Abstract
Phase separation of biomolecules leading to the formation of assemblies with distinct material properties has recently emerged as a new paradigm underlying subcellular organization. The discovery that disordered proteins, long associated with aggregation in neurodegenerative disease, are also implicated in driving liquid phase separation has galvanized significant interest in exploring the relationship between misregulated phase transitions and disease. This review summarizes recent work linking liquid phase separation to neurodegeneration, highlighting a pathological role for altered phase behavior and material properties of proteins assembled via liquid phase separation. The techniques that recent and current work in this area have deployed are also discussed, as is the potential for these discoveries to promote new research directions for investigating the molecular etiologies of neurodegenerative diseases.
Keywords: neurodegeneration, protein aggregation, intrinsically disordered protein, Alzheimer disease, biomaterials, amyloid, Parkinson disease, amyotrophic lateral sclerosis (ALS) (Lou Gehrig disease), material properties, phase separation
Introduction
Over a century of research has sought to identify the origins of protein aggregation associated with neurodegenerative disease (1). First reported in 1911 by Dr. Alois Alzheimer (2), protein aggregates have been associated with an array of neurodegenerative diseases, including Alzheimer's disease (AD),2 Parkinson's disease, ALS, frontotemporal dementia (FTD), and traumatic brain injury (3, 4). Determining the biogenesis of protein aggregation and the mechanisms by which protein misfolding or misregulation impacts pathology is crucial for the development of efficacious therapeutic solutions. The emerging paradigm of intracellular phase separation provides a new framework for studying the longstanding and widespread problem of misregulated protein self-assembly in neurodegenerative disease.
Pathological aggregation
Neurodegenerative disease is broadly defined as the degeneration and loss of neuronal function—either in the brain in cases of dementia, such as AD, or in motor neurons in neuromuscular disorders, such as ALS. Whereas neurodegenerative diseases encompass a diverse spectrum of clinical and pathological presentations, they share striking features, including the predominant risk factor of aging and the aggregation of disordered proteins in the nervous system (5, 6). Disordered proteins, including tau and Aβ in AD, α-synuclein in Parkinson's disease, huntingtin protein in Huntington's disease, and FUS/TDP43 in ALS, pathologically self-assemble into insoluble fibers that further aggregate into the plaques, tangles, or inclusions characteristic of each disorder (3, 4).
The pathological hallmarks shared by this diverse disease class imply a common, systemic origin and mechanism of toxicity. Given these shared features, a major focus of study has been the misregulation of cellular pathways associated with aging that could affect protein health or homeostasis (i.e. proteostasis). Such processes include the misregulation of the proteostasis machinery, protein clearance, posttranslational modifications, and protein damage due to oxidative stress (3, 4). Despite much progress, many important questions remain, including how soluble disordered proteins transform to insoluble structured fibers, how aggregation pathology propagates throughout the brain, and why certain neurons are particularly susceptible to aggregation. A fundamental advance in recent years has been the accruing evidence suggesting that cellular toxicity precedes the formation of fibrous aggregates (3, 4, 7). These findings suggest that fibrous protein deposits may not be a useful therapeutic target—they may simply “report” on or even protect against an otherwise misregulated/dysfunctional process. Significant effort has thus been made to identify events or protein states that promote toxicity before the formation of fibrous aggregates, such as aberrant protein conformational changes (8, 9), or the formation of toxic soluble oligomeric species (7, 10–12). The identification of the events that result in protein aggregation would provide important clues to the mysterious origins of pathological aggregation.
Phase separation and biomolecular condensates
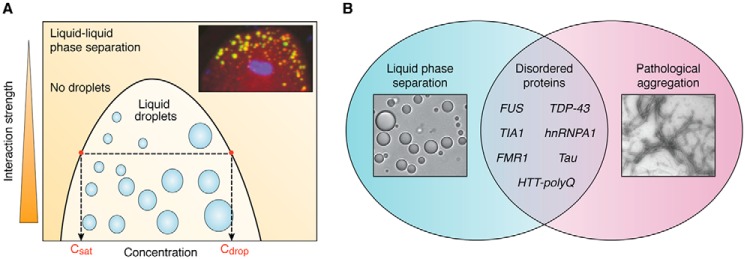
Phase separation has recently emerged as a new paradigm underlying the intracellular assembly of proteins and RNA with emergent collective material properties (thoroughly reviewed in Refs. 13–16). Liquid–liquid phase separation, or the reversible process of a homogenous fluid de-mixing into two distinct liquid phases (Fig. 1A), has been particularly recognized as a new driving force for cellular self-assembly. The advent of this new “phase” in cell biology (16) was pioneered with the demonstrated liquid-like properties of membraneless organelles, including the apparent wetting, fusion, and dynamic exchange of internal components of P granules, (germ granules native to Caenorhabditis elegans) (17), stress granules (18), and the nucleolus (19). In addition to membraneless organelles, various cellular functions, including signaling, cytoskeletal organization, and transcriptional regulation, have been shown to be accompanied by phase separation into condensed states with a range of material properties and are increasingly referred to as biomolecular condensates (15).
Figure 1.
Disordered proteins drive liquid phase separation and neurodegenerative aggregation. A, in classic liquid–liquid phase separation, a coexistence curve, or binodal, delineates the phase-separating region. At a given interaction strength, any concentration above the saturation concentration (Csat) gives rise to phase separation into liquid droplets made up of a higher concentration (Cdrop). Inset, P granules in the early C. elegans embryo (adapted from Ref. 20). B, list of disordered proteins implicated in both liquid phase separation and pathological aggregation into fibers in neurodegenerative diseases.
Whereas many of the molecular and mechanistic details underlying the assembly and dynamics of biomolecular condensates remain to be determined, the role of multivalent protein–protein and protein–RNA interactions has been a dominant theme. Multivalent interactions of varying strength between disordered proteins of low sequence complexity (20–24), modular structured domains (25–27), and combinations of these two archetypes give rise to condensed phases with a spectrum of material properties from liquids to solids.
The discovery that disordered proteins or domains are important drivers of liquid phase separation (28) (Fig. 1) builds on a growing appreciation of the diverse roles that disordered proteins play in the cellular milieu (28, 29). Disordered proteins that have been identified as drivers of the assembly of membraneless organelles include the P granule proteins LAF-1 (20), PGL-3 (30), and MEG proteins (31, 32); the nucleolar protein Fib1 (33, 34); and stress granule proteins FUS (35, 36), TDP-43 (37), and hnRNPA1 (22, 24). These stress granule proteins are further implicated in the pathology of ALS and FTD, where they are found in fibrous form. In fact, low complexity sequences of RNA-binding proteins that associate with condensates have generally been linked to neurodegenerative disease (38, 39). Additionally, proteins long implicated in neurodegenerative aggregation, such as tau (40–42), FMRP (43), and huntingtin protein (44), have recently been demonstrated to undergo liquid phase separation.
