The title compound features an intramolecular hydrogen bond involving the acidic H atom bound to the cage C atom and an ortho-F atom of the heptafluorotolyl substituent.
Keywords: crystal structure, carborane, intramolecular F⋯H hydrogen-bond
Abstract
The molecular structure of the title compound 1-(2′,3′,5′,6′-tetrafluoro-4′-trifluoromethylphenyl)-closo-1,2-dicarbadodecaborane, C9H11B10F7, features an intramolecular ortho-F⋯H2 hydrogen bond [2.11 (2) Å], which is responsible for an orientation of the heptafluorotolyl substituent in which the plane of the aryl ring nearly eclipses the C1—C2 cage connectivity.
Chemical context
Carborane chemistry continues to be an area of intense academic interest but also one that has both potential and real applications in a wide variety of fields, with a particular blossoming of such applications over the last two decades (Grimes, 2016 ▸). Two important factors driving studies into the synthesis and properties of novel carborane compounds for a vast array of applications are the high chemical and thermal stabilities of such species and the relative ease of their derivatization. Several years ago we described a family of doubly substituted closo-C2B10 carboranes bearing fluorinated aryl groups (Tricas et al., 2011 ▸). Our comprehensive (synthetic, spectroscopic, structural, electrochemical and computational) study focused primarily on the stabilization of the reduced form of the carboranes by the presence of the strongly electron-withdrawing fluoroaryl groups, and the study has attracted considerable attention from those working in the related field of carborane photophysics (e.g. Van Nghia et al., 2018 ▸; Marsh et al., 2018 ▸). Very recently we have reported the first examples of substituted carboranes as components of intermolecular frustrated Lewis pairs (FLPs; Benton et al., 2018 ▸). In this field the ability to fine-tune the Lewis acidity or basicity of a functional group on a carborane support by the electron-withdrawing or electron-donating characteristics of a second substituent on the carborane is of potential importance in using these FLPs as catalysts. Herein we report the synthesis and crystal structure of [1-(4′-F3CC6F4)-closo-1,2-C2B10H11], a singly substituted fluoroaryl carborane with the potential for further derivatization.
Structural commentary
H atoms bound to C in closo carboranes are protonic in nature (Grimes, 2016 ▸) and the strongly electron-withdrawing nature of the perfluorotolyl substituent on C1 renders the H atom on C2 in [1-(4′-F3CC6F4)-closo-1,2-C2B10H11] particularly protonic, as evidenced by its high-frequency 1H NMR chemical shift (δ 4.88 ppm). This makes the C1H1 unit a strong hydrogen-bond donor and results in the most striking feature of the structure (Fig. 1 ▸), the intramolecular hydrogen bond between F12 and H2. Molecular dimensions for the hydrogen bond are given in Table 1 ▸ and are complemented by the near-tetrahedral angle C12—F12⋯H2 = 108.4 (6) °. This hydrogen bond is responsible for the orientation of the 4′-F3CC6F4 substituent with respect to the carborane in the solid state, defined by the torsion angle C2—C1—C11—C12 = 9.6 (2)°, in which the plane of the aryl ring almost eclipses the C1—C2 connectivity.
Figure 1.
The molecular structure of [1-(4′-F3CC6F4)-closo-1,2-C2B10H11] with key atoms labelled. Displacement ellipsoids are drawn at the 50% probability level, except for H atoms. The hydrogen bond between F12 and H2 is shown as a dotted line.
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C2—H2⋯F12 | 0.91 (2) | 2.11 (2) | 2.7436 (19) | 126 (2) |
The only other [1-(ortho-F-aryl)-closo-1,2-C2B10H11] species to have been studied crystallographically is that with a 2′-fluoro-4′-(9′′-phenanthrenyl) substituent (Tu et al., 2017 ▸). In this species there is an intermolecular F⋯CcageH hydrogen-bond, 2.091 (4) Å, between the two crystallographically independent molecules in the asymmetric fraction of the unit cell, although the situation is somewhat complicated by partial disorder of both F atoms. The C1—C2 distance in [1-(4′-F3CC6F4)-closo-1,2-C2B10H11], 1.660 (2) Å, stands good comparison with that in [1-Ph-closo-1,2-C2B10H11] [α polymorph, 1.640 (5) Å, Brain et al., 1996 ▸; β polymorph, 1.649 (2) Å, Thomas et al., 1996 ▸]. Dimensions within the 4′-F3CC6F4 substituent are fully consistent with those in [1-(4′-F3CC6F4)-2-Ph-closo-1,2-C2B10H10], [1,2-(4′-F3CC6F4)2-closo-1,2-C2B10H10], [1,7-(4′-F3CC6F4)2-closo-1,7-C2B10H10] and [1,12-(4′-F3CC6F4)2-closo-1,12-C2B10H10] (Tricas et al., 2011 ▸).
Supramolecular features
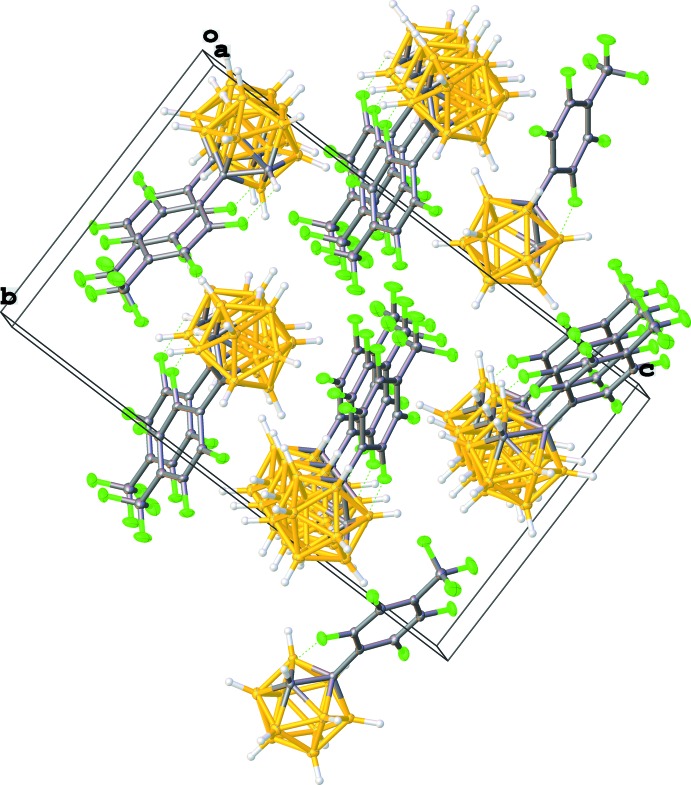
Molecules pack in ribbons parallel to the crystallographic a axis, but there are no significant intermolecular contacts either within or between these ribbons. A view of the crystal packing along [100] is shown in Fig. 2 ▸.
Figure 2.
Unit cell of [1-(4′-F3CC6F4)-closo-1,2-C2B10H11] in a view along [100].
Database survey
A search of the Cambridge Structural Database (CSD, 2019 release; Groom et al., 2016 ▸) yielded 384 examples of [C-aryl-closo-1,2-C2B10] carboranes. However, this number drops to 63 if the second cage C atom is not substituted, i.e. structures of the type [1-aryl-closo-1,2-C2B10H11]. Furthermore, there are only two reported structural studies of cases where the aryl ring is at least partially fluorinated, the aforementioned 2′-fluoro-4′-(9′′-phenanthrenyl) species (Tu et al., 2017 ▸) and [1-(4′-C6H4F)-closo-1,2-C2B10H11] (Clegg, 2016 ▸). Removing the condition that the second cage C atom is not substituted affords 19 further examples of fluoroaryl derivatives of [closo-1,2-C2B10H11]. There are only three examples where a 4′-F3CC6F4 substituent is attached to a [closo-1,2-C2B10] cage, two of which result from our laboratories (Tricas et al., 2011 ▸) and the other from Lee et al. (2017 ▸).
