Abstract
Objective: In this work, the relationship between octamer binding transcription factor 4 (OCT-4) expression and the clinicopathological features of cervical cancer (CC) is evaluated in detail.
Methods: The library databases Pubmed, Embase, Cochrane library, Wan Fang and Chinese National Knowledge Infrastructure (CNKI) were searched for research related to these concepts published from the time the databases were established until May 2018. The obtained studies are screened, extracted, and evaluated according to the inclusion and exclusion criteria, and meta-analysis is carried out via RevMan 5.3.
Results: Ten case–control studies, including 408 cases of CC, 164 cases of cervical intraepithelial neoplasia (CIN), and 148 cases of normal cervix, are included in the analysis. Results show that OCT-4 levels are statistically significantly different between the CC and normal cervical tissue groups (odds ratio (OR) = 15.59, 95% confidence interval (CI): 8.70, 27.94), the CC and CIN groups (OR = 5.64, 95% CI: 3.23, 9.86), the CIN and normal cervical tissues groups (OR = 7.13, 95% CI: 2.41, 21.05), and the CC well/moderately differentiated and poorly differentiated groups (OR = 0.44, 95% CI: 0.24, 0.81). OCT-4 is not statistically significantly different between CIN I + II and CIN III tissues (OR = 0.40, 95% CI: −0.02, 0.81), the CC lymphatic and non-lymphatic metastasis groups (OR = 1.93, 95% CI: 0.83, 4.47), the FIGO I and FIGO II groups (OR = 0.79, 95% CI: 0.29, 2.13), and the adenocarcinoma and squamous cell carcinoma groups (OR = 1.55, 95% CI: 0.70, 3.44).
Conclusions: The available evidence suggests that OCT-4 expression is associated with CC malignancy and histological differentiation. This finding, however, is subject to quantitative studies and quality tests.
Keywords: Cervical cancer, Meta-analysis, OCT-4, Systematic review, Tumor stem cells
Introduction
Cervical cancer (CC) ranks first in women’s reproductive system malignancies among developing countries, accounting for more than 50% [1]. While mortality rates have shown a downward trend since the implementation of the anti-cancer census, tumor recurrence, metastasis, and treatment non-compliance continue to adversely seriously impact patient prognosis [2]. Exploration of the pathogenesis of CC is of great significance finding new drug targets. Some cells have growth patterns and biological characteristics similar to the basic characteristics of stem cells in tumors. Cancer stem cells (CSCs), for example, feature self-renewal, proliferation, differentiation potential and other abilities [3]. In addition, CSCs play a key role in the occurrence, growth, recurrence and metastasis of CC [4,5]. Therefore, identification of CSC colonies and expression markers may be helpful in predicting the progress of CC and finding new therapeutic targets [6].
At the developmental stage, the inner cell mass of embryonic blastocysts can produce pluripotent stem cells called embryonic stem cells (ESCs). ESCs have unrestricted replication ability and can be divided into functional cell types [7]. Great progress has been made on research into the transcriptional signaling pathways of ESCs, including octamer binding transcription factor 4 (OCT-4), sexcribed regions Y-box 2 (Sox 2), Kruppel-like factor 4 (Klf4) and so on. These factors are involved in epithelial stem cell differentiation and tumor invasion [6,8].
OCT-4, also known as OCT-3 or OCT3/4, POU5F1, is a member of the POU transcription factor family (Pit-Oct-Unc (POU)-domain transcription factor family) [9]. As a universal stem cell marker, it is an important regulator in maintaining ESCs and self-renewal. OCT-4 participates in multi-directional differentiation regulation in embryonic development and maintains adult stem cell pluripotency [10]. The transcription factor is expressed not only in embryonic and germ cell tumors [11], but also, to a certain extent, in non-reproductive system tumor cells and tissues, such as lung cancer [12], prostate cancer [13], breast cancer [14] and so on.
At present, studies on the relationship between OCT-4 expression and CC or its clinicopathological features are limited to case studies with small samples, and the conclusions obtained are often diverse. Whether OCT-4 can be used as a surface marker of CC stem cells as a new direction for stem cell-targeted therapy is a popular research topic. In the present study, the literature at home and abroad was collected, and the correlation between OCT-4 expression level and CC was evaluated by meta-analysis to provide evidence-based reference for CC treatment.
Materials and methods
Literature search strategy
We followed Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines [15] to conduct this systematic review. The selected databases, including Pubmed, Embase, Cochrane library, Wan Fang and Chinese National Knowledge Infrastructure (CNKI), were searched to identify suitable research regarding OCT-4 expression and the clinicopathological features of CC in case–control studies published from when the database was established up to May 2018. The search strategy included the terms Octamer binding transcription factor 4, OCT-4, OCT-3, OCT-3/4, and CC, cervical carcinoma, cervical intraepithelial neoplasia (CIN). The corresponding Chinese characters were used in Chinese databases, and the search languages included English and Chinese. Wherever possible, we traced references that had been incorporated into the literature and manually obtained the relevant conference proceedings to identify potential information that had not already been retrieved. Unpublished literature was not retrieved.
Inclusion and exclusion criteria
Studies were included in the meta-analysis if they met the following conditions: evaluated the correlation between OCT-4 expression and clinicopathological features of CC, all cases had complete clinical and pathological data before drawing without radiotherapy or chemotherapy, immunohistochemistry (IHC) methods were used to determine OCT-4 expression, and the results were expressed as cell or intensity scores. Studies were excluded if the OCT-4 detection method for IHC positive criteria were inconsistent. Studies on animals, human xenografts and the CC cell line were also excluded. Studies were excluded if they were review, summary, systematic evaluation, the reader letter and so on.
