Abstract
Transformation by oncogenic Ras profoundly alters actin cytoskeleton organization. We investigated Ras-dependent signaling pathways involved in cytoskeleton disruption by transfecting normal rat kidney (NRK) cells with different Ras mutants. RasV12S35, a mutant known to activate specifically the Raf/MAPK pathway, led to stress fiber and focal contact disruption, whereas the adherens junctions remained intact. Next, we found that pharmacological inhibition of MEK was sufficient to restore the cytoskeletal defects of ras-transformed NRK cells, including assembly of stress fibers and focal contacts, but it did not induce reorganization of the cell-cell junctions. Investigating the mechanism underlying this phenotypic reversion, we found that the sustained MAPK signaling resulting from Ras-transformation down-regulated the expression of ROCKI and Rho-kinase, two-Rho effectors required for stress fiber formation, at the post-transcriptional level. On MEK inhibition, ROCKI/Rho-kinase expression and cofilin phosphorylation were increased, demonstrating that the Rho-kinase/LIM-kinase/cofilin pathway was functionally restored. Finally, using dominant negative or constitutively active mutants, we demonstrated that expression of ROCKI/Rho-kinase was both necessary and sufficient to promote cytoskeleton reorganization in NRK/ras cells. These findings further establish the Ras/MAPK pathway as the critical pathway involved in cytoskeleton disruption during Ras-transformation, and they suggest a new mechanism, involving alteration in ROCKI/Rho-kinase expression, by which oncogenic Ras can specifically target the actin-based cytoskeleton and achieve morphological transformation of the cells.
INTRODUCTION
Oncogenic transformation is characterized not only by deregulated growth control but also by pronounced morphological changes resulting from alterations in the organization of the actin cytoskeleton and adhesive interactions. Changes in the organization of actin filaments are highly correlated with anchorage-independent growth and tumorigenicity, suggesting a fundamental role for actin fibers in cell growth control (reviewed in Pawlak and Helfman, 2001). Alterations in actin filament structure are associated with decreased expression of numerous cytoskeletal proteins (Button et al., 1995). Forced re-expression of these proteins by transfection, including α-actinin (Gluck et al., 1993; Nikolopoulos et al., 2000), profilin (Janke et al., 2000), vinculin (Rodriguez-Fernandez et al., 1992), and tropomyosin (Prasad et al., 1993, 1999; Takenaga and Masuda, 1994; Gimona et al., 1996; Braverman et al., 1996; Janssen and Mier, 1997) can reduce or abrogate the transformed phenotype. Although the mechanisms by which these proteins contribute to growth control remain to be fully elucidated, these studies demonstrate that changes in the expression of specific structural components of the actin cytoskeleton can contribute to transformation.
Small GTPases of the Ras superfamily control a wide spectrum of cellular processes by switching between inactive GDP- and active GTP-bound states. When bound to GTP, these proteins regulate cell behavior by binding to effector molecules and by altering their localization, protein-protein interactions, and activity. Small GTPase proteins can be divided into subfamilies as follows: members of the Ras subfamily regulate cell proliferation and differentiation, whereas members of the Rho subfamily were first identified as regulators of the actin cytoskeleton but also affect gene expression and proliferation (Bar-Sagi and Hall, 2000). The importance of the Ras subfamily of genes in the control of cell proliferation is demonstrated by the high frequency of mutations, among the highest of any gene in human cancers, that activate Ras in tumors (Hunter, 1997). Ras is known to activate multiple effectors, including Raf, which in turn activates the mitogen-activated protein kinase kinase (MEK) and mitogen-activated protein kinase (MAPK) cascade, the phosphatidylinositol 3-kinase (PI 3-K), and the RalGDS family of guanine nucleotide exchange factors for Ral GTPases (Katz and McCormick, 1997). A major task is to discern which effector pathway contributes to which aspect of the transformed phenotype (Shields et al., 2000).
In addition to structural components such as tropomyosins (TM), organization of actin filaments is under the control of the Rho subfamily of small GTPases: Rho, Rac, and Cdc42 (Bishop and Hall, 2000). Among these, Rho regulates the assembly of actin stress fibers and focal contacts through activation of the downstream effectors mDia and the closely related kinases ROCKI/Rho-kinase (Amano et al., 1997; Watanabe et al., 1999). Activation of Rho-kinase by Rho is implicated in stress fiber and in focal contact formation (Leung et al., 1996; Amano et al., 1997). Rho-kinase increases the phosphorylation of myosin light chains and thus increases acto-myosin–based contractility, by directly phosphorylating myosin light chains and by negatively regulating myosin phosphatase (Kureishi et al., 1997; reviewed in Fukata et al., 2001). The resulting contractile forces are thought to contribute to the formation of stress fibers and focal contacts (Burridge et al. 1997, Helfman et al., 1999). Rho-kinase also activates LIM-kinase, which subsequently phosphorylates cofilin and thereby inhibits its actin-depolymerizing activity, thus contributing to actin fiber stabilization (Yang et al., 1998; Maekawa et al., 1999; Bamburg et al., 1999; Ohashi et al., 2000).
Rho-dependent signaling is required for transformation by oncogenic Ras (Khosravi-Far et al., 1995; Qiu et al., 1995; Zhong et al., 1997). Although Rho activation is known to promote the development of stress fibers and focal contacts, Ras-transformed fibroblasts generally exhibit a loss of these structural elements characteristic of Rho activity. These results have led to the interpretation that Rho activity may be reduced in Ras-transformed cells. Accordingly, activated Rho can restore stress fibers in Ras-transformed Rat1 fibroblasts, suggesting that Rho can be inactivated in these Ras-transformed cells (Izawa et al., 1998). By contrast, inhibition of Rho-kinase was shown to block Ras transformation in NIH3T3 cells (Sahai et al., 1999) and to attenuate invasiveness of tumor cells (Itoh et al., 1999). Thus, the relationship between Ras- and Rho-dependent signaling pathways during transformation is still unclear.
Application of MEK inhibitors was reported to cause morphological reversion of Ras-transformed fibroblasts (Fukazawa and Uehara, 2000; Reuveni et al., 2000). The signaling events associated with this phenotypic reversion, however, have not been fully investigated. In the present study, we have investigated the signaling pathways activated by Ras, as well as the mechanism by which oncogenic Ras can specifically target the actin-based cytoskeleton and thereby achieve morphological transformation of the cells.
MATERIALS AND METHODS
Antibodies and Reagents
The mouse monoclonal anti-vinculin, anti-β-actin, anti–c-myc (clone 9E10), and anti-tropomyosin (TM) (clone TM311) antibodies (mAbs), as well as the rabbit polyclonal anti-β catenin antibody, were purchased from Sigma Chemical (St. Louis, MO). The mouse anti-Rho-kinase and anti-ROCKI mAbs were obtained from Transduction Laboratories (Lexington, KY). The mouse antiphosphorylated MAPK (Tyr204) mAb and the rabbit polyclonal anti-MAPK were from Santa Cruz Biotechnology (Santa Cruz, CA). The rabbit polyclonal anticofilin was from Cytoskeleton (Denver, CO). The rabbit serum recognizing cofilin when phosphorylated by LIM-kinase was kindly provided by J. R. Bamburg (Colorado State University, Fort Collins, CO). The rabbit polyclonal antiphospho-Akt (Ser473) was from Cell Signaling Technology (Beverly, MA). The mAb 12CA5 to the HA tag was produced and purified by the Antibody Facility of Cold Spring Harbor Laboratory (Cold Spring Harbor, NY). Secondary antibodies Cy3-conjugated goat anti-mouse and goat anti-rabbit IgG were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA). Oregon green-conjugated phalloidin was from Molecular Probes (Eugene, OR). PD098059, U0126, and LY294002 were from Calbiochem (La Jolla, CA), and Yoshitomi Pharmaceutical Industries (Osaka, Japan) kindly provided Y27632. All chemicals and reagents were obtained from Sigma, unless otherwise indicated, and all tissue culture reagents were from Life Technologies (Gaithersburg, MD).
Cell Culture and Drug Treatments
Normal and v-Ki-ras–transformed normal rat kidney (NRK) cells were from ATCC (NRK ATCC CRL 1570 and 1569, respectively). Cells were maintained in DMEM containing 5% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin in a humidified air (5% CO2) atmosphere, at 37°C. Cells from subconfluent dishes were treated with DMSO alone or with various concentrations of PD098059, U0126, LY294002, or Y27632 (all prepared as 50 mM stock in DMSO and stored at −20°C) before being fixed for immunofluorescence or lysed for Western blotting or RNA extraction.
