Abstract
Cyclic nucleotide–gated (CNG) channels produce the initial electrical signal in mammalian vision and olfaction. They open in response to direct binding of cyclic nucleotide (cAMP or cGMP) to a cytoplasmic region of the channel. However, the conformational rearrangements occurring upon binding to produce pore opening (i.e. gating) are not well understood. SthK is a bacterial CNG channel that has the potential to serve as an ideal model for structure–function studies of gating but is currently limited by its toxicity, native cysteines, and low open probability (Po). Here, we expressed SthK in giant Escherichia coli spheroplasts and performed patch-clamp recordings to characterize SthK gating in a bacterial membrane. We demonstrated that the Po in cAMP is higher than has been previously published and that cGMP acts as a weak partial SthK agonist. Additionally, we determined that SthK expression is toxic to E. coli because of gating by cytoplasmic cAMP. We overcame this toxicity by developing an adenylate cyclase–knockout E. coli cell line. Finally, we generated a cysteine-free SthK construct and introduced mutations that further increase the Po in cAMP. We propose that this SthK model will help elucidate the gating mechanism of CNG channels.
Keywords: electrophysiology, potassium channel, cyclic nucleotide, allosteric regulation, channel activation, bacteria, cyclic nucleotide gating, prokaryotic ion channel, voltage-gated ion channel, allostery
Introduction
Cyclic nucleotide–gated (CNG)2 channels mediate signal transduction in the visual and olfactory systems (1–3). Although they belong to the voltage-gated ion channel superfamily, CNG channels are only weakly voltage-dependent and instead open (gate) upon direct binding of cyclic nucleotide (cNMP) to a cytoplasmic region of the channel (4, 5). In this manner, CNG channels transduce changes in secondary messenger concentrations into alterations in membrane voltage and intracellular Ca2+.
CNG channels assemble as a 4-fold symmetric tetramer with an ion-conducting pore along the 4-fold axis. Each subunit is composed of six transmembrane (TM) helices followed by a large cytoplasmic region (6–8). In the TM region, S1–S4 comprise the voltage-sensing domain, and S5 and S6 comprise the pore domain with the selectivity filter between S5 and S6 (Fig. 1A). Immediately following the TM region is a C-linker domain composed of six helices (A′–F′) followed by a cyclic nucleotide–binding domain (CNBD), which contains four helices (A–C and P) and a β-roll (Fig. 1A) (6–8). The C-linker region contains extensive intersubunit contacts with the A′ and B′ helices of one subunit resting upon the C′ and D′ helices of the adjacent subunit (clockwise if looking from the extracellular side) in an “elbow on the shoulder” configuration (Fig. 1A) (7, 9).
Figure 1.

SthK structure and allosteric gating scheme. A, cartoon representation of SthK cryo-EM structure (Protein Data Bank (PDB) code 6CJU) with the functional domains labeled. cAMP is displayed as spheres. PD, pore domain; VSD, voltage-sensing domain. B, MWC gating scheme with C and O representing closed and open states, respectively. cAMP is indicated as a. Equilibrium constants are shown near arrows.
Like other allosteric proteins, CNG channels transmit a conformational change from the ligand-binding site in each subunit (CNBD) to an active site (pore gate) (Fig. 1A). This allosteric regulation can be envisioned in a gating scheme based on Monod, Wyman, and Changeux (MWC) (10) where a concerted channel opening transition is driven by an increase in affinity for the ligand when the pore gate is open. In this scheme, the opening conformational change of the channel in the absence of ligand is represented by the equilibrium constant L0 (Fig. 1B). The dissociation constant (KD) describes binding of ligand (a) to the closed conformation of a single subunit, and each binding event causes the opening equilibrium to increase by a factor (f). Hence, the fully liganded (four bound cAMP molecules) opening equilibrium constant (L) is equal to L0f4. Microscopic reversibility dictates that the conformational change occurring in the CNBD increases the affinity for the ligand by the same factor, f. Therefore, the allosteric conformational change is energetically driven by a higher affinity for the ligand when the channel is in the open state (KD/f) relative to when it is in the closed state (KD) (Fig. 1B) (10–12). For retinal CNG channels, cGMP and cAMP both bind with a similar affinity to the closed channel (KD); however, cGMP binds with a much higher affinity to the open channel (KD/f). As a consequence, the open probability (Po) in saturating cGMP is nearly 1, whereas the Po in saturating cAMP is between 0.01 and 0.1 (13, 14).
The conformational change occurring in the CNBD to produce an f-fold change in affinity and channel activation has been elucidated largely due to structural and biochemical experiments on a fragment comprising the C-linker and CNBD of the related hyperpolarization-activated cyclic nucleotide–gated channel HCN2 (9, 15–17). Agonists initially bind to the β-roll and then induce a movement of the C-helix toward the β-roll where residues on the C-helix form contacts with the cNMP (6, 9, 18). These contacts of the C-helix with the cNMP represent open state–dependent interactions, which drive the opening conformational change of the pore. In retinal CNG channels, the C-helix forms stronger interactions with cGMP than with cAMP to produce a greater value of f and a higher Po (19, 20).
Despite the detailed knowledge of the conformational change occurring in the CNBD, the conformational changes occurring in the C-linker and TM region are largely unknown due, in part, to the lack of a suitable biochemical model for a full-length CNG channel. Recently, a family of prokaryotic CNG (pCNG) channel homologues was discovered that could serve as a model for studying full-length CNG channels in a purified system (21, 22). However, several properties prevent pCNG channels from serving as an ideal model system for studying gating. First, SthK, the pCNG for which electrophysiology is most amenable, reportedly has a low Po except at very depolarized voltages. At 0 mV, the voltage for most structural experiments of purified protein, SthK has been shown to have an ∼10% Po in saturating cAMP, thus limiting studies of the conformational transitions in the C-linker and TM region (22). Indeed, a recent structure of cAMP-bound SthK and a structure of another cAMP-bound pCNG, LliK, both show the channel in a closed state (8, 23). Second, expression of SthK is toxic to Escherichia coli, which limits the amount of channel protein that can be purified. Finally, a cysteine-free version of SthK is not currently available and is required for site-specific labeling through cysteine modification. Site-specific labeling is a valuable tool for studying conformational dynamics with cysteine modification/cross-linking, double electron–electron resonance spectroscopy, and fluorescence spectroscopy.
Here, we optimize SthK as a model system for studying CNG channel gating. We performed both macroscopic and single-channel patch-clamp recording of SthK expressed in bacterial spheroplasts. We characterized the cNMP dependence and voltage dependence of SthK in a bacterial membrane, developed a method to reduce the toxicity of SthK expression, and engineered a Cys-free SthK construct with a high Po.
Results
Expression and patch-clamp recording of SthK in giant E. coli spheroplasts
Although it is often easy to express eukaryotic channels in mammalian cells or Xenopus oocytes and record ionic currents, many bacterial channels are not expressed at high levels in these systems. To overcome this problem, we have expressed SthK in E. coli and then converted the cells into giant spheroplasts for patch-clamp recording (24–26). E. coli expressing full-length WT SthK with a C-terminal GFP tag (wtSthK) were treated with the antibiotic cephalexin, which blocked the final stage of binary fission and generated long “snake-like” cells with an interconnected cytoplasm (Fig. 2A). Then the cell walls were degraded, allowing the cells to relax into spheroplasts, which are mainly composed of naked inner membrane and are amenable to patch-clamp recording. Expression of wtSthK was visualized by fluorescence of the C-terminal GFP tag (Fig. 2B).
