Abstract
The type-I LacdiNAc (LDN; GalNAcβ1–3GlcNAc) has rarely been observed in mammalian cells except in the O-glycan of α-dystroglycan, in contrast to type-II LDN structures (GalNAcβ1–4GlcNAc) in N- and O-glycans that are present in many mammalian glycoproteins, such as pituitary and hypothalamic hormones. Although a β1,3-N-acetylgalactosaminyltransferase 2 (B3GALNT2; type-I LDN synthase) has been cloned, the function of type-I LDN in mammalian cells is still unclear, as its carrier protein(s) has not been identified. In this study, using HeLa cells, we demonstrate that inhibition of Golgi-resident glycosyltransferase increases the abundance of B3GALNT2-synthesized type-I LDN structures, recognized by Wisteria floribunda agglutinin (WFA). Using isotope-coded glycosylation site–specific tagging (IGOT)–LC/MS analysis of Lec8 Chinese hamster cells lacking galactosylation and of cells transfected with the B3GALNT2 gene, we identified the glycoproteins that carry B3GALNT2-generated type-I LDN in their N-glycans. Our results further revealed that LDN presence on low-density lipoprotein receptor-related protein 1 and nicastrin depends on B3GALNT2, indicating the occurrence of type-I LDN in vivo in mammalian cells. Our analysis also uncovered that most of the identified glycoproteins localize to intracellular organelles, particularly to the endoplasmic reticulum. Whereas B4GALNT3 and B4GALNT4 synthesized LDN on extracellular glycoproteins, B3GALNT2 primarily transferred LDN to intracellular glycoproteins, thereby clearly delineating proteins that carry type-I or type-II LDNs. Taken together, our results indicate the presence of mammalian glycoproteins carrying type-I LDN on N-glycans and suggest that type-I and type-II LDNs have different roles in vivo.
Keywords: N-linked glycosylation, glycosyltransferase, carbohydrate structure, glycoprotein, endoplasmic reticulum (ER), Golgi, B3GALNT2, LC/MS analysis, type-I LacdiNAc, WFA, Wisteria floribunda agglutinin (WFA), intracellular glycoproteins, endoplasmic reticulum, Golgi
Introduction
LacdiNAc (LDN)2 has been studied as a unique disaccharide unit, a competitor of LacNAc, and a modulator of hormones secreted from the hypothalamus (1). Sulfated LDN has been reported to play essential roles in the regulation of circulatory half-life of pituitary glycoprotein hormones (2, 3). Ohkura et al. (4) have demonstrated that most of the LDN-carrying glycoproteins are in the culture medium rather than on the cell membrane of Madin-Darby canine kidney cells and HEK293T cells, which contain high B4GALNT activity. Recently, Sugahara et al. (5) also identified many LDN-carrying glycoproteins in the cultured medium of HEK293T cells, indicating the general prevalence of LDN on glycoproteins in addition to secreted hormones. Because only LDN (GalNAcβ1–4GlcNAc) structures had been observed in mammalian cells until recently, the glycoproteins identified have been assumed to carry LDN (GalNAcβ1–4GlcNAc). Because the LDN structure found in O-mannose glycans on α-dystroglycan contains GalNAcβ1–3GlcNAc (6) hereafter we refer to it as type-I LDN to distinguish it from the previously identified structure (i.e. type-II LDN).
The type-I LDN, GalNAcβ1–3GlcNAc, on glycoproteins can be synthesized by β1,3-N-acetylgalactosaminyltransferase 2 (B3GALNT2), which has already been cloned and characterized. Hiruma et al. (7) demonstrated that the B3GALNT2 enzyme transfers a GalNAc residue to a hydroxyl group at position 3 of GlcNAc via a β1–3 linkage in vitro, although this type-I LDN was not found in mammalian cells until recently. Yoshida-Moriguchi et al. (8) demonstrated that phosphorylation occurs in type-I LDN glycan formed on O-mannose of α-dystroglycan. In addition, mutations in the B3GALNT2 gene cause deficiency in the synthesis of laminin-binding glycans, and individuals with such mutations develop α-dystroglycanopathy, suggesting that type-I LDN glycan synthesized by B3GALNT2 is a key structure in laminin-binding glycans (9). In contrast, type-II LDN, GalNAcβ1–4GlcNAc, on glycoproteins is synthesized by two enzymes, β1,4-N-acetylgalactosaminyl transferases 3 and 4 (B4GALNT3 and B4GALNT4) (10, 11). Until now, it has been unclear whether the type-I LDN structures have a function similar to that of type-II LDN structures.
To elucidate the function of type-I LDN glycans, it is necessary to identify molecules which carry these glycans. Hiruma et al. (7) have previously confirmed that B3GALNT2 can synthesize type-I LDN N-glycan in vitro, which points to the existence of type-I LDN N-glycans in vivo, although no glycoproteins with type-I LDN N-glycan (hereafter “carrier protein”) have been reported. Furthermore, it is likely that the type-I LDN structures on N-glycans are present in many cellular glycoproteins, because B3GALNT2 is ubiquitously expressed in many tissues (7). To date, no method for specific detection of type-I LDN has been established because of the lack of specific probes (such as lectin or antibodies) that can distinguish between type-I and type-II LDNs.
In this study, our first aim was to develop a method for identifying carrier proteins. We screened various lectins and selected Wisteria floribunda agglutinin (WFA) to detect type-I LDN. By means of Lec8 cells and B3GALNT2-transfected Lec8 cells, glycopeptides captured by a WFA column were identified by a highly sensitive LC/MS-based proteomic method. Overexpression and siRNA experiments showed the possibility of existence of glycoproteins with type-I LDN N-glycan in mammalian cells, which has never been reported. The candidate glycoproteins carrying type-I LDN N-glycans were mostly intracellular glycoproteins, and our results revealed a difference in the acceptor molecule preference between B3GALNT2 and B4GALNT3 or B4GALNT4, suggesting that type-I and type-II LDN glycans perform distinct functions.
Results
Detection of type-I LacdiNAc glycan synthesis patient
To detect LDN glycans, WFA was used as reported previously (12). Because it was not clear whether other lectins recognize type-I LDN better than WFA does, we initially employed lectin microarray analysis of whole-cell lysates prepared from HeLa cells and HeLa cells stably expressing B3GALNT2. In addition to WFA, Bauhinia purpurea lectin and Solanum tuberosum lectin recognized the glycoproteins from B3GALNT2-transfected cells, better than those from HeLa cells (data not shown), suggesting that these three lectins can bind to type-I LDN glycans. In agreement with these data, the cloned WFA was found to strongly bind to both type-I and type-II LDN probably because a terminal GalNAc is one of the recognition sites of WFA (12, 13).
To further investigate the carrier molecules of type-I LDN glycans, we detected glycoproteins by WFA blotting. As described above, WFA recognizes both type-I and type-II LDNs. To confirm the products of B3GALNT2 selectively, we tried to inhibit other glycosyltransferases such as B4GALNT3 and B4GALNT4. Given that B3GALNT2 mainly localizes in the endoplasmic reticulum (ER), and B4GALNT3 and B4GALNT4 and B4GALT1 localize in the Golgi apparatus, brefeldin A (BFA), a selective inhibitor of protein transport from the ER to Golgi apparatus (14), was used to assess the activity of B3GALNT2.
In the HeLa cells expressing B3GALNT2 (HeLa+B3GalNT2-GFP), amounts of WFA-binding glycoproteins increased in the presence of BFA (Fig. 1A, lanes 1 and 4), but the amounts of wheat germ agglutinin (WGA)–binding glycoproteins did not. Indeed, siRNA-B3GALNT2 attenuated the stimulatory effect of BFA treatment, indicating that the type-I LDN glycans were synthesized by B3GALNT2 localized in the ER (Fig. 1, A and B, lanes 3 and 6). Moreover, most of the WFA-binding glycans were located on N-glycans because they were removed by peptide N-glycanase F (PNGaseF) (Fig. 1C, lanes 6 and 9) but not by endoglycosidase H (EndoH) (Fig. 1C, lanes 5 and 8). These results suggested that B3GALNT2-dependent type-I LDNs on complex type N-glycans can be recognized by WFA and their synthesis is not suppressed by inhibiting ER to Golgi protein transport (BFA(+)).
