Abstract
Background
Blacks harbor more cardiovascular risk factors than whites, but experience less atrial fibrillation (AF). Conversely, whites may have a lower risk of heart failure (CHF). N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) levels are higher in whites, predict incident AF, and have diuretic effects in the setting of increased ventricular diastolic pressures, potentially providing a unifying explanation for these racial differences.
Methods and Results
We used data from the CHS (Cardiovascular Health Study) to determine the degree to which baseline NT‐proBNP levels mediate the relationships between race and incident AF and CHF by comparing beta estimates between models with and without NT‐proBNP. The ARIC (Atherosclerosis Risk in Communities) study was used to assess reproducibility. Among 4731 CHS (770 black) and 12 418 ARIC (3091 black) participants, there were 1277 and 1253 incident AF events, respectively. Whites had higher baseline NT‐proBNP (CHS: 40% higher than blacks; 95% CI, 29–53; ARIC: 39% higher; 95% CI, 33–46) and had a greater risk of incident AF compared with blacks (CHS: adjusted hazard ratio, 1.60; 95% CI, 1.31–1.93; ARIC: hazard ratio, 1.93; 95% CI, 1.57–2.27). NT‐proBNP levels explained a significant proportion of the racial difference in AF risk (CHS: 36.2%; 95% CI, 23.2–69.2%; ARIC: 24.6%; 95% CI, 14.8–39.6%). Contrary to our hypothesis, given an increased risk of CHF among whites in CHS (adjusted hazard ratio, 1.20; 95% CI, 1.05–1.47) and the absence of a significant association between race and CHF in ARIC (adjusted hazard ratio, 1.07; 95% CI, 0.94–1.23), CHF‐related mediation analyses were not performed.
Conclusions
A substantial portion of the relationship between race and AF was statistically explained by baseline NT‐proBNP levels. No consistent relationship between race and CHF was observed.
Keywords: atrial fibrillation arrhythmia, congestive heart failure, mechanisms, mediation, natriuretic peptide, NT‐proBNP
Subject Categories: Atrial Fibrillation, Electrophysiology, Biomarkers, Heart Failure
Short abstract
See Editorial by Richards
Clinical Perspective
What Is New?
The paradoxical observation that whites exhibit a higher risk of atrial fibrillation than blacks despite generally harboring fewer conventional atrial fibrillation risk factors may be partially explained by higher NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) levels in whites.
What Are the Clinical Implications?
Better understanding the effects of NT‐proBNP in the atria may elucidate why higher NT‐proBNP levels are associated with atrial fibrillation and may provide a potential therapeutic target for atrial fibrillation prevention.
Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia in the United States and the leading cause of embolic stroke.1, 2 Hypertension and congestive heart failure (CHF) are 2 of the most important risk factors for development of AF.3, 4 However, despite the finding that these and other AF risk factors are more common in blacks,5, 6, 7, 8, 9, 10, 11 whites have a greater risk for AF,12, 13, 14, 15 as do American blacks with greater European ancestry.16 The pathophysiology underlying this paradox is unknown. Similarly, in some populations, blacks also have a substantially higher risk for CHF than whites,6 but the mechanism underlying this difference is not completely understood.
Of interest, AF is associated with higher ANP (atrial natriuretic peptide) levels.17, 18 Evidence that genetic aberrations in the ANP gene are associated with AF19 raises the question as to whether abnormalities in ANP expression may cause AF (rather than reflect an atrial myopathy secondary to AF). Furthermore, serum levels of NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) have been closely correlated with ANP,17, 20, 21, 22 have similarly been shown to predict incident AF,17, 23, 24, 25 and are known to have diuretic effects that might relieve volume overload in the setting of CHF.26, 27, 28 Recent evidence demonstrates that NT‐proBNP levels are lower in blacks.29 Therefore, it is plausible that NT‐proBNP levels might mediate the discordant race‐AF and race‐CHF associations. Specifically, the higher baseline level of natriuretic peptides in whites may have a direct atrial effect that increases AF susceptibility. We hypothesized that higher levels of NT‐proBNP at baseline in the absence of elevated filling pressures, by the diuretic action of this protein, may also render whites less prone to CHF.
Understanding these relationships might help to elucidate the mechanisms underlying both of these important diseases and may point to specific prediction strategies and novel treatment approaches informed by racial background.
We sought to quantify the racial differences in incident AF and CHF, and then determine the degree to which NT‐proBNP levels may mediate the racial differences between blacks and whites in incident AF and CHF.
Methods
Using the CHS (Cardiovascular Health Study) and then subsequently the ARIC (Atherosclerosis Risk in Communities) Study population as an independent replication cohort, we measured the degree to which NT‐proBNP level at baseline may explain the differential relationship between race and incident AF and CHF in prospective, population‐based cohort studies. The authors will make the methods (codes for the statistical analysis) available to any researcher for purposes of reproducing the results. The data belong to CHS and ARIC, and the authors therefore do not have the authority to share the study data with investigators outside the University of California, San Francisco (San Francisco, CA); however, investigators can submit an application to obtain the data directly from the CHS or the ARIC Study using their established processes.
Recruitment, characterization, and outcome ascertainment in the CHS and ARIC have been previously published.30, 31 Each cohort's research protocol was approved by its respective institutional review board, and all participants provided written informed consent. The specific methods of the CHS and ARIC are summarized below. Criteria for baseline ascertainment of hypertension, coronary heart disease, CHF, and diabetes mellitus for each cohort are described in Table S1.
