Abstract
Background:
Hyperinsulinemic normoglycemia augments myocardial glucose uptake and utilization. We tested the hypothesis that hyperinsulinemic normoglycemia reduces 30-day mortality and morbidity after cardiac surgery.
Methods:
This dual-center, parallel group, superiority trial randomized cardiac surgical patients between August 2007 and March 2015 at the Cleveland Clinic and Royal Victoria Hospital to intraoperative glycemic management with: 1)hyperinsulinemic normoglycemia, a fixed high-dose insulin and concomitant variable glucose infusion titrated to glucose concentrations of 80–110 mg·dL−1; or 2)standard glycemic management, low-dose insulin infusion targeting glucose >150 mg·dL−1. The primary outcome was a composite of 30-day mortality, mechanical circulatory support, infection, renal or neurologic morbidity. Interim analyses were planned at each 12.5% enrollment of maximum 2,790 patients.
Results:
At the third interim analysis (N=1,439; hyperinsulinemic normoglycemia 709, standard glycemic management 730; 52% planned maximum), the efficacy boundary was crossed and study stopped per protocol. Time-weighted average glucose concentration (means±SDs) with hyperinsulinemic normoglycemia was 108±20 versus 150±33 mg·dL−1 with standard glycemic management, P<0.001. At least one component of the composite outcome occurred in 49(6.9%) patients receiving hyperinsulinemic normoglycemia versus 82(11.2%) receiving standard glucose management (P<efficacy boundary 0.0085); estimated relative risk (95%interim-adjusted CI) 0.62(0.39,0.97), P=0.0043. There was a treatment-by-site interaction (P=0.063); relative risk for the composite outcome was 0.49(0.26,0.91, P=0.0007, N=921) at Royal Victoria Hospital, but 0.96(0.41,2.24, P=0.89, N=518) at the Cleveland Clinic. Severe hypoglycemia (<40 mg·dL−1) occurred in 6(0.9%) patients.
Conclusions:
Intraoperative hyperinsulinemic normoglycemia reduced mortality and morbidity after cardiac surgery. Providing exogenous glucose while targeting normoglycemia may be preferable to simply normalizing glucose concentrations.
9. Summary Statement:
This clinical trial randomized 1,439 cardiac surgical patients to hyperinsulinemic normoglycemia versus standard glucose management and found that morbidity and mortality were reduced in patients who received hyperinsulinemic normoglycemia.
Introduction
Hyperglycemia is associated with mortality and morbidity in critically ill and cardiac surgical patients.1–3 Consistent with these observations, intensive treatment of hyperglycemia aimed at normoglycemia reduced morbidity and mortality in a single center randomized trial of critically ill surgical patients, most of whom had recent cardiac surgery.4 Pediatric critically ill patients, most of whom had cardiac surgery and received intensive insulin therapy aimed at normoglycemia, similarly experienced reduced morbidity and mortality.5 Other trials, however, found that treatment of hyperglycemia with conventional insulin infusions aimed at normoglycemia either provided no benefit6,7 or increased mortality.8,9 Complications resulted, at least in part, from hypoglycemia.10
Disparities in reported outcomes may be related to whether or not sufficient glucose was provided. Outcomes in normoglycemic patients were generally favorable in trials where glucose was supplemented, either intravenously or nutritionally.4,5,11,12 In contrast, outcomes were unfavorable when normoglycemia was produced only by insulin administration.6–9 Provision of insulin and exogenous glucose while avoiding hyperglycemia, promotes myocardial glucose uptake and utilization, augments myocardial efficiency, and increases cardiac output13–18 — all of which may improve outcomes by increasing systemic perfusion and end-organ function. Cardiac surgical patients may especially benefit from normoglycemia with supplemental glucose because intraoperative myocardial ischemia and reperfusion injury are common.12,19,20 An additional benefit of normoglycemia is a reduced risk of perioperative infection.21–23
Hyperinsulinemic normoglycemia is a well-established glycemic management technique in which exogenous glucose is combined with intensive insulin therapy to target normoglycemia.24–26 Application of this technique in cardiac surgical patients aims to improve myocardial and end-organ function. Concurrent potassium supplementation is provided to avoid hypokalemia from insulin-induced cellular uptake of potassium.27 The hyperglycemic normoglycemia technique thus bears a resemblance to glucose-insulin-potassium (GIK) therapy, 12,28,29 except that normoglycemia is targeted. Normalization of glucose concentrations with the hyperinsulinemic normoglycemia technique may also reduce postoperative infections.
This investigation tested the hypothesis that intraoperative hyperinsulinemic normoglycemia improves a composite of 30-day postoperative mortality and serious cardiac, renal, neurologic, and infectious complications in patients recovering from cardiac surgery.
Materials and Methods
This dual-center, randomized, parallel-group, unblinded, superiority trial was approved by the Institutional Review Boards at the Cleveland Clinic, Cleveland, Ohio and Royal Victoria Hospital, Montreal, Quebec, and registered at ClinicalTrials.gov (NCT00524472) on August 31, 2007. Written, informed consent was obtained from each participant.
Adults between 18 and 90 years old scheduled for elective coronary artery bypass grafting (CABG), valve repair or replacement, or a combination of these procedures with cardiopulmonary bypass between August 2007 and April 2015 were screened for inclusion by research personnel. Exclusion criteria included off-pump cardiac surgery, anticipated hypothermic circulatory arrest, elevated baseline cardiac troponin I (>0.5 ng·l−1, Montreal) or troponin T (>0.1 ng·ml−1, Cleveland), kidney disease requiring renal replacement therapy, or active infection requiring ongoing antibiotic therapy. Sub-investigations, unrelated to the primary outcome, have previously been published.19,30–35
Randomization and masking
Study participants were randomly assigned (1:1) to hyperinsulinemic normoglycemia or standard glycemic management. Randomization was performed by the Plan procedure in SAS software, a web-based system, and was stratified by center (Cleveland versus Montreal), cardiac surgical procedure (coronary artery bypass grafting, valve repair/replacement, or combined procedure) and history of diabetes (type 2/diet-controlled versus type 1). Block size within each stratum randomly ranged from four to 16 patients. Allocation was initially concealed in sealed, sequentially numbered envelopes, and later in a web-based system, both accessed shortly before induction of anesthesia.
It was not feasible to blind anesthesia and surgical personnel to the intraoperative glucose management strategy; however, primary outcomes and postoperative clinical and laboratory results were evaluated by research personnel blinded to group allocation.
Procedures
Anesthesia and Surgery
Standard anesthesia monitors were supplemented by central venous or pulmonary artery catheters and transesophageal echocardiography. Midazolam, etomidate, thiopental, propofol, sufentanil and/or fentanyl, volatile anesthetics, and a depolarizing or non-depolarizing muscle relaxant were given during induction and maintenance of anesthesia. Surgery was performed through a full midline sternotomy or minimally invasive upper hemisternotomy, and routine strategies for conduct of cardiopulmonary bypass were followed.
Intermittent antegrade and retrograde administration of Buckberg’s cardioplegia mixed in 5% dextrose was used exclusively in Cleveland until December 2012; thereafter del Nido cardioplegia, a non-glucose containing solution administered as a single anterograde infusion, was occasionally used for isolated valve repair/replacement without CABG. In Montreal, intermittent anterograde and/or retrograde St. Thomas cardioplegia, a non-glucose containing solution was administered. Intravenous vasoactive infusions and antibiotic medications were mixed in 5% dextrose in Cleveland and normal saline solution in Montreal.
During separation from cardiopulmonary bypass, epinephrine was infused for low cardiac index (<2.0 L∙min−1∙m−2) and/or norepinephrine or vasopressin were infused for low systemic vascular resistance (<700 dyn∙sec∙cm−5) to maintain mean arterial pressure >80 mmHg and cardiac index >2.0 L∙min−1∙m−2. Milrinone was infused when cardiac output was low and refractory to routine pharmacologic hemodynamic support. If a pulmonary artery catheter was not present, transesophageal echocardiography was used to assess myocardial contractility and determine whether inotropic versus vasopressor support was needed.
Glucose management
Intraoperative glucose management with hyperinsulinemic normoglycemia involved a fixed-dose insulin infusion of 5 mU·kg−1.min−1 with a concomitant variable glucose (dextrose 20%) infusion supplemented with potassium (40 mEq·L−1) and phosphate (30 mmol·L−1) as previously described.24 The glucose infusion was initiated at approximately 40–60 mL·hr−1 when serum glucose concentration was approximately 110 mg·dL−1 or less, and manually titrated to target glucose concentrations of 80–110 mg·dL−1 every 10 to 15 minutes throughout surgery. Additional boluses of insulin were given for blood glucose >110 mg·dL−1. Arterial blood glucose concentrations were measured with an Accu-Check (Roche Diagnostics, Switzerland) glucose monitor. At sternal closure, the insulin infusion was reduced to 1 mU·kg−1.min−1 and converted to a standard low-dose insulin infusion upon Intensive Care Unit (ICU) admission. After ICU arrival, the glucose infusion was decreased by 25 – 50% every 20 min when the blood glucose was >110 mg·dL−1. When the infusion was at 20 cc·hr−1 or less and blood glucose was >110 mg·dL−1, the infusion was discontinued. Blood glucose concentrations were followed for 45 – 60 min after discontinuation of the dextrose infusion to ensure that hypoglycemia was avoided.
Standard glucose management involved a conventional low-dose insulin infusion titrated to blood glucose concentrations measured by arterial blood gas analysis every 30 – 90 minutes throughout surgery. This low-dose insulin infusion was initiated for blood glucose concentration >120 mg·dL−1 before initiation of cardiopulmonary bypass or >150 mg·dL−1 during or after cardiopulmonary bypass, at a rate based on patient weight and current glucose concentration. Subsequent adjustments were based on a sliding scale of current blood glucose concentration and the change from the previous measurement. Supplemental boluses of insulin were given with acute increases (>30 mg·dL−1) in blood glucose. The insulin protocol for patients assigned to standard glucose management is listed in Appendix 1.
