Colistin is a last-resort antibiotic that is used to treat severe infections caused by MDR and extensively drug-resistant (XDR) bacteria. The World Health Organization (WHO) has designated colistin as a “highest priority critically important antimicrobial for human medicine” (WHO, Critically Important Antimicrobials for Human Medicine, 5th revision, 2017, https://www.who.int/foodsafety/publications/antimicrobials-fifth/en/), as it is often one of the only therapies available for treating serious bacterial infections in critically ill patients. Plasmid-borne mcr genes that confer resistance to colistin pose a threat to public health at an international scale, as they can be transmitted via horizontal gene transfer and have the potential to spread globally. Therefore, the establishment of a complete reference of mcr genes that can be used to screen for plasmid-mediated colistin resistance is essential for developing effective control strategies.
KEYWORDS: Salmonella enterica, antibiotic resistance, colistin, mcr genes, mcr-9, mobilized colistin resistance, multidrug resistance, plasmid-mediated resistance
ABSTRACT
Mobilized colistin resistance (mcr) genes are plasmid-borne genes that confer resistance to colistin, an antibiotic used to treat severe bacterial infections. To date, eight known mcr homologues have been described (mcr-1 to -8). Here, we describe mcr-9, a novel mcr homologue detected during routine in silico screening of sequenced Salmonella genomes for antimicrobial resistance genes. The amino acid sequence of mcr-9, detected in a multidrug-resistant (MDR) Salmonella enterica serotype Typhimurium (S. Typhimurium) strain isolated from a human patient in Washington State in 2010, most closely resembled mcr-3, aligning with 64.5% amino acid identity and 99.5% coverage using Translated Nucleotide BLAST (tblastn). The S. Typhimurium strain was tested for phenotypic resistance to colistin and was found to be sensitive at the 2-mg/liter European Committee on Antimicrobial Susceptibility Testing breakpoint under the tested conditions. mcr-9 was cloned in colistin-susceptible Escherichia coli NEB5α under an IPTG (isopropyl-β-d-thiogalactopyranoside)-induced promoter to determine whether it was capable of conferring resistance to colistin when expressed in a heterologous host. Expression of mcr-9 conferred resistance to colistin in E. coli NEB5α at 1, 2, and 2.5 mg/liter colistin, albeit at a lower level than mcr-3. Pairwise comparisons of the predicted protein structures associated with all nine mcr homologues (Mcr-1 to -9) revealed that Mcr-9, Mcr-3, Mcr-4, and Mcr-7 share a high degree of similarity at the structural level. Our results indicate that mcr-9 is capable of conferring phenotypic resistance to colistin in Enterobacteriaceae and should be immediately considered when monitoring plasmid-mediated colistin resistance.
OBSERVATION
Until recently, bacterial resistance to colistin, a last-resort antibiotic reserved for treating severe infections, was thought to be acquired solely via chromosomal point mutations (1). However, in 2015, plasmid-mediated colistin resistance gene mcr-1 was described in Escherichia coli (1). Mcr-1 is a phosphoethanolamine transferase that modifies cell membrane lipid A head groups with a phosphoethanolamine residue, reducing affinity to colistin (2). Since then, seven additional mcr homologues (mcr-2 to -8) have been identified in Enterobacteriaceae (3–9). Here, we report novel mcr homologue mcr-9, which was identified in a Salmonella enterica serotype Typhimurium (S. Typhimurium) genome.
In silico identification of mcr-9 in an MDR S. Typhimurium genome.