Cardiovascular Health Study
CHS is a prospective, community‐based cohort study designed to follow patients aged ≥65 years to assess cardiovascular risk factors and outcomes, the methods for which have previously been described.31 AF and CHF ascertainment was complete through 2008, during which time medical records were obtained for all hospitalizations.31 Prevalent AF was identified from the baseline ECG,32 and incident AF was identified from study visit ECGs and hospital discharge diagnoses. Incident CHF was identified through self‐report, hospital diagnosis codes, and in‐ and outpatient medical records adjudicated by the CHS Cardiac Events Subcommittee.33
Levels of NT‐proBNP were measured from blood samples taken at study entry. Methods were the same for both the main cohort (1989–1990) and in the additional cohort of black participants (1992–1993). Serum was initially frozen and stored at −70°C to −80°C until testing, which occurred between 2007 and 2008. All testing was performed in a lab certified by the Clinical Laboratory Improvement Amendments and accredited by the College of American Pathologists. A US Food and Drug Administration–approved immunoassay was used for NT‐proBNP measurement on an Elecsys 2010 instrument (Roche Diagnostics, Indianapolis, IN). The coefficient of variation for the NT‐proBNP assay was 2% to 5% during the testing period, and the analytical measurement range for NT‐proBNP was 5 to 35 000 pg/mL. The core laboratory reported data to the central data repository and was blinded to patient outcomes.23, 34
ARIC Study
The ARIC Study is a prospective study of 15 792 primarily white and black middle‐aged participants recruited from 4 communities in the United States between 1987 and 1989, as described previously.30 Prevalent AF was identified from the intake ECG, and incident AF was identified from study visit ECGs, hospital discharge diagnoses, and death certificates through 2013.35 Prevalent CHF was ascertained through medication review, self‐report questionnaire, and physical exam. Incident CHF was diagnosed through annual phone interviews about interim hospitalizations, from hospital discharge diagnosis codes, and death certificates, also through 2013.36
Using serum samples from visit 2 (1990–1992) stored at −70°C in EDTA, NT‐proBNP levels were measured in ARIC using a sandwich immunoassay method (Roche Diagnostics) on a Roche Elecsys 2010 Analyzer in 2012–2013. The measuring range of the assay was 5 to 35 000 pg/mL, and the reliability coefficient for blinded replicate measurements was 0.99 (n=418 pairs).37
Statistical Analysis
We conducted 2 separate analyses with AF and CHF as respective outcomes. After assessing baseline NT‐proBNP levels at study entry (CHS) and visit 2 (ARIC), and excluding patients with prevalent AF or CHF, we determined the association between white or black race and incident AF or CHF, in respective analyses, and then the degree to which the differences were statistically explained by baseline NT‐proBNP level.
To deal with the right skewness of NT‐proBNP and the nonlinearity of its association with cardiovascular events, NT‐proBNP values were log2‐transformed for analysis. After excluding patients with baseline AF or CHF, analyses in both the CHS and ARIC were adjusted for the following covariates ascertained during the baseline visit: age, sex, body mass index, diabetes mellitus, hypertension, coronary artery disease, left ventricular hypertrophy, chronic kidney disease (in CHS), creatinine (in ARIC), smoking status, alcohol consumption, level of education, and income.
Cox proportional hazards regression models adjusted for the above covariates were then used to estimate the race‐AF and race‐CHF associations. Proportional hazard assumptions were tested and confirmed using log‐log survival plots, comparison of Kaplan–Meier and predicted survival plots, and tests for the correlation of scaled Schoenfeld residuals with time.
The degree to which baseline NT‐proBNP may explain the differential racial association with AF or CHF was calculated as the percentage change in the point estimate for race after addition of NT‐proBNP to the multivariate Cox model for AF.38 CIs for the percentage change were obtained using bootstrap resampling. Because there was no evidence that whites exhibited a lower risk of CHF in CHS or ARIC after adjustment, no mediation analysis for NT‐proBNP was performed in either cohort for the CHF analyses.
Results
CHS Participants and NT‐proBNP by Race
Of the 5888 participants in CHS, 4731 were included in the analyses after exclusions. Participant characteristics at baseline are shown in Table 1. On average, blacks had lower income and less education than whites. Blacks had more hypertension and diabetes mellitus at baseline, were more likely to have smoked, and consumed less alcohol at baseline. Whites exhibited a higher baseline NT‐proBNP than blacks (Table 1), and after log transformation and multivariable adjustment, whites had a 40% higher NT‐proBNP than blacks (95% CI, 29–53; P<0.001; unadjusted, 29%; 95% CI, 19–41; P<0.001; Figure 1).
Table 1.
Baseline Participant Characteristics by Race in CHS and ARICa
| CHS (n=4731) | ARIC (n=12 418) | |||||
|---|---|---|---|---|---|---|
| Black (n=770) | White (n=3961) | P Value | Black (n=3091) | White (n=9327) | P Value | |
| Age, mean (SD), y | 72.6 (5.6) | 72.4 (5.4) | 0.37 | 53.4 (5.8) | 54.2 (5.7) | <0.001 |
| Male, n (%) | 281 (37) | 1559 (39) | 0.14 | 1055 (37) | 4275 (46) | <0.001 |
| Annual income, n (%) | <0.001 | <0.001 | ||||
| <$12 000 | 365 (51) | 781 (20) | 1012 (37) | 531 (6) | ||
| ≥$12 000 | 357 (49) | 2926 (80) | 1745 (63) | 8406 (94) | ||
| Education, n (%) | <0.001 | <0.001 | ||||
| ≤ High schoolb | 531 (70) | 2511 (64) | 1988 (67) | 5681 (62) | ||
| ≥ Some college | 228 (30) | 1438 (36) | 965 (33) | 3555 (38) | ||
| NT‐proBNP level, median (Q1, Q3), pg/mL | 88.0 (43.2, 183.3) | 113.6 (60.0, 215.0) | <0.001 | 44.2 (23.0, 87.2) | 56.7 (31.3, 99.1) | <0.001 |
| Body mass index, mean (SD), kg/m2 | 28.5 (5.5) | 26.2 (4.4) | <0.001 | 29.4 (6.0) | 26.8 (4.7) | <0.001 |
| Hypertension, n (%) | 564 (73) | 2167 (55) | <0.001 | 1331 (43) | 1784 (19) | <0.001 |
| Diabetes mellitus, n (%) | 176 (23) | 531 (13) | <0.001 | 483 (17) | 736 (8) | <0.001 |
| Coronary artery disease, n (%) | 126 (16) | 605 (15) | 0.44 | 88 (3) | 418 (5) | 0.001 |
| Left ventricular hypertrophy, n (%)c | 226 (29) | 622 (16) | <0.001 | 158 (6) | 79 (1) | <0.001 |
| Chronic kidney disease, n (%) | 113 (15) | 540 (14) | 0.44 | ··· | ··· | ··· |
| Creatinine, mean (SD), mg/dL | ··· | ··· | ··· | 1.13 (0.5) | 1.08 (0.2) | <0.001 |
| Ever smoker, n (%) | 122 (16) | 439 (11) | <0.001 | 1518 (51) | 5403 (59) | <0.001 |
| Alcoholic drinks/week, median (Q1, Q3) | 0 (0, 0.25) | 0.02 (0, 1.27) | 0.0001 | ··· | ··· | ··· |
| Ever drinker, n (%) | ··· | ··· | ··· | 1625 (55) | 7568 (82) | <0.001 |
| Incident atrial fibrillation, n (%) | 146 (19) | 1131 (29) | <0.001 | 212 (7) | 1041 (11) | <0.001 |
| Incident heart failure, n (%) | 215 (28) | 1139 (29) | 0.64 | 550 (19) | 1195 (13) | <0.001 |
NT‐proBNP indicates N‐terminal pro‐B‐type natriuretic peptide; Q1, Q3, first and third quartiles; CHS, Cardiovascular Health Study; ARIC, Atherosclerosis Risk in Communities Study.
