Abstract
Variation in size may influence the abundance of visitors and reproductive allocation for cushion plants in the extreme alpine environments. To assess effects of plant size on the abundance of main visitors and reproductive allocation in Thylacospermum caespitosum populations at two altitudes, the abundance of the visitors, visiting frequency, total number of flowers, number of fruits, number of unseeded flowers, and reproductive allocation were investigated during the period of reproductive growth. Concurrently, the effects of plant size on the visitors' contributions to fruit setting rate were assessed by a bagging experiment. Our results showed that flies (Musca domestica and Dasyphora asiatica) were the main pollinating insects of T. caespitosum, and they could obvious facilitate (p < 0.05) the fruit setting rate of this cushion plant. Seed set and floral visitation were significantly influenced (p < 0.001) by plant size. Moreover, the reproductive allocation and fruit setting rate of T. caespitosum was influenced (p < 0.001) by plant size. More biomass was allocated to reproduction in plants of greater diameter. There is an increase in reproductive success (increases of fruit number with increase in plant size) in relation to plant size. In conclusion, the extent of M. domestica and D. asiatica to facilitate the fruit setting rate mainly depended on the size of T. caespitosum. Size‐dependent reproductive allocation occurred in T. caespitosum and was the chief factor affecting the contribution of flies to fruit setting rate. These traits reflect reproductive fitness of T. caespitosum related to plant size in extreme alpine environments.
Keywords: cushion plants, flies, ontogeny, pollination, reproductive allocation, seed set
1. INTRODUCTION
Insect visitors can enhance pollination and fruit set of plants, and plants can offer rewards for visitor insects (Bailes, Ollerton, Pattrick, & Glover, 2015). Most flowering plants need visitors to complete their reproductive cycle (Zhu, Yang, & Li, 2013), and attracting multiple visitors may benefit plants by increasing seed set. Visitors usually get rewards from flowering plants, of which food resources (such as nectar, pollen, oil, or other substances) are the most common rewards for the visitor (Simpson & Neff, 1983). Additionally, some flowers act as a shelter for floral visitors, offering protection from predators and cold climate (Sapir, Shmida, & Ne'eman, G., 2006; Seymour, White, & Gibernau, 2003; Zhu et al., 2013). In fact, there are flexible relationships between the majority of flowering plants and their visitors (Waser, Chittka, Price, Williams, & Ollerton, 1996). These flexible relationships might be related to the characteristics and habits of different taxonomic/functional groups and the local ecological context (Fenster, Armbruster, Wilson, Dudash, & Thomson, 2004; Ollerton, Killick, Lamborn, Watts, & Whiston, 2007). This relationship between plants and insect visitors is well known in agricultural crops (Deguines, Julliard, Flores, & Fontaine, 2016; Garibaldi et al., 2015; Klein, Steffan‐Dewenter, Buchori, & Tscharntke, 2002; Orford, Vaughan, & Memmott, 2015; Parsche, Fründ, & Tscharntke, 2011) and some wild plants (such as catchfly, invasive species, and other species; Brown, Lynch, & Zilberman, 2002; Flanagan, Mitchell, & Karron, 2011; Lance, Bailey, Lindsay, & Cobb, 2017; Mustajärvi, Siikamäki, Rytkönen, & Lammi, 2001; Yang, Ferrari, & Shea, 2010), which from agricultural and wild ecosystem. However, relationships between cushion plants and insect visitors in extreme alpine environments are not well understood.
Traditionally, bees, hoverflies, and butterflies are frequently studied as pollinators in agricultural and conservation research; wild and managed bees are well documented as effective pollinators in agricultural and other ecosystems (Orford et al., 2015). However, many flies, bumblebees, and Lepidoptera species are known contributors to pollination in alpine ecosystems (Arnold, Savolainen, & Chittka, 2009; Galen, 1996). In the subnival belt, the high altitude, low temperatures, overcast conditions, short growing season, unstable substrate, intense radiation, and relatively unpredictable weather and high winds are all challenging for insect visitors (Cavieres, Quiroz, Molina‐Montenegro, Muñoz, & Pauchard, 2005; Körner, 2003). Thus, the levels of diversity and activity of insect visitors are reduced due to the harsh climatic conditions in alpine ecosystems, and pollination rates are accordingly inherently low (Torres‐Díaz et al., 2011). These climatic characteristics also progressively reduce pollen availability in alpine plants (Bingham & Orthner, 1998). Alpine plants have developed solutions to deal with low visitor rates and reduction in pollen availability, such as self‐compatible (Liu et al., 2017; Sosenski, Ramos, Domínguez, Boege, & Fornoni, 2017) plants are an evolutionary solution for alpine and arctic plants to deal with low visitor numbers; thus, they will face lower risk of extinction due to low supply of pollen from compatible plants (García‐Camacho & Totland, 2009; Muñoz & Arroyo, 2006). Alpine plants in the subnival belt demonstrate a high frequency of asexual reproduction (Morgan, Wilson, & Knight, 2005; Reid, Hooper, Molenda, & Lortie, 2014). This reproductive strategy benefits alpine plants in harsh climatic conditions with low diversity and activities of pollinators (Milla, Giménez‐Benavides, Escudero, & Reich, 2009). Theoretical and empirical studies have predicted that severe environmental conditions (climatic conditions and insufficient visitors) lead to greater levels of asexual reproduction compared to lower alpine elevations, where high metabolic costs are invested in sexual reproduction relative to asexual reproduction (Chen, Li, Yang, & Sun, 2017; Schuster & Longton, 1983, Stark, Mishler, & McLetchie, 2000). Alpine plants can also compensate for the scarcity of visitors in alpine habitats by increasing the flowering phase and flower longevity (Blionis & Vokou, 2002; Fabbro & Körner, 2004).
Although alpine plants benefit from self‐compatible, asexual reproduction, longer flowering phase, and flower longevity to compensate for the low diversity and activity of pollinators, many alpine species are strongly dependent on scarce insect visitors to increase their seed set. Alpine flowers (Saxifraga oppositifolia, Dryas integrifolia, and Salix arctica) that are frequently visited by insects (pollinated primarily by flies and bumblebees) are totally or partially dependent on them for seed set (Kevan, 1972; Peeters & Totland, 1999). Here, we investigated whether and how strongly floral visitors influenced seed set in an alpine plant species, and what plant traits determined visitor abundance. High allocation to the production of reproductive structures also dictates the extent to which floral visitors pollinate flowers (Campbell & Halama, 1993). Producing (more nectar) may increase visitation rates as well as pollination success, and these in turn result in greater fruit and seed production (Mattila & Kuitunen, 2000; Sletvold, Tye, & Ågren, 2017). Moreover, environmental conditions can affect reproductive allocation patterns in plants. Variation in environmental conditions during reproduction may result in differences in ovule number, germination rates, growth rates of pollen tubes, and seed production (Jennersten, 1991; Young & Stanton, 1990). Alpine plants, hereafter cushion plants, tend toward a highly compact growth form that slowly (ca. 0–7 cm per year) grows along the ground, forming dense mats of vegetation. These slow‐growing species often have very long‐lived leaves (many cushions can grow for centuries or persist for additional centuries; Chen et al., 2017; Forbis & Doak, 2004; le Roux & McGeoch, 2004; Molau, 1997). Previous studies have shown that some cushion species may need 20 years of vegetative growth in order to begin reproduction for the remainder of their life history (Molau, 1997; Morris & Doak, 1998). These characteristics indirectly reflect that plant size may influence reproductive allocation in cushion plants. Thus, studies examining reproductive allocation need to consider the potential effect of size‐related variation in cushion species. After all, study of size dependence of fitness components is usually the only way to apply life history theory predictions to most herbaceous perennials due to the difficulty in evaluating the age of these plants except where long‐term demographic studies are conducted (Shabir, Nawchoo, & Wani, 2017).
