Abstract
Human leucocyte antigen-G (HLA-G) plays an important role in the progression of human cancers. A growing number of published studies have investigated the correlation between the HLA-G 3′ untranslated region (3′UTR) 14-bp insertion/deletion (Ins/Del) polymorphism and the associated cancer risk in different populations. However, results from previous studies are inconclusive and inconsistent for the different type of cancers. Therefore, we undertook a meta-analysis to assess the effects of the HLA-G 14-bp Ins/Del polymorphism on cancer risk. A systematic literature search was conducted in PubMed, Web of Science, CNKI, VIP, and Wanfang databases to obtain relevant studies up to 28 January 2019. The pooled odds ratios (ORs) and corresponding 95% confidence intervals (CIs) were used. Twenty-five published case–control studies comprising 4981 cases and 6391 controls were included in the current meta-analysis. The results of the overall analysis revealed that the HLA–G 14–bp Ins/Ins genotype and Ins allele were associated with the total cancer risk in the homozygote comparison model (Ins/Ins vs. Del/Del: OR = 0.80, CI = 0.64–1.00; P=0.049) and the allelic comparison model (Ins vs. Del: OR = 0.89, CI = 0.81–0.99; P=0.035), with a protective role. Further subgroup analyses indicated that the HLA–G 14–bp Ins/Del polymorphism was associated with the risk of breast cancer and oesophageal cancer (EC), and significant risk of cancer was also observed in Mixed populations and population-based (PB). The results of our meta-analysis show that the HLA–G 14-bp Ins/Del polymorphism plays an important role in cancer risk, particularly in breast cancer and esophageal cancer in Mixed populations. Additional case–control studies with different types of cancer spanning different ethnicities are needed to extend the present findings.
Keywords: Cancer, Human leukocyte antigen-G, Meta-analysis, Polymorphism
Introduction
The incidence and mortality of cancer are increasing worldwide, and cancer has been a major human health problem that creates a large economic burden in both developed and undeveloped countries. According to reported statistics, there were approximately 1688780 new cancer diagnoses, and 600920 cases resulting in mortality due to malignant tumours in the United States in the year 2017 [1]. In 2015, there were nearly 4292000 new cancer diagnoses and 2814000 cancer-related deaths in China [2]. Although the underlying mechanism of carcinogenesis is not completely deciphered, a number of studies have demonstrated that the occurrence of cancer is a complicated process, which includes various environmental factors and genetic susceptibilities [3]. Accumulating evidence has shown that individual genetic susceptibility plays a significant role in the occurrence of a tumour. Moreover, the relationship between polymorphisms and cancer risk has been confirmed for many genes [4,5]. Several lines of evidence have indicated that the progression of a tumour could be related to immunoevasion. Human leucocyte antigen (HLA) may play a critical role in the development and progression of cancer by mediating immune responses [6].
HLA-G, a non-classical HLA class I molecule, is known for its suppressive function and has seven different isoforms. Of the seven isoforms, four have membrane-bound forms (HLA-G1 to HLA-G4) and three have soluble forms (HLA-G5, HLA-G6, and HLA-G7) [7]. Differing from the classic HLA class I molecules, HLA-G is characterised by its restricted tissue distribution, low rate of polymorphism, and immunosuppressive properties [8]. The aberrant expression of HLA-G has been considered a mechanism in a wide variety of tumours that helps the tumour cells escape immunosurveillance [9]. HLA-G has been shown to act as a negative regulator of the human immune response by several mechanisms, including the inhibition of the cytotoxic effects of T lymphocytes and natural killer (NK) cells, as well as the prevention of antigen recognition and anti-proliferative responses of CD4+ T cells [10]. Accumulating evidence has shown that HLA-G is highly expressed in a variety of tumour tissues, including breast cancer [11], cervical cancer [12], hepatocellular carcinoma (HCC) [13], oesophageal carcinoma (EC) [14], thyroid carcinoma [15], lung cancer [14], gastric cancer [14], colorectal cancer (CRC) [14], and renal cell carcinoma [16]. These studies show that HLA-G may play a pivotal role in the occurrence and progression of malignant tumours.
The human HLA-G gene, comprising eight exons and seven introns, is located on chromosome 6p21.3. Several published studies have indicated that some polymorphisms of the HLA-G gene are related to cancer development [17]. The 14-bp insertion/deletion (Ins/Del) polymorphism in exon 8 of the 3′ untranslated region (3′UTR) of HLA-G is the most widely studied polymorphism. It has been demonstrated that the HLA-G 3′UTR 14-bp Ins/Del variation implicates the stability and isoform splicing patterns of HLA-G mRNA [18]. The Ins allele is associated with the decreased expression of HLA-G, while the Del allele is associated with the increased expression of HLA-G [19]. After Castelli et al. [20] first assessed the correlation between the HLA-G 14-bp Ins/Del variation and bladder cancer in 2008, a growing number of molecular epidemiological case–control studies have been carried out in different populations to investigate the association of the HLA-G 14-bp Ins/Del variant with different types of cancers [11,13,21–24]. However, the results of the published articles varied and even contradicted each other. To identify these findings, four meta-analyses of the association between the HLA-G 14-bp Ins/Del variation and cancer risk were carried out several years ago [25–28]. Although all four meta-analyses reached the same conclusion, that there was no relationship between the HLA-G 14-bp Ins/Del polymorphism and the risk of overall cancer, the results of their stratified analyses were inconsistent. Due to the relatively small sample sizes included in the previous meta-analyses, all these meta-analyses lacked sufficient statistical power. Since these reports, many new case–control studies have explored the correlation between the HLA-G 14-bp Ins/Del polymorphism and the risk of different types of cancer; however, the results of these subsequent studies were still inconclusive. Therefore, an updated meta-analysis including all of the currently identified studies was performed to explore the precise association of the HLA-G 14-bp Ins/Del polymorphism with cancer susceptibility.
Materials and methods
Search strategy
A systematic literature search with no language limitation was conducted in PubMed, Web of Science, CNKI, VIP, and Wanfang databases to obtain all eligible studies published before 28 January 2019. The relevant search keywords included: (HLA-G OR ‘Human leukocyte antigen-G’) AND (mutation OR polymorphism OR genotype OR variation) AND (carcinoma OR cancer OR malignancy OR adenocarcinoma OR neoplasm OR neoplasia OR tumour OR tumour). In addition, other relevant articles were acquired by searching the reference lists of the reviews and studies selected from the search parameters described above.
Inclusion and exclusion criteria
Published articles fulfilling the following criteria were included: (i) articles published in English or Chinese; (ii) studies that evaluated the correlation between HLA-G 14-bp Ins/Del polymorphism and cancer risk; (iii) studies that designed as case–control or cohort studies; and (iv) studies that contained sufficient data for genotype distribution estimation or the overall odds ratio (ORs) and 95% confidence intervals (CIs). Articles were excluded based on the following criteria: (i) case reports, not case–control studies, letters, comment articles, reviews or meta-analyses; (ii) lacking sufficient data; and (iii) duplicated publications or samples.
Data extraction
Two investigators (Y.J. and J.L.) independently collected data from the eligible articles in accordance with the inclusion criteria above. Data extracted from all of the selected studies included the following information: the first author, publication year, country, study population ethnicity, cancer type, sources of controls, genotyping method, number of cases and controls for the 14-bp Ins/Del genotypes of HLA-G, and results of the Hardy–Weinberg equilibrium (HWE) test in controls. In cases of inconsistent evaluations, all investigators were consulted to obtain a consensus of inclusion or exclusion of the study in the present meta-analysis.
Methodological quality assessment
The quality of the included studies was appraised according to the Newcastle–Ottawa Scale (NOS) by two independent investigators. Each study had a calculated score based on three criteria including selection, comparability, and exposure (maximum score = 9 points). The score of a study must be higher than 5 to be included in the present meta-analysis (http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp) [29]. Any discrepancies were settled by all investigators through discussion.