Years of enrollment and ascertainment of baseline characteristics for the Cardiovascular Health Study (whites: 1989–1990; blacks: 1992–1993) and Atherosclerosis Risk in Communities study (1987–1989).
Includes some or graduation from vocational school.
By electrocardiographic criteria.
Figure 1.
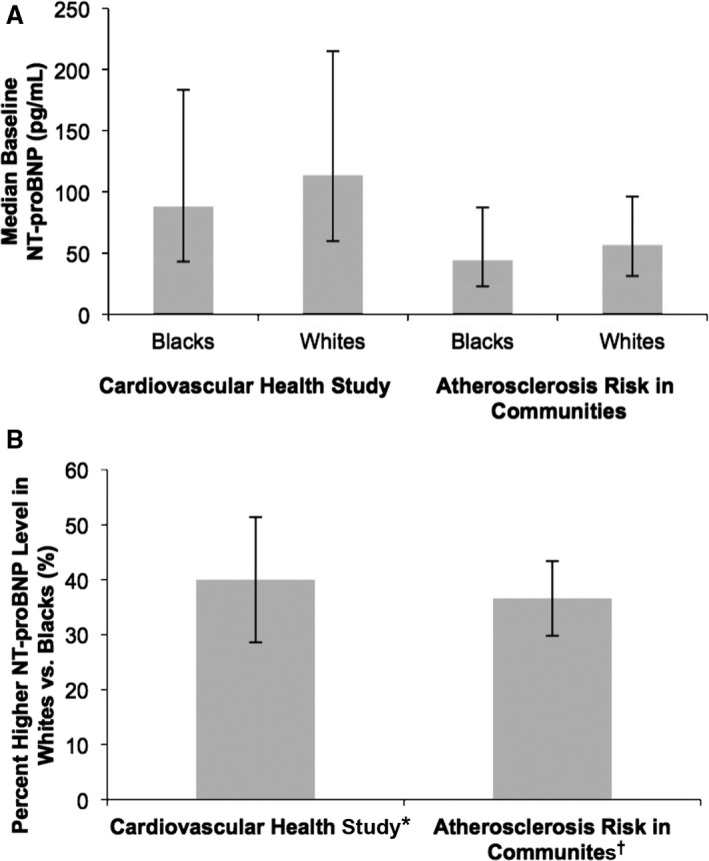
(A) Baseline NT‐proBNP levels and (B) adjusted relative relationship between NT‐proBNP level in whites versus blacks, on average, in the Cardiovascular Health Study and Atherosclerosis Risk in Communities study. (A) p<0.001 blacks vs. whites in both cohorts. (B) p<0.001 percent difference in baseline NT‐proBNP in both cohorts. *Adjustment in the model was made for age, gender, body mass index, hypertension, diabetes, coronary artery disease, left ventricular hypertrophy, chronic kidney disease, ever smoker, drinks per week, education, and income. †Adjustment in the model was made for age, gender, body mass index, hypertension, diabetes, coronary artery disease, left ventricular hypertrophy, creatinine, ever smoker, ever drinker, education, and income.
ARIC Participants and NT‐proBNP by Race
After exclusion, 12 418 ARIC participants were included in these analyses. Their characteristics are shown in Table 1. In ARIC, blacks were, on average, younger than whites, a greater proportion were female, and blacks had less education and lower annual income. Black participants had a higher body mass index, on average, and a higher prevalence of baseline hypertension, diabetes mellitus, and left ventricular hypertrophy. Whites had more coronary artery disease at baseline and were more likely to have ever smoked or consumed alcohol. Whites had higher NT‐proBNP at baseline than blacks (Table 1) and, with log transformation and multivariable adjustment, 39% higher baseline NT‐proBNP than blacks, on average (95% CI, 33–46; P<0.001; unadjusted, 55% higher NT‐proBNP than blacks, on average; 95% CI, 54–56; P<0.001; Figure 1).
AF in the CHS
CHS participants were followed for a mean of 11.8 years in the AF analysis, accumulating 1277 incident AF events. Whites had a greater risk of incident AF both before (hazard ratio [HR], 1.32; 95% CI, 1.12–1.58; P=0.001) and after multivariable adjustment (HR, 1.60; 95% CI, 1.31–1.93; P<0.001; Figure 2). Furthermore, each doubling of NT‐proBNP increased the risk of incident AF by 46% (95% CI, 40–52; P<0.001) after adjusting for age, sex, body mass index, hypertension, diabetes mellitus, coronary artery disease, left ventricular hypertrophy, chronic kidney disease, ever smoking, drinks per week, education, and income.
Figure 2.
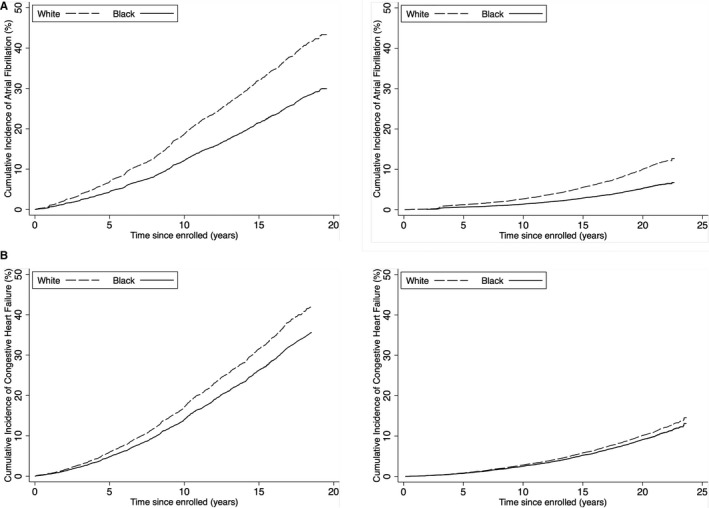
Cumulative incidence of (A) atrial fibrillation and (B) heart failure in the Cardiovascular Health Study and Atherosclerosis Risk in Communities Study, by race. *Adjustment in the model was made for age, sex, body mass index, hypertension, diabetes mellitus, coronary artery disease, left ventricular hypertrophy, chronic kidney disease, ever smoker, drinks per week, education, and income. †Adjustment in the model was made for age, sex, body mass index, hypertension, diabetes mellitus, coronary artery disease, left ventricular hypertrophy, creatinine, ever smoker, ever drinker, education, and income.
