Abstract
Objective
African American patients with systemic lupus erythematosus (SLE) are at high risk for poor outcomes. Both patient characteristics and the severity of the disease may influence physician-patient interactions, which in turn can impact disease outcomes. We aimed to examine whether patient perceptions of interpersonal processes of care (i.e. physician-patient interactions) varied by demographic characteristics, disease activity, and/or depression in African American patients with SLE.
Methods
The Georgians Organized Against Lupus (GOAL) is a cohort drawn from a population-based registry of people with SLE. We conducted a cross-sectional analysis of patient-reported data collected in 2016–17 among 698 African American participants (out of 863 GOAL participants). We assessed physician-patient interactions (communication, patient-centered decision making, and physician interpersonal style) through the Interpersonal Processes of Care survey (IPC-29), disease activity through the Systemic Lupus Activity Questionnaire, and depression through the Patient Health Questionnaire-9. Mean scores of the IPC-29 scales were compared by gender, age and educational attainment with Wilcoxon rank-sum 2-sample test or Kruskal Wallis test. We conducted linear trend test to examine demographic-adjusted scores of IPC across severity of disease activity and depression, and multivariate logistic regression analyses to examine the association of disease activity and depression with suboptimal IPC scores.
Results
Overall, the lowest mean scores were observed for the patient-centered decision making domain, and specifically about how often doctors assessed patients’ problems to follow recommendations and treatment among females compared with males (mean scores 3.13 ± 1.42 and 3.64 ± 1.38, respectively; p=0.015). Mean scores for the assumed socioeconomic level subdomain (how often doctors make assumptions about a patient’s socioeconomic level) were worse in individuals aged 18–34 (mean score 1.59 ± 0.94), compared to those aged 35–55 (mean score 1.47 ± 0.94; p=0.033). Patients with some college or higher educational attainment reported poorer mean scores for most communication and interpersonal style scales than those who reported high-school or less. We found significant linear trends of poorer scores for all communication scales across more severe disease activity and depression symptoms, and poorer scores for all interpersonal style scales across more severe disease activity. Multivariate models revealed that while depression was associated with suboptimal quality of both communication (OR 1.20; 95% CI 1.04–1.39) and interpersonal style (OR 1.12; 95% CI 1.01–1.25), disease activity only increased the odds of suboptimal interpersonal style (OR 1.13; 95% CI 1.03–1.25).
Conclusion
In the African American population with SLE, suboptimal interactions with providers may be explained in part by the mental and physical symptoms of the patient, regardless of age, gender and education. In addition to standard of care treatment, SLE patients with more severe disease activity and depression might need provider-based interventions focused on communication and interpersonal style.
Introduction
A growing body of evidence indicates that physician-patient interactions have a substantial impact on patient satisfaction, as well as self-management and outcomes of chronic conditions (1, 2). Moreover, patient-reported interpersonal processes of care are increasingly utilized as quality measures for health plans, clinics, and providers (3, 4).
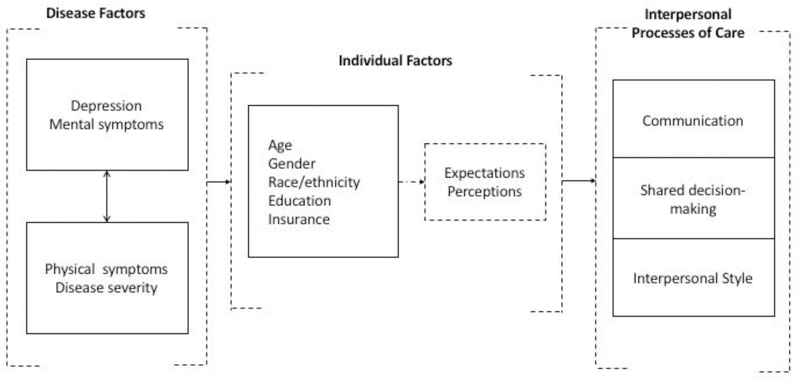
Recent studies have shown that the quality of interpersonal processes of care vary across sociodemographic groups, suggesting that health disparities in people with chronic conditions can be explained in part by those processes (5–7). Moreover, a growing body of research suggests that patient’s health condition can also influence individual perceptions and expectations about providers’ interactions. For instance, findings from several studies indicate that depression, a frequent and often underdiagnosed problem among patients with chronic diseases(8), influence patients’ perceptions of provider interpersonal style(9), patients’ disposition to discuss self-management(10), and patients’ satisfaction with care(11). Moreover, people with chronic conditions and depression have been found to perceive their encounters differently than those without depression(12). As an example, HIV patients with depression have reported a less personable style by their providers than those without depression(13). Providers, on the other hand, have reported lower positive regard for patients with more depressive symptoms compared to those with less depressive symptoms(13). Moreover, patients from socioeconomically disadvantaged groups are more likely to experience communication challenges with their providers as result of multiple factors, including complex health problems, depression, low self-efficacy, and health literacy(14). Figure 1 depicts the relationships between disease, individual factors and IPC that support the theoretical framework for this study.
Figure 1.
Conceptual model of the relationships between disease-related and individual factors with interpersonal processes of care.
SLE is a multisystem chronic disease that disproportionately strikes women of childbearing age and people of color(15, 16). The severity of SLE ranges from mild to life-threatening and its course is characterized for a wide variety of symptoms that wax and wane often unpredictably. Compared to whites, African Americans with SLE have more severe phenotypes and worse outcomes, including more active disease, poorer physical and mental health, and lower life expectancy(17–19). Although psychiatric morbidity is high in SLE, African American patients are less likely than their white counterparts to be diagnosed with depression(20). Moreover, in African American patients with SLE, depression has been found to be associated with greater perceptions of racism in the healthcare encounter(21). SLE patients with depression might be less likely to perceive personable or effective communication as a a consequence of their depression, or may recall communication with their physicians differently than those without depression. Moreover, SLE patients with active disease might experience pain and emotional distress related to their flare symptoms, and their expectations might focus primarily on emotions and symptoms relief, whereas physicians may focus on assessment of the cause of patients’ symptoms. Differences in physician and patient perceptions or expectations may lead to poor physician-patient interactions(22, 23).
Little is known, however, about the extent to which depression, disease activity and sociodemographic characteristics may influence physician-patient interactions in the African American population with SLE.
Because studies focused on high-risk populations can advance our understanding of the relationship between an individual’s characteristics and healthcare services, we aimed to examine physician-patient interactions across demographic subgroups in a large African American SLE cohort from the Southeastern US. Moreover, we explored the association between both disease activity and depression with physician-patient interactions. We hypothesized that among African American patients with SLE, more severe self-reported disease activity and depression symptoms would have a greater negative impact on patients’ interactions with their providers.
Methods
Study Design and Population
We used a cross-sectional design to describe the demographic differences of physician-patient interactions within African American patients with SLE, and to analyze the associations of increasing severity of disease activity and depression symptoms with physician-patient interactions in that population. Data were obtained from the ongoing Georgian Organized Against Lupus (GOAL), a predominantly African American cohort of individuals with SLE. Details of GOAL recruitment and data collection have been published previously(24). Briefly, the primary source of GOAL participants is the Georgia Lupus Registry (GLR). GLR is a retrospective population-based registry funded by the Centers for Disease Control and Prevention designed to more accurately estimate of the incidence and prevalence of SLE in Atlanta, Georgia, where there is a large and socioeconomically evenly distributed black/white population. The GLR was implemented through a pivotal partnership between the Georgia Department of Public Health (GA DPH) and Emory University, which enabled Emory investigators to use the state public health surveillance exemption to the Health Insurance Portability and Accountability Act to acquire access to protected health information without requiring individual patient consent, minimizing case ascertainment bias (25). In 2007 the GA DPH allowed Emory University investigators to contact GLR ascertained SLE patients for further recruitment into a longitudinal (GOAL) cohort. Thus, adult lupus patients who received medical care at community- and university-based practices were recruited by mail, by telephone, and in person to complete annual self-administered surveys. Eligible participants were adults (aged ≥18 years) with a documented diagnosis of SLE [≥4 revised American College of Rheumatology (ACR) criteria (26), or 3 ACR criteria with a diagnosis of SLE by the patient’s treating board-certified rheumatologist].