Size‐dependent variation in reproductive allocation is a common phenomenon in many plant species (Samson & Werk, 1986). Plant size is widely used in the prediction of current and future reproductive output, and the relative advantages or disadvantages of reproducing at different sizes (Stearns, 1992; Sletvold, 2002). Ollerton and Lack (1998) indicated that plant size not only directly influences individual plant fecundity but also can indirectly affect reproductive output. The largest plants are commonly the most fecund, and size is closely correlated with total flower production in populations (Weiner, 1988; Herrera, 1993). In fact, the effect of plant size on reproductive output has been broadly discussed in extreme environments (Aarssen, 2015), such as desert and saline environments (Waller, 1988; Aguilar, Ashworth, Galetto, & Aizen, 2006; del Carmen Mandujano, Carrillo‐Angeles, Martínez‐Peralta, & Golubov, 2010; Benassi et al., 2011; Chacoff et al., 2012; Paasch, Mishler, Nosratinia, Stark, & Fisher, 2015; Salguero‐Gómez et al., 2016). Moreover, although alpine plants benefit from asexual reproduction to compensate for the low diversity and activity of pollinators, many cushion species are entirely dependent on sexual reproduction (Chen et al., 2017). Thylacospermum caespitosum (Caryophyllaceae) represents this type of cushion species. But plant size influences the abundance of floral visitors and biomass allocation for the cushion plant under an extreme alpine environment remains unknown. Here, we investigated whether the fruit setting rate promoted by visitors was related to the size of T. caespitosum. We also evaluated the effects of plant size on the abundance of the main visitors, visiting frequency, total number of flowers, number of fruits, number of unseeded flowers, reproductive biomass ratio, stem‐leaf biomass ratio, root biomass ratio, and fruit setting rate. We aimed to answer the following questions: (a) Who are the main visitors of T. caespitosum? (b) How do cushion plants (T. caespitosum) in extreme environments reproduce as they increase in size? And (c) how do flower visitors interact with T. caespitosum to influence fitness?
2. METHODS
2.1. Study site and study species
The study site was situated in the headwater region of the Urumqi River in the eastern Tianshan Mountains, China. The site is part of the Xinjiang Uygur Autonomous Region. The elevation varies from about 4,100 m to 4,300 m. At the study site, mean annual temperature ranges from ca. 5 during the day and −4°C at night; however, temperature as low as −10 may occur during the vegetative growth season (Liu et al., 2017). As Figure 1 shown, T. caespitosum is a perennial plant that has a woody taproot and forms very dense and solid cushions (Dvorský et al., 2013). Thylacospermum caespitosum is one of the most representative high‐alpine cushion plants in Asian high mountain regions. It is distributed along rocky slopes and crevices from 3,600–6,000 m asl. Thylacospermum caespitosum is found in China (Gansu, Qinghai, Sichuan, Xinjiang, and Xizang), India, Kazakhstan, Kyrgyzstan, Nepal, and Sikkim (Flora of China Editorial Committee, 1999). Thylacospermum caespitosum plants have been studied to understand their ameliorating effects on the harsh environments they inhabit (extreme altitude and dry conditions) and their role as nurse plants for other plant species in alpine ecosystems (Bello et al., 2011; Chen et al., 2015; Dvorský et al., 2013; Michalet et al., 2016).
Figure 1.

Flank view of a Thylacospermum caespitosum in the eastern Tianshan Mountains, China
In the study region, T. caespitosum was distributed in the range of 3,500–4,100 m elevation. Meantime T. caespitosum is foundation species of cushion vegetation in this region. Thylacospermum caespitosum exhibited four main flowering stages as follows (data from our observations in 2015): bud differentiation stage (10 May–20 May), initial flowering stage (20 May–10 June), full‐bloom stage (10 June–10 July), and flower fading stage (after 10 July). Experiments were conducted from May 2016 to October 2016 at the study site.
2.2. Effects of plant size on insect visitation rate
Two populations of T. caespitosum were used: a low elevation population (3,600 m) and a high elevation population (3,900 m). The two populations were about 5 km apart. In order to assess the effects of plant size on insect visitation, 20 flowering plants in each of the two populations were selected to monitor floral visitors. In each population, the selected plants were assigned to the following four plant size classes (each size have five flowering plants): R1: diameter of the plant ranged from 10 to 20 cm; R2: diameter of the plant ranged from 20 to 40 cm; R3: diameter of the plant ranged from 40 to 60 cm; and R4: diameter of the plant ranged from 60 to 80 cm. Floral visitor observation was performed for 5 days on every selected plant during full‐bloom stage (according to our observations, the full‐bloom stage of T. caespitosum lasted for 4–6 days). Visitor observations were carried out between 10:00 and 14:00, which was the peak time for visitors in the extreme alpine environment. Each observation on each flowering plant lasted for 30 min one day. The species of visiting insects, number of visiting insects, and visiting frequency were recorded for both populations. The different days for one plant were averaged for one replicate. Thus there are 5 replicates at per size class, and 20 plants per population (N = 40).
2.3. Effects of plant size on reproductive allocation
The selected plants used to determine visitation rate were used to count flowers (during flowering) and fruits (after flowering) of T. caespitosum by using circular PVC (polyvinyl chloride) piles with 10 cm radius (157 cm2). The centers of PVC piles coincided with the cushion plant. With these data, we could measure the total number of flowers, number of fruits, and number of unseeded flowers. At the same time, all of the above plants were collected at the end of the growing season to estimate biomass allocation (g). All samples were dried at 70°C for 48 hr and weighed (Ploschuk, Slafer, & Ravetta, 2005). Plant biomass was divided into reproductive biomass (all reproductive structures), stem‐leaf weight biomass, and root biomass.
2.4. Effects of plant size on visitors' contributions to fruit setting rate
Sixty flowering plants in each of the two populations were selected to assess the effect of visitors on fruit setting rate of T. caespitosum. In each population, the selected plants were assigned four classes as shown in Section 2.2. For each population, we randomly selected 15 plants in each of the four size classes (N = 60 for each population) and assigned them to one of two treatments: open pollination or exclusion of insect visitors. Twenty flowers (10 flowers for open pollination and 10 for exclusion of insect visitors on each plant) were randomly selected per plant, and we randomly divided the flowers into three sampling sessions. Thus, the 15 plants in each size class were divided into three groups (each group included five plants, with a total of 50 flowers marked as open pollination and 50 as exclusion of insect visitors). One group represented one repetition. Flowers in the open pollination treatment had no interference with regard to floral visitors, whereas flowers in the exclusion treatment were covered with nylon mesh bags to prevent visitation. We collected all the fruits before dehiscence to count fruit setting rate at harvest time. To evaluate the net outcome of the association between visitors and fruit setting rate of plants, we used the relative interaction index RII (Armas, Ordiales, & Pugnaire, 2004). RIIvisitor was used to quantify the intensity of the direct visitors' effects on fruit setting rate of T. caespitosum. RIIvisitor is bounded between −1 and 1, with negative values indicating a negative influence of visitors on fruit setting rate, positive values indicating positive influence, and larger absolute values indicating stronger influence of the visitors.
The variables are as follows: V1, mean fruit setting rate with open pollination; V2, mean fruit setting rate with exclusion of floral visitors.
2.5. Statistical analysis
All experimental results were presented as the mean ± standard error. To test the effect of plant size on abundance of main visitors and reproductive allocation, all parameters (number of flies, visiting frequency, total number of flowers, number of fruits, number of unseeded flowers, flower weight ratio, stem‐leaf weight ratio, root weight ratio, fruit setting rate, and RIIvisitor) were, respectively, evaluated by the generalized linear mixed model (GLMM), where plant number and population were random factors and plant size was fixed factor. Poisson error structure was used in the model. Analyses were performed using the MASS package of R 3.5.1 (R Development Core Team, 2011). Moreover, a paired sample test was used to evaluate differences in fruit setting rate between V1 and V2, and statistical analyses were performed using SPSS ver. 16.0 (SPSS, Chicago, Illinois, USA).
3. RESULTS
Results of field investigations showed that flies (Musca domestica and Dasyphora asiatica) were the main flower‐visiting insects for T. caespitosum (Figures 2 and 3a). Relative abundance of flies was >93% of visitors for all plant sizes of the two populations (Figure 3a). Although T. caespitosum has small flowers (about 2.0–3.5 mm; Figure 2c), the flowers were abundant once in reproductive stage. For example, the number of flowers reached 66.0 and 64.6 per 100 cm2 for the two largest plant size classes in the lower altitude population and 60.4 and 62.4 per 100 cm2 in the same size classes in the higher altitude population (Figures 2d and 4).