Statistical analysis
We conducted this meta-analysis based on the checklists and guidelines according to PRISMA [30]. The HWE was assessed for each study in the control groups using a Chi-square test, and every study with a calculated P less than 0.05 was considered a significant disequilibrium. ORs with 95% CIs were adopted to assess the strength of the relationship between the HLA-G 14 bp Ins/Del polymorphism and the risk of cancer in the homozygote comparisons (Ins/Ins vs. Del/Del), heterozygote comparisons (Ins/Del vs. Del/Del), dominant model (Ins/Del + Ins/Ins vs. Del/Del), recessive model (Ins/Ins vs. Ins/Del + Del/Del), and allelic comparisons (Ins vs. Del). Stratified analyses were carried out based on ethnicity (Asian, African, Caucasian, and Mixed population), type of cancer (publication with only one case–control study was merged as ‘other cancers’), and source of controls (hospital-based and population-based (PB)). Differences based on a Z-test were regarded as statistically significant if the P<0.05. The heterogeneity within each study was measured by a Cochran’s Q statistical test and the I2 test [31]. A random-effects model was applied to measure the pooled OR when the I2 value > 50%. Otherwise, a fixed-effects model was adopted according to the heterogeneity [32]. Sensitivity analysis was performed to assess the effect of each study on the pooled OR by removing each publication one by one to examine the stability of the overall results. Begg’s funnel plot test and Egger’s tests were applied to assess the potential publication bias [33,34]. All statistical analyses were conducted by STATA 12.0 software (version 12.0; STATA Corp. College Station, TX, U.S.A.). All of the tests were two-sided, and a P-value <0.05 was accepted as statistically significant.
Results
Characteristics of eligible studies
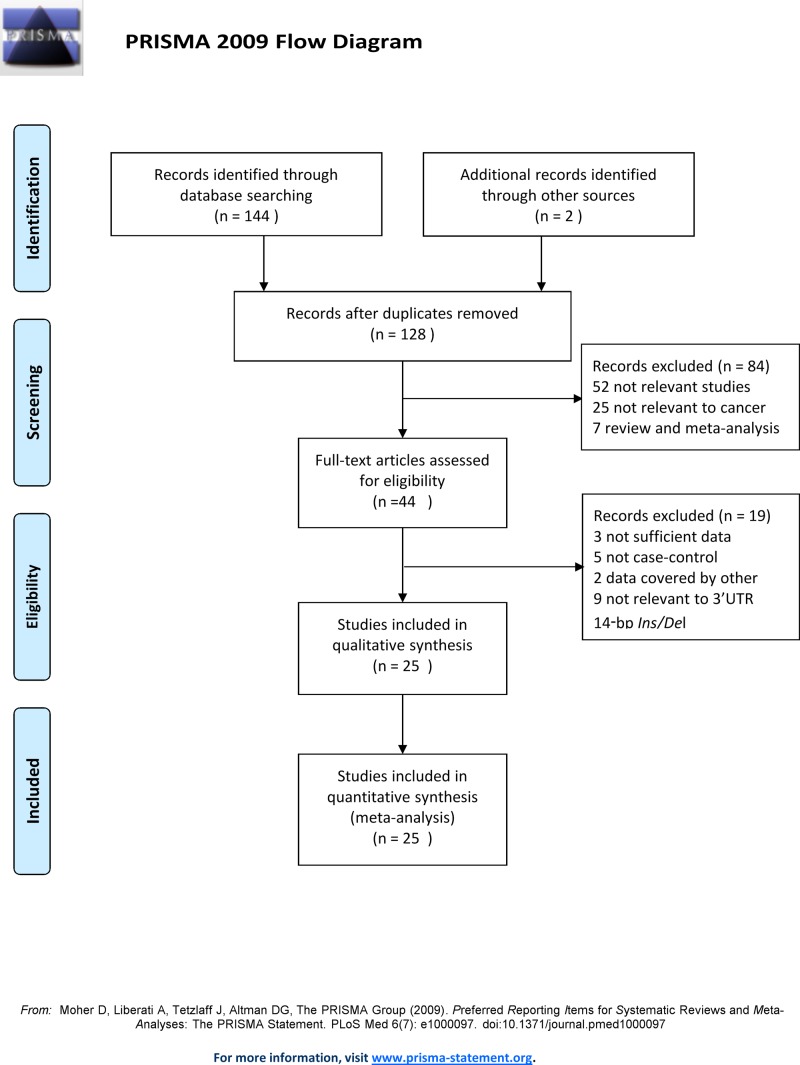
Figure 1 demonstrates the flow chart of the study selection process. After a systematic literature search in the databases mentioned above and a manual search in other sources, a total of 146 candidate articles were acquired. Eighteen search results were excluded as duplicates. Of the remaining 128 articles, 84 were removed after examining the titles and abstracts, resulting in a total of 44 articles. Among the 81 excluded studies, 52 were studies that were obviously irrelevant, 25 were not related to cancer, and 7 were reviews or meta-analyses. After carefully viewing the full text of the 44 potential studies to include in the meta-analysis, 19 of them were removed based on the following reasons: 3 did not have sufficient data, 5 were not case–control studies, 2 data were covered by other studies, and 9 were not relevant to the HLA-G 14 bp Ins/Del polymorphism. Finally, the remaining 25 eligible studies were included in the meta-analysis according to the inclusion and exclusion criteria [11,13,20–24,35–52]. A total of 4981 cases and 6391 controls are included in the current meta-analysis. The characteristics of the included case–control studies are displayed in Table 1. All studies were published between 2008 and 2018. With the exception of two publications reported in Chinese, all studies were written in English. Among all 25 studies, 10 studies were conducted in Asian populations, 7 in Caucasian populations, 6 in Mixed populations, and 2 in African populations. There were 11 different types of tumours in our study including: EC (n=2), non-small cell lung cancer (NSCLC) (n=2), breast cancer (n=5), cervical cancer (n=4), HCC (n=3), non-Hodgkin’s lymphoma (NHL) (n=2), thyroid cancer (n=2), prostate cancer (n=1), CRC (n=1), head and neck squamous cell carcinoma (HNSCC) (n=1), neuroblastoma (n=1), and bladder cancer (n=1). There were 12 PB studies and 13 hospital-based studies. All included studies used polymerase chain reaction (PCR) as the genotyping method with the exception of one study [39] that used DNA-PAGE. With the exception of one study [42], the genotype distributions of controls in all eligible studies did not deviate from the HWE. The distribution of genotypes and allele frequencies of the HLA-G 14 bp Ins/Del polymorphism in the cases and controls are provided in Table 2. Supplementary Table 1 demonstrated that the included studies were reliable based on the methodological quality.
Figure 1. The flow diagram of the included and excluded studies.
Table 1. Characteristics of eligible case–control studies included in this meta-analysis.
