Abstract
Epigenetic mechanisms control gene activity and the development of an organism. The epigenome includes DNA methylation, histone modifications, and RNA-mediated processes, and disruption of this balance may cause several pathologies and contribute to obesity and type 2 diabetes (T2D). This Review summarizes epigenetic signatures obtained from human tissues of relevance for metabolism—i.e., adipose tissue, skeletal muscle, pancreatic islets, liver, and blood—in relation to obesity and T2D. Although this research field is still young, these comprehensive data support not only a role for epigenetics in disease development, but also epigenetic alterations as a response to disease. Genetic predisposition, as well as aging, contribute to epigenetic variability, and several environmental factors, including exercise and diet, further interact with the human epigenome. The reversible nature of epigenetic modifications holds promise for future therapeutic strategies in obesity and T2D.
Keywords: epigenetics, DNA methylation, histone modifications, type 2 diabetes, obesity, aging, exercise, diet, physical activity, prediction
Epigenetic factors are suggested to contribute to metabolic dysfunctions. In this Review, Ling and Rönn summarize evidence for altered DNA methylation, both as a cause and a consequence of human obesity and type 2 diabetes. As epigenetic alterations are dynamic in nature, they may also provide targets for drug development.
Main Text
Introduction
The study of epigenetics and its involvement in metabolic diseases is still a young research field, but it is now attracting a lot of attention and growing at a fast pace. Methodological improvements, with crucial progress each year, have contributed to the current interest and advancements in the field.
Epigenetics has been defined as heritable changes in gene function that take place without a change in the DNA sequence. This classical definition suggests that epigenetics is a heritable trait. However, despite the knowledge that the epigenetic pattern is generally copied through mitotic cell divisions, the heritability through generations is less well established. Epigenetic inheritance through generations as well as the impact of genetic variation on epigenetics are addressed later in this review. Notably, at present, it is common to analyze epigenetic modifications in non-dividing cells, and hence these modifications would not be heritable but may still affect cell-specific gene expression and function. The epigenome includes DNA methylation, histone modifications, and non-coding RNAs, which can regulate cell differentiation, cell-specific gene expression, parental imprinting, X chromosome inactivation, as well as genomic stability and structure. DNA methylation takes place on a cytosine, mainly in CG context or the so-called CpG sites, and to a less extent in non-CG context. Methyltransferases, i.e., DNMT1, DNMT3A, and DNMT3B are responsible for attaching methyl groups to DNA during replication and de novo methylation. They use S-adenosyl-L-methionine (SAM) as the methyl donor. There is passive demethylation, e.g., if the activity of DNMTs or the amount of methyl donors is low during replication, and active demethylation, e.g., by ten eleven translocation (TET) enzymes, which may oxidize methyl-groups to hydroxymethylation followed by repair. Moreover, targeted passive demethylation can also occur, when a methylated cytosine is first oxidized to hydroxymethyl-cytosine by TET enzymes, and it stays as such until the next S-phase, when the hydroxymethyl-cytosine is not recognized by DNMT1 and thus becomes an unmethylated cytosine on the newly synthesized strand. Thereby, cis-regulatory elements can be targeted by TET enzymes for loss of methylation at specific loci.
Today, researchers have found hundreds of post-translational modifications including acetylation, methylation, phosphorylation, and ubiquitylation on amino terminus or tails of histones. A large number of enzymes are responsible for adding and removing histone modifications, contributing to a complex epigenetic regulation (Keating and El-Osta, 2015, Zhang et al., 2015). Non-coding RNAs have been reviewed elsewhere and will not be covered in the present review (Esguerra et al., 2018, Wendt et al., 2018). There is a close relationship between chromatin formation, histone modifications, DNA methylation, and gene activity. In most studies performed in human volunteers it is and will continue to be hard to determine what comes first. Regardless, the information of DNA methylation status will guide us in biology, as it may either influence or indicate gene activity. Differential variability in DNA methylation is commonly seen as a result of epigenetic dysregulation, associated with several phenotypes, pathologies, and an adverse environment. One issue that is important to remember when interpreting the results of DNA methylation is that many of the so-called epigenome-wide association studies are based on an array investigating approximately 450,000 CpG sites, which is about 1.5% of all the CpG sites of the human genome. Keeping that in mind, there is likely many more discoveries to be made now as sequencing-based methods of higher coverage are becoming more available and cost efficient, or in other words, a large proportion of the human epigenome is still a black box, waiting to be discovered. Additionally, as oppose to genetic association studies, epigenetic studies face additional challenges, the most important being the fact that the epigenome is tissue specific, or even cell specific, and that it changes over time.
This review will focus on human studies, which have analyzed DNA methylation in relation to obesity and type 2 diabetes (T2D). However, it will also include some studies that have analyzed histone modifications and the chromatin structure in human tissues of importance for obesity and T2D, as well as some animal studies of interest for this review.
Epigenetics of Obesity
The prevalence of obesity is increasing at a rate that cannot be explained by genetic factors; rather environmental factors are the likely driver. Considering the rate of the obesity epidemic, it is an appealing thought that our genes are programmed to store fat or to store as much as possible of all the excess energy the body is exposed to. Given the worldwide improvement in living standards, in combination with an abundance of fast food and other high-energy sources, it is hence not difficult to understand the challenge for our bodies to become accustomed to the new environment.
Epigenetics is one of the mechanisms linking environmental factors to altered gene activity and thereby an obvious link between the rapid change in eating habits and the observed obesity phenotypes. As part of the epigenome, DNA methylation may also be a mechanism linking obesity to clinical conditions. Several studies have investigated genome-wide (array-based) DNA methylation and its association with obesity or related phenotypes in human tissues.
Already, in 2013, an epigenetic association study of obesity was published, investigating DNA methylation in peripheral blood leukocytes using the Infinium Human Methylation 450K Beadchip (Illumina, San Diego, CA, USA) (Xu et al., 2013). This study identified several CpG sites associated with obesity in a young cohort (Table 1) and also showed that the variance of DNA methylation was greater in the obese cases than in the lean controls. This study also indicated that both differential methylation and differential variability could predict obesity with around 70% confidence (Figure 1). A year later, Dick et al. (2014) used the same Illumina 450k array to investigate association with BMI in whole blood, identifying five CpG sites (Table 1). Among these, three CpG sites are located within HIF3A, and the increased methylation with increased BMI was also confirmed in adipose tissue (Figure 1). HIF3A encodes hypoxia-inducible factor 3 subunit alpha, which is part of a group of heterodimeric transcription factors that regulate responses to low oxygen (hypoxia). In another large-scale study investigating DNA methylation in CD4+ T cells, eight CpG sites were associated with BMI and five with waist circumference (Table 1) (Aslibekyan et al., 2015). These included four CpG sites, e.g., annotated to CPT1A, CD38, and PHGDH, which were associated with both phenotypes (Figure 1). CPT1A encodes the enzyme carnitine palmitoyltransferase 1A, which takes part in carnitine-dependent transport across the mitochondrial membrane when oxidation of long-chain fatty acids is initiated and is important for several metabolic processes. DNA methylation in intron 1 of CPT1A was inversely associated with both BMI and waist circumference (Aslibekyan et al., 2015), a gene for which genetic variation previously has been linked to obesity (Lemas et al., 2012). In addition, for CD38, the direction of association was confirmed in all three studies and for both phenotypes, here with a positive association, in a CpG site close to the transcription start site (Aslibekyan et al., 2015). CD38 also has several links to metabolic traits, e.g., CD38-deficient mice were shown to have a higher metabolic rate and were resistant to obesity induced by a high-fat diet (Barbosa et al., 2007). In another study, Demerath et al. (2015) presented numerous CpG sites where DNA methylation in leukocytes associated with BMI or waist circumference (Table 1). Of these, several were located in genes also found in previous studies of different cohorts or ethnicity, including HIF3A, CPT1A, and ABCG1 (Figure 1). Of interest for obesity, the protein encoded by ABCG1 is involved in macrophage cholesterol and phospholipids transport. Additionally, 16 CpG sites replicated in adipose tissue, including CPT1A (Demerath et al., 2015). Another well-powered study of Illumina 450k DNA methylation in whole blood and the association with obesity identified 94 CpG sites associated with BMI and 49 with waist circumference (Sayols-Baixeras et al., 2017) (Table 1). Importantly, they could show that these sites explain 26% and 29%, respectively, of the heritability of these traits. The largest study to date, combining 450k DNA methylation data from over 10,000 whole blood samples, identified 187 CpG sites significantly associated with BMI (Wahl et al., 2017) (Table 1). Using Mendelian randomization, they could show that alterations in DNA methylation in blood are most often the result of obesity but sometimes also the cause, a finding that was also tested and verified in adipose tissue. Importantly, the DNA methylation sites associated with obesity predicted future risk of T2D, which is a major clinical condition associated with obesity (Wahl et al., 2017). Mendelian randomization was also used to infer causal relations between BMI and differential methylation and gene expression in whole blood cells (Mendelson et al., 2017). Again, more CpG sites showed support of being secondary to obesity than the opposite, but this study also provides evidence for DNA methylation sites that contribute to increased BMI. Interestingly, they also showed that 18% of the interindividual variation in BMI could be assigned to the replicated CpGs with differential methylation in blood. Of the 87 CpG sites robustly associated with BMI (Table 1), gene expression was altered for ten genes, e.g., ABCG1, SREBF1, and CPT1A, and overrepresented in lipid metabolism pathways (Mendelson et al., 2017) (Figure 1). SREBF1 encodes a transcription factor involved in lipid metabolism and may be a target for prevention of coronary artery disease. Adding to the growing numbers of CpG sites significantly associated with BMI and waist circumference (Table 1), Dhana et al. (2018) reported novel associations with both phenotypes found in MS12 and LARS2 while also confirming previous associations of e.g., ABCG1, SREBF1, and CPT1A. MSI2, encoding the Musashi RNA binding protein 2 gene, has a role in post-transcriptional gene regulation and has been associated with eating behavior. Another 29 novel CpG sites associated with obesity were recently described (Table 1) and also replicated in neutrophils to show that the results from peripheral blood leukocytes are not due to altered cell-type composition (Wang et al., 2018). This study further suggests a direct link between DNA methylation, gene expression, and obesity status for several genes, including SOCS3 where obesity was associated with decreased methylation and increased gene expression (Wang et al., 2018) (Figure 1). SOCS3 expression is upregulated in obesity and has been shown to induce both insulin and leptin resistance, with implications for blood glucose control and energy homeostasis (Pedroso et al., 2018). The Illumina 450k array has also been used to investigate alterations in DNA methylation associated with childhood obesity (Table 1) (Fradin et al., 2017, Huang et al., 2015, Rzehak et al., 2017).
Table 1.
Epigenetic Association Studies of Obesity
| Reference | Sample Size Discovery (Replication) | Phenotype | Tissue | Identified Number of CpG Sites | Additional Information |
|---|---|---|---|---|---|
| Xu et al., 2013 | 96 | BMI ≥30 versus BMI <25 | peripheral blood leukocytes | 23,305 FDR | age: 14–21 years |
| Dick et al., 2014 | 459 | BMI | whole blood | 5 FDR | – |
| 339 | BMI | 3Bonf | |||
| 1,789 | BMI | 3Bonf | |||
| Aslibekyan et al., 2015 | 991 | BMI | CD4+ T cells | 8 Bonf | – |
| waist circumference | 5 Bonf | ||||
| 1,935 | BMI | whole blood | 4Bonf | ||
| 2,105 | BMI | whole blood | 2Bonf | ||
| waist circumference | 3Bonf | ||||
| Demerath et al., 2015 | 2,097 | BMI | peripheral blood leukocytes | 76 Bonf | – |
| waist circumference | 164 Bonf | ||||
| 2,377+991 | BMI | whole blood/CD4+ T cells | 37Bonf | ||
| 991 | waist circumference | CD4+ T cells | 8Bonf | ||
| Sayols-Baixeras et al., 2017 | 641/2,515 | BMI | whole blood/whole blood | 94 Bonf | results are based on a meta-analysis of discovery and replication cohorts |
| waist circumference | 49 Bonf | ||||
| Wahl et al., 2017 | 5,387 | BMI | whole blood | 278 Bonf | ∗meta-analysis |
| 4,874 | BMI | whole blood | 187p < 0.05/*Bonf | ||
| Mendelson et al., 2017 | 3,743 | BMI | whole blood | 135 Bonf | – |
| 4,055 | BMI | whole blood/CD4+ T cells | 83Bonf | ||
| Dhana et al., 2018 | 1,450 | BMI | whole blood | 14 Bonf | – |
| waist circumference | 26 Bonf | ||||
| 2,097 | BMI | whole blood | 12 | ||
| waist circumference | 13 | ||||
| Wang et al., 2018 | 700 | BMI/obese versus lean | peripheral blood leukocytes | 76 Bonf | age: 14–36 years |
| 2,097 | BMI | peripheral blood leukocytes | 54 | – | |
| 188 | obese versus lean | neutrophils | 37 | ||
| Xu et al., 2018 | 510 | BMI | whole blood | 20 FDR | – |
| Rönn et al., 2015 | 96 | BMI (males) | subcutaneous adipose tissue | 33,058 FDR | – |
| 94 | BMI (females) | subcutaneous adipose tissue | 39,533 FDR | ||
| Benton et al., 2015 | 15 | before versus after gastric by-pass (women) | omental adipose tissue | 15 Bonf | – |
| subcutaneous adipose tissue | 3,601 Bonf | ||||
| Dahlman et al., 2015 | 30 | post-obese versus never obese (women) | isolated fat cells | 8,504 FDR_1% | – |
| Fradin et al., 2017 | 37 | obesity (BMI Z score > 2.5 versus lean controls) | whole blood | 31 FDR | age: 3–13 years |
| Rzehak et al., 2017 | 374 | BMI | whole blood | 212 FDR | age: 5.5 years |
| Huang et al., 2015 | 149 | BMI | whole blood | 129 (p<0.05; Δ>10%) | age: 9–14 years |
All studies are based on the Illumina 450k array. Results from replication(s) within the studies (where applicable) are marked in italic.
