Abstract
Maternal separation causes depression and anxiety. Exercise ameliorates maternal separation-induced depression. In this study, we investigated the effect of treadmill exercise on anxiety-like behavior in relation with glycogen synthase kinase 3 beta (GSK3β)/β-catenin pathway using maternal separation rat pups. For this study, elevated plus maze test, immunohistochemistry for serotonin (5-hydroxytryptamine, 5-HT), tryptophan hydroxylase (TPH), and western blot for total GSK3β (t-GSK3β), phosphorylated GSK3β (p-GSK3β), total β-catenin (t-β-catenin), and phosphorylated β-catenin (p-β-catenin) were conducted. The rat pups in the exercise groups were scheduled to run on a treadmill for 30 min once a day for 10 days, starting on postnatal day 21. For the rat pups in the fluoxetine-treated group, fluoxetine was orally administrated once a day for 10 consecutive days, starting on postnatal day 21. Anxiety-like behavior was appeared in the rat pups by maternal separation. Maternal separation suppressed 5-HT and TPH expression in the dorsal raphe. Maternal separation suppressed phosphorylation of GSK3β and increased phosphorylation of β-catenin in the hippocampus. However, treadmill exercise and fluoxetine treatment alleviated anxiety and increased 5-HT and TPH expression in the dorsal raphe. Treadmill exercise and fluoxetine treatment also enhanced GSK3β phosphorylation and suppressed β-catenin phosphorylation in the hippocampus. In this study, alleviating effect of treadmill exercise on maternal separation-induced anxiety appeared through enhancing 5-HT expression and GSK3β phosphorylation, and then inhibiting β-catenin phosphorylation. These results showed that treadmill exercise relieves anxiety through GSK3β/β-catenin pathway. Treadmill exercise showed similar ameliorating effect on anxiety-like behavior as fluoxetine.
Keywords: Maternal separation, Anxiety, Exercise, Serotonin, Glycogen synthase kinase 3β, β-Catenin
INTRODUCTION
Close relationship with parents is important factor for the development of offspring. Maternal separation causes emotional, behavioral, cognitive, and socialization problems to offspring. Maternal separation is one of the animal models of depression, and depression induces a functional alteration of various neurotransmitters (Baek et al., 2012; Yadid et al., 2000).
Among the neurotransmitters related with depression and anxiety, serotonin (5-hydroxy trigramamine, 5-HT) is involved in feeding, sleep, diet, sleep, and mood control (Bannai et al., 2007; Ji et al., 2017). Tryptophan hydroxylase (TPH) is known as a speed-limiting enzyme for the production of 5-HT, and the level of TPH has been used as an indicator of 5-HT synthesis. Depression is associated with down-regulation of 5-HT in the dorsal raphe, and 5-HT controls neural stabilization and alleviates depressive behavior (Baek et al., 2012; Roh et al., 2016).
5-HT regulates glycogen synthase kinase 3 beta (GSK3β), and GSK3β is serine/threonine kinase which modulates glycogen biosynthesis (Latapy et al., 2012). Impaired GSK3β activity occurs under the conditions of dysregulation in serotonergic activity, such as mood disorders (Li et al., 2004). Inhibition of GSK3β exerted an anti-depressive effect, and then GSK3β inhibitors have been considered as the antidepressants (Kaidanovich-Beilin et al., 2004). GSK3β is associated with the serotonin-sensitive anxiety and social behavior (Latapy et al., 2012).
The transcription factor β-catenin is the marker of GSK3β inactivation, because cytoplasmic level of β-catenin is increased by inhibition of the GSK3β (Gould et al., 2004). GSK3β/β-catenin signaling plays an important role in stress-related depression, and this pathway contributes to the anti-depressive effect in the mood regulation brain region (Chen et al., 2012).