| First author | Year | Country | Ethnicity | Cancer Type | Source of controls | Genotyping method | Number (case/control) | HWE | NOS score |
|---|---|---|---|---|---|---|---|---|---|
| Gao et al. [21] | 2011 | China | Asian | EC | HB | PCR | 132/254 | Yes | 6 |
| Xu et al. [35] | 2017 | China | Asian | NSCLC | PB | PCR | 113/150 | Yes | 8 |
| Zidi et al. [36] | 2016 | Tunisia | African | Breast cancer | PB | PCR | 104/83 | Yes | 8 |
| Zambra et al. [37] | 2016 | Brazil | Mixed | Prostate cancer | HB | PCR | 187/129 | Yes | 7 |
| Yang et al. [22] | 2014 | Taiwan | Asian | Cervical cancer | HB | PCR | 315/400 | Yes | 7 |
| Silva et al. [38] | 2013 | Brazil | Mixed | Cervical cancer | HB | PCR | 55/50 | Yes | 7 |
| Agnihotri et al. [39] | 2017 | India | Asian | HNSCC | PB | DNA-PAGE | 383/383 | Yes | 8 |
| Wisniewski et al. [40] | 2015 | Poland | Caucasian | NSCLC | PB | PCR | 319/465 | Yes | 8 |
| Teixeira et al. [41] | 2013 | Brazil | Mixed | HCC | PB | PCR | 109/202 | Yes | 7 |
| Haghi et al. [42] | 2015 | Iran | Asian | Breast cancer | PB | PCR | 227/255 | No | 7 |
| Garziera et al. [43] | 2016 | Italy | Caucasian | CRC | PB | PCR | 308/294 | Yes | 8 |
| Chen et al. [44] | 2012 | China | Asian | EC | HB | PCR | 239/467 | Yes | 7 |
| Tawfeek et al. [45] | 2018 | Egypt | African | NHL | PB | PCR | 150/100 | Yes | 8 |
| Dardano et al. [23] | 2012 | Italy | Caucasian | Thyroid cancer | HB | PCR | 183/245 | Yes | 7 |
| Ramos et al. [11] | 2014 | Brazil | Mixed | Breast cancer | HB | PCR | 80/191 | Yes | 7 |
| Eskandari-Nasab et al. [46] | 2013 | Iran | Asian | Breast cancer | PB | PCR | 236/203 | Yes | 8 |
| Lau et al. [24] | 2011 | Australia | Caucasian | Neuroblastoma | PB | PCR | 153/404 | Yes | 8 |
| Kim et al. [47] | 2013 | Korea | Asian | HCC | HB | PCR | 270/91 | Yes | 7 |
| Jiang et al. [13] | 2011 | China | Asian | HCC | PB | PCR | 318/599 | Yes | 8 |
| Jeong et al. [48] | 2014 | Korea | Asian | Breast cancer | HB | PCR | 80/80 | Yes | 7 |
| Ferguson et al. [49] | 2012 | Canada | Caucasian | Cervical cancer | HB | PCR | 539/833 | Yes | 7 |
| Bortolotti et al. [50] | 2014 | Italy | Caucasian | Cervical cancer | HB | PCR | 100/100 | Yes | 7 |
| Castelli et al. [20] | 2008 | Brazil | Mixed | Bladder cancer | PB | PCR | 80/107 | Yes | 8 |
| Bielska et al. [51] | 2015 | Poland | Caucasian | NHL | HB | PCR | 207/150 | Yes | 7 |
| de Figueiredo-Feitosa et al. [52] | 2017 | Brazil | Mixed | Thyroid cancer | PB | PCR | 94/156 | Yes | 8 |
Abbreviation: HB, hospital-based.
Table 2. HLA-G 14–bp Ins/Del polymorphism genotype distribution and allele frequency in cases and controls.
| First author | Year | Genotype (n) | Allele frequency (n) | HWE | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | Control | Case | Control | |||||||||||
| Total | Del/Del | Ins/Del | Ins/Ins | Total | Del/Del | Ins/Del | Ins/Ins | Del | Ins | Del | Ins | |||
| Gao et al. [21] | 2011 | 132 | 54 | 66 | 12 | 254 | 77 | 128 | 46 | 174 | 90 | 282 | 220 | 0.852 |
| Xu et al. [35] | 2017 | 113 | 52 | 44 | 17 | 150 | 51 | 75 | 24 | 148 | 78 | 177 | 123 | 0.919 |
| Zidi et al. [36] | 2016 | 104 | 31 | 52 | 20 | 83 | 20 | 42 | 20 | 114 | 92 | 82 | 82 | 0.975 |
| Zambra et al. [37] | 2016 | 187 | 85 | 83 | 19 | 129 | 45 | 58 | 26 | 253 | 121 | 148 | 110 | 0.656 |
| Yang et al. [22] | 2014 | 315 | 169 | 110 | 36 | 400 | 188 | 176 | 36 | 448 | 182 | 552 | 248 | 0.850 |
| Silva et al. [38] | 2013 | 55 | 11 | 29 | 15 | 50 | 19 | 19 | 12 | 51 | 59 | 57 | 43 | 0.283 |
| Agnihotri et al. [39] | 2017 | 383 | 82 | 212 | 89 | 383 | 122 | 175 | 86 | 376 | 390 | 419 | 347 | 0.876 |
| Wisniewski et al. [40] | 2015 | 319 | 111 | 160 | 48 | 465 | 157 | 231 | 77 | 382 | 256 | 545 | 385 | 0.311 |
| Teixeira et al. [41] | 2013 | 109 | 49 | 44 | 16 | 202 | 70 | 87 | 45 | 142 | 76 | 227 | 177 | 0.205 |
| Haghi et al. [42] | 2015 | 227 | 56 | 127 | 44 | 255 | 52 | 154 | 49 | 239 | 215 | 258 | 252 | 0.004 |
| Garziera et al. [43] | 2016 | 308 | 97 | 138 | 73 | 294 | 114 | 122 | 58 | 332 | 284 | 350 | 238 | 0.059 |
| Chen et al. [44] | 2012 | 239 | 86 | 123 | 30 | 467 | 155 | 237 | 70 | 295 | 183 | 547 | 377 | 0.412 |
| Tawfeek et al. [45] | 2018 | 150 | 40 | 102 | 8 | 100 | 18 | 44 | 38 | 182 | 118 | 80 | 120 | 0.707 |
| Dardano et al. [23] | 2012 | 183 | 47 | 96 | 40 | 245 | 84 | 110 | 51 | 190 | 176 | 278 | 212 | 0.409 |
| Ramos et al. [11] | 2014 | 80 | 18 | 54 | 8 | 191 | 57 | 98 | 36 | 90 | 70 | 212 | 170 | 0.867 |
| Eskandari-Nasab et al. [46] | 2013 | 236 | 80 | 106 | 50 | 203 | 49 | 91 | 63 | 266 | 206 | 189 | 217 | 0.368 |
| Lau et al. [24] | 2011 | 153 | 66 | 58 | 29 | 404 | 146 | 194 | 64 | 190 | 116 | 486 | 322 | 0.973 |
