Abstract
Background: Depression is the commonest psychological problem that affects a woman during her perinatal period worldwide. The risk of prenatal depression increases as the pregnancy progresses and clinically significant depressive symptoms are common in the mid and late trimester. There is a paucity of research on depression during the prenatal period in India. Given this background, the present study aimed to assess the prevalence of prenatal depression and its associated risk factors among pregnant women in Bangalore, Southern India.
Methods: The study was nested within an on-going cohort study. The study participants included 280 pregnant women who were attending the antenatal clinic at Jaya Nagar General Hospital (Sanjay Gandhi Hospital) in Bangalore. The data was collected by using a structured questionnaire which included. Edinburgh Postnatal Depression Scale (EPDS) to screen for prenatal depression.
Results: The proportion of respondents who screened positive for prenatal depression was 35.7%. Presence of domestic violence was found to impose a five times higher and highly significant risk of developing prenatal depression among the respondents. Pregnancy related anxiety and a recent history of catastrophic events were also found to be a positive predictors of prenatal depression.
Conclusion: The high prevalence of prenatal depression in the present study is suggestive of its significance as a public health problem. Health care plans therefore can include screening and diagnosis of prenatal depression in the antenatal care along with other health care facilities provided.
Keywords: prenatal depression, pregnant women, domestic violence, marital discord, social support, pregnancy related anxiety, Banglaore
Introduction
The relationship between a pregnant woman and her developing fetus is possibly the most earnest and overwhelming but perplexing of all human relationships. Pregnancy entails physiological, hormonal and psychological changes which could increase the probability of mental and emotional changes resulting in depression, anxiety or psychological distress in the pregnant mother (1).
Maternal and Child Health Programmes in developing countries are commonly focused upon improving the nutritional status and less importance is given toward a woman's emotional and mental health during and after pregnancy (2, 3). Poor mental health of the woman during pregnancy could have profound consequences for the mother and her child in terms of adverse pregnancy outcomes and offspring development (4–7). Most of the existing data, research, and practice policies with regard to perinatal mental disorders center on the postnatal period and there is less research related depression during pregnancy (8, 9).
Depression is the most common psychological problem that affects a woman worldwide during the perinatal period (3, 10). About 15 % of women are known to be depressed at some point during their lifetime and more predominantly during pregnancy and after childbirth (11). The risk of prenatal depression increases significantly as the pregnancy progresses and clinically significant depressive symptoms are common in the mid and late trimester (12). The prevalence rates of prenatal depression differ between high, middle and low—income countries. Studies from various countries around the world show a prevalence rate ranging from as low as 4% to as high as 81% (13–16). The prevalence rate is reported to be lower in high income countries like Australia 7% (17), Hong Kong 4.4% (18), Finland 7.7% (19), and higher in many of the low-income countries like Pakistan 64.6% (20), Bangladesh 18% (13), Nigeria 24.5% (14), and Ethiopia 24.94% (15). The prevalence of depression in India is varies from 9.18% in one study to 36.7 % reported in another study (21, 22).
Even though prenatal depression is an important public health problem, most studies related to maternal depression are focused on post-natal depression and its outcomes; hence there is paucity of research on depression during the prenatal period, especially from India (8). The importance of screening for depression during pregnancy is that prenatal depression, if not treated and diagnosed early, may continue as postnatal depression (23–25) later on and could also result in an adverse influence on birth outcomes and offspring development. Given this background, the present study aimed to assess the prevalence of prenatal depression and its associated risk factors among pregnant women in Bangalore, Southern India.
Materials and Methods
Study Setting and Participants
The study sample included of pregnant women who were attending the antenatal clinic at Jaya Nagar General Hospital (also known as Sanjay Gandhi Hospital), which is a public sector hospital in Bangalore. The study was nested within an ongoing cohort study, the study protocol of which was published earlier (26). The eligibility criteria included women above or equal to 18 years of age, with confirmed pregnancy of < 6 months (< 24 weeks) and having no obstetric or medical complication in the present pregnancy. The study analyzed the data of 280 pregnant women who had enrolled and completed the baseline visit for the study between August 2017 and April 2018.
Data Collection
Data was obtained from the pregnant women by means of an interview, after obtaining written informed consent. A participant information sheet that explained the purpose and nature of the study was issued to those who were willing to participate in the study. The respondents were ensured about privacy and confidentiality of data. The interview process employed the use of a structured questionnaire installed in an Android tablet App. The App included questions about socio-demographic data, obstetric history, medical history and measures for depression, social support, marital discord, domestic violence, and pregnancy related anxiety described below. Data related history of any mental illness and recent catastrophic event was also recorded. Calibrated instruments were used to measure height and weight and calculate the Body Mass Index (BMI). Data on hemoglobin estimation was obtained from hospital records. Depression, being the outcome variable was measured using Edinburg Postnatal Depression Scale (EPDS).
Study Measures
Depression
EPDS is a widely used 10-item self-reporting instrument, specifically designed for assessing both prenatal as well as postnatal depression. It has a sensitivity of 86%, specificity of 78% and positive predictive value of 73% (27). EPDS has been validated for detecting depression in both antepartum and postpartum mothers in many countries. This scale consists of 10 short questions with a choice of four answers that closely reflects about how she was feeling over the past 7 days. Scores are recorded as 0, 1, 2, and 3 according symptom severity. Certain question items (i.e., 3, 2, 1, and 0) are scored in a reverse manner. Respondents who score 13 and above are likely to be suffering from depression and should seek medical attention.
Social Support
The Multidimensional Scale of Perceived Social Support Scale (MSPSS) used to measure social support includes 12 questions, and is validated for use in the South Asian population (28, 29). These questions directly address the adequacy of social support and have a 7-point rating scale ranging from “very strongly disagree” to “very strongly agree.” The scale assesses the perceptions of social support adequacy from three specific sources: family, friends, and “significant other.” A score of < 2 is considered as low support, a score of 3–5 as moderate support while score of more than five indicates high support.
