Abstract
Wildfires are increasing in incidence and severity across coniferous forests of the western United States, leading to changes in forest structure and wildlife habitats. Knowledge of how species respond to fire‐driven habitat changes in these landscapes is limited and generally disconnected from our understanding of adaptations that underpin responses to fire.
We aimed to investigate drivers of occupancy of a diverse bat community in a fire‐altered landscape, while identifying functional traits that underpinned these relationships.
We recorded bats acoustically at 83 sites (n = 249 recording nights) across the Plumas National Forest in the northern Sierra Nevada over 3 summers (2015–2017). We investigated relationships between fire regime, physiographic variables, forest structure and probability of bat occupancy for nine frequently detected species. We used fourth‐corner regression and RLQ analysis to identify ecomorphological traits driving species–environment relationships across 17 bat species. Traits included body mass; call frequency, bandwidth, and duration; and foraging strategy based on vegetation structure (open, edge, or clutter).
Relationships between bat traits and fire regime were underpinned by adaptations to diverse forest structure. Bats with traits adapting them to foraging in open habitats, including emitting longer duration and narrow bandwidth calls, were associated with higher severity and more frequent fires, whereas bats with traits consistent with clutter tolerance were negatively associated with fire frequency and burn severity. Relationships between edge‐adapted bat species and fire were variable and may be influenced by prey preference or habitat configuration at a landscape scale.
Predicted increases in fire frequency and severity in western US coniferous forests are likely to shift dominance in the bat community to open‐adapted species and those able to exploit postfire resource pulses (aquatic insects, beetles, and snags). Managing for pyrodiversity within the western United States is likely important for maintaining bat community diversity, as well as diversity of other biotic communities.
Keywords: acoustic, community ecology, ecomorphology, fire ecology, fourth‐corner, RLQ, traits, western United States
1. INTRODUCTION
Anthropogenic climate change, drought, fire suppression, and human land use change have led to increases in fire frequency and severity across the globe (Stephens et al., 2014). Changes in fire regime have been particularly evident in the western United States where climate change, land use change, and suppression of more frequent, low severity fires characteristic of historical conditions (Mallek, Safford, Viers, & Miller, 2013; Safford & Stevens, 2017) have led to increases in the number and extent of high severity fires (Dennison, Brewer, Arnold, & Moritz, 2014; Miller & Safford, 2012). With the continued effects of climate change, the western United States is projected to experience 24%–169% increases in annual area burned and an increase in fire season length by 23 days by the mid‐21st century (Yue, Mickley, Logan, & Kaplan, 2013). Although many forests in the western United States are maintained by fire regimes that promote forest heterogeneity (Baker, 2012,2014) and animals have evolved with fire (Pausas & Parr, 2018), comparatively little is known about the implications of rapidly changing fire regimes for managing and maintaining forest biodiversity in the western United States.
Bats are diverse, high trophic level predators that are impacted directly by fire through mortality and injury and indirectly by alteration in roost and foraging habitat availability, and prey communities (Carter, Ford, & Menzel, 2000; Perry, 2012). Fires may influence bat foraging via several mechanisms, mediated by bat ecomorphological traits (Perry, 2012). Fires can substantially alter forest structure by reducing clutter (structurally complex vegetation) and increasing open space, sometimes in the long term (Beaty & Taylor, 2008). Forest structure is an important determinant of insectivorous bat assemblages (Blakey, Law, Kingsford, & Stoklosa, 2017), as bats have diverse morphological and call adaptations for a range of forests from cluttered to open in structure (Schnitzler, Moss, & Denzinger, 2003). For example, a large‐bodied bat with narrow (high aspect ratio) wings and a long duration, low‐frequency call is well adapted to forage on fast prey in open spaces, but has difficulty maneuvering and detecting prey in cluttered habitat (Denzinger & Schnitzler, 2013). In contrast, clutter‐adapted bats can differentiate prey from surrounding vegetation using high frequency, wide bandwidth calls, and maneuver well in small spaces with low aspect ratio wings (Sleep & Brigham, 2003). However, some of these attributes (e.g., slow flight speed) may result in clutter‐adapted bats being relatively more susceptible to predation in open habitats (Lima & O'Keefe, 2013). At a broader landscape scale, fires create high contrast edges, a habitat particularly favored by many foraging bats (Gonsalves, Law, Webb, & Monamy, 2012; Morris, Miller, & Kalcounis‐Rueppell, 2010). Thus, fires that create openings in the landscape and reduce vegetation clutter also may lead to increased foraging opportunities for both open‐ and edge‐adapted foraging bats, while reducing foraging opportunities for bats with clutter‐adapted foraging strategies (Armitage & Ober, 2012; Inkster‐Draper, Sheaves, Johnson, & Robson, 2013). Further, fire can stimulate insect prey production, leading bats to shift foraging behavior to capitalize on abundant postfire insects (Doty, Stawski, Law, & Geiser, 2016; Lacki, Cox, Dodd, & Dickinson, 2009; Malison & Baxter, 2010). Bat adaptations for flight and foraging are likely to influence responses to shifting fire regimes; however, the links between fire regime attributes and bat traits have not been studied.
We evaluated relationships between bat occupancy, bat traits, forest structure, and fire regime in California's Sierra Nevada mountains, where bat diversity is high (17 species; Pierson, Rainey, & Corben, 2001) and frequency and extent of wildfire are increasing (Miller & Safford, 2012). We used a two‐stage approach to investigate relationships between bats and fire. We first tested for relationships between bat occupancy and fire regime and forest structure variables influenced by fire regime. Next, we evaluated ecomorphological traits underpinning these relationships using two trait–environment analyses (fourth‐corner regression and RLQ analysis). We evaluated three fire regime variables in our study: burn severity, years since fire, and fire return interval (FRI). We predicted that bats would show diverse associations to fire regime and forest structure, with open‐ and edge‐adapted bats positively associated with higher severity, more frequent and more recent fires, and clutter‐adapted bats showing the opposite relationship.
2. METHODS
2.1. Study area
We surveyed bats in Plumas National Forest (463,770 ha), within the Sierra Nevada mountain range, in northern California (40°00′01″N 120°40′05″W; Figure 1). The Forest spans an elevation gradient of 311–2,433 m, and has dry and warm summers and cool, wet winters. Mean annual precipitation is high for California (1,036 ± 306 mm), and mean temperature is 10.1 ± 0.9°C., ranging from a mean of 1.3 ± 2.4°C in January to a mean of 19.3 ± 1.5°C in July (1895–2017; Western Regional Climate Center, 2017). Forest communities in Plumas National Forest are dominated by lower and upper montane vegetation such as ponderosa pine (Pinus ponderosa) mixed conifer, white fir (Abies concolor) mixed conifer, and red fir (Abies magnifica), with meadows and montane chaparral present in lower abundance (Fites‐Kaufman, Rundel, Stephenson, & Weixelman, 2007). Additional common tree species include Douglas‐fir (Pseudotsuga menziesii), Jeffrey pine (Pinus jeffreyi), incense cedar (Calocedrus decurrens), and oak (Quercus spp.; Fites‐Kaufman et al., 2007). Large‐scale stand‐replacing fires occur regularly in the Plumas National Forest, with five extensive (>20,000 ha) fires within the last 20 years (USDA Forest Service, 2017).
Figure 1.
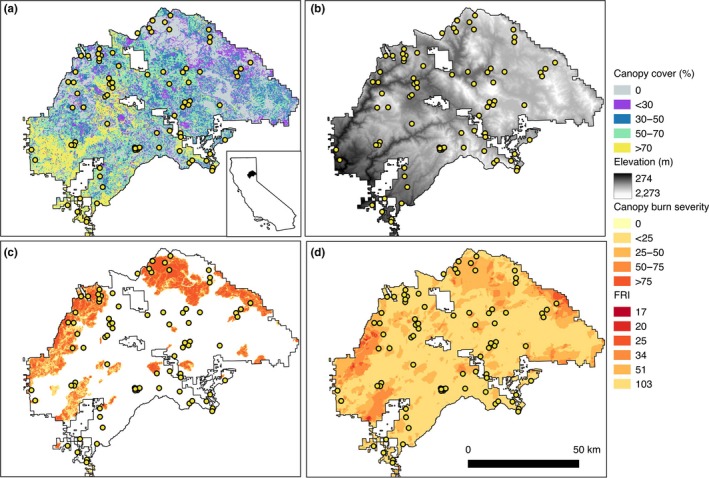
Bats were sampled acoustically at 83 sites (circles) within Plumas National Forest in the Sierra Nevada mountain range, northern California, USA (see inset). Maps show the National Forest boundary with (a) canopy cover (%), (b) digital elevation model (10 m resolution), (c) burn severity (percentage change in canopy cover after fires that burned during 1987–2015), and (d) average fire return interval between 1908 and 2010
2.2. Bat surveys
We randomly selected sampling sites across Plumas National Forest, after removing areas with slopes >15% to improve accessibility of sites (7.8% of the study area). We sampled each site over three consecutive nights, recording echolocating bats using Pettersson D500x bat detectors (Pettersson Elektronik, Uppsala, Sweden). Surveys took place over portions of three summers, corresponding to bat lactation season and the period of greatest bat activity (8 June–31 July 2015, 31 May–8 August 2016, and 12 June–24 July 2017), and individual sites were not revisited in multiple years. At each site, we placed one bat detector with its microphone in a weatherproof PVC tube angled 90° from vertical at a height of 2 m. Detectors were programed to sample from before sunset to after sunrise (7:30p.m.–6:30a.m. Pacific Daylight Time) for three consecutive nights and we did not sample during rainfall or strong wind. We used automated acoustic analysis software (SonoBat 4.2.2, SonoBat, Arcata, CA, US) to identify recorded calls to species where possible. We used the SonoVet tool to manually check calls that had been identified to species by the software to ensure a high level of confidence in identification (Russo & Voigt, 2016). If calls identified to species were not of sufficient quality with features that enabled us to confidently separate them from similar species, that species was not recorded as present for that night at that site. While this approach may increase the probability of false negatives, it reduces false positives, which are more problematic for model inference (Miller et al., 2011). Our final bat dataset spanned 249 survey nights (83 sites for three nights each) and included detections of all 17 bat species known to occur in the region.