Exercise is known to increased brain 5-HT level and exercise acts as noninvasive treatment for depression (Baek et al., 2012; Ji et al., 2017; Lee and Baek, 2017). Fluoxetine is the most widely used antidepressant acting as one of the serotonin reuptake inhibitors (SSRIs) (Ferguson, 2001). In the present study, we investigated the effect of treadmill exercise on the maternal separation-induced anxiety in relation with GSK3β/β-catenin pathway. For this study, elevated plus maze test was conducted for the determination of anxiety level. Immunohistochemistry for the expression of 5-HT and TPH expression and western blot for the expression of GSK3β and β-catenin were performed. We compared the effect of treadmill running on anxiety with fluoxetine.
MATERIALS AND METHODS
Experimental animals
The pregnant female Sprague-Dawley rats, weighing 280±10 g (10 weeks old), were used, and the day of delivery was designated postnatal day 0. This experiment study was approved by the Institutional Care and Use Committee of Kyung Hee University (KHUASP[SE]-17-099). On the postnatal day 14, the off spring randomly divided into 5 groups (n=8 in each group): the control group, the exercise group, the maternal separation group, the maternal separation and exercise group, and the maternal separation and fluoxetine-treated group.
Treadmill exercise protocol and treatment of fluoxetine
The rat pups in the exercise groups were forced to run on motorized treadmill (Columbus Instruments, Columbus, OH, USA) for 30 min once a day for 10 days, stating on the postnatal day 21. Exercise load consisted of treadmill at a speed 2 m/min for the first 30 min, without inclination. For the rat pups in the fluoxetine-treated group, 200 mL of fluoxetine (5 mg/kg; Sigma Chemical Co. St. Louis, MO, USA) was orally administrated once a day for 10 consecutive days, starting on the postnatal day 21.
Elevated plus maze test
The elevated plus maze test was performed on the postnatal day 30, according to a previously described method (Ko et al., 2013). The plus maze consisted of black acrylic with two open arms (50 cm×10 cm×36 cm) and two closed arms (50 cm×10 cm×36 cm), arranged so that the two arms were opposite and connected by a central platform (10 cm×10 cm). The whole plus maze was elevated 60 cm above the floor and illuminated by a 100-W light bulb fixed 2 m above the maze floor. During the test, each rat pup was placed on the central platform of the maze with their head facing an open arm. The latency time and number of entry in the open arms were collected during 7 min.
Tissue preparation
The animals were sacrificed after last treadmill exercise. After anesthetizing with Zoletil 50 (10 mg/kg intraperitoneally; Vibac Laboratories, Carros, France), 50 mM phosphate-buffered saline perfused to the rat pups, and then the rat pups were subsequently fixed with freshly prepared 500 mM phosphate buffer (pH, 7.4) containing 4% paraformaldehyde. The brains of the rat pups were removed and fixed in the same fixative overnight, and then transferred into a 30% sucrose solution for cryoprotection. Serial coronal sections with 40 μm thickness were obtained using a freezing microtome (Leica, Nussloch, Germany).
Immunohistochemistry
Immunohistochemistry was conducted to evaluate the 5-HT and TPH expression in the dorsal raphe, according to the previously described method (Ji et al., 2017; Moon et al., 2018). Free-floating tissue sections were incubated overnight with mouse anti-TPH or rabbit anti-5-HT antibody (1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA, USA). The sections were treated with biotinylated, anti-mouse TPH secondary antibody or with biotinylated anti-rabbit 5-HT secondary antibody (1:200; Vector Laboratories, Burlingame, CA, USA) during 1 hr. Next, avidin-biotin-peroxidase complex (Vector Laboratories) was treated to the section during 1 hour at 25°C. The sections were treated with a reaction mixture consisting of 0.03% 3,3′-diaminobenzidine and 0.03% hydrogen peroxide during 5 min. After the slides were dried under the room conditions, Permount (Fisher Scientific, Fair Lawn, NJ, USA) was used for the coverslips mounting.