Marital Discord
The Revised Dyadic Adjustment Scale (30, 31) measures seven dimensions of relationship among partners within three categories: decision making, values and affection. It consists of 14 items in which the respondents can rate their relationship on a 6-point scale. Scores range from 0 to 69; higher the score greater, is the relationship and vice versa. The cut- off score was taken as 48.
Spouse Physical and Sexual Violence
Spouse physical and sexual violence was measured using the Modified Conflict Tactics Scale (32). It is effective and useful in measuring domestic violence in diverse cultural settings. The scale has 9 questions wherein the respondents affirm whether domestic violence was present or absent.
Socio Economic Scale
The socio-economic class of the respondents was measured by the Modified Kuppuswamy Socio Economic Scale (33). The scale uses education, occupation of the head of the family and monthly family income to calculate socio-economic status. The scores awarded to education and occupation of the head of the family remains unchanged. Revised Consumer Price Index–IW (industrial workers) is used to calculate the monthly income range. The socio-economic status is classified as upper class, upper middle class, lower middle class, upper lower class and lower class.
Pregnancy Related Anxiety
The 10-item Pregnancy Related Anxiety Questionnaire (PRAQ) was used to screen for pregnancy anxiety (34). It appears to have good psychometric and predictive validity for child-birth and childhood outcomes. Each item is scored on a 4-point scale with cut-off scores of 28 and 24 for nulliparous and multiparous women and the internal consistency (Cronbach's alpha) of PRAQ was seen to be 0.79. A score of more than 28 was considered as anxious.
Statistical Analysis
Data were retrieved from the data server. This was followed by data cleaning and analysis using SPSS version 22. Descriptive statistics such as percentage, means and standard deviation were used to summarize the socio demographic data. An EPDS score of 13 and above pointed toward the likelihood of presence of depression. The independent variables were categorized to analyze the association between each independent and outcome variable using a bivariate analysis to calculate the Crude Odd's Ratio with 95% Confidence Interval. Those variables that were associated at a P-value of < 0.2 in the bivariate analysis were entered into a multivariate logistic regression model to calculate the Adjusted Odd's Ratio and to eliminate the effects of confounding. Variables with a P-value of < 0.05 in the multivariate analysis were considered to be significant.
Ethical Considerations
The study was approved by the Ethical Committee of Indian Institute of Public Health Bangalore campus (IIPHHB/TRCIEC/118/2017). Written informed consent was obtained from the pregnant mothers and they were assured of confidentiality and privacy of records.
Results
Socio Demographic Characteristics of the Respondents
Table 1 shows the frequency distribution of socio demographic characteristics of the respondents. Of the 280 pregnant mothers, majority (72.9%) of them belonged to the age group of more than 20 years, the mean age of the respondents being 23.02 ± 3.40 years. Over two-thirds among them (72.1%) were Muslim and 40.4% had completed High school. While 92.1% were housewives, the spouses of over half of the respondents (51.8%) were semi- skilled workers. According to the Kuppuswamy Socio economic status scale, more than half of the respondents (57.5%) belonged to Upper Lower class. Nearly seventy percent of the pregnant mothers had no blood relationship with their husbands, where as a notable 13.2% said that their husbands were a first cousin from their mother's side.
Table 1.
Socio demographic characteristics of the study participants (N = 280).
| Socio demographic characteristics | Frequency (n = 280) | Percentage (%) |
|---|---|---|
| AGE GROUP (IN YEARS) | ||
| ≤ 20 | 76 | 27.1 |
| >20 | 204 | 72.9 |
| RELIGION | ||
| Hinduism | 73 | 26.1 |
| Christianity | 05 | 1.8 |
| Islam | 202 | 72.1 |
| EDUCATIONAL QUALIFICATION OF THE RESPONDENTS | ||
| Illiterate | 07 | 2.5 |
| Primary school | 08 | 2.9 |
| Middle school | 77 | 27.5 |
| High school | 113 | 40.4 |
| PUC or diploma | 52 | 18.6 |
| Graduate | 23 | 8.2 |
| EDUCATIONAL QUALIFICATION OF THE HUSBANDS | ||
| Illiterate | 36 | 12.9 |
| Primary school | 20 | 7.1 |
| Middle school | 71 | 25.4 |
| High school | 95 | 33.9 |
| PUC or diploma | 36 | 12.9 |
| Graduate and post-graduate | 22 | 7.8 |
| OCCUPATION OF THE RESPONDENTS | ||
| Unskilled worker | 11 | 3.9 |
| Semi-skilled worker | 10 | 3.6 |
| Clerical or farmer | 01 | 0.4 |
| Housewife | 258 | 92.1 |
| OCCUPATION OF THE HUSBANDS | ||
| Unemployed | 01 | 0.4 |
| Unskilled worker | 96 | 34.3 |
| Semi-skilled worker | 145 | 51.8 |
| Skilled worker | 35 | 12.5 |
| Clerical or farmer | 01 | 0.4 |
| Semi professional | 02 | 0.7 |
| SOCIO ECONOMIC STATUS | ||
| Upper middle class | 36 | 12.9 |
| Lower middle class | 83 | 29.6 |
| Upper Lower class | 161 | 57.5 |
Prevalence and Magnitude of Prenatal Depression Among the Pregnant Women
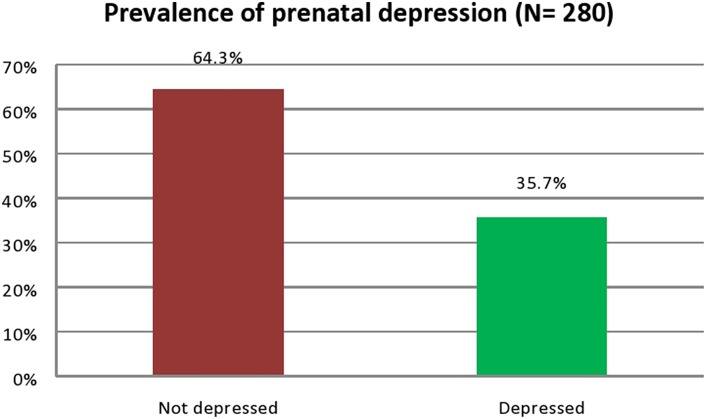
Of the 280 pregnant mothers, the proportion of those who screened positive for prenatal depression was 35.7% (100) suggesting a high probability of clinical depression (Figure 1). The mean EPDS score among the respondents was 10.61± 7.48.