2.3. Nonfire environmental variables
We used three types of nonfire environmental variables in our occupancy models: detection covariates, physiographic variables, and forest structure variables. Detection covariates included variables that were likely to influence nightly fluctuations in bat activity: nightly weather (minimum and maximum temperature) and moon phase (illuminated fraction and moon illuminance; Saldaña‐Vázquez & Munguía‐Rosas, 2013; Turbill, 2008). Physiographic (elevation, slope, distance to water, and percent rock cover) and forest structure (canopy cover, mean tree diameter, stand basal area, trees per ha, distance to open area, and distance to forest edge) variables were used to characterize habitat relationships for each bat species, prior to explicitly examining effects of fire. For field measurements, site was defined as a 100 by 100‐m plot centered on the bat detector, encompassing the likely range of detection distances for bats in the study area.
We used the closest three National Oceanic and Atmospheric Administration weather stations to each site (with a maximum distance of 40 km) to measure daily maximum and minimum temperature. We used the rnoaa v0.7.0 R package to access climate data (Chamberlain et al., 2017). We avoided sampling on rainy nights; precipitation was recorded during seven (8%) sampling periods only with one sampling night recording >5 mm rainfall (9.8 mm). Moon illuminance and fraction illuminated were calculated with methods described by Upham and Hafner (Upham & Hafner, 2013) using the US Naval Observatory Multi‐year Interactive Computer Almanac (MICA) v2.2.2 (United States Naval Observatory, 2011) and the oce v0.9‐20 R package (Kelley, Richards, & Layton, 2016).
We extracted elevation at each site from a 10 m digital elevation model (US Geological Survey, 2017) and calculated slope using the eight pixels surrounding each site location using the raster v 2.6‐7 package in R (Hijmans et al., 2017). We characterized water availability by extracting the distance to water bodies and perennial streams using the NHD Plus v2 dataset (McKay et al., 2012). Overall, we included four water variables: distance to water body (mapped lakes, wetlands, dams, and reservoirs), distance to perennial stream, distance to (any) stream, and distance to (any) water (all aforementioned). We estimated percent rock cover at each site within a 100 by 100‐m plot centered on each bat detector as the average percentage of exposed rock along four transects, starting at the detector and extending for 50 m in each cardinal direction. In the same plots, we estimated canopy cover using a concave densitometer directly above the detector and at four locations 50 m from the detector in each cardinal direction; the five densitometer measurements were averaged at each site. We used a wedge prism to count and estimate the diameter at breast height (DBH) of standing (live or dead) trees within 50‐m of the detector. To partially account for annual and seasonal variation in occupancy (due to breeding phenology and/or migration), we included day of season (1–71), starting with the earliest date of bat recordings across the three seasons, 3 May (1), up to the latest date of bat recordings across the three seasons, 9 August (71), as well as survey year.
2.4. Fire regime variables
We included three variables describing variation in fire regime in our study area: years since fire, fire return interval, and canopy burn severity. We used current fire return interval (FRI; Safford, VandeWater, & Clark, 2016), calculated by dividing number of years the dataset spanned (107 years, 1908–2015) by the number of fires in that period plus one. As there were only three values of FRI within our sites, we treated this variable as both a continuous and categorical variable (frequent = 36, regular = 54 and rare = 107 years). Years since fire was also obtained from Safford et al. (2016), with areas that never burned during the 107‐year period allotted the value 107 (values ranged from 3 to 107 years). Vegetation burn severity calibrated to percent change in canopy cover (hereafter: “burn severity”) was obtained from another USDA Forest Service vector product (USDA Forest Service, 2017) for all fires in the study area between 1987 and 2017, and was estimated using methods described in Miller and Thode (2007). The burn severity product included six categories of percent change in canopy cover, 0 = unburned, 1 = burned but no change in canopy, 2 = <25%, 3 = 25%–50%, 4 = 50%–75%, 5 = >75% burned. As there were not sufficient spread of data across categories (e.g., burn severity of three only had three values) to treat this as a categorical variable, we calculated means of the percentage ranges and treated it as a continuous variable (0, 12.5, 37.5, 62.5, 87.5). Burn severities were unavailable for six of 83 sites that burned between 34 and 89 years prior to the study and were treated as having a burn severity of zero. Although there were more unburned (n = 51) than burned (n = 32) sites in our study and sites were not stratified across fire regime or forest structure variables, we believe variability across the study landscape was sufficient for testing relationships. Five small fires burned within Plumas National Forest during the study (2015–2017), which we consider unlikely to affect study findings given these fires were not close to sampled sites (the closest site was 6 km to one of the burned areas) and covered a small proportion of the study area (<2%). Years since fire and FRI were highly correlated (R = 0.95), whereas burn severity was moderately negatively correlated with TSLF (−0.69) and FRI (−0.61).
2.5. Relationships between fire regime, forest structure, and bat species occupancy
We used single‐season occupancy modeling (MacKenzie et al., 2002) and an information theoretic approach to model selection (Burnham & Anderson, 2002) to evaluate predictors of bat occupancy. We first identified the physiographic and forest structure variables influencing occupancy for each species, comparing models of all possible combinations of uncorrelated physiographic and forest structure variables, ranked by AICc values. We also evaluated whether temporal variation among periods influenced bat occupancy probability by including two covariates in the model selection process (day of season and year). We avoided collinearity by including only the best‐fitting variable among correlated sets (e.g., distance to waterbody, perennial, stream, and water). Initial best models were selected for each species, retaining the highest ranking model based on Akaike's information criterion adjusted for sample sizes (AICc). Next, we identified detection covariates important for each bat species by comparing these initial best models plus all combinations of detection covariates, retaining the model with the combination of detection covariates that minimized AICc. Moon fraction and moon illuminance were correlated (R = 0.75), thus we only included the variable more strongly correlated with detection for each species. The resulting top‐ranking models containing detection and site covariates were considered “biological null models.” Next, we assessed whether bat occupancy was influenced by fire regime by adding each of the three fire regime variables, separately, to the base model. For years since fire, both linear and curvilinear relationships were plausible so we evaluated both linear and quadratic (2nd order polynomial) fits and retained the variable with the lowest AICc. For FRI, we also retained the variable (continuous or categorical) with the lowest AICc. For each species, we then had four models. The biological null models contained only detection, physiographic, and forest structure variables (nonfire regime variables). Three separate models contained the base model and one of the fire regime variables, which included: burn severity, years since fire (either linear or quadratic fit), and FRI (either continuous or categorical). Fire variables were fit separately rather than together, given correlations among fire variables. We ranked the four models for each species and retained the top‐ranking model using AICc as the full model. Where fire models did not outcompete biological null models by >2 ΔAICc, the fire variable was considered uninformative and the biological null model was retained as the full model (Arnold, 2010). Here and elsewhere, we considered differences statistically significant at α < 0.05. The fit of final models were checked using Dunn–Smyth residuals (Warton, Stoklosa, Guillera‐Arroita, MacKenzie, & Welsh, 2017) and using a parametric bootstrap (n = 104 bootstraps) of Pearson's chi‐square test (MacKenzie & Bailey, 2004). We used the unmarked v0.12‐2 package (Fiske & Chandler, 2011) to fit occupancy models and the MuMIn v1.40.4 package (Barton, 2015) for model selection.
2.6. Relationships between fire regime, forest structure, and bat traits
For each of the 17 bat species detected, we compiled data on five “ecomorphological” traits likely to correspond with adaptations to different forest structure and fire regime variables (Table 1). Call traits included characteristic call frequency (Fc), call bandwidth (BW), and call duration (Dur) and were taken from summaries of western United States bat call characteristics included in Sonobat (SonoBat 4.2.2, SonoBat, Arcata, CA, US). We used one morphological trait (body mass in g), as previously published estimates were available for all species in our study, and body mass is highly correlated with other morphological traits such as forearm length (mm; R = 0.95), wing loading (R = 0.97), and wing aspect ratio (R = 0.87; sources listed in Table 1). We categorized foraging strategy into one of three broad groups: open‐adapted foragers, edge‐adapted foragers, and clutter (structurally complex vegetation)‐adapted foragers, based on previously reported foraging behavior (sources listed in Table 1). While these foraging strategies are broad classifications that have been found to be strongly related to three‐dimensional forest structure (Blakey et al., 2017), they do not preclude bats from using a variety of structures while foraging (Denzinger & Schnitzler, 2013).
Table 1.
