfluidics, Circulating tumor cells, Pancreatic cancer
1. Introduction
Cancer metastasis is responsible for the majority (>90%) of all cancer-related deaths. Blood-borne metastases arise after the dissemination of cancer cells into the bloodstream from a tumor site. Tumor cells that are identified in transit within the bloodstream are referred to as circulating tumor cells, or CTCs [1]. CTCs are present in rare numbers, estimated to be between 1 and 100 CTCs per billion normal blood cells in the circulation of patients with advanced disease [2]. Consequently, CTC isolation, identification, and characterization require extremely sensitive and specific analytical methods as well as novel technologies.
In this chapter, CTC sample analysis is demonstrated with a mixer-based microfluidic device. The device is patterned with herringbone (or chevron) structures to disrupt flow streamlines and induce chaotic mixing, maximizing collisions and interactions between target cells in the sample and functionalized device surfaces. The device design was inspired by several groups [3–5], and was geometrically optimized for enhanced capture efficiency and purity in our previous work [6]. The device consists of a polydimethylsiloxane (PDMS) layer on the top, engraved with the desired fluidic structure, permanently bonded to a standard microscope glass slide (as a bottom layer). Other amorphous materials, such as cyclic olefin copolymer (COC), can be used to create a similar microfluidic design.
Blood samples were first prepared by using density gradient centrifugation. Ficoll-based centrifugation methods separate blood components into different layers based on their density, size, and mass, in order to extract cells of interest while reducing the amount of nontarget cells. Typically, erythrocytes (i.e., red blood cells), polymorph nuclear leukocytes (PMNLs), and platelets are separated in the pellet while mononuclear cells (MNCs), including tumor cells, are kept in the interphase often referred to as the buffy coat. Ficoll is a density gradient medium used to separate the low-density buffy coat cells from the bottom pellet. Other similar methods have been developed for the extraction of rare cell populations from whole blood, such as OncoQuick® (Greiner Bio-One, Monroe, NC) and RosetteSep™ (StemCell Technologies, Vancouver, Canada) enrichment systems.
Following blood preparation, the final sample suspension is introduced into a microfluidic device with immobilized epithelial cell adhesion molecule (EpCAM) antibodies. At the end of the capture experiment, a detection antibody mixture is introduced, after cell fixation and permeabilization, to visualize marker expression or lack thereof. CTC enumeration is then performed, based on the FDA (Food and Drug Administration)-approved method for defining a CTC: an EpCAM-antibody-captured cell that has confirmed positive expression for cytokeratins (CK), positive staining with 4′,6-diamidino-2-phenylindole (DAPI), and negative for CD45 (i.e., EpCAM+CK+DAPI+CD45−) [1, 7]. This marker combination effectively confirms the cell to be of an epithelial origin and excludes blood cell contamination. An automated sample stage with a microscope as well as fluorescence quantification and counting software is used to help minimize operator’s manual intervention and increase enumeration reliability and reproducibility.
The protocol detailed in this chapter has been used to process and enumerate CTCs from whole blood samples of patients with advanced pancreatic cancer who are under active palliative treatment. The sensitivity of our methods and techniques is highlighted by our ability to consistently detect low number CTCs in blood from patients with pancreatic cancer, which are otherwise difficult to detect using the FDA-approved method (CellSearch®) [7].
2. Materials and Equipment
2.1. Materials for Device Design and Mold Preparation
CAD software (e.g., AutoCAD).
Photomask with microfluidic designs, either chrome photomask or high resolution transparency photomask (≥2500 dpi) (see Note 1).
One blank 100-mm diameter silicon wafer (see Note 2).
Teflon tweezers, for handling silicon wafers.
Acetone (Fisher Scentific, Hampton, NH) (see Note 3).
Three Pyrex® 3140 dishes, 125 × 65 mm (Corning Inc., Corning, NY).
Isopropanol (IPA), in a wash bottle (Fisher Scientific, Hampton, NH).
Piranha etch solution (H2SO4 and H2O2) (see Note 4).
Buffered oxide etch (BOE) 6:1 solution (Mallinckrodt Baker, Inc., Phillipsburg, NJ).
Deionized (DI) water, in a wash bottle.
A commercial furnace capable of reaching 125 °C.
Hexamethyldisilazane (HMDS), with a hot plate to heat/evaporate HMDS.
A commercial spin coater (see Note 5).
SU-8 negative photoresist (MicroChem, Newtown, MA) (see Note 6).
SU-8 developer (MicroChem, Newtown, MA).
Cotton swabs.
Mask aligner and UV exposure equipment (see Note 7).
Filtered, pressurized inert gas pistol (e.g., air or nitrogen).
Desiccator connected to a vacuum line.
Trichloro(1H,1H,2H,2H-perfluorooctyl)silane (Sigma-Aldrich, St. Louis, MO).
Small weigh boat, or a small vial.
2.2. Materials for Device Fabrication
Sylgard 184 kit: silicone elastomer base and curing agent (Dow Corning, Midland, MI).
Weight boat.
Scale.
Wooden stirrers (see Note 8).
Large petri dish (>100 mm), or tin foil (see Note 9).
Desiccator connected to a vacuum line with compatible polymer tubing.
A commercial oven capable of 70 °C.
Razor blade or scalpel.
Polymethylmethacrylate (PMMA) sheet, or any other clean plastic sheet.
Micropuncher.
Glass microscope slides, 75 mm × 25 mm (Fisher Scientific, Hampton, NH).
Pyrex® 3140 dish, 125 mm × 65 mm (Corning Inc., Corning, NY).
Detergent.
Ionized water.
Benchtop ultrasonic cleaner.
Acetone, in a wash bottle.
DI water, in a wash bottle.
Ethanol, in a wash bottle.
Inert gas pistol (e.g., air or nitrogen).
Corona discharger (see Note 10).
Parafilm M™ wrapping film (Fisher Scientific, Hampton, NH).
