Abstract
Although isocyanide-based multicomponent reactions were proven to be simple, elegant and facile strategies for the synthesis of highly valuable nitrogen-containing heterocycles, their asymmetric versions accessing to optically active nitrogen heterocyclic compounds are rather limited. Here, we illustrate that, relying on the enantioselective addition of simple isocyanides to C=C bonds, several isocyanide-based multicomponent reactions are realized in the presence of a chiral MgII-N,Nʹ-dioxide catalyst. In the reaction among isocyanide, TMSN3, and alkylidene malonate, three- or four-component reactions could be precisely controlled by modulating reaction conditions, supplying two types of enantioenriched tetrazole-derivatives in moderate to high yields. Possible catalytic cycles via a key zwitterionic intermediate, and the vital roles of H2O or excess ligand are provided based on control experiments. Moreover, taking advantage of this zwitterionic intermediate as a 1,3-dipole, an enantioselective dearomative [3+2] annulation reaction of nonactivated isoquinolines is achieved, furnishing chiral 1,2-dihydroisoquinolines in good to excellent results.
Subject terms: Asymmetric catalysis, Asymmetric synthesis, Synthetic chemistry methodology
Asymmetric isocyanide-based multicomponent reactions are elegant, yet challenging, strategies to access valuable N-heterocycles. Here, the authors employ a chiral Mg(II) -N,N′-dioxide catalyst in three- or four-component reactions to obtain chiral tetrazoles and devise a dearomative [3+2] annulation reaction of isoquinolines.
Introduction
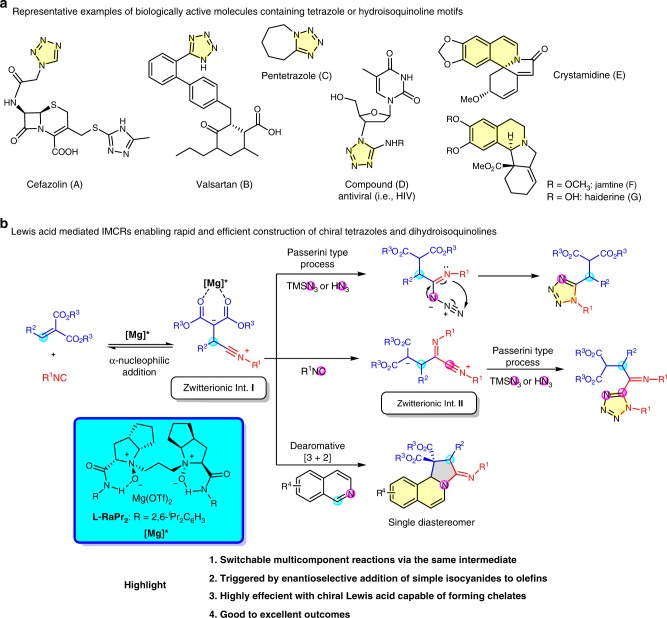
Nitrogen-containing heterocycles are the most popular structural components of pharmaceuticals and agrochemicals1–3. Among the vast array of such compounds, tetrazole and hydroisoquinoline motifs are particularly interesting and have been included in top 25 most commonly utilized nitrogen heterocycles in pharmaceuticals3. The substances with tetrazole motifs exhibit various types of biological properties (Fig. 1a)4–7. For instance, 1,5-disubstituted tetrazoles, as bioisosteres of the cis-amide bond in peptides, display similar physicochemical properties but enhancement of metabolic stability in living systems7. Hydroisoquinoline skeleton is widely represented in bioactive alkaloids, such as jamtine, haiderine, and crystamidine (Fig. 1a)8–10. In consequence, the development of synthetic approaches to tetrazole and reduced isoquinoline derivatives, especially enantioenriched ones having a variety of peripheral functional groups, would therefore be great of importance for the exploration and modification of new bioactive molecules or drugs. In this regard, great endeavors have been continuously devoted for the synthesis of these valuable compounds over the past two decades11–14.
Fig. 1.
Representative active substances and our strategies based on IMCRs for their construction. a Active substances with tetrazole and hydroisoquinoline motifs. b Our strategies to asymmetric synthesis of tetrazole and dihydroisoquinoline derivatives by IMCRs
Isocyanide-based multicomponent reactions (IMCRs) represent one of the most efficient methods for the construction of heterocycles, wherein a cascade of elementary chemical reactions occur in a uniquely ordered manner under the same reaction conditions, allowing rapid assembly of complex molecules in one pot from simple starting materials with remarkable synthetic efficiency and high atom economy15–22. In this context, many IMCRs accessing to 1,5-disubstituted tetrazoles and 1,2-dihydroisoquinolines were reported in the past decade20–25. However, in sharp contrast, enantioselective versions of IMCRs are relatively limited and most of studies are focused on the classic Passerini reaction and Ugi reaction initiated by nucleophilic addition of isocyanides to C=X bonds (X=O, or NR′)15,26. Among the approaches explored to date, asymmetric syntheses of enantioenriched 1,5-disubstituted tetrazoles and 1,2-dihydroisoquinolines are rare. To the best of our knowledge, the only example of catalytic enantioselective synthesis of chiral tetrazoles via Passerini-type reaction was reported in 2008 by Wang and Zhu27. In this work, key for success was the use of chiral [(salen)AlIIIMe] catalyst with a single coordination site, and moderate to high e.r. values were achieved with aliphatic aldehydes to construct 5-(1-hydroxyalkyl)tetrazoles. Mechanistically, the component of aldehyde in Passerini-type reaction could be replaced by the substrates with polar C=C bond to give a zwitterionic intermediate I (Fig. 1b), which potentially participates in various subsequent multicomponent processes23. Although dearomative [3+2] annulation reaction based on this hypothesis would appear to be highly attractive for the synthesis of chiral 1,2-dihydroisoquinolines, the asymmetric version of this reaction remains elusive28–30. Intrigued by the above hypothesis and good performance of N,Nʹ-dioxide-based chiral catalysts showed in enantioselective addition to α,β-unsaturated carbonyl compounds31–34, we envisioned that our chiral N,Nʹ-dioxide/metal complex could probably be suitable to catalyze the kinds of multicomponent reactions mentioned above. Herein, we describe the asymmetric IMCRs initiated by the enantioselective addition of simple isocyanides to olefins (Fig. 1b). Interestingly, through adjusting reaction conditions, we are able to accurately control the number (1 or 2)35-38 of isocyanide molecules participated in the reaction of alkylidene malonates, isocyanides, and TMSN3, leading to two types of enantioenriched tetrazole derivatives 4 and 5, respectively, in moderate to high yields. Moreover, taking advantage of the same zwitterionic intermediate I as a 1,3-dipole, an enantioselective formal [3+2] cycloaddition reaction with isoquinolines is also realized, furnishing the corresponding 1,2-dihydroisoquinolines in good to excellent results. This catalytic protocol also represents a rare example of asymmetric direct dearomatization of nonactivated isoquinoline39.
