The streptococci are increasingly recognized as a core component of the cystic fibrosis (CF) lung microbiome, yet the role that they play in CF lung disease is unclear. The presence of the Streptococcus milleri group (SMG; also known as the anginosus group streptococci [AGS]) correlates with exacerbation when these microbes are the predominant species in the lung.
KEYWORDS: Streptococcus, Streptococcus milleri group, cystic fibrosis, exacerbation, polymicrobial
ABSTRACT
The streptococci are increasingly recognized as a core component of the cystic fibrosis (CF) lung microbiome, yet the role that they play in CF lung disease is unclear. The presence of the Streptococcus milleri group (SMG; also known as the anginosus group streptococci [AGS]) correlates with exacerbation when these microbes are the predominant species in the lung. In contrast, microbiome studies have indicated that an increased relative abundance of streptococci in the lung, including members of the oral microflora, correlates with impacts on lung disease less severe than those caused by other CF-associated microflora, indicating a complex role for this genus in the context of CF. Recent findings suggest that streptococci in the CF lung microenvironment may influence the growth and/or virulence of other CF pathogens, as evidenced by increased virulence factor production by Pseudomonas aeruginosa when grown in coculture with oral streptococci. Conversely, the presence of P. aeruginosa can enhance the growth of streptococci, including members of the SMG, a phenomenon that could be exacerbated by the fact that streptococci are not susceptible to some of the frontline antibiotics used to treat P. aeruginosa infections. Collectively, these studies indicate the necessity for further investigation into the role of streptococci in the CF airway to determine how these microbes, alone or via interactions with other CF-associated pathogens, might influence CF lung disease, for better or for worse. We also propose that the interactions of streptococci with other CF pathogens is an ideal model to study clinically relevant microbial interactions.
CYSTIC FIBROSIS LUNG INFECTIONS ARE POLYMICROBIAL AND INCLUDE STREPTOCOCCI
Patients with cystic fibrosis (CF) have a thick, dehydrated mucus in their airway, which reduces mucociliary clearance and allows for colonization by bacteria. The lung infections in CF patients have been demonstrated to be polymicrobial and complex (1–3), and correlations between certain lung pathogens and declining lung function have been established, as demonstrated for Pseudomonas aeruginosa (4), despite the current high level of antibiotic treatment and eradication strategies (5). Historically, Streptococcus spp. isolated from airway-derived sputum samples were considered to be oropharyngeal contaminants from the process of expectoration (6) or were not isolated at all in clinical laboratories (7). However, streptococci are increasingly recognized as members of the CF lung microbiome, having been cultured from and identified through 16S rRNA gene sequencing of bacteria from literally thousands of sputum and lavage samples (1, 2, 6–21). Streptococci have also been found in multiple protected brush samples collected using a bronchoscope (11); using such protected brushes greatly reduces the risk of contamination by oral flora and supports the conclusion that these microbes are indeed found in the lower airway in patients with CF. As outlined below, specialized medium is required for selecting for the growth of streptococci (7, 22); thus, these organisms can often be missed during routine plating of clinical samples from patients with CF (20). Finally, given the proximity of the oral cavity and the lower airway, it is not surprising to find in the lung microbes that have been seeded from the oral microbiota (23–28). Thus, the presence of Streptococcus in the CF airway available to interact with P. aeruginosa and other organisms should not come as a surprise.
Here, we explore the complex role of streptococci in CF-associated airway infections (Fig. 1), in particular, the observation that streptococci, in a species-dependent fashion, either may be associated with a lower disease burden than typical CF pathogens (2, 9, 12, 21, 29) or, alternatively, may contribute to pulmonary exacerbations (1, 6, 17–19). We also review recent studies indicating that streptococci influence and can be influenced by other microbes in the context of a polymicrobial infection in patients with CF.
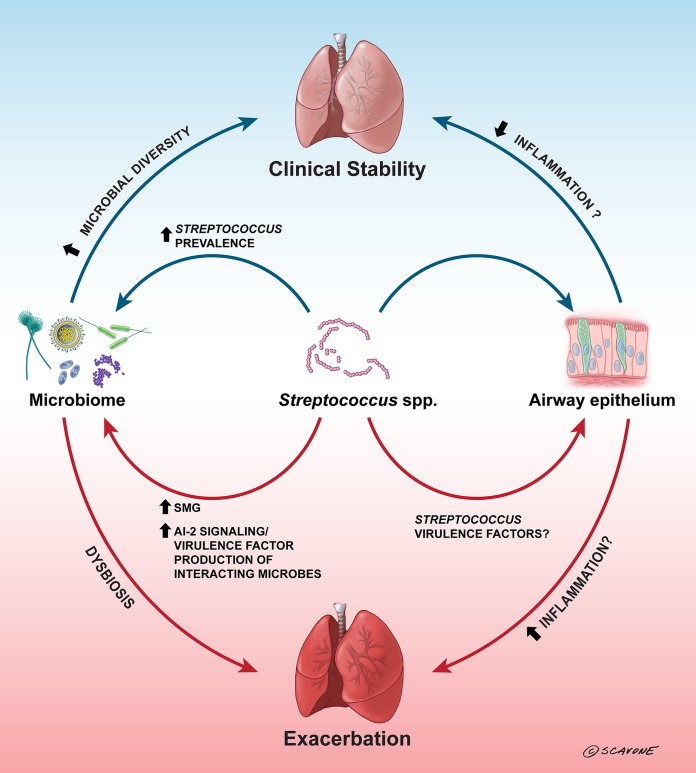
FIG 1.
Streptococci can influence cystic fibrosis airway disease. In CF airways, an increased prevalence of Streptococcus spp. has been correlated with clinical stability, lower inflammation, and/or less severe outcomes (2, 9, 12, 21, 29), but it is unclear whether this is due to direct interactions with the airway epithelium, is due to interactions between the streptococci and the microbiome that lead to increased microbial diversity, or is a consequence of less damage to the airway. Oral streptococci have been demonstrated to inhibit P. aeruginosa growth through the production of hydrogen peroxide and reactive nitrogenous intermediates (49–51); by inhibiting P. aeruginosa, streptococci could open up niches for increased microbial diversity in the lung, which could aid clinical stability. In contrast, the predominance of SMG isolates has been correlated with exacerbation (1, 7, 17, 30), and this may be due to interactions between the SMG isolates and other CF pathogens, such as P. aeruginosa. Oral streptococci have been demonstrated to stimulate increased virulence factor production by P. aeruginosa (43–45) through AI-2 signaling (43), and this could lead to dysbiosis in the lung and eventual exacerbation. The arrows indicate both direct and indirect interactions that are suggested to occur in the CF airways. The blue arrows indicate generally positive effects, the red arrows indicate generally negative effects, and the arrows are labeled with the factors (if known) involved in these effects. (Copyright William Scavone, Kestrel Studio; reproduced with permission.)