The newfound role for disordered proteins in liquid phase separation in general and the evidence for both liquid and fiber formation for specific neuronal proteins introduces compelling new questions about the relationship between phase separation and neurodegeneration. What role, if any, does liquid phase separation play in neurodegeneration? Under what conditions will a disordered protein assume a liquid or fibrous state, and what is the relationship between these distinct material states? The previously unrealized potential for disordered proteins to drive formation into reversible dynamic liquid phases adds an important new dimension to research questions focused on unearthing the origins of pathological aggregation associated with disease. Here we review recent work on the capacity for neuronal proteins to phase separate and discuss the potential for these discoveries to impact new directions in neurodegeneration research. Included first is an outline of the approaches commonly used to probe condensate properties.
Probing material properties
Probing material properties of condensates has been and continues to be crucial in realizing the role of phase separation in pathology and its corresponding potential as a therapeutic target. Summarized here are current approaches to probe the material properties of liquids and the divergence from viscous liquid behavior.
Shape/morphology
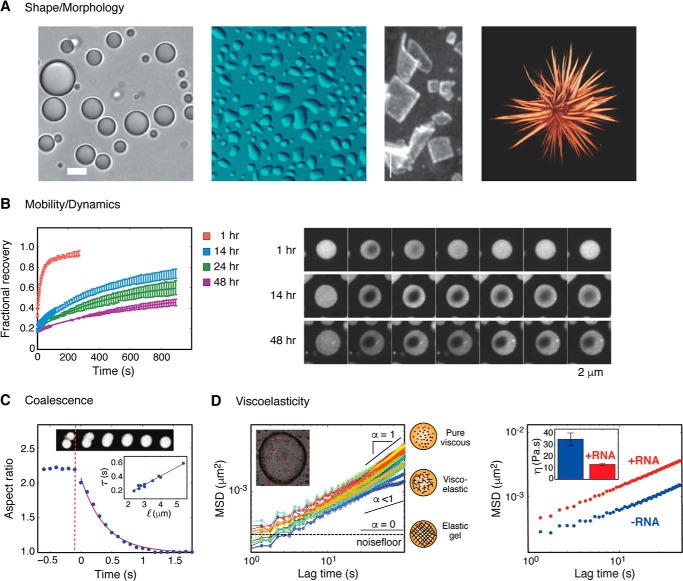
Pure viscous liquid droplets take on spherical shapes in solution, resulting from the energetic need to relax interfacial tension at the liquid–liquid interface (45). Observance of spherical morphologies of protein assemblies has been used frequently to identify the presence of liquid material states (14, 15). Conversely, divergence from a liquid state has been dramatically depicted by the extreme morphological shape change of P granule proteins that can form square solid sheets (46) or the maturation of FUS droplets into a “starburst” morphology (47) (Fig. 2).
Figure 2.
Probing material properties. A, from left to right, LAF-1 droplets form spherical droplets on a pluronic F127-treated glass surface (20); LAF-1 droplets deform upon wetting untreated glass surface; P granule protein CAR-1 forms square sheet structures upon silencing CGH1 expression (46); three-dimensional rendering of FUS protein droplets after maturation (47). B, FRAP experiments of FUS-IDR droplets demonstrating decreasing recovery and dynamics as a function of time (22). C, coalescence of LAF-1 droplet fusion is measured by the relaxation of the aspect ratio to 1 (20). Inset, timescale of fusion scales linearly with size of droplets (20). D, microrheology of condensates. Left, MSD versus lag time for LAF1 droplets. Diffusive exponent α reflects the viscoelasticity of a material (see “Viscoelasticity”) (20). Right, RNA can tune LAF-1 droplet viscosity (20). Error bars, S.D.
However, shape alone is insufficient for determining the material character of condensates. For instance, liquids are not always spherical, as they can deform upon wetting different surfaces, such as membranes in the case of P granules in the adult C. elegans germ line (17) or glass slides with varying surface treatments (34) (Fig. 2). Furthermore, when droplets approach the resolution limit, as is common for small condensates in vivo, accurately resolving droplet morphology becomes increasingly challenging. Additionally, if a system transitions from a liquid to solid state, as has been suggested for balbiani bodies (48) and centrosomal SPD-5 condensates (49), it may retain a spherical shape despite no longer being a pure viscous liquid.
Molecular dynamics, exchange, and mobility
Molecules in viscous liquids are free to diffuse rapidly within droplets and undergo constant exchange between the droplet and bulk solution for liquid systems in thermodynamic equilibrium. Fluorescence recovery after photobleaching (FRAP) allows for the quantification of molecular dynamics, exchange, and mobility within droplets, and is by far the most widely used technique to probe condensate properties both in vitro and in vivo. Quantifying the rate of fluorescence recovery within a droplet allows for the estimation of an effective diffusion coefficient of the molecule probed. A decrease in the recovery rate of a molecule could indicate changes in material properties as well as oligomerization events or altered binding (50). Incomplete fluorescence recovery, or a mobile fraction less than one, reports on any immobile molecules that are not free to exchange and could indicate a divergence from liquid behavior (22, 24, 36, 42, 47, 51, 52) (Fig. 2).
It is important to note that fluorescence recovery rates are only a proxy for liquidity of a material and depend strongly on the probe being monitored. Viscosity is a collective material property of a network of molecules and may not always be directly coupled to molecular diffusion rates. This was recently demonstrated by the length scale dependence of diffusion within LAF1 viscous droplets (53). This work showed that for particles larger than 14 nm in radius, diffusion scaled with particle size, in accordance with the Stokes–Einstein relationship for the given droplet viscosity. However, for molecules smaller than 14 nm, diffusion no longer correlated with particle size and was no longer coupled to the viscosity within the droplet.
Coalescence
Coalescence, or fusion dynamics of droplets, can provide additional information about their material properties. For liquid droplets diffusing in a medium of lower viscosity, the timescale of fusion scales with the droplet size and the ratio of viscosity over surface tension, also known as the inverse capillary velocity (54). Importantly, single-exponential fusion kinetics as well as a linear scaling relationship between time and length are expected for viscous liquids (55). Linear scaling of droplet coalescence has been demonstrated for P granules (17), nucleoli (19), droplets of LAF1 (20), and Whi3 (52). Conversely, arrested coalescence or incomplete fusion, as indicated by aspherical shapes or large aspect ratios, signifies divergence from pure liquid materials. As this method offers only an approximation of the ratio of viscosity to surface tension, additional measurements are necessary to define either viscosity or surface tension and their respective changes.
