Abstract
Exosomes are nanovesicles secreted from various types of cells and can be isolated from various bodily fluids, such as blood and urine. The number and molecular contents, including proteins and RNA of exosomes, have been shown to reflect their parental cell origins, characteristics and biological behaviors. An increasing number of studies have demonstrated that exosomes play a role in the course of tumorigenesis, diagnosis, treatment and prognosis, although its precise functions in tumors are still unclear. Moreover, owing to a lack of a standard approach, exosomes and its contents have not yet been put into clinical practice successfully. This review aims to summarize the current knowledge on exosomes and its contents in esophageal cancer as well as the current limitations/challenges in its clinical application, which may provide a basis for an all-around understanding of the implementation of exosomes and exosomal contents in the surveillance and therapy of esophageal cancer.
Keywords: Exosome, Esophageal cancer, Tumorigenesis, Biomarker, Therapeutic potential
Core tip: The exosome is a popular area of current research. Numerous studies have shown that exosomes may play an important role in the progression of esophageal cancer, but have not yet been applied to the clinic. This review systemically summarized the current knowledge on exosomes and its contents in esophageal cancer and pointed out the current limitations in its clinical application. This paper may provide a basis for an all-around understanding of the implementation of exosomes in esophageal cancer.
INTRODUCTION
Exosomes were first discovered by Johnstone and Pan while studying the transformation from reticulocyte to mature red blood cells in 1987. Originally, exosomes were considered a form of waste discharge in the process of reticulocytes differentiating into mature red blood cells. Interestingly, further studies found that exosomes from intracellular multivesicular bodies that fuse with cell membranes and then released into the extracellular matrix are membrane nanoscale vesicles with a diameter of 50-150 nm and have a lipid bilayer membrane structure[1]. Moreover, studies have found that exosomes can be secreted from a variety of cells including red blood cells, T cells, B cells, dendritic cells and tumor cells[2-5] and are widely distributed in various body fluids, such as blood, urine, saliva and milk.
Exosomes contain various types of biologically active substances, including proteins, lipids, DNA fragments and RNA, such as microRNA (miR), circular RNAs (circRNAs), long non-coding RNA and mRNA[6]. Researchers found that these biologically active substances contained within the exosomes may participate in the immune response, antigen presentation, intercellular communication, transport of proteins and RNA and many other physiological processes[7-9]. It suggested that exosomes are a crucial intercellular organelle and communication media. Thus, exosomes may play a pivotal role in the diagnosis and therapy of various diseases including tumors.
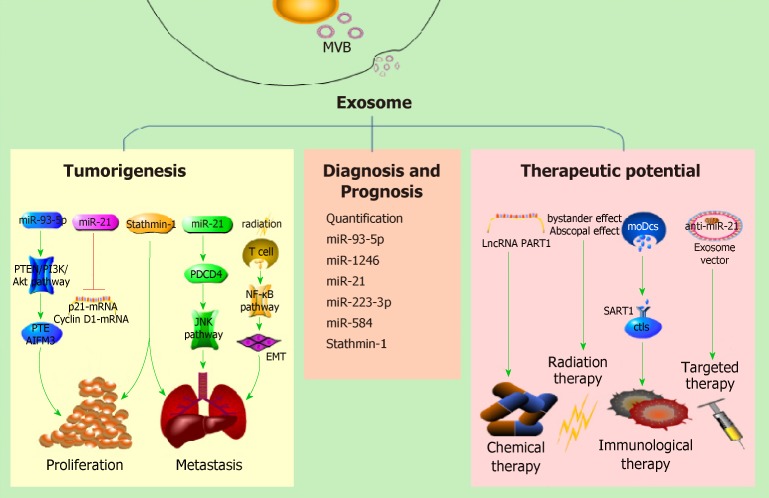
There were 455800 newly diagnosed esophageal cancer (EC) patients and 400200 deaths worldwide in 2012[10]. Just in China, there were approximately 477900 new cases and 375000 deaths in 2015[11]. Recent studies have shown that exosomes secreted from various cells including tumor cells, irradiated T cells[12] or tumor associated fibroblasts[13] and exosomal contents may play important roles in EC development. Therefore, exosomes may be of value as a diagnostic/prognostic tool and as a therapeutic target in EC (Figure 1).
Figure 1.
The role of exosomes in esophageal cancer. CTLs: Cytotoxic T lymphocytes; MVB: Intracellular multivesicular body. Red line and cross bar: Inhibit; Green arrow: Promote.
PubMed, MEDLINE advanced search builder and Geenmedical was used for this review’s literature search. The terms searched were “exosome” and “esophageal cancer,” and the time period was restricted from 2008 to 2018. Then we further screened the retrieved articles according to (1) the formation and regulation of exosomes and (2) the research progress of exosomes in EC. Cross references were also screened for papers relevant to this paper. Here we reviewed relevant articles from the last 10 years with the aim of summarizing the function of exosomes in tumorigenesis, diagnosis and treatment of EC (Table 1).
Table 1.
The function of exosomes in esophageal cancer
| Function | Exosome or its cargo | Pathway | Expression | Ref. |
| Tumorigenesis | miR-93-5p | PTEN/PI3K/Akt | up | [15] |
| miR-25 | miR/PTEN mRNA | up | [16] | |
| miR-210 | miR/AIFM3 mRNA | |||
| Ir-T cell-derived exosomes | NF-κB | up | [9] | |
| miR-21 | JNK | up | [29,30] | |
| Stathmin-1 | [44] | |||
| Diagnosis and prognosis | Quantification of exosomes | [50] | ||
| miR-93-5p | up | [15] | ||
| miR-1246 | up | [55] | ||
| miR-21 | up | [29] | ||
| miR-223-3p | down | [57,58] | ||
| miR-584 | down | [58] | ||
| Stathmin-1 | up | [44] | ||
| Therapy | lncRNA PART1 | up | [12] | |
| moDcs-derived exosomes | [65] |
Ir-T cell: Irradiated T cells; JNK: c-Jun N-terminal kinase; lncRNA: Long non-coding RNA; miR: MicroRNA; moDcs: Monocyte-derived dendritic cells.