Synthesis and crystallization
Under dry N2 and using anhydrous, degassed solvents, [closo-1,2-C2B10H12] (0.75 g, 5.2 mmol) was dissolved in a 1:1 mixture of toluene and diethyl ether (40 mL). The colourless solution was cooled to 273 K before n-BuLi (3.58 mL of a 1.6 M solution in hexanes, 5.73 mmol, 1.1 equiv.) was added dropwise over the course of 2 min. whilst stirring vigorously. The solution was warmed to room temperature and changed from colourless to yellow. After further stirring for 1 h the solution was cooled to 273 K, resulting in a white suspension. Whilst stirring vigorously, octafluorotoluene (0.74 mL, 5.2 mmol, 1.0 equiv.) was added dropwise over the course of 1 min., causing the solution to turn from yellow to deep red. The solution was stirred for 4 h at room temperature and then quenched with saturated [NH4]Cl (aq., 20 mL). The organic layer was isolated and the aqueous phase extracted with Et2O (3 × 20 mL). The organic phases were combined and reduced in volume in vacuo to yield a brown residue. Products were isolated by column chromatography on silica eluting with 313–333 K petroleum ether to give both the target compound [1-(4′-F3CC6F4)-closo-1,2-C2B10H11] (R f = 0.27, 0.57 g, 30% yield) and the disubstituted species [1,2-(4′-F3CC6F4)2-closo-1,2-C2B10H10] (R f = 0.37, 0.33 g, 11% yield, Tricas et al., 2011 ▸) as colourless solids once evacuated to dryness.
C9H11B10F7 requires; C 30.0, H 3.08. Found; C 30.5, H 2.83%. 1H NMR (CDCl3, 400.1 MHz, 298 K, δ): 4.88 (br. s, 1H, CH cage). 11B{1H} NMR (CDCl3, 128.4 MHz, 298 K, δ): −0.32 (1B), −1.80 (1B), −8.06 (2B), −9.62 (2B), −11.17 (2B), −12.89 (2B). 19F NMR (CDCl3, 376.5 MHz, 298 K, δ): −56.72 (t, 3F, J FF = 21.3 Hz, CF 3), −135.17 (br. s, 2F, F ortho), −137.26 (m, 2F, F meta). Crystals of [1-(4′-F3CC6F4)-closo-1,2-C2B10H11] suitable for a single-crystal X-ray diffraction study were grown from the slow evaporation of a 313–333 K petroleum ether solution of the product.
Refinement
Crystal data, data collection and structure refinement details are summarized in Table 2 ▸. The cage C atom (C2) not carrying the substituent was distinguished from B atoms by both the Vertex–Centroid Distance (McAnaw et al., 2013 ▸) and Boron–Hydrogen Distance (McAnaw et al., 2014 ▸) methods. Cage H atoms were located from difference-Fourier maps and allowed positional refinement, with U iso(H) = 1.2U eq(B or C). Five poorly fitting reflections were omitted which marginally decreased the R-factor and standard uncertainties from the previous refinement.
Table 2. Experimental details.
| Crystal data | |
| Chemical formula | C9H11B10F7 |
| M r | 360.28 |
| Crystal system, space group | Orthorhombic, P212121 |
| Temperature (K) | 120 |
| a, b, c (Å) | 6.7872 (2), 11.6926 (3), 19.4863 (5) |
| V (Å3) | 1546.43 (7) |
| Z | 4 |
| Radiation type | Mo Kα |
| μ (mm−1) | 0.14 |
| Crystal size (mm) | 0.30 × 0.21 × 0.10 |
| Data collection | |
| Diffractometer | Rigaku Oxford Diffreaction SuperNova |
| Absorption correction | Multi-scan (CrysAlis PRO; Rigaku OD, 2018 ▸) |
| T min, T max | 0.907, 1.000 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 40258, 5615, 5190 |
| R int | 0.041 |
| (sin θ/λ)max (Å−1) | 0.768 |
| Refinement | |
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.039, 0.092, 1.15 |
| No. of reflections | 5615 |
| No. of parameters | 268 |
| H-atom treatment | Only H-atom coordinates refined |
| Δρmax, Δρmin (e Å−3) | 0.32, −0.24 |
| Absolute structure | Flack x determined using 1991 quotients [(I +)−(I −)]/[(I +)+(I −)] (Parsons et al., 2013 ▸) |
| Absolute structure parameter | −0.03 (14) |
Supplementary Material
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2056989019004067/lh5894sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989019004067/lh5894Isup2.hkl
Supporting information file. DOI: 10.1107/S2056989019004067/lh5894Isup3.mol
CCDC reference: 1905663
Additional supporting information: crystallographic information; 3D view; checkCIF report
Acknowledgments
We thank Dr G. Nicol (University of Edinburgh) for the data collection.
supplementary crystallographic information
Crystal data
| C9H11B10F7 | Dx = 1.547 Mg m−3 |
| Mr = 360.28 | Mo Kα radiation, λ = 0.71073 Å |
| Orthorhombic, P212121 | Cell parameters from 14204 reflections |
| a = 6.7872 (2) Å | θ = 3.6–32.3° |
| b = 11.6926 (3) Å | µ = 0.14 mm−1 |
| c = 19.4863 (5) Å | T = 120 K |
| V = 1546.43 (7) Å3 | Block, colourless |
| Z = 4 | 0.30 × 0.21 × 0.10 mm |
| F(000) = 712 |
Data collection
| Rigaku Oxford Diffreaction SuperNova diffractometer | 5615 independent reflections |
| Radiation source: micro-focus sealed X-ray tube, SuperNova (Mo) X-ray Source | 5190 reflections with I > 2σ(I) |
| Mirror monochromator | Rint = 0.041 |
| Detector resolution: 5.1574 pixels mm-1 | θmax = 33.1°, θmin = 3.2° |
| ω scans | h = −10→10 |