Figure 2.
SthK expression and patch-clamp recording in E. coli spheroplasts. A, method for preparing E. coli spheroplasts. B, bright-field and fluorescence images of spheroplasts expressing GFP-tagged SthK. C, top, non-leak-subtracted currents in the absence of cNMP from inside-out patches of SthK-expressing spheroplasts in response to the voltage protocol at the top. Middle and bottom, leak-subtracted currents in the presence of 1 mm cAMP or 5 mm cGMP.
To record wtSthK currents, we formed inside-out patches from wtSthK-expressing spheroplasts using the patch-clamp technique (27). In the absence of cNMP, most patches show no currents from intrinsic channels and a small, ohmic leak current (Fig. 2C, top). Perfusion of the cytoplasmic face of the patch with cAMP produced maximal ionic currents at −120 mV between 50 pA and 3 nA. With voltage steps, a small time-dependent increase in the current occurred upon stepping to depolarized potentials, and a current decline occurred upon stepping to hyperpolarized potentials (Fig. 2C). These kinetic features indicate a slight depolarization dependence to activation, which has previously been observed in SthK in Xenopus oocytes and artificial lipid bilayers (21, 22). Furthermore, inward currents were larger and noisier at hyperpolarized voltages, suggesting a larger single-channel conductance and lower Po at hyperpolarized potentials (Fig. 2C). Finally, perfusion of a solution containing cGMP elicited no macroscopic currents (Fig. 2C, bottom). These results indicate that spheroplast patch-clamp recording is a feasible method for studying wtSthK gating and permeation.
Cyclic nucleotide dependence of wtSthK in the bacterial membrane
We first measured the cAMP dependence of wtSthK in the bacterial membrane using macroscopic currents. By varying the concentration of cAMP perfused onto the patch and measuring the steady-state current evoked at −60 mV, we obtained a dose-response curve with a K1/2 of 1.5 ± 0.4 μm and Hill coefficient (h) of 1.5 ± 0.1 (n = 3) (Fig. 3A). These values differ significantly from the K1/2 of 17 μm and h of 3 reported in artificial bilayers and the K1/2 of 3.7 μm and h of 1.3 reported in Xenopus oocytes (21, 22).
Figure 3.
Cyclic nucleotide-dependent gating of wtSthK. A, dose-response curve of wtSthK channels for cAMP. The solid black line represents fit of the Hill equation with K1/2 = 1.5 μm and h = 1.5. Points and error bars represent mean ± S.E., respectively, from three patches. B, single-channel currents from a patch containing 150–200 wtSthK channels held at −60 mV in the absence of cNMP (upper trace) and in the presence of 5 mm cGMP (lower trace). The inset shows zoom of an ∼1-s region. Data were filtered at 0.5 kHz for display. C, concentration jump from 0 to 1 mm cAMP while holding the patch at −40 mV. The inset shows an ∼500-ms region. The thin red line represents fit of a single exponential with a time constant of 11.0 ms.
Previous studies have disagreed on whether cGMP acts as an inhibitor (i.e. f ≤ 1) or as a weak partial agonist (f is small but >1) (Fig. 1B) (21, 22). To determine the efficacy of cGMP activation of SthK, we held a patch containing 150–200 wtSthK channels at −60 mV and perfused it with 5 mm cGMP while continuously recording for ∼1 min (Fig. 3B). On this time scale, a number of small inward current spikes were visible that were not seen in the absence of cyclic nucleotide (Fig. 3B). Zooming in on one of these spikes shows a clear 50–100-ms single-channel open burst (Fig. 3B). Idealizing the trace gave an estimate of Po in cGMP between 10−6 and 10−5. These results support the conclusion that cGMP acts as a very weak partial agonist, although likely with lower efficacy than shown previously (22). This conclusion is further supported by our results from mutant channels below.
It has previously been reported that SthK exhibits a slow component of the activation time course indicative of mode shift or hysteresis (21, 22). By measuring macroscopic currents in inside-out patches with a rapid perfusion system, we performed concentration-jump experiments to assess whether we could observe evidence of a slower conformational change. In a patch held at −40 mV, currents elicited by a jump from 0 to 1 mm cAMP (∼1000× K1/2) were fit to a single exponential with no sign of a slower component on the 1–10-s timescale (Fig. 3C). The ∼10–15-ms time constant measured for these concentration jumps was likely limited by the rate of perfusion and does not accurately represent the time course of channel activation. However, our results show no sign of a slow component of activation at these saturating cAMP concentrations.
Voltage dependence of wtSthK in the bacterial membrane
We next characterized the voltage dependence of wtSthK in a bacterial membrane using macroscopic currents. We recorded currents in saturating cAMP in response to a family of voltage pulses and measured the steady-state conductance from the leak-subtracted instantaneous tail currents at +100 mV (Fig. 4A). The resulting normalized conductance–voltage (G–V) curve displayed a shallow sigmoidal shape with a minimal value of 0.58 at −120 mV, indicating that voltage can reduce the Po by ∼40% of its maximal value but cannot completely close the channel (Fig. 4B). Fitting this G–V curve with the Boltzmann equation yielded a V1/2 of 22 ± 7.4 mV and an e-fold change every 46.6 mV (Fig. 4B). This weak voltage dependence corresponds to a zδ of 0.55 ± 0.02 equivalent electronic charges (n = 3), similar to the value of 0.8 charges previously reported based on single-channel Po recordings in artificial bilayers (22). This G–V curve suggests the channel opening transition (L) is voltage-independent but coupled to a voltage sensor that moves the equivalent of 0.5 electronic charges across the membrane per wtSthK tetramer.
Figure 4.
Voltage dependence of wtSthK gating. A, leak-subtracted current family for wtSthK in the presence of 1 mm cAMP. B, normalized G–V curve calculated from the instantaneous tail current at +100 mV (black points). The solid blue line represents fit of a Boltzmann equation with V1/2 = 22 and zδ = 0.55. Red points represent Po from single-channel recordings (right axis). Points and error bars represent mean ± S.E., respectively, from three to 10 patches. C, representative single-channel recording of wtSthK in 1 mm cAMP at −60 mV. An all-points histogram is shown on the right. The blue line represents fit with a sum of Gaussians with Po = 0.63 for this patch. D, representative single-channel recording of wtSthK in 1 mm cAMP at +60 mV. An all-points histogram is shown on the right. The blue line represents fit with a sum of Gaussians with Po = 0.91 for this patch. E, leak-subtracted single-channel voltage ramp from −120 to +120 mV in 500 ms.
To determine the Po more directly, we recorded single-channel currents at −60 and +60 mV. The current traces showed bursting behavior with longer closures between bursts and rapid flickering closures within a burst (Fig. 4, C and D). All-points histograms of the trace were fit with the sum of two Gaussians, indicating a Po of 0.61 ± 0.046 at −60 mV (n = 10) and 0.90 ± 0.018 at +60 mV (n = 5). This Po is consistent with our G–V curve (Fig. 4B) and is substantially higher than what has been previously reported for SthK in Xenopus oocytes or artificial membranes (21, 22).
A single-channel voltage ramp from −120 to +120 mV revealed a strong inward rectification. The amplitude for inward currents at hyperpolarized voltages was about twice as large as the outward currents at depolarized voltages (Fig. 4E). A ∼3-fold higher conductance was seen for inward currents at −100 mV compared with outward currents at +100 mV in artificial membranes (22). However, the single-channel conductance we observed was about 3- and 2-fold smaller for inward and outward currents, respectively (22). This effect could be due to a difference in the expression system, rapid block by the 20 mm Mg2+ in our recording solutions, or reduced ion diffusion caused by the 500 mm sucrose in our recording solutions.
