Abstract
Introduction
To inform the WHO Guideline on self-care interventions, we conducted a systematic review of the impact of ovulation predictor kits (OPKs) on time-to-pregnancy, pregnancy, live birth, stress/anxiety, social harms/adverse events and values/preferences.
Methods
Included studies had to compare women desiring pregnancy who managed their fertility with and without OPKs, measure an outcome of interest and be published in a peer-reviewed journal. We searched for studies on PubMed, CINAHL, LILACS and EMBASE through November 2018. We assessed risk of bias assessed using the Cochrane tool for randomised controlled trials (RCTs) and the Evidence Project tool for observational studies, and conducted meta-analysis using random effects models to generate pooled estimates of relative risk (RR).
Results
Four studies (three RCTs and one observational study) including 1487 participants, all in high-income countries, were included. Quality of evidence was low. Two RCTs found no difference in time-to-pregnancy. All studies reported pregnancy rate, with mixed results: one RCT from the 1990s among couples with unexplained or male-factor infertility found no difference in clinical pregnancy rate (RR: 1.09, 95% CI 0.51 to 2.32); two more recent RCTs found higher self-reported pregnancy rates among OPK users (pooled RR: 1.40, 95% CI 1.08 to 1.80). A small observational study found higher rates of pregnancy with lab testing versus OPKs among women using donor insemination services. One RCT found no increase in stress/anxiety after two menstrual cycles using OPKs, besides a decline in positive affect. No studies measured live birth or social harms/adverse events. Six studies presented end-users’ values/preferences, with almost all women reporting feeling satisfied, comfortable and confident using OPKs.
Conclusion
A small evidence base, from high-income countries and with high risk of bias, suggests that home-based use of OPKs may improve fertility management when attempting to become pregnant with no meaningful increase in stress/anxiety and with high user acceptability.
Systematic review registration number
PROSPERO registration number CRD42019119402.
Keywords: systematic review, ovulation predictor kits, fertility management, infertility
Key questions.
What is already known?
Better timing of condom-less intercourse can improve the probability of achieving pregnancy; ovulation predictor kits (OPKs) are accurate and commercially available.
What are the new findings?
Three randomised controlled trials (RCTs) show evidence that OPKs may increase pregnancy rates.
One RCT found slight or no increase in stress/anxiety from using OPKs.
Ovulation predictor kids are generally acceptable to women and improve fertility awareness.
What do the new findings imply?
Home ovulation testing appears to be an acceptable intervention that can increase pregnancy rates, improve reproductive health knowledge and encourage shared decision-making for couples who are attempting to achieve pregnancy.
Introduction
Global estimates show that approximately 15%–25% of couples are unable to become pregnant despite attempting for 5 years or more.1 2 Infertility is typically diagnosed if a woman is unable to become pregnant after 12 months of regular condom-less intercourse,3 although this timeframe may vary by age (eg, advanced maternity), anatomical abnormalities (eg, tubal occlusion, fibroids, physical disabilities) or disease (eg, HIV, endometriosis, oncofertility).4 Clinically, 20%–30% of infertility cases are attributable to the male partner,5 a similar percentage to the female partner and up to 50% to both partners.6 Couples diagnosed as infertile, or women who require assistance to become pregnant, may turn to medically assisted reproduction using various diagnostics and interventions, including ovulation induction, ovarian stimulation and ovulation triggering, as well as procedures using processed semen of a partner or donor such as intrauterine, intracervical and intravaginal insemination or egg collection with external in vitro fertilisation.7 However, many such options are unaffordable or inaccessible to women or couples in resource-constrained settings.
Better timing of condom-less intercourse or, if required or desired, the self-intravaginal insemination of semen, during the woman’s fertile window can be supported using ovulation predictor kits (OPKs). OPKs are readily available in many settings worldwide without prescription, including at pharmacies, drugstores, convenience stores and supermarkets, as well as online websites which ship globally. These kits do not predict ovulation, per se, but rather the surge of luteinising hormone (LH) that precedes ovulation while also tracking corresponding oestrogen levels.8 OPKs do not directly pinpoint a peak fertility day and may need multiple pregnancy attempts within the appropriate timeframe during a woman’s menstrual cycle. OPKs are fairly accurate at predicting ovulation and the fertile window.9 10 They increase fertility awareness about when ovulation should occur during the woman’s cycle and may alert her to potential menstrual cycle abnormalities. Women with HIV sero-discordant partners could use OPKs to time intercourse and limit exposure to condom-less sex in order to reduce the risk of transmission of sexually transmitted infections, including HIV.11 12 Single women, women who wish to observe specific religious or cultural traditions, migrant/irregular workers or couples in unconsummated marriages (for instance, due to male erectile dysfunction or physical disabilities) may wish to use OPKs to appropriately time condom-less intercourse or attempt self-intravaginal insemination.13
Home-based use of OPKs has the potential to increase autonomy and agency for women globally. Increasing reproductive health awareness by using OPKs could yield greater knowledge concerning ovulation and a woman’s fertile window. Use of OPKs through a human rights-based approach could be particularly empowering for women and girls who face barriers to enacting decisions in relation to their sexuality, reproduction, health and well-being. A 2015 Cochrane review by Manders et al examined randomised controlled trial (RCT) evidence for the effectiveness of timed intercourse using both OPKs and other fertility awareness methods on the outcomes of live birth, pregnancy and adverse events, including stress.8 They found that that timed intercourse using OPKs may increase pregnancy rates compared with intercourse without ovulation prediction, with no difference in stress. In order to develop normative guidance by the WHO on self-care interventions for sexual and reproductive health and rights, we conducted a systematic review of available evidence of effectiveness and values and preferences surrounding the use of home-based, self-initiated ovulation prediction kits for fertility management to attempt pregnancy.
Methods
We conducted this systematic review in accordance to Preferred Reporting Items for Systematic review and Meta-Analyses (PRISMA) guidelines.14
Research question and inclusion criteria
Should home-based OPKs be made available as an additional approach for fertility management?
Population: Women attempting to become pregnant.
Intervention: Fertility management that includes home-based OPKs.
Comparison: Fertility management that does not include home-based OPKs (clinician-led assessment of ovulation only or no ovulation prediction).
Outcomes: (1) Time-to-pregnancy; (2) Pregnancy; (3) Live birth; (4) Stress/anxiety; (5) Social harms/adverse events (eg, device-related issues, coercion, violence [eg, intimate-partner violence, violence from family members or community members ], psychosocial harm, self-harm, suicide, stigma, discrimination).
