Host-adapted bacterial pathogens such as NTHi cannot survive out of their host environment and have evolved host-specific mechanisms to obtain nutrients and evade the immune response. Relatively few of these host adaptations have been characterized at the molecular level. NTHi utilizes sialic acid as a nutrient and also incorporates this sugar into LOS, which is important in biofilm formation and immune evasion. In the present study, we showed that NTHi has evolved to preferentially utilize the Neu5Ac form of sialic acid. This adaptation is due to the substrate preference of the enzyme CMP-Neu5Ac synthetase, which synthesizes the activated form of Neu5Ac for macromolecule biosynthesis. This adaptation allows NTHi to evade killing by a human antibody response against the nonhuman sialic acid Neu5Gc.
KEYWORDS: Haemophilus influenzae, bacterial metabolism, glycobiology, microbial pathogenesis, sialic acid
ABSTRACT
Nontypeable Haemophilus influenzae (NTHi) is a Gram-negative bacterial pathogen that is adapted exclusively to human hosts. NTHi utilizes sialic acid from the host as a carbon source and as a terminal sugar on the outer membrane glycolipid lipooligosaccharide (LOS). Sialic acid expressed on LOS is critical in NTHi biofilm formation and immune evasion. There are two major forms of sialic acids in most mammals, N-acetylneuraminic acid (Neu5Ac) and N-glycolylneuraminic acid (Neu5Gc), the latter of which is derived from Neu5Ac. Humans lack the enzyme to convert Neu5Ac to Neu5Gc and do not express Neu5Gc in normal tissues; instead, Neu5Gc is recognized as a foreign antigen. A recent study showed that dietary Neu5Gc can be acquired by NTHi colonizing humans and then presented on LOS, which acts as an antigen for the initial induction of anti-Neu5Gc antibodies. Here we examined Neu5Gc uptake and presentation on NTHi LOS. We show that, although Neu5Gc and Neu5Ac are utilized equally well as sole carbon sources, Neu5Gc is not incorporated efficiently into LOS. When equal amounts of Neu5Gc and Neu5Ac are provided in culture media, there is ∼4-fold more Neu5Ac incorporated into LOS, suggesting a bias in a step of the LOS biosynthetic pathway. CMP-Neu5Ac synthetase (SiaB) was shown to have ∼4,000-fold-higher catalytic efficiency for Neu5Ac than for Neu5Gc. These data suggest that NTHi has adapted preferential utilization of Neu5Ac, thus avoiding presentation of the nonhuman Neu5Gc in the bacterial cell surface. The selective pressure for this adaptation may represent the human antibody response to the Neu5Gc xenoantigen.
INTRODUCTION
Haemophilus influenzae is a host-adapted human pathogen that is categorized into typeable strains that express a polysaccharide capsule (serotypes a to f) and nontypeable (noncapsulated) H. influenzae (NTHi) strains (1). H. influenzae is carried asymptomatically in the upper respiratory tract of 40% to 80% of healthy humans (2). Capsulated H. influenzae strains typically cause invasive diseases such as meningitis, while NTHi strains are responsible for acute and chronic infections of the respiratory tract, such as middle ear infection in children (3), exacerbations of chronic obstructive pulmonary disease (COPD) in the elderly (4), and community-acquired pneumonia (5). Since the introduction of a vaccine against H. influenzae serotype b (Hib), the incidence of invasive infection caused by NTHi has increased significantly worldwide (6, 7). NTHi is now a major cause of severe invasive disease in neonates and in children who have significant comorbidities (8, 9). Invasive NTHi infections are fatal in ∼10% of children between 2 and 4 years of age and in ∼17% of children under the age of 1 (10, 11). The increase in invasive disease caused by NTHi is likely due to many factors, including increased numbers of vulnerable patient populations, rather than being solely due to Hib vaccine-induced strain replacement (6).
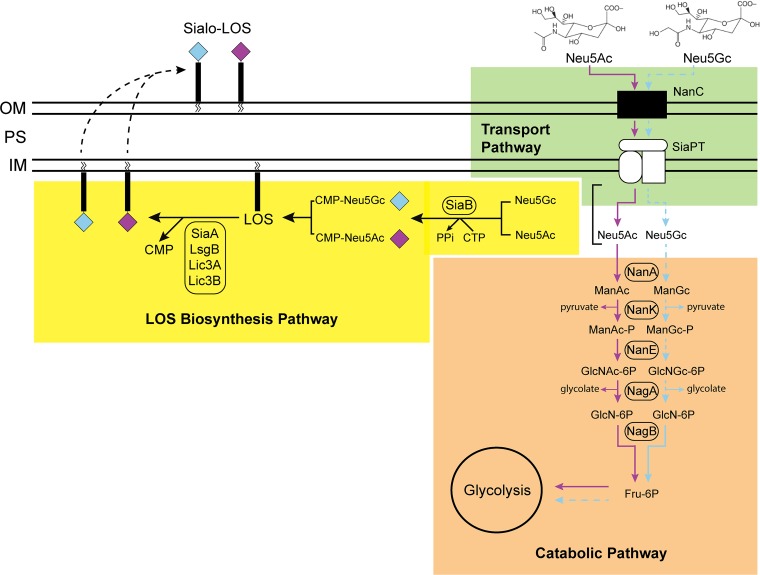
Sialic acids are a diverse group of carboxylated nine carbon-backbone sugars (12, 13) with N-glycolylneuraminic acid (Neu5Gc) and its precursor N-acetylneuraminic acid (Neu5Ac) being the two most abundant sialic acids found on the mammalian cell surface. Neu5Gc is present in many mammals, including the great apes, with one notable exception being humans (14, 15), owing to a mutation in the CMP-Neu5Ac hydroxylase enzyme CMAH (16–19), with the result that humans produce only Neu5Ac. Absence of Neu5Gc in humans was first discovered after investigation of the basis for “serum sickness,” a condition that results from the presence of Hanganutziu-Deicher (HD) antibodies (anti-Neu5Gc antibodies), which recognize Neu5Gc antigens in animal serum administered to human patients (20–22). Neu5Gc is present at very low or nondetectable levels in normal human body fluids and on tissue surfaces (18) but can be found in certain cancers (23–29), and anti-Neu5Gc antibodies have been proposed to be a biomarker to detect cancer (30, 31). However, recent studies have revealed Neu5Gc to be present in or on more human tissue types than initially thought, such as vascular endothelium (32), carcinomas (33), placental tissues (34), epithelial cells lining body organs (27), gangliosides (35) and on endothelia during vascular inflammation leading to atherosclerosis (32). It is thought that Neu5Gc from dietary sources such as red meat and dairy products is the source of this xenosugar on normal human tissue (27, 36). However, incorporation of Neu5Gc onto the cell surface has been demonstrated to mediate chronic inflammation as a result of the presence of anti-Neu5Gc antibodies in human serum, causing “xenosialitis” (37, 38). The source of these anti-Neu5Gc antibodies has been proposed to be NTHi colonization during infancy (39), as NTHi is able to decorate the termini of its LOS with sialic acids. This molecular mimicry of the host allows immune evasion (40, 41) and is also required for virulence (42) and biofilm formation (41, 43). NTHi cannot synthesize sialic acids and must scavenge exogenous sialic acid from the host (44). If Neu5Gc is present in the host from the diet, it may be acquired by NTHi along with Neu5Ac and either be incorporated into the LOS through the LOS biosynthetic pathway via the CMP-Neu5Ac synthetase, namely, sialic acid synthetase (SiaB), or serve as a carbon source via the catabolic pathway encoded by the nan genes (Fig. 1) (45).
FIG 1.