Western blot analysis
Western blot analysis was performed, according to the previous method (Cho et al., 2018; Wu et al., 2015). The right hemisphere was homogenized on ice, and lysed in a lysis buffer containing 50 mM HEPES (pH 7.5), 150 mM NaCl, 10% glycerol, 1% Triton X-100, 1 mM PMSF, 1 mM EGTA, 1.5 mM MgCl2·6H2O, 1 mM sodium orthovanadate, and 100 mM sodium fluoride. Protein content was measured using a Bio-Rad colorimetric protein assay kit (Bio-Rad, Hercules, CA, USA). Protein samples (30 μg) were separated on sodium dodecyl sulfate-polyacrylamide gel and transferred onto a nitrocellulose membrane. Mouse GSK3β antibody, mouse phosphorylated GSK3β (p-GSK3β) antibody, mouse β-catenin antibody, and mouse phosphorylated β-catenin (p-β-catenin) antibody (1:1,000; Santa Cruz Biotechnology) were used as the primary antibodies. Horseradish peroxidase-conjugated anti-mouse antibody (1:2,000; Vector Laboratories) for GSK3β, p-GSK3β, β-catenin, and p-β-catenin were used as secondary antibodies. Using a cold pack and prechilled buffer, membrane transfer was conducted at 4°C. Enhanced chemiluminescence detection kit (Santa Cruz Biotechnology) was used for band detection.
Data analysis
The numbers of 5-HT and TPH-positive cells in the dorsal raphe were counted hemilaterally through a light microscope (Olympus, Tokyo, Japan). The area of the dorsal raphe was measured using an Image-Pro plus computer-assisted image analysis system (Media Cybernetics Inc., Silver Spring, MD, USA) attached to a light microscope (Olympus). Molecular Analyst (ver. 1.4.1, Bio-Rad) was used for analyzing of detected bands.
Differences among the groups were evaluated using SPSS Statistics ver. 21.0 (IBM Co., Armonk, NY, USA) by the one-way analysis of variance followed by Duncan post hoc test. All values are expressed as the mean±standard error of the mean. Statistically significant differences were established at P<0.05.
RESULTS
Anxiety-like behavior
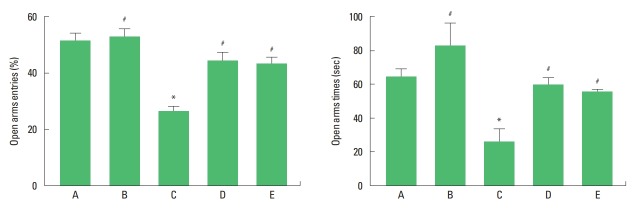
Anxiety-like behavior is presented in Fig. 1. Maternal separation decreased the number of entry and the time of latency in the open arms (P<0.05). Treadmill exercise and fluoxetine treatment increased the number of entry and the time of latency in the maternal separation rat pups (P<0.05).
Fig. 1.
Anxiety-like behavior in the elevated plus maze test. Left panel: percentage of open arms entries. Right panel: latency of open arms. A, control group; B, exercise group; C, maternal separation group; D, maternal separation and exercise group; E, maternal separation and fluoxetine-treated group. *P<0.05 compared to the control group. #P<0.05 compared to the maternal separation group.
Expression of 5-HT and TPH in the dorsal raphe
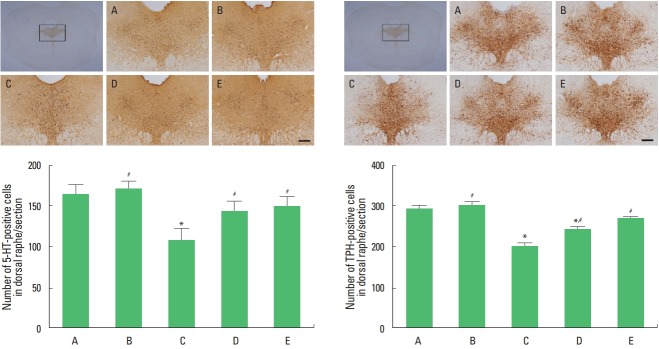
Expression of 5-HT and TPH in the dorsal raphe is presented in Fig. 2. Maternal separation decreased the number of 5-HT-positive and TPH-positive cells in the dorsal raphe (P<0.05). Treadmill exercise and fluoxetine treatment increased the number of 5-HT-positive and TPH-positive cells in the maternal separation rat pups (P<0.05).
