Due to its multifaceted lifestyle, Staphylococcus aureus needs a complex regulatory network to connect environmental signals with cellular physiology. One particular transcription factor, named σB (SigB), is involved in the general stress response and the expression of virulence factors. For many years, great confusion has existed about the role of σB in the regulation of the biofilm lifestyle in S. aureus. Our study demonstrated that σB is not necessary for exopolysaccharide-dependent biofilms and, even more, that S. aureus produces stronger biofilms in the absence of σB. The increased accumulation of exopolysaccharide correlates with higher stability of the proteins responsible for its synthesis. The present findings reveal an additional regulatory layer to control biofilm exopolysaccharide synthesis under stress conditions.
KEYWORDS: Biofilm, PNAG, SigB, Staphylococcus aureus, ica operon
ABSTRACT
Staphylococcus aureus clinical strains are able to produce at least two distinct types of biofilm matrixes: biofilm matrixes made of the polysaccharide intercellular adhesin (PIA) or poly-N-acetylglucosamine (PNAG), whose synthesis is mediated by the icaADBC locus, and biofilm matrixes built of proteins (polysaccharide independent). σB is a conserved alternative sigma factor that regulates the expression of more than 100 genes in response to changes in environmental conditions. While numerous studies agree that σB is required for polysaccharide-independent biofilms, controversy persists over the role of σB in the regulation of PIA/PNAG-dependent biofilm development. Here, we show that genetically unrelated S. aureus σB-deficient strains produced stronger biofilms under both static and flow conditions and accumulated higher levels of PIA/PNAG exopolysaccharide than their corresponding wild-type strains. The increased accumulation of PIA/PNAG in the σB mutants correlated with a greater accumulation of the IcaC protein showed that it was not due to adjustments in icaADBC operon transcription and/or icaADBC mRNA stability. Overall, our results reveal that in the presence of active σB, the turnover of Ica proteins is accelerated, reducing the synthesis of PIA/PNAG exopolysaccharide and consequently the PIA/PNAG-dependent biofilm formation capacity.
IMPORTANCE Due to its multifaceted lifestyle, Staphylococcus aureus needs a complex regulatory network to connect environmental signals with cellular physiology. One particular transcription factor, named σB (SigB), is involved in the general stress response and the expression of virulence factors. For many years, great confusion has existed about the role of σB in the regulation of the biofilm lifestyle in S. aureus. Our study demonstrated that σB is not necessary for exopolysaccharide-dependent biofilms and, even more, that S. aureus produces stronger biofilms in the absence of σB. The increased accumulation of exopolysaccharide correlates with higher stability of the proteins responsible for its synthesis. The present findings reveal an additional regulatory layer to control biofilm exopolysaccharide synthesis under stress conditions.
INTRODUCTION
The dangerous community- and hospital-acquired pathogen Staphylococcus aureus is the leading cause of a variety of diseases ranging from moderate skin and soft tissue infections to very serious diseases, such as septic shock, toxic shock syndrome, and necrotizing pneumonia (1). The enormous pathogenic competence of S. aureus depends on its capacity to grow under a wide variety of environmental conditions, including those encountered in almost every organ of the human body (lung, heart, blood, bone, skin, muscles, eye, joints, and intestinal tract), and to produce a large array of toxins and enzymes in order to evade the immune system. To grow under different environmental conditions, S. aureus makes use of an efficient signal transduction system that facilitates the integration of environmental stimuli and adjusts the cellular physiology in response (1). One key factor of such a signal transduction system is the alternative σB (SigB) transcription factor (2–8). σB directly or indirectly regulates processes including cell wall maintenance, intermediary metabolism, membrane transport, and virulence under environmental stresses, such as alkaline and acidic pH, heat shock, hydrogen peroxide, cell wall antibiotics, and entry into stationary phase (5, 7, 9, 10).
One important strategy used by S. aureus to grow and survive in host tissues is the adoption of the biofilm lifestyle, where bacteria grow encased in a self-produced extracellular matrix mostly composed of polysaccharides, proteins, and nucleic acids (11–13). Living inside the biofilm provides protection against desiccation, diffusion of antibiotics and other compounds, and the host immune system, among others (14). Given that biofilm development implies changes in the expression levels of hundreds of genes, it is not surprising that σB has been considered an optimal candidate for connecting environmental conditions with the development of the biofilm phenotype. However, confusion exists regarding the role of σB in regulating S. aureus biofilm development. Some studies suggest that σB is necessary for S. aureus biofilm development (15–17), whereas other studies have found that S. aureus is still able to form biofilms in the absence of σB (18, 19).
These seemingly contradictory findings might be explained by the capacity of S. aureus to produce biofilm matrixes with different compositions. Some S. aureus strains produce a proteinaceous biofilm matrix that is composed of a variety of cell wall proteins and extracellular DNA (eDNA) (20–26). In this case, the presence of σB is required to build the biofilm matrix (17, 19, 27, 28). This phenotype has been related to increased expression of the regulatory RNA, RNAIII, in the absence of σB, which elevates the expression of extracellular proteases and alters the activity of murein hydrolases. Alternatively, S. aureus is able to produce a polysaccharide-dependent biofilm that is mediated by the production of the polysaccharide intercellular adhesin (PIA) or poly-N-acetylglucosamine (PNAG). PIA/PNAG synthesis depends on the expression of icaADBC-encoded enzymes whose expression is mainly repressed at a transcriptional level by the IcaR protein (29–31). Some studies have reported that σB positively regulates icaADBC expression at a transcriptional level (16, 32). On the other hand, other studies have revealed that the absence of σB does not affect PIA/PNAG production and biofilm formation capacity (18). It was therefore of interest to determine the role of σB in PNAG-mediated biofilm formation by S. aureus.
In this study, we evaluated the consequences of the absence of σB in several genetically unrelated S. aureus strains that produce PNAG-dependent biofilms by analyzing (i) icaADBC transcription, (ii) ica mRNA stability, (iii) Ica protein production, and (iv) PIA/PNAG accumulation. The results revealed that S. aureus σB mutants produce higher levels of PIA/PNAG than wild-type strains by accumulating, at least, higher levels of IcaC protein, thus confirming that σB is a repressor of PIA/PNAG synthesis and exopolysaccharide-dependent biofilms.
RESULTS
Impact of σB mutation on PNAG-dependent biofilm phenotypes in S. aureus.
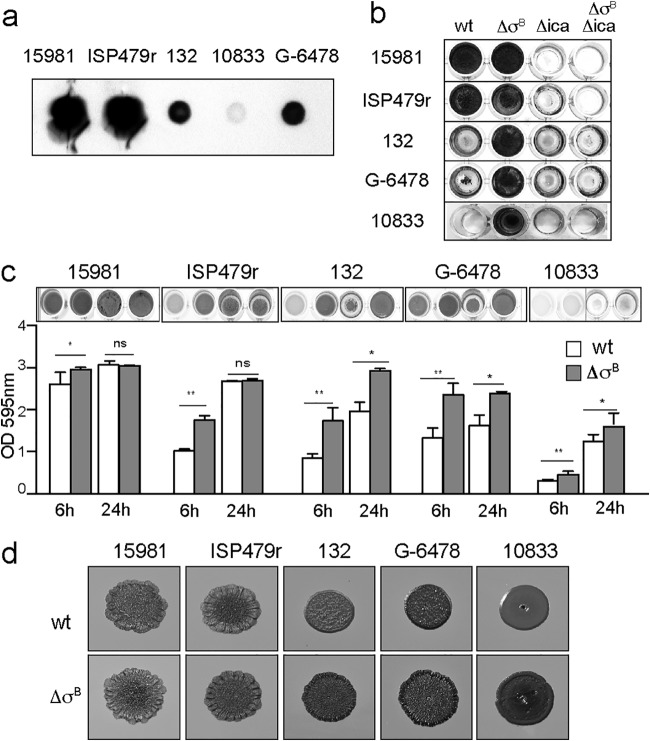
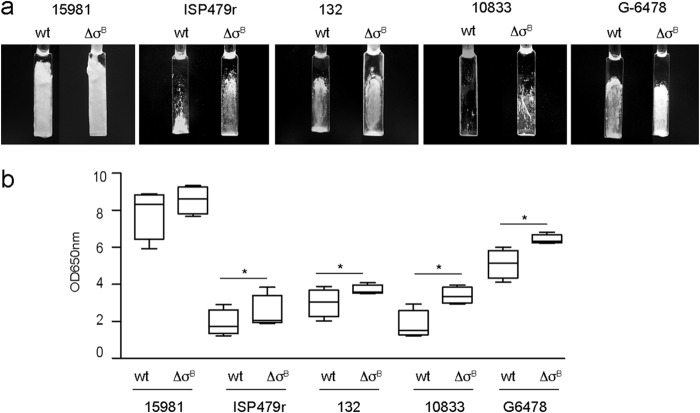
To elucidate the role of σB in S. aureus PNAG-dependent biofilms, we selected five genetically unrelated clinical isolates that produce biofilms of different intensities depending on the levels of PIA/PNAG exopolysaccharide detected by dot blot analysis using anti-PNAG-specific polyclonal antisera (Fig. 1a). Under the conditions tested, the biofilm formation capacities of these strains were primarily dependent on PIA/PNAG exopolysaccharide, since deletion of the icaADBC genes completely inhibited biofilm development (Fig. 1b). Then, we constructed a σB mutant of each strain and compared the biofilm formation capacities of the σB mutants to those of the corresponding wild-type strains. The results showed that, after 6 h of growth, all the S. aureus σB mutants displayed a higher capacity to form a biofilm than wild-type strains (P < 0.05) (Fig. 1c). Interestingly, these differences remained after 24 h of growth when weak biofilm-forming strains (132, G-6478, and 10833) (P < 0.05) were analyzed. In contrast, such differences disappeared in the strong biofilm-forming strains (15981 and ISP479r), probably due to saturation in the quantity of biofilm cells (Fig. 1c). To confirm the role of σB mutation in biofilm enhancement, σB mutants of the strong biofilm former S. aureus 15981 and the weak biofilm former S. aureus 132 were complemented with the σB gene using the pSK9 plasmid. σB complementation led to the restoration of biofilm levels shown by wild-type strains (see Fig. S1 in the supplemental material). Another phenotype associated with biofilm development in S. aureus is colony morphology on Congo red agar plates. In agreement with the biofilm formation phenotype, σB mutants of the weak biofilm-forming strains produced rougher colonies than their corresponding wild-type strains, which showed a smooth phenotype. In the case of the strong biofilm formers, wild-type strains already showed a rough colony morphology, and thus, the phenotype was not affected by the absence of σB (Fig. 1d). Finally, we examined whether the absence of σB affects biofilm development under flow culture conditions using microfermentors, where σB is expected to be active. After 24 h of incubation, visual inspection of the biofilms that had developed on the surfaces of the slides inside the microfermentors revealed that the majority of S. aureus σB mutants produced a significantly higher biofilm biomass than their corresponding wild-type strains (P < 0.05) (Fig. 2). Taken together, these results indicated that deletion of σB enhances the PIA/PNAG-dependent biofilm formation capacity of clinical S. aureus strains.