FDR, false discovery rate < 0.05; Bonf, Bonferroni correction for multiple testing; FDR_1%, FDR < 0.01.
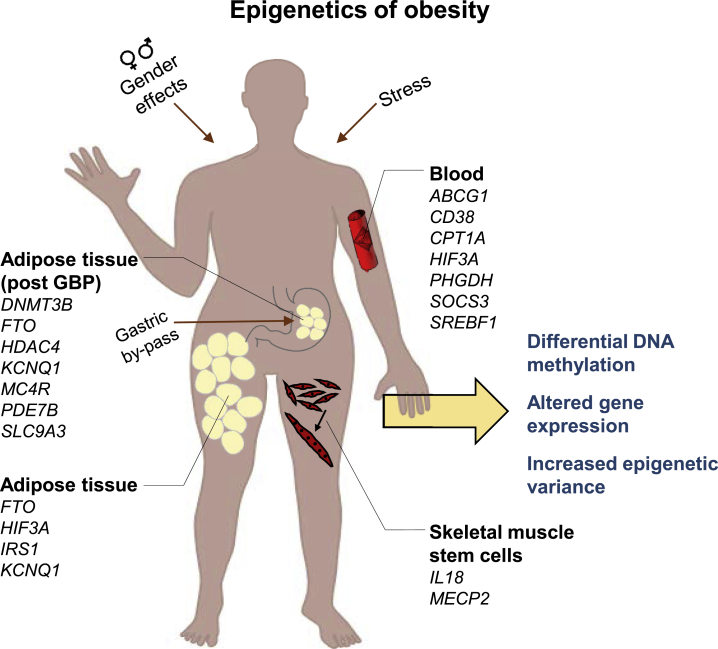
Figure 1.
Obesity Is Associated with Differential DNA Methylation and Increased Epigenetic Variability
The figure illustrates tissues and genes with observed alterations in DNA methylation due to obesity and related phenotypes (BMI, waist circumference), some of which are also associated with gene expression. Alterations in DNA methylation are more often a result of obesity than the opposite. GBP, gastric by-pass.
With a slightly different perspective, Xu et al. (2018) investigated accumulative stress modulation of DNA methylation in whole blood and its effects on obesity. First, DNA methylation of 20 CpG sites was found to be significantly associated with BMI (Table 1). Among them, interaction between stress and DNA methylation of one CpG site in SOCS3, the one that was also identified by Wang et al. (2018), was associated with BMI (Figure 1) (Xu et al., 2018).
Several studies have also investigated the association between DNA methylation and obesity-related traits in adipose tissue, an endocrine organ and the body’s main energy reservoir. Using Illuimna’s 450k array, Rönn et al. (2015) identified thousands of CpG sites in subcutaneous adipose tissue (SAT) of 96 males, which were associated with BMI. Interestingly, as many as 2,825 genes were identified where both DNA methylation and gene expression associated with BMI, including FTO and IRS1. In the female cohort of this study, we could also replicate all three HIF3A CpG sites reported by Dick et al. (2014). However, in the male cohort, two other CpG sites of HIF3A, also intragenic, were the only ones significantly associated with BMI (Rönn et al., 2015) (Figure 1). Another study identified differential methylation in both omental and SAT in response to gastric by-pass and associated weight loss (Benton et al., 2015) (Figure 1). One intergenic CpG site overlapped between the tissues, and another two genes showed differential methylation in both tissues (PDE7B and SLC9A3), however, not for the same CpG loci. In SAT, KCNQ1 had six significant CpG sites, all with lower DNA methylation after weight loss, and several other genes previously associated with obesity and related traits also displayed altered DNA methylation. In support of this differential methylation observed in SAT, DNA methyl-transferases (DNMT3A and 3L) showed decreased methylation after weight loss, as well as MBD4, encoding a protein that binds specifically to methylated cytosines. In addition, three genes encoding histone deacetylases, HDAC4, 7, and 10, displayed differential methylation in response to gastric by-pass and weight loss (Benton et al., 2015). To follow up on these results, Dahlman et al. (2015) compared DNA methylation in isolated fat cells from women 2 years after gastric by-pass with weight-matched women who had never been obese. Of the 8,504 CpG sites with differential methylation, 27% were linked to adipogenesis, which could be a mechanism for the higher number of fat cells observed in obese and post-obese individuals (Dahlman et al., 2015). Prospective studies comparing the DNA methylome from various tissues before and after weight loss have shown small but widespread changes across the genome and suggested that baseline DNA methylation can be used as a biomarker for the outcome, i.e., the weight loss response (Aronica et al., 2017).
In a recent study by Davegårdh et al. (2017), array-based DNA methylation and gene expression were investigated in muscle stem cells from obese and non-obese individuals before and after differentiation. Among obese subjects, methylation of 147,161 CpG sites was significantly altered during differentiation from myoblasts to myotubes, compared to 39,572 sites in non-obese subjects. These abnormal epigenetic changes, found e.g. in MECP2, IL18, ENHO, and PLCB1 as well as in genes of pathways involved in the immune response, provide one explanation for how obesity may impair myogenesis and thereby affect muscle regeneration and function (Figure 1).
What we have learned from the observational studies is that obesity and related phenotypes induce epigenetic dysregulation, seen as increased variability in DNA methylation. Obesity is considered a heterogeneous disease, which could influence the outcome, together with different genetic background, gender, and tissue specificity. The latter is an important limitation, as many studies are performed on blood cells, which most likely do not contribute to obesity. To increase the likelihood for obtaining epigenetic results that are biological meaningful, the tissue to study should be carefully selected. Furthermore, when interpreting the published associations between obesity and DNA methylation in blood, it should be kept in mind that some studies include overlapping cohorts to maximize sample size, thereby increasing power. However, DNA methylation data from human adipose tissue, an organ of importance for obesity, supports a role for epigenetics in the disease pathogenesis. Together, these studies support an impact of BMI on epigenetic variation of candidate genes for both obesity and T2D, which seems to affect gene expression and metabolism.
Epigenetics of Type 2 Diabetes in Humans
Only a decade ago, the first epigenetics studies were performed in pancreatic islets and skeletal muscle from patients with T2D (Barrès et al., 2009, Ling et al., 2008). Although these initial studies only analyzed DNA methylation of candidate genes or parts of the genome, they identified altered methylation patterns in humans with T2D compared with non-diabetic controls, which clearly supports a role for epigenetics in the growing incidence of diabetes. Since 2008, there have been technical advances and an increasing interest in epigenetics of T2D driving this research field forward.
T2D is characterized by chronically elevated blood glucose levels, which develop due to insulin resistance in combination with impaired insulin secretion. It is well established that aging, a sedentary lifestyle, and obesity contribute to insulin resistance in target tissues including skeletal muscle, liver, and adipose tissue. Pancreatic islet function decreases after long-term exposure to elevated lipid and glucose levels (Hall et al., 2014, Hall et al., 2018). Because of aging populations and an increasing prevalence of obesity, the number of patients with T2D is increasing at alarming rates worldwide, and the current prevalence of 422 million people is expected to rise to 592 million in 2035. Of note, 5 million people die from diabetes every year, most often because of cardiovascular events (Cho et al., 2018). Subsequently, there is a huge need to dissect the molecular mechanisms such as epigenetics, which may cause diabetes.
To understand if epigenetics contributes to disease, one must identify epigenetic alterations in diseased versus healthy people. However, once such alterations are identified, it needs to be determined if they cause or if they are secondary to disease. Furthermore, since the epigenetic patterns are cell specific, it is essential to study tissues of importance for a certain disease, e.g., pancreatic islets, skeletal muscle, adipose tissue, and the liver for T2D. Indeed, numerous human epigenetic case-control studies for T2D have been performed in these tissues; some of these results will be summarized here (Figure 2). These studies have been dependent on technical and bioinformatic advances in tools used to analyze the epigenome.
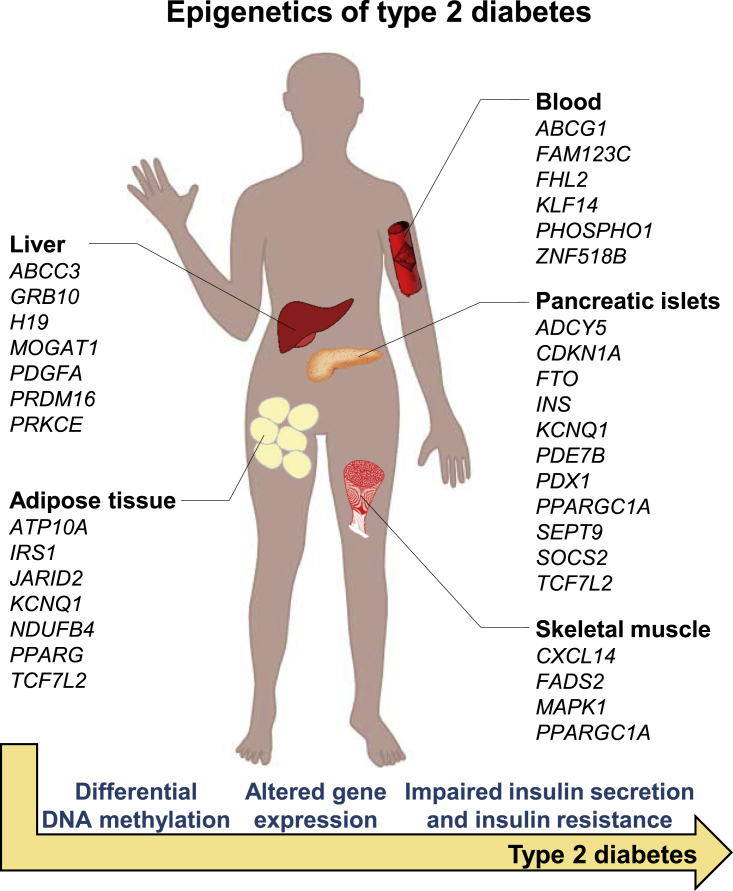
Figure 2.
Type 2 Diabetes Is Associated with Differential DNA Methylation in Human Tissues
The figure illustrates tissues and genes with observed alterations in DNA methylation in subjects with type 2 diabetes compared with non-diabetic controls. Some of these genes also show differential gene expression and have been shown to functionally affect diabetes-related phenotypes such as insulin secretion. DNA methylation of the genes indicated in blood has been associated with future risk of type 2 diabetes in prospective cohorts.
Initial studies analyzed DNA methylation of candidate genes for T2D such as INS (encoding insulin), PDX1, PPARGC1A (encoding PGC1α), and GLP1R (encoding the GLP-1 receptor) in human pancreatic islets from donors with T2D and non-diabetic controls (Hall et al., 2013, Ling et al., 2008, Yang et al., 2011, Yang et al., 2012). Islets from T2D donors were found to have increased DNA methylation and decreased expression of these key genes, which were associated with impaired insulin secretion (Figure 2). In addition, high glucose and glycated hemoglobin (HbA1c) seemed to directly increase DNA methylation of these genes (Hall et al., 2018, Yang et al., 2011, Yang et al., 2012).
Development of Illumina’s Infinium arrays made it possible to analyze methylation of numerous CpG sites simultaneously. This technology was used to analyze methylation of ∼27,000 and ∼450,000 CpG sites, respectively, in pancreatic islets from T2D and non-diabetic donors (Table 2) (Dayeh et al., 2014, Volkmar et al., 2012). Dayeh et al. (2014) found altered DNA methylation of 1,649 CpG sites annotated to 843 genes in islets from 15 T2D cases versus 34 controls. Out of these genes, 102 also exhibited differential gene expression in the islets from T2D donors. CDKN1A, PDE7B, and SEPT9 belong to the genes with decreased DNA methylation and increased gene expression in T2D islets (Figure 2). Functional studies of CDKN1A and PDE7B demonstrated direct negative effects of promoter methylation on the transcriptional activity of these genes. To mimic the situation of T2D, these three genes were overexpressed in clonal β cells, which resulted in decreased glucose-stimulated insulin secretion. Overexpression of CDKN1A, encoding a potent cyclin-dependent kinase inhibitor that regulates cell-cycle progression to G1, also decreased cell proliferation in the clonal β cells (Dayeh et al., 2014). In diabetic islets, Dayeh et al. (2014) also found differential DNA methylation of CpG sites annotated to several candidate genes for T2D and obesity, as identified by genome-wide association studies (GWASs), such as ADCY5, FTO, HHEX, IRS1, KCNQ1, PPARG, and TCF7L2 (Figure 2) (Dayeh et al., 2014).
Table 2.
Epigenetic Association Studies in Type 2 Diabetes (T2D) Case-Control Cohorts, Based on Illumina’s Infinium Arrays
| Reference | Sample Size | Tissue | Identified Number of CpG Sites | Additional Information |
|---|---|---|---|---|
| Dayeh et al., 2014 | 15 cases, 34 controls | human pancreatic islets | 1,649 FDR < 5%, Δbeta>5% | – |
| Volkmar et al., 2012 | 5 cases, 11 controls | human pancreatic islets | 276 p < 0.01, Δbeta>15% | – |
| Abderrahmani et al., 2018 | 96 cases, 96 controls | liver | 1 Bonf | only women |
| Kirchner et al., 2016 | 8 obese T2D, 7 obese, 7 controls | liver | 5,834 FDR<25% | three-group comparison; only men |
| Nilsson et al., 2014 | 14 MZ twin pairs | subcutaneous adipose tissue | 0 FDR<5% | MZ twins discordant for T2D |
| 28 cases, 28 controls | subcutaneous adipose tissue | 15,627 FDR < 15% | ||
| Nilsson et al., 2015 | 35 cases, 60 controls | liver | 251 FDR < 5% | – |
| Ribel-Madsen et al., 2012 | 11 MZ twin pairs | skeletal muscle | 1 Perm | MZ twins discordant for T2D |
| 5 MZ twin pairs | subcutaneous adipose tissue | 7 Perm |
FDR, false discovery rate; Bonf, Bonferroni correction for multiple testing; Perm, Permutation correction; MZ, monozygotic.