To be included in the effectiveness (PICO) review, an article had to (1) have a comparative study design examining women who managed their fertility using home-based OPKs to women who did not manage their fertility using home-based OPKs (ie, clinician-led assessment only or no ovulation prediction), (2) evaluate one or more of the outcomes listed above and (3) be published in a peer-reviewed journal.
Inclusion was not restricted by location of the intervention. No language or publication date restrictions were used in the search.
OPKs could include both urine-based and serum-based kits and any modality (stick, monitor, digital, electronic slip that connects to a phone and so on). In order to focus on OPKs as a specific biomedical and biochemical intervention, the other behavioural and non-biochemical methods of ovulation prediction, such as calendar/standard day/fertility awareness methods, basal body temperature monitoring, Billings/cervical mucus monitoring methods, use of fertility beads and so on, were not included.15–17
Search strategy and screening process
We searched the electronic databases PubMed, CINAHL, LILACS and EMBASE through 21 November 2018, using search terms for ovulation and prediction. The full search strategy is available in online supplementary file 1. Secondary reference searching was also conducted on all included articles and on studies included in the Manders et al Cochrane review.8 We searched for ongoing RCTs were searched through clinicaltrials.gov, the WHO International Clinical Trials Registry Platform, the Pan African Clinical Trials Registry and the Australian New Zealand Clinical Trials Registry.
bmjgh-2019-001403supp001.pdf (324.1KB, pdf)
After title/abstract screening, full-text articles were obtained of all potential studies. Two reviewers independently assessed all full-text articles for study inclusion eligibility and resolved differences through consensus.
Data extraction and analysis
Two reviewers independently extracted data and conducted the risk of bias assessments. Standardised data extraction forms included fields for study citation, objectives, location, population characteristics, description of the OPK, description of any additional intervention components, study design, sample size, follow-up periods and loss to follow-up, analytic approach, reported numerical outcomes, results and limitations.
For RCTs, risk of bias was assessed using the Cochrane Collaboration’s tool for assessing risk of bias.18 For observational studies that presented comparative data, study rigour was assessed using the Evidence Project risk of bias tool for intervention evaluations.19
Data were analysed according to coding categories and outcomes, following the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach used by WHO for guideline development.20 If studies included other methods in conjunction with OPKs (ie, a multifactorial intervention), we planned to compare the intervention group to a control group receiving the same other methods but excluding OPK. Where multiple studies reported the same outcome, we conducted meta-analysis using random effects models to generate pooled risk ratios (RR) using the programme Comprehensive Meta-Analysis.18 Where studies reported both intention-to-treat and per-protocol analyses, we used intention-to-treat data. Where comparative event data were reported at multiple timepoints, we used data from the final timepoint. Heterogeneity was assessed using I-squared statistics, and funnel plots were created to examine the potential for publication bias if there were a sufficient number of included studies. Data from RCTs and non-randomised studies were meta-analysed separately.
Values and preferences review
The same search was used to identify studies presenting information on end-users’ values and preferences for home ovulation testing with OPKs. Studies were included if they presented primary data examining individuals’ values and preferences regarding OPKs. These studies could be qualitative or quantitative in nature, but had to present primary data collection; opinion pieces and review articles were excluded. Values and preferences literature also underwent data extraction by two reviewers using standardised forms to collect information on study location, study population, study design and key findings. Results were summarised qualitatively.
Patient and public involvement
A patient representative from the USA contributed to the systematic review protocol and is a coauthor on this review. Patients were also involved in a global survey of values and preferences and in focus group discussions with vulnerable communities conducted to inform the WHO Guideline on self-care interventions, and thus play a significant role in the overall recommendation informed by this review.
Results
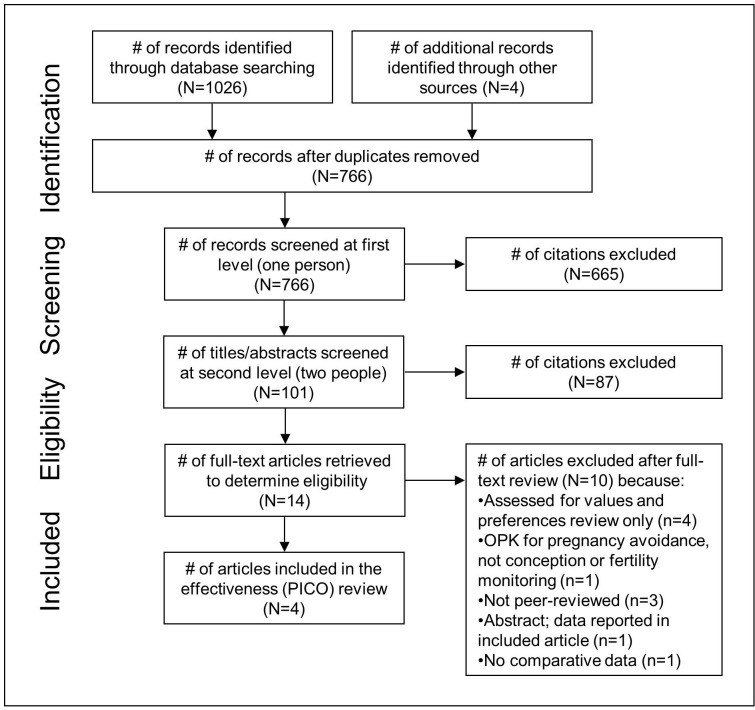
Electronic database searching retrieved 1026 results, and an additional four were identified through searching trial registries, contacting experts in the field and secondary searching (figure 1). After removing duplicates, there were 766 unique citations. After initial screening of titles and abstracts, 101 citations remained. After two independent reviewers screened in duplicate and gained consensus, 14 articles were pulled for full-text review. Of these, four were included in the effectiveness (PICO) review21–24 and seven in the values and preferences review.21 23–28
Figure 1.
PRISMA flow diagram of the different phases of a systematic review. OPK, ovulation predictor kit; PRISMA, Preferred Reporting Items for Systematic review and Meta-Analyses.
Study characteristics
Table 1 presents summary characteristics of the four included studies presenting evidence on benefits and harms. The four studies included 1487 total women (or couples); individual study sample sizes ranged from 117 to 1000. Articles were published between 1992 and 2013 for studies taking place between 1991 and 2010. All studies were conducted in high-income countries. Two studies (one each in the UK and the USA) recruited general population women nationwide, and two studies (one each in Scotland and Australia) recruited women undergoing fertility treatment or investigation. Participants' ages ranged from 18 to 43.