Results
Optimization of reaction conditions
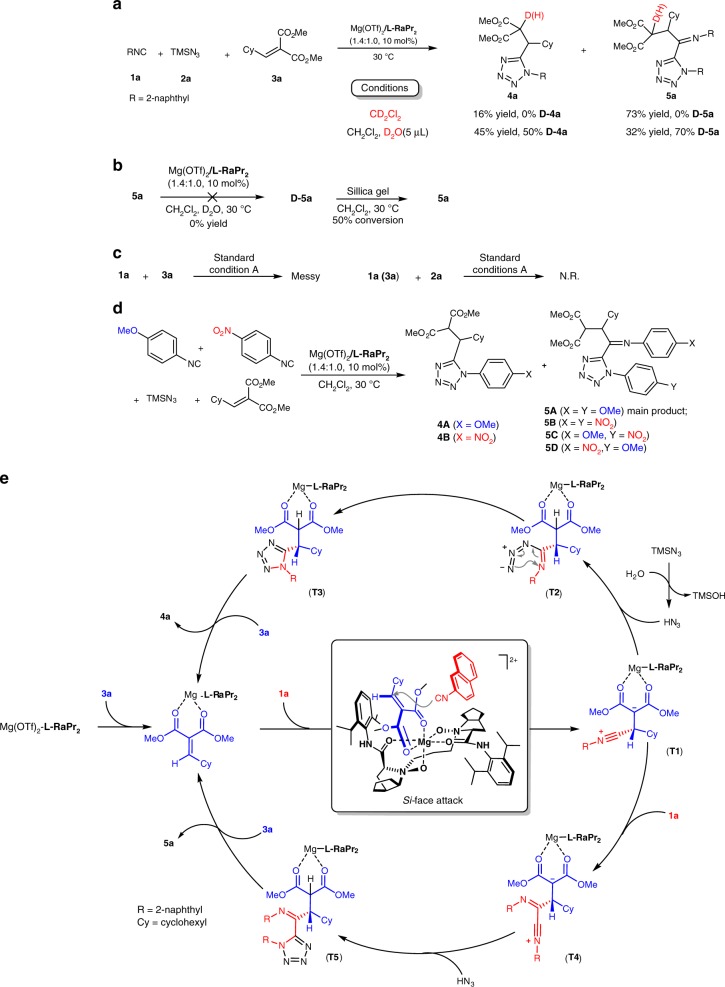
Our initial attempts involved using dimethyl 2-(cyclohexylmethylene) malonate as the model substrate together with 2-naphthyl isocyanide, TMSN3 to optimize the reaction conditions. First, the investigation of metal salts was carried out in the presence of chiral N,N′-dioxide ligand L-PrPr2. It was found that only Mg(OTf)2/L-PrPr2 complex could promote the reaction smoothly, affording two different 1,5-disubstituted tetrazole derivatives. One was assigned to be compound 4a via one molecular isocyanide-involved three-component reaction (29% yield, 82:18 e.r.) and the other was compound 5a incorporated bimolecular isonitrile via a four-component reaction (53% yield, 92.5:7.5 e.r. For details, see Supplementary Figs. 1–7). But messy mixture was obtained with metal salts as Sc(OTf)3 (for details, see Supplementary Table 1). Encouraged by these results, various chiral N,N′-dioxide ligands coordinating with Mg(OTf)2 were evaluated (Table 1, entries 1–4). Chiral N,N′-dioxide ligands had a significant effect on the distribution of the products and e.r. values. When employing L-pipecolic acid-derived L-PiPr2 as the ligand, interestingly, only four-component reaction product 5a was obtained in 97% yield with lower e.r. value (entry 2; 82:18 e.r. vs 92.5:7.5 e.r.). Changing the chiral backbone of the ligand to L-ramipril acid-derived L-RaPr2, higher e.r. values were achieved for both product 5a (46% yield, 96:4 e.r. vs 92.5:7.5 e.r.) and the product 4a (38% yield, 83.5:16.5 e.r. vs 82:18 e.r. For details, see Supplementary Table 2). No better results were afforded with further increase of the steric hindrance of aniline in ligand (entry 4). Next, other parameters were systematically screened. Noteworthily, the ratio of Mg(OTf)2 to L-RaPr2 displayed a considerable influence on the product distribution (entries 5–6). With excessive amount of metal salt (MgII/L-RaPr2 = 1.5/1.0), four-component product 5a was formed dominantly in 88% yield with 94:6 e.r. (entry 5). On the contrary, three-component product 4a prevails (entry 6; 53% yield with 88:12 e.r. for 4a, and 25% yield with 95.5:4.5 e.r. for 5a) with increasing the amount of L-RaPr2 (MgII/L-RaPr2 = 1.0/1.5). After fine-tuning, the optimized conditions for four-component reaction were set to be Mg(OTf)2/L-RaPr2 (1.4/1.0, 10 mol%) as the catalyst in CH2ClCH2Cl at 30 °C for 3 h, and the desired product 5a was provided in 91% yield with 94.5:5.5 e.r. (conditions A, entry 7). To our delight, it was found that four-component reaction was almost suppressed at lower reaction temperature (−40 °C), and the three-component product 4a was isolated as the main product (entry 8; 57% yield with 94.5:5.5 e.r.). However, the reactivity dropped dramatically and extending the reaction time was needed to get moderate yield (entry 9; 73% yield). Fortunately, the addition of 5 Å molecular sieve could accelerate the reaction without any impact on the enantioselectivity (entry 10; 91% yield with 95:5 e.r.). After adjustment of temperature and ratio of components, the optimized conditions for three-component product 4a entailed the use of the Mg(OTf)2/L-RaPr2 (1.0/1.5, 10 mol%) as the catalyst and 5 Å MS as the additive in CH2Cl2 at −40 °C for 2 days, then −20 °C for 3 days (conditions B, entry 10).
Table 1.