Figure 1.
Prevalence of prenatal depression among the pregnant women (N = 280).
Association of Prenatal Depression With Socio-Demographic Characteristics
The association of socio-demographic factors like age group, educational qualification, occupation, and socio-economic status of the respondents with depression was non-significant on bivariate analysis (p-value >0.05). This is seen from Table 2.
Table 2.
Association of socio demographic characteristics with depression among the pregnant women (N = 280).
| Socio demographic Characteristics | Depressed mothers (>13) (n = 100) | Non-depressed mothers (<13) (n = 180) | Bivariate analysis-crude odd's ratio (95% CI) | P-value |
|---|---|---|---|---|
| AGE GROUP (IN YEARS) | ||||
| ≤ 20 | 29 (29%) | 47 (26.1%) | 1.156 (0.670–1.994) | 0.603 |
| >20 | 71 (71%) | 133 (73.9%) | 1 | |
| EDUCATIONAL QUALIFICATION OF THE RESPONDENTS | ||||
| < High school | 77 (77.0%) | 126 (70.0%) | 0.710 (0.403–1.250) | 0.236 |
| ≥High school | 23 (23.0%) | 54 (30.0%) | 1 | |
| EDUCATIONAL QUALIFICATION OF THE HUSBANDS | ||||
| < High school | 49 (49%) | 78 (43.3%) | 1.256 (0.769–2.052) | 0.362 |
| ≥High school | 51 (51%) | 102 (56.7%) | 1 | |
| OCCUPATION OF THE RESPONDENTS | ||||
| Working | 08 (8.0%) | 15 (8.3%) | 1.045 (0.427–2.559) | 0.922 |
| Housewife | 92 (92.0%) | 165 (91.7%) | 1 | |
| OCCUPATION OF THE HUSBANDS | ||||
| Skilled worker | 12 (12%) | 26 (14.4%) | 1 | |
| Semi/unskilled worker | 88 (88%) | 154 (85.6%) | 1.238 (0.595–2.575) | 0.568 |
| SOCIO ECONOMIC STATUS | ||||
| Upper middle class | 12 (12.0%) | 24 (13.3%) | 1 | |
| Lower middle class | 26 (26.0%) | 57 (31.7%) | 0.912 (0.396–2.100) | 0.829 |
| Upper lower class | 62 (62.0%) | 99 (55.0%) | 1.253 (0.584–2.686) | 0.563 |
Association of Prenatal Depression With Obstetric History (Table 3)
Table 3.
Association of depression with obstetric history of the pregnant women (N = 280).
| Obstetric history | Depressed mothers (>13) (n = 100) | Non-depressed mothers (<13) (n = 180) | Bivariate analysis crude OR (95% CI) | P-value | Multivariate analysis adjusted OR (95% CI) | P-value |
|---|---|---|---|---|---|---|
| GRAVIDITY | ||||||
| Primigravida | 37 (37.0%) | 81 (45.0%) | 1 | 1 | ||
| Multigravida | 63 (63.0%) | 99 (55.0%) | 1.393 (0.844–2.299) | 0.195 | 1.386 (0.793–2.424) | 0.252 |
| PARITY | ||||||
| Primipara | 43 (43.0%) | 86 (47.8%) | 1 | |||
| Multipara | 57 (57.0%) | 94 (52.2%) | 1.213 (0.741–1.984) | 0.442 | ||
| HISTORY OF ABORTION | ||||||
| Yes | 25 (25.0%) | 37 (20.6%) | 0.776 (0.435–1.385) | 0.391 | ||
| No | 75 (75.0%) | 143 (79.4%) | 1 | |||
| PREGNANCY UNPLANNED | ||||||
| Yes | 52 (52.0%) | 74 (41.1%) | 1.552 (0.949–2.538) | 1.604 (0.925–2.782) | ||
| No | 48 (48.0%) | 106 (58.9%) | 1 | 0.080 | 1 | 0.092 |
On bivariate analysis, the number of pregnancies (gravida) and unplanned pregnancy showed an association with depression at a P-value of < 0.2. However, there was no significant association observed from multivariate logistic regression analysis.
Association Between Social Support, Marital Discord, Domestic Violence, Prenatal Anxiety, Consanguinity, and Catastrophic Events With Prenatal Depression (Table 4)
Table 4.
Association of maternal depression with social support, marital discord, and domestic violence among the pregnant mothers (N = 280).