Table 1.
Description of included studies and reported outcomes for the PICO review
| Author | Study location and population characteristics | Intervention description | Study methods | Reported outcomes |
| Anderson et al, 199621 | Scotland (Edinburgh) Women using donor insemination services Age: average 32–33 |
Intervention: Home ovulation test kit of participant's choice: all kits used a quantitative colour change which was compared with a reference colour, equivalent to LH concentration of 2.5 IU/L) Urine specimen collected at home Device: Conceive (Quidel, San Diego, USA), Clearplan (Unipath, Bedford, UK) or Predictor (Schefaro International BP, Rotterdam, Netherlands) Control: Urinary fertility monitoring with lab LH surge testing |
Study design: Prospective cohort study Sample size: 117 (Intervention: 64, Control: 53) Length of follow-up: 6 cycles |
2. Pregnancy |
| Leader et al, 199222 | Australia (Sydney) Couples with unexplained infertility or whose fertility was thought to be due to reduced sperm motility Age: range 21–43 |
Intervention: Commercially available urinary fertility monitor (levels of E3G and LH to estimate fertility status); provided OPK and given instructions on usage Urine specimen collected at fertility clinic (donor insemination) Device: Clearplan (Fisons Consumer Health, Sydney) Control: Information about best time of menstrual cycle to achieve pregnancy |
Study design: RCT Sample size: 160 (Intervention: 80, Control: 80) Length of follow-up: 3 cycles |
2. Pregnancy |
| Robinson et al, 200723 | USA (national) Women attempting to become pregnant <24 months Age: range 21–40 Women were excluded if using hormonal birth control or fertility drugs that contained hCG or LH, had a medical condition that presented a risk if they became pregnant or had been attempting to become pregnant >2 years. |
Intervention: Commercially available urinary fertility monitor (levels of E3G and LH to estimate fertility status); provided OPK and given instructions on usage Urine specimen collected at home Device: Clearblue Easy Fertility Monitor (Unipath, Waltham, Massachusetts, USA) Control: No specific instructions given; free to use aids to attempt pregnancy other than the intervention, including other home ovulation tests |
Study design: RCT Sample size: 1000 (Intervention: 500, Control: 500) Length of follow-up: 3 cycles |
|
| Tiplady et al, 201324 | UK (national) Women attempting to become pregnant <12 months and who had regular menstruation Age: range 18–40 Women were excluded if they had used hormonal contraception in the last 3 months, were currently undergoing fertility treatment or investigation, had previously been diagnosed as infertile, had a history of depression, anxiety or panic attacks or were dependent on either drugs or alcohol. Women who had previously used ovulation tests were not excluded from participating. |
Intervention: Commercially available urinary fertility monitor (levels of E3G and LH to estimate fertility status); provided OPK and given instructions on usage Urine specimen collected at home Device: Clearblue Digital Home Ovulation Test (SPD Swiss Precision Diagnostics, GmbH) Control: Given the 2010 National Institute for Health and Clinical Excellence guidelines for increasing the chances of achieving pregnancy (intercourse every 2–3 days); asked not to use any additional methods to time when ovulation occurs (ie, ovulation testing, basal body temperature) |
Study design: RCT Sample size: 210 (Intervention: 115: Control:95) Length of follow-up: 2 cycles |
|
E3G, estrone-3-glucuronide; LH, luteinising hormone; OPK, ovulation predictor kit; RCT, randomised controlled trial.
All studies included a study arm where women used a commercially available home ovulation prediction kit: one study allowed intervention group participants to choose one of three available home-based OPKs (Conceive, Clearplan or Predictor),21 and the rest used a single product (Clearblue Digital Home Ovulation Test, also known as Clearblue Easy Fertility Monitor or Clearplan).22–24 Three studies had a control group where participants were given general information about fertility and ways to spontaneously improve achieving pregnancy: one gave instructions via telephone about the best time during the menstrual cycle to achieve pregnancy;22 another allowed control group members to use other aids to attempt pregnancy including home ovulation tests other than the intervention;23 the third asked participants not to use any additional methods to determine ovulation timing.24 One study had its control group send urine samples for lab LH testing to time donor insemination.21
All studies measured the outcome pregnancy (indicated by self-report via home-based pregnancy test, except for one study which confirmed pregnancy via ultrasound22), but follow-up periods ranged from two to six menstrual cycles. Because of incomplete data for the third cycle, one study only analysed data from the first two cycles.23 Two studies measured time-to-pregnancy, and one study reported on stress/anxiety. No studies presented comparative data on the outcomes of live birth or social harms/adverse events.
Three RCTs and one prospective cohort study were included. Risk of bias among RCTs was generally high. Blinding was impossible, given the intervention. All participants knew whether they were in the intervention or control group, increasing risk of performance bias. The self-reported pregnancy outcome may have suffered from detection bias, as lack of blinding could lead to greater awareness of fertility, and increased frequency of pregnancy testing, and thus greater rates of positive pregnancy tests. Couples using OPKs may also have had increased frequency of intercourse, which may have impacted the likelihood of pregnancy and time-to-pregnancy. There is high risk of publication bias, given the small number and small sample size of included studies. In addition, two studies were funded by the OPK manufacturer,23 24 and one study had its intervention delivered by the manufacturer;21 because of the commercial nature of OPKs, there may be some concern that data yielding negative results have not been published. The single observational study was of moderate quality. Table 2 presents an assessment of risk of bias for each study. The GRADE table for this review with quality assessment by outcome is available in online supplementary file 2.
Table 2.
Quality assessment of included studies
| Cochrane Risk of Bias Tool (for RCTs) | |||||||
| Type of bias | Selection | Performance | Detection | Attrition | Reporting | Other biases | |
| Author Year | Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | |
| Leader et al, 199222 | Low | Low | High* | Low | Low | Unclear | None |
| Robinson et al, 200723 | Low | Low | High* | High† | High‡ | High§ | None |
| Tiplady et al, 201324 | Low | Low | High* | High† | Low | Unclear | High¶ |
| Evidence Project Study Risk of Bias Tool (for non-RCTs) | |||||||||
| Author Year | Study design includes | Comparison groups equivalent at baseline on | Random assignment (group or individual) to the intervention | Participants randomly selected for assessment | Control for potential confounders | Follow-up rate ≥75% | |||
| Preintervention and postintervention data | Control or comparison group | Cohort | Socio demographics |
Outcome measures | |||||
| Anderson et al, 199621 | No | Yes | Yes | Yes | Yes | No | No | No | Yes |
*Blinding of participants and personnel not possible, based on the intervention.