Upon ICU admission, both groups transitioned to the same standardized postoperative insulin treatment protocol in the ICU. This involved measurement of blood glucose by arterial blood gas analysis approximately every two hours with adjustment of insulin infusion to maintain serum glucose <150 mg·dL−1 on postoperative day one and <120 mg·dL−1 on day two and later. In 2009 following publication of the NICE-SUGAR trial9, the postoperative glucose target increased to <180 mg/dL.
Severe and moderate hypoglycemia was defined as blood glucose less than 40 and 60 mg·dL−1, respectively. Hypoglycemia was treated by administration of 20% dextrose (25100 ml). A summary of major protocol changes that occurred since initiation of this investigation are found in Appendix 2.
Outcomes
The primary outcome was a collapsed composite (any versus none) of the following major postoperative complications occurring within 30 days of surgery, including 1) all-cause postoperative mortality; 2) failure to wean from cardiopulmonary bypass or postoperative low cardiac index (<1.8 L·min−1.m−2) requiring mechanical circulatory support with intraaortic balloon counter-pulsation, ventricular assist device, and/or extracorporeal mechanical oxygenation; 3) serious postoperative infection including any of the following infectious complications: mediastinitis, sternal wound infection requiring surgical debridement, sepsis, or pneumonia requiring mechanical ventilatory support; 4) acute postoperative kidney injury requiring renal replacement therapy; and 5) new postoperative focal (aphasia, decrease in limb function, hemiparesis) or global (diffuse encephalopathy with >24 hours of severely altered mental status or failure to awaken postoperatively) neurologic deficit.
The secondary outcomes included postoperative atrial fibrillation, defined as the occurrence of new onset postoperative atrial fibrillation following cardiac surgery, duration of hospitalization (days) and ICU stay (days), and one year all-cause mortality.
We also recorded a composite of minor postoperative complications within the first 30 days including mechanical ventilation >72 hours, low cardiac index (cardiac index <1.8 l·min−1.m−2 despite adequate fluid replacement (lack of hemodynamic response to repeated fluid administration of crystalloid or colloid intravascular solutions) and high dose inotropic support for >4 hours), acute kidney injury (increase in creatinine >100%), hospitalization >30 days, and all-cause hospital readmission within 30 days. Detailed definitions of the primary and secondary outcomes are listed in Appendix 3.
Statistical analysis
Balance on baseline characteristics between randomized groups was assessed using the standardized difference (STD = difference in means or proportions divided by pooled standard deviation). Imbalance was defined as a STD >0.2 in absolute value,36 and such variables were adjusted for in all analyses.
We assessed the effect of hyperinsulinemic normoglycemia versus standard therapy on the primary outcome (any complication) using Cochran-Mantel-Haenszel chi-square analysis, adjusting for clinical site. Results were reported as the estimated relative risk and interim analysis adjusted 95% confidence interval. We assessed the interaction between treatment effect and site using the Breslow-Day test for homogeneity of odds ratios. We also assessed the treatment-by-component interaction overall and within site using multivariate (one record per component per subject) generalized estimating equation “distinct-effects” logistic models.37
Groups were compared on binary secondary outcomes using the same methods as for the primary outcome. The treatment effect on time-to-event secondary outcomes (i.e., duration of mechanical ventilation, ICU and hospital stay [time to discharge alive]) were assessed using Cox proportional hazards models adjusting for site. For patients who died during the index hospitalization (N = 22), the hospital stay was assigned to be the longest observed hospital stay + 1 day, and censored at that time (i.e., not discharged alive). “Time to discharge alive” was not done used for ICU length of stay because the exact date/time of death was not recorded (just whether in-hospital or not). Median (95% CI) survival time was estimated from Kaplan-Meier curves.
Intraoperative time-weighted mean glucose concentration was calculated across measurements for each patient using the trapezoidal method and equal to the area under the curve divided by the total glucose reading time.
The significance level for each hypothesis was 0.05 and all tests were 2-sided. Confidence intervals were adjusted for the group sequential design (using confidence coefficient of 2.63) to maintain overall study alpha of 0.05 for combined sites and 0.025 overall (confidence coefficient of 2.86) within sites. Significance criterion for treatment-by-site interaction was set at 0.10 a priori. Bonferroni correction was performed while assessing each individual component of the composite primary outcome, with the significant level of 0.0017 (i.e., 0.0085/5 components = 0.0017) with 99.83% confidence intervals (CI), and 0.00084 (i.e., 0.0042/5) with 99.92% CI within site. SAS 9.2 (Carey, NC) or East 5.3 (Cytel Corporation) were used for all analyses.
Sample size calculations
A maximum of 2,790 patients was required to detect a 30% relative reduction in the composite of any major complications (i.e. any versus none) from an expected 15% incidence of complications in the standard group at the overall 0.05 significance level with 90% power. Interim analyses to assess efficacy and futility on the primary outcome of the occurrence of any major complication were planned at each 12.5% of the maximum planned enrolment in this group sequential design (N = 349, 697, 1046, 1394, 1743, 2091, and 2440). Patient recruitment continued while the interim analyses were performed, thus the timing of the interim analyses varied slightly from the original plan. We used the alpha (Type I error) and beta (Type II error) spending approach of Hwang et al.38 with parameters gamma = −2 for efficacy and gamma = −3 for futility.
Results
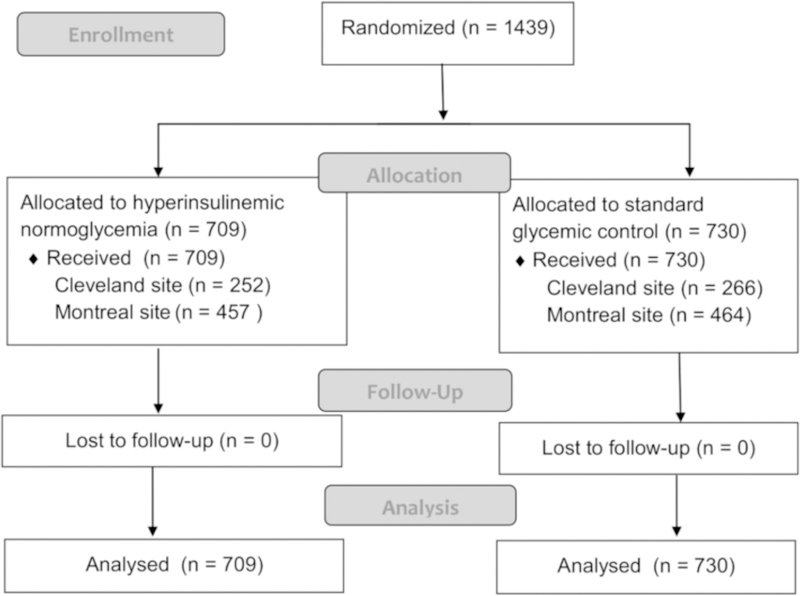
Patients were recruited from August 17, 2007 until March 30, 2015; 1,439 patients were randomly assigned to hyperinsulinemic normoglycemia (N = 709) and standard glycemic management (N = 730), with 518 in Cleveland and 931 in Montreal (Figure 1). The number of patients that were screened for this investigation was not available. At the third interim analysis with N = 1,439 (52% of maximum enrolment; patient recruitment continued during data analysis, thus the third interim analysis was later than initially planned), the treatment effect of hyperinsulinemic normoglycemia on the primary outcome crossed the predefined efficacy boundary for the combined sites, and the study was stopped as per the protocol. The P value boundaries for efficacy and futility were P <0.0085 and P ≥ 0.803, respectively.
Figure 1.
Patient flow diagram
Randomized groups were well-balanced (absolute standardized difference <0.20) on all preoperative patient demographics, clinical characteristics, preoperative echocardiographic measurements, and perioperative variables (Table 1). One year survival data were unavailable on 104 patients (hyperinsulinemic normoglycemia N = 56; standard glycemic management N = 48).
Table 1.