FIG 1.
Mutation of σB in unrelated biofilm-positive S. aureus strains enhances biofilm formation. (a) Dot blot analysis of PNAG accumulation in different S. aureus strains. Cell surface extracts of biofilm cultures were spotted onto nitrocellulose filters. PNAG production was detected with anti-S. aureus PNAG antiserum. (b) Biofilm formation of the unrelated S. aureus strains and their respective mutants in σB (ΔσB), icaADBC genes (Δica), and ΔσB Δica. Bacteria were grown on polystyrene microtiter plates for 24 h. wt, wild type. (c) Biofilm formation of the wild-type S. aureus strains and their respective σB mutants on polystyrene microtiter plates after 6 h and 24 h. The bacterial cells were stained with crystal violet, and biofilms were quantified by solubilizing the crystal violet with alcohol-acetone and determining the absorbance at 595 nm. The error bars represent the standard deviations of the results of three independent experiments. *, P < 0.05; **, P < 0.01. (d) Colony morphologies of biofilm-positive strains and their isogenic σB mutants on Congo red agar after 24 h of incubation.
FIG 2.
Influence of σB deletion on biofilm formation in continuous-flow microfermentors. (a) Biofilm development of the wild-type strain (wt) and its isogenic ΔσB mutants on glass slides of microfermentors after 24 h. (b) Quantification of biofilms adhering to glass slides. The glass slides were placed into 10 ml of PBS. Cells were removed from the slides by vigorous vortexing. The optical density of the solution was measured at 650 nm (OD650). The biofilm formation of S. aureus 10833 wt and ΔσB was represented as 10 times the OD650 of the glass slides. The box and whisker plot indicates high and low values, medians, and interquartile ranges. Each group contained 4 or 5 microfermentors. Statistical differences were determined with Mann-Whitney tests. The asterisks indicate differences in competition indexes greater than 1 (P < 0.05).
σB mutants accumulate higher levels of PIA/PNAG exopolysaccharide.
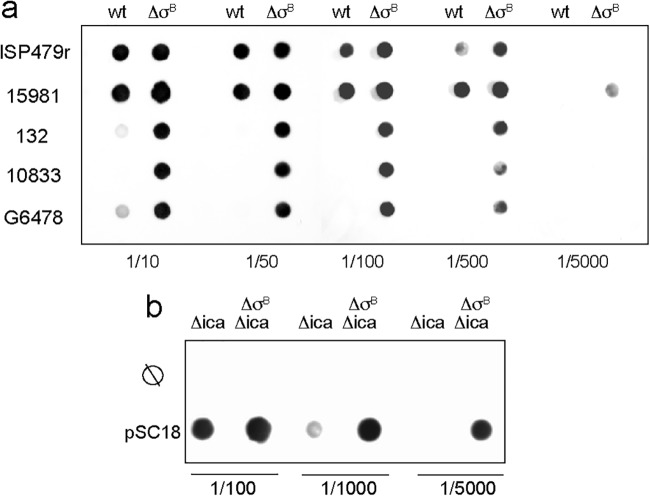
We next asked whether the increased biofilm formation capacity shown by S. aureus σB mutants was due to enhanced accumulation of PIA/PNAG exopolysaccharide. Thus, we compared the levels of PIA/PNAG exopolysaccharide produced by the wild-type and σB mutants and found that in all cases, the σB mutants produced higher levels of PNAG than the corresponding wild-type strains (Fig. 3a). To unequivocally demonstrate that the enhanced capacity to produce a biofilm was indeed due to greater accumulation of PIA/PNAG, we generated a nonpolar deletion of the icaADBC operon in the σB mutants. The resulting ΔσB Δica double mutants lost the capacity to form a biofilm in microtiter plates (Fig. 1b). To further confirm the positive role of σB in PNAG production, we complemented S. aureus 15981 Δica and ΔσB Δica mutant strains with a plasmid carrying the icaRADBC module and found that restoration of PNAG production was even greater in the ΔσB Δica double mutant than in the Δica single-mutant strain. Overall, these data indicated that S. aureus accumulates higher levels of PIA/PNAG exopolysaccharide in the absence of σB.
FIG 3.
Analysis of PNAG accumulation in σB mutants. (a) Dot-blot analysis of PNAG accumulation in S. aureus wild-type and ΔσB mutant strains. Cell surface extracts of biofilm cultures were treated with proteinase K and spotted onto nitrocellulose filters at different dilutions (1:10 to 1:5,000). PNAG production was detected with anti-S. aureus PNAG antiserum. (b) PNAG levels of the S. aureus 15981 Δica mutant and the ΔσB Δica double mutant complemented with pSC18 plasmid, which carries the ica locus. For dot blot analysis, samples diluted 1:100, 1:1,000, and 1:5,000 were spotted onto nitrocellulose membranes, and PNAG production was detected with an anti-PNAG antiserum.
σB does not regulate icaADBC expression at a transcriptional level.
The discovery that σB mutants accumulate higher levels of PNAG than wild-type strains raises the possibility that σB negatively regulates icaADBC transcription. To analyze this hypothesis, the promoter region of the ica operon was fused to gfpmut2, generating plasmid pCN52-Pica_gfp (33). The plasmids were introduced into S. aureus 15981 and 132 and their corresponding σB mutants, and expression of green fluorescent protein (GFP) was determined by Western blotting at the exponential (Exp) and stationary (ON) phases. Despite the fact that σB mutants accumulated higher levels of PIA/PNAG during the exponential and stationary phases (Fig. 4a), there were no significant differences in GFP expression between wild-type strains and σB-deficient strains (Fig. 4b). These results suggested that regulation of the icaADBC operon by σB does not occur at the transcriptional level. To address this issue, we next replaced, by allelic exchange, the native ica promoter (from positions −57 to +1) in the chromosome of S. aureus wild-type strains 15981 and 132 and their corresponding σB mutant strains using the constitutive Phyper promoter (Phyp-ica) (see Fig. S2 in the supplemental material) (34) and analyzed biofilm formation and PIA/PNAG production by the resulting strains. The results revealed that, even when ica operon expression is placed under the control of a constitutive promoter, σB-deficient strains still accumulate higher levels of PIA/PNAG and produce stronger biofilms than their corresponding wild-type strains (Fig. 4c and d). Taken together, these data showed that σB affects ica expression at a posttranscriptional level.
FIG 4.