Optimization of the reaction conditions
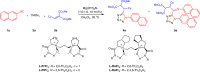
| |||||||
|---|---|---|---|---|---|---|---|
| 4a | 5a | ||||||
| entry | Ligand (L) | Mg(OTf)2: L | T (°C) | Yield (%)a | e.r.b | Yield (%)a | e.r.b |
| 1 | L-PrPr 2 | 1.0:1.0 | 35 | 29 | 82:18 | 53 | 92.5:7.5 |
| 2 | L-PiPr 2 | 1.0:1.0 | 35 | – | – | 97 | 82:18 |
| 3 | L-RaPr 2 | 1.0:1.0 | 35 | 38 | 83.5:16.5 | 46 | 96:4 |
| 4 | L-RaPr 3 | 1.0:1.0 | 35 | 36 | 72:28 | 43 | 86.5:13.5 |
| 5 | L-RaPr 2 | 1.5:1.0 | 35 | – | – | 88 | 94:6 |
| 6 | L-RaPr 2 | 1.0:1.5 | 35 | 53 | 88:12 | 25 | 95.5:4.5 |
| 7c | L-RaPr 2 | 1.4:1.0 | 30 | – | – | 91 | 94.5:5.5 |
| 8d | L-RaPr 2 | 1.0:1.5 | −40 | 57 | 94.5:5.5 | – | – |
| 9d,e | L-RaPr 2 | 1.0:1.5 | −40 | 73 | 95:5 | – | – |
| 10f | L-RaPr 2 | 1.0:1.5 | −40 then −20 | 91 | 95:5 | – | – |
Unless otherwise noted, all reactions were carried out with 1a (0.10 mmol), 2a (0.12 mmol), 3a (0.10 mmol), and Mg(OTf)2/L-RaPr2 (1.0:1.0, 10 mol%) in CH2Cl2 (1.0 mL) at 35 °C for 3 h
aIsolated yield
bDetermined by CSP-HPLC analysis
cCarried out with 1a (0.20 mmol), 2a (0.24 mmol), 3a (0.20 mmol), and Mg(OTf)2/L-RaPr2 (1.4:1.0, 10 mol%) in CH2ClCH2Cl (1.0 mL) at 30 °C for 3 h
d−40 °C for 7 days
eWith 5 Å MS (10 mg) as the additive
fCarried out with 1a (0.10 mmol), 2a (0.15 mmol), 3a (0.15 mmol), 5 Å MS (10 mg), and Mg(OTf)2/L-RaPr2 (1.0/1.5, 10 mol%) in CH2Cl2 (1.0 mL) at −40 °C for 2 days, then −20 °C for 3 days
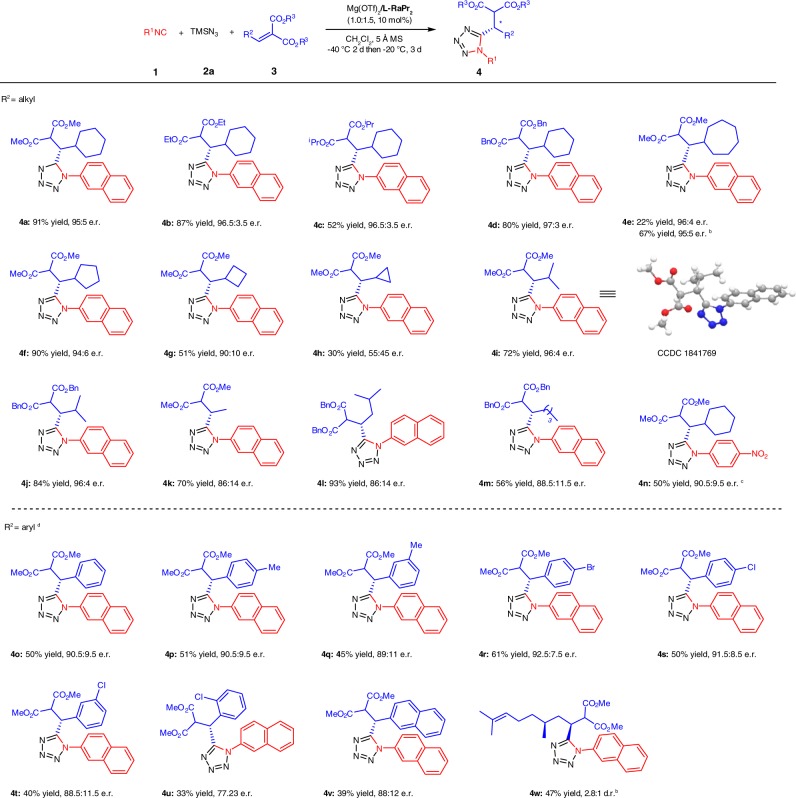
Substrate scope of three-component tetrazoles
With the standard reaction conditions in hand (Table 1, entry 10), the substrate scope of three-component reaction was explored. As depicted in Fig. 2, a wide range of alkylidene malonates were investigated. We first examined the effect of the ester moiety. Aside from dimethyl 2-(cyclohexylmethylene)malonate, diethyl, diisopropyl or dibenzyl 2-(cyclohexylmethylene)malonates were also suitable substrates, delivering the corresponding products 4a–4d with equally good enantioselectivities (95:5–97:3 e.r.). Nevertheless, the reactivity gradually decreased with the increase of steric hindrance of the ester group (52–91%). It was noteworthy that the size of ring of β-cyclic alkyl substituents showed an apparent influence on the reactivities and enantioselectivities (4e–4h). Of the rings investigated, seven-membered one led to the lowest yield (22%) and excellent enantioselectivity (96:4 e.r.). Raising the reaction temperature to −10 °C, a higher yield (67%) with maintained enantioselectivity was achieved for the product 4e. With the decrease of ring size from five-membered to three-membered, both yields and e.r. values of the products 4f–4h decreased remarkably. The substrates derived from isobutyl aldehyde, were found to be applicable in this reaction, yielding the corresponding products 4i and 4j in good yields (72 and 84%) and high enantioselectivities (96:4 e.r.). We also tested with other linear or branched aliphatic substituted ones, and the corresponding products 4k–4m were afforded in good to excellent yields (56–93%) with slightly lower enantioselectivities (86:14–88.5:11.5 e.r.). Next, the tolerance of isocyanide in three-component reaction was examined. Compared with 2-naphthyl isocyanide 1a, the reaction of 4-nitrophenyl isocyanide could also furnish the target product (4n) in medium yield (50%) and good enantioselectivity (90.5:9.5 e.r.) at 0 °C.
Fig. 2.
Substrate scope of asymmetric synthesis three-component tetrazoles. Unless otherwise noted, all reactions were carried out with 1 (0.10 mmol), 2a (0.15 mmol), 3 (0.15 mmol), 5 Å MS (10 mg), and Mg(OTf)2/L-RaPr2 (1.0/1.5, 10 mol%) in CH2Cl2 (1.0 mL) at −40 °C for 2 days, then −20 °C for 3 days. Isolated yield and e.r. was determined by CSP-HPLC analysis. bPerformed at −10 °C. cPerformed at 0 °C with Mg(OTf)2/L-RaPr2 (1.0/1.0, 10 mol%). dCarried out with 1 (0.10 mmol), 2a (0.15 mmol), 3 (0.10 mmol), H2O (5 µL), and Mg(OTf)2/L-RaPr2 (1.0/1.0, 10 mol%) in CH2Cl2 (1.0 mL) at 30 °C for 2 days
Initial attempt of using aromatic aldehyde-derived methylene malonates in the reaction system was unsuccessful and three-component product was obtained in extremely lower yield (for details, see Supplementary Table 5). It was interesting that the addition of water not only accelerated the rate of reaction but also suppressed four-component reaction process. Thus, different aryl-substituted alkylidene malonates were subjected to the reaction. The positions of substituent at aromatic ring of alkylidene malonate also significantly affected the outcomes of the reaction. Para-substituted substrates gave the desired products (4p, 4r, and 4s) with both higher yields and enantioselectivities than those of ortho- or meta-substituted ones (4q, 4t, and 4u). Fused-ring-substituted alkylidene malonate provided the corresponding product (4v) in 39% yield and 88:12 e.r. (–)-Citronellal-derived substrate yielded the corresponding product 4w in 47% yield and 2.8:1 d.r. at −10 °C. The absolute configuration of the product 4i was explicitly determined to be S-configuration by single-crystal X-ray diffraction analysis.