†Blinding of outcome assessment not possible for self-reported pregnancy (via positive pregnancy test).
‡Unexplained high dropout rate (35%): 191 non-responders in the OPK group and 144 in the control group.
§Unreported outcome (live birth). Study reported results from two menstrual cycles, instead of from the prespecified three cycles (‘Although women were recruited to the study for three cycles, insufficient evaluable data were provided for the third cycle of the study, and therefore data were analysed for the first two complete cycles following confirmation that the participants were not pregnant at baseline. The reason for the limited third-cycle data was thought to be related to confusion on the part of the participants regarding returning data at the end of cycle 3.’).
¶A second (biased, ratio 2:1) cohort was recruited into the OPK group to increase the power of the data for the outcome stress, because of higher pregnancy rates in the OPK group.
OPK, ovulation predictor kit; RCTs, randomised controlled trials.
bmjgh-2019-001403supp002.docx (22.2KB, docx)
For each of the main outcomes, results are presented below and in table 1.
Time to pregnancy
Two RCTs measured time-to-pregnancy, indicated by positive pregnancy tests.23 24 There was no evidence of a statistically significant difference in time-to-pregnancy in either study.
In one study among the general population, 46 of 500 participants in the OPK group (9.2%) became pregnant during the first menstrual cycle, compared with 27 of 500 (5.4%) in the control group; during the second cycle, another 23 in the OPK group became pregnant (cumulatively 22.8%) and another 23 in the control group (cumulatively 10%).23
The other study, whose general population participants had been assessed within a fertility clinic for their likely ability to become pregnant through condom-less intercourse and were presumed fertile, found pregnancies among women at the beginning of the first menstrual cycle (22 of 87 in the OPK group [25.2%] compared with 13 of 68 in the control group [19.1%]); after the first cycle, 30 of 55 women using OPKs were found pregnant (54.5%) compared with 9 of 54 (16.6%) in the control group and after the second cycle, 7 of 44 women using OPKs were found pregnant (15.9%) compared with 6 of 43 (14.0%) in the control group.24 Pre-cycle 1 pregnancies were included in this study, as participants were sent study materials after recruitment and randomisation and may have become pregnant by the first timepoint (day 6 of cycle 1).
Pregnancy
All included studies reported pregnancy rates, whether clinical or self-reported pregnancy (table 3).
Table 3.
Summary of pregnancy outcomes
| Pregnancy outcome | # effect sizes | RR | 95% CI | P value (RR) | Q statistic | P value (Q) | I-squared |
| Pregnancy (clinical and self-reported) | 3 RCTs | 1.36 | 1.07 to 1.73 | 0.01 | 0.43 | 0.81 | 0.00 |
| Pregnancy (clinical only) | 1 RCT | 1.09 | 0.51 to 2.32 | 0.85 | – | – | – |
| Pregnancy (self-reported only) | 2 RCTs | 1.40 | 1.08 to 1.80 | 0.01 | 0.06 | 0.81 | 0.00 |
| Pregnancy (clinical only) | 1 observational | 0.35 | 0.15 to 0.86 | 0.01 | – | – | – |
OPK, ovulation predictor kit; RCT, randomised controlled trial; RR, risk ratio comparing using OPKs to not using OPKs for fertility management.
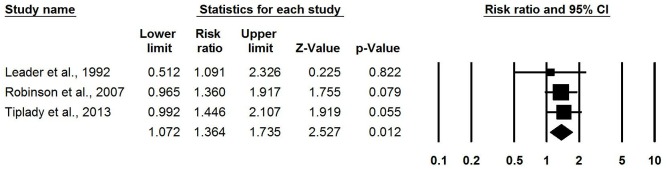
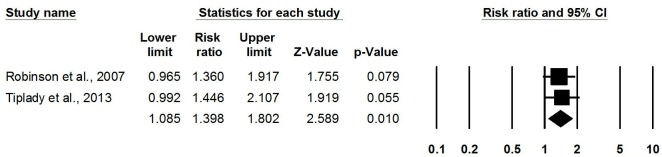
Meta-analysis of three RCTs found that using a home-based OPK for timing intercourse was associated with higher pregnancy rates than not using OPKs (pooled RR: 1.36, 95% CI 1.07 to 1.73) (figure 2).22–24 One RCT published in 1992 among couples with unexplained or male-factor infertility measuring clinical pregnancy (confirmed by ultrasound) found no difference in pregnancy rate (RR: 1.09, 95% CI 0.51 to 2.32).22 Meta-analysis of two more recent RCTs (one conducted 2001–2002 and the other in 2010) among the general population measuring pregnancy via home-based pregnancy tests found higher pregnancy rates when using OPKs (pooled RR: 1.40, 95% CI 1.08 to 1.80) (figure 3).23 24
Figure 2.
Meta-analysis of likelihood of pregnancy (clinical and self-reported) from RCTs comparing OPKs to no OPKs. OPKs, ovulation predictor kits; RCTs, randomised controlled trials.
Figure 3.
Meta-analysis of likelihood of pregnancy (self-reported only) from RCTs comparing OPKs to no OPKs. OPKs, ovulation predictor kits; RCTs, randomised controlled trials.
One prospective cohort study published in 1996 treated 174 cycles in 64 home-testing participants and 110 cycles in 53 lab-testing participants with donor insemination.21 The clinical pregnancy rate in home-tested cycles was significantly reduced when using OPKs compared with lab-testing (RR: 0.35, 95% CI 0.15 to 0.86).
Stress/anxiety
One RCT reported levels of stress/anxiety stratified by OPK use.24 The study measured stress in five different ways, using scales to self-report stress, positive/negative affect and general health, as well as two biochemical measures (urinary cortisol and estrone-3-glucuronide (E3G)). Across all measurement approaches, there was no evidence of difference in stress, besides a statistically significant decrease in positive affect score, at the final study timepoint (beginning of menstrual cycle 3) between the group using an OPK and the control group (table 4).
Table 4.