| Absorption correction: multi-scan (CrysAlis PRO; Rigaku OD, 2018) | k = −17→17 |
| Tmin = 0.907, Tmax = 1.000 | l = −28→29 |
| 40258 measured reflections |
Refinement
| Refinement on F2 | Hydrogen site location: difference Fourier map |
| Least-squares matrix: full | Only H-atom coordinates refined |
| R[F2 > 2σ(F2)] = 0.039 | w = 1/[σ2(Fo2) + (0.0409P)2 + 0.2387P] where P = (Fo2 + 2Fc2)/3 |
| wR(F2) = 0.092 | (Δ/σ)max < 0.001 |
| S = 1.15 | Δρmax = 0.32 e Å−3 |
| 5615 reflections | Δρmin = −0.24 e Å−3 |
| 268 parameters | Absolute structure: Flack x determined using 1991 quotients [(I+)-(I-)]/[(I+)+(I-)] (Parsons et al., 2013) |
| 0 restraints | Absolute structure parameter: −0.03 (14) |
| Primary atom site location: dual |
Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| C1 | 0.4255 (2) | 0.75630 (14) | 0.63450 (7) | 0.0127 (3) | |
| C2 | 0.3379 (2) | 0.84949 (14) | 0.69108 (8) | 0.0144 (3) | |
| H2 | 0.239 (3) | 0.825 (2) | 0.7189 (11) | 0.017* | |
| B3 | 0.5657 (3) | 0.79020 (16) | 0.70696 (9) | 0.0150 (3) | |
| H3 | 0.569 (4) | 0.731 (2) | 0.7447 (11) | 0.018* | |
| B4 | 0.6746 (3) | 0.78014 (17) | 0.62414 (9) | 0.0152 (3) | |
| H4 | 0.760 (3) | 0.7102 (19) | 0.6185 (11) | 0.018* | |
| B5 | 0.4984 (3) | 0.82791 (16) | 0.56196 (9) | 0.0157 (3) | |
| H5 | 0.481 (3) | 0.7834 (19) | 0.5125 (11) | 0.019* | |
| B6 | 0.2792 (3) | 0.86920 (16) | 0.60596 (9) | 0.0156 (3) | |
| H6 | 0.137 (3) | 0.848 (2) | 0.5881 (11) | 0.019* | |
| B7 | 0.5140 (3) | 0.93737 (17) | 0.72239 (9) | 0.0173 (3) | |
| H7 | 0.498 (3) | 0.960 (2) | 0.7758 (11) | 0.021* | |
| B8 | 0.7317 (3) | 0.89674 (17) | 0.67875 (10) | 0.0172 (3) | |
| H8 | 0.868 (4) | 0.904 (2) | 0.7027 (11) | 0.021* | |
| B9 | 0.6915 (3) | 0.92010 (17) | 0.58946 (9) | 0.0174 (3) | |
| H9 | 0.810 (3) | 0.940 (2) | 0.5576 (11) | 0.021* | |
| B10 | 0.4473 (3) | 0.97497 (17) | 0.57793 (10) | 0.0191 (3) | |
| H10 | 0.411 (4) | 1.030 (2) | 0.5388 (11) | 0.023* | |
| C11 | 0.3333 (2) | 0.63954 (14) | 0.62898 (7) | 0.0133 (3) | |
| B11 | 0.3386 (3) | 0.98580 (16) | 0.66084 (10) | 0.0178 (3) | |
| H11 | 0.223 (4) | 1.039 (2) | 0.6735 (11) | 0.021* | |
| F12 | 0.06277 (15) | 0.67898 (9) | 0.70430 (5) | 0.0194 (2) | |
| C12 | 0.1573 (2) | 0.60768 (14) | 0.66176 (8) | 0.0142 (3) | |
| B12 | 0.5930 (3) | 1.01793 (17) | 0.65039 (10) | 0.0185 (3) | |
| H12 | 0.642 (4) | 1.101 (2) | 0.6567 (12) | 0.022* | |
| F13 | −0.09989 (16) | 0.48161 (9) | 0.68394 (5) | 0.0216 (2) | |
| C13 | 0.0708 (3) | 0.50228 (14) | 0.65191 (8) | 0.0160 (3) | |
| C14 | 0.1543 (3) | 0.41874 (14) | 0.61068 (8) | 0.0171 (3) | |
| F15 | 0.4241 (2) | 0.37422 (9) | 0.53809 (6) | 0.0269 (3) | |
| C15 | 0.3300 (3) | 0.44732 (14) | 0.57878 (8) | 0.0177 (3) | |
| F16 | 0.58167 (16) | 0.57283 (9) | 0.55240 (5) | 0.0215 (2) | |
| C16 | 0.4149 (3) | 0.55452 (14) | 0.58724 (8) | 0.0157 (3) | |
| F17 | −0.1264 (2) | 0.31645 (11) | 0.57675 (7) | 0.0366 (3) | |
| F18 | 0.0274 (2) | 0.25723 (10) | 0.66597 (6) | 0.0341 (3) | |
| F19 | 0.1512 (2) | 0.23058 (11) | 0.56619 (7) | 0.0382 (3) | |
| C141 | 0.0518 (3) | 0.30462 (15) | 0.60420 (9) | 0.0227 (4) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C1 | 0.0121 (6) | 0.0145 (7) | 0.0115 (6) | 0.0011 (5) | 0.0001 (5) | 0.0002 (5) |
| C2 | 0.0142 (6) | 0.0147 (7) | 0.0143 (6) | −0.0005 (6) | 0.0025 (6) | −0.0028 (5) |
| B3 | 0.0150 (7) | 0.0177 (8) | 0.0125 (7) | 0.0003 (6) | −0.0021 (6) | −0.0009 (6) |
| B4 | 0.0119 (7) | 0.0192 (8) | 0.0145 (7) | 0.0015 (7) | 0.0001 (6) | −0.0002 (6) |
| B5 | 0.0157 (7) | 0.0186 (8) | 0.0128 (7) | 0.0018 (7) | −0.0001 (6) | 0.0017 (6) |
| B6 | 0.0141 (8) | 0.0162 (8) | 0.0164 (8) | 0.0034 (6) | −0.0007 (6) | 0.0001 (6) |
| B7 | 0.0176 (8) | 0.0184 (8) | 0.0158 (7) | −0.0029 (7) | 0.0014 (6) | −0.0030 (6) |
| B8 | 0.0139 (8) | 0.0199 (8) | 0.0179 (8) | −0.0027 (7) | 0.0001 (6) | −0.0021 (7) |
| B9 | 0.0153 (8) | 0.0201 (8) | 0.0167 (8) | −0.0007 (7) | 0.0025 (6) | 0.0010 (6) |
| B10 | 0.0204 (8) | 0.0186 (8) | 0.0183 (8) | 0.0024 (7) | 0.0002 (7) | 0.0040 (6) |
| C11 | 0.0138 (6) | 0.0152 (7) | 0.0111 (6) | 0.0004 (6) | −0.0010 (5) | −0.0009 (5) |
| B11 | 0.0175 (8) | 0.0150 (7) | 0.0208 (8) | 0.0016 (7) | 0.0012 (7) | −0.0014 (7) |
| F12 | 0.0153 (4) | 0.0195 (5) | 0.0234 (5) | −0.0007 (4) | 0.0064 (4) | −0.0067 (4) |
| C12 | 0.0150 (7) | 0.0159 (7) | 0.0116 (6) | 0.0012 (6) | 0.0001 (5) | −0.0020 (5) |
| B12 | 0.0171 (8) | 0.0171 (8) | 0.0213 (8) | −0.0013 (7) | 0.0020 (7) | −0.0011 (7) |
| F13 | 0.0180 (5) | 0.0231 (5) | 0.0239 (5) | −0.0056 (4) | 0.0047 (4) | −0.0001 (4) |
| C13 | 0.0164 (7) | 0.0178 (7) | 0.0137 (6) | −0.0014 (6) | 0.0002 (6) | 0.0007 (5) |
| C14 | 0.0230 (8) | 0.0152 (7) | 0.0131 (6) | −0.0013 (6) | −0.0033 (6) | −0.0001 (5) |
| F15 | 0.0349 (6) | 0.0197 (5) | 0.0262 (5) | 0.0019 (5) | 0.0096 (5) | −0.0102 (4) |
| C15 | 0.0244 (8) | 0.0156 (7) | 0.0130 (6) | 0.0021 (6) | 0.0018 (6) | −0.0028 (5) |