MDR S. Typhimurium strain HUM_TYPH_WA_10_R9_3274 (NCBI RefSeq accession no. GCF_002091095.1) was isolated from a patient in Washington State in 2010 (10). It had previously been tested for resistance to a panel of 12 antimicrobials that did not include colistin (10). ABRicate version 0.8 (https://github.com/tseemann/abricate) identified 20 antimicrobial resistance (AMR) genes in the HUM_TYPH_WA_10_R9_3274 assembly using the ResFinder database (accessed 11 June 2018) (11) and minimum identity and coverage thresholds of 75 and 50% (10), respectively, none of which had been previously described to confer colistin resistance (see Table S1 in the supplemental material). Four plasmid replicons, including IncHI2 and IncHI2A, were detected with at least 80% identity and 60% coverage using ABRicate and PlasmidFinder (accessed 11 June 2018 [Table S1]) (12).
Antimicrobial resistance (AMR) genes and plasmid replicons detected in the assembly of HUM_TYPH_WA_10_R9_3274. Download Table S1, DOCX file, 0.1 MB (22.3KB, docx) .
Copyright © 2019 Carroll et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
To detect mcr-9 in the HUM_TYPH_WA_10_R9_3274 assembly, all colistin resistance-conferring nucleotide sequences available in ResFinder (52 sequences, accessed 22 January 2019 [see Table S2 in the supplemental material]) were translated into amino acid sequences using EMBOSS Transeq (reading frame 1 [https://www.ebi.ac.uk/Tools/st/emboss_transeq/]). The implementation of Translated Nucleotide BLAST (tblastn) (13) in BTyper version 2.3.2 (14) selected mcr-3.17 as the highest-scoring mcr allele, which aligned to mcr-9 with 64.5% amino acid identity and 99.5% coverage (Table S1).
Comparison of mcr-9 to previously published mcr homologues using Protein BLAST (blastp). Download Table S2, DOCX file, 0.1 MB (21.2KB, docx) .
Copyright © 2019 Carroll et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
MUSCLE version 3.8.31 (15) was used to construct alignments of the amino acid sequence of mcr-9 (NCBI protein accession no. WP_001572373.1) and the following: (i) the 52 mcr amino acid sequences from ResFinder (53 sequences [Table S2]), (ii) the top 100 hits produced when mcr-9 was queried against NCBI’s non-redundant protein (nr) database using the Protein BLAST (blastp) web server (https://blast.ncbi.nlm.nih.gov/Blast.cgi?PAGE=Proteins [accessed 22 January 2019]; 152 sequences excluding mcr-9’s self-match [see Table S3 in the supplemental material]), and (iii) amino acid sequences of 61 putative phosphoethanolamine transferases used in other papers describing novel mcr genes (4, 5, 8, 9) (213 sequences [see Table S4 in the supplemental material]). For each alignment, RAxML version 8.2.12 (16) was used to construct a phylogeny using the PROTGAMMAAUTO method and 1,000 bootstrap replicates.
Top 100 hits obtained by querying mcr-9 against NCBI’s non-redundant protein sequence (nr) database using Protein BLAST (blastp). Download Table S3, DOCX file, 0.1 MB (27KB, docx) .
Copyright © 2019 Carroll et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Comparison of mcr-9 to putative phosphoethanolamine transferases used in other papers describing novel mcr genes. Download Table S4, DOCX file, 0.1 MB (22.9KB, docx) .
Copyright © 2019 Carroll et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
The amino acid sequence of mcr-9 most closely resembled those of mcr-3 and mcr-7 (Fig. 1A; see Fig. S1 in the supplemental material). However, the S. Typhimurium isolate in which mcr-9 was detected was not resistant to colistin at the >2-mg/liter European Committee on Antimicrobial Susceptibility Testing (EUCAST [http://www.eucast.org]) breakpoint when a broth microdilution method was used to determine the colistin MIC (see Table S5 in the supplemental material).