Overview of the sialic acid catabolic and LOS biosynthesis pathways of NTHi for sialic acids Neu5Ac (purple) and Neu5Gc (blue). Depending on its needs, NTHi can negate toxic building up of sialic acid by funneling excess sugar through the catabolic pathway or can convert sialic acid to an activated state for LOS sialylation. On the inner membrane (IM), the biosynthesis pathway converts sialic acid to CMP-sialic acid, which acts as an electron donor to drive the transfer of sialic acids as terminal sugars to LOS. The sialylated LOS is flipped through the periplasmic space (PS) onto the outer membrane (OM).
In the present study, we examined the uptake and presentation of Neu5Gc and Neu5Ac on the NTHi cell surface to better understand the mechanism of NTHi induction of anti-Neu5Gc antibodies in humans.
RESULTS
Neu5Gc is efficiently utilized as a carbon source.
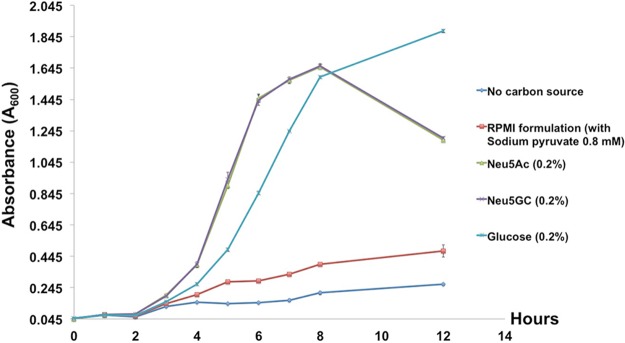
The utilization of Neu5Ac and its metabolic fate have been characterized in NTHi previously (46), but a study of the utilization of Neu5Gc by NTHi has not yet been carried out, although NTHi has been proposed to be a key antigen in the generation of anti-Neu5Gc antibodies (39). It was proposed that generation of these anti-Neu5Gc antibodies against NTHi requires incorporation of Neu5Gc from human serum acquired from the diet into the LOS by NTHi (39), likely through promiscuity of the LOS biosynthesis machinery or catabolic pathway (Fig. 1). We hypothesized that there may be a bias in sialic acid utilization by NTHi that drives incorporation of the relatively scarce Neu5Gc into NTHi LOS. For example, in cases of inefficient catabolism of Neu5Gc as a carbon source (relative to Neu5Ac), the lower rate of flux may generate a pool of Neu5Gc that is available for activation by addition of CMP and may thereby drive its preferential incorporation into LOS (Fig. 1). In fact, growth of NTHi strain 2019 using sialic acid-free chemically defined RPMI 1640 medium supplemented with 1% (vol/vol) hemin and 20 µg/ml NAD (sRPMI medium) (47) and supplemented with either Neu5Ac or Neu5Gc as the sole carbon source revealed that there was no difference in the growth rates of NTHi on these distinct sialic acids (Fig. 2).
FIG 2.
Growth of NTHi strain 2019 in sialic acid-free RPMI media. The medium was supplemented with a sole carbon source, and bacterial growth was monitored for 12 h. The growth curves of Neu5Ac and Neu5Gc were found to be nearly identical and are superimposed.
Preferential addition of Neu5Ac or Neu5Gc on NTHi LOS.
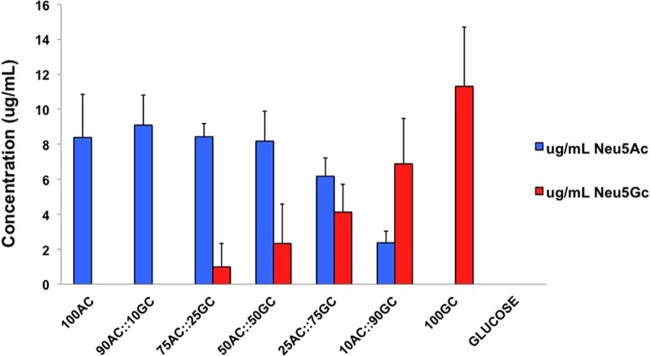
On the basis of the results presented in Fig. 2, we concluded that Neu5Ac and Neu5Gc are utilized equally well by NTHi as a sole carbon source, and previous work demonstrated that the sialic acid transporter SiaP bound Neu5Ac and Neu5Gc with similar affinities (48). This indicated that the transport and catabolic pathways were unbiased in the utilization of these two sialic acids (Fig. 1). To investigate the potential for bias in the incorporation of Neu5Ac or Neu5Gc in the LOS biosynthesis pathway, NTHi strain 2019 was grown on sialic acid-free sRPMI media supplemented with various molar ratios of Neu5Ac and Neu5Gc (Fig. 3). NTHi LOS was purified from these cultures, and the amount of Neu5Ac and Neu5Gc present on LOS was determined by sugar analysis using high performance anion-exchange chromatography with amperometric detection. These results demonstrated a clear preference for incorporation of Neu5Ac over Neu5Gc. For example, when an equal 50:50 ratio of Neu5Ac/Neu5Gc was provided, 4-fold more Neu5Ac was present in LOS than Neu5Gc (n = 3; P = 0.0210 [using Student's t test]).
FIG 3.
Quantified sialic acid (indicated in micrograms per milliliter) of highly pure NTHi LOS grown on RPMI media supplemented with various molarity ratios of Neu5Ac and Neu5Gc. At a 1:1 ratio, the amount of Neu5Ac measured was found to be significantly (4-fold) greater than that of Neu5Gc (n = 3, P = 0.0210). The P value was calculated using a two-tailed unpaired Student's t test.
Preference for sialic acid Neu5Ac of NTHi is due to CMP-Neu5Ac synthetase (SiaB).
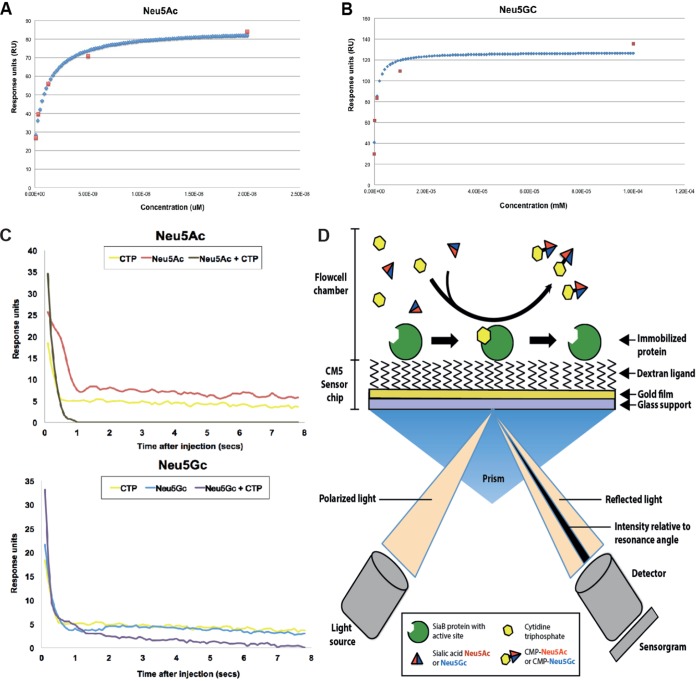
There are several candidate enzymes in the sialic acid NTHi catabolic pathway that could be responsible for this Neu5Ac bias, such as the CMP-sialic acid synthetase (SiaB), which activates sialic acid by adding CMP to form the nucleotide sugar required for enzymatic addition of sialic acid to LOS (49), or the CMP-sialyltransferases SiaA, LsgB, Lic3A, and Lic3B, all of which add sialic acid as the terminal sugar of LOS (40, 50) (Fig. 1). We therefore purified SiaB, which activates these sialic acids to the CMP-sugar form, thus allowing their incorporation into LOS, to determine if the bias for Neu5Ac was occurring at this activation step. Surface plasmon resonance (SPR) analysis was used to determine the SiaB dissociation constant (KD) values for Neu5Ac and Neu5Gc. This analysis revealed a 600-fold-higher affinity of SiaB for Neu5Ac than for Neu5Gc (Table 1; see also Fig. 4A and B). Having established this substrate binding preference, we devised a novel, on-chip SiaB activity assay using SPR to obtain full enzyme kinetics of SiaB against both Neu5Gc and Neu5Ac. An illustration of the SPR-based kinetic assay is shown in Fig. 4D. These data revealed a 4,000-fold-higher catalytic efficiency (kcat/Km) of SiaB for Neu5Ac than for Neu5Gc and thereby revealed that the preferential incorporation of Neu5Ac into LOS (Table 1) is due to more efficient activation with respect to the CMP sugar by SiaB.