Viscoelasticity
Materials exist on a viscoelastic spectrum, from liquids with only a viscous component to solids with only an elastic component. A material that has both viscous and elastic components is considered to be viscoelastic. The viscoelasticity of materials can be accurately measured using methods of rheology, the study of how materials flow or deform in response to pressure or stress. In particle tracking microrheology, small micrometer-sized particles are embedded into materials, and their mean squared displacement (MSD) is calculated and plotted as a function of lag time. The diffusive exponent, α, derived from the slope of a log–log plot of MSD versus lag time, can be used to quantify the viscoelasticity of the material (56, 57), where α ranges from 0 (for elastic solids) to 1 (for viscous fluids), with intermediate values indicating a viscoelastic material (Fig. 2). Microrheology has been used to directly measure the viscosity of droplets composed of the P granule protein LAF1 (20) as well as the polyglutamine (polyQ) protein Whi3 (52) and nucleolar proteins (34).
Microrheology is a powerful tool to directly quantify viscoelastic properties of droplets in vitro; however, it can be more challenging to perform accurate microrheological measurements within small condensates in vivo. Nanorheology, a modified fluorescence correlation spectroscopy technique that measures diffusion of smaller sub-micrometer particles, has recently been used to measure viscosity of protein droplets (53); this and related imaging-based technologies have the potential to be powerful tools for measurements in vivo. Further development of rheological techniques and their application to biomolecular condensates will offer important insight into the role of changing material properties in neurodegenerative disease. Microrheology measurements could, for example, be used to quantify the liquid–solid transition of condensates, as has been analogously used in measuring the gel point of stereotypical gel systems, such as acrylamide (58).
Reversibility and/or presence of insoluble aggregates
Another way to probe the properties of droplets is to probe the reversibility or presence of aggregates in solution over time or as a function of mutation/perturbation. The dissolution of phase-separated liquid droplets can be achieved by reversing the phase-separating condition (i.e. by changing the salt, temperature, or pH or “cycling” those parameters). Incomplete reversibility to a single soluble phase signals a divergence from a liquid state. This phenomenon has been referred to as “maturation” or even a “liquid to solid transition” (44, 47). Additionally, droplet dissolution by 1,6-hexanediol is often used to probe droplet properties (24, 42, 44, 59, 60). This aliphatic alcohol is thought to disrupt transient hydrophobic interactions between aromatic residues (61) sometimes present within liquid phases, but fails to disrupt the more stable interactions present in other fibrous forms. However, it is not actually clear precisely which interactions 1,6-hexanediol disrupts, and responses to this compound do not directly report on the presence or absence of a liquid phase.
The presence of insoluble aggregates within droplets can be further confirmed in a number of ways. A simple centrifugation assay after dissolution of the condensed liquid phase, can report on the formation of any insoluble material. EM can be used to analyze the aggregate structures in more detail and/or confirm the presence of nanoscale fibers. Additionally, small fluorescent molecular probes like thioflavin S or T, which report on the presence of cross-β-sheet structures, are often used to discern ordered fibers from amorphous aggregates (24, 42, 47, 60). It is important to note that these probes are quite prone to false negatives and positives (62), and careful controls along with complementary techniques should be applied.
Phase separation of neuronal proteins
Stress granule proteins
Stress granule–related proteins have provided the first link between phase-separated compartments and neurological disease. The proteins FUS, TDP-43, hnRNPA1, and TIA1 and the C9orf72 translational dipeptide repeats GR/PR, are each implicated in the pathology of ALS and FTD with demonstrated roles in phase separation.
FUS
Fused in sarcoma (FUS) protein is an RNA binding protein that associates with liquid-like stress granules (SGs) and is further implicated in chromosomal translocations in cancer and aggregation in ALS/FTD (63). In 2015, Patel et al. (47) demonstrated that FUS-GFP can phase-separate into spherical droplets, capable of fusion and with fast and complete recovery by FRAP. They further showed that introduction of the ALS disease mutation G156E to their FUS-GFP construct resulted in dramatic changes to the material properties of the droplets over time, illustrated by significant loss of mobile fraction by FRAP and the appearance, upon shaking, of nonspherical structures (Fig. 2). Murakami et al. (36) further showed that reversibility of FUS aggregates in vitro and in vivo is compromised by ALS/FTD mutations, consequently leading to the sequestering of additional proteins within the aggregates. Together, these studies suggest that changing of material properties from reversible liquid states to less soluble states (i.e. “maturation” or a liquid-to-solid transition) could contribute to disease. Additionally, phosphorylation of FUS (64) has been shown to disrupt both FUS phase separation and aggregation, suggesting a potential treatment pathway for FUS assembly regulation.
TDP-43
Aggregates of TAR-DNA–binding protein of 43 kDa (TDP-43) are impressively found in ∼97% of all cases of ALS and 45% of FTD cases (65, 66), but the connection between TDP-43 aggregation and toxicity remains unclear. The C-terminal domain of TDP-43 has been shown to phase-separate into droplets with rapid dynamics by FRAP (37). Probing several disease mutants, the authors found that most mutants inhibited phase separation, demonstrated by a shift to the right in the phase diagram, aside from A321V, which exhibits a shift to the left. Interestingly, all disease mutants showed enhanced “conversion” to aggregates over time, as seen by a loss of reversibility upon temperature cycling.
hnRNPA1
Heterogeneous nuclear ribonucleoproteins (hnRNPs) form cytoplasmic inclusions that are pathologically deposited in ALS/FTD patients. Molliex et al. (24) demonstrated that hnRNPA1 can form liquid droplets in a concentration-dependent manner. They show that fibrillization of the disease-causing mutation D262V is promoted by phase separation using a combination of temperature cycling and ThT staining (24). Similarly, Lin et al. (22) demonstrated the accumulation of aggregates in hnRNPA1 droplets over time that are further exacerbated by the amyloid-enhancing D262V mutation.
TIA1
T cell–restricted intracellular antigen-1 (TIA1) is an SG component with a demonstrated role in SG assembly (67, 68). The disordered domain of TIA1 has been shown to undergo liquid phase separation in the presence of RNA (22). Using the full-length TIA1, Mackenzie et al. (51) showed that disease mutations in TIA1 (P362L, A381T, and E384K) promote phase separation, demonstrated as a shift to the left in the phase diagram; decrease mobile fraction in TIA1 droplets by FRAP; and decrease droplet reversibility. In vivo, they found that TIA1 mutations alter stress granule dynamics, in that they inhibit the rate and completion of disassembly. Interestingly, stress-induced colocalization of TDP-43 into granules accelerates aggregation of TIA1 within SGs.