EXOSOME AND ESOPHAGEAL CANCER TUMORIGENESIS
Recent studies found that exosomes derived from many cancer cell types, including glioblastoma[14], tongue cancer[15], thyroid carcinoma[15], breast cancer[17], EC[18], etc, may play a crucial part in the course of tumor formation and cancer metastasis by transfer of exo-miRNA, exo-circRNA, exo-DNA and exo-protein.
Promotion of tumor proliferation
Researchers have found that exosomes are involved in tumor proliferation by transferring exo-microRNA. MiR-93-5p[18] can be transferred between EC9706 cells by exosomes and may downregulate the expression of p21 and Cyclin D1 through the PTEN/PI3K/Akt pathway to promote the proliferation of EC cells. Ke et al[19] found that the overexpression of miR-25 and miR-210 in EV–Co-Cultured (EV: the extracellular vesicle secreted from esophageal adenocarcinoma (EA) cells) gastroids decreased the mRNA levels of PTEN and AIFM3, respectively, which are known tumor suppressor genes[20,21].
Promotion of invasion and metastasis of tumors
Exosomes and epithelial-mesenchymal transition: A large number of studies have indicated that epithelial-mesenchymal transition (EMT) is closely related to cancer metastasis[22-26]. Recent studies found that exosomes may be involved in the course of tumor metastasis by EMT. Min et al[12] found that exosomes derived from irradiated T cells showed the potential role of promoting metastasis of TE13 cells in vitro, possibly by promoting EMT through regulating β-catenin and the NF-κB/snail pathway. A recent study demonstrated that overexpression of exosomal circPRMT5 in urothelial carcinoma of the bladder tissues could also promote the EMT process in vivo and in vitro through the miR-30c sponge[27]. However, exosomal circRNA has not been reported in the pathogenesis of EC. Thus, further studies on the relationship of exo-circRNA and EMT in tumors is needed.
MiR-21: A study has shown a high expression of miR-21 in EC cells and the exosomes secreted by them, and the expression of miR-21 is higher in exosomes than in their donor cells[28,29]. Exosome-shuttling miR-21 overexpression from parental cells could target programmed cell death 4 protein thus activating its downstream c-Jun N-terminal kinase signaling pathway and distinctly promote the migration and invasion of recipient cells.
Stathmin-1: Stathmin-1 is a microtubule-destabilizing cytosolic phosphoprotein that plays an important role in tumor cell proliferation and migration and may be regulated by miR-34a[30,31], miR-223[32] and miR-193b[33]. It is associated with cancer metastasis and poor prognosis in osteosarcoma[31,34], prostate cancer[35,36], head and neck squamous cell carcinoma[37], hepatocellular carcinoma[32], colorectal cancer[33,38], gallbladder carcinoma[39] and non-small-cell lung cancer[40]. In esophageal squamous cell carcinoma (ESCC), stathmin-1 is also vital for ESCC invasiveness and predicts a poor prognosis[41]. Knockdown stathmin-1 expression enhanced the sensitivity of ESCC cell line to docetaxel and radiation[42]. Stathmin-1 can be delivered by exosomes and then promote the division, growth, migration, and invasion of esophageal squamous cells[43].
Others: In addition, studies demonstrated that the downregulation of the ECRG4 gene in serum exosomes of oral squamous cell carcinoma patients was closely related to tumor growth in vitro and in vivo[44]. MiR-221-3p from cervical squamous cell carcinoma exosomes transferred into human lymphatic endothelial cells to accelerate lymphangiogenesis and lymphatic metastasis via downregulating vasohibin-1[45], but their relationship in EC requires further research.
EXOSOMES AS BIOMARKERS FOR EARLY DIAGNOSIS AND PROGNOSTIC PREDICTION OF ESOPHAGEAL CANCER
Studies have confirmed that the number or contents (including RNA and proteins) of exosomes in the circulating blood are of use as a biomarker for cancers, such as lung cancer[46], prostate cancer[47], colorectal cancer[48], as well as EC[18,28,43,49].
Exosome quantification
Matsumoto et al[49] established mouse subcutaneous tumor models by injecting human ESCC cell line (TE2-CD63-GFP) and detected tumor-derived exosomes from the plasma in the mouse model. They also recruited 66 ESCC patients and 20 non-malignant patients to measure exosomes separated from 100 µL of each individual’s plasma and analyzed the relationship between the prognosis of ESCC cases and the plasma exosome amount. Results showed that ESCC patients had more exosomes than in non-malignant patients. The 3-year overall survival rate of patients with higher levels of exosomes was 79.2%. However, the 3-year overall survival rate of patients with lower levels of exosomes was 47.2%. The molecular mechanism and the major donor cell require further research.
Exosome microRNA
MiRNAs, a large family of small, noncoding RNAs, are enriched in exosomes and could regulate the expression of their target genes. MiRNAs are relatively stable, which can in part represent the level of miRNAs in its donor cell. Numerous studies have indicated that aberrant expression levels of exosomal miRNAs are closely related to the onset of multiple diseases, including cancer. MiR-21, miR-25, miR-93, miR-192, and miR-210 are widely studied oncogenic miRNAs, and have been studied as biomarkers for some kinds of human cancers[50,51].
MiR-93-5p: MicroRNA-93-5p can be transferred between EC9706 cells by exosomes and promote the proliferation of recipient EC9706 cells. Liu et al[18] also found that the expression of miR-93-5p was statistically different (P = 0.035) between EC patients and healthy participants (the former being 1.39 times higher than the latter). The upregulation of plasma miR-93-5p in ESCC patients increased the risk of EC. The Cancer Genome Atlas data analysis also showed that miR-93-5p expression differs in tissues and is associated with patient survival. Therefore, miR-93-5p may be a plasma biomarker for the diagnosis and prognosis of EC.