Expression Vectors and Transient and Stable Transfections
Plasmids pDCR-Ha-Ras (G12V, T35S), pDCR-Ha-Ras (G12V, Y40C), and pDCR-Ha-Ras (G12V, E37G), in which HA-tagged Ras proteins were expressed under the control of CMV promoter were a generous gift of M. A. White (University of Texas, Southwestern Medical Center, Dallas, TX). pEF-BOS-myc-Rho-kinase-CAT and pEF-BOS-myc-Rho-kinase-RB/PH(TT) constructs, encoding myc-tagged mutants of Rho-kinase, were kindly provided by K. Kaibuchi (Nara Institute of Science and Technology, Nara, Japan). For stable expression of the Ras effector loop mutants, NRK cells were transfected by the calcium phosphate procedure. Transfected cells were selected in G418-containing medium (500 μg/ml) for 2 weeks, and drug-resistant colonies were pooled and analyzed, as described in RESULTS. For transient transfections, cells were grown on glass coverslips in DMEM containing 5% FBS. After 1 day of culture, cells were transfected with plasmid DNA with Lipofectamine PLUS reagent (Life Technologies) according to the manufacturer's protocol. At 20 h post-transfection, cells were eventually treated with DMSO, PD098059, or U0126 for 30 h or with Y27632 for 1 h. Coverslips were then fixed and stained for immunofluorescence.
Immunofluorescence
Cells grown on glass coverslips were fixed with 3% paraformaldehyde for 15 min, permeabilized with 0.2% Triton X-100 in PBS for 15 min, then blocked for 30 min with 1% BSA at room temperature. Incubations with primary antibodies against vinculin (1:400), TM (1:100), β-catenin (1:500), HA-tag (1:800), or myc-tag (1:500) were conducted at room temperature for 1 h. After washing, cells were incubated with Cy3-conjugated secondary antibodies (1:500) for 45 min. To stain actin, fixed cells were incubated with Oregon green-conjugated phalloidin. Cells were finally stained with 4′6-diamidino-2-phenylindole (DAPI), and coverslips were mounted using Prolong Antifade (Molecular Probes). Samples were examined and pictures acquired on a Zeiss Axiophot microscope equipped with a Photometrics SenSys (Oberkochen, Germany) cooled CCD camera using Image 2.0.5 software (Oncor, Gaithersburg, MD). All photographs were taken at the same magnification.
Western Blot
Control and treated cells were washed with ice-cold PBS containing 1 mM sodium orthovanadate before direct extraction in 2% SDS Laemmli sample buffer. Lysates were clarified by centrifugation (16,000 g, 15 min at 4°C), and protein concentrations were measured by bicinchoninic acid protein assay (Bio-Rad, Hercules, CA). Equal amounts of proteins were resolved by SDS-PAGE and were transferred to nitrocellulose membrane (Schleicher & Schuell, Keene, NH). The membrane was blocked in 5% nonfat dried milk or 2% BSA in Tris-buffered saline plus 0.1% Tween 20 and incubated with primary antibodies for 1 h, followed by incubation with appropriate horseradish peroxidase-conjugated secondary antibodies for 1 h at room temperature. Immunoreactive bands were detected by chemiluminescence (NEN, Boston, MA), according to the manufacturer's instructions.
Rho-GTP Pull-down Assay
Measurement of GTP-bound Rho was performed using the Rho Activation Assay kit (Upstate Biotechnology), following the manufacter's instructions. Briefly, the RhoA-binding domain of Rhotekin expressed as a GST-fusion protein was used to affinity precipitate GTP-bound Rho from cells lysed in 50 mM Tris, pH 7.2, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 500 mM NaCl, 10 mM MgCl2 and a cocktail of protease inhibitors (Roche). Precipitated Rho-GTP was then detected by immunoblot analysis, using a polyclonal anti-Rho (-A, -B, -C) antibody (Upstate Biotechnology).
Semiquantitative RT-PCR
Total RNAs were extracted with Trizol reagent (Life Technologies). cDNA synthesis and PCR amplification were performed with Superscript One-Step RT-PCR (Life Technologies), using 0.2 μg RNA. Rho-kinase cDNAs were amplified with sense primer (5′-ATGTCGACTGGGGACAGTTTTGAGACT) and antisense primer (5′-CTATAGATTTCTTCTTTGATTTCCCTC) for 15, 20, and 25 cycles, to remain within the exponential phase of amplification. As an internal control, rat GAPDH cDNA was also amplified with sense primer (5′-GTTCCAGTATGATTCTACCCACGG) and antisense primer (5′-ATGAGCCCTTCCACGATGCCAAAG). Ten-microliter aliquots of the PCR reaction were size-separated on a 2% agarose gel, photographed, and analyzed by densitometry using AlphaImager 2200 v5.5 software (Alpha Innotech Corp., San Leandro, CA).
RESULTS
Constitutive Activation of the Ras/MAPK Pathway Is Sufficient to Disrupt the Actin Cytoskeleton in NRK Cells
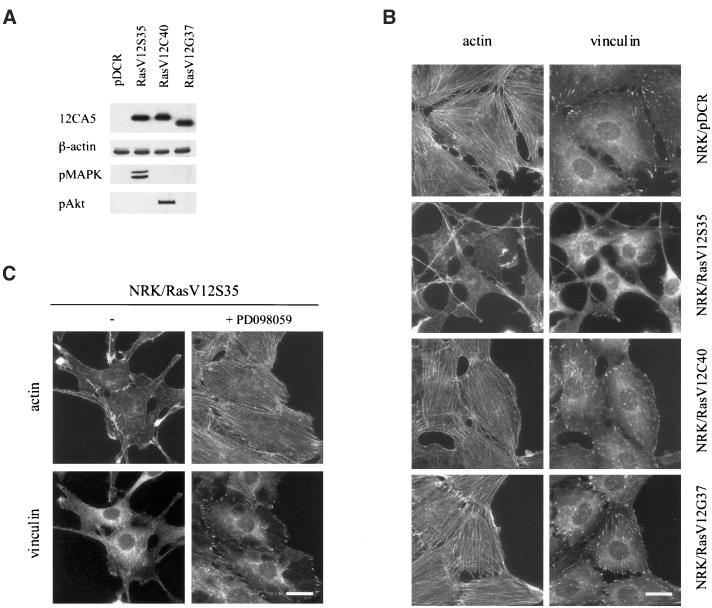
Whereas untransformed NRK cells have a flat morphology and well-developed stress fibers, NRK/ras cells (rat fibroblasts transformed by v-Ki-ras) are round and are devoid of stress fibers and focal contacts. To assess the relative contribution of the various downstream effectors of Ras, we analyzed different Ras effector loop mutants for their ability to induce cytoskeleton disruption in NRK cells. Untransformed NRK cells were transfected with three different Ras effector loop mutants: RasV12S35, which activates almost exclusively the Raf pathway; RasV12C40, which activates exclusively the PI 3-K pathway; and RasV12G37, which activates RalGDS (White et al., 1995). Stable cell lines were established for each construct. Expression and specificity of the transfected constructs were confirmed by Western-blot analysis using antibodies to the HA epitope tag, active phospho-MAPK, which is downstream of Raf, and phospho-Akt, which is downstream of PI 3-K. Figure 1A shows that RasV12S35 increased only MAPK phosphorylation, whereas RasV12C40 increased only Akt phosphorylation. RasV12G37 had no activatory effect on either kinase, confirming that this mutant does not activate either pathway.
Figure 1.
Activation of the Ras/MAPK pathway disrupts the actin cytoskeleton and focal contacts in NRK cells. NRK cells were transfected with cDNAs encoding various mutant forms of Ras or empty vector (pDCR). G418-resistant clones were pooled and grown onto coverslips for immunofluorescence staining, or extracts were prepared for Western blotting. (A) Whole-cell extracts from NRK/pDCR, NRK/RasV12S35, NRK/RasV12C40, and NRK/RasV12G37 cells were immunoblotted with mAb 12CA5 (recognizing the HA tag) and anti-β-actin to ensure equal loading. The same lysates were analyzed with antiphospho-MAPK (pMAPK) or antiphospho-Akt (pAkt) antibodies. (B) NRK cells grown on glass coverslips were fixed. Actin stress fibers and focal contacts were visualized by indirect immunofluorescence using phalloidin and antivinculin mAb, respectively. Bar, 20 μm. (C) NRK/RasV12S35 cells were treated with 0.1% DMSO or 50 μM PD098059 for 48h, then fixed and stained with phalloidin and antivinculin mAb. Bar, 20 μm.