C9orf72
Expansion of hexanucleotide repeat GGGCC in the intron of chromosome 9 ORF 72 (C9ORF72) is the most common cause of FTD and ALS, serving as evidence for the connection between the pathologies of the two neurological diseases (69, 70). One potential consequence of this expansion lies in the misregulated translation of this sequence, which produces dipeptide repeats GR and PR (71). In 2016, Lee et al. (59) found that expression and colocalization of GR and PR dipeptides with nucleoli and SGs can impair dynamics and mobile fraction in living cells by FRAP. They further showed that GR/PR peptides colocalize with hnRNPA1 and TIA1 droplets in vitro and promote phase separation by decreasing the saturation concentration. Boeynaems et al. (72) further showed that PR peptides can alone phase-separate into dynamic liquids capable of fusion and recovery by FRAP.
Other neurodegenerative proteins
More recently, additional neuronal proteins not previously known to be associated with liquid compartments have also been demonstrated to undergo liquid phase separation. These include tau (40–42, 73, 74), FMRP (43, 72), and huntingtin protein (44).
Tau
Tau is a neuronal protein that pathologically aggregates into fibers and tangles associated with a long list of tauopathies, from Alzheimer's disease to traumatic brain injury (75). Recent work has found that in the presence of macromolecular crowder, phosphorylation, and/or RNA, tau can phase-separate into liquid droplets. Wegmann et al. (42) have shown that full-length tau (tau441-GFP) forms droplets in cultured neurons that exhibit rapid recovery after photobleaching. In vitro, they show that phosphorylated tau, naturally phosphorylated from expression in insect cells, forms droplets that become less dynamic over time. They further show that increased phosphorylation levels of tau, as well as the addition of co-factors known to increase tau aggregation, such as heparin and RNA, induce or enhance droplet formation.
Similarly, the microtubule-binding region fragment of tau, K18, has been shown to form droplets that are enhanced by increasing the temperature to 37 °C (40). Phosphorylation also enhances K18 phase separation by lowering the saturation concentration and the apparent rate of droplet formation. Both studies demonstrated the formation of ThS (42) and ThT (40) positive aggregates in their respective droplet systems. Another fragment of tau, d187, has been shown to form droplets in an RNA-dependent manner (41). Using the insect cell–expressed phosphorylated tau441-GFP in the presence of 10% dextran, Hernández-Vega et al. (74) further showed that tau phase separation can enhance the organization of tubulin and MT polymerization, suggesting a potential functional role for tau phase separation.
Huntingtin
Recent work by Peskett et al. (44) has shown that HTT exon 1 with extended polyQ repeats can form assemblies with distinct morphologies in mammalian cells and yeast. They show that polyQ expansions can form two types of assemblies with distinct intensity features they refer to as “dim” and “bright” as well as distinct physical properties. The dim assemblies appear to be more consistent with a liquid state, in that they recover almost completely and within seconds by FRAP, can undergo fusion, and are reversible by 1,6-hexanediol, whereas the “bright” assemblies exhibit significantly slower recovery times with very low mobile fractions, are 1,6-hexanediol–insensitive, often have asymmetrical structures or spikes that protrude from their center of mass, and appear by electron tomography to contain a web of fibrous nanostructures.
In vitro, they were able to show that polyQ25 could form droplets in the presence of 10% dextran that could fuse, recover rapidly by FRAP, and were sensitive to 1,6-hexanediol. Interestingly, they note that these droplets rapidly matured, showing fibrous protrusions and partial resistance to 1,6-hexanediol dissolution in just 30 min of incubation.
Novel therapeutic targets
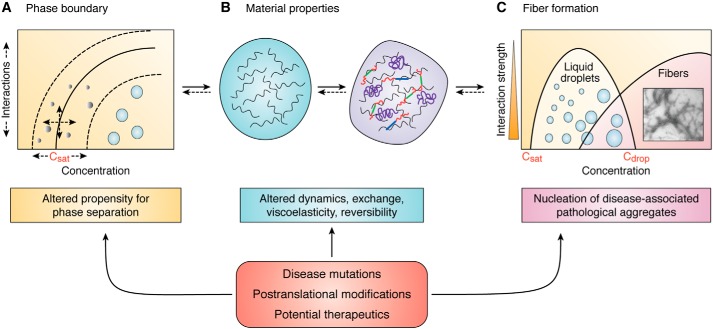
These recent discoveries position liquid phase separation as a compelling novel component in the neurodegeneration pathway and consequently a new avenue for therapeutic strategies. However, the unanswered questions already surrounding the area of pathological aggregation are equally present here. For instance, whether or not protein fibers promote, protect, or are simply an artifact of toxicity should frame interpretations of data that aim to parse the connections between liquids and fibers. Additionally, whether or not liquid phases contribute to loss of function, gain of dysfunction, or both remains to be determined. Furthermore, various cases of reversible and/or functional amyloid fibers have been reported (76, 77), further challenging assumptions about the pathological status of protein fibers. In light of these questions, it can be useful to break down the current evidence for the role of liquid phase separation in neurodegeneration into three effective modes of action: those that influence (a) propensity for phase separation, (b) material properties of phase-separated droplets, and (c) nucleation of fibers (Fig. 3). This framework further delineates three potential pathways for therapeutic intervention.
Figure 3.
Regulating and targeting liquid phase separation. Three modes of action currently link phase separation to pathology with the potential to serve as pathways for intervention. A, the phase boundary, a metric for the propensity to phase-separate, is defined by the saturation concentration and the relative interaction strengths between molecules. B, material properties of condensed phases include viscoelasticity, reversibility of exchange, and the dynamics and mobility of molecules within and across droplets. C, fiber formation may be nucleated within droplets, potentially giving rise to pathology. These three modes of action, which are sensitive to disease-associated conditions, highlight distinct avenues for regulation and therapeutic targeting of liquid phase separation in disease.
Propensity for phase separation
Phase separation is extremely sensitive to perturbations, including protein concentration, cellular/environmental conditions, and the relative abundance of cofactors or binding partners. The demonstrated capacity for neuronal proteins to phase-separate raises new possibilities for assembly (mis)regulation in vivo. A shift in a phase boundary of a protein or group of biomolecules that enhances (left shift) or inhibits (right shift) phase separation could have significant consequences for the function/dysfunction of neuronal proteins. ALS disease mutations have been shown to both inhibit (TDP43 (37)) and enhance (TDP4-3 and A231V (37) and TIA1 (51)) phase separation; post-translational modifications, such as phosphorylation, have been shown to inhibit phase separation in the case of FUS (64) but enhance phase separation in the case of tau (42). Interestingly, in both cases, the effect is consistent with inhibiting or enhancing aggregation. Independent of the potential relationship between liquids and fibers or the fate of the phase-separated state, the liquid phase diagram and its tunable phase boundary could serve as a useful assay in screening effective drug candidates.