MiR-1246: MiR-1246 is another cancer associated miRNA dysregulated in many malignant tumors[52,53]. Takeshita et al[54] used ESCC cell lines (including TE1, TE2, TE4, TE6 and TE11) to evaluate the diagnostic and prognostic values of the exo-miR-1246 and estimated the miR-1246 level in peripheral blood of ESCC patients. In the serum samples of ESCC patients, the sensitivity and specificity of miR-1246 were 57.4% and 67.4%, respectively. The area under the curve of receiver operating characteristic curve was 0.665 when setting the optimal cut-off value to 1.15 for squamous cell carcinoma (SCC)-Ag. Furthermore, the miR-1246 expression level of the lymph nodes in adjacent stations was apparently higher than that of the distant lymph nodes.
MiR-21: One microarray data analysis[28] showed that a total of 15 miRNAs were upregulated in the plasma of ESCC patients compared with healthy control participants. They were hsa-miR-16-5p, hsa-miR-130a-3p, hsa-miR-15a-5p, hsa-miR-144-3p, hsa-miR-19b-3p, hsa-miR-5196-5p, hsa-miR-25a-3p, hsa-miR1914-3p, hsa-miR-93-5p, hsa-miR-107, hsa-miR-3911, hsa-miR-21-5p, hsa-let-7d-3p, hsa-let7i-5p and hsa-miR-1290. In contrast, four miRNAs including hsa-miR-1238-3p, hsa-miR-6069, hsa-miR-191-3p, hsa-miR-4665-3p and hsa-miR-937-5p were downregulated. One case-control study on the correlation between exosome-shuttling miR-21 and EC morbidity indicated that the relative expression of miR-21 was 2.95 times higher in EC patient plasma compared to healthy controls. Furthermore, conditional logistic regression analysis showed that the higher miR-21 was expressed, the higher the EC incidence risk was (odds ratio: 1.107; 95% confidence interval: 1.012-1.21; P = 0.026). The area under the curve value was 0.60 to show the diagnostic value of exosome-shuttling miR-21 in ESCC patients.
MiR-223-3p: Exo-miRNAs are also important biomarkers for EA diagnosis and progression[55]. Warnecke-Eberz et al[56] first isolated exosomes from serum of EA patients and compared exosomal miRNA profiles in matching primary tumors with adjacent tissues. Results showed that a total of eight miRNAs (miR-126-5p, miR-146a-5p, miR-192-5p, miR-196b-5p, miR-223-3p, miR-223-5p, miR-409-3p and miR-483-5p) were significantly overexpressed. Conversely, ten miRNAs (miR-22-3p, miR-23b-5p, miR-27b-3p, miR-149-5p, miR-203-5p, miR-224-5p, miR-452-5p, miR-671-3p, miR-944-5p and miR-1201-5p) were significantly downregulated. They also detected that miR-223-3p was overexpressed in T2-staged adenocarcinoma patients and was higher than that in T3 tumors. There was no statistical difference in the overexpression of miR-223-5p and miR-483-5p in EA and ESCC. This result is consistent with the research of Zhou et al[57].
MiR-584: A four-stage study[57] including screening, training, testing and validating identified that miR-106a, miR-18a, miR-20b, miR-486-5p and miR-584 were upregulated, but miR-223-3p was downregulated in the plasma of patients with ESCC. MiR-584 was also overexpressed in ESCC tissues. This result is consistent with the data in The Cancer Genome Atlas database. Furthermore, exosome miR-584 in plasma was consistently dysregulated. Therefore, miR-584 could play an important role in the early diagnosis of ESCC.
Exosome proteins
Stathmin-1 was abundant in exosomes and could enter peripheral blood loaded by exosomes[43]. Yan et al[43] found that the serum levels of stathmin-1 in ESCC patients with lymph node metastasis were significantly higher compared with the cases without lymph node metastasis. As the stage increased, its sensitivity improved accordingly. They also measured the serum levels of stathmin-1 in patients with various cancers. When setting the diagnostic critical value to 4.47ng/mL, the positive rates of stathmin-1 were 81.0% (295/364) for ESCC, 68.8% (33/48) for head and neck squamous cell carcinoma, 71.2% (37/52) for lung squamous cell carcinoma, 50.9% (27/53) for lung adenocarcinoma, 27.1% (16/59) for gastric cancer, 43.9% (25/57) for colorectal cancer and 45.3% (24/53) for hepatocellular carcinoma. The area under the curve value of stathmin-1 for SCC was over 0.8, but it was lower than 0.7 for other types of cancer. Thus, stathmin-1 showed excellent diagnostic capability for SCC and may be a serology biomarker for SCC in the clinic, especially for ESCC.
EXOSOMES AND ESOPHAGEAL CANCER THERAPEUTIC POTENTIAL
Chemotherapy
Kang et al[15] demonstrated that long non-coding RNA PART1, as a competitive endogenous RNA, regulated and transported by exosomes, took part in the formation of drug resistance in ESCC patients via the STAT1-long non-coding RNA PART-Bcl-2 pathway in a gefitinib drug-resistant cell line. We hypothesize that the level of PART1 in exosomes is a promising diagnostic serological biomarker to evaluate the clinical benefits of gefitinib therapy in ESCC patients.
