Abstract
Background
The JROAD‐DPC (Japanese Registry of All Cardiac and Vascular Diseases Diagnosis Procedure Combination) is a nationwide claims database comprised of the Japanese DPC/Per Diem Payment System. This study aimed to investigate the relationship between prescription rates of guideline‐directed medications in each hospital and in‐hospital mortality among patients with acute myocardial infarction.
Methods and Results
A total of 61 838 Japanese patients from 741 hospitals with acute myocardial infarction between 2012 and 2013 were enrolled. The relationship between prescription rates of 4 guideline‐directed medications for acute myocardial infarction and in‐hospital mortality was analyzed. There were variations in the prescription ratio of β‐blockers on admission (median prescription rate 23% [interquartile range 11% to 38%]) and at discharge (51% [36% to 63%]), and of angiotensin converting enzyme/receptor blocker (60% [47% to 70%]). The highest prescription rate quartile of each medication was associated with a significantly lower mortality compared with the lowest prescription rate quartile (aspirin on admission, incidence rate ratio 0.67 [95% CI 0.61‐0.74], P<0.001; aspirin at discharge, incidence rate ratio 0.50 [95% CI 0.46‐0.55], P<0.001; β‐blocker on admission, 0.83 [0.76‐0.92], P<0.001; β‐blocker at discharge, 0.78 [0.71‐0.85], P<0.001; angiotensin converting enzyme/receptor blocker, 0.68 [0.62‐0.75], P<0.001; statin, 0.63 [0.57‐0.70], P<0.001). The composite prescription score was inversely associated with in‐hospital mortality (β coefficient=−0.48, P<0.001) and was closer to the plateau in the high‐score range (median mortality for composite prescription scores of 6, 15, and 24 were 10.6%, 6.8%, and 4.6%, respectively).
Conclusions
The prescription rates of guideline‐directed medications for treatment of Japanese acute myocardial infarction patients were inversely associated with in‐hospital mortality.
Keywords: acute myocardial infarction, medication, quality indicators
Subject Categories: Quality and Outcomes
Clinical Perspective
What Is New?
Prescription rates of guideline‐recommended medications during hospitalization are related to in‐hospital prognosis during the course of care for Japanese patients with acute myocardial infarction.
Composite prescription scores inversely correlated with in‐hospital mortality rates; as the composite prescription score increases, it appears to be closer to the plateau in the high‐score area.
What Are the Clinical Implications?
Quality indicators related to the prescription rates of guideline‐recommended drugs for acute myocardial infarction may be useful even in countries with performance rates of percutaneous coronary intervention as high as in Japan.
Our result suggests that improvement of prescription rates for guideline‐directed medications may improve the prognosis with acute myocardial infarction patients, especially in hospitals with low prescription rates.
Introduction
Despite the implementation of aggressive medical management and early reperfusion, acute myocardial infarction (AMI) remains the leading cause of death in the world and in Japan.1, 2, 3 Studies have demonstrated that outcomes of AMI can be improved with appropriate treatments that have been summarized into guidelines and performance standards as quality indicators.4, 5, 6, 7, 8, 9 However, it is known that the prognosis for AMI varies greatly among regions and hospitals. Under these circumstances, assessments of the process of care play an important role in management of AMI and are targets of hospital quality improvement initiatives (eg, the American Heart Association's GWTG [Get With The Guidelines] program).8, 9, 10 The GWTG program has improved the quality of AMI care with important implications in the United States and the United Kingdom.11 However, different healthcare expenditures could influence the compliance with GWTG standards in countries other than the United States.
The JROAD‐DPC (Japanese Registry of All Cardiac and Vascular Diseases Diagnosis Procedure Combination), launched by the Japanese Circulation Society, is a nationwide claim database using data from the Japanese DPC/Per Diem Payment System.12, 13 Data from DPC/Per Diem Payment System list the lump sum medical expenses evaluated based on diagnostic and procedural costs beginning in 2002. In this study we investigated whether there are relationships between the prescription rate of guideline‐directed medication in each hospital and in‐hospital mortality among patients with AMI, especially with regard to aspirin, β‐blockers, angiotensin‐converting enzyme inhibitor/angiotensin receptor blockers (ACEI/ARB), and statins using data from 2012 to 2013 in the nationwide JROAD‐DPC database. Association of a composite prescription score (CPS) and in‐hospital mortality was also investigated.
Methods
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
The JROAD‐DPC database was created by combining JROAD data derived from a Japanese Circulation Society national survey to assess the clinical activity of each Japanese institution with cardiovascular beds and to provide adequate feedback to teaching hospitals for improving the patient‐care database14 and the DPC, which is a mixed‐case patient classification system launched in 2002 by the Ministry of Health, Labor, and Welfare of Japan.15 The DPC database contains patient demographics and several disease‐specific data for each patient. An attending physician is responsible for clinical data entry for each patient. Drugs and procedures are recorded based on receipt data of medical care.16
The JROAD‐DPC database includes 1 422 703 health records from 794 certificated hospitals that were collected in 2012 and 2013. These data include 61 838 patients with AMI (from 710 hospitals). We examined the prescription ratio of guideline‐directed drugs for each hospital. Guideline‐directed medications for AMI include aspirin on admission and discharge, β‐blocker on admission and discharge, ACEI/ARB at discharge, and statin at discharge. Prescription on admission was defined as prescription by the end of the second hospital day. We categorized hospitals into quartiles based on the prescription rate of each drug (first quartile [Q1], second quartile [Q2], third quartile [Q3], and fourth quartile [Q4]) and investigated the relationships between the quartiles of the prescription rates and mortality rates. For each of the 4 guideline‐directed medications for AMI, a CPS was created by giving points ranging from 1 to 4 from the lower quartile of the prescription rates, with the scores ranging from 6 to 24 points. We investigated the association between CPS and mortality at each hospital and the relationship of CPS with hospital‐level variation.