Bat species detected in Plumas National Forest, Sierra Nevada, CA, with percentage of the 83 sites in which each species was recorded (%), total nights detected (n), and mean detection (ρ) and occupancy (ψ) probabilities for top‐ranked models (Appendices S1 and S2)
| Scientific name | Common name | Sp code | % | n | ρ | ψ | Fc kHz | BW kHz | Dur ms | Mass g | Foraging strategy |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Myotis californicus | California myotis | Myca | 83 | 160 | 0.77 ± 0.03 | 0.92 ± 0.04 | 49.1 | 54.3 | 3.8 | 4.2 | Edge (Frick, Hayes, & Heady, 2009) |
| Myotis evotis | Western long‐eared myotis | Myev | 74 | 114 | 0.58 ± 0.04 | 0.89 ± 0.05 | 34.3 | 50.4 | 3.7 | 7.3 | Clutter (Faure, Fullard, & Barclay, 1990) |
| Lasionycteris noctivagans | Silver‐haired bat | Lano | 53 | 92 | 0.67 ± 0.05 | 0.58 ± 0.07 | 26.5 | 16.1 | 9.2 | 10.6 | Edge (Barclay, 1986) |
| Eptesicus fuscus | Big brown bat | Epfu | 42 | 66 | 0.55 ± 0.06 | 0.53 ± 0.01 | 28.2 | 29.4 | 7.8 | 15.9 | Edge (Frick et al., 2009) |
| Tadarida brasiliensis | Mexican free‐tailed bat | Tabr | 37 | 59 | 0.55 ± 0.17 | 0.43 ± 0.08 | 25.5 | 8.2 | 11.5 | 12.5 | Open (Frick et al., 2009) |
| Lasiurus cinereus | Hoary bat | Laci | 29 | 32 | 0.27 ± 0.06 | 0.47 ± 0.12 | 20.1 | 6.3 | 11.0 | 33.0 | Open (Barclay, 1986) |
| Myotis lucifugus | Little brown bat | Mylu | 29 | 48 | 0.46 ± 0.08 | 0.29 ± 0.09 | 40.8 | 36.4 | 6.0 | 7.1 | Edge (Burles, Brigham, Ring, & Reimchen, 2008; Ratcliffe & Dawson, 2003) |
| Myotis thysanodes | Fringed myotisb | Myth | 24 | 30 | 0.38 ± 0.08 | 0.26 ± 0.08 | 24.5 | 52.6 | 3.9 | 8.4 | Clutter (O'Farrell & Studier, 1980) |
| Myotis yumanensis | Yuma myotis | Myyu | 19 | 28 | 0.29 ± 0.08 | 0.25 ± 0.08 | 49.2 | 44.4 | 5.5 | 5.2 | Edge (Frick et al., 2009) |
| Antrozous pallidus | Pallid bata, b | Anpa | 19 | 21 | NA | NA | 28.0 | 28.3 | 6.8 | 17.3 | Clutter (Frick et al., 2009) |
| Myotis volans | Long‐legged myotis | Myvo | 18 | 18 | NA | NA | 41.6 | 52.7 | 4.8 | 10.4 | Edge (Frick et al., 2009) |
| Lasiurus blossevillii | Western red bata | Labl | 10 | 13 | NA | NA | 38.9 | 15.8 | 10.7 | 12.5 (Harvey, Altenbach, & Best, 2011) | Edge (Frick et al., 2009) |
| Corynorhinus townsendii | Townsend's long‐eared bata, b | Coto | 5 | 6 | NA | NA | 23.4 | 21.1 | 4.6 | 10.2 | Clutter (Fellers & Pierson, 2002; Segura‐Trujillo, Lidicker, & Álvarez‐Castañeda, 2016) |
| Myotis ciliolabrum | Small‐footed myotis | Myci | 4 | 5 | NA | NA | 44.3 | 54.5 | 3.2 | 4.9 (Barclay & Brigham, 1991) | Edge (Holloway & Barclay, 2001) |
| Parastrellus hesperus | Canyon bat | Pahe | 5 | 5 | NA | NA | 45.9 | 15.2 | 5.5 | 4.4 | Edge (Segura‐Trujillo et al., 2016) |
| Eumops perotis californicus | Western mastiff bata | Eupe | 2 | 3 | NA | NA | 10.4 | 10.4 | 15.4 | 53.5 | Open (Best, Kiser, & Freeman, 1996) |
| Euderma maculatum | Spotted bata | Euma | 2 | 2 | NA | NA | 10.0 | 4.9 | 3.2 | 17.9 (Barclay & Brigham, 1991) | Clutter (Segura‐Trujillo et al., 2016) |
Sparse detections for eight species precluded modeling detection and occupancy probabilities. Call traits included characteristic call frequency (Fc), call bandwidth (BW), and call duration (Dur) and were obtained from summaries of western United States bat call characteristics included in Sonobat (SonoBat 4.2.2, SonoBat, Arcata, CA, US). We used body mass from the PanTHERIA database (Jones et al., 2009) except where indicated and foraging strategy was taken from multiple sources.
California Species of special Concern by California Department of Fish and Wildlife.
USDA Forest Service, Pacific Southwest Region, 2013, Regional Forester's Sensitive Species (https://www.fs.usda.gov/detail/r5/plants-animals/wildlife/).
We investigated consensus between two methods to identify how bat ecomorphological traits related to forest structure and fire regime variables: a model‐based fourth‐corner analysis (Brown et al., 2014; Legendre, Galzin, & Harmelin‐Vivien, 1997) and an ordination‐based RLQ analysis (Dolédec, Chessel, Ter Braak, & Champely, 1996). These methods allowed us to explore specific ecomorphological traits underpinning species–environment relationships. Both methods used three matrices: environmental data for each site (R), species occurrence for each site (L), and species' traits (Q). The model‐based fourth‐corner analysis predicted presence (species recorded during at least one sampling night), as a function of explanatory variables (forest structure and fire regime variables), species traits and the interaction between environmental variables and species traits (Brown et al., 2014). The coefficients for the interaction between environmental variables and species traits were the “fourth‐corner” terms (Brown et al., 2014), allowing quantification of specific trait–environment relationships. As these methods do not allow for repeated sampling, we converted our data to presence–absence format, by assigning sites where a species was recorded during at least one night as one, and coding sites where the species was never recorded over the three nights as zero. While we recognize that “absences” may depict a lack of detection rather than true absences, mean detection probabilities were high (>50%) making it likely that most species were detected within the 3‐night period (Table 1). We used the binomial family to fit the models and employed a LASSO penalty (Hastie, Tibshirani, & Friedman, 2009) for automatic model selection, which removed all fourth‐corner interactions not improving model fit (Brown et al., 2014). We used the mvabund v.3.13.1 package to fit fourth‐corner regression models (Wang, Naumann, Wright, & Warton, 2012).
The RLQ analysis approaches the same “fourth‐corner” problem by performing a simultaneous ordination of the three matrices, producing an overview of trait–environment relationships, visualized in scores (eigenvalues) across two axes (Dray et al., 2014). The strength and direction of associations between traits and environment (forest structure and fire regime variables) can be interpreted from the size and sign of the eigenvalue. We modeled a set of uncorrelated (|R| < 0.7; Dormann et al., 2013) forest structure and fire regime variables including the following: mean tree diameter, tree basal area, trees per ha, canopy cover, burn severity, and FRI. To test significance of trait–environment relationships, we used an analysis of deviance with row‐resampling and 104 bootstrap iterations for the fourth‐corner regression and a Monte Carlo permutation test with 106 iterations for the RLQ analysis. We used the ade4 v1.7‐10 package to perform RLQ analysis (Dray & Dufour, 2007).
3. RESULTS
3.1. Relationships between fire regime, forest structure, and bat species occupancy
During 249 recording nights (June‐August, 2015–2017), we documented 17 bat species, including five listed as species of special concern by the California Department of Fish and Wildlife (Antrozous pallidus, Corynorhinus townsendii, Euderma maculatum, Lasiurus blossevillii, and Eumops perotis californicus; California Department of Fish & Wildlife Natural Diversity Database, 2017) and three designated as Forest Service sensitive species (A. pallidus, C. townsendii, and Myotis thysanodes; Table 1). For nine species with sufficient detections to allow modeling of occupancy and detection probabilities (detected >10% of nights), final models of three species contained fire regime variables (Myotis evotis, Myotis lucifugus, Eptesicus fuscus: years since fire; Appendices S1 and S2). Of these, M. lucifugus and E. fuscus showed a statistically significant relationship with years since fire (Figure 2a,b, Appendices S1 and S2). Probability of M. lucifugus occupancy decreased with years since fire (Figure 2a), whereas E. fuscus occupancy probability increased with years since fire until approximately 54 years, and then began to decrease (Figure 2b).
Figure 2.