TABLE 1.
Comparison of the CMP-Neu5Ac synthetase enzyme kinetic parameters of NTHi 2019 from this study with those of other organisms and with data from Bravo et al. (56) and from Mizanur and Pohl (57)a
| Source organism (MW, Da) |
Km (μM) |
Vmax (μM/min) |
Kcat/Km (μM−1 min−1) |
Source or reference |
|---|---|---|---|---|
| NTHi strain 2019 (24,800) | ||||
| Neu5Ac | 0.143 × 10−3 ± 0.012 | 1.8 ± 0.6 | 315 | This study |
| Neu5Gc | 85.6 × 10−3 ± 5.3 | 0.26 ± 0.04 | 0.076 | This study |
| Clostridium thermocellum (26,000) | ||||
| Neu5Ac | 130 ± 10 | 9 ± 1 | 1.0 | 57 |
| Neu5Gc | 160 ± 10 | 7 ± 1 | 0.6 | 57 |
| Pasteurella haemolytica (43,000) | ||||
| Neu5Ac | 1,820 ± 200 | 197 ± 29 | NA | 56 |
Km for the donor molecule CTP (not shown) was measured to be 200 μM. MW, molecular weight; NA, not assessed.
FIG 4.
Enzyme kinetic measurements of NTHi SiaB (CMP-Neu5Ac synthase) enzyme determined using glycan array and surface plasmon resonance. (A) The KD value against sialic acid Neu5Ac was calculated to be 1.2 nM. Red dots represent dilution concentrations. Blue dots represent the best-fit curve. (B) The KD value against sialic acid Neu5Gc was calculated to be 1 μM. Red dots represent dilution concentrations. Blue dots represent the best-fit curve. (C) Km and Vmax of SiaB against sialic acids Neu5Ac (top) and Neu5Gc (bottom) measured using surface plasmon resonance (Table 1). (D) Illustration showing the principle of the SPR assay to measure SiaB protein activity. SiaB protein was anchored through amine coupling onto a layer of gold containing dextran ligand. Both CTP and Neu5Ac or Neu5Gc are injected into the flowcell chamber. CTP binds to the active site of SiaB followed by Neu5Ac/Neu5Gc binding to form the CMP-Neu5Ac-Neu5Gc complex released from SiaB. The KD value against CTP (not shown) was found to be >200 μM.
The presence of Neu5Gc on the surface of NTHi leads to increased opsonophagocytic killing.
In order to examine if incorporation of Neu5Ac or Neu5Gc into NTHi LOS leads to differences in killing using an anti-Neu5Gc antibody, we grew NTHi strain 2019 in chemically defined RPMI media containing either Neu5Gc or Neu5Ac. Opsonophagocytic killing assays of these strains performed with affinity-purified, human anti-Neu5Gc monospecific antibodies demonstrated that presentation of Neu5Gc on NTHi LOS resulted in a statistically significant increase (P = <0.05 at all serum dilutions [Student's t test]) in killing by those antisera at all antibody dilutions (Fig. 5).
FIG 5.
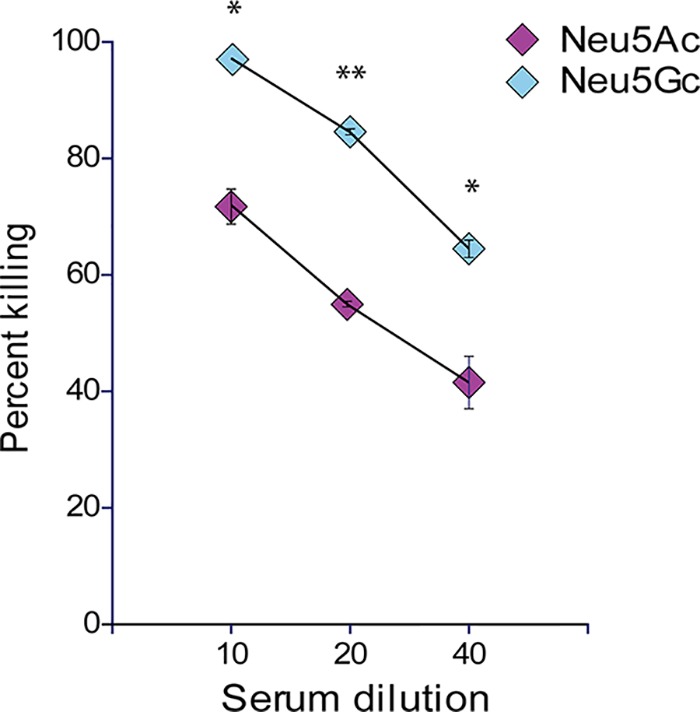
Anti-Neu5Gc-dependent opsonophagocytic killing of NTHi. NTHi strain 2019 was grown with either Neu5Ac (red diamonds) or Neu5Gc (blue diamonds) as a sole carbon source, and opsonophagocytic killing assays were carried out using affinity-purified human anti-Neu5Gc IgGs. Percent killing analyses were carried out by comparisons to a complement-only control. *, P = <0.05; **, P = <0.005 (calculated using Student's t test).
DISCUSSION
Recent work has demonstrated a higher incidence of cancer in Neu5Gc-fed mice that have also generated an anti-Neu5Gc immune response (38). It was also proposed that generation of these anti-Neu5Gc xenoautoantibodies in humans derives from an immune response to NTHi displaying Neu5Gc on LOS on the bacterial cell surface early in life (39). These findings support the idea of the importance of understanding the molecular basis of Neu5Gc display on NTHi LOS. As dietary Neu5Gc is transitorily present and relatively low in concentration compared to the abundant Neu5Ac present in the human body, we hypothesized that there may be a bias in NTHi favoring incorporation of Neu5Gc as the terminal sugar on LOS.
Our data on utilization of Neu5Gc and Neu5Ac as carbon sources show that there is no bias in uptake and catabolism of these sugars. This observation is consistent with the results of a previous study that showed that the sialic acid transporter SiaP has a minor preference for binding Neu5Ac versus Neu5Gc (48), but this is likely not the major factor in NTHi presenting Neu5Ac over Neu5Gc on LOS. Our studies examining the relative levels of incorporation of Neu5Ac and Neu5Gc into LOS revealed a bias against the incorporation of Neu5Gc into LOS. Our data support the hypothesis that this preferential incorporation occurs at a crucial step in the NTHi LOS sialylation pathway, where CMP is added to the free sialic acids by SiaB to form the activated CMP-sialic acid required for incorporation into NTHi LOS.