Generation and functional characterization of a cysteine-free SthK construct
The ability to perform site-specific labeling of cysteine residues through thiol-reactive probes, such as maleimides, enables spectroscopic experiments such as fluorescence or electron paramagnetic resonance (EPR). To optimize the SthK construct as a biochemical model for studying CNG channel gating, we mutated the two native cysteines to generate a Cys-free SthK construct (cfSthK). Based on an alignment with other pCNG channels, Cys-153, located near the extracellular end of S5, was mutated to Val, and Cys-387, located on the β-roll >15 Å from the ligand-binding site, was mutated to Ser. The resulting cfSthK has very similar properties to wtSthK. Perfusion of cAMP produced large currents, whereas perfusion of cGMP did not produce measurable currents (Fig. 5A). Furthermore, a cAMP dose-response curve yielded values of K1/2 = 1.1 ± 0.1 μm and h = 1.5 ± 0.04 (n = 3), both of which are similar to wtSthK (Fig. 5B).
Figure 5.
Properties of Cys-free SthK. A, leak-subtracted current family for cfSthK in the presence of 1 mm cAMP (red, top) and 5 mm cGMP (green, bottom). The voltage protocol is the same as in Fig. 2C. B, dose-response curve of cfSthK channels for cAMP. The solid black line represents fit of the Hill equation with K1/2 = 1.1 μm and h = 1.5. The dashed black line represents cAMP dose response for wtSthK. Points and error bars represent mean ± S.E., respectively, from three patches. C, representative single-channel recordings for cfSthK at −60 mV in 1 mm cAMP. An all-points histogram is shown on the right. The blue line represents fit with a sum of Gaussians to give Po = 0.58 for this patch.
In addition, single channels of cfSthK also behave similarly to single channels of wtSthK. A single-channel recording at −60 mV in saturating cAMP again showed bursting behavior (Fig. 5C). Fitting an all-points histogram gave a Po of 0.50 ± 0.041 (n = 5). This cfSthK background served as the foundation for our subsequent experiments.
Overcoming toxicity of SthK expression
The ability to express and purify large quantities of protein is an important feature of a model system for structure–function studies. Unfortunately, expression of wtSthK and cfSthK was toxic to E. coli. When C43 cells containing cfSthK under an IPTG-inducible promoter were induced in mid-log phase, the OD600 remained relatively constant over the next 10 h (Fig. 6A). This suggests that cfSthK expression either halts growth or kills cells at a similar rate as division, resulting in a constant steady-state density and low overall expression.
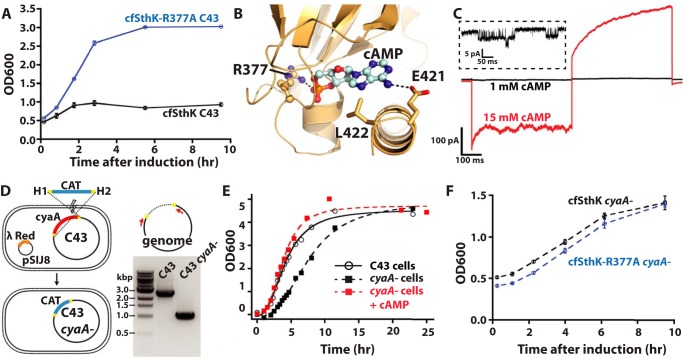
Figure 6.
Toxicity of cfSthK expression in E. coli. A, growth curves for C43 cells with expression of cfSthK or cfSthK-R377A. Points and error bars represent mean ± S.D., respectively, from three cultures. B, crystal structure of SthK CNBD bound to cAMP (PDB code 4D7T). cAMP and residues in direct contact with cAMP are shown as sticks. C, leak-subtracted currents in 1 (black trace) and 15 mm (red trace) cAMP from a patch expressing cfSthK-R377Q. The inset shows single-channel openings at −60 mV in the presence of 1 mm cAMP. D, left, cartoon showing the strategy for making C43 cyaA− cells. Right, gel showing colony PCR of the region between H1 and H2 of C43 and C43 cyaA− cells. E, growth curves comparing nontransformed C43 cyaA− cells in the absence and presence of cAMP with nontransformed C43 cells. F, growth curves for C43 cyaA− cells with expression of cfSthK or cfSthK-R377A. Points and error bars represent mean ± S.D., respectively, from three cultures.
We hypothesized that the toxicity of cfSthK expression is due to binding of cAMP and subsequent opening of the channel in the bacteria during expression. SthK has a higher apparent affinity (∼1 μm) for cAMP than does E. coli cAMP receptor protein (∼20 μm), suggesting that physiological cAMP concentrations could activate SthK during expression (28). To test this possibility, we mutated the conserved Arg-377. This Arg residue is found in the CNBD where it forms salt-bridge and hydrogen-bond interactions with the phosphate of cAMP (9, 29) (Fig. 6B). In CNG and HCN channels, mutation of this residue dramatically decreases the apparent affinity for cNMP (30, 31). When inside-out patches from E. coli expressing the cfSthK-R377Q mutant were perfused with 15 mm cAMP, substantial current was observed but with very large voltage-dependent current relaxation, suggesting that this cAMP concentration was not saturating (Fig. 6C). Indeed, perfusion with 1 mm cAMP produced only single openings (Fig. 6C, inset). These experiments demonstrate that mutation of Arg-377 dramatically decreased the apparent affinity for cAMP with a K1/2 shifted at least 1000-fold to a value greater than 1 mm. This K1/2 for cfSthK-R377Q is likely to be outside the range of cAMP concentrations in E. coli. Interestingly, E. coli cells transformed with a plasmid carrying the more dramatic cfSthK-R377A mutant and induced at mid-log phase continue to grow to more than 3× the OD600 reached by cfSthK-expressing cells (Fig. 6A). This dramatic rescue suggests that the observed toxicity is indeed due to cAMP-dependent gating of SthK during expression.
Although mutation of Arg-377 presents an opportunity for producing cfSthK on a larger scale, the high cAMP concentration required to activate the cfSthK-R377A channels diminishes its usefulness as a model system for CNG channels. Therefore, we pursued an alternative strategy to reduce SthK toxicity by producing a strain of C43 E. coli cells lacking adenylate cyclase (cyaA), the enzyme responsible for cAMP synthesis. Deletion of cyaA from the C43 genome was accomplished using oligonucleotide-mediated recombination (32). Successful loss of cyaA, with replacement by the gene encoding chloramphenicol resistance (CAT), was confirmed by colony PCR using locus-specific primers (Fig. 6D). This new strain, termed C43 cyaA−, displayed a somewhat slower growing phenotype that was rescued by addition of cAMP to the growth medium, consistent with previous reports of cyaA disruption in E. coli (Fig. 6E) (33, 34).
To test for toxicity of cfSthK expression in the adenylate cyclase knockout, C43 cyaA− cells were transformed with either cfSthK or cfSthK-R377A, and expression was induced at mid-log phase. The growth of C43 cells expressing cfSthK was rescued by the cyaA− knockout, showing similar growth rates as well as similar final densities as C43 cyaA− cells expressing cfSthK-R377A (Fig. 6F). These results further indicate that SthK expression toxicity is indeed due to endogenous cAMP gating the channel during growth. More importantly, this strategy allows the production of large quantities of SthK with a high affinity for cAMP.