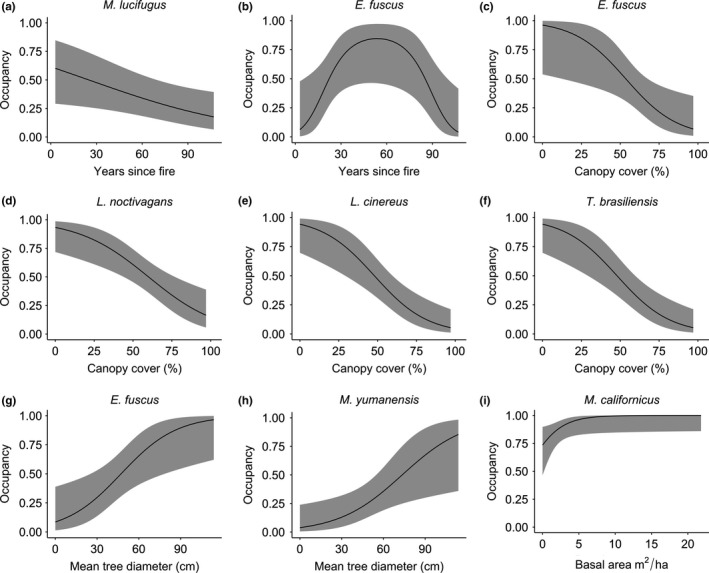
Relationships between forest structure and fire regime variables (Appendix S2) and predicted occupancy probabilities of seven bat species in the Sierra Nevada, California. Models are single‐season occupancy models and fitted lines are shown with 95% confidence intervals (shaded area). All other covariates (aside from the focal variable) were fixed to mean values to produce the figures presented herein. All relationships plotted were statistically significant (α < 0.05)
Forest structure variables were important predictors of bat species occupancy, with 7 of 9 species related to at least one forest structure variable (Appendix S2). Probability of occupancy decreased with increasing canopy cover for two edge‐adapted and two open‐adapted species (E. fuscus, Lasionycteris noctivagans, Lasiurus cinereus, and Tadarida brasiliensis, respectively; Figure 2c–f; Appendix S2). Basal area was positively associated with an edge‐adapted bat (Myotis californicus; Figure 2i; Appendix S2), and mean tree diameter was positively related to occupancy probability for two edge‐adapted species: Myotis yumanensis and E. fuscus (Figure 2g,h; Appendix S2). Among the physiographic variables, M. evotis and M. lucifugus were more likely to occur at higher elevations, M. californicus at lower elevations and M. thysanodes more likely to occur on gentler slopes (Appendix S2). For seven of nine species, daily maximum temperature was a predictor of bat detection probability, with greater detection probabilities at higher temperatures (Appendix S2). Day of season was not a predictor of occupancy probability for any of the species; however, year was a predictor of occupancy of L. cinereus, with occupancy probability higher in 2015 than in 2016, though no other pairwise comparisons were significant (Appendix S2).
3.2. Relationships between fire regime, forest structure, and bat traits
Traits of the 17 bat species in the study area showed trends consistent with ecomorphological theory. Bats with lower frequency calls generally also had narrower call bandwidth, longer call duration, and larger body mass, with open‐adapted foraging strategies more likely (Figure 3). Clutter‐adapted bats varied in call frequency, bandwidth, and body mass, but all had relatively short call duration (Figure 3). Edge‐adapted bats also showed variation in call traits; however, all had body mass below 16 g (Figure 3) and call frequencies above 25 kHz.
Figure 3.
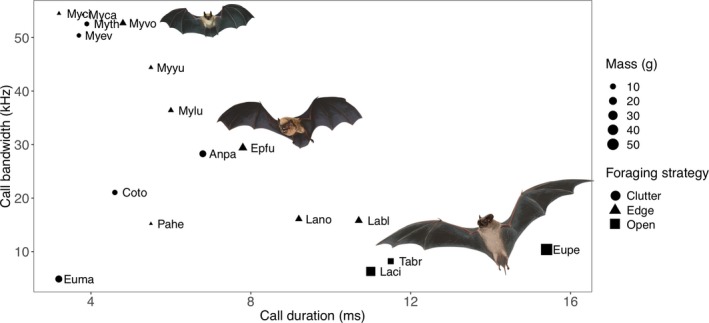
Four ecomorphological traits for 17 bat species detected in Plumas National Forest, based on values from the literature (see Table 1). Squares represent open‐adapted, triangles depict edge‐adapted and circles represent clutter‐adapted bat species. Species codes are given in Table 1
Traits of 17 bat species were related to fire regime and forest structure variables based on our fourth‐corner regression model (Deviance = 90.95, p < 0.0001) and the RLQ (Monte Carlo permutation test: p < 0.0001). The first axis of the RLQ analysis (Figure 4b) explained 97.8% of the co‐structure of traits with forest structure and fire regime variables. Strength of predictors of the trait‐environment relationship is indicated by number of associations and darkness of color in the fourth‐corner analysis (Figure 4a) and magnitude of axis 1 eigenvalue (x‐axis) in the RLQ analysis (Figure 4b). Strongest predictors of the trait‐environment relationships among forest structure and fire regime variables were canopy cover, trees per ha, tree basal area, burn severity and FRI, while strongest trait predictors were call bandwidth, call duration and foraging strategy (Figure 4).
Figure 4.
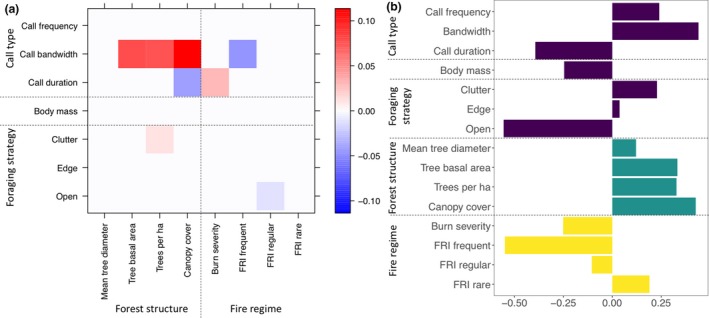
Relationships between 5 traits of 17 bat taxa and four forest structure and two fire regime variables based on (a) fourth‐corner regression and (b) RLQ analysis. In the fourth‐corner regression (a), the strength and direction of relationships between traits (y‐axis) and forest structure and fire regime variables (x‐axis) is indicated by color (red: positive and blue: negative). For example, in the fourth‐corner regression (a), bats with longer duration calls are associated with higher burn severity. In the RLQ analysis (b), the x‐axis shows eigenvalues for Axis 1 and the y‐axis shows five bat traits (purple), four forest structure variables (green), and two fire regime variables (yellow). The strength and direction of Axis 1 eigenvalues of the RLQ analysis (b) indicate how traits covary with forest structure and fire regime variables. For example, in the RLQ analysis (b) open‐adapted bats were most strongly associated with frequent fires (FRI frequent)
Overall, relationships between traits and forest structure were consistent with predictions based on bat ecomorphology (Figure 5). The fourth‐corner analysis (Figure 4a) indicated bats with broader call bandwidth and, to a lesser extent shorter calls and clutter‐adapted foraging strategy, were associated with more cluttered sites (higher canopy cover, basal area and trees per ha or mean tree diameter; Figure 4a). All of these relationships were supported by the RLQ analysis, which further indicated open‐adapted bats with greater body mass and lower call frequencies were negatively associated with forest clutter (Figure 4b). Canopy cover, trees per ha and tree basal area showed the strongest trait‐environment relationships across both analyses, with mean tree diameter showing the weakest relationships with bat traits (Figure 4). There was some consensus between both analyses in the relationships between bat traits and fire regime; bats with longer duration calls were associated with greater burn severities and narrower bandwidth calls were associated with more frequent fires. The weaker negative relationship between open‐adapted foraging bats and regularly occurring fire in the fourth‐corner analysis was not supported by the RLQ analysis, which showed a weak positive relationship between open‐adapted bats and regular fire (Figure 4b). Relationships between bat traits and fire identified by the RLQ analysis, but not the fourth‐corner analysis, included a positive association between open‐adapted bats, frequent fire, and burn severity, and a negative relationship for clutter‐adapted bats. There was no evidence for relationships between edge‐adapted foraging bats and fire or forest structure in either analysis (Figure 4). In the RLQ, rare fires were associated with cluttered forest structure, while frequent fires with higher burn severity were associated with more open forest structure (Figure 4b).
Figure 5.
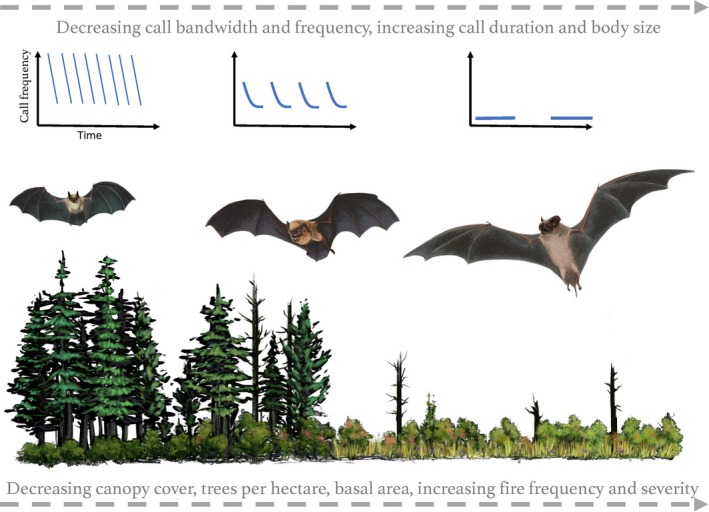
Illustration of ecomorphological relationships revealed in this study. As habitats change across a gradient of increasing burn severity and frequency and decreasing clutter (left to right), larger bats with narrower bandwidth, lower frequency and longer duration calls are more likely to occupy the area. From left to right, representatives from three bat foraging strategies are shown: clutter‐adapted (Myotis thysanodes), edge‐adapted (Eptesicus fuscus), and open‐adapted (Tadarida brasiliensis). Body sizes are not to scale
4. DISCUSSION
Results indicate variable responses to fire regime and forest structure within a diverse montane forest bat community, underpinned by ecomorphological traits of individual species. Bat adaptations to forest structure largely explained the relationships between fire regime and bat traits. Greater burn severities, more frequent fires, and hence more open forests (lower canopy cover and basal area) favored bats with traits adapting them to foraging in open habitats, consistent with previous research (Armitage & Ober, 2012; Buchalski, Fontaine, Heady, Hayes, & Frick, 2013; Cox, Willcox, Keyser, & Vander Yacht, 2016; Inkster‐Draper et al., 2013). Conversely, our trait–environment analysis indicated that bats with clutter‐adapted traits were negatively associated with burn severity and frequency of fires, although the literature reports positive or no effect of burn severity (Buchalski et al., 2013; Lacki, Dodd, Skowronski, Dickinson, & Rieske, 2017) and no effect of fire frequency (Armitage & Ober, 2012). These patterns mirrored results from bird studies in which aerial insectivores adapted to open and edge habitats are more abundant in burned forest while gleaning species (adapted to cluttered habitats) are more abundant in unburned forest (Kotliar et al., 2002) and are negatively associated with burn severity (Azeria et al., 2011).