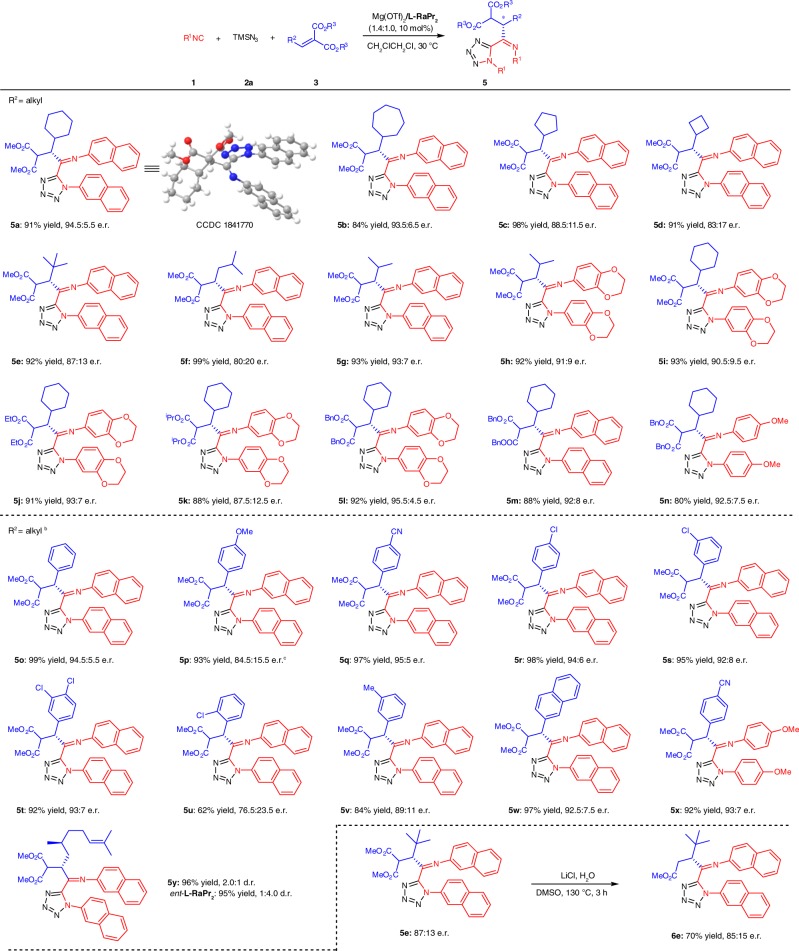
Substrate scope of four-component tetrazoles
Having established the optimized conditions for the synthesis of four-component products (Table 1, entry 7), we evaluated the generality of this transformation (Fig. 3). The ring size of alkylidene malonates appeared to have a similar influence on the enantioselectivities with that of three-component reaction, and the substrates with smaller rings were prone to give lower enantioselectivities (5a–5d). Other branched aldehyde-derived substrates were suitable as well to afford the desired product 5e–5h in high yields (92–99% yield) with moderate to good e.r. values (80:20–93:7 e.r.). In addition, the ester group in alkylidene malonates could be extended to others (5i–5l). Highest e.r. value was obtained with Bn group. It should be pointed out that the aromatic aldehyde-derived substrates were applicable, delivering the corresponding products (5o–5x) in good yields, however, the enantioselectivities depended on the electronic nature and position of the substituent on aryl group of the alkylidene malonates. Higher enantioselectivities were observed with substrates containing electron-withdrawing groups than those containing electron-donating ones (5o–5v). Similarly, compared with the meta- and ortho-substituted substrates, para-substituted ones gave the desired products with higher enantioselectivities (5r–5u). Naphthyl substituted alkylidene malonate was also tolerated to yield the product (5w) in good results. Regarding to (–)-Citronellal-derived substrate, higher diastereoselectivity was obtained with ent-L-RaPr2 as ligand (1:4.0 d.r. vs 2.0:1 d.r.). Notably, the screening of isocyanides indicated that the electronic properties of substituents on aromatic rings of isocyanide displayed a profound effect on the course of the reaction (shown in 5l–5n), and replacing the electron-donating substituents with the electron-withdrawing group at para-position of the phenyl ring, for example, nitro group, only three-component product was detected rather than the desired four-component product. The structure of the racemic adduct s-trans-5a was confirmed by single-crystal X-ray diffraction analysis. Next, derivatization of the product 5e was conducted. One of the ester groups could be easily removed in the presence of LiCl and H2O with maintained enantioselectivity (6e; 70% yield, 85:15 e.r.). It is worth noting that imide moiety was very stable even under high temperature.
Fig. 3.
