Short abstract
See Article by Laufs et al
Keywords: Editorials, bempedoic acid, statin, statin intolerance, statin‐associated muscle symptoms, statin‐associated adverse effects
Subject Categories: Cardiovascular Disease
A confluence of data from epidemiological and mendelian randomization studies and clinic trials support low‐density lipoprotein cholesterol (LDL‐C) as a causal risk factor for atherosclerotic cardiovascular disease (ASCVD).1 Lifestyle modification with diet and exercise remains one of the most important interventions in treating dyslipidemia. However, patients at higher risk for ASCVD events may require pharmacotherapy. For the treatment of elevated blood cholesterol levels, statins are the most established and cost‐effective therapy in both secondary and primary prevention patients. With 28 large randomized controlled trials and their meta‐analyses, the evidence supporting statins as the first‐line cholesterol‐lowering agent for ASCVD risk reduction is iron clad.2, 3, 4 However, although statins are generally well tolerated, some patients have adverse effects to therapy and others do not respond fully.5
The most common statin‐associated adverse effect is musculoskeletal symptoms, which may range from mild (aches) to severe (rhabdomyolysis). Potential risk factors for statin‐associated muscle symptoms include advanced age, high physical activity, drugs affecting statin metabolism, renal insufficiency, heavy alcohol consumption, and genetic predisposition. More important, statin‐associated muscle symptoms are the most frequent adverse effects leading to statin nonadherence and discontinuation.6 In patients who cannot tolerate their current statin regimen, strategies include either adjusting dosages, frequency, or type of statins, with or without the addition of nonstatin agents, or transitioning to nonstatin agents altogether.
Fortunately, the arsenal of nonstatin therapy at a clinician's disposal has expanded in recent years. Ezetimibe and PCSK9 (proprotein convertase subtilisin/kexin type 9) serine protease inhibitors have been validated in large randomized controlled trials to lower LDL‐C and also lower ASCVD events.7, 8, 9 Although these trials were all conducted on a background of statin therapy, their efficacy in patients who cannot take full‐dose statins may be cautiously extrapolated. At the same time, both ezetimibe and PCSK9 inhibitors are relatively well tolerated, supporting these agents over older medications, such as bile acid–binding resins (Figure). Yet, both ezetimibe and PCSK9 inhibitors have their limitations. Ezetimibe has modest LDL‐C–lowering efficacy in the range of 13% to 20%.4 PCSK9 inhibitors have more robust LDL‐C–lowering effects (43%–64%), but they are administered as an injection, have not been studied for long‐term safety and efficacy, and, at their current prices, may not be cost‐effective.4
Figure 1.
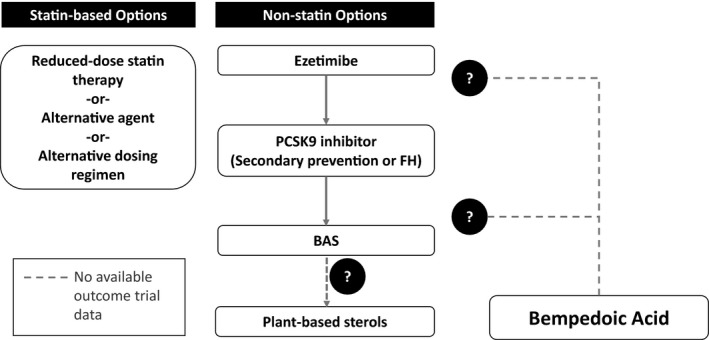
Therapeutic options in patients with confirmed statin‐associated adverse effects. Therapies may be used alone or in combination on the basis of degree of atherosclerotic cardiovascular disease risk, amount of low‐density lipoprotein cholesterol lowering desired, and patient preference. Where bempedoic acid falls within this schema will depend on findings of the ongoing cardiovascular outcome trial. BAS indicates bile acid sequestrant; FH, familial hypercholesterolemia; PCSK9, proprotein convertase subtilisin/kexin type 9.