| Kim et al. [47] | 2013 | 270 | 159 | 93 | 18 | 91 | 61 | 28 | 2 | 411 | 129 | 150 | 32 | 0.841 |
| Jiang et al. [13] | 2011 | 318 | 187 | 113 | 18 | 599 | 304 | 241 | 54 | 487 | 149 | 849 | 349 | 0.822 |
| Jeong et al. [48] | 2014 | 80 | 54 | 21 | 5 | 80 | 44 | 32 | 4 | 129 | 31 | 120 | 40 | 0.837 |
| Ferguson et al. [49] | 2012 | 539 | 184 | 242 | 113 | 833 | 272 | 399 | 162 | 610 | 468 | 943 | 723 | 0.770 |
| Bortolotti et al. [50] | 2014 | 100 | 49 | 40 | 11 | 100 | 38 | 40 | 22 | 138 | 62 | 116 | 84 | 0.201 |
| Castelli et al. [20] | 2008 | 80 | 28 | 37 | 15 | 107 | 35 | 50 | 22 | 93 | 67 | 120 | 94 | 0.868 |
| Bielska et al. [51] | 2015 | 207 | 49 | 91 | 67 | 150 | 33 | 89 | 28 | 189 | 225 | 155 | 145 | 0.071 |
| de Figueiredo-Feitosa et al. [52] | 2017 | 94 | 34 | 47 | 13 | 156 | 61 | 65 | 30 | 115 | 73 | 187 | 125 | 0.255 |
Meta-analysis results
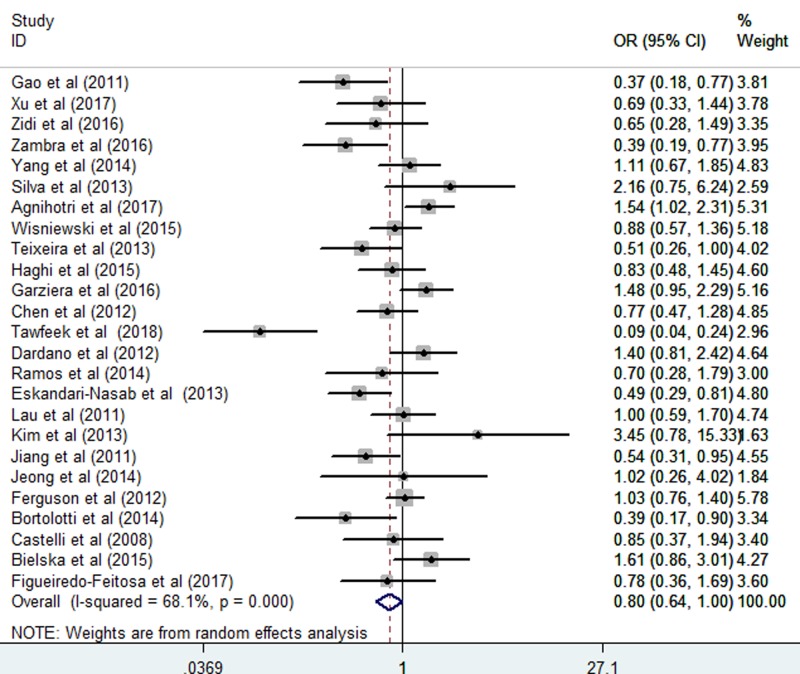
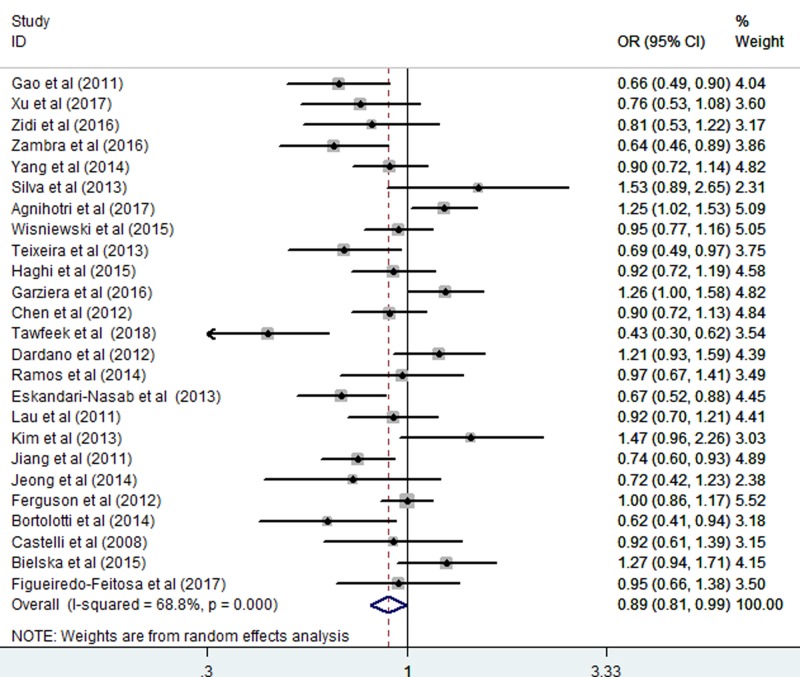
The relationship between the HLA-G 14 bp Ins/Del polymorphism and cancer risk was assessed. The results revealed that the HLA-G 14 bp Ins/Del polymorphism was significantly associated with cancer risk in the homozygote comparison (Ins/Ins vs. Del/Del: OR = 0.80, CI = 0.64–1.00; P=0.049, Figure 2 and Table 3) and allelic comparison (Ins vs. Del: OR = 0.89, CI = 0.81–0.99; P=0.035, Figure 3 and Table 3). However, no significant association with cancer risk was found in other models including: Ins/Del vs. Del/Del: OR = 0.93, CI = 0.81–1.06; P=0.267; Ins/Del + Ins/Ins vs.Del/Del: OR = 0.82, CI = 0.68–1.01; P=0.056; and Ins/Ins vs. Ins/Del + Del/Del: OR = 0.89, CI = 0.78–1.02; P=0.107 (Table 3). The random-effects model was used due to the significant heterogeneity of the included studies.
Figure 2. Forest plots of the HLA-G 14-bp Ins/Del polymorphism and cancer risk (homozygote comparisons: Ins/Ins vs. Del/Del).
Table 3. Analysis of the HLA-G 14 bp Ins/Del polymorphism and risk of cancer.
| Variables | n | Homozygote (Ins/Ins vs. Del/Del) | Heterozygote (Ins/Del vs. Del/Del) | Dominant (Ins/Del + Ins/Ins vs. Del/Del) | Recessive (Ins/Ins vs. Ins/Del + Del/Del) | Allelic (Ins vs. Del) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | Phet | OR (95% CI) | Phet | OR (95% CI) | Phet | OR (95% CI) | Phet | OR (95% CI) | Phet | ||
| Total | 25 | 0.80 (0.64–1.00) | 0.000 | 0.93 (0.81–1.06) | 0.000 | 0.82 (0.68–1.01) | 0.000 | 0.89 (0.78–1.02) | 0.000 | 0.89 (0.81–0.99) | 0.000 |
| Ethnicity | |||||||||||
| Asian | 10 | 0.81 (0.58–1.12) | 0.003 | 0.84 (0.67–1.06) | 0.001 | 0.87 (0.74–1.02) | 0.068 | 0.83 (0.66–1.04) | 0.000 | 0.87 (0.75–1.02) | 0.001 |
| African | 2 | 0.25 (0.04–1.62) | 0.003 | 0.92 (0.57–1.48) | 0.585 | 0.26 (0.03–2.08) | 0.000 | 0.67 (0.43–1.05) | 0.641 | 0.59 (0.32–1.08) | 0.026 |
| Mixed | 6 | 0.67 (0.49–0.92) | 0.146 | 1.11 (0.77–1.59) | 0.060 | 0.64 (0.48–0.86) | 0.489 | 0.99 (0.69–1.43) | 0.034 | 0.85 (0.72–0.99) | 0.083 |
| Caucasian | 7 | 1.09 (0.91–1.29) | 0.084 | 0.96 (0.77–1.19) | 0.044 | 1.12 (0.87–1.43) | 0.039 | 0.99 (0.81–1.21) | 0.053 | 1.03 (0.90–1.19) | 0.035 |
| Type of cancer | |||||||||||
| EC | 2 | 0.56 (0.28–1.15) | 0.104 | 0.86 (0.65–1.13) | 0.409 | 0.66 (0.45–0.96) | 0.156 | 0.80 (0.61–1.03) | 0.223 | 0.81 (0.67–0.97) | 0.118 |
| NSCLC | 2 | 0.83 (0.57–1.20) | 0.583 | 0.79 (0.47–1.31) | 0.094 | 0.90 (0.64–1.23) | 0.918 | 0.80 (0.51–1.23) | 0.124 | 0.90 (0.75–1.07) | 0.288 |
| Breast cancer | 5 | 0.65 (0.48–0.89) | 0.656 | 0.82 (0.65–1.05) | 0.108 | 0.74 (0.57–0.96) | 0.346 | 0.77 (0.61–0.97) | 0.201 | 0.82 (0.70–0.94) | 0.423 |
| Other cancers | 5 | 1.00 (0.64–1.58) | 0.009 | 1.04 (0.70–1.56) | 0.002 | 1.03 (0.84–1.26) | 0.082 | 1.03 (0.70–1.52) | 0.001 | 0.99 (0.78–1.26) | 0.004 |
| Cervical cancer | 4 | 0.94 (0.62–1.54) | 0.068 | 0.90 (0.64–1.26) | 0.061 | 1.06 (0.85–1.32) | 0.127 | 0.90 (0.65–1.24) | 0.054 | 0.94 (0.74–1.18) | 0.052 |
| HCC | 3 | 0.75 (0.34–1.65) | 0.057 | 0.83 (0.67–1.04) | 0.195 | 0.78 (0.40–1.53) | 0.103 | 0.85 (0.56–1.30) | 0.042 | 0.88 (0.59–1.31) | 0.012 |
| NHL | 2 | 0.40 (0.03–6.47) | 0.000 | 0.81 (0.53–1.22) | 0.336 | 0.45 (0.02–9.65) | 0.000 | 0.77 (0.52–1.14) | 0.316 | 0.75 (0.26–2.15) | 0.000 |
| Thyroid cancer | 2 | 1.15 (0.74–1.79) | 0.223 | 1.26 (0.84–1.90) | 0.616 | 0.92 (0.63–1.36) | 0.291 | 1.35 (0.97–1.88) | 0.407 | 1.11 (0.89–1.39) | 0.294 |
| Source of control | |||||||||||
| HB | 12 | 0.90 (0.68–1.23) | 0.002 | 0.94 (0.77–1.14) | 0.012 | 0.91 (0.67–1.23) | 0.001 | 0.93 (0.77–1.12) | 0.009 | 0.94 (0.81–1.09) | 0.001 |
| PB | 13 | 0.72 (0.53–0.99) | 0.000 | 0.92 (0.75–1.12) | 0.003 | 0.76 (0.58–1.00) | 0.000 | 0.86 (0.71–1.05) | 0.000 | 0.85 (0.73–0.99) | 0.000 |
Significant results (P<0.05) are highlighted in bold. Abbreviation: HB, hospital-based.