Fig. 2.
5-Hydroxytryptamine (5-HT) and tryptophan hydroxylase (TPH) expression in the dorsal raphe. Left panel: photomicrographs of 5-HT-positive cells. Right panel: photomicrographs of TPH-positive cells. The scale bar represents 200 μm. □: Counting areas of the dorsal raphe. A, control group; B, exercise group; C, maternal separation group; D, maternal separation and exercise group; E, maternal separation and fluoxetine-treated group. *P<0.05 compared to the control group. #P<0.05 compared to the maternal separation group.
Expression of t-GSK3β and p-GSK3β in the hippocampus
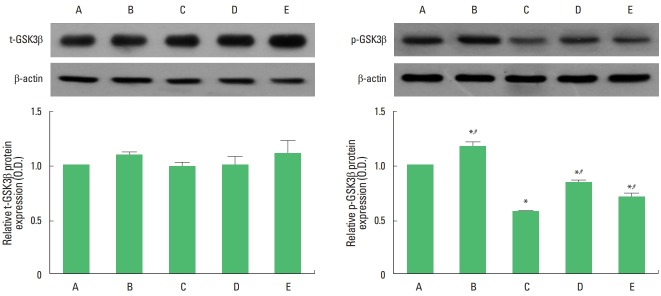
Expression of t-GSK3β and p-GSK3β is presented in Fig. 3. Expression of t-GSK3β was not changed by maternal separation. However, maternal separation decreased the expression of p-GSK3β in the hippocampus (P<0.05). Treadmill exercise and fluoxetine treatment increased the expression of p-GSK3β in the maternal separation rat pups (P<0.05).
Fig. 3.
Effect of treadmill exercise on total glycogen synthase kinase 3 beta (t-GSK3β) and phosphorylated GSK3β (p-GSK3β) expression in the hippocampus. Left panel: representative expression of t-GSK3β in the hippocampus. Right panel: representative expression of p-GSK3β in the hippocampus. A, control group; B, exercise group; C, maternal separation group; D, maternal separation and exercise group; E, maternal separation and fluoxetine-treated group. *P<0.05 compared to the control group. #P<0.05 compared to the maternal separation group.
Expression of t-β-catenin and p-β-catenin in the hippocampus
The expression of t-β-catenin and p-β-catenin is presented in Fig. 4. Expression of t-β- was decreased by maternal separation (P<0.05). Treadmill exercise and fluoxetine treatment increased t-β-catenin expression in the maternal separation rat pups (P<0.05). Expression of p-β-catenin was increased by maternal separation (P<0.05). Treadmill exercise and fluoxetine treatment suppressed p-β-catenin expression in the maternal separation rat pups.
Fig. 4.
Total β-catenin (t-β-catenin) and phosphorylated β-catenin (p-β-catenin) expression in the hippocampus. Left panel: representative expression of t-β-catenin. Right panel: representative expression of p-β-catenin. A, control group; B, exercise group; C, maternal separation group; D, maternal separation and exercise group; E, maternal separation and fluoxetine-treated group. *P<0.05 compared to the control group. #P<0.05 compared to the maternal separation group.
DISCUSSION
Exercise is known to ameliorate depressive symptoms induced by maternal separation (Baek et al., 2012; Wu et al., 2017). The elevated plus maze is a test for the determination of anxiety level (Ko et al., 2013). In the present study, shorter latency time and less open arms entries were observed in the maternal separation rat pups, showing that maternal separation increased anxiety level. However, treadmill exercise and fluoxetine treatment lengthened latency time and increased open arms entries, showing that treadmill running reduced anxiety level. Previous studies suggested that exercise ameliorates anxiety-like behavior (Ko et al., 2013; Motaghinejad et al., 2017).