Bempedoic Acid: What We Know
It is this gap that bempedoic acid hopes to bridge. Bempedoic acid is an oral medication that targets ATP citrate lyase. ATP citrate lyase acts upstream of hydroxymethylglutaryl–coenzyme A reductase, catalyzing the production of acetyl coenzyme A, the precursor of the mevalonate pathway of cholesterol synthesis.10 Like statins, the downstream effects of bempedoic acid are the upregulation of LDL receptors and increased uptake of circulating LDL particles. However, unlike statins, bempedoic acid is a prodrug and converted in the liver by long‐chain acyl–coenzyme A synthetase 1. As this form of acyl–coenzyme A synthetase is predominantly hepatic and not expressed in skeletal muscle, it is thought that the active drug is localized to the liver, with fewer effects on the musculoskeletal system.10
Earlier‐phase randomized controlled trials of bempedoic acid demonstrated a dose‐dependent lowering of LDL‐C as well as lowering of LDL particle number, apolipoprotein B, non–high‐density lipoprotein cholesterol, and high‐sensitivity C‐reactive protein.11, 12 In the completed phase 3 trial, CLEAR (Cholesterol Lowering via Bempedoic Acid and ACL‐Inhibiting Regimen) Tranquility, bempedoic acid, 180 mg/d, when added to ezetimibe, reduced LDL‐C by 28.5% compared with ezetimibe alone and was overall well tolerated.13 Meanwhile, the phase 3 safety trial, CLEAR Harmony, showed no significant differences between treatment arm and placebo with respect to any adverse events and serious adverse events after 52 weeks, when added to a background of maximally tolerated statin therapy; however, adverse events leading to discontinuation were higher in the treatment arm (10.9% versus 7.1%; P=0.005). There were no significant differences in cardiovascular events or mortality reported between treatment and placebo groups. A lower rate of new‐onset or worsening diabetes mellitus was observed in the bempedoic acid arm compared with placebo (3.3% versus 5.4%; P=0.02), although there was a higher rate of gout (1.2% versus 0.3%; P=0.03).14
CLEAR Serenity Trial: What We Have Learned
In the present phase 3 trial, CLEAR Serenity, Laufs et al, in this issue of the Journal of the American Heart Association (JAHA), assessed the efficacy and safety of bempedoic acid in patients with hypercholesterolemia (LDL‐C=155.6±38.8 mg/dL in the placebo group and 158.5±40.4 mg/dL in the bempedoic acid group) who could not tolerate statin therapy.15 Statin intolerance was defined as the inability to tolerate at least 2 statins, 1 at a low daily dose (rosuvastatin, 5 mg; atorvastatin, 10 mg; simvastatin, 10 mg; lovastatin, 20 mg; pravastatin, 40 mg; fluvastatin, 40 mg; or pitavastatin, 2 mg), because of an adverse event that began or increased on statin and resolved or improved when statin was discontinued. In this study, 345 patients were randomized 2:1 to either bempedoic acid, 180 mg, or placebo daily over a period of 24 weeks, with a primary end point of mean percentage change from baseline of LDL‐C at 12 weeks. Both primary and secondary prevention patients were included with 2% of the patients having a history of heterozygous familial hypercholesterolemia. Approximately 8.4% were taking low‐dose statin therapy, and one third were taking nonstatin therapies (ezetimibe or fish oil). At 12 weeks, treatment with bempedoic acid significantly lowered LDL‐C, −21.4% (95% CI, −25.1% to −17.7%) more from baseline compared with placebo, with effect continuing through the duration for the study. Heterogeneity of effect was observed with diabetes mellitus status (P=0.012), with diabetic patients appearing to have less LDL‐C reduction compared with nondiabetic patients at 12 weeks. Bempedoic acid also lowered non–high‐density lipoprotein cholesterol (−14.8%), apolipoprotein B (−15.0%), and high‐sensitivity C‐reactive protein (−24.3%). Muscle‐related adverse events occurred less frequently in the bempedoic acid group compared with placebo (12.8% versus 16.2%). However, when comparing the treatment arm with placebo, there appeared to be higher rates of overall adverse events (64.1% versus 56.8%), serious adverse events (6.0% versus 3.6%), and adverse events leading to treatment discontinuation (18.4% versus 11.7%) in the bempedoic acid group. There were a further 9 major adverse cardiovascular events (3.8%) that occurred in the bempedoic acid group compared with 0 events in the placebo group.