Figure 3. Forest plots of the HLA-G 14–bp Ins/Del polymorphism and cancer risk (allelic comparisons: Ins vs. Del).
In the stratified analysis shown in Table 3, we explored the association between the HLA-G 14-bp Ins/Del variation and cancer risk in different ethnicities. The results showed a decreased cancer risk in Mixed populations based on three genetic models (Ins/Ins vs. Del/Del: OR = 0.67, CI = 0.49–0.92, P=0.014; Ins/Del + Ins/Ins vs.Del/Del: OR = 0.64, CI = 0.48–0.86, P=0.003; and Ins vs. Del: OR = 0.85, CI = 0.72–0.99, P=0.034). In a stratified analysis based on the cancer types, we found that the HLA-G 14-bp Ins/Del polymorphism was significantly associated with a reduced EC risk in the dominant model (Ins/Del + Ins/Ins vs.Del/Del: OR = 0.66, CI = 0.45–0.96, P=0.029) and in the allelic comparisons model (Ins vs. Del: OR = 0.81, CI = 0.67–0.97, P=0.022). Similar results were found in breast cancer based on all genetic models except for the heterozygote comparisons (Ins/Ins vs. Del/Del: OR = 0.65, CI = 0.48–0.89, P=0.007; Ins/Del + Ins/Ins vs.Del/Del: OR = 0.74, CI = 0.57–0.96, P=0.022; Ins/Ins vs. Ins/Del + Del/Del: OR = 0.77, CI = 0.61–0.97, P=0.024; and Ins vs. Del: OR = 0.82, CI = 0.70–0.94, P=0.006). In subgroups formed according to source of the controls, significantly decreased risks were observed in the PB analysis in the homozygote comparisons model (Ins/Ins vs. Del/Del: OR = 0.72, CI = 0.53–0.99, P=0.047), the dominant model (Ins/Del + Ins/Ins vs.Del/Del: OR = 0.76, CI = 0.58–1.00, P=0.048) and the allelic comparisons model (Ins vs. Del: OR = 0.85, CI = 0.73–0.99, P=0.040).
Test of heterogeneity
A Q test and I2 statistic were assessed to evaluate the heterogeneity among the selected studies. High heterogeneity was observed across studies, as well as in some subgroup analyses, as tested by random-effects analysis. Moreover, we evaluated the heterogeneity of all genetic models in regard to different ethnicities, cancer types, and the source of the controls. However, the observed heterogeneity could not be completely explained by different ethnicities, types of cancer, or the source of the controls (data not shown).
Sensitivity analyses
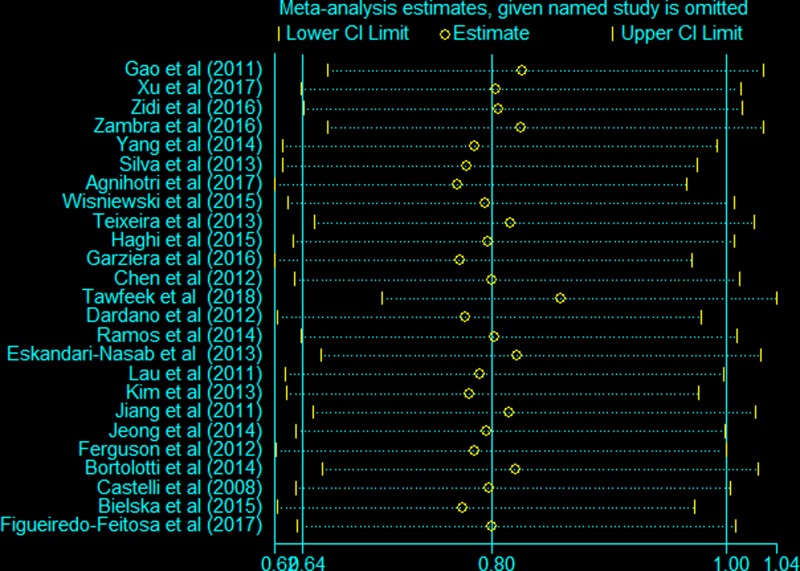
Sensitivity analysis was carried out to examine the influence of each eligible study on the pooled ORs by the sequential removal of each individual study form the analysis. The individual removal procedure affected the pooled ORs, indicating the instability and unreliability of our findings for the homozygote comparisons (Figure 4). Sensitivity analyses of other genetic models yielded similar results (Supplementary Figure S1).
Figure 4. Sensitivity analysis of the HLA-G 14-bp Ins/Del polymorphism and cancer risk (homozygote comparisons: Ins/Ins vs. Del/Del).
Publication bias
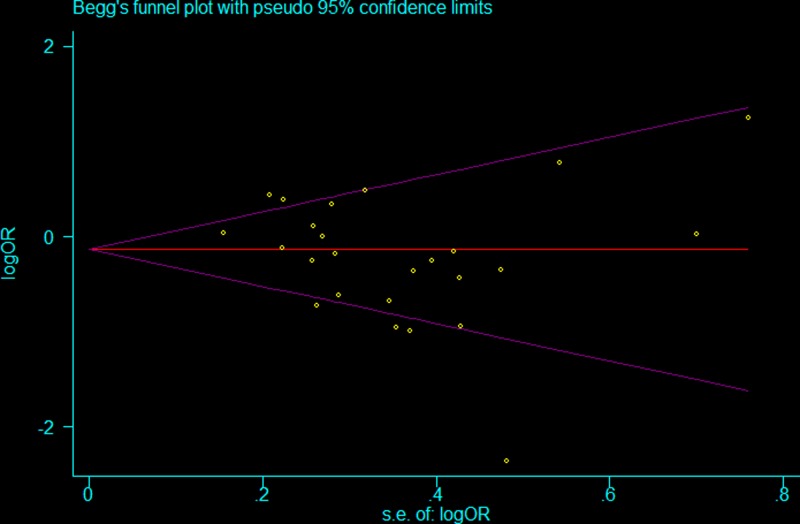
Begg’s and Egger’s tests were conducted to explore the potential for publication bias in assessment of the relationship between the HLA-G 14 Ins/Del polymorphism and cancer risk in all genetic models. No asymmetry was observed in the Begg’s funnel plots, and neither Begg’s rank correlation nor Egger’s regression showed publication bias among the studies (Figure 5 and Supplementary Table S2).
Figure 5. Funnel plot assessing evidence of publication bias (homozygote comparisons: Ins/Ins vs. Del/Del).
Discussion
A well-characterised distinguishing feature of malignant tumours is their ability to evade antitumour immune destruction, which has proven to be a major contributor to tumorigenesis [53]. HLA-G is an important complex molecule that plays an important role in facilitating tumour escape from immune surveillance by its immunosuppressive function on T and NK cells [10], and the aberrant expression of HLA-G has been reported to be related to a variety of tumours [11–16]. The expression level of the HLA-G protein is related to HLA-G gene polymorphisms. The Ins allele has been shown to decrease the expression of HLA-G, and the Del allele has been shown to elevate the expression of HLA-G [19]. To date, a number of studies have explored the relationship between the HLA-G gene polymorphisms and the risk of cancer. Among the HLA-G gene polymorphisms, the HLA-G 14-bp Ins/Del polymorphism is the most widely explored. Up to now, multiple published case–control studies have investigated the underlying correlation between the HLA-G 14-bp Ins/Del polymorphism and cancer risk. However, the biological role of the HLA-G 14-bp Ins/Del polymorphism in the development of cancer remains poorly understood. Considering the inconsistent or even contradictory previously published results, and the fact that individual case–control studies may have been statistically underpowered, we assessed the effect of the polymorphism in the risk of cancer in the present meta-analysis. The present analysis includes all eligible studies to precisely explore the association of the HLA-G 14-bp Ins/Del polymorphism with cancer susceptibility.