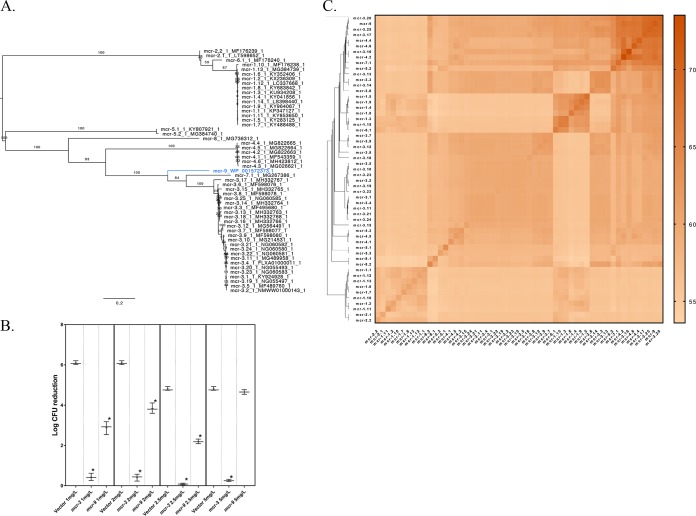
FIG 1.
(A) Comparison of mcr-9 to all previously described mcr homologues, based on amino acid sequence. The maximum likelihood phylogeny was constructed using RAxML version 8.2.12 with the amino acid sequences of novel mobilized colistin resistance gene mcr-9 (in blue) and all previously described mcr genes (mcr-1 to -8 [in black]). The phylogeny is rooted at the midpoint, with branch lengths reported in substitutions per site. Branch labels correspond to bootstrap support percentages out of 1,000 replicates. (B) Colistin killing assay of E. coli NEB5α harboring a pLIV2 empty vector (negative control), mcr-3 (positive control), or mcr-9, expressed under the control of the IPTG-controlled SPAC/lacOid promoter. Cells were grown in MH-II (Mueller-Hinton II) medium with IPTG to the mid-exponential phase. Colistin was added at concentrations of 0, 1, 2, 2.5, or 5 mg/liter, and the bacteria were incubated at 37°C for 1 h. The samples were diluted in phosphate-buffered saline (PBS) and plated on LB agar plates for the determination of CFU. Log CFU reduction was calculated by comparing CFU after each treatment to CFU levels obtained at 0 mg/liter colistin, using three independent biological replicates. Asterisks denote significant differences compared to empty vector treatment (P < 0.05 by Student's t test relative to the concentration's respective negative control after a Bonferroni correction). (C) Similarity matrix (composed of Dali Z-scores) of all previously described Mcr groups (Mcr-1 to -8) and Mcr-9, based on protein structure. The Dali server was used to perform all-against-all comparisons of 3D structural models based on all mcr homologues (Fig. 2A); for this analysis, amino acid sequences of mcr-5.3 and mcr-8.2, which were not available in ResFinder, were additionally included from the National Database of Antibiotic Resistant Organisms (NDARO).
Maximum likelihood phylogenies constructed using the amino acid sequences of novel mobilized colistin resistance gene mcr-9 (in blue) plus all (n = 52) previously described mcr genes (mcr-1, -2, -3, -4, -5, -6, -7, and -8) available in ResFinder (accessed 22 January 2019 [in pink]), as well as (A) the 100 top hits produced when mcr-9 was queried against NCBI’s non-redundant protein sequence (nr) database using the Protein BLAST (blastp) web server (accessed 22 January 2019) and default parameters (152 total sequences; mcr-9’s self-match was excluded, as it was already present in the nr database), as well as (B) amino acid sequences of 61 putative phosphoethanolamine transferases used in other papers describing novel mcr genes (213 total sequences). RAxML version 8.2.12 was used to construct the phylogenies, which were annotated with FigTree version 1.4.3. The phylogenies are rooted at the midpoint, with branch lengths reported in substitutions per site. Branch labels correspond to bootstrap support percentages out of 1,000 replicates. Download FIG S1, PDF file, 0.1 MB (118.1KB, pdf) .
Copyright © 2019 Carroll et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
MIC profiles of colistin against Salmonella strains tested in this study. Download Table S5, DOCX file, 0.1 MB (17.7KB, docx) .