35-mm petri dishes (Corning Inc., Corning, NY).
2.3. Materials for Device Functionalization
Female luer-to-barb adapters (IDEX Health & Science, Oak Harbor, WA).
Tubing: 0.0625-in. outer diameter, 0.008-in. inner diameter (IDEX Health & Science, Oak Harbor, WA) (see Note 11).
Sterile 3-mL Luer-Lock syringes (Becton Dickinson, Franklin Lakes, NJ).
Anhydrous ethanol.
Dulbecco’s phosphate buffered saline (DPBS) free of Ca2+/Mg2+ ions (Fisher Scientific, Hampton, NH).
Bovine serum albumin (BSA) (MP Biomedicals, Solon, OH) (see Note 12).
Two commercial programmable syringe pumps with infuse function (see Note 13).
Vacuum line with compatible polymer tubing.
Precision vacuum regulator (0–30″ Hg).
One truncated 1-mL pipette tip.
Avidin (Invitrogen, Carlsbad, CA).
Biotinylated anti-EpCAM (anti-human CD326, clone 1B7, eBioscience, San Diego, CA) (see Note 14).
2.4. Materials for Sample Processing
Ficoll-Paque centrifugation media (GE Healthcare Life Sciences, Marlborough, MA) (see Note 15).
Alcohol, 100% (Thomas Scientific, Swedesboro, NJ).
Cotton swabs.
Sterile 5-mL Luer-Lock syringe with needle (Becton Dickinson, Franklin Lakes, NJ).
Sterile 50-mL centrifuge tubes (Corning Inc., Corning, NY).
Sterile 10-mL disposable serological pipettes (Fisher Scientific, Hampton, NH).
Serological pipette controller (Fisher Scientific, Hampton, NH).
Centrifuge with swing-out rotor.
Two commercial programmable syringe pumps with infuse function (see Note 13).
DPBS free of Ca2+/Mg2+ ions (Fisher Scientific, Hampton, NH).
Fetal bovine serum (FBS) (Fisher Scientific, Hampton, NH).
Sterile 1-mL syringes (Becton Dickinson, Franklin Lakes, NJ).
Polytetrafluoroethylene (PTFE) micromagnetic stirrer bar, 5 mm length × 2 mm diameter (Cowie, Wilmington, DE).
Basic magnetic stirrer (Fisher Scientific, Hampton, NH).
Female luer-to-barb adapters (IDEX Health & Science, Oak Harbor, WA).
Tubing: 0.0625-inch outer diameter, 0.008-in. inner diameter (IDEX Health & Science, Oak Harbor, WA) (see Note 11).
Sterile 2-mL microcentrifuge tubes.
Sterile 3-mL Luer-Lock syringes (Becton Dickinson, Franklin Lakes, NJ).
Paraformaldehyde (PFA), 96%, extra pure (Acros Organics, Morris Plains, NJ).
Triton X-100,98%, molecular biology grade (Amersham Biosciences, Sweden).
Anti-cytokeratin-FITC (Cytokeratin conjugated with fluorescein isothiocyanate, clone CAM 5.2, BD Biosciences, San Jose, CA) (see Note 14).
Anti-CD45-PE (CD45 conjugated with phycoerythrin, clone HI30, BD Biosciences, San Jose, CA) (see Note 14).
DAPI (4′,6-diamidino-2-phenylindole, Invitrogen, Carlsbad, CA) (see Note 16).
2.5. Materials for CTC Detection
Fluorescence microscopy setup: inverted IX71 Olympus microscope, 10× and 20× objectives, xenon lamp, fluorescent filters to match the dye-conjugated antibodies (see Note 17), computer with CellSens software (Olympus, Center Valley, PA) or another software; optional: automatic or semiautomatic stage (Prior, Rockland, MA).
3. Methods
3.1. Design and Mold Preparation
Design and draw the microfluidic architecture in CAD software (e.g., AutoCAD).
Submit the CAD file to a commercial supplier for a photomask order (see Note 1).
Place a silicon wafer in an acetone bath inside a polyethylene beaker for 5 min to remove organic materials. Occasional stirring is recommended.
Wash the silicon wafer with IPA for 20 s using a wash bottle.
Using Teflon tweezer, immerse the wafer substrate into a Pyrex® dish with piranha wet etch (H2SO4 and H2O2) for 5 min. Wash the wafer with running DI water for 2 min. Transfer the wafer into a different Pyrex® dish with BOE and leave it in for 20 s. Rinse the wafer thoroughly for 30 s with a DI water wash bottle. Use an inert gas pistol to make the wafer surface dry.
Bake the wafer at 125 °C in the oven for 10 min.
Place the wafer in the HMDS hot plate and release HMDS for 30 s. Incubate the wafer within the HMDS for 2 min. Remove the wafer off the hot plate and wait at least 2 min to let the wafer cool off before moving on to the next step. Alternatively, you may treat the wafer with HMDS via evaporation in a Pyrex® dish.
Place the wafer on a headway spinner and dispense 4 mL of SU-8 photoresist on the top of the wafer.
Spin the wafer at 500 revolutions per minute (RPM) for 10 s, with an acceleration of 100 RPM per second.
Increase the spinner speed to 2250 RPM (~288 × g) for 30 s, with an acceleration of 300 RPM per second.
Clean the underside and the edges of the wafer with a cotton swab wetted with SU-8 developer to remove excess SU-8 photoresist buildup. This step is to avoid contaminating the hot plate and mask aligner equipment with photoresist. Dedicated spin coaters have edge bead removal (EBR) and can be programmed to perform this step automatically after spin coating (see Note 18).
Soft bake: place the wafer on the hot plate at 65 °C for 3 min. Remove the wafer from the hot plate and allow it to cool down to room temperature. Place the wafer on a hot plate, at 95 °C for 6 min (see Note 19).