Figure 1.
WFA recognizes LacdiNAc synthesized by B3GALNT2. A, lectin blotting (LB, upper panel) or immunoblotting (IB, lower panel) of whole-cell lysates (10 μg) from HeLa+B3GALNT2-GFP cells cultured with DMSO (BFA(−); lanes 1-3) or 15 μg/ml BFA (BFA(+); lanes 4-6) for 2 days. HeLa+B3GALNT2-GFP cells were treated with nontargeting siRNA (siCont; lanes 2 and 5) or B3GALNT2-targeting siRNA (siRNA; lanes 3 and 6) for 2 days before BFA treatment. WFA (left) or WGA (right) were used for LB. An anti-GFP antibody (left) to detect B3GALNT2-GFP or an anti-GAPDH antibody (right) were used for IB. B, WFA-captures (left) or WGA-captured glycoproteins (right) from whole-cell lysates used in A were detected by WFA (left) or WGA (right), respectively. C, WFA-blotting of WFA-captured glycoproteins treated with EndoH (lanes 2, 5, and 8) or PNGaseF (lanes 3, 6, and 9) from HeLa+GFP cells (lanes 1–3) or HeLa+B3GALNT2-GFP cells (lanes 4–9) cultured with DMSO (BFA(−), lanes 1–6) or BFA (BFA(+), lanes 7–9).
Capturing type-I LDN glycan-carrying proteins from Lec8 cells
To determine the function of type-I LDN, we aimed to identify the carrier glycoproteins of type-I LDN-bearing N-glycans. HeLa cells highly express B3GALNT2 but barely express B4GALNT3 and B4GALNT4.3 As shown above in Fig. 1A, WFA signals did not disappear from a HeLa cell lysate after treatment with siRNA-B3GALNT2. WFA preferentially binds to the terminal GalNAc of LDN; however, it also weakly binds to LacNAc (LN) (13). Thus, it is likely that WFA-binding glycoproteins in HeLa cells are both LDN- and LN-carrying glycoproteins. To avoid contamination with LN-carrying glycoproteins, we used Lec8 cells, which are derived from a Chinese hamster ovary (CHO) and lack the modification with Gal. Some studies have shown that Lec8 cells do not contain LDN glycans (15, 16). We confirmed that the RNA expression levels of B4GALNT3 and B4GALNT4 in our Lec8 cells were very low as compared with those of B3GALNT2 (B3GALNT2/GAPDH = 0.03, B4GALNT3/GAPDH = 0.0002, B4GALNT4/GAPDH = 0.001, data not shown).
We prepared cell lysates from Lec8 cells and the Lec8 cells stably expressing B3GALNT2 tagged with the Myc peptide. As expected, B3GALNT2 expression up-regulated WFA-binding glycoproteins (Fig. 2A, lanes 1–4), whereas the expression of WGA-binding glycoproteins did not change (Fig. 2A, lanes 5–8). In line with the WFA blotting data, the number and amounts of glycoproteins captured by WFA beads increased as evidenced by silver staining (Fig. 2B), suggesting that the capture of glycoproteins by WFA from Lec8 cells would be useful for identifying the glycoproteins carrying type-I LDN glycans.
Figure 2.
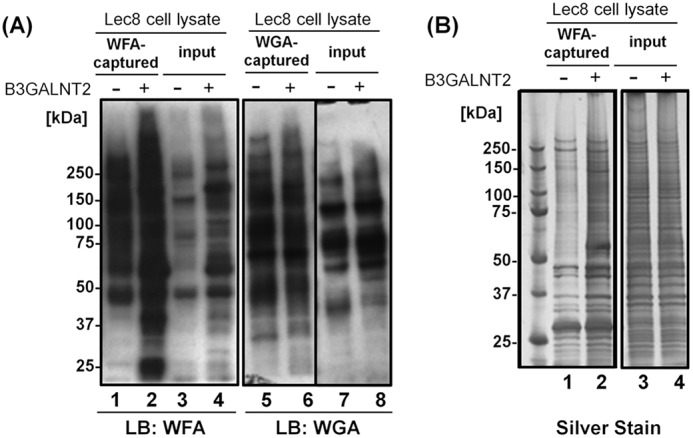
WFA(+) proteins in B3GALNT2-transfected cells. A, lectin blotting of WFA-captured (lanes 1 and 2) or WGA-captured (lanes 5 and 6) glycoproteins or whole-cell lysates (input; lanes 3, 4, 7, and 8) from Lec8 cells (lanes 1, 3, 5, and 7) or B3GALNT2-myc-His-transfected Lec8 cells (lanes 2, 4, 6, and 8). WFA (lanes 1–4) or WGA (lanes 5–8) were used as described in “Experimental procedures” (lectin blot). B, silver staining of WFA-captured glycoproteins (lanes 1 and 2) or whole-cell lysates (input; lanes 3 and 4) from Lec8 cells (lanes 1 and 3) or B3GALNT2-myc-His–transfected Lec8 cells (lanes 2 and 4).
Identification of type-I LDN carrier glycoproteins in Lec8 cells
To determine the glycoproteins carrying type-I LDN on N-glycans, oligopeptides captured by WFA beads were analyzed by an LC/MS-based method (IGOT) (17, 18) (see “Experimental procedures”). We prepared samples twice, and a list of glycopeptides identified in the two experiments was generated (Table S1). As summarized in Fig. 3A, 152 glycoproteins were identified in Lec8 cells, whereas 306 glycoproteins were identified in the B3GALNT2-transfected Lec8 cells. One hundred forty glycoproteins were common for the two lists, and 166 glycoproteins were identified only in the B3GALNT2-overexpressing cells, which potentially carry type-I and not type-II LDN.
Figure 3.
IGOT-LC/MS analysis of WFA capture proteins. A, comparison of WFA-binding glycoproteins identified by IGOT-LC/MS analysis between Lec8 cells (gray, Lec8, 152) and B3GALNT2-transfected Lec8 cells (black, +B3GALNT2, 306). A total of 140 glycoproteins were in the overlap between the two sets. (The results show combined data on proteins identified in the first and second experiments. Because proteins identified by the Mascot search included a few isoforms, deferent isoforms corresponding to the same gene name were assumed to be one protein). B and C, the identified proteins were classified based on the Cell Part members (B) or Organelle members (C) within the Cellular Component categorized by a bioinformatic protein classification system, PANTHER. The numbers of genes classified from WFA(+)-glycoproteins of Lec8 cells (light gray, WFA(+)_Lec8) or B3GALNT2-transfected Lec8 cells (black, WFA(+)_+B3GALNT2) are shown as solid bars. As controls, the bars from Amide-80-column(+)-proteins of Lec8 cells (white, Amide(+)_Lec8) or B3GALNT2-transfected Lec8 cells (dark gray, Amide(+)_+B3GalNT2) are also indicated.
Next, the characteristics of the candidate glycoproteins that carry type-I LDN in Lec8 cells were investigated by classifying them according to their cellular localization based on human Gene Ontology analysis (PANTHER v.13.1; Fig. 3, B and C). The glycoproteins that bound to the amide column (Amide(+)), which represent total glycoproteins, were mainly intracellular and plasma membrane glycoproteins. B3GALNT2 overexpression increased the amount of intracellular glycoproteins, with LDN N-glycan being more than 2-fold more abundant than that in Lec8 cells. When intercellular glycoproteins were next classified by organelles, they turned out to be mainly located in the ER or Golgi apparatus. B3GALNT2 remarkably increased the amount of the ER-associated glycoproteins that are LDN carriers (Fig. 3C), including the glycoproteins for glycosylphosphatidylinositol (GPI) anchor biogenesis and protein folding and/or refolding. All the collected data suggested that B3GALNT2 can synthesize LDN on numerous intracellular glycoproteins, which are primarily associated with the ER.