Copyright © 2019 Carroll et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
mcr-9 confers resistance to colistin when cloned into colistin-susceptible E. coli NEB5α.
Coding regions of mcr-9 and mcr-3 were cloned under the control of an IPTG (isopropyl-β-d-thiogalactopyranoside)-induced SPAC/lacOid promoter and expressed in E. coli NEB5α (see Text S1 in the supplemental material). Colistin killing assays (Fig. 1B; see Fig. S2 in the supplemental material) were performed by incubating E. coli harboring the empty pLIV2 vector (negative control), pLIV2 with mcr-3 (positive control), or pLIV2 with mcr-9 with different concentrations of colistin (0, 1, 2, 2.5, and 5 mg/liter). E. coli cells harboring the empty vector failed to survive at all tested colistin concentrations >0 mg/liter. While mcr-3 expression conferred clinical levels of colistin resistance (i.e., beyond the 2-mg/liter EUCAST breakpoint) in E. coli at all tested concentrations, mcr-9 expression conferred clinical resistance at 1, 2, and 2.5 mg/liter, but not 5 mg/liter of colistin (Fig. 1B; Fig. S2).
Detailed descriptions of experimental methods. Download Text S1, DOCX file, 0.1 MB (21KB, docx) .
Copyright © 2019 Carroll et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Selected images associated with the colistin killing assay of E. coli NEB5α harboring a pLIV2 empty vector (negative control), mcr-3 (positive control), or mcr-9, expressed under the control of the IPTG-controlled SPAC/lacOid promoter. Cells were grown in MH-II (Mueller-Hinton II) medium with IPTG to the mid-exponential phase. Colistin was added at concentrations of 0, 1, 2, 2.5, or 5 mg/liter, and the bacteria were incubated at 37°C for 1 h. The samples were diluted in PBS and plated on LB agar plates for the determination of CFU. Download FIG S2, PDF file, 2.4 MB (2.4MB, pdf) .
Copyright © 2019 Carroll et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Mcr-3, Mcr-4, Mcr-7, and Mcr-9 are highly similar at the structural level.
Three-dimensional (3D) structural models of all nine Mcr homologues (Fig. 2A) based on EptA (2) were constructed using the Phyre2 server (17) and visualized using UCSF Chimera (18). Congruent with the phylogeny based on their amino acid sequences (Fig. 1A), comparisons of different Mcr protein models using Dali (19) revealed that Mcr-3, Mcr-4, Mcr-7, and Mcr-9 were closely related at the structural level (Fig. 1C).
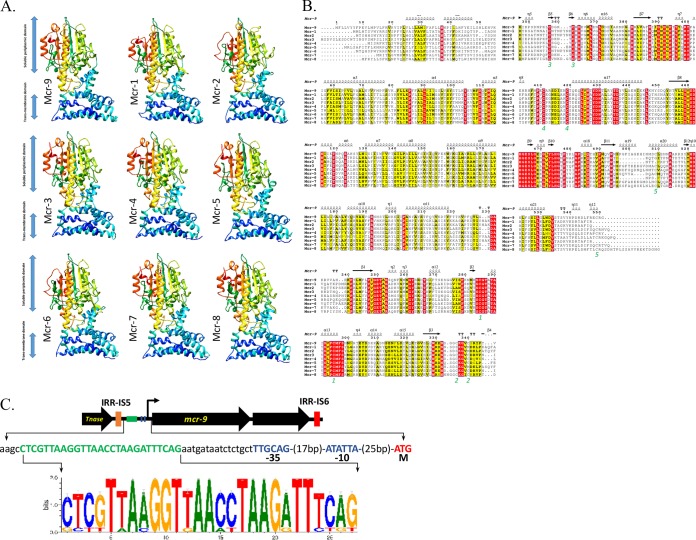
FIG 2.