Depression or anxiety is associated with imbalance of neurotransmitters, such as serotonin, norepinephrine, and dopamine in the brain. Among them, 5-HT is closely related with the depression and anxiety (Baek et al., 2012; Ji et al., 2017; Motaghinejad et al., 2017). TPH is an enzyme that regulates 5-HT synthesis, and serotonin impairment in the suicide might be due to the hypofunction of serotonin synthesizing enzyme (Boldrini et al., 2005). In the present study, expression of 5-HT and TPH in the dorsal raphe was decreased by induction of maternal separation. However, treadmill exercise and fluoxetine treatment increased the expression of 5-HT and TPH in the dorsal raphe, suggesting that treadmill exercise and fluoxetine treatment restored the maternal separation-induced decrease of 5-HT and TPH expression. Our results confirmed that treadmill exercise and fluoxetine treatment alleviated maternal separation-induced anxiety status.
GSK3β inhibitors produce a weak anti-depressant-like effect and a strong anti-mania-like effect in bipolar disorder (Rowe et al., 2007). Inhibition of GSK3β in the hippocampus and striatum has anxiolytic and pro-social effect (Latapy et al., 2012). In the present study, t-GSK-β level in the hippocampus was not changed by maternal separation, however, the expression of p-GSK3β was decreased by maternal separation. The expression of 5-HT is closely related to the p-GSK3β expression. GSK3β is inhibited by SSRIs which used as antidepressants, and GSK3β is closely related to depression patients (Duman and Voleti, 2012; Inkster et al., 2009). In the hippocampus, fluoxetine regulates neurogenesis via up-regulating p-GSK3β, and this effect depends on 5-HT1A receptor (Hui et al., 2014; Li et al., 2004). In our study, treadmill exercise and fluoxetine treatment increased the expression of p-GSK3β in the hippocampus, demonstrating that treadmill exercise and fluoxetine enhances GSK3β phosphorylation via up-regulating 5-HT level.
GSK3β phosphorylation inhibits β-catenin decomposition by proteasome (Hwang et al., 2016). Inhibition of GSK3β activates β-catenin (Sen et al., 2009), and regular exercise inhibits stress-induced p-β-catenin expression (Leem et al., 2018). In the present study, expression of t-β-catenin in the hippocampus was decreased by maternal separation, in contrast, treadmill exercise and fluoxetine treatment increased t-β-catenin expression. Expression of p-β-catenin was increased by maternal separation, meanwhile, treadmill exercise and fluoxetine treatment suppressed p-β-catenin. In our study, treadmill exercise and fluoxetine regulate β-catenin activation via down-regulating β-catenin phosphorylation.
We demonstrated that treadmill exercise exerts alleviating effect on maternal separation-induced anxiety through enhancing 5-HT expression and GSK3β phosphorylation, and then inhibiting β-catenin phosphorylation. Treadmill exercise showed similar ameliorating effect on anxiety-like behavior as fluoxetine. Treadmill exercise acts as a useful strategy for the treatment of anxiety through GSK3β/β-catenin pathway.
ACKNOWLEDGMENTS
This work was supported by 2017 Sangmyung University Research Foundation of Korea.