Baseline and surgical characteristics by treatment and site. Data are presented as N (%), Mean ± SD or Median [1st, 3rd quartiles]
| Combined Sites | Cleveland Clinic | Royal Victoria Hospital | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Factor | Hyperinsulinemic Normoglycemia (N=709) | Standard Therapy (N=730) | ASD | Hyperinsulinemic Normoglycemia (N=252) | Standard Therapy (N=266) | ASD | Hyperinsulinemic Normoglycemia (N=457) | Standard Therapy (N=464) | ASD |
| Demographics | |||||||||
| Age | 66 ± 11c | 66 ± 11b | 0.02 | 66 ± 13a | 66 ± 12a | −0.03 | 67 ± 11c | 66 ± 10b | 0.05 |
| Female | 189 (27)b | 184 (26)a | 0.04 | 76 (30)a | 72 (27)a | 0.07 | 113 (26)b | 112 (25)a | 0.02 |
| White race | 596 (87)b | 615 (86)a | 0.01 | 245 (97)a | 248 (93)a | 0.19 | 351 (81)b | 367 (82)a | −0.04 |
| Body mass index | 28.5 ± 5.7b | 28.3 ± 5.4a | 0.03 | 29.6 ± 6.1a | 28.9 ± 5.7a | 0.11 | 27.9 ± 5.4b | 28.0 ± 5.2a | −0.01 |
| Medical History | |||||||||
| Diabetes | 226 (32)a | 249 (34)a | −0.05 | 69 (27)a | 74 (28)a | −0.01 | 157 (34)a | 175 (38)a | −0.07 |
| COPD/Asthma | 107 (16)b | 85 (12)a | 0.10 | 41 (16)a | 34 (13)a | 0.10 | 66 (15)b | 51 (11)a | 0.11 |
| Pulmonary Hypertension | 101 (15)b | 102 (14)a | 0.01 | 57 (23)a | 66 (25)a | −0.05 | 44 (10)b | 36 (8)a | 0.07 |
| Stroke | 41 (6)b | 32 (5)b | 0.07 | 23 (9)a | 19 (7)a | 0.07 | 18 (4)b | 13 (3)b | 0.07 |
| Hypertension | 533 (77)b | 561 (79)a | 0.03 | 160 (63)a | 171 (64)a | 0.02 | 373 (86)b | 390 (87)a | 0.04 |
| Heart failure | 146 (21)b | 137 (19)a | 0.05 | 77 (31)a | 73 (27)a | 0.07 | 69 (16)b | 64 (14)a | 0.047 |
| Myocardial infarction | 193 (28)b | 173 (24)a | 0.09 | 59 (23)a | 62 (23)a | 0.002 | 134 (31)b | 111 (25)a | 0.13 |
| Dialysis | 4 (1)b | 4 (1)a | 0.003 | 2 (1)a | 3 (1)a | 0.03 | 2 (0)b | 1 (0)a | 0.04 |
| Peripheral vascular disease | 51 (7)b | 42 (6)a | 0.06 | 32 (13)a | 28 (11)a | 0.07 | 19 (4)b | 14 (3)a | 0.06 |
| Smoking | 197 (29)b | 180 (25)a | 0.05 | 109 (43)a | 117 (44)a | 0.01 | 88 (20)b | 63 (14)a | 0.16 |
| ASA physical status | 0.08 | 0.14 | 0.02 | ||||||
| 2 | 2 (0)b | 0 (0)a | 2 (1)a | 0 (0)a | 0(0)a | 0(0)a | |||
| 3 | 334 (49) | 349 (49) | 46 (18) | 48 (18) | 288 (66) | 301 (67) | |||
| 4 | 348 (51) | 363 (51) | 201 (80) | 215 (81) | 147 (34) | 148 (33) | |||
| 5 | 2 (0) | 1 (0) | 2 (1) | 1 (0) | 0(0) | 0(0) | |||
| Preoperative medications | |||||||||
| ACE Inhibitor | 266 (39)b | 262 (37)b | 0.03 | 96 (38)a | 96 (36)a | 0.04 | 170 (39)b | 166 (38)b | 0.03 |
| Antiarrhythmic | 56 (8)b | 67 (10)b | 0.05 | 34 (13)a | 44 (17)a | 0.09 | 22 (5)b | 23 (5)b | 0.01 |
| Beta-blocker | 434 (63)b | 452 (64)a | 0.01 | 116 (46)a | 134 (50)a | 0.09 | 318 (73)b | 318 (71)a | 0.03 |
| Calcium Blocker | 128 (19)b | 147 (21)b | 0.05 | 32 (13)a | 47 (18)a | 0.14 | 96 (22)b | 100 (23)b | 0.01 |
| Cox-2 Inhibitor | 13 (2)d | 3 (0)d | 0.14 | 6 (3)c | 1 (0)c | 0.19 | 7 (2)b | 2 (0)b | 0.12 |
| Statin | 475 (69)b | 486 (69)b | 0.004 | 130 (52)a | 145 (55)a | 0.06 | 345 (79)b | 341 (78)b | 0.04 |
| Steroid | 33 (5)b | 23 (3)b | 0.08 | 11 (4)a | 16 (6)a | 0.07 | 22 (5)b | 7 (2)b | 0.20 |
| Anti-diabetic drugs | 145 (21)b | 144 (20)a | 0.02 | 6 (2)a | 6 (2)a | 0.008 | 139 (32)b | 138 (31)a | 0.02 |
| Sulfonylureas or Meglitinides | 52 (8)d | 58 (9)d | 0.02 | 21 (10)c | 18 (8)c | 0.071 | 31 (7)b | 40 (9)b | 0.07 |
| Biguanides (metformin) | 126 (20)d | 124 (19)d | 0.02 | 24 (12)c | 29 (13)c | 0.05 | 102 (23)b | 95 (21)b | 0.05 |
| Thiazolidinediones | 16 (3)d | 11 (2)d | 0.06 | 7 (3)c | 4 (2)c | 0.10 | 9 (2)b | 7 (2)b | 0.04 |
| Insulin | 70 (11)d | 68 (10)d | 0.02 | 24 (12)c | 26 (12)c | 0.005 | 46 (11)b | 42 (9)b | 0.04 |
| Preoperative echocardiographie measurements | |||||||||
| Mitral regurgitation severity | 0.17 | 0.25 | 0.29 | ||||||
| 0 | 97 (27)g | 89 (24)c | 74 (37)c | 77 (36)c | 0.04 | 23 (14)f | 12 (8)g | ||
| + 1 | 85 (23) | 86 (23) | 42 (21) | 39 (18) | 0.09 | 43 (27) | 47 (31) | ||
| +2 | 78 (22) | 66 (18) | 34 (17) | 23 (11) | 0.09 | 44 (28) | 43 (28) | ||
| +3 | 46 (13) | 47 (13) | 21 (10) | 31 (14) | 25 (16) | 16 (11) | |||
| + 4 | 56 (15) | 79 (22) | 31 (15) | 46 (21) | 25 (16) | 33 (22) | |||
| Mitral stenosis | 8 (2)g | 6 (2)c | 0.04 | 6 (3)c | 5 (2)c | 0.04 | 170 (39)b | 1 (1)g | 0.05 |
| Aortic regurgitation severity | 0.18 | 0.17 | 0.36 | ||||||
| 0 | 187 (48)g | 194 (51)c | 142 (57)a | 164 (62)a | 45 (33)h | 30 (26)h | |||
| +1 | 77 (20) | 91 (24) | 42 (17) | 42 (16) | 35 (25) | 49 (42) | |||
| +2 | 66 (17) | 46 (12) | 38 (15) | 28 (11) | 28 (20) | 18 (16) | |||
| +3 | 32 (8) | 24 (6) | 17 (7) | 14 (5) | 15 (11) | 10 (9) | |||
| +4 | 27 (7) | 25 (7) | 12 (5) | 16 (6) | 15 (11) | 9 (8) | |||
| Aortic stenosis | 225 (47)b | 201 (43)b | 0.08 | 119 (47)a | 111 (42)a | 0.11 | 106 (47)b | 90 (45)b | 0.04 |
| LV ejection fraction | 0.13 | 0.07 | 0.20 | ||||||
| LVEF > 60% | 246 (37)c | 257 (37)c | 89 (39)b | 91 (36)a | 157 (36)b | 166 (38)b | |||
| LVEF 50 – 59% | 236 (36) | 238 (34) | 91 (39) | 106 (42) | 145(34) | 132 (30) | |||
| LVEF 45 – 49% | 3 (0) | 3 (0) | 3 (1) | 3 (1) | |||||
| LVEF 40 – 44% | 54 (8) | 65 (9) | 10 (4) | 13 (5) | 44 (10) | 52 (12) | |||
| LVEF 35 – 39% | 34 (5) | 54 (8) | 11 (5) | 12 (5) | 23 (5) | 42 (10) | |||
| LVEF <35% | 89 (13) | 76 (11) | 27 (12) | 28 (11) | 62(14) | 48 (11) | |||
| Preoperative laboratory measurements | |||||||||
| BUN (mg/dL) | 20.9 ± 16.7e | 21.0 ± 17.1e | 0.006 | 22 ± 11a | 21.5 ± 10.2a | 0.05 | 20 ± 20e | 21 ± 21e | 0.03 |
| Creatinine (mg/dL) | 96.7 ± 45.6b | 97.1 ± 52.5b | 0.007 | 104 ± 66a | 101 ± 62a | 0.05 | 92 ± 27b | 95 ± 46b | 0.06 |
| Hematocrit (mg/dL) | 40 ± 8b | 40 ± 8b | 0.03 | 40 ± 5a | 41 ± 5a | 0.09 | 40 ± 9b | 40 ± 10b | 0.007 |
| Perioperative variables | |||||||||
| Cardiac surgical procedure CABG (No valve) | 298 (42) | 311 (43) | 0.02 | 85 (34) | 87 (33) | 0.05 | 213 (47) | 224 (48) | 0.03 |
| Valve (No CABG) | 272 (38) | 281 (38) | 97 (38) | 109 (41) | 175 (38) | 172 (37) | |||
| CABG + Valve | 138 (19) | 138 (19) | 70 (28) | 70 (26) | 68 (15) | 68 (15) | |||
| Previous Cardiac Surgery | 94 (14)b | 84 (12)a | 0.06 | 69 (27) | 68 (26) | 0.04 | 25 (6)b | 16 (4) a | 0.10 |
| Surgical variables | |||||||||
| Duration of surgery (min) | 260 [200, 341]f | 273 [210, 351]f | 0.08 | 376 [311, 444]c | 358 [305, 423]c | 0.1 | 215 [170, 250]e | 220 [180, 270]e | 0.14 |
| Duration of cardiopulmonary bypass (min) | 96 [77, 126]d | 99 [77, 126]d | 0.01 | 93 [77, 120]c | 94 [74, 116]c | 0.04 | 99 [77, 130]b | 101 [79, 130]b | 0.04 |
| Duration of aortic cross-clamp (min) | 77 [60, 104]d | 79 [61, 103]d | 0.02 | 71 [58, 93]c | 74 [57, 93]c | 0.001 | 82 [61, 108]b | 84 [64, 107]b | 0.03 |
| Cardioplegia | 0.07 | 0.12 | 0 | ||||||
| St. Thomas | 457 (64) | 464 (64) | 0 (0) | 0(0) | 457 (100) | 464 (100) | |||
| Buckberg’s | 246 (35) | 257 (35) | 246 (98) | 257 (97) | 0(0) | 0 (0) | |||
| Del Nido | 5 (1) | 9 (1) | 5 (2) | 9 (3) | 0(0) | 0 (0) | |||
| Microplegia | 1 (0) | 0 (0) | 1 (0) | 0 (0) | 0(0) | 0(0) | |||
Data are represented as N (%), mean ± SD, or median [25th, 75th percentiles]. CC = Cleveland Clinic; RVH = Royal Victoria Hospital; COPD = Chronic obstructive pulmonary disease; ASA = American Society of Anesthesiologists (ASA) physical status classification system; ACE inhibitor: Angiotensin converting enzyme inhibitor; LVEF = Left ventricular ejection fraction; CABG = Coronary artery bypass grafting. LV = Left ventricular tASD = Absolute standardized difference (difference in means or proportions divided by standard deviation); imbalance defined as absolute value of ASD > 0.20 (small effect size).
Missing data points:
1 ~19
20 ~29
35~51
70~79
150~162
190~200
231~299
300~514.