Synthesis of type-I LDN N-glycans in Lec8 cells
Because the above finding that B3GALNT2 synthesizes LDN on N-glycans (in addition to the known synthesis on O-mannose glycans) in cells was novel, we next verified whether B3GALNT2 actually synthesizes LDN on N-glycans. The N-glycans released from WFA-captured glycopeptides were analyzed by MALDI-TOF MS (Fig. 4). In Fig. 4, the obtained peak (m/z) is indicated by the number of hexose (Hex), N-acetylhexosamine (HexNAc), and deoxyhexose (dHex) units without the trimannosyl-chitobiose core (three Man and two GlcNAc residues) calculated from the mass. As presented in Fig. 4A, the peaks of N-glycans from Lec8 cells were obtained, and the intensities of major peaks were found to be similar. In contrast, B3GALNT2 expression increased the intensities of glycans (021), (031), and (041) compared with a peak of glycan (011) (Fig. 4B), suggesting that the composition of WFA-binding glycans was changed by B3GALNT2 overexpression. Because glycan (011) consisted of zero mannose units (Hex), one GlcNAc (HexNAc), and one fucose (dHex) unit on a trimannosyl-chitobiose core, this structure could be an acceptor for B3GALNT2. According to the analysis of glycopeptides in B3GALNT2-transfected cells, the frequency of glycan (021) with two HexNAc units was more than double that of glycan (011). Similarly, glycans (031) and (041) with multiple HexNAc residues increased in quantity, suggesting that the amount of the HexNAc-HexNAc structure was increased by B3GALNT2 expression (Fig. 4B). In fact, the peak of (031) was subjected to tandem MS (MS/MS) analysis, detecting the fragment ion with m/z 527.25 corresponding to the LDN fragment (data not shown). These results revealed that the WFA-captured glycopeptides contained type-I LDN glycans, which were synthesized and up-regulated upon B3GALNT2 overexpression, suggesting that the identified glycoproteins could be carrier molecules of type-I LDN in Lec8 cells.
Figure 4.
A comparison of N-glycan profiles by MALDI-TOF-MS analysis. A and B, MALDI-TOF-MS analysis of N-glycans from Lec8 cells (A, red, Lec8) and B3GALNT2-transfected Lec8 cells (B, green, +B3GALNT2). A peak height of (011)-N-glycan is indicated as a height reference (dotted square, dotted line). Note, increases of HexNAc-containing structures (021, 031, and 041) were remarkable in B3GALNT2-transfected Lec8 cells (red rectangles). The glycan composition of major peaks containing hexose (Hex), N-acetylhexosamines (HexNAc), and deoxyhexose (dHex) is indicated at the top of a peak (Hex, HexNAc, dHex). The numbers were obtained after subtraction of the trimannosyl-chitobiose core structure (Man3-GlcNAc2) of N-glycan. a.u.: arbitrary units.
Preferences of B3GALNT2 during the synthesis of type-I LDN in cells
In other studies, type-II LDN glycans have been detected in the secreted glycoproteins found in conditioned media and serum samples (5). Nonetheless, the glycoproteins identified here as type-I carriers were mainly intracellular glycoproteins (Table S1 and Fig. 4), suggesting that the type-I LDN-carrying glycoproteins differ from type-II LDN-carrying glycoproteins. To test this hypothesis, we compared the acceptor preferences between type-I LDN synthase (B3GALNT2) and type-II LDN synthase (B4GALNT3 and B4GALNT4) when they were transiently expressed in cells. B4GALNT3 and B4GALNT4 increased the amount of WFA-binding glycoproteins in the cultured medium, in contrast to B3GALNT2 (Fig. 5A); this result confirmed the previously published findings and the above data on the transient expression as well. Conversely, B3GALNT2 up-regulated WFA-binding glycoproteins in the cell lysate as compared with the control cells, and BFA treatment further increased the amount of WFA-binding intracellular glycoproteins (Fig. 5B), confirming that B3GALNT2 has a preference for intracellular glycoproteins over extracellular glycoproteins. Although B4GALNT3 and B4GALNT4 also up-regulated WFA-binding intracellular glycoproteins, most of them were assumed to be secreted proteins. This is because BFA treatment, which can inhibit protein transport and secretion pathways, decreased WFA-positive signals in intracellular glycoproteins (Fig. 5B, lanes 3 and 4 versus lanes 7 and 8).
Figure 5.
B3GALNT2 targets intercellular proteins but not secreted proteins. A and B, WFA-blotting of WFA-captured glycoproteins from the cultured medium (A, secreted) or whole-cell lysates (B, intracellular) of HeLa cells transfected with mock (lane 1), B3GALNT2 (lane 2), B4GALNT3 (lane 3), or B4GALNT4 (lane 4) plasmid DNA. HeLa transfectants were treated with DMSO (BFA(−), lanes 1–4) or 15 μg/ml BFA (BFA(+), lanes 5–8) for 2 days after transfection in (B). C, immunoblotting of WFA-captured glycoproteins (lanes 1–6) or whole-cell lysates (lanes 7–12) from untreated HeLa cells (lanes 1 and 7) or HeLa transfectants (lanes 2–6, 8-12). HYOU1, PIGS, ADGRG1, and NCSTN were detected. Amounts of WFA-captured HYOU1 and PIGS increased significantly when B3GALNT2s were transfected into the cells (lanes 2 and 4).
Next, we further examined the acceptor preference toward the candidate glycoproteins for type-I LDN glycans. The WFA-captured glycoproteins in the cell lysate and cultured medium were detected by Western blotting based on candidate glycoproteins. For this comparative analysis, we selected the glycoproteins that were frequently identified by the IGOT analysis of the glycopeptides captured by WFA from B3GALNT2-transfected cells. Hypoxia up-regulated protein 1 (HYOU1) and phosphatidylinositol glycan anchor biosynthesis class S (PIGS) are ER-resident glycoproteins associated with protein folding and/or refolding and GPI anchor biogenesis, respectively (19, 20). Adhesion G protein–coupled receptor G1 (ADGRG1) and nicastrin (NCSTN) were chosen as membrane glycoproteins and are a collagen III receptor and a component of γ-secretase, respectively (21, 22). In HeLa cells, all four glycoproteins in the WFA-captured fraction were up-regulated by B3GALNT2 transfection without changes in the total expression levels of the protein (Fig. 5C). In contrast, although B4GALNT3 and B4GALNT4 increased the amount of WFA-captured NCSTN, they did not increase the amounts of WFA-captured HYOU1, PIGS, and ADGRG1 (Fig. 5C). These results clearly indicated that the type-I LDN-carrying glycoproteins differ from type-II LDN-carrying glycoproteins because the acceptor specificity differed between type-I and type-II synthases.
Type-I LDN carrier glycoproteins present in cell lines
To obtain further evidence that the identified glycoproteins were modified by B3GALNT2 in Lec8 cells, we examined lectin-captured glycoproteins by Western blotting (Fig. 6). First, PGAP1 (GPI inositol deacylase) was chosen as an ER-associated glycoprotein (23), along with PIGS and HYOU1. As a plasma membrane-associated glycoprotein, LRP1 (low-density lipoprotein receptor-related protein 1) was also analyzed (24) because LRP1 is one of highly N-glycosylated glycoproteins. In Lec8 cells, all five proteins, PIGS, PGAP1, HYOU1, NCSTN, and LRP1, were up-regulated in the WFA-captured fractions of B3GALNT2 transfectants (Fig. 6, lanes 3 and 4) without an increase in the total expression of the target molecules (lanes 5 and 6), in contrast to the absence of differences (except for HYOU1) in the WGA-captured fractions (lanes 1 and 2). The reason why HYOU1 was enriched by the WGA capture is unclear. These results suggested that glycoproteins identified by IGOT-LC/MS contain type-I LDN synthesized by B3GALNT2.
Figure 6.

Up-regulation of LacdiNAc(+)-glycoproteins in B3GALNT2-transfected Lec8 cells. Immunoblotting of WGA-captured (lanes 1 and 2) or WFA-captured (lanes 3 and 4) glycoproteins or whole-cell lysates (lanes 5 and 6) from Lec8 cells (lanes 1, 3, and 5) or B3GALNT2-transfected Lec8 cells (lanes 2, 4, and 6). PIGS, PGAP1, HYOU1, NCSTN, and LRP1 were analyzed.