Among the different cell lines, the NRK/RasV12S35 cells exhibited spindle-shape morphology and grew in multiple layers. The distribution of actin filaments and vinculin was examined by indirect immunofluorescence (Figure 1B). This analysis showed that the actin cytoskeleton and focal contacts were disrupted in NRK/RasV12S35 cells. In contrast, both the NRK/RasV12C40 and NRK/RasV12G37 cell lines retained organized stress fibers and abundant cell-matrix contacts characteristic of control NRK cells (Figure 1B). To further demonstrate that activation of a MEK-dependent pathway was responsible for the observed loss of actin fibers and focal contact, NRK/RasV12S35 cells were treated with 50 μM of the MEK inhibitor PD098059 (Dudley et al., 1995). This treatment leads to restoration of stress fibers and focal contacts (Figure 1C). These results demonstrate that activation of the Ras/MAPK pathway is both necessary and sufficient to disrupt actin stress fibers and focal contacts in NRK cells.
Inhibition of MEK Restores Actin Cytoskeleton Organization and Focal Contact Assembly in Ras-transformed NRK Cells
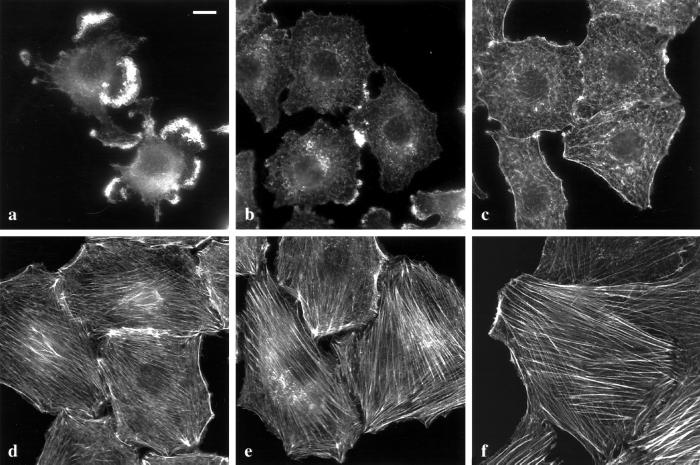
We next tested whether inhibition of the Ras/MAPK pathway could reverse the phenotype of NRK cells transformed by a fully activated oncogenic Ras (NRK/ras cells). We found that NRK/ras cells treated for 24 h with 50 μM PD098059 reacquired a fibroblastic morphology and formed a flat, nonoverlapping monolayer. To examine cytoskeletal changes associated with this morphological reversion, actin was stained with fluorescence-labeled phalloidin. Stress fibers are noticeably absent from NRK/ras cells compared with the well-developed actin bundles observed in untransformed NRK cells (Figure 2a, compared with 2f). On treatment with PD098059, actin organization changed from patches in rounded cells (Figure 2a) to a diffuse network in flat cells (10 h, Figure 2b) and then to cortical fibers surrounding cells with a more polarized morphology (24 h, Figure 2c). After 48 h of PD098059 treatment, actin filaments were reorganized into stress fibers extending through the entire cellular body (Figure 2d). Bundling of actin eventually increased further at 72 h, thus resembling stress fibers of untransformed NRK cells (Figure 2e, compared with 2f).
Figure 2.
Reorganization of the actin cytoskeleton in NRK/ras cells upon treatment with PD098059. NRK/ras cells were treated with 0.1% DMSO (a) or 50 μM PD098059 for 10 h (b), 24 h (c), 48 h (d), or 72 h (e), then fixed and stained with Oregon green-conjugated phalloidin. Untransformed NRK cells are shown in (f) for comparison. Bar, 20 μm.
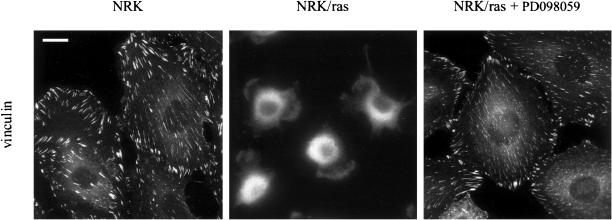
Ras-transformed NRK cells also exhibit disrupted focal contacts. Immunofluorescence labeling with anti-vinculin antibodies showed that NRK cells contained well-developed focal contacts, whereas the vinculin staining in NRK/ras cells exhibited a diffuse cytoplasmic pattern (Figure 3). A Western-blot analysis showed that the level of expression of vinculin was not affected in NRK/ras cells. Treatment of NRK/ras cells with 50 μM PD098059 for 48 h led to the assembly of vinculin-containing focal contacts (Figure 3). These focal contacts were almost indistinguishable from those of control NRK cells. This result suggests that constitutive activation of MEK prevents the assembly of focal contacts and that inhibition of MEK is sufficient to reverse this effect.
Figure 3.
Assembly of focal contacts in NRK/ras cells treated with PD098059. NRK and NRK/ras cells are compared with NRK/ras cells treated with 50 μM PD098059 for 48 h. Fixed cells were stained with anti-vinculin mAb, followed by Cy3-conjugated antimouse antibody. Bar, 20 μm.
To eliminate the possibility that PD098059 may restore actin stress fibers and focal contacts by acting on targets other than MEK1/2, we used the unrelated MEK inhibitor U0126 (Favata et al., 1998). Consistent with the effects being MEK-dependent, treatment of NRK/ras cells with 25 μM of U0126 led to effects similar to those of PD098059.
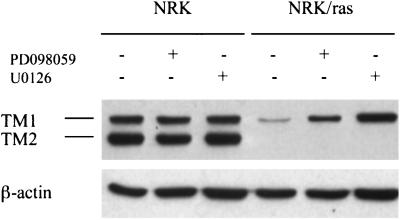
Alterations in actin filament structure are correlated with decreased expression of numerous cytoskeletal proteins (Button et al., 1995). It was previously shown that Ras-transformed NRK cells have substantially reduced levels of high-molecular-weight TM isoforms (Matsumura et al., 1983). Because these actin-associated proteins are involved in stabilization of the actin cytoskeleton, we asked whether restoration of stress fibers in cells treated with MEK inhibitors was associated with changes in the level of TM expression level. Treatment of NRK/ras cells by either 50 μM PD098059 or 25 μM U0126 for 48 h specifically enhanced TM-1 expression (Figure 4). The expression level of TM-2 was not affected. Immunofluorescence staining showed that the TM-1 was incorporated into the newly assembled stress fibers (our unpublished results). Taken together, these results demonstrate that the Ras/MAPK pathway plays a pivotal role in the regulation of actin cytoskeleton organization in Ras-transformed NRK cells.
Figure 4.
MEK inhibition causes elevation of TM-1 expression level. Whole cell extracts of NRK and NRK/ras cells maintained in the absence (−) or presence (+) of 50 μM PD098059 or 25 μM U0126 for 48 h were immunoblotted using anti-TM or anti-β-actin antibodies.