There is clinical need and potential for additional nonstatin LDL‐C–lowering options for patients who cannot tolerate statin therapy, especially in the primary prevention setting, where there is currently less evidence on the efficacy that favors any of the nonstatin medications and where the cost‐benefit would likely preclude use of PCSK9 inhibitors. Meanwhile, the pairing of bempedoic acid with ezetimibe remains an intriguing regimen. Although LDL‐C lowering in both medications is modest, their combined effect may, in theory, approach that of high‐intensity statins and, thus, may also be an option in secondary prevention patients and patients with familial hypercholesterolemia unable to tolerate high‐intensity statin therapy or those in whom further LDL‐C reduction is desired.16 Furthermore, both bempedoic acid and ezetimibe have been shown to lower high‐sensitivity C‐reactive protein.7, 11, 13 The additional anti‐inflammatory property could be advantageous when compared with PCSK9 inhibitors, which are powerful LDL‐C–lowering agents but lack significant effect on inflammation. Thus, results of the CLEAR Serenity trial by Laufs et al15 suggest that bempedoic acid has the potential to fill this niche. This potential, however, is built on the assumption that bempedoic acid can demonstrate efficacy and acceptable safety in its ongoing cardiovascular outcomes trial: CLEAR Outcomes (ClinicalTrials.gov Identifier: NCT02993406). A shared target pathway and mechanism of action between bempedoic acid and statin leads some credence to this assumption. Yet, outcome trial evidence will be invaluable to establish bempedoic acid as an effective, stand‐alone, nonstatin agent.
The CLEAR Serenity trial also raises questions about bempedoic acid. First, the baseline LDL‐C levels in the study were high, which may exaggerate the percentage reduction of LDL‐C. It is somewhat reassuring that previous phase 3 trials with the 180‐mg/d dose, which had lower LDL‐C baselines, showed a similar range in percentage reduction of LDL‐C: 18.1% in the CLEAR Harmony trial (baseline LDL‐C=102–104 mg/dL on a background of maximally tolerated statins) and 28.5% in the CLEAR Tranquility trial (baseline LDL‐C=123–130 mg/dL on a background of ezetimibe).13, 14 There was further a sizable percentage of premature discontinuation (24.8% in the bempedoic acid group versus 16.2% in the placebo group), which seems high for a study of this duration. Also, although the rate of muscle symptoms was observed to be lower with bempedoic acid compared with placebo (12.8% versus 16.2%), the percentage of discontinuation attributable to adverse events observed with bempedoic acid (18.4%) was higher compared with placebo (11.7%). Finally, the occurrence of 9 adjudicated major adverse cardiac events in the treatment arm compared with 0 in the placebo arm is concerning. As the authors pointed out, this may simply be a statistical anomaly as previous studies, including the phase 3 safety trial, did not show a treatment‐related increase in cardiovascular events or mortality in the bempedoic acid group.14
Hope or Hype? What Else Is Needed?
In conclusion, the jury is still out on bempedoic acid. Although statins are generally well tolerated, adverse effects associated with statin therapy are not uncommon. As such, there remains a need for nonstatin agents, which will provide clinicians with more flexibility in the treatment of hypercholesterolemia and ASCVD. The biological rationale of bempedoic acid is sound, and contemporary results on its efficacy are favorable, especially when considering reduction in high‐sensitivity C‐reactive protein and versatility when used in combination with ezetimibe or low‐dose statin therapy. However, whether bempedoic acid reduces the risk of ASCVD events remains unproved, and the present study raises questions about the tolerability of bempedoic acid. Therefore, results of the CLEAR Serenity trial should be viewed with cautious optimism and further reinforce the importance of the ongoing cardiovascular outcome trial, both to clarify the clinical effectiveness of bempedoic acid and to soothe any concerns about its safety.
Disclosures
Virani has grant funding from the Department of Veteran Affairs, the American Heart Association, the American Diabetes Association, and the Drs Tahira and Nuruddin Jooma Fund; honorarium from the American College of Cardiology (Associate Editor for Innovations ACC.org) and the National Lipid Association; and serves on the steering committee for the PALM (Patient and Provider Assessment of Lipid Management) Registry at the Duke Clinical Research Institute (no financial remuneration). Jia has no disclosures to report.
J Am Heart Assoc. 2019;8:e012352 DOI: 10.1161/JAHA.119.012352.
The opinions expressed in this article are not necessarily those of the editors or of the American Heart Association.