Data extraction
The following information was extracted from the literature: the title, first author names, the year of publication, country, the general situation of the included cases; the detection method of OCT-4; IHC methodology (primary antibody source), the clinical features of CC: lymph node metastasis, pathological grade, histological differentiation, tissue type and clinical stage. Whether all the literature was included was determined by two reviewers. They calculated the score according to the principle independently. All documents were included in the decision by two reviewers. If any differences arise, they may be decided by discussion, or consultation with the third reviewer.
Quality assessment
The quality of the methodology of each included observational study was assessed using the Newcastle–Ottawa Quality Assessment Scale (NOS) [12].The NOS includes three aspects for cohort studies: selection, comparability and exposure or outcome. The full score was nine stars, and the high-quality study was considered as a study whose score was greater than or equal to six [16–18].
Statistical analysis
The meta-analysis was performed by Review Manager Version 5.3 software (Cochrane Collaboration, Software Update, Oxford, United Kingdom). The association between OCT-4 expression and the clinical parameters of CC were quantitatively determined by calculating the pooled odds ratio (OR) and its 95% confidence interval (CI).
Cochran’s Q test and the I2 statistic was performed to assess the heterogeneity among studies with a significance level of α = 0.1. A fixed-effect model used to calculate parameters in meta-analysis, when P>0.1and I2 < 50%, whereas a random-effect model should used when P<0.1 and I2 > 50%. P<0.1 and I2< 50%, however, within the acceptable range, a fixed-effect model should used to calculate parameters in meta-analysis. If P>0.1 and I2 > 50%, the random-effect model can be used [19]. Publication bias was quantitatively assessed by using Egger’s test, and these operations were completed by STATA software (Stata Corporation, version 12.0,College Station, TX, U.S.A.). All P-values were two-tailed, P<0.05 was considered statistically significant publication bias.
Results
Literature search results and study characteristics
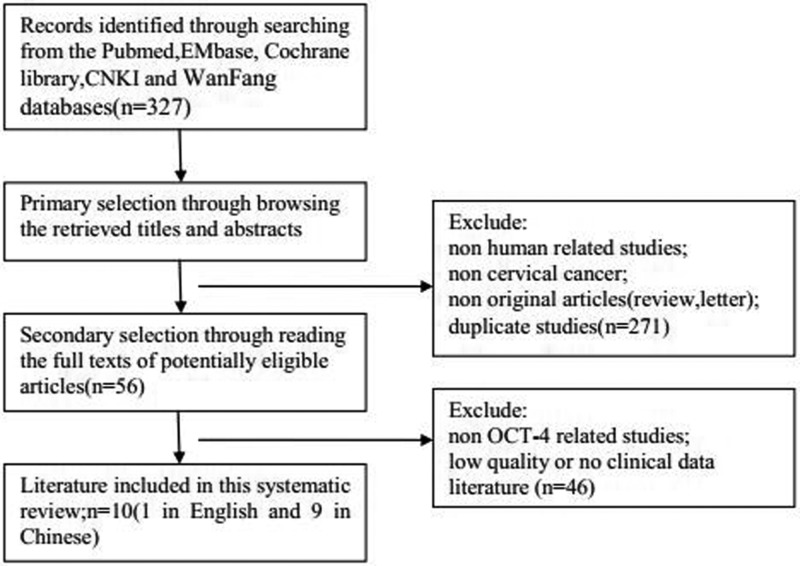
A total of 327 articles were obtained from the preliminary examination. According to the above inclusion criteria and exclusion criteria, the frequencies of OCT-4 protein expression in CC were evaluated in ten case–control studies (Figure 1).
Figure 1. Flow chart of literature search and selection schema.
Ten studies [20–29] included 408 CC cases, nine studies [20–26,28,29] reported the expression of OCT-4 in CC and normal cervical tissues, four studies [23,24,26,27] reported OCT-4 expression in the CC tissue and CIN tissue groups, three studies [23,24,26] reported OCT-4 expression in the CIN tissue and normal cervical tissues, three studies [24,26,27] reported OCT-4 expression in the CIN Ⅰ + Ⅱ tissue and CIN Ⅲ tissues, six studies [21,22,24,26,28,29] reported the expression of OCT-4 in CC with different degrees of differentiation. Three studies [21,27,29] reported the expression of OCT-4 in different lymph node metastases, and three studies [21,25,29] reported the expression of OCT-4 in different stages of CC, eight studies [20,22–28] reported the expression of OCT-4 in CC with different pathological grades. The basic characteristics and quality of each study are shown in Table 1 and 2.
Table 1. Basic characteristics of included studies.
| First author | Year | Time to collect cases (year) | Country | Antibodies | NCM | CIN | CC tissue | Evaluation index | NOS | Reference | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SCC | AC | ||||||||||
| Cao | 2010 | 2006–2007 | China | Santa Cruz | 12 | NA | 22 | 8 | a | 7 | [20] |
| Zhao | 2011 | 2005–2010 | China | Santa Cruz | 10 | 20 | 30 | NA | a, b, c | 7 | [21] |
| Deng | 2012 | 2009–2010 | China | Bioss Antibodies | 9 | NA | NA | 46 | a, b | 7 | [22] |
| Wan | 2012 | 2010–2011 | China | Epitomics | 10 | 30 | 35 | 5 | a, b | 7 | [23] |
| Huang | 2013 | 2011–2012 | China | Dongsheng Biotech Co. Ltd | 20 | 30 | 14 | 6 | a, b | 7 | [24] |
| Zhang | 2013 | 2008–2012 | China | Cell Signaling | 28 | NA | 10 | 42 | a, b | 7 | [25] |
| Tang | 2013 | 2010–2012 | China | Santa Cruz | 10 | 30 | 16 | 4 | a, b | 7 | [26] |
| Yan | 2015 | 2005–2014 | China | Fuzhou Maixin Biotech. Co. Ltd | NA | 55 | NA | 66 | b, c | 7 | [27] |
| Liu | 2015 | 2008–2010 | China | Abcam | 20 | NA | 42 | 10 | a, b | 7 | [28] |
| Jing | 2014 | 2011–2012 | China | Santa Cruz | 28 | NA | NA | 43 | a, b, c | 8 | [29] |
a, The expression of OCT-4 in normal cervical tissues and CC tissues. b, OCT-4 expression in cervical tissues with different degrees of differentiation. c, OCT-4 expression in CC of different lymphatic metastases.