The Ras/MAPK Pathway Is Not Implicated in Cell-Cell Contact Disruption
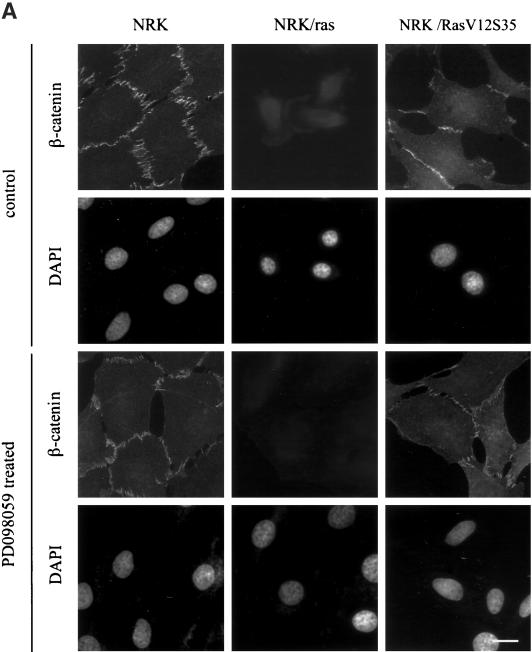
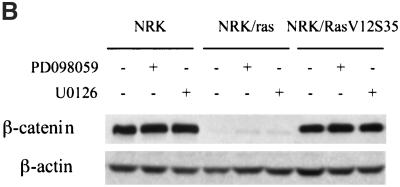
Transformation of NRK cells by Ras also resulted in the disruption of β-catenin containing cell-cell contacts. Immunofluorescence labeling with anti-β-catenin showed that NRK cells formed strong cell-cell junctions, whereas NRK/ras cells showed only a faint and diffuse staining (Figure 5A). The addition of PD098059 for 48 h did not restore β-catenin distribution in NRK/ras cells (Figure 5A). Further treatments with PD098059 or U0126 at various concentrations, for various times, failed to restore β-catenin–containing cell-cell contacts, suggesting that MEK does not play a role in the distribution of β-catenin in NRK/ras cells. Consistently, NRK/RasV12S35 cells still exhibited β-catenin–containing cell-cell contacts (Figure 5A) despite a strong activation of MEK in these cells, as shown by the constitutive phosphorylation of MAPK (see Figure 1A). In addition to monitoring the localization and assembly of β-catenin into cell-cell contacts, we measured its expression level by immunoblotting. Consistent with the immunofluorescence data, Figure 5B shows a down-regulation of β-catenin expression in NRK/ras cells that was not restored upon MEK inhibition. The expression level of β-catenin in NRK/RasV12S35 cells was shown to be normal compared with that of NRK cells (Figure 5B). These results suggest that the Ras/MAPK pathway specifically targets stress fibers and focal contacts during transformation of NRK cells by Ras but is not implicated or not sufficient to disrupt cell-cell junctions.
Figure 5.
Activation of the Ras/MAPK pathway is not implicated in β-catenin–containing cell-cell junction disruption. NRK, NRK/ras, and NRK/RasV12S35 cells were treated with DMSO, PD098059, or U0126, then fixed and stained for immunofluorescence or lysed for Western blot analysis. (A) Cells were treated with 0.1% DMSO (control) or 50 μM PD098059 for 48 h and fixed. Cell-cell junctions were visualized using anti–β-catenin antibody, whereas nuclei were stained with DAPI. Bar, 20 μm. (B) Whole-cell extracts of cells maintained in the absence (−) or presence (+) of 50 μM PD098059 or 25 μM U0126 for 48 h were immunoblotted using anti-β–catenin or anti-β–actin antibodies.
ROCKI/Rho-Kinase Signaling Is Compromised in NRK/ras Cells and Is Restored Upon MEK Inhibition by a Posttranscriptional Mechanism
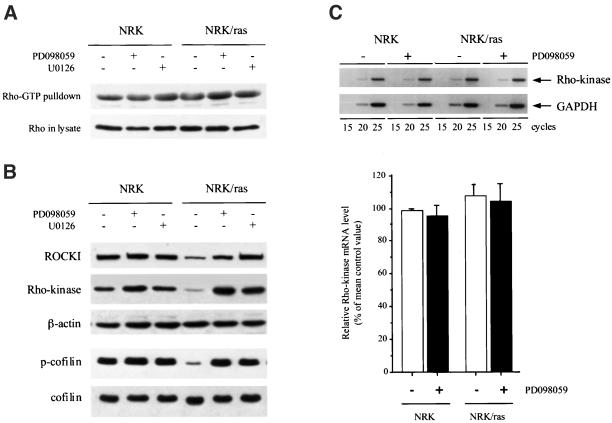
The Rho family of small GTPases, Rho, Rac, and Cdc42, plays a central role in regulating actin organization through downstream effectors, which activities are controlled by interactions with active GTP-bound forms of the Rho family (Bishop and Hall, 2000). ROCKI/Rho-kinase are serine/threonine kinases involved in Rho-mediated actin reorganization, i.e., formation of stress fibers and focal contacts (Leung et al., 1996; Amano et al., 1997). To examine whether the morphological changes induced by MEK inhibitors were accompanied by modifications in Rho activation, the activity of endogenous RhoA was measured in NRK and NRK/ras cells, using a pull-down assay that only capture the active GTP-bound form of the GTPase (Ren et al., 1999). There was little difference between Rho-GTP levels in parental versus Ras-transformed cells, as well as between untreated versus MEK inhibitor-treated cells (Figure 6A), demonstrating that neither the loss of stress fibers in Ras-transformed cells nor the restoration of stress fibers induced by MEK inhibitors were associated with changes in Rho-GTP levels.
Figure 6.
Restoration of ROCKI/Rho-kinase expression and activity upon MEK inhibitor treatment of NRK/ras cells. (A) NRK and NRK/ras cells were maintained in the absence (−) or presence (+) of 50 μM PD098059 or 25 μM U0126 for 48 h. Rho-GTP levels were then analyzed (representative of two experiments). (B) Cells were treated as indicated, and whole cell extracts were immunoblotted using anti-ROCKI, anti-Rho-kinase, and anti-β-actin antibodies (top panels). The same extracts were probed with an antiphospho-cofilin antibody, followed by anti-cofilin antibody, after the membrane was stripped (bottom panels). (C) Rho-kinase mRNA expression in NRK and NRK/ras cells upon treatment with PD098059. Rho-kinase cDNAs were amplified by PCR for 15, 20, or 25 cycles from RNAs of NRK and NRK/ras cells incubated in the presence (+) or in the absence (−) of 50 μM PD098059 for 48 h. As a control, rat GAPDH cDNA was also amplified (top panel). Densitometry analysis of Rho-kinase cDNAs amplified for 20 cycles. Data are representative of three independent experiments (mean ± SD). Relative Rho-kinase mRNA levels, normalized to GAPDH levels, are expressed vs. control NRK, which is arbitrarily set to 100% (bottom panel).
Despite normal levels of Rho-GTP, NRK/ras cells are devoid of stress fibers, suggesting that Rho-GTP was no longer coupled to formation of actin stress fibers in these cells. Interestingly, we found that expression of both ROCKI and Rho-kinase was greatly decreased in NRK/ras cells compared with untransformed NRK cells (Figure 6B). On treatment with PD098059 or U0126, Rho-kinase expression returned to levels similar to that seen in NRK cells (Figure 6B). The levels of ROCKI and Rho-kinase were also examined in the Ras-effector mutant expressing cells, especially the NRK/Ras V12S35 cell line. No changes were observed compared with the control parental cells, suggesting that activation of MEK is required but not sufficient to induce down-regulation of ROCKI/Rho-kinase expression. ROCKI and Rho-kinase activates LIM-kinase, which subsequently phosphorylates cofilin and thereby inhibits its actin-depolymerizing activity (Yang et al., 1998; Maekawa et al., 1999; Bamburg et al., 1999; Ohashi et al., 2000). Concomitant with the restoration of Rho-kinase expression, we found that cofilin phosphorylation was induced in NRK/ras cells upon treatment with either MEK inhibitor, suggesting that the Rho-kinase/LIM-kinase/cofilin pathway was functionally restored (Figure 6B). Phosphorylation of the myosin light chain, another target of ROCKI/Rho-kinase (Kureishi et al., 1997; Fukata et al., 2001), was also increased upon ROCKI/Rho-kinase expression up-regulation (our unpublished results).
We next examined whether down-regulation of Rho-kinase in NRK/ras cells was controlled at the transcriptional level. Rho-kinase mRNA levels in NRK and NRK/ras cells were examined by semiquantitative RT-PCR analysis. As shown in Figure 6C, steady-state levels of Rho-kinase mRNA were similar in NRK and NRK/ras cells, suggesting that the down-regulation of Rho-kinase expression in NRK/ras cells is controlled at the protein level. Up-regulation of Rho-kinase expression in NRK/ras cells treated with PD098059 also appeared to be regulated at the protein level, because the Rho-kinase mRNA level did not change significantly upon treatment with PD098059 (Figure 6C).