Engineering a cfSthK construct with higher opening favorability
Previous reports have shown that SthK has an energetically unfavorable opening transition (L in Fig. 1B) as indicated by a low Po at saturating cAMP concentrations, reported to be 0.14 at +100 mV and 0.65 at +200 mV (21, 22). As demonstrated earlier, SthK displays weak depolarization-dependent activation, indicating that the Po at 0 mV is substantially lower (Fig. 4B). Many structural experiments including cryoelectron microscopy (cryo-EM) and double electron–electron resonance spectroscopy are currently only feasible at 0 mV. Under these conditions, the ensemble of channels on an EM grid or in a cuvette will assume an equilibrium distribution with a vast majority of SthK channels in the closed state (Fig. 1B). This point is most clearly illustrated in the recently published structure of SthK, which shows essentially identical closed conformations for the apo-, cAMP-bound, and cGMP-bound SthK channel (8). Although we observed a higher Po of wtSthK in bacterial membranes than has been previously reported, our Po estimate of 0.73 at 0 mV (Fig. 4B) suggests that alternative approaches may be necessary to study the opening conformational transition using purified SthK.
To increase the Po for SthK, we made four mutations in the cfSthK background. On the D′ helix of the C-linker, Arg-284 and Glu-290 were both mutated to Gln (Fig. 7A, right) based on a report that mutation of the equivalent residues in bovine retinal CNG channels (bCNGA1) to the corresponding residues in the Caenorhabditis elegans CNG channel (TAX-4) substantially increases the efficacy of its partial agonist, cAMP (35). On the C-helix of the CNBD, Leu-422 was mutated to Gln based on a report that a similar mutation in bCNGA1 also increases cAMP efficacy (Fig. 7A, right) (19). Finally, Ala-208 on S6 was mutated to Val (Fig. 7A, left). This mutation was discovered serendipitously and found to increase maximal Po in cfSthK. Incidentally, the equivalent residue in TAX-4 is also a Val (6). Thus, the resulting construct contains three mutations to the corresponding residues in TAX-4, which has a very favorable opening transition and an open-channel cryo-EM structure (6, 35) We named this construct cfSthK-3QV to reflect the three mutations to Gln and the one mutation to Val.
Figure 7.
Characterization of cfSthK-3QV construct. A, cartoon representation of SthK (PDB code 6CJQ in the middle and left panels and PDB code 4D7T on the right) showing locations of three Gln residues and one Val residue in the 3QV construct. B, representative single-channel recording of cfSthK-3QV in 1 mm cAMP. An all-points histogram is shown on the right. The blue line represents fit to a sum of Gaussians with Po = 0.92 for this patch. C, left, leak-subtracted currents in cAMP (red traces) and cGMP (green traces) for cfSthK-L422Q and cfSthK-3QV channels. Right, currents at −60 mV in cGMP relative to cAMP for cfSthK-L422Q, cfSthK-3Q, and cfSthK-3QV. Bars and error bars represent mean ± S.E., respectively, for three patches. D, dose-response curves for cfSthK-3QV in cAMP (red circles) and cGMP (green squares). The solid black line represents Hill fit with K1/2 = 32 nm and h = 2.2 and with K1/2 = 5.5 μm and h = 1.9 for cAMP and cGMP, respectively. The dashed black line represents cAMP dose response for wtSthK. Points and error bars represent mean ± S.E., respectively, for three patches. E, growth curves of C43 and C43 cyaA− cells with cfSthK and cfSthK-3QV. Expression of the indicated SthK construct was induced between an OD600 of 0.4 and 0.6. Points and error bars represent mean ± S.D., respectively, for three cultures.
Strikingly, single-channel recording of the cfSthK-3QV construct revealed a very high Po even at −60 mV (Fig. 7B). Similar to wtSthK, the traces of cfSthK-3QV showed both longer-lived closures and very brief closures. However, the longer closures were much shorter than those observed in wtSthK and cfSthK (Figs. 4C and 5C). Fitting an all-points histogram gave a value of 0.92 ± 0.007 for Po at −60 mV (n = 4) (Fig. 7B).
To determine the extent by which the cfSthK-3QV construct increased favorability of the opening transition (L in Fig. 1B), we measured the activation by the weak partial agonist, cGMP, because Po with a partial agonist is more sensitive to changes in L than Po with a full agonist (36, 37). Of the four mutations added to cfSthK that comprise the cfSthK-3QV construct, L422Q is the only mutation of a residue in the CNBD that interacts with cNMP in the binding site (Fig. 7A) and, hence, might change agonist specificity. Therefore, we compared cfSthK-L422Q and SthK-3QV channels, which interact with the cAMP and cGMP through identical residues. Strikingly, the fractional activation by cGMP relative to cAMP at −60 mV increases dramatically between these two channels, from 2.5 ± 0.57% for cfSthK-L422Q (n = 3) to 89 ± 1.1% for SthK-3QV (n = 3) (Fig. 7C). This large fractional activation by cGMP further supports the conclusion that cGMP is a partial agonist on SthK channels, not an antagonist.
The dramatic increase in fractional activation between these two channels reflects a large energetic change in the opening favorability produced by the A208V, R284Q, and E290Q mutations. To determine the magnitude of energetic change, we calculated LcGMP for each channel. For cfSthK-L422Q, the 2.5% fractional activation by cGMP corresponds to LcGMP ≈ 0.025 (Fig. 7C). For cfSthK-3QV, making the conservative estimate that Po = 0.92 in saturating cAMP, we calculated a Po in cGMP of ≈0.84, which corresponds to LcGMP ≈ 5.3 (Fig. 7, B and C). This analysis suggests that the three mutations outside the CNBD introduced to generate the 3QV construct (i.e. not including L422Q) increased the liganded opening equilibrium constant, L, by at least 200-fold, corresponding to a ΔΔG of −3.2 kcal/mol. The cfSthK-3Q construct (i.e. lacking the A208V mutation) exhibited an intermediate level of cGMP fractional activation, indicating that the C-linker mutations and the S6 mutation both contribute substantially to the increased opening favorability of cfSthK-3QV (Fig. 7C, right).
The large increase in L was further apparent in the dose-response curve for cAMP in cfSthK-3QV. Although wtSthK produced a K1/2 value of 1.5 μm, the K1/2 of cfSthK-3QV was shifted by about 50-fold to a value of 32 ± 5 nm with h = 2.2 ± 0.2 (n = 3) (Fig. 7D). Furthermore, the large increase in L for the cfSthK-3QV channel allowed a dose-response curve to be acquired for cGMP (Fig. 7D). The cGMP dose response can be fit with K1/2 = 5.5 ± 0.3 μm and h = 1.9 ± 0.1 (n = 3). Notably, the observation that mutations known to increase opening favorability in CNG channels also do so in SthK further suggests that SthK is mechanistically similar to CNG channels and is a viable model to study CNG gating (6, 19, 35).
The combination of higher cAMP apparent affinity, higher cGMP efficacy, and higher Po at hyperpolarized voltages made cfSthK-3QV expression highly toxic to E. coli. C43 cells transformed with cfSthK-3QV showed greatly diminished growth in culture, even compared with cfSthK (Fig. 7E). However, cultures of cfSthK-3QV in C43 cyaA− cells still grew at a similar rate and reached a similar density as cultures expressing cfSthK (Fig. 7E). These results demonstrate that the strategy of eliminating cAMP from the E. coli cytoplasm is still sufficient to reduce the apparent toxicity of cfSthK-3QV expression.