Abbreviations: AC, adenocarcinoma; NA, not available; NCE, normal cervical tissue; SCC, squamous cell carcinoma.
Table 2. The publication bias of OCT-4 expression in CC.
| Clinicopathological features | t | 95% CI | P |
|---|---|---|---|
| Well/moderately vs. poorly differentiated | 1.47 | −7.509–8.524 | 0.280 |
| Lymphatic vs. non-lymphatic metastasis | −0.56 | −53.025–48.559 | 0.676 |
| FIGO I vs. FIGO II | 0.62 | −25.033–27.614 | 0.645 |
| SCC vs. AC | 3.33 | 0.580–6.354 | 0.029 |
Abbreviations: AC, adenocarcinoma; SCC, squamous cell carcinoma.
Results of the meta-analysis
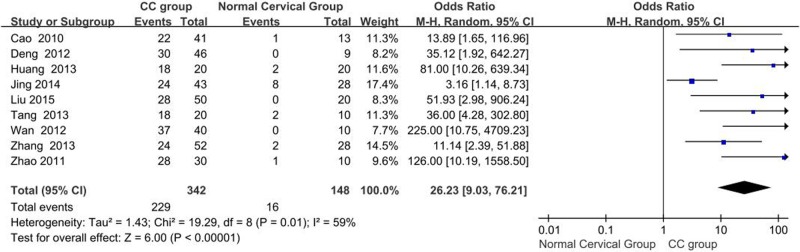
Differences in OCT-4 protein expression: CC tissues vs. normal cervical tissues
A total of nine studies reporting OCT-4 expression in CC tissues and normal cervical tissues were obtained. I2 estimates showed heterogeneity (I2 = 59%, χ2 = 19.29, P=0.01) among the studies, and the random-effects model was used in the meta-analysis. The results showed that expression of OCT-4 protein in CC is significantly higher than that in normal cervical tissues (OR = 26.23, 95% CI: 9.03–76.21, P<0.00001, Figure 2).
Figure 2. Meta analysis of the expression of OCT-4 protein in CC tissues vs. normal cervical tissues.
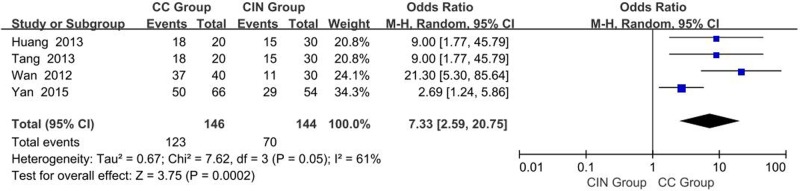
Differences in OCT-4 protein expression : CC tissue vs. CIN tissue
A total of four studies reporting OCT-4 expression in CC tissue and CIN tissue groups were obtained. I2 estimates showed heterogeneity (I2 = 61%, χ2 = 7.62, P=0.05) among the studies, and the random-effects model was used in the meta-analysis. The results showed that expression of OCT-4 protein in CC tissues is significantly higher than that in CIN tissues (OR = 7.33, 95% CI: 2.59–20.75, P=0.0002, Figure 3).
Figure 3. Meta analysis of the expression of OCT-4 protein in CC tissues vs. CIN tissues.
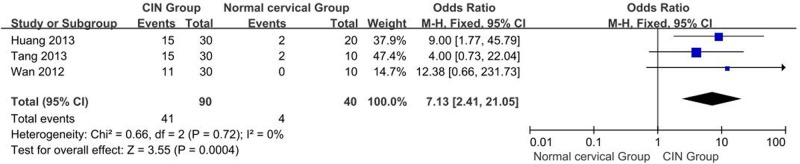
Differences in OCT-4 protein expression : CIN tissue vs. normal cervical tissues
A total of three studies reporting OCT-4 expression in normal cervical tissues and CIN tissues were obtained. I2 estimate indicated has no heterogeneity (I2 = 0%, χ2 = 0.66, P=0.72) among the studies, and the fixed-effects model used in this meta-analysis. The results showed that expression of OCT-4 protein in CIN tissues is significantly higher than that in normal cervical tissues (OR = 7.13, 95% CI: 2.41–21.05, P=0.0004, Figure 4).
Figure 4. Meta analysis of the expression of OCT-4 protein in normal cervical tissues vs. CIN tissues.
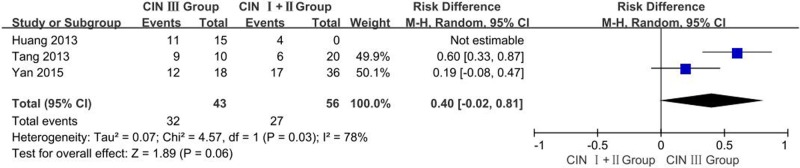
Differences in OCT-4 protein expression: CIN Ⅰ + Ⅱ tissue vs. CIN Ⅲ tissue
A total of three studies reported OCT-4 expression in the CIN Ⅰ + Ⅱ tissue and CIN Ⅲ tissue groups. The I2estimate indicated has heterogeneity (I2 = 78%, χ2 = 4.57, P=0.03) among the studies, and the random-effects model used in this meta-analysis. The results showed that the expression of OCT-4 protein in CIN Ⅲ tissues was higher than that in CIN Ⅰ + Ⅱ tissues, however, the difference was not significantly (OR = 0.40, 95% CI: −0.02–0.81, P=0.06, Figure 5).