Calculate the exposure time based on the equipment’s power setting and an exposure dose of 160 mJ/cm2 (see Note 20). Set the wavelength of the exposure to 365 nm.
The photoresist-coated wafer is inserted into the mask aligner for UV exposure.
Post exposure bake (PEB): place the wafer on a hot plate at 95 °C for 6 min. The design pattern will become visible on the layer surface about 1 min into the PEB. If no visible latent image is seen during or after PEB, there was insufficient energy exposure, heating, or both.
Repeat steps 8–13 to coat a second layer of photoresist. Use the MA6 alignment machine to align the second-layer photomask with the exposed first layer feature. The success of alignment is determined by overlapping of alignment marks in the photomask and alignment marks in the first layer. After alignment, UV exposure is applied to the wafer.
Repeat step 15.
Submerge the wafer in SU-8 developer in a Pyrex® dish. Leave the wafer in the developer for 6–9 min (see Note 21). Wash the developed wafer with running SU-8 developer for 20 s, using a wash bottle. Rinse the resist layer with IPA for another 20 s.
Dry the wafer surface with a filtered, pressurized inert gas pistol. During the drying, white marks indicate an incomplete development. If the developing time is significantly longer or shorter than the indicated time, the problem may have occurred during the SU-8 spin coating or energy exposure steps.
The resolution of the master’s feature sizes can be measured using a profilometer (see Note 22).
Place the master in a desiccator next to a small weigh boat (or a small vial) containing 2–3 drops of trichloro (1H,1H,2H,2H-perfluorooctyl) silane. Connect the desiccator to a vacuum line and evacuate the air from the desiccator. Incubate the master in the desiccator for at least 30 min to allow the silane to evaporate and form a monolayer on the surface of the master. Silanizing the master is important for preventing the PDMS from adhering to the master during PDMS device fabrication. The master is now ready to be used for PDMS device fabrication.
3.2. Device Fabrication
Fill a weigh boat with 10 parts of Sylgard 184 monomer base and 1 part of the curing agent, by weight (see Note 23).
Use a wooden stirrer to mix the two components thoroughly, for at least 3 min. Small bubbles will form in the mixture and can be used as guides to determine the extent of mixing. Ensure that the mixture is homogeneous, since incomplete mixing may affect the mechanical properties of the cured PDMS.
Place the master in a petri dish and pour the polymer mixture to the desired thickness. Alternatively, you can also encompass the master using tin foil to form a tin foil boat. In either case, you should ensure that the bottom-side of the silicon wafer is flat; an uneven surface may cause the wafer to break more easily when cutting PDMS out of it, in later steps.
Place the petri dish in a desiccator connected to a vacuum line and turn on the vacuum. Incubate the master in the vacuum for 20–30 min, or until most bubbles have disappeared or risen from the surface of the master. Degassing time will vary depending on the amount of PDMS and the width of the container. Turn off the vacuum and vent the desiccator. This step can be repeated to fully remove all bubbles from the PDMS mixture.
Place the master layered with PDMS in an oven at 70 ° C, and cure for at least 1 h (see Note 24).
Take the master out of the oven and allow it to cool down to room temperature. Cut out the desired PDMS pieces using a razor blade, or scalpel, and place the PDMS on a clean plastic sheet with the features facing down. The PDMS piece should be cut to be the same size as a microscope glass slide (3 in. × 1 in.). Be careful not to apply too much pressure on the master, as it may break.
Use a micro-puncher to create holes in the PDMS pieces to serve as inlets and outlets; the holes should be punched at each end of the fluidic channels. Ensure that the punched holes are equal or slightly smaller than the outer diameter of the tubing you use in Subheading 3.3.
Place the PDMS pieces and standard microscope glass slides in a glass dish (e.g., Pyrex®) with a solution of detergent and ionized water. Place the container in an ultrasonic cleaning bath for at least 30 min to wash off any debris on the PDMS microfluidic features and glass slides.
Rinse the glass slides thoroughly with DI water to remove any remaining detergent. Use an acetone wash bottle to rinse the slides for 10 s, followed by a DI water rinse for 30 s. Rinse off the glass slide with ethanol for 10 s and dry it off with an inert gas pistol. Place the prepared microscope glass slides in a covered hood to avoid dust particles and other debris from settling on their surface.
Rinse a PDMS piece with DI water for at least 1 min, ensuring that the detergent has been washed off completely. Rinse off the PDMS piece with ethanol for 10 s using a wash bottle, focusing on the feature side. Dry off the PDMS piece with an inert gas pistol and place it inside a covered hood, to avoid debris from falling on the feature side of the PDMS pieces.
Treat the feature side of a PDMS piece and one side of a microscope glass slide with a corona discharger for 5 min (each). Align the corona-treated PDMS piece, feature side down, against the corona-treated microscope glass slide and apply pressure evenly from one end to the other, to ensure proper bonding and to avoid bubbles between the glass and the PDMS. You may also treat the PDMS-glass interface around the device to further enhance the bonding at the edges (see Note 25).
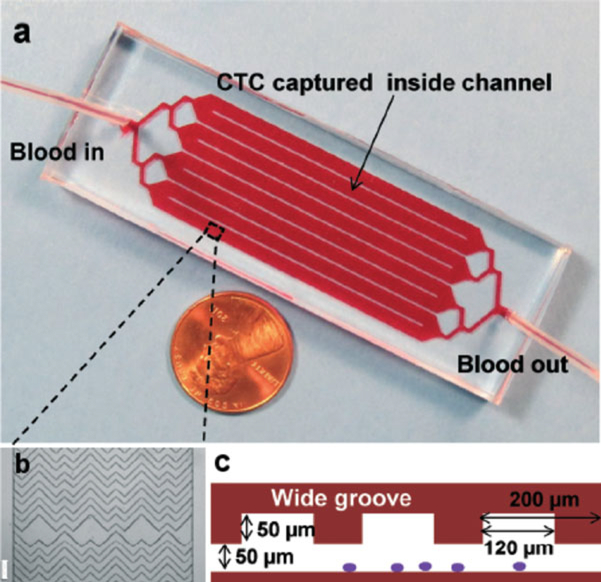
Place the device in an oven at 70 °C for 1 h to strengthen the permanent PDMS–glass bond. The microfluidic device and its design is shown in Fig. 1.