| F16 | 0.0204 (5) | 0.0231 (5) | 0.0211 (5) | −0.0007 (4) | 0.0087 (4) | −0.0055 (4) |
| C16 | 0.0167 (7) | 0.0169 (7) | 0.0137 (6) | 0.0007 (6) | 0.0025 (6) | −0.0005 (5) |
| F17 | 0.0359 (7) | 0.0292 (6) | 0.0447 (7) | −0.0129 (5) | −0.0165 (6) | 0.0038 (6) |
| F18 | 0.0577 (9) | 0.0216 (5) | 0.0229 (5) | −0.0110 (6) | −0.0015 (6) | 0.0062 (4) |
| F19 | 0.0506 (8) | 0.0214 (6) | 0.0425 (7) | −0.0066 (6) | 0.0098 (7) | −0.0142 (5) |
| C141 | 0.0311 (10) | 0.0181 (8) | 0.0188 (7) | −0.0056 (7) | −0.0030 (7) | 0.0000 (6) |
Geometric parameters (Å, º)
| C1—C2 | 1.660 (2) | B7—B11 | 1.782 (3) |
| C1—B3 | 1.748 (2) | B7—B12 | 1.773 (3) |
| C1—B4 | 1.726 (2) | B8—H8 | 1.04 (2) |
| C1—B5 | 1.716 (2) | B8—B9 | 1.782 (3) |
| C1—B6 | 1.743 (2) | B8—B12 | 1.789 (3) |
| C1—C11 | 1.506 (2) | B9—H9 | 1.04 (2) |
| C2—H2 | 0.91 (2) | B9—B10 | 1.792 (3) |
| C2—B3 | 1.723 (3) | B9—B12 | 1.779 (3) |
| C2—B6 | 1.721 (2) | B10—H10 | 1.03 (2) |
| C2—B7 | 1.690 (2) | B10—B11 | 1.781 (3) |
| C2—B11 | 1.699 (3) | B10—B12 | 1.796 (3) |
| B3—H3 | 1.01 (2) | C11—C12 | 1.405 (2) |
| B3—B4 | 1.779 (2) | C11—C16 | 1.399 (2) |
| B3—B7 | 1.782 (3) | B11—H11 | 1.03 (2) |
| B3—B8 | 1.767 (3) | B11—B12 | 1.779 (3) |
| B4—H4 | 1.01 (2) | F12—C12 | 1.3394 (18) |
| B4—B5 | 1.792 (3) | C12—C13 | 1.378 (2) |
| B4—B8 | 1.772 (3) | B12—H12 | 1.03 (2) |
| B4—B9 | 1.774 (3) | F13—C13 | 1.338 (2) |
| B5—H5 | 1.10 (2) | C13—C14 | 1.386 (2) |
| B5—B6 | 1.784 (3) | C14—C15 | 1.386 (3) |
| B5—B9 | 1.780 (3) | C14—C141 | 1.510 (2) |
| B5—B10 | 1.782 (3) | F15—C15 | 1.3294 (19) |
| B6—H6 | 1.06 (2) | C15—C16 | 1.389 (2) |
| B6—B10 | 1.769 (3) | F16—C16 | 1.3372 (19) |
| B6—B11 | 1.779 (3) | F17—C141 | 1.330 (2) |
| B7—H7 | 1.08 (2) | F18—C141 | 1.335 (2) |
| B7—B8 | 1.770 (3) | F19—C141 | 1.324 (2) |
| C2—C1—B3 | 60.65 (10) | B12—B7—B3 | 108.66 (13) |
| C2—C1—B4 | 108.82 (13) | B12—B7—H7 | 131.8 (13) |
| C2—C1—B5 | 109.28 (12) | B12—B7—B11 | 60.04 (11) |
| C2—C1—B6 | 60.71 (10) | B3—B8—B4 | 60.34 (10) |
| B4—C1—B3 | 61.60 (10) | B3—B8—B7 | 60.50 (11) |
| B4—C1—B6 | 113.49 (12) | B3—B8—H8 | 118.9 (13) |
| B5—C1—B3 | 113.44 (12) | B3—B8—B9 | 108.31 (13) |
| B5—C1—B4 | 62.76 (10) | B3—B8—B12 | 108.60 (14) |
| B5—C1—B6 | 62.08 (10) | B4—B8—H8 | 121.7 (13) |
| B6—C1—B3 | 113.34 (12) | B4—B8—B9 | 59.89 (10) |
| C11—C1—C2 | 119.58 (13) | B4—B8—B12 | 107.99 (13) |
| C11—C1—B3 | 119.30 (12) | B7—B8—B4 | 108.21 (13) |
| C11—C1—B4 | 123.05 (14) | B7—B8—H8 | 120.4 (13) |
| C11—C1—B5 | 120.23 (12) | B7—B8—B9 | 107.47 (13) |
| C11—C1—B6 | 115.29 (13) | B7—B8—B12 | 59.76 (11) |
| C1—C2—H2 | 117.1 (15) | B9—B8—H8 | 124.2 (13) |
| C1—C2—B3 | 62.21 (10) | B9—B8—B12 | 59.77 (11) |
| C1—C2—B6 | 62.01 (9) | B12—B8—H8 | 123.0 (13) |
| C1—C2—B7 | 112.67 (13) | B4—B9—B5 | 60.56 (10) |
| C1—C2—B11 | 112.60 (12) | B4—B9—B8 | 59.78 (11) |
| B3—C2—H2 | 115.4 (15) | B4—B9—H9 | 118.6 (13) |
| B6—C2—H2 | 116.6 (14) | B4—B9—B10 | 108.56 (13) |
| B6—C2—B3 | 115.76 (12) | B4—B9—B12 | 108.33 (13) |
| B7—C2—H2 | 119.7 (14) | B5—B9—B8 | 108.29 (13) |
| B7—C2—B3 | 62.93 (11) | B5—B9—H9 | 121.5 (13) |
| B7—C2—B6 | 115.47 (13) | B5—B9—B10 | 59.85 (11) |
| B7—C2—B11 | 63.45 (11) | B8—B9—H9 | 119.8 (12) |
| B11—C2—H2 | 120.2 (15) | B8—B9—B10 | 108.59 (13) |
| B11—C2—B3 | 115.93 (13) | B10—B9—H9 | 124.2 (13) |
| B11—C2—B6 | 62.67 (10) | B12—B9—B5 | 108.27 (13) |
| C1—B3—H3 | 116.6 (13) | B12—B9—B8 | 60.30 (11) |
| C1—B3—B4 | 58.57 (9) | B12—B9—H9 | 123.2 (13) |
| C1—B3—B7 | 104.36 (12) | B12—B9—B10 | 60.38 (11) |
| C1—B3—B8 | 104.81 (12) | B5—B10—B9 | 59.74 (11) |
| C2—B3—C1 | 57.14 (9) | B5—B10—H10 | 121.3 (13) |
| C2—B3—H3 | 115.2 (14) | B5—B10—B12 | 107.46 (13) |
| C2—B3—B4 | 103.69 (12) | B6—B10—B5 | 60.31 (10) |
| C2—B3—B7 | 57.65 (10) | B6—B10—B9 | 107.91 (13) |
| C2—B3—B8 | 103.45 (13) | B6—B10—H10 | 120.6 (14) |
| B4—B3—H3 | 127.5 (13) | B6—B10—B11 | 60.16 (11) |
| B4—B3—B7 | 107.38 (13) | B6—B10—B12 | 107.93 (13) |
| B7—B3—H3 | 122.7 (13) | B9—B10—H10 | 122.7 (14) |
| B8—B3—H3 | 134.0 (14) | B9—B10—B12 | 59.47 (11) |
| B8—B3—B4 | 59.97 (11) | B11—B10—B5 | 107.93 (13) |
| B8—B3—B7 | 59.82 (11) | B11—B10—B9 | 107.16 (13) |
| C1—B4—B3 | 59.83 (10) | B11—B10—H10 | 122.0 (13) |
| C1—B4—H4 | 116.4 (13) | B11—B10—B12 | 59.65 (11) |
| C1—B4—B5 | 58.35 (10) | B12—B10—H10 | 122.9 (14) |
| C1—B4—B8 | 105.56 (13) | C12—C11—C1 | 124.15 (14) |
| C1—B4—B9 | 104.88 (12) | C16—C11—C1 | 121.42 (14) |
| B3—B4—H4 | 113.0 (12) | C16—C11—C12 | 114.37 (15) |
| B3—B4—B5 | 108.39 (13) | C2—B11—B6 | 59.27 (10) |
| B5—B4—H4 | 124.2 (12) | C2—B11—B7 | 58.03 (10) |
| B8—B4—B3 | 59.69 (10) | C2—B11—B10 | 104.42 (13) |
| B8—B4—H4 | 124.3 (13) | C2—B11—H11 | 119.0 (13) |
| B8—B4—B5 | 108.19 (14) | C2—B11—B12 | 103.91 (13) |
| B8—B4—B9 | 60.34 (11) | B6—B11—B7 | 108.20 (13) |
| B9—B4—B3 | 108.15 (13) | B6—B11—B10 | 59.59 (11) |