Homologues of CMP-Neu5Ac synthetase SiaB are found in eukaryotes and prokaryotes (51). CMP-sialic acid synthetases have been well studied and characterized in a range of pathogenic bacteria such as Neisseria meningitidis (52), Escherichia coli (53), Streptococcus agalactiae (54), Haemophilus ducreyi (55), Pasteurella haemolytica (now Mannheimia haemolytica) (56), and Pasteurella multocida (52) and in nonpathogenic species such as Clostridium thermocellum (57). Here we found a marked substrate preference for Neu5Ac over Neu5Gc by SiaB of NTHi, indicating exquisite adaptation to the Neu5Ac sialic acid produced by the human host. The only other CMP synthetase enzymes that have been analyzed kinetically for Neu5Ac or Neu5Gc preferences are those from P. haemolytica (56) and C. thermocellum (57). Neither of these enzymes shows a preference for Neu5Ac or Neu5Gc substrates (see Table 1). P. haemolytica is a respiratory pathogen infecting mostly domestic cattle (cows and sheep, both of which are able to produce both Neu5Ac and Neu5Gc). Nonpathogenic C. thermocellum is an anaerobic thermophilic bacterium which lives in a wide variety of habitats, including the human gut. As neither of these organisms is human adapted, it would be expected that they would not contain a SiaB homologue that favors Neu5Ac over Neu5Gc, as they are likely to encounter both these sugars in their niches. In contrast, our demonstration that NTHi has evolved to use either Neu5Ac or Neu5Gc as a carbon source to maximize diversity in nutrient-restricted environments such as the human host but shows preferential utilization of human-exclusive sialic acid Neu5Ac in LOS biosynthesis is evidence of NTHi-human coevolution. NTHi has evolved to avoid the incorporation of Neu5Gc into LOS, and this has likely been driven by immunoevasion of the human immune response to Neu5Gc.
There have been many studies of the mechanisms used by human-adapted pathogens to acquire and utilize nutrients that are abundant in the host, for example, acquisition of iron from human transferrin (58–60) and hemoglobin (61); acquisition of heme from human hemoglobin-haptoglobin (61), complex hemoglobin (62), and myoglobin (63); and utilization of NAD (64–66). Many human-adapted bacterial pathogens also coat themselves with human-derived molecules in order to evade the immune system; for example, Campylobacter jejuni adds GD1a-like epitopes in LOS, which mimic human gangliosides (67); Neisseria meningitidis expresses LOS with terminal lacto-N-neotetraose (68, 69), the same structure present on paragloboside, the precursor to the ABH antigens on human blood cells; and Helicobacter pylori lipopolysaccharide (LPS) contains fucosylated glycans that mimic the Lewis X and Y antigens also found on human blood cells (70). A number of human-adapted bacterial pathogens also specifically target Neu5Ac in preference to Neu5Gc; for example, Streptococcus pneumoniae preferentially recognizes and utilizes Neu5Ac (71), and the toxin produced by Salmonella enterica serovar Typhi has a much higher affinity for cells with Neu5Ac-containing glycan structures than for those containing Neu5Gc (72). We recently reported that two major adhesins of NTHi, the HMW1/2 proteins, preferentially bind Neu5Ac sialic acid-containing glycans over Neu5Gc-containing glycans (73), demonstrating that NTHi preferentially binds glycans present in the human host. Our findings reported in this study indicate the molecular basis for a further host adaptation in NTHi. While sialic acid uptake and the presence of catabolic enzymes allow NTHi to utilize either Neu5Ac or Neu5Gc as a carbon source for growth, the exquisite preference of SiaB for Neu5Ac allows NTHi to limit the level of Neu5Gc on the cell surface and to avoid killing by the highly abundant anti-Neu5Gc antibodies found in human serum. The presence of human antibodies specific for Neu5Gc-containing structures is likely the driving force for this adaptation and demonstrates that NTHi is highly adapted to the human host.
MATERIALS AND METHODS
Bacterial strains, media, and culture conditions.
NTHi strain 2019 is a human clinical isolate (74). NTHi was grown on brain heart infusion (BHI) agar (Oxoid, United Kingdom) supplemented (sBHI) with 1% (vol/vol) hemin (Sigma) and 20 μg/ml NAD+ (Sigma) or on chocolate agar prepared by adding 40 ml of fresh defibrinated horse blood to 400 ml of blood agar base no. 2 (Oxoid) and heating until the blood lysed. NTHi cultures were incubated at 37°C for 16 h in the presence of 5% (vol/vol) CO2. Sialic acid sole carbon source growth experiments were carried out in chemically defined media as described previously (47). For sialic acid-free chemically defined media, an RPMI 1640 (Sigma) formulation was used and supplemented as described previously (46). Escherichia coli BL21(DE3) was grown on Luria-Bertani (LB) broth (Oxoid), with 100 μg/ml ampicillin (Sigma) where appropriate, at 37°C with 200 rpm shaking.
Growth experiments with sialic acid as the sole carbon source.
NTHi strain 2019 was first grown on sBHI agar for 16 h as described above. Bacterial plate growth was collected, suspended in sBHI broth, and centrifuged at 5,000 rpm for 5 min to collect the cell pellet. The cell pellet was washed and grown in sialic acid-free chemically defined RPMI 1640 medium supplemented with 1% (vol/vol) hemin and 20 µg/ml NAD (sRPMI) as described previously (47). Optical density (OD) was normalized for cultures with a starting growth level of an OD at 600 nm (OD600) of 0.05. Cultures were supplemented with 100 μM sialic acid Neu5Ac or Neu5Gc (Inalco), 20% glucose (Sigma), or sodium pyruvate (Sigma) as the sole carbon source, where applicable. Growth was measured as a function of OD600 over 12 h at 37°C.
NTHi LOS purification and preparation.
NTHi strain 2019 was first grown on chocolate agar for 16 h as described above and then restreaked onto sRPMI medium plates supplemented with differing molar ratios of the sialic acids Neu5Ac and Neu5Gc. Plates were then incubated for 16 h at 37°C in the presence of 5% (vol/vol) CO2. Bacterial growth was collected and resuspended in nuffer A (60 mM Tris base, 10 mM EDTA, 2% SDS, pH 6.8). LOS was purified using a proteinase K/hot phenol method as described previously (75, 76). Purified LOS was resuspended in ultrapure chromatography-grade water (Sigma), frozen at −80 °C, and lyophilized overnight using a freeze dryer.
NTHi LOS sialic acid analysis.
Following quantification of the dry weight of each freeze-dried purified LOS sample, a 1 mg/ml stock of each sample was prepared in ultrapure chromatography-grade water. A subsample (80 µl) was subjected to acid hydrolysis using trifluoroacetic acid (3.35 M). At the end of the reaction, the sample volume was dried under vacuum and the residue reconstituted using an internal standard, ketodeoxynonulosonic acid (100 µl, 100 µM). The analysis was carried out at the Australian Proteome Analysis Facility (APAF; Macquarie University, Sydney, Australia) using an instrument incorporating high-pH anion-exchange chromatography with pulsed amperometric detection (HPAEC-PAD) fitted with a CarboPAC PA1 guard column (2 by 50 mm) connected to a CarboPAC PA1 column (2 by 250 mm) held at 25°C as described previously (77). The sample (20 µl) was injected into the HPAEC-PAD instrument and analyzed using a separation gradient of 60 to 300 mM sodium acetate–sodium hydroxide (100 mM), at a flow rate of 0.5 ml/min. The analytes detected were quantified using internal calibration as described previously (78).