Summary of stress outcomes
| Stress outcome (from one RCT) | OPK group | Control group | Mean difference | 95% CI | # effect sizes | Study design | Total n | p value | ||||
| Mean | SD | n | Mean | SD | n | |||||||
| PSS | 17.76 | 6.48 | 37 | 15.78 | 6.25 | 40 | 1.98 | −0.91 to 4.87 | 1 | RCT | 77 | 0.18 |
| PANAS: positive affect | 29.75 | 10.24 | 36 | 34.26 | 8.06 | 38 | −4.51 | −8.77 to −0.25 | 1 | RCT | 74 | 0.04 |
| PANAS: negative affect | 17.55 | 6.97 | 38 | 16.90 | 6.64 | 40 | 0.65 | −2.42 to 3.72 | 1 | RCT | 78 | 0.67 |
| SF-12: physical attributes | 41.86 | 4.00 | 38 | 41.12 | 3.14 | 40 | 0.74 | −0.88 to 2.36 | 1 | RCT | 78 | 0.37 |
| SF-12: mental attributes | 46.40 | 7.15 | 38 | 46.15 | 5.11 | 40 | 0.25 | −2.54 to 3.04 | 1 | RCT | 78 | 0.86 |
| Cortisol:creatinine ratio | 139.30 | 59.03 | 37 | 156.23 | 89.44 | 38 | −16.90 | −51.87 to 18.07 | 1 | RCT | 75 | 0.34 |
| E3G:creatinine ratio | 101.59 | 52.34 | 37 | 95.24 | 52.43 | 38 | 6.35 | −17.76 to 30.46 | 1 | RCT | 75 | 0.60 |
SF-12: Short-Form-12 Health Survey. It is a short, reliable, validated generic questionnaire for functional health status and outcomes, with both physical and mental health composite scores. Higher scores indicate higher health-related quality of life (range 0–100).
E3G:creatinine ratio: ratio of estrone-3-glucuronide (ng/mL) to creatinine (g/dL). E3G is an oestrogen marker associated with depression and anxiety, where a higher ratio indicates higher depression/anxiety.
PANAS: The Positive and Negative Affect Schedule (PANAS) comprises 10 positive affects (interested, excited, strong, enthusiastic, proud, alert, inspired, determined, attentive, active) and 10 negative affects (distressed, upset, guilty, scared, hostile, irritable, ashamed, nervous, jittery, afraid), where higher scores indicate stronger emotion (range 10–50).
Higher scores indicate higher stress, based on perceptions of how unpredictable, uncontrollable and overloaded participants find their lives (range 0–40).
OPK, ovulation predictor kit; PSS, perceived stress scale; RCT, randomised controlled trial; n, sample size.
Values and preferences
Six studies in seven articles reported end-users' values and preferences regarding OPKs, three of which related to studies reported above for the PICO review (table 5).21 23–28
Table 5.
Description of included studies for the values and preferences review
| Author | Study location | Study population | Study design | OPK |
| Anderson et al, 199621 | Scotland (Edinburgh) | Couples using donor insemination services who had used OPKs (not lab-based urinary LH testing) to time insemination | Prospective cohort study (acceptability questionnaire mailed after completing study) Sample size: 40 couples |
Conceive, Clearplan or Predictor |
| Ayoola et al, 201526 | USA (Grand Rapids, Michigan) | Women attempting to become pregnant Women were 18–39 years old, low-income, racial/ethnic minorities, medically underserved and living in urban areas. Most had at least one previously unplanned pregnancy. |
Cross-sectional survey Sample size: 22 women |
Ovulation test strips in ‘Knowing Your Body’ kit |
| Kopitzke et al, 199127 | USA (Lexington, Kentucky) | Female patients at infertility clinics Women were 26–40 years old (mean age 33). Median family income was $47 000. Women had participated in a previous study of endometriosis and infertility. Couples had been attempting to become pregnant for an average of 3.2 years (range 2–15 years). |
Cross-sectional study (mail survey) Sample size: 26 women |
LH urine ovulation kit (not specified) |
| Robinson et al, 200723 | USA (national) | Women attempting to become pregnant <24 months Women were 21–40 years old (maximum 15% of total participants in the 35–40 age group) with a partner age 21–50. Women were excluded if using hormonal birth control or fertility drugs that contained hCG or LH, had a medical condition that presented a risk if they became pregnant or had been attempting to become pregnant >2 years. |
RCT (consumer satisfaction questionnaire mailed after completing study) Sample size: 305 women |
Clearblue Easy Fertility Monitor |
| Severy et al, 200625 | USA (Gainesville, Florida, and Raleigh, North Carolina) | Couples attempting to become pregnant <12 months reporting concern over lack of success. Women were 18–44 years old, in a mutually monogamous relationship with a male partner and English-literate. Women had never undergone infertility investigation or treatment, regular cycles (21–42 days), at least 3 months after stopping hormonal contraception, last pregnancy or breastfeeding, at least 13 cycles after a last Depo-Provera injection Women were excluded if using hormonal medication or had liver or kidney disease, polycystic ovarian syndrome or any medical condition that would put the volunteer at risk if she were to become pregnant. |
Prospective cohort study (acceptability assessments every cycle for four cycles) Sample size: 61 couples |
Clearplan Easy Fertility Monitor |
| Jones et al, 201528 and Tiplady et al, 201324 |
UK (national) | Women attempting to become pregnant <12 months Women were 18–40 years old who had regular menstruation and wished to become pregnant. Women were excluded if they had used hormonal contraception in the last 3 months, were currently undergoing fertility treatment or investigation, had previously been diagnosed as infertile, had a history of depression, anxiety or panic attacks or were dependent on either drugs or alcohol. Women who had previously used ovulation tests were not excluded from participating. |
RCT (individual semistructured telephone interviews with all participants after completing study) Sample size: 210 women (qualitative analysis reached saturation after coding 18 interviews each from OPK and control group) |
Clearblue Digital Home Ovulation Test |
LH, luteinising hormone; OPK, ovulation predictor kit; RCT, randomised controlled trial.
Three articles reported results from women from the general population attempting to become pregnant who had participated in an RCT. In the USA, women who used the OPK were sent a consumer satisfaction questionnaire.23 OPK users found the device to be easy or very easy to use (90%) and convenient or very convenient (80%). Most believed the OPK was accurate or very accurate (73%) and were confident or very confident that it identified their fertile days (71%).
In the UK, another RCT reported qualitative findings from follow-up semistructured telephone interviews, reported in two articles.24 28 All participants from both OPK and control groups found OPKs appealing. Women who had used OPKs found the digital ovulation tests easy to use and understand, especially compared with visual read tests. Half of the OPK group felt that they better understood changes in their bodies after using the OPK and self-reported not feeling any pressure or stress from the test. Almost all OPK users said the OPK met their expectations and would consider purchasing and using this product in future.
