Abstract
A potential mechanism of cytotoxicity attributed to Alzheimer’s Aβ peptides postulates that their aggregation disrupts membrane structure causing uncontrollable permeation of Ca2+ ions. To gain molecular insights into these processes, we have performed all-atom explicit solvent replica exchange with solute tempering molecular dynamics simulations probing aggregation of the naturally occurring Aβ fragment Aβ25-35 within the DMPC lipid bilayer. To compare the impact produced on the bilayer by Aβ25-35 oligomers and monomers, we used as a control our previous simulations, which explored binding of Aβ25-35 monomers to the same bilayer. We found that compared to monomeric species aggregation results in much deeper insertion of Aβ25-35 peptides into the bilayer hydrophobic core causing more pronounced disruption in its structure. Aβ25-35 peptides aggregate by incorporating monomer-like structures with stable C-terminal helix. As a result the Aβ25-35 dimer features unusual helix head-to-tail topology supported by a parallel off-registry interface. Such topology affords further growth of an aggregate by recruiting additional peptides. Free energy landscape reveals that inserted dimers represent the dominant equilibrium state augmented by two metastable states associated with surface bound dimers and inserted monomers. Using the free energy landscape we propose the pathway of Aβ25-35 binding, aggregation, and insertion into the lipid bilayer.
Subject terms: Membrane biophysics, Computational biology and bioinformatics
Introduction
Amyloid-β (A) peptides are the natural products of cellular proteolysis resulting from cleavage of transmembrane amyloid precursor proteins (APP) by β and γ secretases. Decades of research have established that these peptides play a central role in the onset and development of Alzheimer’s disease (AD), a neurodegenerative condition leading to memory loss and cognitive disfunction. An important physical feature of AD is an appearance of extracellular neuritic plaques or amyloid fibrils composed of aggregated Aβ peptides. These remarkably ordered aggregates rich in β-structure1,2 predominantly involve two, 40- and 42-residue, peptide species, Aβ1-40 and Aβ1-42. Being derived from transmembrane and extracellular regions of APP, these amyloidogenic peptides contain a highly polar N-terminus, a mixed polar/apolar middle section, and an exclusively hydrophobic C-terminus. Importantly, they demonstrate high in vitro and in vivo cytotoxicity against various cells including neurons and are capable of degrading intercellular synapses3–6. Traditionally, Aβ fibrils were considered the primary AD neurotoxic species, but recent studies probing correlations with dementia symptoms have pointed to soluble oligomers as the most potent cytotoxic Aβ forms7,8. Aggregation of Aβ peptides represents the core of the amyloid cascade hypothesis9, which postulates that Aβ aggregated species trigger a variety of biochemical pathways eventually leading to neuronal death. Among those are direct interactions of Aβ peptides with neuronal membranes or their binding to microglial and neuronal cellular receptors causing oxidative stress, inflammatory response, and altered calcium homeostasis.
A spectrum of in vivo Aβ species is remarkably diverse and includes various N- and/or C-termini truncated peptides10. One of them is an 11-mer peptide fragment Aβ25-35 shown in Fig. 1a, which is composed of APP regions embedded in the membrane (29–35) and exposed to the membrane-water interface (25–28)11. In vivo Aβ25-35 is localized in neurons of the subiculum and entorhinal cortex12. This peptide represents an apparent functional domain of the full-length Aβ responsible for its amyloidogenic and cytotoxic properties3,13. Consequently, Aβ25-35 is the shortest Aβ fragment retaining some of the amyloidogenic and cytotoxic properties of the full-length peptide11,14–16. In particular, Aβ25-35 demonstrates remarkable speed of aggregation and almost immediate, without aging, cytotoxicity in vitro3,13. For example, fresh Aβ25-35 peptides form sediments within an hour, which is even faster than Aβ1-423, whereas mature Aβ25-35 fibrils have been reported to appear within 12 hours after incubation17. Interestingly, rapid development of cytotoxicity may implicate the Aβ25-35 monomers or small oligomers. Indeed, monomeric Aβ25-35 is known to produce apoptotic signals leading to cellular death15. Behavioral studies have shown that Aβ25-35 peptides cause amnesia and memory deficits in mice models18,19. Because of its small size and properties reminiscent of the full-length peptides, Aβ25-35 has been a target of numerous experimental and in silico investigations15,20–23.
Figure 1.
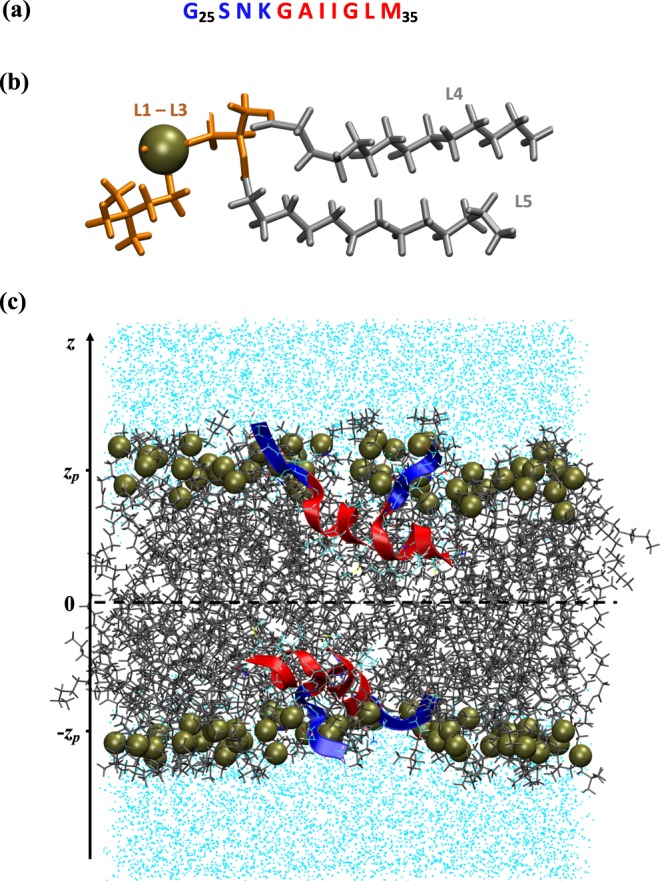
Simulation model for investigating Aβ25-35 aggregation in the DMPC lipid bilayer. (a) The sequence of Aβ25-35 peptide. N-terminal R3 and C-terminal R4 regions are shown in blue and red, respectively. (b) DMPC lipid consists of five structural groups (see Methods). The polar lipid headgroups L1–L3 are shown in orange, and the fatty acid tails L4 and L5 constituting the hydrophobic core are shown in grey. Phosphorus atom is represented as a tan sphere. (c) A snapshot of the DMPC bilayer with two inserted Aβ25-35 dimers in an ID state (see Results). Lipids and water are in grey and cyan, respectively, and phosphorus atoms are displayed as tan spheres. R3 (blue) and R4 (red) regions in Aβ25-35 peptides are distinguished. The centers of mass of phosphorus atoms in each leaflet occur, on average, at ±ZP (zP = 17.37 Å).
Interactions of Aβ peptides with cellular membranes may represent one of the primary mechanisms of their cytotoxicity24–29. Consequently, binding of Aβ25-35 peptides to lipid bilayers has been extensively examined experimentally. In particular, calorimetric studies revealed strong affinity of Aβ25-35 peptides to anionic POPC/POPG lipid bilayers30, whereas neutron diffraction data indicated that Aβ25-35 penetrates and structurally perturbs POPC/POPS bilayers31. Notably, the extent of bilayer structural distortion has reportedly exceeded even that observed for the full-length Aβ1-40 or Aβ1-42 peptides. Aβ25-35 binds not only to anionic, but also to zwitterionic lipid bilayers. Electron paramagnetic resonance studies have found that this peptide becomes inserted into the DLPC bilayer, positioning at the boundary between the hydrophobic core and hydrophilic headgroup region32. Similar conclusions have been reached in an earlier X-ray diffraction investigation33.