NTHi 2019 CMP-Neu5Ac synthetase (SiaB) protein expression and purification.
siaB from NTHi strain 2019 was amplified using Thermococcus kodakaraensis (KOD) polymerase according to the instructions of the manufacturer (TaKaRa Bio) and primers siaB-F (5′-GACGACGACAAGATGAAAATAATAATGACAAGAATTGCAATT-3′) and siaB-R (5′-GAGGAGAAGCCCGGGAATTCTTTTGAAATTAAACTTTCGG-3′) and was cloned into overexpression vector pET51-Ek/LIC (Merck Millipore) according to manufacturer’s instructions so as to be in-frame with an N-terminal 6×His tag. (Sequences in each primer used for ligation-independent cloning [LIC] are underlined.) Clones were confirmed by sequencing using BigDye Terminator 3.1 (Thermo Fisher) according to the manufacturer’s instructions. The resulting construct was designated pET51::2019siaB. SiaB overexpression was induced with 0.1 mM isopropyl β-d-1-thiogalactopyranoside (IPTG) (Sigma), and the cultures were grown at 37°C with shaking at 200 rpm for 4 h. Overexpressed cultures were pelleted at 5,000 rpm for 15 min at 4°C, and the pellet was then resuspended in lysis buffer (50 mM NaH2PO4, 300 mM NaCl, pH 7) containing protease inhibitor cocktail (Roche), lysozyme (0.2 mg/ml), and DNase (10 µg/ml). Cells were lysed using a TissueLyser (Qiagen; five rounds of 1 min at 50 oscillations−1 s−1/1 min on ice). Lysate was clarified by centrifugation at 10,000 rpm for 30 min. Supernatant was decanted and mixed with 1/3 volume of lysis buffer containing 20 mM imidazole (Sigma) and was applied to a column containing Talon resin (Clontech). The column was washed with lysis buffer containing 20 mM imidazole (5 column volumes), and SiaB was eluted with lysis buffer containing 500 mM imidazole. Following analysis of fractions by SDS-PAGE, fractions containing pure SiaB were pooled and dialyzed against lysis buffer (5 liters) overnight at 4°C and were concentrated using Amersham centrifugal concentrators (Merck Millipore) according to the manufacturer’s instructions. Protein concentration was determined using a bicinchoninic acid (BCA) protein assay (Thermo Fisher).
Enzyme assay and kinetics of SiaB using surface plasmon resonance (SPR).
The enzyme assay used in this study was modified from methods previously described (79). Purified SiaB was immobilized onto a Series S CM5 sensor chip by amine coupling. Briefly, the carboxy dextran surface was prepared by injection of NHS (N-hydroxysuccinimide)/EDC [1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide)] followed by the SiaB protein at 100 µg/ml. The protein was cross-linked by the addition of ethanolamine. Flow cell 1 served as a reference cell and was prepared under the same conditions but without immobilized protein. CTP and sialic acids (Neu5Ac and Neu5Gc) were diluted in buffer (20 mM MgCl2, 0.2 M MOPS [morpholinepropanesulfonic acid], pH 8.1) (57) at concentrations ranging from 100 µM to 0.05 nM. Single-cycle and multicycle kinetic experiments were performed at 37°C using a Biacore T100 system (GE Healthcare). Affinity constants (KD) for CTP, Neu5Ac, and Neu5Gc were determined by the use of analysis software. Enzyme kinetic parameters Km, Vmax, and Kcat/Km were calculated for Neu5Ac and Neu5Gc (Table 1).
Opsonophagocytic killing assays.
Opsonophagocytic killing assays were carried out as described previously (80). NTHi strain 2019 was grown in supplemented RPMI medium with either Neu5Ac or Neu5Gc, with the two strains being compared in the same experiment. Experiments were carried out in duplicate using a 1:10 to 1:40 dilution of primary anti-Neu5Gc IgG antibody that had been subjected to affinity purification from human intravenous immunoglobulin (IVIG) (31). Percent killing at each dilution was calculated by determining the ratio of the bacterial colony count at each dilution to that of the complement-only serum control.
ACKNOWLEDGMENTS
This project was funded by NHMRC Program grants 565526 and 1071659 and by NHMRC Principal Research Fellowship 1138466 (to M.P.J.), by NIAID grants AI AI024616 (to M.A.A.) and R01 AI081887 (to S.J.B.), by American Heart Association Grant-in-Aid 17GRNT33630171 (to S.J.B.), and by NIGMS grant GM32373 (to A.V.). M.P.J. was also supported by a University of Iowa Helen C. Levitt Visiting Professorship. This work was also partially supported by the European Union H2020 Program grants (ERC-2016-STG-716220) (to V.P.-K.). We thank the Australian Proteome Analysis Facility (APAF), Macquarie University, for their service in sugar analysis. This research was facilitated by access to APAF as supported under the Australian Government's National Collaborative Research Infrastructure Strategy (NCRIS). We acknowledge Griffith University Australia for providing a PhD scholarship to P.S.K.N.
P.S.K.N. conducted all NTHi sialic acid-related experiments, including growth experiments, LOS purification (with M.A.A.), and analysis of quantified LOS sialic acid. C.J.D. and L.E.H.-T. performed all surface plasmon resonance experiments. S.J.B. and L.E.W. carried out opsonophagocytic killing assays. T.M. generated affinity-purified human anti-Neu5Gc IgGs. M.P.J. conceived the study, partly in discussions with M.A.A., A.V., and V.P.-K. P.S.K.N., J.M.A., and M.P.J. wrote the manuscript. J.M.A. and A.V. edited the manuscript. All of us read and approved the manuscript.
We declare that we have no competing interests.
This manuscript is dedicated to the memory of Dr. Stephen J. Barenkamp M.D. who passed away 17 March 2019.
Footnotes
Citation Ng PSK, Day CJ, Atack JM, Hartley-Tassell LE, Winter LE, Marshanski T, Padler-Karavani V, Varki A, Barenkamp SJ, Apicella MA, Jennings MP. 2019. Nontypeable Haemophilus influenzae has evolved preferential use of N-acetylneuraminic acid as a host adaptation. mBio 10:e00422-19. https://doi.org/10.1128/mBio.00422-19.
Contributor Information
Brian J. Akerley, University of Mississippi Medical Center.
Larry S. McDaniel, University of Mississippi Medical Center.
REFERENCES
- 1.Pittman M. 1931. Variation and type specificity in the bacterial species Hemophilus influenzae. J Exp Med 53:471–492. doi: 10.1084/jem.53.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turk DC. 1984. The pathogenicity of Haemophilus influenzae. J Med Microbiol 18:1–16. doi: 10.1099/00222615-18-1-1. [DOI] [PubMed] [Google Scholar]
- 3.Murphy TF, Faden H, Bakaletz LO, Kyd JM, Forsgren A, Campos J, Virji M, Pelton SI. 2009. Nontypeable Haemophilus influenzae as a pathogen in children. Pediatr Infect Dis J 28:43–48. doi: 10.1097/INF.0b013e318184dba2. [DOI] [PubMed] [Google Scholar]
- 4.Sethi S, Evans N, Grant BJB, Murphy TF. 2002. New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. N Engl J Med 347:465–471. doi: 10.1056/NEJMoa012561. [DOI] [PubMed] [Google Scholar]
- 5.Johnson RH. 1988. Community-acquired pneumonia: etiology, diagnosis, and treatment. Clin Ther 10:568–573. [PubMed] [Google Scholar]