Figure 2.

Thylacospermum caespitosum (a) and its visitors (b and c) and fruit (d)
Figure 3.
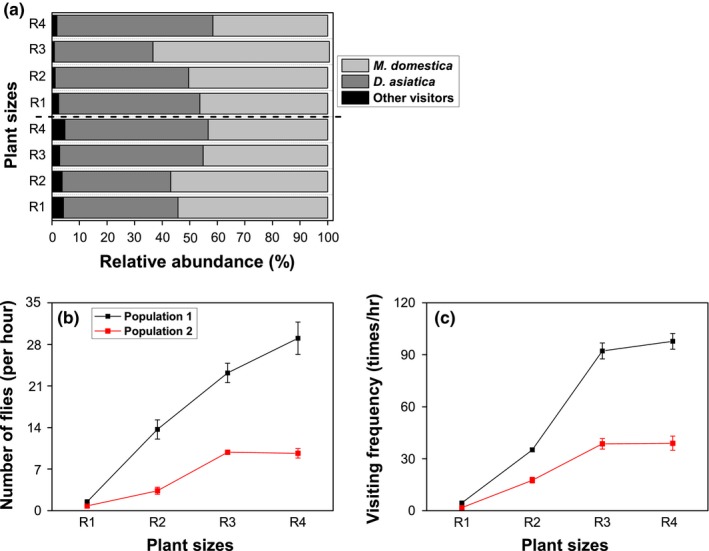
Changes in relative abundance of visitors (a), fly abundance (b), and visiting frequency (c) in relation to different sizes of Thylacospermum caespitosum plants at two different altitudes. Error bars indicate the standard error of the means (n = 5)
Figure 4.
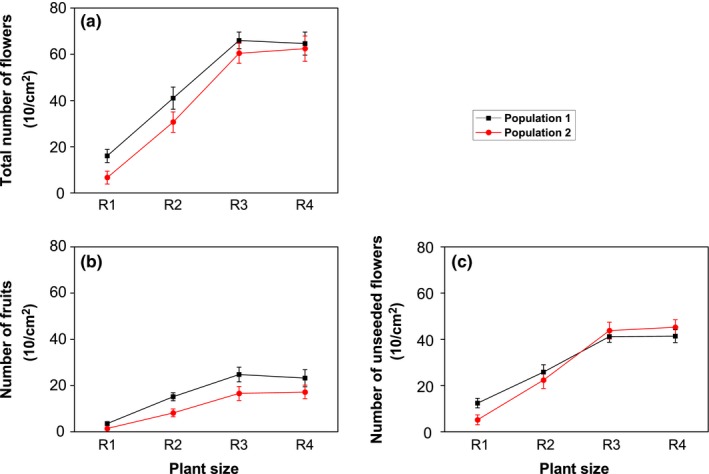
Change in number of flowers (100 cm2), number of fruits (100 cm2), and number of unseeded flowers (100 cm2) in relation to different sizes of Thylacospermum caespitosum plants at two different altitudes. Error bars indicate the standard error of the means (n = 5)
As shown in Table 1, number of flies and visiting frequency of T. caespitosum were dramatically (p < 0.001) influenced by plant size. For the lower altitude population, fly abundance and visited flowers were increased significantly with larger plant size (Figure 3b,c). When compared with R1, number of flies and visiting frequency were increased by 8.0 (R2) times, 14.3 (R3) times, and 18.1 (R4) times in the low altitude population; and 6.8 (R2) times, 19.6 (R3) times, and 20.8 (R4) times in the lower altitude population, respectively (Figure 3b,c). Similar results were observed in the high altitude population. These results indicated that the size of T. caespitosum was one of the most important factors influencing the number of flies and visited flowers. The greater the plant size, the more flowers were visited by flies.
Table 1.
Effects of plant size on abundance of main visitors and reproductive allocation in Thylacospermum caespitosum
| Fixed effect variables | Model parameter | |||
|---|---|---|---|---|
| β ± SE | 95% CI | t | p | |
| Number of flies (hr) | 0.90 ± 0.10 | −0.00007 to 0.00003 | 8.89 | <0.001 |
| Visiting frequency (times hr) | 2.53 ± 0.30 | −0.830 to 0.031 | 8.36 | <0.001 |
| Total number of flowers (100 cm2) | 0.45 ± 0.05 | −1.972 to 1.658 | 8.32 | <0.001 |
| Number of fruits (100 cm2) | 0.50 ± 0.09 | −1.667 to 1.178 | 5.50 | <0.001 |
| Number of unseeded flowers (100 cm2) | 0.43 ± 0.05 | −2.088 to 2.360 | 8.43 | <0.001 |
| Flower weight ratio (%) | 0.42 ± 0.08 | −1.468 to 1.524 | 5.19 | <0.001 |
| Stem‐leaf weight ratio (%) | −0.01 ± 0.01 | −1.733 to 1.877 | −0.70 | 0.4867 |
| Root weight ratio (%) | −0.02 ± 0.01 | −3.069 to 2.200 | −1.54 | 0.1307 |
| V1 (%) | 0.27 ± 0.06 | −1.034 to 0.721 | 4.70 | <0.001 |
| V2 (%) | 0.02 ± 0.05 | −1.837 to 1.671 | 0.43 | 0.6724 |
| RIIvisitor | 0.54 ± 0.07 | −1.119 to 0.672 | 8.01 | <0.001 |
Plant size had a great effect on total number of flowers, number of fruits, and number of unseeded flowers of T. caespitosum (p < 0.001, Table 1). For the lower altitude population, total number of flowers, number of fruits, and number of unseeded flowers number increased significantly (p < 0.05) when compared with R1 (Figure 4). When compared with R1, the total number of flowers, number of fruits, and number of unseeded flowers increased by 1.6 (R2) times, 3.1 (R3) times, 3.0 (R4) times (Figure 4). Similar changes were observed in the high altitude population (Figure 4). These results indicated that the size of T. caespitosum influences total number of flowers, number of fruits, and number of unseeded flowers.
The reproductive biomass ratio of T. caespitosum was significantly affected (p < 0.001) by plant size, but stem‐leaf weight ratio and root weight ratio not be affected (p > 0.05, Table 1). For the lower altitude population of T. caespitosum, the flower biomass ratio was significantly increased (p < 0.05) by 2.3 (R2) times, 4.0 (R3) times, and 3.5 (R4) times compared to R1 (Figure 5). Similarly, flower weight ratio significantly increased (p < 0.05) by 2.4 (R2) times, 4.9 (R3) times, and 4.9 (R4) when compared with R1 for high altitude populations. These results indicated that reproductive allocation of T. caespitosum was clearly influenced by plant size.
Figure 5.
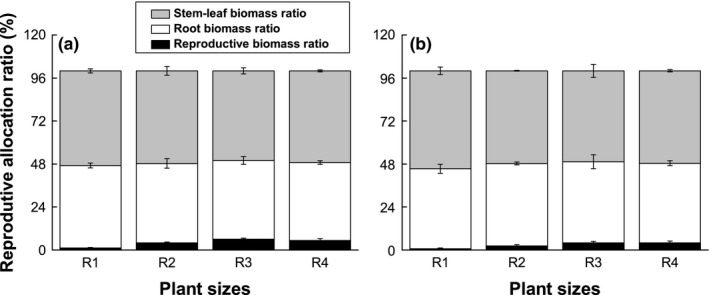
Changes in flower weight ratio, stem‐leaf weight ratio, and root weight ratio in relation to different sizes of Thylacospermum caespitosum plants at two different altitudes (a, low elevation and b, high elevation). Error bars indicate the standard error of the means (n = 5)
Fruit setting rate during open pollination (V1) was significantly affected (p < 0.001) by size of T. caespitosum plants (p < 0.001, Table 1). For the lower altitude population of T. caespitosum, V1 was significantly increased (p < 0.05) by 2.1 (R3) times and 2.2 (R4) times compared with R1 (Figure 6a). Similarly, V1 was significantly increased (p < 0.05) by 2.2 (R3) times and 2.1 (R4) times when compared with R1 for the high altitude population. However, fruit setting rate during the exclusion of insect visitors (V2) was not affected by plant size in the two populations (p > 0.05, Table 1). V1 was significantly higher (p < 0.05) than V2 except for R1 in the low and high elevation populations (Figure 6a,b). Moreover, plant size has a great effect on RII of T. caespitosum (p < 0.001, Table 1). RII increased with the increase in plant size, with R3 and R4 significantly higher (p < 0.05) than R1 and R2 (Figure 6c,d). These results indicated that flies may facilitate fruit setting in T. caespitosum, which was especially obvious in larger plants.