Mediation analysis demonstrated that the percent of racial differences in AF risk statistically explained by NT‐proBNP was ≈36% (Table 2).
Table 2.
Mediation Analysis Results for the Percent Effect Explained by NT‐proBNP of the Racial Difference Between Blacks and Whites in Incident AF
| Predictor | Outcome Measure | Estimate | Cardiovascular Health Studya | Atherosclerosis Risk in Communitiesb | ||
|---|---|---|---|---|---|---|
| Effect (95% CI) | P Value | Effect (95% CI) | P Value | |||
| White race (vs black race) | Baseline NT‐proBNP | Relative difference (% greater) | 40.1 (28.6–52.5) | <0.001 | 39.4 (32.7–46.4) | <0.001 |
| NT‐proBNPc | Incident AF | Hazard ratio | 1.39 (1.33–1.45) | <0.001 | 1.56 (1.50–1.63) | <0.001 |
| White race (vs black race) | Incident AF | Hazard ratio, excluding NT‐proBNP from adjustment | 1.60 (1.31–1.93) | <0.001 | 1.93 (1.57–2.27) | <0.001 |
| White race (vs black race) | Incident AF | Hazard ratio, including NT‐proBNP in adjustment | 1.34 (1.11–1.63) | 0.002 | 1.64 (1.35–2.00) | <0.001 |
| White race (vs black race) | Incident AF | Percent effect explained by NT‐proBNP | 36.2 (23.2–69.2) | <0.001 | 24.6 (14.8–39.6) | <0.001 |
AF indicates atrial fibrillation; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
Adjustment in the model was made for age, sex, body mass index, hypertension, diabetes mellitus, coronary artery disease, left ventricular hypertrophy, chronic kidney disease, ever smoker, drinks per week, education, and income.
Adjustment in the model was made for age, sex, body mass index, hypertension, diabetes mellitus, coronary artery disease, left ventricular hypertrophy, creatinine, ever smoker, ever drinker, education, and income.
The model of NT‐proBNP as a predictor reflects the effect of a doubling of NT‐proBNP.
AF in the ARIC
Follow‐up for a mean of 18.2 years yielded 1253 incident AF events. As in CHS, whites in ARIC had a greater risk of incident AF (unadjusted HR, 1.53; 95% CI, 1.32–1.77; P<0.001; adjusted HR, 1.93; 95% CI, 1.57–2.27; P<0.001; Figure 2). After adjustment, a doubling of baseline NT‐proBNP was associated with an increased risk of incident AF by over 50% (adjusted, 56%; 95% CI, 50–63%; P<0.001; unadjusted, 62%; 95% CI, 56–68; P<0.001).
Comparing models with and without NT‐proBNP included, differences in baseline NT‐proBNP statistically explained nearly 25% of the increase in incident AF among whites (Table 2).
CHF in CHS
Participants were followed for a mean of 10.9 years, with 1354 incident CHF events. Before adjustment, white race was associated with lower incidence of CHF (HR, 0.87; 95% CI, 0.75–1.01; P=0.07). After adjustment, contrary to what had been hypothesized, there was an increased risk of incident CHF with white race (HR, 1.20; 95% CI, 1.05–1.47; P=0.01; Figure 2). Sequential addition of each covariate into the unadjusted model was performed, and the increased risk of CHF in whites after adjustment could not be attributed to any single variable alone; however, diabetes mellitus, hypertension, and income exhibited the largest effects (Table S2). Furthermore, in the CHS cohort as a whole, there was an increased risk of CHF with doubling of baseline NT‐proBNP (45% increased risk; 95% CI, 41–52; P<0.001; adjusted, 34% increased risk; 95% CI, 29–40; P<0.001)—again, contrary to our hypothesis that higher baseline NT‐proBNP in the absence of clinical AF or CHF would have a protective association with CHF. Because there was no increased risk of incident CHF with black race after adjustment, a mediation analysis was not performed.
CHF in ARIC
Study participants were followed for a mean of 18.8 years, accumulating 1783 incident CHF diagnoses. In an unadjusted Cox proportional hazard model, white race was associated with a lower incidence of CHF (HR, 0.66; 95% CI, 0.59–0.73; P<0.001; Figure 2). This association disappeared with adjustment, resulting in no association between race and incident CHF (HR, 1.07; 95% CI, 0.94–1.23; P=0.32). Covariates were individually added to the unadjusted model, and the addition of income alone eliminated the statistical racial difference in incident CHF (Table S3). No other single variable alone eliminated statistical significance in the model. Similar to the finding in CHS, there was in increased risk of CHF with doubling of baseline NT‐proBNP (unadjusted increased risk, 62%; 95% CI, 56–68; P<0.001; adjusted, 58%; 95% CI, 51–64; P<0.001). Because there was no association of race with incident CHF in ARIC, a mediation analysis for NT‐proBNP was not performed.
Discussion
Utilizing baseline NT‐proBNP levels before the diagnoses of AF or CHF, we found that higher NT‐proBNP statistically explained a significant proportion of the racial difference noted in incident AF in 2 large, community‐based studies. However, contrary to our hypothesis, higher baseline NT‐proBNP levels in the absence of clinical AF or CHF were not protective against incident CHF in either CHS or ARIC after taking potential confounders into account, and there was no disparate racial association with incident CHF.