The Illumina arrays have also been used to analyze DNA methylation in human adipose tissue, liver, and skeletal muscle from subjects with T2D and non-diabetic controls (Table 2) (Abderrahmani et al., 2018, Kirchner et al., 2016, Nilsson et al., 2014, Nilsson et al., 2015, Nitert et al., 2012, Ribel-Madsen et al., 2012). These studies identified numerous CpG sites with altered DNA methylation in target tissues from patients with T2D, supporting the role of epigenetics in the pathogenesis of diabetes (Figure 2). However, in-line with genetic studies, the effect size of each CpG site was quite modest. This is of no surprise since T2D is a complex, polygenic, and multifactorial disease, and it would be unlikely to find methylation of a few CpG sites that had a large effect size on T2D.
State-of-the-art array-based epigenetic methods used in the described studies only cover a modest part (∼1.5%) of the methylome. To generate a full picture of the epigenome, whole-genome analyses of the spectrum of epigenetic marks are required, e.g., whole-genome bisulfite sequencing (WGBS) for analysis of DNA methylation, chromatin immunoprecipitation followed by sequencing (ChIP-seq) for analysis of histone modifications, and assay for transposase-accessible chromatin using sequencing (ATAC-seq) for analysis of chromatin accessibility. WGBS was used to analyze methylation of ∼2.4 × 107 CpG sites (83% of all CpG sites in the human genome) in islets from T2D subjects and controls (Volkov et al., 2017). This analysis identified 25,820 differentially methylated regions (DMRs) in the T2D islets. Interestingly, two of the most significant DMRs covered PDX1, a key transcription factor in islets that regulates insulin expression (Figure 2). Additionally, there was an overlap between T2D candidate genes identified by DIAGRAM (Scott et al., 2017) and the T2D-associated islet DMRs, including 159 DMRs annotated to 43 T2D genes, such as ADCY5, TCF7L2, and KCNQ1. Furthermore, 457 genes, including NR4A3, PARK2, PID1, and SOCS2, had both DMRs and expression changes in T2D islets (Figure 2). When some of these candidate genes were either overexpressed or silenced in cultured β cells to represent the situation in diabetic islets, insulin secretion was impaired, thus connecting epigenetic mechanisms to islet dysfunction (Volkov et al., 2017). As expected, islet methylation levels were linked to certain histone marks, and combinations of these different epigenetic marks are likely part of the machinery controlling gene activity and chromatin structure. Moreover, methylated DNA immunoprecipitation (MeDIP)-seq was used to study methylation in blood from monozygotic twin pairs discordant for T2D (Yuan et al., 2014). Here, the strongest replicated signal was annotated to MALT1, which encodes a protein involved in insulin and glycemic pathways and related to taurocholate levels in blood.
Notably, genome-wide histone modifications have so far only been analyzed in pancreatic islets of non-diabetic subjects (Bhandare et al., 2010, Pasquali et al., 2014, Stitzel et al., 2010), whereas other studies performed in blood cells have included subjects with T2D (Hou et al., 2011, Paneni et al., 2015). In addition, histone modifications have been analyzed in monocytes cultured in normal and high glucose (Miao et al., 2007). The same applies for analysis of the chromatin structure, for example by ATAC-seq, where mainly samples from non-diabetic people have been used (Ackermann et al., 2016, Thurner et al., 2018, Varshney et al., 2017). Therefore, there is a large need for further epigenome-wide studies in tissues from subjects with T2D. Some of these studies should be performed in sorted cell fractions and/or using single-cell analyses.
Epigenetic changes in patients with diabetes may eventually contribute to vascular complications. Indeed, there is a growing body of literature linking epigenetic modifications to diabetic complications such as retinopathy, diabetic kidney disease, stroke, and myocardial infarction (Agardh et al., 2015, Bell et al., 2010b, Berdasco et al., 2017, Chen et al., 2016, Nakatochi et al., 2017).
Together, these pioneering studies have identified epigenetic alterations in tissues from patients with T2D, indeed showing a link between epigenetics and diabetes in humans. Numerous of the epigenetic changes were also associated with differential gene expression. Functional follow-up studies suggest that epigenetics may contribute to phenotypes characterizing diabetes. However, future studies need to test if the identified epigenetic alterations are causative for diabetes and phenotypes related to the disease.
Diet and Epigenetics in Relation to Human Obesity and T2D
Several lines of evidence indicate that epigenetics have important effects on obesity and T2D in humans. The next question is how do epigenetic alterations arise in obese and diabetic people? To start with, our diet contains methionine and folate, which provide us with methyl donors, e.g., SAM through the methionine cycle (Ducker and Rabinowitz, 2017, Duncan et al., 2013). Ducker and Rabinowitz recently reviewed “One-Carbon Metabolism in Health and Disease,” which is why we refer to their work for more details in this field (Ducker and Rabinowitz, 2017). DNA and histone methyltransferases (DNMTs and e.g., SET7/9 and KMT2D) can attach methyl groups to our DNA and histone tails. To achieve an optimal gene regulation, this process needs to be balanced throughout the genome and in all human cells. SAM and its product S-adenosylhomocysteine (SAH) levels have been used as indicators of methylation capacity (Caudill et al., 2001). However, while plasma levels of methionine, folate, SAM, SAM/SAH ratio, B12, and homocysteine have been used as markers of methylation capacity and risk of disease, plasma levels may not reflect their corresponding status within tissues and the cell nucleus (Duncan et al., 2013). Unfortunately, it has been difficult to obtain simultaneous plasma and tissue measurements for many of these metabolites over time under various nutrient inputs.
Nevertheless, it has been shown that subjects with T2D have reduced levels of folate in the circulation (Nilsson et al., 2015). Additionally, these folate levels correlated positively with the average degree of DNA methylation of 236 CpG sites with significantly decreased methylation in liver biopsies from T2D versus control subjects (Nilsson et al., 2015). Moreover, a higher dietary intake of folate was prospectively associated with a lower risk of diabetes in women (Hong et al., 2017). When a subclinical folate deficiency was created in postmenopausal women, plasma homocysteine increased and lymphocyte DNA methylation decreased (Jacob et al., 1998). Evidence from an animal study supports the importance of folate, as folic acid reduced the fat mass and serum glucose levels as well as improved insulin resistance in mice fed a high-fat diet (Li et al., 2018). Additionally, it altered the DNA methylation pattern of genes associated with obesity and T2D in adipose tissue of these mice. Intake of saturated fatty acids may also affect the epigenome, as it was shown that exposure to high levels of palmitate increased histone acetyl transferase (HAT) activity and altered histone acetylation in clonal β cells (Malmgren et al., 2013). Indeed, the intracellular metabolism provides us with acetyl groups, which can be attached to or removed from histone tails by HATs or HDACs, respectively, and thereby regulate gene activity (Keating and El-Osta, 2015). This fundamental knowledge suggests that the unhealthy diet of many obese and diabetic people may affect their epigenome and thereby disease pathogenesis.
Several intervention studies have been performed to dissect the impact of different diets on the human epigenome. These interventions include high-fat diet overfeeding and fasting studies, as well as a randomized control trial of saturated fatty acids (SFAs) versus polyunsaturated fatty acids (PUFAs) (Brøns et al., 2010, Gillberg et al., 2016, Hjort et al., 2017, Jacobsen et al., 2012, Jacobsen et al., 2014, Perfilyev et al., 2017) (Figure 3). A 5-day high-fat diet overfeeding, mimicking the diet seen in many obese people, changed both the gene expression and methylation patterns in human skeletal muscle and adipose tissue (Brøns et al., 2010, Gillberg et al., 2016, Jacobsen et al., 2012, Jacobsen et al., 2014). Importantly, it seemed easier to induce methylation changes by overfeeding than to reverse them by a control diet.
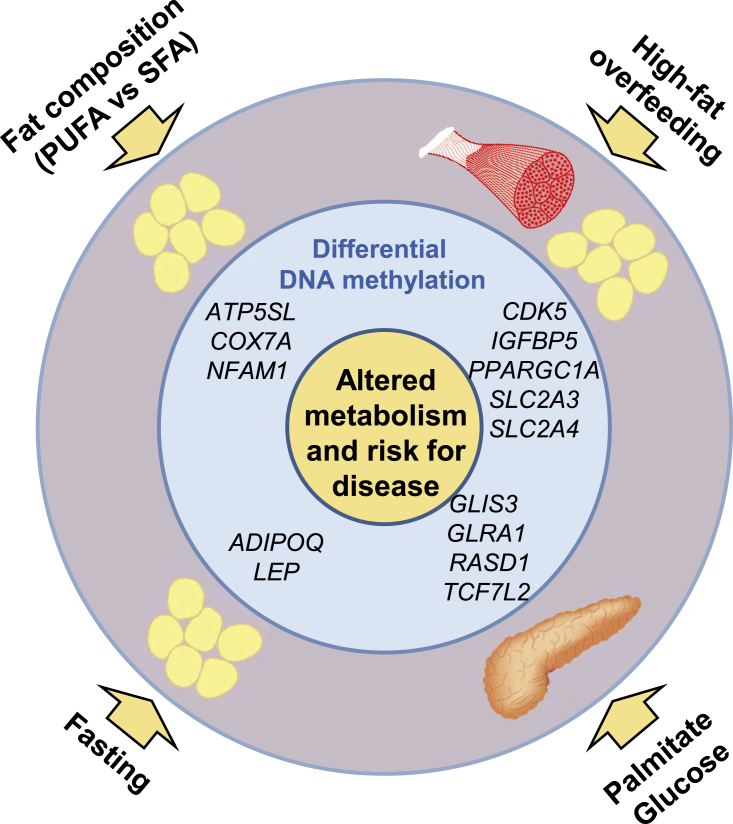
Figure 3.
Different Diets and Nutrients Are Associated with Differential DNA Methylation in Human Tissues Including Adipose Tissue, Skeletal Muscle, and Pancreatic Islets
The figure illustrates tissues and genes with observed alterations in DNA methylation in response to human diet interventions (overfeeding either polyunsaturated fatty acids [PUFAs] or saturated fatty acids [SFAs] for 7 weeks; 5-day high-fat overfeeding; or 36-h fasting), as well as human pancreatic islets cultured in either 1 M palmitate or 16.7 mM glucose versus control for 48 h. Some of these genes also show differential expression, and the diets and nutrients further affect metabolism and clinical phenotypes.
The interest in fasting and its profound health benefits has increased over recent years (Di Francesco et al., 2018). People born with a low birth weight (LBW) have an increased risk of metabolic diseases, and they respond differently to prolonged fasting than normal birth weight (NBW) people (Jørgensen et al., 2015). Leptin and adiponectin are expressed and secreted from adipocytes. These two adipokines control food intake and insulin sensitivity. Obese and T2D subjects have higher leptin and lower adiponectin levels compared to healthy controls, which contribute to dysregulation of their satiety and insulin resistance. Hjort et al. (2017) recently studied the effects of 36-h fasting on DNA methylation and expression of the genes encoding leptin and adiponectin (LEP and ADIPOQ) in human adipose tissue from healthy young men born with an LBW or NBW. Fasting increased methylation of CpG sites annotated to both LEP and ADIPOQ in NBW men but had no effect in LBW men (Figure 3). This may be due to lower epigenetic flexibility in LBW men; they had a higher degree of LEP and ADIPOQ methylation at baseline compared with NBW men, and their methylation did not increase further in response to fasting. Moreover, serum leptin levels decreased with 36-h fasting in both birth weight groups, while serum adiponectin levels did not change. In contrast, ADIPOQ mRNA levels increased in LBW men, whereas LEP expression did not change with fasting. It is possible that the timing, 36-h fasting, contributed to the differences seen in expression and secretion of these adipokines.
Not only the amount but also the type of dietary fat has significant effects on metabolic pathways involved in obesity and T2D (Ludwig et al., 2018). Visceral obesity and ectopic fat accumulation, both risk factors for diabetes and cardiovascular disease, are influenced by the dietary fat composition (Rosqvist et al., 2014). A randomized controlled trial, including a 7-week diet of either excessive SFAs or PUFAs, resulted in similar weight gain between the two groups; however, the diet high in SFAs was associated with elevated liver and visceral fat accumulation compared with PUFA. Notably, in adipose tissue, DNA methylation of 4,875 CpG sites was affected differently between the two diets (Perfilyev et al., 2017) (Figure 3). In addition, the methylation level in blood samples of preadolescents has been associated with the intake of PUFA or SFA (Voisin et al., 2015a).
To study the impact of dietary factors on the epigenome of human pancreatic islets, these were exposed to high levels of glucose or palmitate in vitro for 48 h (Hall et al., 2014, Hall et al., 2018). First, both glucose and palmitate exposure impaired glucose-stimulated insulin secretion. Second, in an attempt to understand the mechanism, it was shown that exposure to high glucose levels altered both methylation and expression of GLRA1, RASD1, VAC14, SLCO5A1, and CHRNA5 (Hall et al., 2018). Remarkably, palmitate had a more pronounced effect on the islets, where 290 genes with differential expression had a corresponding change in DNA methylation, for example, TCF7L2 and GLIS3. Interestingly, out of the genes with altered expression due to palmitate treatment in human islets, 67 were also associated with BMI and 37 were differentially expressed in T2D patients (Hall et al., 2014).
Thus, dietary factors seem to alter the epigenome in several human tissues of importance for metabolism and may thereby affect gene expression and the pathogenesis of obesity and T2D.
Physical Activity and Epigenetics
Lifestyle interventions, including regular exercise, are known to prevent or delay incidence of T2D in individuals with an increased risk for the disease (Knowler et al., 2002, Tuomilehto et al., 2001). Exercise has a beneficial effect not only on glucose homeostasis and whole-body energy balance but also on the immune system. As for other environmental factors, epigenetics is thought to mediate these external stimuli to partly regulate gene expression and phenotypic outcome. In humans, several studies have investigated how exercise, acute or long-term, influences DNA methylation in skeletal muscle and adipose tissue (Barrès et al., 2012, Fabre et al., 2018, Nitert et al., 2012, Rönn et al., 2013).