Figure 6.
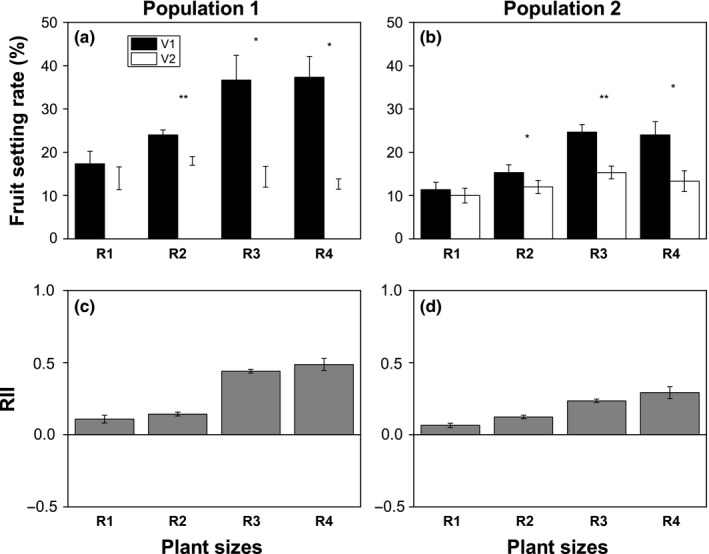
Fruit setting rate during open pollination (V1) and isolation from insect visitors (V2) in two populations (a, low elevation and b, high elevation): mean relative interaction index (RII) of visitor effects on two pollinations of Thylacospermum caespitosum (c, low elevation and d, high elevation). Error bars indicate the standard error of the means (n = 3). *represents differences among two populations, * p < 0.05, ** p < 0.01
4. DISCUSSION
Insect visitor diversity, abundance, and activity decrease dramatically in extreme alpine environments relative to tropical (or lowland) regions due to harsh climatic conditions (Körner, 2003; Medan et al., 2002). Scarcity of insect visitors adversely affects plant reproduction (Kuijper & Pen, 2014). However, alpine flowers are frequently visited by insects and are totally or partially dependent on them for seed set (Kevan, 1972; Peeters & Totland, 1999). Our results indicated that flies were the major visiting insects for T. caespitosum, and they may promote fruit setting of this cushion plant (especially in larger plants; Figures 2, 3 and 6). But bees, hoverflies, and butterflies (taxa frequently studied as visitors in agricultural and conservation contexts) were not observed in our study. In fact, more researchers are realizing that the contributions of visitors other than bees (such as nonsyrphid Diptera) have been underexplored despite their potential to contribute to plant reproduction and stability in the face of environmental change (Rader et al., 2016; Tiusanen, Hebert, Schmidt, & Roslin, 2016). Lefebvre, Fontaine, Villemant, and Daugeron (2014) indicated that flies (flies represent more than 60% of all visitors, with 54% of them being Empidinae) widely replaced bees as the main flower visitors in a subalpine meadow in the French Alps, and among them the Empidinae might play a key role in pollination. Kevan (1972) and Peeters and Totland (1999) showed that alpine flowers most frequently visited by flies and bumblebees are totally or partially dependent on them for seed set.
We then ask the question, why were floral visitors predominantly flies? On one hand, nonsyrphid dipteran visitors are dominant at high altitudes and latitudes, including alpine and subarctic ecosystems where bees are less abundant, mainly due to the loss of habitat from anthropogenic land use change and intensification (Elberling & Olesen, 1999; Shrestha et al., 2016; Vanbergen et al., 2014; Williams & Osborne, 2009). The proportion of Muscidae species usually increases with altitude, but the proportions of Syrphidae and bumblebees decreases (Pont, 1993). On the other hand, the scarcity of flower visitors with long proboscises in the alpine ecosystem may have opened a niche for flies and put a selection premium (extra charges in selection process) on a longer proboscis and more shallow flowers (Elberling & Olesen, 1999). This may indirectly help flies become predominant floral visitors of T. caespitosum. Moreover, our results were consistent with the previous view that flies (Muscidae) are probably the most important flower visitors above the timberline and may facilitate (The greater the plant size, the more flowers were visited by flies and the higher the fruit setting rate in T. caespitosum) the pollination of alpine plants (Larson, Kevan, & Inouye, 2001; Orford et al., 2015). In fact, flies (and other nonbee taxa) often have broader temporal activity ranges and can provide pollination services at different times of the day compared with bees and in weather conditions when bees are unable to forage (Howlett, 2012; McCall & Primack, 1992; Rader, Edwards, Westcott, Cunningham, & Howlett, 2013). Rader et al. (2016) indicated that nonbee insect (including flies) visitors play a significant role in global crop production and potentially make pollination services more robust to changes in land use. Thus, we conclude that floral visitors with the capacity to pollinate T. caespitosum over a broader temporal range (primarily nonbee taxa) have a stronger influence on their reproductive fitness than those with a more restricted temporal range. This is significant because it demonstrates how abiotic conditions select for particular pollinators, and how this may have a cascading effect on a long‐lived plant's fitness. Seed set and floral visitation of T. caespitosum were influenced by plant size. One possible reason is that small plants have less investment in reproduction relative to large individuals. Plant size influences several important fitness components (flowering probability, reproductive allocation, and fecundity) of herbaceous perennial plants (Msndez & Karlsson, 2004). This size‐dependent reproductive allocation (large plants generally have large reproductive investment) is common in alpine plants (Sun et al., 2014). Our results support the presence of more flowers and greater reproductive allocation in larger T. caespitosum plants (Figures 3 and 4; Table 1). Large reproductive investment means more materials will be used to attract insects for pollination. Moreover, difficulty in allocating nonstructural carbohydrates (as a main reward to visitors) to reproduction exists in extreme conditions. Under harsh environments, nonstructural carbohydrates can help plants to survive (Chapin, Schulze, & Mooney, 1990; Dietze et al., 2014). Theses carbohydrates correlate with resistance to extreme conditions (Hartmann, Ziegler, Kolle, & Trumbore, 2013; O'Brien, Leuzinger, Philipson, Tay, & Hector, 2014; Sala, Woodruff, & Meinzer, 2012; Slewinski, 2012; Tattini, Gucci, Romani, Baldi, & Everard, 1996), where nonstructural carbohydrates accumulate in plant tissue for use in cryoprotection, desiccation protection, and maintenance of turgor pressure and ionic composition (Ögren, Nilsson, & Sundblad, 1997; Myers & Kitajima, 2007; Bansal & Germino, 2008, 2009; Bansal, Reinhardt, & Germino, 2011). It is not cost‐effective for visitors to visit small plants in extreme alpine conditions because they will receive less reward in the process. Thus, differences in reproductive allocation with plant size may cause seed set and floral visitation in T. caespitosum.