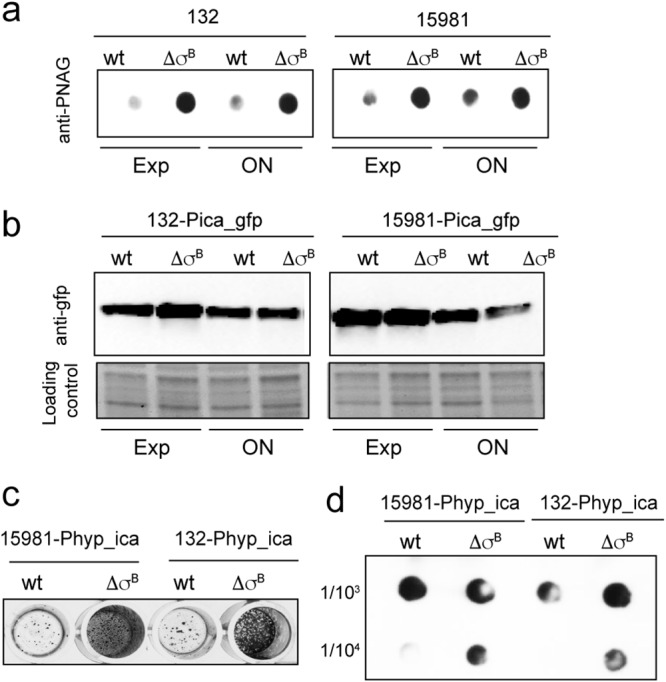
Analysis of ica operon expression in σB mutants. (a) PNAG accumulation at different growth stages: exponential (Exp) and stationary (ON). Samples diluted 1:100 or 1:5,000 were spotted onto nitrocellulose membranes. PNAG production was detected with anti-PNAG antiserum. (b) Effects of σB deletion on ica promoter activity in S. aureus strains 15981 and 132 (wt) and their respective ΔσB mutants carrying pCN52-Pica_gfp at exponential and stationary phases. Expression of GFP under the control of the ica promoter (Pica_gfp) was determined by Western blotting using monoclonal antibodies. (c) Biofilm formation by S. aureus 15981 Phyp_ica and 132 Phyp_ica and their respective σB mutants on polystyrene microtiter plates after 4 h of incubation. The bacterial cells were stained with crystal violet. (d) PNAG accumulation in cell extracts of S. aureus 15981 Phyp_ica and 132 Phyp_ica and their respective σB mutants. PNAG production was detected by dot blot analysis using anti-S. aureus PNAG antiserum.
Role of σB in posttranscriptional regulation of the ica operon.
To investigate whether σB posttranscriptionally regulates ica expression, we used plasmid pHRG to express the 5ʹ untranslated region (UTR) sequence of the ica operon under the control of the constitutive promoter Phyper fused in frame with gfpmut2 (pHRG_5ʹUTRica) (Table 1). The plasmid was introduced into S. aureus wild-type strains 15981 and 132 and their respective σB mutants, and the expression of GFP was determined by Western blotting. Absence of σB did not lead to significant changes in GFP expression, suggesting that σB does not act on icaADBC mRNA translation (Fig. 5a). Then, we investigated whether σB affects icaADBC mRNA levels. For that, we examined icaADBC mRNA in S. aureus 15981 and 132 wild-type strains and in their corresponding σB mutants by Northern blotting using a riboprobe specific for icaA and icaC mRNAs. The results showed no differences in ica mRNA transcript levels and mRNA processing between wild-type and σB mutant strains (Fig. 5b). Collectively, these results suggest that σB upregulates PIA/PNAG expression without affecting icaADBC mRNA stability.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant characteristicsa | Reference |
|---|---|---|
| S. aureus strains | ||
| 15981 | Biofilm-positive clinical strain | 18 |
| ISP479r | ISP479 rsbU-positive strain | 59 |
| 12313 | Biofilm-positive clinical strain | This study |
| 132 | Biofilm-positive clinical strain; MRSA clinical strain | 23 |
| G-6478 | Biofilm-positive clinical strain; MRSA clinical strain | 23 |
| 10833 | Clumping factor-positive variant of Newman D2C | 29 |
| 12313 ΔσB | 12313 with deleted σB gene | This study |
| 10833 ΔσB | 10833 with deleted σB gene | This study |
| ISP479r ΔσB | ISP479r with deleted σB gene | 18 |
| ISP479r Δica | ISP479r with deleted icaADBC operon | This study |
| ISP479r ΔσB Δica | ISP479r σB mutant with deleted icaADBC operon | This study |
| G-6478 ΔσB | G-6478 with deleted σB gene | This study |
| G-6478Δica | G-6478 with deleted icaADBC operon | This study |
| G-6478 ΔσB Δica | G-6478 σB mutant with deleted icaADBC operon | This study |
| 132 ΔσB | 132 with deleted σB gene | This study |
| 132 Δica | 132 with deleted icaADBC operon | 23 |
| 132 ΔσBΔica | 132 σB mutant with deleted icaADBC operon | This study |
| 15981 ΔσB | 15981 σB mutant | 18 |
| 15981 Δica | 15981 with deleted icaADBC operon | 56 |
| 15981 ΔσB Δica | 15981 σB mutant with deleted icaADBC operon | This study |
| 15981 icaC-3×flag | 15981 with a 3×flag-tagged icaC gene | This study |
| 15981 ΔσB icaC-3×flag | 15981 σB mutant with a 3×flag-tagged icaC gene | This study |
| 132 icaC-3×flag | 132 with a 3×flag-tagged icaC gene | This study |
| 132 ΔσB icaC-3×flag | 132 σB mutant with a 3×flag-tagged icaC gene | This study |
| 15981_Pica-gfp | 15981 with pCN52-Pica | This study |
| 15981 ΔσB_Pica-gfp | 15981 σB mutant with pCN52-Pica | This study |
| 132_Pica-gfp | 132 with pCN52-Pica | This study |
| 132 ΔσB_Pica-gfp | 132 σB mutant with pCN52-Pica | This study |
| 15981_Phyp-ica | 15981 constitutively expressing the ica operon | This study |
| 15981 ΔσB_Phyp-ica | 15981 σB mutant constitutively expressing the ica operon | This study |
| 132_Phyper-ica | 132 constitutively expressing the ica operon | This study |
| 132 ΔσB_Phyp-ica | 132 σB mutant constitutively expressing the ica operon | This study |
| 15981 RNAIII | 15981 transformed with plasmid pALC2073 RNAIII | This study |
| 15981 ΔσB RNAIII | 15981 σB transformed with plasmid pALC2073 RNAIII | This study |
| 132 RNAIII | 132 transformed with plasmid pALC2073 RNAIII | 23 |
| 132ΔσB RNAIII | 132 σB transformed with plasmid pALC2073 RNAIII | This study |
| Plasmids | ||
| pMAD | E. coli-S. aureus shuttle vector | 60 |
| pSK9 | Plasmid carrying the σB gene | 16 |
| pHRG | pCN47 derivative plasmid used for posttranscriptional fusions to GFP | This study |
| pCN52-Pica_gfp | Plasmid with GFP expressed under the control of the Pica promoter | This study |
| pCN52-Phyp_gfp | Plasmid with GFP expressed under the control of the Phyper promoter | This study |
| pHRG-5ʹUTRica | Plasmid carrying a posttranscriptional fusion of ica with GFP | This study |
MRSA, methicillin-resistant Staphylococcus aureus.
FIG 5.