NT‐proBNP and AF
The paradoxical racial association with AF in various populations has been a point of interest in the literature.12, 39, 40, 41, 42 The cardiac risk factors for AF have been well defined in multiple population studies and have similarly been shown to be more common in blacks than whites.5, 6, 7, 8, 9 Despite this, whites experience a greater risk of AF,12 as do blacks with a greater proportion of European ancestry.16
A potential explanation for this paradox—that, despite fewer independent risk factors for AF, whites have a higher risk for AF than blacks—may be mechanistically related to natriuretic peptides, higher levels of which have been associated with incident AF17, 18, 23, 43 and circulating levels of which are higher in whites than blacks.29, 44 Presence of AF has been associated with higher natriuretic peptides both acutely and chronically,45, 46, 47, 48 and natriuretic peptide levels have been shown to decrease after cardioversion of AF, illustrating that AF itself may increase natriuretic peptide levels.49, 50 However, because higher levels of baseline natriuretic peptide predict incident AF, the relationship likely goes “both ways.”23 More‐elevated levels of natriuretic peptide have been associated with atrial dysfunction,51 atrial fibrosis,52 and a shorter atrial effective refractory period,53 all characteristics known to enhance the probability of AF and even mechanistically serve as a cause.54, 55, 56 In fact, in a case of familial AF, a mutation in the gene expressing ANP was mechanistically linked to the AF phenotype.19 In the current study, despite substantial differences in absolute baseline NT‐proBNP levels between the CHS and ARIC (likely related to the differences in age between the cohorts), the relationships between every doubling of NT‐proBNP levels and increased risk of AF were remarkably similar.
Consistent with our current findings, whites have previously been shown to exhibit higher levels of natriuretic peptide levels than blacks, and the difference does not appear to be related to more heart failure or volume overload.29 Therefore, there is presumably some genetic predisposition to releasing more NT‐proBNP independent of myocardial stretch or left ventricular end‐diastolic pressure. Similarly, whites have a higher risk of AF compared with blacks unrelated to more‐established cardiovascular risk factors,12, 13, 14 and blacks with more European ancestry (determined by genotyping) have a higher risk of AF,16 suggesting that the race‐AF relationship is likely genetically mediated. Our current mediation analysis is consistent with the notion that propensity to have a higher NT‐proBNP level at baseline may explain some of the differential risk of AF by race. This itself, in turn, raises the question as to whether natriuretic peptides may have a direct causal relationship with AF.
NT‐proBNP and CHF
In designing the present study, we had hypothesized that higher levels of baseline NT‐proBNP, while potentially imposing increased risk of AF for whites, may also mediate the differential racial association with CHF observed in some populations.6 The general theory is that a higher NT‐proBNP level, on average, would help maintain diuresis and therefore help to mitigate volume overload. In fact, we observed no increased risk of CHF in blacks versus whites after adjustment in either CHS or ARIC, and so the extent to which baseline NT‐proBNP may mediate the differential association was rendered moot in statistical analyses. Indeed, although whites had greater risk for CHF in CHS, there was no such association with CHF in ARIC. These observations themselves demonstrate that the apparent racial propensity to differential NT‐proBNP levels does not translate into consistent differences in CHF incidence. It is possible that the higher prevalence of CHF risk factors among blacks is counterbalanced by a protective effect of NT‐proBNP, resulting in a net equivalence of incident CHF.
Our findings regarding race and CHF are also consistent with previous literature. In the MESA (Multiethnic Study of Atherosclerosis), Bahrami et al concluded that blacks have more incident CHF than whites; however, after adjustment for hypertension and/or diabetes mellitus, that difference was attenuated.6 Interestingly, data from the CARDIA (Coronary Artery Risk Development in Young Adults) study clearly showed that young blacks were at a substantially higher risk than whites for CHF,57 more consistent with our findings in ARIC (a younger population than that of CHS), where, after adjustment, risk for CHF in whites and blacks was statistically equivalent. This may suggest that there is an important interaction between age and race that is relevant to CHF pathophysiology.
This study had several limitations. CHS and ARIC rely on intake questionnaires for prevalence of diseases at baseline, although review of medical records and surveys of treating physicians were performed at baseline to identify prevalent cardiovascular disease in CHS. The current study lacked echocardiography data or other direct measurements of filling pressures to determine potential underlying mechanistic relationships to baseline NT‐proBNP levels. Self‐report of baseline comorbidities may limit the sensitivity and specificity of disease prevalence at baseline, particularly with AF, which cannot necessarily be refuted by ECG. The effects of the bias this creates cannot be determined, but nonetheless the sensitivity and specificity of the presence of diagnoses at baseline is likely similar between the predictor groups in this study and therefore may not affect comparative results. Although NT‐proBNP was measured in blood samples at study entry, and those with prevalent AF or CHF were excluded from the study, there may have been occult AF or presence of certain comorbidities at baseline, such as undetected hypertension, that may have increased baseline NT‐proBNP measurements and similarly increased the risk of AF and CHF in those patients. However, although this may have biased the analysis toward an increased risk of AF and/or CHF in patients with higher NT‐proBNP, it would not appear to affect or explain the results of the mediation analysis. Follow‐up in both CHS and ARIC included multiple visits, review of intervening medical records, and serial ECGs; however, it remains possible that some AF remained undetected. Nonetheless, more‐sensitive monitoring should generally affect the incidence of AF similarly in both whites and blacks, but again should not affect the mediation analysis. Whereas the statistical model of the study is meant to address the possible role NT‐proBNP may play in mediation of the differential relationship between race and AF, the statistical model is merely suggestive and not definitive of mediation or a causal relationship. Also, there may be unmeasured confounders not captured by data available in CHS and ARIC, although both of these cohorts include robust lifestyle and demographic information, and all relevant covariates were included in multivariable models. Finally, because this is an observational study, the relationships described should be interpreted as associations, and no conclusions regarding causality can be made.
Conclusion
A substantial portion of the strong and paradoxical relationship between white race and AF was explained by higher baseline NT‐proBNP levels in both a middle‐aged and older adult population. Conversely, despite the higher NT‐proBNP levels in whites, no consistent relationship between race and CHF was observed between the 2 cohorts. Given previous literature demonstrating an atrial remodeling effect of natriuretic peptides, these data draw attention to whether natriuretic peptides may have a direct casual effect on AF, suggesting that targeting such pathways to prevent or treat AF may prove fruitful.
Sources of Funding
This research was supported by contracts HHSN268201200036C, HHSN268200800007C, HHSN268201800001C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, and N01HC85086 and grants U01HL080295 and U01HL130114 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by R01AG023629 from the National Institute on Aging (NIA). Additional support was provided by grant N01HC35129 (Gottdiener). A full list of principal CHS investigators and institutions can be found at CHS‐NHLBI.org. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The Atherosclerosis Risk in Communities study has been funded in whole or in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract Nos. HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700004I, and HHSN268201700005I. Dr Selvin was supported by NIH/NIDDK grants K24DK106414 and R01DK089174. Reagents for the NT‐proBNP assays were donated by the Roche Diagnostics Corporation. Additional support was provided by the American Heart Association through grant 16EIA26410001 to Dr Alonso.