Radiation therapy
Radiotherapy is one of the main treatment methods for EC. Several studies have shown that exosomes derived from the exposed cells in the microenvironment could increase the curative effect of radiotherapy through the bystander effect and abscopal effect[58-61]. For example, exosomes derived from mesenchymal stem cells are the main determinant in enhancing radiation effects in the metastatic spread of melanoma cells. More often than not the reason might be that exosome-derived factors could be involved in the bystander and abscopal effects when combining radiotherapy with mesenchymal stem cells[62]. Recently, Bruton et al[63] presented an unusual case of the abscopal effect in EA with distant metastasis. After palliative radiation therapy to this patient, they observed a complete response of the irradiated tissues, the primary tumor and adjacent lymph nodes, as well as non-irradiated distant lymph nodes. One year later, the patient is still cancer-free. This case inspires the hope that advanced understanding of the abscopal effect of radiation therapy increases the effect of EA and improves patient outcomes.
Immunological therapy
An in vitro study[64] revealed that immunotherapy, which was based on dendritic cells, could generate monocyte-derived dendritic cells (moDcs). The moDcs were powerful enough to induce cytotoxic T lymphocytes. Advanced study demonstrated that SART1 peptide-specific cytotoxic T lymphocytes could be induced by moDcs-derived exosomes, which had antigen presenting ability. Therefore, exosomes may play a potent part in the immunotherapy of EC.
Targeted therapy
Targeted therapy, also known as bio-missile, is a treatment for established cancer-causing sites at the cellular and molecular level. As is well known, exosomes are cell-derived natural nanometric vesicles, widely existing in body fluids and participate in the processes of many diseases, including cancer. There are many advantages of exosome-based nanometric vehicles: non-toxic, safe, non-immunogenic and programmable to have a strong delivery capacity and targeting specificity. Therefore, some scholars proposed to make exosomes a biological carrier to deliver anti-cancer drugs and genes for cancer stem cell-targeted therapy[65,66]. Jc Bose et al[66] found that delivery of anti-miR-21 could inhibit the overexpression of endogenous oncogenic miR-21 in tumor cells. Exosome-mediated anti-miR-21 delivery attenuates doxorubicin resistance in breast cancer cells. This makes the killing-cell efficiency of doxorubicin three times higher than that with doxorubicin alone. They also verified the biodistribution in vivo, tumor specific accumulation of anti-miR-21 loaded TEV-GIONs (gold-iron oxide nanoparticles) and its theranostic property. In summary, exosomes are expected to be used as targeted therapeutic vectors for various cancers, including EC in the near future.
OTHER ROLES OF EXOSOME IN ESOPHAGEAL CANCER
A study[13] demonstrated that dysregulated miR-33a, miR-1, miR-326, miR-133a/b, miR-548h, miR-603, miR-141-5p, miR-429, miR-26a and miR-548k contained in exosomes from tumor associated fibroblasts next to EC cells were all involved with stromal remodeling including endocytosis, adhesion, gap and tight junction, focal adhesion, actin cytoskeleton regulation and ubiquitin-mediated proteolysis in tumor microenvironments through targeting different mRNA molecules.
CONCLUSION
Exosomes are another newly discovered vehicle of efficient cell communication in addition to classical intercellular communication (including signaling molecules, membrane binding, channels, etc.). It is an important component of the tumor microenvironment and plays a complex role in the progression and treatment of EC.
To date, the research on exosomes in EC has been successful, but some problems still need to be solved in the clinical diagnosis and treatment of EC. Although studies have shown that the number and molecular contents of exosomes in patients with EC may be helpful for the diagnosis, there are still some questions worth thinking about and require more efforts to solve. Which part of the blood or which content is more sensitive? Is the RNA and protein of the circulating exosomes more sensitive than the corresponding circulating RNA and protein? What should the diagnostic criteria related to exosomes be? Last but not least, the same miRNA plays the opposite role in different cancers. For example, miR-93-5p can promote the formation of EC, lacrimal adenoid cystic carcinoma[67,68] and hepatoma development[69], but it was proved that miR-93-5P may inhibit epithelial ovarian carcinoma tumorigenesis and progression by targeting Ras homolog gene family member C[70]. Therefore, we should consider comorbidities when analyzing the cause of tumor formation. In conclusion, we need to analyze the role and specific mechanisms of exosomes in the formation, monitoring and treatment of EC in a multifaceted way.
Footnotes
Conflict-of-interest statement: Authors declare no conflict of interests for this article.
Manuscript source: Unsolicited manuscript
Peer-review started: December 29, 2018
First decision: January 18, 2019
Article in press: March 9, 2019
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Aurello P, Khuroo MS S-Editor: Wang JL L-Editor: Filipodia E-Editor: Wu YXJ
Contributor Information
Lin-Lin Su, Department of Radiotherapy, The Second Hospital of Hebei Medical University, Shijiazhuang 050000, Hebei Province, China.
Xiao-Jing Chang, Department of Radiotherapy, The Second Hospital of Hebei Medical University, Shijiazhuang 050000, Hebei Province, China.
Huan-Di Zhou, Department of Radiotherapy, The Second Hospital of Hebei Medical University, Shijiazhuang 050000, Hebei Province, China; Department of Central Laboratory, The Second Hospital of Hebei Medical University, Shijiazhuang 050000, Hebei Province, China.
Liu-Bing Hou, Department of Radiotherapy, The Second Hospital of Hebei Medical University, Shijiazhuang 050000, Hebei Province, China; Department of Central Laboratory, The Second Hospital of Hebei Medical University, Shijiazhuang 050000, Hebei Province, China.
Xiao-Ying Xue, Department of Radiotherapy, The Second Hospital of Hebei Medical University, Shijiazhuang 050000, Hebei Province, China. xxy0636@163.com.