Figure 5. Meta analysis of the expression of OCT-4 protein in CIN Ⅰ + Ⅱ tissues vs. CIN Ⅲ tissues.
Differential expression of OCT-4 in CC
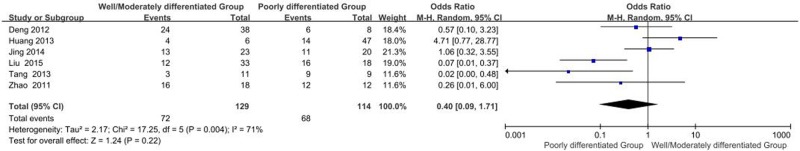
Differential expression of OCT-4 in CC: poorly differentiated vs. well and moderately differentiated
A total of six studies reporting OCT-4 expression in the CC with different degrees of differentiation. I2 estimates showed no heterogeneity among the studies (I2 = 71%, χ2 = 17.25, P=0.004), and the random-effects model was used for this meta-analysis. It was indicated that the expression level of OCT-4 protein in poorly differentiated CC is higher than in well-differentiated and moderately differentiated CC, however, the difference is not statistically significant (OR = 0.40, 95% CI: 0.09–1.71, P=0.22, Figure 6).
Figure 6. Meta analysis of expression of OCT-4 protein in CC tissues with different degrees of differentiation.
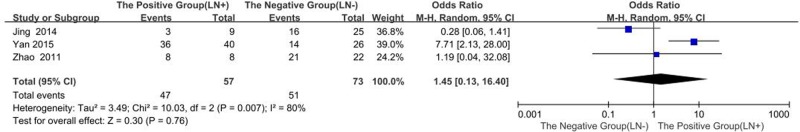
Differential expression of OCT-4 in CC with lymph node metastases: positive group vs. negative group
A total of three studies reporting OCT-4 overexpression in positive and negative lymph node metastases groups of CC tissues. I2 estimate showed heterogeneity among the studies (I2 = 80%, χ2 = 10.03, P=0.007), and the random-effects model was used for this meta-analysis. The results showed that there is no significant difference in OCT-4 protein expression in patients with lymph node metastases compared with those without (OR = 1.45, 95% CI: 0.13–16.40, P=0.76, Figure 7). It is speculated that the expression of OCT-4 protein in CC is not significantly associated with the occurrence of lymph node metastasis.
Figure 7. Meta-analysis of expression of OCT-4 protein in CC tissues with positive group vs. negative group lymph node metastases.
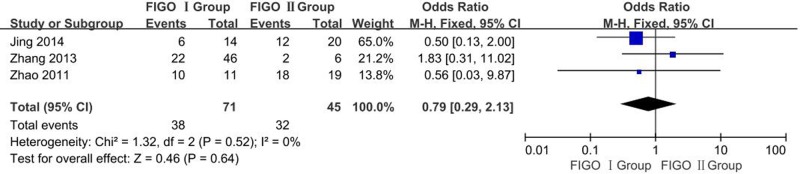
Differential expression of OCT-4 in different stages of CC: FIGO I vs. FIGO II
A total of three studies reporting OCT-4 overexpression in FIGO I and FIGO II of CC tissues. I2 estimate showed heterogeneity among the studies (I2 = 0%, χ2 = 1.32, P=0.52), and the fixed-effects model was used for this meta-analysis. There is no significant difference in the expression of OCT-4 protein among different FIGO stages of CC (OR = 0.79, 95% CI: 0.29–2.13, P=0.64, Figure 8).
Figure 8. Meta analysis of differences in expression of OCT-4 protein in different stages of CC.
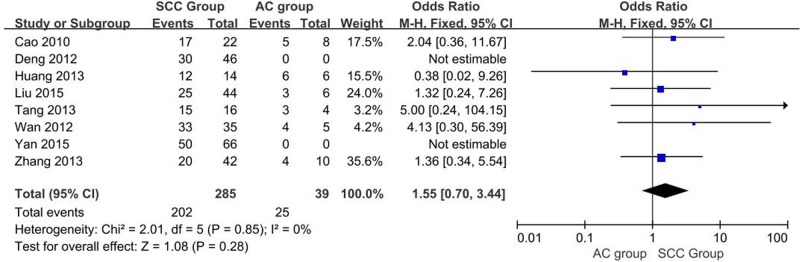
Differential expression of OCT-4 in CC squamous cell carcinoma vs. adenocarcinoma
A total of eight studies reporting OCT-4 overexpression in different pathological types of CC tissues. I2 estimate revealed significant heterogeneity among the studies (I2 = 0%, χ2 = 2.01, P=0.85), and the fixed-effects model was used for this meta-analysis. There is no significant difference in the expression of OCT-4 protein between squamous cell carcinoma (SCC) and adenocarcinoma; (AC) (OR = 1.55, 95% CI: 0.70–3.44, P=0.28, Figure 9).
Figure 9. Meta analysis of differences in expression of OCT-4 protein in CC tissues with different pathological types.
Publication bias
No publication bias in studies including in the literature related to lymphatic metastasis, differentiation and FIGO staging (P>0.05), whereas studies on the pathological type had publication bias (P<0.05).
Discussion
CC is a common gynecological tumor that threatens women’s health. CSCs are characterized by self-renewal, multi-directional differentiation, radiotherapy and chemotherapy resistance, high tumorigenicity and so on, and may affect the invasion, metastasis, and recurrence of CC [30–32].