Disclosures
None.
Supporting information
Table S1. Diagnostic Criteria for Baseline Cardiac Risk Factors at Study Entry in the Cardiovascular Health Study and Atherosclerosis Risk in Communities Cohort
Table S2. Results of Cox Proportional Hazards Model With White vs Black Race as a Predictor of Incident Heart Failure, Adjusting for Individual Covariates in the Cardiovascular Health Study
Table S3. Results of Cox Proportional Hazards Model With White vs Black Race as a Predictor of Incident Heart Failure, Adjusting for Individual Covariates in the Atherosclerosis Risk in Communities Study
Acknowledgments
The authors thank the staff and participants of the CHS and ARIC studies for their important contributions.
(J Am Heart Assoc. 2019;8:e010868 DOI: 10.1161/JAHA.118.010868.)
References
- 1. Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, de Ferranti SD, Ferguson JF, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Lutsey PL, Mackey JS, Matchar DB, Matsushita K, Mussolino ME, Nasir K, O'Flaherty M, Palaniappan LP, Pandey A, Pandey DK, Reeves MJ, Ritchey MD, Rodriguez CJ, Roth GA, Rosamond WD, Sampson UKA, Satou GM, Shah SH, Spartano NL, Tirschwell DL, Tsao CW, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics—2018 update: a report from the American Heart Association. Circulation. 2018;137:e67–e492. [DOI] [PubMed] [Google Scholar]
- 2. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW; ACC/AHA Task Force Members . 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130:e199–e267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Krahn AD, Manfreda J, Tate RB, Mathewson FA, Cuddy TE. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba Follow‐Up Study. Am J Med. 1995;98:476–484. [DOI] [PubMed] [Google Scholar]
- 4. Schnabel RB, Sullivan LM, Levy D, Pencina MJ, Massaro JM, D'Agostino RB Sr, Newton‐Cheh C, Yamamoto JF, Magnani JW, Tadros TM, Kannel WB, Wang TJ, Ellinor PT, Wolf PA, Vasan RS, Benjamin EJ. Development of a risk score for atrial fibrillation (Framingham Heart Study): a community‐based cohort study. Lancet. 2009;373:739–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. US Renal Data System, USRDS 2009 Annual Data Report: Atlas of Chronic Kidney Disease and End‐Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2009.
- 6. Bahrami H, Kronmal R, Bluemke DA, Olson J, Shea S, Liu K, Burke GL, Lima JA. Differences in the incidence of congestive heart failure by ethnicity: the Multi‐Ethnic Study of Atherosclerosis. Arch Intern Med. 2008;168:2138–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Burt VL, Whelton P, Roccella EJ, Brown C, Cutler JA, Higgins M, Horan MJ, Labarthe D. Prevalence of hypertension in the US adult population. Results from the Third National Health and Nutrition Examination Survey, 1988–1991. Hypertension. 1995;25:305–313. [DOI] [PubMed] [Google Scholar]
- 8. Kalogeropoulos A, Georgiopoulou V, Kritchevsky SB, Psaty BM, Smith NL, Newman AB, Rodondi N, Satterfield S, Bauer DC, Bibbins‐Domingo K, Smith AL, Wilson PW, Vasan RS, Harris TB, Butler J. Epidemiology of incident heart failure in a contemporary elderly cohort: the Health, Aging, and Body Composition Study. Arch Intern Med. 2009;169:708–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shai I, Jiang R, Manson JE, Stampfer MJ, Willett WC, Colditz GA, Hu FB. Ethnicity, obesity, and risk of type 2 diabetes in women: a 20‐year follow‐up study. Diabetes Care. 2006;29:1585–1590. [DOI] [PubMed] [Google Scholar]
- 10. Brancati FL, Whelton PK, Kuller LH, Klag MJ. Diabetes mellitus, race, and socioeconomic status. A population‐based study. Ann Epidemiol. 1996;6:67–73. [DOI] [PubMed] [Google Scholar]
- 11. Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dewland TA, Olgin JE, Vittinghoff E, Marcus GM. Incident atrial fibrillation among Asians, Hispanics, blacks, and whites. Circulation. 2013;128:2470–2477. [DOI] [PubMed] [Google Scholar]
- 13. Borzecki AM, Bridgers DK, Liebschutz JM, Kader B, Kazis LE, Berlowitz DR. Racial differences in the prevalence of atrial fibrillation among males. J Natl Med Assoc. 2008;100:237–245. [DOI] [PubMed] [Google Scholar]
- 14. Psaty BM, Manolio TA, Kuller LH, Kronmal RA, Cushman M, Fried LP, White R, Furberg CD, Rautaharju PM. Incidence of and risk factors for atrial fibrillation in older adults. Circulation. 1997;96:2455–2461. [DOI] [PubMed] [Google Scholar]
- 15. Marcus GM, Whooley MA, Glidden DV, Pawlikowska L, Zaroff JG, Olgin JE. Interleukin‐6 and atrial fibrillation in patients with coronary artery disease: data from the Heart and Soul Study. Am Heart J. 2008;155:303–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Marcus GM, Alonso A, Peralta CA, Lettre G, Vittinghoff E, Lubitz SA, Fox ER, Levitzky YS, Mehra R, Kerr KF, Deo R, Sotoodehnia N, Akylbekova M, Ellinor PT, Paltoo DN, Soliman EZ, Benjamin EJ, Heckbert SR; Candidate‐Gene Association Resource (CARe) Study . European ancestry as a risk factor for atrial fibrillation in African Americans. Circulation. 2010;122:2009–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chang IC, Chen LY, Chong JP, Austin E, Quay CN, Gong L, Mark Richards A, Ling LH. Plasma mid‐regional pro‐atrial natriuretic peptide and N‐terminal pro‐brain natriuretic peptide improve discrimination of lone atrial fibrillation. Int J Cardiol. 2015;188:10–12. [DOI] [PubMed] [Google Scholar]
- 18. Mandalenakis Z, Eriksson H, Welin L, Caidahl K, Dellborg M, Rosengren A, Lappas G, Hedner J, Johansson S, Svardsudd K, Hansson PO. Atrial natriuretic peptide as a predictor of atrial fibrillation in a male population study. The Study of Men Born in 1913 and 1923. Int J Cardiol. 2014;171:44–48. [DOI] [PubMed] [Google Scholar]