Insulin administration and treatment effects
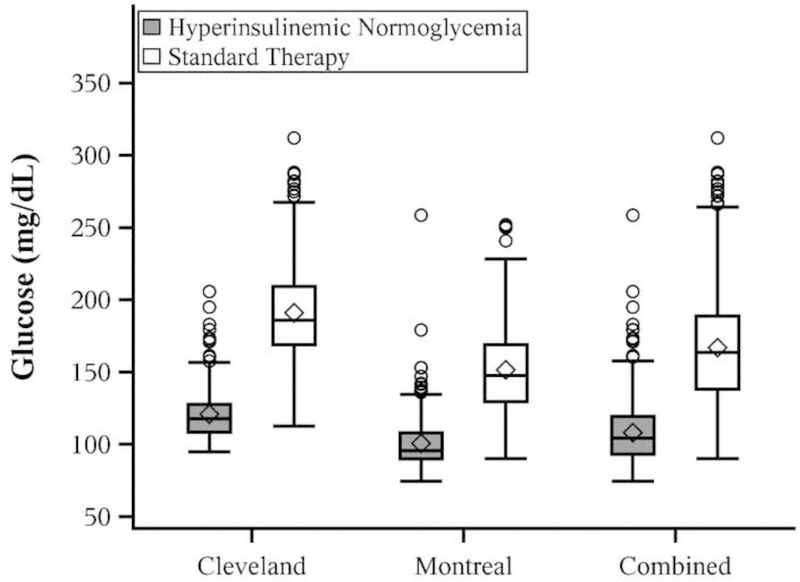
Overall mean ± SD time-weighted average glucose concentration was 108 ± 20 mg·dL−1 with hyperinsulinemic normoglycemia versus 150 ± 33 mg·dL−1 with standard glycemic management. The Cleveland site had higher time-weighted average glucose concentration than Montreal overall, as well as within each treatment (all P<0.001). In Cleveland, glucose concentration was 121 ± 19 mg·dL−1 with hyperinsulinemic normoglycemia and 171 ± 31 mg·dL−1 with standard glycemic management. At the Royal Victoria Hospital, patients in the hyperinsulinemic normoglycemia group had glucose concentrations of 101 ± 17 mg·dL−1 versus 136 ± 26 mg·dL−1 with standard glycemic management. Reduction in mean time-weighted average intraoperative glucose concentration was similar at each site, with the estimated ratio of means (hyperinsulinemic normoglycemia/standard) (95% CI) being 0.71 (0.69, 0.73) in Cleveland versus 0.74 (0.72, 0.76) in Montreal. The overall effect for combined sites was 0.73 (0.72, 0.74; Figure 2).
Figure 2.
Boxplots comparing randomized groups on time weighted intraoperative glucose concentrations overall and within site. All = combined sites; HN = hyperinsulinemic normoglycemia. Box shows the interquartile range; horizontal line marks the median; whiskers extend to high and low values within 1.5 interquartile range of the box; circles are values beyond 1.5 interquartile range of the box; diamond shows the mean.
Moderate hypoglycemia (glucose concentration <60 mg·dL−1) occurred in 91 (13%) of the hyperinsulinemic normoglycemia group and severe hypoglycemia (<40 mg·dL−1) occurred in 6 (0.9%). The average duration of a hypoglycemic episode in the hyperinsulinemic normoglycemic group was 9 (range 3–16) minutes. Only one patient in the conventional insulin infusion group had severe hypoglycemia, lasting 29 minutes.
Primary results
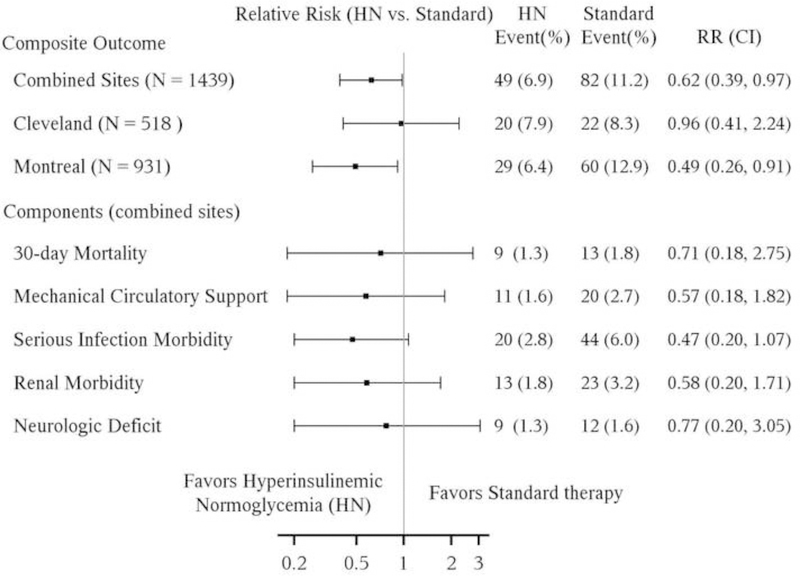
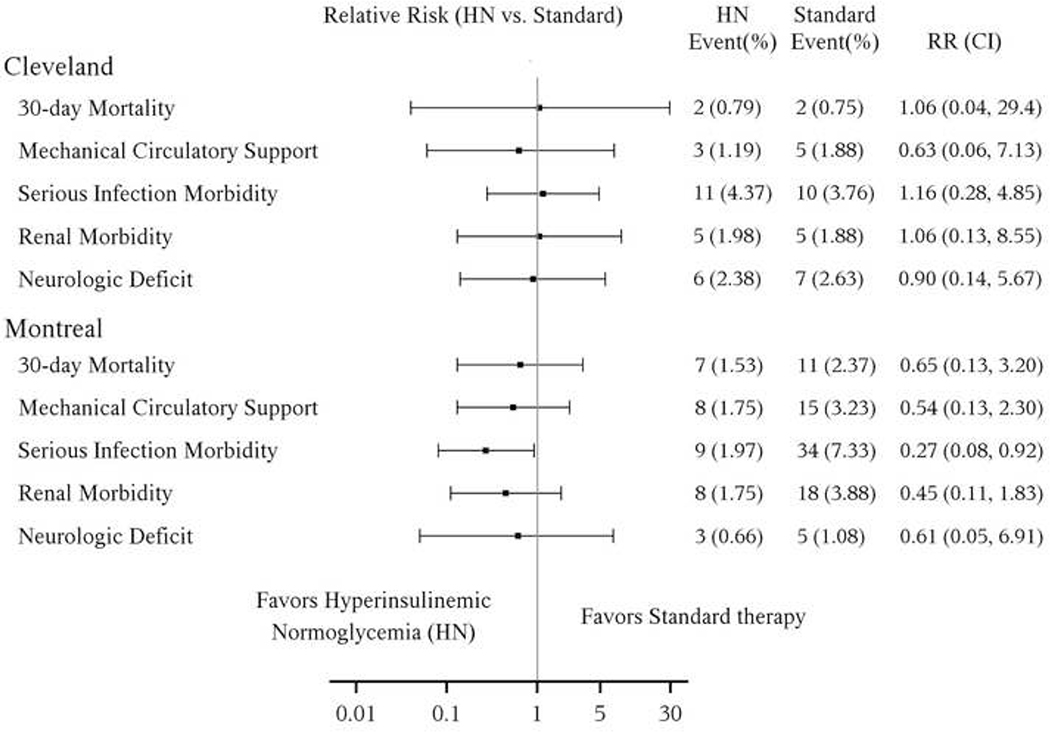
At least one component of the composite outcome occurred in 49 (6.9%) of patients receiving hyperinsulinemic normoglycemia versus 82 (11.2%) receiving standard glucose management (P< efficacy boundary of 0.0085) for an estimated relative risk (95% interim-adjusted CI) of 0.62 (0.39, 0.97), P = 0.0043 (Figure 3a). However, there was a strong treatment-by-site interaction (P = 0.063, less than the a priori criterion of 0.10); the relative risk for the composite outcome was 0.49 (0.26, 0.91, P = 0.0007, N = 921) at the Royal Victoria Hospital, Montreal, but 0.96(0.41, 2.24, P = 0.89, N = 518) at the Cleveland Clinic. Proportions and relative risks for the individual major complications in the combined sites are shown in Figure 3a and by individual site in Figure 3b. There was no evidence of treatment-by component interaction overall (P=0.84), for Cleveland (P = 0.96) or Montreal (P = 0.52), and thus inference within components was statistically unnecessary. Nevertheless, after adjusting for multiple comparisons across components, only serious infection morbidity in Montreal was significantly affected by intervention.
Figure 3.
Comparison of the hyperinsulinemic normoglycemia (HN) and standard therapy group on the composite outcome of any major morbidity/30-day mortality and individual components of the composite outcome at combined sites (Figure 3a) and within individual sites (Figure 3b). CI=confidence interval; Interaction P-value (treatment-by-site) = 0.063. Confidence intervals adjusted for group sequential design (using confidence coefficient of 2.633) to maintain overall study alpha of 0.05 for combined sites and confidence coefficient of 2.86 within sites. P-values for combined sites: significant if P < 0.0085 for efficacy (with 99.15% CI); P-values for each site: significant if P < 0.0042 for efficacy (with 99.58% CI); P-values for each component: significant if P < 0.0042/5 = 0.00084 (with 99.92% CI) using Bonferroni correction.
Secondary results
Time-to-event secondary outcomes for the combined sites are shown in Table 2; no differences were found on any of the five outcomes. Secondary outcomes are shown by site in Appendix 4; no differences were found in any secondary outcome within site. Although there was a significant treatment-by-site interaction for ICU length of stay (P = 0.046), the treatment effect was not significant for either site; the hyperinsulinemic normoglycemia group was an estimated 1.24 (0.98, 1.57) times more likely to be discharged earlier than in the standard group in Montreal (P = 0.0026, not significant after Bonferroni correction), and 0.99 (0.74, 1.33) at the Cleveland Clinic (P = 0.89). Similarly, the treatment-by-site interaction was significant for hospital stay (P = 0.07), but the treatment effect was not significant at either site. There were no differences between groups on other secondary outcomes, including the composite of minor complications, postoperative atrial fibrillation, or one-year mortality (i.e. all P >0.0085, Table 2).