The population-based cohort was further enriched through recruitment of community patients (via internet, the Lupus Foundation of America GA Chapter, and referrals by partner community rheumatologists). In addition, GOAL enrolled patients receiving treatment at the Emory Rheumatology Clinic and the Lupus Clinic located at the Grady Memorial Hospital. While Emory and community practices serve predominantly insured patients, Grady Memorial Hospital is the only safety net hospital for a large indigent population from metropolitan Atlanta and the state of Georgia.
Beginning in 2011–2012, participants completed annual surveys, which collect self-reported data on sociodemographics, disease outcomes, and healthcare. Surveys were designed for individuals with limited health literacy and targeted an eighth-grade reading level. Flexible administration modes (self- or interviewer-administered) and delivery methods (mail, telephone, and in-person) were available. As in similar studies(27), the average time to complete the GOAL survey was below 1 hour. In this study, we focused on self-reported African American respondents of the 2016–2017 GOAL survey, coincident with the introduction of a tool to measure physician-patient interactions. The Emory University Institutional Review Board, Grady Health System Research Oversight Committee, and the Georgia Department of Public Health Institutional Review Board approved the study protocol. All GOAL participants gave informed signed consent.
Measures
Physician-patient Interactions
Physician-patient interactions were measured using the 29-item Interpersonal Processes of Care (IPC) survey(6). The IPC-29 is a patient-reported instrument that measures three general domains (communication, decision making, and interpersonal style) through 12 subdomains (first-order factors), which in turn are classified in 7 scales (second-order factors) (Table 1). Scales and subdomains represent either ‘positive’ (e.g. elicited concerns, responded) or ‘negative’ (e.g. lack of clarity) constructs. For each item, participants are asked how often that type of care had been provided, using a 5-point Likert scale (1=never; 2=rarely; 3=sometimes; 4=usually; 5=always). Each scale and subdomain is scored separately by adding the total score within the scale or subdomain and dividing by the number of questions. Possible scores range from 1 to 5. A higher score indicates higher frequency of the labeled construct (e.g. more frequent reports of elicited concerns, responded [positive construct], or more frequent reports of lack of clarity [negative construct]. The tool has been validated in socioeconomically and ethnically diverse populations of adults from general medicine practices, and has been used to measure quality of care in ethnic minorities with chronic conditions(1, 6, 28). The IPC-29 has rendered unbiased mean comparison across groups and moderate to high reliability (range 0.61–0.91) for within groups comparisons(6).
Table 1.
Description of the Interpersonal Processes of Care (IPC-29)
| Scales/subdomains | How frequently… |
|---|---|
| 1. Communication | |
| Hurried communication (−) | Doctors are hard to understand, ignored patient’s concerns, were bothered by patient’s questions, or distracted |
| Lack of clarity (−) | Doctors spoke quickly/used complex words |
| Hurried, distracted (−) | Doctors ignored patient’s concerns, were distracted or bothered |
| Elicited concerns, responded (+) | Doctors heard patient’s concerns and took them seriously |
| Explained results, meds (+) | Doctors explained results and medications |
| Explained results (+) | Doctors explained tests and physical examination results |
| Explained medications (+) | Doctors explained what would happen without the medicines and their side effects |
| 2. Decision Making | |
| Patient-centered decision making (+) | Doctors asked patient and worked out treatment together |
| Asked patient (+) | Doctors asked about patient’s difficulties to follow up recommendations |
| Decided together (+) | Doctors asked patient’s preferences about treatment and worked out together treatment plan |
| 3. Interpersonal Style | |
| Compassionate, respectful (+) | Doctors provided emotional support, were compassionate and respectful |
| Emotional support, comp (+) | Doctors were compassionate and expressed concern about patient’s feelings |
| Respectful (+) | Doctors respected and treated patient as an equal |
| Discrimination (−) | Doctors made assumptions or discriminated |
| Assumed socioeconomic status (−) | Doctors made assumptions about patient’s level of education or income |
| Discriminated due to race (−) | Patient perceived discrimination or inattentiveness of doctors due to patient’s race or ethnicity |
| Disrespectful office staff (−) | Office staff were negative or rude, gave patient a hard time, talked down to patient |
Domains are underlined, scales are in italic font, subdomains are indented
Sociodemographic Characteristics
Demographics (age, gender, educational attainment and insurance status) were collected using self-reported questionnaires.
Disease Activity
Disease activity was assessed with the Systemic Lupus Activity Questionnaire (SLAQ), a validated tool used extensively to measure patient-reported disease activity in SLE populations (23). The SLAQ includes 24 questions that are scored from 0 to 3 based on symptoms rated as “no problem”, “mild”, “moderate”, or “severe”. SLAQ scoring ranges from 0 to 47 with higher scores indicating greater SLE-related disease activity. Cutoff scores to categorically classify disease activity have not been previously defined; consequently, we measured severity of disease activity as an ordinal variable using three ranges of SLAQ scores that were evenly distributed in our sample as follows: (i) none to mild activity (SLAQ score 0 to10; n=219); (ii) moderate activity (SLAQ score 11 to 19; n=246); and (iii) severe activity (SLAQ score 20 or higher; n=233).
Depression Symptoms
Depression symptoms were assessed with the 9-question Patient Health Questionnaire (PHQ-9), a tool that has been validated among individuals with rheumatologic disorders (29). The PHQ-9 has been extensively used in epidemiology to measure depression and severity of depression symptoms (30, 31). PHQ-9 scores range from 0–27. We assessed depression symptoms as an ordinal variable using previously established categories of minimal (PHQ-9 score 0–4), mild (PHQ-9 score 5–9) and moderate to severe (PHQ-9 score ≥10).
SLE-related Factors
We assessed disease duration and organ damage, which can potentially be confounders in the association between interpersonal processes of care and disease activity or depression. Disease duration was assessed as a continuous variable (in years since diagnosis). Organ damage accumulated over time as consequence of disease activity, comorbidities or side effects of medications used to treat lupus, was measured with the self-administered version of the Brief Index of Lupus Damage (SA-BILD) survey, which has been validated in our own cohort (32, 33).
Statistical Analysis
For descriptive analyses, patient characteristics were summarized using frequency and percentage for categorical variables, and mean and standard deviation (SD) for continuous variables. We used the Wilcoxon rank-sum 2-sample test or Kruskal Wallis test to compare the mean scores of the major domains of the IPC-29 by gender, age and educational attainment.
Linear trend tests were conducted to examine the means of IPC-29 scores within communication, decision-making and interpersonal style scales, across severe disease activity and depression symptoms. For the linear trends analyses, we calculated both crude and adjusted means. Adjustment variables included sociodemographics (age, gender, education, disease duration, insurance), organ damage, and either depression symptoms (when disease activity was the testing group) or disease activity (when depression symptoms was the testing group).