| B9—B4—H4 | 132.0 (13) | B6—B11—H11 | 116.0 (13) |
| B9—B4—B5 | 59.87 (11) | B6—B11—B12 | 108.23 (13) |
| C1—B5—B4 | 58.89 (10) | B7—B11—H11 | 122.5 (13) |
| C1—B5—H5 | 117.4 (12) | B10—B11—B7 | 108.13 (13) |
| C1—B5—B6 | 59.70 (10) | B10—B11—H11 | 125.1 (13) |
| C1—B5—B9 | 105.07 (12) | B12—B11—B7 | 59.71 (11) |
| C1—B5—B10 | 105.72 (12) | B12—B11—B10 | 60.59 (11) |
| B4—B5—H5 | 121.0 (12) | B12—B11—H11 | 129.4 (13) |
| B6—B5—B4 | 108.42 (12) | F12—C12—C11 | 121.58 (14) |
| B6—B5—H5 | 117.4 (12) | F12—C12—C13 | 116.01 (14) |
| B9—B5—B4 | 59.57 (11) | C13—C12—C11 | 122.40 (14) |
| B9—B5—H5 | 128.9 (12) | B7—B12—B8 | 59.59 (11) |
| B9—B5—B6 | 107.79 (13) | B7—B12—B9 | 107.47 (14) |
| B9—B5—B10 | 60.41 (11) | B7—B12—B10 | 107.89 (14) |
| B10—B5—B4 | 108.22 (13) | B7—B12—B11 | 60.24 (11) |
| B10—B5—H5 | 126.0 (12) | B7—B12—H12 | 120.2 (13) |
| B10—B5—B6 | 59.49 (11) | B8—B12—B10 | 108.13 (13) |
| C1—B6—B5 | 58.22 (9) | B8—B12—H12 | 122.4 (14) |
| C1—B6—H6 | 116.7 (13) | B9—B12—B8 | 59.94 (11) |
| C1—B6—B10 | 105.12 (13) | B9—B12—B10 | 60.16 (11) |
| C1—B6—B11 | 105.05 (12) | B9—B12—H12 | 124.1 (14) |
| C2—B6—C1 | 57.27 (9) | B10—B12—H12 | 122.3 (13) |
| C2—B6—B5 | 103.54 (12) | B11—B12—B8 | 107.95 (13) |
| C2—B6—H6 | 119.8 (12) | B11—B12—B9 | 107.79 (13) |
| C2—B6—B10 | 104.01 (13) | B11—B12—B10 | 59.76 (11) |
| C2—B6—B11 | 58.06 (10) | B11—B12—H12 | 119.9 (14) |
| B5—B6—H6 | 122.8 (12) | C12—C13—C14 | 122.48 (15) |
| B10—B6—B5 | 60.20 (11) | F13—C13—C12 | 117.73 (14) |
| B10—B6—H6 | 130.7 (12) | F13—C13—C14 | 119.80 (15) |
| B10—B6—B11 | 60.25 (11) | C13—C14—C15 | 116.21 (15) |
| B11—B6—B5 | 107.92 (13) | C13—C14—C141 | 118.91 (16) |
| B11—B6—H6 | 125.7 (13) | C15—C14—C141 | 124.88 (16) |
| C2—B7—B3 | 59.42 (10) | C14—C15—C16 | 121.42 (15) |
| C2—B7—H7 | 115.0 (13) | F15—C15—C14 | 121.74 (16) |
| C2—B7—B8 | 104.70 (13) | F15—C15—C16 | 116.84 (15) |
| C2—B7—B11 | 58.52 (11) | C15—C16—C11 | 123.09 (15) |
| C2—B7—B12 | 104.55 (13) | F16—C16—C11 | 121.11 (15) |
| B3—B7—H7 | 114.5 (13) | F16—C16—C15 | 115.80 (14) |
| B3—B7—B11 | 108.96 (13) | F17—C141—C14 | 111.14 (15) |
| B8—B7—B3 | 59.68 (11) | F17—C141—F18 | 107.02 (17) |
| B8—B7—H7 | 127.8 (13) | F18—C141—C14 | 110.39 (14) |
| B8—B7—B11 | 108.64 (13) | F19—C141—C14 | 112.96 (16) |
| B8—B7—B12 | 60.65 (11) | F19—C141—F17 | 107.84 (15) |
| B11—B7—H7 | 120.4 (13) | F19—C141—F18 | 107.23 (16) |
| C1—C2—B3—B4 | 37.23 (11) | B5—B6—B11—C2 | −95.07 (13) |
| C1—C2—B3—B7 | 139.34 (13) | B5—B6—B11—B7 | −62.62 (16) |
| C1—C2—B3—B8 | 99.11 (12) | B5—B6—B11—B10 | 38.15 (12) |
| C1—C2—B6—B5 | −36.99 (11) | B5—B6—B11—B12 | 0.60 (17) |
| C1—C2—B6—B10 | −99.16 (13) | B5—B9—B10—B6 | 37.70 (12) |
| C1—C2—B6—B11 | −139.86 (13) | B5—B9—B10—B11 | 101.12 (14) |
| C1—C2—B7—B3 | −38.66 (12) | B5—B9—B10—B12 | 138.37 (13) |
| C1—C2—B7—B8 | 1.77 (17) | B5—B9—B12—B7 | 63.77 (16) |
| C1—C2—B7—B11 | 104.88 (14) | B5—B9—B12—B8 | 101.08 (14) |
| C1—C2—B7—B12 | 64.68 (16) | B5—B9—B12—B10 | −37.22 (12) |
| C1—C2—B11—B6 | 38.07 (12) | B5—B9—B12—B11 | 0.22 (17) |
| C1—C2—B11—B7 | −104.99 (14) | B5—B10—B11—C2 | 2.09 (18) |
| C1—C2—B11—B10 | −2.38 (18) | B5—B10—B11—B6 | −38.21 (13) |
| C1—C2—B11—B12 | −65.08 (16) | B5—B10—B11—B7 | 62.68 (17) |
| C1—B3—B4—B5 | 33.61 (12) | B5—B10—B11—B12 | 100.14 (15) |
| C1—B3—B4—B8 | 134.37 (13) | B5—B10—B12—B7 | −63.30 (16) |
| C1—B3—B4—B9 | 97.01 (13) | B5—B10—B12—B8 | −0.31 (17) |
| C1—B3—B7—C2 | 34.40 (11) | B5—B10—B12—B9 | 36.98 (12) |
| C1—B3—B7—B8 | −98.99 (13) | B5—B10—B12—B11 | −100.94 (14) |
| C1—B3—B7—B11 | 2.00 (16) | B6—C1—C2—B3 | −146.76 (13) |
| C1—B3—B7—B12 | −61.82 (16) | B6—C1—C2—B7 | −107.80 (14) |
| C1—B3—B8—B4 | −39.12 (11) | B6—C1—C2—B11 | −38.34 (13) |
| C1—B3—B8—B7 | 98.22 (13) | B6—C1—B3—C2 | 31.37 (12) |
| C1—B3—B8—B9 | −1.92 (17) | B6—C1—B3—B4 | −105.08 (14) |
| C1—B3—B8—B12 | 61.45 (15) | B6—C1—B3—B7 | −3.25 (17) |
| C1—B4—B5—B6 | 34.71 (12) | B6—C1—B3—B8 | −65.28 (16) |
| C1—B4—B5—B9 | 134.96 (13) | B6—C1—B4—B3 | 104.84 (14) |
| C1—B4—B5—B10 | 97.73 (13) | B6—C1—B4—B5 | −37.07 (13) |
| C1—B4—B8—B3 | 39.91 (12) | B6—C1—B4—B8 | 65.00 (15) |
| C1—B4—B8—B7 | 1.53 (17) | B6—C1—B4—B9 | 2.23 (16) |
| C1—B4—B8—B9 | −98.53 (13) | B6—C1—B5—B4 | 141.27 (13) |
| C1—B4—B8—B12 | −61.69 (15) | B6—C1—B5—B9 | 102.08 (14) |
| C1—B4—B9—B5 | −38.56 (11) | B6—C1—B5—B10 | 39.17 (12) |
| C1—B4—B9—B8 | 99.67 (13) | B6—C1—C11—C12 | −59.64 (19) |
| C1—B4—B9—B10 | −1.49 (16) | B6—C1—C11—C16 | 117.44 (16) |
| C1—B4—B9—B12 | 62.52 (15) | B6—C2—B3—C1 | −32.51 (13) |
| C1—B5—B6—C2 | 36.54 (11) | B6—C2—B3—B4 | 4.73 (18) |
| C1—B5—B6—B10 | 135.11 (13) | B6—C2—B3—B7 | 106.83 (15) |
| C1—B5—B6—B11 | 96.93 (13) | B6—C2—B3—B8 | 66.60 (15) |
| C1—B5—B9—B4 | 38.86 (12) | B6—C2—B7—B3 | −107.29 (14) |
| C1—B5—B9—B8 | 1.55 (17) | B6—C2—B7—B8 | −66.86 (17) |
| C1—B5—B9—B10 | −99.78 (13) | B6—C2—B7—B11 | 36.25 (13) |