| Maternal characteristics | Depressed mothers (>13) (n = 100) | Non-depressed mothers (<13) (n = 180) | Bivariate analysis crude OR (95% CI) | P-value | Multivariate analysis adjusted OR (95% CI) | P-value |
|---|---|---|---|---|---|---|
| SOCIAL SUPPORT | ||||||
| High support (1–2.9) | 44 (44.0%) | 94 (52.2%) | 1 | 1 | ||
| Moderate support (3–5) | 24 (24.0%) | 54 (30.0%) | 0.949 (0.521–1.729) | 0.865 | 0.839 (0.436–1.616) | 0.600 |
| Low support (5.1–7) | 32 (32.0%) | 32 (17.8%) | 2.136 (1.164–3.919) | 0.014 | 1.785 (0.915–3.481) | 0.089 |
| MARITAL DISCORD | ||||||
| No | 32 (32.0%) | 79 (43.9%) | 1 | 1 | ||
| Yes | 68 (68.0%) | 101 (56.1%) | 1.662 (0.995–2.776) | 0.052 | 1.517 (0.862–2.671) | 0.149 |
| SPOUSE PHYSICAL AND SEXUAL VIOLENCE | ||||||
| Yes | 11 (11.0%) | 04 (2.2%) | 5.438 (1.684–17.564) | 0.005 | 5.916 (1.703–20.558) | 0.005* |
| No | 89 (89.0%) | 176 (97.8%) | 1 | 1 | ||
| PREGNANCY RELATED ANXIETY | ||||||
| Absent | 47 (47.0%) | 109 (60.6%) | 1 | 1 | ||
| Present | 53 (53.0%) | 71 (39.4%) | 1.731 (1.057–2.836) | 0.029 | 2.016 (1.134–3.587) | 0.017* |
| CONSANGUINITY | ||||||
| No | 77 (77.0%) | 121 (67.2%) | 1 | |||
| Yes | 23 (23.0%) | 59 (32.8%) | 0.613 (0.350–1.073) | 0.086 | 0.728 (0.397–1.334) | 0.304 |
| CATASTROPHIC EVENTS | ||||||
| No | 64 (31.4%) | 140 (68.6%) | 1 | 1 | ||
| Yes | 36 (47.4%) | 40 (68.6%) | 1.969 (1.149–3.374) | 0.014 | 2.148 (1.203–3.837) | 0.010* |
Positively significant according to multivariable analysis.
Association with low social support and presence of marital discord was significant on bivariate analysis but not in multivariate logistic regression. Presence of domestic violence was found to impose a five times higher and highly significant risk of developing prenatal depression among the respondents (COR = 5.438; 95% CI: 1.6–17.5, AOR = 5.916; 95% CI: 1.7–20.5). Pregnancy related anxiety was also found to be a positive predictor of prenatal depression (COR = 1.731; 95% CI: 1.05–2.8, AOR = 2.016; 95% CI: 1.13–3.5). The blood relationship with the husband did not show any significant association with prenatal depression on bivariate analysis and multivariable analysis. Presence of catastrophic events over the past 1 year imposed a two times higher and significant risk of developing prenatal depression among the respondents (COR = 1.969; 95% CI: 1.14–3.37, AOR = 2.148; 95% CI: 1.20–3.83, p-value = 0.010). History of mental illness was not included in the analysis because only one respondent had history of this kind and was undergoing treatment with medications.
Association of Prenatal Depression With Physiologic Parameters (Table 5)
Table 5.
Association of prenatal depression with physiological parameters (N = 280).
| Physiological parameters | Depressed mothers (>13) (n = 100) | Non-depressed mothers (<13) (n = 180) | Bivariate analysis crude OR (95% CI) | P-value | Multivariate analysis adjusted OR (95% CI) | P-value |
|---|---|---|---|---|---|---|
| BODY MASS INDEX | ||||||
| Normal | 56 (56.0%) | 96 (53.3%) | 1 | |||
| Underweight | 12 (12.0%) | 21 (11.7%) | 0.980 (0.448–2.141) | 0.959 | ||
| Obese | 32 (32.0%) | 63 (35.0%) | 0.871 (0.508–1.491) | 0.614 | ||
| ANEMIA | ||||||
| Present | 41 (41.0%) | 54 (30.0%) | 1.621 (0.973–2.701) | 0.063 | 1.586 (0.912–2.756) | 0.102 |
| Absent | 59 (59.0%) | 126 (70.0%) | 1 | 1 | ||
Table 5 shows the association between physiologic parameters with prenatal depression. Presence of anemia (COR = 1.621; 95% CI: 0.9–2.7, AOR = 1.586; 95% CI: 0.91–2.75) showed some strong association with prenatal depression although this was not statistically significant. No association found between BMI and depression.
Discussion
In this study we have measured the prevalence of prenatal depression among pregnant women and its association with certain risk factors such as socio-demographic characteristics, obstetric history, social support, marital discord, spouse physical and sexual violence, and physiologic measurements which included body mass index and hemoglobin level.
The mean age of respondents was 23.02 ± 3.40 years, which reflects upon the Indian cultural tradition of early marriage and parenthood. The prevalence of depression during pregnancy was 37.8% which is suggestive of a high probability of depression (using an EPDS cutoff score ≥13) among the respondents. The prevalence of prenatal depression makes it a significant public health issue in the study region. EPDS has been validated for use in India and Karnataka (26). Our study used a cut off score of more than or equal to 13 to identify women with depression; this yields a sensitivity of 100% and specificity of 84.9% in Indian settings (27). Another study from Karnataka showed an almost similar prevalence of 36.8% (21) whereas George et al., Ajinkya et al., and Bavle et al. observed much lower prevalence rates of 16.3% in coastal south India (35), 9.18% in Navi Mumbai (22), and 12.3% in Bangalore (36), respectively. This difference could be attributed to diversity in the socio-economic status, socio-cultural and psychosocial factors such as social support which might vary across different regions in the country. Moreover, this study was conducted in a public sector hospital setting, which in itself could pose as a risk factor and predictor for prenatal depression (28) due to inadequate quality of care in such settings.
In our study, majority of the study participants belonged to the low income group. Although we could not document a significant association with socio-economic status, the risk of depression during, and after pregnancy is higher among the socially disadvantaged group (10, 37, 38). It is hypothesized that low income increases the likelihood of poor living conditions, financial struggle and influences interpersonal relationships which could lead to psychosocial stress. Over a third of the study participants were high school graduates though over 90% were not working; however there was no association of education and occupation with depression. Bavle et al. (36) in their study among pregnant women in Bangalore observed that being educated but not employed outside the house could predispose to depression during pregnancy. Study findings from other low income settings point toward a significant association of a woman's occupation with depression: women who were housewives or employed in the private sector or as a laborer or merchant business were prone to get depressed during pregnancy (39, 40). Other socio-demographic factors such as age, husband's education, and occupation did not predict the occurrence of depression in the present study even though some studies have identified young age as a risk factor (41, 42). In Asian settings, having an unemployed or uneducated husband increases the probability of depression (43, 44).