Relationships between fire and individual species occupancy were neutral or positive in our study, consistent with other studies of effects of wildfire on bat activity in United States and Australia (Buchalski et al., 2013; Law, Doty, Chidel, & Brassil, 2018). Low correlation between edge‐adapted foraging strategy and fire and forest structure variables (Figure 4) was likely due to variation in individual species' relationships to fire regime and forest structure variables (Figure 2). Previous studies of M. lucifugus and E. fuscus reported no effect of fire on activity (Austin, Silvis, Ford, Muthersbaugh, & Powers, 2018; Loeb & Waldrop, 2008; Silvis, Gehrt, & Williams, 2016), but that work focused on prescribed fire, which likely burned at relatively lower severity than much of the wildfire that occurred within our study area. The positive association between M. lucifugus occupancy and recent fires may be influenced by the species' preference for aquatic prey, as pulses in aquatic productivity can be stimulated by fire (Malison & Baxter, 2010; Roby & Azuma, 1995). Varying effects of years since fire and forest structure on edge‐adapted bats like E. fuscus may reflect responses to larger scale configuration of fire habitat, for example, availability and juxtaposition of edges and forest openings (Loeb & O'Keefe, 2011; Morris et al., 2010). Adding to the complexity of edge‐adapted bats' relationships with fire, a study of bat activity (rather than occupancy) indicated increased use of habitats with greater burn severities in the Sierra Nevada for several edge and clutter‐adapted bats including medium‐high frequency calling A. pallidus, M. thysanodes, and undifferentiated Myotis spp. (Buchalski et al., 2013). Whereas generalizations can be made between adaptations to forest structure and effects of fire regime in the western United States, the variable relationships of edge‐adapted bat species to fire regime variables may indicate that unexplored complexities in the relationships persist. Alternatively, given edge‐adapted foragers have fewer specialized traits, when compared to open‐ and clutter‐adapted bats, they may employ more flexible foraging strategies and adapt to a greater variety of conditions (Denzinger & Schnitzler, 2013). Finally, study scale and observation error associated with fire regime variables may obscure existing relationships between bats and burned landscapes.
Predicted increases in the number and extent of high severity fires in coniferous forests of the western United States (Dennison et al., 2014; Miller & Safford, 2012) are likely to increase foraging opportunities for open‐ and edge‐adapted bats with relatively longer duration and narrower bandwidth calls (e.g., E. perotis, L. cinereus, T. brasiliensis, L. noctivagans). However, increases in fire frequency and severity may reduce foraging opportunities for clutter‐adapted bats and bats with shorter duration and wider bandwidth calls (e.g., C. townsendii, M. evotis, M. thysanodes, Myotis ciliolabrum, Myotis volans, M. californicus), including species that are most at risk from the spread of white‐nose syndrome in the Western United States (Weller et al., 2018). Increasing fire frequency and severity could have positive short‐term (5 years postfire) effects for bats that forage on aquatic insects (Malison & Baxter, 2010) such as M. lucifugus and M. yumanensis via increased postfire pulses of aquatic productivity. Similarly, the primary prey for E. fuscus, A. pallidus, and Parastrellus hesperus are beetles, which are abundant in postfire landscapes (Kral, Limb, Harmon, & Hovick, 2017). However, moths (Lepidoptera) are the primary prey of the remaining 12 species in our study and may be negatively affected by fire (Armitage & Ober, 2012; Kral et al., 2017). Myotis evotis, the only species in our study area for which roosting preferences with relation to fire have been studied, selected roosts away from burned areas (Snider, Cryan, & Wilson, 2013). High severity fire destroys canopy, hence reducing roost availability for foliage roosting species like L. cinereus and L. blossevillii; fire may also cause direct mortality of these species if it occurs during winter when they may roost within leaf litter (Johnston & Whitford, 2009; Perry & McDaniel, 2015). However, many of the species in our study area use snags, hollows, crevices, and exfoliating bark to roost, structures that may be created or enhanced by fire (Johnson, Edwards, Ford, & Gates, 2009; O'Keefe & Loeb, 2017).
Bat community diversity in the increasingly fire‐prone forests of the western United States likely will benefit from management for mixed‐severity fire and pyrodiverse landscapes, which also has been shown to be important for bird communities in the region (Kelly & Brotons, 2017; Tingley, Ruiz‐Gutiérrez, Wilkerson, Howell, & Siegel, 2016). Variable severity fire creates a mosaic of forest gaps and edges (Comfort, Clark, Anthony, Bailey, & Betts, 2016), which are high‐quality foraging habitat for many bat species (Gonsalves et al., 2012; Loeb et al., 2011; Morris et al., 2010) including the 12 open‐ and edge‐adapted species in our study as well as some clutter‐adapted bats. Low severity fire may promote growth of larger trees, by thinning smaller trees (Brown, Mutch, Spoon, & Wakimoto, 1995), and may increase habitat quality for small (M. californicus and M. yumanensis) and medium‐sized (E. fuscus) edge‐adapted bats (Figure 2, Appendix S2). Years since fire is also an important predictor of bat occupancy in our study area (Figure 2, Appendix S2). Natural postfire succession following variable severity fire facilitates development of structurally heterogeneous environments at the landscape scale with pockets of cluttered forest interspersed, and initial (<15 years) natural regeneration after high severity fire in the Sierra Nevada creates heterogeneous land‐cover patterns (Hanson, 2018). However, rarely burned areas also are important for clutter‐adapted bats in our study area, four of which are of conservation concern (Table 1). Pyrodiversity also is likely to promote bat prey diversity (Kral et al., 2017) and roosting opportunities (Johnson et al., 2009; O'Keefe & Loeb, 2017). Managing for diverse fire regimes, at the appropriate scale, facilitates habitat development and maintenance for species where roosting and foraging habitats diverge (Azeria et al., 2011). Although pyrodiversity does not always lead to increased wildlife biodiversity (Pastro, Dickman, & Letnic, 2011; Taylor et al., 2012), it is likely to benefit wildlife including bats, birds, and insects within the fire‐prone forests of the western United States (Buchalski et al., 2013; Ponisio et al., 2016; Tingley et al., 2016). Management strategies aiming for mixed‐severity fire also are more likely to be consistent with historical fire regimes in the Western United States (Baker, 2014).
A better understanding of the links between fire regimes and wildlife traits can aid in the development and selection of management options that maximize biodiversity in the fire‐prone forests of the western United States. These forests host diverse animal assemblages, adapted to a variety of fire‐mediated habitat conditions (Buchalski et al., 2013; Roberts, Kelt, Van Wagtendonk, Miles, & Meyer, 2015; Rochester et al., 2010; White et al., 2016), making it challenging for forest managers to decide where to allocate limited management resources. Identifying relationships between fire regime and species functional traits allows forest managers to: a) identify which fire characteristics or treatments are most important for particular species and broader communities; b) manage forests with fire for large numbers of species concurrently; c) predict how future changes in fire regimes might influence community diversity; and d) through the use of traits and not species, compare relationships between bats and fire across regions and internationally.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
Rachel V. Blakey developed the paper concept, wrote the document, conducted analysis, and produced figures (with illustrations provided by Lauren Helton, see Acknowledgements). Elisabeth B. Webb & Dylan C. Kesler secured funding, conceptualized the experimental and fieldwork design, developed the paper concept, and provided editorial and scientific input on the manuscript. Rodney B. Siegel developed the paper concept and provided editorial and scientific input on the manuscript. Derek Corcoran supervised field data collection and provided editorial input on a draft version. Matthew Johnson provided funding, scoping and development of overarching project, and editorial and scientific input on the manuscript.
Supporting information
ACKNOWLEDGMENTS
This study was funded by USDA Forest Service Plumas National Forest. We have no competing interests to declare. We thank D. Arsenault, J. Buchanan, A. Martinez, W. Castellino, J. Lee, A. Enos, B. Graevs E. Ingalls, H. Wall, E. Deal, and G. Graells for helping with field data collection. We also thank G. Rotert who helped recover files from a corrupted hard drive, J. Szewczak for assisting with call identification, W. Frick for reviewing the trait table, and M. MacPherson and the MO Cooperative Fish & Wildlife Research Unit writing group for insightful edits of the draft manuscript. L. Helton produced the beautiful bat and habitat illustrations for Figures 3 and 5. The Missouri Cooperative Fish and Wildlife Research Unit is jointly sponsored by the Missouri Department of Conservation, the University of Missouri, the US Fish and Wildlife Service, the US Geological Survey, and the Wildlife Management Institute. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government. This is contribution 622 of The Institute for Bird Populations.
Blakey RV, Webb EB, Kesler DC, Siegel RB, Corcoran D, Johnson M. Bats in a changing landscape: Linking occupancy and traits of a diverse montane bat community to fire regime. Ecol Evol. 2019;9:5324–5337. 10.1002/ece3.5121
DATA ACCESSIBILITY
Bat detection data are archived on the USDA Forest Service Nature Resource Information System (https://www.fs.fed.us/nrm/index.shtml).