THE STREPTOCOCCUS MILLERI GROUP AS AN AGENT OF EXACERBATION
Several studies have characterized the correlation between the Streptococcus milleri group (SMG; also known as the anginosus group streptococci [AGS]) and pulmonary exacerbation (1, 7, 17, 30). These studies demonstrate that when SMG isolates are the numerically dominant pathogens in the lung, patients experience an exacerbation that will not resolve until the levels of SMG in the airway have been adequately reduced through antibiotic therapy (1, 7, 17, 30). Utilizing a semiselective agar medium for the detection of SMG isolates, Sibley and colleagues found that these microbes have an overall prevalence of ∼40% in their patient population and that in cases where the patient was hospitalized for a pulmonary exacerbation, SMG isolates were observed to reach numerical dominance (>107 CFU per milliliter of sputum) (7). Another group demonstrated that nalidixic acid and sulfamethazine (NAS) agar also allowed for semiselective quantification of SMG isolates from sputum samples; these investigators isolated members of the SMG from 6 out of 10 sputum samples from patients with CF (31). Together, these data suggest that the members of the SMG represent significant (and underappreciated) pathogens in CF airway disease. Furthermore, it was demonstrated that in 73% and 29% of cases, respectively, P. aeruginosa and Staphylococcus aureus were found to cocolonize patients with SMG isolates, indicating that this group of streptococci may interact with these better-known CF pathogens, which could affect the virulence and growth of these pathogens (7).
IS THERE A ROLE FOR TYPICAL PATHOGENIC STREPTOCOCCI IN CF?
As discussed above, streptococci found in the CF airway are typically associated with the normal oral flora. However, recent studies suggest that pathogenic streptococci that are typically associated with acute infections of the respiratory tract can also be found in the airways of patients with CF. For example, 15 of 318 (4.7%) adults examined in a retrospective study were culture positive for group A streptococcus (GAS) in their sputum, and 7/15 of these patients were suffering from an exacerbation at the time of the positive culture (32), analogous to the observations made for SMG isolates and exacerbation described above. In another study, ∼20% of the 212 children with CF studied were shown to have detectable Streptococcus pneumoniae when oropharyngeal swab specimens were analyzed by quantitative PCR (33). Interestingly, Dennis and colleagues showed that mucoid variants of S. pneumoniae could be isolated from the sputum of children with CF (32). Furthermore, while these mucoid strains showed reduced initial biofilm formation in vitro compared to nonmucoid strains, they eventually formed more robust biofilms and were more virulent in a mouse model of CF. Thus, the phenotypes of the mucoid and nonmucoid S. pneumoniae strains mirror those of mucoid and nonmucoid P. aeruginosa strains (34, 35). The mucoid phenotype of the S. pneumoniae isolates was due to capsule production, and these strains switched to a nonmucoid phenotype, likely via phase variation, while growing in biofilms (32, 36), which is likely their mode of growth in the context of a CF-related infection.
The interaction of P. aeruginosa with S. pneumoniae has not been investigated in any depth, but one report suggests a mechanism whereby P. aeruginosa could promote the growth or persistence of S. pneumoniae. The LasB elastase, whose production can be stimulated by streptococci, as discussed below, was shown using in vitro studies to reduce the efficacy of alveolar macrophages in the clearing of both P. aeruginosa and S. pneumoniae by ∼2-fold (37). These data indicated that P. aeruginosa could modify the airway environment to promote the persistence of S. pneumoniae. Therefore, we would suggest that future studies of P. aeruginosa-Streptococcus interactions include S. pneumoniae and GAS, as well as the more typical CF-associated streptococcal species.
STREPTOCOCCI ARE ASSOCIATED WITH DISEASE LESS SEVERE THAN THAT CAUSED BY TRADITIONAL CF PATHOGENS
Five recent microbiome studies indicate that colonization of the CF lung by oral Streptococcus spp. may be associated with a disease burden less severe than that associated with typical CF pathogens (2, 9, 12, 21, 29). Work from our group has indicated that an increased relative abundance of members of the genus Streptococcus in the lung, determined by 16S rRNA gene sequencing, correlates with clinical stability (2). Another study utilizing 16S rRNA gene sequencing indicated that the relative abundance of streptococci was significantly lower in patients with CF in the lowest quartile of forced expiratory volume in 1 s (FEV1), a key marker of lung function (9); thus, a low relative abundance of streptococci (and, thus, a higher abundance of other CF pathogens) was associated with poorer lung function. It has also been demonstrated that the relative abundance of streptococci increases when patients undergo CF transmembrane conductance regulator modulation therapy with ivacaftor, while the relative abundance of P. aeruginosa declines (12). In another study, Acosta et al., studying a cohort of 104 patients with CF (18 to 22 years of age), showed that higher levels of Streptococcus were associated with a decreased likelihood of progressing to early end-stage lung disease (29). Furthermore, Coburn et al., examining 269 patients over a 60-year age range, identified Streptococcus spp. to be part of the core lung microbiota (as assessed via analysis of sputum) and observed a greater degree of dominance of this microbe in younger patients (<25 years of age) and in patients with less severe disease (21). The same study (21) observed that when Streptococcus spp. were the dominant organisms, the overall diversity of the community was higher in both pediatric and adult patients. This finding is consistent with that of a longitudinal study of CF patients reported by Zhao and colleagues, especially for the samples from early in life (38). Finally, a separate study suggests that the increased diversity in CF airway communities is beneficial, as low diversity was associated with more rapid lung function decline over the following 5-year period (29). Perhaps this increased diversity helps to mitigate airway damage directly or by effectively competing against traditional CF pathogens, such as P. aeruginosa and Burkholderia. Zemanick et al. made similar observations studying a cohort of 136 pediatric and 10 adult patients with CF across several centers (14), with Streptococcus spp. more frequently being the dominant microbe among younger subjects (<6 years old) and with an inverse correlation between airway inflammation and the levels of Streptococcus spp. being seen. Importantly, these investigators found that the lower levels of inflammatory markers associated with the presence of Streptococcus spp. remained even after controlling for the relative abundance of Pseudomonas via a multivariate regression analysis; we take these data to suggest that it is the presence of Streptococcus, rather than the absence of Pseudomonas, that is associated with lower airway inflammation. Finally, metatranscriptome analysis of a cohort of patients with a second disease associated with chronic bacterial infections in the airway, chronic obstructive pulmonary disease (COPD), showed that while patients with the highest levels of transcriptionally active Streptococcus spp. had higher bacterial loads, they were less likely to suffer from an exacerbation (39).
Together, these studies indicate that the streptococci may influence disease progression through a mechanism that is not currently understood but that may function by mitigating airway inflammation and/or damage. While the absence of any bacterial load in the airway is preferred, in the context of CF, these studies suggest in the aggregate that if microbes are present, the less severe outcome appears to be associated with a high relative abundance of streptococci. It is important to note here that we are not suggesting that Streptococcus should be considered a probiotic to treat CF-associated infections but, rather, are noting several clinical associations wherein patients with higher loads of Streptococcus, compared to other CF pathogens' appear to have a lower disease burden. Of course, given the correlative nature of these studies, it is equally possible that less damaged lungs better support high relative levels of streptococci.
The data presented here, combined with the findings of the studies described above associating SMG members and S. pneumoniae with exacerbation in CF, paint a complicated picture of how streptococci impact the host in the context of polymicrobial CF airway infections. Thus, in future studies examining the role of Streptococcus spp. in disease, it is of utmost importance to understand which species of these microbes are present, as well as which strains should be chosen for use in any in vitro studies.