Among the obstetric history variables, unplanned pregnancy increased the odds of depression on bivariate analysis. However, no significant association was observed on multivariate logistic regression analysis. Other studies show that the chance of getting depressed is higher in case of an unplanned pregnancy (15, 37, 45). Similarly multigravidity appeared to be risk factor for depression on bivariate analysis but not on logistic regression analysis although some studies do report a significant relationship (36, 46).
In this study, among the psychosocial factors, presence of spouse physical and sexual violence and pregnancy related anxiety were significant risk factors for prenatal depression in the multivariable analysis. Earlier research has also reported a strong relationship between domestic violence and the risk of depression in pregnancy in high as well as middle to low—income settings (47, 48). Moderate and low social support were significantly related on bivariate but not on multivariate analysis. The linkage between poor social support and prenatal depression has been well-documented (49, 50). Low social support may increase mental stress by inducing feelings of insecurity, predispose toward substance abuse (51), and promote interpersonal conflict (52). The findings from the present study are concurrent with the study results reported by Nongrum et al. India (53), George et al. in Southern India (35), Silva et al. in Brazil (42), and Bernard et al. in Jamaica (54). Depression and anxiety show frequent co-existence and anxiety may emerge as a strong predictor for depression (24, 37). Mohamad et al. (55) and Edward et al. (56) also demonstrated that anxiety strongly increased the risk of suffering from depression during pregnancy. Even a history of mental illness can pose as a risk factor for depression (57, 58); however in our study only one respondent appeared to have such a history. Marital discord appeared predict presence of depression on bivariate but not on multivariate analysis; other studies report that this is e a well-established risk factor due to its influence on social support (59, 60). Likewise consanguinity seemed to be associated with depression only on bivariate testing. Consanguineous marriages are fairly prevalent in South India and clinical observations have reported a high prevalence of depression in such communities (61) which could be genetically driven (62). A major catastrophic event in the past 1 year was an important risk factor which was significantly associated with prenatal depression in this study; this is consistent with the study results reported by Leigh et al. (63) and Shakeel et al. (58). Another study reported that negative life events may lead to persistent higher levels of depressive symptoms since positive life events can decrease the severity of depression over time (64).
Among the physiologic measurements, anemia was significantly associated with depression on bivariate although not so on multivariate analysis. This is in agreement with the study findings reported by Lukose et al. (65); however Yilmaz et al. depicted the existence of such an association between depressive symptoms and anemia in the third trimester of pregnancy (66). Body mass index was not linked with the risk of prenatal depression in the present study. Research done in other countries reportedly point toward an interconnection between obesity and depression (67, 68). The causal pathway could include inflammation (62), hormonal imbalance (69), or sleep disturbance (70).
Study Strengths And Limitations
This study focuses on prenatal depression which has received less attention than postnatal depression. All the instruments/scales used to measure the study variables had good psychometric properties. Our study had few a limitations. Antenatal care at such hospitals is mostly availed by pregnant women from the lower and middle—income groups in a community. Hence the findings from this study cannot be extrapolated to pregnant women belonging to the high income group as there could be variations in the psychosocial factors and standard of living. As a part of the cohort study protocol, we excluded women with high risk pregnancies and those with a history of intake of steroidal medication over the past 1 year; this could limit the generalizability of the study findings. Adverse obstetric complications during pregnancy can modulate the mental health of a woman during pregnancy. In the south-east Asian context, conflict with in-laws is also a significant risk factor, although this item was not recorded in the present study but will be included in future data collection. We used the EPDS scale which is a self –reporting screening measure for identifying women at risk for depression. Even though EPDS has a high sensitivity and specificity and can be easily administered by a trained health worker, it is important to confirm the presence of depression by using a structured clinical interview to confirm diagnosis.
Conclusion
The present study showed a high prevalence of prenatal depression which is suggestive of its public health importance in the study region. Spouse physical and sexual violence, pregnancy related anxiety and a history of catastrophic events were important predictors of prenatal depression. Obstetric practice should include screening and diagnosis of prenatal depression as a part of routine antenatal care in low and middle—income countries.
Ethics Statement
This study was carried out in accordance with the recommendations of the Ethical Committee of Indian Institute of Public Health Bangalore campus (IIPHHB/TRCIEC/118/2017) with written informed consent from all subjects. All subjects gave written informed consent in accordance with the Declaration of Helsinki. The protocol was approved by the Ethical committee of Indian Institute of Public Health Bangalore campus.
Author Contributions
AN and MK: conceptualization. BS, SV, and JV: formal analysis. AN and GM: funding acquisition. CM: methodology. AN and SB: writing-original draft preparation.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to extend our sincere thanks to the staffs of Jaya Nagar General Hospital for supporting us during data collection.
Footnotes
Funding. This project is funded by Welcome Trust DBT India Alliance (Clinical and Public Health Research Fellowship), grant number IA/CPHI/16/1/502634.