Discussion
Prokaryotic channels have the potential to provide major insights into the gating mechanism of CNG channels. Here, we attempted to establish SthK as a model system for studying CNG gating.
First, we characterized the functional properties of wtSthK in E. coli membranes. SthK has been characterized previously using different systems, either expressed in Xenopus oocytes or reconstituted in artificial membranes (21, 22). The functional properties that we measured for SthK in bacterial spheroplasts exhibit a number of important differences from the properties reported previously. Most notably, the Po that we observed at saturating cAMP concentration (0.61 and 0.90 at −60 and +60 mV, respectively) was much higher than measured in oocytes (0.14 at +100 mV) or artificial membranes (0.65 at +200 mV) (21, 22). Assuming a simple closed–open equilibrium, our Po corresponds to nearly a 2 kcal/mol stabilization of the open state. Furthermore, the correspondence between the single-channel Po and the relative conductance at +60 and −60 mV suggests that Po approaches 1 at highly depolarized voltages (Fig. 4B). This more favorable opening transition is also reflected in a higher apparent affinity for cAMP (1.5 μm) compared with oocytes (3.7 μm) and artificial membranes (17 μm) (21, 22). These results indicate that wtSthK displays a much more favorable opening transition in bacterial membranes.
There are a number of possible explanations for the observed difference in Po. Although our wtSthK construct contains the native SthK C-helix sequence, the construct used previously for reconstitution and structural experiments contained a C-helix truncation that resulted in the C-helix mutations E421L and L422E (8, 22). These residues directly interact with cAMP in the X-ray crystal structure of the SthK C-linker/CNBD fragment (see Fig. 6B) (29). Furthermore, the C-helix is thought to form an open state–dependent interaction with the cNMP (Fig. 6B). This interaction is thought to be largely responsible for the f-fold change in cAMP affinity that drives the opening transition, L (Fig. 1B). Therefore, disruption of this interaction could lower f and alter gating. Furthermore, several studies in CNG and HCN channels have shown that a negatively charged residue in the position equivalent to 422 substantially reduces cAMP efficacy (19, 38, and 45). The ∼2 kcal/mol change in gating favorability is on the order of previously reported energetic interactions in this region (19). Consequently, it seems plausible that the differences in maximum Po could be due, in part, to differences in C-helix sequence. However, in oocytes, a low Po was reported for the full-length wtSthK construct, which suggests that other factors are also at work (21).
A second notable difference in our studies is that our recording solutions include 20 mm Mg2+ whereas neither of the other two studies included Mg2+ in their recording solutions (21, 22). Mg2+ was required to inhibit endogenous E. coli channels in the spheroplasts. Rapid Mg2+ block could potentially explain the lower single-channel conductance observed in our studies (Fig. 4E) compared with previous studies (22). However, it is unknown whether Mg2+ blocks SthK or whether the block is voltage-dependent and/or state-dependent. If Mg2+ block is open state–dependent, it would increase the apparent Po of the channel by mass action. The value for L in the presence of Mg2+ would increase to L(1 + M) with L representing the closed–open equilibrium constant in the absence of block and M representing the open–block equilibrium constant (Fig. 1B). However, a ∼26-fold increase in L would require that M be ≈25. In which case, the single-channel current would be reduced to of that previously measured. Therefore, this mechanism alone could not account for an increase in L of this magnitude.
A third possible explanation for the differences is that the bacterial membrane itself may enhance opening favorability. An 86Rb+ uptake assay showed that reconstitution of SthK in proteoliposomes containing 20% cardiolipin (CL) appeared to increase SthK activity (22). CL is a charged lipid that is composed of two phosphatidylglycerols linked by an additional glycerol molecule. The previous recordings of SthK were done in oocytes or bilayers that contained little or no CL. The E. coli inner membrane, however, contains about 5–10% CL (39, 40). CL is known to regulate bacterial inner-membrane proteins as well as mitochondrial inner-membrane proteins (41–43). Additionally, many ion channels are strongly regulated by charged lipids such as phosphatidylinositol phosphates (44). This suggests that the ∼2 kcal/mol increase in opening favorability could indeed come, in part, from interaction with a charged lipid such as CL.
A feature of SthK on which previous studies disagree pertains to the effect of cGMP binding. In oocytes, no SthK current was elicited in the presence of cGMP, and cGMP inhibited activation of the channels by cAMP (21). These authors concluded that cGMP acts as a competitive inhibitor by binding to the CNBD but not promoting activation of the channel (f ≤ 1) (Fig. 1B). In artificial membranes, cGMP was also reported to inhibit cAMP-induced currents. However, these authors observed a significant single-channel Po in the presence of cGMP alone. Therefore, they concluded that cGMP acts as a weak partial agonist on SthK (f is small but >1) (Fig. 1B) (22). Our results also indicate that cGMP acts as a partial agonist of SthK in bacterial membranes, but likely with a lower efficacy than previously reported. This difference in cGMP efficacy may result from the C-helix mutations in the previous study at positions known to affect the relative activation by cAMP and cGMP. Indeed, a Glu at the position equivalent to 422 has been shown to dramatically increase the cGMP activation of both mammalian CNG and HCN channels (19, 45).
The ideal biochemical model for structure–function studies of CNG channel gating would be a channel that is Cys-free, is nontoxic, and has a high Po in the presence of ligand. We have produced such a model in the cfSthK-3QV construct. Removal of the two native Cys residues did not noticeably alter the gating properties (Fig. 5). Generation of the C43 cyaA− E. coli strain eliminated the toxicity of SthK expression (Fig. 6). Finally, addition of mutations in SthK (cfSthK-3QV) substantially increased the Po (Fig. 7). Importantly, the cfSthK-3QV construct could still be grown in the C43 cyaA− cells. This construct provides a foundation for future experiments of CNG channel gating.
Experimental procedures
Bacterial spheroplasts
The production of bacterial spheroplasts was done as described previously with some modification (24–26). SthK constructs (UniProt accession no. E0RR11) were cloned into a pCGFP vector (46). E. coli C43 cells were transformed with the indicated construct and streaked onto a 2× YT plate containing 100 μg/ml carbenicillin. A single colony was picked from a freshly transformed plate and inoculated into 10 ml of 2× YT medium containing 100 μg/ml carbenicillin. The culture was incubated at 37 °C with 220 rpm shaking until the OD600 reached ∼0.3. 1 ml of this culture was then diluted into 10 ml of fresh 2× YT prewarmed at 42 °C and containing 100 μg/ml carbenicillin and 60 μg/ml cephalexin (from a 10 mg/ml stock in H2O). The culture was incubated at 42 °C with 180 rpm shaking for 1.5 h. To make spheroplasts for single-channel recordings, the culture was then moved to 37 °C and shaken at 150 rpm for 20 min. Then 0.4 mm IPTG was added, and the culture was incubated for an additional ∼20 min at 37 °C. To make spheroplasts for macroscopic recordings, the culture was removed from the 42 °C incubator and placed at 19 °C with 150 rpm shaking for 20 min. Then 0.4 mm IPTG was added, and the culture was incubated overnight at 19 °C with 150 rpm shaking. 1 ml of culture was removed and spun at 5000 rpm at 4 °C for 6 min. The supernatant was discarded. All of the following steps were performed at room temperature. The pellet was gently resuspended in 500 μl of 0.8 m sucrose. The following solutions were added in succession (inverting tube several times after each addition): 30 μl of 1 m Hepes, pH 7.4; 24 μl of 0.5 mg/ml lysozyme (freshly prepared in H2O); 6 μl of 5 mg/ml DNase I (freshly prepared in H2O); and 6 μl of 125 mm EDTA, pH 8. Spheroplast formation was monitored under the microscope (40× objective). After ∼4–7 min, 100 ml of stop solution (0.7 m sucrose, 20 mm MgCl2, 10 mm Hepes, pH 7.4) was added, and the tube was inverted multiple times. The sample was aliquoted into 50-μl fractions and placed directly into a −80 °C freezer for storage.