In 2012, Nitert et al. discovered altered DNA methylation of genes in the MAPK, insulin, and calcium signaling pathways in skeletal muscle from men with a family history of T2D (Nitert et al., 2012). This study also showed that long-term exercise alters DNA methylation, for example, of genes such as MEF2A, RUNX1, NDUFC2, and THADA, important in T2D and muscle physiology. Furthermore, in vitro methylation of these promoter regions confirmed their ability to silence gene expression. The same year, Barrès et al. showed that a single bout of exercise decreases promoter DNA methylation of PPARGC1A, PDK4, and PPARD in human skeletal muscle while increasing gene activity of these key substrate metabolism genes (Barrès et al., 2012). Also in adipose tissue, the epigenetic signature is altered in response to exercise (Fabre et al., 2018, Rönn et al., 2013). In men with a previous low level of physical activity, a 6-month exercise intervention altered the level of DNA methylation of numerous CpG sites, covering a total of 7,663 genes throughout the genome (Rönn et al., 2013). These include candidate genes for obesity and T2D such as CREB4, GRB14, TUB, FTO, ADCY5, TCF7L2, and KCNQ1. Importantly, for one-third of the gene regions with absolute methylation changes >5%, a simultaneous change in mRNA expression was also observed, and these included candidate genes such as HDAC4 and NCOR2, both with an increase in DNA methylation and a corresponding decrease in mRNA expression after exercise. Functional experiments in vitro confirmed that these two genes affect lipogenesis in adipocytes, concluding that epigenetics may be a link between exercise and adipocyte metabolism (Rönn et al., 2013). In addition, acute exercise points to epigenomic and transcriptomic changes in adipocyte-specific genes (Fabre et al., 2018). This study also suggests that the epigenetic response to exercise is more evident in the trained versus untrained state, whereas the transcriptional response to acute exercise is reduced after a period of endurance training.
Of importance for exercise physiology, a candidate gene approach for 5′ adenosine monophosphate-activated protein kinase (AMPK) showed that 4 weeks of extensive exercise induced altered promoter methylation of PRKAA2, the catalytic subunit of AMPK, in human blood (King-Himmelreich et al., 2016). This finding was verified in mice skeletal muscle, where increased DNA methylation was associated with reduced mRNA and protein expression.
It is now evident that both acute and long-term exercise significantly impact DNA methylation in a highly gene- and tissue-specific manner. Future studies should address if epigenetics can explain the individual response to certain exercise and what the mechanisms would be. One hypothesis is that altered baseline DNA methylation would impair the ability to respond to muscle contractions by further altering the methylome, and consequently, the epigenetic regulation of gene expression due to exercise would be diminished. Indeed, one study in human skeletal muscle recently showed associations between exercise response, based on phosphocreatine recovery rate, and altered DNA methylation and expression levels (Stephens et al., 2018). The results point to epigenomic and transcriptomic alterations in glutathione and mitochondrial metabolism, as well as in insulin signaling, and some of the epigenetic differences were also observed in myogenic progenitor cells.
Age and Epigenetics
Aging is associated with increased abdominal obesity, insulin resistance, and T2D. A better understanding of age-related molecular mechanisms that predispose to obesity and T2D may help develop new therapies and prevention strategies. Although DNA methylation is considered a stable epigenetic mark, the epigenome itself is not stable over time. The reprogramming during embryonic development is the most pronounced period, but the epigenetic drift is strongly associated with aging, where the genome gains and loses methylation stochastically over time (Fraga et al., 2005). In 2005, Fraga et al. found that the epigenome was similar in cells from young monozygotic twin pairs, whereas it was different in elderly twin pairs, supporting a pronounced effect of age on DNA methylation. Since then, numerous studies have investigated the impact of aging on the epigenome in human tissues of importance for obesity and T2D including adipose tissue, skeletal muscle, the liver, and pancreatic islets (Bacos et al., 2016, Bysani et al., 2017, Ling et al., 2007, Rönn et al., 2008, Rönn et al., 2015). Interestingly, DNA methylation of 3,470 sites changed significantly with aging in three different cell types i.e., adipose tissue, liver, and blood (Bysani et al., 2017), and methylation of some CpG sites seems to be affected in the same way in all studied tissues, e.g., FHL2, ELOVL2, and KLF14. FHL2 or four and a half LIM domains 2 has been implicated in Wnt signaling and diabetic kidney disease (Li et al., 2015). ELOVL2 encodes an ELOVL fatty acid elongase, which seems to regulate lipid metabolism in β cells by channeling palmitate toward β-oxidation instead of ceramide synthesis (Bellini et al., 2018). Additionally, KLF14 encodes a transcription factor, and single nucleotide polymorphisms (SNPs) associated with KLF14 expression increase the risk of T2D and affect high-density lipoprotein (HDL) cholesterol levels (Small et al., 2011). Moreover, obesity was found to influence age-driven epigenetic changes, which provides a molecular link between aging and obesity (Almén et al., 2014). How these age-related epigenetic changes take place remains to be elucidated. For example, what makes a certain methyl transferase change DNA methylation with age at a certain CpG site in several different tissues?
Interaction between Genetics and Epigenetics in Relation to Obesity and Diabetes
As DNA methylation mainly occurs on the nucleotide cytosine (C) when followed by a guanine (G), it is obvious that genetic variation that removes or introduces such a CG dinucleotide can and will affect the possibility for DNA methylation in this position. Notable, ∼25% of all SNPs introduce or take away a CpG site. In 2007, we identified an SNP annotated to NDUFB6, which introduces a CpG site and thereby affects DNA methylation and gene expression in human skeletal muscle in an age-related manner (Ling et al., 2007). However, apart from this direct effect of genetic variants on DNA methylation, it is less clear if genetic variants also affect DNA methylation more distant, and what would be the mechanism for this. It is also not known to what extent this occurs throughout the genome and how it contributes to clinical phenotypes.
In 2013, we further showed that 48% of the genetic variants associated with T2D, at that point, indeed introduced or removed a CpG site (Dayeh et al., 2013). As expected, all analyzed CpG-SNPs were associated with DNA methylation, and for 37.5% the SNPs also affected methylation of surrounding CpG sites. This study was performed in human pancreatic islets, and some of the T2D CpG-SNPs were also associated with gene expression, alternative splicing, and insulin secretion (Dayeh et al., 2013). This study was extended, and in 2014, we presented a genome-wide picture of associations between genetic and epigenetic variation in human pancreatic islets (Olsson et al., 2014). Numerous SNP-CpG pairs in cis were identified as well as in trans (here defined as a distance >500 kb between the SNP and CpG site); the mechanism for the latter remains to be explained. These associations in cis include reported diabetes genes, e.g., ADCY5, KCNJ11, HLA-DQA1, INS, PDX1, and GRB10. We further identified SNP-CpG pairs that associated with gene expression and insulin secretion, and causal inference tests (CIT) pointed to DNA methylation as the potential mediator of genetic association with gene expression and insulin secretion. For example, the CIT identified causal relationships between SNPs, DNA methylation, and expression of genes annotated to the human leukocyte antigen (HLA) region, which are strongly linked to type 1 diabetes. Genes involved in glutathione metabolism, including GSTT2 and GSTT1, were also identified by CIT. This study identified several candidate genes, and the ones that were functionally tested (GPX7, GSTT1, and SNX19) indeed showed effects on key biological processes such as proliferation and apoptosis in pancreatic β cells (Olsson et al., 2014). A DNA methylation quantitative trait locus (mQTL) analysis, based on the Illumina 450k array, was also performed in human adipose tissue (Volkov et al., 2016). More than 100,000 significant mQTLs were identified, again highlighting the tight connection between genetic variation and DNA methylation. These mQTLs include obesity, lipid, and T2D locus identified by GWASs, e.g., POMC, APOA5, CETP, FADS2, GCKR, SORT1, and LEPR. Numerous SNPs in significant mQTLs were also associated with adipose tissue gene expression, BMI, lipid traits, glucose, and insulin levels; phenotypes which may be predisposed to obesity and T2D. Once again, it was shown that genetic variants mediate the effect on metabolic traits including BMI and HbA1c via altered levels of DNA methylation (Volkov et al., 2016).
Bell et al. (2010a) performed an integrative genetic and epigenetic analysis of T2D risk loci, where they identified haplotype-specific methylation in the FTO region. After a thorough investigation, the most significant variation of methylation based on genetic background was found in a genomic region of 7.7 kb, harboring a putative enhancer region. Hence, epigenetic analysis may be one way to narrow down the large LD blocks identified in GWASs and to find the functional element connecting genotype to phenotype (Bell et al., 2010a). A publication a year later confirmed that FTO risk variants alter the epigenome (Almén et al., 2012). Here, another FTO risk allele was associated with DNA methylation of five CpG sites, annotated to six different genes. In an attempt to understand how SNPs identified from GWASs act to influence body mass, data for 52 obesity-associated SNPs were connected to Illumina 450k array methylation data in blood from 355 individuals (Voisin et al., 2015b). This study revealed 28 SNPs that affected a total of 107 CpG sites, of which 35% were located in promoter regions.
The more we learn, the more there is to understand, and for future genomic studies we need to integrate as many aspects of the DNA and chromatin structure as possible. Gene regulation is a highly complex process starting from the nucleotide sequence, adding on DNA methylation, small interfering RNA molecules and histone modifications, with the epigenetic modifications also being affected by both genetic and environmental influences. Importantly, results from CIT suggest that epigenetic mechanisms may cause phenotypes related to diabetes and obesity in humans.
Epigenetic Inheritance Related to Obesity and Diabetes
It is an interesting and challenging hypothesis that epigenetic inheritance contributes to evolution. With the rapid change of our environment during the last decades, changes to our epigenome provide adaptation faster than through genetic mutations, while it is reversible and can be gradually lost. Intergenerational inheritance refers to parental effects where exposures before or during pregnancy may affect the germ cells or the offspring and its reproductive cells. In that way, environmental factors may affect three generations at a time (Figure 4). Hence, to define true transgenerational epigenetic inheritance, i.e., persistent changes that are not due to the original exposure directly, the phenotype must be observed also in the fourth generation. To our knowledge, there is limited, if any, research providing evidence for transgenerational epigenetic inheritance in humans, and the fact that epigenetic changes are dynamic and transient in nature adds another challenge to this research field. Looking beyond human studies, there are still limited data on true transgenerational epigenetic effects, which requires transmission from F0 to F3 or further, and these have mainly been seen in C. elegans (Perez and Lehner, 2019). However, several studies have made attempts to study transfer of epigenetic mechanisms through some generations and link these to obesity and T2D.
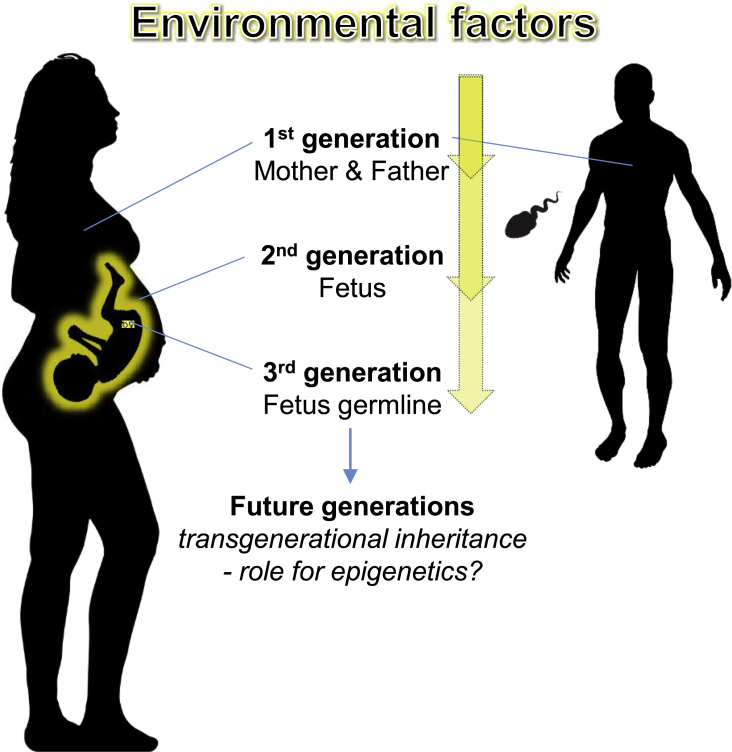
Figure 4.
Illustration of How the Environment Affects Three Generations at a Time
At time of conception, the environment influences the parents, including the spermatozoa methylome and the in utero environment and eventually has the possibility to affect both the fetus and its reproductive cells. Evidence for transgenerational epigenetic inheritance in humans, which requires four generations or more, is still sparse.
Epigenetic mechanisms are necessary in the regulation of cell differentiation, but when it comes to reproduction, the epigenetic pattern was originally thought to be completely reset at certain stages of early development. In rodents, it has been clearly demonstrated that the epigenetic states are not entirely reprogrammed (Morgan et al., 1999, Rakyan et al., 2003). In comparison to human studies, rodents have a short generation span, and in these studies, the influence of genetic background as well as environmental factors can be controlled. An epigenetic modification at the agouti locus in mice affects gene expression and causes yellow fur, obesity, and diabetes. The epigenetic state of this locus has been shown to be inherited between generations, through the female germline (Morgan et al., 1999). There are also examples of paternal transmission of epigenetic information, where offspring to male mice on a high-fat diet display altered metabolic phenotypes, including obesity and β cell dysfunction, and gene expression in the pancreatic islet (Ng et al., 2010).