Although experimental techniques are indispensable for studying the interactions of Aβ25-35 with cellular membranes, they cannot resolve underlying atomistic details. Nonetheless, this information is critical for understanding the mechanisms of Aβ aggregation within the lipid environment and disruption of bilayer structure, which together are likely to lead to Aβ cytotoxicity. To gain insights into associated molecular mechanisms, we have used replica exchange with solute tempering (REST) simulations to investigate interactions of Aβ25-35 monomer with a DMPC lipid bilayer34. We discovered that the monomer binds to the membrane adopting two coexisting states: a stable state bound to the surface polar headgroups and a less stable state embedded in the hydrophobic core. A moderate free energy barrier separates both states, and it is therefore likely that the Aβ25-35 monomer frequently transitions between surface-bound and inserted conformations. Although in the inserted state the peptide induces considerable bilayer disruption, the overall effect on the DMPC bilayer integrity is minimal due to the dominance of surface bound state. In this paper, we extend our previous all-atom REST investigations to probe the aggregation of Aβ25-35 peptides within the DMPC bilayer (Fig. 1). We show that Aβ25-35 peptides readily aggregate into dimers and in contrast to monomeric species penetrate deep into the DMPC bilayer causing extensive damage to its structure. By computing the free energy landscape we reconstruct the pathway of Aβ25-35 binding and aggregation.
Results
Aggregation does not substantially reorganize Aβ25-35 structure
We first investigated the changes in the secondary structure of Aβ25-35 peptides caused by aggregation. (It is important to make a note about terminology. Because dimeric states occur with the probability of 0.64 ± 0.07 (see Models and Methods), for brevity we collectively refer to the peptides sampled in our simulations as dimers unless we specifically distinguish dimeric and monomeric subpopulations). Figure 2 presents the helical propensities 〈H(i)〉 for amino acids i in Aβ25-35 dimers binding to the DMPC bilayer. As a control, we use 〈H(i)〉 computed in our previous REST simulations probing binding of Aβ25-35 monomers to the same bilayer34. Aggregation promotes helical structure increasing the number of amino acids adopting stable helix (〈H(i)〉 > 0.5) from three in Aβ25-35 monomers to five in the dimers. Overall, Aβ25-35 helix content 〈H〉 increases from 0.31 ± 0.03 to 0.39 ± 0.03, although a more pronounced rise is seen in the C-terminal R4 region (from 0.39 ± 0.02 to 0.54 ± 0.02). The analysis of β-turn and random coil structure is given in Supplementary Information. Thus, aggregation moderately increases helical propensity, particularly in the C-terminus, with concurrent reduction in β-turn conformations, whereas random coil propensity remains largely unaffected. Aggregation causes Aβ25-35 extension manifested in the increase of the end-to-end distance r1N from 14.4 ± 0.4 to 15.4 ± 0.4 Å. Computation of intrapeptide contacts in Supplementary Information shows that aggregation stabilizes few local interactions, particularly Gly29-Ile32 and Gly29-Gly33, reflecting the enhancement of helical structure. Nonetheless, taken together, the analysis of secondary and tertiary structure does not reveal significant structural reorganization in Aβ25-35 peptides bound to the DMPC bilayer caused by aggregation.
Figure 2.
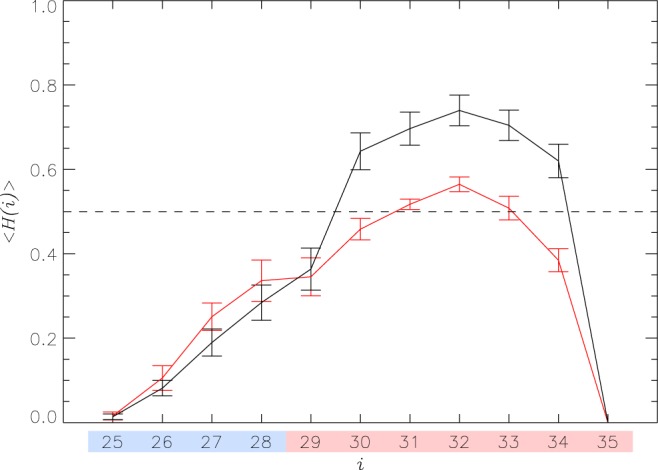
Helical propensities 〈H(i)〉 for Aβ25-35 amino acids i. Data for Aβ25-35 dimers and monomers34 are shown in black and red, respectively. Vertical bars show sampling errors. Regions R3 and R4 are colored according to Fig. 1a.
Aggregation promotes deeper penetration of Aβ25-35 into the DMPC bilayer
Enhanced cytotoxicity of Aβ oligomers can be related to their deeper insertion into lipid bilayers compared to Aβ monomers. To investigate this possibility, we have computed the probabilities P(z; i) for amino acids i to occur at a distance z from the bilayer midplane. Their distribution shown in Fig. 3a suggests that most amino acids in Aβ25-35 dimer are inserted in the DMPC bilayer. To substantiate this observation, we computed the average locations of amino acids i along the bilayer normal, 〈z(i)〉, and compared them between Aβ25-35 dimers and monomers34. With the exception of first three N-terminal amino acids all others in the dimer are inserted into the bilayer, because they occur, on average, below the position of the center of mass of phosphorous atoms, i.e., 〈z(i)〉 < zP. In contrast, all amino acids in Aβ25-35 monomers reside within the bilayer headgroup region (zp < z < zP + 6.5 Å), i.e., they are classified as surface bound.
Figure 3.
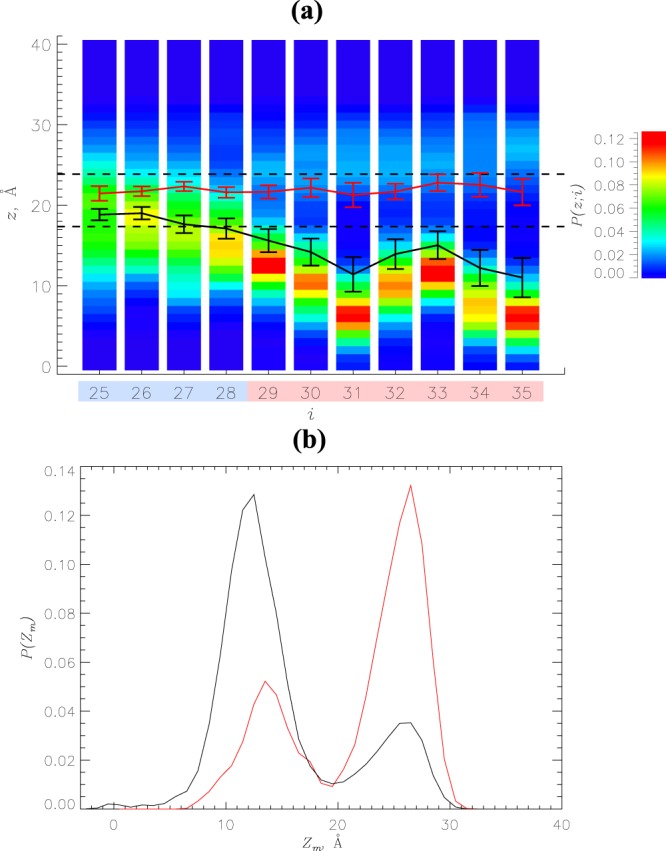
Binding of Aβ25-35 dimers to the DMPC bilayer. (a) Probabilities P(z; i) for amino acids i in Aβ25-35 dimers to occur at the distance z from the bilayer midplane. The scale on the right color codes P(z; i). Black and red lines represent the average positions of amino acids in Aβ25-35 dimers and monomers34, 〈z(i)〉, with sampling errors. The dashed line at zP separates the bilayer hydrophobic core from its surface region, whereas the dashed line at zp + 6.5 Å distinguishes the bilayer surface region and solvent. Regions R3 and R4 are colored according to Fig. 1a. (b) Probability distributions P(Zm) of the positions of the center of mass of Aβ25-35 peptides Zm along the bilayer normal. Peptide inserted and surface bound states are separated by the minimum midpoint in bimodal P(Zm) distributions. Data in black and red correspond to Aβ25-35 dimers and monomers.
Amino acid positions in the bilayer analyzed above suggest different binding propensities of Aβ25-35 dimers and monomers. To check this assertion, we computed the probability distributions P(Zm) of the position of the center of mass of Aβ25-35 peptide Zm along the bilayer normal. The distributions P(Zm) computed for the dimers and monomers presented in Fig. 3b are bimodal. The peptides from Aβ25-35 dimer are inserted in the bilayer with the probability of 0.79 ± 0.08, while the probability of binding to the bilayer surface is only 0.21 ± 0.08. For Aβ25-35 monomers the respective probabilities are almost opposite being 0.31 ± 0.07 and 0.69 ± 0.0734. Thus, aggregation dramatically shifts the distribution of Aβ25-35 states from predominantly surface bound for the monomers to overwhelmingly inserted for the dimers. The dependence of Aβ25-35 helical propensity on insertion depth is analyzed in Supplementary Information.