Furthermore, we conducted multivariate logistic regression analyses to evaluate the impact of disease activity and depression on “suboptimal” quality of physician-patient interactions. We summarized scores of each of three broad IPC domains: communication, patient-centered decision making, and physician interpersonal style using 16 out of the 29 IPC questions, as depicted in the appendix and suggested by Stewart and colleagues(6, 7). Reverse codes were calculated for ‘negative’ constructs, such as lack of clarity, discriminated due to race/ethnicity. To be consistent with prior studies in patients with chronic conditions, scores of 5 were deemed to represent “optimal” quality of IPC, and scores 1–4 represented “suboptimal” quality of IPC (28). For each model, the outcomes were “suboptimal” communication, patient-centered decision making and provider’s interpersonal style. Disease activity and depression were analyzed as continuous variables and the three models were adjusted for sociodemographics (age, gender, education, and insurance status), and disease-related factors (disease duration and cumulative organ damage). We reported the adjusted odds ratio for each model with 95% confidence intervals (CIs). An increment of 0.5 SD (4 units for SLAQ and 3 units for PHQ-9), which is a commonly-used definition of minimal important difference in patient-reported outcomes (34), was used to estimate the adjusted ORs in the models. P values ≤ 0.05 were considered statistically significant for all tested associations. Additionally, we conducted sensitivity analyses by adding healthcare facility to the set of control variables used in the trend and multivariate analyses. All statistical analyses were performed with SAS software, version 9.4 (SAS Institute, Cary, NC, USA).
Results
Description of the Study Population
Out of 863 respondents to the 2016–17 survey, 698 (80.9%) who self-identified as African Americans were selected for this study. Of these 698 individuals, 359 (51.4%) were recruited directly from the population-based GLR. The remaining 339 participants were recruited from the Grady Lupus Clinic, the Emory Rheumatology Clinic, or community rheumatology practices. We did not find significant differences in age at diagnosis between GOAL participants consented through GLR (mean 32.2, SD 11.7 years old) and those further enrolled from other sources (mean 32.3, SD 11.7 years old). However, because those participants enrolled from GLR have been in the cohort for a longer time, the proportion of individuals younger than 35 years old at the time of this study was 8.6% and 34.8% for GLR and other sources, respectively (p<0.0001). Similarly, GLR-enrolled participants reported significantly longer disease duration (mean 21.0 years, SD 9.1) compared to those recruited from other sources (mean 9.3 years, SD 7.4), p< 0.0001. We did not find any significant differences in gender, educational attainment, or unemployment between participants enrolled from GLR compared to other sources. However, patients recruited from GLR were less likely to be uninsured (n=23, 6.4%) compared to those from other sources (n=65, 19.3%), p <0.0001.
As shown in Table 2, the mean age of our overall sample was 47.5 (SD 13.7) years and 650 (93.1%) participants were women. Educational attainment was evenly distributed; 12.7% of the sample was uninsured. Unemployment or disability was reported by 284 participants (42.0%). Mean disease activity was moderate with a score of 16.0 (SD 8.6) and 608 (87.1%) participants had some organ damage (SA-BILD ≥1). Mean PHQ-9 score was 7.6 (SD 6.1), with 212 (30.4%) participants endorsing mild depression symptoms and 225 (32.2%) moderate to severe depression symptoms.
Table 2.
Description of the African American Cohort (n=698)
| Characteristic | Statistic |
|---|---|
| Age at survey (years), mean ± SD | 47.5 ± 13.7 |
| Age group (years), n (%) | |
| 18–34 | 149 (21.3) |
| 35–54 | 322 (46.1) |
| 55+ | 227 (32.5) |
| Disease duration (years), mean ± SD | 15.3 ± 10.2 |
| Gender (female), n (%) | 650 (93.1) |
| Education (years), mean ± SD | 14.3 ± 2.8 |
| Educational attainment, n (%) | |
| High school or less | 241 (34.8) |
| Some college | 243 (35.1) |
| Some college | 208 (30.1) |
| Uninsured, n (%) | 88 (12.7) |
| Insurance*, n (%) | 607 (87.3) |
| Medicaid | 122 (17.6) |
| Medicare/Medicaid | 85 (12.2) |
| Medicare | 172 (24.7) |
| Private insurance | 228 (32.8) |
| Unemployed or disabled, n (%) | 284 (42.0) |
| Disease activity score, mean ± SD | 16.0 ± 8.6 |
| Severity of disease activity, n (%) | |
| None-mild (SLAQ score 0–10) | 219 (31.4) |
| Moderate (SLAQ score 11–19) | 246 (35.2) |
| Severe (SLAQ score ≥20) | 233 (33.4) |
| Depression (PHQ score), mean ± SD | 7.6 ± 6.1 |
| Severity of depression symptoms, n (%) | |
| Minimal (PHQ-9 score 0–4) | 261 (37.4) |
| Mild (PHQ-9 score 5–9) | 212 (30.4) |
| Moderate to severe (PHQ-9 score >10) | 225 (32.2) |
| Organ damage (SA-BILD score), median (IQR) | 3 (1–4) |
| Organ damage ≥ 1, n (%) | 608 (87.1) |
3 cases with missing data.
IPC-29 by Demographics
Table 3 depicts mean IPC-29 scores, overall and by demographic characteristics. Overall, the lowest (best) mean scores within negative constructs were observed in discriminated due to race/ethnicity (1.32, SD 0.71) and the highest (worse) in lack of clarity (1.89, SD 0.89) of the interpersonal style and communication domains, respectively. Overall, the lowest (worse) and highest (best) mean scores within positive constructs were found in asked patient (3.16, SD 1.42) and respectful interpersonal style (4.27, SD 0.94).
Table 3.
Differences of Interpersonal Processes of Care Means by Demographic Characteristics in African American Patients with SLE
| Gender | Age | Educational attainment | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IPC Domains/scales/subdomains | Overall | Female n=650 | Male n=48 | P value | 18–34 n=149 | 35–54 n=322 | 55+ n=227 | P value | ≤ HS n=241 | Some College n=243 | College+ n=208 | P value |
| 1. Communication | ||||||||||||