At present, there are also a number of studies supporting intergenerational epigenetic inheritance in humans. Observations from the Pima Native Americans have shown that offspring from mothers with T2D during pregnancy, as compared to non-diabetic mothers, develop both T2D and obesity at a higher risk (45 versus 1.4% and 58 versus 17%, respectively) (Pettitt et al., 1983, Pettitt et al., 1988). This effect seems, partly, to be independent of genetics. Importantly, these findings were followed by a study of siblings born before and after their mothers were diagnosed with T2D, limiting the impact of genetic and external environmental factors. Here, the risk of T2D and obesity was significantly higher in the siblings born after the mother developed T2D, strongly suggesting the intrauterine exposure to mediate the risk independent of genetic background (Dabelea et al., 2000). There is also strong epidemiological data linking prenatal exposure to famine with persistent epigenetic differences, as well as increased risk of disease, later in life (Heijmans et al., 2008, Roseboom et al., 2006). Early gestation seems to be the most vulnerable period and is associated with obesity among other common diseases, while glucose intolerance was seen in individuals exposed to famine during any time of gestation (Roseboom et al., 2006). Differential DNA methylation of several genes important for metabolic disease, e.g., IGF2, INSIGF, and LEP, were reported and were, apart from stage of gestation, also dependent on sex of the exposed fetus (Heijmans et al., 2008, Tobi et al., 2009). Another study supporting epigenetic inheritance is the Överkalix study, where food supply during the child’s slow growth period was linked to common diseases and mortality over three generations (Kaati et al., 2002). Here, it was shown that if the paternal grandfather had an abundance of food during this period, diabetes mortality increased.
To understand the link between maternal hyperglycemia and offspring obesity, an epigenetic candidate gene approach was undertaken to investigating DNA methylation levels of LEP, a gene regulating energy balance (Allard et al., 2015). Using Mendelian randomization, this study showed that maternal hyperglycemia decreases LEP DNA methylation levels in offspring and is associated with higher cord blood leptin levels. This suggests that epigenetic regulation induced by the in utero environment may contribute to increased adiposity later in life. Another human study investigated how pre-pregnancy BMI affected the offspring epigenome and adiposity (Sharp et al., 2015). Interestingly, methylation of a number of CpG sites (n = 28) were significantly altered in cord blood from offspring of obese mothers but many more (n = 1,621) when the mother was underweight. Paternal BMI seemed to be of less importance according to the results from this study, as did gestational weight gain (Sharp et al., 2015). Taking this approach forward to a large, well-powered replicated study, Sharp et al. (2017) presented robust associations between maternal BMI and DNA methylation of 86 sites in blood from newborns. Of these, the signal was persistent in adolescents for 72 sites; however, causal inference was only observed for 8 of the sites. In another study, Hjort et al. (2018) found differential DNA methylation of 76 sites in the blood of 93 offspring to women with gestational diabetes mellitus (GDM) compared with 95 controls. While most of these methylation differences were because of confounding by maternal pre-pregnancy BMI, methylation changes of 13 sites were independently associated with maternal GDM.
As evidence for paternal transmission of epigenetic information is emerging, it seems like the developing embryo is controlled not only by maternal influences. The spermatozoa, similar to the somatic cells, has an epigenome that is dynamic and influenced by environmental factors, which likely affect the phenotype of the next generation (Donkin and Bèrres, 2018). For example, DNA methylation and the expression of sncRNAs is altered in spermatozoa from obese men, and interestingly, the sperm DNA methylome is remodeled after bariatric surgery (Donkin et al., 2016). This study points to genes known from genetic association studies of obesity, including FTO, MC4R, BDNF, and CHST8. Also, exercise has the ability to reprogram the sperm methylome in humans (Denham et al., 2015), whether these changes are inherited and beneficial for the offspring remains to be established.
How Can Epigenetics Contribute to How We Practice Medicine in the Future?
As described in this review, epigenetic modifications in key tissues seem to contribute to development of obesity and T2D (Figures 1 and 2). It is hence possible that current knowledge in epigenetics could be translated into the clinic to prevent further increase in disease prevalence and to develop new therapies. Regular exercise and healthy diets are known to prevent the development of T2D in individuals at risk for disease (Knowler et al., 2002, Tuomilehto et al., 2001). However, identification of subjects with a high risk of developing T2D and obesity is a criterion for disease prevention. Epigenetic biomarkers may be used for the prediction of obesity and T2D in future medicine. These biomarkers should be developed in easily accessible human cells such as blood (Gillberg and Ling, 2015). Such epigenetic biomarkers are already in clinical use for prediction in cancer patients (Oussalah et al., 2018). Moreover, several studies have analyzed DNA methylation in the blood of prospective cohorts for T2D (Bacos et al., 2016, Chambers et al., 2015, Dayeh et al., 2016, Wahl et al., 2017). Interestingly, methylation of the ABCG1 locus was associated with an increased risk, whereas methylation of PHOSPHO1 was associated with a decreased risk for future T2D in several studies (Chambers et al., 2015, Dayeh et al., 2016) (Figure 2). Moreover, the ABCG1 site showed increased methylation in both adipose tissue and blood from the diabetic twins in monozygotic twin pairs discordant for T2D, while methylation of PHOSPHO1 was decreased in skeletal muscle of diabetic versus control twins (Dayeh et al., 2016). A different study showed that blood-based epigenetic biomarkers reflect age-associated DNA methylation changes in human pancreatic islets and associate with future insulin secretion and T2D (Bacos et al., 2016). Here, aging was found to increase methylation of sites annotated to KLF14, FHL2, ZNF518B, and FAM123C in both human pancreatic islets and blood (Figure 2). Moreover, increased methylation in blood of the same sites was associated with higher insulin secretion and decreased risk of T2D ∼10 years later in prospective cohorts. Perfilyev et al. also found association between DNA methylation in adipose tissue at baseline and weight increase in response to extra energy intake (Perfilyev et al., 2017). Epigenetic biomarkers that translate from target tissue to blood may subsequently be of clinical relevance since they can be readily analyzed in patients and high-risk populations for diabetes. However, new studies identifying epigenetic markers with a better predictive capacity are needed and these should perform better than current factors known to predict diabetes.
The reversible nature of epigenetic modifications, together with recent advances in epigenome-targeting methods, provides us with new opportunities for novel epigenetic therapies (Liu et al., 2016). If one could identify causal epigenetic mechanisms in obesity and T2D, these could be targeted for novel treatments. In a recent study, Ou et al. (2019) targeted demethylation at the CDKN1C locus, a cell-cycle regulator, to repress p57 expression and induce β-cell replication. Together these studies support future epigenetic editing attempts in obesity and T2D treatment.
Several pharmacological agents already exist with effects on DNA methylation and histone modifications, including DNMT inhibitors (e.g., Aza) and histone deacetylase inhibitors (HDACi, e.g., VPA and TSA). Moreover, epigenetic drugs have been tested with good results, e.g., for leukemia where Aza is first-line treatment for higher-risk myelodysplastic syndromes, and BET and IDH1/2 inhibitors, which are in clinical trials to treat acute myeloid leukemia (AML). Epigenetic drugs may also affect key tissues for obesity and T2D. Notably, there is a growing body of literature showing that HDACs regulate glucose homeostasis and islet function (Lenoir et al., 2011, Lundh et al., 2015, Mihaylova et al., 2011, Rönn et al., 2013). An HDACi, MC1568, was recently found to improve insulin secretion in pancreatic islets from human donors with T2D (Daneshpajooh et al., 2018). MC1568 inhibits HDAC7, which has increased expression and decreased DNA methylation in pancreatic islets from diabetic versus non-diabetic donors (Daneshpajooh et al., 2017). Overexpression of HDAC7 in clonal β cells and rat islets resulted in impaired glucose-stimulated insulin secretion and mitochondrial dysfunction. However, the chronic nature of T2D implies lifelong use of drugs, and it is important to carefully weigh the benefits against the risks. Low specificity and global action of HDACi may lead to side effects. Therefore, development of selective epigenetic drugs may represent an avenue for future therapeutic purposes.
Discussion and Conclusions
Almost all the knowledge we have concerning epigenetic dysregulation in relation to human obesity and T2D has emerged during the last 10 years. With that in mind, the advancements are tremendous, and for future perspectives, the research field holds unlimited possibilities. Larger cohorts are needed, suggestively through worldwide collaborations, and because of the tissue- or even cell-specific nature of epigenetic information, more human biopsies are desirable. Several studies report small, but significant, changes on a group level, whereas a paired study design is harder to find. As metabolic diseases are heterogeneous in nature, the interindividual variation is large, and several small changes may act together to influence the phenotype. The use of single-cell types would allow to better connect the epigenome to the transcriptome and to define the extent of alterations needed in DNA methylation to affect gene expression. Moreover, mQTL studies and CITs have linked SNPs via significant differences in DNA methylation to differential gene expression in human tissues.
It is an exciting observation that many of the regions that display altered DNA methylation in response to environmental influences or in certain pathologies, are often seen in more than one tissue and are observed under several medical conditions. This suggests that some regions of our DNA and chromatin is less stable, hence more vulnerable to external stimuli, which results in epigenetic dysregulation or variability. Thereby, collaborations across research fields could further improve our understanding of the human epigenome and also guide in method development. Within the near future, genome-wide sequencing is needed to fully dissect the DNA methylome in combination with other epigenetic modifications; however, in the long run, the aim should be to dissect regions with large epigenetic variability and with shown function in gene expression and phenotype transmission. Thus, targeted methods may be developed that maximize the epigenetic information obtained, in spite of reduced coverage, cost, and statistical penalties.
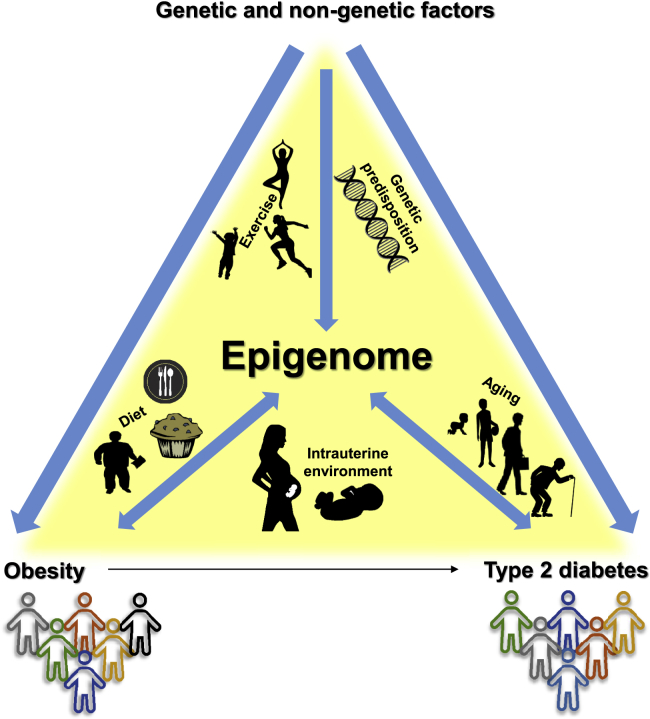
There is now enough evidence to establish that the human epigenome contributes to diseases and also interacts with and responds to various physiological conditions (Figure 5). Aging and genetic variation are both important contributors to the epigenetic variability seen in individuals affected by obesity or T2D. Furthermore, the in utero environment and external factors such as physical activity and availability of nutrients affect the epigenome. Most important, the transient and reversible nature of epigenetic modifications provides an open field for discovery of targets for future prediction and therapeutic concepts in obesity and T2D.
Figure 5.
Interactions between the Environment, the Epigenome, and Obesity and Type 2 Diabetes
Several genetic and non-genetic factors contribute to the development of obesity and type 2 diabetes. The same factors, e.g., exercise, genetic predisposition, diet, intrauterine environment, and aging, are also known to influence the human epigenome. Furthermore, the epigenome may both contribute to disease and interact with and respond to various physiological conditions of heterogeneous diseases.
Acknowledgments
This work was supported by grants from the Swedish Foundation for Strategic Research for IRC15-0067, Swedish Research Council, Region Skåne (ALF), Knut and Alice Wallenberg Foundation, Novo Nordisk Foundation, EFSD/Lilly Fellowship, Söderberg Foundation, the Swedish Diabetes foundation, Diabetes Wellness Sweden, the Swedish Heart and Lung foundation, Påhlsson Foundation, the Royal Physiographic Society of Lund, Hjelt Diabetes Foundation, EXODIAB and Linné grant (B31 5631/2006), and the European Research Council (PAINTBOX no. 725840).
Author Contributions
C.L. and T.R. wrote the paper and prepared the figures.