Interviewees also discussed perceived pros and cons of home ovulation testing.24 28 The most frequently mentioned advantages of OPKs were understanding their menstrual cycle and pinpointing ovulation time, which participants felt increased their likelihood of getting pregnant. They liked their increased knowledge and decreased stress, as well as the OPK's digital charting/tracking and precision, saying that this ability to plan pregnancy attempts with their partner made them feel proactive and more relaxed. Women felt the OPKs provided emotional support, giving reassurance and providing a helping hand (both to become pregnant and to alert the couple when something went wrong). Some believed OPKs also improved relationships with their partner, building teamwork and decreasing stress. Some participants also noticed that OPKs helped them identify anovular cycles, prompting them to seek medical assistance earlier—a useful benefit of negative or abnormal results.
Most interviewees could not think of any disadvantages.24 28 However, some women mentioned detrimental effects on their sexual life and relationship with their partner, saying that sex became less spontaneous, exciting and romantic and that there were increased expectations and pressure. Some also described emotional consequences after prolonged OPK usage, such as dependence, obsession, self-doubt, demotivation and difficult-to-resolve questions/uncertainties. Though some participants reported stress when using OPKs, some reported stress when attempting to become pregnant without OPKs; overall, many felt that using OPKs decreased stress. Finally, women who became pregnant while using OPKs seemed to remember less how the test affected their sex life, compared with women who did not become pregnant.
One study in the USA among 61 couples attempting pregnancy25 found that, at baseline, women, compared with their male partners, expressed greater preference for the monitor and believed it would be more informative about the women's reproductive processes (multivariate analysis of variance [ANOVA] F(10,93)=4.779, p<0.0001). Curiously, initial acceptability was higher among couples who eventually became pregnant (multivariate ANOVA F(4,79)=2.50, p<0.05). All participants liked the OPK, expected it to improve their chance of becoming pregnant and expected the OPK to increase their knowledge concerning their female bodies and about the process of reproduction. Among those who did not become pregnant, product acceptability and expectations of becoming pregnant decreased across four cycles while using an OPK, while feelings of sadness/anger/loss and strain/blame in the relationship increased.
As part of a prospective cohort study in Scotland in the 1990s,21 researchers administered an acceptability questionnaire to the 63 couples who had used home ovulation testing to time donor insemination and received 40 responses (63%). Of these, 73% thought the kits expensive, 86% found the kits easy to use and 75% found the result easy to interpret. 81% expressed confidence in their use, but a third would have liked further assistance in using the kits. Only half would prefer home testing in future cycles. Even with the convenience of home testing, 67% felt that undergoing treatment with donor insemination significantly interfered with their working lives. A retrospective study published in 1991 among 26 infertility patients in the USA found that timing intercourse via OPKs could lead to emotional distress.27 However, they also stated that other events related to losing a pregnancy (eg, ectopic pregnancy, miscarriage) or not becoming pregnant (eg, negative pregnancy test, menses onset, seeing a pregnant woman) were more emotionally difficult than using the OPKs.
Finally, as part of a recent feasibility study in the USA on a ‘Knowing your Body’ (family planning/fertility awareness) kit which included ovulation test strips, a survey was conducted among a convenience sample of low-income, racial/ethnic minority, urban, medically underserved women.26 Ninety-one per cent had used the OPKs included in the kit; of these, 77% were extremely or very confident that they could properly use the test strip to know when they were ovulating and, in addition, 73% were comfortable using the OPK.
Discussion
Fertility management when attempting a desired pregnancy is an important issue for women and couples globally. This review evaluated the benefits and risks of using OPKs as a self-care intervention to time conception compared with provider testing or no ovulation prediction. Two RCTs measured time-to-pregnancy and found no difference in time-to-pregnancy between OPK and non-OPK users.23 24 Three RCTs found either no difference in clinical pregnancy rate22 or higher self-reported pregnancy rates23 24 among women using home-based OPKs. One observational study from the mid-1990s found reduced clinical pregnancy rates among women who used OPKs to time a donor insemination.21 One study reporting several indicators for stress/anxiety found no difference in stress as measured by questionnaires or biochemical markers, though they did find one decrease in positive affect.24 No included studies reported comparative data on live birth or other social harms/adverse events.
In terms of values and preferences, women who had used or heard of OPKs were usually highly satisfied: they found the kits easy to use and understand, convenient and accurate. Almost all women were confident in their ability to use the kit and in its ovulation predictive ability. Users appreciated knowing more about their menstrual cycle and timing to attempt pregnancy, which decreased stress, provided emotional support and enabled teamwork with their partner. However, especially in those who did not achieve pregnancy, an overdependence on OPKs could decrease spontaneity and foster obsession and doubt. Most participants stated that they would use OPKs again in the future. The one study with less confidence about OPKs took place in the 1990s; the value of these participants' opinions on ease of use and ease of interpretation is limited, given changes in the OPKs now available. Though some reported stress and increasing dissatisfaction from prolonged use of OPKs, especially among couples who did not achieve pregnancy, OPK usage caused less emotional distress than other pregnancy (loss)-related events.
Strengths and limitations
This systematic review included multiple study designs: both randomised trials and observational studies. For comprehensiveness, we searched multiple databases and conducted secondary and hand searching, and we did not exclude studies based on language or location or publication date. Screening and data extraction were conducted in duplicate and resolved via consensus. Where possible, we used meta-analysis when presenting quantitative findings. Another strength is our combination of quantitative evidence on benefits and harms with more qualitative values and preferences.
However, this review is limited in the depth and quality of its evidence base. Overall, the quality of evidence was low to very low, as blinding was impossible (potentially leading to detection and reporting biases). One RCT with 1453 participants reporting pregnancies, live birth and stress/anxiety29 could not be analysed because its results have not yet been presented by intervention group; its findings could potentially impact the results of this review. Three of the included studies were very small, with 210 or fewer total participants each.21 22 24 Only two studies reported clinical pregnancy rates, both conducted in the 1990s.21 22 It is unclear if the current OPKs are more accurate and effective than those used decades ago. Stress and anxiety were only reported in one study from a high-income country,24 and no other comparative reports of social harms/adverse events were found. Further research is needed to assess the evidence on these outcomes in both high-resource and lower-resource settings, and within different sociocultural environments which may place a higher value on pregnancy and childbearing.