References
- DiPietro JA. Psychological and psychophysiological considerations regarding the maternal-fetal relationship. Infant Child Dev. (2010) 19:27–38. 10.1002/icd.651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A, Patel V, Maselko J, Kirkwood B. The neglected ‘m’ in MCH programmes–why mental health of mothers is important for child nutrition. Trop Med Int Health. (2008) 13:579–83. 10.1111/j.1365-3156.2008.02036.x [DOI] [PubMed] [Google Scholar]
- Bowen A, Muhajarine N. Antenatal depression. Can. Nurse. (2006) 102:26–30. [PubMed] [Google Scholar]
- Grote NK, Bridge JA, Gavin AR, Melville JL, Iyengar S, Katon WJ. A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Arch Gen Psychiatry. (2010) 67:1012–24. 10.1001/archgenpsychiatry.2010.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Accortt E, Cheadle A, Dunkel SC. Prenatal depression and adverse birth outcomes: an updated systematic review. Matern Child Health J. (2014) 19 1306–37. 10.1007/s10995-014-1637-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Bergh B, Mulder E, Mennes M, Glover V. Antenatal maternal anxiety and stress and the neurobehavioural development of the fetus and child: links and possible mechanisms. A review. Neurosci Biobehav Rev. (2005) 29:237–58. 10.1016/j.neubiorev.2004.10.007 [DOI] [PubMed] [Google Scholar]
- Plant DT, Pawlby S, Sharp D, Zunszain PA, Pariante CM. Prenatal maternal depression is associated with offspring inflammation at 25 years: a prospective longitudinal cohort study. Transl Psychiatry. (2016) 6:e936. 10.1038/tp.2015.155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satyanarayana VA, Lukose A, Srinivasan K. Maternal mental health in pregnancy and child behavior. Indian J Psychiatry. (2011) 53:351–61. 10.4103/0019-5545.91911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard L, Molyneaux E, Dennis C, Rochat T, Stein A, Milgrom J. Non- psychotic mental disorders in the perinatal period. Lancet. (2014) 384:1775–88. 10.1016/S0140-6736(14)61276-9 [DOI] [PubMed] [Google Scholar]
- Fatoye FO, Adeyemi AB, Oladimeji B. Emotional distress and its correlates among Nigerian women in late pregnancy. J Obstet Gynecol. (2004) 24:504–9. 10.1080/01443610410001722518 [DOI] [PubMed] [Google Scholar]
- Rochat T, Tomlinson M, Newell M, Stein A. Depression among pregnant women testing for HIV in rural South Africa: implications for VCT. In:9th International AIDS Impact Conference. Botswana: (2009). [Google Scholar]
- Manikkam I, Burns JK. Antenatal depression and its risk factors: an urban prevalence study in KwaZulu-Natal. South Afr Med J. (2012) 102:940–4. 10.7196/samj.6009 [DOI] [PubMed] [Google Scholar]
- Nasreen H, Kabir Z, Forsell Y, Edhborg M. Prevalence and associated factors of depressive and anxiety symptoms during pregnancy: a population based study in rural Bangladesh. BMC Womens Health. (2011) 111:22 10.1186/1472-6874-11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson O, Ajayi I. Prevalence of antenatal depression and associated risk factors among pregnant women attending clinics in Abeokuta North Local Government Area, Nigeria. Depres Res Treat. (2016) 2016:4518979. 10.1155/2016/4518979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biratu A, Haile D. Prevalence of antenatal depression and associated factors among pregnant women in Addis Ababa, Ethiopia: a cross-sectional study. Reprod Health. (2015) 12:99. 10.1186/s12978-015-0092-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafri SAM, Ali M, Ali R, Shaikh S, Abid M, Aamir IS. Prevalence of depression among Pregnant Women attending Antenatal Clinics in Pakistan. Acta Psychopathol. (2017) 3:54 10.4172/2469-6676.100126 [DOI] [Google Scholar]
- Eastwood J, Ogbo F, Hendry A, Noble J, Page A. The impact of antenatal depression on perinatal outcomes in Australian women. PLoS ONE. (2017) 12:e0169907. 10.1371/journal.pone.0169907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DT, Chan SS, Sahota DS, Yip AS, Tsui M, Chung TK. A prevalence study of antenatal depression among Chinese women. J Affect Disord. (2004) 82:93–9. 10.1016/j.jad.2003.10.003 [DOI] [PubMed] [Google Scholar]
- Pajulo M, Savonlahti E, Sourander A, Helenius H, Piha J. Antenatal depression, substance dependency and social support. J Affect Disord. (2001) 65:9–17. 10.1016/S0165-0327(00)00265-2 [DOI] [PubMed] [Google Scholar]
- Humayun A, Haider I, Imran N, Iqbal H, Humayun N. Antenatal depression and its predictors in Lahore, Pakistan. East Mediterran Heath J. (2013) 19:327–32. 10.26719/2013.19.4.327 [DOI] [PubMed] [Google Scholar]
- Pai Keshava K, Shruthi SH, Hulegar AA, Sandeep KR. Prevalence of antenatal depression and gender preference: A cross sectional study among Mangalore population, Karnataka, India. J Pharm Biomed Sci. (2013) 29:1011–4. [Google Scholar]
- Ajinkya S, Jadhav PR, Srivastava NN. Depression during pregnancy: prevalence and obstetric risk factors among pregnant women attending a tertiary care hospital in Navi Mumbai. Ind Psychiatry J. (2013) 22:37–40. 10.4103/0972-6748.123615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heron J, O'Connor TG, Evans J, Golding J, Glover V, ALSPAC Study Team The course of anxiety and depression through pregnancy and the pospartum in a community sample. J Affect Disord. (2004) 80:65–73. 10.1016/j.jad.2003.08.004 [DOI] [PubMed] [Google Scholar]
- Verreault N, Da Costa D, Marchand A, Ireand K, Drista M, Khalife S. Rates and risk factors associated with depressive sympotms during pregnancy and with postpartum onset. J Psychosom Obset Gynaecol. (2014) 35:84–91. 10.3109/0167482X.2014.947953 [DOI] [PubMed] [Google Scholar]