To map residue interactions with lipids, we computed the contact map 〈Cl(i, k)〉, which reports the formation of contacts between amino acids i and lipid groups k. Figure S4a shows that in the Aβ25-35 dimers the N-terminal R3 amino acids bind exclusively to the DMPC headgroups L1–L3. In contrast, the C-terminal R4 amino acids Ile31, Ile32, Leu34, and Met35 mostly interact with the fatty acid tails L4 and L5, whereas Gly29 and Gly33 exclusively bind to the headgroups. This pattern of amino acid-lipid interactions reflects the formation of C-terminus helix, in which the Gly-rich face of the helix is positioned toward the DMPC headgroups and water as seen in Fig. 3a. For comparison, the contact map 〈Cl(i, k)〉m for Aβ25-35 monomers34 shown in Fig. S4b demonstrates that binding interactions are largely restricted to the peptide N-terminus and DMPC headgroups. Analysis of the difference in the number of contacts with lipids per amino acid 〈ΔCl(i)〉 shown in Fig. S4c leads to two observations. First, aggregation strengthens all amino acid - lipids interactions resulting in the net increase of the number of peptide-bilayer contacts from 12.3 ± 1.1 to 18.9 ± 1.6. Second, the amino acids displaying the largest gains in interactions with the DMPC bilayer are Lys28 (), Ala30 (0.9 ± 0.3), Val34 (0.9 ± 0.2), Asn27 (0.7 ± 0.2), and Met35 (0.6 ± 0.2). As a result among all amino acids cationic Lys28 binds most tightly to the DMPC bilayer, i.e., it forms the largest number of contacts with lipids (). In summary, aggregation promotes (i) hydrophobic contacts of Aβ25-35 C-terminus with fatty acid tails and (ii) electrostatic interactions between cationic Lys28 and the anionic lipid phosphate group.
Aggregation facilitates disruption of DMPC bilayer
If aggregation promotes deeper penetration of Aβ25-35 peptides into the DMPC bilayer, it is then expected to enhance bilayer disruption. To examine changes in the bilayer structure occurring in response to Aβ25-35 dimer binding, we plot in Fig. 4 the number density nl(r, z) of DMPC heavy atoms as a function of the distance r to the peptide center of mass and the distance z to the bilayer midplane. The cross-sectional profile nl(r, z) shows a deep lipid density void created by aggregated Aβ25-35 species, which is muted for Aβ25-35 monomers34. To quantify aggregation impact, we compared the bilayer thickness D in the distant and proximal regions (see Models and Methods for definitions). Due to binding of Aβ25-35 monomer the DMPC bilayer thins by ΔD = 4.0 ± 1.5 Å34. In contrast, binding of Aβ25-35 dimers increases ΔD more than four-fold to 17.0 ± 0.9 Å. To illustrate the drop in lipid density, we compared the DMPC surface number densities ns in the distant and proximal regions. Monomer binding decreases ns by one-third, from 0.015 ± 0.000 Å−2 to 0.010 ± 0.001 Å−2 34. In contrast, the corresponding decrease caused by Aβ25-35 dimer is three-fold, from 0.015 ± 0.000 Å−2 to 0.005 ± 0.001 Å−2. Similar conclusion follows from the analysis of volume number density of lipid heavy atoms nl, which, in response to Aβ25-35 monomer binding, decreases about 25% from 0.034 ± 0.000 in the distant region to 0.025 ± 0.001 Å−3 in the center of binding footprint. For comparison, corresponding changes caused by Aβ25-35 dimer are almost three-fold, from 0.034 ± 0.000 to 0.013 ± 0.002 Å−3. We show in Supplementary Information that Aβ25-35 dimer has a stronger disordering impact on DMPC fatty acid tails than monomeric peptides. Thus, taken together Aβ25-35 dimers cause a strikingly stronger disruption in the bilayer structure than the monomers.
Figure 4.
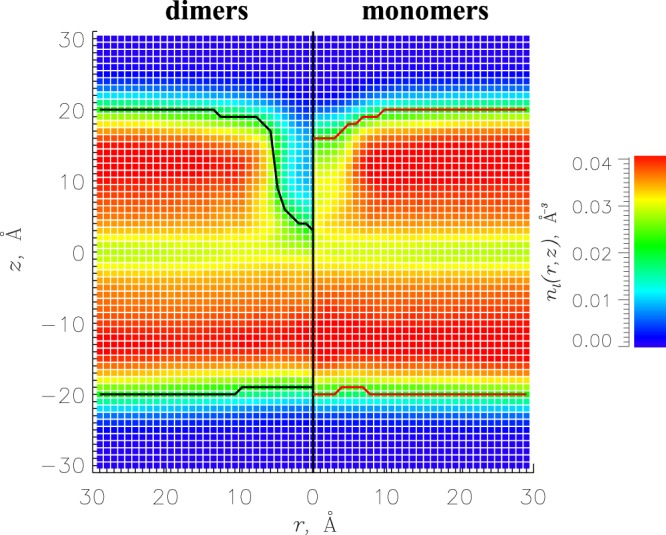
The number density of DMPC heavy atoms nl(r, z) as a function of the distance r to the peptide center of mass and the distance z to the bilayer midplane. The density cross-sections for Aβ25-35 dimers and monomers34 are given on the left and right sections of the panel. Continuous black and red lines mark the bilayer boundaries zb(r). The figure shows that Aβ25-35 dimer induces a deep lipid density void as opposed to Aβ25-35 monomers.
Free energy landscape of Aβ25-35 aggregation
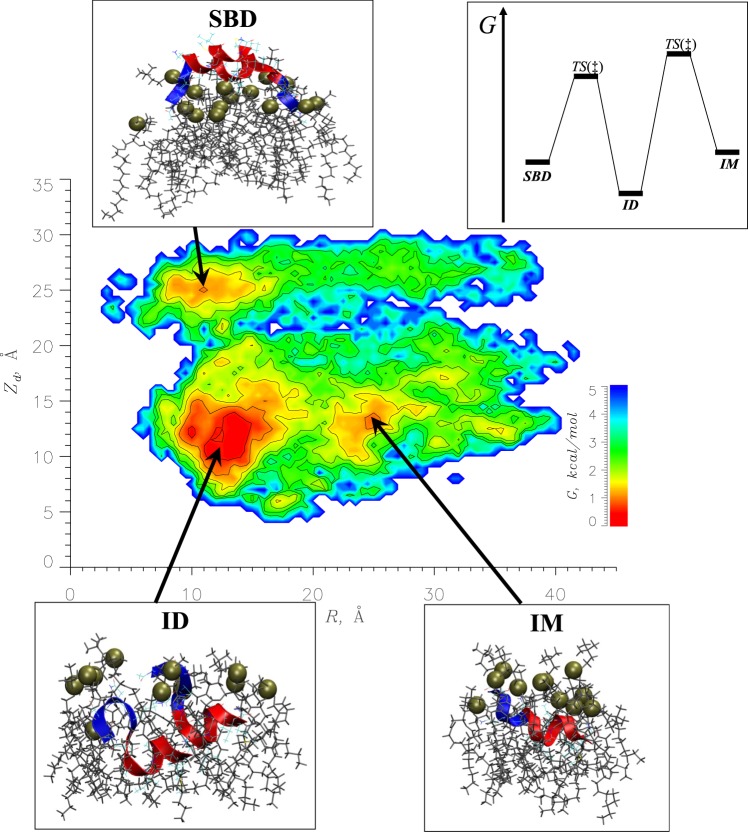
A mechanism of Aβ25-35 aggregation and binding to the DMPC bilayer can be gleaned from the free energy landscape. To this end, we computed the free energy of Aβ25-35 peptides using the probability to observe Aβ25-35 peptides with their centers of mass separated by the distance R and the center of mass of both peptides located at the distance Zd from the bilayer midplane. The resulting free energy landscape in Fig. 5 reveals three distinct basins or states listed in Table 1. The lowest free energy state ID is associated with Aβ25-35 dimers inserted into the bilayer (Zd ~ 12 Å and R ~ 12 Å). The probability for Aβ25-35 peptides to participate in ID is 0.55 ± 0.7. The second state SBD separated from ID by the free energy gap ΔG = 0.8 kcal/mol represents Aβ25-35 dimers bound to the bilayer surface (Zd ~ 25 Å and R ~ 11 Å). This state has the probability of 0.12 ± 0.05. Finally, the third state IM separated from ID by the free energy gap ΔG = 0.9 kcal/mol is populated by the dissociated Aβ25-35 peptides inserted in the bilayer (Zd ~ 13 Å and R ~ 25 Å). The probability of IM is 0.11 ± 0.05. The free energy barriers separating the three states are given in Table 1. Their values indicate that the inserted dimers ID are surrounded by the highest free energy barriers (Δ/mol), whereas the inserted monomers IM and surface bound dimers SBD reside in more shallow free energy basins (Δ/mol). Notably, there is no direct low free energy path in Fig. 5 between IM and SBD states, which may interconvert only by passing through ID serving as intermediate. To provide structural description of states we analyzed Aβ25-35 structures used in computing the free energies of states (Table 1).