Table 2.
Comparison of the Hyperinsulinemic Normoglycemia vs. Standard Therapy groups on Secondary Outcomes
| Outcomes | Hyperinsulinemic Normoglycemia (N = 709) | Standard Therapy (N = 730) | ||||
|---|---|---|---|---|---|---|
| N | Median (95% CI) | N | Median (95%CI) | HR (99.83%CI)* | P-value* | |
| ICU stay (hrs) | 0.046b | |||||
| 649 | 25(24.9, 26.3) | 671 | 27 (25.2, 27.3) | 1.13 (0.95, 1.35) | 0.025 | |
| Hospital stay (days)** | 0.07 b | |||||
| 686 | 8 [6, 12] | 713 | 8 [6, 12] | 1.05 (0.89, 1.25) | 0.35 | |
| N | Event (%) | N | Event (%) | RR (99.83%CI)* | ||
| Postoperative atrial fibrillation | 0.51 b | |||||
| 709 | 209 (29) | 730 | 235 (32) | 0.92 (0.75,1.13) | 0.29 | |
| Any minor complicationa | 0.21 b | |||||
| 709 | 194(27) | 730 | 227(31) | 0.95 (0.87,1.04) | 0.13 | |
| 1-year mortality | 0.13 b | |||||
| 653 | 32 (5) | 682 | 22 (3) | 1.52 (0.74,3.11) | 0.12 | |
Confidence intervals and P values from Cox proportional hazards for ICU stay and Hospital stay, and Cochran-Mantel-Haenszel test for binary outcomes. Confidence intervals adjusted for group sequential design using confidence coefficient of 2.633 for combined sites in order to maintain overall study alpha of 0.05. Significant if P < 0.0085 /6 = 0.0017 using Bonferroni correction.
The observed longest hospital stay + 1 day was assigned to patients who died during hospitalization (N = 22).
Minor complications include any one of the following: a prolonged requirement for mechanical ventilation (>72 hours), low cardiac index (cardiac index <1.8 l/min/m2 despite adequate fluid replacement and high dose inotropic support for >4 hours), acute kidney injury (increase in creatinine >100%), prolonged hospitalization (>30 days), and all-cause hospital readmission within 30 days).
treatment × site P-value.
Discussion
Hyperinsulinemic normoglycemia reduced the composite outcome of 30-day mortality and serious complications by nearly 40% (confidence interval 3–61%) in patients having cardiac surgery across our two clinical sites. Our results are broadly consistent with previous work showing that normoglycemia reduces various complications when supplemental glucose is provided.4,5,11,12
The fixed high-dose insulin infusion technique contrasts with most previous trials in which only insulin was given to maintain normoglycemia. Insulin is cardioprotective independent of glucose concentrations.39 Insulin administration during reperfusion reduces myocardial infarction via Akt and p70s6 kinase-dependent signaling pathways28,39 and may improve myocardial metabolic and functional recovery after cardioplegic arrest.40,41 Laboratory investigations similarly report myocardial benefit from provision of glucose and insulin.42
The protective effects of enhanced myocardial glucose uptake and utilization may be especially beneficial during cardiac surgery because it might counteract myocardial dysfunction consequent to cardioplegic arrest and ischemia and reperfusion injury. Previous studies that largely enrolled cardiac surgical patients (N = 1548 and 700) similarly demonstrated benefit, although intensive insulin therapy targeting normoglycemia with supplemental glucose was initiated following surgery.4,5 One other investigation (N = 371), however, examined the benefit of intraoperative glucose control during cardiac surgery and reported worse outcomes with intensive insulin therapy,8 although a standard insulin infusion, rather than hyperinsulinemic normoglycemia, was evaluated.
Hyperinsulinemic normoglycemia resembles GIK therapy which provided myocardial protection and improved left ventricular function in some,14,28 but not all,43 investigations. Both approaches are thought to provide cardioprotective benefits by increasing myocardial glucose uptake and improving coupling of glycolysis and glucose utilization.42,44,45 However, hyperinsulinemic normoglycemia differs from GIK in avoiding hyperglycemia which is consistently associated with worse outcomes.3,46 Variable degrees of hyperglycemia may explain why GIK demonstrated benefit in some investigations12,28 but not in others.29,47,48
Aside from the overall significant benefit of hyperinsulinemic normoglycemia, the most striking aspect of our results is that the benefit was apparently restricted to one study site. We considered several potential explanations. Although glycemic management was standardized, cardioplegia at the Cleveland Clinic contained glucose whereas it did not in Montreal; thus both groups at the Cleveland Clinic received exogenous glucose during cardioplegic arrest. It is possible that the usual provision of glucose-containing cardioplegia provided significant myocardial protection and reduced low cardiac output syndrome and mechanical circulatory support in all patients at the Cleveland Clinic, regardless of randomized group.
The need for mechanical circulatory support was low (<2%) in all groups that received glucose from either hyperinsulinemic normoglycemia or cardioplegia administration. Only the standard glycemic management group in Montreal did not receive exogenous glucose during cardioplegic arrest and also demonstrated the highest need for mechanical circulatory support. Consistent with this theory, a previously reported sub-investigation49 from Montreal provided evidence of cardio-protection and improved myocardial function in patients who received hyperinsulinemic normoglycemia, but not standard glucose management. In contrast, myocardial function at the Cleveland Clinic was not different between groups.30
Glucose concentrations for both randomized groups were higher in Cleveland than in Montreal, presumably because patients at the Cleveland Clinic were given cardioplegia with glucose and medications were mixed with glucose. Higher glucose concentrations at the Cleveland Clinic may explain the lack of difference between groups, whereas the effect was profound at the Royal Victoria Hospital.50 Results for the primary outcome were clearly centered around the null hypothesis at the Cleveland Clinic, with a relative risk estimate of 0.96. However, because Cleveland contributed only about a third of the patients, the site-specific 95% confidence intervals for the primary outcome range from a 59% reduction to a 2.2-fold increase in the composite outcome, which does not allow a firm negative conclusion.
Hyperinsulinemic normoglycemia reduced serious postoperative infection only in Montreal. Others similarly reported a nearly 50% reduction in blood stream and sternal wound infections in cardiac surgical and critically ill patients who received intensive insulin therapy.4,22 Hyperglycemia impairs leukocyte function, increasing risk of infection,51,52 and our results are consistent with these observation. The Cleveland site however, received no benefit from hyperinsulinemic normoglycemia. The incidence of postoperative infectious complications in the Cleveland control group was half of the incidence of infection in Montreal. It is therefore possible that infection risk at the Cleveland Clinic was already low so that hyperinsulinemic normoglycemia provided little additional benefit.
Hypoglycemia, which has been closely linked to adverse outcomes in other investigations, rarely occurred in our study. We attribute the low incidence of hypoglycemia to the profound stress counter-regulatory response and insulin resistant state that ensues with cardiac surgery and during the conduct of cardiopulmonary bypass. But it is also due to frequent blood glucose measurements (generally every 10–15 min) and close titration of glucose by dedicated investigators.
We could not blind anesthesia or surgical personnel to intraoperative glycemic management; however, most outcomes occurred several hours to days postoperatively and were recorded by research personnel who were blinded to treatment assignment. Our investigation cannot determine whether the benefit of hyperinsulinemic normoglycemia was due to the administration of high-dose insulin with glucose supplementation versus benefits of normoglycemia; thus the observed benefits may have resulted from more intensive glucose, rather than the concomitant provision of supplemental glucose. The study stopped after slightly more than 50% of the planned patients were enrolled, but it was not “stopped early” for logistical or other non-statistical reasons; enrollment was stopped per protocol by the Executive Committee because results at a planned interim analysis met a priori efficacy criteria. Our “group sequential” design protected the type I error at 5% and the type II error at 10% for the primary analyses. That said, as is true with any such design which crosses a boundary and thus (legitimately) stops enrollment before the maximum is reached, our confidence intervals would have been somewhat narrower had we continued.
In summary, hyperinsulinemic normoglycemia in patients having cardiac surgery reduced a composite of postoperative morbidity and mortality. Because previous investigations targeting normoglycemia in the absence of exogenous glucose supply found no benefit, targeting normoglycemia while providing exogenous glucose may be preferable to simply normalizing blood glucose concentrations.
6. Acknowledgements:
We thank Ann Wright, Department of Anesthesia, Royal Victoria Hospital, McGill University, for her review of this manuscript.
10. Funding Statement: This investigation was supported by The National Institutes of Health (NIH) National Heart, Lung and Blood Institute (NHLBI), Bethesda, Maryland K23 HL093065 (Andra Duncan, M.D.), the Departments of Cardiothoracic Anesthesia and Outcomes Research at the Cleveland Clinic, and the Department of Anesthesia at the Royal Victoria Hospital, McGill University.
Appendix 1. Cleveland Clinic Operating Room Insulin Therapy Protocol
Blood Glucose Goal: 70 – 150 mg/dL. Regular Insulin 100 units/100 ml in 0.9% normal saline in a concentration of 1 unit/ml will be used.
- Starting Insulin: Start if pre CPB blood glucose > 120, and if on pump or post pump blood glucose > 150.
- Bolus dose: 0.03 units/kg (maximum bolus is 3 units)
- Initiate continuous infusion: initial rate 0.03 units/kg/hr (maximum initial rate is 3 units/hr)
- See Table 1 (Insulin Infusion adjustment) for adjustment of insulin rate.
Blood glucose monitoring: Measure blood glucose between 30 – 60 minutes during surgery. (This recommendation was changed to 60 – 90 min in 2009).
- Hypoglycemia protocol:
- If blood glucose ≤ 60 mg/dL: stop insulin infusion, give 25 – 50 mL of 50% dextrose solution, obtain blood glucose level every 30 minutes until blood glucose > 80 mg/dL for three consecutive levels, and then check blood glucose every 30 – 60 minutes.
- If blood glucose 60 – 70 mg/dL, or 71 – 85 mg/dL and decreasing: stop insulin infusion, obtain blood glucose level every 30 minutes until blood glucose > 85 mg/dL for three consecutive measurements, then check blood glucose every hour.