- Tachibana Y, Koizumi T, Takehara K, Kakee N, Tsujii H, Mori R, et al. Antenatal risk factors of postpartum depression at 20 weeks of gestation in Japanese sample: psychosocial perspectives from a Cohort study in Tokyo. PLoS ONE. (2015) 10:e0142410. 10.1371/journal.pone.0142410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath A, Murthy G, Babu G, Di Renzo G. Effect of prenatal exposure to maternal cortisol and psychological distress on infant development in Bengaluru, southern India: a prospective cohort study. BMC Psychiatry. (2017) 17:255. 10.1186/s12888-017-1424-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression: development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. (1987) 150:782–6. [DOI] [PubMed] [Google Scholar]
- Zimet GD, Dahlem NW, Zimet SG, Farley GK. The multidimensional scale of perceived social support. J Pers Assess. (1988) 52:30–41. [DOI] [PubMed] [Google Scholar]
- Ng CG, AmerSiddiq AN, Aida SA, Zainal NZ, Koh OH. Validation of the Malay version of the Multidimensional Scale of Perceived Social Support (MSPSS-M) among a group of medical students in Faculty of Medicine, University Malaya. Asian J Psychiatr. (2010) 3:3–6. 10.1016/j.ajp.2009.12.001 [DOI] [PubMed] [Google Scholar]
- Busby DM, Christensen C, Crane DR, Larson JH. A revision of the dyadic adjustment scale for use with distressed and non-distressed couples: construct hierarchy and multidimensional scales. J Marital Family Ther. (1995) 21:289–308. [Google Scholar]
- Crane DR, Middleton KC, Bean RA. Establishing criterion scores for the Kansas marital satisfaction scale and the revised dyadic adjustment scale. Am J Family Ther. (2000) 28:53–60. 10.1080/019261800261815 [DOI] [Google Scholar]
- Straus M, Gelles R. Physical Violence in 8145 American Families: Risk Factors and Adaptations to Violence. Family Relations. New Bmnswick, NJ: Transaction Publishing; (1990), 49–73. [Google Scholar]
- Shaikh Z, Pathak R. Revised Kuppuswamy and B G Prasad socio-economic scales for 2016. Int J Community Med Public Health. (2017) 4:997–9. 10.18203/2394-6040.ijcmph20171313 [DOI] [Google Scholar]
- Huizink A, Delforterie M, Scheinin N, Tolvanen M, Karlsson L, Karlsson H. Adaption of pregnancy anxiety questionnaire–revised for all pregnant women regardless of parity: PRAQ-R2. Arch Womens Mental Health. (2015) 19:125–32. 10.1007/s00737-015-0531-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George C, Lalitha A, Antony A, Jacob K. Antenatal depression in costal South India: Prevalence and risk factors in the community. Int J Soc Psychiatry. (2015) 62:141–7. 10.1177/0020764015607919 [DOI] [PubMed] [Google Scholar]
- Bavle AD, Chandahalli AS, Phatak AS, Rangaiah N, Kuthandahalli SM, Nagendra PN. Antenatal depression in a tertiary care Hospital. Indian J Psychol Med. (2016) 38:31–5. 10.4103/2F0253-7176.175101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adewuya AO, Ola BA, Aloba OO, Dada AO, Fasoto OO. Prevalence and correlates of depression in late pregnancy among Nigerian women. Depress Anxiety. (2007) 24:15–21. 10.1002/da.20221 [DOI] [PubMed] [Google Scholar]
- Black MM, Baqu AH, Zaman K, McNary SW, Le K, El Arifeen S, et al. Depressive symptoms among rural Bangladeshi mothers: implications for infant development. J Child Psychol Psychiatry. (2007) 48:764–72. 10.1111/j.1469-7610.2007.01752.x [DOI] [PubMed] [Google Scholar]
- Ayele T, Azale T, Alemu K, Abdissa Z, Mulat H, Fekadu A. Prevalence and associated factors of antenatal depression among women attending antenatal care service at gondar university hospital, northwest Ethiopia. PLoS ONE. (2016) 11:e0155125. 10.1371/journal.pone.0155125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemta W. Prevalence and factors associated with antenatal depression among women following antenatal care at Shashemane health facilities, South Ethiopia. Ann Glob Health. (2015) 81:90 10.1016/j.aogh.2015.02.709 [DOI] [Google Scholar]
- Lancaster CA, Gold KJ, Flynn HA, Yoo H, Marcus SM, Davis MM. Risk factors for depressive symptoms during pregnancy: a systematic review. Am J Obstetr Gynecol. (2010) 202:5–14. 10.1016/j.ajog.2009.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MMJ, Leite EPRC, Nogueira DA, Clapis MJ. Depression in pregnancy. Prevalence and associated factors. Invest. Educ. Enferm. (2016) 34:342–50. 10.17533/udea.iee.v34n2a14 [DOI] [PubMed] [Google Scholar]
- Rahman A, Creed F. Outcome of prenatal depression and risk factors associated with persistence in the first postnatal year: prospective study from Rawalpindi, Pakistan. J Affect Disord. (2007) 100:115–21. 10.1016/2Fj.jad.2006.10.004t [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andajani-Sutjahjo S, Manderson L, Astbury J. Complex emotions, complex problems: understanding the experiences of perinatal depression among new mothers in urban Indonesia. Cult Med Psychiatr. (2007) 31:101–22. 10.1007/s11013-006-9040-0 [DOI] [PubMed] [Google Scholar]
- Martha A, Mesfin T, Tadese A, Dessalegn B. Prevalence and predictors of antenatal depressive symptoms among women attending Adama Hospital Antenatal Clinic, Adama, Ethiopia. Int J Nurs Midwifery. (2017) 9:58–64. 10.5897/IJNM2016.0239 [DOI] [Google Scholar]
- DiPietro JA, Costigan KA, Sipsma HL. Continuity in self-report measures of maternal anxiety, stress, and depressive symptoms from pregnancy through two years postpartum. J Psychosom Obstet Gynaecol. (2008) 29:115–24. 10.1080/01674820701701546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazmararian JA, Lazorick S, Spitz AM, Ballard TJ, Saltzman LE, Marks JS. Prevalence of violence against pregnant women. JAMA. (1996) 275:1915–20. [PubMed] [Google Scholar]