In this meta-analysis, we evaluated the HLA-G 14-bp Ins/Del polymorphism and cancer risk relationship with all qualified case–control studies. In total, 4981 cases and 6391 controls were included. By quantificatively analysing the integrated data, the results of our present meta-analysis revealed that the HLA-G 14-bp Ins/Del polymorphism is significantly associated with the susceptibility of overall cancer. There were a larger number of studies that had evaluated the correlation between the HLA-G 14-bp Ins/Del polymorphism and the susceptibility to different types of cancer. However, the conclusions were paradoxical. Gao et al. [21] carried out a case–control study and found that the HLA-G 14-bp Ins/Del variant was associated with an elevated risk of EC. Similar results were found in other types of cancer, including thyroid cancer [52], breast cancer [46], and cervical cancer [22], among others. However, a few studies reported the opposite result, that the HLA-G 14-bp Ins/Del polymorphism could decrease the risk of some types of cancer. Additionally, some studies showed that the HLA-G 14-bp Ins/Del polymorphism did not play a role in cancer susceptibility. Furthermore, results from studies on the correlation between the HLA-G 14-bp Ins/Del polymorphism in the same types of cancer were inconsistent. For example, the study conducted by Teixeira et al. [41] demonstrated that individuals with the HLA-G 14-bp Ins/Del polymorphism had significantly increased risk for the occurrence of HCC, while Kim et al. [47] showed no relationship between the HLA-G 14-bp Ins/Del variant and HCC susceptibility; however, Jiang et al. [13] indicated that this variation may actually be a protective factor in HCC susceptibility. To address this controversy and to obtain a more accurate conclusion, several meta-analyses have been carried out several years ago [25–28]. Inconsistent with our present study, all of the previous meta-analyses reached the same conclusion: there was no relationship between the HLA-G 14-bp Ins/Del polymorphism and the risk of overall cancer. A latest meta-analysis of 21 published case–control studies with 3815 cases and 5802 controls was performed by Almeida et al. in 2018 [54]; however, they assessed the relationship between the HLA-G 14 bp Ins/Del polymorphism and the risk of cancer only in the allelic comparisons (Ins vs. Del), and no positive results were found. Our results demonstrated, for the first time, a significant relationship between the HLA-G 14-bp Ins/Del polymorphism and a decreased overall cancer risk. Compared with previous meta-analyses, our study included a larger sample size, a wider variety of cancer types, and a more diverse sample population. Hence, our results are persuasive based on their adequate statistical power.
Significant heterogeneity among the studies was shown in our results; we performed stratified analyses in terms of ethnicity, types of cancer, and sources of controls. In the subgroup analysis based on ethnicity, an obviously decreased cancer susceptibility was demonstrated in Mixed populations alone but not in Asian, African, or Caucasian populations. This discrepancy in cancer risk may be interpreted by geographic climate, daily lifestyle, ethnic diversity, dietary habits, as well as differences in alleles and genotypes in various ethnic populations. When carrying out stratified analysis by cancer type, we found that the HLA-G 14 bp Ins/Del polymorphism was significantly associated with a reduced EC and breast cancer risk, but we failed to find a significant risk association in other types of cancer. This result may be explained by the inherent heterogeneity of tumorigenic development in diverse cancer types [55]. Due to the relatively small sample size of each cancer type, inadequate statistical power may also be a factor in lacking a significant polymorphism–cancer risk relationship in these other cancer types. When we evaluated the HLA-G 14-bp Ins/Del polymorphism–cancer risk association according to source of the control, a significantly decreased risk was observed in PB controls but not in hospital-based controls; this result further verifies that the HLA-G 14-bp Ins/Del polymorphism is a potential protective factor for cancer. Previously, published meta-analyses also performed subgroup analysis to explore the association between the HLA-G 14 bp Ins/Del variant and risk of developing cancer; some significant results were reported and are partially in line with the conclusions from our present study. Zhang and Wang [25] conducted a meta-analysis in 2014 and found that the polymorphism was associated with risk of developing HCC in a subgroup analysis by cancer type. This finding was not in accordance with our result; however, only two case–control studies of HCC were included in their study. Li et al. [26] revealed a significant association between the HLA-G 14 bp Ins/Del variant and both breast cancer and PB control subgroup analyses, which is in agreement with the conclusions from our study. In 2015, Ge et al. [28] demonstrated the significant association in Asian populations and in breast cancer subgroups in stratified analyses. Inconsistent with their results, we found no association between the HLA-G 14 bp Ins/Del polymorphism and cancer risk in Asian populations in the present study. However, compared with their meta-analysis that included only six case–control studies on Asian populations, the results of our study, which involved ten case–control trials, have more adequate and more robust statistical power.
Despite our efforts to assess the association between the HLA–G 14–bp Ins/Del variant and the risk of cancer, there are several limitations we must account for in the present meta-analysis that may impact the objectivity of the findings. First, only unadjusted estimates were used to assess the strength of the relationship between the HLA–G 14–bp Ins/Del variant and the risk of developing cancer. The analysis cannot account for confounding factors such as life habit, environment factors, gene–gene interactions, gene–environment interactions, and even different variant loci in the same gene factors. Second, there may be a selection bias in our study, since only published case–control studies written in Chinese or English were included in our meta-analysis. Some potential eligible studies may have been excluded, because they were not detected, published, or because they were written in other languages. Third, although the total sample sizes of our meta-analysis were relatively large, the sample sizes of some stratified analyses were extremely small. There were not enough appropriate studies in some subgroups, weakening the statistical power to investigate the real relationship between the HLA-G 14-bp Ins/Del polymorphism and cancer risk. Fourth, because of the high heterogeneity in our present meta-analysis, the reliability of the findings may be weakened. Despite the application of the random-effects model in our meta-analysis, the findings on the overall cancer susceptibility should be taken cautiously. Fifth, the result of our meta-analysis should be interpreted with caution and needs to be confirmed by more case–control studies, because the sensitivity analyses indicated that deletion of certain individual study had an impact on the reliability of our results. Larger sample sizes and well-designed case–control experiments with various types of cancer in diverse ethnicities are needed to further verify the relationship between the HLA–G 14-bp Ins/Del variant and cancer risk.
In summary, the pooled results of our meta-analysis demonstrated that the HLA–G 14-bp Ins/Del polymorphism may play an important role in decreasing cancer susceptibility, especially in breast cancer and oesophageal cancer (EC), in the Mixed populations. The results allowed us to hypothesise that the HLA–G 14-bp Ins/Del variant may be a potential protective factor of cancer. Larger sample sizes and well-designed case–control experiments with various types of cancer in different ethnicities are needed to further verify our findings.
Supporting information
Supplementary Figure 1.
Supplemental Table 1. Methodological quality of the included studies according to the Newcastle-Ottawa Scale.
Supplemental Table 2. The results of Begg’s and Egger’s tests for the publication bias.
Acknowledgments
We are indebted to all the people who helped with our present meta-analysis.
Abbreviations
- CI
confidence interval
- CRC
colorectal cancer
- EC
oesophageal cancer/carcinoma
- HCC
hepatocellular carcinoma
- HLA
human leucocyte antigen
- HWE
Hardy–Weinberg equilibrium
- Ins/Del
insertion/deletion
- NK
natural killer
- OR
odds ratio
- PB
population-based
- 3′UTR
3′ untranslated region
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Author Contribution
Y.J. and L.L. conceived the study. Y.J. and J.L. searched the databases and extracted the data. Y.-E.W. and X.Z. analysed the data. Y.J. and Y.W. wrote the draft of the paper. Y.J. and L.L. reviewed the manuscript.
Funding
The authors declare that there are no sources of funding to be acknowledged.