Fluorescence imaging
Spheroplast imaging was performed on a Nikon Eclipse TE2000-E microscope with a 60× water immersion objective (numerical aperture, 1.2). Images were acquired on an Evolve 512 EMCCD camera (Photometrics) using the program MetaMorph (Molecular Devices). The images were analyzed in ImageJ (National Institutes of Health).
Electrophysiology
Symmetrical solutions containing 150 mm KCl, 20 mm MgCl2, 500 mm sucrose, 10 mm Hepes, pH 7.4, were used both in the pipette and in the bath. Patch pipettes were pulled from borosilicate glass tubes without polishing to an open pipette resistance of 2–4 megaohms for macroscopic recordings and 5–8 megaohms for single-channel recordings. Spheroplasts were thawed from −80 °C, and 12–20 μl was added to the bath and allowed to settle to the bottom for ∼20 min. A gigaohm seal was formed on the membrane. Then the pipette was brought away from the bottom of the dish, and the head stage was flicked to excise the patch in an inside-out configuration. Spheroplasts were patched within 1 h after addition to the dish.
Data were acquired using an Axopatch 200A amplifier with Patchmaster software (HEKA Elektronik). For single-channel recordings, the data were sampled at 20 kHz and low-pass filtered at 2 kHz. For recordings at +60 mV, data were further filtered at 1 kHz. For macroscopic recordings, the data were sampled at 10 kHz and low-pass filtered at 2 kHz. Single-channel currents were recorded at a holding potential of −60 mV unless otherwise indicated. Perfusion was achieved through an RSC-100 rapid change solution changer (Biologic). For dose-response curves, steady-state currents were measured at −60 mV. Macroscopic currents recorded in the presence of cAMP and cGMP were leak-subtracted using identical voltage protocols in the absence of ligand to remove leak and capacitance currents.
Data analysis
Data were analyzed using Igor (Wavemetrics), QuB express (47), and Microsoft Excel. Dose-response curves were fit with the Hill equation,
| (Eq. 1) |
where Imax represents the maximal current in saturating cAMP, K1/2 represents the concentration of cNMP producing half-maximal current, and h represents the Hill coefficient.
G–V curves were fit with the Boltzmann equation,
| (Eq. 2) |
where Imax represents the maximal leak-subtracted current measured 2.5 ms after stepping to +100 mV, zδ represents the equivalent charge movement, F represents Faraday's constant, R represents the universal gas constant, and T represents absolute temperature.
Single-channel recordings were analyzed by the accumulation of all data points into a histogram. Histograms from patches containing a single channel were fit with a sum of two Gaussians.
| (Eq. 3) |
Currents from patches containing two or more channels were fit with polynomial distributions, Equation 4 for two channels and Equation 5 for three channels,
| (Eq. 4) |
| (Eq. 5) |
where C scales the amplitude of the Gaussians to the number of counts, i represents the single-channel current, σ represents the variance, and Po represents the single-channel open probability.
The opening equilibrium constant, L, was calculated using the following equation.
| (Eq. 6) |
The change in free energy of opening was calculated using the following equation,
| (Eq. 7) |
where R represents the universal gas constant, T represents the absolute temperature, and L1 and L2 represent equilibrium constants.
Generation of cyaA− E. coli
Adenylate cyclase–deficient E. coli were generated by oligonucleotide-mediated recombination as described previously (Table 1) (32). C43(DE3) E. coli (Lucigen) were transformed with temperature-sensitive helper plasmid pSIJ8, which encodes the λRed recombinase proteins Gam, Beta, and Exo under control of the araBAD promoter as well as a rhamnose-inducible flippase recombinase (FLP) (48). Transformed cells were grown in 2× YT medium at 30 °C with 100 μg/ml carbenicillin until reaching an OD600 of ≈0.4, at which point the λRed proteins were induced with 0.25% l-arabinose. Induced cells were grown for an additional 30 min before making electrocompetent by concentrating ∼100-fold and washing (four times) with 10% glycerol. Aliquots of λRed-induced electrocompetent cells were stored at −80 °C until use.
Table 1.
Strains, plasmids, and primers used in cyaA knockout
The chloramphenicol resistance cassette, flanked by flippase recognition target sequences (FRT-cat-FRT), was PCR-amplified from plasmid pKD3 using KOD polymerase (Novagen) and primers containing 36–38-nucleotide extensions homologous to regions immediately flanking the cyaA gene locus of C43. The resulting ∼1.1-kbp PCR product was gel-purified.
3 μl (141 ng) of FRT-cat-FRT dsDNA was mixed with 50 μl of electrocompetent C43 cells expressing the λRed system and transferred to a chilled electroporation cuvette with a 1-mm gap. Cells were shocked using a Bio-Rad GenePulser (1.8 kV, 25 microfarads, 250 ohms) and recovered in 0.5 ml of 2× YT medium at 30 °C for 2 h. Cells were plated on LB agar containing 100 μg/ml carbenicillin and 10 μg/ml chloramphenicol and incubated at 30 °C. Colonies were visible after ∼3 days at 30 °C, and successful recombinants were inoculated into 4 ml of 2× YT with 100 μg/ml carbenicillin and 20 μg/ml chloramphenicol. After overnight growth at 30 °C, cultures were streaked onto plates containing 100 μg/ml carbenicillin and 20 μg/ml chloramphenicol and again grown at 30 °C. This cycle of colony purification was then repeated a second time. Loss of the cyaA gene and simultaneous gain of the FRT-cat-FRT cassette was confirmed by colony PCR using JumpStart Taq Ready Mix (2×) (Sigma) and locus-specific as well as insert-specific primer pairs. Finally, removal of the helper plasmid pSIJ8 was achieved by overnight growth at 37 °C in 2× YT + 20 μg/ml chloramphenicol. Cells from the resulting strain, termed C43 cyaA−, were made electrocompetent and stored in aliquots at −80 °C.
Growth curves
For growth analysis, bacteria were grown in 10-ml cultures of 2× YT medium in 50-ml Mini Bioreactor tubes (Corning) with 220 rpm shaking. Optical densities were recorded at 600 nm from 10-fold dilutions of bacterial cultures using a Beckmann DU-800 spectrophotometer. Untransformed C43 and C43 cyaA− cells in the absence of antibiotics were grown at 37 °C, whereas SthK-transformed bacteria were grown at 32 °C and included 50 μg/ml kanamycin. Bacteria were selected from single colonies and grown overnight to saturation. The following day, the cultures were diluted to OD600 < 0.05, induced with 0.5 mm IPTG when OD600 reached 0.45–0.65, and moved to 20 °C. Alternatively, SthK-transformed colonies were resuspended directly from plates and diluted to OD600 ≈ 0.4 in 2× YT medium containing 50 μg/ml kanamycin and 1 mm IPTG, and growth was monitored at 32 °C.