STREPTOCOCCI IMPACT P. AERUGINOSA BIOLOGY, VIABILITY, AND VIRULENCE FACTOR PRODUCTION
There are several studies that indicate that streptococci and P. aeruginosa cocolonize patients with CF (1, 2, 8, 10), and thus, these microbes likely have the opportunity to interact with each other, although additional studies are sorely needed to understand the extent of this interaction in vivo. In vitro studies also demonstrate that mucoid and nonmucoid P. aeruginosa strains can coaggregate with a variety of oral streptococci (40, 41), and our group showed that P. aeruginosa and Streptococcus constellatus could form mixed microcolonies in vitro when these microbes formed biofilms on a CF patient-derived airway cell line (42). These data both indicate the potential for these different microbes to physically interact and provide a mechanism whereby these oral microbes might be carried deeper into the airway via their physical association with P. aeruginosa. Recent studies have just begun to explore how such interactions might impact the respective microbes in the context of polymicrobial infections, and we review such studies below.
One study demonstrated that an oropharyngeal Streptococcus isolate interacts with P. aeruginosa through AI-2 signaling and that this signal induces transcription of the P. aeruginosa virulence factors elastase, phenazines, and rhamnolipids, resulting in increased P. aeruginosa-mediated virulence in a rat agar bead model of chronic lung infection (43). In contrast, the oropharyngeal Streptococcus isolate did not contribute to lung disease in monoculture in the rat lung. These results are further supported by additional studies that demonstrate increased elastase, phenazine, and rhamnolipid production by P. aeruginosa strains exposed to streptococci, including Streptococcus anginosus (44, 45). Interestingly, two lasR mutant variants of P. aeruginosa from patients with CF showed more robust pyocyanin and elastase production and associated host damage and inflammation when grown in coculture with several SMG members (44), indicating that coculture with another microbial species can (at least in part) bypass the quorum sensing (QS) defect of the lasR mutant strains. Analogous observations have been made for P. aeruginosa lasR mutants growing in coculture with the fungus Candida albicans (46), indicating that the loss of QS signaling observed for pure strains of P. aeruginosa isolated from the CF airway may not accurately reflect the situation in the context of an in vivo, polymicrobial infection. Furthermore, a recent study also demonstrated that S. anginosus can stimulate P. aeruginosa to convert from a mucoid phenotype to a nonmucoid, high-pyocyanin-producing phenotype in an in vitro, hypercapnic (10% CO2) environment; this phenotype is associated with the reduced survival of Galleria mellonella wax moths, which are used as an insect model of pathogenesis, during infection (47, 48). Taken together, these data call into question the strengths of the conclusions that can be drawn regarding the virulence potential of strains in the CF airway based solely on in vitro clinical culture data assaying single species.
In contrast to the positive interaction described above, some interactions between P. aeruginosa and streptococci can be detrimental to P. aeruginosa. For example, oral streptococci, such as S. oralis, S. sanguinis, and S. gordonii, have been shown to produce H2O2, which can inhibit P. aeruginosa when the streptococci are established as primary colonizers (49, 50). Interestingly, these interactions are influenced by environmental conditions, such as (i) aerobic conditions, when S. oralis can cocolonize with P. aeruginosa (49); (ii) conditions in which streptococci can use H2O2 production to outcompete P. aeruginosa in 10% CO2 (49); or (iii) environments wherein S. parasanguinis, S. gordonii, and S. sanguinis can produce H2O2, which reacts with excess nitrite in the medium to form reactive nitrogenous intermediates (RNI) that inhibit P. aeruginosa growth (50, 51). Together, these studies indicate that the local environment might play a large role in the outcome of interactions among these microbial species, which is of great relevance to a CF airway, which includes anoxic and hypoxic environments (52–54), as well as the more typical normoxic environment. It is also important to note that there is clearly variability in the observations made regarding interactions as a function of which isolate of P. aeruginosa and/or a Streptococcus sp. is used; therefore, investigators should strongly consider using multiple isolates of P. aeruginosa (mucoid and nonmucoid) and multiple Streptococcus species (and isolates of those species) to better understand the generality of any finding related to polymicrobial interactions.
As mentioned above, Scoffield and Wu (50) documented that S. parasanguinis, S. gordonii, and S. sanguinis can inhibit the growth of P. aeruginosa when provided with nitrite; it is the interaction of the nitrite plus the Streptococcus-derived peroxide that results in the generation of RNI. Given the evidence that P. aeruginosa participates in dissimilatory nitrogen metabolism in environments like the CF airway (55–57) and the evidence for nitrate and denitrification in CF patient sputum (58–62), there is a clear potential for the generation of RNI in the context of these polymicrobial infections. Further supporting their model, in a subsequent report (51), Scoffield and Wu showed that a P. aeruginosa strain defective for its nitrite reductase (nirS) showed increased sensitivity to nitrite-mediated killing, likely due to the accumulation of nitrite in the culture (51) and, presumably, increased RNI production. Interestingly, these investigators identified a mutation in the permease component of an ABC transporter (PA3252) that was resistant to this killing by S. parasanguinis (50), indicating the possibility that the relationship between these microbes has the potential to evolve in the context of chronic, long-term CF airway infections. These investigators did show that S. parasanguinis could protect Drosophila from a P. aeruginosa infection, indicating an important proof of principle that such interactions may indeed occur in vivo.
Finally, it is likely that other metabolic interactions in the CF airway might drive changes in the interaction between P. aeruginosa and Streptococcus. As an example, Flynn and colleagues (63) showed that P. aeruginosa can only inefficiently utilize the mucins found in abundance in the CF airway, but they demonstrated that some anaerobes, including Streptococcus, as well as Prevotella and Veillonela, can degrade mucins and generate small fatty acids that can be utilized by P. aeruginosa for enhanced growth. Oral streptococci have previously been shown to effectively degrade salivary mucins (64). In particular, Hunter and colleagues, using growth and mutational studies in vitro and measuring the gene expression of P. aeruginosa in sputum, noted that propionate is one such carbon/energy source produced by anaerobes and utilized by P. aeruginosa (63). Indeed, this report and others have detected propionate in lavage and sputum samples from the CF airway (63, 65, 66). There are likely additional such interactions between these (and other) microbes driven by metabolic cross feeding that will be uncovered going forward.
P. AERUGINOSA IMPACTS STREPTOCOCCAL VIABILITY AND GROWTH
Just as streptococci can impact P. aeruginosa, the converse is true as well. A recent report from our group demonstrated that P. aeruginosa limits Streptococcus growth, likely via iron sequestration, in minimal medium coculture conditions (67). Iron availability in the airway has been demonstrated to range from 0.02 μM in healthy controls to levels as high as 8 μM in CF patients, and the measured patient-to-patient variability is quite high in CF patient sputum (68–71). Furthermore, the extent of iron bioavailable to streptococci is unclear and difficult to determine. Given the potentially high concentration of iron in the CF airway, it is not clear if P. aeruginosa and streptococci are competing for iron in the CF lung and, if so, when or where.
P. aeruginosa has also been demonstrated to suppress S. constellatus biofilm formation through the secretion of monorhamnolipids and β-hydroxyalkanoyl-β-hydroxyalkanoic acids (HAAs) (42). This suppression of biofilm formation can be alleviated by treatment of CF patients with the maintenance antibiotic tobramycin. These data indicate that the CF airway environment and therapeutics used in the context of CF can also modulate microbe-microbe interactions directly, as described above, and perhaps indirectly via altering the airway environment via modulating iron levels, for example.