| C1—B5—B9—B12 | −62.32 (16) | B6—C2—B7—B12 | −3.95 (18) |
| C1—B5—B10—B6 | −39.27 (12) | B6—C2—B11—B7 | −143.06 (13) |
| C1—B5—B10—B9 | 98.67 (13) | B6—C2—B11—B10 | −40.46 (12) |
| C1—B5—B10—B11 | −1.13 (17) | B6—C2—B11—B12 | −103.15 (14) |
| C1—B5—B10—B12 | 61.81 (16) | B6—B5—B9—B4 | 101.33 (13) |
| C1—B6—B10—B5 | 38.42 (11) | B6—B5—B9—B8 | 64.01 (16) |
| C1—B6—B10—B9 | 0.97 (16) | B6—B5—B9—B10 | −37.31 (12) |
| C1—B6—B10—B11 | −98.94 (13) | B6—B5—B9—B12 | 0.15 (17) |
| C1—B6—B10—B12 | −61.87 (16) | B6—B5—B10—B9 | 137.94 (13) |
| C1—B6—B11—C2 | −34.17 (11) | B6—B5—B10—B11 | 38.14 (13) |
| C1—B6—B11—B7 | −1.71 (17) | B6—B5—B10—B12 | 101.08 (14) |
| C1—B6—B11—B10 | 99.06 (13) | B6—B10—B11—C2 | 40.29 (12) |
| C1—B6—B11—B12 | 61.50 (15) | B6—B10—B11—B7 | 100.88 (14) |
| C1—C11—C12—F12 | −3.8 (2) | B6—B10—B11—B12 | 138.35 (14) |
| C1—C11—C12—C13 | 175.70 (15) | B6—B10—B12—B7 | 0.34 (18) |
| C1—C11—C16—C15 | −177.46 (15) | B6—B10—B12—B8 | 63.34 (16) |
| C1—C11—C16—F16 | 2.2 (2) | B6—B10—B12—B9 | 100.62 (14) |
| C2—C1—B3—B4 | −136.45 (13) | B6—B10—B12—B11 | −37.30 (12) |
| C2—C1—B3—B7 | −34.62 (11) | B6—B11—B12—B7 | −100.86 (14) |
| C2—C1—B3—B8 | −96.65 (13) | B6—B11—B12—B8 | −63.82 (16) |
| C2—C1—B4—B3 | 39.38 (12) | B6—B11—B12—B9 | −0.51 (17) |
| C2—C1—B4—B5 | −102.53 (13) | B6—B11—B12—B10 | 37.12 (12) |
| C2—C1—B4—B8 | −0.46 (16) | B7—C2—B3—C1 | −139.34 (13) |
| C2—C1—B4—B9 | −63.23 (14) | B7—C2—B3—B4 | −102.11 (13) |
| C2—C1—B5—B4 | 101.79 (14) | B7—C2—B3—B8 | −40.23 (12) |
| C2—C1—B5—B6 | −39.48 (12) | B7—C2—B6—C1 | 103.31 (15) |
| C2—C1—B5—B9 | 62.60 (15) | B7—C2—B6—B5 | 66.33 (16) |
| C2—C1—B5—B10 | −0.31 (17) | B7—C2—B6—B10 | 4.15 (17) |
| C2—C1—B6—B5 | 136.52 (13) | B7—C2—B6—B11 | −36.54 (14) |
| C2—C1—B6—B10 | 97.15 (13) | B7—C2—B11—B6 | 143.06 (13) |
| C2—C1—B6—B11 | 34.51 (12) | B7—C2—B11—B10 | 102.61 (14) |
| C2—C1—C11—C12 | 9.6 (2) | B7—C2—B11—B12 | 39.91 (12) |
| C2—C1—C11—C16 | −173.29 (14) | B7—B3—B4—C1 | −96.50 (13) |
| C2—B3—B4—C1 | −36.56 (11) | B7—B3—B4—B5 | −62.89 (16) |
| C2—B3—B4—B5 | −2.95 (17) | B7—B3—B4—B8 | 37.87 (12) |
| C2—B3—B4—B8 | 97.81 (13) | B7—B3—B4—B9 | 0.51 (17) |
| C2—B3—B4—B9 | 60.45 (15) | B7—B3—B8—B4 | −137.34 (13) |
| C2—B3—B7—B8 | −133.39 (13) | B7—B3—B8—B9 | −100.14 (14) |
| C2—B3—B7—B11 | −32.40 (12) | B7—B3—B8—B12 | −36.78 (12) |
| C2—B3—B7—B12 | −96.22 (14) | B7—B8—B9—B4 | −101.31 (14) |
| C2—B3—B8—B4 | −98.20 (12) | B7—B8—B9—B5 | −63.66 (16) |
| C2—B3—B8—B7 | 39.14 (11) | B7—B8—B9—B10 | −0.21 (18) |
| C2—B3—B8—B9 | −61.01 (15) | B7—B8—B9—B12 | 37.39 (13) |
| C2—B3—B8—B12 | 2.36 (15) | B7—B8—B12—B9 | −137.90 (14) |
| C2—B6—B10—B5 | 97.77 (13) | B7—B8—B12—B10 | −100.52 (14) |
| C2—B6—B10—B9 | 60.32 (15) | B7—B8—B12—B11 | −37.33 (12) |
| C2—B6—B10—B11 | −39.59 (12) | B7—B11—B12—B8 | 37.04 (12) |
| C2—B6—B10—B12 | −2.52 (16) | B7—B11—B12—B9 | 100.35 (14) |
| C2—B6—B11—B7 | 32.45 (12) | B7—B11—B12—B10 | 137.98 (14) |
| C2—B6—B11—B10 | 133.22 (14) | B8—B3—B4—C1 | −134.37 (13) |
| C2—B6—B11—B12 | 95.67 (14) | B8—B3—B4—B5 | −100.76 (15) |
| C2—B7—B8—B3 | −40.30 (12) | B8—B3—B4—B9 | −37.35 (13) |
| C2—B7—B8—B4 | −1.99 (17) | B8—B3—B7—C2 | 133.39 (13) |
| C2—B7—B8—B9 | 61.26 (16) | B8—B3—B7—B11 | 100.99 (14) |
| C2—B7—B8—B12 | 98.65 (14) | B8—B3—B7—B12 | 37.17 (13) |
| C2—B7—B11—B6 | −32.94 (12) | B8—B4—B5—C1 | −97.42 (13) |
| C2—B7—B11—B10 | −96.00 (14) | B8—B4—B5—B6 | −62.71 (16) |
| C2—B7—B11—B12 | −133.85 (13) | B8—B4—B5—B9 | 37.53 (12) |
| C2—B7—B12—B8 | −98.91 (14) | B8—B4—B5—B10 | 0.31 (17) |
| C2—B7—B12—B9 | −61.44 (16) | B8—B4—B9—B5 | −138.23 (13) |
| C2—B7—B12—B10 | 2.03 (17) | B8—B4—B9—B10 | −101.17 (14) |
| C2—B7—B12—B11 | 39.45 (12) | B8—B4—B9—B12 | −37.15 (13) |
| C2—B11—B12—B7 | −39.07 (12) | B8—B7—B11—C2 | 96.17 (14) |
| C2—B11—B12—B8 | −2.03 (16) | B8—B7—B11—B6 | 63.23 (16) |
| C2—B11—B12—B9 | 61.28 (15) | B8—B7—B11—B10 | 0.17 (18) |
| C2—B11—B12—B10 | 98.91 (13) | B8—B7—B11—B12 | −37.68 (12) |
| B3—C1—C2—B6 | 146.76 (13) | B8—B7—B12—B9 | 37.47 (13) |
| B3—C1—C2—B7 | 38.96 (13) | B8—B7—B12—B10 | 100.94 (14) |
| B3—C1—C2—B11 | 108.42 (14) | B8—B7—B12—B11 | 138.36 (13) |
| B3—C1—B4—B5 | −141.90 (13) | B8—B9—B10—B5 | −100.80 (14) |
| B3—C1—B4—B8 | −39.84 (12) | B8—B9—B10—B6 | −63.10 (17) |
| B3—C1—B4—B9 | −102.61 (13) | B8—B9—B10—B11 | 0.31 (18) |
| B3—C1—B5—B4 | 36.27 (13) | B8—B9—B10—B12 | 37.56 (13) |
| B3—C1—B5—B6 | −105.00 (14) | B8—B9—B12—B7 | −37.31 (13) |
| B3—C1—B5—B9 | −2.92 (17) | B8—B9—B12—B10 | −138.30 (14) |
| B3—C1—B5—B10 | −65.83 (16) | B8—B9—B12—B11 | −100.85 (14) |
| B3—C1—B6—C2 | −31.35 (12) | B9—B4—B5—C1 | −134.96 (13) |
| B3—C1—B6—B5 | 105.17 (14) | B9—B4—B5—B6 | −100.25 (14) |
| B3—C1—B6—B10 | 65.80 (16) | B9—B4—B5—B10 | −37.23 (12) |
| B3—C1—B6—B11 | 3.16 (17) | B9—B4—B8—B3 | 138.43 (13) |
| B3—C1—C11—C12 | 80.50 (19) | B9—B4—B8—B7 | 100.05 (14) |
| B3—C1—C11—C16 | −102.42 (17) | B9—B4—B8—B12 | 36.83 (12) |