Plant size is one of the major biotic factors that determined the amount of energy available for reproduction and seed development. Generally, large plants usually have large reproductive outputs (for example seeds or flowers) and have been confirmed in alpine plants (Rees & Venable, 2007; Sun et al., 2014; Venable & Rees, 2009). Large reproductive investment means more materials will be used to attract insects for pollination. Our results showed that the total number of flowers, the number of fruits, number of unseeded flowers, and reproductive allocation of T. caespitosum were clearly influenced by plant size (Figures 3 and 4; Table 1). He et al. (2008) found that the biomass of reproductive organs and allocation of resources to reproduction in three Oxytropis species from the Qinghai‐Tibet Plateau increased with increases in plant size. Similar positive relationships between reproductive allocation and plant size have also been found in Polygonum macrophyllum (Meng, Wang, Liu, & Zhu, 2011). Our results similarly showed that the total number of flowers, the number of fruits, number of unseeded flowers, and reproductive allocation were significantly increased (p < 0.05) with increases in plant size in two populations of T. caespitosum (Figures 4 and 5). This can be simply explained by the fact that larger T. caespitosum produced more reproductive biomass than smaller ones. This may be attributed to plant modular architecture, as relatively large individuals within a population have more vegetative and reproductive modules than smaller individuals (Weiner, 1988; Niklas, 1993). Underlying size‐dependent reproductive allocation is an adaptation of T. caespitosum to extreme environments. Reproductive allometry related to plant size is thought to be due to environmental variability and is interpreted as an adaptive strategy of plant growth and allocation (Bonser & Aarssen, 2009). Allocating more biomass to functions that maximize vegetative growth is more common under harsh environments (Shabir et al., 2017). For small individuals, large resource investment in reproduction may have a negative influence on future reproduction, growth, or survival (Obeso, 2002; Shibata & Kudo, 2016). Inversely, large resource investment in vegetative growth may lead to positive effects on future reproduction, especially in the harsh alpine environment where conditions (high altitude, low temperatures, overcast conditions, short growing season, unstable substrate, intense radiation, and relatively unpredictable weather and high winds) are not advantageous to plant survival. This type of allocation therefore implies trade‐offs for T. caespitosum in harsh alpine environments because resources allocated to one function or organ are not available to other functions or organs (Weiner, 2004).
In brief, flies are the main visitors of T. caespitosum, and floral visitors with the capacity to pollinate T. caespitosum over a broader temporal range (primarily nonbee taxa) have a stronger influence on T. caespitosum reproductive fitness than those with a more restricted temporal range. This is significant because it demonstrates how abiotic conditions select for particular pollinators, and how this may have a cascading effect on a long‐lived plant's fitness. Differences in reproductive allocation with plant size may cause seed set and floral visitation in T. caespitosum. Larger plants produce disproportionately more flower and fruit. This size‐dependent allocation of resources implies trade‐offs by T. caespitosum in harsh alpine environments and reflects cushions survival strategies.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
Ruiming Zhao designed and performed the study. Hua Zhang performed statistical analysis. Lizhe An conceived and financed this study. All the authors participated in writing the manuscript.
Supporting information
ACKNOWLEDGMENTS
This work was supported by the Key Program National Natural Science Foundation of China (41830321, 31230014); the National Science Foundation of China (31770432); the National Basic Research Program of China (973 Program) (2013CB429904); the Science and Technology Partnership Program, Ministry of Science and Technology of China (KY201501008); the National Science and Technology Major Project in Gansu (17ZD2WA017)
Zhao R, Zhang H, An L. Plant size influences abundance of floral visitors and biomass allocation for the cushion plant Thylacospermum caespitosum under an extreme alpine environment. Ecol Evol. 2019;9:5501–5511. 10.1002/ece3.5147
DATA ACCESSIBILITY
We agree to make our data publicly available in a relevant repository.
REFERENCES
- Aarssen, L. W. (2015). Body size and fitness in plants: revisiting the selection consequences of competition. Perspectives in Plant Ecology, Evolution and Systematics, 17(3), 236–242. 10.1016/j.ppees.2015.02.004. [DOI] [Google Scholar]
- Aguilar, R. , Ashworth, L. , Galetto, L. , & Aizen, M. A. (2006). Plant reproductive susceptibility to habitat fragmentation: Review and synthesis through a meta‐analysis. Ecology Letters, 9(8), 968–980. 10.1111/j.1461-0248.2006.00927.x. [DOI] [PubMed] [Google Scholar]
- Armas, C. , Ordiales, R. , & Pugnaire, F. I. (2004). Measuring plant interactions: A new comparative index. Ecology, 85(10), 2682–2686. 10.1890/03-0650 [DOI] [Google Scholar]
- Arnold, S. E. , Savolainen, V. , & Chittka, L. (2009). Flower colours along an alpine altitude gradient, seen through the eyes of fly and bee pollinators. Arthropod‐Plant Interactions, 3(1), 27–43. 10.1007/s11829-009-9056-9 [DOI] [Google Scholar]
- Bailes, E. J. , Ollerton, J. , Pattrick, J. G. , & Glover, B. J. (2015). How can an understanding of plant–pollinator interactions contribute to global food security? Current Opinion in Plant Biology, 26, 72–79. 10.1016/j.pbi.2015.06.002 [DOI] [PubMed] [Google Scholar]
- Bansal, S. , & Germino, M. J. (2008). Carbon balance of conifer seedlings at timberline: Relative changes in uptake, storage, and utilization. Oecologia, 158(2), 217. [DOI] [PubMed] [Google Scholar]
- Bansal, S. , & Germino, M. J. (2009). Temporal variation of nonstructural carbohydrates in montane conifers: similarities and differences among developmental stages, species and environmental conditions. Tree Physiology, 29(4), 559–568. 10.1093/treephys/tpn045 [DOI] [PubMed] [Google Scholar]
- Bansal, S. , Reinhardt, K. , & Germino, M. J. (2011). Linking carbon balance to establishment patterns: comparison of whitebark pine and Engelmann spruce seedlings along an herb cover exposure gradient at treeline. Plant Ecology, 212(2), 219–228. 10.1007/s11258-010-9816-8. [DOI] [Google Scholar]
- Bello, F. D. , Doležal, J. , Dvorský, M. , Chlumská, Z. , Řeháková, K. , Klimešová, J. , & Klimeš, L. (2011). Cushions of Thylacospermum caespitosum (Caryophyllaceae) do not facilitate other plants under extreme altitude and dry conditions in the north–west Himalayas. Annals of Botany, 108(3), 567–573. 10.1093/aob/mcr183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benassi, M. , Stark, L. R. , Brinda, J. C. , McLetchie, D. N. , Bonine, M. , & Mishler, B. D. (2011). Plant size, sex expression and sexual reproduction along an elevation gradient in a desert moss. The Bryologist, 114(2), 277–289. 10.1639/0007-2745-114.2.277. [DOI] [Google Scholar]
- Bingham, R. A. , & Orthner, A. R. (1998). Efficient pollination of alpine plants. Nature, 391(6664), 238–239. 10.1038/34564. [DOI] [Google Scholar]