Synthesis of type-I LDN N-glycans on glycoproteins in cell lines
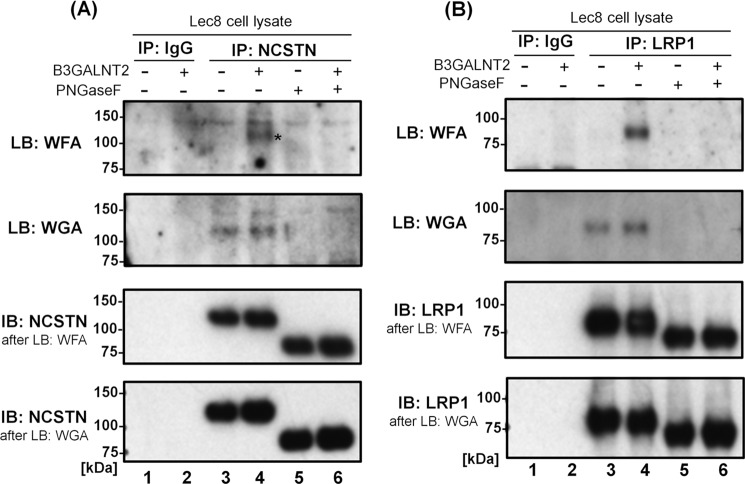
Next, to further assess the modification of type-I LDN N-glycans by B3GALNT2 on the identified glycoproteins, NCSTN (Fig. 7A) and LRP1 (Fig. 7B) were immunoprecipitated from Lec8 cells and from the B3GALNT2-transfected Lec8 cells, followed by lectin blotting with WFA or WGA. NCSTN and LRP1 were chosen as two highly N-glycosylated glycoproteins. WFA signals on the immunoprecipitated proteins NCSTN and LRP1 were substantial only in B3GALNT2-transfected Lec8 cells and were faint in Lec8 cells (Fig. 7, A and B, lanes 3 and 4). WGA signals were detected but were almost the same between the two cell lines. Additionally, the amount of immunoprecipitated glycoproteins was not affected by stable expression of B3GALNT2 (Fig. 7, A and B, lanes 3 and 4). Moreover, the WFA and WGA signals were eliminated by PNGaseF treatment (Fig. 7, A and B, lanes 5 and 6), indicating that the glycan synthesized by the expressed B3GALNT2 was present on N-glycans of these glycoproteins. These results confirmed that the identified glycoproteins carried type-I LDN glycans synthesized by B3GALNT2 on N-glycans in HeLa and Lec8 cells.
Figure 7.
Confirmation of LacdiNAc(+)-N-glycans on a candidate protein. A and B, immunoprecipitated NCSTN (A) or immunoprecipitated LRP1 (B) was examined by lectin blotting (LB) with WFA for LacdiNAc or WGA. Data from groups “control IgG” (lanes 1 and 2) and “protein-specific IgG” (lanes 3–6) were compared. N-glycosylation instances were removed by PNGaseF (lanes 5 and 6), and the amounts of target proteins were confirmed by immunoblotting (IB, lanes 3–6).
Intrinsic type-I LDN carrier glycoproteins in HeLa cells
The above results showed that B3GALNT2 was capable of synthesizing type-I LDN on N-glycan when the enzyme was overexpressed by transfection. Given that B3GALNT2 is highly expressed endogenously in many cell lines and tissues (7), it is likely that type-I LDN glycans are present in some cell lines at a low or normal expression level without transfection of B3GALNT2. Thus, we examined the glycans on endogenous NCSTN and LRP1 expressed by HeLa cells because these two proteins were found to contain WFA-binding glycans without the transfection. Transfection of siRNA-B3GALNT2 into HeLa cells dramatically down-regulated NCSTN and LRP1 in the WFA-captured fraction, without decreasing the expression of NCSTN and LRP1 in the whole-cell lysates (Fig. 8). This result proved that NCSTN and LRP1 are the glycoproteins containing the type-I LDN synthesized by endogenous B3GALNT2.
Figure 8.

The presence of type-I LacdiNAc synthesized by endogenous B3GALNT2. Immunoblotting of WFA-captured glycoproteins (lanes 1–3) or whole-cell lysates (lanes 4–6) from HeLa cells treated with nontargeting siRNA (siCont; lanes 1 and 4) or B3GALNT2-targeting siRNA (siRNA_#1, lanes 2 and 5; siRNA_#1–3, lanes 3 and 6) for 4 days. Amounts of NCSTN and LRP1 bearing WFA(+)-glycans decreased depending on siRNA treatment (lanes 2 and 3) without a decrease in the total amounts of NCSTN and LRP1 (lanes 5 and 6).
Discussion
Even after type-I LDN, i.e. GalNAcβ1–3GlcNAc, was found in O-mannose glycans (8), the presence and function of type-I LDNs in N-glycans have been unclear probably because specific probes and methods for detection of type-I LDN have not been available. To identify the carrier molecules and to examine the ability of B3GALNT2 to synthesize type-I LDN on N-glycans, without the use of specific probes, we chose a strategy based on Lec8 cells lacking galactose addition during glycan synthesis and stably expressing B3GALNT2, followed by WFA capture of LDN+glycopeptides and LC/MS analysis. Our results revealed that LDN was attached to more than 100 glycoproteins after B3GALNT2 overexpression, in addition to 100 glycoproteins in Lec8 cells that endogenously express B3GALNT2 (Fig. 3). Because Lec8 cells barely express B4GALNT3 and B4GALNT4 at the mRNA level, we assumed that the LDN structure found in Lec8 cells before transfection may be type-I LDN. Collectively, our results point to the general presence of type-I LDN unless competition with LacNAc synthesis takes place. Of note, we confirmed that the WFA-binding glycoproteins identified by means of stable expression of B3GALNT2 were intracellular, especially the ER-resident glycoproteins, in agreement with the finding that B3GALNT2 mainly localizes in the ER and partly in the Golgi apparatus (9). Indeed, when the cells were treated with BFA (an inhibitor of protein transport from the ER to Golgi apparatus), the amount of LDN structures in N-glycans increased in intracellular glycoproteins (Figs. 1 and 5). Because these type-I LDNs were located on complex-type N-glycans, which are insensitive to EndoH, it is likely that GalNAc was attached to GlcNAc at the terminal end of N-glycans, and the modified glycoproteins were delivered to intracellular organelles rather than secreted from the cell. Nevertheless, it should be noted that artificial overexpression of B3GALNT2 is different from endogenous conditions. The genetic manipulations of B3GALNT2 may cause aberrant modification of substrates.
Some studies identified secreted glycoproteins as the carrier molecules of type-II LDN (4, 5), in accordance with our finding that B4GALNT3 and B4GALNT4 synthesized LDN on extracellular glycoproteins, but B3GALNT2 did not (Fig. 5). These results promptly raised the question about the differences between type-I and type-II LDNs from the standpoint of the enzymes responsible for their synthesis. First, the distinctive localization of B3GALNT2 and B4GALNT3 and B4GALNT4 should be one of the reasons for their different preferences for acceptor molecules. B4GALNT3 and B4GALNT4 localize in Golgi (25) and synthesize type-II LDN on the membrane, and the affected glycoproteins are secreted (26–30); thus, sulfated type-II LDN can be involved in the clearance of LDN-carrying molecules (2, 3). Although the role of type-I LDN found on intracellular glycoproteins has not yet been elucidated, B3GALNT2 is mainly present in the ER and has a chance to interact with the glycoproteins at the early stage of a protein synthesis pathway. According to our results, HYOU1 and PIGS, which are ER-resident glycoproteins, were affected only by B3GALNT2 (not by B4GALNT3 and B4GALNT4) (Fig. 5). Furthermore, many of the LDN+glycoproteins were captured by WFA without desialylation. In general, one of the major terminal glycosylation types in N-glycans is sialylation, which takes place in trans-Golgi and often inhibits lectin binding. Thus, the binding of WFA to glycoproteins modified by B3GALNT2 indicates that the glycoproteins with type-I LDN on N-glycans may exit Golgi before sialylation. Therefore, it would be interesting to determine whether the type-I LDN synthesis on N-glycans is involved in the selection of pathways of protein delivery to intracellular organelles.