| B3—C2—B6—C1 | 32.57 (13) | B9—B5—B6—C1 | −97.39 (13) |
| B3—C2—B6—B5 | −4.42 (17) | B9—B5—B6—C2 | −60.85 (15) |
| B3—C2—B6—B10 | −66.59 (16) | B9—B5—B6—B10 | 37.72 (12) |
| B3—C2—B6—B11 | −107.29 (15) | B9—B5—B6—B11 | −0.46 (17) |
| B3—C2—B7—B8 | 40.43 (12) | B9—B5—B10—B6 | −137.94 (13) |
| B3—C2—B7—B11 | 143.54 (13) | B9—B5—B10—B11 | −99.80 (14) |
| B3—C2—B7—B12 | 103.34 (13) | B9—B5—B10—B12 | −36.86 (12) |
| B3—C2—B11—B6 | 107.02 (14) | B9—B8—B12—B7 | 137.90 (14) |
| B3—C2—B11—B7 | −36.04 (12) | B9—B8—B12—B10 | 37.38 (12) |
| B3—C2—B11—B10 | 66.56 (16) | B9—B8—B12—B11 | 100.58 (14) |
| B3—C2—B11—B12 | 3.86 (17) | B9—B10—B11—C2 | −60.88 (16) |
| B3—B4—B5—C1 | −34.20 (12) | B9—B10—B11—B6 | −101.18 (14) |
| B3—B4—B5—B6 | 0.50 (17) | B9—B10—B11—B7 | −0.30 (18) |
| B3—B4—B5—B9 | 100.75 (14) | B9—B10—B11—B12 | 37.17 (13) |
| B3—B4—B5—B10 | 63.53 (16) | B9—B10—B12—B7 | −100.28 (14) |
| B3—B4—B8—B7 | −38.38 (12) | B9—B10—B12—B8 | −37.29 (12) |
| B3—B4—B8—B9 | −138.43 (13) | B9—B10—B12—B11 | −137.92 (13) |
| B3—B4—B8—B12 | −101.60 (14) | B10—B5—B6—C1 | −135.11 (13) |
| B3—B4—B9—B5 | −101.17 (14) | B10—B5—B6—C2 | −98.57 (13) |
| B3—B4—B9—B8 | 37.07 (12) | B10—B5—B6—B11 | −38.18 (12) |
| B3—B4—B9—B10 | −64.10 (16) | B10—B5—B9—B4 | 138.64 (13) |
| B3—B4—B9—B12 | −0.08 (17) | B10—B5—B9—B8 | 101.33 (14) |
| B3—B7—B8—B4 | 38.31 (12) | B10—B5—B9—B12 | 37.46 (12) |
| B3—B7—B8—B9 | 101.56 (14) | B10—B6—B11—C2 | −133.22 (14) |
| B3—B7—B8—B12 | 138.95 (13) | B10—B6—B11—B7 | −100.77 (14) |
| B3—B7—B11—C2 | 32.75 (12) | B10—B6—B11—B12 | −37.56 (13) |
| B3—B7—B11—B6 | −0.19 (17) | B10—B9—B12—B7 | 100.99 (14) |
| B3—B7—B11—B10 | −63.25 (17) | B10—B9—B12—B8 | 138.30 (14) |
| B3—B7—B11—B12 | −101.10 (14) | B10—B9—B12—B11 | 37.45 (12) |
| B3—B7—B12—B8 | −36.76 (13) | B10—B11—B12—B7 | −137.98 (14) |
| B3—B7—B12—B9 | 0.71 (18) | B10—B11—B12—B8 | −100.94 (14) |
| B3—B7—B12—B10 | 64.18 (17) | B10—B11—B12—B9 | −37.62 (12) |
| B3—B7—B12—B11 | 101.60 (14) | C11—C1—C2—B3 | 109.06 (15) |
| B3—B8—B9—B4 | −37.40 (12) | C11—C1—C2—B6 | −104.18 (15) |
| B3—B8—B9—B5 | 0.26 (18) | C11—C1—C2—B7 | 148.02 (14) |
| B3—B8—B9—B10 | 63.71 (17) | C11—C1—C2—B11 | −142.52 (14) |
| B3—B8—B9—B12 | 101.31 (15) | C11—C1—B3—C2 | −109.50 (15) |
| B3—B8—B12—B7 | 37.09 (12) | C11—C1—B3—B4 | 114.05 (16) |
| B3—B8—B12—B9 | −100.81 (14) | C11—C1—B3—B7 | −144.12 (14) |
| B3—B8—B12—B10 | −63.42 (16) | C11—C1—B3—B8 | 153.86 (14) |
| B3—B8—B12—B11 | −0.23 (17) | C11—C1—B4—B3 | −108.18 (15) |
| B4—C1—C2—B3 | −39.81 (12) | C11—C1—B4—B5 | 109.92 (15) |
| B4—C1—C2—B6 | 106.95 (13) | C11—C1—B4—B8 | −148.02 (14) |
| B4—C1—C2—B7 | −0.85 (17) | C11—C1—B4—B9 | 149.21 (13) |
| B4—C1—C2—B11 | 68.61 (16) | C11—C1—B5—B4 | −114.20 (16) |
| B4—C1—B3—C2 | 136.45 (13) | C11—C1—B5—B6 | 104.53 (15) |
| B4—C1—B3—B7 | 101.83 (14) | C11—C1—B5—B9 | −153.40 (14) |
| B4—C1—B3—B8 | 39.80 (12) | C11—C1—B5—B10 | 143.70 (14) |
| B4—C1—B5—B6 | −141.27 (13) | C11—C1—B6—C2 | 111.15 (14) |
| B4—C1—B5—B9 | −39.19 (12) | C11—C1—B6—B5 | −112.33 (14) |
| B4—C1—B5—B10 | −102.10 (14) | C11—C1—B6—B10 | −151.70 (13) |
| B4—C1—B6—C2 | −99.18 (14) | C11—C1—B6—B11 | 145.66 (13) |
| B4—C1—B6—B5 | 37.34 (13) | C11—C12—C13—F13 | −178.03 (14) |
| B4—C1—B6—B10 | −2.04 (17) | C11—C12—C13—C14 | 2.1 (2) |
| B4—C1—B6—B11 | −64.68 (16) | B11—C2—B3—C1 | −103.11 (14) |
| B4—C1—C11—C12 | 153.91 (15) | B11—C2—B3—B4 | −65.87 (16) |
| B4—C1—C11—C16 | −29.0 (2) | B11—C2—B3—B7 | 36.23 (13) |
| B4—B3—B7—C2 | 95.46 (13) | B11—C2—B3—B8 | −4.00 (16) |
| B4—B3—B7—B8 | −37.93 (12) | B11—C2—B6—C1 | 139.86 (13) |
| B4—B3—B7—B11 | 63.06 (16) | B11—C2—B6—B5 | 102.87 (14) |
| B4—B3—B7—B12 | −0.76 (17) | B11—C2—B6—B10 | 40.70 (12) |
| B4—B3—B8—B7 | 137.34 (13) | B11—C2—B7—B3 | −143.54 (13) |
| B4—B3—B8—B9 | 37.20 (12) | B11—C2—B7—B8 | −103.11 (14) |
| B4—B3—B8—B12 | 100.56 (14) | B11—C2—B7—B12 | −40.20 (13) |
| B4—B5—B6—C1 | −34.37 (12) | B11—B6—B10—B5 | 137.36 (13) |
| B4—B5—B6—C2 | 2.17 (16) | B11—B6—B10—B9 | 99.91 (14) |
| B4—B5—B6—B10 | 100.74 (14) | B11—B6—B10—B12 | 37.08 (13) |
| B4—B5—B6—B11 | 62.56 (16) | B11—B7—B8—B3 | −101.53 (14) |
| B4—B5—B9—B8 | −37.31 (13) | B11—B7—B8—B4 | −63.22 (17) |
| B4—B5—B9—B10 | −138.64 (13) | B11—B7—B8—B9 | 0.02 (17) |
| B4—B5—B9—B12 | −101.18 (14) | B11—B7—B8—B12 | 37.41 (13) |
| B4—B5—B10—B6 | −101.08 (13) | B11—B7—B12—B8 | −138.36 (13) |
| B4—B5—B10—B9 | 36.86 (12) | B11—B7—B12—B9 | −100.89 (14) |
| B4—B5—B10—B11 | −62.94 (17) | B11—B7—B12—B10 | −37.42 (13) |
| B4—B5—B10—B12 | 0.00 (17) | B11—B10—B12—B7 | 37.64 (13) |
| B4—B8—B9—B5 | 37.66 (13) | B11—B10—B12—B8 | 100.63 (14) |
| B4—B8—B9—B10 | 101.10 (14) | B11—B10—B12—B9 | 137.92 (13) |
| B4—B8—B9—B12 | 138.70 (14) | F12—C12—C13—F13 | 1.5 (2) |
| B4—B8—B12—B7 | 101.01 (14) | F12—C12—C13—C14 | −178.38 (14) |
| B4—B8—B12—B9 | −36.89 (12) | C12—C11—C16—C15 | −0.1 (2) |