Material properties
The material properties of protein phases, such as viscoelasticity and internal diffusivity, are also sensitive to perturbations and highly tunable by condition and cofactors. We are just beginning to discern the role of these material states in regulating the components within. In the case of SGs, there is increasing evidence for the impact of changing dynamics on pathology. Overall, disease mutations in FUS (36, 47), TDP-43 (37, 51), hnRNPA1 (22, 24), and TIA1 (51) give rise to changes in material properties, including a decrease in fluidity, mobile fraction, coalescence, and internal diffusivity. Additionally, longer polyQ protein variants that are associated with disease appear to form assemblies with distinct morphologies and material properties (44). Changes in granule material properties can have direct consequences for both loss and gain of function. Decreasing dynamics would impact interactions and reactions associated with granule function, whereas loss of reversibility may impact the native availability of a particular protein in the cytoplasmic or nucleoplasmic bulk. Furthermore, as in the case of FUS, such changes can lead to the aberrant sequestration of additional proteins within the assembly with the potential for innumerable functional consequences. Together, this suggests that devising methods to modulate the material properties of granules could present a useful therapeutic strategy.
Fiber formation
It is an intriguing possibility that enhanced propensity to liquid phase-separate, increased duration or interactions in a liquid state, and/or modulated liquid phase properties can contribute to the formation of fibers. Evidence for this now exists for SG proteins as well as tau and huntingtin. Phase separation could enhance the formation of fibers by providing an unstable oversaturated highly concentrated environment. This begs the question of how liquid states, if that unstable, are normally maintained, suggesting the potential involvement of stabilizing cofactors or chaperones. Fiber formation could in turn contribute to material property changes with aberrant functional consequences, such as unwanted sequestration as described above. Strategies to preserve liquid properties or otherwise inhibit conditions that result in the nucleation of fibers, could prove to be effective. Whereas the precise relationship between fluids and fibers still needs to be determined, the growing evidence linking phase separation to the classic pathological hallmark of neurodegeneration holds significant potential for impactful discoveries.
Conclusion
The previously unrealized potential for intracellular proteins to assemble via phase separation into soft condensed material states provides a new lens through which to probe the origins of pathological aggregation associated with neurodegeneration. The evidence in support of a relationship between droplet properties, protein aggregation, and neurodegenerative pathology is rapidly growing; however, significant future work is necessary to determine the molecular and regulatory mechanisms underlying these processes. The new paradigm of phase separation coupled with the advancement of techniques to probe this new phenomenon in and out of cells has the potential to drive new therapeutic directions for treating many devastating neurodegenerative diseases.
Acknowledgments
I thank Clifford P. Brangwynne, Elizabeth Rhoades, and Stephanie Weber for providing useful feedback on early manuscript drafts and Rachel Fisher, Charles J. McDonald, and Alfredo Vidal Ceballos for proofreading the manuscript.
This article is part of the thematic series, Phase separation of RNA-binding proteins in physiology and disease. The authors declares that she has no conflicts of interest with the contents of this article.
- AD
- Alzheimer's disease
- FTD
- frontotemporal dementia
- FRAP
- fluorescence recovery after photobleaching
- SG
- stress granule
- hnRNP
- heterogeneous nuclear ribonucleoprotein
- polyQ
- polyglutamine.
References
- 1. Goedert M., and Spillantini M. G. (2006) A century of Alzheimer's disease. Science 314, 777–781 10.1126/science.1132814 [DOI] [PubMed] [Google Scholar]
- 2. Alzheimer A. (1911) Concerning unsual medical cases in old age. Z. Gesamte Neurol. Psy. 4, 356–385 10.1007/BF02866241 [DOI] [Google Scholar]
- 3. Chiti F., and Dobson C. M. (2006) Protein misfolding, functional amyloid, and human disease. Annu. Rev. Biochem. 75, 333–366 10.1146/annurev.biochem.75.101304.123901 [DOI] [PubMed] [Google Scholar]
- 4. Knowles T. P., Vendruscolo M., and Dobson C. M. (2014) The amyloid state and its association with protein misfolding diseases. Nat. Rev. Mol. Cell Biol. 15, 384–396 10.1038/nrm3810 [DOI] [PubMed] [Google Scholar]
- 5. Wyss-Coray T. (2016) Ageing, neurodegeneration and brain rejuvenation. Nature 539, 180–186 10.1038/nature20411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aguzzi A., and O'Connor T. (2010) Protein aggregation diseases: pathogenicity and therapeutic perspectives. Nat. Rev. Drug. Discov. 9, 237–248 10.1038/nrd3050 [DOI] [PubMed] [Google Scholar]
- 7. Bucciantini M., Giannoni E., Chiti F., Baroni F., Formigli L., Zurdo J., Taddei N., Ramponi G., Dobson C. M., and Stefani M. (2002) Inherent toxicity of aggregates implies a common mechanism for protein misfolding diseases. Nature 416, 507–511 10.1038/416507a [DOI] [PubMed] [Google Scholar]
- 8. Elbaum-Garfinkle S., and Rhoades E. (2012) Identification of an aggregation-prone structure of tau. J. Am. Chem. Soc. 134, 16607–16613 10.1021/ja305206m [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Trexler A. J., and Rhoades E. (2010) Single molecule characterization of alpha-synuclein in aggregation-prone states. Biophys. J. 99, 3048–3055 10.1016/j.bpj.2010.08.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Campioni S., Mannini B., Zampagni M., Pensalfini A., Parrini C., Evangelisti E., Relini A., Stefani M., Dobson C. M., Cecchi C., and Chiti F. (2010) A causative link between the structure of aberrant protein oligomers and their toxicity. Nat. Chem. Biol. 6, 140–147 10.1038/nchembio.283 [DOI] [PubMed] [Google Scholar]
- 11. Haass C., and Selkoe D. J. (2007) Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid beta-peptide. Nat. Rev. Mol. Cell Biol. 8, 101–112 10.1038/nrm2101 [DOI] [PubMed] [Google Scholar]