Second, although B3GALNT2 is expressed strongly in many cell types and tissues as compared with glycosyltransferases (according to Ref. 7), the expression of B4GALNT3 and B4GALNT4 is relatively limited in some tissues. For instance, B4GALNT3 expression is weak in the stomach, colon, and testes, and B4GALNT4 expression is weak in the brain, colon, and ovaries (10, 11). In our experiments, we chose HeLa and Lec8 cells, which do not express B4GALNT3 and B4GALNT4 strongly (10, 11 and this study), to reduce the contamination with type-II LDN in the process of identifying type-I LDN carrier molecules. Expression differences between the type-I LDN synthase and type-II LDN synthase are also important. Our results indicate that the secreted glycoproteins carrying type-I LDN are minor products as compared with the intracellular glycoproteins, but some glycoproteins, such as NCSTN, could be modified via attachment of both type-I and type-II LDNs (Fig. 5), indicating the presence of membrane glycoproteins or secreted glycoproteins (with type-I LDN in the glycoproteins identified here) that are type-II LDN carrier glycoproteins. Indeed, comparative analysis of Sugahara et al. data (5) and the current data showed that the identified N-glycosylation sites are different in the pool of the same glycoprotein as presented in Table S2 (e.g. PLTP, CLU, PSAP, ICAM5, and LGALS3BP), suggesting that acceptor specificity differs between B3GALNT2 and B4GALNT3 or B4GALNT4. Further experiments will be needed to characterize the acceptor specificity in detail.
In this work, we demonstrated that endogenous type-I LDN-carrying molecules can be present in cultured mammalian cells, but it is necessary to confirm the presence of type-I LDN N-glycan in vivo. As described above, we need to develop probes or methods to distinguish between type-I and type-II LDNs in the future. For instance, because WFA was recently cloned and its structure was reported (12, 13), introducing mutations into WFA may alter structure of the domain binding to the carbohydrate and generate new lectins specific to type-I LDNs. This strategy has been applied to the earthworm 29 lectin, Agrocybe cylindracea galectin, and peanut agglutinin and successfully yielded new lectins that have glycan specificity different from that of the original lectins, as described elsewhere (31, 32). Alternatively, simultaneous determination of both peptide sequences and glycan structures including linkage information is required. Nonetheless, the current technology involving MS/MS can distinguish disaccharides of type-I and type-II LDNs by linkage-specific fragmentation (33) but not for the N-glycan with type-I and type-II LDNs yet.
The finding of mutations in the B3GALNT2 gene of patients with congenital muscular disorders (9) has shed light on the function of B3GALNT2. The structure of laminin-binding glycans on O-mannose and more than 10 enzymes, including B3GALNT2, that are involved in α-dystroglycanopathy have been documented (34). The type-I LDN attached to O-mannose is required for phosphorylation by POMK (8) and is further modified via attachment of ribitol by FKTN (35), indicating that B3GALNT2 is one of the key enzymes synthesizing laminin-binding glycans. Our results uncovered a new role of B3GALNT2 in the type-I LDN synthesis on N-glycans. Of note, the glycoproteins modified with type-I LDN turned out to be mainly present as intracellular glycoproteins, and our data suggest that the number of such glycoproteins will increase when a sensitive and comprehensive analysis involving probes specific to type-I LDN is conducted. If our hypothesis is correct, the effect of the mutations in B3GALNT2 in the affected patients may be associated with N-glycosylated glycoproteins but not limited to laminin-binding glycans on α-dystroglycan.
Because α-dystroglycanopathy is classified by the common defect that eliminates or down-regulates the glycans binding to laminin, mutations in B3GALNT2 actually are the cause of α-dystroglycanopathy as reported elsewhere (9). Moreover, phenotypes of α-dystroglycanopathy differ gene by gene, and patients with B3GALNT2 mutations have severe symptoms (36, 37). Considering our finding that B3GALNT2 can modify intracellular glycoproteins, especially in the ER (in addition to O-mannose on α-dystroglycan), the defects of B3GALNT2 may affect glycoproteins regulating a function inside the cell. It would be especially interesting to investigate the molecular defects of the glycoproteins carrying the type-I LDN in cultured cells and in patients.
Thus, we identified candidate molecules carrying type-I LDN on N-glycans, which was synthesized by B3GALNT2. Our results revealed that type-I LDN carrier proteins are mainly intracellular glycoproteins, indicating a difference in acceptor preferences between B3GALNT2 and B4GALNT3 or B4GALNT4. Consequently, we propose that type-I and type-II LDNs (which are present on different glycoproteins) have distinct functions.
Experimental procedures
Reagents
Complementary DNAs encoding B3GALNT2, B4GALNT3, and B4GALNT4 were subcloned into a mammalian expression vector containing the Kozak sequence and the FLAG tag at the carboxyl terminus of the glycosyltransferases3 or the pcDNA3.1/myc-His vector (Thermo Fisher Scientific). B3GALNT2 was fused with GFP at the carboxyl terminus and was designated as B3GALNT2-GFP, as described previously (38). Chemical reagents such as a protease inhibitor mixture, BFA, lectins, GalNAc, PNGaseF, and EndoH were purchased from Merck, FUJIFILM Wako, Vector, Takara Bio, or New England Biolabs unless stated otherwise. An anti-PIGS antibody and anti-PGAP1 antibody were purchased from Proteintech; an anti-HYOU1 antibody and anti-LRP1 antibody from GeneTex, and an anti-NCSTN antibody from Santa Cruz Biotechnology. An HRP-conjugated anti-rabbit IgG antibody and an HRP-conjugated anti-goat IgG antibody were acquired from Dako and employed as secondary antibodies.
Cell culture
HeLa cells and Lec8 cells were obtained from the American Type Culture Collection (ATCC) or Japanese Collection of Research Bioresources (JCRB) Cell Bank. HeLa cells were grown in RPMI 1640 supplemented with penicillin, streptomycin, and 10% fetal bovine serum at 37 °C and 5% CO2. Lec8 cells were grown in DME/high glucose supplemented with glutamine, penicillin, streptomycin, and 10% fetal bovine serum. Stable transfectants expressing GFP, B3GALNT2-GFP, or B3GALNT2-myc-His were selected by cultivation with neomycin (500 μg/ml) after transfection with the Lipofectamine® LTX Reagent with PlusTM Reagent (Thermo Fisher Scientific). Control siRNA or human B3GALNT2-targeting siRNAs was transfected into HeLa cells by means of Lipofectamine RNAiMAX (Thermo Fisher Scientific). To inhibit protein transport from the ER to Golgi apparatus, 15 μg/ml BFA was added to the cell culture medium for 2 days of incubation, and the cells and/or conditioned media were collected for lectin capture.
Preparation of whole-cell extracts and secreted proteins for immunoprecipitation
Transient transfection of cDNAs into HeLa or Lec8 cells was performed using the Lipofectamine® LTX Reagent with PlusTM Reagent (Thermo Fisher Scientific). Whole-cell extracts were prepared by lysing 107 cells in 300 μl of lysis buffer (1% of Nonidet P-40, a protease inhibitor mixture, 1 mm phenylmethylsulfonyl fluoride, 2 mm EDTA, 10% glycerol, 137 mm NaCl, and 20 mm Tris-HCl, pH 8.0). The lysates were centrifuged at 19,280 × g and 4 °C for 30 min, and the supernatant was subjected to the following experiments. The secreted proteins were obtained from the cultured media 2 days after replacement of the regular medium with a serum-free medium (RPMI 1640, Thermo Fisher Scientific).