In contrast to the negative interactions described above, recent studies have found that P. aeruginosa can also enhance Streptococcus growth through one or more currently undescribed pathways (42, 44, 47, 48, 67). For example, our group demonstrated that P. aeruginosa clinical and laboratory strains can enhance the growth of multiple oral Streptococcus spp., including members of the SMG, through a currently unknown mechanism (67). These data indicate that the presence of P. aeruginosa can promote the growth and/or persistence of streptococci in the context of the CF airway.
Interestingly, a recent study using a metagenome-based approach argued that Streptococcus spp. replicated their genomes (a surrogate for growth) 40- to 60-fold faster than P. aeruginosa (72). This finding would be consistent with the published observations that coculture of Streptococcus with P. aeruginosa enhances the growth of the Streptococcus with no obvious benefit to P. aeruginosa growth (42, 67).
Furthermore, Scoffield and colleagues investigated the increased biofilm formation of S. parasanguinis cocultured with a mucoid P. aeruginosa strain and found that S. parasanguinis is able to bind to P. aeruginosa-produced alginate using the streptococcal surface adhesin BapA1 in vitro or BapA1 and Fap1 in a Drosophila melanogaster coinfection model (73).
Taken together, these studies indicate that, at least in some contexts, P. aeruginosa can enhance Streptococcus species growth or biofilm formation; however, the mechanisms underlying such interactions are still poorly understood. Thus, a significant effort directed toward understanding the mechanisms underlying P. aeruginosa-Streptococcus interactions will likely be fruitful going forward.
INTERACTIONS BETWEEN STREPTOCOCCI AND OTHER CF-ASSOCIATED PATHOGENS
Very few studies have investigated the interactions between streptococci and other pathogens in the context of CF. For example, anaerobic Gram-negative bacteria that produce β-lactamases, in particular, Bacteroides spp., have been shown to protect GAS from penicillin in a mouse model of infection (74), and thus, it is possible that other β-lactamase-producing microbes could exert a similar protective effect for other streptococci (75, 76).
Additionally, there is a dearth of investigations into multispecies interactions (i.e., the interactions of 3 or more microbes) in the context of CF, but two interesting studies have investigated how polymicrobial interactions among S. anginosus, S. aureus, and P. aeruginosa affect antimicrobial susceptibility in a multispecies biofilm (77, 78). The first study indicates that S. anginosus biofilm cells can be protected from cell wall-active antibiotics when grown in a multispecies biofilm with P. aeruginosa and S. aureus or when S. anginosus monoculture biofilms are treated with S. aureus supernatant (77), indicating that S. aureus is able to protect S. anginosus from these antibiotics. Additionally, these investigators demonstrated that S. aureus is sensitized to both cell wall-active antibiotics and others antibiotics, like tobramycin, in a multispecies biofilm with P. aeruginosa and S. anginosus (77). P. aeruginosa antibiotic sensitivity is relatively unaffected during polymicrobial culture (77), a finding consistent with the findings of our studies (79). In a subsequent study, the same team explored the mechanism whereby coculture in a community could render S. anginosus biofilm cells protected from the cell wall-active antibiotic vancomycin (78). Tavernier et al. reported (78) that in the presence of P. aeruginosa, S. anginosus produces a thicker cell wall, which has previously been described to be a mechanism by which S. aureus is protected from treatment with vancomycin (80–82). Consistent with this observation, coculture of S. anginosus with P. aeruginosa resulted in the upregulation of 36 genes in the cell wall synthesis and recycling functional category, the largest such category of genes upregulated in S. anginosus during growth in coculture, compared to their regulation in a monoculture of S. anginosus.
Together, these studies indicate that the interactions among streptococci and other CF pathogens can be complex, and the possible outcomes of these interactions in the CF lung microenvironment are not always obvious. However, given that ∼30% of patients with CF have both S. aureus and P. aeruginosa in their airway (83, 84) and SMG members were found to cocolonize patients infected with P. aeruginosa and S. aureus (2, 7, 85), such polymicrobial interactions are likely not uncommon. Additionally, interactions with streptococci are not limited to the lower airways, and a multitude of studies have investigated polymicrobial interactions with streptococci in the oral microbiome (reviewed in reference 86). These oral microbiome studies may provide some insights into how streptococci could interact with microbes in the CF airway. However, further research is required to fully understand how streptococci might interact with other CF lung pathogens and how these interactions may modulate microbial growth or biofilm formation and/or impact the host airway.
CONCLUSIONS
The role that streptococci play in the CF lung needs to be investigated further, because the current evidence indicates that the predominance of the Streptococcus milleri group correlates with exacerbations, while the increased relative abundance of other streptococci may correlate with a lower disease burden in CF patients. It is currently unclear how these complex relationships between streptococci and outcomes in CF patients are mediated.
For the detrimental interactions, it is unclear whether these effects are due directly to the interactions between Streptococcus spp. and the host or whether streptococci are able to negatively influence patient health indirectly through interactions with cocolonizing microbes within the lung (Fig. 1). The data thus far indicate that oral streptococci can potentiate virulence factor production of P. aeruginosa in vitro and in vivo (43–45, 47, 48).
In contrast, as described above, younger and healthier patients earlier in the disease stage are found to have a greater diversity in the airway microbiome, including more streptococci, and, thus, a lower abundance of typical CF pathogens. It is possible that the streptococci found in the airway are able to reduce the relative abundance of P. aeruginosa through the production of hydrogen peroxide (49) and reactive nitrogenous species (50, 51), as described in in vitro coculture studies. Alternatively, oral streptococci may actively reduce inflammation, a conclusion consistent with a recent report (87). Finally, streptococci may exert their impact via a niche exclusion mechanism, that is, by simply preventing high-level colonization of the airway by P. aeruginosa or other CF pathogens. Additional studies are required to determine the nature of the influence that the streptococci have on the CF airway.
Finally, it is currently unknown what kinds of interactions streptococci may have with CF lung pathogens other than P. aeruginosa, such as S. aureus, Stenotrophomonas, Burkholderia, or others. All of these interactions can potentially impact host health, as well as the responsiveness of these microbes to therapeutics. It is clear that for polymicrobial interactions, such as those in CF patients, that we must move beyond the one-bug mind-set. The observation that streptococci can have differential impacts on patient outcomes adds an additional layer of complexity to any analysis. We would argue, however, that the large available data sets on the airway microbiota in CF patients during clinical stability and disease exacerbation, plus the rich clinical data available, provide a unique opportunity to study polymicrobial interactions and their impact on the host using streptococci as model organisms.
Given the well-documented nature of polymicrobial infections in the context of CF (1–3), we suggest that interactions of streptococci with other CF-related pathogens is an emerging model system that may be used to provide an understanding of the molecular mechanisms of microbe-microbe interactions; such studies can exploit the abundant extant data regarding the environmental, nutritional, and microbial context of the CF lung. While other interactions have been studied in depth, particularly between P. aeruginosa and S. aureus and their negative impacts on CF patients (84), we see that one advantage of using streptococci as a model system is, as described above, the participation of these microbes in processes with varied impacts on the host. We also argue that the most productive approach to dissecting such interactions is to leverage multiple techniques, including microbiome studies, in vitro models, clinical studies, the latest omics (metagenomics, metabolomics, proteomics) technologies, and modeling, and such data have been generated for sputum, lavage, and brush samples from the CF airway. Finally, the ability to generate complementary data from laboratory experimental models and the analysis of large clinical data sets make such an approach particularly powerful.