References
- 1.Siegel R.L., Miller K.D. and Jemal A. (2017) Cancer statistics. CA Cancer J. Clin. 67, 7–30 10.3322/caac.21387 [DOI] [PubMed] [Google Scholar]
- 2.Chen W., Zheng R., Baade P.D., Zhang S., Zeng H., Bray F.. et al. (2016) Cancer statistics in China, 2015. CA Cancer J. Clin. 66, 115–132 10.3322/caac.21338 [DOI] [PubMed] [Google Scholar]
- 3.Pharoah P.D., Dunning A.M., Ponder B.A. and Easton D.F. (2004) Association studies for finding cancer-susceptibility genetic variants. Nat. Rev. Cancer 4, 850–860 10.1038/nrc1476 [DOI] [PubMed] [Google Scholar]
- 4.Köberle B., Koch B., Fischer B.M. and Hartwig A. (2016) Single nucleotide polymorphisms in DNA repair genes and putative cancer risk. Arch. Toxicol. 90, 2369–2388 10.1007/s00204-016-1771-2 [DOI] [PubMed] [Google Scholar]
- 5.Hu J., Liu C., Yin Q., Ying M., Li J., Li L.. et al. (2014) Association between the CYP1A2-164 A/C polymorphism and colorectal cancer susceptibility: a meta-analysis. Mol. Genet. Genomics 289, 271–277 10.1007/s00438-013-0806-0 [DOI] [PubMed] [Google Scholar]
- 6.Powell A.G., Horgan P.G. and Edwards J. (2012) The bodies fight against cancer: is human leucocyte antigen (HLA) class 1 the key? J. Cancer Res. Clin. Oncol. 138, 723–728 10.1007/s00432-012-1192-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rouas-Freiss N., Moreau P., LeMaoult J. and Carosella E.D. (2014) The dual role of HLA-G in cancer. J. Immunol. Res. 10.1155/2014/359748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donadi E.A., Castelli E.C., Arnaiz-Villena A., Roger M., Rey D. and Moreau P. (2011) Implications of the polymorphism of HLA-G on its function, regulation, evolution and disease association. Cell. Mol. Life Sci. 68, 369–395 10.1007/s00018-010-0580-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sheu J. and Shih I.M. (2010) HLA-G and immune evasion from cancer cells. J. Formos. Med. Assoc. 109, 248–257 10.1016/S0929-6646(10)60050-2 [DOI] [PubMed] [Google Scholar]
- 10.Loustau M., Wiendl H., Ferrone S. and Carosella E.D. (2013) HLA-G 2012 conference: the 15-year milestone update. Tissue Antigens 81, 127–136 10.1111/tan.12053 [DOI] [PubMed] [Google Scholar]
- 11.Ramos C.S., Gonçalves A.S., Marinho L.C., Gomes Avelino M.A., Saddi V.A. and Lopes A.C. (2014) Analysis of HLA-G gene polymorphism and protein expression in invasive breast ductal carcinoma. Hum. Immunol. 75, 667–672 10.1016/j.humimm.2014.04.005 [DOI] [PubMed] [Google Scholar]
- 12.Li X.J., Zhang X., Lin A., Ruan Y.Y. and Yan W.H. (2012) Human leukocyte antigen-G (HLA-G) expression in cervical cancer lesions is associated with disease progression. Hum. Immunol. 73, 946–949 10.1016/j.humimm.2012.07.041 [DOI] [PubMed] [Google Scholar]
- 13.Jiang Y., Chen S., Jia S., Zhu Z., Gao X., Dong D.. et al. (2011) Association of HLA-G 3′ UTR 14-bp insertion/deletion polymorphism with hepatocellular carcinoma susceptibility in a Chinese population. DNA Cell Biol. 30, 1027–1032 10.1089/dna.2011.1238 [DOI] [PubMed] [Google Scholar]
- 14.Cao M., Yie S.M., Liu J., Ye S.R., Xia D. and Gao E. (2011) Plasma soluble HLA-G is a potential biomarker for diagnosis of colorectal, gastric, esophageal and lung cancer. Tissue Antigens 78, 120–128 10.1111/j.1399-0039.2011.01716.x [DOI] [PubMed] [Google Scholar]
- 15.Meneghini A.J. and Wastowski I.J. (2013) Association between the HLA-G molecule and lymph node metastasis in papillary thyroid cancer. Hum. Immunol. 74, 447–451 10.1016/j.humimm.2012.12.012 [DOI] [PubMed] [Google Scholar]
- 16.Li B.L., Lin A., Zhang X.J., Zhang X., Zhang J.G., Wang Q.. et al. (2009) Characterization of HLA-G expression in renal cell carcinoma. Tissue Antigens 74, 213–221 10.1111/j.1399-0039.2009.01302.x [DOI] [PubMed] [Google Scholar]
- 17.Eskandari-Nasab E., Hashemi M., Hasani S.S., Omrani M., Taheri M. and Mashhadi M.A. (2013) Association between HLA-G 3′UTR 14-bp ins/del polymorphism and susceptibility to breast cancer. Cancer Biomarkers 13, 253–259 10.3233/CBM-130364 [DOI] [PubMed] [Google Scholar]
- 18.Rousseau P., Le Discorde M., Mouillot G., Marcou C., Carosella E.D. and Moreau P. (2003) The 14 bp deletion-insertion polymorphism in the 3′ UT region of the HLA-G gene influences HLA-G mRNA stability. Hum. Immunol. 64, 1005–1010 10.1016/j.humimm.2003.08.347 [DOI] [PubMed] [Google Scholar]
- 19.Chen X.Y., Yan W.H., Lin A., Xu H.H., Zhang J.G. and Wang X.X. (2008) The 14 bp deletion polymorphisms in HLA-G gene play an important role in the expression of soluble HLA-G in plasma. Tissue Antigens 72, 335–341 10.1111/j.1399-0039.2008.01107.x [DOI] [PubMed] [Google Scholar]
- 20.Castelli E.C., Mendes-Junior C.T., Viana de Camargo J.L. and Donadi E.A. (2008) HLA-G polymorphism and transitional cell carcinoma of the bladder in a Brazilian population. Tissue Antigens 72, 149–157 10.1111/j.1399-0039.2008.01091.x [DOI] [PubMed] [Google Scholar]
- 21.Gao X.J., Chen Y., Aheli M., Wang H.J., Deng Y.C. and Zhang H.X. (2011) Relationship between HLA-G genetic polymorphism and the susceptibility of esophageal carcinoma in Kazakh nationality in Xinjiang. Chin. J. Public Health 27, 288–291 [Google Scholar]
- 22.Yang Y.C., Chang T.Y., Chen T.C., Lin W.S., Chang S.C. and Lee Y.J. (2014) Human leucocyte antigen-G polymorphisms are associated with cervical squamous cell carcinoma risk in Taiwanese women. Eur. J. Cancer 50, 469–474 10.1016/j.ejca.2013.10.018 [DOI] [PubMed] [Google Scholar]
- 23.Dardano A., Rizzo R., Polini A., Stignani M., Tognini S., Pasqualetti G.. et al. (2012) Soluble human leukocyte antigen-g and its insertion/deletion polymorphism in papillary thyroid carcinoma: novel potential biomarkers of disease? J. Clin. Endocrinol. Metab. 97, 4080–4086 10.1210/jc.2012-2231 [DOI] [PubMed] [Google Scholar]
- 24.Lau D.T., Norris M.D., Marshall G.M., Haber M. and Ashton L.J. (2011) HLA-G polymorphisms, genetic susceptibility, and clinical outcome in childhood neuroblastoma. Tissue Antigens 78, 421–427 10.1111/j.1399-0039.2011.01781.x [DOI] [PubMed] [Google Scholar]
- 25.Zhang S.L. and Wang H.T. (2014) Association between HLA-G 14-bp insertion/deletion polymorphism and cancer risk: a meta-analysis. J. BUON 19, 567–572 [PubMed] [Google Scholar]
- 26.Li T., Huang H., Liao D., Ling H., Su B. and Cai M. (2015) Genetic polymorphism in HLA-G 3′UTR 14-bp ins/del and risk of cancer: a meta-analysis of case-control study. Mol. Genet. Genomics 290, 1235–1245 10.1007/s00438-014-0985-3 [DOI] [PubMed] [Google Scholar]
- 27.Li T., Huang H., Liao D., Ling H., Su B. and Cai M. (2015) Lack of association between the HLA-G 3′UTR 14-bp ins/del polymorphism and cancer risk: a meta-analysis of case-control study. Hum. Immunol. 15, 564–569 10.1016/j.humimm.2015.11.011 [DOI] [PubMed] [Google Scholar]