| B4—B8—B12—B10 | 0.50 (17) | C12—C11—C16—F16 | 179.54 (14) |
| B4—B8—B12—B11 | 63.69 (16) | C12—C13—C14—C15 | −0.8 (2) |
| B4—B9—B10—B5 | −37.37 (12) | C12—C13—C14—C141 | 178.50 (15) |
| B4—B9—B10—B6 | 0.33 (17) | B12—B7—B8—B3 | −138.95 (13) |
| B4—B9—B10—B11 | 63.74 (17) | B12—B7—B8—B4 | −100.64 (14) |
| B4—B9—B10—B12 | 100.99 (14) | B12—B7—B8—B9 | −37.39 (13) |
| B4—B9—B12—B7 | −0.39 (18) | B12—B7—B11—C2 | 133.85 (13) |
| B4—B9—B12—B8 | 36.92 (12) | B12—B7—B11—B6 | 100.91 (14) |
| B4—B9—B12—B10 | −101.38 (14) | B12—B7—B11—B10 | 37.85 (13) |
| B4—B9—B12—B11 | −63.93 (16) | B12—B8—B9—B4 | −138.70 (14) |
| B5—C1—C2—B3 | −106.66 (13) | B12—B8—B9—B5 | −101.05 (14) |
| B5—C1—C2—B6 | 40.10 (12) | B12—B8—B9—B10 | −37.60 (13) |
| B5—C1—C2—B7 | −67.70 (16) | B12—B9—B10—B5 | −138.37 (13) |
| B5—C1—C2—B11 | 1.76 (17) | B12—B9—B10—B6 | −100.67 (14) |
| B5—C1—B3—C2 | 99.73 (14) | B12—B9—B10—B11 | −37.25 (13) |
| B5—C1—B3—B4 | −36.72 (13) | B12—B10—B11—C2 | −98.05 (14) |
| B5—C1—B3—B7 | 65.11 (16) | B12—B10—B11—B6 | −138.35 (14) |
| B5—C1—B3—B8 | 3.09 (17) | B12—B10—B11—B7 | −37.46 (13) |
| B5—C1—B4—B3 | 141.90 (13) | F13—C13—C14—C15 | 179.35 (14) |
| B5—C1—B4—B8 | 102.07 (14) | F13—C13—C14—C141 | −1.4 (2) |
| B5—C1—B4—B9 | 39.29 (12) | C13—C14—C15—F15 | 179.88 (15) |
| B5—C1—B6—C2 | −136.52 (13) | C13—C14—C15—C16 | −0.9 (2) |
| B5—C1—B6—B10 | −39.37 (12) | C13—C14—C141—F17 | 61.8 (2) |
| B5—C1—B6—B11 | −102.01 (13) | C13—C14—C141—F18 | −56.8 (2) |
| B5—C1—C11—C12 | −130.74 (16) | C13—C14—C141—F19 | −176.85 (15) |
| B5—C1—C11—C16 | 46.3 (2) | C14—C15—C16—C11 | 1.4 (3) |
| B5—B4—B8—B3 | 101.10 (14) | C14—C15—C16—F16 | −178.31 (15) |
| B5—B4—B8—B7 | 62.72 (16) | F15—C15—C16—C11 | −179.38 (15) |
| B5—B4—B8—B9 | −37.33 (12) | F15—C15—C16—F16 | 1.0 (2) |
| B5—B4—B8—B12 | −0.50 (17) | C15—C14—C141—F17 | −119.01 (19) |
| B5—B4—B9—B8 | 138.23 (13) | C15—C14—C141—F18 | 122.40 (19) |
| B5—B4—B9—B10 | 37.07 (12) | C15—C14—C141—F19 | 2.4 (2) |
| B5—B4—B9—B12 | 101.08 (14) | C16—C11—C12—F12 | 178.93 (14) |
| B5—B6—B10—B9 | −37.45 (12) | C16—C11—C12—C13 | −1.6 (2) |
| B5—B6—B10—B11 | −137.36 (13) | C141—C14—C15—F15 | 0.7 (3) |
| B5—B6—B10—B12 | −100.28 (14) | C141—C14—C15—C16 | 179.88 (16) |
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C2—H2···F12 | 0.91 (2) | 2.11 (2) | 2.7436 (19) | 126 (2) |
Funding Statement
This work was funded by Engineering and Physical Sciences Research Council grant .
References
- Benton, A., Copeland, Z., Mansell, S. M., Rosair, G. M. & Welch, A. J. (2018). Molecules, 23, 3099. [DOI] [PMC free article] [PubMed]
- Brain, P. T., Cowie, J., Donohoe, D. J., Hnyk, D., Rankin, D. W. H., Reed, D., Reid, B. D., Robertson, H. E., Welch, A. J., Hofmann, M. & Schleyer, P. von R. (1996). Inorg. Chem. 35, 1706–1708. [DOI] [PubMed]
- Clegg, W. (2016). Private Communication (refcode CCDC 1505580). CCDC, Cambridge, England.
- Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. (2009). J. Appl. Cryst. 42, 339–341.
- Grimes, R. N. (2016). Carboranes, 3rd ed. Amsterdam: Elsevier.
- Groom, C. R., Bruno, I. J., Lightfoot, M. P. & Ward, S. C. (2016). Acta Cryst. B72, 171–179. [DOI] [PMC free article] [PubMed]
- Lee, Y. H., Lee, H. D., Ryu, J. Y., Lee, J. & Lee, M. H. (2017). J. Organomet. Chem. 846, 81–87.
- Marsh, A. V., Cheetham, N. J., Little, M., Dyson, M., White, A. J. P., Beavis, P., Warriner, C. N., Swain, A. C., Stavrinou, P. N. & Heeney, M. (2018). Angew. Chem. Int. Ed. 57, 10640–10645. [DOI] [PMC free article] [PubMed]
- McAnaw, A., Lopez, M. E., Ellis, D., Rosair, G. M. & Welch, A. J. (2014). Dalton Trans. 43, 5095–5105. [DOI] [PubMed]
- McAnaw, A., Scott, G., Elrick, L., Rosair, G. M. & Welch, A. J. (2013). Dalton Trans. 42, 645–664. [DOI] [PubMed]
- Parsons, S., Flack, H. D. & Wagner, T. (2013). Acta Cryst. B69, 249–259. [DOI] [PMC free article] [PubMed]
- Rigaku OD (2018). CrysAlis PRO. Rigaku Corporation, Oxford, England.
- Sheldrick, G. M. (2015a). Acta Cryst. A71, 3–8.
- Sheldrick, G. M. (2015b). Acta Cryst. C71, 3–8.
- Thomas, Rh. Ll., Rosair, G. M. & Welch, A. J. (1996). Acta Cryst. C52, 1024–1026.
- Tricas, H., Colon, M., Ellis, D., Macgregor, S. A., McKay, D., Rosair, G. M., Welch, A. J., Glukhov, I. V., Rossi, F., Laschi, F. & Zanello, P. (2011). Dalton Trans. 40, 4200–4211. [DOI] [PubMed]
- Tu, D., Leong, P., Guo, S., Yan, H., Lu, C. & Zhao, Q. (2017). Angew. Chem. Int. Ed. 56, 11370–11374. [DOI] [PubMed]
- Van Nghia, N., Oh, J., Sujith, S., Jung, J. & Lee, M. H. (2018). Dalton Trans. 47, 17441–17449. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2056989019004067/lh5894sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989019004067/lh5894Isup2.hkl
Supporting information file. DOI: 10.1107/S2056989019004067/lh5894Isup3.mol
CCDC reference: 1905663
Additional supporting information: crystallographic information; 3D view; checkCIF report