Protect the inlet and outlet of the PDMS device from debris by covering them with Parafilm. Store the PDMS devices in 35mm petri dishes until use. Alternatively, continue to the next step without covering the device if it is to be used at the time. Starting the steps in Subheading 3.3, immediately after the PDMS-glass bonding will make the initial device fill easier, since bubbles will not form as often in recently treated hydrophilic PDMS channels.
Repeat steps 10–13 with other PDMS pieces.
Fig. 1.
(a) The microfluidic chip, consisting of a single inlet that bifurcates into eight parallel channels that bifurcate into an outlet (each channel is 2.1 mm wide and 50 mm long). (b) Micrograph (4× magnification in bright field) of the staggered chevron grooves within a channel, showing their asymmetry and periodicity; scale bar = 200 μm. (c) Cross-sectional view of the grooves within the microchannels, with a channel depth of 50 μm and a groove depth of 50 μm; the groove pitch is 200 μm and the groove width is 120 μm. Adapted with permission from [6]
3.3. Device Functionalization
Attach luer-to-barb adapters to 10 in. of tubing. Insert the adapter-tubing complex to the ends of three sterile 3-mL Luer-lock syringes.
Fill one sterile 3-mL Luer-lock syringe with anhydrous ethanol, another sterile 3-mL syringe with DPBS, and a third sterile 3-mL syringe with 2% BSA in DPBS. When a syringe is loaded on the pump, you should expel air bubbles within the syringe and tubing prior to inserting the tubing into a device’s inlet hole.
Microchannels are easily filled with ethanol due to its hydro-philicity. Load the pump with the syringe filled with anhydrous ethanol. Place a few drops of ethanol at the inlet hole of a prepared device, and the ethanol solution should fill most of the device up via capillary forces. Insert the tubing into the inlet hole, and wash the microchannels with three ethanol washing steps at a high flow rate (900 μL at 2 μL/s) (see Note 26). Repeat the three washing steps but with DPBS buffer this time (900 μL at 2 μL/s) (see Note 27).
At this point in time, you should make sure there are no bubbles housed within the microdevice. If there are no bubbles, move on to the next step. If there are bubbles, you can try removing them via extra washing steps or by tapping the top of the PDMS piece lightly during a washing step. If bubbles still persist, you may use higher flow rates (i.e., 10 μL/s) or use manual force to push the fluid in the syringe into the device. However, be cautious since a higher flow rate may cause device debonding if irreversible bonding between the PDMS piece and the glass slide is not strong enough.
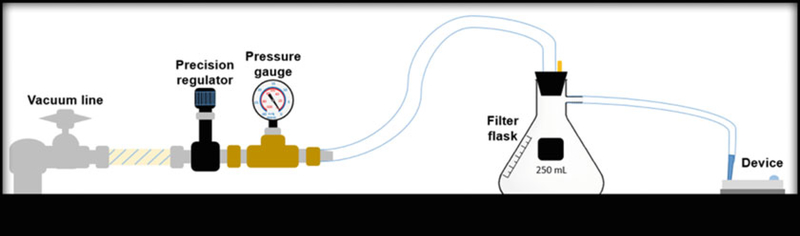
A 1-mL pipette tip, with 2 cm of the tip cut off (truncated), is inserted inside the end of the tubing which is connected to a filter flask. The flask is connected to a pressure gauge and a precision vacuum regulator (0–30″ Hg) that is then connected to a vacuum line. This setup, depicted in Fig. 2 below, is used to introduce low volume reagents (100 μL) into the microfluidic devices.
Use a pipette to remove excess liquid from the inlet hole, leaving enough liquid for fluid continuity to avoid the introduction of bubbles into the device. Place a large droplet (100 μL) of avidin solution (1 mg/mL) at the device inlet. Place the truncated syringe tip attached to the vacuum line at the device outlet to begin withdrawing the avidin solution into the microchannels. Ensure that the vacuum pressure is very low (close to 0 mmHg) during this step. Incubate the device at room temperature for 15 min (see Note 28).
After the avidin incubation is completed, rinse the device with three DPBS washes at a medium flow rate (900 μL at 1.5 μL/s).
Incubate the device with a 100-μL solution of 20 μg/mL biotinylated anti-EpCAM and 1% BSA in DPBS at room temperature for 15 min.
Rinse the device with three medium-flow rate washes (900 μL at 1.5 μL/s) with a solution of 1% BSAin DPBS. Incubate the BSA solution in the device for at least 20 min. The device is now ready for sample introduction. If the sample is not ready to be processed, the device can be stored at room temperature. If the device needs to be stored longer than 2 h, it should be stored at 4 °C to preserve antibody immobilization.
Fig. 2.
Vacuum-enabled reagent introduction setup. The vacuum line is directly connected to a precision vacuum regulator and pressure gauge, which are then connected to a filter flask that is attached to tubing with a truncated pipette tip at the end. The pipette tip is pressed up against the microfluidic device inlet/outlet to withdraw the reagent solution into the device
3.4. Sample Processing
Invert the Ficoll-Paque bottle several times to ensure a homogeneous mix. Snap off the propylene cap to expose the rubber stopper. Disinfect the rubber stopper with alcohol on a cotton swab and allow it to dry. Puncture the rubber stopper with the needle of a 5-mL syringe, and transfer 10 mL of the Ficoll-Paque media to a sterile 50-mL centrifuge tube.
Invert the vacutainer several times and remove the cap to draw out the 10-mL patient blood sample (see Note 29), using a sterile 10-mL serological pipette, and transfer it to a sterile 50mL centrifuge tube; keep the serological pipette in the vacutainer after use.