ROCKI/Rho-Kinase Is Both Necessary and Sufficient to Promote Assembly of Stress Fibers in NRK/ras Cells
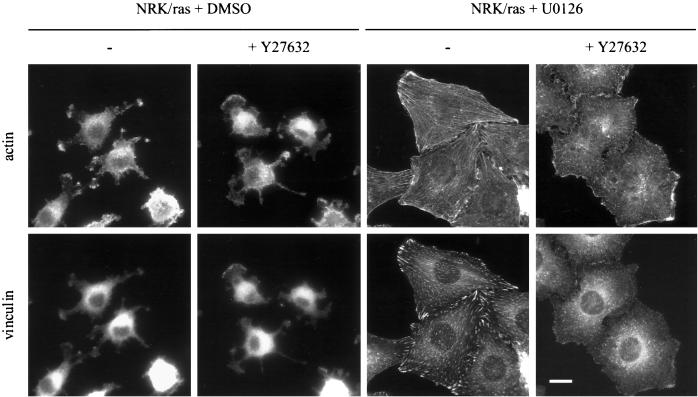
To determine whether ROCKI/Rho-kinase was involved in the morphological changes induced by inhibition of MEK in NRK/ras cells, we used the specific ROCKI/Rho-kinase inhibitor Y27632 (Kuwahara et al., 1999). Treatment of NRK/ras cells with Y27632 had no effect on cell morphology (Figure 7), suggesting that the low level of ROCKI/Rho-kinase expressed in NRK/ras cells has no apparent role in maintaining their transformed morphology. Stress fibers were induced in NRK/ras cells by treatment with U0126 for 48 h, then cells were further incubated with 10 μM Y27632 for 1 h. Inhibition of ROCKI/Rho-kinase resulted in disassembly of stress fibers and focal contacts (Figure 7), suggesting that formation of these structures was under the control of a ROCKI/Rho-kinase–dependent pathway that was restored upon MEK inhibition.
Figure 7.
Assembly of stress fibers in NRK/ras cells requires Rho-kinase activity. NRK/ras cells were treated with 0.1% DMSO or 25 μM U0126 for 48 h. Cells were then further incubated in the absence (−) or presence (+) of 10 μM Y27632 for 1 h and fixed. Actin and vinculin were labeled as described in Figure 1. Bar, 20 μm.
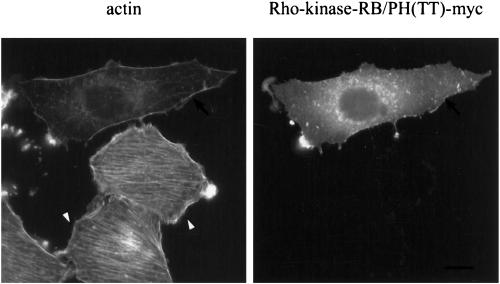
To further demonstrate the requirement for ROCKI/Rho-kinase activity in the morphological reversion induced by MEK inhibitors, we expressed a dominant-negative form of Rho-kinase, Rho-kinase-RB/PH(TT) (Amano et al., 1997). Consistent with the decreased levels of Rho-kinase expression, NRK/ras cells transfected with Rho-kinase-RB/PH(TT) in the absence of MEK inhibitors showed essentially no change in morphology. NRK/ras cells transfected with Rho-kinase-RB/PH(TT) were then treated with PD098059 or U0126. This treatment failed to restore stress fibers or focal contacts in the cells expressing the dominant negative form of ROCKI/Rho-kinase (Figure 8). This result demonstrates that elevation of Rho-kinase expression is absolutely required for the restoration of actin fibers in NRK/ras cells treated with MEK inhibitors.
Figure 8.
Overexpression of a dominant-negative Rho-kinase prevents assembly of stress fibers in NRK/ras cells treated with MEK inhibitors. NRK/ras cells were transiently transfected with pEF-Bos-myc-Rho-kinase-RB/PH(TT). After 24 h, cells were treated with 25 μM U0126 for 30 h, then fixed. Actin was stained as described in Figure 1. Note that nontransfected cells (arrowhead) form stress fibers, whereas Rho-kinase-RB/PH(TT)–transfected cells (arrow) are devoid of stress fibers. Bar, 20 μm.
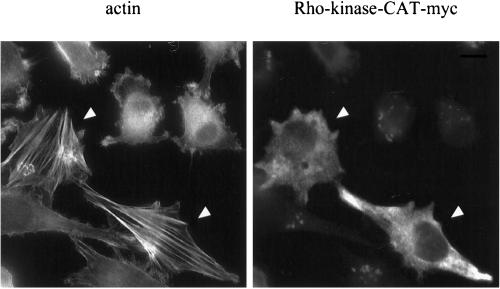
Finally, we determined whether expression of Rho-kinase was sufficient to reverse the Ras-transformed phenotype. Transfection of NRK cells with a wild-type Rho-kinase produced thick stress fibers but had no effect in NRK/ras cells. Expression of a constitutively active form of Rho-kinase, Rho-kinase-CAT (Amano et al., 1997) in NRK/ras cells restored the formation of stress fibers, unlike neighboring cells that do not express the construct (Figure 9). The extent of the reversion was only partial, however, because stress fibers were very thick and cells did not spread, suggesting that other pathways are cooperating with Rho-kinase to correctly assemble actin fibers when NRK/ras cells are treated with MEK inhibitors. Taken together, these results demonstrate that Rho-kinase activity is both necessary and sufficient to promote assembly of stress fibers and focal contacts in NRK/ras cells.
Figure 9.
Expression of constitutively active Rho-kinase induces stress fibers in NRK/ras cells. NRK/ras cells were transfected with pEF-Bos-myc-Rho-kinase-CAT plasmid (Rho-kinase-CAT). Cells were fixed 24 h after transfection and stained with phalloidin or myc antibodies as described in Figure 1. Bar, 20 μm.
DISCUSSION
Among the most striking manifestations of the transformed phenotype are the severe disruption of the actin cytoskeleton and the loss of cell-matrix and cell-cell contacts frequently associated with altered patterns of cytoskeletal protein expression. These cellular changes contribute directly to the poor adhesiveness of transformed cells, their enhanced motility, and their ability to grow in an anchorage-independent manner (Hunter, 1997; Pawlak and Helfman, 2001). It is therefore important to determine the molecular mechanisms responsible for these cellular changes.
Oncogenic Ras has been shown to dramatically affect actin organization, assembly of focal contacts, and the formation of adherens junctions and tight junctions (Izawa et al., 1998; Potempa and Ridley, 1998; Chen et al., 2000; Reuveni et al., 2000). Ras is known to activate several signaling pathways and could, in principle, exert its cytoskeletal effects via several distinct pathways. PI 3-K was reported to be a Ras effector (Rodriguez-Viciana et al., 1994), mediating signals from Ras to the actin cytoskeleton (Rodriguez-Viciana et al., 1997) and to adherens junctions (Potempa and Ridley, 1998). In this study, we show that expression of the Ras mutant RasV12C40, which strongly activates the PI 3-K pathway, was not sufficient to disrupt stress fibers and focal contacts in NRK cells. In addition, inhibition of PI 3-K by LY294002 or wortmannin failed to restore actin microfilaments or focal contacts in NRK/ras cells, further suggesting that activation of PI 3-K is not required for Ras-induced disruption of the cytoskeleton in NRK cells.
The present study rather points to the MEK/MAPK pathway as the main route mediating oncogenic Ras effects on stress fibers and focal contacts. Several lines of evidence support this conclusion. First, transfection of the Ras mutant RasV12S35, which selectively and strongly activates the Ras/MAPK pathway, induces disruption of stress fibers and focal contacts in untransformed NRK cells. Second, we showed that application of two structurally unrelated MEK inhibitors, namely PD098059 and U0126, induced complete recovery of stress fibers in NRK/ras cells, with a concomitant assembly of vinculin-containing focal contacts. Taken together, these results strongly support the notion that the Ras/MAPK pathway is a major pathway through which morphological transformation occurs in these cells. Further support comes from a recent report showing that the phenotypic reversion of Ras-transformed Rat1 fibroblasts induced by the Ras farnesylation inhibitor HR12 is mediated by the Ras/MAPK pathway (Reuveni et al., 2000).
Formation of adherens junctions is also dramatically affected by oncogenic Ras, thus allowing transformed cells to migrate (Potempa and Ridley, 1998; Reuveni et al., 2000). Reuveni et al. (2000) showed that assembly of cell-cell junctions is restored in Rat1/ras cells upon HR12 treatment, most likely through inhibition of MEK. In our system, however, we demonstrated that the Ras/MAPK pathway is not implicated in regulation of this cellular structure. Application of MEK inhibitors did not restore β-catenin–containing cell-cell contacts or β-catenin expression in NRK/ras cells. In addition, untransformed NRK cells transfected with the RasV12S35 mutant retained expression of β-catenin and correct localization at cell-cell junctions. The divergent results between our study and the work of Reuveni and colleagues (2000) may be due to differences in cell types used in these studies (Rat1 vs. NRK) or type of transformation (Ha-ras vs. Ki-ras). Another possibility is that the ras-farnesylation inhibitor HR12 used by Reuveni and colleagues (2000) has a yet uncharacterized effect on another pathway implicated in cell-cell junction regulation in addition to its demonstrated effect as a MEK inhibitor. In epithelial MDCK cells, activation of both MAPK and PI 3-K pathways by Ras is required for cell-cell junction disassembly (Potempa and Ridley, 1998). We tested this hypothesis in our model by treating NRK/ras cells with PD098059 together with LY294002. This treatment failed to restore cell-cell junctions (our unpublished results), strengthening the fact that epithelial and fibroblastic cells exhibit quite different phenotypes upon transformation by Ras.