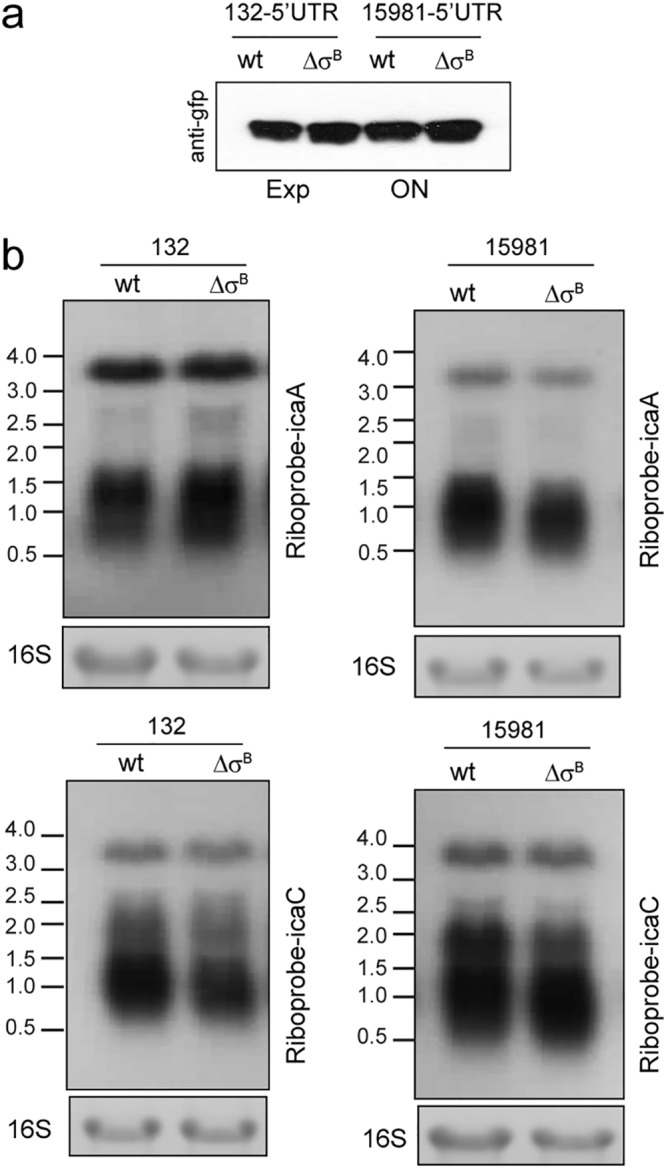
Analysis of the posttranscriptional regulation of the ica operon by σB. (a) Fusion of the 5ʹ UTR of the ica operon to gfpmut2 under the control of the Phyper promoter. Levels of GFP were determined in the S. aureus 132 and 15981 wild-type strains and their respective σB mutants by Western blotting using monoclonal antibodies against GFP. (b) Northern blot analysis of RNA harvested from S. aureus 15981 and 132 wild-type strains and their corresponding σB mutants. The strains were grown in TSB-gluc at 37°C until exponential phase. The lower gels show 16S ribosome bands stained with ethidium bromide as a loading control. The blots were probed with a riboprobe specific for icaA and icaC transcripts. The positions of RNA standards in kilobases are indicated.
σB controls Ica protein levels.
Next, we investigated the possibility that σB modulates Ica protein levels. To do so, we tagged the last protein (IcaC) encoded by the icaADBC operon in the wild-type strains 15981 and 132 and in their σB mutants. The resulting IcaC-3×Flag strains retained the capacity to form a biofilm, indicating that addition of the 3×Flag epitope did not impair IcaC functionality (see Fig. S3 in the supplemental material). Then, the IcaC-3×Flag protein was detected by immunoblotting with commercial anti-3×Flag antibodies. Quantification of immunodetected bands showed that levels of IcaC-3×Flag were significantly higher in σB mutants than in wild-type strains (Fig. 6a).
FIG 6.
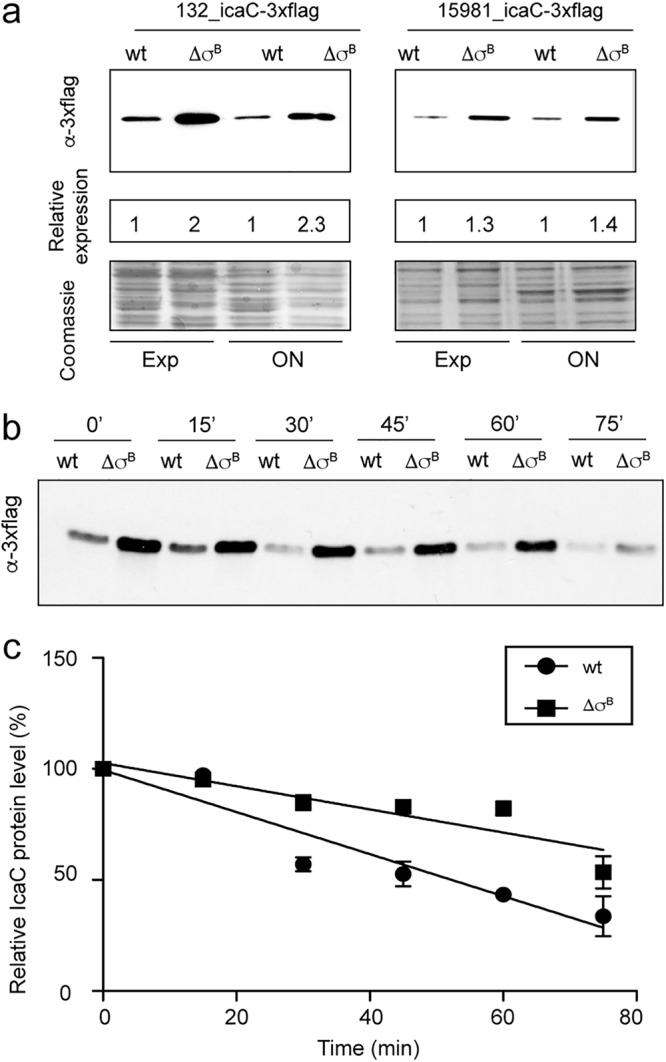
σB influence on IcaC protein levels. (a) Immunodetection of IcaC-tagged protein in the wt and the σB mutants of S. aureus 15981 and 132 at exponential phase. Whole-cell bacterial lysates were subjected to electrophoretic separation in an SDS-12% polyacrylamide gel. The proteins were transferred onto a nitrocellulose membrane and probed with anti-Flag M2 MAb conjugated with peroxidase. Relative quantification of the Flag protein was obtained using the ImageJ program. A Coomassie-stained gel portion is shown as a loading control. (b) Immunodetection of IcaC-tagged protein upon blocking protein biosynthesis in exponentially growing cells. (c) Relative IcaC levels upon blocking protein biosynthesis as an average of the results of two independent experiments; the error bars indicate standard deviations.
To gain insight into the σB-mediated accumulation of the IcaC protein, we analyzed IcaC stability by blocking de novo protein biosynthesis in exponentially growing wild-type and σB mutant cells. Analysis of IcaC levels by Western blotting showed that the protein remained relatively constant after transcriptional inhibition and that gradual degradation occurred after 30 min (Fig. 6b). Densitometry of this progression showed slower decay of the IcaC protein in the σB mutant than in the wild-type strain (Fig. 6c). Together, these results suggest that IcaC protein turnover decreases in the absence of σB.
DISCUSSION
The transition from a planktonic single-cell lifestyle to a biofilm-associated community in S. aureus requires a complex and highly regulated process that needs to be globally coordinated. Increasing evidence indicates that the stress sigma factor σB is a key element for biofilm formation in several bacterial species, including Listeria monocytogenes, Bacillus subtilis, and Bacillus cereus (35). However, the contribution of σB to regulation of the biofilm formation process of S. aureus is still a matter of debate (17, 32, 36–42). There is wide agreement that σB is necessary for building PNAG-independent biofilms. However, the role of σB in PNAG-dependent biofilms is more arguable. Early reports claimed that disruption of σB impaired PNAG synthesis, whereas other reports claimed that deletion of σB increased aggregation and did not affect PNAG-dependent biofilm development (3, 16, 18, 32). Another source of confusion has been the fact that σB participation is crucial for icaADBC expression in the closely related species Staphylococcus epidermidis, where σB downregulates the expression of icaR (37). Consequently, S. epidermidis σB mutants produce high levels of IcaR, which results in the repression of PNAG production (37, 40, 41).
With the aim of clarifying the role of S. aureus σB in the regulation of PNAG synthesis, we analyzed the consequences of σB deletion in five genetically unrelated strains. Deletions were generated by double crossover without the insertion of antibiotic resistance markers to avoid undesirable polar effects. The results unambiguously showed that σB is dispensable for PNAG synthesis in S. aureus and, indeed, that σB mutants displayed a higher capacity to accumulate PNAG on the cell wall and produce a biofilm.
How does σB affect PNAG synthesis in S. aureus? The simplest and most plausible explanation was that σB directly or indirectly regulates ica operon transcription. However, our results refuted this hypothesis. Evaluation of the ica promoter activity in wild-type and σB mutant strains showed no differences between the strains. Furthermore, the expression of PNAG remained higher in the absence of σB than in the wild type after replacement of the ica native promoter by a constitutive Phyper promoter, indicating that σB controls ica operon expression without affecting ica transcriptional levels. These results agreed with previous transcriptome analyses of wild-typeS. aureus and its corresponding σB mutant in which the ica genes were never identified as members of the σB regulon (9, 43, 44).