Figure 5.
The free energy of Aβ25-35 dimers G(Zd, R) as a function of the distance R between the peptides’ centers of mass and the distance Zd between the dimer center of mass and the bilayer midplane. The contours are drawn with the 0.5 kcal/mol increment for the free energies G ≤ 5 kcal/mol. Three low free energy states, ID, SBD, and IM, are identified and the representative structures from their most populated conformational clusters are shown. The colors of R3 and R4 regions follow Fig. 1a. Lipids with phosphorous atoms represented by tan spheres, which surround Aβ25-35 peptides, are shown. The diagram summarizing the low free energy and transition states included in Table 1 is shown in the upper right corner.
Table 1.
Aβ25-35 low free energy states.
| State k | P(k)a | G(k)b, kcal/mol | k → lc | G†, kcal/mol |
|---|---|---|---|---|
| ID | 0.55 ± 0.07 | 0.0 ± 0.2 | ID → IM | 4.2 |
| ID → SBD | 3.7 | |||
| SBD | 0.12 ± 0.05 | 0.8 ± 0.5 | SBD → ID | 2.9 |
| IM | 0.11 ± 0.05 | 0.9 ± 0.3 | IM → ID | 3.3 |
aFraction of Aβ25-35 peptides included in a state k. To compute P(k) we considered all structures in a basin with the free energies , where Gk,min is the minimum free energy in k and is the free energy of transition state along the minimum free energy path from k.
bTo compute the free energy of k, G(k), we integrated G(Zd, R) within the interval [Gk,min, Gk,min + 0.5 kcal/mol].
cTransition from state k to state l.
Inserted dimers
Clustering of ID dimers with the cut-off R0 = 4.4 Å (see Models and Methods) revealed one dominant cluster ID1 shown in Fig. 5, which includes 86% of ID dimers. The distinctive feature of ID1 is the formation of parallel out-of-registry aggregation interface composed of the sequence regions Gly33-Met35 and Gly29-Ile31, which are linked by three stable (>0.40) hydrophobic interpeptide amino acid contacts - Gly29-Gly33 (), Ala30-Leu34 (0.69), Ile31-Met35 (0.50). In addition, ID1 aggregation interface involves, on an average, 1.7 backbone hydrogen bonds. Importantly, the helical conformations in ID1 peptides predominantly occur in the C-terminal R4 region (), while being sparse in the N-terminus (). Due to such distribution of helical structure, Aβ25-35 peptides in a dimer are arranged in a helix head-to-tail tandem as shown in the inset to Fig. 6. Further analysis of ID1 shows that the “idle” aggregation interfaces in the left or right peptides are available to add new peptides. Indeed, the probability that all the three interface amino acids have dangling hydrogen bond donors (or acceptors) is 0.64. Rarely, ID dimers populate the second cluster ID2, which includes 9% of structures. The aggregation interface of this cluster implicates a T-like arrangement of peptides, in which interactions are formed between R3 region in one peptide and both, R3 and R4, regions in the other. Interestingly, the structures of ID Aβ25-35 peptides are similar to the inserted monomers I sampled in our previous REST simulations, which studied binding of isolated Aβ25-35 monomers to the DMPC bilayer34 (see Supplementary Information).
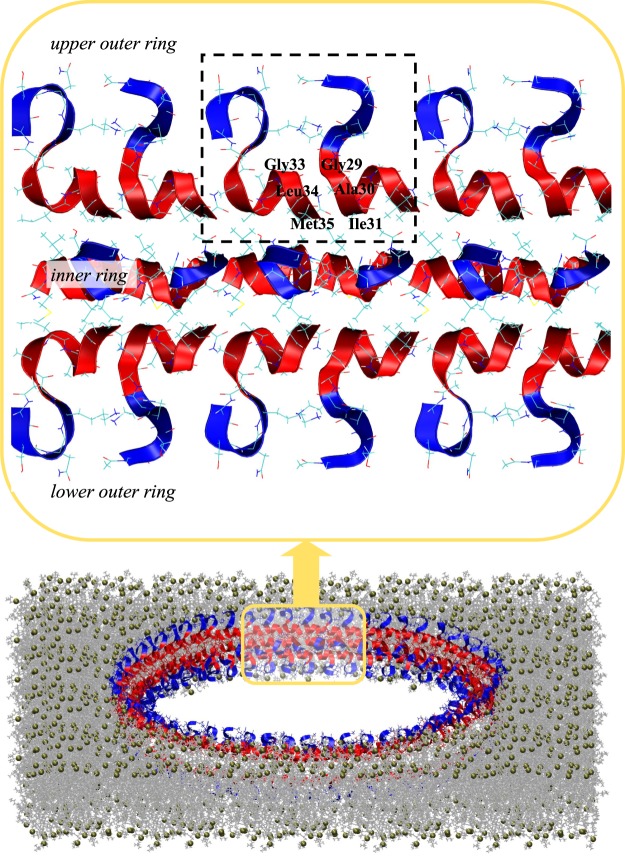
Figure 6.
A hypothetical structure of Aβ25-35 annular oligomer or pore in the lipid bilayer formed by three concentric rings of peptides. There are two outer rings placed in the upper and lower bilayer leaflets interacting via their hydrophobic C-termini (in red) with the bilayer core. The peptides N-termini (in blue) occur next to the bilayer surface interacting with the lipid headgroups. The third inner ring is shifted closer to the pore center and its polar N-termini are directed toward the pore center creating its polar lining. The inset magnifies the six peptides from each of the three rings. As building blocks the rings utilize ID Aβ25-35 dimers enclosed by dashed lines. The amino acids participating in the dimer parallel aggregation interface are identified. The bilayer representation follows that used in Fig. 1c. The oligomer has a diameter of 20 nm as measured by AFM36.
Surface bound dimers
Aβ25-35 dimers SBD bound to the bilayer surface and clustered with the cut-off R0 = 5.1 Å populate two distinct clusters, SBD1 and SBD2, representing 57 and 31% of SBD structures. The distinct feature of the most populated cluster SBD1 shown in Fig. 5, which sets it apart from ID dimers, is the aggregation interface, in which two helical peptides are docked side-by-side in antiparallel arrangement. This interface is supported by four stable () hydrophobic side chain contacts - Ile31-Leu34 (), Ile31-Met35 (0.65), Ile31-Ile31 (0.45), and Ile32-Leu34 (0.43).
Inserted monomers
Clustering inserted monomers IM with the cut-off R0 = 2.5 Å produced two populated clusters, IM1 and IM2 (Fig. 5), which encompass 67 and 21% of structures. The common feature of IM clusters is the formation of stable helical structure in R4, but they differ with respect to the occurrence of stable interactions between R3 and R4 regions (specifically, the contact Asn27-Ala30 is present in IM1 and absent in IM2). Importantly, the characteristics of IM are similar to those of inserted monomers I sampled in our previous REST simulations34 (See Supplementary Information).