- 19. Hodgson‐Zingman DM, Karst ML, Zingman LV, Heublein DM, Darbar D, Herron KJ, Ballew JD, de Andrade M, Burnett JC Jr, Olson TM. Atrial natriuretic peptide frameshift mutation in familial atrial fibrillation. N Engl J Med. 2008;359:158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Eckstein J, Potocki M, Murray K, Breidthardt T, Ziller R, Mosimann T, Klima T, Hoeller R, Moehring B, Sou SM, Rubini Gimenez M, Morgenthaler NG, Mueller C. Direct comparison of mid‐regional pro‐atrial natriuretic peptide with N‐terminal pro B‐type natriuretic peptide in the diagnosis of patients with atrial fibrillation and dyspnoea. Heart. 2012;98:1518–1522. [DOI] [PubMed] [Google Scholar]
- 21. Richards M, Di Somma S, Mueller C, Nowak R, Peacock WF, Ponikowski P, Mockel M, Hogan C, Wu AH, Clopton P, Filippatos GS, Anand I, Ng L, Daniels LB, Neath SX, Shah K, Christenson R, Hartmann O, Anker SD, Maisel A. Atrial fibrillation impairs the diagnostic performance of cardiac natriuretic peptides in dyspneic patients: results from the BACH Study (Biomarkers in ACute Heart Failure). JACC Heart Fail. 2013;1:192–199. [DOI] [PubMed] [Google Scholar]
- 22. Schnabel RB, Wild PS, Wilde S, Ojeda FM, Schulz A, Zeller T, Sinning CR, Kunde J, Lackner KJ, Munzel T, Blankenberg S. Multiple biomarkers and atrial fibrillation in the general population. PLoS One. 2014;9:e112486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Patton KK, Ellinor PT, Heckbert SR, Christenson RH, DeFilippi C, Gottdiener JS, Kronmal RA. N‐terminal pro‐B‐type natriuretic peptide is a major predictor of the development of atrial fibrillation: the Cardiovascular Health Study. Circulation. 2009;120:1768–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Patton KK, Heckbert SR, Alonso A, Bahrami H, Lima JA, Burke G, Kronmal RA. N‐terminal pro‐B‐type natriuretic peptide as a predictor of incident atrial fibrillation in the Multi‐Ethnic Study of Atherosclerosis: the effects of age, sex and ethnicity. Heart. 2013;99:1832–1836. [DOI] [PubMed] [Google Scholar]
- 25. Sinner MF, Stepas KA, Moser CB, Krijthe BP, Aspelund T, Sotoodehnia N, Fontes JD, Janssens AC, Kronmal RA, Magnani JW, Witteman JC, Chamberlain AM, Lubitz SA, Schnabel RB, Vasan RS, Wang TJ, Agarwal SK, McManus DD, Franco OH, Yin X, Larson MG, Burke GL, Launer LJ, Hofman A, Levy D, Gottdiener JS, Kaab S, Couper D, Harris TB, Astor BC, Ballantyne CM, Hoogeveen RC, Arai AE, Soliman EZ, Ellinor PT, Stricker BH, Gudnason V, Heckbert SR, Pencina MJ, Benjamin EJ, Alonso A. B‐type natriuretic peptide and C‐reactive protein in the prediction of atrial fibrillation risk: the CHARGE‐AF Consortium of community‐based cohort studies. Europace. 2014;16:1426–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. de Bold AJ, Borenstein HB, Veress AT, Sonnenberg H. A rapid and potent natriuretic response to intravenous injection of atrial myocardial extract in rats. Life Sci. 1981;28:89–94. [DOI] [PubMed] [Google Scholar]
- 27. Tikkanen I, Fyhrquist F, Metsarinne K, Leidenius R. Plasma atrial natriuretic peptide in cardiac disease and during infusion in healthy volunteers. Lancet. 1985;2:66–69. [DOI] [PubMed] [Google Scholar]
- 28. Yoshimura M, Yasue H, Morita E, Sakaino N, Jougasaki M, Kurose M, Mukoyama M, Saito Y, Nakao K, Imura H. Hemodynamic, renal, and hormonal responses to brain natriuretic peptide infusion in patients with congestive heart failure. Circulation. 1991;84:1581–1588. [DOI] [PubMed] [Google Scholar]
- 29. Gupta DK, Claggett B, Wells Q, Cheng S, Li M, Maruthur N, Selvin E, Coresh J, Konety S, Butler KR, Mosley T, Boerwinkle E, Hoogeveen R, Ballantyne CM, Solomon SD. Racial differences in circulating natriuretic peptide levels: the Atherosclerosis Risk in Communities Study. J Am Heart Assoc. 2015;4:e001831 DOI: 10.1161/JAHA.115.001831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC Investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 31. Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A, O’Leary H, Psaty BM, Rautaharju P, Tracy RP, Weiler PG. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. [DOI] [PubMed] [Google Scholar]
- 32. Furberg CD, Psaty BM, Manolio TA, Gardin JM, Smith VE, Rautaharju PM. Prevalence of atrial fibrillation in elderly subjects (the Cardiovascular Health Study). Am J Cardiol. 1994;74:236–241. [DOI] [PubMed] [Google Scholar]
- 33. Psaty BM, Delaney JA, Arnold AM, Curtis LH, Fitzpatrick AL, Heckbert SR, McKnight B, Ives D, Gottdiener JS, Kuller LH, Longstreth WT Jr. Study of cardiovascular health outcomes in the era of claims data: the Cardiovascular Health Study. Circulation. 2016;133:156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. deFilippi CR, Christenson RH, Gottdiener JS, Kop WJ, Seliger SL. Dynamic cardiovascular risk assessment in elderly people. The role of repeated N‐terminal pro‐B‐type natriuretic peptide testing. J Am Coll Cardiol. 2010;55:441–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Alonso A, Agarwal SK, Soliman EZ, Ambrose M, Chamberlain AM, Prineas RJ, Folsom AR. Incidence of atrial fibrillation in whites and African‐Americans: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2009;158:111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study). Am J Cardiol. 2008;101:1016–1022. [DOI] [PubMed] [Google Scholar]