- 12. Winner B., Jappelli R., Maji S. K., Desplats P. A., Boyer L., Aigner S., Hetzer C., Loher T., Vilar M., Campioni S., Tzitzilonis C., Soragni A., Jessberger S., Mira H., Consiglio A., et al. (2011) In vivo demonstration that α-synuclein oligomers are toxic. Proc. Natl. Acad. Sci. U.S.A. 108, 4194–4199 10.1073/pnas.1100976108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Boeynaems S., Alberti S., Fawzi N. L., Mittag T., Polymenidou M., Rousseau F., Schymkowitz J., Shorter J., Wolozin B., Van Den Bosch L., Tompa P., and Fuxreiter M. (2018) Protein phase separation: a new phase in cell biology. Trends Cell Biol. 28, 420–435 10.1016/j.tcb.2018.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shin Y., and Brangwynne C. P. (2017) Liquid phase condensation in cell physiology and disease. Science 357, eaaf4382 10.1126/science.aaf4382 [DOI] [PubMed] [Google Scholar]
- 15. Banani S. F., Lee H. O., Hyman A. A., and Rosen M. K. (2017) Biomolecular condensates: organizers of cellular biochemistry. Nat. Rev. Mol. Cell Biol. 18, 285–298 10.1038/nrm.2017.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dolgin E. (2018) What lava lamps and vinaigrette can teach us about cell biology. Nature 555, 300–302 10.1038/d41586-018-03070-2 [DOI] [PubMed] [Google Scholar]
- 17. Brangwynne C. P., Eckmann C. R., Courson D. S., Rybarska A., Hoege C., Gharakhani J., Jülicher F., and Hyman A. A. (2009) Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science 324, 1729–1732 10.1126/science.1172046 [DOI] [PubMed] [Google Scholar]
- 18. Wippich F., Bodenmiller B., Trajkovska M. G., Wanka S., Aebersold R., and Pelkmans L. (2013) Dual specificity kinase DYRK3 couples stress granule condensation/dissolution to mTORC1 signaling. Cell 152, 791–805 10.1016/j.cell.2013.01.033 [DOI] [PubMed] [Google Scholar]
- 19. Brangwynne C. P., Mitchison T. J., and Hyman A. A. (2011) Active liquid-like behavior of nucleoli determines their size and shape in Xenopus laevis oocytes. Proc. Natl. Acad. Sci. U.S.A. 108, 4334–4339 10.1073/pnas.1017150108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Elbaum-Garfinkle S., Kim Y., Szczepaniak K., Chen C. C., Eckmann C. R., Myong S., and Brangwynne C. P. (2015) The disordered P granule protein LAF-1 drives phase separation into droplets with tunable viscosity and dynamics. Proc. Natl. Acad. Sci. U.S.A. 112, 7189–7194 10.1073/pnas.1504822112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nott T. J., Petsalaki E., Farber P., Jervis D., Fussner E., Plochowietz A., Craggs T. D., Bazett-Jones D. P., Pawson T., Forman-Kay J. D., and Baldwin A. J. (2015) Phase transition of a disordered nuage protein generates environmentally responsive membraneless organelles. Mol. Cell 57, 936–947 10.1016/j.molcel.2015.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lin Y., Protter D. S., Rosen M. K., and Parker R. (2015) Formation and maturation of phase-separated liquid droplets by RNA-binding proteins. Mol. Cell 60, 208–219 10.1016/j.molcel.2015.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kato M., Han T. W., Xie S., Shi K., Du X., Wu L. C., Mirzaei H., Goldsmith E. J., Longgood J., Pei J., Grishin N. V., Frantz D. E., Schneider J. W., Chen S., Li L., et al. (2012) Cell-free formation of RNA granules: low complexity sequence domains form dynamic fibers within hydrogels. Cell 149, 753–767 10.1016/j.cell.2012.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Molliex A., Temirov J., Lee J., Coughlin M., Kanagaraj A. P., Kim H. J., Mittag T., and Taylor J. P. (2015) Phase separation by low complexity domains promotes stress granule assembly and drives pathological fibrillization. Cell 163, 123–133 10.1016/j.cell.2015.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li P., Banjade S., Cheng H. C., Kim S., Chen B., Guo L., Llaguno M., Hollingsworth J. V., King D. S., Banani S. F., Russo P. S., Jiang Q. X., Nixon B. T., and Rosen M. K. (2012) Phase transitions in the assembly of multivalent signalling proteins. Nature 483, 336–340 10.1038/nature10879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Su X., Ditlev J. A., Hui E., Xing W., Banjade S., Okrut J., King D. S., Taunton J., Rosen M. K., and Vale R. D. (2016) Phase separation of signaling molecules promotes T cell receptor signal transduction. Science 352, 595–599 10.1126/science.aad9964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Banjade S., and Rosen M. K. (2014) Phase transitions of multivalent proteins can promote clustering of membrane receptors. Elife 3, 10.7554/eLife.04123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Uversky V. N. (2017) Intrinsically disordered proteins in overcrowded milieu: membrane-less organelles, phase separation, and intrinsic disorder. Curr. Opin Struct. Biol. 44, 18–30 10.1016/j.sbi.2016.10.015 [DOI] [PubMed] [Google Scholar]
- 29. Forman-Kay J. D., and Mittag T. (2013) From sequence and forces to structure, function, and evolution of intrinsically disordered proteins. Structure 21, 1492–1499 10.1016/j.str.2013.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Saha S., Weber C. A., Nousch M., Adame-Arana O., Hoege C., Hein M. Y., Osborne-Nishimura E., Mahamid J., Jahnel M., Jawerth L., Pozniakovski A., Eckmann C. R., Jülicher F., and Hyman A. A. (2016) Polar positioning of phase-separated liquid compartments in cells regulated by an mRNA competition mechanism. Cell 166, 1572–1584.e16 10.1016/j.cell.2016.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Smith J., Calidas D., Schmidt H., Lu T., Rasoloson D., and Seydoux G. (2016) Spatial patterning of P granules by RNA-induced phase separation of the intrinsically-disordered protein MEG-3. Elife 5, e21337 10.7554/eLife.21337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang J. T., Smith J., Chen B. C., Schmidt H., Rasoloson D., Paix A., Lambrus B. G., Calidas D., Betzig E., and Seydoux G. (2014) Regulation of RNA granule dynamics by phosphorylation of serine-rich, intrinsically disordered proteins in C. elegans. Elife 3, e04591 10.7554/eLife.04591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Berry J., Weber S. C., Vaidya N., Haataja M., and Brangwynne C. P. (2015) RNA transcription modulates phase transition-driven nuclear body assembly. Proc. Natl. Acad. Sci. U.S.A. 112, E5237–E5245 10.1073/pnas.1509317112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Feric M., Vaidya N., Harmon T. S., Mitrea D. M., Zhu L., Richardson T. M., Kriwacki R. W., Pappu R. V., and Brangwynne C. P. (2016) Coexisting liquid phases underlie nucleolar subcompartments. Cell 165, 1686–1697 10.1016/j.cell.2016.04.