Regulation of the expression of the pgaABCD operon, homologous to icaADBC in Escherichiacoli, takes place at a posttranscriptional level. The small RNA-binding protein CsrA represses pga gene expression and the production of PGA by binding to pgaA mRNA. This prevents the 30S ribosome subunit from binding, thus affecting pgaABCD mRNA stability and accelerating mRNA degradation (45). Inspired by these findings, we asked whether σB might posttranscriptionally regulate ica operon expression through the activation of an RNA-binding protein or a small RNA that would bind to the ica mRNA, destabilizing the ica transcript. However, analysis of GFP expression by the use of a posttranscriptional fusion of the 31-nucleotide (nt) untranslated leader of the icaA coding sequence with GFP showed no differences in GFP levels between the wild type and σB mutants. Furthermore, Northern blotting analysis with riboprobes specific for icaA and icaC did not detect any differences in ica mRNA levels and mRNA integrity between the wild type and σB mutants. Instead, we found that the IcaC protein is somewhat more stable in σB mutants than in the corresponding wild-type strains. How does σB translationally regulate Ica proteins levels? Analysis of transcriptome sequencing (RNA-seq) data obtained in a previous study (43) that compared S. aureus 15981 wild-type and σB mutant strains showed the existence of at least 18 small RNAs (sRNAs) whose expression depended on σB. We are currently exhaustively studying whether PNAG production depends on any σB-regulated sRNAs, which might affect icaADBC mRNA translation. Another possible explanation is that specific proteases that may affect Ica protein levels are actually repressed in the σB mutant. This possibility seems in principle unlikely because the majority of proteases are in fact induced when σB is inactive. However, following the analogy of IcaADBC machinery to PgaABCD machinery in order to synthesize poly-N-acetylglucosamine (PIA/PNAG/Pga), we cannot exclude the possibility that the IcaC protein adopts a conformation in the absence of σB that makes the protein less susceptible to proteases. In the case of PgaABCD, U. Jenal and colleagues nicely showed that PIA/PNAG synthesis is allosterically controlled through c-di-GMP binding to the membrane-anchored PgaCD complex (46). When the concentration of c-di-GMP decreases, PgaD is no longer able to interact with c-di-GMP and is rapidly removed by proteolysis, and thus, the synthesis of PIA/PNAG is inhibited. S. aureus contains only one gene encoding a protein with a conserved GGDEF domain, designated GdpS (47). Different studies have shown that GdpS contributes to staphylococcal biofilm formation (47–49). However, the mechanisms by which GdpS regulates biofilm formation remains unclear. Recombinant GdpS protein is unable to synthesize quantifiable levels of c-di-GMP in vitro, and c-di-GMP has not been detected in S. aureus extracts, as judged by liquid chromatography-tandem mass spectrometry (LC–MS-MS) (50). Thus, despite the absence of evidence indicating the presence of c-di-GMP in the cytoplasm of S. aureus, it is tempting to speculate that c-di-GMP, or another signaling molecule whose level in the bacterial cytoplasm depends on the presence of σB, might interact with Ica proteins and affect their accessibility to proteases.
The findings of this study, together with previous results on the effect of σB on S. aureus multicellular behavior, are summarized in Fig. 7. Under environmental conditions where σB is active, it represses the synthesis of proteases and nuclease production and also suppresses PIA/PNAG synthesis, enabling the formation of a polysaccharide-independent biofilm. When σB is not active, overexpression of extracellular proteases and nucleases (Nuc) reduces the production of a protein/DNA-mediated biofilm. On the other hand, PIA/PNAG accumulation and the subsequent PIA/PNAG-mediated biofilm development take place through the increase of Ica protein levels controlled at a posttranslational level. Lastly, σB can also indirectly affect biofilm production by generating a proper ratio of biofilm-negative variants through regulation of the transposition activity of the insertion sequence IS256, which preferentially inserts within the icaC gene (51). Interestingly, in all these regulatory processes, σB regulates the synthesis of PIA/PNAG indirectly by affecting the expression of other factors that ultimately alter PIA/PNAG levels. Thus, hierarchical regulation has to exist to coordinate all these confluent mechanisms and efficiently transmit environmental signals to multicellular behavior through the activity of σB.
FIG 7.
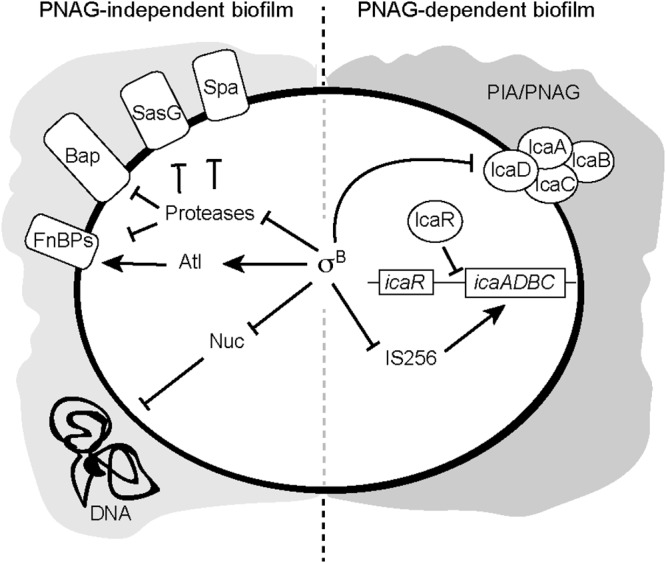
Proposed model of σB regulatory effect on S. aureus biofilm formation. The transcriptional regulator σB induces PNAG-independent biofilm formation by repressing extracellular proteases, by inhibiting nuclease secretion, and/or by the activation of Atl. On the other hand, σB regulates PNAG-dependent multicellular behavior by modulating PNAG levels by at least repressing IS256 and translationally regulating Ica enzymes.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The strains and plasmids used in this study are listed in Table 1. S. aureus strains 15981, 132, G-6478, and 10833 were isolated from nosocomial infections at the Microbiology Department of the University Clinics of Navarra (Spain) (Table 1). 132 is a methicillin-resistant S. aureus strain. When grown in Trypticase soy broth (TSB) supplemented with 3% NaCl, S. aureus 132 produces an exopolysaccharidic biofilm matrix (23). ISP479r is a derivative of ISP479 with a functional rsbU gene. E. coli XL1-Blue cells were routinely grown in Luria-Bertani (LB) broth or on LB agar (Pronadisa, Spain) with appropriate antibiotics. S. aureus strains were cultured on Trypticase soy agar (TSA), in TSB supplemented with 0.25% glucose (TSB-gluc), or in TSB supplemented with 3% NaCl when indicated. Media were supplemented with appropriate antibiotics at the following concentrations: erythromycin (Em), 10 μg ml−1 or 1.5 μg ml−1; ampicillin (Am), 100 μg ml−1; and chloramphenicol (Cm), 20 μg ml−1.
Manipulation of DNA.
Restriction endonucleases (Thermo Scientific) were used according to the manufacturer's specifications. PCR products were amplified with Phusion high-fidelity DNA polymerase (Thermo Scientific). Oligonucleotides were obtained from Stab Vida Corporation. σB and ica deletions were performed as previously described (18) using plasmids pMADsigAD and pMADicaAD (23). To construct S. aureus strains constitutively expressing icaADBC, the ica promoter from positions +1 to −50 was replaced by the Phyper promoter (52). For that, 2 PCR fragments of 500 bp that flank this region were amplified using primers IcaR5 (GGATCCAAAAGGATGCTTTCAAATACC)/IcaR3 (GCATGCTTGAAGGATAAGATTATTGATA) and IcaOp5 (CAACCTAACTAACGAAAGGTAG)/IcaOp3 (GAATTCTAGGATTACCTGTAACT). The IcaOp5 primer contains 55 nt of the Phyper promoter and the SphI restriction enzyme sequence. Then, the two PCR fragments were cloned into the pMAD plasmid, giving pMAD-pHyper-ica. Allelic exchange was performed as previously described (18).
Colonial morphology on Congo red agar.
The colony morphology of S. aureus was analyzed using Congo red agar plates (53, 54). Congo red agar was prepared as follows: 30 g/liter of Trypticase soy (Pronadisa), 15 g/liter of agar (Pronadisa), 0.8 g/liter of Congo red stain (Sigma), and 20 g/liter of sucrose. The Congo red stain and the sucrose solution were autoclaved separately (121°C for 20 min and 115°C for 15 min, respectively). S. aureus strains were streaked on Congo red agar and were incubated at 37°C for 24 h. S. aureus biofilm-positive variants display a rough colony morphology when grown on this medium, whereas biofilm-negative variants exhibit a smooth colony morphology.
Biofilm formation assays.
A biofilm formation assay in microtiter wells was performed as described previously (18). Briefly, strains were grown overnight at 37°C and diluted 1:40 in growth medium. The cell dilutions were used to inoculate sterile 96-well polystyrene microtiter plates. After 6 or 24 h of incubation at 37°C, the wells were gently rinsed three times with water, dried, and stained with 0.1% crystal violet for 15 min. The wells were rinsed again, and the crystal violet was solubilized in 200 μl of ethanol-acetone (80:20 [vol/vol]). The optical density at 595 nm was determined using a microplate reader (Multiskan EX; Labsystems). All experiments were performed in triplicate. A two-tailed Student t test was used to determine the difference in biofilm thickness between the wild type and the σB mutants. Biofilm formation under flow conditions was performed using microfermentors (55). Bacteria (108) from an overnight preculture were used to inoculate the microfermentors. After 24 h of incubation at 37°C, biofilm development was recorded with a Nikon Coolpix 950 digital camera and quantified by resuspension of the cells attached on the Pyrex slides in 10 ml of TSB. The optical density of the suspension was determined at 650 nm. All experiments were performed in triplicate.