| Hurried communication (−) | 1.72 ± 0.75 | 1.75 ± 0.76 | 1.44 ± 0.61 | 0.0014 | 1.73 ± 0.69 | 1.74 ± 0.76 | 1.70 ± 0.79 | 0.54 | 1.62 ± 0.71 | 1.81 ± 0.82 | 1.75 ± 0.72 | 0.044 |
| Lack of clarity (−) | 1.89 ± 0.89 | 1.90 ± 0.90 | 1.64 ± 0.83 | 0.025 | 1.93 ± 0.83 | 1.91 ± 0.91 | 1.81 ± 0.91 | 0.14 | 1.84 ± 0.90 | 1.96 ± 0.96 | 1.84 ± 0.81 | 0.44 |
| Hurried, distracted (−) | 1.62 ± 0.80 | 1.64 ± 0.81 | 1.31 ± 0.54 | 0.0027 | 1.60 ± 0.73 | 1.62 ± 0.80 | 1.62 ± 0.83 | >0.99 | 1.47 ± 0.74 | 1.70 ± 0.86 | 1.69 ± 0.77 | 0.0002 |
| Elicited concerns, responded (+) | 4.15 ± 0.90 | 4.14 ± 0.91 | 4.35 ± 0.75 | 0.15 | 4.14 ± 0.85 | 4.11 ± 0.93 | 4.22 ± 0.89 | 0.31 | 4.25 ± 0.84 | 4.09 ± 0.97 | 4.12 ± 0.88 | 0.16 |
| Explained results, meds (+) | 3.86 ± 1.08 | 3.84 ± 1.08 | 4.08 ± 0.95 | 0.14 | 3.87 ± 1.10 | 3.84 ± 1.09 | 3.87 ± 1.04 | 0.92 | 4.13 ± 0.98 | 3.70 ± 1.14 | 3.73 ± 1.06 | <0.0001 |
| Explained results (+) | 4.14 ± 1.08 | 4.14 ± 1.07 | 4.25 ± 1.09 | 0.28 | 4.03 ± 1.12 | 4.11 ± 1.09 | 4.27 ± 1.01 | 0.13 | 4.36 ± 0.98 | 3.99 ± 1.14 | 4.06 ± 1.07 | <0.0001 |
| Explained medications (+) | 3.57 ± 1.28 | 3.55 ± 1.29 | 3.91 ± 1.21 | 0.057 | 3.72 ± 1.24 | 3.57 ± 1.31 | 3.48 ± 1.26 | 0.18 | 3.89 ± 1.19 | 3.43 ± 1.33 | 3.39 ± 1.25 | <0.0001 |
| 2. Decision Making | ||||||||||||
| PC decision making (+) | 3.17 ± 1.29 | 3.14 ± 1.29 | 3.63 ± 1.26 | 0.01 | 3.32 ± 1.29 | 3.16 ± 1.32 | 3.09 ± 1.25 | 0.22 | 3.29 ± 1.27 | 3.16 ± 1.32 | 3.08 ± 1.27 | 0.24 |
| Asked patient (+) | 3.16 ± 1.42 | 3.13 ± 1.42 | 3.64 ± 1.38 | 0.015 | 3.27 ± 1.42 | 3.16 ± 1.45 | 3.10 ± 1.37 | 0.50 | 3.30 ± 1.39 | 3.18 ± 1.45 | 3.01 ± 1.40 | 0.089 |
| Decided together (+) | 3.19 ± 1.39 | 3.16 ± 1.39 | 3.60 ± 1.37 | 0.029 | 3.35 ± 1.37 | 3.18 ± 1.42 | 3.09 ± 1.34 | 0.17 | 3.26 ± 1.42 | 3.14 ± 1.37 | 3.18 ± 1.37 | 0.61 |
| 3. Interpersonal Style | ||||||||||||
| Compassionate, respectful (+) | 4.15 ± 0.91 | 4.13 ± 0.92 | 4.44 ± 0.68 | 0.034 | 4.17 ± 0.92 | 4.11 ± 0.94 | 4.20 ± 0.86 | 0.75 | 4.30 ± 0.83 | 4.06 ± 1.00 | 4.09 ± 0.88 | 0.014 |
| Emotional support, comp (+) | 4.08 ± 0.99 | 4.06 ± 1.00 | 4.35 ± 0.81 | 0.072 | 4.07 ± 1.00 | 4.01 ± 1.04 | 4.17 ± 0.91 | 0.37 | 4.16 ± 0.97 | 3.99 ± 1.06 | 4.07 ± 0.92 | 0.18 |
| Respectful (+) | 4.27 ± 0.94 | 4.24 ± 0.95 | 4.57 ± 0.66 | 0.027 | 4.31 ± 0.93 | 4.26 ± 0.95 | 4.25 ± 0.93 | 0.60 | 4.49 ± 0.77 | 4.16 ± 1.03 | 4.12 ± 0.96 | <0.0001 |
| Discrimination (−) | 1.42 ± 0.72 | 1.42 ± 0.72 | 1.35 ± 0.81 | 0.21 | 1.47 ± 0.79 | 1.36 ± 0.69 | 1.46 ± 0.72 | 0.11 | 1.29 ± 0.65 | 1.43 ± 0.77 | 1.55 ± 0.73 | <0.0001 |
| Assumed SES (−) | 1.52 ± 0.94 | 1.52 ± 0.94 | 1.52 ± 1.04 | 0.69 | 1.59 ± 0.94 | 1.47 ± 0.94 | 1.55 ± 0.96 | 0.033 | 1.37 ± 0.88 | 1.49 ± 0.96 | 1.73 ± 0.96 | <0.0001 |
| Discriminated due to race (−) | 1.32 ± 0.71 | 1.34 ± 0.70 | 1.17 ± 0.72 | 0.0093 | 1.35 ± 0.81 | 1.28 ± 0.64 | 1.38 ± 0.71 | 0.056 | 1.22 ± 0.65 | 1.37 ± 0.76 | 1.40 ± 0.70 | 0.0004 |
| Disrespectful office staff (−) | 1.39 ± 0.73 | 1.39 ± 0.73 | 1.34 ± 0.74 | 0.28 | 1.43 ± 0.81 | 1.36 ± 0.67 | 1.41 ± 0.75 | 0.92 | 1.28 ± 0.66 | 1.44 ± 0.75 | 1.46 ± 0.77 | 0.0013 |
Domains are underlined, scales are in italic font, subdomains are indented. A higher score indicates higher frequency of the labeled construct. Abbreviations HS: high school; meds: medications; PC: patient-centered; comp: compassionate; SES: socioeconomic status. P-values are calculated with Wilcoxon Rank-sum test or Kruskal Wallis test.
When IPC-29 mean scores were analyzed by demographics (Table 3), we found that females reported significantly higher mean scores for all negative communication constructs (hurried communication [F: 1.75, SD 0.76; M: 1.44, SD 0.61], lack of clarity [F: 1.90; SD 0.90; M: 1.64, SD 0.83] and hurried, distracted [F: 1.64, SD 0.81; M:1.31, SD 0.54]), compared to males. All positive constructs of the patient-centered decision-making and interpersonal style domains were also significantly lower in females compared to males, with the exception of the emotional support subdomain. Significantly higher mean scores for the discriminated due to race or ethnicity subdomain were observed in females (1.34, SD 0.70), compared to males (1.17, SD 0.72).
IPC-29 scores did not show significant differences by age groups, except for the assumed SES subdomain within the discrimination scale. The youngest (18–34) group reported statistically significant higher mean score (1.59, SD 0.94) for assumed SES (patient perceived that doctors made assumptions about his/her education or income) compared to those aged 35–54 (mean score 1.47, SD 0.94).
Significant differences of mean scores by educational attainment were observed for both positive and negative constructs of the communication and interpersonal style domains, but not for patient-centered decision making. Compared with participants who reported some college or higher education, those with high school or less showed significantly higher mean scores in the explained results and medications scale, the compassionate, respectful scale, and the respectful subdomain. The lowest scores within positive constructs were for the patient-centered decision making domain. In contrast, the respectful subdomain showed the highest mean scores, with a significant higher mean among those who achieved lower education (mean score 4.49, SD 0.77), compared to those with some college (mean score 4.16, SD 1.03), and college or higher (mean score 4.12, SD 0.96). Participants who achieved greater educational attainment reported highest scores for the discrimination constructs.
IPC-29 by Disease Activity
IPC mean scores across disease activity categories are depicted in Table 4. When crude scores were analyzed, a significant trend of increasingly higher mean scores from mild to moderate and to severe disease activity was found for all negative IPC-29 scales and subdomains. A significant association in the opposite direction was found for all positive constructs, with the exception of decided together in the patient-centered decision-making domain.
Table 4.