Footnotes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- Baek SS, Jun TW, Kim KJ, Shin MS, Kang SY, Kim CJ. Effects of postnatal treadmill exercise on apoptotic neuronal cell death and cell proliferation of maternal-separated rat pups. Brain Dev. 2012;34:45–56. doi: 10.1016/j.braindev.2011.01.011. [DOI] [PubMed] [Google Scholar]
- Bannai M, Fish EW, Faccidomo S, Miczek KA. Anti-aggressive effects of agonists at 5-HT1B receptors in the dorsal raphe nucleus of mice. Psychopharmacology (Berl) 2007;193:295–304. doi: 10.1007/s00213-007-0780-5. [DOI] [PubMed] [Google Scholar]
- Boldrini M, Underwood MD, Mann JJ, Arango V. More tryptophan hydroxylase in the brainstem dorsal raphe nucleus in depressed suicides. Brain Res. 2005;1041:19–28. doi: 10.1016/j.brainres.2005.01.083. [DOI] [PubMed] [Google Scholar]
- Chen YC, Tan QR, Dang W, Wang HN, Zhang RB, Li ZY, Lin H, Liu R. The effect of citalopram on chronic stress-induced depressive-like behavior in rats through GSK3β/β-catenin activation in the medial prefrontal cortex. Brain Res Bull. 2012;88:338–344. doi: 10.1016/j.brainresbull.2012.03.004. [DOI] [PubMed] [Google Scholar]
- Cho JW, Jung SY, Kim DY, Chung YR, Choi HH, Jeon JW, Han JH. PI3K-Akt-Wnt pathway is implicated in exercise-induced improvement of short-term memory in cerebral palsy rats. Int Neurourol J. 2018;22(Suppl 3):S156–164. doi: 10.5213/inj.1836224.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Voleti B. Signaling pathways underlying the pathophysiology and treatment of depression: novel mechanisms for rapid-acting agents. Trends Neurosci. 2012;35:47–56. doi: 10.1016/j.tins.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson JM. SSRI Antidepressant medications: adverse effects and tolerability. Prim Care Companion J Clin Psychiatry. 2001;3:22–27. doi: 10.4088/pcc.v03n0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould TD, Chen G, Manji HK. In vivo evidence in the brain for lithium inhibition of glycogen synthase kinase-3. Neuropsychopharmacology. 2004;29:32–38. doi: 10.1038/sj.npp.1300283. [DOI] [PubMed] [Google Scholar]
- Hui J, Zhang J, Kim H, Tong C, Ying Q, Li Z, Mao X, Shi G, Yan J, Zhang Z, Xi G. Fluoxetine regulates neurogenesis in vitro through modulation of GSK-3β/β-catenin signaling. Int J Neuropsychopharmacol. 2014;18(5):pyu099. doi: 10.1093/ijnp/pyu099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang SY, Deng X, Byun S, Lee C, Lee SJ, Suh H, Zhang J, Kang Q, Zhang T, Westover KD, Mandinova A, Lee SW. Direct targeting of β-catenin by a small molecule stimulates proteasomal degradation and suppresses oncogenic Wnt/β-catenin signaling. Cell Rep. 2016;16:28–36. doi: 10.1016/j.celrep.2016.05.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inkster B, Nichols TE, Saemann PG, Auer DP, Holsboer F, Muglia P, Matthews PM. Association of GSK3beta polymorphisms with brain structural changes in major depressive disorder. Arch Gen Psychiatry. 2009;66:721–728. doi: 10.1001/archgenpsychiatry.2009.70. [DOI] [PubMed] [Google Scholar]
- Ji ES, Lee JM, Kim TW, Kim YM, Kim YS, Kim K. Treadmill exercise ameliorates depressive symptoms through increasing serotonin expression in postpartum depression rats. J Exerc Rehabil. 2017;13:130–135. doi: 10.12965/jer.1734968.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaidanovich-Beilin O, Milman A, Weizman A, Pick CG, Eldar-Finkelman H. Rapid antidepressive-like activity of specific glycogen synthase kinase-3 inhibitor and its effect on beta-catenin in mouse hippocampus. Biol Psychiatry. 2004;55:781–784. doi: 10.1016/j.biopsych.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Ko IG, Kim SE, Kim TW, Ji ES, Shin MS, Kim CJ, Hong MH, Bahn GH. Swimming exercise alleviates the symptoms of attention-deficit hyperactivity disorder in spontaneous hypertensive rats. Mol Med Rep. 2013;8:393–400. doi: 10.3892/mmr.2013.1531. [DOI] [PubMed] [Google Scholar]