Author contributions
J. L. W. M., E. G. B. E., and W. N. Z. conceptualization; J. L. W. M. and E. G. B. E. data curation; J. L. W. M. and E. G. B. E. formal analysis; J. L. W. M. and E. G. B. E. investigation; J. L. W. M. and E. G. B. E. methodology; J. L. W. M. writing-original draft; J. L. W. M., E. G. B. E., and W. N. Z. writing-review and editing; W. N. Z. funding acquisition.
Acknowledgments
We thank Ximena Optiz-Araya for technical assistance, Yoni Haitin and Zachary M. James for preliminary experiments on SthK, Galen E. Flynn and Gucan Dai for helpful comments on the manuscript, and Sharona E. Gordon and all members of the W. N. Z. laboratory for helpful advice and support.
This work was supported by National Institutes of Health Grants R01EY010329, R01MH102378, and R01GM127325; National Institutes of Health Cardiovascular Pathology Training Grant T32HL007312 (to J. L. W. M.); and the Raymond and Beverly Sackler Scholars Program and National Institutes of Health Vision Training Grant T32EY007031 (to E. G. B. E.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- CNG
- cyclic nucleotide–gated
- CNBD
- cyclic nucleotide–binding domain
- cNMP
- cyclic nucleotide (cAMP or cGMP)
- Po
- open probability
- TM
- transmembrane
- MWC
- Monod, Wyman, and Changeux
- HCN
- hyperpolarization-activated cyclic nucleotide–gated
- pCNG
- prokaryotic CNG
- h
- Hill coefficient
- G–V
- conductance–voltage
- cf
- Cys-free
- IPTG
- isopropyl 1-thio-β-d-galactopyranoside
- CAT
- chloramphenicol resistance gene
- CL
- cardiolipin
- FRT
- flippase recognition target sequence.
References
- 1. Burns M. E., and Baylor D. A. (2001) Activation, deactivation, and adaptation in vertebrate photoreceptor cells. Annu. Rev. Neurosci. 24, 779–805 10.1146/annurev.neuro.24.1.779 [DOI] [PubMed] [Google Scholar]
- 2. Kaupp U. B. (2010) Olfactory signalling in vertebrates and insects: differences and commonalities. Nat. Rev. Neurosci. 11, 188–200 10.1038/nrn2789 [DOI] [PubMed] [Google Scholar]
- 3. DeMaria S., and Ngai J. (2010) The cell biology of smell. J. Cell Biol. 191, 443–452 10.1083/jcb.201008163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Craven K. B., and Zagotta W. N. (2006) CNG and HCN channels: two peas, one pod. Annu. Rev. Physiol. 68, 375–401 10.1146/annurev.physiol.68.040104.134728 [DOI] [PubMed] [Google Scholar]
- 5. Zagotta W. N., and Siegelbaum S. A. (1996) Structure and function of cyclic nucleotide-gated channels. Annu. Rev. Neurosci. 19, 235–263 10.1146/annurev.ne.19.030196.001315 [DOI] [PubMed] [Google Scholar]
- 6. Li M., Zhou X., Wang S., Michailidis I., Gong Y., Su D., Li H., Li X., and Yang J. (2017) Structure of a eukaryotic cyclic-nucleotide-gated channel. Nature 542, 60–65 10.1038/nature20819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. James Z. M., and Zagotta W. N. (2018) Structural insights into the mechanisms of CNBD channel function. J. Gen. Physiol. 150, 225–244 10.1085/jgp.201711898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rheinberger J., Gao X., Schmidpeter P. A., and Nimigean C. M. (2018) Ligand discrimination and gating in cyclic nucleotide-gated ion channels from apo and partial agonist-bound cryo-EM structures. eLife 7, e39775 10.7554/eLife.39775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zagotta W. N., Olivier N. B., Black K. D., Young E. C., Olson R., and Gouaux E. (2003) Structural basis for modulation and agonist specificity of HCN pacemaker channels. Nature 425, 200–205 10.1038/nature01922 [DOI] [PubMed] [Google Scholar]
- 10. Monod J., Wyman J., and Changeux J.-P. (1965) On the nature of allosteric transitions: a plausible model. J. Mol. Biol. 12, 88–118 10.1016/S0022-2836(65)80285-6 [DOI] [PubMed] [Google Scholar]
- 11. Tibbs G. R., Goulding E. H., and Siegelbaum S. A. (1997) Allosteric activation and tuning of ligand efficacy in cyclic-nucleotide-gated channels. Nature 386, 612–615 10.1038/386612a0 [DOI] [PubMed] [Google Scholar]
- 12. Auerbach A. (2003) Life at the top: the transition state of AChR gating. Sci. STKE 2003, re11 10.1126/stke.2003.188.re11 [DOI] [PubMed] [Google Scholar]
- 13. Sunderman E. R., and Zagotta W. N. (1999) Mechanism of allosteric modulation of rod cyclic nucleotide-gated channels. J. Gen. Physiol. 113, 601–620 10.1085/jgp.113.5.601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Matulef K., and Zagotta W. N. (2003) Cyclic nucleotide-gated ion channels. Annu. Rev. Cell Dev. Biol. 19, 23–44 10.1146/annurev.cellbio.19.110701.154854 [DOI] [PubMed] [Google Scholar]
- 15. Puljung M. C., DeBerg H. A., Zagotta W. N., and Stoll S. (2014) Double electron–electron resonance reveals cAMP-induced conformational change in HCN channels. Proc. Natl. Acad. Sci. U.S.A. 111, 9816–9821 10.1073/pnas.1405371111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. DeBerg H. A., Brzovic P. S., Flynn G. E., Zagotta W. N., and Stoll S. (2016) Structure and energetics of allosteric regulation of HCN2 ion channels by cyclic nucleotides. J. Biol. Chem. 291, 371–381 10.1074/jbc.M115.696450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Akimoto M., Zhang Z., Boulton S., Selvaratnam R., VanSchouwen B., Gloyd M., Accili E. A., Lange O. F., and Melacini G. (2014) A mechanism for the auto-inhibition of hyperpolarization-activated cyclic nucleotide-gated (HCN) channel opening and its relief by cAMP. J. Biol. Chem. 289, 22205–22220 10.1074/jbc.M114.572164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Matulef K., Flynn G. E., and Zagotta W. N. (1999) Molecular rearrangements in the ligand-binding domain of cyclic nucleotide-gated channels. Neuron 24, 443–452 10.1016/S0896-6273(00)80857-0 [DOI] [PubMed] [Google Scholar]
- 19. Varnum M. D., Black K. D., and Zagotta W. N. (1995) Molecular mechanism for ligand discrimination of cyclic nucleotide-gated channels. Neuron 15, 619–625 10.1016/0896-6273(95)90150-7 [DOI] [PubMed] [Google Scholar]
- 20. Sunderman E. R., and Zagotta W. N. (1999) Sequence of events underlying the allosteric transition of rod cyclic nucleotide-gated channels. J. Gen. Physiol. 113, 621–640 10.1085/jgp.113.5.621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brams M., Kusch J., Spurny R., Benndorf K., and Ulens C. (2014) Family of prokaryote cyclic nucleotide-modulated ion channels. Proc. Natl. Acad. Sci. U.S.A. 111, 7855–7860 10.1073/pnas.1401917111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schmidpeter P. A. M., Gao X., Uphadyay V., Rheinberger J., and Nimigean C. M. (2018) Ligand binding and activation properties of the purified bacterial cyclic nucleotide–gated channel SthK. J. Gen. Physiol. 150, 821–834 10.1085/jgp.201812023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. James Z. M., Borst A. J., Haitin Y., Frenz B., DiMaio F., Zagotta W. N., and Veesler D. (2017) CryoEM structure of a prokaryotic cyclic nucleotide-gated ion channel. Proc. Natl. Acad. Sci. U.S.A. 114, 4430–4435 10.1073/pnas.1700248114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kuo M. M., Saimi Y., Kung C., and Choe S. (2007) Patch clamp and phenotypic analyses of a prokaryotic cyclic nucleotide-gated K+ channel using Escherichia coli as a host. J. Biol. Chem. 282, 24294–24301 10.1074/jbc.M703618200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Santos J. S., Lundby A., Zazueta C., and Montal M. (2006) Molecular template for a voltage sensor in a novel K+ channel. I. Identification and functional characterization of KvLm, a voltage-gated K+ channel from Listeria monocytogenes. J. Gen. Physiol. 128, 283–292 10.1085/jgp.200609572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Martinac B., Rohde P. R., Cranfield C. G., and Nomura T. (2013) Patch clamp electrophysiology for the study of bacterial ion channels in giant spheroplasts of E. coli. Methods Mol. Biol. 966, 367–380 10.1007/978-1-62703-245-2_23 [DOI] [PubMed] [Google Scholar]