- 37. Lutsey PL, Alonso A, Selvin E, Pankow JS, Michos ED, Agarwal SK, Loehr LR, Eckfeldt JH, Coresh J. Fibroblast growth factor‐23 and incident coronary heart disease, heart failure, and cardiovascular mortality: the Atherosclerosis Risk in Communities study. J Am Heart Assoc. 2014;3:e000936 DOI: 10.1161/JAHA.114.000936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lin DY, Fleming TR, De Gruttola V. Estimating the proportion of treatment effect explained by a surrogate marker. Stat Med. 1997;16:1515–1527. [DOI] [PubMed] [Google Scholar]
- 39. Marcus GM, Olgin JE, Whooley M, Vittinghoff E, Stone KL, Mehra R, Hulley SB, Schiller NB. Racial differences in atrial fibrillation prevalence and left atrial size. Am J Med. 2010;123:375.e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Koopman JJ, van Bodegom D, Westendorp RG, Jukema JW. Scarcity of atrial fibrillation in a traditional African population: a community‐based study. BMC Cardiovasc Disord. 2014;14:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rader F, Van Wagoner DR, Ellinor PT, Gillinov AM, Chung MK, Costantini O, Blackstone EH. Influence of race on atrial fibrillation after cardiac surgery. Circ Arrhythm Electrophysiol. 2011;4:644–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jensen PN, Thacker EL, Dublin S, Psaty BM, Heckbert SR. Racial differences in the incidence of and risk factors for atrial fibrillation in older adults: the Cardiovascular Health Study. J Am Geriatr Soc. 2013;61:276–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hussein AA, Saliba WI, Martin DO, Shadman M, Kanj M, Bhargava M, Dresing T, Chung M, Callahan T, Baranowski B, Tchou P, Lindsay BD, Natale A, Wazni OM. Plasma B‐type natriuretic peptide levels and recurrent arrhythmia after successful ablation of lone atrial fibrillation. Circulation. 2011;123:2077–2082. [DOI] [PubMed] [Google Scholar]
- 44. Choi EY, Bahrami H, Wu CO, Greenland P, Cushman M, Daniels LB, Almeida AL, Yoneyama K, Opdahl A, Jain A, Criqui MH, Siscovick D, Darwin C, Maisel A, Bluemke DA, Lima JA. N‐terminal pro‐B‐type natriuretic peptide, left ventricular mass, and incident heart failure: Multi‐Ethnic Study of Atherosclerosis. Circ Heart Fail. 2012;5:727–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ellinor PT, Low AF, Patton KK, Shea MA, Macrae CA. Discordant atrial natriuretic peptide and brain natriuretic peptide levels in lone atrial fibrillation. J Am Coll Cardiol. 2005;45:82–86. [DOI] [PubMed] [Google Scholar]
- 46. Silvet H, Young‐Xu Y, Walleigh D, Ravid S. Brain natriuretic peptide is elevated in outpatients with atrial fibrillation. Am J Cardiol. 2003;92:1124–1127. [DOI] [PubMed] [Google Scholar]
- 47. Therkelsen SK, Groenning BA, Kjaer A, Svendsen JH, Boje Jensen G. ANP and BNP in atrial fibrillation before and after cardioversion—and their relationship to cardiac volume and function. Int J Cardiol. 2008;127:396–399. [DOI] [PubMed] [Google Scholar]
- 48. Tsuchida K, Tanabe K. Influence of paroxysmal atrial fibrillation attack on brain natriuretic peptide secretion. J Cardiol. 2004;44:1–11. [PubMed] [Google Scholar]
- 49. Wozakowska‐Kaplon B. Effect of sinus rhythm restoration on plasma brain natriuretic peptide in patients with atrial fibrillation. Am J Cardiol. 2004;93:1555–1558. [DOI] [PubMed] [Google Scholar]
- 50. Yamada T, Murakami Y, Okada T, Okamoto M, Shimizu T, Toyama J, Yoshida Y, Tsuboi N, Ito T, Muto M, Kondo T, Inden Y, Hirai M, Murohara T. Plasma atrial natriuretic peptide and brain natriuretic peptide levels after radiofrequency catheter ablation of atrial fibrillation. Am J Cardiol. 2006;97:1741–1744. [DOI] [PubMed] [Google Scholar]
- 51. Arima M, Kanoh T, Kawano Y, Oigawa T, Yamagami S, Matsuda S. Plasma levels of brain natriuretic peptide increase in patients with idiopathic bilateral atrial dilatation. Cardiology. 2002;97:12–17. [DOI] [PubMed] [Google Scholar]
- 52. Cao H, Xue L, Wu Y, Ma H, Chen L, Wang X, Zhu Q, Dai N, Chen Y. Natriuretic peptides and right atrial fibrosis in patients with paroxysmal versus persistent atrial fibrillation. Peptides. 2010;31:1531–1539. [DOI] [PubMed] [Google Scholar]
- 53. Crozier I, Richards AM, Foy SG, Ikram H. Electrophysiological effects of atrial natriuretic peptide on the cardiac conduction system in man. Pacing Clin Electrophysiol. 1993;16:738–742. [DOI] [PubMed] [Google Scholar]
- 54. Moe GK. On the multiple wavelet hypothesis of atrial fibrillation. Arch Int Pharmacodyn Ther. 1962;140:183–188. [Google Scholar]
- 55. Frustaci A, Chimenti C, Bellocci F, Morgante E, Russo MA, Maseri A. Histological substrate of atrial biopsies in patients with lone atrial fibrillation. Circulation. 1997;96:1180–1184. [DOI] [PubMed] [Google Scholar]
- 56. Verheule S, Sato T, Everett T IV, Engle SK, Otten D, Rubart‐von der Lohe M, Nakajima HO, Nakajima H, Field LJ, Olgin JE. Increased vulnerability to atrial fibrillation in transgenic mice with selective atrial fibrosis caused by overexpression of TGF‐beta1. Circ Res. 2004;94:1458–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bibbins‐Domingo K, Pletcher MJ, Lin F, Vittinghoff E, Gardin JM, Arynchyn A, Lewis CE, Williams OD, Hulley SB. Racial differences in incident heart failure among young adults. N Engl J Med. 2009;360:1179–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Diagnostic Criteria for Baseline Cardiac Risk Factors at Study Entry in the Cardiovascular Health Study and Atherosclerosis Risk in Communities Cohort
Table S2. Results of Cox Proportional Hazards Model With White vs Black Race as a Predictor of Incident Heart Failure, Adjusting for Individual Covariates in the Cardiovascular Health Study
Table S3. Results of Cox Proportional Hazards Model With White vs Black Race as a Predictor of Incident Heart Failure, Adjusting for Individual Covariates in the Atherosclerosis Risk in Communities Study