(A) Structural models of all published Mcr proteins (Mcr-1 to -8) and Mcr-9, based on lipooligosaccharide phosphoethanolamine transferase EptA. Models were constructed using the Phyre2 server, and structures were viewed and edited using UCSF Chimera. Structural models show conservation of two EptA domains: transmembrane-anchored and soluble periplasmic domains. (B) Location of Mcr-9 secondary structure elements within the alignment of Mcr amino acid sequences, constructed using the ESPript 3 server. The top track denotes Mcr-9 secondary structure elements (alpha helixes and beta sheets). Green digits below the alignment denote cysteine residues forming a disulfide bridge (e.g., 1 forms a bridge with 1, 2 with 2, etc.). Within the amino acid sequence alignment itself, a strict identity (i.e., identical amino acid residue at a site) is denoted by a red box and a white character. A yellow box around an amino acid residue denotes similarity across groups, where groups were defined using the default “all” specification in ESPript 3 (ESPript 3 total score [TSc] > in-group threshold [ThIn]), while a residue in boldface denotes similarity within a group (ESPript 3 in-group score [ISc] > ThIn). (C) Organization of the mcr-9 locus in S. Typhimurium. An unknown function cupin fold metalloprotein is encoded by the gene downstream of mcr-9 (unlabeled black arrow). The mcr-9 locus is flanked by two different terminal repeat sequences (IRR) from the IS5 (orange box) and IS6 (red box) families. The mcr-9 upstream region contains highly conserved putative −35 and −10 σ70-dependent promoter elements (blue boxes and blue text). Moreover, the mcr-9 promoter region contains an inverted repeat motif (green box, green text, and sequence logo) that is conserved in more than 95% of 321 mcr-9 genes, as shown by the sequence logo (constructed using WebLogo) (24).
Proteins encoded by mcr-1 to -9 revealed high levels of conservation for both the membrane-anchored domain and the soluble catalytic domain (Fig. 2A). Interestingly, analyses of structural models of the nine Mcr homologues using the ESPript 3 server (20) showed that both amino acids and structural elements were conserved on the C-terminal catalytic domain, while only structural elements were conserved on the membrane-anchored N-terminal domain (Fig. 2B).
Numerous genera of Enterobacteriaceae harbor mcr-9 on IncHI2 plasmids.
blastp searches of mcr-9 against NCBI’s nr database revealed that mcr-9 was present in multiple genera of Enterobacteriaceae (Table S3). The 10 highest-scoring hits in the nr database matched mcr-9 with at least 99% amino acid identity (including mcr-9 characterized here [Table S3 and Fig. S1A]); the amino acid identities of the remaining hits with high query coverage (> 90%) dropped below 88% identity (Table S3 and Fig. S1A). mcr-9 was detected in 335 genomes linked to NCBI identical protein groups (IPGs) associated with the 10 highest-scoring protein accession numbers (accessed 23 January 2019 [see Tables S3 and S6 in the supplemental material]). Analysis of the mcr-9 promoter region in 321 of these genomes (Text S1) showed conserved putative σ70 family-dependent −35 and −10 regions and an inverted repeat (Fig. 2C). The conserved DNA motif in the mcr-9 promoter is likely a recognition sequence for a transcription regulator, suggesting that additional factors or induction/derepression conditions might be needed for full expression of wild-type mcr-9. Promoter variation (21) and testing conditions (22, 23) have been shown to influence mcr expression and the colistin MIC, which may explain why the S. Typhimurium strain queried here was colistin susceptible under the tested conditions.
Location of mcr-9 on contigs for 335 genome assemblies. Download Table S6, DOCX file, 0.1 MB (103.1KB, docx) .