PNAG detection.
PNAG production in S. aureus strains was detected as described previously (56). Briefly, overnight cultures were diluted 1:100 in growth medium. The cell suspensions were used to inoculate sterile 24-well polystyrene microtiter plates (Costar). After incubation, the cells were centrifuged, and the pellets were resuspended in 0.5 M EDTA (pH 8.0) to obtain the same density for each sample. The cells were incubated for 5 min at 100°C, and 40 μl of the supernatant was incubated with 10 μl of proteinase K (20 mg/ml; Sigma) for 30 min at 37°C. After addition of 10 μl of Tris-buffered saline (20 mM Tris-HCl, 150 mM NaCl [pH 7.4]) containing 0.01% bromophenol blue, 5 μl of sample dilutions was spotted on a nitrocellulose filter using a Bio-Dot microfiltration apparatus (Bio-Rad), blocked overnight, and incubated for 2 h with an anti-S. aureus PNAG antibody diluted 1:20,000 (57). Bound antibodies were detected with a peroxidase-conjugated goat anti-rabbit immunoglobulin G (IgG) antibody.
Generation of Ica transcriptional and posttranscriptional fusions with GFP.
To obtain an ica transcriptional fusion, we amplified the ica promoter using primers AU59 (ATGCCTGCAGGTCGACTTTTTATAACCCCCTACTGAAAATTAATCACACT)/AU76 (ACGAATTCGAGCTCGGTACCTTTCTTTACCTACCTTTCGTTAGTTAGGTTG) and cloned it using an In-fusion cloning kit (Clontech) in the pCN52 plasmid, giving plasmid pCN52-Pica_gfp. To obtain a posttranscriptional fusion, the 5ʹ UTR of ica was amplified using primers IcaA.Td5.EcoRI (GAATTCCAACCTAACTAACGAAAGGTAG) and IcaA.RBS.3.SpeI (ACTAGTCAATTTCTTTACCTACCTTTCGT) and cloned into plasmid pHRG. We constructed pHRG, a derivative pCN47 plasmid (33) in which the Phyper promoter and GFP lacking its ribosomal binding site were cloned using SphI and EcoRI and SpeI and AscI, respectively. The Ica 5ʹ UTR was fused in frame with gfpmut2 without its ribosomal binding site and constitutively expressed under the control of the Phyper promoter.
Immunoblot analysis.
Overnight cultures of S. aureus strains with pCN52-Pica_gfp or pHRG-5ʹUTRica plasmids were diluted 1:100 and grown in growth medium at 37°C under static conditions. Samples were obtained at the exponential and late stationary growth phases. Cells were resuspended in phosphate-buffered saline (PBS) and lysed using a FastPrep apparatus. Supernatants from total protein extracts were recovered and analyzed by SDS-PAGE and Western blotting, as detailed below. A volume of Laemmli buffer was added to the samples and boiled for 5 min; 4 μg of protein was used for SDS-PAGE analysis with 12% precast gels (Bio-Rad). The gels were stained with 0.25% Coomassie brilliant blue R250 (Sigma) as loading controls. For Western blot analysis, protein extracts were blotted onto Hybond-ECL nitrocellulose membranes (Amersham Biosciences). Anti-GFP antibodies (Living Color A.v. monoclonal antibody JL-8; Clontech) were diluted 1:2,500 with 0.1% PBS-Tween–5% skim milk. Alkaline phosphatase-conjugated goat anti-mouse immunoglobulin G (Sigma) diluted 1:5,000 in 0.1% PBS-Tween–5% skim milk was used as a secondary antibody, and the subsequent chemiluminescence reaction was recorded.
RNA extraction and Northern blotting.
Bacteria were grown in 100 ml of growth medium at 37°C under shaking conditions for 4 h. The cultures were centrifuged, and the pellets were frozen in liquid nitrogen and stored at −80°C. Total RNA from the bacterial pellets was extracted by using the TRIzol reagent method as described previously (58). Briefly, the bacterial pellets were resuspended in 400 μl of solution A (10% glucose, 12.5 mM Tris [pH 7.6], 10 mM EDTA). The cells were transferred to lysing matrix B tubes (MP Biomedicals) containing 500 μl of acid phenol (Ambion) and mechanically lysed by using a Fastprep apparatus (BIO101). After lysis, the tubes were centrifuged, and the aqueous phase was transferred to 2-ml tubes containing 1 ml of TRIzol, mixed, and incubated for 5 min at room temperature. Chloroform (100 μl) was added, mixed gently, and incubated for 3 min at room temperature. The aqueous phase was transferred into a 2-ml tube containing 200 μl of chloroform, mixed, and incubated for 5 min at room temperature. The tubes were centrifuged, and RNA contained in the aqueous phase was precipitated by addition of isopropanol. Northern blotting was performed as described previously (58). Briefly, 11 μg of total RNA was separated in precast agarose gels (Sigma). RNAs were blotted onto Nytran membranes (0.2-μm pore size; Sigma), UV cross-linked, prehybridized in Ultrahyb solution (Ambion) at 65°C, and labeled with strand-specific riboprobes specific for icaA: AU52 (TAATACGACTCACTATAGGGTATCCACGTAAATGCAATTTCC)/AU53 (TGGAAGTTCAGATAATACAGC) and icaC AU54 (TAATACGACTCACTATAGGGGTATGATATTGCGTGAATTC)/AU55 (TCACGATACCGTGCTACAC). The membranes were washed, and autoradiography images were registered at different exposure times for each gene.
Protein tagging and immunodetection analysis.
Transfer of the 3×Flag sequence into IcaC was performed by recombination using plasmid pMADicaC-3×Flag. To construct pMADicaC-3×Flag, the region of icaC corresponding to the C-terminal region of IcaC was amplified using primers CFlag1 (GCAAATGGAGACTATTGG) and CFlag2 (ATAAGCATTAATGTTCAATTTA). The CFlag2 primer contains 66 nt coding for the 3×Flag sequence. Strains containing IcaC with 3×Flag were grown in growth medium for 5 h (Exp) and 24 h (ON). Then, the cells were harvested by centrifugation, and the pellets were resuspended in proportional quantities of PBS buffer containing lysostaphin (12.5 μg/ml; Sigma) and DNase I for 2 h. A volume of Laemmli buffer was added before loading the samples in SDS-12% PAGE. Proteins were transferred onto nitrocellulose membranes (Hybond; Amersham Biosciences), and 3×Flag fusion proteins were immunodetected by the use of anti-Flag M2 monoclonal antibodies (MAbs) conjugated with peroxidase (Sigma). Densitometry analysis of the detected bands was performed using ImageJ (http://rsbweb.nih.gov/ij/).
Translation block experiment.
To assess the in vivo stability of IcaC-3×Flag, overnight cultures of strains S. aureus 132 icaC-3×Flag and ΔσB icaC-3×Flag were diluted 1:100 in fresh TSB-NaCl medium, and the cultures were grown to exponential phase at 37°C. Then, protein synthesis was inhibited at time point zero by the addition of 200 μg/ml rifampin and 500 μg/ml lincomycin. Samples were harvested at the indicated times after translation inhibition, and IcaC levels were analyzed by immunoblotting using anti-Flag M2 MAbs conjugated with peroxidase (Sigma). Band intensities were quantified using ImageJ software and normalized to levels present 5 min after translation inhibition for each strain.
Supplementary Material
ACKNOWLEDGMENTS
We express our gratitude to T. Maira-Litrán, Harvard Medical School of Boston, for providing us the anti-PNAG antiserum, W. Ziebuhr for pSK9, and Friedrich Götz for pSC18.
Work in the Laboratory of Microbial Pathogenesis is funded by the Spanish Ministry of Science, Innovation and Universities (grants SAF2015-74267-JIN and BIO2017-83035-R [Agencia Española de Investigación/Fondo Europeo de Desarrollo Regional, European Union]).
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JB.00098-19.