Crude and Adjusted Scores of Interpersonal Processes of Care Across Disease Activity in African American Patients with SLE
| IPC Characteristic | Crude mean (95%CI) of IPC | Adjusted* mean (95%CI) of IPC | ||||||
|---|---|---|---|---|---|---|---|---|
| Mild DA n=219 | Moderate DA n=246 | Severe DA n=233 | P value | Mild DA n=219 | Moderate DA n=246 | Severe DA n=233 | P value | |
| 1.Communication | ||||||||
| Hurried communication (−) | 1.49 (1.39–1.59) | 1.71 (1.62–1.80) | 1.96 (1.87–2.06) | <0.0001 | 1.49 (1.34–1.64) | 1.63 (1.49–1.78) | 1.78 (1.61–1.94) | 0.0008 |
| Lack of clarity (−) | 1.66 (1.54–1.78) | 1.91 (1.80–2.02) | 2.07 (1.96–2.19) | <0.0001 | 1.69 (1.51–1.87) | 1.84 (1.66–2.01) | 1.88 (1.69–2.08) | 0.064 |
| Hurried, distracted (−) | 1.38 (1.27–1.48) | 1.57 (1.48–1.67) | 1.89 (1.79–1.99) | <0.0001 | 1.36 (1.20–1.51) | 1.50 (1.34–1.65) | 1.70 (1.53–1.88) | 0.0001 |
| Elicited concerns, responded (+) | 4.37 (4.25–4.49) | 4.18 (4.07–4.29) | 3.92 (3.81–4.04) | <0.0001 | 4.36 (4.18–4.54) | 4.22 (4.04–4.40) | 4.06 (3.86–4.26) | 0.0043 |
| Explained results, meds (+) | 4.03 (3.89–4.17) | 3.89 (3.76–4.03) | 3.65 (3.52–3.79) | 0.0002 | 4.04 (3.82–4.25) | 3.95 (3.73–4.16) | 3.77 (3.53–4.01) | 0.032 |
| Explained results (+) | 4.30 (4.16–4.44) | 4.25 (4.12–4.38) | 3.89 (3.75–4.02) | <0.0001 | 4.24 (4.02–4.45) | 4.24 (4.02–4.45) | 3.95 (3.72–4.19) | 0.022 |
| Explained medications (+) | 3.77 (3.60–3.94) | 3.54 (3.38–3.70) | 3.43 (3.26–3.59) | 0.0047 | 3.84 (3.59–4.10) | 3.66 (3.41–3.91) | 3.59 (3.31–3.88) | 0.095 |
| 2. Decision Making | ||||||||
| PC decision making (+) | 3.30 (3.13–3.47) | 3.17 (3.01–3.33) | 3.05 (2.89–3.22) | 0.044 | 3.34 (3.08–3.60) | 3.26 (3.00–3.52) | 3.18 (2.90–3.47) | 0.30 |
| Asked patient (+) | 3.28 (3.09–3.47) | 3.20 (3.03–3.38) | 3.01 (2.83–3.19) | 0.048 | 3.39 (3.10–3.68) | 3.36 (3.08–3.65) | 3.22 (2.90–3.54) | 0.31 |
| Decided together (+) | 3.32 (3.14–3.51) | 3.15 (2.98–3.33) | 3.10 (2.92–3.28) | 0.090 | 3.28 (2.99–3.56) | 3.17 (2.89–3.45) | 3.15 (2.84–3.46) | 0.43 |
| 3. Interpersonal Style | ||||||||
| Compassionate, respectful (+) | 4.41 (4.29–4.53) | 4.15 (4.04–4.27) | 3.91 (3.80–4.03) | <0.0001 | 4.41 (4.23–4.59) | 4.20 (4.03–4.38) | 4.06 (3.86–4.25) | 0.0007 |
| Emotional support, com (+) | 4.35 (4.22–4.48) | 4.09 (3.97–4.21) | 3.81 (3.68–3.93) | <0.0001 | 4.30 (4.10–4.50) | 4.10 (3.90–4.29) | 3.92 (3.70–4.14) | 0.0008 |
| Respectful (+) | 4.49 (4.37–4.61) | 4.25 (4.14–4.37) | 4.07 (3.95–4.19) | <0.0001 | 4.56 (4.38–4.75) | 4.37 (4.18–4.55) | 4.25 (4.04–4.45) | 0.0036 |
| Discriminated (−) | 1.27 (1.17–1.36) | 1.41 (1.32–1.50) | 1.57 (1.48–1.66) | <0.0001 | 1.28 (1.14–1.43) | 1.40 (1.26–1.55) | 1.51 (1.35–1.67) | 0.0078 |
| Assumed SES (−) | 1.40 (1.27–1.52) | 1.47 (1.35–1.59) | 1.69 (1.57–1.82) | 0.0008 | 1.42 (1.23–1.61) | 1.50 (1.31–1.69) | 1.68 (1.47–1.89) | 0.017 |
| Discriminated due to race (−) | 1.15 (1.06–1.24) | 1.35 (1.26–1.43) | 1.46 (1.37–1.55) | <0.0001 | 1.16 (1.02–1.30) | 1.30 (1.16–1.44) | 1.34 (1.19–1.50) | 0.023 |
| Disrespectful office staff (−) | 1.22 (1.12–1.31) | 1.42 (1.33–1.51) | 1.52 (1.42–1.61) | <0.0001 | 1.26 (1.12–1.41) | 1.40 (1.26–1.55) | 1.39 (1.23–1.55) | 0.13 |
Domains are underlined, scales are in italic font, subdomains are indented. A higher score indicates higher frequency of the labeled construct.
Adjusted by age, disease duration, gender, education, insurance status, organ damage, and depression. DA: disease activity; P values are calculated with linear trend test.
We then adjusted the scores by sociodemographic characteristics, organ damage and severity of depression symptoms. Adjusted mean scores were slightly lower for moderate and severe disease activity categories. The increasingly higher scores across more severe disease activity categories remained significant for most negative constructs of the communication and interpersonal style domains, except for the lack of clarity subdomain and the disrespectful staff scale. The highest scores within negative constructs were for hurried communication, with means of 1.49, 1.63, and 1.78 for patients with mild, moderate and severe disease activity, respectively (p=0.0008); followed by assumed socioeconomic status (SES), with means of 1.42, 1.50, and 1.68 in those with mild, moderate and severe disease activity, respectively (p=0.017). The trend of increasingly greater mean scores for positive constructs across less severe disease activity categories remained statistically significant for all communication scales and subdomains, except explained medications. Similarly, all interpersonal style constructs showed a trend of increasingly higher scores in those individuals with milder disease activity, compared with those in the moderate and severe categories. Patient-centered decision-making scales showed the lowest means for positive constructs of the three major domains, but the linear trend test did not show any statistical significance across activity severity.
IPC-29 by Depression Symptoms
IPC-29 crude and adjusted scores across severity of depression symptoms are depicted in Table 5. We observed a statistically significant trend of progressively higher crude means of all negative IPC-29 constructs as the severity of depression symptoms increased. For instance, means for lack of clarity were 1.70, 1.82, and 2.17 for patients with minimal, mild, and moderate-to-severe depression, respectively (p<0.0001). We also found a significant association in the opposite direction for all positive constructs, with the exception of the subdomain decided together, in the patient-centered decision-making domain. The positive constructs that showed the lowest means were also those in the patient-centered decision making domain and it was a significant trend of lower scores in patients with more severe depression (p=0.049).
Table 5.