References
- 1.Malla B, Zaugg K, Vassella E, Aebersold DM, Dal Pra A. Exosomes and Exosomal MicroRNAs in Prostate Cancer Radiation Therapy. Int J Radiat Oncol Biol Phys. 2017;98:982–995. doi: 10.1016/j.ijrobp.2017.03.031. [DOI] [PubMed] [Google Scholar]
- 2.Ibrahim SH, Hirsova P, Tomita K, Bronk SF, Werneburg NW, Harrison SA, Goodfellow VS, Malhi H, Gores GJ. Mixed lineage kinase 3 mediates release of C-X-C motif ligand 10-bearing chemotactic extracellular vesicles from lipotoxic hepatocytes. Hepatology. 2016;63:731–744. doi: 10.1002/hep.28252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Segura E, Nicco C, Lombard B, Véron P, Raposo G, Batteux F, Amigorena S, Théry C. ICAM-1 on exosomes from mature dendritic cells is critical for efficient naive T-cell priming. Blood. 2005;106:216–223. doi: 10.1182/blood-2005-01-0220. [DOI] [PubMed] [Google Scholar]
- 4.Regev-Rudzki N, Wilson DW, Carvalho TG, Sisquella X, Coleman BM, Rug M, Bursac D, Angrisano F, Gee M, Hill AF, Baum J, Cowman AF. Cell-cell communication between malaria-infected red blood cells via exosome-like vesicles. Cell. 2013;153:1120–1133. doi: 10.1016/j.cell.2013.04.029. [DOI] [PubMed] [Google Scholar]
- 5.Yu S, Cao H, Shen B, Feng J. Tumor-derived exosomes in cancer progression and treatment failure. Oncotarget. 2015;6:37151–37168. doi: 10.18632/oncotarget.6022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 7.Bang C, Thum T. Exosomes: new players in cell-cell communication. Int J Biochem Cell Biol. 2012;44:2060–2064. doi: 10.1016/j.biocel.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Miksa M, Wu R, Dong W, et al. Immature dendritic cell-derived exosomes rescue septic animals via milk fat globule epidermal growth factor-factor VIII [corrected] J Immunol. 2009;183:5983–5990. doi: 10.4049/jimmunol.0802994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. 2014;14:195–208. doi: 10.1038/nri3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 11.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 12.Min H, Sun X, Yang X, Zhu H, Liu J, Wang Y, Chen G, Sun X. Exosomes Derived from Irradiated Esophageal Carcinoma-Infiltrating T Cells Promote Metastasis by Inducing the Epithelial-Mesenchymal Transition in Esophageal Cancer Cells. Pathol Oncol Res. 2018;24:11–18. doi: 10.1007/s12253-016-0185-z. [DOI] [PubMed] [Google Scholar]
- 13.Smith RA, Lam AK. Liquid Biopsy for Investigation of Cancer DNA in Esophageal Adenocarcinoma: Cell-Free Plasma DNA and Exosome-Associated DNA. Methods Mol Biol. 2018;1756:187–194. doi: 10.1007/978-1-4939-7734-5_17. [DOI] [PubMed] [Google Scholar]
- 14.Skog J, Würdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, Curry WT, Jr, Carter BS, Krichevsky AM, Breakefield XO. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang M, Ren M, Li Y, Fu Y, Deng M, Li C. Exosome-mediated transfer of lncRNA PART1 induces gefitinib resistance in esophageal squamous cell carcinoma via functioning as a competing endogenous RNA. J Exp Clin Cancer Res. 2018;37:171. doi: 10.1186/s13046-018-0845-9. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Mitomo S, Maesawa C, Ogasawara S, Iwaya T, Shibazaki M, Yashima-Abo A, Kotani K, Oikawa H, Sakurai E, Izutsu N, Kato K, Komatsu H, Ikeda K, Wakabayashi G, Masuda T. Downregulation of miR-138 is associated with overexpression of human telomerase reverse transcriptase protein in human anaplastic thyroid carcinoma cell lines. Cancer Sci. 2008;99:280–286. doi: 10.1111/j.1349-7006.2007.00666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Melo SA, Sugimoto H, O'Connell JT, Kato N, Villanueva A, Vidal A, Qiu L, Vitkin E, Perelman LT, Melo CA, Lucci A, Ivan C, Calin GA, Kalluri R. Cancer exosomes perform cell-independent microRNA biogenesis and promote tumorigenesis. Cancer Cell. 2014;26:707–721. doi: 10.1016/j.ccell.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu MX, Liao J, Xie M, Gao ZK, Wang XH, Zhang Y, Shang MH, Yin LH, Pu YP, Liu R. miR-93-5p Transferred by Exosomes Promotes the Proliferation of Esophageal Cancer Cells via Intercellular Communication by Targeting PTEN. Biomed Environ Sci. 2018;31:171–185. doi: 10.3967/bes2018.023. [DOI] [PubMed] [Google Scholar]
- 19.Ke X, Yan R, Sun Z, Cheng Y, Meltzer A, Lu N, Shu X, Wang Z, Huang B, Liu X, Wang Z, Song JH, Ng CK, Ibrahim S, Abraham JM, Shin EJ, He S, Meltzer SJ. Esophageal Adenocarcinoma-Derived Extracellular Vesicle MicroRNAs Induce a Neoplastic Phenotype in Gastric Organoids. Neoplasia. 2017;19:941–949. doi: 10.1016/j.neo.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feng X, Jiang J, Shi S, Xie H, Zhou L, Zheng S. Knockdown of miR-25 increases the sensitivity of liver cancer stem cells to TRAIL-induced apoptosis via PTEN/PI3K/Akt/Bad signaling pathway. Int J Oncol. 2016;49:2600–2610. doi: 10.3892/ijo.2016.3751. [DOI] [PubMed] [Google Scholar]