Altogether, our results demonstrate that in NRK/Ras cells, organization of the actin-based cytoskeleton is mainly under the control of the Ras/MAPK pathway. Although the mechanism by which MEK or MAPK delivers the signal to the cytoskeleton is not fully understood, our results provide important new insights. We found that activation of MEK in NRK/ras cells induces alterations in ROCKI/Rho-kinase–dependent pathways, which are implicated in actin stress fiber organization without changes in the activation status of Rho. During the preparation of this manuscript, a similar conclusion was reached by Sahai et al. (2001) in Ras-transformed Swiss-3T3 cells. However, although Sahai et al. reported a modification in the subcellular localization of ROCKI/Rho-kinase that is thought to bring ROCKI/Rho-kinase away from its substrates, we observed that NRK/ras cells have a greatly reduced level of both ROCKI and Rho-kinase. This decreased expression was a direct consequence of sustained MAPK signaling, because inhibition of MEK restored the expression of ROCKI/Rho-kinase. We further demonstrated that ROCKI/Rho-kinase expression is controlled in NRK/ras cells at the protein level (Figure 6). Although the most prominent role assigned to MEK/MAPK activity is related to transcriptional regulation, recent reports have suggested a role for this pathway in post-transcriptional events related to cell adhesion. MEK was found to localize to the cell periphery (Kranenburg et al., 1999), while MAPK was shown to be targeted to focal contacts upon activation (Fincham et al., 2000). The translocation of MEK or MAPK to focal contacts may then serve to direct specificity toward appropriate downstream targets at cellular adhesion (Fincham et al., 2000). Changes in the phosphorylation state of cytoskeleton components may provide a regulatory mechanism through which MAPK could affect cytoskeleton organization. To date, however, there are no reports of direct phosphorylation of ROCK/Rho-kinase by MEK/MAPK. Interestingly, induction of calpain activity downstream of MEK/MAPK was recently demonstrated in EGFR signaling and this activation was linked to focal contacts disassembly (Glading et al., 2000). Calpains also degrade Focal Adhesion Kinase (FAK) upon transformation by v-Src (Carragher et al., 2001). Based on these experiments, one could hypothesized that MEK-induced activation of proteases such as calpains could be implicated in ROCKI/Rho-kinase degradation during Ras transformation.
Importantly, we demonstrated that not only expression of ROCKI/Rho-kinase was restored but also its activity, because we showed that phosphorylation of cofilin by LIM-kinase and phosphorylation of myosin light chain were both induced upon MEK inhibition. Phosphorylation of cofilin by LIMK induces a stabilization of actin filaments through inhibition of its actin-depolymerizing activity (Yang et al., 1998; Maekawa et al., 1999; Bamburg et al., 1999; Ohashi et al., 2000), whereas the phosphorylation of myosin light chains increases myosin activity (Amano et al., 1996). Together, these events directly contribute to the assembly of stress fibers (Bishop and Hall, 2000). Conversely, suppression of these signaling pathways most likely accounts for the loss of stress fibers in Ras-transformed cells. We have also demonstrated that up-regulation of ROCKI/Rho-kinase expression induced by MEK inhibition is critical for the concomitant restoration of the actin cytoskeleton. Both the ROCKI/Rho-kinase inhibitor Y27632, and a dominant negative mutant of Rho-kinase, Rho-kinase-RB/PH(TT) (Amano et al., 1997) abolished the effects of the PD098059 treatment, demonstrating that Rho-kinase is indeed required for the process of actin stress fiber assembly and suggesting that no other pathway can compensate for the defect in Rho-kinase expression. Although previous studies have reported a phenotypic reversion of ras-transformed cells upon PD098059 treatment, our study provides a molecular mechanism through which this inhibitor acts.
These experiments prompted us to check whether overexpression of Rho-kinase in NRK/ras cells could be sufficient to reverse their transformed phenotype. Expression of a wild-type Rho-kinase failed to induce stress fibers, possibly because the protein expressed by this exogenous construct is prone to degradation like the endogenous Rho-kinase. A constitutively active mutant of Rho-kinase was able to induce stress fibers in NRK/ras cells, demonstrating that down-regulation of Rho-kinase expression is a critical step for the acquisition of a transformed phenotype during Ras transformation of NRK cells. However, compared with the effect of MEK inhibitors, this mutant fails to completely reverse cells to a normal morphology, suggesting that other proteins cooperate with Rho-kinase during PD098059-induced reversion. Rho-kinase is thought to cooperate with an additional Rho effector, mDia, in the control of stress fiber formation (Nakano et al., 1999; Watanabe et al., 1999). We do not know at this point, however, whether mDia is affected by Ras-transformation of NRK cells.
Cellular transformation by Ras is associated with a marked decrease in the expression of several actin-binding proteins (Button et al., 1995), including high-molecular-weight TMs. Alterations in TM expression seem to be central to the transformed phenotype, because reexpression of these proteins can reverse several aspects of the transformed phenotype, including actin microfilament assembly, contact inhibition, and anchorage-dependent cell growth, but the underlying mechanisms are incompletely understood (reviewed in Pawlak and Helfman, 2001). Treatment of c-Jun–transformed FR3T3 rat cells with PD098059 reversed down-regulation of TM-2 (Ljundahl et al., 1998). By contrast, the ras-induced down-regulation of TMs in NIH3T3-ras cells was found to be MEK independent, because treatment with PD098059 had little effect on TM levels (Janssen et al., 1998). Interestingly, we report here that inhibition of MEK in NRK/ras cells totally alleviated repression of TM-1 expression, suggesting that the decreased TM-1 level observed in NRK/ras cells is a direct result of Ras signaling pathway. TMs are actin-binding proteins know to protect actin stress fibers from the action of severing proteins, such as gelsolin (Ishikawa et al., 1989; Pittenger et al., 1994). It is thus likely that TMs will cooperate with ROCKI/Rho-kinase to properly assemble and stabilize the actin cytoskeleton during PD098059 treatment of NRK/ras cells. Obviously, the involvement of other proteins that were not investigated in this study is not excluded.
In summary, our results demonstrate that in NRK/Ras cells, organization of the actin cytoskeleton is mainly under the control of the Ras/MAPK pathway and that chronic activation of this pathway during Ras transformation specifically targets formation of stress fibers and focal contacts but is not implicated in cell-cell junction regulation. Our study also provides a mechanism, involving down-regulation of structural components of the cytoskeleton and inhibition of ROCKI/Rho-kinase–dependent pathways, by which oncogenic Ras can achieve morphological transformation of the cells. Although several studies have reported that Rho can cooperate with Ras to transform cells (Khosravi-Far et al., 1995; Qiu et al., 1995; Zhong et al., 1997), down-regulation of ROCKI/Rho-kinase expression may represent a way to uncouple Rho from stress fiber formation without affecting its other biological functions, such as regulation of proliferation and migration (Olson et al., 1998; Banyard et al., 2000). This would explain why Ras-transformed cells generally exhibit a loss of stress fibers and focal contacts, which are characteristic of Rho activity in normal cells. Whether MAPK signals to the cytoskeleton directly by phosphorylating cytoskeletal components and/or cytoskeleton regulators, such as ROCKI/Rho-kinase, or indirectly by controlling their protein levels through transcription, translation, or degradation is under investigation.
ACKNOWLEDGMENTS
We are grateful to James R. Bamburg for the phospho-specific cofilin antibody and Kozo Kaibuchi for the Rho-kinase expression plasmids. We thank Esteban Araya and Alyssa Carlberg for excellent technical support. The assistance of Eric Julien in many aspects of our work is especially acknowledged. D.M.H. was supported by a grant from the National Cancer Institute (CA-83182).