For immunoprecipitation, whole-cell lysates were mixed with a primary antibody against NCSTN or LRP1 and incubated for 30 min at 4 °C, followed by collection of antibody-bound proteins on ProG-agarose beads (GE Healthcare) for NCSTN or ProA-agarose beads (GE Healthcare) for LRP1. After 30 min mixing at 4 °C, the beads were precipitated, washed with PBS, and resuspended in sample buffer containing SDS and 2-mercaptoethanol.
Similarly, the glycoproteins were precipitated with lectin beads. The samples were mixed with WFA-agarose beads or WGA-agarose beads for 30 min at 4 °C. N-glycans on the glycoproteins captured by lectin beads and antibody beads were removed by incubation of the beads with 1.0 milliunits of PNGaseF or EndoH overnight at 37 °C.
Western blot and lectin blot analyses and silver staining
The proteins were separated by SDS-PAGE and transferred onto a polyvinylidene difluoride membrane (Immobilon-P, EMD Millipore) for Western blotting or lectin blotting. Alternatively, the protein samples were subjected to silver staining (EzStain Silver, ATTO). For Western blotting, the membrane was blocked with skim milk and incubated with a diluted primary antibody for 30 min at room temperature, followed by an HRP-conjugated secondary antibody for 30 min at room temperature. For lectin blotting, the membrane was blocked with 3% BSA and incubated with diluted biotin-conjugated WFA (1:50,000). After three washes with TBS containing 0.05% Tween 20 (TBST), the membrane was incubated with diluted HRP-conjugated streptavidin (1:50,000, GE Healthcare) for 30 min at room temperature. After extensive washes with TBST three times, the HRP reaction was visualized by means of an enhanced chemiluminescence system (ECL, Perkin Elmer).
WFA HPLC on a WFA affinity column for purification of glycopeptides from whole–Lec8 cell extracts
A whole–Lec8 cell lysate was solubilized in 6 ml of 0.5 m Tris-HCl, pH 8.5, containing 7 m guanidine-HCl and 10 mm EDTA. The disulfide bonds of proteins were reduced by the addition of DTT, and the resulting cysteine residues were alkylated with iodoacetamide as described previously (5). An aliquot of the protein samples was digested with lysyl endopeptidase (FUJIFILM Wako) and N-tosyl phenylalanyl chloromethyl ketone-treated trypsin (Thermo Fisher Scientific) as described elsewhere (5).
The protease-digested peptide mixture was dissolved in 75% acetonitrile in H2O containing 0.1% TFA, and then purified by hydrophilic interaction LC on a TSK gel Amide-80 column (4.6 mm internal diameter (i.d.) × 50 mm, Tosoh) as described previously (5). Next, desialylation of the eluted glycopeptides was carried out by heat treatment under acidic conditions at 80 °C for 1 h. The desialylated glycopeptides were dried in a centrifugal concentrator in vacuum and dissolved in 10 mm Tris-HCl, pH 7.5.
WFA affinity HPLC was performed as described in Ref. 5. WFA-agarose (Vector Laboratories) was packed into a TriconTM 5/20 Column (5.0 mm i.d. × 20 mm; GE Healthcare) in 10 mm Tris-HCl, pH 7.5. The desialylated glycopeptides were loaded onto the WFA affinity column. After a wash, bound glycopeptides were eluted with a buffer (10 mm Tris-HCl, pH 7.5) containing 10 mm GalNAc.
The WFA-bound glycopeptides were next desalted by reverse-phase chromatography on a Mightysil RP-18GP column (2.0 mm i.d. × 50 mm; 3 μm particles; Kanto Chemical), with monitoring of the absorbance of the eluted fractions at 215 nm via a Shimadzu UV detector, SPD-20.
Nano-LC/MS/MS analysis of 18O-labeled glycopeptides
N-glycosylated peptides were labeled specifically with 18O via the IGOT reaction as described previously (17, 18). The mixture of deglycosylated 18O-labeled peptides was analyzed by an automated nanoflow HPLC system coupled online to an LTQ Orbitrap Velos mass spectrometer (Thermo Fisher Scientific) via a nano electrospray ion source as described elsewhere (5, 18). The peptides were separated sequentially on a reverse-phase C18 tip column (150 μm i.d. × 70 mm; Nikkyo Technos, Japan) via a 5–35% linear gradient of acetonitrile in 0.1% (v/v) formic acid at a flow rate of 300 nl/min.
WFA-bound or Amide-80–bound glycopeptide fractions were analyzed to compare their peptide composition. Raw data files were converted to Mascot Generic Format (MGF) files in the Proteome Discoverer software (ver. 2.2, Thermo Fisher Scientific) and then processed by the Mascot algorithm (ver. 2.5, Matrix Science) to assign the data to peptides from the National Center for Biotechnology Information (NCBI) RefSeq Chinese Hamster (Cricetulus griseus) protein sequence database (67,449 entries as of July 4, 2018). The database search was performed with the parameters described previously (5). All results of the peptide search were exported to a CSV (comma separated values) file and processed in Microsoft Excel as documented in Ref. 5.
N-glycans were released and derivatized according to the method described previously (5, 39), with slight modifications. Briefly, the released glycans were premethylated with methyl iodide, and then were spotted along with a dried matrix (10 mg/ml of 2,5-dihydroxybenzoic acid (proteomics grade, FUJIFILM Wako) in 30% ethanol, 0.5 μl) onto a stainless steel sample plate for MALDI-MS (MTP 384 target plate ground steel BC; Bruker Daltonics). The mass spectra were acquired by MALDI-MS (Ultraflex TOF-TOF; Bruker Daltonics, Bremen, Germany) in reflectron positive-ion mode. The spectra were analyzed in the flexAnalysis software (ver. 2.0, Bruker Daltonics). MS/MS spectra were obtained by AXIMA QIT-TOF MS (Shimadzu) as described elsewhere (5).
Bioinformatic analysis of the identified proteins
The proteins were classified based on their molecular functions and biological processes according to Gene Ontology information (40) using protein annotation via an evolutionary relationship classification system, PANTHER (http://www.pantherdb.org).4 Because PANTHER does not deal with CHO genes, we converted protein gene ID (GI) accession numbers (from the identified CHO proteins) to official gene symbols on the website of DAVID Bioinformatics Resources v.6.8 (https://david.ncifcrf.gov/conversion.jsp) (41).
Author contributions
T. N., K. A., and H. K. data curation; T. N., K. A., and H. K. formal analysis; T. N. and K. A. investigation; T. N. and K. A. writing-original draft; K. A., T. S., and H. N. conceptualization; K. A. and H. N. writing-review and editing; T. S. resources; T. S. and H. K. methodology; H. N. supervision; H. N. funding acquisition.
Supplementary Material
Acknowledgments
We thank Dr. Akira Togayachi, Dr. Hiroaki Tateno, and Dr. Jun Iwaki (AIST) for helpful discussion and Mika Fujita, Azusa Tomioka, Masako Sukegawa, and Shigeko Tsujikawa (AIST) for technical assistance.
This work was supported by the Japan Society for the Promotion of Science, grants-in-aid for Scientific Research, KAKENHI Grant numbers JP13313213 and JP15641201. The authors declare that they have no conflicts of interest with the contents of this article.
This article contains Tables S1 and S2.
K. Angata, T. Nakane, T. Sato, S. Furukawa, S. Tsujikawa, H. Sawaki, M. Sogabe, T. Kubota, and H. Narimatsu, manuscript in preparation.
Please note that the JBC is not responsible for the long-term archiving and maintenance of this site or any other third party hosted site.
- LDN
- LacdiNAc structure
- ADGRG1
- adhesion G protein–coupled receptor G1
- B3GALNT2
- β1,3-N-acetylgalactosaminyltransferase 2
- B4GALNT3/4
- β1,4-N-acetylgalactosaminyl transferase 3/4
- BFA
- brefeldin A
- CHO
- Chinese hamster ovary
- EndoH
- endoglycosidase H
- ER
- endoplasmic reticulum
- GPI
- glycosylphosphatidylinositol
- Hex
- hexose
- HYOU1
- hypoxia up-regulated protein 1
- i.d.