Two recent RCTs presented pregnancy rates and time-to-pregnancy based on positive pregnancy tests from the first two menstrual cycles.23 24 Self-reported pregnancy rates early in pregnancy unconfirmed by ultrasound have somewhat limited clinical relevance, given the risk for early miscarriage, but these results still support the potential for OPKs to improve timed intercourse for achieving pregnancy. Short follow-up across studies (2–6 cycles) limited assessment of differences between the intervention and control groups, especially since pregnancy is a relatively rare event (particularly in couples defined as infertile—12 months of attempting—or when infertility may be due to a functional disability). This follow-up length also precluded assessment of clinically important outcomes such as live birth, but maintenance of a pregnancy resulting in a live birth can be due to many factors unrelated to an ability to become pregnant. In addition, couples' expectations and perceived stress could be mitigated if they realised it commonly takes more than 2–6 cycles to become pregnant; some studies claim an 85% chance of becoming pregnant within 12 cycles.30 31
All studies used urinary fertility monitoring, and most used the same commercially available OPK (Clearblue). No studies were found on the effectiveness of other modes or types of OPK. Participant populations varied by study, which may affect pooled results: two studies focused on couples undergoing fertility treatment or investigation,21 22 while the others recruited women and couples attempting pregnancy from the general population.23 24 The control groups in each study also differed: some received general recommendations on timing condom-less intercourse,22–24 one allowed participants to use other pregnancy aids,23 one requested participants to abstain from other ovulation-timing methods24 and one collected urine specimens for lab testing.21 Additional high-powered RCTs that provide all participants with menstrual cycle and reproduction-timing education, provide information about realistic expectations on chance of pregnancy per cycle attempts and also give participants a fertility assessment prior to study inclusion in order to ensure potential capacity to become pregnant and carry a child to term (fecundability) through appropriately timed condom-less intercourse, would strengthen the evidence base. If vaginal insemination is used with donor semen, these participant data need to be appropriately disaggregated. If women are found to have a lower ovarian reserve or if men are found with lower quality semen parameters, these participant data also need to be appropriated disaggregated. All included studies took place in high-income countries; thus, additional research evidence is needed from participants from lower-resource settings.
It is challenging to determine the effectiveness of OPKs, given the paucity of evidence for benefits and harms. Generally, our results on effectiveness agree with the 2015 review by Manders et al,8 which found that urinary fertility monitoring to time intercourse could lead to higher pregnancy rates with no increase in stress.
Given that over 186 million couples worldwide suffer from infertility and childlessness2 and Global Burden of Disease and Disability data indicate global levels of primary and secondary infertility have hardly changed between 1990 and 2010,1 it is imperative to find innovative solutions to support couples with safe, affordable, acceptable and accessible options to ensure that all women, including infertile women and couples, are able to plan for their fertility goals and achieve pregnancy safely. Findings presented in this review have been used to inform the development of WHO recommendations for self-care interventions for sexual and reproductive health and rights relating to use of home-based OPKs. The benefits and harms, and values and preferences, of this intervention have been considered along with resource use, human rights and feasibility issues to inform the recommendation. We hope that additional research regarding the outcomes important to decision-makers will be conducted to provide the evidence required to fill the gaps identified in the current literature.
Conclusion
Ensuring that policies and programmes support the desire of women’s and couples’ sexual and reproductive desires and rights requires innovative approaches. Home-based use of OPKs has the potential to empower couples and women who are attempting to become pregnant. As an additional resource for fertility management, OPKs may increase the likelihood of becoming pregnant and generally have not been found to cause additional stress/anxiety especially among those who do become pregnant. OPKs have been found highly acceptable by end-users. They also improve end-users' fertility awareness and knowledge, enabling women to know their bodies better. However, more research is needed to assess time-to-pregnancy, pregnancy rates, stress/anxiety, social harms/adverse events and values and preferences using a diverse set of OPKs (beyond Clearblue), especially in low-resource settings (eg, low-income and middle-income countries), and with longer follow-up time periods. Further research for understanding the complex influence of social constructs, treatment and technology availability and service integration on the realisation of desired pregnancies among vulnerable populations will be particularly important.
Acknowledgments
We thank Laura Ferguson for her contribution to conceptualising this review and Nandi Siegfried for her thoughtful comments on the protocol. We also thank our Johns Hopkins Bloomberg School of Public Health graduate students (Eric Rodriguez, Molly Petersen, Po-Yu Teresa Chiang and Priyanka Mysore) for their assistance in searching trial registries, screening citations and extracting data.
Footnotes
Handling editor: Soumyadeep Bhaumik
Contributors: MN and SVdP conceptualised the study. CEK and PTY designed the protocol, with feedback from SVdP, TM and LB. PTY ran the search and oversaw screening. PTY extracted data and assessed risk of bias. PTY and CEK conducted meta-analysis. PTY drafted the manuscript. All authors reviewed the draft, provided critical review and read and approved the final manuscript. The corresponding author, as guarantor, accepts full responsibility for the finished article, has access to any data, and controlled the decision to publish. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: We gratefully acknowledge the UNDP-UNFPA-UNICEF-WHO-World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP) and the Children's Investment Fund Foundation (CIFF).
Disclaimer: HRP was involved in the study design, but both funders played no part in the decision to submit the article for publication, nor in the collection, analysis and interpretation of data. All authors had full access to all of the data (including statistical reports and tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data comes from articles in the peer-reviewed literature. Extracted data are available on request to the corresponding author.