Discussion
Although specific causes of cytotoxicity of Aβ aggregated species remain unknown, several potential mechanisms have been proposed, including enhanced production of oxygen reactive species, activation of toll-like receptors leading to local inflammation, and increased permeation of Ca2+ ions through neuronal membranes35. The latter mechanism assumes that aggregation of Aβ peptides results in formation of structured pores in the bilayer, which were characterized experimentally27,36–38, and/or induces sufficient destabilization in interlipid interactions causing increased ion permeability through transient pores39. Conversely, this mechanism implies that monomeric Aβ species are less cytotoxic due to their limited impact on the bilayer structure. Because our all-atom explicit solvent simulations compared the binding of Aβ25-35 monomers34 and dimers to the DMPC bilayer, they are well positioned to glean molecular insights into putative mechanisms of cytotoxicity.
First, we have demonstrated that aggregation promotes deeper insertion of Aβ25-35 peptides into the zwitterionic DMPC bilayer. Indeed, all except three N-terminal amino acids in the Aβ25-35 dimer are localized in the bilayer hydrophobic core, whereas all amino acids in the Aβ25-35 monomer are bound to the DMPC bilayer surface. The probability distribution P(Zm) of the positions of Aβ25-35 peptide center of mass along the bilayer normal is bimodal due to the coexistence of surface-bound and inserted states. Strikingly, aggregation shifts their distribution away from being predominantly surface bound to overwhelmingly inserted (almost 80%). These results are in good agreement with the available experimental data, which implicate Aβ25-35 binding to uncharged liposomes33, model zwitterionic POPC membranes40, and zwitterionic DLPC multilamellar vesicles32. Furthermore, Dante et al.40 have found that upon binding to the POPC bilayer Aβ25-35 at 3 mol% concentration resides in two states as detected by the location of deuterated D-Leu34. It is notable that in 86% of Aβ25-35 states this residue is positioned 14 Å away from the bilayer midplane, which is consistent with the distance of 13 Å predicted by our simulations (Fig. 3a). In remaining 14% of states D-Leu34 is located at the distance of 27 Å from the bilayer midplane indicating that it is likely to be surface bound or unbound. A coexistence of inserted and surface bound Aβ25-35 states has been observed for the weakly anionic 97:3 DMPC/DMPS bilayer and 3 mol% of Aβ25-3541. Those findings are qualitatively consistent with our results, which were obtained at 4 mol% of peptide34. As a result of deeper penetration into the bilayer all amino acids form stronger interactions with lipids. However, it is the hydrophobic C-terminus amino acids Ala30 and Leu34 and cationic Lys28 that demonstrate the largest gain in binding interactions. Therefore, in agreement with the aggregation mechanisms proposed by Bokvist et al., a combination of hydrophobic effect and electrostatic interactions drives an increase in binding affinity of Aβ25-35 oligomers26.
Second, our findings indicate that Aβ25-35 aggregation leads to stronger disruption in the bilayer structure. This conclusion follows from a pronounced lipid density void, which emerges in the DMPC bilayer when the inserted Aβ dimer displaces lipids. Indeed, aggregation increases the extent of bilayer thinning ΔD more than four-fold compared to monomeric species. As a result, Aβ25-35 dimer reduces the thickness of the DMPC bilayer almost 40%, from 40 Å42 to ≈24 Å. Simultaneously, a three-fold drop in lipid surface number density is observed within the Aβ25-35 dimer binding footprint, which is far more dramatic than that caused by Aβ25-35 monomers34. We point out that the thinning observed in our simulations is not related to hydrophobic mismatch occurring between the thickness of hydrophobic lipid core and the length of protein hydrophobic transmembrane region. Instead, it is created by Aβ25-35 peptides displacing lipids from the volume of bilayer and effectively plugging the lipid void. We believe this was the reason why X-ray scattering experiments did not detect a decrease in bilayer thickness upon binding of Aβ25-35 at various concentrations43. Our analysis of carbon-deuterium order parameter and lipid tilt angles revealed that disordering in DMPC fatty acid tails increases with aggregation. The same trend in tilt angles has been seen experimentally for POPC/DMPS bilayer as a function of peptide concentration43.
Our third result pertains to the structure of the Aβ25-35 dimer. The free energy landscape in Fig. 5 has revealed that Aβ25-35 dimers inserted into the DMPC bilayer represent the most thermodynamically stable state ID gathering more than half of Aβ25-35 peptides. Structurally, this state is homogeneous as an overwhelming fraction of dimers can be grouped into a single dominant cluster ID1 shown in Fig. 5. The centroid of this cluster exhibits a dimer, which utilizes a parallel out-of-registry aggregation interface composed of the sequence regions Gly33-Met35 in the left peptide and Gly29-Ile31 in the right peptide (the inset to Fig. 6). The interface draws its stability from three stable hydrophobic contacts (Gly29-Gly33, Ala30-Leu34, Ile31-Met35) and backbone hydrogen bonds. Thus, the structure of Aβ25-35 dimer uses an unusual helix head-to-tail tandem of peptides, which differs from Aβ fibril conformations rich in β-structure44 or the structures of C99 homodimers in the lipid bilayers. The NMR-resolved conformations and molecular dynamics simulations of the latter exhibit two helices associated via their sides, which depending on the environment may or may not involve Gly-Gly heptad interactions45–47. However, similar to C99 dimerization, Aβ25-35 dimer incorporates peptides in monomer-like conformations. Our analysis in Supplementary Information (Fig. S6) confirms that the distributions of helical structure and intrapeptide interactions are similar between ID dimers, inserted IM monomers, and Aβ25-35 inserted monomers I sampled in our previous REST simulations34. Thus, Aβ25-35 aggregates by recruiting monomeric peptides without radical reorganization in their structure. This outcome is in line with recent X-ray scattering experiments, which showed Aβ25-35 peptide to retain its monomeric α-helical structures up to the concentration of 3 mol%, which is almost identical to that in our simulations (4 mol%)43. It is likely that the helix head-to-tail topology of Aβ25-35 dimer is relevant for aggregation of antimicrobial peptides48. Similar to Aβ25-35 these short peptides typically adopt an amphipathic helical structure upon binding to anionic bacterial membranes. It is conceivable that their aggregation proceeds via head-to-tail assembly of monomeric units as shown in inset to Fig. 6.
An intriguing feature of the ID dimer is that it readily affords recruitment of new peptides in the same monomer-like conformations by adding them either to the left or right available aggregation interfaces (the inset to Fig. 6). Therefore, the Aβ25-35 dimers identified in our simulations may represent building blocks, which by replicating their structure may grow into larger annular aggregates detected by recent AFM studies36. Since Aβ25-35 dimers efficiently displace lipids, one can expect that such annular structures with a diameter of about 20 nm36 and constructed of dozens of Aβ25-35 dimeric units may form stable pores for ion permeation as shown in Fig. 6. Such hypothetical pore may be constructed of several concentric rings of Aβ25-35 peptides. Note that Aβ25-35 dimers utilize not only helix head-to-tail ID topology, but also helix side-by-side antiparallel aggregation interface in SBD. Taking into account the amphiphilic character of Aβ25-35 dimer, one can envision that in the inner ring the polar N-termini of the peptides are pointed toward the center of the pore, whereas the hydrophobic C-terminal helices are bound to the helices of the peptides from the two outer rings in antiparallel fashion as in SBD. Therefore, the proposed structure of a pore features a polar lining made of N-terminal regions of Aβ25-35, which should stabilize a pore through favorable hydration.
Finally, REST sampling enabled us to construct in Fig. 5 the free energy landscape G(Zd, R) governing Aβ binding and aggregation. It shows that the peptides populate three states or basins. The most thermodynamically stable state ID populated by inserted dimers and discussed above is supplemented by two metastable states - inserted IM monomers and SBD dimers bound to the surface of the DMPC bilayer. Because the states IM and SBD are separated from ID by the free energy gaps of ~1 kcal/mol and the free energy barriers surrounding these states are modest (4 kcal/mol), Aβ25-35 peptides may interconvert between these states while still predominantly sampling ID. Interestingly, Fig. 5 does not implicate an existence of stable Aβ25-35 monomers bound to the DMPC bilayer surface suggesting that such species rapidly aggregate forming a state SBD. Absence of monomeric surface bound species also demonstrates that DMPC lipid bilayer acts as an aggregation catalyst. Because the free energy landscape G(Zd, R) does not feature a low free energy path between IM and SBD, we expect the following scheme to depict Aβ25-35 kinetics:
| 1 |
Eq. (1) suggests the aggregation pathway at low Aβ25-35 concentrations. The peptides bind to the DMPC bilayer as monomers28 and rapidly aggregate into SBD dimers on the bilayer surface. Next, SBD Aβ25-35 dimers penetrate the DMPC bilayer becoming inserted dimers ID. While the peptides overwhelmingly retain ID dimeric form, they may transiently dissociate into inserted monomers IM or move to the bilayer surface to sample SBD dimers. Direct insertion of surface bound monomers into the bilayer is disfavored, because according to scenario (1) these species are redirected toward forming surface bound dimers. It is important to point out that Aβ25-35 aggregation does not involve radical restructuring of monomeric peptides, which are integrated largely intact into dimers.