- 21.Delettre C, Yuste VJ, Moubarak RS, Bras M, Lesbordes-Brion JC, Petres S, Bellalou J, Susin SA. AIFsh, a novel apoptosis-inducing factor (AIF) pro-apoptotic isoform with potential pathological relevance in human cancer. J Biol Chem. 2006;281:6413–6427. doi: 10.1074/jbc.M509884200. [DOI] [PubMed] [Google Scholar]
- 22.Krebs AM, Mitschke J, Lasierra Losada M, Schmalhofer O, Boerries M, Busch H, Boettcher M, Mougiakakos D, Reichardt W, Bronsert P, Brunton VG, Pilarsky C, Winkler TH, Brabletz S, Stemmler MP, Brabletz T. The EMT-activator Zeb1 is a key factor for cell plasticity and promotes metastasis in pancreatic cancer. Nat Cell Biol. 2017;19:518–529. doi: 10.1038/ncb3513. [DOI] [PubMed] [Google Scholar]
- 23.Fici P, Gallerani G, Morel AP, Mercatali L, Ibrahim T, Scarpi E, Amadori D, Puisieux A, Rigaud M, Fabbri F. Splicing factor ratio as an index of epithelial-mesenchymal transition and tumor aggressiveness in breast cancer. Oncotarget. 2017;8:2423–2436. doi: 10.18632/oncotarget.13682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gibbons DL, Creighton CJ. Pan-cancer survey of epithelial-mesenchymal transition markers across the Cancer Genome Atlas. Dev Dyn. 2018;247:555–564. doi: 10.1002/dvdy.24485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malek R, Wang H, Taparra K, Tran PT. Therapeutic Targeting of Epithelial Plasticity Programs: Focus on the Epithelial-Mesenchymal Transition. Cells Tissues Organs. 2017;203:114–127. doi: 10.1159/000447238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okubo K, Uenosono Y, Arigami T, Yanagita S, Matsushita D, Kijima T, Amatatsu M, Uchikado Y, Kijima Y, Maemura K, Natsugoe S. Clinical significance of altering epithelial-mesenchymal transition in metastatic lymph nodes of gastric cancer. Gastric Cancer. 2017;20:802–810. doi: 10.1007/s10120-017-0705-x. [DOI] [PubMed] [Google Scholar]
- 27.Chen X, Chen RX, Wei WS, Li YH, Feng ZH, Tan L, Chen JW, Yuan GJ, Chen SL, Guo SJ, Xiao KH, Liu ZW, Luo JH, Zhou FJ, Xie D. PRMT5 Circular RNA Promotes Metastasis of Urothelial Carcinoma of the Bladder through Sponging miR-30c to Induce Epithelial-Mesenchymal Transition. Clin Cancer Res. 2018;24:6319–6330. doi: 10.1158/1078-0432.CCR-18-1270. [DOI] [PubMed] [Google Scholar]
- 28.Liao J, Liu R, Shi YJ, Yin LH, Pu YP. Exosome-shuttling microRNA-21 promotes cell migration and invasion-targeting PDCD4 in esophageal cancer. Int J Oncol. 2016;48:2567–2579. doi: 10.3892/ijo.2016.3453. [DOI] [PubMed] [Google Scholar]
- 29.Liao J, Liu R, Yin L, Pu Y. Expression profiling of exosomal miRNAs derived from human esophageal cancer cells by Solexa high-throughput sequencing. Int J Mol Sci. 2014;15:15530–15551. doi: 10.3390/ijms150915530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chakravarthi BVSK, Chandrashekar DS, Agarwal S, Balasubramanya SAH, Pathi SS, Goswami MT, Jing X, Wang R, Mehra R, Asangani IA, Chinnaiyan AM, Manne U, Sonpavde G, Netto GJ, Gordetsky J, Varambally S. miR-34a Regulates Expression of the Stathmin-1 Oncoprotein and Prostate Cancer Progression. Mol Cancer Res. 2018;16:1125–1137. doi: 10.1158/1541-7786.MCR-17-0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vetter NS, Kolb EA, Mills CC, Sampson VB. The Microtubule Network and Cell Death Are Regulated by an miR-34a/Stathmin 1/βIII-Tubulin Axis. Mol Cancer Res. 2017;15:953–964. doi: 10.1158/1541-7786.MCR-16-0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Imura S, Yamada S, Saito YU, Iwahashi S, Arakawa Y, Ikemoto T, Morine Y, Utsunomiya T, Shimada M. miR-223 and Stathmin-1 Expression in Non-tumor Liver Tissue of Patients with Hepatocellular Carcinoma. Anticancer Res. 2017;37:5877–5883. doi: 10.21873/anticanres.12033. [DOI] [PubMed] [Google Scholar]
- 33.Guo F, Luo Y, Mu YF, Qin SL, Qi Y, Qiu YE, Zhong M. miR-193b directly targets STMN1 and inhibits the malignant phenotype in colorectal cancer. Am J Cancer Res. 2016;6:2463–2475. [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao C, Li H, Wang L, Sun W. An Immunohistochemical Study of Stathmin 1 Expression in Osteosarcoma Shows an Association with Metastases and Poor Patient Prognosis. Med Sci Monit. 2018;24:6070–6078. doi: 10.12659/MSM.910953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Correction: "miR-34a Regulates Expression of the Stathmin-1 Oncoprotein and Prostate Cancer Progression". Mol Cancer Res. 2018;16:1205–1206. doi: 10.1158/1541-7786.MCR-18-0519. [DOI] [PubMed] [Google Scholar]
- 36.Aksoy A, Artas G, Sevindik OG. Predictive value of stathmin-1 and osteopontin expression for taxan resistance in metastatic castrate-resistant prostate cancer. Pak J Med Sci. 2017;33:560–565. doi: 10.12669/pjms.333.12559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu H, Deng WW, Yang LL, Zhang WF, Sun ZJ. Expression and phosphorylation of Stathmin 1 indicate poor survival in head and neck squamous cell carcinoma and associate with immune suppression. Biomark Med. 2018;12:759–769. doi: 10.2217/bmm-2017-0443. [DOI] [PubMed] [Google Scholar]