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Burke K. A., Janke A. M., Rhine C. L., and Fawzi N. L. (2015) Residue-by-residue view of in vitro FUS granules that bind the C-terminal domain of RNA polymerase II. Mol. Cell 60, 231–241 10.1016/j.molcel.2015.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Murakami T., Qamar S., Lin J. Q., Schierle G. S., Rees E., Miyashita A., Costa A. R., Dodd R. B., Chan F. T., Michel C. H., Kronenberg-Versteeg D., Li Y., Yang S. P., Wakutani Y., Meadows W., et al. (2015) ALS/FTD mutation-induced phase transition of FUS liquid droplets and reversible hydrogels into irreversible hydrogels impairs RNP granule function. Neuron 88, 678–690 10.1016/j.neuron.2015.10.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Conicella A. E., Zerze G. H., Mittal J., and Fawzi N. L. (2016) ALS mutations disrupt phase separation mediated by α-helical structure in the TDP-43 low-complexity C-terminal domain. Structure 24, 1537–1549 10.1016/j.str.2016.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. King O. D., Gitler A. D., and Shorter J. (2012) The tip of the iceberg: RNA-binding proteins with prion-like domains in neurodegenerative disease. Brain Res. 1462, 61–80 10.1016/j.brainres.2012.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ramaswami M., Taylor J. P., and Parker R. (2013) Altered ribostasis: RNA-protein granules in degenerative disorders. Cell 154, 727–736 10.1016/j.cell.2013.07.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ambadipudi S., Biernat J., Riedel D., Mandelkow E., and Zweckstetter M. (2017) Liquid–liquid phase separation of the microtubule-binding repeats of the Alzheimer-related protein Tau. Nat. Commun. 8, 275 10.1038/s41467-017-00480-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang X., Lin Y., Eschmann N. A., Zhou H., Rauch J. N., Hernandez I., Guzman E., Kosik K. S., and Han S. (2017) RNA stores tau reversibly in complex coacervates. PLoS Biol. 15, e2002183 10.1371/journal.pbio.2002183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wegmann S., Eftekharzadeh B., Tepper K., Zoltowska K. M., Bennett R. E., Dujardin S., Laskowski P. R., MacKenzie D., Kamath T., Commins C., Vanderburg C., Roe A. D., Fan Z., Molliex A. M., Hernandez-Vega A., et al. (2018) Tau protein liquid-liquid phase separation can initiate tau aggregation. EMBO J. 37, e98049 10.15252/embj.201798049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vernon R. M., Chong P. A., Tsang B., Kim T. H., Bah A., Farber P., Lin H., and Forman-Kay J. D. (2018) Pi-Pi contacts are an overlooked protein feature relevant to phase separation. Elife 7, e31486 10.7554/eLife.31486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Peskett T. R., Rau F., O'Driscoll J., Patani R., Lowe A. R., and Saibil H. R. (2018) A liquid to solid phase transition underlying pathological huntingtin exon1 aggregation. Mol. Cell 70, 588–601.e6 10.1016/j.molcel.2018.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hyman A. A., Weber C. A., and Jülicher F. (2014) Liquid-liquid phase separation in biology. Annu. Rev. Cell Dev. Biol. 30, 39–58 10.1146/annurev-cellbio-100913-013325 [DOI] [PubMed] [Google Scholar]
- 46. Hubstenberger A., Noble S. L., Cameron C., and Evans T. C. (2013) Translation repressors, an RNA helicase, and developmental cues control RNP phase transitions during early development. Dev. Cell 27, 161–173 10.1016/j.devcel.2013.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Patel A., Lee H. O., Jawerth L., Maharana S., Jahnel M., Hein M. Y., Stoynov S., Mahamid J., Saha S., Franzmann T. M., Pozniakovski A., Poser I., Maghelli N., Royer L. A., Weigert M., et al. (2015) A liquid-to-solid phase transition of the ALS protein FUS accelerated by disease mutation. Cell 162, 1066–1077 10.1016/j.cell.2015.07.047 [DOI] [PubMed] [Google Scholar]
- 48. Boke E., and Mitchison T. J. (2017) The balbiani body and the concept of physiological amyloids. Cell Cycle 16, 153–154 10.1080/15384101.2016.1241605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Woodruff J. B., Ferreira Gomes B., Widlund P. O., Mahamid J., Honigmann A., and Hyman A. A. (2017) The centrosome is a selective condensate that nucleates microtubules by concentrating tubulin. Cell 169, 1066–1077.e10 10.1016/j.cell.2017.05.028 [DOI] [PubMed] [Google Scholar]
- 50. Reits E. A., and Neefjes J. J. (2001) From fixed to FRAP: measuring protein mobility and activity in living cells. Nat. Cell Biol. 3, E145–E147 10.1038/35078615 [DOI] [PubMed] [Google Scholar]
- 51. Mackenzie I. R., Nicholson A. M., Sarkar M., Messing J., Purice M. D., Pottier C., Annu K., Baker M., Perkerson R. B., Kurti A., Matchett B. J., Mittag T., Temirov J., Hsiung G. R., Krieger C., et al. (2017) TIA1 mutations in amyotrophic lateral sclerosis and frontotemporal dementia promote phase separation and alter stress granule dynamics. Neuron 95, 808–816 e809 10.1016/j.neuron.2017.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhang H., Elbaum-Garfinkle S., Langdon E. M., Taylor N., Occhipinti P., Bridges A. A., Brangwynne C. P., and Gladfelter A. S. (2015) RNA controls PolyQ protein phase transitions. Mol. Cell 60, 220–230 10.1016/j.molcel.2015.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wei M. T., Elbaum-Garfinkle S., Holehouse A. S., Chen C. C., Feric M., Arnold C. B., Priestley R. D., Pappu R. V., and Brangwynne C. P. (2017) Phase behaviour of disordered proteins underlying low density and high permeability of liquid organelles. Nat. Chem. 9, 1118–1125 10.1038/nchem.2803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Eggers J., Lister J. R., and Stone H. A. (1999) Coalescence of liquid drops. J. Fluid Mech. 401, 293–310 10.1017/S002211209900662X [DOI] [Google Scholar]
- 55. Ceballos A. V., McDonald C. J., and Elbaum-Garfinkle S. (2018) Methods and strategies to quantify phase separation of disordered proteins. Methods Enzymol. 611, 31–50 10.1016/bs.mie.2018.09.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mason T. G., and Weitz D. A. (1995) Linear viscoelasticity of colloidal hard sphere suspensions near the glass transition. Phys. Rev. Lett. 75, 2770–2773 10.1103/PhysRevLett.75.2770 [DOI] [PubMed] [Google Scholar]
- 57. Wirtz D. (2009) Particle-tracking microrheology of living cells: principles and applications. Annu. Rev. Biophys. 38, 301–326 10.1146/annurev.biophys.050708.133724 [DOI] [PubMed] [Google Scholar]
- 58. Larsen T. H., and Furst E. M. (2008) Microrheology of the liquid-solid transition during gelation. Phys. Rev. Lett. 100, 146001 10.1103/PhysRevLett.100.146001 [DOI] [PubMed] [Google Scholar]