- 28.Ge Y.Z., Ge Q., Li M.H., Shi G.M., Xu X., Xu L.W.. et al. (2014) Association between human leukocyte antigen-G 14-bp insertion/deletion polymorphism and cancer risk: a meta-analysis and systematic review. Hum. Immunol. 75, 827–832 10.1016/j.humimm.2014.06.004 [DOI] [PubMed] [Google Scholar]
- 29.Stang A. (2010) Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 25, 603–605 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 30.Knobloch K., Yoon U. and Vogt P.M. (2010) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int. J. Surg. 8, 336–341 10.1016/j.ijsu.2010.02.007 [DOI] [PubMed] [Google Scholar]
- 31.Higgins J.P., Thompson S.G., Deeks J.J. and Altman D.G. (2003) Measuring inconsistency in meta-analyses. BMJ 327, 557–560 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Higgins J.P. and Thompson S.G. (2002) Quantifying heterogeneity in a meta-analysis. Stat. Med. 21, 1539–1558 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 33.Begg C.B. and Mazumdar M. (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50, 1088–1101 10.2307/2533446 [DOI] [PubMed] [Google Scholar]
- 34.Egger M., Davey Smith G., Schneider M. and Minder C. (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu D.P., Gan L.H., Liu J.M., Lv D.Q., Lin J. and Yan W.H. (2017) HLA-G 14 bp insertion/deletion polymorphism is a prognostic factor for non-small-cell lung cancer. Chin. J. Microbiol. Immunol. 37, 361–368 [Google Scholar]
- 36.Zidi I., Dziri O., Zidi N., Sebai R., Boujelebene N., Ben Hassine A.. et al. (2016) Association of HLA-G +3142 C>G polymorphism and breast cancer in Tunisian population. Immunol. Res. 64, 961–968 [DOI] [PubMed] [Google Scholar]
- 37.Zambra F.M., Biolchi V., de Cerqueira C.C., Brum I.S., Castelli E.C. and Chies J.A. (2016) Immunogenetics of prostate cancer and benign hyperplasia-the potential use of an HLA-G variant as a tag SNP for prostate cancer risk. HLA 87, 79–88 10.1111/tan.12741 [DOI] [PubMed] [Google Scholar]
- 38.Silva I.D., Muniz Y.C., Sousa M.C., Silva K.R., Castelli E.C., Filho J.C.. et al. (2013) HLA-G 30′UTR polymorphisms in high grade and invasive cervico-vaginal cancer. Hum. Immunol. 74, 452–458 10.1016/j.humimm.2012.11.025 [DOI] [PubMed] [Google Scholar]
- 39.Agnihotri V., Gupta A., Kumar R., Upadhyay A.D., Dwivedi S., Kumar L.. et al. (2017) Promising link of HLA-G polymorphism, tobacco consumption and risk of Head and Neck Squamous Cell Carcinoma (HNSCC) in North Indian population. Hum. Immunol. 78, 172–178 10.1016/j.humimm.2016.12.007 [DOI] [PubMed] [Google Scholar]
- 40.Wiśniewski A., Kowal A., Wyrodek E., Nowak I., Majorczyk E., Wagner M.. et al. (2015) Genetic polymorphisms and expression of HLA-G and its receptors, KIR2DL4 and LILRB1, in non-small cell lung cancer. Tissue Antigens 85, 466–475 10.1111/tan.12561 [DOI] [PubMed] [Google Scholar]
- 41.Teixeira A.C., Mendes-Junior C.T., Souza F.F., Marano L.A., Deghaide N.H., Ferreira S.C.. et al. (2013) The 14bp-deletion allele in the HLA-G gene confers susceptibility to the development of hepatocellular carcinoma in the Brazilian population. Tissue Antigens 81, 408–413 10.1111/tan.12097 [DOI] [PubMed] [Google Scholar]
- 42.Haghi M., Hosseinpour Feizi M.A., Sadeghizadeh M. and Lotfi A.S. (2015) 14-bp insertion/deletion polymorphism of the HLA-G gene in breast cancer among women from North Western Iran. Asian Pac. J. Cancer Prev. 16, 6155–6158 10.7314/APJCP.2015.16.14.6155 [DOI] [PubMed] [Google Scholar]
- 43.Garziera M., Catamo E., Crovella S., Montico M., Cecchin E., Lonardi S.. et al. (2016) Association of the HLA-G 30′UTR polymorphisms with colorectal cancer in Italy: a first insight. Int. J. Immunogenet. 43, 32–39 10.1111/iji.12243 [DOI] [PubMed] [Google Scholar]
- 44.Chen Y., Gao X.J., Deng Y.C. and Zhang H.X. (2012) Relationship between HLA-G gene polymorphism and the susceptibility of esophageal cancer in Kazakh and Han nationality in Xinjiang. Biomarkers 17, 9–15 10.3109/1354750X.2011.633242 [DOI] [PubMed] [Google Scholar]
- 45.Tawfeek G.A. and Alhassanin S. (2018) HLA-G gene polymorphism in egyptian patients with non-hodgkin lymphoma and its clinical outcome. Immunol. Invest. 47, 315–325 10.1080/08820139.2018.1430826 [DOI] [PubMed] [Google Scholar]
- 46.Eskandari-Nasab E., Hashemi M., Hasani S.S., Omrani M., Taheri M. and Mashhadi M.A. (2013) Association between HLA-G 3′UTR 14-bp ins/del polymorphism and susceptibility to breast cancer. Cancer Biomark. 13, 253–259 10.3233/CBM-130364 [DOI] [PubMed] [Google Scholar]
- 47.Kim S.K., Chung J.H., Jeon J.W., Park J.J., Cha J.M., Joo K.R.. et al. (2013) Association between HLA-G 14-bp insertion/deletion polymorphism and hepatocellular carcinoma in Korean patients with chronic Hepatitis B viral infection. Hepatogastroenterology 60, 796–798 [DOI] [PubMed] [Google Scholar]
- 48.Jeong S., Park S., Park B.W., Park Y., Kwon O.J. and Kim H.S. (2014) Human Leukocyte Antigen-G (HLA-G) polymorphism and expression in breast cancer patients. PLoS ONE 9, e98284 10.1371/journal.pone.0098284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ferguson R., Ramanakumar A.V., Koushik A., Coutlée F., Franco E. and Roger M. (2012) Human leukocyte antigen G polymorphism is associated with an increased risk of invasive cancer of the uterine cervix. Int. J. Cancer 131, E312–E319 10.1002/ijc.27356 [DOI] [PubMed] [Google Scholar]
- 50.Bortolotti D., Gentili V., Rotola A., Di Luca D. and Rizzo R. (2014) Implication of HLA-G 3′; untranslated region polymorphisms in human papillomavirus infection. Tissue Antigens 83, 113–118 10.1111/tan.12281 [DOI] [PubMed] [Google Scholar]
- 51.Bielska M., Bojo M., Klimkiewicz-Wojciechowska G., Jesionek-Kupnicka D., Borowiec M., Kalinka-Warzocha E.. et al. (2015) Human leukocyte antigen-G polymorphisms influence the clinical outcome in diffuse large B-cell lymphoma. Genes Chromosomes Cancer 54, 185–193 10.1002/gcc.22235 [DOI] [PubMed] [Google Scholar]
- 52.de Figueiredo-Feitosa N.L., Martelli Palomino G., Cilião Alves D.C., Mendes Junior C.T., Donadi E.A. and Maciel L.M. (2017) HLA-G 3′ untranslated region polymorphic sites associated with increased HLA-G production are more frequent in patients exhibiting differentiated thyroid tumours. Clin. Endocrinol. (Oxf.) 86, 597–605 10.1111/cen.13289 [DOI] [PubMed] [Google Scholar]
- 53.Hanahan D. and Weinberg R.A. (2011) Hallmarks of cancer: the next generation. Cell 144, 646–674 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 54.Almeida B.S., Muniz Y.C.N., Prompt A.H., Castelli E.C., Mendes-Junior C.T. and Donadi E.A. (2018) Genetic association between HLA-G 14-bp polymorphism and diseases: a systematic review and meta-analysis. Hum. Immunol. 79, 724–735 10.1016/j.humimm.2018.08.003 [DOI] [PubMed] [Google Scholar]
- 55.Vogelstein B., Papadopoulos N., Velculescu V.E., Zhou S., Diaz L.A. Jr and Kinzler K.W. (2013) Cancer genome landscapes. Science 339, 1546–1558 10.1126/science.1235122 [DOI] [PMC free article] [PubMed] [Google Scholar]