Substrate scope of asymmetric synthesis four-component tetrazoles. Unless otherwise noted, all reactions were carried out with 1 (0.20 mmol), 2a (0.24 mmol), 3 (0.20 mmol), and Mg(OTf)2/L-RaPr2 (1.4/1.0, 10 mol%) in CH2ClCH2Cl (1.0 mL) at 30 °C for 3 h. Isolated yield and e.r. was determined by CSP-HPLC analysis. bCarried out with 1 (0.20 mmol), 2a (0.20 mmol), 3 (0.15 mmol). c20 mol% of NaBArF4 {sodium tetrakis[3,5-bis(trifluoromethyl)phenyl]borate} was added
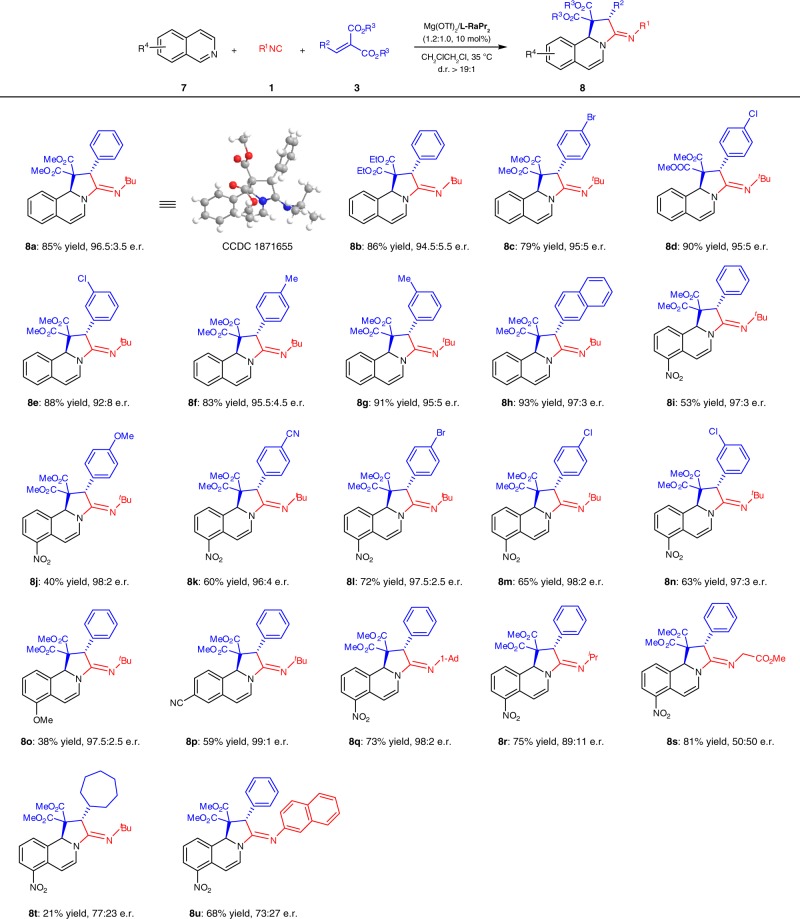
Substrate scope of 1,2-dihydroisoquinoline derivatives
As illustrated in Fig. 1b, the nucleophilic addition of isocyanides to polar C=X or C=C bonds affords zwitterionic intermediates, which theoretically could be used as 1,3-dipoles. However, comparing with classic Passerini and Ugi reactions, where these zwitterionic intermediates were captured by carboxylic acid, the utility of these species in cycloaddition reactions is rather limited. To the best of our knowledge, only sporadic racemic versions of such reactions were reported to date23–25. Encouraged by the good performance of our chiral MgII-N,N′-dioxide Lewis acid catalyst for the synthesis of 1,5-disubstituted tetrazole derivatives, we assumed if the zwitterionic intermediate I was captured by imine, another hetereo-cyclic amidine-type of product could be obtained. Many kinds of combinations with different electrophiles, isocyanides, and dipolarophiles were tested. The trick is how to control a cascade of elementary chemical reactions occurring in a uniquely ordered manner, since two reactants are electrophiles. It should be a better choice if the reactivity of dipolarophile is lower than the electrophile for the isocyanide-initiated addition step. Finally, phenyl-substituted alkylidene malonate 3o, isoquinoline 7a, and tert-butyl isocyanide 1e were employed in the model reaction to optimize the reaction conditions. We are glad to find that by slight modification the four-component reaction conditions as Mg(OTf)2/L-RaPr2 (1.2/1.0, 10 mol%) in CH2ClCH2Cl (0.5 mL) at 35 °C for 2 days, the desired cycloaddition product 8a was produced as a single diastereomer in 85% yield with 96.5:3.5 e.r. (for details, see Supplementary Tables 7–9). Under such conditions, the substrates with respect to alkylidene malonates were evaluated. As shown in Fig. 4, a broad range of alkylidene malonates bearing different ester groups or β-aryl substituents reacted smoothly to deliver the corresponding products with good yields and high enantioselectivities (8a–8h; 79–93% yield, 92:8–97:3 e.r.). Comparatively, aliphatic aldehyde-derived alkylidene malonate was less efficient, yielding 8t in much lower yield and enantioselectivity (21% yield, 77:23 e.r.). On the other hand, other substituted isoquinolines were suitable as well regardless of electronic nature of the substitutions. The desired cycloaddition products (8i–8p) were isolated in excellent enantioselectivities (96:4–99:1 e.r.) with slightly lower yields (40–72%). Next, the screening of isocyanides implied that the steric hindrance exhibited a considerable effect on the e.r. values of the product; the more bulk 1-adamantanyl isocyanide gave 98:2 e.r., while the less bulk isocyanides afford the product (8r and 8s) with much lower enantioselectivities. In addition, switching to aromatic isocyanide resulted in a rapid drop of e.r. value (8r). The absolute configuration of the product 8a was determined to be (2S, 10bS, E) by single-crystal X-ray diffraction analysis. It should be noted that this transformation was a simultaneous dearomative process of isoquinolines, delivering fused polycyclic 1,2-dihydroisoquinoline-based amidine derivatives with a single diastereomer in all cases.
Fig. 4.
Substrate scope of asymmetric synthesis 1,2-dihydroisoquinoline derivatives. All reactions were carried out 7 (0.10 mmol), 1 (0.15 mmol), 3 (0.15 mmol), and Mg(OTf)2/L-RaPr2 (1.2:1.0, 10 mol%) in CH2ClCH2Cl (0.5 mL) at 35 °C for 2 days. Isolated yield and e.r. was determined by CSP-HPLC analysis
Control experiments and proposed catalytic cycle
In order to get insight into the reaction mechanism, we carried out several control experiments. The first one was deuterium experiment under four-component reaction conditions (Table 1, entry 7) to understand the source of proton. As reflected in Fig. 5a, when deuterated dichloromethane was used as the solvent, the four-component product 5a without deuterium was obtained as the major product (4a: 16% yield, 5a: 73% yield). While the addition of a small amount of D2O (5 μL) dramatically affected the ratio of two products. In this case, three-component product 4a (with 50% deuterium) was isolated as the main product instead of 5a (with 70% deuterium). In addition, the percent of deuterated 5a decreased from 70 to 35% by treatment with silicone gel (Fig. 5b). These results not only indicated that the proton came from a trace amount of water or work up with silicone gel but also highlighted the remarkable influence of water on the pathway. With this observation, HN3 was supposed to be involved in both three-component reaction pathway and four-component reaction pathway. Next, we evaluated the possible transformations of each two reactants. It showed that in the presence of a chiral Lewis acid catalyst, no reaction occurred from TMSN3 2a with isocyanide 1a or alkylidene malonate 3a. Isocyanide 1a could react with 3a rapidly, generating a messy mixture. These results implied that the reaction is triggered by the addition of isocyanide 1a to 3a catalyzed by chiral Lewis acid. Furthermore, we subjected an equal amount of two electron-different isocyanides for the multicomponent reaction, and the characteristic signal of main product 5A was detected by NMR analysis and the molecular ion peaks of other products 4A, 4B, 5B, 5C, and 5D can be detected by high resolution time of flight mass spectrometry (Fig. 5c). The cross-reaction products 5C and 5D confirmed again that the intermediate generated from the addition of isocyanide to alkylidene involved in the initial step of both three-component reaction and four-component reaction.