The discovery of CSCs provides new possibilities for diagnosing and treating CC. Scholars have conducted extensive research on CSCs related to CC. In 2012, Lopez et al. [33] found cancer-initiating cell subsets with self-renewal ability in the CC cell lines HeLa, SiHa, Ca Ski and C-4I, which can express the characteristic markers of stem cells, epithelial mesenchymal transition (EMT) and radioresistance. Liu et al. [34] explored the functional relationship between endogenous nuclear protein SOX2 expression and cervical cancer CSCs, and revealed that isolating CSCs from CC somatic tumors is feasible. Ortiz-Sánchez et al. [35] confirmed that the expression of CK-17, p63+, AII+, CD49f+ in CC stem cells and activity of aldehyde dehydrogenase (ALDHbright) are high. Tyagi et al. [36] confirmed that CSCs isolated from CC have self-renewal ability and could express the markers OCT-4, Sox2, Nanog, Lrig1 and CD133. Javed et al. [37] found that CD133 positive in CC stem cells, and increased expression in recurrent CC, increased CD133-positive tumor cell formation, and up-regulated EMT-related markers.
OCT-4 expression is closely related to the occurrence of malignant tumors and affects their rapid development, high metastasis rate, and poor prognosis, thus suggesting that the protein can predict the occurrence and development of tumors and be regarded as a prognosis indicator [38,39]. OCT-4 plays a dual role in the non-differentiated cell stages. In addition to maintaining cells in the non-differentiated stage, OCT-4 can differentiate cells into other types. While knockout of OCT-4 results in the disappearance of, cell differentiation and stem cell characteristics, the transcription factor retains its self-renewal and infinite amplification abilities, thereby resulting in the occurrence of tumors [40,41].
CC is related to HPV infection, especially high-risk HPV16 and HPV18 [42]. Liu et al. [43] predicted that HPV infection may accelerate the up-regulation of OCT-4 expression to trigger CC, and suggested that OCT-4 may present a new type of targeted therapeutic approach for CC. His team [44] further proved that HPV16 is the key factor triggering the occurrence and development of CC. The expression of OCT-4 is up-regulated in CC cells and may be activated by HPV16 infection; in fact, its expression level in HPV16-infected CC cells is higher than that in non-infected patients [45].
Kim et al. [46] found that OCT-4 expression in CC cells is higher than that in the normal cervix, and that OCT-4 overexpression is associated with space invasion of CC lymph vessels. Compared with the low expression of OCT-4, progression-free survival (PFS) and overall survival (OS) are worse in 5 years in the overexpression group, thereby suggesting that OCT-4 is an independent risk factor of CC and that patients with OCT-4 overexpression suffer from poor prognoses. OCT-4 increases progressively from the normal cervix to CIN and then to CC. Shen et al. [47] showed that OCT-4 overexpression in the radiation-resistant group is considerably higher than that in the radiation-sensitive group and that PFS in the OCT-4 overexpression group decreases. Thus, OCT-4 protein could predict radiation resistance in locally advanced cervical SCC (LACSCC) patients and poor survival rates. Considering these findings, OCT-4 is an independent prognostic factor of CC, and may represent a potential target for CC treatment [48].
Many miRNAs, such as miR-145, miR-302, miR-125b, miR-430, miR-200, miR-335, are associated with OCT genes [49]. OCT-4 induces miR-125b expression in certain means, thereby inhibiting BAK1 expression and, ultimately, the apoptosis of CC cells. Multiple miRNAs are involved in the regulation of tumor growth, metastasis of tumor, and chemoradiotherapy sensitivity by interacting with OCT-4 [50]. Yan et al. [42] confirmed that miR-145 could improve the sensitivity of CC radiation by inhibiting OCT-4 expression; this finding suggesting that cyclin D1 positively regulates OCT-4 and mediates the function of miR-145.
In the present study, the clinicopathological features of OCT-4 and CC were collected for statistical analysis and results indicated that OCT-4 expression is higher in CC than in CIN and normal tissues. This result is consistent with the findings of Huang et al. [21,23,24], who suggested that OCT-4 overexpression increases the risk of CC. Among different clinical and pathological features, OCT-4 expression shows different results. Yan et al. [27] believed that OCT-4 expression has significant meaning for CC lymphatic metastasis, and increases the risk of poor prognosis. Zhao et al. [21] and Ji et al. [29] reported that OCT-4 expression is not involved in CC lymphatic metastasis. Deng et al. [21–23,25,27,28] found that OCT-4 expression is significantly related to histological differentiation; Jing et al. [24,26,29], however, denied this argument. Meta-analysis showed that the lower differentiation of CC, the higher the expression of OCT-4, which indicates that OCT-4 overexpression is closely related to the occurrence and histological differentiation of CC. By contrast, OCT-4 expression is not significantly related to lymphatic metastasis, FIGO stage, and pathological type.
The meta-analysis features publication bias as follows. (i) Although Korean researcher Kim [41] reported that OCT-4 is expressed in CC, the relevant data could not be extracted and included. The study area was in China, and a simple model of population was applied; thus, extrapolation of findings is limited. (ii) Both Chinese and English literature were included in this work, but the gray literature was insufficient. This limitation may result in missed negative results and publication bias. (iii) The included studies were of moderate quality, which may obscure the unknown defects of the design. (iv) The inclusion criteria of certain studies were minimal, and the conclusions must be further verified. (v) The studies all applied the IHC method to detect OCT-4 expression, but the antibody manufacturers, dilution concentration, and judgment criteria were not identical, which may affect the results.