- Blionis, G. J. , & Vokou, D. (2002). Structural and functional divergence of Campanula spatulata subspecies on Mt Olympos (Greece). Plant Systematics and Evolution, 232(1–2), 89–105. 10.1007/s006060200029. [DOI] [Google Scholar]
- Bonser, S. P. , & Aarssen, L. W. (2009). Interpreting reproductive allometry: Individual strategies of allocation explain size‐dependent reproduction in plant populations. Perspectives in Plant Ecology, Evolution and Systematics, 11(1), 31–40. 10.1016/j.ppees.2008.10.003 [DOI] [Google Scholar]
- Brown, C. , Lynch, L. , & Zilberman, D. (2002). The economics of controlling insect‐transmitted plant diseases. American Journal of Agricultural Economics, 84(2), 279–291. 10.1111/1467-8276.00297. [DOI] [Google Scholar]
- Campbell, D. R. , & Halama, K. J. (1993). Resource and pollen limitations to lifetime seed production in a natural plant population. Ecology, 74(4), 1043–1051. 10.2307/1940474 [DOI] [Google Scholar]
- Cavieres, L. A. , Quiroz, C. L. , Molina‐Montenegro, M. A. , Muñoz, A. A. , & Pauchard, A. (2005). Nurse effect of the native cushion plant Azorella monantha on the invasive non–native Taraxacum officinale in the high–Andes of central Chile. Perspectives in Plant Ecology, Evolution and Systematics, 7(3), 217–226. 10.1016/j.ppees.2005.09.002 [DOI] [Google Scholar]
- Chacoff, N. P. , Vázquez, D. P. , Lomáscolo, S. B. , Stevani, E. L. , Dorado, J. , & Padrón, B. (2012). Evaluating sampling completeness in a desert plant–pollinator network. Journal of Animal Ecology, 81(1), 190–200. 10.1111/j.1365-2656.2011.01883.x. [DOI] [PubMed] [Google Scholar]
- Chapin, F. S. III , Schulze, E. D. , & Mooney, H. A. (1990). The ecology and economics of storage in plants. Annual Review of Ecology and Systematics, 21(1), 423–447. 10.1146/annurev.es.21.110190.002231 [DOI] [Google Scholar]
- Chen, J. , Li, Y. , Yang, Y. , & Sun, H. (2017). How cushion communities are maintained in alpine ecosystems: A review and case study on alpine cushion plant reproduction. Plant Diversity., 21(39), 221–228. 10.1016/j.pld.2017.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, J. , Schöb, C. , Zhou, Z. , Gong, Q. , Li, X. , Yang, Y. , … Sun, H. (2015). Cushion plants can have a positive effect on diversity at high elevations in the Himalayan Hengduan Mountains. Journal of Vegetation Science, 26(4), 768–777. 10.1111/jvs.12275 [DOI] [Google Scholar]
- Deguines, N. , Julliard, R. , De Flores, M. , & Fontaine, C. (2016). Functional homogenization of flower visitor communities with urbanization. Ecology and Evolution, 6(7), 1967–1976. 10.1002/ece3.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Carmen Mandujano, M. , Carrillo‐Angeles, I. , Martínez‐Peralta, C. , & Golubov, J. (2010). Reproductive biology of Cactaceae. In Desert plants (pp. 197–230). Berlin, Heidelberg: Springer. [Google Scholar]
- Dietze, M. C. , Sala, A. , Carbone, M. S. , Czimczik, C. I. , Mantooth, J. A. , Richardson, A. D. , & Vargas, R. (2014). Nonstructural carbon in woody plants. Annual Review of Plant Biology, 65, 667–687. 10.1146/annurev-arplant-050213-040054 [DOI] [PubMed] [Google Scholar]
- Dvorský, M. , Doležal, J. , Kopecký, M. , Chlumská, Z. , Janatková, K. , Altman, J. , … Řeháková, K. (2013). Testing the stress–gradient hypothesis at the roof of the world: Effects of the cushion plant Thylacospermum caespitosum on species assemblages. PLoS ONE, 8(1), e53514 10.1371/journal.pone.0053514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elberling, H. , & Olesen, J. M. (1999). The structure of a high latitude plant–flower visitor system: The dominance of flies. Ecography, 22(3), 314–323. 10.1111/j.1600-0587.1999.tb00507.x [DOI] [Google Scholar]
- Fabbro, T. , & Körner, C. (2004). Altitudinal differences in flower traits and reproductive allocation. Flora‐Morphology, Distribution, Functional Ecology of Plants, 199(1), 70–81. 10.1078/0367-2530-00128 [DOI] [Google Scholar]
- Fenster, C. B. , Armbruster, W. S. , Wilson, P. , Dudash, M. R. , & Thomson, J. D. (2004). Pollination syndromes and floral specialization. Annual Review of Ecology, Evolution, and Systematics, 35, 375–403. [Google Scholar]
- Flanagan, R. J. , Mitchell, R. J. , & Karron, J. D. (2011). Effects of multiple competitors for pollination on bumblebee foraging patterns and Mimulus ringens reproductive success. Oikos, 120(2), 200–207. 10.1111/j.1600-0706.2010.18777.x [DOI] [Google Scholar]
- Flora of China Editorial Committee (1999). Flora of China. Beijing, China: Science Press. [Google Scholar]
- Forbis, T. A. , & Doak, D. F. (2004). Seedling establishment and life history trade–offs in alpine plants. American Journal of Botany, 91(7), 1147–1153. 10.3732/ajb.91.7.1147 [DOI] [PubMed] [Google Scholar]
- Galen, C. (1996). Rates of floral evolution: Adaptation to bumblebee pollination in an alpine wildflower. Polemonium Viscosum. Evolution, 50(1), 120–125. [DOI] [PubMed] [Google Scholar]
- García‐Camacho, R. , & Totland, Ø. (2009). Pollen limitation in the alpine: A meta–analysis. Arctic, Antarctic, and Alpine Research, 41(1), 103–111. 10.1657/1523-0430-41.1.103 [DOI] [Google Scholar]
- Garibaldi, L. A. , Bartomeus, I. , Bommarco, R. , Klein, A. M. , Cunningham, S. A. , Aizen, M. A. , … Morales, C. L. (2015). EditorDITOR'Ss CHchoiceOICE: REVIEWreview: Trait matching of flower visitors and crops predicts fruit set better than trait diversity. Journal of Applied Ecology, 52(6), 1436–1444. [Google Scholar]
- Hartmann, H. , Ziegler, W. , Kolle, O. , & Trumbore, S. (2013). Thirst beats hunger–declining hydration during drought prevents carbon starvation in Norway spruce saplings. New Phytologist, 200(2), 340–349. 10.1111/nph.12331 [DOI] [PubMed] [Google Scholar]
- He, Y. P. , Fei, S. M. , Duan, Y. W. , Liu, J. Q. , Chen, X. M. , & Jiang, J. M. (2008). The influences of meadow degradation on the resource allocation of Oxytropis plants in Haibei Region. Journal of Sichuan Forestry Science and Technology, 29, 9–16. [Google Scholar]
- Herrera, C. M. (1993). Selection on complexity of corolla outline in a hawkmoth‐pollinated violet. Evolutionary Trends in Plants, 7, 5501. [Google Scholar]
- Howlett, B. G. (2012). Hybrid carrot seed crop pollination by the fly Calliphora vicina (Diptera: Calliphoridae). Journal of Applied Entomology, 136(6), 421–430. 10.1111/j.1439-0418.2011.01665.x [DOI] [Google Scholar]
- Jennersten, O. (1991). Cost of reproduction in Viscaria vulgaris (Caryophyllaceae): A field experiment. Oikos, 197–204. 10.2307/3545337 [DOI] [Google Scholar]
- Kevan, P. G. (1972). Insect pollination of high arctic flowers. The Journal of Ecology, 831–847. 10.2307/2258569 [DOI] [Google Scholar]
- Klein, A. M. , Steffan‐Dewenter, I. , Buchori, D. , & Tscharntke, T. (2002). Effects of land–use intensity in tropical agroforestry systems on coffee flower–visiting and trap–nesting bees and wasps. Conservation Biology, 16(4), 1003–1014. 10.1046/j.1523-1739.2002.00499.x [DOI] [Google Scholar]
- Körner, C. H. (2003). Alpine plant life. Berlin, Germany: Springer. [Google Scholar]
- Kuijper, B. , & Pen, I. (2014). Conflict over condition‐dependent sex allocation can lead to mixed sex‐determination systems. Evolution, 68(11), 3229–3247. 10.1111/evo.12513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lance, R. F. , Bailey, P. , Lindsay, D. L. , & Cobb, N. S. (2017). Precipitation and the robustness of a plant and flower‐visiting insect network in a xeric ecosystem. Journal of Arid Environments, 144, 48–59. 10.1016/j.jaridenv.2017.03.015 [DOI] [Google Scholar]