- 59. Lee K. H., Zhang P., Kim H. J., Mitrea D. M., Sarkar M., Freibaum B. D., Cika J., Coughlin M., Messing J., Molliex A., Maxwell B. A., Kim N. C., Temirov J., Moore J., Kolaitis R. M., et al. (2016) C9orf72 dipeptide repeats impair the assembly, dynamics, and function of membrane-less organelles. Cell 167, 774–788.e17 10.1016/j.cell.2016.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kroschwald S., Maharana S., Mateju D., Malinovska L., Nüske E., Poser I., Richter D., and Alberti S. (2015) Promiscuous interactions and protein disaggregases determine the material state of stress-inducible RNP granules. Elife 4, e06807 10.7554/eLife.06807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ribbeck K., and Görlich D. (2002) The permeability barrier of nuclear pore complexes appears to operate via hydrophobic exclusion. EMBO J. 21, 2664–2671 10.1093/emboj/21.11.2664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Malmos K. G., Blancas-Mejia L. M., Weber B., Buchner J., Ramirez-Alvarado M., Naiki H., and Otzen D. (2017) ThT 101:a primer on the use of thioflavin T to investigate amyloid formation. Amyloid 24, 1–16 10.1080/13506129.2017.1304905 [DOI] [PubMed] [Google Scholar]
- 63. Deng H., Gao K., and Jankovic J. (2014) The role of FUS gene variants in neurodegenerative diseases. Nat. Rev. Neurol. 10, 337–348 10.1038/nrneurol.2014.78 [DOI] [PubMed] [Google Scholar]
- 64. Monahan Z., Ryan V. H., Janke A. M., Burke K. A., Rhoads S. N., Zerze G. H., O'Meally R., Dignon G. L., Conicella A. E., Zheng W., Best R. B., Cole R. N., Mittal J., Shewmaker F., and Fawzi N. L. (2017) Phosphorylation of the FUS low-complexity domain disrupts phase separation, aggregation, and toxicity. EMBO J. 36, 2951–2967 10.15252/embj.201696394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ling H., Kara E., Bandopadhyay R., Hardy J., Holton J., Xiromerisiou G., Lees A., Houlden H., and Revesz T. (2013) TDP-43 pathology in a patient carrying G2019S LRRK2 mutation and a novel p.Q124E MAPT. Neurobiol. Aging 34, 2889.e5–9 10.1016/j.neurobiolaging.2013.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Arnold E. S., Ling S. C., Huelga S. C., Lagier-Tourenne C., Polymenidou M., Ditsworth D., Kordasiewicz H. B., McAlonis-Downes M., Platoshyn O., Parone P. A., Da Cruz S., Clutario K. M., Swing D., Tessarollo L., Marsala M., et al. (2013) ALS-linked TDP-43 mutations produce aberrant RNA splicing and adult-onset motor neuron disease without aggregation or loss of nuclear TDP-43. Proc. Natl Acad. Sci. U.S.A. 110, E736–E745 10.1073/pnas.1222809110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kedersha N., Cho M. R., Li W., Yacono P. W., Chen S., Gilks N., Golan D. E., and Anderson P. (2000) Dynamic shuttling of TIA-1 accompanies the recruitment of mRNA to mammalian stress granules. J. Cell Biol. 151, 1257–1268 10.1083/jcb.151.6.1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Gilks N., Kedersha N., Ayodele M., Shen L., Stoecklin G., Dember L. M., and Anderson P. (2004) Stress granule assembly is mediated by prion-like aggregation of TIA-1. Mol. Biol. Cell 15, 5383–5398 10.1091/mbc.e04-08-0715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. DeJesus-Hernandez M., Mackenzie I. R., Boeve B. F., Boxer A. L., Baker M., Rutherford N. J., Nicholson A. M., Finch N. A., Flynn H., Adamson J., Kouri N., Wojtas A., Sengdy P., Hsiung G. Y. R., Karydas A., et al. (2011) Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron 72, 245–256 10.1016/j.neuron.2011.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Renton A. E., Majounie E., Waite A., Simón-Sánchez J., Rollinson S., Gibbs J. R., Schymick J. C., Laaksovirta H., van Swieten J. C., Myllykangas L., Kalimo H., Paetau A., Abramzon Y., Remes A. M., Kaganovich A., et al. (2011) A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron 72, 257–268 10.1016/j.neuron.2011.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zu T., Liu Y., Bañez-Coronel M., Reid T., Pletnikova O., Lewis J., Miller T. M., Harms M. B., Falchook A. E., Subramony S. H., Ostrow L. W., Rothstein J. D., Troncoso J. C., and Ranum L. P. W. (2013) RAN proteins and RNA foci from antisense transcripts in C9ORF72 ALS and frontotemporal dementia. Proc. Natl. Acad. Sci. U.S.A. 110, E4968–E4977 10.1073/pnas.1315438110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Boeynaems S., Bogaert E., Kovacs D., Konijnenberg A., Timmerman E., Volkov A., Guharoy M., De Decker M., Jaspers T., Ryan V. H., Janke A. M., Baatsen P., Vercruysse T., Kolaitis R. M., Daelemans D., Taylor J. P., Kedersha N., Anderson P., Impens F., Sobott F., Schymkowitz J., Rousseau F., Fawzi N. L., Robberecht W., Van Damme P., Tompa P., and Van Den Bosch L. (2017) Phase separation of C9orf72 dipeptide repeats perturbs stress granule dynamics. Mol. Cell 65, 1044–1055.e5 10.1016/j.molcel.2017.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Zhou L., McInnes J., Wierda K., Holt M., Herrmann A. G., Jackson R. J., Wang Y. C., Swerts J., Beyens J., Miskiewicz K., Vilain S., Dewachter I., Moechars D., De Strooper B., Spires-Jones T. L., et al. (2017) Tau association with synaptic vesicles causes presynaptic dysfunction. Nat. Commun. 8, 15295 10.1038/ncomms15295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hernández-Vega A., Braun M., Scharrel L., Jahnel M., Wegmann S., Hyman B. T., Alberti S., Diez S., and Hyman A. A. (2017) Local nucleation of microtubule bundles through tubulin concentration into a condensed tau phase. Cell Rep. 20, 2304–2312 10.1016/j.celrep.2017.08.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Arendt T., Stieler J. T., and Holzer M. (2016) Tau and tauopathies. Brain Res. Bull. 126, 238–292 10.1016/j.brainresbull.2016.08.018 [DOI] [PubMed] [Google Scholar]
- 76. Audas T. E., Audas D. E., Jacob M. D., Ho J. J. D., Khacho M., Wang M., Perera J. K., Gardiner C., Bennett C. A., Head T., Kryvenko O. N., Jorda M., Daunert S., Malhotra A., Trinkle-Mulcahy L., et al. (2016) Adaptation to stressors by systemic protein amyloidogenesis. Dev. Cell 39, 155–168 10.1016/j.devcel.2016.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Berchowitz L. E., Kabachinski G., Walker M. R., Carlile T. M., Gilbert W. V., Schwartz T. U., and Amon A. (2015) Regulated formation of an amyloid-like translational repressor governs gametogenesis. Cell 163, 406–418 10.1016/j.cell.2015.08.060 [DOI] [PMC free article] [PubMed] [Google Scholar]