Fig. 5.
Control experiments and proposed catalytic cycle. a Deuterium labeling experiments. b Control experiments for 5a and D-5a. c Control experiments for two materials. d NMR spectra and HRMS for reaction involving two different isocyanides. e Proposed catalytic cycle for IMCRs
Based on the control experiments and the previous works27,40,41, a possible catalytic cycle along with a transition-state model were proposed to elucidate the reaction processes and the chiral induction. As depicted in Fig. 5e, at the outset of the reaction, the N,N′-dioxide L-RaPr2 and the bidentate alkylidene malonate 3a coordinated to the MgII center to form a complex with octahedral geometry. Since the Re face of alkylidene malonate 3a was shielded by the bulk neighboring amide group of the ligand, 2-naphthyl isocyanide 1a attacked the Si face of 3a to form the intermediate T1, which could undergo two different pathways. One is three-component reaction process, in which the intermediate T1 was trapped HN3 formed in situ42 to give the intermediate T2, which subsequently underwent cyclization to afford T3. Then, the catalyst released from the product 4a to close this catalytic cycle. The other four-component process started from the intermediate T1, trapping by another molecule of 2-naphthyl isocyanide 1a to afford the zwitterionic Intermediate T4, which was subsequently reacted with TMSN3 or HN3 in a similar manner to yield the corresponding tetrazole product 5a.
As showing in Fig. 5e, the reaction pathways were determined by the trap of the intermediate T1, with either N3− or another molecule of isocyanide. Regarding our experimental outcomes, several factors were found to be crucial. The first one is the electronic nature of isocyanides. According to the observations mentioned in Figs. 2 and 3, electron-rich isocyanides with higher nucleophilicity were prone to underwent four-component reaction, whereas electron-poor isocyanides is favorable for three-component reaction. The reaction conditions were also critical. The addition of either N,N′-dioxide L-RaPr2 or water is conducive to three-component reaction. In this process, excess amount of the N,N′-dioxide L-RaPr2 could serve as a Lewis base to activated TMSN3 by coordination, which facilitated the nucleophilic addition of N3− to intermediate T1. In addition, in the presence of water, HN3 was formed in situ, which was proved to be an efficient strategy in Passerini tetrazole reaction27,43. Regarding to excessive metal-promoted four-component reaction pathway, we also screened MgII with other counteranions (ClO4− or NTf−) or other metal salts [LiOTf, NaOTf, and Ca(OTf)2], it seemed that OTf− anion was beneficial to four-component reaction37.
Discussion
Triggered by the enantioselective addition of isocyanides to C=C bonds, several enantioselective isocyanide-based multicomponent reactions were realized by using chiral MgII-N,N′-dioxide Lewis acid catalyst, providing a direct and efficient access to highly valuable chiral 1,5-disubstituted tetrazoles and 1,2-dihydroisoquinolines. On one hand, we accomplished the asymmetric MCRs of isocyanides, TMSN3 with alkylidene malonates. With modification of reaction conditions, both one molecule of isocyanide insertion process and two molecules of isocyanide incorporated pathway could be accurately regulated, furnishing two types of enantioenriched 1,5-disubstituted tetrazoles with good outcomes. On the other hand, the zwitterionic intermediate resulted from the addition of isocyanides to C=C bonds was successfully employed as a 1,3-dipole in the example of enantioselective dearomative [3+2] annulation reaction of isoquinolines, affording chiral fused polycyclic 1,2-dihydroisoquinoline-based amidine derivatives in good to excellent results (up to 93% yield, >19:1 d.r., 99:1 e.r.). It is expected that our findings in this work could stimulate further exploration in asymmetric IMCRs. Further studies on applying this catalyst system to other IMCRs are currently underway in our lab.
Methods
General procedure for asymmetric alkyl substituted three-component tetrazoles
To an oven-dried tube under nitrogen atmosphere were added Mg(OTf)2 (0.010 mmol, 10 mol%), L-RaPr2 (0.015 mmol, 15 mol%), 2-(cyclohexylmethylene)malonate 3a (0.15 mmol), 5 Å MS (10 mg), and CH2Cl2 (1.0 mL). The mixture was stirred in CH2Cl2 (1.0 mL) at 35 °C for 0.5 h. Subsequently, TMSN3 2a (0.15 mmol) and 2-naphthyl isocyanide 1a (0.10 mmol) were added at −40 °C. The mixture was stirred at −40 °C for 2 days then at −20 °C for 3 days, and directly purified by flash chromatography on silica gel (eluent: petroleum ether/ethyl acetate = 9/1) to afford the desired product 4a (91% yield, 95:5 e.r.).
General procedure for asymmetric aryl substituted three-component tetrazoles
To an oven-dried tube under nitrogen atmosphere were added Mg(OTf)2 (0.010 mmol, 10 mol%), L-RaPr2 (0.010 mmol, 10 mol%), dimethyl 2-benzylidenemalonate 3o (0.10 mmol), 5 μL H2O, and CH2Cl2 (1.0 mL). The mixture was stirred in CH2Cl2 (1.0 mL) at 35 °C for 0.5 h. Subsequently, TMSN3 2a (0.15 mmol) and 2-naphthyl isocyanide 1a (0.10 mmol) were added at 30 °C. The mixture was stirred at 30 °C for 48 h, and directly purified by flash chromatography on silica gel (eluent: petroleum ether/ethyl acetate = 9/1) to afford the desired product 4o (50% yield, 90.5:9.5 e.r.).
General procedure for asymmetric four-component tetrazoles
To an oven-dried tube under nitrogen atmosphere were added Mg(OTf)2 (0.014 mmol, 14 mol%), L-RaPr2 (0.010 mmol, 10 mol%), 2-(cyclohexylmethylene)malonate 3a (0.20 mmol), and CH2ClCH2Cl (1.0 mL). The mixture were stirred in CH2ClCH2Cl (1.0 mL) at 35 °C for 0.5 h. Subsequently, TMSN3 2a (0.24 mmol) and 2-naphthyl isocyanide 1a (0.10 mmol) were added at 30 °C. The mixture was stirred at 30 °C for 3 h, and directly purified by flash chromatography on silica gel (eluent: petroleum ether/ethyl acetate = 9/1) to afford the desired product 5a (91% yield, 94.5:5.5 e.r.).
General procedure for asymmetric 1,2-dihydroisoquinoline derivatives
To an oven-dried tube were added Mg(OTf)2 (0.012 mmol, 12 mol%), L-RaPr2 (0.010 mmol, 10 mol%), dimethyl 2-benzylidenemalonate 3o (0.15 mmol), and CH2ClCH2Cl (0.5 mL). The mixture was stirred in CH2ClCH2Cl (0.5 mL) at 35 °C for 0.5 h. Subsequently, isoquinoline 6a (0.10 mmol) and tert-butyl isocyanide 7a (0.15 mmol) were added at 35 °C. The mixture was stirred at 35 °C for 48 h, and directly purified by flash chromatography on silica gel (eluent: petroleum ether/ethyl acetate = 9/1) to afford the desired product 8a (85% yield, 96.5:3.5 e.r.).