In conclusion, OCT-4 expression is higher in CC than in CIN and normal tissues. Such a result is related to histological differentiation and is not significantly correlated with lymphatic metastasis, FIGO stage, and pathological type, which indicates that OCT-4 may play important role in the occurrence and progression of CC. The findings also suggest OCT-4 may serve as a prognostic indicator of CC and potential target of stem cell therapy. However, rigorous, less biased, and high-quality randomized controlled studies based on NOS standards are needed for further confirmation of the present findings.
Abbreviations
- CC
cervical cancer
- CI
confidence interval
- CIN
cervical intraepithelial neoplasia
- CSC
cancer stem cell
- EMT
epithelial mesenchymal transition
- ESC
embryonic stem cell
- IHC
immunohistochemistry
- NOS
Newcastle–Ottawa Quality Assessment Scale
- OCT-4
octamer binding transcription factor 4
- OR
odds ratio
- PFS
progression-free survival
- SCC
squamous cell carcinoma
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
This work was supported by the Foundation of Taihe Hospital [grant number 2014JJXM031].
Author Contribution
Conceptualization: Zi-ye Gao and Sheng-bao Li. Formal analysis: Zi-ye Gao and Xiao-bo Liu. Investigation: Zi-ye Gao, Xiao-bo Liu, and Feng-mei Yang. Methodology: Zi-ye Gao, Xiao-bo Liu, Feng-mei Yang, and Bo Gao. Resources: Zi-ye Gao, Xiao-bo Liu, and Bo Gao. Software: Zi-ye Gao, Xiao-bo Liu, Ling Liu, and Jin-zhang Zhao. Supervision: Bo Gao and Sheng-bao Li; Writing – original draft: Zi-ye Gao and Xiao-bo Liu. Writing – review and editing: Zi-ye Gao, Xiao-bo Liu, Jin-zhang Zhao, and Bo Gao. All authors read and approved the final manuscript.
References
- 1.Rahman M.F., Akhter S.N., Alam M.J., Sarker A.S., Uddin M.J., Bashar A.. et al. (2016) Detection of cervical cancer through visual inspection of cervix with acetic acid (VIA) and colposcopy at Mymensingh Medical College Hospital. Mymensingh Med. J. 25, 402–409 [PubMed] [Google Scholar]
- 2.Park K.J., Braschi-Amirfarzan M., DiPiro P.J., Giardino A.A., Jagannathan J.P., Howard S.A.. et al. (2016) Multimodality imaging of locally recurrent and metastatic cervical cancer: emphasis on histology, prognosis, and management. Abdom. Radiol. 41, 2496–2508 10.1007/s00261-016-0825-5 [DOI] [PubMed] [Google Scholar]
- 3.Visvader J.E. and Lindeman G.J. (2008) Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat. Rev. Cancer 8, 755–768 10.1038/nrc2499 [DOI] [PubMed] [Google Scholar]
- 4.Luo C.L., Liu Y.Q., Wang P., Song C.H., Wang K.J., Dai L.P.. et al. (2016) The effect of quercetin nanoparticle on cervical cancer progression by inducing apoptosis, autophagy and anti-proliferation via JAK2 suppression. Biomed. Pharmacother. 82, 595–605 10.1016/j.biopha.2016.05.029 [DOI] [PubMed] [Google Scholar]
- 5.Kumazawa S., Kajiyama H., Umezu T., Mizuno M., Suzuki S., Yamamoto E.. et al. (2014) Possible association between stem-like hallmark and radioresistance in human cervical carcinoma cells. J. Obstet. Gynaecol. Res. 40, 1389–1398 10.1111/jog.12357 [DOI] [PubMed] [Google Scholar]
- 6.Gwak J.M., Kim M., Kim H.J., Jang M.H. and Park S.Y. (2017) Expression of embryonal stem cell transcription factors in breast cancer: Oct4 as an indicator for poor clinical outcome and tamoxifen resistance. Oncotarget 8, 36305–36318 10.18632/oncotarget.16750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bibikova M., Laurent L.C., Ren B., Loring J.F. and Fan J.B. (2008) Unraveling epigenetic regulation in embryonic stem cells. Cell Stem Cell 2, 123–134 10.1016/j.stem.2008.01.005 [DOI] [PubMed] [Google Scholar]
- 8.Hu Q. and Rosenfeld M.G. (2012) Epigenetic regulation of human embryonic stem cells. Front. Genet. 3, 238 10.3389/fgene.2012.00238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun L., Liu T., Zhang S., Guo K. and Liu Y. (2017) Oct4 induces EMT through LEF1/beta-catenin dependent WNT signaling pathway in hepatocellular carcinoma. Oncol. Lett. 13, 2599–2606 10.3892/ol.2017.5788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zangrossi S., Marabese M., Broggini M., Giordano R., D’Erasmo M., Montelatici E.. et al. (2007) Oct-4 expression in adult human differentiated cells challenges its role as a pure stem cell marker. Stem Cells 25, 1675–1680 10.1634/stemcells.2006-0611 [DOI] [PubMed] [Google Scholar]
- 11.Yang Y., Wang Y., Yin C. and Li X. (2014) Clinical significance of the stem cell gene Oct-4 in cervical cancer. Tumour Biol. 35, 5339–5345 10.1007/s13277-014-1696-4 [DOI] [PubMed] [Google Scholar]
- 12.Li S.J., Huang J., Zhou X.D., Zhang W.B., Lai Y.T. and Che G.W. (2016) Clinicopathological and prognostic significance of Oct-4 expression in patients with non-small cell lung cancer: a systematic review and meta-analysis. J. Thorac. Dis. 8, 1587–1600 10.21037/jtd.2016.06.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qi Y.F., Wu L., Li Z.Q., Wu M.L., Wang H.F., Chan K.Y.. et al. (2016) Nodal signaling modulates the expression of Oct-4 via nuclear translocation of beta-catenin in lung and prostate cancer cells. Arch. Biochem. Biophys. 608, 34–41 10.1016/j.abb.2016.07.003 [DOI] [PubMed] [Google Scholar]