References
- Abderrahmani A., Yengo L., Caiazzo R., Canouil M., Cauchi S., Raverdy V., Plaisance V., Pawlowski V., Lobbens S., Maillet J. Increased hepatic PDGF-AA signaling mediates liver insulin resistance in obesity-associated type 2 diabetes. Diabetes. 2018;67:1310–1321. doi: 10.2337/db17-1539. [DOI] [PubMed] [Google Scholar]
- Ackermann A.M., Wang Z., Schug J., Naji A., Kaestner K.H. Integration of ATAC-seq and RNA-seq identifies human alpha cell and beta cell signature genes. Mol. Metab. 2016;5:233–244. doi: 10.1016/j.molmet.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agardh E., Lundstig A., Perfilyev A., Volkov P., Freiburghaus T., Lindholm E., Rönn T., Agardh C.D., Ling C. Genome-wide analysis of DNA methylation in subjects with type 1 diabetes identifies epigenetic modifications associated with proliferative diabetic retinopathy. BMC Med. 2015;13:182. doi: 10.1186/s12916-015-0421-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allard C., Desgagné V., Patenaude J., Lacroix M., Guillemette L., Battista M.C., Doyon M., Ménard J., Ardilouze J.L., Perron P. Mendelian randomization supports causality between maternal hyperglycemia and epigenetic regulation of leptin gene in newborns. Epigenetics. 2015;10:342–351. doi: 10.1080/15592294.2015.1029700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almén M.S., Jacobsson J.A., Moschonis G., Benedict C., Chrousos G.P., Fredriksson R., Schiöth H.B. Genome wide analysis reveals association of a FTO gene variant with epigenetic changes. Genomics. 2012;99:132–137. doi: 10.1016/j.ygeno.2011.12.007. [DOI] [PubMed] [Google Scholar]
- Almén M.S., Nilsson E.K., Jacobsson J.A., Kalnina I., Klovins J., Fredriksson R., Schiöth H.B. Genome-wide analysis reveals DNA methylation markers that vary with both age and obesity. Gene. 2014;548:61–67. doi: 10.1016/j.gene.2014.07.009. [DOI] [PubMed] [Google Scholar]
- Aronica L., Levine A.J., Brennan K., Mi J., Gardner C., Haile R.W., Hitchins M.P. A systematic review of studies of DNA methylation in the context of a weight loss intervention. Epigenomics. 2017;9:769–787. doi: 10.2217/epi-2016-0182. [DOI] [PubMed] [Google Scholar]
- Aslibekyan S., Demerath E.W., Mendelson M., Zhi D., Guan W., Liang L., Sha J., Pankow J.S., Liu C., Irvin M.R. Epigenome-wide study identifies novel methylation loci associated with body mass index and waist circumference. Obesity (Silver Spring) 2015;23:1493–1501. doi: 10.1002/oby.21111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacos K., Gillberg L., Volkov P., Olsson A.H., Hansen T., Pedersen O., Gjesing A.P., Eiberg H., Tuomi T., Almgren P. Blood-based biomarkers of age-associated epigenetic changes in human islets associate with insulin secretion and diabetes. Nat. Commun. 2016;7:11089. doi: 10.1038/ncomms11089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa M.T., Soares S.M., Novak C.M., Sinclair D., Levine J.A., Aksoy P., Chini E.N. The enzyme CD38 (a NAD glycohydrolase, EC 3.2.2.5) is necessary for the development of diet-induced obesity. FASEB J. 2007;21:3629–3639. doi: 10.1096/fj.07-8290com. [DOI] [PubMed] [Google Scholar]
- Barrès R., Osler M.E., Yan J., Rune A., Fritz T., Caidahl K., Krook A., Zierath J.R. Non-CpG methylation of the PGC-1alpha promoter through DNMT3B controls mitochondrial density. Cell Metab. 2009;10:189–198. doi: 10.1016/j.cmet.2009.07.011. [DOI] [PubMed] [Google Scholar]
- Barrès R., Yan J., Egan B., Treebak J.T., Rasmussen M., Fritz T., Caidahl K., Krook A., O'Gorman D.J., Zierath J.R. Acute exercise remodels promoter methylation in human skeletal muscle. Cell Metab. 2012;15:405–411. doi: 10.1016/j.cmet.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Bell C.G., Finer S., Lindgren C.M., Wilson G.A., Rakyan V.K., Teschendorff A.E., Akan P., Stupka E., Down T.A., Prokopenko I. Integrated genetic and epigenetic analysis identifies haplotype-specific methylation in the FTO type 2 diabetes and obesity susceptibility locus. PLoS One. 2010;5:e14040. doi: 10.1371/journal.pone.0014040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell C.G., Teschendorff A.E., Rakyan V.K., Maxwell A.P., Beck S., Savage D.A. Genome-wide DNA methylation analysis for diabetic nephropathy in type 1 diabetes mellitus. BMC Med. Genomics. 2010;3:33. doi: 10.1186/1755-8794-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellini L., Campana M., Rouch C., Chacinska M., Bugliani M., Meneyrol K., Hainault I., Lenoir V., Denom J., Véret J. Protective role of the ELOVL2/docosahexaenoic acid axis in glucolipotoxicity-induced apoptosis in rodent beta cells and human islets. Diabetologia. 2018;61:1780–1793. doi: 10.1007/s00125-018-4629-8. [DOI] [PubMed] [Google Scholar]
- Benton M.C., Johnstone A., Eccles D., Harmon B., Hayes M.T., Lea R.A., Griffiths L., Hoffman E.P., Stubbs R.S., Macartney-Coxson D. An analysis of DNA methylation in human adipose tissue reveals differential modification of obesity genes before and after gastric bypass and weight loss. Genome Biol. 2015;16:8. doi: 10.1186/s13059-014-0569-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berdasco M., Gómez A., Rubio M.J., Català-Mora J., Zanón-Moreno V., Lopez M., Hernández C., Yoshida S., Nakama T., Ishikawa K. DNA methylomes reveal biological networks involved in human eye development, functions and associated disorders. Sci. Rep. 2017;7:11762. doi: 10.1038/s41598-017-12084-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandare R., Schug J., Le Lay J., Fox A., Smirnova O., Liu C., Naji A., Kaestner K.H. Genome-wide analysis of histone modifications in human pancreatic islets. Genome Res. 2010;20:428–433. doi: 10.1101/gr.102038.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brøns C., Jacobsen S., Nilsson E., Rönn T., Jensen C.B., Storgaard H., Poulsen P., Groop L., Ling C., Astrup A. Deoxyribonucleic acid methylation and gene expression of PPARGC1A in human muscle is influenced by high-fat overfeeding in a birth-weight-dependent manner. J. Clin. Endocrinol. Metab. 2010;95:3048–3056. doi: 10.1210/jc.2009-2413. [DOI] [PubMed] [Google Scholar]
- Bysani M., Perfilyev A., de Mello V.D., Rönn T., Nilsson E., Pihlajamäki J., Ling C. Epigenetic alterations in blood mirror age-associated DNA methylation and gene expression changes in human liver. Epigenomics. 2017;9:105–122. doi: 10.2217/epi-2016-0087. [DOI] [PubMed] [Google Scholar]
- Caudill M.A., Wang J.C., Melnyk S., Pogribny I.P., Jernigan S., Collins M.D., Santos-Guzman J., Swendseid M.E., Cogger E.A., James S.J. Intracellular S-adenosylhomocysteine concentrations predict global DNA hypomethylation in tissues of methyl-deficient cystathionine beta-synthase heterozygous mice. J. Nutr. 2001;131:2811–2818. doi: 10.1093/jn/131.11.2811. [DOI] [PubMed] [Google Scholar]
- Chambers J.C., Loh M., Lehne B., Drong A., Kriebel J., Motta V., Wahl S., Elliott H.R., Rota F., Scott W.R. Epigenome-wide association of DNA methylation markers in peripheral blood from Indian Asians and Europeans with incident type 2 diabetes: a nested case-control study. Lancet Diabetes Endocrinol. 2015;3:526–534. doi: 10.1016/S2213-8587(15)00127-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Miao F., Paterson A.D., Lachin J.M., Zhang L., Schones D.E., Wu X., Wang J., Tompkins J.D., Genuth S. Epigenomic profiling reveals an association between persistence of DNA methylation and metabolic memory in the DCCT/EDIC type 1 diabetes cohort. Proc. Natl. Acad. Sci. USA. 2016;113:E3002–E3011. doi: 10.1073/pnas.1603712113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho N.H., Shaw J.E., Karuranga S., Huang Y., da Rocha Fernandes J.D., Ohlrogge A.W., Malanda B. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018;138:271–281. doi: 10.1016/j.diabres.2018.02.023. [DOI] [PubMed] [Google Scholar]
- Dabelea D., Hanson R.L., Lindsay R.S., Pettitt D.J., Imperatore G., Gabir M.M., Roumain J., Bennett P.H., Knowler W.C. Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity: a study of discordant sibships. Diabetes. 2000;49:2208–2211. doi: 10.2337/diabetes.49.12.2208. [DOI] [PubMed] [Google Scholar]
- Dahlman I., Sinha I., Gao H., Brodin D., Thorell A., Rydén M., Andersson D.P., Henriksson J., Perfilyev A., Ling C. The fat cell epigenetic signature in post-obese women is characterized by global hypomethylation and differential DNA methylation of adipogenesis genes. Int. J. Obes. (Lond) 2015;39:910–919. doi: 10.1038/ijo.2015.31. [DOI] [PubMed] [Google Scholar]
- Daneshpajooh M., Bacos K., Bysani M., Bagge A., Ottosson Laakso E., Vikman P., Eliasson L., Mulder H., Ling C. HDAC7 is overexpressed in human diabetic islets and impairs insulin secretion in rat islets and clonal beta cells. Diabetologia. 2017;60:116–125. doi: 10.1007/s00125-016-4113-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneshpajooh M., Eliasson L., Bacos K., Ling C. MC1568 improves insulin secretion in islets from type 2 diabetes patients and rescues beta-cell dysfunction caused by Hdac7 upregulation. Acta Diabetol. 2018;55:1231–1235. doi: 10.1007/s00592-018-1201-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davegårdh C., Broholm C., Perfilyev A., Henriksen T., García-Calzón S., Peijs L., Hansen N.S., Volkov P., Kjøbsted R., Wojtaszewski J.F. Abnormal epigenetic changes during differentiation of human skeletal muscle stem cells from obese subjects. BMC Med. 2017;15:39. doi: 10.1186/s12916-017-0792-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayeh T., Tuomi T., Almgren P., Perfilyev A., Jansson P.A., de Mello V.D., Pihlajamäki J., Vaag A., Groop L., Nilsson E. DNA methylation of loci within ABCG1 and PHOSPHO1 in blood DNA is associated with future type 2 diabetes risk. Epigenetics. 2016;11:482–488. doi: 10.1080/15592294.2016.1178418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayeh T., Volkov P., Salö S., Hall E., Nilsson E., Olsson A.H., Kirkpatrick C.L., Wollheim C.B., Eliasson L., Rönn T. Genome-wide DNA methylation analysis of human pancreatic islets from type 2 diabetic and non-diabetic donors identifies candidate genes that influence insulin secretion. PLoS Genet. 2014;10:e1004160. doi: 10.1371/journal.pgen.1004160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayeh T.A., Olsson A.H., Volkov P., Almgren P., Rönn T., Ling C. Identification of CpG-SNPs associated with type 2 diabetes and differential DNA methylation in human pancreatic islets. Diabetologia. 2013;56:1036–1046. doi: 10.1007/s00125-012-2815-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demerath E.W., Guan W., Grove M.L., Aslibekyan S., Mendelson M., Zhou Y.H., Hedman Å.K., Sandling J.K., Li L.A., Irvin M.R. Epigenome-wide association study (EWAS) of BMI, BMI change and waist circumference in African American adults identifies multiple replicated loci. Hum. Mol. Genet. 2015;24:4464–4479. doi: 10.1093/hmg/ddv161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denham J., O'Brien B.J., Harvey J.T., Charchar F.J. Genome-wide sperm DNA methylation changes after 3 months of exercise training in humans. Epigenomics. 2015;7:717–731. doi: 10.2217/epi.15.29. [DOI] [PubMed] [Google Scholar]
- Dhana K., Braun K.V.E., Nano J., Voortman T., Demerath E.W., Guan W., Fornage M., van Meurs J.B.J., Uitterlinden A.G., Hofman A. An epigenome-wide association study of obesity-related traits. Am. J. Epidemiol. 2018;187:1662–1669. doi: 10.1093/aje/kwy025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Francesco A., Di Germanio C., Bernier M., de Cabo R. A time to fast. Science. 2018;362:770–775. doi: 10.1126/science.aau2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick K.J., Nelson C.P., Tsaprouni L., Sandling J.K., Aïssi D., Wahl S., Meduri E., Morange P.E., Gagnon F., Grallert H. DNA methylation and body-mass index: a genome-wide analysis. Lancet. 2014;383:1990–1998. doi: 10.1016/S0140-6736(13)62674-4. [DOI] [PubMed] [Google Scholar]
- Donkin I., Barrès R. Sperm epigenetics and influence of environmental factors. Mol. Metab. 2018;14:1–11. doi: 10.1016/j.molmet.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donkin I., Versteyhe S., Ingerslev L.R., Qian K., Mechta M., Nordkap L., Mortensen B., Appel E.V., Jørgensen N., Kristiansen V.B. Obesity and bariatric surgery drive epigenetic variation of spermatozoa in humans. Cell Metab. 2016;23:369–378. doi: 10.1016/j.cmet.2015.11.004. [DOI] [PubMed] [Google Scholar]
- Ducker G.S., Rabinowitz J.D. One-carbon metabolism in health and disease. Cell Metab. 2017;25:27–42. doi: 10.1016/j.cmet.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan T.M., Reed M.C., Nijhout H.F. The relationship between intracellular and plasma levels of folate and metabolites in the methionine cycle: a model. Mol. Nutr. Food Res. 2013;57:628–636. doi: 10.1002/mnfr.201200125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esguerra J.L.S., Nagao M., Ofori J.K., Wendt A., Eliasson L. MicroRNAs in islet hormone secretion. Diabetes Obes. Metab. 2018;20:11–19. doi: 10.1111/dom.13382. [DOI] [PubMed] [Google Scholar]