Abbreviations used:
- MAPK
mitogen-activated protein kinase
- MEK
mitogen-activated protein kinase kinase
- NRK
normal rat kidney
- PI 3-K
phosphatidylinositol 3-kinase
- TM
tropomyosin
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.01–02-0302. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.01–02-0302
REFERENCES
- Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase. J Biol Chem. 1996;271:20246–20249. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- Amano M, Chihara K, Kimura K, Fukata Y, Nakamura N, Matsuura Y, Kaibuchi K. Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science. 1997;275:1308–1311. doi: 10.1126/science.275.5304.1308. [DOI] [PubMed] [Google Scholar]
- Bamburg JR, McGough A, Ono S. Putting a new twist on actin: ADF/cofilins modulate actin dynamics. Trends Cell Biol. 1999;9:364–370. doi: 10.1016/s0962-8924(99)01619-0. [DOI] [PubMed] [Google Scholar]
- Banyard J, Anand-Apte B, Symons M, Zetter BR. Motility and invasion are differentially modulated by Rho family GTPases. Oncogene. 2000;19:580–591. doi: 10.1038/sj.onc.1203338. [DOI] [PubMed] [Google Scholar]
- Bar-Sagi D, Hall A. Ras and Rho GTPases : a family reunion. Cell. 2000;103:227–238. doi: 10.1016/s0092-8674(00)00115-x. [DOI] [PubMed] [Google Scholar]
- Bishop AL, Hall A. Rho GTPases and their effector proteins. Biochem J. 2000;348:241–255. [PMC free article] [PubMed] [Google Scholar]
- Braverman RH, Cooper HL, Lee HS, Prasad GL. Anti-oncogenic effects of tropomyosin: isoform specificity and importance of protein coding sequences. Oncogene. 1996;13:537–545. [PubMed] [Google Scholar]
- Burridge K, Chrzanowska-Wodnicka M, Zhong C. Focal adhesion assembly. Trends Cell Biol. 1997;7:342–347. doi: 10.1016/S0962-8924(97)01127-6. [DOI] [PubMed] [Google Scholar]
- Button E, Shapland C, Lawson D. Actin, its associated proteins and metastasis. Cell Motil Cytoskeleton. 1995;30:247–251. doi: 10.1002/cm.970300402. [DOI] [PubMed] [Google Scholar]
- Carragher NO, Fincham VJ, Riley D, Frame MC. Cleavage of focal adhesion kinase by different proteases during Src-regulated transformation and apoptosis. J Biol Chem. 2001;276:4270–4275. doi: 10.1074/jbc.M008972200. [DOI] [PubMed] [Google Scholar]
- Chen Y, Lu G, Schneeberger EE, Goodenough DA. Restoration of tight junction structure and barrier function by down-regulation of the mitogen-activated protein kinase pathway in ras-transformed Madin-Darby canine kidney cells. Mol Biol Cell. 2000;11:849–862. doi: 10.1091/mbc.11.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley DT, Pang L, Decker SJ, Bridges AJ, Saltiel AR. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc Natl Acad Sci USA. 1995;92:7686–7689. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favata MF, Horiuchi KY, Manos EJ, Daulerio AJ, Stradley DA, Feeser WS, Van Dyk DE, Pitts WJ, Earl RA, Hobbs F, Copeland RA, Magolda RL, Scherle PA, Trzaskos JM. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem. 1998;273:18623–18632. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- Fincham VJ, James M, Frame MC, Winder SJ. Active ERK/MAP kinase is targeted to newly forming cell-matrix adhesions by integrin engagement and v-Src. EMBO J. 2000;19:2911–2923. doi: 10.1093/emboj/19.12.2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukata Y, Amano M, Kaibuchi K. Rho-Rho-kinase pathway in smooth muscle contraction and cytoskeletal reorganization of non-muscle cells. Trends in Pharmacol Sci. 2001;22:32–39. doi: 10.1016/s0165-6147(00)01596-0. [DOI] [PubMed] [Google Scholar]
- Fukazawa H, Uehara Y. U0126 reverses Ki-ras-mediated transformation by blocking both mitogen-activated protein kinase and p70 S6 kinase pathways. Cancer Res. 2000;60:2104–2107. [PubMed] [Google Scholar]
- Gimona M, Kazzaz J, Helfman DM. Forced expression of tropomyosin 2 or 3 in v-Ki-ras-transformed fibroblasts results in distinct phenotypic effects. Proc Natl Acad Sci USA. 1996;93:9618–9623. doi: 10.1073/pnas.93.18.9618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glading A, Chang P, Lauffenburger DA, Wells A. Epidermal growth factor receptor activation of calpain is required for fibroblast motility and occurs via an ERK/MAP kinase signaling pathway. J Biol Chem. 2000;275:2390–2398. doi: 10.1074/jbc.275.4.2390. [DOI] [PubMed] [Google Scholar]
- Gluck U, Kwiatkowski, Ben-Ze'ev A. Suppression of tumorigenicity in simian virus 40-transformed 3T3 cells transfected with α-actinin. Proc Natl Acad Sci USA. 1993;90:383–387. doi: 10.1073/pnas.90.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfman DM, Levy ET, Berthier C, Shtutman M, Riveline D, Grosheva I, Lachish-Zalait A, Elbaum M, Bershadsky AD. Caldesmon inhibits nonmuscle cell contractility and interferes with the formation of focal adhesions. Mol Biol Cell. 1999;10:3097–3112. doi: 10.1091/mbc.10.10.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T. Oncoprotein networks. Cell. 1997;88:333–346. doi: 10.1016/s0092-8674(00)81872-3. [DOI] [PubMed] [Google Scholar]
- Ishikawa R, Yamashiro S, Matsumura F. Differential modulation of actin-severing activity of gelsolin by multiple isoforms of cultured rat cell tropomyosin: potentiation of protective ability of tropomyosins by 83-kDa nonmuscle caldesmon. J Biol Chem. 1989;264:7490–7497. [PubMed] [Google Scholar]
- Itoh K, Yoshioka K, Akedo H, Uehata M, Ishizaki T, Narumiya S. An essential part for Rho-associated kinase in the transcellular invasion of tumor cells. Nature Med. 1999;5:221–225. doi: 10.1038/5587. [DOI] [PubMed] [Google Scholar]
- Izawa I, Amano M, Chihara K, Yamamoto T, Kaibuchi K. Possible involvement of the inactivation of the Rho-Rho-kinase pathway in oncogenic Ras-induced transformation. Oncogene. 1998;17:2863–2871. doi: 10.1038/sj.onc.1202213. [DOI] [PubMed] [Google Scholar]
- Janke J, Schluter K, Jandrig B, Theile M, Kolbe K, Arnold W, Grinstein E, Schwartz A, Estevez-Schwarz L, Schlag PM, Jockusch BM, Scerneck S. Suppression of tumorigenicity in breast cancer cells by the microfilament protein profilin 1. J Exp Med. 2000;191:1675–1685. doi: 10.1084/jem.191.10.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen RAJ, Mier JW. Tropomyosin-2 cDNA lacking the 3′ untranslated region riboregulator induces growth inhibition of v-Ki-ras-transformed fibroblasts. Mol Biol Cell. 1997;8:897–908. doi: 10.1091/mbc.8.5.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen RAJ, Veenstra KG, Jonasch P, Mier JW. Ras- and Raf-induced down-modulation of non-muscle tropomyosin are MEK-independent. J Biol Chem. 1998;273:32182–32186. doi: 10.1074/jbc.273.48.32182. [DOI] [PubMed] [Google Scholar]
- Katz ME, McCormick F. Signal transduction from multiple Ras effectors. Curr Opin Genet Dev. 1997;7:75–79. doi: 10.1016/s0959-437x(97)80112-8. [DOI] [PubMed] [Google Scholar]
- Khosravi-Far R, Solski PA, Clark GJ, Kinch MS, Der CJ. Activation of Rac1, RhoA and mitogen-activated protein kinases is required for ras transformation. Mol Cell Biol. 1995;15:6443–6453. doi: 10.1128/mcb.15.11.6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranenburg O, Verlan I, Moolenaar WH. Dynamin is required for the activation of mitogen-activated protein (MAP) kinase by MAP kinase kinase. J Biol Chem. 1999;274:35301–35304. doi: 10.1074/jbc.274.50.35301. [DOI] [PubMed] [Google Scholar]