REFERENCES
- 1.Haag AF, Bagnoli F. 2017. The role of two-component signal transduction systems in Staphylococcus aureus virulence regulation. Curr Top Microbiol Immunol:145–198. doi: 10.1007/82_2015_5019. [DOI] [PubMed] [Google Scholar]
- 2.Kazmierczak MJ, Wiedmann M, Boor KJ. 2005. Alternative sigma factors and their roles in bacterial virulence. Microbiol Mol Biol Rev 69:527–543. doi: 10.1128/MMBR.69.4.527-543.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kullik I, Giachino P, Fuchs T. 1998. Deletion of the alternative sigma factor sigmaB in Staphylococcus aureus reveals its function as a global regulator of virulence genes. J Bacteriol 180:4814–4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan PF, Foster SJ, Ingham E, Clements MO. 1998. The Staphylococcus aureus alternative sigma factor sigmaB controls the environmental stress response but not starvation survival or pathogenicity in a mouse abscess model. J Bacteriol 180:6082–6089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horsburgh MJ, Aish JL, White IJ, Shaw L, Lithgow JK, Foster SJ. 2002. SigmaB modulates virulence determinant expression and stress resistance: characterization of a functional rsbU strain derived from Staphylococcus aureus 8325-4. J Bacteriol 184:5457–5467. doi: 10.1128/JB.184.19.5457-5467.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giachino P, Engelmann S, Bischoff M. 2001. Sigma(B) activity depends on RsbU in Staphylococcus aureus. J Bacteriol 183:1843–1852. doi: 10.1128/JB.183.6.1843-1852.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pané-Farré J, Jonas B, Hardwick SW, Gronau K, Lewis RJ, Hecker M, Engelmann S. 2009. Role of RsbU in controlling SigB activity in Staphylococcus aureus following alkaline stress. J Bacteriol 191:2561–2573. doi: 10.1128/JB.01514-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tuchscherr L, Bischoff M, Lattar SM, Noto Llana M, Pförtner H, Niemann S, Geraci J, Van de Vyver H, Fraunholz MJ, Cheung AL, Herrmann M, Völker U, Sordelli DO, Peters G, Löffler B. 2015. Sigma factor SigB is crucial to mediate Staphylococcus aureus adaptation during chronic infections. PLoS Pathog 11:e1004870. doi: 10.1371/journal.ppat.1004870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bischoff M, Dunman P, Kormanec J, Macapagal D, Murphy E, Mounts W, Berger-Bächi B, Projan S. 2004. Microarray-based analysis of the Staphylococcus aureus sigmaB regulon. J Bacteriol 186:4085–4099. doi: 10.1128/JB.186.13.4085-4099.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jonsson I-M, Arvidson S, Foster S, Tarkowski A. 2004. Sigma factor B and RsbU are required for virulence in Staphylococcus aureus-induced arthritis and sepsis. Infect Immun 72:6106–6111. doi: 10.1128/IAI.72.10.6106-6111.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Götz F. 2002. Staphylococcus and biofilms. Mol Microbiol 43:1367–1378. doi: 10.1046/j.1365-2958.2002.02827.x. [DOI] [PubMed] [Google Scholar]
- 12.O'Gara JP. 2017. Into the storm: chasing the opportunistic pathogen Staphylococcus aureus from skin colonisation to life-threatening infections. Environ Microbiol 19:3823–3833. doi: 10.1111/1462-2920.13833. [DOI] [PubMed] [Google Scholar]
- 13.Otto M. 2018. Staphylococcal biofilms. Microbiol Spectr 6:1–17. doi: 10.1128/microbiolspec.GPP3-0023-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flemming H-C, Wingender J, Szewzyk U, Steinberg P, Rice SA, Kjelleberg S. 2016. Biofilms: an emergent form of bacterial life. Nat Rev Microbiol 14:563–575. doi: 10.1038/nrmicro.2016.94. [DOI] [PubMed] [Google Scholar]
- 15.Bateman BT, Donegan NP, Jarry TM, Palma M, Cheung AL. 2001. Evaluation of a tetracycline-inducible promoter in Staphylococcus aureus in vitro and in vivo and its application in demonstrating the role of sigB in microcolony formation. Infect Immun 69:7851–7857. doi: 10.1128/IAI.69.12.7851-7857.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rachid S, Ohlsen K, Wallner U, Hacker J, Hecker M, Ziebuhr W. 2000. Alternative transcription factor sigma(B) is involved in regulation of biofilm expression in a Staphylococcus aureus mucosal isolate. J Bacteriol 182:6824–6826. doi: 10.1128/JB.182.23.6824-6826.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martí M, Trotonda MP, Tormo-Más MA, Vergara-Irigaray M, Cheung AL, Lasa I, Penadés JR. 2010. Extracellular proteases inhibit protein-dependent biofilm formation in Staphylococcus aureus. Microbes Infect 12:55–64. doi: 10.1016/j.micinf.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Valle J, Toledo-Arana A, Berasain C, Ghigo J-M, Amorena B, Penadés JR, Lasa I. 2003. SarA and not SigmaB is essential for biofilm development by Staphylococcus aureus. Mol Microbiol 48:1075–1087. doi: 10.1046/j.1365-2958.2003.03493.x. [DOI] [PubMed] [Google Scholar]
- 19.Houston P, Rowe SE, Pozzi C, Waters EM, O'Gara JP. 2011. Essential role for the major autolysin in the fibronectin-binding protein-mediated Staphylococcus aureus biofilm phenotype. Infect Immun 79:1153–1165. doi: 10.1128/IAI.00364-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cucarella C, Solano C, Valle J, Amorena B, Lasa I, Penadés JR. 2001. Bap, a Staphylococcus aureus surface protein involved in biofilm formation. J Bacteriol 183:2888–2896. doi: 10.1128/JB.183.9.2888-2896.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rice KC, Mann EE, Endres JL, Weiss EC, Cassat JE, Smeltzer MS, Bayles KW. 2007. The cidA murein hydrolase regulator contributes to DNA release and biofilm development in Staphylococcus aureus. Proc Natl Acad Sci U S A 104:8113–8118. doi: 10.1073/pnas.0610226104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boles BR, Horswill AR. 2008. Agr-mediated dispersal of Staphylococcus aureus biofilms. PLoS Pathog 4:e1000052. doi: 10.1371/journal.ppat.1000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vergara-Irigaray M, Valle J, Merino N, Latasa C, García B, Ruiz de Los Mozos I, Solano C, Toledo-Arana A, Penadés JR, Lasa I. 2009. Relevant role of fibronectin-binding proteins in Staphylococcus aureus biofilm-associated foreign-body infections. Infect Immun 77:3978–3991. doi: 10.1128/IAI.00616-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Merino N, Toledo-Arana A, Vergara-Irigaray M, Valle J, Solano C, Calvo E, Lopez JA, Foster TJ, Penadés JR, Lasa I. 2009. Protein A-mediated multicellular behavior in Staphylococcus aureus. J Bacteriol 191:832–843. doi: 10.1128/JB.01222-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O'Neill E, Pozzi C, Houston P, Smyth D, Humphreys H, Robinson DA, O'Gara JP. 2007. Association between methicillin susceptibility and biofilm regulation in Staphylococcus aureus isolates from device-related infections. J Clin Microbiol 45:1379–1388. doi: 10.1128/JCM.02280-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Corrigan RM, Rigby D, Handley P, Foster TJ. 2007. The role of Staphylococcus aureus surface protein SasG in adherence and biofilm formation. Microbiology 153:2435–2446. doi: 10.1099/mic.0.2007/006676-0. [DOI] [PubMed] [Google Scholar]
- 27.Lauderdale KJ, Boles BR, Cheung AL, Horswill AR. 2009. Interconnections between Sigma B, agr, and proteolytic activity in Staphylococcus aureus biofilm maturation. Infect Immun 77:1623–1635. doi: 10.1128/IAI.01036-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kiedrowski MR, Kavanaugh JS, Malone CL, Mootz JM, Voyich JM, Smeltzer MS, Bayles KW, Horswill AR. 2011. Nuclease modulates biofilm formation in community-associated methicillin-resistant Staphylococcus aureus. PLoS One 6:e26714. doi: 10.1371/journal.pone.0026714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cramton SE, Gerke C, Schnell NF, Nichols WW, Götz F. 1999. The intercellular adhesion (ica) locus is present in Staphylococcus aureus and is required for biofilm formation. Infect Immun 67:5427–5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McKenney D, Pouliot KL, Wang Y, Murthy V, Ulrich M, Döring G, Lee JC, Goldmann DA, Pier GB. 1999. Broadly protective vaccine for Staphylococcus aureus based on an in vivo-expressed antigen. Science 284:1523–1527. doi: 10.1126/science.284.5419.1523. [DOI] [PubMed] [Google Scholar]