REFERENCES
- Armitage, D. W. , & Ober, H. K. (2012). The effects of prescribed fire on bat communities in the longleaf pine sandhills ecosystem. Journal of Mammalogy, 93, 102–114. 10.1644/11-MAMM-A-169.1 [DOI] [Google Scholar]
- Arnold, T. W. (2010). Uninformative parameters and model selection using Akaike's information criterion. Journal of Wildlife Management, 74, 1175–1178. 10.2193/2009-367 [DOI] [Google Scholar]
- Austin, L. V. , Silvis, A. , Ford, W. M. , Muthersbaugh, M. , & Powers, K. E. (2018). Bat activity following restoration prescribed burning in the central Appalachian upland and riparian habitats. Natural Areas Journal, 38, 183–195. 10.3375/043.038.0208 [DOI] [Google Scholar]
- Azeria, E. T. , Ibarzabal, J. , Hébert, C. , Boucher, J. , Imbeau, L. , & Savard, J. L. (2011). Differential response of bird functional traits to post‐ fire salvage logging in a boreal forest ecosystem. Acta Oecologica, 37, 220–229. 10.1016/j.actao.2011.02.005 [DOI] [Google Scholar]
- Baker, W. L. (2012). Implications of spatially extensive historical data from surveys for restoring dry forests of Oregon's eastern Cascades. Ecosphere, 3(3), art23 10.1890/ES11-00320.1 [DOI] [Google Scholar]
- Baker, W. L. (2014). Historical forest structure and fire in Sierran mixed‐conifer forests reconstructed from General Land Office survey data. Ecosphere, 5, 5324–70. 10.1890/ES14-00046.1 [DOI] [Google Scholar]
- Barclay, R. M. R. (1986). The echolocation calls of hoary (Lasiurus cinereus) and silver‐haired (Lasionycteris noctivagans) bats as adaptations for long‐ versus short‐range foraging strategies and the consequences for prey selection. Canadian Journal of Zoology, 64, 2700–2705. 10.1139/z86-394 [DOI] [Google Scholar]
- Barclay, R. M. R. , & Brigham, R. M. (1991). Prey detection, dietary niche breadth, and body size in bats: Why are aerial insectivorous bats so small? The American Naturalist, 137, 693–703. 10.1086/521238 [DOI] [Google Scholar]
- Barton, K. (2015). Package ‘MuMIn' [Internet]. [cited 15 Sep 2015]. Retrieved from: https://cran.r-project.org/package=MuMIn
- Beaty, R. M. , & Taylor, A. H. (2008). Fire history and the structure and dynamics of a mixed conifer forest landscape in the northern Sierra Nevada, Lake Tahoe Basin, California, USA. Forest Ecology and Management, 255, 707–719. 10.1016/j.foreco.2007.09.044 [DOI] [Google Scholar]
- Best, T. L. , Kiser, W. M. , & Freeman, P. W. (1996). Eumops perotis. Mammalian Species, 534, 5324–8. 10.2307/3504077 [DOI] [Google Scholar]
- Blakey, R. V. , Law, B. S. , Kingsford, R. T. , & Stoklosa, J. (2017). Terrestrial laser scanning reveals below‐canopy bat trait relationships with forest structure. Remote Sensing of Environment, 198, 40–51. 10.1016/j.rse.2017.05.038 [DOI] [Google Scholar]
- Brown, A. M. , Warton, D. I. , Andrew, N. R. , Binns, M. , Cassis, G. , & Gibb, H. (2014). The fourth‐corner solution – Using predictive models to understand how species traits interact with the environment. Methods in Ecology and Evolution, 5, 344–352. 10.1111/2041-210X.12163 [DOI] [Google Scholar]
- Brown, J. K. , Mutch, R. W. , Spoon, C. W. , & Wakimoto, R. H. (1995). Proceedings: Symposium on Fire in Wilderness and Park Management, Missoula, MT, March 30‐April 1, 1993. Ogden, UT: Intermountain Research Station. [Google Scholar]
- Buchalski, M. R. , Fontaine, J. B. , Heady, P. A. , Hayes, J. P. , & Frick, W. F. (2013). Bat Response to Differing Fire Severity in Mixed‐Conifer Forest California, USA. PLoS One, 8(3), e57884 10.1371/journal.pone.0057884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burles, D. W. , Brigham, R. M. , Ring, R. A. , & Reimchen, T. E. (2008). Diet of two insectivorous bats, Myotis lucifugus and Myotis keenii, in relation to arthropod abundance in a temperate Pacific Northwest rainforest environment. Canadian Journal of Zoology, 86, 1367–1375. 10.1139/Z08-125 [DOI] [Google Scholar]
- Burnham, K. P. , & Anderson, D. R. (2002). Model selection and inference: A practical information‐theoretic approach (2nd ed.). New York, NY: Springer‐Verlag. [Google Scholar]
- California Department of Fish and Wildlife Natural Diversity Database . (2017). Special Animals List.
- Carter, T. C. , Ford, W. M. , & Menzel, M. A. (2000). Fire and Bats in the Southeast and Mid‐Atlantic: More Questions Than Answers? The role of fire in nongame wildfire managment and community restoration: Traditional uses and new directions Proceedings of a special workshop. Nashville, Tennessee: United States Department of Agriculture, Forest Service, Northeastern Research Station, General Technical Report NE‐288. pp. 139–143.
- Chamberlain, S. , Anderson, B. , Salmon, M. , Erickson, A. , Potter, N. , Stachelek, J. , … Edmund, H. (2017). Package “rnoaa” [Internet]. [cited 27 Mar 2018]. doi:10.2307/2533043>.
- Comfort, E. J. , Clark, D. A. , Anthony, R. G. , Bailey, J. , & Betts, M. G. (2016). Quantifying edges as gradients at multiple scales improves habitat selection models for northern spotted owl. Landscape Ecology, 31, 1227–1240. 10.1007/s10980-015-0330-1 [DOI] [Google Scholar]
- Cox, M. R. , Willcox, E. V. , Keyser, P. D. , & Vander Yacht, A. L. (2016). Bat response to prescribed fire and overstory thinning in hardwood forest on the Cumberland Plateau, Tennessee. Forest Ecology and Management, 359, 221–231. 10.1016/j.foreco.2015.09.048 [DOI] [Google Scholar]
- Dennison, P. E. , Brewer, S. C. , Arnold, J. D. , & Moritz, M. A. (2014). Large wildfire trends in the western United States, 1984–2011. Geophysical Research Letters, 41, 2928–2933. 10.1002/2014gl059576 [DOI] [Google Scholar]
- Denzinger, A. , & Schnitzler, H.‐U. (2013). Bat guilds, a concept to classify the highly diverse foraging and echolocation behaviors of microchiropteran bats. Frontiers in Physiology, 4, 164 10.3389/fphys.2013.00164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolédec, S. , Chessel, D. , Ter Braak, C. J. F. , & Champely, S. (1996). Matching species traits to environmental variables: A new three‐table ordination method. Environmental and Ecological Statistics, 3, 143–166. 10.1007/bf02427859 [DOI] [Google Scholar]
- Dormann, C. F. , Elith, J. , Bacher, S. , Buchmann, C. , Carl, G. , Carré, G. , … Lautenbach, S. (2013). Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography, 36, 027–046. 10.1111/j.1600-0587.2012.07348.x [DOI] [Google Scholar]
- Doty, A. C. , Stawski, C. , Law, B. S. , & Geiser, F. (2016). Post‐wildfire physiological ecology of an Australian microbat. Journal of Comparative Physiology B, 186, 937–946. 10.1007/s00360-016-1003-3 [DOI] [PubMed] [Google Scholar]
- Dray, S. , Choler, P. , Dolédec, S. , Peres-Neto, P. R. , Thuiller, W. , Pavoine, S. , & ter Braak, C. J. F. (2014). Combining the fourth-corner and the RLQ methods for assessing trait responses to environmental variation. Ecology, 95(1), 14–21. [DOI] [PubMed] [Google Scholar]
- Dray, S. , & Dufour, A. (2007). The ade4 Package: Implementing the duality diagram for ecologists. Journal of Statistical Software, 22, 5324–20. 10.18637/jss.v022.i04 [DOI] [Google Scholar]
- Faure, P. A. , Fullard, J. H. , & Barclay, R. M. R. (1990). The response of tympanate moths to the echolocation calls of a substrate gleaning bat, Myotis evotis. Journal of Comparative Physiology A, 166, 843–849. 10.1007/BF00187331 [DOI] [Google Scholar]
- Fellers, G. M. , & Pierson, E. D. (2002). Habitat use and foraging behavior of Townsend's big‐eared bat (Corynorhinus townsendii) in coastal California. Journal of Mammalogy, 83, 167–177. 10.1093/jmammal/83.1.167 [DOI] [Google Scholar]
- Fiske, I. J. , & Chandler, R. B. (2011). Unmarked: An R package for fitting hierarchical models of wildlife occurrence and abundance. Journal of Statistical Software, 43, 5324–23. 10.1002/wics.10 [DOI] [Google Scholar]
- Fites‐Kaufman, J. , Rundel, P. , Stephenson, N. , & Weixelman, D. A. (2007). Montane and subalpine vegetation of the sierra nevada and cascade ranges. Terr Veg Calif. 456–501. 10.1525/california/9780520249554.003.0017 [DOI]
- Frick, W. F. , Hayes, J. P. , & Heady, P. A. (2009). Nestedness of desert bat assemblages: Species composition patterns in insular and terrestrial landscapes. Oecologia, 158, 687–697. 10.1007/s00442-008-1168-x [DOI] [PubMed] [Google Scholar]
- Gonsalves, L. , Law, B. , Webb, C. , & Monamy, V. (2012). Are vegetation interfaces important to foraging insectivorous bats in endangered coastal saltmarsh on the Central Coast of New South Wales? Pacific Conservation Biology, 18, 282–292. 10.1071/pc120282 [DOI] [Google Scholar]
- Hanson, C. T. (2018). Landscape heterogeneity following high‐severity fire in California's forests. Wildlife Society Bulletin, 42, 264–271. 10.1002/wsb.871 [DOI] [Google Scholar]
- Harvey, M. J. , Altenbach, J. S. , & Best, T. L. (2011). Bats of the United States and Canada . Baltimore, MD: The Johns Hopkins University Press. [Google Scholar]
- Hastie, T. , Tibshirani, R. , & Friedman, J. (2009). The elements of statistical learning (2nd ed.). New York, NY: Springer‐Verlag. [Google Scholar]
- Hijmans, R. J. , van Etter, J. , Cheng, J. , Mattiuzzi, M. , Summer, M. , Greenberg, J. A. , … Ghosh, A. (2017). Package “raster” [Internet]. [cited 2 Mar 2018]. Retrieved from: https://cran.r-project.org/web/packages/raster/raster.pdf
- Holloway, G. L. , & Barclay, R. M. R. (2001). Myotis ciliolabrum. Mammalian Species, 1410, 5324–5. 10.2307/0.670.1 [DOI] [Google Scholar]
- Inkster‐Draper, T. E. , Sheaves, M. , Johnson, C. N. , & Robson, S. K. (2013). Prescribed fire in eucalypt woodlands: Immediate effects on a microbat community of northern Australia. Wildlife Research, 40, 70–76. 10.1071/WR12133 [DOI] [Google Scholar]
- Johnson, J. B. , Edwards, J. W. , Ford, W. M. , & Gates, J. E. (2009). Roost tree selection by northern myotis (Myotis septentrionalis) maternity colonies following prescribed fire in a Central Appalachian Mountains hardwood forest. Forest Ecology and Management, 258, 233–242. 10.1016/j.foreco.2009.04.008 [DOI] [Google Scholar]
- Johnston, D. S. , & Whitford, S. (2009). Seasonal range maps for western red bats (Lasiurus blossevillii) in California and wintering western red bat in red gum eucalyptus (Eucalyptus camaldulensis) leaf litter. Western Bat Working Group Biannual Conference Austin, Texas.