- 38.Tan HT, Chung MCM. Label-Free Quantitative Phosphoproteomics Reveals Regulation of Vasodilator-Stimulated Phosphoprotein upon Stathmin-1 Silencing in a Pair of Isogenic Colorectal Cancer Cell Lines. Proteomics. 2018;18:e1700242. doi: 10.1002/pmic.201700242. [DOI] [PubMed] [Google Scholar]
- 39.Wang J, Yao Y, Ming Y, Shen S, Wu N, Liu J, Liu H, Suo T, Pan H, Zhang D, Ding K, Liu H. Downregulation of stathmin 1 in human gallbladder carcinoma inhibits tumor growth in vitro and in vivo. Sci Rep. 2016;6:28833. doi: 10.1038/srep28833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang J, Fu J, Pan Y, Zhang X, Shen L. Silencing of miR-1247 by DNA methylation promoted non-small-cell lung cancer cell invasion and migration by effects of STMN1. Onco Targets Ther. 2016;9:7297–7307. doi: 10.2147/OTT.S111291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ni PZ, He JZ, Wu ZY, Ji X, Chen LQ, Xu XE, Liao LD, Wu JY, Li EM, Xu LY. Overexpression of Stathmin 1 correlates with poor prognosis and promotes cell migration and proliferation in oesophageal squamous cell carcinoma. Oncol Rep. 2017;38:3608–3618. doi: 10.3892/or.2017.6039. [DOI] [PubMed] [Google Scholar]
- 42.Suzuki S, Yokobori T, Altan B, Hara K, Ozawa D, Tanaka N, Sakai M, Sano A, Sohda M, Bao H, Fukuchi M, Miyazaki T, Kaira K, Asao T, Kuwano H. High stathmin 1 expression is associated with poor prognosis and chemoradiation resistance in esophageal squamous cell carcinoma. Int J Oncol. 2017;52:1184–1190. doi: 10.3892/ijo.2017.3899. [DOI] [PubMed] [Google Scholar]
- 43.Yan L, Dong X, Gao J, Liu F, Zhou L, Sun Y, Zhao X. A novel rapid quantitative method reveals stathmin-1 as a promising marker for esophageal squamous cell carcinoma. Cancer Med. 2018;7:1802–1813. doi: 10.1002/cam4.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mao L, Li X, Gong S, Yuan H, Jiang Y, Huang W, Sun X, Dang X. Serum exosomes contain ECRG4 mRNA that suppresses tumor growth via inhibition of genes involved in inflammation, cell proliferation, and angiogenesis. Cancer Gene Ther. 2018;25:248–259. doi: 10.1038/s41417-018-0032-3. [DOI] [PubMed] [Google Scholar]
- 45.Zhou CF, Ma J, Huang L, Yi HY, Zhang YM, Wu XG, Yan RM, Liang L, Zhong M, Yu YH, Wu S, Wang W. Cervical squamous cell carcinoma-secreted exosomal miR-221-3p promotes lymphangiogenesis and lymphatic metastasis by targeting VASH1. Oncogene. 2019;38:1256–1268. doi: 10.1038/s41388-018-0511-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fleitas T, Martínez-Sales V, Vila V, Reganon E, Mesado D, Martín M, Gómez-Codina J, Montalar J, Reynés G. Circulating endothelial cells and microparticles as prognostic markers in advanced non-small cell lung cancer. PLoS One. 2012;7:e47365. doi: 10.1371/journal.pone.0047365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tavoosidana G, Ronquist G, Darmanis S, Yan J, Carlsson L, Wu D, Conze T, Ek P, Semjonow A, Eltze E, Larsson A, Landegren UD, Kamali-Moghaddam M. Multiple recognition assay reveals prostasomes as promising plasma biomarkers for prostate cancer. Proc Natl Acad Sci U S A. 2011;108:8809–8814. doi: 10.1073/pnas.1019330108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Silva J, Garcia V, Rodriguez M, Compte M, Cisneros E, Veguillas P, Garcia JM, Dominguez G, Campos-Martin Y, Cuevas J, Peña C, Herrera M, Diaz R, Mohammed N, Bonilla F. Analysis of exosome release and its prognostic value in human colorectal cancer. Genes Chromosomes Cancer. 2012;51:409–418. doi: 10.1002/gcc.21926. [DOI] [PubMed] [Google Scholar]
- 49.Matsumoto Y, Kano M, Akutsu Y, Hanari N, Hoshino I, Murakami K, Usui A, Suito H, Takahashi M, Otsuka R, Xin H, Komatsu A, Iida K, Matsubara H. Quantification of plasma exosome is a potential prognostic marker for esophageal squamous cell carcinoma. Oncol Rep. 2016;36:2535–2543. doi: 10.3892/or.2016.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kinoshita T, Yip KW, Spence T, Liu FF. MicroRNAs in extracellular vesicles: potential cancer biomarkers. J Hum Genet. 2017;62:67–74. doi: 10.1038/jhg.2016.87. [DOI] [PubMed] [Google Scholar]
- 51.Bigagli E, Luceri C, Guasti D, Cinci L. Exosomes secreted from human colon cancer cells influence the adhesion of neighboring metastatic cells: Role of microRNA-210. Cancer Biol Ther. 2016;17:1–8. doi: 10.1080/15384047.2016.1219815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhai LY, Li MX, Pan WL, Chen Y, Li MM, Pang JX, Zheng L, Chen JX, Duan WJ. In Situ Detection of Plasma Exosomal MicroRNA-1246 for Breast Cancer Diagnostics by a Au Nanoflare Probe. ACS Appl Mater Interfaces. 2018;10:39478–39486. doi: 10.1021/acsami.8b12725. [DOI] [PubMed] [Google Scholar]
- 53.Wei C, Li Y, Huang K, Li G, He M. Exosomal miR-1246 in body fluids is a potential biomarker for gastrointestinal cancer. Biomark Med. 2018;12:1185–1196. doi: 10.2217/bmm-2017-0440. [DOI] [PubMed] [Google Scholar]