ACKNOWLEDGMENTS
We thank K. Antosca and J. Madan for their helpful comments on the manuscript.
This work was supported by a Molecular and Cellular Biology at Dartmouth training grant (T32GM008704) and the Munck-Pfefferkorn Fund, the Cystic Fibrosis Foundation (OTOOLE16GO), and NIH (R37 AI83256-06) to G.A.O.
REFERENCES
- 1.Sibley CD, Parkins MD, Rabin HR, Duan K, Norgaard JC, Surette MG. 2008. A polymicrobial perspective of pulmonary infections exposes an enigmatic pathogen in cystic fibrosis patients. Proc Natl Acad Sci U S A 105:15070–15075. doi: 10.1073/pnas.0804326105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Filkins LM, Hampton TH, Gifford AH, Gross MJ, Hogan DA, Sogin ML, Morrison HG, Paster BJ, O'Toole GA. 2012. Prevalence of streptococci and increased polymicrobial diversity associated with cystic fibrosis patient stability. J Bacteriol 194:4709–4717. doi: 10.1128/JB.00566-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Filkins LM, O'Toole GA. 2015. Cystic fibrosis lung infections: polymicrobial, complex, and hard to treat. PLoS Pathog 11:e1005258. doi: 10.1371/journal.ppat.1005258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harun SN, Wainwright C, Klein K, Hennig S. 2016. A systematic review of studies examining the rate of lung function decline in patients with cystic fibrosis. Paediatr Respir Rev 20:55–66. doi: 10.1016/j.prrv.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Langton Hewer SC, Smyth AR. 2017. Antibiotic strategies for eradicating Pseudomonas aeruginosa in people with cystic fibrosis. Cochrane Database Syst Rev 4:CD004197. doi: 10.1002/14651858.CD004197.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sibley CD, Rabin H, Surette MG. 2006. Cystic fibrosis: a polymicrobial infectious disease. Future Microbiol 1:53–61. doi: 10.2217/17460913.1.1.53. [DOI] [PubMed] [Google Scholar]
- 7.Sibley CD, Grinwis ME, Field TR, Parkins MD, Norgaard JC, Gregson DB, Rabin HR, Surette MG. 2010. McKay agar enables routine quantification of the 'Streptococcus milleri' group in cystic fibrosis patients. J Med Microbiol 59:534–540. doi: 10.1099/jmm.0.016592-0. [DOI] [PubMed] [Google Scholar]
- 8.Tunney MM, Field TR, Moriarty TF, Patrick S, Doering G, Muhlebach MS, Wolfgang MC, Boucher R, Gilpin DF, McDowell A, Elborn JS. 2008. Detection of anaerobic bacteria in high numbers in sputum from patients with cystic fibrosis. Am J Respir Crit Care Med 177:995–1001. doi: 10.1164/rccm.200708-1151OC. [DOI] [PubMed] [Google Scholar]
- 9.Flight WG, Smith A, Paisey C, Marchesi JR, Bull MJ, Norville PJ, Mutton KJ, Webb AK, Bright-Thomas RJ, Jones AM, Mahenthiralingam E. 2015. Rapid detection of emerging pathogens and loss of microbial diversity associated with severe lung disease in cystic fibrosis. J Clin Microbiol 53:2022–2029. doi: 10.1128/JCM.00432-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Acosta N, Whelan FJ, Somayaji R, Poonja A, Surette MG, Rabin HR, Parkins MD. 2017. The evolving cystic fibrosis microbiome: a comparative cohort study spanning 16 years. Ann Am Thorac Soc 14:1288–1297. doi: 10.1513/AnnalsATS.201609-668OC. [DOI] [PubMed] [Google Scholar]
- 11.Hogan DA, Willger SD, Dolben EL, Hampton TH, Stanton BA, Morrison HG, Sogin ML, Czum J, Ashare A. 2016. Analysis of lung microbiota in bronchoalveolar lavage, protected brush and sputum samples from subjects with mild-to-moderate cystic fibrosis lung disease. PLoS One 11:e0149998. doi: 10.1371/journal.pone.0149998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ronan NJ, Einarsson GG, Twomey M, Mooney D, Mullane D, NiChroinin M, O'Callaghan G, Shanahan F, Murphy DM, O'Connor OJ, Shortt CA, Tunney MM, Eustace JA, Maher MM, Elborn JS, Plant BJ. 2018. CORK study in cystic fibrosis: sustained improvements in ultra-low-dose chest CT scores after CFTR modulation with ivacaftor. Chest 153:395–403. doi: 10.1016/j.chest.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Maeda Y, Elborn JS, Parkins MD, Reihill J, Goldsmith CE, Coulter WA, Mason C, Millar BC, Dooley JS, Lowery CJ, Ennis M, Rendall JC, Moore JE. 2011. Population structure and characterization of viridans group streptococci (VGS) including Streptococcus pneumoniae isolated from adult patients with cystic fibrosis (CF). J Cyst Fibros 10:133–139. doi: 10.1016/j.jcf.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 14.Zemanick ET, Wagner BD, Robertson CE, Ahrens RC, Chmiel JF, Clancy JP, Gibson RL, Harris WT, Kurland G, Laguna TA, McColley SA, McCoy K, Retsch-Bogart G, Sobush KT, Zeitlin PL, Stevens MJ, Accurso FJ, Sagel SD, Harris JK. 2017. Airway microbiota across age and disease spectrum in cystic fibrosis. Eur Respir J 50:1700832. doi: 10.1183/13993003.00832-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kramná L, Dřevínek P, Lin J, Kulich M, Cinek O. 2018. Changes in the lung bacteriome in relation to antipseudomonal therapy in children with cystic fibrosis. Folia Microbiol (Praha) 63:237–248. doi: 10.1007/s12223-017-0562-3. [DOI] [PubMed] [Google Scholar]
- 16.Mahboubi MA, Carmody LA, Foster BK, Kalikin LM, VanDevanter DR, LiPuma JJ. 2016. Culture-based and culture-independent bacteriologic analysis of cystic fibrosis respiratory specimens. J Clin Microbiol 54:613–619. doi: 10.1128/JCM.02299-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parkins MD, Sibley CD, Surette MG, Rabin HR. 2008. The Streptococcus milleri group—an unrecognized cause of disease in cystic fibrosis: a case series and literature review. Pediatr Pulmonol 43:490–497. doi: 10.1002/ppul.20809. [DOI] [PubMed] [Google Scholar]
- 18.Sibley CD, Sibley KA, Leong TA, Grinwis ME, Parkins MD, Rabin HR, Surette MG. 2010. The Streptococcus milleri population of a cystic fibrosis clinic reveals patient specificity and intraspecies diversity. J Clin Microbiol 48:2592–2594. doi: 10.1128/JCM.00414-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grinwis ME, Sibley CD, Parkins MD, Eshaghurshan CS, Rabin HR, Surette MG. 2010. Characterization of Streptococcus milleri group isolates from expectorated sputum of adult patients with cystic fibrosis. J Clin Microbiol 48:395–401. doi: 10.1128/JCM.01807-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zachariah P, Ryan C, Nadimpalli S, Coscia G, Kolb M, Smith H, Foca M, Saiman L, Planet PJ. 2018. Culture-independent analysis of pediatric bronchoalveolar lavage specimens. Ann Am Thorac Soc 15:1047–1056. doi: 10.1513/AnnalsATS.201802-146OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coburn B, Wang PW, Diaz Caballero J, Clark ST, Brahma V, Donaldson S, Zhang Y, Surendra A, Gong Y, Tullis ED, Yau YC, Waters VJ, Hwang