- Larson, B. M. H. , Kevan, P. G. , & Inouye, D. W. (2001). Flies and flowers: Taxonomic diversity of anthophiles and pollinators. The Canadian Entomologist, 133(4), 439–465. 10.4039/Ent133439-4 [DOI] [Google Scholar]
- le Roux, P. C. , & McGeoch, M. A. (2004). The use of size as an estimator of age in the subantarctic cushion plant, Azorella selago (Apiaceae). Arctic, Antarctic, and Alpine Research, 36(4), 509–517. 10.1657/1523-0430(2004)36\509:TUOSAA]2.0.CO;2 [DOI] [Google Scholar]
- Lefebvre, V. , Fontaine, C. , Villemant, C. , & Daugeron, C. (2014). Are empidine dance flies major flower visitors in alpine environments? A case study in the Alps. France. Biology Letters, 10(11), 20140742 10.1098/rsbl.2014.0742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, G. , Zhao, L. , Li, R. , Wu, T. , Jiao, K. , & Ping, C. (2017). Permafrost warming in the context of step–wise climate change in the Tien Shan Mountains, China. Permafrost and Periglacial Processes, 28(1), 130–139. 10.1002/ppp.1885 [DOI] [Google Scholar]
- Mattila, E. , & Kuitunen, M. T. (2000). Nutrient versus pollination limitation in Platanthera bifolia and Dactylorhiza incarnata (Orchidaceae). Oikos, 89(2), 360–366. 10.1034/j.1600-0706.2000.890217.x [DOI] [Google Scholar]
- McCall, C. , & Primack, R. B. (1992). Influence of flower characteristics, weather, time of day, and season on insect visitation rates in three plant communities. American Journal of Botany, 434–442. 10.1002/j.1537-2197.1992.tb14571.x [DOI] [Google Scholar]
- Medan, D. , Montaldo, N. H. , Devoto, M. , Maniese, A. , Vasellati, V. , Roitman, G. G. , & Bartoloni, N. H. (2002). Plant‐pollinator relationships at two altitudes in the Andes of Mendoza, Argentina. Arctic, Antarctic, and Alpine Research, 34(3), 233–241. 10.1080/15230430.2002.12003490 [DOI] [Google Scholar]
- Meng, L. H. , Wang, Z. K. , Liu, C. Y. , & Zhu, W. L. (2011). Reproductive allocation of an alpine perennial, Polygonum macrophyllum . Acta Botanica Boreali‐Occidentalia Sinica, 6, 018. [Google Scholar]
- Michalet, R. , Schöb, C. , Xiao, S. , Zhao, L. , Chen, T. , An, L. Z. , & Callaway, R. M. (2016). Beneficiary feedback effects on alpine cushion benefactors become more negative with increasing cover of graminoids and in dry conditions. Functional Ecology, 30(1), 79–87. [Google Scholar]
- Milla, R. , Giménez‐Benavides, L. , Escudero, A. , & Reich, P. B. (2009). Intra‐and interspecific performance in growth and reproduction increase with altitude: A case study with two Saxifraga species from northern Spain. Functional Ecology, 23(1), 111–118. [Google Scholar]
- Molau, U. (1997). Responses to natural climatic variation and experimental warming in two tundra plant species with contrasting life forms: Cassiope tetragona and Ranunculus nivalis . Global Change Biology, 3(S1), 97–107. [Google Scholar]
- Morgan, M. T. , Wilson, W. G. , & Knight, T. M. (2005). Plant population dynamics, pollinator foraging, and the selection of self–fertilization. The American Naturalist, 166(2), 169–183. 10.1086/431317 [DOI] [PubMed] [Google Scholar]
- Morris, W. , & Doak, D. (1998). Life history of the long–lived gynodioecious cushion plant Silene acaulis (Caryophyllaceae), inferred from size–based population projection matrices. American Journal of Botany, 85(6), 784–784. [PubMed] [Google Scholar]
- Msndez, M. , & Karlsson, P. S. (2004). Between–population variation in size–dependent reproduction and reproductive allocation in Pinguiculavulgaris (Lentibulariaceae) and its environmental correlates. Oikos, 104(1), 59–70. [Google Scholar]
- Muñoz, A. A. , & Arroyo, M. T. (2006). Pollen limitation and spatial variation of reproductive success in the insect–pollinated shrub Chuquiraga oppositifolia (Asteraceae) in the Chilean Andes. Arctic, Antarctic, and Alpine Research, 38(4), 608–613. 10.1657/1523-0430(2006)38\608:PLASVO]2.0.CO;2 [DOI] [Google Scholar]
- Mustajärvi, K. , Siikamäki, P. , Rytkönen, S. , & Lammi, A. (2001). Consequences of plant population size and density for plant–pollinator interactions and plant performance. Journal of Ecology, 89(1), 80–87. 10.1046/j.1365-2745.2001.00521.x [DOI] [Google Scholar]
- Myers, J. A. , & Kitajima, K. (2007). Carbohydrate storage enhances seedling shade and stress tolerance in a neotropical forest. Journal of Ecology, 95(2), 383–395. 10.1111/j.1365-2745.2006.01207.x [DOI] [Google Scholar]
- Niklas, K. J. (1993). Ontogenetic–response models and the evolution of plant size. Evolutionary Trends in Plants, 7, 42–48. [Google Scholar]
- O'Brien, M. J. , Leuzinger, S. , Philipson, C. D. , Tay, J. , & Hector, A. (2014). Drought survival of tropical tree seedlings enhanced by non‐structural carbohydrate levels. Nature Climate Change, 4(8), 710 10.1038/nclimate2281 [DOI] [Google Scholar]
- Obeso, J. R. (2002). The costs of reproduction in plants. New Phytologist, 155(3), 321–348. 10.1046/j.1469-8137.2002.00477.x [DOI] [PubMed] [Google Scholar]
- Ögren, E. , Nilsson, T. , & Sundblad, L. G. (1997). Relationship between respiratory depletion of sugars and loss of cold hardiness in coniferous seedlings over‐wintering at raised temperatures: Indications of different sensitivities of spruce and pine. Plant, Cell & Environment, 20(2), 247–253. [Google Scholar]
- Ollerton, J. , Killick, A. , Lamborn, E. , Watts, S. , & Whiston, M. (2007). Multiple meanings and modes: On the many ways to be a generalist flower. Taxon, 56(3), 717–728. 10.2307/25065855 [DOI] [Google Scholar]
- Ollerton, J. , & Lack, A. (1998). Relationships between flowering phenology, plant size and reproductive success in shape Lotus corniculatus (Fabaceae). Plant Ecology, 139(1), 35–47. [Google Scholar]
- Orford, K. A. , Vaughan, I. P. , & Memmott, J. (2015). The forgotten flies: the importance of non–syrphid Diptera as pollinators. Proceedings of the Royal Society of London B: Biological Sciences, 282(1805), 20142934 10.1098/rspb.2014.2934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paasch, A. E. , Mishler, B. D. , Nosratinia, S. , Stark, L. R. , & Fisher, K. M. (2015). Decoupling of sexual reproduction and genetic diversity in the female‐biased Mojave Desert moss Syntrichia caninervis (Pottiaceae). International Journal of Plant Sciences, 176(8), 751–761. [Google Scholar]
- Parsche, S. , Fründ, J. , & Tscharntke, T. (2011). Experimental environmental change and mutualistic vs. antagonistic plant flower–visitor interactions. Perspectives in Plant Ecology, Evolution and Systematics, 13(1), 27–35. 10.1016/j.ppees.2010.12.001 [DOI] [Google Scholar]
- Peeters, L. , & Totland, Ø. (1999). Wind to insect pollination ratios and floral traits in five alpine Salix species. Canadian Journal of Botany, 77(4), 556–563. [Google Scholar]
- Ploschuk, E. L. , Slafer, G. A. , & Ravetta, D. A. (2005). Reproductive allocation of biomass and nitrogen in annual and perennial Lesquerella crops. Annals of Botany, 96(1), 127–135. 10.1093/aob/mci158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pont, A. C. (1993). Observations on anthophilous Muscidae and other Diptera (Insecta) in Abisko National Park. Sweden. Journal of Natural History, 27(3), 631–643. 10.1080/00222939300770361 [DOI] [Google Scholar]