Supplementary information
Acknowledgements
The authors acknowledgements financial support from the National Natural Science Foundation of China (grant nos. 21890723, 21772127, and 21625205), and the National Program for Support of Top-Notch Young Professionals, and the Fundamental Research Funds for the Central Universities.
Author contributions
Q.X. performed the experiments. Y.S.C. repeated data. S.X.D. participated in structure characterization and discussion. X.M.F. and X.H.L. supervised the project. X.M.F., X.H.L., S.X.D., and Q.X. co-wrote the manuscript.
Data availability
The X-ray crystallographic coordinates for structures 4i, 5a, and 8a reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers 1841769, 1841770, and 1871655. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. All other data are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Journal peer review information: Nature Communications thanks Santanu Mukherjee, Michael Doyle and other anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xiaohua Liu, Email: liuxh@scu.edu.cn.
Xiaoming Feng, Email: xmfeng@scu.edu.cn.
Supplementary information
Supplementary Information accompanies this paper at 10.1038/s41467-019-09904-5.
References
- 1.Mordini A, Reginato G, Calamante M, Zani L. Two- and three-dimensional rings in drugs. Chem. Biol. Drug. Des. 2014;83:450–461. doi: 10.1111/cbdd.12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Properzi R, Marcantoni E. Construction of heterocyclic structures by trivalent cerium salts promoted bond forming reactions. Chem. Soc. Rev. 2014;43:779–791. doi: 10.1039/C3CS60220F. [DOI] [PubMed] [Google Scholar]
- 3.Vitaku E, Smith DT, Njardarson JT. Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among U.S. FDA approved pharmaceuticals. J. Med. Chem. 2014;57:10257–10274. doi: 10.1021/jm501100b. [DOI] [PubMed] [Google Scholar]
- 4.Wittenberger SJ. Recent developments in tetrazole chemistry. A review. Org. Prep. Proced. Int. 1994;26:499–531. doi: 10.1080/00304949409458050. [DOI] [Google Scholar]
- 5.Herr RJ. 5-Substituted-1H-tetrazoles as carboxylic acid isosteres: medicinal chemistry and synthetic methods. Bioorg. Med. Chem. 2002;10:3379–3393. doi: 10.1016/S0968-0896(02)00239-0. [DOI] [PubMed] [Google Scholar]
- 6.Sarvary A, Maleki A. A review of syntheses of 1,5-disubstituted tetrazole derivatives. Mol. Divers. 2015;19:189–212. doi: 10.1007/s11030-014-9553-3. [DOI] [PubMed] [Google Scholar]
- 7.Zabrocki J, Smith GD, Dunbar JB, Ijima H, Marshall GR. Conformational mimicry. 1. 1,5-Disubstituted tetrazole ring as a surrogate for the cis amide bond. J. Am. Chem. Soc. 1988;110:5875–5880. doi: 10.1021/ja00225a045. [DOI] [Google Scholar]
- 8.Ahmad VU, Rahman A, Rasheed T, Rehman H. Jamtine-N-oxide-a new isoquinoline alkaloid from Cocculus hirsutus. Heterocycles. 1987;26:1251–1255. doi: 10.3987/R-1987-07-1841. [DOI] [Google Scholar]
- 9.Ahmad VU, Iqbal S. Haiderine, a new isoquinoline alkaloid from Cocculus hirsutus. Nat. Prod. Lett. 1993;2:105–109. doi: 10.1080/10575639308043793. [DOI] [Google Scholar]
- 10.Ito K, Haruna M, Jinno Y, Furukawa H. Studies on the Erythrina alkaloids. XI. Alkaloids of Erythrina crystagalli LINN. Structure of a new alkaloid, crystamidine. Chem. Pharm. Bull. 1976;24:52–55. doi: 10.1248/cpb.24.52. [DOI] [Google Scholar]
- 11.Chrzanowska M, Rozwadowska MD. Asymmetric synthesis of isoquinoline alkaloids. Chem. Rev. 2004;104:3341–3370. doi: 10.1021/cr030692k. [DOI] [PubMed] [Google Scholar]
- 12.Chrzanowska M, Grajewska A, Rozwadowska MD. Asymmetric synthesis of isoquinoline alkaloids: 2004–2015. Chem. Rev. 2016;116:12369–12465. doi: 10.1021/acs.chemrev.6b00315. [DOI] [PubMed] [Google Scholar]
- 13.Koldobskii GI. Strategies and prospects in functionalization of tetrazoles. Russ. J. Org. Chem. 2006;42:469–486. doi: 10.1134/S1070428006040014. [DOI] [Google Scholar]
- 14.Roh J, Vávrová K, Hrabálek A. Synthesis and functionalization of 5-substituted tetrazoles. Eur. J. Org. Chem. 2012;2012:6101–6118. doi: 10.1002/ejoc.201200469. [DOI] [Google Scholar]
- 15.Wang Q, Wang DX, Wang MX, Zhu J. Still unconquered: enantioselective Passerini and Ugi multicomponent reactions. Acc. Chem. Res. 2018;51:1290–1300. doi: 10.1021/acs.accounts.8b00105. [DOI] [PubMed] [Google Scholar]
- 16.Dömling A. Recent developments in isocyanide based multicomponent reactions in applied chemistry. Chem. Rev. 2006;106:17–89. doi: 10.1021/cr0505728. [DOI] [PubMed] [Google Scholar]
- 17.Gulevich AV, Zhdanko AG, Orru RVA, Nenajdenko VG. Isocyanoacetate derivatives: synthesis, reactivity, and application. Chem. Rev. 2010;110:5235–5331. doi: 10.1021/cr900411f. [DOI] [PubMed] [Google Scholar]
- 18.Qiu G, Ding Q, Wu J. Recent advances in isocyanide insertion chemistry. Chem. Soc. Rev. 2013;42:5257–5269. doi: 10.1039/c3cs35507a. [DOI] [PubMed] [Google Scholar]
- 19.Giustiniano M, et al. To each his own: isonitriles for all flavors. Functionalized isocyanides as valuable tools in organic synthesis. Chem. Soc. Rev. 2017;46:1295–1357. doi: 10.1039/C6CS00444J. [DOI] [PubMed] [Google Scholar]
- 20.Sadjadi S, Heravi MM. Recent application of isocyanides in synthesis of heterocycles. Tetrahedron. 2011;67:2707–2752. doi: 10.1016/j.tet.2011.01.086. [DOI] [Google Scholar]
- 21.Sadjadi S, Heravi MM, Nazari N. Isocyanide-based multicomponent reactions in the synthesis of heterocycles. RSC Adv. 2016;6:53203–53272. doi: 10.1039/C6RA02143C. [DOI] [Google Scholar]