- 54.Takeshita N, Hoshino I, Mori M, Akutsu Y, Hanari N, Yoneyama Y, Ikeda N, Isozaki Y, Maruyama T, Akanuma N, Komatsu A, Jitsukawa M, Matsubara H. Serum microRNA expression profile: miR-1246 as a novel diagnostic and prognostic biomarker for oesophageal squamous cell carcinoma. Br J Cancer. 2013;108:644–652. doi: 10.1038/bjc.2013.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bansal A, Gupta V, Wang K. MicroRNA Expression Signatures During Malignant Progression From Barrett's Esophagus. J Cell Biochem. 2016;117:1288–1295. doi: 10.1002/jcb.25497. [DOI] [PubMed] [Google Scholar]
- 56.Warnecke-Eberz U, Chon SH, Hölscher AH, Drebber U, Bollschweiler E. Exosomal onco-miRs from serum of patients with adenocarcinoma of the esophagus: comparison of miRNA profiles of exosomes and matching tumor. Tumour Biol. 2015;36:4643–4653. doi: 10.1007/s13277-015-3112-0. [DOI] [PubMed] [Google Scholar]
- 57.Zhou X, Wen W, Zhu J, Huang Z, Zhang L, Zhang H, Qi LW, Shan X, Wang T, Cheng W, Zhu D, Yin Y, Chen Y, Zhu W, Shu Y, Liu P. A six-microRNA signature in plasma was identified as a potential biomarker in diagnosis of esophageal squamous cell carcinoma. Oncotarget. 2017;8:34468–34480. doi: 10.18632/oncotarget.16519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Al-Mayah AH, Irons SL, Pink RC, Carter DR, Kadhim MA. Possible role of exosomes containing RNA in mediating nontargeted effect of ionizing radiation. Radiat Res. 2012;177:539–545. doi: 10.1667/rr2868.1. [DOI] [PubMed] [Google Scholar]
- 59.Al-Mayah A, Bright S, Chapman K, Irons S, Luo P, Carter D, Goodwin E, Kadhim M. The non-targeted effects of radiation are perpetuated by exosomes. Mutat Res. 2015;772:38–45. doi: 10.1016/j.mrfmmm.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 60.Golden EB, Demaria S, Schiff PB, Chachoua A, Formenti SC. An abscopal response to radiation and ipilimumab in a patient with metastatic non-small cell lung cancer. Cancer Immunol Res. 2013;1:365–372. doi: 10.1158/2326-6066.CIR-13-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reynders K, Illidge T, Siva S, Chang JY, De Ruysscher D. The abscopal effect of local radiotherapy: using immunotherapy to make a rare event clinically relevant. Cancer Treat Rev. 2015;41:503–510. doi: 10.1016/j.ctrv.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.de Araujo Farias V, O'Valle F, Serrano-Saenz S, Anderson P, Andrés E, López-Peñalver J, Tovar I, Nieto A, Santos A, Martín F, Expósito J, Oliver FJ, de Almodóvar JMR. Exosomes derived from mesenchymal stem cells enhance radiotherapy-induced cell death in tumor and metastatic tumor foci. Mol Cancer. 2018;17:122. doi: 10.1186/s12943-018-0867-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bruton Joe M, Truong PT. Abscopal Effect after Palliative Radiation Therapy for Metastatic Adenocarcinoma of the Esophagus. Cureus. 2018;10:e3089. doi: 10.7759/cureus.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Narita M, Kanda T, Abe T, Uchiyama T, Iwafuchi M, Zheng Z, Liu A, Kaifu T, Kosugi S, Minagawa M, Itoh K, Takahashi M. Immune responses in patients with esophageal cancer treated with SART1 peptide-pulsed dendritic cell vaccine. Int J Oncol. 2015;46:1699–1709. doi: 10.3892/ijo.2015.2846. [DOI] [PubMed] [Google Scholar]
- 65.Wang J, Zheng Y, Zhao M. Exosome-Based Cancer Therapy: Implication for Targeting Cancer Stem Cells. Front Pharmacol. 2017;7:533. doi: 10.3389/fphar.2016.00533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jc Bose R, Uday Kumar S, Zeng Y, Afjei R, Robinson E, Lau K, Bermudez A, Habte F, Pitteri SJ, Sinclair R, Willmann JK, Massoud TF, Gambhir SS, Paulmurugan R. Tumor Cell-Derived Extracellular Vesicle-Coated Nanocarriers: An Efficient Theranostic Platform for the Cancer-Specific Delivery of Anti-miR-21 and Imaging Agents. ACS Nano. 2018;12:10817–10832. doi: 10.1021/acsnano.8b02587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liang L, Zhao L, Zan Y, Zhu Q, Ren J, Zhao X. MiR-93-5p enhances growth and angiogenesis capacity of HUVECs by down-regulating EPLIN. Oncotarget. 2017;8:107033–107043. doi: 10.18632/oncotarget.22300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hao J, Jin X, Shi Y, Zhang H. miR-93-5p enhance lacrimal gland adenoid cystic carcinoma cell tumorigenesis by targeting BRMS1L. Cancer Cell Int. 2018;18:72. doi: 10.1186/s12935-018-0552-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang X, Liao Z, Bai Z, He Y, Duan J, Wei L. MiR-93-5p Promotes Cell Proliferation through Down-Regulating PPARGC1A in Hepatocellular Carcinoma Cells by Bioinformatics Analysis and Experimental Verification. Genes (Basel) 2018;9:E51. doi: 10.3390/genes9010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen X, Chen S, Xiu YL, Sun KX, Zong ZH, Zhao Y. RhoC is a major target of microRNA-93-5P in epithelial ovarian carcinoma tumorigenesis and progression. Mol Cancer. 2015;14:31. doi: 10.1186/s12943-015-0304-6. [DOI] [PMC free article] [PubMed] [Google Scholar]