With a new sterile 10-mL serological pipette, add 10 mL (or equivalent volume to the blood sample) of Ca2+/Mg2+-free DPBS to the blood sample in the 50-mL tube; keep the serological pipette in the DPBS container. Use 2 mL of the diluting DPBS solution to rinse out any remaining blood from the walls of the vacutainer and the cap, and transfer it to the blood sample in the 50-mL tube. Use the same serological pipette (blood-treated) from the vacutainer to mix the blood and buffer via pipetting.
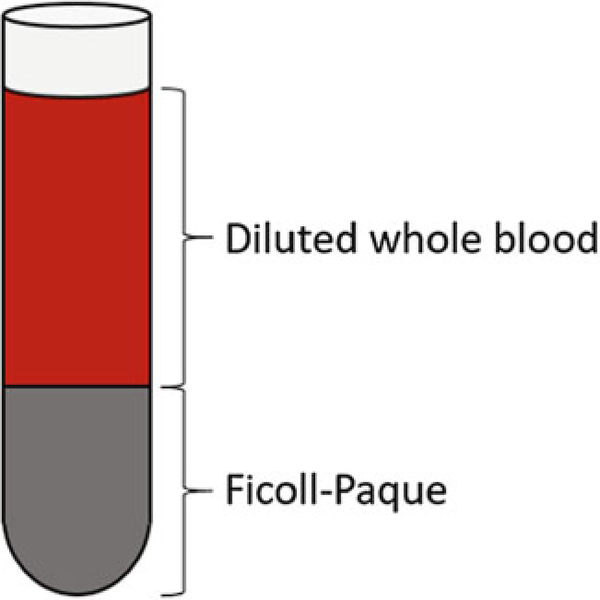
Very slowly and carefully layer the diluted blood sample on top of the Ficoll-Paque media; make sure to not mix the blood and the Ficoll-Paque solution. The Ficoll media and diluted blood should be separate layers, as shown in Fig. 3. If you’re using a pipette controller with a speed setting, use the gravity (G) setting. Use 2 mL of the diluting DPBS solution to rinse out any remaining blood from the walls of the 50-mL tube (that contained the diluted blood) as well as from the serological pipette, and add it on top of the blood sample already layered on top of the Ficoll-Paque solution.
Centrifuge the tube at 800 × g for 30 min at 18 °C (see Note 30) with slow acceleration and no breaks. Make sure to balance the rotor with a 50-mL centrifuge tube filled with the same volume of water, or with a second blood sample processed in parallel.
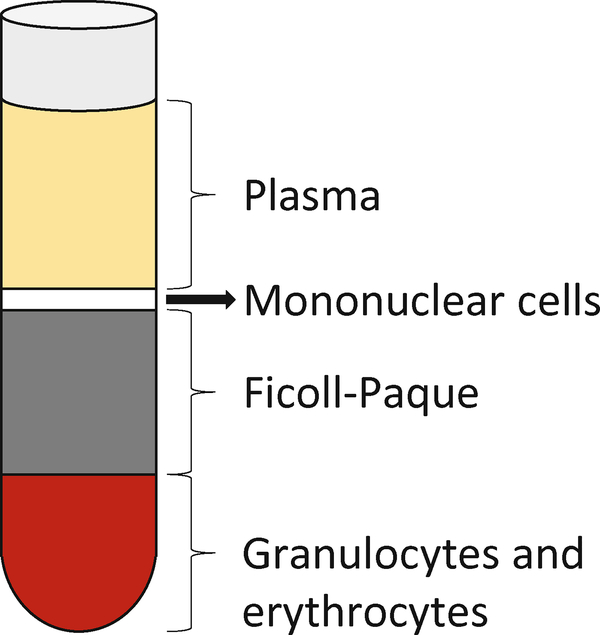
After centrifugation, the 50-mL sample tube should be layered as it is depicted in Fig. 4. Withdraw the upper layer containing plasma and platelets, using the blood-treated serological pipette, and transfer to a different tube for storage and analysis, or dispose of it properly (see Note 31). Leave some of the upper plasma layer above the undisturbed layer of mononuclear cells (see Note 32).
Using the blood-treated serological pipette, transfer the layer of mononuclear cells to a new sterile, BSA-treated 50-mL centrifuge tube. You can withdraw some of the Ficoll-Paque layer, leaving some of the layer above the bottom hematocrit (see Note 33).
Using the diluting DPBS solution, fill up the sample tube to the 50-mL mark. Use pipetting to homogenize the sample, then cap the centrifuge tube. Centrifuge at 300 × g for 10 min at 18 °C (max acceleration and breaks). Make sure to balance the rotor.
Discard the supernatant (see Note 31). Add 30 mL of 2% FBS in Ca2+/Mg2+-free DPBS. Use pipetting to ensure the sample is thoroughly mixed and the cell pellet has been fully resuspended. Cap the tube and centrifuge it at 300 × gfor 10 min at 18 °C (max acceleration and breaks). Make sure to balance the rotor.
Discard the supernatant (see Note 31). Resuspend the cell pellet in 1 mL of 2% BSA in DPBS (equivalent to 100 μL of buffer for every 1 mL of whole blood prepared) and transfer it to a sterile 2-mL microcentrifuge tube (see Note 34). Make sure the 2-mL centrifuge tube has a relatively flat bottom, to facilitate sample withdrawal in the following step.
To avoid cell settling in the 1-mL sample syringe, a micromagnetic stirring bar is placed inside. A magnetic stir-plate is placed next to the syringe pump, so that the end of the syringe is above the plate. The mixing within the syringe keeps the cells in suspension while the sample is pumped through the device.