- Gazmararian JA, Petersen R, Spitz AM, Goodwin MM, Saltzman LE, Marks JS. Violence and reproductive health: current knowledge and future research directions. Matern Child Health. (2000) 4:79–84. 10.1023/A:1009514119423 [DOI] [PubMed] [Google Scholar]
- Westdahl C, Milan S, Magriples U, Kershaw TS, Rising SS, Ickovics JR. Social support and social conflict as predictors of prenatal depression. Obstetr Gynecol. (2007) 110:134–40. 10.1097/2F01.AOG.0000265352.61822.1b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J, Jiang X, Yao J, Li X, Liu X, Pang M. Depression, social support, and coping styles among pregnant women after the Lushan Earthquake in Ya'an, China. PLoS ONE. (2015) 10:e0135809. 10.1371/journal.pone.0135809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahluwalia IB, Mack KA, Mokdad A. Mental and physical distress and high-risk behaviors among reproductive-age women. Obstet Gynecol. (2004) 104:477–83. 10.1097/01.AOG.0000137920.58741.26 [DOI] [PubMed] [Google Scholar]
- Grimstad H, Schei B, Backe B, Jacobsen G. Interpersonal conflict and physical abuse in relation to pregnancy and infant birth weight. J Womens Health Gend Based Med. (1999) 8:847–53. 10.1089/152460999319165 [DOI] [PubMed] [Google Scholar]
- Nongrum R, Thomas E, Lionel J, Jacob KS. Domestic violence as a risk factor for maternal depression and neonatal outcomes: a hospital-based cohort study. Indian J Psychol Med. (2014) 36:179–81. 10.4103/2F0253-7176.130989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard O, Gibson R, McCaw-Binns A, Reece J, Coore-Desai C, Shakespeare-Pellington S, et al. Antenatal depressive symptoms in Jamaica associated with limited perceived partner and other social support: a cross-sectional study. PLoS ONE. (2018) 13:e0194338. 10.1371/journal.pone.0194338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamad Yusuff AS, Tang L, Binns CW, Lee AH. Prevalence of antenatal depressive symptoms among women in Sabah, Malaysia. J Matern Fetal Neonatal Med. (2015) 29:1170–4. 10.3109/14767058.2015.1039506 [DOI] [PubMed] [Google Scholar]
- Edwards B, Galletly C, Semmler-Booth T, Dekker G. Antenatal psychosocial risk factors and depression among women living in socioeconomically disadvantaged suburbs in Adelaide, South Australia. Aust N Z J Psychiatr. (2008) 42:45–50. 10.1080/00048670701732673t [DOI] [PubMed] [Google Scholar]
- Faisal-Cury A, Menezes P, Araya R, Zugaib M. Common mental disorders during pregnancy: prevalence and associated factors among low-income women in Sao Paulo, Brazil: depression and anxiety during pregnancy. Arch Womens Ment Health. (2009) 12:335–43. 10.1007/s00737-009-0081-6 [DOI] [PubMed] [Google Scholar]
- Shakeel N, Eberhard-Gran M, Sletner L, Slinning K, Martinsen EW, Holme I, et al. A prospective cohort study of depression in pregnancy, prevalence and risk factors in a multi-ethnic population. BMC Pregnancy Childbirth. (2015) 15:5. 10.1186/s12884-014-0420-0t [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau Y, Keung DW. Correlates of depressive symptomatology during the second trimester of pregnancy among Hong Kong Chinese. Soc Sci Med. (2007) 64:1802–11. 10.1016/j.socscimed.2007.01.001 [DOI] [PubMed] [Google Scholar]
- Crawford DW, Houts RM, Huston TL, George LJ. Compatibility, leisure, satisfaction in marital relationship. J Marriage Family. (2002) 64:433–49. 10.1111/j.1741-3737.2002.00433.x [DOI] [Google Scholar]
- Rao TSS. The Genetics of affective disorder-a pedigree study. Indian J Psychiatry. (1993) 35:127–30. [PMC free article] [PubMed] [Google Scholar]
- Rao TSS, Prabhakar AK, Jagannatha Rao KS, Sambamurthy K, Asha MR, Ram D, et al. Relationship between consanguinity and depression in a south Indian population. Indian J Psychiatry. (2009) 51:50–2. 10.4103/2F0019-5545.44906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh B, Milgrom J. Risk factors for antenatal depression, postnatal depression and parenting stress. BMC Psychiatr. (2008) 8:24. 10.1186/2F1471-244X-8-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zayas LH, Jankowski KRB, McKee MD. Prenatal and postpartum depression among low-income Dominican and Puerto Rican women. Hisp J Behav Sci. (2003) 25:370–85. 10.1177/2F0739986303256914 [DOI] [Google Scholar]
- Lukose A, Ramthal A, Thomas T, Bosch R, Kurpad AV, Duggan C, et al. Nutritional factors associated with antenatal depressive symptoms in the early stage of pregnancy among urban South Indian women. Matern Child Health J. (2014) 18:161–70. 10.1007/s10995-013-1249-2 [DOI] [PubMed] [Google Scholar]
- Yilmaz E, Yilmaz Z, Çakmak B, Gültekin IB, Çekmez Y, Mahmutoglu S, et al. Relationship between anemia and depressive mood in the last trimester of pregnancy. J Matern Fetal Neonatal Med. (2017) 30:977–82. 10.1080/14767058.2016.1194389 [DOI] [PubMed] [Google Scholar]
- Schuette SA, Kominiarek MA, Wisner KL, Massey SH. Pre-pregnancy body mass index and third-trimester depressive symptoms in a healthy privately insured sample. AJP Rep. (2018) 8:e13–7. 10.1055/s-0038-1625974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castanon N, Lasselin J, Capuron L. Neuropsychiatric comorbidity in obesity: role of inflammatory processes. Front Endocrinol. (2014) 5:74. 10.3389/fendo.2014.00074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph JJ, Golden SH. Cortisol dysregulation: the bidirectional link between stress, depression, and type 2 diabetes mellitus. Ann N Y Acad Sci. (2017) 1391:20–34. 10.1111/nyas.13217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broussard JL, Van Cauter E. Disturbances of sleep and circadian rhythms: novel risk factors for obesity. Curr Opin Endocrinol Diabetes Obes. (2016) 23:353–9. 10.1097/MED.0000000000000276 [DOI] [PMC free article] [PubMed] [Google Scholar]