- Resuming insulin infusion:
- Restart at half the previous rate when blood glucose rises above 150 mg/dL.
Table 1:
Insulin Infusion Adjustment (Do not adjust insulin rate every hour - only make adjustments to the insulin rate every two hours)
| Blood Glucose | If BG DECREASES≥ 30 mg/dl since last level | If BG isSTABLE (change in BG < 30 mg/dl) since last level | If BG INCREASES ≥ 30 mg/dl since last level |
|---|---|---|---|
| ≤ 60 | Stop insulin infusion See Hypoglycemia Protocol | Stop insulin infusion See Hypoglycemia Protocol | –– |
| 61 – 70 | Stop insulin infusion See Hypoglycemia Protocol | Stop insulin infusion/ See Hypoglycemia Protocol | –– |
| 71–85 | Stop insulin infusion See Hypoglycemia Protocol | Decrease rate by 50% | |
| 86 – 100 | Decrease rate by 50% | Decrease rate by 50% | –– |
| 101 – 115 | Decrease rate by 50% | Continue current rate | –– |
| 116 – 150 | Decrease rate by 50% | Increase rate by 25% | Increase rate by 25% |
| 151 – 200 | Decrease rate by 25% | Increase rate by 25% | Bolus 2 units/ Increase rate by 25% |
| 201 – 250 | Continue current rate | Bolus 2 units/ Increase rate by 25% | Bolus 4 units/ Increase rate by 25% |
| 251 – 300 | Continue current rate | Bolus 4 units/ Increase rate by 50% | Bolus 6 units/ Increase rate by 50% |
| 301 – 350 | Continue current rate | Bolus 6 units/ Increase rate by 50% | Bolus 8 units/ Increase rate by 50% |
| 351 – 400 | Continue current rate | Bolus 8 units/ Increase rate by 50% | Bolus 10 units/ Increase rate by 50% |
| > 400 | Notify staff anesthesiologist* | Notify staff anesthesiologist* | Notify staff anesthesiologist* |
(Note: if insulin rate is ≥ 30 units/hr* notify staff anesthesiologist)
Severe hyperglycemia will be treated per anesthesiologist’s discretion.
Appendix 2. Hyperinsulinemic Normoglycemia versus Conventional Insulin Infusion for Intra-operative Glucose Management in Patients having Cardiac Surgery: A Randomized Clinical Trial
Summary of Major protocol changes from original protocol (date July 23, 2006)
| Date | Protocol Change | Rationale |
|---|---|---|
| 07/23/2006 | • Original protocol | |
| 11/19/2007 | • Inclusion criteria were broadened. Initial inclusion criteria were changed from patients having mitral valve surgery with CABG to all cardiac surgeries requiring cardiopulmonary bypass | An increase in patient enrollment was needed. |
| 05/07/2008 (delirium) 07/17/2008 (echocardiographic and left atrial tissue analysis) 4/28/2008 (quality of life - deleted DASI) 05/07/2008 (quality of life, add SF-12) |
The following secondary outcomes were limited to a single center sub-investigations (Cleveland Clinic only) and are thus not reported in this manuscript • Postoperative delirium • Echocardiographic-measurement of left ventricular function • Postoperative quality of life measured by a health survey • Analysis of left atrial tissue |
These sub-investigations answered site-specific questions. |
| 05/07/2008 | Changed measurement of follow-up of endpoints, including all-cause mortality, from 15 – 30 days to 1 and 3 months (30 and 90 days). | This change allowed better synchronization and efficiency of data collection with other timepoints. All 15-day outcomes continued to be captured at 30 days. |
| 9/29/2008 (hospital readmission) 01/29/2009 (additional secondary outcomes) |
We revised the secondary outcomes including a “composite of minor outcomes” which including prolonged intubation, low cardiac index, renal insufficiency, prolonged hospitalization, hospital readmission | This revised secondary outcome captured perioperative data that had a lesser, but still important, impact on postoperative course. |
| 01/29/2009 | The following exclusion criteria were added: • Active infection including patients with endocarditis or infected pacemaker leads • Any infection requiring long-term antibiotics (>14 days) • Kidney disease requiring renal replacement therapy |
To identify patients who required renal replacement therapy and serious infection, which are components of the primary composite outcome, we excluded patients who required renal replacement therapy or had severe infection at time of enrollment. |
| 01/29/2009 | The following primary outcomes were deleted: 1) Low cardiac index 2) Perioperative myocardial infarction 3) Prolonged intubation (>72 hours) 4) Anuria (urine output < 0.5 cc/kg/8 hr) |
Changes to the primary outcome were made to improve measures of postoperative recovery. These outcomes were problematic because of 1) lack of standardized definition for perioperative myocardial infarction 2) inadequate documentation of low cardiac index and anuria 3) heterogeneity of patients who experience prolonged intubation |
| 01/29/2009 | The following secondary outcomes were removed: dysrhythmias, hyperlactatemia, postoperative troponin I or T, inotropic support |
Secondary outcomes were revised to select more important clinical endpoints |
| 01/29/2009 | The sample size was increased to 2790 patients and the number of interim analyses was changed from 3 to 7 | The sample size estimate was re-adjusted to power the study for a 30% reduction in risk of complications (previously a 60% reduction). |
Appendix 3.
Appendix 3A.
Primary outcome: Definitions of the components of a composite of major post-operative complications occurring within 30 days after surgery
| Major complications | Requirements for acceptance |
|---|---|
| Death within 30 days | All-cause mortality identified during initial hospitalization or during 30 day follow-up. |
| Post-operative mechanical circulatory support | Failure to wean from CPB or post-operative low cardiac index (CI <1.8 l/min/m2) conditions requiring circulatory support with either intra-aortic balloon pump, ventricular assist device, and/or extracorporeal mechanical oxygenation during or post-CPB or during post-operative course. |
| Serious Infection Morbidity | Post-operative course complicated by one of the following: 1) sepsis with evidence of acute organ dysfunction. Sepsis is recognized as a clinical syndrome that may be defined by infection highly suspected (clinical syndrome pathognomonic for infection) or proven (by culture, stain, or polymerase chain reaction) and presence of two or more of the following systemic inflammatory response syndrome (SIRS) criteria: heart rate > 90 beats per minute (tachycardia); body temperature < 36 °C or > 38 °C (hypothermia or fever); respiratory rate > 20 breaths per minute or, a PaCO2 less than 32 mmHg (tachypnea or hypocapnia due to hyperventilation); white blood cell count < 4000 cells/mm3 or > 12000 cells/mm3, or greater than 10% band forms (immature white blood cells) (leukopenia, leukocytosis, or bandemia)124; 2) mediastinitis (sternal click, open sternal wound, drainage from mediastinal incision, with fever, and including positive cultures along with elevated white blood cell count and the institution of antimicrobial therapy and reexploration with operative note diagnosing mediastinitis or sternectomy with muscle flap grafts to the affected area or diagnosis by physician of mediastinitis); 3) sternal wound infection (sternal wound infection other than mediastinitis, documented with positive cultures, requiring surgical intervention); or 4) pneumonia (fever > 38°C, elevation in white blood cell count, increase in sputum production, infiltrate in chest x-ray > 24 hours, positive sputum culture) requiring mechanical ventilation. |
| Renal morbidity | Post-operative requirement for renal dialysis. Patients with pre-operative requirement for dialysis are excluded. |
| Neurologic deficit | New post-operative focal (aphasia, decrease in limb function, or hemiparesis confirmed by clinical findings and/or computed tomographic scan) or global neurologic deficit (diffuse encephalopathy with greater than 24 hours of severely altered mental status, and/or failure to awaken post-operatively). |
Appendix 3B.
Secondary Outcomes
| Secondary Outcomes | Requirements for Acceptance |
|---|---|
| Composite of minor outcomes | The occurrence of one of more of the minor complications listed in Table 3 occurring within 30 days of surgery |
| Postoperative atrial fibrillation | The occurrence of new-onset postoperative atrial fibrillation following cardiac surgery occurring within 30 days of surgery. Patients who had paroxysmal or persistent atrial fibrillation prior to surgery are excluded. |
| Duration of Hospitalization | Days from day of surgery to hospital discharge. |
| Duration of Intensive Care Unit Stay | Days from day of surgery to discharge from intensive care unit. |
| All-cause mortality at one year | All-cause mortality identified during one-year follow-up. |
Appendix 3C.
Components of the composite of the minor outcomes (a secondary outcome).
| Prolonged intubation | Endotracheal intubation and mechanical ventilation required for >72 hours post-operatively, measured from arrival in intensive care unit following surgery until weaning from mechanical ventilation and endotracheal extubation. Additional periods of time where reintubation and mechanical ventilation is required are included. |
|---|---|
| Low cardiac index | Cardiac index <1.8 l/min/m2 despite adequate fluid replacement and high dose inotropic support for >4 hours |
| Renal insufficiency | Postoperative increase in baseline creatinine >100%. Baseline creatinine was defined as the preoperative measurement immediately prior to surgery. |
| Prolonged hospitalization | Hospitalization following surgery > 30 days |
| Hospital readmission | Postoperative complications requiring readmission to a hospital for any reason identified during 30 day followup. |
Appendix 4. Treatment effect on secondary composite outcome by site
| Site Complications | Hyperinsulinemic Normoglycemia | Standard Therapy | Relative Risk (99.92% CI)* | P-value |
|---|---|---|---|---|
| Cleveland Clinic, Cleveland | N = 252 | N = 266 | ||
| Postoperative atrial fibrillation | 108 (44) | 128 (50) | 0.89 (0.64,1.23) | 0.23 |
| Duration of hospitalization (days) | 7 [5, 12] | 7 [5, 11] | 0.93 (0.69, 1.25)* | 0.40 |
| Intensive care unit stay (hours) | 41(27.8,46.2) | 32 (27.7, 5.2) | 0.99 (0.74, 1.33)* | 0.89 |
| One-year all-cause mortality | 18 (7) | 8 (3) | 2.38 (0.59, 9.52) | 0.031 |
| Any minor complication c | 119 (47) | 127 (48) | 0.99 (0.72, 1.36) | 0.89 |
| Royal Victoria Hospital, Montreal | N = 457 | N = 464 | ||
| Postoperative atrial | ||||
| fibrillation | 101(22) | 107(23) | 0.96 (0.64, 1.44) | 0.73 |
| Duration of hospitalization(days) | 8 [7, 11] | 9 [7, 13] | 1.13 (0.90, 1.42)* | 0.066 |
| Intensive care unitstay (hours) | 24(22.8,24.5) | 24(23.6,25.0) | 1.24 (0.98, 1.57)* | 0.0026 |
| One-year all-cause mortality | 14 (3) | 14 (3) | 1.04 (0.30, 3.59) | 0.92 |
| Any minor complication c | 81 (18) | 110 (24) | 0.77 (0.49, 1.21) | 0.051 |
Data are presented as median [25th, 75th percentiles] for length of ICU stay and hospital stay, event (%) for binary outcomes.