Copyright © 2019 Carroll et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Of the 335 genomes in which mcr-9 was detected, 65 had at least one plasmid replicon (detected using ABRicate and PlasmidFinder as described above) present on the same contig as mcr-9; in 59 of these 65 genomes, IncHI2 and/or IncHI2A replicons were detected on the same contig as mcr-9 (Table S6). In 32 of the 37 closed genomes in which it was detected, mcr-9 was harbored on a plasmid (Table S6). These results indicate that mcr-9 has the potential to reduce susceptibility to colistin, up to and beyond the EUCAST breakpoint, and can be found extrachromosomally in multiple species of Enterobacteriaceae, making it a relevant threat to public health. Future studies querying the plasmids that harbor mcr-9 (e.g., transferability, stability, and copy number variation) will offer further insight into the potential role that mcr-9 plays in the dissemination of colistin resistance worldwide.
Accession number(s).
The nucleotide and amino acid sequences of mcr-9 are available under NCBI reference sequence accession no. NZ_NAAN01000063.1 (NCBI protein accession no. WP_001572373.1).
ACKNOWLEDGMENTS
This material is based on work supported by the National Science Foundation (NSF) Graduate Research Fellowship Program under grant no. DGE-1650441, with additional funding provided by an NSF Graduate Research Opportunities Worldwide (GROW) grant through a partnership with the Swiss National Science Foundation (SNF).
We thank Julie Siler (Cornell University) for providing colistin resistance testing materials.
Footnotes
Citation Carroll LM, Gaballa A, Guldimann C, Sullivan G, Henderson LO, Wiedmann M. 2019. Identification of novel mobilized colistin resistance gene mcr-9 in a multidrug-resistant, colistin-susceptible Salmonella enterica serotype Typhimurium isolate. mBio 10:e00853-19. https://doi.org/10.1128/mBio.00853-19.
Contributor Information
Mark S. Turner, University of Queensland.
Gregory Siragusa, Eurofins.
David White, University of Tennessee.
REFERENCES
- 1.Liu Y-Y, Wang Y, Walsh TR, Yi L-X, Zhang R, Spencer J, Doi Y, Tian G, Dong B, Huang X, Yu L-F, Gu D, Ren H, Chen X, Lv L, He D, Zhou H, Liang Z, Liu J-H, Shen J. 2016. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis 16:161–168. doi: 10.1016/S1473-3099(15)00424-7. [DOI] [PubMed] [Google Scholar]
- 2.Anandan A, Evans GL, Condic-Jurkic K, O’Mara ML, John CM, Phillips NJ, Jarvis GA, Wills SS, Stubbs KA, Moraes I, Kahler CM, Vrielink A. 2017. Structure of a lipid A phosphoethanolamine transferase suggests how conformational changes govern substrate binding. Proc Natl Acad Sci U S A 114:2218–2223. doi: 10.1073/pnas.1612927114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xavier BB, Lammens C, Ruhal R, Kumar-Singh S, Butaye P, Goossens H, Malhotra-Kumar S. 2016. Identification of a novel plasmid-mediated colistin-resistance gene, mcr-2, in Escherichia coli, Belgium, June 2016. Euro Surveill 21:30280. doi: 10.2807/1560-7917.ES.2016.21.27.30280. [DOI] [PubMed] [Google Scholar]
- 4.Yin W, Li H, Shen Y, Liu Z, Wang S, Shen Z, Zhang R, Walsh TR, Shen J, Wang Y. 2017. Novel plasmid-mediated colistin resistance gene mcr-3 in Escherichia coli. mBio 8:e00543-17. doi: 10.1128/mBio.00543-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carattoli A, Villa L, Feudi C, Curcio L, Orsini S, Luppi A, Pezzotti G, Magistrali CF. 2017. Novel plasmid-mediated colistin resistance mcr-4 gene in Salmonella and Escherichia coli, Italy 2013, Spain and Belgium, 2015 to 2016. Euro Surveill 22:30589. doi: 10.2807/1560-7917.ES.2017.22.31.30589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borowiak M, Fischer J, Hammerl JA, Hendriksen RS, Szabo I, Malorny B. 2017. Identification of a novel transposon-associated phosphoethanolamine transferase gene, mcr-5, conferring colistin resistance in d-tartrate fermenting Salmonella enterica subsp. enterica serovar Paratyphi B. J Antimicrob Chemother 72:3317–3324. doi: 10.1093/jac/dkx327. [DOI] [PubMed] [Google Scholar]