- 6.Langereis JD, de Jonge MI. 2015. Invasive disease caused by nontypeable Haemophilus influenzae. Emerg Infect Dis 21:1711–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agrawal A, Murphy TF. 2011. Haemophilus influenzae infections in the H. influenzae type b conjugate vaccine era. J Clin Microbiol 49:3728–3732. doi: 10.1128/JCM.05476-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gkentzi D, Slack MP, Ladhani SN. 2012. The burden of nonencapsulated Haemophilus influenzae in children and potential for prevention. Curr Opin Infect Dis 25:266–272. doi: 10.1097/QCO.0b013e32835310a4. [DOI] [PubMed] [Google Scholar]
- 9.Naito S, Takeuchi N, Ohkusu M, Takahashi-Nakaguchi A, Takahashi H, Imuta N, Nishi J, Shibayama K, Matsuoka M, Sasaki Y, Ishiwada N. 2018. Clinical and bacteriologic analysis of nontypeable Haemophilus influenzae strains isolated from children with invasive diseases in Japan from 2008 to 2015. J Clin Microbiol 56:e00141-18. doi: 10.1128/JCM.00141-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ladhani S, Slack MPE, Heath PT, von Gottberg A, Chandra M, Ramsay ME. 2010. Invasive Haemophilus influenzae disease, Europe, 1996–2006. Emerg Infect Dis 16:455–463. doi: 10.3201/eid1603.090290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collins S, Vickers A, Ladhani SN, Flynn S, Platt S, Ramsay ME, Litt DJ, Slack MPE. 2016. Clinical and molecular epidemiology of childhood invasive nontypeable Haemophilus influenzae disease in England and Wales. Pediatr Infect Dis J 35:e76–e84. doi: 10.1097/INF.0000000000000996. [DOI] [PubMed] [Google Scholar]
- 12.Angata T, Varki A. 2002. Chemical diversity in the sialic acids and related alpha-keto acids: an evolutionary perspective. Chem Rev 102:439–469. doi: 10.1021/cr000407m. [DOI] [PubMed] [Google Scholar]
- 13.Schauer R. 1982. Chemistry, metabolism, and biological functions of sialic acids. Adv Carbohydr Chem Biochem 40:131–234. doi: 10.1016/S0065-2318(08)60109-2. [DOI] [PubMed] [Google Scholar]
- 14.Varki A. 2001. N-Glycolylneuraminic acid deficiency in humans. Biochimie 83:615–622. doi: 10.1016/S0300-9084(01)01309-8. [DOI] [PubMed] [Google Scholar]
- 15.Schauer R, Srinivasan GV, Coddeville B, Zanetta J-P, Guérardel Y. 2009. Low incidence of N-glycolylneuraminic acid in birds and reptiles and its absence in the platypus. Carbohydr Res 344:1494–1500. doi: 10.1016/j.carres.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 16.Irie A, Suzuki A. 1998. CMP-N-Acetylneuraminic acid hydroxylase is exclusively inactive in humans. Biochem Biophys Res Commun 248:330–333. doi: 10.1006/bbrc.1998.8946. [DOI] [PubMed] [Google Scholar]
- 17.Irie A, Koyama S, Kozutsumi Y, Kawasaki T, Suzuki A. 1998. The molecular basis for the absence of N-glycolylneuraminic acid in humans. J Biol Chem 273:15866–15871. doi: 10.1074/jbc.273.25.15866. [DOI] [PubMed] [Google Scholar]
- 18.Muchmore EA, Diaz S, Varki A. 1998. A structural difference between the cell surfaces of humans and the great apes. Am J Phys Anthropol 107:187–198. doi:. [DOI] [PubMed] [Google Scholar]
- 19.Chou HH, Takematsu H, Diaz S, Iber J, Nickerson E, Wright KL, Muchmore EA, Nelson DL, Warren ST, Varki A. 1998. A mutation in human CMP-sialic acid hydroxylase occurred after the homo-pan divergence. Proc Natl Acad Sci U S A 95:11751–11756. doi: 10.1073/pnas.95.20.11751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Merrick JM, Zadarlik K, Milgrom F. 1978. Characterization of the Hanganutziu-Deicher (serum-sickness) antigen as gangliosides containing N-glycolylneuraminic acid. Int Arch Allergy Immunol 57:477–480. doi: 10.1159/000232140. [DOI] [PubMed] [Google Scholar]
- 21.Nowak JA, Jain NK, Stinson MW, Merrick JM. 1986. Interaction of bovine erythrocyte N-glycolylneuraminic acid-containing gangliosides and glycoproteins with a human Hanganutziu-Deicher serum. Mol Immunol 23:693–700. doi: 10.1016/0161-5890(86)90079-9. [DOI] [PubMed] [Google Scholar]
- 22.Higashi H, Naiki M, Matuo S, Okouchi K. 1977. Antigen of "serum sickness" type of heterophile antibodies in human sera: identification as gangliosides with N-glycolylneuraminic acid. Biochem Biophys Res Commun 79:388–395. doi: 10.1016/0006-291X(77)90169-3. [DOI] [PubMed] [Google Scholar]
- 23.Narayanan S. 1994. Sialic acid as a tumor marker. Ann Clin Lab Sci 24:376–384. [PubMed] [Google Scholar]
- 24.Sillanaukee P, Ponnio M, Jaaskelainen IP. 1999. Occurrence of sialic acids in healthy humans and different disorders. Eur J Clin Invest 29:413–425. doi: 10.1046/j.1365-2362.1999.00485.x. [DOI] [PubMed] [Google Scholar]
- 25.Ikuta K, Nishi Y, Shimizu Y, Higashi H, Kitamoto N, Kato S, Fujita M, Nakano Y, Taguchi T, Naiki M. 1982. Hanganutziu-Deicher type-heterophile antigen-positive cells in human cancer tissues demonstrated by membrane immunofluorescence. Biken J 25:47–50. [PubMed] [Google Scholar]
- 26.Higashi H, Nishi Y, Fukui Y, Ikuta K, Ueda S, Kato S, Fujita M, Nakano Y, Taguchi T, Sakai S, et al. 1984. Tumor-associated expression of glycosphingolipid Hanganutziu-Deicher antigen in human cancers. Gan 75:1025–1029. [PubMed] [Google Scholar]
- 27.Tangvoranuntakul P, Gagneux P, Diaz S, Bardor M, Varki N, Varki A, Muchmore E. 2003. Human uptake and incorporation of an immunogenic nonhuman dietary sialic acid. Proc Natl Acad Sci U S A 100:12045–12050. doi: 10.1073/pnas.2131556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Higashi H, Sasabe T, Fukui Y, Maru M, Kato S. 1988. Detection of gangliosides as N-glycolylneuraminic acid-specific tumor-associated Hanganutziu-Deicher antigen in human retinoblastoma cells. Jpn J Cancer Res 79:952–956. doi: 10.1111/j.1349-7006.1988.tb00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakarai H, Saida T, Shibata Y, Irie RF, Kano K. 1987. Expression of heterophile, Paul-Bunnell and Hanganutziu-Deicher antigens on human melanoma cell lines. Int Arch Allergy Immunol 83:160–166. doi: 10.1159/000234349. [DOI] [PubMed] [Google Scholar]