- Fabre O., Ingerslev L.R., Garde C., Donkin I., Simar D., Barrès R. Exercise training alters the genomic response to acute exercise in human adipose tissue. Epigenomics. 2018;10:1033–1050. doi: 10.2217/epi-2018-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fradin D., Boëlle P.Y., Belot M.P., Lachaux F., Tost J., Besse C., Deleuze J.F., De Filippo G., Bougnères P. Genome-wide methylation analysis identifies specific epigenetic marks in severely obese children. Sci. Rep. 2017;7:46311. doi: 10.1038/srep46311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraga M.F., Ballestar E., Paz M.F., Ropero S., Setien F., Ballestar M.L., Heine-Suñer D., Cigudosa J.C., Urioste M., Benitez J. Epigenetic differences arise during the lifetime of monozygotic twins. Proc. Natl. Acad. Sci. USA. 2005;102:10604–10609. doi: 10.1073/pnas.0500398102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillberg L., Ling C. The potential use of DNA methylation biomarkers to identify risk and progression of type 2 diabetes. Front. Endocrinol. (Lausanne) 2015;6:43. doi: 10.3389/fendo.2015.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillberg L., Perfilyev A., Brøns C., Thomasen M., Grunnet L.G., Volkov P., Rosqvist F., Iggman D., Dahlman I., Risérus U. Adipose tissue transcriptomics and epigenomics in low birthweight men and controls: role of high-fat overfeeding. Diabetologia. 2016;59:799–812. doi: 10.1007/s00125-015-3852-9. [DOI] [PubMed] [Google Scholar]
- Hall E., Dayeh T., Kirkpatrick C.L., Wollheim C.B., Dekker Nitert M., Ling C. DNA methylation of the glucagon-like peptide 1 receptor (GLP1R) in human pancreatic islets. BMC Med. Genet. 2013;14:76. doi: 10.1186/1471-2350-14-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall E., Dekker Nitert M., Volkov P., Malmgren S., Mulder H., Bacos K., Ling C. The effects of high glucose exposure on global gene expression and DNA methylation in human pancreatic islets. Mol. Cell. Endocrinol. 2018;472:57–67. doi: 10.1016/j.mce.2017.11.019. [DOI] [PubMed] [Google Scholar]
- Hall E., Volkov P., Dayeh T., Bacos K., Rönn T., Nitert M.D., Ling C. Effects of palmitate on genome-wide mRNA expression and DNA methylation patterns in human pancreatic islets. BMC Med. 2014;12:103. doi: 10.1186/1741-7015-12-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijmans B.T., Tobi E.W., Stein A.D., Putter H., Blauw G.J., Susser E.S., Slagboom P.E., Lumey L.H. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc. Natl. Acad. Sci. USA. 2008;105:17046–17049. doi: 10.1073/pnas.0806560105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjort L., Jørgensen S.W., Gillberg L., Hall E., Brøns C., Frystyk J., Vaag A.A., Ling C. 36 h fasting of young men influences adipose tissue DNA methylation of LEP and ADIPOQ in a birth weight-dependent manner. Clin. Epigenetics. 2017;9:40. doi: 10.1186/s13148-017-0340-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjort L., Martino D., Grunnet L.G., Naeem H., Maksimovic J., Olsson A.H., Zhang C., Ling C., Olsen S.F., Saffery R. Gestational diabetes and maternal obesity are associated with epigenome-wide methylation changes in children. JCI Insight. 2018;3 doi: 10.1172/jci.insight.122572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S.M., Woo H.W., Kim M.K., Kim S.Y., Lee Y.H., Shin D.H., Shin M.H., Chun B.Y., Choi B.Y. A prospective association between dietary folate intake and type 2 diabetes risk among Korean adults aged 40 years or older: the Korean Multi-Rural Communities Cohort (MRCohort) Study. Br. J. Nutr. 2017;118:1078–1088. doi: 10.1017/S0007114517003087. [DOI] [PubMed] [Google Scholar]
- Hou C., Zhao M., Li X., Li Y.J., Lin Y., Lu Q.J., Zhou Z.G. Histone H3 acetylation of tumor necrosis factor-alpha and cyclooxygenase-2 in patients with type 2 diabetes. Zhonghua Yi Xue Za Zhi. 2011;91:1805–1808. [PubMed] [Google Scholar]
- Huang R.C., Garratt E.S., Pan H., Wu Y., Davis E.A., Barton S.J., Burdge G.C., Godfrey K.M., Holbrook J.D., Lillycrop K.A. Genome-wide methylation analysis identifies differentially methylated CpG loci associated with severe obesity in childhood. Epigenetics. 2015;10:995–1005. doi: 10.1080/15592294.2015.1080411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob R.A., Gretz D.M., Taylor P.C., James S.J., Pogribny I.P., Miller B.J., Henning S.M., Swendseid M.E. Moderate folate depletion increases plasma homocysteine and decreases lymphocyte DNA methylation in postmenopausal women. J. Nutr. 1998;128:1204–1212. doi: 10.1093/jn/128.7.1204. [DOI] [PubMed] [Google Scholar]
- Jacobsen S.C., Brøns C., Bork-Jensen J., Ribel-Madsen R., Yang B., Lara E., Hall E., Calvanese V., Nilsson E., Jørgensen S.W. Effects of short-term high-fat overfeeding on genome-wide DNA methylation in the skeletal muscle of healthy young men. Diabetologia. 2012;55:3341–3349. doi: 10.1007/s00125-012-2717-8. [DOI] [PubMed] [Google Scholar]
- Jacobsen S.C., Gillberg L., Bork-Jensen J., Ribel-Madsen R., Lara E., Calvanese V., Ling C., Fernandez A.F., Fraga M.F., Poulsen P. Young men with low birthweight exhibit decreased plasticity of genome-wide muscle DNA methylation by high-fat overfeeding. Diabetologia. 2014;57:1154–1158. doi: 10.1007/s00125-014-3198-8. [DOI] [PubMed] [Google Scholar]
- Jørgensen S.W., Brøns C., Bluck L., Hjort L., Færch K., Thankamony A., Gillberg L., Friedrichsen M., Dunger D.B., Vaag A.A. Metabolic response to 36 hours of fasting in young men born small vs appropriate for gestational age. Diabetologia. 2015;58:178–187. doi: 10.1007/s00125-014-3406-6. [DOI] [PubMed] [Google Scholar]
- Kaati G., Bygren L.O., Edvinsson S. Cardiovascular and diabetes mortality determined by nutrition during parents’ and grandparents’ slow growth period. Eur. J. Hum. Genet. 2002;10:682–688. doi: 10.1038/sj.ejhg.5200859. [DOI] [PubMed] [Google Scholar]
- Keating S.T., El-Osta A. Epigenetics and metabolism. Circ. Res. 2015;116:715–736. doi: 10.1161/CIRCRESAHA.116.303936. [DOI] [PubMed] [Google Scholar]
- King-Himmelreich T.S., Schramm S., Wolters M.C., Schmetzer J., Möser C.V., Knothe C., Resch E., Peil J., Geisslinger G., Niederberger E. The impact of endurance exercise on global and AMPK gene-specific DNA methylation. Biochem. Biophys. Res. Commun. 2016;474:284–290. doi: 10.1016/j.bbrc.2016.04.078. [DOI] [PubMed] [Google Scholar]
- Kirchner H., Sinha I., Gao H., Ruby M.A., Schönke M., Lindvall J.M., Barrès R., Krook A., Näslund E., Dahlman-Wright K. Altered DNA methylation of glycolytic and lipogenic genes in liver from obese and type 2 diabetic patients. Mol. Metab. 2016;5:171–183. doi: 10.1016/j.molmet.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowler W.C., Barrett-Connor E., Fowler S.E., Hamman R.F., Lachin J.M., Walker E.A., Nathan D.M., Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemas D.J., Wiener H.W., O'Brien D.M., Hopkins S., Stanhope K.L., Havel P.J., Allison D.B., Fernandez J.R., Tiwari H.K., Boyer B.B. Genetic polymorphisms in carnitine palmitoyltransferase 1A gene are associated with variation in body composition and fasting lipid traits in Yup'ik Eskimos. J. Lipid Res. 2012;53:175–184. doi: 10.1194/jlr.P018952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenoir O., Flosseau K., Ma F.X., Blondeau B., Mai A., Bassel-Duby R., Ravassard P., Olson E.N., Haumaitre C., Scharfmann R. Specific control of pancreatic endocrine beta- and delta-cell mass by class IIa histone deacetylases HDAC4, HDAC5, and HDAC9. Diabetes. 2011;60:2861–2871. doi: 10.2337/db11-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S.Y., Huang P.H., Tarng D.C., Lin T.P., Yang W.C., Chang Y.H., Yang A.H., Lin C.C., Yang M.H., Chen J.W. Four-and-a-half LIM domains Protein 2 is a coactivator of Wnt signaling in diabetic kidney disease. J. Am. Soc. Nephrol. 2015;26:3072–3084. doi: 10.1681/ASN.2014100989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Tang R., Ma F., Ouyang S., Liu Z., Wu J. Folic acid supplementation alters the DNA methylation profile and improves insulin resistance in high-fat-diet-fed mice. J. Nutr. Biochem. 2018;59:76–83. doi: 10.1016/j.jnutbio.2018.05.010. [DOI] [PubMed] [Google Scholar]
- Ling C., Del Guerra S., Lupi R., Rönn T., Granhall C., Luthman H., Masiello P., Marchetti P., Groop L., Del Prato S. Epigenetic regulation of PPARGC1A in human type 2 diabetic islets and effect on insulin secretion. Diabetologia. 2008;51:615–622. doi: 10.1007/s00125-007-0916-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling C., Poulsen P., Simonsson S., Rönn T., Holmkvist J., Almgren P., Hagert P., Nilsson E., Mabey A.G., Nilsson P. Genetic and epigenetic factors are associated with expression of respiratory chain component NDUFB6 in human skeletal muscle. J. Clin. Invest. 2007;117:3427–3435. doi: 10.1172/JCI30938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X.S., Wu H., Ji X., Stelzer Y., Wu X., Czauderna S., Shu J., Dadon D., Young R.A., Jaenisch R. Editing DNA methylation in the mammalian genome. Cell. 2016;167:233–247. doi: 10.1016/j.cell.2016.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig D.S., Willett W.C., Volek J.S., Neuhouser M.L. Dietary fat: from foe to friend? Science. 2018;362:764–770. doi: 10.1126/science.aau2096. [DOI] [PubMed] [Google Scholar]
- Lundh M., Galbo T., Poulsen S.S., Mandrup-Poulsen T. Histone deacetylase 3 inhibition improves glycaemia and insulin secretion in obese diabetic rats. Diabetes Obes. Metab. 2015;17:703–707. doi: 10.1111/dom.12470. [DOI] [PubMed] [Google Scholar]
- Malmgren S., Spégel P., Danielsson A.P., Nagorny C.L., Andersson L.E., Nitert M.D., Ridderstråle M., Mulder H., Ling C. Coordinate changes in histone modifications, mRNA levels, and metabolite profiles in clonal INS-1 832/13 beta-cells accompany functional adaptations to lipotoxicity. J. Biol. Chem. 2013;288:11973–11987. doi: 10.1074/jbc.M112.422527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson M.M., Marioni R.E., Joehanes R., Liu C., Hedman Å.K., Aslibekyan S., Demerath E.W., Guan W., Zhi D., Yao C. Association of body mass index with DNA methylation and gene expression in blood cells and relations to cardiometabolic disease: a Mendelian randomization approach. PLoS Med. 2017;14:e1002215. doi: 10.1371/journal.pmed.1002215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao F., Wu X., Zhang L., Yuan Y.C., Riggs A.D., Natarajan R. Genome-wide analysis of histone lysine methylation variations caused by diabetic conditions in human monocytes. J. Biol. Chem. 2007;282:13854–13863. doi: 10.1074/jbc.M609446200. [DOI] [PubMed] [Google Scholar]
- Mihaylova M.M., Vasquez D.S., Ravnskjaer K., Denechaud P.D., Yu R.T., Alvarez J.G., Downes M., Evans R.M., Montminy M., Shaw R.J. Class IIa histone deacetylases are hormone-activated regulators of FOXO and mammalian glucose homeostasis. Cell. 2011;145:607–621. doi: 10.1016/j.cell.2011.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan H.D., Sutherland H.G., Martin D.I., Whitelaw E. Epigenetic inheritance at the agouti locus in the mouse. Nat. Genet. 1999;23:314–318. doi: 10.1038/15490. [DOI] [PubMed] [Google Scholar]
- Nakatochi M., Ichihara S., Yamamoto K., Naruse K., Yokota S., Asano H., Matsubara T., Yokota M. Epigenome-wide association of myocardial infarction with DNA methylation sites at loci related to cardiovascular disease. Clin. Epigenetics. 2017;9:54. doi: 10.1186/s13148-017-0353-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng S.F., Lin R.C., Laybutt D.R., Barres R., Owens J.A., Morris M.J. Chronic high-fat diet in fathers programs beta-cell dysfunction in female rat offspring. Nature. 2010;467:963–966. doi: 10.1038/nature09491. [DOI] [PubMed] [Google Scholar]
- Nilsson E., Jansson P.A., Perfilyev A., Volkov P., Pedersen M., Svensson M.K., Poulsen P., Ribel-Madsen R., Pedersen N.L., Almgren P. Altered DNA methylation and differential expression of genes influencing metabolism and inflammation in adipose tissue from subjects with type 2 diabetes. Diabetes. 2014;63:2962–2976. doi: 10.2337/db13-1459. [DOI] [PubMed] [Google Scholar]
- Nilsson E., Matte A., Perfilyev A., de Mello V.D., Käkelä P., Pihlajamäki J., Ling C. Epigenetic alterations in human liver from subjects with type 2 diabetes in parallel with reduced folate levels. J. Clin. Endocrinol. Metab. 2015;100:E1491–E1501. doi: 10.1210/jc.2015-3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitert M.D., Dayeh T., Volkov P., Elgzyri T., Hall E., Nilsson E., Yang B.T., Lang S., Parikh H., Wessman Y. Impact of an exercise intervention on DNA methylation in skeletal muscle from first-degree relatives of patients with type 2 diabetes. Diabetes. 2012;61:3322–3332. doi: 10.2337/db11-1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson A.H., Volkov P., Bacos K., Dayeh T., Hall E., Nilsson E.A., Ladenvall C., Rönn T., Ling C. Genome-wide associations between genetic and epigenetic variation influence mRNA expression and insulin secretion in human pancreatic islets. PLoS Genet. 2014;10:e1004735. doi: 10.1371/journal.pgen.1004735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou K., Yu M., Moss N.G., Wang Y.J., Wang A.W., Nguyen S.C., Jiang C., Feleke E., Kameswaran V., Joyce E.F. Targeted demethylation at the CDKN1C/p57 locus induces human beta cell replication. J. Clin. Invest. 2019;129:209–214. doi: 10.1172/JCI99170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oussalah A., Rischer S., Bensenane M., Conroy G., Filhine-Tresarrieu P., Debard R., Forest-Tramoy D., Josse T., Reinicke D., Garcia M. Plasma mSEPT9: a novel circulating cell-free DNA-based epigenetic biomarker to diagnose hepatocellular carcinoma. EBioMedicine. 2018;30:138–147. doi: 10.1016/j.ebiom.2018.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paneni F., Costantino S., Battista R., Castello L., Capretti G., Chiandotto S., Scavone G., Villano A., Pitocco D., Lanza G. Adverse epigenetic signatures by histone methyltransferase Set7 contribute to vascular dysfunction in patients with type 2 diabetes mellitus. Circ. Cardiovasc. Genet. 2015;8:150–158. doi: 10.1161/CIRCGENETICS.114.000671. [DOI] [PubMed] [Google Scholar]