Crude and Adjusted Scores of Interpersonal Processes of Care Across Depression Symptoms in African American Patients with SLE
| IPC Characteristic | Crude mean (95%CI) of IPC | Adjusted* mean (95%CI) of IPC | ||||||
|---|---|---|---|---|---|---|---|---|
| Minimal Depression n=249 | Mild Depression n=193 | Mod-Severe Depression n=213 | P value | Minimal Depression n=249 | Mild Depression n=193 | Mod-Severe Depression n=213 | P value | |
| 1. Communication | ||||||||
| Hurried communication (−) | 1.53 (1.44–1.62) | 1.68 (1.58–1.77) | 1.99 (1.90–2.09) | <0.0001 | 1.55 (1.40–1.70) | 1.58 (1.43–1.73) | 1.84 (1.68–1.99) | 0.0004 |
| Lack of clarity (−) | 1.70 (1.59–1.80) | 1.82 (1.70–1.93) | 2.17 (2.05–2.28) | <0.0001 | 1.72 (1.53–1.90) | 1.74 (1.55–1.92) | 2.02 (1.84–2.21) | 0.0016 |
| Hurried, distracted (−) | 1.42 (1.33–1.51) | 1.58 (1.48–1.69) | 1.87 (1.77–1.97) | <0.0001 | 1.44 (1.28–1.60) | 1.47 (1.31–1.63) | 1.71 (1.55–1.87) | 0.0014 |
| Elicited concerns, responded (+) | 4.31 (4.20–4.42) | 4.22 (4.10–4.33) | 3.91 (3.79–4.03) | <0.0001 | 4.27 (4.08–4.45) | 4.29 (4.11–4.48) | 4.04 (3.85–4.23) | 0.022 |
| Explained results, meds (+) | 4.04 (3.91–4.17) | 3.90 (3.75–4.04) | 3.61 (3.47–3.75) | <0.0001 | 4.01 (3.79–4.23) | 3.97 (3.76–4.19) | 3.71 (3.48–3.93) | 0.010 |
| Explained results (+) | 4.34 (4.21–4.47) | 4.22 (4.07–4.36) | 3.85 (3.71–3.99) | <0.0001 | 4.23 (4.01–4.45) | 4.23 (4.01–4.45) | 3.90 (3.68–4.13) | 0.0054 |
| Explained medications (+) | 3.74 (3.59–3.90) | 3.58 (3.40–3.75) | 3.38 (3.21–3.55) | 0.0019 | 3.79 (3.53–4.06) | 3.72 (3.46–3.98) | 3.52 (3.25–3.79) | 0.05 |
| 2. Decision Making | ||||||||
| PC decision making (+) | 3.22 (3.06–3.37) | 3.32 (3.14–3.49) | 2.98 (2.82–3.15) | 0.049 | 3.26 (2.99–3.52) | 3.42 (3.16–3.69) | 3.09 (2.82–3.36) | 0.25 |
| Asked patient (+) | 3.24 (3.06–3.41) | 3.26 (3.07–3.46) | 2.98 (2.80–3.17) | 0.050 | 3.34 (3.04–3.63) | 3.44 (3.15–3.74) | 3.16 (2.86–3.46) | 0.27 |
| Decided together (+) | 3.20 (3.03–3.37) | 3.38 (3.19–3.57) | 2.99 (2.81–3.17) | 0.089 | 3.18 (2.89–3.47) | 3.40 (3.11–3.68) | 3.01 (2.72–3.30) | 0.27 |
| 3. Interpersonal Style | ||||||||
| Compassionate, respectful (+) | 4.32 (4.21–4.42) | 4.23 (4.11–4.35) | 3.89 (3.77–4.01) | <0.0001 | 4.25 (4.07–4.44) | 4.32 (4.13–4.50) | 4.04 (3.85–4.23) | 0.033 |
| Emotional support, com (+) | 4.26 (4.14–4.38) | 4.16 (4.03–4.29) | 3.79 (3.66–3.91) | <0.0001 | 4.15 (3.95–4.35) | 4.20 (4.00–4.40) | 3.91 (3.70–4.11) | 0.024 |
| Respectful (+) | 4.40 (4.29–4.51) | 4.34 (4.22–4.47) | 4.04 (3.92–4.16) | <0.0001 | 4.40 (4.21–4.60) | 4.49 (4.30–4.67) | 4.24 (4.04–4.43) | 0.10 |
| Discriminated (−) | 1.33 (1.24–1.41) | 1.37 (1.27–1.46) | 1.57 (1.47–1.66) | 0.0002 | 1.39 (1.24–1.54) | 1.33 (1.18–1.48) | 1.50 (1.34–1.65) | 0.18 |
| Assumed SES (−) | 1.44 (1.33–1.55) | 1.49 (1.36–1.62) | 1.65 (1.52–1.77) | 0.016 | 1.54 (1.34–1.73) | 1.47 (1.28–1.66) | 1.60 (1.40–1.80) | 0.57 |
| Discriminated due to race (−) | 1.22 (1.14–1.30) | 1.25 (1.16–1.35) | 1.51 (1.42–1.60) | <0.0001 | 1.23 (1.09–1.38) | 1.19 (1.05–1.33) | 1.41 (1.26–1.56) | 0.027 |
| Disrespectful office staff (−) | 1.25 (1.16–1.34) | 1.34 (1.24–1.44) | 1.60 (1.51–1.69) | <0.0001 | 1.27 (1.12–1.42) | 1.30 (1.15–1.45) | 1.53 (1.38–1.68) | 0.0012 |
Domains are underlined, scales are in italic font, subdomains are indented. A higher score indicates higher frequency of the labeled construct.
Adjusted by age, disease duration, gender, education, insurance, organ damage, and disease activity.
Mod: moderate; Sev: severe. P values are calculated with linear trend test.
When means were adjusted for socio-demographics and disease-related factors, communication scores of most positive and negative constructs remained statistically significant as depression symptoms increased in severity, with the exception of the explained medications subdomain. (Table 5). Adjusted mean scores within the decision-making domain were not statistically significant between the different levels of depression. After means were adjusted, the inverse trend of greater severity of depression symptoms and positive interpersonal style constructs remained statistically significant only for the compassionate/respectful scale and the emotional support subdomains. Within the interpersonal style domain, severity of depression symptoms remained statistically associated with adjusted scores of the discrimination due to race subdomain and the disrespectful staff scale, but not for the assumed SES subdomain.
Effect of Depression and Disease Activity on Suboptimal IPC
Table 6 depicts the independent effects of depression and disease activity on suboptimal communication (Model 1), shared decision making (Model 2) and interpersonal style (Model 3). Model 1 rendered a statistically significant association of depression but not disease activity with suboptimal communication. After adjustment for potential confounders, per 3-unit increase of the depression score, the odds of suboptimal communication increased in 20% (OR 1.20; 95% CI 1.04–1.39; p=0.012). Neither depression nor disease activity had a significant effect on suboptimal patient-centered decision making (Model 2). However, both disease activity and depression were found to be significantly associated with suboptimal interpersonal style (Model 3). After adjustment for confounders, per 4-unit increase of the disease activity score, the odds of interpersonal style increased in 13% (OR 1.13; 95% CI 1.03–1.25; p=0.014), and per 3-unit increase of the depression score, the odds of interpersonal style increased in 12% (OR 1.12; 95% CI 1.01–1.25; p=0.03).
Table 6.
Multivariate Models of the Effect of Disease Activity and Depression on Suboptimal Quality of IPC in African American Patients with SLE
| Variable | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| Suboptimal Communication | Suboptimal Patient-centered Decision Making | Suboptimal Interpersonal Style | ||||
| OR (95%CI) | P Value | OR (95%CI) | P Value | OR (95%CI) | P Value | |
| Disease activity (per 4 unit↑) | 1.09 (0.96–1.24) | 0.18 | 1.09 (0.98–1.22) | 0.12 | 1.13 (1.03–1.25) | 0.014 |
| Depression (per 3 unit↑) | 1.20 (1.04–1.39) | 0.012 | 1.08 (0.96–1.21) | 0.22 | 1.12 (1.01–1.25) | 0.03 |
Proportional odds ratios (ORs) are adjusted for age, gender, disease duration, education, insurance status, and accrual organ damage
Sensitivity Analyses
We added healthcare facility (Grady, Emory, private practices, and multiple facilities) as a control variable in the trend and multivariate analyses. The trends of increasingly worse IPC with either more severe disease activity or more severe depression remained the same after controlling for facility (Supplementary Tables 1 and 2). Similarly, the significant association between disease activity and suboptimal interpersonal style, as well as between depression and suboptimal communication and interpersonal style remained the same after controlling the analysis for healthcare facility (Supplementary Table 3).