Hazard ratios
Confidence intervals adjusted for group sequential design using confidence coefficient of 2.633 for combined sites and 2.86 within sites in order to maintain overall study alpha of 0.05.
Chi-square test for binary outcomes, and Cox proportional hazards model for length of ICU stay and hospital stay. Bonferroni correction: significant if P < 0.0042/5 = 0.00084 within site.
included mechanical ventilation >72 hours, low cardiac index, acute kidney injury, hospitalization >30 days, all-cause hospital readmission within 30 days.
Footnotes
Conflicts of Interest: Andra Duncan receives funding from Fresenius Kabi for research unrelated to the current investigation.
Clinical Trial Registration: www.ClinicalTrials.gov (NCT00524472).
- Carvalho G et al. Cardioprotective effects of glucose and insulin administration while maintaining normoglycemia (GIN therapy) in patients undergoing coronary artery bypass grafting. J Clin Endocrinol Metab. 2011;96:1469–77.
- Duncan AE et al. Hyperinsulinemic normoglycemic does not meaningfully improve myocardial performance during cardiac surgery: A randomized trial. Anesthesiology. 2015;123:272–87. (This subinvestigation examined the effect of hyperinsulinemic normoglycemia on echocardiographically-measured myocardial strain).
- Saager L et al. Intraoperative tight glucose control using hyperinsulinemic normoglycemic increases delirium after cardiac surgery. Anesthesiology. 2015;122:1214–23.
- Abd-Elsayed A et al. Hyperinsulinemic normoglycemia decreases glucose variability during cardiac surgery. Journal of anesthesia. 2017;31:185–192.
- Sato H et al. High-dose insulin administration improves left ventricular function after coronary artery bypass graft surgery. J Cardiothorac Vasc Anesth. 2011;25:1086–91.
- Schricker T et al. Intraoperative maintenance of normoglycemia with insulin and glucose preserves verbal learning after cardiac surgery. PLoS One. 2014;9:e99661.
- Sato H, Carvalho G, Sato T, Lattermann R, Matsukawa T and Schricker T. The association of preoperative glycemic control, intraoperative insulin sensitivity, and outcomes after cardiac surgery. J Clin Endocrinol Metab. 2010;95:4338–44.
References
- 1.Capes SE, Hunt D, Malmberg K, Gerstein HC: Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet 2000; 355: 773–8 [DOI] [PubMed] [Google Scholar]
- 2.Capes SE, Hunt D, Malmberg K, Pathak P, Gerstein HC: Stress Hyperglycemia and Prognosis of Stroke in Nondiabetic and Diabetic Patients: A Systematic Overview. Stroke 2001; 32: 2426–2432 [DOI] [PubMed] [Google Scholar]
- 3.Outtara A, Lecomte P, Le Manach Y, Landi M, Jacqueminet S, Platonov I, Bonnet N, Riou B, Coriat P: Poor intraoperative blood glucose control is associated with a worsened hospital outcome after cardiac surgery in diabetic patients. Anesthesiology 2005; 103: 687–694 [DOI] [PubMed] [Google Scholar]
- 4.Van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R: Intensive insulin therapy in critically ill patients. N Engl J Med 2001; 345: 1359–1367 [DOI] [PubMed] [Google Scholar]
- 5.Vlasselaers D, Milants I, Desmet L, Wouters PJ, Vanhorebeek I, van den Heuvel I, Mesotten D, Casaer MP, Meyfroidt G, Ingels C, Muller J, Van Cromphaut S, Schetz M, Van den Berghe G: Intensive insulin therapy for patients in paediatric intensive care: a prospective, randomised controlled study. Lancet 2009; 373: 547–56 [DOI] [PubMed] [Google Scholar]
- 6.Brunkhorst FM, Engel C, Bloos F, Meier-Hellmann A, Ragaller M, Weiler N, Moerer O, Gruendling M, Oppert M, Grond S, Olthoff D, Jaschinski U, John S, Rossaint R, Welte T, Schaefer M, Kern P, Kuhnt E, Kiehntopf M, Hartog C, Natanson C, Loeffler M, Reinhart K, German Competence Network S: Intensive insulin therapy and pentastarch resuscitation in severe sepsis. New England Journal of Medicine 2008; 358: 125–39 [DOI] [PubMed] [Google Scholar]
- 7.Preiser JC, Devos P, Ruiz-Santana S, Mélot C, Annane D, Groeneveld J, Iapichino G, Leverve X, Nitenberg G, Singer P, Wernerman J, Joannidis M, Stecher A, Chioléro R: A prospective randomised multi-centre controlled trial on tight glucose control by intensive insulin therapy in adult intensive care units: The Glucontrol study. Intensive Care Med 2009; 35: 1738–48 [DOI] [PubMed] [Google Scholar]
- 8.Gandhi GY, Nuttall GA, Abel MD, Mullany CJ, Schaff HV, O’Brien PC, Johnson MG, Williams AR, Cutshall SM, Mundy LM, Rizza RA, McMahon MM: Intensive intraoperative insulin therapy versus conventional glucose management during cardiac surgery: A randomized trial. Ann Intern Med 2007; 146: 233–43 [DOI] [PubMed] [Google Scholar]
- 9.Investigators N-SS, Finfer S, Chittock DR, Su SY, Blair D, Foster D, Dhingra V, Bellomo R, Cook D, Dodek P, Henderson WR, Hebert PC, Heritier S, Heyland DK, McArthur C, McDonald E, Mitchell I, Myburgh JA, Norton R, Potter J, Robinson BG, Ronco JJ: Intensive versus conventional glucose control in critically ill patients. New England Journal of Medicine 2009; 360: 1283–97 [DOI] [PubMed] [Google Scholar]
- 10.Finfer S, Liu B, Chittock DR, Norton R, Myburgh JA, McArthur C, Mitchell I, Foster D, Dhingra V, Henderson WR, Ronco JJ, Bellomo R, Cook D, McDonald E, Dodek P, Hebert PC, Heyland DK, Robinson BG: Hypoglycemia and risk of death in critically ill patients. N Engl J Med 2012; 367: 1108–18 [DOI] [PubMed] [Google Scholar]
- 11.Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, Van Wijngaerden E, Bobbaers H, Bouillon R: Intensive insulin therapy in the medical ICU. N Engl J Med 2006; 354: 449–61 [DOI] [PubMed] [Google Scholar]
- 12.Lazar HL, Chipkin SR, Fitzgerald CA, Bao Y, Cabral H, Apstein CS: Tight Glycemic Control in Diabetic Coronary Artery Bypass Graft Patients Improves Perioperative Outcomes and Decreases Recurrent Ischemic Events. Circulation 2004; 109: 1497–1502 [DOI] [PubMed] [Google Scholar]
- 13.Kjellman UW, Bjork K, Dahlin A, Ekroth R, Kirno K, Svensson G, Wernerman J: Insulin(GIK) improves myocardial metabolism in patients during blood cardioplegia. Scand Cardiovasc J 2000; 34: 321–30 [DOI] [PubMed] [Google Scholar]
- 14.Lazar HL: Enhanced preservation of acutely ischemic myocardium and improved clinical outcomes using glucose-insulin-potassium (GIK) solutions. Am J Cardiol 1997; 80: 90A–93A [DOI] [PubMed] [Google Scholar]
- 15.Schipke JD, Friebe R, Gams E: Forty years of glucose-insulin-potassium (GIK) in cardiac surgery: a review of randomized, controlled trials. Eur J Cardiothorac Surg 2006; 29: 479–85 [DOI] [PubMed] [Google Scholar]
- 16.Stanley WC, Lopaschuk GD, Hall JL, McCormack JG: Regulation of myocardial carbohydrate metabolism under normal and ischaemic conditions. Potential for pharmacological interventions. Cardiovascular Research 1997; 33: 243–57 [DOI] [PubMed] [Google Scholar]
- 17.Stanley WC, Recchia FA, Lopaschuk GD: Myocardial substrate metabolism in the normal and failing heart. Physiological Reviews 2005; 85: 1093–129 [DOI] [PubMed] [Google Scholar]
- 18.Zhou L, Huang H, McElfresh TA, Prosdocimo DA, Stanley WC: Impact of anaerobic glycolysis and oxidative substrate selection on contractile function and mechanical efficiency during moderate severity ischemia. Am J Physiol Heart Circ Physiol 2008; 295: H939–H945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carvalho G, Pelletier P, Albacker T, Lachapelle K, Joanisse DR, Hatzakorzian R, Lattermann R, Sato H, Marette A, Schricker T: Cardioprotective effects of glucose and insulin administration while maintaining normoglycemia (GIN therapy) in patients undergoing coronary artery bypass grafting. J Clin Endocrinol Metab 2011; 96: 1469–77 [DOI] [PubMed] [Google Scholar]
- 20.Lazar HL, Philippides G, Fitzgerald C, Lancaster D, Shemin RJ, Apstein C: Glucose-insulin-potassium solutions enhance recovery after urgent coronary artery bypass grafting. Journal of Thoracic & Cardiovascular Surgery 1997; 113: 354–60; discussion 360–2 [DOI] [PubMed] [Google Scholar]
- 21.de Vries FE, Gans SL, Solomkin JS, Allegranzi B, Egger M, Dellinger EP, Boermeester MA: Meta-analysis of lower perioperative blood glucose target levels for reduction of surgical-site infection. Br J Surg 2017; 104: e95–e105 [DOI] [PubMed] [Google Scholar]