- 31.Maira-Litrán T, Kropec A, Abeygunawardana C, Joyce J, Mark G, Goldmann DA, Pier GB. 2002. Immunochemical properties of the staphylococcal poly-N-acetylglucosamine surface polysaccharide. Infect Immun 70:4433–4440. doi: 10.1128/IAI.70.8.4433-4440.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cerca N, Brooks JL, Jefferson KK. 2008. Regulation of the intercellular adhesin locus regulator (icaR) by SarA, SigmaB, and IcaR in Staphylococcus aureus. J Bacteriol 190:6530–6533. doi: 10.1128/JB.00482-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Charpentier E, Anton AI, Barry P, Alfonso B, Fang Y, Novick RP. 2004. Novel cassette-based shuttle vector system for Gram-positive bacteria. Appl Environ Microbiol 70:6076–6085. doi: 10.1128/AEM.70.10.6076-6085.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Balestrino D, Hamon MA, Dortet L, Nahori M-A, Pizarro-Cerda J, Alignani D, Dussurget O, Cossart P, Toledo-Arana A. 2010. Single-cell techniques using chromosomally tagged fluorescent bacteria to study Listeria monocytogenes infection processes. Appl Environ Microbiol 76:3625–3636. doi: 10.1128/AEM.02612-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nagórska K, Hinc K, Strauch MA, Obuchowski M. 2008. Influence of the SigmaB stress factor and yxaB, the gene for a putative exopolysaccharide synthase under SigmaB control, on biofilm formation. J Bacteriol 190:3546–3556. doi: 10.1128/JB.01665-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Conlon KM, Humphreys H, O'Gara JP. 2002. icaR encodes a transcriptional repressor involved in environmental regulation of ica operon expression and biofilm formation in Staphylococcus epidermidis. J Bacteriol 184:4400–4408. doi: 10.1128/JB.184.16.4400-4408.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Knobloch JK-M, Jäger S, Horstkotte MA, Rohde H, Mack D. 2004. RsbU-dependent regulation of Staphylococcus epidermidis biofilm formation is mediated via the alternative sigma factor SigmaB by repression of the negative regulator gene icaR. Infect Immun 72:3838–3848. doi: 10.1128/IAI.72.7.3838-3848.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Conlon KM, Humphreys H, O'Gara JP. 2004. Inactivations of rsbU and sarA by IS256 represent novel mechanisms of biofilm phenotypic variation in Staphylococcus epidermidis. J Bacteriol 186:6208–6219. doi: 10.1128/JB.186.18.6208-6219.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beenken KE, Blevins JS, Smeltzer MS. 2003. Mutation of sarA in Staphylococcus aureus limits biofilm formation. Infect Immun 71:4206–4211. doi: 10.1128/IAI.71.7.4206-4211.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jefferson KK, Pier DB, Goldmann DA, Pier GB. 2004. The teicoplanin-associated locus regulator (TcaR) and the intercellular adhesin locus regulator (IcaR) are transcriptional inhibitors of the ica locus in Staphylococcus aureus. J Bacteriol 186:2449–2456. doi: 10.1128/JB.186.8.2449-2456.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cue D, Lei MG, Luong TT, Kuechenmeister L, Dunman PM, O'Donnell S, Rowe S, O'Gara JP, Lee CY. 2009. Rbf promotes biofilm formation by Staphylococcus aureus via repression of icaR, a negative regulator of icaADBC. J Bacteriol 191:6363–6373. doi: 10.1128/JB.00913-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tormo MA, Martí M, Valle J, Manna AC, Cheung AL, Lasa I, Penadés JR. 2005. SarA is an essential positive regulator of Staphylococcus epidermidis biofilm development. J Bacteriol 187:2348–2356. doi: 10.1128/JB.187.7.2348-2356.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lasa I, Toledo-Arana A, Dobin A, Villanueva M, de los Mozos IR, Vergara-Irigaray M, Segura V, Fagegaltier D, Penadés JR, Valle J, Solano C, Gingeras TR. 2011. Genome-wide antisense transcription drives mRNA processing in bacteria. Proc Natl Acad Sci U S A 108:20172–20177. doi: 10.1073/pnas.1113521108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mäder U, Nicolas P, Depke M, Pané-Farré J, Débarbouillé M, van der Kooi-Pol MM, Guérin C, Dérozier S, Hiron A, Jarmer H, Leduc A, Michalik S, Reilman E, Schaffer M, Schmidt F, Bessières P, Noirot P, Hecker M, Msadek T, Völker U, van Dijl JM. 2016. Staphylococcus aureus transcriptome architecture: from laboratory to infection-mimicking conditions. PLoS Genet 12:e1005962. doi: 10.1371/journal.pgen.1005962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang X, Dubey AK, Suzuki K, Baker CS, Babitzke P, Romeo T. 2005. CsrA post-transcriptionally represses pgaABCD, responsible for synthesis of a biofilm polysaccharide adhesin of Escherichia coli. Mol Microbiol 56:1648–1663. doi: 10.1111/j.1365-2958.2005.04648.x. [DOI] [PubMed] [Google Scholar]
- 46.Steiner S, Lori C, Boehm A, Jenal U. 2013. Allosteric activation of exopolysaccharide synthesis through cyclic di-GMP-stimulated protein-protein interaction. EMBO J 32:354–368. doi: 10.1038/emboj.2012.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Holland LM, O'Donnell ST, Ryjenkov DA, Gomelsky L, Slater SR, Fey PD, Gomelsky M, O'Gara JP. 2008. A staphylococcal GGDEF domain protein regulates biofilm formation independently of cyclic dimeric GMP. J Bacteriol 190:5178–5189. doi: 10.1128/JB.00375-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fischer A, Kambara K, Meyer H, Stenz L, Bonetti E-J, Girard M, Lalk M, François P, Schrenzel J. 2014. GdpS contributes to Staphylococcus aureus biofilm formation by regulation of eDNA release. Int J Med Microbiol 304:284–299. doi: 10.1016/j.ijmm.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 49.Ishihara Y, Hyodo M, Hayakawa Y, Kamegaya T, Yamada K, Okamoto A, Hasegawa T, Ohta M. 2009. Effect of cyclic bis(3’-5’)diguanylic acid and its analogs on bacterial biofilm formation. FEMS Microbiol Lett 301:193–200. doi: 10.1111/j.1574-6968.2009.01825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Corrigan RM, Abbott JC, Burhenne H, Kaever V, Gründling A. 2011. c-di-AMP is a new second messenger in Staphylococcus aureus with a role in controlling cell size and envelope stress. PLoS Pathog 7:e1002217. doi: 10.1371/journal.ppat.1002217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Valle J, Vergara-Irigaray M, Merino N, Penadés JR, Lasa I. 2007. SigmaB regulates IS256-mediated Staphylococcus aureus biofilm phenotypic variation. J Bacteriol 189:2886–2896. doi: 10.1128/JB.01767-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Quisel JD, Burkholder WF, Grossman AD. 2001. In vivo effects of sporulation kinases on mutant Spo0A proteins in Bacillus subtilis. J Bacteriol 183:6573–6578. doi: 10.1128/JB.183.22.6573-6578.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Deighton M, Pearson S, Capstick J, Spelman D, Borland R. 1992. Phenotypic variation of Staphylococcus epidermidis isolated from a patient with native valve endocarditis. J Clin Microbiol 30:2385–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Götz F, Verheij HM, Rosenstein R. 1998. Staphylococcal lipases: molecular characterisation, secretion, and processing. Chem Phys Lipids 93:15–25. doi: 10.1016/S0009-3084(98)00025-5. [DOI] [PubMed] [Google Scholar]
- 55.Ghigo JM. 2001. Natural conjugative plasmids induce bacterial biofilm development. Nature 412:442–445. doi: 10.1038/35086581. [DOI] [PubMed] [Google Scholar]
- 56.Toledo-Arana A, Merino N, Vergara-Irigaray M, Débarbouillé M, Penadés JR, Lasa I. 2005. Staphylococcus aureus develops an alternative, ica-independent biofilm in the absence of the arlRS two-component system. J Bacteriol 187:5318–5329. doi: 10.1128/JB.187.15.5318-5329.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maira-Litrán T, Kropec A, Goldmann DA, Pier GB. 2005. Comparative opsonic and protective activities of Staphylococcus aureus conjugate vaccines containing native or deacetylated staphylococcal poly-N-acetyl-beta-(1-6)-glucosamine. Infect Immun 73:6752–6762. doi: 10.1128/IAI.73.10.6752-6762.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu JM, Livny J, Lawrence MS, Kimball MD, Waldor MK, Camilli A. 2009. Experimental discovery of sRNAs in Vibrio cholerae by direct cloning, 5S/tRNA depletion and parallel sequencing. Nucleic Acids Res 37:e46. doi: 10.1093/nar/gkp080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pattee PA. 1981. Distribution of Tn551 insertion sites responsible for auxotrophy on the Staphylococcus aureus chromosome. J Bacteriol 145:479–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Arnaud M, Chastanet A, Débarbouillé M. 2004. New vector for efficient allelic replacement in naturally nontransformable, low-GC-content, gram-positive bacteria. Appl Environ Microbiol 70:6887–6891. doi: 10.1128/AEM.70.11.6887-6891.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.