Discussion
This study examined patient perceptions of interpersonal processes of care (IPC) amongst African American individuals with SLE. Our findings underscore substantial differences of IPC by gender and educational attainment, and suggest that the severity of both disease activity and depression might impact specific processes during medical encounters in this population.
Compared to men, women reported more frequent negative experiences related to their provider’s communication (e.g. hurried, lack of clarity, distracted). Moreover, more women than men reported that their providers engaged less frequently in patient-centered decision making, and were less compassionate and respectful. As prior data suggest that female doctors engage in more positive and longer discussion with their patients than male doctors (35, 36), further research is needed to determine whether gender differences in communication scores in the African American SLE population might be mediated by unmet expectations related to the gender of the physician. Women in our study also reported more discrimination or inattentiveness of providers due their race or ethnicity than males, which raises the question about whether African American females perceive more discrimination or they are more exposed to objective experiences of healthcare discrimination. An alternative explanation is that physicians spend more time with men because outcomes in this group are deemed to be worse.
With the exception of a worse mean score for assumed socioeconomic status (interpersonal style domain) in younger individuals, no substantial differences were observed across IPC by age. Our data are consistent with those from a predominantly white SLE cohort(37), which reported no significant differences by age in patient-reported quality of communication. Further research is needed to examine whether younger African American subjects with SLE may perceive more discrimination, or be more frequently exposed to true experiences of discrimination in the healthcare, compared to their white counterparts(38).
Patients who achieved some college or higher education were more likely to report worse scores for physician-patient communication and interpersonal style, compared to those with high school or less. These findings contrast with prior reports of less effective communication between providers and patients of lower educational level(39). Our data suggest that in the African American population, the balance between patient’s expectations and satisfaction about healthcare interactions may be more difficult to obtain among the more educated patients. Whether a provider’s subconscious bias about “lower” education level within this racial group might play a role on those findings warrants further investigation. Although patient-centered decision making scales also tended to be worse in patients who achieved higher education, no significant differences were observed by education attainment.
Our results also indicate that patient’s perceptions of IPC can be influenced by disease activity and depression symptoms. We found statistically significant trends of increasingly worse adjusted scores for most communication and interpersonal style constructs across mild, moderate, and severe disease activity. Greater severity of depression symptoms was also significantly associated with increasingly worse adjusted scores of IPC for the hurried communication, explained results/medications, and disrespectful office staff scales.
Disease activity and depression were independently associated with suboptimal quality of overall provider’s interpersonal style, and depression was also associated with suboptimal quality of overall physician-patient communication, after adjusting for demographics, permanent organ damage and disease duration. Interestingly, neither disease activity nor depression were significantly associated with suboptimal quality patient-centered decision-making.
To our knowledge, this is the first study of IPC in a large African American cohort with SLE. Our data indicate that, as in other chronic conditions, depression in SLE may have a negative impact on physician-patient communication(12, 28). Because depression is highly prevalent in African American patients with SLE, depression screening and proper management may result not only in better mental outcomes but may also contribute to improve physician-patient interactions. Moreover, we found that both disease activity and depression are related to patients’ negative perceptions about physicians’ interpersonal style. These findings suggest that in addition to standard of care treatment, SLE patients with depression and active disease might need provider-based interventions focused on communication and interpersonal style. Our results also indicate that there is room for improvement in the decision-making process, which on average was reported to be shared only ‘sometimes’ between providers and patients, throughout all demographic and disease severity subgroups.
Our study has limitations. First, the cross-sectional design does not allow one to establish a cause-effect relationship and we cannot rule out that poor quality of physician-patient interactions leads to worse outcomes in the African American population with SLE, as it was described in predominantly white populations(37, 40). However, findings from studies in people with other chronic conditions support an influence of both sociodemographic and disease-related factors on IPC, possibly mediated by patient’s perceptions and expectations(10–13). Second, IPC responses are subject to social desirability bias, and participants cared for at Grady or Emory facilities, which are affiliated with the Emory study investigators, might be more susceptible to that bias than those cared for at other practices. However, those biases have been minimized by several methodological strategies, including the IPC questions that assess patients’ experiences with their doctors in general without pointing to any specific provider or clinical setting, the use of mail as the primary delivery mode of the GOAL survey, and the lack of participant’s identifier in the survey. Moreover, results from sensitivity analyses remained the same after controlling for the source of care. Third, we were not able to take into account physician and patient factors that might play a role in patient-reported IPC, such as demographic characteristics of the provider, length and complexity of encounters, or patient health literacy and trust in the healthcare system.
Conclusion
This study indicates that in the African American population with SLE, patient perceptions of providers’ communication and interpersonal style vary widely by demographics and disease characteristics. Among African American patients with SLE, women and those with higher educational attainment reported poorer communication and less interpersonal style of care. Moreover, African American individuals with SLE who had greater disease activity and more severe depression symptoms reported poorer communication and less personable involvement by their doctors. These findings suggest that patients with more severe disease may need more attention, effective communication and agreeable style by providers. Recognizing areas for improvement, as well as demographic and disease-related disparities in patient’s perceptions of IPC are fundamental to design tailored interventions to provide better quality of healthcare and improve outcomes in high-risk populations with SLE.
Supplementary Material
Acknowledgements
We thank the participants of the GOAL study.
Funding
Supported by Human Genome Science Inc. and GlaxoSmithKline (GHO-11–3366) and the Centers for Disease Control and Prevention (CDC) grant U01DP005119.
Footnotes
Declaration of Conflicting Interests
Drenkard C and Lim SS report a grant from GlaxoSmithKline (GSK) during the conduct of the study. GSK had no role in the study design or in the collection, analysis, or the decision to submit the manuscript for publication. Publication of this article was not contingent upon approval by GlaxoSmithKline.
Bao G and Lewis TT declare no conflicts of interest.