The aggregation scenario depicted above is broadly consistent with the three-stage aggregation pathway proposed by Cuco et al., which includes adsorption, nucleation, and penetration49. In that hypothetical pathway, nucleation of adsorbed peptides promotes their penetration into the bilayer in agreement with our prediction that aggregation drastically increases the population of inserted Aβ25-35 species in Fig. 3b. The aggregation scenario suggested by our simulations should only be valid at low peptide concentrations, which favor α-helical Aβ25-35 structures43, whereas higher peptide concentrations lead to β-barrel aggregates37,38. The effects of bilayer composition and post-translational peptide modifications on Aβ25-35 aggregation and Ca2+ permeation are discussed in Supplementary Information. In addition, the free energy landscape in Fig. 5 may underestimate the slope toward aggregated surface bound species due to neglect of bilayer curvature and associated surface tension effects43. However, these neglected factors are expected to disfavor the formation of surface bound monomers even further than predicted by our findings.
In summary, our all-atom explicit solvent REST simulations provide the first, to our knowledge, description of Aβ peptide de novo aggregation mediated by a lipid bilayer. The microscopic details concerning the mechanism of Aβ binding, aggregation, and insertion into the bilayer together with the disruption in the bilayer structure are expected to advance our understanding of the molecular causes of Aβ cytotoxicity.
Models and Methods
Simulation system
Our simulation system consisted of four Aβ25-35 peptides, dimyristoyl phosphatidylcholine (DMPC) bilayer, water, and counterions (Fig. 1). The all-atom CHARMM22 force field with CMAP corrections50 and the all-atom CHARMM36 force field51 were used to represent peptides and lipids, respectively. For water we used a modified TIP3P model52. Neutral acetylated and amidated groups were used to cap the peptides. A pair of Aβ25-35 monomers was placed on each side of the bilayer, each leaflet of which was formed by 50 DMPC molecules. The solvent was comprised of 4344 water molecules, and four chloride counterions were added. The total number of atoms was 25,480, and the initial unit cell dimensions were approximately 56 Å × 56 Å × 78 Å. The design of our simulation system affords probing simultaneous binding of two Aβ25-35 dimers and reduces the possibility of the development of bilayer curvature53.
Replica exchange simulations
To sample the conformational ensemble we used isobaric-isothermal replica exchange with solute tempering (REST) molecular dynamics simulations54. Because REST formalism is documented elsewhere54,55, we provide here its brief outline. In all, the temperatures of replicas were distributed geometrically from T0 = 330 K to TR−1 = 430 K. An exchange between replicas r and r + 1 occurs with the probability , where , , H is the enthalpy, and X defines system coordinates. Solvent-solvent and solute-solvent interactions in replica r with the temperature Tr were scaled by the factors Tr/T0 and (Tr/T0)1/2, respectively. This scaling excludes solvent-solvent energy contributions from and reduces the number of replicas without affecting the temperature range or exchange rates. Aβ peptides and ions were treated as solute, whereas lipids and water were considered as solvent. Replica exchanges were attempted every 2 ps succeeding with the probability of 0.28.
To perform REST simulations we used the program NAMD56 with REST implementation57 and in-house scripts for managing replicas and exchanges. Periodic boundary conditions were utilized. Covalent bonds associated with hydrogen atoms were constrained by the SHAKE algorithm. Electrostatic interactions were computed using Ewald summations, and van der Waals interactions were smoothly switched off from 8 to 12 Å. Underdamped Langevin dynamics with a damping coefficient γ = 5 ps−1 was used to control temperature, and the Nose-Hoover Langevin piston method with piston period and decay of 200 and 100 fs, respectively, was used to set pressure at 1 atm. The x and y dimensions of the system were coupled, and the z dimension along the bilayer normal fluctuated independently. An integration timestep of 1 fs was used. To preserve bilayer integrity at high REST temperatures we applied harmonic restraints to the centers of mass of phosphorus atoms in each leaflet as described58. Another set of restraints at z periodic boundaries prevented aggregation of Aβ25-35 dimers34. Impact of these restraints is negligible and has been discussed previously34.
To prepare starting structures for REST simulations we used the conformations of Aβ25-35 peptides, which were inserted or surface bound to the DMPC bilayer34. These initial structures, in which the peptides were temporarily constrained along the bilayer normal, were equilibrated at 440 K for 30 ns. The procedure generated diverse structures, in which peptides form random interpeptide interactions and half of them were surface bound to the bilayer and half were inserted. These starting conformations were used to initialize six REST trajectories, which produced 2.88 μs of sampling (360 ns per replica). By monitoring REST convergence (see Supplementary Information), we excluded 40 ns of sampling per replica in each trajectory as non-equilibrated. Thus, the total equilibrium sampling is reduced to 0.96 μs or 1.92 μs per dimer.
Computation of structural probes
To facilitate analysis we divided Aβ25-35 peptide into the polar N-terminal R3 and the hydrophobic C-terminal R4 regions (Fig. 1a). A DMPC lipid consists of five structural groups as shown in Fig. 1b. These include choline (L1) and phosphate (L2) groups, glycerol backbone (L3) and two fatty acid tails (L4 and L5). Intra- and intermolecular interactions were detected using the positions of centers of mass of amino acid side chains and lipid structural groups. A contact between them occurs if the distance between their centers of mass is less than 6.5 Å. Aβ25-35 dimer is formed if at least one interpeptide contact is established. Aβ25-35 secondary structure was assigned using the program STRIDE59, and α-, 310-, or π helices were combined into a helical state.
To probe Aβ25-35 penetration into the bilayer, we defined the probability P(z; i) for an amino acid i to occur at a distance z from the bilayer midplane. An amino acid is considered inserted if it resides below the average position of the center of mass of phosphorus atoms zP. Similarly, an amino acid is bilayer surface bound if it occurs within the lipid headgroup region (zp < z < zP + 6.5 Å). The number density nl(r, z) of DMPC heavy atoms with respect to the distance r to the Aβ25-35 peptide center of mass and the distance z to the bilayer midplane mapped the impact of peptides on the bilayer structure. Using nl(r, z) we defined the bilayer boundary zb(r) and thickness D as described34. To examine lipid disordering, carbon-deuterium order parameter SCD was computed for carbons 2 through 14 in the sn − 2 fatty acid tails. We also computed the tilt angle γ measured between the bilayer normal and the vector connecting the first and last carbons in sn − 2 fatty acid tail. DMPC lipids were divided into distant occurring at the distance r > 20 Å from the Aβ25-35 peptide center of mass and proximal occurring within the center of binding footprint (, where 〈Rg〉 is Aβ25-35 radius of gyration). All structural probes are reported as thermodynamic averages computed for the wild-type replica at T0 = 330 K and denoted with angular brackets 〈..〉. Slightly elevated temperature facilitated conformational sampling and comparisons with our previous Aβ25-35 monomer simulations34.
Conformational clustering
Clustering of peptide conformations was performed using the method of Daura et al.60 implemented in VMD61. To this end, we first computed the free energy landscape of Aβ25-35 dimers G(Zd, R) as a function of the z-position of the center of mass of two peptides Zd and the distance between their centers of mass R. Clustering was applied separately to the structures populating each of the three G(Zd, R) basins (see Table 1 and Fig. 5). For each pair of Aβ25-35 dimers from ID or SBD aggregated states, we computed RMSD between backbone Cα atoms after dimer alignment and permuting the identities of peptides. In the dissociated basin IM, RMSD values were computed between peptide monomers. Dimer or monomer clusters were defined by the RMSD cutoff value R0. To select R0, we examined clustering at various cutoffs selecting only populated clusters, each of which must represent at least 5% of basin structures and together they must capture at least 50% of basin structures. Then R0 is the largest RMSD cutoff that occurs prior to a major reorganization of clusters such as when two smaller distinct clusters merge into one larger cluster. The clusters were assumed distinct if they exhibit different aggregation interfaces or peptide conformations. Structural properties of a cluster are reported as averages over the conformations populating a cluster.