Use the 1-mL sample syringe to withdraw the final cell suspension from the 2-mL microcentrifuge tube. Connect a luer-to-barb adapter attached via tubing to the syringe, and secure the syringe on the pump. Place the magnetic stir-plate below the tip of the sample syringe; you may need to raise the height of the stir-plate to move it closer to the syringe tip.
Set your syringe pump to inject 800 μL (equivalent to 8 mL of whole blood, see Note 35) at 1 μL/s (equivalent to 3.6 μL/h). Insert the tubing from the sample syringe into the inlet hole of the microdevice (see Note 26). Start your pump to introduce the sample into the antibody-functionalized device. Collect the outlet stream by directing it to a microcentrifuge tube via tubing; the outlet stream solution can then be analyzed separately, reprocessed in another device, or treated as waste (see Note 31).
Wash the device with three high-flow rate DPBS washes (900 μL at 2 μL/s). You should treat all outlet streams, following this washing step, as biohazardous waste (see Note 31).
Fill a sterile 3-mL syringe with a solution of 4% PFA in DPBS, and attach a luer-to-barb adapter and tubing. Introduce 300 μL of the 4% PFA solution at 1 μL/s and incubate for 10 min. After the incubation time, wash the device with three high-flow rate DPBS washes (900 μL at 2 μL/s).
Fill a sterile 3-mL syringe with a solution of 0.2% Triton X-100 in DPBS, and attach a luer-to-barb adapter and tubing. Introduce 300 μL of the 0.2% Triton X-100 solution at 1 μL/s and incubate for 10 min. After the incubation time, wash the device with three high-flow rate washes (900 μL at 2 μL/s) using 2% BSA in DPBS solution. Incubate the BSA solution in the device for at least 20 min.
Using sterile pipette tips in a covered hood, create a fluorescent antibody cocktail by mixing 10 μL of 12.5 μg/mL anti-CD45-PE, 10 μL of 25 μg/mL anti-CK-FITC, and 80 μL of 300 nM DAPI.
Introduce the 100-μL fluorescent antibody solution into the device using the vacuum line (refer to steps 5 and 6 from Subheading 3.3). Incubate at room temperature for 30 min, shielded from light, and rinse out the device with three high-flow rate DPBS washes (900 μL at 2 μL/s).
Fig. 3.
Diluted blood sample layered over the Ficoll-Paque solution
Fig. 4.
Blood sample separated into gradients via centrifugation. The bottom-most layer is the hematocrit, composed mainly of red blood cells. Above the hematocrit lies the Ficoll-Paque solution, which serves to separate the hematocrit from the desired mononuclear cell layer. Above the mononuclear cells are plasma
3.5. CTC Detection
Turn on the computer and fluorescence light source (see Note 36). Place the microdevice in the glass microscope slide slot.
Use the bright field channel, with 4× magnification, to inspect the microchannels and bonding of the PDMS to the glass. Use the automatic scanning function to take an overview image of the entire device’s microchannel area (see Note 37). This bright field overview image will serve as a map to guide the operator through the device’s fluidic boundaries.
Switch to the FITC fluorescent channel and use the automatic scanning function to image the entire device’s microchannel area with 10 × magnification.
Repeat step 3 using the DAPI and CY3 fluorescent channels.
Using the imaging software (see Note 38), the DAPI, FITC, and CY3 fluorescent overview images are overlaid. The images are ready to be analyzed.
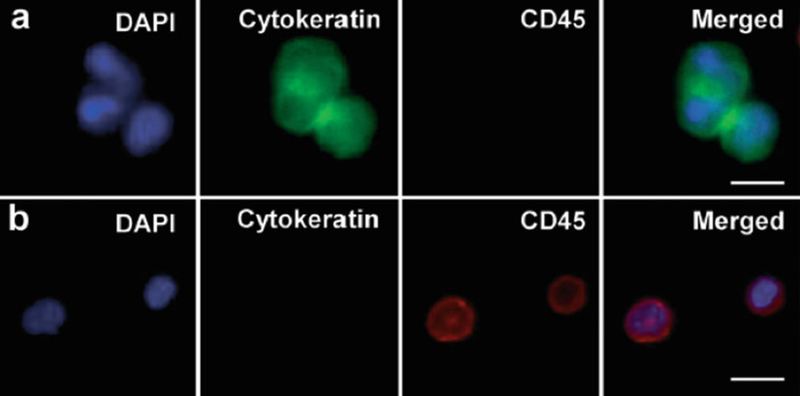
During analysis of the overlaid fluorescent channels, the FDA-approved definition of a CTC is used. The cell must be DAPI-positive, CK-positive (FITC-green), and CD45-negative (PE-red) (see Note 39) as shown in Fig. 5. Cell’s morphological features such as cell size and shape, preserved cell nucleus, entire nucleus housed within cytoplasm, and increased nuclear to cytoplasmic (N/C) ratio are also considered.
Fig. 5.
Fluorescent microscope images (40× magnification) of CTCs captured from patient blood samples; separate microscope channels (DAPI, FITC, and CY3, respectively) as well as the merged image. (a) Representative image of three CTCs (DAPI+, Cytokeratin+, and CD45−). (b) Images of nonspecifically captured white blood cells (DAPI+, Cytokeratin−, and CD45+). Scale bar = 10 μm. Reproduced with permission from [6]
Table 1.
Fluorescent filter selection used. All filters were purchased from Chroma Technology Corporation (Bellows Falls, VT)
| Fluorophore | DAPI | FITC | CY3 |
|---|---|---|---|
| Excitation (EX) | AT350/50× | HQ480/40× | ET545/25× |
| Beam splitter (BS) | T400lp | Q505lp | T565lpxr |
| Emission (EM) | ET460/50m | ET525/30m | ET605/70m |
| Set catalog # | 49000 | 49011+q505lp | 49004 |
Acknowledgments
We acknowledge financial support from the National Cancer Institute (NCI-K25CA149080) and the University of Florida Health Cancer Center (UFHCC) for Pilot Project Award, and the University of Florida Division of Sponsored Research for Preparatory Grant and Opportunity Fund. Jose Varillas is supported in part by the NIH TL1 grant under the University of Florida Clinical and Translational Science Awards (TL1TR001428 and UL1TR001427) and the Robert C. Pittman fellowship of the Nanoscience Institute for Medical and Engineering Technology (NIMET).