- Latapy C, Rioux V, Guitton MJ, Beaulieu JM. Selective deletion of forebrain glycogen synthase kinase 3β reveals a central role in serotonin- sensitive anxiety and social behaviour. Philos Trans R Soc Lond B Biol Sci. 2012;367:2460–2474. doi: 10.1098/rstb.2012.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Baek SS. Role of exercise on molecular mechanisms in the regulation of antidepressant effects. J Exerc Rehabil. 2017;13:617–620. doi: 10.12965/jer.1735188.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leem YH, Kato M, Chang H. Regular exercise and creatine supplementation prevent chronic mild stress-induced decrease in hippocampal neurogenesis via Wnt/GSK3β/β-catenin pathway. J Exerc Nutrition Biochem. 2018;22:1–6. doi: 10.20463/jenb.2018.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Zhu W, Roh MS, Friedman AB, Rosborough K, Jope RS. In vivo regulation of glycogen synthase kinase-3β (GSK3β) by serotonergic activity in mouse brain. Neuropsychopharmacology. 2004;29:1426–1431. doi: 10.1038/sj.npp.1300439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon EJ, Ko IG, Kim SE, Jin JJ, Hwang L, Kim CJ, An H, Lee BJ, Yi JW. Dexmedetomidine ameliorates sleep deprivation-induced depressive behaviors in mice. Int Neurourol J. 2018;22(Suppl 3):S139–146. doi: 10.5213/inj.1836228.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motaghinejad O, Motaghinejad M, Motevalian M, Rahimi-Sharbaf F, Beiranvand T. The effect of maternal forced exercise on offspring pain perception, motor activity and anxiety disorder: the role of 5-HT2 and D2 receptors and CREB gene expression. J Exerc Rehabil. 2017;13:514–525. doi: 10.12965/jer.1734992.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh JH, Ko IG, Kim SE, Lee JM, Ji ES, Kim JH, Chang HK, Lee SK, Kim KH. Treadmill exercise ameliorates intracerebral hemorrhage-induced depression in rats. J Exerc Rehabil. 2016;12:299–307. doi: 10.12965/jer.1632692.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe MK, Wiest C, Chuang DM. GSK-3 is a viable potential target for therapeutic intervention in bipolar disorder. Neurosci Biobehav Rev. 2007;31:920–931. doi: 10.1016/j.neubiorev.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen B, Styner M, Xie Z, Case N, Rubin CT, Rubin J. Mechanical loading regulates NFATc1 and β-catenin signaling through a GSK3beta control node. J Biol Chem. 2009;284:34607–34617. doi: 10.1074/jbc.M109.039453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Chen J, Chen C, Wang W, Wen L, Gao K, Chen X, Xiong S, Zhao H, Li S. Wnt/β-catenin coupled with HIF-1α/VEGF signaling pathways involved in galangin neurovascular unit protection from focal cerebral ischemia. Sci Rep. 2015;5:16151. doi: 10.1038/srep16151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CC, Hung CJ, Lin SY, Wang YY, Chang CY, Chen WY, Liao SL, Raung SL, Yang CP, Chen CJ. Treadmill exercise alleviated prenatal buprenorphine exposure-induced depression in rats. Neurochem Int. 2017;110:91–100. doi: 10.1016/j.neuint.2017.09.012. [DOI] [PubMed] [Google Scholar]
- Yadid G, Nakash R, Deri I, Tamar G, Kinor N, Gispan I, Zangen A. Elucidation of the neurobiology of depression: insights from a novel genetic animal model. Prog Neurobiol. 2000;62:353–378. doi: 10.1016/s0301-0082(00)00018-6. [DOI] [PubMed] [Google Scholar]