- Jones, K. E. , Bielby, J. , Cardillo, M. , Fritz, S. A. , O'Dell, J. , Orme, C. D. L. , … Purvis, A. (2009). PanTHERIA: A species‐level database of life history, ecology, and geography of extant and recently extinct mammals. Ecology. 90, 2648 10.1890/08-1494.1 [DOI] [Google Scholar]
- Kelley, D. , Richards, C. , & Layton, C. (2016). Package ‘oce' [Internet]. Retrieved from: https://cran.r-project.org/web/packages/oce/oce.pdf
- Kelly, L. T. , & Brotons, L. (2017). Using fire to promote biodiversity. Science, 355, 1264–1265. 10.1126/science.aam7672 [DOI] [PubMed] [Google Scholar]
- Kotliar, N. B. , Hejl, S. J. , Hutto, R. L. , Saab, V. , Melcher, C. , & McFadzen, M. E. (2002). Effects of fire and post‐fire salvage logging on avian communities in conifer‐dominated forests of the western United States. Studies in Avian Biology, 25, 49–64. [Google Scholar]
- Kral, K. C. , Limb, R. F. , Harmon, J. P. , & Hovick, T. J. (2017). Arthropods and fire: Previous research shaping future conservation. Rangeland Ecology and Management, 70, 589–598. 10.1016/j.rama.2017.03.006 [DOI] [Google Scholar]
- Lacki, M. J. , Cox, D. R. , Dodd, L. E. , & Dickinson, M. B. (2009). Response of northern bats (Myotis septentrionalis) to prescribed fires in eastern Kentucky forests. Journal of Mammalogy, 90, 1165–1175. 10.1644/08-MAMM-A-349.1 [DOI] [Google Scholar]
- Lacki, M. J. , Dodd, L. E. , Skowronski, N. S. , Dickinson, M. B. , & Rieske, L. K. (2017). Relationships among burn severity, forest canopy structure and bat activity from spring burns in oak‐hickory forests. International Journal of Wildland Fire, 26, 963–972. 10.1071/WF16159 [DOI] [Google Scholar]
- Law, B. S. , Doty, A. , Chidel, M. , & Brassil, T. (2018). Bat activity before and after a severe wildfire in Pilliga forests: Resilience influenced by fire extent and landscape mobility? Austral Ecology, 43, 706–718. 10.1111/aec.12617 [DOI] [Google Scholar]
- Legendre, P. , Galzin, R. G. , & Harmelin‐Vivien, M. L. (1997). Relating behavior to habitat: Solutions to the fourth‐corner problem. Ecology, 78, 547–562. 10.2307/2266029 [DOI] [Google Scholar]
- Lima, S. L. , & O'Keefe, J. M. (2013). Do predators influence the behaviour of bats? Biological Reviews, 88, 626–644. 10.1111/brv.12021 [DOI] [PubMed] [Google Scholar]
- Loeb, S. C. , & O'Keefe, J. M. (2011). Bats and gaps: The role of early successional patches in the roosting and foraging ecology of bats In Greenberg C., Collins B., & Thompson F., III (Eds.). Sustaining Young Forest Communities (pp. 167–189). Dordrecht, The Netherlands: Springer; 10.1007/978-94-007-1620-9 [DOI] [Google Scholar]
- Loeb, S. C. , & Waldrop, T. A. (2008). Bat activity in relation to fire and fire surrogate treatments in southern pine stands. Forest Ecology and Management, 255, 3185–3192. 10.1016/j.foreco.2007.10.060 [DOI] [Google Scholar]
- MacKenzie, D. I. , & Bailey, L. L. (2004). Assessing the fit of site‐occupancy models. Journal of Agricultural, Biological, and Environmental Statistics, 9, 300–318. 10.1198/108571104X3361 [DOI] [Google Scholar]
- MacKenzie, D. I. , Nichols, J. D. , Lachman, G. B. , Droege, S. , Andrew Royle, J. , & Langtimm, C. A. (2002). Estimating site occupancy rates when detection probabilities are less than one. Ecology, 83(8), 2248–2255.x. [Google Scholar]
- Malison, R. L. , & Baxter, C. V. (2010). The fire pulse: Wildfire stimulates flux of aquatic prey to terrestrial habitats driving increases in riparian consumers. Canadian Journal of Fisheries and Aquatic Sciences, 67, 570–579. 10.1139/F10-006 [DOI] [Google Scholar]
- Mallek, C. , Safford, H. , Viers, J. , & Miller, J. (2013). Modern departures in fire severity and area vary by forest type, Sierra Nevada and southern Cascades, California, USA. Ecosphere, 4, 5324–28. 10.1890/ES13-00217.1 [DOI] [Google Scholar]
- McKay, L. , Bondelid, T. , Dewald, T. , Johnston, J. , Moore, R. , & Rea, A. (2012). NHDPlus Version 2 User Guide.
- Miller, D. A. , Nichols, J. D. , Mcclintock, B. T. , Campbell, E. H. , Bailey, L. L. , & Weir, L. A. (2011). Improving occupancy estimation when two types of observational error occur: Non‐detection and species misidentification. Ecology, 92, 1422–1428. 10.2307/23035095 [DOI] [PubMed] [Google Scholar]
- Miller, J. D. , & Safford, H. (2012). Trends in wildfire severity: 1984 to2010 in the Sierra Nevada, Modoc Plateau, and southern Cascades, California, Usa. Fire Ecology, 8, 41–57. 10.4996/fireecology.0803041 [DOI] [Google Scholar]
- Miller, J. D. , & Thode, A. E. (2007). Quantifying burn severity in a heterogeneous landscape with a relative version of the delta Normalized Burn Ratio (dNBR). Remote Sensing of Environment, 109, 66–80. 10.1016/j.rse.2006.12.006 [DOI] [Google Scholar]
- Morris, A. D. , Miller, D. A. , & Kalcounis‐Rueppell, M. C. (2010). Use of forest edges by bats in a managed pine forest landscape. Journal of Wildlife Management, 74, 26–34. 10.2193/2008-471 [DOI] [Google Scholar]
- O'Farrell, M. J. , & Studier, E. H. (1980). Myotis thysanodes. Mammalian Species, 137(137), 5324–5. 10.2307/3503773 [DOI] [Google Scholar]
- O'Keefe, J. M. , & Loeb, S. C. (2017). Indiana bats roost in ephemeral, fire‐dependent pine snags in the southern Appalachian Mountains, USA. Forest Ecology and Management, 391, 264–274. 10.1016/j.foreco.2017.01.036 [DOI] [Google Scholar]
- Pastro, L. A. , Dickman, C. R. , & Letnic, M. (2011). Burning for biodiversity or burning biodiversity? Prescribed burn vs. wildfire impacts on plants, lizards, and mammals. Ecological Applications, 21, 3238–3253. 10.1890/10-2351.1 [DOI] [Google Scholar]
- Pausas, J. G. , & Parr, C. L. (2018). Towards an understanding of the evolutionary role of fire in animals. Evolutionary Ecology, 32, 113–125. 10.1007/s10682-018-9927-6 [DOI] [Google Scholar]
- Perry, R. W. (2012). A Review of Fire Effects on Bats and Bat Habitat in the Eastern Oak Region. Proceedings of the 4th Fire in Eastern Oak Forest Conference; 2011 May 17–19 Spring Field, MO, 2011 May 17–19 Spring Field, MO. Gen Tech.: 170–191.