Footnotes
Turnaround time for transparency printing service is normally 3–5 business days (inμg/mLcluding the shipping time by a professional printing shop), while a chrome photomask may take a week or two. Either one of these options may work, depending on the smallest feature size of the microfluidic design.
It is not recommended to use test quality wafers since their deformities are often severe enough to disrupt silicon master fabrication.
Waste disposal regulations should be followed with all chemicals, bio-reagents, and sharps; always protect yourself by wearing personal protective equipment (PPE).
Piranha solutions are used to remove organic residues from substrates; the solution is highly corrosive and a powerful oxidizer. Acid piranha is a 3:1 mixture of concentrated sulfuric acid (H2SO4) and hydrogen peroxide (H2O2), respectively. When preparing, always add the peroxide to the acid very slowly. Whenever handling piranha, only use glass containers. Prepare only as much as is needed, do not store any piranha solution. Piranha solution must be handled on a solvent bench with good ventilation. Proper protection clothes (nonflammable), thick rubber gloves, and helmet must be used during preparation. Piranha solution waste should be disposed of properly in a glass container; they should not be disposed of before cooling down to room temperature. Heated piranha solution in the waste container may also cause an explosion. Caution: if the H2O2 concentration is at 50% or greater, an explosion could occur.
A headway photoresist spinner was used.
SU-8 2035 photoresist is recommended. However, other photoresists with different viscosities will work as well (e.g., 2025, 2050, and 2075). The steps in Subheading 3.1 are specific to using SU-8 2035 photoresist for generating a two-layer silicon wafer master, in which each layer is 50 μm thick.
The Karl Suss MA6 is used. The SU-8 photoresist is in hard contact with the photomask. The photomask should be cleaned prior to this step to ensure there are no dust particles or other artifacts that may lead to an imperfect contact.
Alternatively, you can use plastic silverware or a pipet tip to stir the PDMS mixture. Plastic silverware is useful for quickly and easily mixing the PDMS mixture. However, they should be thoroughly cleaned if they are reused, as PDMS can cure at room temperature and may contaminate your next batch.
Another type of container may be used; wider containers may be better since they expose a larger surface of the contained fluid to air, which would aid in degassing the PDMS. Alternatively, you may use tin foil to wrap the silicon master and enclose the PDMS prepolymer solution.
The BD-10AS high frequency generator (Electro-Technic Products, Chicago, IL) was used. This lightweight handheld high frequency generator is meant for intermittent use, not to be operated for more than 10 min at a time. Alternatively, an oxygen plasma chamber or a UV ozone machine may be used under proper conditions. Please follow all safety regulations and read equipment instructions prior to use; high voltage equipment may cause harm to operator if used improperly.
FEP (Fluorinated Ethylene Propylene) tubing is mostly used for low-pressure microfluidics since it exhibits desired properties such as biocompatibility, flexibility, optical clarity, and resistance to most chemicals. Other tubing, such as fluorinated polymer tubing may also be used.
Chromatopur™ bovine albumin, low IgG, immunoassay grade was used. It is recommended that all pipette tips, centrifuge tubes, syringes, micromagnetic stir bars, and devices should be treated or rinsed with a solution of 2% BSA in DPBS prior to use with whole blood or the final sample suspension. BSA solutions should be aliquoted and stored at −20 °C; they may be stored at 4 °C for a limited length of time. Do not use BSA solution that has become turbid, as it will cause issues during Subheading 3.3.
KDS Legato (KD Scientific, Holliston, MA) and PHD Ultra (Harvard Apparatus, Holliston, MA) syringe pump series were used. These pumps have dual-syringe capability, allowing for 2 devices to be functionalized or used at one time.
Antibody clone selection is very important in CTC analysis. The clones used in our study have been previously shown to consistently work in clinical and translational studies.
It is recommended to use Ficoll-Paque PREMIUM of 1.077 g/mL (± 0.001 g/mL).
It is recommended to use the dilactate salt form of DAPI as it is more water soluble than the dihydrochloride salt. Use caution when using DAPI since it is a known mutagen and should be handled with care. The dye must be disposed of safely and in accordance with the applicable local regulations.
There is a range of options for fluorescent dye and filter selection. For CTC applications, FITC- and PE-conjugated antibodies are most commonly used. It is important to ensure that there is minimal overlap in fluorescent excitation and emission between dyes and filters used. The filter selection is shown below in Table 1.
There is a buildup of photoresist at the wafer edges during spinning. By removing any edge bead, the photomask can be placed into contact with the silicon wafer, resulting in the best resolution using contact lithography.
Soft bake includes 65 °C and 95 °C heating step. The 65 °C heating step helps smoothen the photoresist layer and promote uniformity. The 95 °C heating step increases solvent evaporation and hardens the photoresist. If the soft bake is too short, it will make the photoresist soft and sticky. The photomask can be contaminated during contact exposure and the photoresist layer can be destroyed. After the soft bake of the SU-8 is complete, the wafer can be kept in a box away from light and then be exposed, baked, and developed within a month. Therefore, the time before exposure is not a critical parameter. However, it is recommended to continue to the next step rather than waiting.
This energy exposure dose is specific to a photoresist layer thickness of 50 μm. This is one of the most important parameters to achieve the desired dimensions of the design. If the exposure time is too short, the photoresist may not be cross-linked over its entire depth, therefore it will lift off during the following development step. If the exposure time is too long, the width of the channels may be increased.
This development time is specific to a photoresist layer thickness of 50 μm.
The Dektak 150 surface profilometer was used.