References
- 1. Mascarenhas MN, Flaxman SR, Boerma T, et al. National, regional, and global trends in infertility prevalence since 1990: a systematic analysis of 277 health surveys. PLoS Med 2012;9:e1001356 10.1371/journal.pmed.1001356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rutstein SO, Shah IH. Infecundity, infertility, and childlessness in developing countries. demographic and health surveys (DHS) comparative reports No. 9. Calverton, MD, USA: ORC Macro and WHO, 2004. Available: https://www.who.int/reproductivehealth/topics/infertility/DHS-CR9.pdf
- 3. Zegers-Hochschild F, Adamson GD, de Mouzon J, et al. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology, 2009. Fertil Steril 2009;92:1520–4. 10.1016/j.fertnstert.2009.09.009 [DOI] [PubMed] [Google Scholar]
- 4. Zegers-Hochschild F, Adamson GD, Dyer S, et al. The International glossary on infertility and fertility care, 2017. Fertil Steril 2017;108:393–406. 10.1016/j.fertnstert.2017.06.005 [DOI] [PubMed] [Google Scholar]
- 5. Agarwal A, Mulgund A, Hamada A, et al. A unique view on male infertility around the globe. Reprod Biol Endocrinol 2015;13 10.1186/s12958-015-0032-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cates W, Farley TM, Rowe PJ. Worldwide patterns of infertility: is Africa different? Lancet 1985;2:596–8. [DOI] [PubMed] [Google Scholar]
- 7. Gunn DD, Bates GW. Evidence-based approach to unexplained infertility: a systematic review. Fertil Steril 2016;105:1566–74. 10.1016/j.fertnstert.2016.02.001 [DOI] [PubMed] [Google Scholar]
- 8. Manders M, McLindon L, Schulze B, et al. Timed intercourse for couples trying to conceive. Cochrane Database Syst Rev 2015;2015 10.1002/14651858.CD011345.pub2 [DOI] [PubMed] [Google Scholar]
- 9. Su H-W, Yi Y-C, Wei T-Y, et al. Detection of ovulation, a review of currently available methods. Bioeng Transl Med 2017;2(3):238–46. 10.1002/btm2.10058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Eichner SF, Timpe EM. Urinary-based ovulation and pregnancy: point-of-care testing. Ann Pharmacother 2004;38:325–31. 10.1345/aph.1D210 [DOI] [PubMed] [Google Scholar]
- 11. Letchumanan M, Coyte PC, Loutfy M. An economic evaluation of conception strategies for heterosexual serodiscordant couples where the male partner is HIV-positive. Antivir Ther 2015;20:613–21. 10.3851/IMP2956 [DOI] [PubMed] [Google Scholar]
- 12. U.S. Department of Health and Human Services Panel on Treatment of Pregnant Women with HIV Infection and Prevention of Perinatal Transmission. Reproductive options for couples in which one or both partners are living with HIV : Office of AIDS Research Advisory Council. Recommendations for the use of antiretroviral drugs in pregnant women with HIV infection and interventions to reduce perinatal HIV transmission in the United States Washington, DC, USA: U.S. Department of Health and Human Services, 2018. [Google Scholar]
- 13. Banerjee K, Singla B. Pregnancy outcome of home intravaginal insemination in couples with unconsummated marriage. J Hum Reprod Sci 2017;10:293–6. 10.4103/jhrs.JHRS_5_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. World Health Organization Medical eligibility criteria for contraceptive use. 5th ed Geneva, Switzerland: WHO, 2015. [PubMed] [Google Scholar]
- 16. World Health Organization Selected practice recommendations for contraceptive use. 3rd ed Geneva, Switzerland: WHO, 2016. [PubMed] [Google Scholar]
- 17. World Health Organization, Johns Hopkins Bloomberg School of Public Health Center for Communication Programs, USAID . Family planning: a global Handbook for providers. 3rd ed, 2018. [Google Scholar]
- 18. Higgins JPT, Green S. Chapter 8.5 The Cochrane Collaboration's tool for assessing risk of bias : Cochrane Handbook for Systematic Reviews of Interventions Version 510 [updated March 2011] [Internet]. London, England: The Cochrane Collaboration, 2011. [Google Scholar]
- 19. Kennedy CE, Fonner VA, Armstrong KA, et al. The evidence project risk of bias tool: assessing study rigor for both randomized and non-randomized intervention studies. Syst Rev 2019;8 10.1186/s13643-018-0925-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang Y, Akl EA, Schünemann HJ. Using systematic reviews in guideline development: the grade approach. Res Synth Methods 2018. 10.1002/jrsm.1313. [Epub ahead of print: 14 Jul 2018]. [DOI] [PubMed] [Google Scholar]
- 21. Anderson RA, Eccles SM, Irvine DS. Home ovulation testing in a donor insemination service. Hum Reprod 1996;11:1674–7. [DOI] [PubMed] [Google Scholar]
- 22. Leader LR, Russell T, Stenning B. The use of Clearplan home ovulation detection kits in unexplained and male factor infertility. Aust N Z J Obstet Gynaecol 1992;32:158–60. 10.1111/j.1479-828X.1992.tb01930.x [DOI] [PubMed] [Google Scholar]
- 23. Robinson JE, Wakelin M, Ellis JE. Increased pregnancy rate with use of the Clearblue easy fertility monitor. Fertil Steril 2007;87:329–34. 10.1016/j.fertnstert.2006.05.054 [DOI] [PubMed] [Google Scholar]
- 24. Tiplady S, Jones G, Campbell M, et al. Home ovulation tests and stress in women trying to conceive: a randomized controlled trial. Human reproduction 2013;28:138–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Severy LJ, Robinson J, Findley-Klein C, et al. Acceptability of a home monitor used to aid in conception: psychosocial factors and couple dynamics. Contraception 2006;73:65–71. [DOI] [PubMed] [Google Scholar]
- 26. Ayoola AB, Slager D, Feenstra C, et al. A feasibility study of women's confidence and comfort in use of a kit to monitor ovulation. J Midwifery Womens Health 2015;60:604–9. 10.1111/jmwh.12347 [DOI] [PubMed] [Google Scholar]
- 27. Kopitzke EJ, Berg BJ, Wilson JF, et al. Physical and emotional stress associated with components of the infertility investigation: perspectives of professionals and patients. Fertil Steril 1991;55:1137–43. 10.1016/S0015-0282(16)54365-9 [DOI] [PubMed] [Google Scholar]
- 28. Jones G, Carlton J, Weddell S, et al. Women's experiences of ovulation testing: a qualitative analysis. Reprod Health 2015;12 10.1186/s12978-015-0103-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pyper C, Bromhall L, Dummett S, et al. The Oxford conception study design and recruitment experience. Paediatr Perinat Epidemiol 2006;20(Suppl 1):51–9. [DOI] [PubMed] [Google Scholar]
- 30. Wang X, Chen C, Wang L, et al. Conception, early pregnancy loss, and time to clinical pregnancy: a population-based prospective study. Fertil Steril 2003;79:577–84. 10.1016/S0015-0282(02)04694-0 [DOI] [PubMed] [Google Scholar]
- 31. Gnoth C, Godehardt D, Godehardt E, et al. Time to pregnancy: results of the German prospective study and impact on the management of infertility. Hum Reprod 2003;18:1959–66. 10.1093/humrep/deg366 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgh-2019-001403supp001.pdf (324.1KB, pdf)
bmjgh-2019-001403supp002.docx (22.2KB, docx)