- R Development Core Team. (2011). R Development Core Team R: A Language and Environment for Statistical Computing 3–900051‐07‐0. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Rader, R. , Bartomeus, I. , Garibaldi, L. A. , Garratt, M. P. D. , Howlett, B. G. , Winfree, R. , … Woyciechowski, M. (2016). Non–bee insects are important contributors to global crop pollination. Proceedings of the National Academy of Sciences of the United States of America, 113(1), 146–151. 10.1073/pnas.1517092112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rader, R. , Edwards, W. , Westcott, D. A. , Cunningham, S. A. , & Howlett, B. G. (2013). Diurnal effectiveness of pollination by bees and flies in agricultural Brassica rapa: Implications for ecosystem resilience. Basic and Applied Ecology, 14(1), 20–27. 10.1016/j.baae.2012.10.011 [DOI] [Google Scholar]
- Rees, M. , & Venable, D. L. (2007). Why do big plants make big seeds? Journal of Ecology, 95(5), 926–936. 10.1111/j.1365-2745.2007.01277.x [DOI] [Google Scholar]
- Reid, A. , Hooper, R. , Molenda, O. , & Lortie, C. J. (2014). Ecological implications of reduced pollen supply in the alpine: A case study using a dominant cushion plant species. F1000Research, 3, 130 10.12688/f1000research.4382.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala, A. , Woodruff, D. R. , & Meinzer, F. C. (2012). Carbon dynamics in trees: Feast or famine? Tree Physiology, 32(6), 764–775. 10.1093/treephys/tpr143 [DOI] [PubMed] [Google Scholar]
- Salguero‐Gómez, R. , Jones, O. R. , Jongejans, E. , Blomberg, S. P. , Hodgson, D. J. , Mbeau‐Ache, C. , … Buckley, Y. M. (2016). Fast–slow continuum and reproductive strategies structure plant life‐history variation worldwide. Proceedings of the National Academy of Sciences, 113(1), 230–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson, D. A. , & Werk, K. S. (1986). Size‐dependent effects in the analysis of reproductive effort in plants. The American Naturalist, 127(5), 667–680. [Google Scholar]
- Sapir, Y. , Shmida, A. , & Ne'eman, G., (2006). Morning floral heat as a reward to the pollinators of the Oncocyclus irises. Oecologia, 147(1), 53–59. 10.1007/s00442-005-0246-6 [DOI] [PubMed] [Google Scholar]
- Schuster, R. M. , & Longton, R. E. (1983). Reproductive biology. New Manual of Bryology, 1, 386–462. [Google Scholar]
- Seymour, R. S. , White, C. R. , & Gibernau, M. (2003). Environmental biology: Heat reward for insect pollinators. Nature, 426(6964), 243–244. [DOI] [PubMed] [Google Scholar]
- Shabir, P. A. , Nawchoo, I. A. , & Wani, A. A. (2017). A performance appraisal of size dependent reproduction and reproductive allocation: A case study of two Inula species from Kashmir Himalaya. Russian Journal of Ecology, 48(5), 440–448. 10.1134/S1067413617050113 [DOI] [Google Scholar]
- Shibata, A. , & Kudo, G. (2016). Size‐dependent sex allocation and reproductive investment in a gynodioecious shrub. AoB Plants, 9(1), plw089 10.1093/aobpla/plw089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha, M. , Lunau, K. , Dorin, A. , Schulze, B. , Bischoff, M. , Burd, M. , & Dyer, A. G. (2016). Floral colours in a world without birds and bees: The plants of Macquarie Island. Plant Biology, 18(5), 842–850. 10.1111/plb.12456 [DOI] [PubMed] [Google Scholar]
- Simpson, B. B. , & Neff, J. L. (1983). Evolution and diversity of floral rewards. Handbook of Experimental Pollination Biology, 142–159. [Google Scholar]
- Sletvold, N. (2002). Effects of plant size on reproductive output and offspring performance in the facultative biennial Digitalis purpurea . Journal of Ecology, 90(6), 958–966. [Google Scholar]
- Sletvold, N. , Tye, M. , & Ågren, J. (2017). Resource–and pollinator–mediated selection on floral traits. Functional Ecology, 31(1), 135–141. [Google Scholar]
- Slewinski, T. L. (2012). Non‐structural carbohydrate partitioning in grass stems: A target to increase yield stability, stress tolerance, and biofuel production. Journal of Experimental Botany, 63(13), 4647–4670. 10.1093/jxb/ers124 [DOI] [PubMed] [Google Scholar]
- Sosenski, P. , Ramos, S. E. , Domínguez, C. A. , Boege, K. , & Fornoni, J. (2017). Pollination biology of the hexaploid self‐compatible species Turnera velutina (Passifloraceae). Plant Biology, 19(2), 101–107. [DOI] [PubMed] [Google Scholar]
- Stark, L. R. , Mishler, B. D. , & McLetchie, D. N. (2000). The cost of realized sexual reproduction: Assessing patterns of reproductive allocation and sporophyte abortion in a desert moss. American Journal of Botany, 87(11), 1599–1608. 10.2307/2656736 [DOI] [PubMed] [Google Scholar]
- Stearns, S. (1992). The evolution of life histories. New York: Oxford University Press. [Google Scholar]
- Sun, H. , Niu, Y. , Chen, Y.‐S. , Song, B. o. , Liu, C.‐Q. , Peng, D.‐L. , … Yang, Y. (2014). Survival and reproduction of plant species in the Qinghai‐Tibet Plateau. Journal of Systematics and Evolution, 52(3), 378–396. 10.1111/jse.12092 [DOI] [Google Scholar]
- Tattini, M. , Gucci, R. , Romani, A. , Baldi, A. , & Everard, J. D. (1996). Changes in non–structural carbohydrates in olive (Olea europaea) leaves during root zone salinity stress. Physiologia Plantarum, 98(1), 117–124. 10.1111/j.1399-3054.1996.tb00682.x [DOI] [Google Scholar]
- Tiusanen, M. , Hebert, P. D. , Schmidt, N. M. , & Roslin, T. (2016). One fly to rule them all‐muscid flies are the key pollinators in the Arctic. Proceedings of the Royal Society B, 283(1839), 20161271 10.1098/rspb.2016.1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres‐Díaz, C. , Gomez‐Gonzalez, S. , Stotz, G. C. , Torres‐Morales, P. , Paredes, B. , Pérez‐Millaqueo, M. , & Gianoli, E. (2011). Extremely long‐lived stigmas allow extended cross‐pollination opportunities in a high Andean plant. PLoS ONE, 6(5), e19497 10.1371/journal.pone.0019497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanbergen, A. J. , Woodcock, B. A. , Gray, A. , Grant, F. , Telford, A. , Lambdon, P. , … Cavers, S. (2014). Grazing alters insect visitation networks and plant mating systems. Functional Ecology, 28(1), 178–189. 10.1111/1365-2435.12191 [DOI] [Google Scholar]
- Venable, D. L. , & Rees, M. (2009). The scaling of seed size. Journal of Ecology, 97(1), 27–31. 10.1111/j.1365-2745.2008.01461.x [DOI] [Google Scholar]
- Waller, D. M. (1988). Plant morphology and reproduction. Plant reproductive ecology: Patterns and strategies, 203–227 [Google Scholar]
- Waser, N. M. , Chittka, L. , Price, M. V. , Williams, N. M. , & Ollerton, J. (1996). Generalization in pollination systems, and why it matters. Ecology, 77(4), 1043–1060. 10.2307/2265575 [DOI] [Google Scholar]
- Weiner, J. A. C. O. B. (1988). The influence of competition on plant reproduction. Plant Reproductive Ecology: Patterns and Strategies, 228–245. [Google Scholar]
- Weiner, J. (2004). Allocation, plasticity and allometry in plants. Perspectives in Plant Ecology, Evolution and Systematics, 6(4), 207–215. 10.1078/1433-8319-00083 [DOI] [Google Scholar]
- Williams, P. H. , & Osborne, J. L. (2009). Bumblebee vulnerability and conservation world–wide. Apidologie, 40(3), 367–387. 10.1051/apido/2009025 [DOI] [Google Scholar]
- Yang, S. , Ferrari, M. J. , & Shea, K. (2010). Pollinator behavior mediates negative interactions between two congeneric invasive plant species. The American Naturalist, 177(1), 110–118. 10.1086/657433 [DOI] [PubMed] [Google Scholar]
- Young, H. J. , & Stanton, M. L. (1990). Influences of floral variation on pollen removal and seed production in wild radish. Ecology, 71(2), 536–547. 10.2307/1940307 [DOI] [Google Scholar]
- Zhu, X. , Yang, J. , & Li, Q. (2013). Alpine Codonopsis convolvulacea (Campanulaceae) provides multiple rewards to its main pollinator. Plant Ecology and Diversity, 6(2), 187–193. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
We agree to make our data publicly available in a relevant repository.