Supplementary information
Author Contributions
A.K.S. and D.K.K. designed research; A.K.S. performed research; A.K.S. analyzed data; and A.K.S. and D.K.K. wrote the paper. All authors reviewed the manuscript.
Data Availability
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-43685-7.
References
- 1.Petkova AT, Yau W-M, Tycko R. Experimental constraints on quaternary structure in Alzheimer’s β-amyloid fibrils. Biochemistry. 2006;45:498–512. doi: 10.1021/bi051952q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Serpell LC. Alzheimer’s amyloid fibrils: Structure and assembly. Biochim Biophys. Acta. 2000;1502:16–30. doi: 10.1016/S0925-4439(00)00029-6. [DOI] [PubMed] [Google Scholar]
- 3.Pike C, Burdick D, Walencewicz A, Glabe C, Cotman C. Neurodegeneration induced by β-amyloid peptides in vitro: The role of peptide assembly state. J. Neurosci. 1993;13:1676–1687. doi: 10.1523/JNEUROSCI.13-04-01676.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams TL, Serpell LC. Membrane and surface interactions of Alzheimer’s Aβ peptide - insights into the mechanism of cytotoxicity. FEBS J. 2011;278:3905–3917. doi: 10.1111/j.1742-4658.2011.08228.x. [DOI] [PubMed] [Google Scholar]
- 5.Sergeant N, et al. Truncated beta-amyloid peptide species in pre-clinical Alzheimers disease as new targets for the vaccination approach. J. Neurochem. 2003;85:1581–1591. doi: 10.1046/j.1471-4159.2003.01818.x. [DOI] [PubMed] [Google Scholar]
- 6.Christensen R, Marcussen A, Wortwein G, Knudsen G, Aznar S. Aβ(1-42) injection causes memory impairment, lowered cortical and serum bdnf levels, and decreased hippocampal 5-ht(2a) levels. Exp. Neurol. 2008;210:164–171. doi: 10.1016/j.expneurol.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 7.Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: Lessons from the Alzheimers amyloid β-peptide. Nature Rev. Mol. Cell. Biol. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- 8.Shankar GM, et al. Amyloid-β protein dimers isolated directly from Alzheimers brains impair synaptic plasticity and memory. Nature Medicine. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hardy J, Higgins GA. Alzheimer’s disease: The amyloid cascade hypothesis. Science. 1992;256:184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- 10.Maddalena A, et al. Cerebrospinal fluid profile of amyloid β peptides in patients with Alzheimer’s disease determined by protein biochip technology. Neurodegener. Dis. 2004;1:231–235. doi: 10.1159/000080991. [DOI] [PubMed] [Google Scholar]
- 11.Millucci L, Ghezzi L, Bernardini G, Santucci A. Conformations and biological activities of amyloid β peptide 25-35. Curr Protein Pept Sci. 2010;11:54–67. doi: 10.2174/138920310790274626. [DOI] [PubMed] [Google Scholar]
- 12.Kaneko, I., Yamada, N., Usui, Y. & Oda, T. Possible involvement of β-amyloids racemized at ser residue in Alzheimer’s disease. In Alzheimer’s Disease: Biology, Diagnose and Therapeutics, 519–528, Chichester (John Wiley & Sons, 1997).
- 13.Pike CJ, et al. Structure-activity analyses of beta-amyloid peptides: Contributions of the beta 25-35 region to aggregation and neurotoxicity. J. Neurochem. 1995;64:253–265. doi: 10.1046/j.1471-4159.1995.64010253.x. [DOI] [PubMed] [Google Scholar]
- 14.Sato K, et al. Correlation among secondary structure, amyloid precursor protein accumulation, and neurotoxicity of amyloid β(25-35) peptide as analyzed by single alanine substitution. J. Biochem. 1995;118:1108–1111. doi: 10.1093/oxfordjournals.jbchem.a124994. [DOI] [PubMed] [Google Scholar]
- 15.Clementi ME, et al. Aβ(31-35) and Aβ(25-35) fragments of amyloid beta-protein induce cellular death through apoptotic signals: Role of the redox state of methionine-35. FEBS. 2005;579:2913–2918. doi: 10.1016/j.febslet.2005.04.041. [DOI] [PubMed] [Google Scholar]
- 16.Tsai H-HG, et al. Location and conformation of amyloid β(25-35) peptide and its sequence-shuffled peptides within membranes: Implications for aggregation and toxicity in PC12 cells. Chem. Med. Chem. 2014;9:1002–1011. doi: 10.1002/cmdc.201400062. [DOI] [PubMed] [Google Scholar]
- 17.Song Y, Li P, Liu L, Bortolini C, Dong M. Nanostructural differentiation and toxicity of amyloid-β25-35 aggregates ensue from distinct secondary conformation. Sci. Rep. 2018;8:765. doi: 10.1038/s41598-017-19106-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tepanichev MY, Moiseeva YV, Lazareva NA, Gulyaeva NV. Studies of the effects of fragment (25-35) of beta-amyloid peptide on the behavior of rats in a radial maze. Neurosci. Behav. Physiol. 2005;35:511–518. doi: 10.1007/s11055-005-0086-1. [DOI] [PubMed] [Google Scholar]
- 19.Limon ID, et al. Amyloid-beta(25-35) impairs memory and increases NO in the temporal cortex of rats. Neurosci. Res. 2009;63:129–137. doi: 10.1016/j.neures.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 20.Frozza F, et al. A comparative study of β-amyloid peptides aβ1-42 and aβ25-35 toxicity in organotypic hippocampal slice cultures. Neurochem. Res. 2009;34:295–303. doi: 10.1007/s11064-008-9776-8. [DOI] [PubMed] [Google Scholar]
- 21.Misiti F, et al. Aβ(31-35) peptide induce apoptosis in pc 12 cells: contrast with aβ(25-35) peptide and examination of underlying mechanisms. Neurochem. Int. 2005;46:575–583. doi: 10.1016/j.neuint.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Tsai HHG, Lee JB, Tseng SS, Pan XA, Shih YC. Folding and membrane insertion of amyloid-beta (25-35) peptide and its mutants: Implications for aggregation and neurotoxicity. Proteins Struct. Funct. Bioinform. 2010;78:1909–1925. doi: 10.1002/prot.22705. [DOI] [PubMed] [Google Scholar]
- 23.Larini L, Shea J-E. Role of β-hairpin formation in aggregation: The self-assembly of the amyloid-β(25-35) peptide. Biophys. J. 2012;103:576–586. doi: 10.1016/j.bpj.2012.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Terzi E, Hölzemann G, Seelig J. Interaction and Alzheimer’s β-amyloid peptide(1-40) with lipid membranes. Biochemistry. 1997;36:14845–14852. doi: 10.1021/bi971843e. [DOI] [PubMed] [Google Scholar]
- 25.Yip CM, McLaurin J. Amyloid-β peptide assembly: a critical step in fibrillogenesis and membrane disruption. Biophys. J. 2001;80:1359–1371. doi: 10.1016/S0006-3495(01)76109-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bokvist M, Lindström F, Watts A, Gröbner G. Two types of Alzheimer’s β-amyloid (1-40) peptide membrane interactions: Aggregation preventing transmembrane anchoring versus accelerated surface fibril formation. J. Mol. Biol. 2004;335:1039–1049. doi: 10.1016/j.jmb.2003.11.046. [DOI] [PubMed] [Google Scholar]
- 27.Quist A, et al. Amyloid ion channels: A common structural link for protein-misfolding disease. Proc. Natl. Acad. Sci. USA. 2005;102:10427–10432. doi: 10.1073/pnas.0502066102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nag S, Chen J, Irudayaraj J, Maiti S. Measurement of the attachment and assembly of small amyloid-β oligomers on live cell membranes at physiological concentrations using single-molecule tools. Biophys. J. 2010;99:1969–1975. doi: 10.1016/j.bpj.2010.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ambroggio EE, et al. Surface behavior and lipid interaction of Alzheimer’s β-amyloid peptide 1-42: A membrane-disrupting peptide. Biophys. J. 2005;88:2706–2713. doi: 10.1529/biophysj.104.055582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Terzi E, Holzemann G, Seelig J. Alzheimer β-amyloid peptide 25-35: Electrostatic interactions with phospholipid membranes. Biochemistry. 1994;33:7434–7441. doi: 10.1021/bi00189a051. [DOI] [PubMed] [Google Scholar]