- 14.Bhatt S., Stender J.D., Joshi S., Wu G. and Katzenellenbogen B.S. (2016) OCT-4: a novel estrogen receptor-alpha collaborator that promotes tamoxifen resistance in breast cancer cells. Oncogene 35, 5722–5734 10.1038/onc.2016.105 [DOI] [PubMed] [Google Scholar]
- 15.Moher D., Liberati A., Tetzlaff J. and Altman D.G. (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339, b2535 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lo C.K., Mertz D. and Loeb M. (2014) Newcastle-Ottawa Scale: comparing reviewers’ to authors’ assessments. BMC Med. Res. Methodol. 14, 45 10.1186/1471-2288-14-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stang A. (2010) Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 25, 603–605 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 18.Wei S., Chen M., Chen N. and Liu L. (2017) Feasibility and safety of robot-assisted thoracic surgery for lung lobectomy in patients with non-small cell lung cancer: a systematic review and meta-analysis. World J. Surg. Oncol. 15, 98 10.1186/s12957-017-1168-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peng J., Qi S., Wang P., Li W., Liu C. and Li F. (2016) Diagnosis and prognostic significance of c-Met in cervical cancer: a meta-analysis. Dis. Markers 2016, 6594016 10.1155/2016/6594016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cao H.Z., Ji J. and Zheng P.S. (2010) Expression of Oct4 gene in cervical cancer. J. Xi’an Jiaotong Univ. (Med. Sci.) 17–21 1671-8259(2010)01-0017-05 [Google Scholar]
- 21.Zhao Y., Sun Z.H., Wu Q., Xu F. and Shen J.H. (2011) Expressions of Nanog and Oct4 in cervical cancer tissues and their clinical significance. Chin. J. Cancer Prev. Treat. 1243–1246 10.16073/j.cnki.cjcpt.2011.16.011 [DOI] [Google Scholar]
- 22.Deng X., Liu S.T., Che G.H., Li Y. and Gao H. (2012) Expressions and clinical significance of SOX-2 and Oct4 in cervical squamous cell carcinoma. Chin. J. Lab. Diagn. 2018–2021 10.3969/j.issn.1007-4287.2012.11.020 [DOI] [Google Scholar]
- 23.Wan L.J., Shang L.X., Wu N., Hu C.Y. and Wang X. (2012) The significance of stem cells key transcriptions factor Oct-4 expression in cervical cancer. Chin. J. Birth Health Heredity 26–28 10.13404/j.cnki.cjbhh.2012.05.031 [DOI] [Google Scholar]
- 24.Huang P., Huang H., Li R༌Chen G.Y., Jing Z.Y. and Xie J.B. (2013) Expression of Oct4 in cervical carcinoma and its association with HPV infection. Lab. Med. Clin. 2655–2656 10.3969/j.issn.1672-9455.2013.20.008 [DOI] [Google Scholar]
- 25.Zhang L., Wei Z.T., Li J.J., G H.N. and Zhang S.L. (2013) Expressions of transcription factor Oct4 and SOX-2 in cervical cancer tissue and their significance. J. Jilin Univ. (Medicine Ed.) 1252–1255 10.7694/jldxyxb20130635 [DOI] [Google Scholar]
- 26.Tang Q.Y., Huang P. and Jin Y. (2013) Expression of stem cell gene Bcl-2 and Oct4 in cervical carcinoma and precancerous tissues and their clinical significance. Hainan Med. J. 625–627 10.3969/j.issn.1003-6350.2012.05.0270 [DOI] [Google Scholar]
- 27.Yan H.M., Yao M.F. and Yao H. (2015) Expressions and clinical significance of SOX2 OCT4 TWIST and YB-1 in squamous cell carcinoma of cervix. Chin. Remed. Clinics 1092–1094 10.11655/zgywylc2015.08.015 [DOI] [Google Scholar]
- 28.Liu H., Fang C.H., Zhang Q.Y., Li K.X., Fan X.M. and Chen J.X. (2015) Expressions of stem cell gene Oct4 in cervical cancer and clinical significance. J. Hebei Med. Univ. 1267–1270 10.3969/j.issn.1007-3205.2015.11.00 [DOI] [Google Scholar]
- 29.Ji J., Wei X. and Wang Y. (2014) Embryonic stem cell markers Sox-2 and OCT4 expression and their correlation with WNT signal pathway in cervical squamous cell carcinoma. Int. J. Clin. Exp. Pathol. 7, 2470–2476 [PMC free article] [PubMed] [Google Scholar]
- 30.Dominguez-Catzin V., Reveles-Espinoza A.M., Sanchez-Ramos J., Cruz-Cadena R., Lemus-Hernandez D. and Garrido E. (2017) HPV16-E2 protein modifies self-renewal and differentiation rate in progenitor cells of human immortalized keratinocytes. Virol. J. 14, 65 10.1186/s12985-017-0736-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang L., Guo H., Yang L., Dong L., Lin C., Zhang J.. et al. (2013) Morusin inhibits human cervical cancer stem cell growth and migration through attenuation of NF-kappaB activity and apoptosis induction. Mol. Cell Biochem. 379, 7–18 10.1007/s11010-013-1621-y [DOI] [PubMed] [Google Scholar]