References
- 1. Musunuru K, Kathiresan S. Surprises from genetic analyses of lipid risk factors for atherosclerosis. Circ Res. 2016;118:579–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cholesterol Treatment Trialists’ (CTT) Collaboration , Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, Peto R, Barnes EH, Keech A, Simes J, Collins R. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta‐analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cholesterol Treatment Trialists’ Collaboration . Efficacy and safety of statin therapy in older people: a meta‐analysis of individual participant data from 28 randomised controlled trials. Lancet. 2019;393:407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, Braun LT, de Ferranti S, Faiella‐Tommasino J, Forman DE, Goldberg R, Heidenreich PA, Hlatky MA, Jones DW, Lloyd‐Jones D, Lopez‐Pajares N, Ndumele CE, Orringer CE, Peralta CA, Saseen JJ, Smith SC Jr, Sperling L, Virani SS, Yeboah J. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APHA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018. Available at: 10.1016/j.jacc.2018.11.002. Accessed March 22, 2019. [DOI] [Google Scholar]
- 5. Miedema MD, Virani SS. Harder‐to‐treat patients: recognizing them and adapting treatment strategies. Am J Cardiol. 2016;118:13A–18A. [DOI] [PubMed] [Google Scholar]
- 6. Zhang H, Plutzky J, Turchin A. Discontinuation of statins in routine care settings. Ann Intern Med. 2013;159:75–76. [DOI] [PubMed] [Google Scholar]
- 7. Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, Darius H, Lewis BS, Ophuis TO, Jukema JW, De Ferrari GM, Ruzyllo W, De Lucca P, Im K, Bohula EA, Reist C, Wiviott SD, Tershakovec AM, Musliner TA, Braunwald E, Califf RM; IMPROVE‐IT Investigators . Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–2397. [DOI] [PubMed] [Google Scholar]
- 8. Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, Sever PS, Pedersen TR; FOURIER Steering Committee and Investigators . Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713–1722. [DOI] [PubMed] [Google Scholar]
- 9. Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, Edelberg JM, Goodman SG, Hanotin C, Harrington RA, Jukema JW, Lecorps G, Mahaffey KW, Moryusef A, Pordy R, Quintero K, Roe MT, Sasiela WJ, Tamby JF, Tricoci P, White HD, Zeiher AM; ODYSSEY OUTCOMES Committees and Investigators . Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379:2097–2107. [DOI] [PubMed] [Google Scholar]
- 10. Bilen O, Ballantyne CM. Bempedoic acid (ETC‐1002): an investigational inhibitor of ATP citrate lyase. Curr Atheroscler Rep. 2016;18:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ballantyne CM, Davidson MH, Macdougall DE, Bays HE, Dicarlo LA, Rosenberg NL, Margulies J, Newton RS. Efficacy and safety of a novel dual modulator of adenosine triphosphate‐citrate lyase and adenosine monophosphate‐activated protein kinase in patients with hypercholesterolemia: results of a multicenter, randomized, double‐blind, placebo‐controlled, parallel‐group trial. J Am Coll Cardiol. 2013;62:1154–1162. [DOI] [PubMed] [Google Scholar]
- 12. Gutierrez MJ, Rosenberg NL, Macdougall DE, Hanselman JC, Margulies JR, Strange P, Milad MA, McBride SJ, Newton RS. Efficacy and safety of ETC‐1002, a novel investigational low‐density lipoprotein‐cholesterol‐lowering therapy for the treatment of patients with hypercholesterolemia and type 2 diabetes mellitus. Arterioscler Thromb Vasc Biol. 2014;34:676–683. [DOI] [PubMed] [Google Scholar]
- 13. Ballantyne CM, Banach M, Mancini GBJ, Lepor NE, Hanselman JC, Zhao X, Leiter LA. Efficacy and safety of bempedoic acid added to ezetimibe in statin‐intolerant patients with hypercholesterolemia: a randomized, placebo‐controlled study. Atherosclerosis. 2018;277:195–203. [DOI] [PubMed] [Google Scholar]
- 14. Ray KK, Bays HE, Catapano AL, Lalwani ND, Bloedon LT, Sterling LR, Robinson PL. Ballantyne CM; on behalf of the CLEAR Harmony Steering Committee. Safety and Efficacy of Bempedoic Acid to Reduce LDL Cholesterol. Engl J Med. 2019;380:1022–1032. [DOI] [PubMed] [Google Scholar]
- 15. Laufs U, Banach M, Mancini GBJ, Gaudet D, Bloedon LT, Sterling LRL, Kelly S, Stroes ESG. Efficacy and safety of bempedoic acid in patients with hypercholesterolemia and statin intolerance. J Am Heart Assoc. 2019;8:e011662 DOI: 10.1161/JAHA.118.011662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Thompson PD, MacDougall DE, Newton RS, Margulies JR, Hanselman JC, Orloff DG, McKenney JM, Ballantyne CM. Treatment with ETC‐1002 alone and in combination with ezetimibe lowers LDL cholesterol in hypercholesterolemic patients with or without statin intolerance. J Clin Lipidol. 2016;10:556–567. [DOI] [PubMed] [Google Scholar]