Pobiner Band Priest J are employees of GSK and own stock and/or stock options in GSK.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Napoles AM, Gregorich SE, Santoyo-Olsson J, O’Brien H, Stewart AL. Interpersonal processes of care and patient satisfaction: do associations differ by race, ethnicity, and language? Health services research. 2009;44(4):1326–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bauer AM, Parker MM, Schillinger D, Katon W, Adler N, Adams AS, et al. Associations between antidepressant adherence and shared decision-making, patient-provider trust, and communication among adults with diabetes: diabetes study of Northern California (DISTANCE).J Gen Intern Med. 2014;29(8):1139–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Safran DG, Karp M, Coltin K, Chang H, Li A, Ogren J, et al. Measuring patients’ experiences with individual primary care physicians. Results of a statewide demonstration project. J Gen Intern Med. 2006;21(1):13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenthal MB, Fernandopulle R, Song HR, Landon B. Paying for quality: providers’ incentives for quality improvement. Health Aff (Millwood). 2004;23(2):127–41. [DOI] [PubMed] [Google Scholar]
- 5.Cuffee YL, Hargraves JL, Rosal M, Briesacher BA, Schoenthaler A, Person S, et al. Reported racial discrimination, trust in physicians, and medication adherence among inner-city African Americans with hypertension. Am J Public Health. 2013;103(11):e55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stewart AL, Napoles-Springer AM, Gregorich SE, Santoyo-Olsson J. Interpersonal processes of care survey: patient-reported measures for diverse groups. Health services research. 2007;42(3 Pt 1):1235–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stewart AL, Nápoles-Springer A, Pérez-Stable EJ. Interpersonal processes of care in diverse populations. The Milbank quarterly. 1999;77(3):305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naranjo DM, Fisher L, Arean PA, Hessler D, Mullan J. Patients with type 2 diabetes at risk for major depressive disorder over time. Ann Fam Med. 2011;9(2):115–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seligman HK, Fernandez A, Stern RJ, Weech-Maldonado R, Quan J, Jacobs EA. Risk factors for reporting poor cultural competency among patients with diabetes in safety net clinics. Med Care. 2012;50(9 Suppl 2):S56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beverly EA, Ganda OP, Ritholz MD, Lee Y, Brooks KM, Lewis-Schroeder NF, et al. Look who’s (not) talking: diabetic patients’ willingness to discuss self-care with physicians. Diabetes Care. 2012;35(7):1466–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall JA, Milburn MA, Roter DL, Daltroy LH. Why are sicker patients less satisfied with their medical care? Tests of two explanatory models. Health Psychol. 1998;17(1):70–5. [DOI] [PubMed] [Google Scholar]
- 12.Schenker Y, Stewart A, Na B, Whooley MA. Depressive symptoms and perceived doctor-patient communication in the Heart and Soul study. J Gen Intern Med. 2009;24(5):550–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jonassaint CR, Haywood C Jr., Korthuis PT, Cooper LA, Saha S, Sharp V, et al. The impact of depressive symptoms on patient-provider communication in HIV care. AIDS Care. 2013;25(9):1185–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duru OK, Gerzoff RB, Selby JV, Brown AF, Ackermann RT, Karter AJ, et al. Identifying risk factors for racial disparities in diabetes outcomes: the translating research into action for diabetes study. Med Care. 2009;47(6):700–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lim SS, Bayakly AR, Helmick CG, Gordon C, Easley KA, Drenkard C. The incidence and prevalence of systemic lupus erythematosus, 2002–2004: The Georgia Lupus Registry. Arthritis Rheumatol. 2014;66(2):357–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Somers EC, Marder W, Cagnoli P, Lewis EE, DeGuire P, Gordon C, et al. Population-based incidence and prevalence of systemic lupus erythematosus: the Michigan Lupus Epidemiology and Surveillance program. Arthritis Rheumatol. 2014;66(2):369–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Odutola J, Ward MM. Ethnic and socioeconomic disparities in health among patients with rheumatic disease. Curr Opin Rheumatol. 2005;17(2):147–52. [DOI] [PubMed] [Google Scholar]
- 18.Nee R, Jindal RM, Little D, Ramsey-Goldman R, Agodoa L, Hurst FP, et al. Racial differences and income disparities are associated with poor outcomes in kidney transplant recipients with lupus nephritis. Transplantation. 2013;95(12):1471–8. [DOI] [PubMed] [Google Scholar]
- 19.Crosslin KL, Wiginton KL. The impact of race and ethnicity on disease severity in systemic lupus erythematosus. Ethn Dis. 2009;19(3):301–7. [PubMed] [Google Scholar]
- 20.Knight AM, Xie M, Mandell DS. Disparities in Psychiatric Diagnosis and Treatment for Youth with Systemic Lupus Erythematosus: Analysis of a National US Medicaid Sample. J Rheumatol. 2016;43(7):1427–33. [DOI] [PubMed] [Google Scholar]
- 21.Vina ER, Hausmann LR, Utset TO, Masi CM, Liang KP, Kwoh CK. Perceptions of racism in healthcare among patients with systemic lupus erythematosus: a cross-sectional study. Lupus science & medicine. 2015;2(1):e000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ha JF, Longnecker N. Doctor-patient communication: a review. Ochsner J. 2010;10(1):38–43. [PMC free article] [PubMed] [Google Scholar]
- 23.Budych K, Helms TM, Schultz C. How do patients with rare diseases experience the medical encounter? Exploring role behavior and its impact on patient-physician interaction. Health Policy. 2012;105(2–3):154–64. [DOI] [PubMed] [Google Scholar]
- 24.Drenkard CRK, Easley K, Bao G. Lim SS. Primary Preventive Services in Patients with Systemic Lupus Erythematosus: Study from a Population-based Sample in Southeast U.S. Semin Arthritis Rheum (submitted under review). 2013. [DOI] [PubMed]
- 25.Lim SS, Bayakly R, Gordon C, Helmick CG, Easley K, Bao G, et al. The Georgia Lupus Registry: The Incidence and Prevalence of Systemic Lupus Erythematosus. Arthritis Rheum. 2011;63(10 [abstract]):S–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40(9):1725. [DOI] [PubMed] [Google Scholar]
- 27.Schmajuk G, Yazdany J, Trupin L, Yelin E. Hydroxychloroquine treatment in a community-based cohort of patients with systemic lupus erythematosus. Arthritis Care Res (Hoboken). 2010;62(3):386–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.White RO, Chakkalakal RJ, Presley CA, Bian A, Schildcrout JS, Wallston KA, et al. Perceptions of Provider Communication Among Vulnerable Patients With Diabetes: Influences of Medical Mistrust and Health Literacy. J Health Commun. 2016;21(sup2):127–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hyphantis T, Kotsis K, Voulgari PV, Tsifetaki N, Creed F, Drosos AA. Diagnostic accuracy, internal consistency, and convergent validity of the Greek version of the patient health questionnaire 9 in diagnosing depression in rheumatologic disorders. Arthritis Care Res (Hoboken). 2011;63(9):1313–21. [DOI] [PubMed] [Google Scholar]
- 30.Shim RS, Baltrus P, Ye J, Rust G. Prevalence, treatment, and control of depressive symptoms in the United States: results from the National Health and Nutrition Examination Survey (NHANES), 2005–2008. Journal of the American Board of Family Medicine : JABFM. 2011;24(1):33–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wittayanukorn S, Qian J, Hansen RA. Prevalence of depressive symptoms and predictors of treatment among U.S. adults from 2005 to 2010. General hospital psychiatry. 2014;36(3):330–6. [DOI] [PubMed] [Google Scholar]
- 32.Yazdany J, Trupin L, Gansky SA, Dall’era M, Yelin EH, Criswell LA, et al. Brief index of lupus damage: a patient-reported measure of damage in systemic lupus erythematosus. Arthritis Care Res (Hoboken). 2011;63(8):1170–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Drenkard C, Yazdany J, Trupin L, Katz PP, Dunlop-Thomas C, Bao G, et al. Validity of a Self-Administered Version of the Brief Index of Lupus Damage in a Predominantly African American Systemic Lupus Erythematosus Cohort. Arthritis Care & Research. 2014;66(6):888–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41(5):582–92. [DOI] [PubMed] [Google Scholar]
- 35.Roter DL, Hall JA, Aoki Y. Physician gender effects in medical communication: a meta-analytic review. JAMA. 2002;288(6):756–64. [DOI] [PubMed] [Google Scholar]
- 36.Hall JA, Irish JT, Roter DL, Ehrlich CM, Miller LH. Gender in medical encounters: an analysis of physician and patient communication in a primary care setting. Health psychology : official journal of the Division of Health Psychology, American Psychological Association. 1994;13(5):384–92. [DOI] [PubMed] [Google Scholar]
- 37.Yelin E, Yazdany J, Tonner C, Trupin L, Criswell LA, Katz P, et al. Interactions between patients, providers, and health systems and technical quality of care. Arthritis Care Res (Hoboken). 2015;67(3):417–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hausmann LR, Jeong K, Bost JE, Ibrahim SA. Perceived discrimination in health care and health status in a racially diverse sample. Med Care. 2008;46(9):905–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brown SJ. Patient-centered communication. Annu Rev Nurs Res. 1999;17:85–104. [PubMed] [Google Scholar]
- 40.Yelin E, Yazdany J, Trupinx L. Relationship Between Process of Care and a Subsequent Increase in Damage in Systemic Lupus Erythematosus. Arthritis care & research. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.