DM, Guttman DS. 2015. Lung microbiota across age and disease stage in cystic fibrosis. Sci Rep 5:10241. doi: 10.1038/srep10241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vandeplassche E, Coenye T, Crabbe A. 2017. Developing selective media for quantification of multispecies biofilms following antibiotic treatment. PLoS One 12:e0187540. doi: 10.1371/journal.pone.0187540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dickson RP, Erb-Downward JR, Martinez FJ, Huffnagle GB. 2016. The microbiome and the respiratory tract. Annu Rev Physiol 78:481–504. doi: 10.1146/annurev-physiol-021115-105238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dickson RP, Huffnagle GB. 2015. The lung microbiome: new principles for respiratory bacteriology in health and disease. PLoS Pathog 11:e1004923. doi: 10.1371/journal.ppat.1004923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whiteson KL, Bailey B, Bergkessel M, Conrad D, Delhaes L, Felts B, Harris JK, Hunter R, Lim YW, Maughan H, Quinn R, Salamon P, Sullivan J, Wagner BD, Rainey PB. 2014. The upper respiratory tract as a microbial source for pulmonary infections in cystic fibrosis. Parallels from island biogeography. Am J Respir Crit Care Med 189:1309–1315. doi: 10.1164/rccm.201312-2129PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dickson RP, Erb-Downward JR, Prescott HC, Martinez FJ, Curtis JL, Lama VN, Huffnagle GB. 2014. Cell-associated bacteria in the human lung microbiome. Microbiome 2:28. doi: 10.1186/2049-2618-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dickson RP, Erb-Downward JR, Freeman CM, Walker N, Scales BS, Beck JM, Martinez FJ, Curtis JL, Lama VN, Huffnagle GB. 2014. Changes in the lung microbiome following lung transplantation include the emergence of two distinct Pseudomonas species with distinct clinical associations. PLoS One 9:e97214. doi: 10.1371/journal.pone.0097214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morris A, Beck JM, Schloss PD, Campbell TB, Crothers K, Curtis JL, Flores SC, Fontenot AP, Ghedin E, Huang L, Jablonski K, Kleerup E, Lynch SV, Sodergren E, Twigg H, Young VB, Bassis CM, Venkataraman A, Schmidt TM, Weinstock GM, Lung H. 2013. Comparison of the respiratory microbiome in healthy nonsmokers and smokers. Am J Respir Crit Care Med 187:1067–1075. doi: 10.1164/rccm.201210-1913OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Acosta N, Heirali A, Somayaji R, Surette MG, Workentine ML, Sibley CD, Rabin HR, Parkins MD. 2018. Sputum microbiota is predictive of long-term clinical outcomes in young adults with cystic fibrosis. Thorax 73:1016–1025. doi: 10.1136/thoraxjnl-2018-211510. [DOI] [PubMed] [Google Scholar]
- 30.Cade A, Denton M, Brownlee KG, Todd N, Conway SP. 1999. Acute bronchopulmonary infection due to Streptococcus milleri in a child with cystic fibrosis. Arch Dis Child 80:278–279. doi: 10.1136/adc.80.3.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Waite RD, Wareham DW, Gardiner S, Whiley RA. 2012. A simple, semiselective medium for anaerobic isolation of anginosus group streptococci from patients with chronic lung disease. J Clin Microbiol 50:1430–1432. doi: 10.1128/JCM.06184-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dennis EA, Coats MT, Griffin S, Pang B, Briles DE, Crain MJ, Swords WE. 2018. Hyperencapsulated mucoid pneumococcal isolates from patients with cystic fibrosis have increased biofilm density and persistence in vivo. Pathog Dis 76:fty073. doi: 10.1093/femspd/fty073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Esposito S, Colombo C, Tosco A, Montemitro E, Volpi S, Ruggiero L, Lelii M, Bisogno A, Pelucchi C, Principi N, Italian Pneumococcal Study Group on Cystic Fibrosis. 2016. Streptococcus pneumoniae oropharyngeal colonization in children and adolescents with cystic fibrosis. J Cyst Fibros 15:366–371. doi: 10.1016/j.jcf.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 34.Hentzer M, Teitzel GM, Balzer GJ, Heydorn A, Molin S, Givskov M, Parsek MR. 2001. Alginate overproduction affects Pseudomonas aeruginosa biofilm structure and function. J Bacteriol 183:5395–5401. doi: 10.1128/JB.183.18.5395-5401.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hay ID, Gatland K, Campisano A, Jordens JZ, Rehm BH. 2009. Impact of alginate overproduction on attachment and biofilm architecture of a supermucoid Pseudomonas aeruginosa strain. Appl Environ Microbiol 75:6022–6025. doi: 10.1128/AEM.01078-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Allegrucci M, Sauer K. 2007. Characterization of colony morphology variants isolated from Streptococcus pneumoniae biofilms. J Bacteriol 189:2030–2038. doi: 10.1128/JB.01369-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bastaert F, Kheir S, Saint-Criq V, Villeret B, Dang PM, El-Benna J, Sirard JC, Voulhoux R, Sallenave JM. 2018. Pseudomonas aeruginosa LasB subverts alveolar macrophage activity by interfering with bacterial killing through downregulation of innate immune defense, reactive oxygen species generation, and complement activation. Front Immunol 9:1675. doi: 10.3389/fimmu.2018.01675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao J, Schloss PD, Kalikin LM, Carmody LA, Foster BK, Petrosino JF, Cavalcoli JD, VanDevanter DR, Murray S, Li JZ, Young VB, LiPuma JJ. 2012. Decade-long bacterial community dynamics in cystic fibrosis airways. Proc Natl Acad Sci U S A 109:5809–5814. doi: 10.1073/pnas.1120577109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ren L, Zhang R, Rao J, Xiao Y, Zhang Z, Yang B, Cao D, Zhong H, Ning P, Shang Y, Li M, Gao Z, Wang J. 2018. Transcriptionally active lung microbiome and its association with bacterial biomass and host inflammatory status. mSystems 3:e00199-18. doi: 10.1128/mSystems.00199-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Komiyama K, Habbick BF, Gibbons RJ. 1987. Interbacterial adhesion between Pseudomonas aeruginosa and indigenous oral bacteria isolated from patients with cystic fibrosis. Can J Microbiol 33:27–32. doi: 10.1139/m87-005. [DOI] [PubMed] [Google Scholar]
- 41.Komiyama K, Gibbons RJ. 1984. Interbacterial adherence between Actinomyces viscosus and strains of Streptococcus pyogenes, Streptococcus agalactiae, and Pseudomonas aeruginosa. Infect Immun 44:86–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Price KE, Naimie AA, Griffin EF, Bay C, O'Toole GA. 2016. Tobramycin-treated Pseudomonas aeruginosa PA14 enhances Streptococcus constellatus 7155 biofilm formation in a cystic fibrosis model system. J Bacteriol 198:237–247. doi: 10.1128/JB.00705-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Duan K, Dammel C, Stein J, Rabin H, Surette MG. 2003. Modulation of Pseudomonas aeruginosa gene expression by host microflora through interspecies communication. Mol Microbiol 50:1477–1491. doi: 10.1046/j.1365-2958.2003.03803.x. [DOI] [PubMed] [Google Scholar]