- 31.Dante S, Hauss T, Dencher NA. Insertion of externally administered amyloid β peptide 25-35 and perturbation of lipid bilayers. Biochemistry. 2003;42:13667–13672. doi: 10.1021/bi035056v. [DOI] [PubMed] [Google Scholar]
- 32.DÉrrico G, et al. Interaction between Alzheimer’s aβ(25-35) peptide and phospholipid bilayers: The role of cholesterol. Biochimica et Biophysica Acta. 2008;1778:2710–2716. doi: 10.1016/j.bbamem.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 33.Mason RP, Estermyer JD, Kelly JF, Mason PE. Alzheimer’s disease amyloid β peptide 25-35 is localized in the membrane hydrocarbon core: X-ray diffraction analysis. Biochem. Biophys. Res. Commun. 1996;222:78–82. doi: 10.1006/bbrc.1996.0699. [DOI] [PubMed] [Google Scholar]
- 34.Smith AK, Klimov DK. Binding of cytotoxic aβ25-35 peptide to the dmpc lipid bilayer. J. Chem. Inform. Model. 2018;58:1053–1065. doi: 10.1021/acs.jcim.8b00045. [DOI] [PubMed] [Google Scholar]
- 35.Krstic D, Knuesel I. Deciphering the mechanism underlying late-onset Alzheimer disease. Nat Rev Neurol. 2013;9:25–34. doi: 10.1038/nrneurol.2012.236. [DOI] [PubMed] [Google Scholar]
- 36.Saponetti MS, et al. Aggregation of aβ(25-35) on DOPC and DOPC/DHA bilayers: An atomic force microscopy study. Plos One. 2014;9:e115780. doi: 10.1371/journal.pone.0115780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kandel N, Zheng T, Huo Q, Tatulian SA. Membrane binding and pore formation by a cytotoxic fragment of amyloid β peptide. J. Physc. Chem. B. 2017;121:10293–10305. doi: 10.1021/acs.jpcb.7b07002. [DOI] [PubMed] [Google Scholar]
- 38.Kandel N, Matos JO, Tatulian SA. Structure of amyloid β25–35 in lipid environment and cholesterol dependent membrane pore formation. Sci. Reports. 2019;9:2689. doi: 10.1038/s41598-019-38749-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shai Y. Mode of action of membrane active antimicrobial peptides. Biopolymers. 2002;66:236–248. doi: 10.1002/bip.10260. [DOI] [PubMed] [Google Scholar]
- 40.Dante S, Hauss T, Dencher NA. β-amyloid 25 to 35 is intercalated in anionic and zwitterionic lipid membranes to different extents. Biophys. J. 2002;83:2610–2616. doi: 10.1016/S0006-3495(02)75271-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dies H, Toppozini L, Rheinstadter MC. The interaction between amyloid-β peptides and anionic lipid membranes containing cholesterol and melatonin. PLoS One. 2014;9:e99124. doi: 10.1371/journal.pone.0099124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lockhart C, Klimov DK. The Alzheimer’s disease Aβ peptide binds to the anionic DMPS lipid bilayer. BBA Biomembranes. 2016;1858:1118–1128. doi: 10.1016/j.bbamem.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 43.Tang J, et al. Amyloid-β25–35 peptides aggregate into cross-β sheets in unsaturated anionic lipid membranes at high peptide concentrations. Soft Matter. 2016;12:3165–3176. doi: 10.1039/C5SM02619A. [DOI] [PubMed] [Google Scholar]
- 44.Paravastua AK, Leapman RD, Yaua W-M, Tycko R. Molecular structural basis for polymorphism in Alzheimers β-amyloid fibrils. Proc. Natl. Acad. Sci. USA. 2008;105:18349–18354. doi: 10.1073/pnas.0806270105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nadezhdin KD, Bocharova OV, Bocharov EV, Arseniev AS. Dimeric structure of transmembrane domain of amyloid precursor protein in micellar environment. FEBS Lett. 2012;586:1687–1692. doi: 10.1016/j.febslet.2012.04.062. [DOI] [PubMed] [Google Scholar]
- 46.Wang H, et al. Molecular determinants and thermodynamics of the amyloid precursor protein transmembrane domain implicated in Alzheimer’s disease. J. Mol. Biol. 2011;408:879–895. doi: 10.1016/j.jmb.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dominguez L, Foster L, Straub JE, Thirumalai D. Impact of membrane lipid composition on the structure and stability of the transmembrane domain of amyloid precursor protein. Proc. Natl. Acad. Sci. USA. 2016;113:E5281–E5287. doi: 10.1073/pnas.1606482113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dathe M, Wieprecht T. Structural features of helical antimicrobial peptides: their potential to modulate activity on model membranes and biological cells. Biochimica et Biophysica Acta. 1999;1462:71–87. doi: 10.1016/S0005-2736(99)00201-1. [DOI] [PubMed] [Google Scholar]
- 49.Cuco A, Serro AP, Farinha JP, Saramago B, da Silva AG. Interaction of the Alzheimer aβ(25–35) peptide segment with model membranes. Coll. Surf. B Biointerfaces. 2016;141:10–18. doi: 10.1016/j.colsurfb.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 50.Buck M, Bouguet-Bonnet S, Pastor RW, MacKerell AD. Importance of the CMAP correction to the CHARMM22 protein force field: Dynamics of hen lysozyme. Biophys. J. 2006;90:L36–L38. doi: 10.1529/biophysj.105.078154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Klauda JB, et al. Update of the CHARMM all-atom additive force field for lipids: validation on six lipid types. J. Phys. Chem. B. 2010;114:7830–7843. doi: 10.1021/jp101759q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.MacKerell AD, et al. All-atom empirical potential for molecular modeling and dynamics studies of proteins. J. Phys. Chem. B. 1998;102:3586–3616. doi: 10.1021/jp973084f. [DOI] [PubMed] [Google Scholar]
- 53.Park S, Beaven AH, Klauda JB, Im W. How tolerant are membrane simulations with mismatch in area per lipid between leaflets? J. Chem. Theor. Comput. 2015;11:3466–3477. doi: 10.1021/acs.jctc.5b00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang L, Friesner RA, Berne BJ. Replica exchange with solute scaling: A more efficient version of replica exchange with solute tempering (REST2) J. Phys. Chem. B. 2011;115:9431–9438. doi: 10.1021/jp204407d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith AK, Lockhart C, Klimov DK. Does replica exchange with solute tempering efficiently sample Aβ peptide conformational ensembles? J. Chem. Theor. Comp. 2016;12:5201–5214. doi: 10.1021/acs.jctc.6b00660. [DOI] [PubMed] [Google Scholar]
- 56.Kalé L, et al. NAMD2: Greater scalability for parallel molecular dynamics. J. Comput. Phys. 1999;151:283–312. doi: 10.1006/jcph.1999.6201. [DOI] [Google Scholar]
- 57.Jo S, Jiang W. A generic implementation of replica exchange with solute tempering (REST2) algorithm in NAMD for complex biophysical simulations. Comput. Phys. Commun. 2015;197:304–311. doi: 10.1016/j.cpc.2015.08.030. [DOI] [Google Scholar]
- 58.Lockhart C, Klimov DK. Alzheimer’s Aβ10-40 peptide binds and penetrates DMPC bilayer: An isobaric-isothermal replica exchange molecular dynamics study. J. Phys. Chem. B. 2014;118:2638–2648. doi: 10.1021/jp412153s. [DOI] [PubMed] [Google Scholar]
- 59.Frishman D, Argos P. Knowledge-based protein secondary structure assignment. Proteins Struct. Funct. Gen. 1995;23:566–579. doi: 10.1002/prot.340230412. [DOI] [PubMed] [Google Scholar]
- 60.Daura X, et al. Peptide folding: When simulation meets experiment. Angew. Chem. Int. Ed. 1999;38:236–240. doi: 10.1002/(SICI)1521-3773(19990115)38:1/2<236::AID-ANIE236>3.0.CO;2-M. [DOI] [Google Scholar]
- 61.Humphrey W, Dalke A, Schulten K. VMD – Visual Molecular Dynamics. J. Mol. Graph. 1996;14:33–38. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.