- internal diameter
- IGOT
- isotope-coded glycosylation site–specific tagging
- LN
- LacNAc structure
- LRP1
- low-density lipoprotein receptor–related protein 1
- MS/MS
- tandem mass spectrometry
- NCSTN
- nicastrin
- PNGaseF
- peptide N-glycanase F
- PGAP1
- GPI inositol deacylase
- PIGS
- phosphatidylinositol glycan anchor biosynthesis class S
- WFA
- Wisteria floribunda agglutinin
- WGA
- wheat germ agglutinin.
References
- 1. Manzella S. M., Hooper L. V., and Baenziger J. U. (1996) Oligosaccharides containing β1,4-linked N-acetylgalactosamine, a paradigm for protein-specific glycosylation. J. Biol. Chem. 271, 12117–12120 10.1074/jbc.271.21.12117 [DOI] [PubMed] [Google Scholar]
- 2. Green E. D., van Halbeek H., Boime I., and Baenziger J. U. (1985) Structural elucidation of the disulfated oligosaccharide from bovine lutropin. J. Biol. Chem. 260, 15623–15630 [PubMed] [Google Scholar]
- 3. Fiete D., Srivastava V., Hindsgaul O., and Baenziger J. U. (1991) A hepatic reticuloendothelial cell receptor specific for SO4-4GalNAc β1,4GlcNAc β1,2Man α that mediates rapid clearance of lutropin. Cell 67, 1103–1110 10.1016/0092-8674(91)90287-9 [DOI] [PubMed] [Google Scholar]
- 4. Ohkura T., Seko A., Hara-Kuge S., and Yamashita K. (2002) Occurrence of secretory glycoprotein-specific GalNAc β1→4GlcNAc sequence in N-glycans in MDCK cells. J Biochem. 132, 891–901 10.1093/oxfordjournals.jbchem.a003302 [DOI] [PubMed] [Google Scholar]
- 5. Sugahara D., Tomioka A., Sato T., Narimatsu H., and Kaji H. (2015) Large-scale identification of secretome glycoproteins recognized by Wisteria floribunda agglutinin: A glycoproteomic approach to biomarker discovery. Proteomics 15, 2921–2933 10.1002/pmic.201400443 [DOI] [PubMed] [Google Scholar]
- 6. Yoshida-Moriguchi T., Yu L., Stalnaker S. H., Davis S., Kunz S., Madson M., Oldstone M. B., Schachter H., Wells L., and Campbell K. P. (2010) O-mannosyl phosphorylation of α-dystroglycan is required for laminin binding. Science 327, 88–92 10.1126/science.1180512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hiruma T., Togayachi A., Okamura K., Sato T., Kikuchi N., Kwon Y. D., Nakamura A., Fujimura K., Gotoh M., Tachibana K., Ishizuka Y., Noce T., Nakanishi H., and Narimatsu H. (2004) A novel human β1,3-N-acetylgalactosaminyltransferase that synthesizes a unique carbohydrate structure, GalNAc β1–3GlcNAc. J. Biol. Chem. 279, 14087–14095 10.1074/jbc.M310614200 [DOI] [PubMed] [Google Scholar]
- 8. Yoshida-Moriguchi T., Willer T., Anderson M. E., Venzke D., Whyte T., Muntoni F., Lee H., Nelson S. F., Yu L., and Campbell K. P. (2013) SGK196 is a glycosylation-specific O-mannose kinase required for dystroglycan function. Science 341, 896–899 10.1126/science.1239951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stevens E., Carss K. J., Cirak S., Foley A. R., Torelli S., Willer T., Tambunan D. E., Yau S., Brodd L., Sewry C. A., Feng L., Haliloglu G., Orhan D., Dobyns W. B., Enns G. M., et al. (2013) Mutations in B3GALNT2 cause congenital muscular dystrophy and hypoglycosylation of α-dystroglycan. Am. J. Hum. Genet. 92, 354–365 10.1016/j.ajhg.2013.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sato T., Gotoh M., Kiyohara K., Kameyama A., Kubota T., Kikuchi N., Ishizuka Y., Iwasaki H., Togayachi A., Kudo T., Ohkura T., Nakanishi H., and Narimatsu H. (2003) Molecular cloning and characterization of a novel human β1,4-N-acetylgalactosaminyltransferase, β4GalNAc-T3, responsible for the synthesis of N,N′-diacetyllactosediamine, GalNAc β1–4GlcNAc. J. Biol. Chem. 278, 47534–47544 10.1074/jbc.M308857200 [DOI] [PubMed] [Google Scholar]
- 11. Gotoh M., Sato T., Kiyohara K., Kameyama A., Kikuchi N., Kwon Y. D., Ishizuka Y., Iwai T., Nakanishi H., and Narimatsu H. (2004) Molecular cloning and characterization of β1,4-N-acetylgalactosaminyltransferases IV synthesizing N,N′-diacetyllactosediamine. FEBS Lett. 562, 134–140 10.1016/S0014-5793(04)00219-4 [DOI] [PubMed] [Google Scholar]
- 12. Haji-Ghassemi O., Gilbert M., Spence J., Schur M. J., Parker M. J., Jenkins M. L., Burke J. E., van Faassen H., Young N. M., and Evans S. V. (2016) Molecular basis for recognition of the cancer glycobiomarker, LacdiNAc (GalNAc[β1→4]GlcNAc), by Wisteria floribunda agglutinin. J. Biol. Chem. 291, 24085–24095 10.1074/jbc.M116.750463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sato T., Tateno H., Kaji H., Chiba Y., Kubota T., Hirabayashi J., and Narimatsu H. (2017) Engineering of recombinant Wisteria floribunda agglutinin specifically binding to GalNAcβ1,4GlcNAc (LacdiNAc). Glycobiology 27, 743–754 10.1093/glycob/cwx038 [DOI] [PubMed] [Google Scholar]
- 14. Sampath D., Varki A., and Freeze H. H. (1992) The spectrum of incomplete N-linked oligosaccharides synthesized by endothelial cells in the presence of brefeldin A. J. Biol. Chem. 267, 4440–4455 [PubMed] [Google Scholar]
- 15. Do K. Y., Do S. I., and Cummings R. D. (1997) Differential expression of LacdiNAc sequences (GalNAc β1–4GlcNAc-R) in glycoproteins synthesized by Chinese hamster ovary and human 293 cells. Glycobiology 7, 183–194 10.1093/glycob/7.2.183 [DOI] [PubMed] [Google Scholar]
- 16. Kawar Z. S., Haslam S. M., Morris H. R., Dell A., and Cummings R. D. (2005) Novel poly-GalNAc β1–4GlcNAc (LacdiNAc) and fucosylated poly-LacdiNAc N-glycans from mammalian cells expressing β1,4-N-acetylgalactosaminyltransferase and α1,3-fucosyltransferase. J. Biol. Chem. 280, 12810–12819 10.1074/jbc.M414273200 [DOI] [PubMed] [Google Scholar]
- 17. Kaji H., Saito H., Yamauchi Y., Shinkawa T., Taoka M., Hirabayashi J., Kasai K., Takahashi N., and Isobe T. (2003) Lectin affinity capture, isotope-coded tagging and mass spectrometry to identify N-linked glycoproteins. Nat. Biotechnol. 21, 667–672 10.1038/nbt829 [DOI] [PubMed] [Google Scholar]
- 18. Sugahara D., Kaji H., Sugihara K., Asano M., and Narimatsu H. (2012) Large-scale identification of target proteins of a glycosyltransferase isozyme by Lectin-IGOT-LC/MS, an LC/MS-based glycoproteomic approach. Sci. Rep. 2, 680 10.1038/srep00680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Easton D. P., Kaneko Y., and Subjeck J. R. (2000) The hsp110 and Grp1 70 stress proteins: Newly recognized relatives of the Hsp70s. Cell Stress Chaperones 5, 276–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ohishi K., Inoue N., and Kinoshita T. (2001) PIG-S and PIG-T, essential for GPI anchor attachment to proteins, form a complex with GAA1 and GPI8. EMBO J. 20, 4088–4098 10.1093/emboj/20.15.4088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Luo R., Jeong S. J., Jin Z., Strokes N., Li S., and Piao X. (2011) G protein-coupled receptor 56 and collagen III, a receptor-ligand pair, regulates cortical development and lamination. Proc. Natl. Acad. Sci. U.S.A. 108, 12925–12930 10.1073/pnas.1104821108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yu G., Nishimura M., Arawaka S., Levitan D., Zhang L., Tandon A., Song Y. Q., Rogaeva E., Chen F., Kawarai T., Supala A., Levesque L., Yu H., Yang D. S., Holmes E., et al. (2000) Nicastrin modulates presenilin-mediated notch/glp-1 signal transduction and βAPP processing. Nature 407, 48–54 10.1038/35024009 [DOI] [PubMed] [Google Scholar]