- 27. Hamill O. P., Marty A., Neher E., Sakmann B., and Sigworth F. J. (1981) Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 391, 85–100 10.1007/BF00656997 [DOI] [PubMed] [Google Scholar]
- 28. Takahashi M., Blazy B., Baudras A., and Hillen W. (1989) Ligand-modulated binding of a gene regulatory protein to DNA. Quantitative analysis of cyclic-AMP induced binding of CRP from Escherichia coli to non-specific and specific DNA targets. J. Mol. Biol. 207, 783–796 10.1016/0022-2836(89)90244-1 [DOI] [PubMed] [Google Scholar]
- 29. Kesters D., Brams M., Nys M., Wijckmans E., Spurny R., Voets T., Tytgat J., Kusch J., and Ulens C. (2015) Structure of the SthK carboxy-terminal region reveals a gating mechanism for cyclic nucleotide-modulated ion channels. PLoS One 10, e0116369 10.1371/journal.pone.0116369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tibbs G. R., Liu D. T., Leypold B. G., and Siegelbaum S. A. (1998) A state-independent interaction between ligand and a conserved arginine residue in cyclic nucleotide-gated channels reveals a functional polarity of the cyclic nucleotide binding site. J. Biol. Chem. 273, 4497–4505 10.1074/jbc.273.8.4497 [DOI] [PubMed] [Google Scholar]
- 31. Chen S., Wang J., and Siegelbaum S. A. (2001) Properties of hyperpolarization-activated pacemaker current defined by coassembly of Hcn1 and Hcn2 subunits and basal modulation by cyclic nucleotide. J. Gen. Physiol. 117, 491–504 10.1085/jgp.117.5.491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Datsenko K. A., and Wanner B. L. (2000) One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U.S.A. 97, 6640–6645 10.1073/pnas.120163297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Perlman R. L., and Pastan I. (1969) Pleiotropic deficiency of carbohydrate utilization in an adenyl cyclase deficient mutant of Escherichia coli. Biochem. Biophys. Res. Commun. 37, 151–157 10.1016/0006-291X(69)90893-6 [DOI] [PubMed] [Google Scholar]
- 34. Brickman E., Soll L., and Beckwith J. (1973) Genetic characterization of mutations which affect catabolite-sensitive operons in Escherichia coli, including deletions of the gene for adenyl cyclase. J. Bacteriol. 116, 582–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Paoletti P., Young E. C., and Siegelbaum S. A. (1999) C-Linker of cyclic nucleotide-gated channels controls coupling of ligand binding to channel gating. J. Gen. Physiol. 113, 17–34 10.1085/jgp.113.1.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gordon S. E., and Zagotta W. N. (1995) A histidine residue associated with the gate of the cyclic nucleotide-activated channels in rod photoreceptors. Neuron 14, 177–183 10.1016/0896-6273(95)90252-X [DOI] [PubMed] [Google Scholar]
- 37. Craven K. B., and Zagotta W. N. (2004) Salt bridges and gating in the COOH-terminal region of HCN2 and CNGA1 channels. J. Gen. Physiol. 124, 663–677 10.1085/jgp.200409178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gordon S. E., Oakley J. C., Varnum M. D., and Zagotta W. N. (1996) Altered ligand specificity by protonation in the ligand binding domain of cyclic nucleotide-gated channels. Biochemistry 35, 3994–4001 10.1021/bi952607b [DOI] [PubMed] [Google Scholar]
- 39. Shibuya I. (1992) Metabolic regulations and biological functions of phospholipids in Escherichia coli. Prog. Lipid Res. 31, 245–299 10.1016/0163-7827(92)90010-G [DOI] [PubMed] [Google Scholar]
- 40. Carranza G., Angius F., Ilioaia O., Solgadi A., Miroux B., and Arechaga I. (2017) Cardiolipin plays an essential role in the formation of intracellular membranes in Escherichia coli. Biochim. Biophys. Acta 1859, 1124–1132 10.1016/j.bbamem.2017.03.006 [DOI] [PubMed] [Google Scholar]
- 41. Planas-Iglesias J., Dwarakanath H., Mohammadyani D., Yanamala N., Kagan V. E., and Klein-Seetharaman J. (2015) Cardiolipin interactions with proteins. Biophys. J. 109, 1282–1294 10.1016/j.bpj.2015.07.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Corey R. A., Pyle E., Allen W. J., Watkins D. W., Casiraghi M., Miroux B., Arechaga I., Politis A., and Collinson I. (2018) Specific cardiolipin–SecY interactions are required for proton-motive force stimulation of protein secretion. Proc. Natl. Acad. Sci. U.S.A. 115, 7967–7972 10.1073/pnas.1721536115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Laage S., Tao Y., and McDermott A. E. (2015) Cardiolipin interaction with subunit c of ATP synthase: solid-state NMR characterization. Biochim. Biophys. Acta 1848, 260–265 10.1016/j.bbamem.2014.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hille B., Dickson E. J., Kruse M., Vivas O., and Suh B.-C. (2015) Phosphoinositides regulate ion channels. Biochim. Biophys. Acta 1851, 844–856 10.1016/j.bbalip.2014.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Flynn G. E., Black K. D., Islas L. D., Sankaran B., and Zagotta W. N. (2007) Structure and rearrangements in the carboxy-terminal region of SpIH channels. Structure 15, 671–682 10.1016/j.str.2007.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kawate T., and Gouaux E. (2006) Fluorescence-detection size-exclusion chromatography for precrystallization screening of integral membrane proteins. Structure 14, 673–681 10.1016/j.str.2006.01.013 [DOI] [PubMed] [Google Scholar]
- 47. Nicolai C., and Sachs F. (2013) Solving ion channel kinetics with the QuB software. Biophys. Rev. Lett. 08, 191–211 10.1142/S1793048013300053 [DOI] [Google Scholar]
- 48. Jensen S. I., Lennen R. M., Herrgård M. J., and Nielsen A. T. (2015) Seven gene deletions in seven days: fast generation of Escherichia coli strains tolerant to acetate and osmotic stress. Sci. Rep. 5, 17874 10.1038/srep17874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Miroux B., and Walker J. E. (1996) Over-production of proteins in Escherichia coli: mutant hosts that allow synthesis of some membrane proteins and globular proteins at high levels. J. Mol. Biol. 260, 289–298 10.1006/jmbi.1996.0399 [DOI] [PubMed] [Google Scholar]