- Perry, R. W. , & McDaniel, V. L. (2015). Temperatures below leaf litter during winter prescribed burns: Implications for litter‐roosting bats. International Journal of Wildland Fire, 24, 544–549. 10.1071/WF14119 [DOI] [Google Scholar]
- Pierson, E. D. , Rainey, W. E. , & Corben, C. J. (2001). Seasonal patterns of bat distribution along an altitudinal gradient in the Sierra Nevada. Sacramento, CA: Report for California Department of Transportation. [Google Scholar]
- Ponisio, L. C. , Wilkin, K. , M'Gonigle, L. K. , Kulhanek, K. , Cook, L. , Thorp, R. , … Kremen, C. (2016). Pyrodiversity begets plant‐pollinator community diversity. Global Change Biology, 22, 1794–1808. 10.1111/gcb.13236 [DOI] [PubMed] [Google Scholar]
- Ratcliffe, J. M. , & Dawson, J. W. (2003). Behavioural flexibility: The little brown bat, Myotis lucifugus, and the northern long‐eared bat, M. septentrionalis, both glean and hawk prey. Animal Behaviour, 66, 847–856. 10.1006/anbe.2003.2297 [DOI] [Google Scholar]
- Roberts, S. L. , Kelt, D. A. , Van Wagtendonk, J. W. , Miles, A. K. , & Meyer, M. D. (2015). Effects of fire on small mammal communities in frequent‐fire forests in California. Journal of Mammalogy, 96, 107–119. 10.1093/jmammal/gyu011 [DOI] [Google Scholar]
- Roby, K. B. , & Azuma, D. L. (1995). Changes in a reach of a northern California stream following wildfire. Environmental Management, 19, 591–600. 10.1007/BF02471970 [DOI] [Google Scholar]
- Rochester, C. J. , Brehme, C. S. , Clark, D. R. , Stokes, D. C. , Hathaway, S. A. , & Fisher, R. N. (2010). Reptile and amphibian responses to large‐scale wildfires in Southern California. Journal of Herpetology, 44, 333–351. 10.1670/08-143.1 [DOI] [Google Scholar]
- Russo, D. , & Voigt, C. C. (2016). The use of automated identification of bat echolocation calls in acoustic monitoring: A cautionary note for a sound analysis. Ecological Indicators, 66, 598–602. 10.1016/j.ecolind.2016.02.036 [DOI] [Google Scholar]
- Safford, H. D. , & Stevens, J. T. (2017). Natural Range of Variation (NRV) for yellow pine and mixed conifer forests in the bioregional assessment area, including the Sierra Nevada, southern Cascades, and Modoc and Inyo National Forests. Gen Tech Rep PSW‐GTR‐2562, 5324–151.
- Safford, H. D. , VandeWater, K. , & Clark, C. (2016). California Fire Return Interval Departure (FRID) map, 2015 version. USDA Forest Service, Pacific Southwest Region, Sacramento and Vallejo, CA. [Internet]. [cited 10 May 2018]. Retrieved from: http://www.fs.usda.gov/main/r5/landmanagement/gis
- Saldaña‐Vázquez, R. A. , & Munguía‐Rosas, M. A. (2013). Lunar phobia in bats and its ecological correlates: A meta‐analysis. Mammalian Biology, 78, 216–219. 10.1016/j.mambio.2012.08.004 [DOI] [Google Scholar]
- Schnitzler, H.‐U. , Moss, C. F. , & Denzinger, A. (2003). From spatial orientation to food acquisition in echolocating bats. Trends in Ecology and Evolution, 18, 386–394. 10.1016/S0169-5347(03)00185-X [DOI] [Google Scholar]
- Segura‐Trujillo, C. A. , Lidicker, W. Z. , & Álvarez‐Castañeda, S. T. (2016). New perspectives on trophic guilds of arthropodivorous bats in North and Central America. Journal of Mammalogy, 97, 644–654. 10.1093/jmammal/gyv212 [DOI] [Google Scholar]
- Silvis, A. , Gehrt, S. D. , & Williams, R. A. (2016). Effects of shelterwood harvest and prescribed fire in upland Appalachian hardwood forests on bat activity. Forest Ecology and Management, 360, 205–212. 10.1016/j.foreco.2015.10.010 [DOI] [Google Scholar]
- Sleep, D. , & Brigham, R. (2003). An experimental test of clutter tolerance in bats. Journal of Mammalogy, 84, 216–224. [DOI] [Google Scholar]
- Snider, E. A. , Cryan, P. M. , & Wilson, K. R. (2013). Roost selection by western long‐eared myotis (Myotis evotis) in burned and unburned piñon–juniper woodlands of southwestern Colorado. Journal of Mammalogy, 94, 640–649. 10.1644/11-MAMM-A-153.1 [DOI] [Google Scholar]
- Stephens, S. L. , Burrows, N. , Buyantuyev, A. , Gray, R. W. , Keane, R. E. , Kubian, R. , … van Wagtendonk, J. W. (2014). Temperate and boreal forest mega‐fires: Characteristics and challenges. Frontiers in Ecology and the Environment, 12, 115–122. 10.1890/120332 [DOI] [Google Scholar]
- Taylor, R. S. , Watson, S. J. , Nimmo, D. G. , Kelly, L. T. , Bennett, A. F. , & Clarke, M. F. (2012). Landscape‐scale effects of fire on bird assemblages: Does pyrodiversity beget biodiversity? Diversity and Distributions, 18, 519–529. 10.1111/j.1472-4642.2011.00842.x [DOI] [Google Scholar]
- Tingley, M. W. , Ruiz‐Gutiérrez, V. , Wilkerson, R. L. , Howell, C. A. , & Siegel, R. B. (2016). Pyrodiversity promotes avian diversity over the decade following forest fire. Proceedings of the Royal Society B: Biological Sciences, 283(1840), 20161703, 10.1098/rspb.2016.1703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turbill, C. (2008). Winter activity of Australian tree‐roosting bats: Influence of temperature and climatic patterns. Journal of Zoology, 276, 285–290. 10.1111/j.1469-7998.2008.00487.x [DOI] [Google Scholar]
- United States Naval Observatory . (2011). MICA: Multiyear interactive computer almanac 1800–2050, version 2.2.2. Richmond, VA: William‐Bell. [Google Scholar]
- Upham, N. S. , & Hafner, J. C. (2013). Do nocturnal rodents in the Great Basin Desert avoid moonlight? Journal of Mammalogy, 94, 59–72. 10.1644/12-mamm-a-076.1 [DOI] [Google Scholar]
- US Geological Survey . (2017) The National Map, 2017, 3DEP products and services: The National Map, 3D Elevation Program [Internet]. [cited 20 Jul 2018]. Retrieved from: https://nationalmap.gov/3DEP/3dep_prodserv.html
- USDA Forest Service . (2017). Vegetation Burn Severity – Canopy Cover [ESRI personal geodatabase] Region 5 North Sierra. [Internet]. [cited 10 May 2018]. Retrieved from: https://www.fs.usda.gov/detail/r5/landmanagement/gis/?cxml:id=stelprd3804880
- Wang, Y. , Naumann, U. , Wright, S. T. , & Warton, D. I. (2012). Mvabund‐ an R package for model‐based analysis of multivariate abundance data. Methods in Ecology and Evolution, 3, 471–474. 10.1111/j.2041-210X.2012.00190.x [DOI] [Google Scholar]
- Warton, D. I. , Stoklosa, J. , Guillera‐Arroita, G. , MacKenzie, D. I. , & Welsh, A. H. (2017). Graphical diagnostics for occupancy models with imperfect detection. Methods in Ecology and Evolution, 8, 408–419. 10.1111/2041-210X.12761 [DOI] [Google Scholar]
- Weller, T. J. , Rodhouse, T. J. , Neubaum, D. J. , Ormsbee, P. C. , Dixon, R. D. , Popp, D. L. , … Navo, K. W. (2018). A review of bat hibernacula across the western United States: Implications for white‐nose syndrome surveillance and management. PLoS One, 13, e0205647 10.1371/journal.pone.0205647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Western Regional Climate Center . (2017). Western US Climate Historical Summaries: Quincy station (047195) [Internet]. [cited 20 Jul 2017]. Retrieved from: https://wrcc.dri.edu/cgi-bin/cliMAIN.pl?ca7195
- White, A. M. , Manley, P. N. , Tarbill, G. l. , Richardson, T. W. , Russell, R. E. , Safford, H. D. , & Dobrowski, S. Z. (2016). Avian community responses to post‐fire forest structure: Implications for fire management in mixed conifer forests. Animal Conservation, 19, 256–264. 10.1111/acv.12237 [DOI] [Google Scholar]
- Yue, X. , Mickley, L. J. , Logan, J. A. , & Kaplan, J. O. (2013). Ensemble projections of wildfire activity and carbonaceous aerosol concentrations over the western United States in the mid‐21st century. Atmospheric Environment, 77, 767–780. 10.1016/j.atmosenv.2013.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Bat detection data are archived on the USDA Forest Service Nature Resource Information System (https://www.fs.fed.us/nrm/index.shtml).