- 44.Whiley RA, Sheikh NP, Mushtaq N, Hagi-Pavli E, Personne Y, Javaid D, Waite RD. 2014. Differential potentiation of the virulence of the Pseudomonas aeruginosa cystic fibrosis Liverpool epidemic strain by oral commensal streptococci. J Infect Dis 209:769–780. doi: 10.1093/infdis/jit568. [DOI] [PubMed] [Google Scholar]
- 45.Sibley CD, Duan K, Fischer C, Parkins MD, Storey DG, Rabin HR, Surette MG. 2008. Discerning the complexity of community interactions using a Drosophila model of polymicrobial infections. PLoS Pathog 4:e1000184. doi: 10.1371/journal.ppat.1000184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cugini C, Morales DK, Hogan DA. 2010. Candida albicans-produced farnesol stimulates Pseudomonas quinolone signal production in LasR-defective Pseudomonas aeruginosa strains. Microbiology 156:3096–3107. doi: 10.1099/mic.0.037911-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Waite RD, Qureshi MR, Whiley RA. 2017. Correction: modulation of behaviour and virulence of a high alginate expressing Pseudomonas aeruginosa strain from cystic fibrosis by oral commensal bacterium Streptococcus anginosus. PLoS One 12:e0176577. doi: 10.1371/journal.pone.0176577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Waite RD, Qureshi MR, Whiley RA. 2017. Modulation of behaviour and virulence of a high alginate expressing Pseudomonas aeruginosa strain from cystic fibrosis by oral commensal bacterium Streptococcus anginosus. PLoS One 12:e0173741. doi: 10.1371/journal.pone.0173741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Whiley RA, Fleming EV, Makhija R, Waite RD. 2015. Environment and colonisation sequence are key parameters driving cooperation and competition between Pseudomonas aeruginosa cystic fibrosis strains and oral commensal streptococci. PLoS One 10:e0115513. doi: 10.1371/journal.pone.0115513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scoffield JA, Wu H. 2015. Oral streptococci and nitrite-mediated interference of Pseudomonas aeruginosa. Infect Immun 83:101–107. doi: 10.1128/IAI.02396-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scoffield JA, Wu H. 2016. Nitrite reductase is critical for Pseudomonas aeruginosa survival during co-infection with the oral commensal Streptococcus parasanguinis. Microbiology 162:376–383. doi: 10.1099/mic.0.000226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Worlitzsch D, Tarran R, Ulrich M, Schwab U, Cekici A, Meyer KC, Birrer P, Bellon G, Berger J, Weiss T, Botzenhart K, Yankaskas JR, Randell S, Boucher RC, Doring G. 2002. Effects of reduced mucus oxygen concentration in airway Pseudomonas infections of cystic fibrosis patients. J Clin Invest 109:317–325. doi: 10.1172/JCI0213870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alvarez-Ortega C, Harwood CS. 2007. Responses of Pseudomonas aeruginosa to low oxygen indicate that growth in the cystic fibrosis lung is by aerobic respiration. Mol Microbiol 65:153–165. doi: 10.1111/j.1365-2958.2007.05772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cowley ES, Kopf SH, LaRiviere A, Ziebis W, Newman DK. 2015. Pediatric cystic fibrosis sputum can be chemically dynamic, anoxic, and extremely reduced due to hydrogen sulfide formation. mBio 6:e00767-15. doi: 10.1128/mBio.00767-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yoon SS, Hassett DJ. 2004. Chronic Pseudomonas aeruginosa infection in cystic fibrosis airway disease: metabolic changes that unravel novel drug targets. Expert Rev Anti Infect Ther 2:611–623. doi: 10.1586/14787210.2.4.611. [DOI] [PubMed] [Google Scholar]
- 56.Yoon SS, Hennigan RF, Hilliard GM, Ochsner UA, Parvatiyar K, Kamani MC, Allen HL, DeKievit TR, Gardner PR, Schwab U, Rowe JJ, Iglewski BH, McDermott TR, Mason RP, Wozniak DJ, Hancock RE, Parsek MR, Noah TL, Boucher RC, Hassett DJ. 2002. Pseudomonas aeruginosa anaerobic respiration in biofilms. Relationships to cystic fibrosis pathogenesis. Dev Cell 3:593–603. doi: 10.1016/S1534-5807(02)00295-2. [DOI] [PubMed] [Google Scholar]
- 57.Palmer KL, Brown SA, Whiteley M. 2007. Membrane-bound nitrate reductase is required for anaerobic growth in cystic fibrosis sputum. J Bacteriol 189:4449–4455. doi: 10.1128/JB.00162-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Palmer KL, Aye LM, Whiteley M. 2007. Nutritional cues control Pseudomonas aeruginosa multicellular behavior in cystic fibrosis sputum. J Bacteriol 189:8079–8087. doi: 10.1128/JB.01138-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kolpen M, Kragh KN, Bjarnsholt T, Line L, Hansen CR, Dalboge CS, Hansen N, Kuhl M, Hoiby N, Jensen PO. 2015. Denitrification by cystic fibrosis pathogens—Stenotrophomonas maltophilia is dormant in sputum. Int J Med Microbiol 305:1–10. doi: 10.1016/j.ijmm.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 60.Line L, Alhede M, Kolpen M, Kuhl M, Ciofu O, Bjarnsholt T, Moser C, Toyofuku M, Nomura N, Hoiby N, Jensen PO. 2014. Physiological levels of nitrate support anoxic growth by denitrification of Pseudomonas aeruginosa at growth rates reported in cystic fibrosis lungs and sputum. Front Microbiol 5:554. doi: 10.3389/fmicb.2014.00554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kolpen M, Kuhl M, Bjarnsholt T, Moser C, Hansen CR, Liengaard L, Kharazmi A, Pressler T, Hoiby N, Jensen PO. 2014. Nitrous oxide production in sputum from cystic fibrosis patients with chronic Pseudomonas aeruginosa lung infection. PLoS One 9:e84353. doi: 10.1371/journal.pone.0084353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Quinn RA, Lim YW, Maughan H, Conrad D, Rohwer F, Whiteson KL. 2014. Biogeochemical forces shape the composition and physiology of polymicrobial communities in the cystic fibrosis lung. mBio 5:e00956-13. doi: 10.1128/mBio.00956-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Flynn JM, Niccum D, Dunitz JM, Hunter RC. 2016. Evidence and role for bacterial mucin degradation in cystic fibrosis airway disease. PLoS Pathog 12:e1005846. doi: 10.1371/journal.ppat.1005846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wickstrom C, Herzberg MC, Beighton D, Svensater G. 2009. Proteolytic degradation of human salivary MUC5B by dental biofilms. Microbiology 155:2866–2872. doi: 10.1099/mic.0.030536-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ghorbani P, Santhakumar P, Hu Q, Djiadeu P, Wolever TM, Palaniyar N, Grasemann H. 2015. Short-chain fatty acids affect cystic fibrosis airway inflammation and bacterial growth. Eur Respir J 46:1033–1045. doi: 10.1183/09031936.00143614. [DOI] [PubMed] [Google Scholar]