- Kureishi Y, Kobayashi S, Amano M, Kimura K, Kanaide H, Nakano T, Kaibuchi K, Itoh M. Rho-associated kinase directly induces smooth muscle contraction through myosin light chain phosphorylation. J Biol Chem. 1997;272:12257–12260. doi: 10.1074/jbc.272.19.12257. [DOI] [PubMed] [Google Scholar]
- Kuwahara K, Saito Y, Nakagawa O, Kishimoto I, Harada M, Ogawa E, Miyamoto Y, Hamanaka I, Kajiyama N, Takahashi N, Izumi T, Kawakami R, Tamura N, Ogawa Y, Nakao K. The effects of the selective ROCK inhibitor, Y27632, on ET-1-induced hypertrophic response in neonatal rat cardiac myocytes: possible involvement of Rho/ROCK pathway in cardiac muscle cell hypertrophy. FEBS Lett. 1999;452:314–318. doi: 10.1016/s0014-5793(99)00680-8. [DOI] [PubMed] [Google Scholar]
- Leung T, Chen X, Manser E, Lim L. The p160 RhoA-binding kinase ROKα is a member of a kinase family and is involved in the reorganization of the cytoskeleton. Mol Cell Biol. 1996;16:5313–5327. doi: 10.1128/mcb.16.10.5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljundahl S, Linger S, Franzen B, Binetruy B, Auer G, Shoshan MC. Down-regulation of tropomyosin-2 expression in c-jun-transformed rat fibroblasts involves induction of a MEK1-dependent autocrine loop. Cell Growth Diff. 1998;9:565–573. [PubMed] [Google Scholar]
- Maekawa M, Ishizaki T, Boku S, Watanabe N, Fujita A, Iwamatsu A, Obinata T, Ohashi K, Mizuno K, Narumyia S. Signaling from Rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science. 1999;285:895–898. doi: 10.1126/science.285.5429.895. [DOI] [PubMed] [Google Scholar]
- Matsumura F, Lin JJ-C, Yamashiro-Matsumura S, Thomas GP, Topp WC. Differential expression of tropomyosin forms in the microfilaments isolated from normal and transformed rat cultured cells. J Biol Chem. 1983;258:13954–13964. [PubMed] [Google Scholar]
- Nakano K, Takaishi K, Kodama A, Mammoto A, Shiozaki H, Monden M, Takai Y. Distinct actions and cooperative roles of ROCK and mDia in Rho small G protein-induced reorganization of the actin cytoskeleton in Madin-Darby canine kidney cells. Mol Biol Cell. 1999;10:2481–2491. doi: 10.1091/mbc.10.8.2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolopoulos SN, Spengler BA, Kisselbach K, Evans AE, Biedler JL, Ross RA. The human non-muscle α-actinin protein encoded by the ACTN4 gene suppresses tumorigenicity of human neuroblastoma cells. Oncogene. 2000;19:380–386. doi: 10.1038/sj.onc.1203310. [DOI] [PubMed] [Google Scholar]
- Ohashi K, Nagata K, Maekawa M, Ishizaki T, Narumyia S, Mizuno K. Rho-associated kinase ROCK activates LIM-kinase 1 by phosphorylation at threonine 508 within the activation loop. J Biol Chem. 2000;275:3577–3582. doi: 10.1074/jbc.275.5.3577. [DOI] [PubMed] [Google Scholar]
- Olson MF, Paterson HF, Marshall CJ. Signals from Ras and Rho GTPases interact to regulate expression of p21Waf1/Cip1. Nature. 1998;394:295–299. doi: 10.1038/28425. [DOI] [PubMed] [Google Scholar]
- Pawlak G, Helfman DM. Cytoskeletal changes in cell transformation and tumorigenesis. Curr Opin Genet Dev. 2001;11:41–47. doi: 10.1016/s0959-437x(00)00154-4. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Kazzaz JA, Helfman DM. Functional properties of non-muscle tropomyosin isoforms. Curr Opin Cell Biol. 1994;6:96–104. doi: 10.1016/0955-0674(94)90122-8. [DOI] [PubMed] [Google Scholar]
- Potempa S, Ridley AJ. Activation of both MAP kinase and phosphatidylinositide 3-kinase by ras is required for hepatocyte growth factor/scatter factor-induced adherens junctions disassembly. Mol Biol Cell. 1998;9:2185–2200. doi: 10.1091/mbc.9.8.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad GL, Fuldner RA, Cooper HL. Expression of transduced tropomyosin 1 cDNA suppresses neoplastic growth of cells transformed by the ras oncogene. Proc Natl Acad Sci USA. 1993;90:7039–7043. doi: 10.1073/pnas.90.15.7039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad GL, Masuelli L, Raj MHG, Harindranath N. Suppression of src-induced transformed phenotype by expression of tropomyosin-1. Oncogene. 1999;18:2027–2031. doi: 10.1038/sj.onc.1202264. [DOI] [PubMed] [Google Scholar]
- Qiu RG, Chen J, McCormick F, Symons M. A role for Rho in Ras transformation. Proc Natl Acad Sci USA. 1995;92:11781–11785. doi: 10.1073/pnas.92.25.11781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X, Kiosses WB, Schwartz MA. Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J. 1999;18:578–585. doi: 10.1093/emboj/18.3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuveni H, Geiger T, Geiger B, Levitzki A. Reversal of the ras-induced transformed phenotype by HR12, a novel ras farnesylation inhibitor, is mediated by the Mek/Erk pathway. J Cell Biol. 2000;151:1179–1192. doi: 10.1083/jcb.151.6.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Fernandez JL, Geiger B, Salomon D, Sabanay I, Zoller M, Ben-Ze'ev A. Suppression of tumorigenicity in transformed cells after transfection with vinculin. J Cell Biol. 1992;119:427–438. doi: 10.1083/jcb.119.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Viciana P, Warne PH, Dhand R, Vanhaesebroek B, Gout I, Fry MJ, Waterfield MD, Downward J. Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature. 1994;370:527–532. doi: 10.1038/370527a0. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Viciana P, Warme PH, Khwaja A, Marte BM, Pappin D, Das P, Waterfield MD, Ridley A, Downward J. Role of phosphoinositide 3-OH kinase in cell transformation and control of the actin cytoskeleton by ras. Cell. 1997;89:457–467. doi: 10.1016/s0092-8674(00)80226-3. [DOI] [PubMed] [Google Scholar]
- Sahai E, Ishizaki T, Narumiya S, Treisman R. Transformation mediated by RhoA requires activity of ROCK kinases. Curr Biol. 1999;9:136–145. doi: 10.1016/s0960-9822(99)80067-0. [DOI] [PubMed] [Google Scholar]
- Sahai E, Olson MF, Marshall CJ. Cross-talk between ras and rho signaling pathways in transformation favors proliferation and increased motility. EMBO J. 2001;20:755–766. doi: 10.1093/emboj/20.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields JM, Pruit K, McFall A, Shaub A, Der CJ. Understanding Ras: ‘it ain’t over 'til it's over.'. Trends Cell Biol. 2000;10:147–154. doi: 10.1016/s0962-8924(00)01740-2. [DOI] [PubMed] [Google Scholar]
- Takenaga K, Masuda A. Restoration of microfilament bundle organization in v-raf-transformed NRK cells after transduction with tropomyosin 2 cDNA. Cancer Lett. 1994;87:47–53. doi: 10.1016/0304-3835(94)90408-1. [DOI] [PubMed] [Google Scholar]
- Watanabe N, Kato T, Fujita A, Ishizaki T, Narumyia S. Cooperation between mDia1 and ROCK in Rho-induced actin reorganization. Nat Cell Biol. 1999;1:136–143. doi: 10.1038/11056. [DOI] [PubMed] [Google Scholar]
- White MA, Nicolette C, Minden A, Polverino A, Van Aelst L, Karin M, Wigler MH. Multiple Ras functions can contribute to mammalian cell transformation. Cell. 1995;80:533–541. doi: 10.1016/0092-8674(95)90507-3. [DOI] [PubMed] [Google Scholar]
- Yang N, Higuchi O, Ohashi K, Nagata K, Wada A, Kangawa K, Nishida E, Mizuno K. Cofilin phosphorylation by LIM-kinase 1 and its role in Rac-mediated actin reorganization. Nature. 1998;393:809–812. doi: 10.1038/31735. [DOI] [PubMed] [Google Scholar]
- Zhong C, Kinch MS, Burridge K. Rho-stimulated contractility contributes to the fibroblastic phenotype of Ras-transformed epithelial cells. Mol Biol Cell. 1997;8:2329–2344. doi: 10.1091/mbc.8.11.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]