- Pasquali L., Gaulton K.J., Rodríguez-Seguí S.A., Mularoni L., Miguel-Escalada I., Akerman İ., Tena J.J., Morán I., Gómez-Marín C., van de Bunt M. Pancreatic islet enhancer clusters enriched in type 2 diabetes risk-associated variants. Nat. Genet. 2014;46:136–143. doi: 10.1038/ng.2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedroso J.A.B., Ramos-Lobo A.M., Donato J., Jr. SOCS3 as a future target to treat metabolic disorders. Hormones (Athens) 2018 doi: 10.1007/s42000-018-0078-5. [DOI] [PubMed] [Google Scholar]
- Perez M.F., Lehner B. Intergenerational and transgenerational epigenetic inheritance in animals. Nat. Cell Biol. 2019;21:143–151. doi: 10.1038/s41556-018-0242-9. [DOI] [PubMed] [Google Scholar]
- Perfilyev A., Dahlman I., Gillberg L., Rosqvist F., Iggman D., Volkov P., Nilsson E., Risérus U., Ling C. Impact of polyunsaturated and saturated fat overfeeding on the DNA-methylation pattern in human adipose tissue: a randomized controlled trial. Am. J. Clin. Nutr. 2017;105:991–1000. doi: 10.3945/ajcn.116.143164. [DOI] [PubMed] [Google Scholar]
- Pettitt D.J., Aleck K.A., Baird H.R., Carraher M.J., Bennett P.H., Knowler W.C. Congenital susceptibility to NIDDM. Role of intrauterine environment. Diabetes. 1988;37:622–628. doi: 10.2337/diab.37.5.622. [DOI] [PubMed] [Google Scholar]
- Pettitt D.J., Baird H.R., Aleck K.A., Bennett P.H., Knowler W.C. Excessive obesity in offspring of Pima Indian women with diabetes during pregnancy. N. Engl. J. Med. 1983;308:242–245. doi: 10.1056/NEJM198302033080502. [DOI] [PubMed] [Google Scholar]
- Rakyan V.K., Chong S., Champ M.E., Cuthbert P.C., Morgan H.D., Luu K.V., Whitelaw E. Transgenerational inheritance of epigenetic states at the murine Axin(Fu) allele occurs after maternal and paternal transmission. Proc. Natl. Acad. Sci. USA. 2003;100:2538–2543. doi: 10.1073/pnas.0436776100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribel-Madsen R., Fraga M.F., Jacobsen S., Bork-Jensen J., Lara E., Calvanese V., Fernandez A.F., Friedrichsen M., Vind B.F., Højlund K. Genome-wide analysis of DNA methylation differences in muscle and fat from monozygotic twins discordant for type 2 diabetes. PLoS One. 2012;7:e51302. doi: 10.1371/journal.pone.0051302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roseboom T., de Rooij S., Painter R. The Dutch famine and its long-term consequences for adult health. Early Hum. Dev. 2006;82:485–491. doi: 10.1016/j.earlhumdev.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Rosqvist F., Iggman D., Kullberg J., Cedernaes J., Johansson H.E., Larsson A., Johansson L., Ahlström H., Arner P., Dahlman I. Overfeeding polyunsaturated and saturated fat causes distinct effects on liver and visceral fat accumulation in humans. Diabetes. 2014;63:2356–2368. doi: 10.2337/db13-1622. [DOI] [PubMed] [Google Scholar]
- Rzehak P., Covic M., Saffery R., Reischl E., Wahl S., Grote V., Weber M., Xhonneux A., Langhendries J.P., Ferre N. DNA-methylation and body composition in preschool children: epigenome-wide-analysis in the European childhood obesity project (CHOP)-study. Sci. Rep. 2017;7:14349. doi: 10.1038/s41598-017-13099-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rönn T., Poulsen P., Hansson O., Holmkvist J., Almgren P., Nilsson P., Tuomi T., Isomaa B., Groop L., Vaag A. Age influences DNA methylation and gene expression of COX7A1 in human skeletal muscle. Diabetologia. 2008;51:1159–1168. doi: 10.1007/s00125-008-1018-8. [DOI] [PubMed] [Google Scholar]
- Rönn T., Volkov P., Davegårdh C., Dayeh T., Hall E., Olsson A.H., Nilsson E., Tornberg A., Dekker Nitert M., Eriksson K.F. A six months exercise intervention influences the genome-wide DNA methylation pattern in human adipose tissue. PLoS Genet. 2013;9:e1003572. doi: 10.1371/journal.pgen.1003572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rönn T., Volkov P., Gillberg L., Kokosar M., Perfilyev A., Jacobsen A.L., Jørgensen S.W., Brøns C., Jansson P.A., Eriksson K.F. Impact of age, BMI and HbA1c levels on the genome-wide DNA methylation and mRNA expression patterns in human adipose tissue and identification of epigenetic biomarkers in blood. Hum. Mol. Genet. 2015;24:3792–3813. doi: 10.1093/hmg/ddv124. [DOI] [PubMed] [Google Scholar]
- Sayols-Baixeras S., Subirana I., Fernández-Sanlés A., Sentí M., Lluís-Ganella C., Marrugat J., Elosua R. DNA methylation and obesity traits: an epigenome-wide association study. The REGICOR study. Epigenetics. 2017;12:909–916. doi: 10.1080/15592294.2017.1363951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott R.A., Scott L.J., Mägi R., Marullo L., Gaulton K.J., Kaakinen M., Pervjakova N., Pers T.H., Johnson A.D., Eicher J.D. An expanded genome-wide association study of type 2 diabetes in Europeans. Diabetes. 2017;66:2888–2902. doi: 10.2337/db16-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp G.C., Lawlor D.A., Richmond R.C., Fraser A., Simpkin A., Suderman M., Shihab H.A., Lyttleton O., McArdle W., Ring S.M. Maternal pre-pregnancy BMI and gestational weight gain, offspring DNA methylation and later offspring adiposity: findings from the Avon longitudinal study of parents and children. Int. J. Epidemiol. 2015;44:1288–1304. doi: 10.1093/ije/dyv042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp G.C., Salas L.A., Monnereau C., Allard C., Yousefi P., Everson T.M., Bohlin J., Xu Z., Huang R.C., Reese S.E. Maternal BMI at the start of pregnancy and offspring epigenome-wide DNA methylation: findings from the pregnancy and childhood epigenetics (PACE) consortium. Hum. Mol. Genet. 2017;26:4067–4085. doi: 10.1093/hmg/ddx290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small K.S., Hedman A.K., Grundberg E., Nica A.C., Thorleifsson G., Kong A., Thorsteindottir U., Shin S.Y., Richards H.B., GIANT Consortium Identification of an imprinted master trans regulator at the KLF14 locus related to multiple metabolic phenotypes. Nat. Genet. 2011;43:561–564. doi: 10.1038/ng.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens N.A., Brouwers B., Eroshkin A.M., Yi F., Cornnell H.H., Meyer C., Goodpaster B.H., Pratley R.E., Smith S.R., Sparks L.M. Exercise response variations in skeletal muscle PCr recovery rate and insulin sensitivity relate to muscle epigenomic profiles in individuals with type 2 diabetes. Diabetes Care. 2018;41:2245–2254. doi: 10.2337/dc18-0296. [DOI] [PubMed] [Google Scholar]
- Stitzel M.L., Sethupathy P., Pearson D.S., Chines P.S., Song L., Erdos M.R., Welch R., Parker S.C., Boyle A.P., Scott L.J. Global epigenomic analysis of primary human pancreatic islets provides insights into type 2 diabetes susceptibility loci. Cell Metab. 2010;12:443–455. doi: 10.1016/j.cmet.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurner M., van de Bunt M., Torres J.M., Mahajan A., Nylander V., Bennett A.J., Gaulton K.J., Barrett A., Burrows C., Bell C.G. Integration of human pancreatic islet genomic data refines regulatory mechanisms at type 2 diabetes susceptibility loci. Elife. 2018;7 doi: 10.7554/eLife.31977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobi E.W., Lumey L.H., Talens R.P., Kremer D., Putter H., Stein A.D., Slagboom P.E., Heijmans B.T. DNA methylation differences after exposure to prenatal famine are common and timing- and sex-specific. Hum. Mol. Genet. 2009;18:4046–4053. doi: 10.1093/hmg/ddp353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuomilehto J., Lindström J., Eriksson J.G., Valle T.T., Hämäläinen H., Ilanne-Parikka P., Keinänen-Kiukaanniemi S., Laakso M., Louheranta A., Rastas M. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N. Engl. J. Med. 2001;344:1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- Wahl S., Drong A., Lehne B., Loh M., Scott W.R., Kunze S., Tsai P.C., Ried J.S., Zhang W., Yang Y. Epigenome-wide association study of body mass index, and the adverse outcomes of adiposity. Nature. 2017;541:81–86. doi: 10.1038/nature20784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Pan Y., Zhu H., Hao G., Huang Y., Barnes V., Shi H., Snieder H., Pankow J., North K. An epigenome-wide study of obesity in African American youth and young adults: novel findings, replication in neutrophils, and relationship with gene expression. Clin. Epigenetics. 2018;10:3. doi: 10.1186/s13148-017-0435-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshney A., Scott L.J., Welch R.P., Erdos M.R., Chines P.S., Narisu N., Albanus R.D., Orchard P., Wolford B.N., Kursawe R. Genetic regulatory signatures underlying islet gene expression and type 2 diabetes. Proc. Natl. Acad. Sci. USA. 2017;114:2301–2306. doi: 10.1073/pnas.1621192114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendt A., Esguerra J.L., Eliasson L. Islet microRNAs in health and type-2 diabetes. Curr. Opin. Pharmacol. 2018;43:46–52. doi: 10.1016/j.coph.2018.08.003. [DOI] [PubMed] [Google Scholar]
- Voisin S., Almén M.S., Moschonis G., Chrousos G.P., Manios Y., Schiöth H.B. Dietary fat quality impacts genome-wide DNA methylation patterns in a cross-sectional study of Greek preadolescents. Eur. J. Hum. Genet. 2015;23:654–662. doi: 10.1038/ejhg.2014.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voisin S., Almén M.S., Zheleznyakova G.Y., Lundberg L., Zarei S., Castillo S., Eriksson F.E., Nilsson E.K., Blüher M., Böttcher Y. Many obesity-associated SNPs strongly associate with DNA methylation changes at proximal promoters and enhancers. Genome Med. 2015;7:103. doi: 10.1186/s13073-015-0225-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkmar M., Dedeurwaerder S., Cunha D.A., Ndlovu M.N., Defrance M., Deplus R., Calonne E., Volkmar U., Igoillo-Esteve M., Naamane N. DNA methylation profiling identifies epigenetic dysregulation in pancreatic islets from type 2 diabetic patients. EMBO J. 2012;31:1405–1426. doi: 10.1038/emboj.2011.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkov P., Bacos K., Ofori J.K., Esguerra J.L., Eliasson L., Rönn T., Ling C. Whole-genome bisulfite sequencing of human pancreatic islets reveals novel differentially methylated regions in type 2 diabetes pathogenesis. Diabetes. 2017;66:1074–1085. doi: 10.2337/db16-0996. [DOI] [PubMed] [Google Scholar]
- Volkov P., Olsson A.H., Gillberg L., Jørgensen S.W., Brøns C., Eriksson K.F., Groop L., Jansson P.A., Nilsson E., Rönn T. A genome-wide mQTL analysis in human adipose tissue identifies genetic variants associated with DNA methylation, gene expression and metabolic traits. PLoS One. 2016;11:e0157776. doi: 10.1371/journal.pone.0157776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K., Zhang X., Wang Z., Hu Y., Sinha R. Epigenome-wide association analysis revealed that SOCS3 methylation influences the effect of cumulative stress on obesity. Biol. Psychol. 2018;131:63–71. doi: 10.1016/j.biopsycho.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Su S., Barnes V.A., De Miguel C., Pollock J., Ownby D., Shi H., Zhu H., Snieder H., Wang X. A genome-wide methylation study on obesity: differential variability and differential methylation. Epigenetics. 2013;8:522–533. doi: 10.4161/epi.24506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B.T., Dayeh T.A., Kirkpatrick C.L., Taneera J., Kumar R., Groop L., Wollheim C.B., Nitert M.D., Ling C. Insulin promoter DNA methylation correlates negatively with insulin gene expression and positively with HbA(1c) levels in human pancreatic islets. Diabetologia. 2011;54:360–367. doi: 10.1007/s00125-010-1967-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B.T., Dayeh T.A., Volkov P.A., Kirkpatrick C.L., Malmgren S., Jing X., Renström E., Wollheim C.B., Nitert M.D., Ling C. Increased DNA methylation and decreased expression of PDX-1 in pancreatic islets from patients with type 2 diabetes. Mol. Endocrinol. 2012;26:1203–1212. doi: 10.1210/me.2012-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan W., Xia Y., Bell C.G., Yet I., Ferreira T., Ward K.J., Gao F., Loomis A.K., Hyde C.L., Wu H. An integrated epigenomic analysis for type 2 diabetes susceptibility loci in monozygotic twins. Nat. Commun. 2014;5:5719. doi: 10.1038/ncomms6719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T., Cooper S., Brockdorff N. The interplay of histone modifications - writers that read. EMBO Rep. 2015;16:1467–1481. doi: 10.15252/embr.201540945. [DOI] [PMC free article] [PubMed] [Google Scholar]