It is recommended to use unpowdered gloves in this and all following steps to avoid contamination of the PDMS.
The curing process will vary, highly dependent on the amount of PDMS, thickness, and surface area exposed. PDMS can be cured between 25 and 150 °C, per the manufacturer. Curing at higher temperatures requires less time, while using lower temperatures requires longer time. Leaving the PDMS to cure overnight is acceptable, as excess time will not affect the results.
PDMS is hydrophobic with a low-energy surface that is nonreactive. Exposing PDMS to corona discharge makes the PDMS surface temporarily hydrophilic and reactive. This allows for irreversible bonding of PDMS to glass, silicon, or another PDMS piece that was also treated with corona discharge.
When inserting tubing to the inlet hole of a device, ensure that all air has been expelled from the syringe, the luer-to-barb connection, and from within the tubing itself. This will prevent unwanted introduction of bubbles into the microdevice.
Each device wash is approximately 3 times the device volume (100 μL × 3). For this device, we utilize three different levels of flow rates. A low flow rate is 1 μL/s, a medium flow rate is 1.5 μL/s, and a high flow rate is 2 μL/s. These flow rate recommendations are highly device-specific.
During this incubation period, you should begin preparing the blood sample if it is available (move onto Subheading 3.4). Anytime the device is kept at room temperature, ensure the inlet and outlet are kept wet to avoid air introduction into the device due to fluid evaporation.
The blood sample should be as fresh as possible and free of clots. Delay in processing blood samples can result in loss of cell viability, lower cell recovery, and higher contamination of red blood cells. If a blood sample can not be processed immediately, it should be stored at room temperature rather than in the fridge (4 °C) to prevent cell loss [7]. An anticoagulant can be added to the blood sample to ensure it is free of clots, though most vacutainers are coated with an anticoagulant.
It is recommended to use a centrifugation temperature of 18 °C by the manufacturer. However, if temperature control is not available for centrifugation, room temperature will work as well.
Dispose of biomedical waste properly. Treat any materials contaminated with blood with bleach for at least 20 min, and rinse with water. You should also treat excess blood sample or sample supernatant with bleach in this manner. Bleach (sodium hypochlorite) can be a hazardous chemical when not handled properly; use proper workplace controls and practices as well as PPE when handling bleach.
You should take extreme caution to avoid disturbing the layer of mononuclear cells. It is also possible to withdraw the layer of mononuclear cells without first removing the upper plasma layer. As an alternative to step 6 (Subheading 3.4), you can transfer the plasma and mononuclear layers together. Drawing excess plasma layer causes unnecessary contamination by platelets and plasma proteins; however, these would be washed away in the following centrifuge washing steps.
The desired mononuclear layer is theoretically separate from the Ficoll-Paque solution below it. However, in practice, some mononuclear cells will be suspended within the Ficoll-Paque solution. Therefore, it is suggested to withdraw as much of the upper layer of the Ficoll-Paque layer without extracting the hematocrit layer below. Keep in mind that drawing excess Ficoll-Paque increases granulocyte contamination.
There will be 50–150 μL of remaining liquid sample at the bottom of the tube when you discard the supernatant. You can use a standard micropipette to measure and take this into account when creating your final 1-mL cell sample. It is advised to treat the 2-mL microcentrifuge tube with 5% BSA solution (in DPBS) for at least 1 h prior to use, in order to reduce unwanted cell losses.
Although 10 mL of the whole blood sample was treated, only 8 mL is introduced into the device. Keep in mind that it is not possible to introduce all of the prepared sample volume within the syringe due to physical constraints; the micro stir bar will block the piston from pushing all of the fluid inside the syringe out. Furthermore, there is some dead volume within the syringe, the luer-to-barb adapter, and the tubing.
Some light sources, such as xenon and mercury lamps, require 5–10 min to stabilize their arcs after the burner is ignited.
If automated or semiautomated scanning hardware and software is not available, manual scanning and inspection can be performed. Individual cell images should be taken and analyzed for marker expression, or lack thereof. The same CTC enumeration guidelines from step 6 (Subheading 3.5) should be used.
Image editing software such as Adobe Photoshop® (Adobe, San Jose, California) has also been used to overlay fluorescent images.
A threshold is typically used to determine whether a specific cell is considered to be positive or negative for the expression of a given marker. This threshold was obtained from previously quantified CTCs.
References
- 1.Yu M, Stott S, Toner M, Maheswaran S, Haber DA (2011) Circulating tumor cells: approaches to isolation and characterization. J Cell Biol 192 (3):373–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hou HW, Warkiani ME, Khoo BL, Li ZR, Soo RA, Tan Ds et al. (2013) Isolation and retrieval of circulating tumor cells using centrifugal forces. Sci Rep 3:1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forbes TP, Kralj JG (2012) Engineering and analysis of surface interactions in a microfluidic herringbone micromixer. Lab Chip 12(15):2634–2637 [DOI] [PubMed] [Google Scholar]
- 4.Stott SL, Hsu CH, Tsukrov DI, Yu M, Miyamoto dT , Waltman BA et al. (2010) Isolation of circulating tumor cells using a microvortex-generating herringbone-chip. Proc Natl Acad Sci U S A 107(43):18392–18397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stroock AD, Dertinger SK, Ajdari A, Mezic I, Stone HA, Whitesides GM (2002) Chaotic mixer for microchannels. Science 295(5555): 647–651 [DOI] [PubMed] [Google Scholar]
- 6.Sheng W, Ogunwobi OO, Chen T, Zhang J, George TJ, Liu C et al. (2014) Capture, release and culture of circulating tumor cells from pancreatic cancer patients using an enhanced mixing chip. Lab Chip 14(1):89–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riethdorf S, Fritsche H, Muller V, Rau T, Schindlbeck C, Rack B et al. (2007) Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: a validation study of the Cell Search system. Clin Cancer Res 13(3):920–928 [DOI] [PubMed] [Google Scholar]