- 30.Padler-Karavani V, Yu H, Cao H, Chokhawala H, Karp F, Varki N, Chen X, Varki A. 2008. Diversity in specificity, abundance, and composition of anti-Neu5Gc antibodies in normal humans: potential implications for disease. Glycobiology 18:818–830. doi: 10.1093/glycob/cwn072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Padler-Karavani V, Hurtado-Ziola N, Pu M, Yu H, Huang S, Muthana S, Chokhawala HA, Cao H, Secrest P, Friedmann-Morvinski D, Singer O, Ghaderi D, Verma IM, Liu Y-T, Messer K, Chen X, Varki A, Schwab R. 2011. Human xeno-autoantibodies against a non-human sialic acid serve as novel serum biomarkers and immunotherapeutics in cancer. Cancer Res 71:3352–3363. doi: 10.1158/0008-5472.CAN-10-4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pham T, Gregg CJ, Karp F, Chow R, Padler-Karavani V, Cao H, Chen X, Witztum JL, Varki NM, Varki A. 2009. Evidence for a novel human-specific xeno-auto-antibody response against vascular endothelium. Blood 114:5225–5235. doi: 10.1182/blood-2009-05-220400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hedlund M, Padler-Karavani V, Varki NM, Varki A. 2008. Evidence for a human-specific mechanism for diet and antibody-mediated inflammation in carcinoma progression. Proc Natl Acad Sci U S A 105:18936–18941. doi: 10.1073/pnas.0803943105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Diaz SL, Padler-Karavani V, Ghaderi D, Hurtado-Ziola N, Yu H, Chen X, Brinkman-Van der Linden ECM, Varki A, Varki NM. 2009. Sensitive and specific detection of the non-human sialic acid N-glycolylneuraminic acid in human tissues and biotherapeutic products. PLoS One 4:e4241. doi: 10.1371/journal.pone.0004241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yin J, Hashimoto A, Izawa M, Miyazaki K, Chen G-Y, Takematsu H, Kozutsumi Y, Suzuki A, Furuhata K, Cheng F-L, Lin C-H, Sato C, Kitajima K, Kannagi R. 2006. Hypoxic culture induces expression of sialin, a sialic acid transporter, and cancer-associated gangliosides containing non-human sialic acid on human cancer cells. Cancer Res 66:2937–2945. doi: 10.1158/0008-5472.CAN-05-2615. [DOI] [PubMed] [Google Scholar]
- 36.Bergfeld AK, Pearce OMT, Diaz SL, Pham T, Varki A. 2012. Metabolism of vertebrate amino sugars with N-glycolyl groups: elucidating the intracellular fate of the non-human sialic acid N-glycolylneuraminic acid. J Biol Chem 287:28865–28881. doi: 10.1074/jbc.M112.363549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Padler-Karavani V, Hedlund M, Yu H, Cao H, Chokhawala H, Karp F, Data A, Chen X, Varki N, Varki A. 2008. Neu5Gc and human anti-Neu5Gc antibodies in inflammation-induced carcinoma progression. Cancer Res 68:5397–5397.18593942 [Google Scholar]
- 38.Samraj AN, Pearce OMT, Läubli H, Crittenden AN, Bergfeld AK, Banda K, Gregg CJ, Bingman AE, Secrest P, Diaz SL, Varki NM, Varki A. 2015. A red meat-derived glycan promotes inflammation and cancer progression. Proc Natl Acad Sci U S A 112:542–547. doi: 10.1073/pnas.1417508112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taylor RE, Gregg CJ, Padler-Karavani V, Ghaderi D, Yu H, Huang S, Sorensen RU, Chen X, Inostroza J, Nizet V, Varki A. 2010. Novel mechanism for the generation of human xeno-autoantibodies against the nonhuman sialic acid N-glycolylneuraminic acid. J Exp Med 207:1637–1646. doi: 10.1084/jem.20100575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hood DW, Cox AD, Gilbert M, Makepeace K, Walsh S, Deadman ME, Cody A, Martin A, Månsson M, Schweda EK, Brisson JR, Richards JC, Moxon ER, Wakarchuk WW. 2001. Identification of a lipopolysaccharide alpha-2,3-sialyltransferase from Haemophilus influenzae. Mol Microbiol 39:341–350. doi: 10.1046/j.1365-2958.2001.02204.x. [DOI] [PubMed] [Google Scholar]
- 41.Greiner LL, Watanabe H, Phillips NJ, Shao J, Morgan A, Zaleski A, Gibson BW, Apicella MA. 2004. Nontypeable Haemophilus influenzae strain 2019 produces a biofilm containing N-acetylneuraminic acid that may mimic sialylated O-linked glycans. Infect Immun 72:4249–4260. doi: 10.1128/IAI.72.7.4249-4260.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bouchet V, Hood DW, Li J, Brisson J-R, Randle GA, Martin A, Li Z, Goldstein R, Schweda EKH, Pelton SI, Richards JC, Moxon ER. 2003. Host-derived sialic acid is incorporated into Haemophilus influenzae lipopolysaccharide and is a major virulence factor in experimental otitis media. Proc Natl Acad Sci U S A 100:8898–8903. doi: 10.1073/pnas.1432026100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Swords WE, Moore ML, Godzicki L, Bukofzer G, Mitten MJ, VonCannon J. 2004. Sialylation of lipooligosaccharides promotes biofilm formation by nontypeable Haemophilus influenzae. Infect Immun 72:106–113. doi: 10.1128/IAI.72.1.106-113.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnston JW, Coussens NP, Allen S, Houtman JCD, Turner KH, Zaleski A, Ramaswamy S, Gibson BW, Apicella MA. 2008. Characterization of the N-acetyl-5-neuraminic acid-binding site of the extracytoplasmic solute receptor (SiaP) of nontypeable Haemophilus influenzae strain 2019. J Biol Chem 283:855–865. doi: 10.1074/jbc.M706603200. [DOI] [PubMed] [Google Scholar]
- 45.Vimr E, Lichtensteiger C, Steenbergen S. 2000. Sialic acid metabolism's dual function in Haemophilus influenzae. Mol Microbiol 36:1113–1123. doi: 10.1046/j.1365-2958.2000.01925.x. [DOI] [PubMed] [Google Scholar]
- 46.Johnston JW, Zaleski A, Allen S, Mootz JM, Armbruster D, Gibson BW, Apicella MA, Munson RS. 2007. Regulation of sialic acid transport and catabolism in Haemophilus influenzae. Mol Microbiol 66:26–39. doi: 10.1111/j.1365-2958.2007.05890.x. [DOI] [PubMed] [Google Scholar]
- 47.Coleman HN, Daines DA, Jarisch J, Smith AL. 2003. Chemically defined media for growth of Haemophilus influenzae strains. J Clin Microbiol 41:4408–4410. doi: 10.1128/JCM.41.9.4408-4410.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Severi E, Randle G, Kivlin P, Whitfield K, Young R, Moxon R, Kelly D, Hood D, Thomas GH. 2005. Sialic acid transport in Haemophilus influenzae is essential for lipopolysaccharide sialylation and serum resistance and is dependent on a novel tripartite ATP-independent periplasmic transporter. Mol Microbiol 58:1173–1185. doi: 10.1111/j.1365-2958.2005.04901.x. [DOI] [PubMed] [Google Scholar]
- 49.Hood DW, Makepeace K, Deadman ME, Rest RF, Thibault P, Martin A, Richards JC, Moxon ER. 1999. Sialic acid in the lipopolysaccharide of Haemophilus influenzae: strain distribution, influence on serum resistance and structural characterization. Mol Microbiol 33:679–692. doi: 10.1046/j.1365-2958.1999.01509.x. [DOI] [PubMed] [Google Scholar]
- 50.Jones PA, Samuels NM, Phillips NJ, Munson RS, Bozue JA, Arseneau JA, Nichols WA, Zaleski A, Gibson BW, Apicella MA. 2002. Haemophilus influenzae type b strain A2 has multiple sialyltransferases involved in lipooligosaccharide sialylation. J Biol Chem 277:14598–14611. doi: 10.1074/jbc.M110986200. [DOI] [PubMed] [Google Scholar]
- 51.Bravo IG, García-Vallvé S, Romeu A, Reglero A. 2004. Prokaryotic origin of cytidylyltransferases and alpha-ketoacid synthases. Trends Microbiol 12:120–128. doi: 10.1016/j.tim.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 52.Li Y, Yu H, Cao H, Muthana S, Chen X. 2012. Pasteurella multocida CMP-sialic acid synthetase and mutants of Neisseria meningitidis CMP-sialic acid synthetase with improved substrate promiscuity. Appl Microbiol Biotechnol 93:2411–2423. doi: 10.1007/s00253-011-3579-6. [DOI] [PubMed] [Google Scholar]
- 53.Yu H, Yu H, Karpel R, Chen X. 2004. Chemoenzymatic synthesis of CMP-sialic acid derivatives by a one-pot two-enzyme system: comparison of substrate flexibility of three microbial CMP-sialic acid synthetases. Bioorg Med Chem 12:6427–6435. doi: 10.1016/j.bmc.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 54.Yu H, Ryan W, Yu H, Chen X. 2006. Characterization of a bifunctional cytidine 5'-monophosphate N-acetylneuraminic acid synthetase cloned from Streptococcus agalactiae. Biotechnol Lett 28:107–113. doi: 10.1007/s10529-005-4955-z. [DOI] [PubMed] [Google Scholar]