- 22.Maleki A, Sarvary A. Synthesis of tetrazoles via isocyanide-based reactions. RSC Adv. 2015;5:60938–60955. doi: 10.1039/C5RA11531K. [DOI] [Google Scholar]
- 23.Mironov MA, Mokrushin VS, Maltsev SS. New method for the combinatorial search of multi component reactions. Synlett. 2003;7:943–946. doi: 10.1055/s-2003-39298. [DOI] [Google Scholar]
- 24.Li M, Qiu ZX, Wen LR, Zhou ZM. Novel regio- and stereo-selectivity: synthesis of dihydropyrrolo[1,2-f] phenanthridines via isocyanide-based multicomponent reaction. Tetrahedron. 2011;67:3638–3648. doi: 10.1016/j.tet.2011.03.085. [DOI] [Google Scholar]
- 25.Sagırli K, Dürüst Y, Kariuki B, Knight DW. A practical isocyanide-based multicomponent synthesis of polysubstituted cyclopentenes. Tetrahedron. 2013;69:69–72. doi: 10.1016/j.tet.2012.10.065. [DOI] [Google Scholar]
- 26.Zhang J, et al. Asymmetric phosphoric acid–catalyzed four-component Ugi reaction. Science. 2018;361:1072–1073. doi: 10.1126/science.aau7497. [DOI] [PubMed] [Google Scholar]
- 27.Yue T, Wang MX, Wang DX, Zhu J. Asymmetric synthesis of 5-(1-hydroxyalkyl)tetrazoles by catalytic enantioselective Passerini-type reactions. Angew. Chem. Int. Ed. 2008;47:9454–9457. doi: 10.1002/anie.200804213. [DOI] [PubMed] [Google Scholar]
- 28.Xu X, Zavalij PZ, Doyle MP. Catalytic asymmetric syntheses of quinolizidines by dirhodium-catalyzed dearomatization of isoquinolinium/pyridinium methylides—the role of catalyst and carbene source. J. Am. Chem. Soc. 2013;135:12439–12447. doi: 10.1021/ja406482q. [DOI] [PubMed] [Google Scholar]
- 29.Guo C, Fleige M, Janssen-Müller D, Daniliuc CG, Glorius F. Switchable selectivity in an NHC-catalysed dearomatizing annulation reaction. Nat. Chem. 2015;7:842–847. doi: 10.1038/nchem.2337. [DOI] [PubMed] [Google Scholar]
- 30.Xu YL, et al. Catalytic asymmetric inverse-electron demand 1,3-dipolar cycloaddition of isoquinolinium methylides with enecarbamates by a chiral N,N’‑dioxide/Ag(I) complex. ACS Catal. 2016;6:589–592. doi: 10.1021/acscatal.5b02178. [DOI] [Google Scholar]
- 31.Liu XH, Lin LL, Feng XM. Chiral N,N’-dioxides: new ligands and organocatalysts for catalytic asymmetric reactions. Acc. Chem. Res. 2011;44:574–587. doi: 10.1021/ar200015s. [DOI] [PubMed] [Google Scholar]
- 32.Liu XH, Lin LL, Feng XM. Chiral N,N’-dioxide ligands: synthesis, coordination chemistry and asymmetric catalysis. Org. Chem. Front. 2014;1:298–302. doi: 10.1039/c3qo00059a. [DOI] [Google Scholar]
- 33.Liu XH, Zheng HF, Xia Y, Lin LL, Feng XM. Asymmetric cycloaddition and cyclization reactions catalyzed by chiral N,N’-dioxide-metal complexes. Acc. Chem. Res. 2017;50:2621–2631. doi: 10.1021/acs.accounts.7b00377. [DOI] [PubMed] [Google Scholar]
- 34.Zheng K, Liu XH, Feng XM. Recent advances in metal-catalyzed asymmetric 1,4-conjugate addition (ACA) of nonorganometallic nucleophiles. Chem. Rev. 2018;118:7586–7656. doi: 10.1021/acs.chemrev.7b00692. [DOI] [PubMed] [Google Scholar]
- 35.Chatani N, Oshita M, Tobisu M, Ishii Y, Murai S. A GaCl3-catalyzed [4+1] cycloaddition of α,β-unsaturated carbonyl compounds and isocyanides leading to unsaturated γ-lactone derivatives. J. Am. Chem. Soc. 2003;125:7812–7813. doi: 10.1021/ja035014u. [DOI] [PubMed] [Google Scholar]
- 36.Winkler JD, Asselin SM. Synthesis of novel heterocyclic structures via reaction of isocyanides with s-trans-enones. Org. Lett. 2006;8:3975–3977. doi: 10.1021/ol061451c. [DOI] [PubMed] [Google Scholar]
- 37.Tobisu M, et al. Brønsted acid catalyzed formal insertion of isocyanides into a C–O bond of acetals. J. Am. Chem. Soc. 2007;129:11431–11437. doi: 10.1021/ja073286h. [DOI] [PubMed] [Google Scholar]
- 38.Soeta T, Miyamoto Y, Fujinami S, Ukaji Y. The Lewis acid-catalyzed [3+1+1] cycloaddition of azomethine ylides with isocyanides. Tetrahedron. 2014;70:6623–6629. doi: 10.1016/j.tet.2014.06.118. [DOI] [Google Scholar]
- 39.Preindl J, Chakrabarty S, Waser J. Dearomatization of electron poor six-membered N-heterocycles through [3+2] annulation with aminocyclopropanes. Chem. Sci. 2017;8:7112–7118. doi: 10.1039/C7SC03197A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao XH, et al. Asymmetric dearomatization of indoles through a michael/friedel-crafts-type cascade to construct polycyclic spiroindolines. Angew. Chem. Int. Ed. 2015;54:4032–4035. doi: 10.1002/anie.201410814. [DOI] [PubMed] [Google Scholar]
- 41.Luo WW, et al. A N,N’-dioxide/Mg(OTf)2 complex catalyzed enantioselective α-addition of isocyanides to alkylidene malonates. Chem. Sci. 2016;7:4736–4740. doi: 10.1039/C6SC00689B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yudin AK. Aziridines and Epoxides in Organic Synthesis, Weinheim: (Wiley-VHC; 2006. [Google Scholar]
- 43.Pharande SG, Escobosa ARC, Gámez-Montaño R. Endogenous water-triggered and ultrasound accelerated synthesis of 1,5-disubstituted tetrazoles via a solvent and catalyst-free Ugi-azide reaction. Green. Chem. 2017;19:1259–1262. doi: 10.1039/C6GC03324E. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The X-ray crystallographic coordinates for structures 4i, 5a, and 8a reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers 1841769, 1841770, and 1871655. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. All other data are available from the corresponding author upon reasonable request.