- 32.Zhang Z., Bu X., Chen H., Wang Q. and Sha W. (2016) Bmi-1 promotes the invasion and migration of colon cancer stem cells through the downregulation of E-cadherin. Int. J. Mol. Med. 38, 1199–1207 10.3892/ijmm.2016.2730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lopez J., Poitevin A., Mendoza-Martinez V., Perez-Plasencia C. and Garcia-Carranca A. (2012) Cancer-initiating cells derived from established cervical cell lines exhibit stem-cell markers and increased radioresistance. BMC Cancer 12, 48 10.1186/1471-2407-12-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu X.F., Yang W.T., Xu R., Liu J.T. and Zheng P.S. (2014) Cervical cancer cells with positive Sox2 expression exhibit the properties of cancer stem cells. PLoS ONE 9, e87092 10.1371/journal.pone.0087092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ortiz-Sanchez E., Santiago-Lopez L., Cruz-Dominguez V.B., Toledo-Guzman M.E., Hernandez-Cueto D., Muniz-Hernandez S.. et al. (2016) Characterization of cervical cancer stem cell-like cells: phenotyping, stemness, and human papilloma virus co-receptor expression. Oncotarget 7, 31943–31954 10.18632/oncotarget.8218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tyagi A., Vishnoi K., Mahata S., Verma G., Srivastava Y., Masaldan S.. et al. (2016) Cervical cancer stem cells selectively overexpress HPV Oncoprotein E6 that controls stemness and self-renewal through upregulation of HES1. Clin. Cancer Res. 22, 4170–4184 10.1158/1078-0432.CCR-15-2574 [DOI] [PubMed] [Google Scholar]
- 37.Javed S., Sharma B.K., Sood S., Sharma S., Bagga R., Bhattacharyya S.. et al. (2018) Significance of CD133 positive cells in four novel HPV-16 positive cervical cancer-derived cell lines and biopsies of invasive cervical cancer. BMC Cancer 18, 357 10.1186/s12885-018-4237-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yun J.H., Kim K.A., Yoo G., Kim S.Y., Shin J.M., Kim J.H.. et al. (2017) Phenethyl isothiocyanate suppresses cancer stem cell properties in vitro and in a xenograft model. Phytomedicine 30, 42–49 10.1016/j.phymed.2017.01.015 [DOI] [PubMed] [Google Scholar]
- 39.Jiang X.D., Luo G., Wang X.H., Chen L.L., Ke X. and Li Y. (2017) [Expression of Oct4 and Sox2 and their clinical significance in tongue squamous cell carcinoma]. Zhong. Kou Qiang Yi Xue Za Zhi 52, 27–33 [DOI] [PubMed] [Google Scholar]
- 40.Cantz T., Key G., Bleidissel M., Gentile L., Han D.W., Brenne A.. et al. (2008) Absence of OCT4 expression in somatic tumor cell lines. Stem Cells 26, 692–697 10.1634/stemcells.2007-0657 [DOI] [PubMed] [Google Scholar]
- 41.Tai M.H., Chang C.C., Kiupel M., Webster J.D., Olson L.K. and Trosko J.E. (2005) Oct4 expression in adult human stem cells: evidence in support of the stem cell theory of carcinogenesis. Carcinogenesis 26, 495–502 10.1093/carcin/bgh321 [DOI] [PubMed] [Google Scholar]
- 42.Yan S., Li X., Jin Q. and Yuan J. (2016) MicroRNA-145 sensitizes cervical cancer cells to low-dose irradiation by downregulating OCT4 expression. Exp. Ther. Med. 12, 3130–3136 10.3892/etm.2016.3731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu D., Zhou P., Zhang L., Wu G., Zheng Y. and He F. (2011) Differential expression of Oct4 in HPV-positive and HPV-negative cervical cancer cells is not regulated by DNA methyltransferase 3A. Tumour Biol. 32, 941–950 10.1007/s13277-011-0196-z [DOI] [PubMed] [Google Scholar]
- 44.Liu D., Zhou P., Zhang L., Zheng Y. and He F. (2012) HPV16 activates the promoter of Oct4 gene by sequestering HDAC1 from repressor complex to target it to proteasomal degradation. Med. Hypotheses 79, 531–534 10.1016/j.mehy.2012.07.012 [DOI] [PubMed] [Google Scholar]
- 45.Liu D., Zhou P., Zhang L.. et al. (2012) HDAC1/DNMT3A-containing complex is associated with suppression of Oct4 in cervical cancer cells. Biochemistry (Mosc.) 77, 934–940 10.1134/S0006297912080159 [DOI] [PubMed] [Google Scholar]
- 46.Kim B.W., Cho H., Choi C.H., Ylaya K., Chung J.Y., Kim J.H.. et al. (2015) Clinical significance of OCT4 and SOX2 protein expression in cervical cancer. BMC Cancer 15, 1015 10.1186/s12885-015-2015-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shen L., Huang X., Xie X., Su J., Yuan J. and Chen X. (2014) High expression of SOX2 and OCT4 indicates radiation resistance and an independent negative prognosis in cervical squamous cell carcinoma. J. Histochem. Cytochem. 62, 499–509 10.1369/0022155414532654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang Y., Wang Y., Yin C. and Li X. (2014) Clinical significance of the stem cell gene Oct-4 in cervical cancer. Tumour Biol. 35, 5339–5345 10.1007/s13277-014-1696-4 [DOI] [PubMed] [Google Scholar]
- 49.Chen C., Meng F., Wan H. and Zhou Q. (2015) [Interaction between microRNAs and OCT4]. Zhong. Fei Ai Za Zhi 18, 55–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Y.D., Cai N., Wu X.L., Cao H.Z., Xie L.L. and Zheng P.S. (2013) OCT4 promotes tumorigenesis and inhibits apoptosis of cervical cancer cells by miR-125b/BAK1 pathway. Cell Death Dis. 4, e760 10.1038/cddis.2013.272 [DOI] [PMC free article] [PubMed] [Google Scholar]