- 55.Tullius MV, Munson RS, Wang J, Gibson BW. 1996. Purification, cloning, and expression of a cytidine 5'-monophosphate N-acetylneuraminic acid synthetase from Haemophilus ducreyi. J Biol Chem 271:15373–15380. doi: 10.1074/jbc.271.26.15373. [DOI] [PubMed] [Google Scholar]
- 56.Bravo IG, Barrallo S, Ferrero MA, Rodríguez-Aparicio LB, Martínez-Blanco H, Reglero Á. 2001. Kinetic properties of the acylneuraminate cytidylyltransferase from Pasteurella haemolytica A2. Biochem J 358:585–598. doi: 10.1042/bj3580585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mizanur RM, Pohl NL. 2007. Cloning and characterization of a heat-stable CMP-N-acylneuraminic acid synthetase from Clostridium thermocellum. Appl Microbiol Biotechnol 76:827–834. doi: 10.1007/s00253-007-1053-2. [DOI] [PubMed] [Google Scholar]
- 58.Morton DJ, Williams P. 1989. Utilization of transferrin-bound iron by Haemophilus species of human and porcine origins. FEMS Microbiol Lett 53:123–127. doi: 10.1111/j.1574-6968.1989.tb03609.x. [DOI] [PubMed] [Google Scholar]
- 59.Gray-Owen SD, Loosmore S, Schryvers AB. 1995. Identification and characterization of genes encoding the human transferrin-binding proteins from Haemophilus influenzae. Infect Immun 63:1201–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schryvers AB, Gray-Owen S. 1992. Iron acquisition in Haemophilus influenzae: receptors for human transferrin. J Infect Dis 165(Suppl 1):S103–S104. doi: 10.1093/infdis/165-Supplement_1-S103. [DOI] [PubMed] [Google Scholar]
- 61.Stull TL. 1987. Protein sources of heme for Haemophilus influenzae. Infect Immun 55:148–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Frangipane ME, Morton DJ, Wooten JA, Pozsgay JM, Stull TL. 1994. Binding of human hemoglobin by Haemophilus influenzae. FEMS Microbiol Lett 118:243–248. doi: 10.1111/j.1574-6968.1994.tb06835.x. [DOI] [PubMed] [Google Scholar]
- 63.Morton DJ, Van Wagoner TM, Seale TW, Whitby PW, Stull TL. 2006. Utilization of myoglobin as a heme source by Haemophilus influenzae requires binding of myoglobin to haptoglobin. FEMS Microbiol Lett 258:235–240. doi: 10.1111/j.1574-6968.2006.00230.x. [DOI] [PubMed] [Google Scholar]
- 64.Reidl J, Schlör S, Kraiss A, Schmidt-Brauns J, Kemmer G, Soleva E. 2000. NADP and NAD utilization in Haemophilus influenzae. Mol Microbiol 35:1573–1581. [DOI] [PubMed] [Google Scholar]
- 65.Kemmer G, Reilly TJ, Schmidt-Brauns J, Zlotnik GW, Green BA, Fiske MJ, Herbert M, Krai A, Schlor S, Smith A, Reidl J. 2001. NadN and e (P4) are essential for utilization of NAD and nicotinamide mononucleotide but not nicotinamide riboside in Haemophilus influenzae. J Bacteriol 183:3974–3981. doi: 10.1128/JB.183.13.3974-3981.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Garavaglia S, Bruzzone S, Cassani C, Canella L, Allegrone G, Sturla L, Mannino E, Millo E, De Flora A, Rizzi M. 2012. The high-resolution crystal structure of periplasmic Haemophilus influenzae NAD nucleotidase reveals a novel enzymatic function of human CD73 related to NAD metabolism. Biochem J 441:131–141. doi: 10.1042/BJ20111263. [DOI] [PubMed] [Google Scholar]
- 67.Nachamkin I, Liu J, Li M, Ung H, Moran AP, Prendergast MM, Sheikh K. 2002. Campylobacter jejuni from patients with Guillain-Barre syndrome preferentially expresses a GD(1a)-like epitope. Infect Immun 70:5299–5303. doi: 10.1128/IAI.70.9.5299-5303.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Michon F, Beurret M, Gamian A, Brisson JR, Jennings HJ. 1990. Structure of the L5 lipopolysaccharide core oligosaccharides of Neisseria meningitidis. J Biol Chem 265:7243–7247. [PubMed] [Google Scholar]
- 69.Gamian A, Beurret M, Michon F, Brisson JR, Jennings HJ. 1992. Structure of the L2 lipopolysaccharide core oligosaccharides of Neisseria meningitidis. J Biol Chem 267:922–925. [PubMed] [Google Scholar]
- 70.Moran AP. 1996. The role of lipopolysaccharide in Helicobacter pylori pathogenesis. Aliment Pharmacol Ther 10(Suppl 1):39–50. doi: 10.1046/j.1365-2036.1996.22164004.x. [DOI] [PubMed] [Google Scholar]
- 71.Hentrich K, Löfling J, Pathak A, Nizet V, Varki A, Henriques-Normark B. 2016. Streptococcus pneumoniae senses a human-like sialic acid profile via the response regulator CiaR. Cell Host Microbe 20:307–317. doi: 10.1016/j.chom.2016.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Deng L, Song J, Gao X, Wang J, Yu H, Chen X, Varki N, Naito-Matsui Y, Galán JE, Varki A. 2014. Host adaptation of a bacterial toxin from the human pathogen Salmonella Typhi. Cell 159:1290–1299. doi: 10.1016/j.cell.2014.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Atack JM, Day CJ, Poole J, Brockman KL, Bakaletz LO, Barenkamp SJ, Jennings MP. 2018. The HMW2 adhesin of non-typeable Haemophilus influenzae is a human-adapted lectin that mediates high-affinity binding to 2-6 linked N-acetylneuraminic acid glycans. Biochem Biophys Res Commun 503:1103–1107. doi: 10.1016/j.bbrc.2018.06.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Campagnari AA, Gupta MR, Dudas KC, Murphy TF, Apicella MA. 1987. Antigenic diversity of lipooligosaccharides of nontypable Haemophilus influenzae. Infect Immun 55:882–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Allen S, Zaleski A, Johnston JW, Gibson BW, Apicella MA. 2005. Novel sialic acid transporter of Haemophilus influenzae. Infect Immun 73:5291–5300. doi: 10.1128/IAI.73.9.5291-5300.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Apicella MA. 2008. Isolation and characterization of lipopolysaccharides. Methods Mol Biol 431:3–13. [DOI] [PubMed] [Google Scholar]
- 77.Hardy MR, Townsend RR. 1988. Separation of positional isomers of oligosaccharides and glycopeptides by high-performance anion-exchange chromatography with pulsed amperometric detection. Proc Natl Acad Sci U S A 85:3289–3293. doi: 10.1073/pnas.85.10.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rohrer JS, Thayer J, Weitzhandler M, Avdalovic N. 1998. Analysis of the N-acetylneuraminic acid and N-glycolylneuraminic acid contents of glycoproteins by high-pH anion-exchange chromatography with pulsed amperometric detection. Glycobiology 8:35–43. doi: 10.1093/glycob/8.1.35. [DOI] [PubMed] [Google Scholar]
- 79.Stocklein WF, Behrsing O, Scharte G, Micheel B, Benkert A, Schössler W, Warsinke A, Scheller FW. 2000. Enzyme kinetic assays with surface plasmon resonance (BIAcore) based on competition between enzyme and creatinine antibody. Biosens Bioelectron 15:377–382. doi: 10.1016/S0956-5663(00)00094-4. [DOI] [PubMed] [Google Scholar]
- 80.Winter LE, Barenkamp SJ. 2014. Antibodies to the HMW1/HMW2 and Hia adhesins of nontypeable Haemophilus influenzae mediate broad-based opsonophagocytic killing of homologous and heterologous strains. Clin Vaccine Immunol 21:613–621. doi: 10.1128/CVI.00772-13. [DOI] [PMC free article] [PubMed] [Google Scholar]