- 7.AbuOun M, Stubberfield EJ, Duggett NA, Kirchner M, Dormer L, Nunez-Garcia J, Randall LP, Lemma F, Crook DW, Teale C, Smith RP, Anjum MF. 2017. mcr-1 and mcr-2 variant genes identified in Moraxella species isolated from pigs in Great Britain from 2014 to 2015. J Antimicrob Chemother 72:2745–2749. doi: 10.1093/jac/dkx286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang YQ, Li YX, Lei CW, Zhang AY, Wang HN. 2018. Novel plasmid-mediated colistin resistance gene mcr-7.1 in Klebsiella pneumoniae. J Antimicrob Chemother 73:1791–1795. doi: 10.1093/jac/dky111. [DOI] [PubMed] [Google Scholar]
- 9.Wang X, Wang Y, Zhou Y, Li J, Yin W, Wang S, Zhang S, Shen J, Shen Z, Wang Y. 2018. Emergence of a novel mobile colistin resistance gene, mcr-8, in NDM-producing Klebsiella pneumoniae. Emerg Microbes Infect 7:122. doi: 10.1038/s41426-018-0124-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carroll LM, Wiedmann M, den Bakker H, Siler J, Warchocki S, Kent D, Lyalina S, Davis M, Sischo W, Besser T, Warnick LD, Pereira RV. 2017. Whole-genome sequencing of drug-resistant Salmonella enterica isolates from dairy cattle and humans in New York and Washington States reveals source and geographic associations. Appl Environ Microbiol 83:e00140-17. doi: 10.1128/AEM.00140-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zankari E, Hasman H, Cosentino S, Vestergaard M, Rasmussen S, Lund O, Aarestrup FM, Larsen MV. 2012. Identification of acquired antimicrobial resistance genes. J Antimicrob Chemother 67:2640–2644. doi: 10.1093/jac/dks261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carattoli A, Zankari E, García-Fernández A, Voldby Larsen M, Lund O, Villa L, Møller Aarestrup F, Hasman H. 2014. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob Agents Chemother 58:3895–3903. doi: 10.1128/AAC.02412-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, Bealer K, Madden TL. 2009. BLAST+: architecture and applications. BMC Bioinformatics 10:421. doi: 10.1186/1471-2105-10-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carroll LM, Kovac J, Miller RA, Wiedmann M. 2017. Rapid, high-throughput identification of anthrax-causing and emetic Bacillus cereus group genome assemblies using BTyper, a computational tool for virulence-based classification of Bacillus cereus group isolates using nucleotide sequencing data. Appl Environ Microbiol 83:e01096-17. doi: 10.1128/AEM.01096-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edgar RC. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelley LA, Mezulis S, Yates CM, Wass MN, Sternberg MJ. 2015. The Phyre2 web portal for protein modeling, prediction and analysis. Nat Protoc 10:845–858. doi: 10.1038/nprot.2015.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. 2004. UCSF Chimera—a visualization system for exploratory research and analysis. J Comput Chem 25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 19.Holm L, Laakso LM. 2016. Dali server update. Nucleic Acids Res 44:W351–W355. doi: 10.1093/nar/gkw357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robert X, Gouet P. 2014. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res 42:W320–W324. doi: 10.1093/nar/gku316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang B, He Y, Ma X, Cai R, Zeng J, Lu Y, Zhang W, Lan K, E S, Tang YW, Kreiswirth BN, Chen C, Chen L. 2018. Promoter variation and gene expression of mcr-1-harboring plasmids in clinical isolates of Escherichia coli and Klebsiella pneumoniae from a Chinese hospital. Antimicrob Agents Chemother 62:e00018-18. doi: 10.1128/AAC.00018-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang H, Miao M, Yan J, Wang M, Tang YW, Kreiswirth BN, Zhang X, Chen L, Du H. 2017. Expression characteristics of the plasmid-borne mcr-1 colistin resistance gene. Oncotarget 8:107596–107602. doi: 10.18632/oncotarget.22538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gwozdzinski K, Azarderakhsh S, Imirzalioglu C, Falgenhauer L, Chakraborty T. 2018. An improved medium for colistin susceptibility testing. J Clin Microbiol 56:e01950-17. doi: 10.1128/JCM.01950-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crooks GE, Hon G, Chandonia JM, Brenner SE. 2004. WebLogo: a sequence logo generator. Genome Res 14:1188–1190. doi: 10.1101/gr.849004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Antimicrobial resistance (AMR) genes and plasmid replicons detected in the assembly of HUM_TYPH_WA_10_R9_3274. Download Table S1, DOCX file, 0.1 MB (22.3KB, docx) .