- 22.Furnary AP, Zerr KJ, Grunkemeier GL, Starr A: Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures. Ann Thorac Surg 1999; 67: 352–362 [DOI] [PubMed] [Google Scholar]
- 23.Furnary AP, Wu Y, Bookin SO: Effect of hyperglycemia and continuous intravenous insulin infusions on outcomes of cardiac surgical procedures: the Portland Diabetic Project. Endocr Pract 2004; 10 Suppl 2: 21–33 [DOI] [PubMed] [Google Scholar]
- 24.Carvalho G, Moore A, Qizilbash B, Lachapelle K, Schricker T: Maintenance of normoglycemia during cardiac surgery. Anesthesia & Analgesia 2004; 99: 319–24 [DOI] [PubMed] [Google Scholar]
- 25.Komada H, Hirota Y, So A, Nakamura T, Okuno Y, Fukuoka H, Iguchi G, Takahashi Y, Sakaguchi K, Ogawa W: Insulin Secretion and Insulin Sensitivity Before and After Surgical Treatment of Pheochromocytoma or Paraganglioma. J Clin Endocrinol Metab 2017; 102: 3400–3405 [DOI] [PubMed] [Google Scholar]
- 26.Niedzwiecki P, Naskret D, Pilacinski S, Pempera M, Uruska A, Adamska A, Zozulinska-Ziolkiewicz D: The Higher the Insulin Resistance the Lower the Cardiac Output in Men with Type 1 Diabetes During the Maximal Exercise Test. Metab Syndr Relat Disord 2017; 15: 252–257 [DOI] [PubMed] [Google Scholar]
- 27.Ferrannini E, Seghieri G, Muscelli E: Insulin and the renin-angiotensin-aldosterone system: influence of ACE inhibition. J Cardiovasc Pharmacol 1994; 24 Suppl 3: S61–9 [PubMed] [Google Scholar]
- 28.Howell NJ, Ashrafian H, Drury NE, Ranasinghe AM, Contractor H, Isackson H, Calvert M, Williams LK, Freemantle N, Quinn DW, Green D, Frenneaux M, Bonser RS, Mascaro JG, Graham TR, Rooney SJ, Wilson IC, Pagano D: Glucose-Insulin-Potassium Reduces the Incidence of Low Cardiac Output Episodes After Aortic Valve Replacement for Aortic Stenosis in Patients With Left Ventricular Hypertrophy: Results From the Hypertrophy, Insulin, Glucose, and Electrolytes (HINGE) Trial. Circulation 2011; 123: 170–177 [DOI] [PubMed] [Google Scholar]
- 29.Seied-Hosseini SM, Pourmoghadas A, Aghadavoudi O, Amini M, Mirmohammad-Sadeghi M, Golabchi A, Hedayatpour B, Haratian E, Ghaem-Maghami N, Khanoom Sharegh L: Efficacy of Glucose-Insulin-Potassium Infusion on Left Ventricular Performance in Type II Diabetic Patients Undergoing Elective Coronary Artery Bypass Graft.Dy. ARYA Atheroscler 2010; 6: 62–8 [PMC free article] [PubMed] [Google Scholar]
- 30.Duncan AE, Kashy BK, Sarwar S, Stenina-Adognravi O, Christoffersen S, Alfirevic A, Sale S, Yang D, Thomas JD, Gillinov AM, Sessler DI: Hyperinsulinemic normoglycemic does not meaningfully improve myocardial performance during cardiac surgery: A randomized trial. Anesthesiology 2015; 123: 272–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saager L, Duncan AE, Yared J-P, Hesler BD, You J, Deogaonkar A, Sessler DI, Kurz A: Intraoperative tight glucose control using hyperinsulinemic normoglycemic increases delirium after cardiac surgery. Anesthesiology 2015; 122: 1214–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abd-Elsayed A, Mascha EJ, Yang D, Sessler DI, Duncan A: Hyperinsulinemic normoglycemia decreases glucose variability during cardiac surgery. J Anesth 2017; 31: 185–192 [DOI] [PubMed] [Google Scholar]
- 33.Sato H, Hatzakorzian R, Carvalho G, Sato T, Lattermann R, Matsukawa T, Schricker T: High-dose insulin administration improves left ventricular function after coronary artery bypass graft surgery. J Cardiothorac Vasc Anesth 2011; 25: 1086–91 [DOI] [PubMed] [Google Scholar]
- 34.Schricker T, Sato H, Beaudry T, Codere T, Hatzakorzian R, Pruessner JC: Intraoperative maintenance of normoglycemia with insulin and glucose preserves verbal learning after cardiac surgery. PLoS One 2014; 9: e99661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sato H, Carvalho G, Sato T, Lattermann R, Matsukawa T, Schricker T: The association of preoperative glycemic control, intraoperative insulin sensitivity, and outcomes after cardiac surgery. J Clin Endocrinol Metab 2010; 95: 4338–44 [DOI] [PubMed] [Google Scholar]
- 36.Austin PC: Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Statistics in Medicine 2009; 28: 3083–3107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mascha EJ, Sessler DI: Statistical grand rounds: design and analysis of studies with binary- event composite endpoints: guidelines for anesthesia research. Anesth Analg 2011; 112: 1461–71 [DOI] [PubMed] [Google Scholar]
- 38.Hwang IK, Shih WJ, De Cani JS: Group sequential designs using a family of type I error probability spending functions. Statistics in Medicine 1990; 9: 1439–45 [DOI] [PubMed] [Google Scholar]
- 39.Jonassen AK, Sack MN, Mjøs OD, Yellon DM: Myocardial protection by insulin at reperfusion requires early administration and is mediated via Akt and p70s6 kinase cell-survival signaling. Circ Res 2001; 89: 1191–8 [DOI] [PubMed] [Google Scholar]
- 40.Rao V, Borger MA, Weisel RD, Ivanov J, Christakis GT, Cohen G, Yau TM: Insulin cardioplegia for elective coronary bypass surgery. J Thorac Cardiovasc Surg 2000; 119: 1176–84 [DOI] [PubMed] [Google Scholar]
- 41.Onorati F, Renzulli A, De Feo M, Santarpino G, Galdieri N, Quarto C, De Santo LD, Cotrufo M: Myocardial protection with insulin cardioplegia: who can really benefit? J Cardiovasc Surg (Torino) 2005; 46: 569–76 [PubMed] [Google Scholar]
- 42.Hafstad AD, Khalid AM, How OJ, Larsen TS, Aasum E: Glucose and insulin improve cardiac efficiency and postischemic functional recovery in perfused hearts from type 2 diabetic (db/db) mice. Am J Physiol Endocrinol Metab 2007; 292: E1288–94 [DOI] [PubMed] [Google Scholar]
- 43.Shim YH, Kweon TD, Lee JH, Nam SB, Kwak YL: Intravenous glucose-insulin-potassium during off-pump coronary artery bypass surgery does not reduce myocardial injury. Acta Anaesthesiol Scand 2006; 50: 954–61 [DOI] [PubMed] [Google Scholar]
- 44.Eberli FR, Weinberg EO, Grice WN, Horowitz GL, Apstein CS: Protective effect of increased glycolytic substrate against systolic and diastolic dysfunction and increased coronary resistance from prolonged global underperfusion and reperfusion in isolated rabbit hearts perfused with erythrocyte suspensions. Circ Res 1991; 68: 466–81 [DOI] [PubMed] [Google Scholar]
- 45.Liu Q, Docherty JC, Rendell JC, Clanachan AS, Lopaschuk GD: High levels of fatty acids delay the recovery of intracellular pH and cardiac efficiency in post-ischemic hearts by inhibiting glucose oxidation. J Am Coll Cardiol 2002; 39: 718–25 [DOI] [PubMed] [Google Scholar]
- 46.Doenst T, Wijeysundera D, Karkouti K, Zechner C, Maganti M, Rao V, Borger MA: Hyperglycemia during cardiopulmonary bypass is an independent risk factor for mortality in patients undergoing cardiac surgery. Journal of Thoracic & Cardiovascular Surgery 2005; 130: 1144–50 [DOI] [PubMed] [Google Scholar]
- 47.Rabi D, Clement F, McAlister F, Majumdar S, Sauve R, Johnson J, Ghali W: Effect of perioperative glucose-insulin-potassium infusions on mortality and atrial fibrillation after coronary artery bypass grafting: a systematic review and meta-analysis. Can J Cardiol 2010; 26: 178–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roh GU, Shim JK, Song JW, Kang HM, Kwak YL: Effect of glucose-insulin-potassium on hyperlactataemia in patients undergoing valvular heart surgery: A randomised controlled study. Eur J Anaesthesiol 2015; 32: 555–62 [DOI] [PubMed] [Google Scholar]
- 49.Albacker TB, Carvalho G, Schricker T, Lachapelle K: Myocardial protection during elective coronary artery bypass grafting using high-dose insulin therapy. Annals of Thoracic Surgery 2007; 84: 1920–7 [DOI] [PubMed] [Google Scholar]
- 50.Srinivasan M, Herrero P, McGill JB, Bennik J, Heere B, Lesniak D, Davila-Roman VG, Gropler RJ: The effects of plasma insulin and glucose on myocardial blood flow in patients with type 1 diabetes mellitus. Journal of the American College of Cardiology 2005; 46: 42–8 [DOI] [PubMed] [Google Scholar]
- 51.Turina M, Fry DE, Polk HC Jr.: Acute hyperglycemia and the innate immune system: clinical, cellular, and molecular aspects. Crit Care Med 2005; 33: 1624–33 [DOI] [PubMed] [Google Scholar]
- 52.Alba-Loureiro TC, Munhoz CD, Martins JO, Cerchiaro GA, Scavone C, Curi R, Sannomiya P: Neutrophil function and metabolism in individuals with diabetes mellitus. Braz J Med Biol Res 2007; 40: 1037–44 [DOI] [PubMed] [Google Scholar]