- 23. Tanaka S., Maeda Y., Tashima Y., and Kinoshita T. (2004) Inositol deacylation of glycosylphosphatidylinositol-anchored proteins is mediated by mammalian PGAP1 and yeast Bst1p. J. Biol. Chem. 279, 14256–14263 10.1074/jbc.M313755200 [DOI] [PubMed] [Google Scholar]
- 24. Van Leuven F., Stas L., Hilliker C., Lorent K., Umans L., Serneels L., Overbergh L., Torrekens S., Moechars D., De Strooper B., and Van den Berghe H. (1994) Structure of the gene (LRP1) coding for the human α2-macroglobulin receptor lipoprotein receptor-related protein. Genomics 24, 78–89 10.1006/geno.1994.1584 [DOI] [PubMed] [Google Scholar]
- 25. Ikehara Y., Sato T., Niwa T., Nakamura S., Gotoh M., Ikehara S. K., Kiyohara K., Aoki C., Iwai T., Nakanishi H., Hirabayashi J., Tatematsu M., and Narimatsu H. (2006) Apical Golgi localization of N,N′-diacetyllactosediamine synthase, β4GalNAc-T3, is responsible for LacdiNAc expression on gastric mucosa. Glycobiology 16, 777–785 10.1093/glycob/cwl005 [DOI] [PubMed] [Google Scholar]
- 26. Dell A., Morris H. R., Easton R. L., Panico M., Patankar M., Oehniger S., Koistinen R., Koistinen H., Seppala M., and Clark G. F. (1995) Structural analysis of the oligosaccharides derived from glycodelin, a human glycoprotein with potent immunosuppressive and contraceptive activities. J. Biol. Chem. 270, 24116–24126 10.1074/jbc.270.41.24116 [DOI] [PubMed] [Google Scholar]
- 27. Smith P. L., and Baenziger J. U. (1988) A pituitary N-acetylgalactosamine transferase that specifically recognizes glycoprotein hormones. Science 242, 930–933 10.1126/science.2460923 [DOI] [PubMed] [Google Scholar]
- 28. Bergwerff A. A., Van Oostrum J., Kamerling J. P., and Vliegenthart J. F. (1995) The major N-linked carbohydrate chains from human urokinase. The occurrence of 4-O-sulfated, (α2–6)-sialylated or (α1–3)-fucosylated N-acetylgalactosamine(β1–4)-N-acetylglucosamine elements. Eur. J. Biochem. 228, 1009–1019 10.1111/j.1432-1033.1995.tb20351.x [DOI] [PubMed] [Google Scholar]
- 29. Sasaki N., Shinomi M., Hirano K., Ui-Tei K., and Nishihara S. (2011) LacdiNAc (GalNAc β1–4GlcNAc) contributes to self-renewal of mouse embryonic stem cells by regulating leukemia inhibitory factor/STAT3 signaling. Stem Cells 29, 641–650 10.1002/stem.615 [DOI] [PubMed] [Google Scholar]
- 30. Che M. I., Huang J., Hung J. S., Lin Y. C., Huang M. J., Lai H. S., Hsu W. M., Liang J. T., and Huang M. C. (2014) β1, 4-N-acetylgalactosaminyltransferase III modulates cancer stemness through EGFR signaling pathway in colon cancer cells. Oncotarget. 5, 3673–3684 10.18632/oncotarget.1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hu D., Tateno H., and Hirabayashi J. (2015) Lectin engineering, a molecular evolutionary approach to expanding the lectin utilities. Molecules 20, 7637–7656 10.3390/molecules20057637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Abo H., Soga K., Tanaka A., Tateno H., Hirabayashi J., and Yamamoto K. (2015) Mutated leguminous lectin containing a heparin-binding like motif in a carbohydrate-binding loop specifically binds to heparin. PLoS One 10, e0145834 10.1371/journal.pone.0145834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xue J., Laine R. A., and Matta K. L. (2015) Enhancing MSn mass spectrometry strategy for carbohydrate analysis: A b2 ion spectral library. J. Proteomics. 112, 224–249 10.1016/j.jprot.2014.07.013 [DOI] [PubMed] [Google Scholar]
- 34. Kanagawa M., and Toda T. (2017) Muscular dystrophy with ribitol-phosphate deficiency: A novel post-translational mechanism in dystroglycanopathy. J. Neuromuscul. Dis. 4, 259–267 10.3233/JND-170255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kanagawa M., Kobayashi K., Tajiri M., Manya H., Kuga A., Yamaguchi Y., Akasaka-Manya K., Furukawa J. I., Mizuno M., Kawakami H., Shinohara Y., Wada Y., Endo T., and Toda T. (2016) Identification of a post-translational modification with ribitol-phosphate and its defect in muscular dystrophy. Cell Rep. 14, 2209–2223 10.1016/j.celrep.2016.02.017 [DOI] [PubMed] [Google Scholar]
- 36. Sframeli M., Sarkozy A., Bertoli M., Astrea G., Hudson J., Scoto M., Mein R., Yau M., Phadke R., Feng L., Sewry C., Fen A. N. S., Longman C., McCullagh G., Straub V., Robb S., Manzur A., Bushby K., and Muntoni F. (2017) Congenital muscular dystrophies in the UK population: Clinical and molecular spectrum of a large cohort diagnosed over a 12-year period. Neuromuscul. Disord. 27, 793–803 10.1016/j.nmd.2017.06.008 [DOI] [PubMed] [Google Scholar]
- 37. Maroofian R., Riemersma M., Jae L. T., Zhianabed N., Willemsen M. H., Wissink-Lindhout W. M., Willemsen M. A., de Brouwer A. P. M., Mehrjardi M. Y. V., Ashrafi M. R., Kusters B., Kleefstra T., Jamshidi Y., Nasseri M., Pfundt R., et al. (2017) B3GALNT2 mutations associated with non-syndromic autosomal recessive intellectual disability reveal a lack of genotype-phenotype associations in the muscular dystrophy-dystroglycanopathies. Genome Med. 9, 118 10.1186/s13073-017-0505-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Angata K., Yen T. Y., El-Battari A., Macher B. A., and Fukuda M. (2001) Unique disulfide bond structures found in ST8Sia IV polysialyltransferase are required for its activity. J. Biol. Chem. 276, 15369–15377 10.1074/jbc.M100576200 [DOI] [PubMed] [Google Scholar]
- 39. Ito H., Kuno A., Sawaki H., Sogabe M., Ozaki H., Tanaka Y., Mizokami M., Shoda J., Angata T., Sato T., Hirabayashi J., Ikehara Y., and Narimatsu H. (2009) Strategy for glycoproteomics: Identification of glyco-alteration using multiple glycan profiling tools. J Proteome Res. 8, 1358–1367 10.1021/pr800735j [DOI] [PubMed] [Google Scholar]
- 40. Holliday G. L., Davidson R., Akiva E., and Babbitt P. C. (2017) Evaluating functional annotations of enzymes using the gene ontology. Methods Mol. Biol. 1446, 111–132 10.1007/978-1-4939-3743-1_9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Anderson D. C., Lapp S. A., Barnwell J. W., and Galinski M. R. (2017) A large scale Plasmodium vivax–Saimiri boliviensis trophozoite-schizont transition proteome. PLoS One 12, e0182561 10.1371/journal.pone.0182561 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.