Copyright © 2019 Carroll et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Comparison of mcr-9 to previously published mcr homologues using Protein BLAST (blastp). Download Table S2, DOCX file, 0.1 MB (21.2KB, docx) .
Copyright © 2019 Carroll et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Top 100 hits obtained by querying mcr-9 against NCBI’s non-redundant protein sequence (nr) database using Protein BLAST (blastp). Download Table S3, DOCX file, 0.1 MB (27KB, docx) .
Copyright © 2019 Carroll et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Comparison of mcr-9 to putative phosphoethanolamine transferases used in other papers describing novel mcr genes. Download Table S4, DOCX file, 0.1 MB (22.9KB, docx) .
Copyright © 2019 Carroll et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Maximum likelihood phylogenies constructed using the amino acid sequences of novel mobilized colistin resistance gene mcr-9 (in blue) plus all (n = 52) previously described mcr genes (mcr-1, -2, -3, -4, -5, -6, -7, and -8) available in ResFinder (accessed 22 January 2019 [in pink]), as well as (A) the 100 top hits produced when mcr-9 was queried against NCBI’s non-redundant protein sequence (nr) database using the Protein BLAST (blastp) web server (accessed 22 January 2019) and default parameters (152 total sequences; mcr-9’s self-match was excluded, as it was already present in the nr database), as well as (B) amino acid sequences of 61 putative phosphoethanolamine transferases used in other papers describing novel mcr genes (213 total sequences). RAxML version 8.2.12 was used to construct the phylogenies, which were annotated with FigTree version 1.4.3. The phylogenies are rooted at the midpoint, with branch lengths reported in substitutions per site. Branch labels correspond to bootstrap support percentages out of 1,000 replicates. Download FIG S1, PDF file, 0.1 MB (118.1KB, pdf) .
Copyright © 2019 Carroll et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
MIC profiles of colistin against Salmonella strains tested in this study. Download Table S5, DOCX file, 0.1 MB (17.7KB, docx) .
Copyright © 2019 Carroll et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Detailed descriptions of experimental methods. Download Text S1, DOCX file, 0.1 MB (21KB, docx) .
Copyright © 2019 Carroll et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Selected images associated with the colistin killing assay of E. coli NEB5α harboring a pLIV2 empty vector (negative control), mcr-3 (positive control), or mcr-9, expressed under the control of the IPTG-controlled SPAC/lacOid promoter. Cells were grown in MH-II (Mueller-Hinton II) medium with IPTG to the mid-exponential phase. Colistin was added at concentrations of 0, 1, 2, 2.5, or 5 mg/liter, and the bacteria were incubated at 37°C for 1 h. The samples were diluted in PBS and plated on LB agar plates for the determination of CFU. Download FIG S2, PDF file, 2.4 MB (2.4MB, pdf) .
Copyright © 2019 Carroll et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Location of mcr-9 on contigs for 335 genome assemblies. Download Table S6, DOCX file, 0.1 MB (103.1KB, docx) .
Copyright © 2019 Carroll et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.