- 66.Mirkovic B, Murray MA, Lavelle GM, Molloy K, Azim AA, Gunaratnam C, Healy F, Slattery D, McNally P, Hatch J, Wolfgang M, Tunney MM, Muhlebach MS, Devery R, Greene CM, McElvaney NG. 2015. The role of short-chain fatty acids, produced by anaerobic bacteria, in the cystic fibrosis airway. Am J Respir Crit Care Med 192:1314–1324. doi: 10.1164/rccm.201505-0943OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Scott JE, Li K, Filkins LM, Zhu B, Kuchma SL, Schwartzman JD, Ga OT. 4 February 2019. Pseudomonas aeruginosa can inhibit growth of streptococcal species via siderophore production. J Bacteriol doi: 10.1128/JB.00014-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moreau-Marquis S, Bomberger JM, Anderson GG, Swiatecka-Urban A, Ye S, O'Toole GA, Stanton BA. 2008. The deltaF508-CFTR mutation results in increased biofilm formation by Pseudomonas aeruginosa by increasing iron availability. Am J Physiol Lung Cell Mol Physiol 295:L25–L37. doi: 10.1152/ajplung.00391.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stites SW, Plautz MW, Bailey K, O'Brien-Ladner AR, Wesselius LJ. 1999. Increased concentrations of iron and isoferritins in the lower respiratory tract of patients with stable cystic fibrosis. Am J Respir Crit Care Med 160:796–801. doi: 10.1164/ajrccm.160.3.9811018. [DOI] [PubMed] [Google Scholar]
- 70.Stites SW, Walters B, O'Brien-Ladner AR, Bailey K, Wesselius LJ. 1998. Increased iron and ferritin content of sputum from patients with cystic fibrosis or chronic bronchitis. Chest 114:814–819. doi: 10.1378/chest.114.3.814. [DOI] [PubMed] [Google Scholar]
- 71.Coss-Bu JA, Sachdeva RC, Bricker JT, Harrison GM, Jefferson LS. 1997. Hemoptysis: a 10-year retrospective study. Pediatrics 100:E7. doi: 10.1542/peds.100.3.e7. [DOI] [PubMed] [Google Scholar]
- 72.Pienkowska K, Wiehlmann L, Tummler B. 23 January 2019. Metagenome—inferred bacterial replication rates in cystic fibrosis airways. J Cyst Fibros doi: 10.1016/j.jcf.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 73.Scoffield JA, Duan D, Zhu F, Wu H. 2017. A commensal streptococcus hijacks a Pseudomonas aeruginosa exopolysaccharide to promote biofilm formation. PLoS Pathog 13:e1006300. doi: 10.1371/journal.ppat.1006300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brook I, Pazzaglia G, Coolbaugh JC, Walker RI. 1983. In-vivo protection of group A beta-haemolytic streptococci from penicillin by beta-lactamase-producing Bacteroides species. J Antimicrob Chemother 12:599–606. doi: 10.1093/jac/12.6.599. [DOI] [PubMed] [Google Scholar]
- 75.Brook I. 2009. The role of beta-lactamase-producing-bacteria in mixed infections. BMC Infect Dis 9:202. doi: 10.1186/1471-2334-9-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Maddocks JL. 1980. Indirect pathogenicity. J Antimicrob Chemother 6:307–309. doi: 10.1093/jac/6.3.307. [DOI] [PubMed] [Google Scholar]
- 77.Tavernier S, Crabbe A, Hacioglu M, Stuer L, Henry S, Rigole P, Dhondt I, Coenye T. 2017. Community composition determines activity of antibiotics against multispecies biofilms. Antimicrob Agents Chemother 61:e00302-17. doi: 10.1128/AAC.00302-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tavernier S, Sass A, De Bruyne M, Baeke F, De Rycke R, Crabbe A, Vandecandelaere I, Van Nieuwerburgh F, Coenye T. 2018. Decreased susceptibility of Streptococcus anginosus to vancomycin in a multispecies biofilm is due to increased thickness of the cell wall. J Antimicrob Chemother 73:2323–2330. doi: 10.1093/jac/dky216. [DOI] [PubMed] [Google Scholar]
- 79.Orazi G, O'Toole GA. 2017. Pseudomonas aeruginosa alters Staphylococcus aureus sensitivity to vancomycin in a biofilm model of cystic fibrosis infection. mBio 8:e00873-17. doi: 10.1128/mBio.00873-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cázares-Domínguez V, Cruz-Córdova A, Ochoa SA, Escalona G, Arellano-Galindo J, Rodríguez-Leviz A, Hernández-Castro R, López-Villegas EO, Xicohtencatl-Cortes J. 2015. Vancomycin tolerant, methicillin-resistant Staphylococcus aureus reveals the effects of vancomycin on cell wall thickening. PLoS One 10:e0118791. doi: 10.1371/journal.pone.0118791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cui L, Iwamoto A, Lian JQ, Neoh HM, Maruyama T, Horikawa Y, Hiramatsu K. 2006. Novel mechanism of antibiotic resistance originating in vancomycin-intermediate Staphylococcus aureus. Antimicrob Agents Chemother 50:428–438. doi: 10.1128/AAC.50.2.428-438.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cui L, Ma X, Sato K, Okuma K, Tenover FC, Mamizuka EM, Gemmell CG, Kim MN, Ploy MC, El-Solh N, Ferraz V, Hiramatsu K. 2003. Cell wall thickening is a common feature of vancomycin resistance in Staphylococcus aureus. J Clin Microbiol 41:5–14. doi: 10.1128/JCM.41.1.5-14.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schwerdt M, Neumann C, Schwartbeck B, Kampmeier S, Herzog S, Görlich D, Dübbers A, Große-Onnebrink J, Kessler C, Küster P, Schültingkemper H, Treffon J, Peters G, Kahl BC. 2018. Staphylococcus aureus in the airways of cystic fibrosis patients—a retrospective long-term study. Int J Med Microbiol 308:631–639. doi: 10.1016/j.ijmm.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 84.Limoli DH, Yang J, Khansaheb MK, Helfman B, Peng L, Stecenko AA, Goldberg JB. 2016. Staphylococcus aureus and Pseudomonas aeruginosa co-infection is associated with cystic fibrosis-related diabetes and poor clinical outcomes. Eur J Clin Microbiol Infect Dis 35:947–953. doi: 10.1007/s10096-016-2621-0. [DOI] [PubMed] [Google Scholar]
- 85.Rogers GB, Hart CA, Mason JR, Hughes M, Walshaw MJ, Bruce KD. 2003. Bacterial diversity in cases of lung infection in cystic fibrosis patients: 16S ribosomal DNA (rDNA) length heterogeneity PCR and 16S rDNA terminal restriction fragment length polymorphism profiling. J Clin Microbiol 41:3548–3558. doi: 10.1128/JCM.41.8.3548-3558.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kolenbrander PE, London J. 1993. Adhere today, here tomorrow: oral bacterial adherence. J Bacteriol 175:3247–3252. doi: 10.1128/jb.175.11.3247-3252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kaci G, Goudercourt D, Dennin V, Pot B, Dore J, Ehrlich SD, Renault P, Blottiere HM, Daniel C, Delorme C. 2014. Anti-inflammatory properties of Streptococcus salivarius, a commensal bacterium of the oral cavity and digestive tract. Appl Environ Microbiol 80:928–934. doi: 10.1128/AEM.03133-13. [DOI] [PMC free article] [PubMed] [Google Scholar]