Ethics Statement
This research plan was designed by the authors and approved by the Institutional Review Board of the National Cerebral and Cardiovascular Center, which waived the requirement for individual informed consent by the “opt‐out” principle. Each hospital anonymized each patient's identification using code change equations made by each hospital in the original DPC data, which were sent to the Ministry of Health, Labor, and Welfare. Patients were notified through the hospital homepage and by posters in each hospital that their information was collected for this study. Patients could opt out from having their information included in the database.
Statistical Analyses
Continuous variables are presented as mean±SD for normally distributed variables; they were compared using the t test. Nonnormally distributed variables are presented as medians (interquartile range [IQR]). They were compared using the Mann‐Whitney U test. Categorical baseline variables were compared using the Fisher exact test or the χ2 test as appropriate. Analysis of variance was used to compare means across multiple groups. Trends among multiple groups were analyzed by the Cochran‐Armitage test. We used the Poisson model to determine the association between each quartile group and in‐hospital mortality. For the adjustment of institutional background variation, we also developed mixed Poisson regression models with each institute being considered a random intercept. We also adjusted for the number of cardiologists, number of hospital beds, and number of patients with AMI in each institute added to the last model. The association between CPS and mortality was analyzed using linear regression. All P<0.05 were considered statistically significant. The analyses were performed using SAS 9.4 (SAS Institute, Cary, NC) and STATA 15 (Stata Corp, College Station, TX).
Results
Baseline Characteristics of AMI Patients
Among 73 436 patients with AMI hospitalized in 827 hospitals between 2012 and 2013, we excluded subjects under the age of 18 years (n=17), those who had died within 24 hours after admission (n=2848), and those without Killip class or prescription information (n=8408). We also excluded hospitals with <5 AMI hospitalizations annually. This resulted in a total of 61 838 patients in 710 hospitals who were included in the present analysis (Figure S1).
Table 1 and Tables S1 through S6 summarize patients’ characteristics on the basis of individual and hospital data. In terms of individual data, mean age was 68.8 years, and 26.2% of patients were women. A total of 64.0% of patients had hypertension, 29.6% had diabetes mellitus, and 2.8% were on hemodialysis. Overall, 11.4% of patients had a Killip classification of 4. Following hospitalization, 85.7% of patients received percutaneous coronary intervention (PCI), 2.6% had coronary arterial bypass grafting, and 0.9% had fibrinolysis treatment; 52.2% participated in cardiac rehabilitation during hospitalization. Importantly, 3917 patients (6.3%) reached the primary end point. According to hospital characteristics, the participation rate for cardiac rehabilitation was widely distributed (IQR 12.5% to 80.6%) (Table 1).
Table 1.
Patient Characteristics
| Patient Base (n=61 838) | Hospital Variation Mean (SD) or Median (IQR) (n=741) | |
|---|---|---|
| Age, y, mean (SD) | 68.84 (13.03) | 69.50 (3.29) |
| Female | 16 197 (26.19) | 26.47 (22.33‐31.03) |
| Body mass index, kg/m2, mean (SD) | 23.10 (3.92) | 23.62 (0.91) |
| Hypertension | 39 625 (64.08) | 62.50 (50.51‐71.55) |
| Diabetes mellitus | 18 307 (29.60) | 28.75 (21.88‐34.94) |
| Smoker (current and ex‐smoker) | 36 684 (59.32) | 57.50 (47.22‐66.67) |
| Hemodialysis | 1759 (2.84) | 1.90 (0‐3.88) |
| Charlson Score, mean (SD) | 2.09 (1.07) | 2.09 (0.34) |
| Killip Class | ||
| I | 30 593 (49.47) | 50.00 (33.33‐62.50) |
| II | 18 701 (30.24) | 30.00 (19.74‐41.38) |
| III | 5524 (8.93) | 7.45 (4.08‐12.11) |
| IV | 7020 (11.35) | 9.88 (5.43‐14.89) |
| CAG | 57 870 (93.58) | 94.44 (89.36‐96.98) |
| PCI | 52 996 (85.70) | 85.94 (78.57‐90.74) |
| CABG | 1634 (2.64) | 0 (0‐3.09) |
| IABP | 9532 (15.41) | 11.76 (6.58‐18.92) |
| PCPS | 1163 (1.88) | 0.66 (0‐2.38) |
| Fibrinolysis | 523 (0.85) | 0 (0‐0.52) |
| Cardiac rehabilitation | 32 305 (52.24) | 48.57 (12.5‐80.58) |
Values presented are given as numbers (percentage) unless stated. CABG indicates coronary arterial bypass grafting; CAG, coronary angiography; IABP, intra‐aortic balloon pump; IQR, interquartile range; PCI, percutaneous coronary intervention; PCPS, percutaneous cardiopulmonary support.
Prescription Rate of Guideline‐Directed Medications
The prescription rate of aspirin was 87% (IQR 82% to 92%) at admission and 80% (IQR 75% to 85%) at discharge, with a small amount of hospital‐level variation (Figure 1A and 1C). In contrast, there were wide variations in the prescription rates of β‐blockers at admission (23%, IQR 11% to 38%) and at discharge (51%, IQR 36% to 63%) (Figure 1B and 1D). The prescription rate of ACEI/ARBs was 52.0% (IQR 40.3% to 62.3%) at discharge (Figure 1E), and that of statins was 80% (IQR 75% to 85%) at discharge (Figure 1F).
Figure 1.
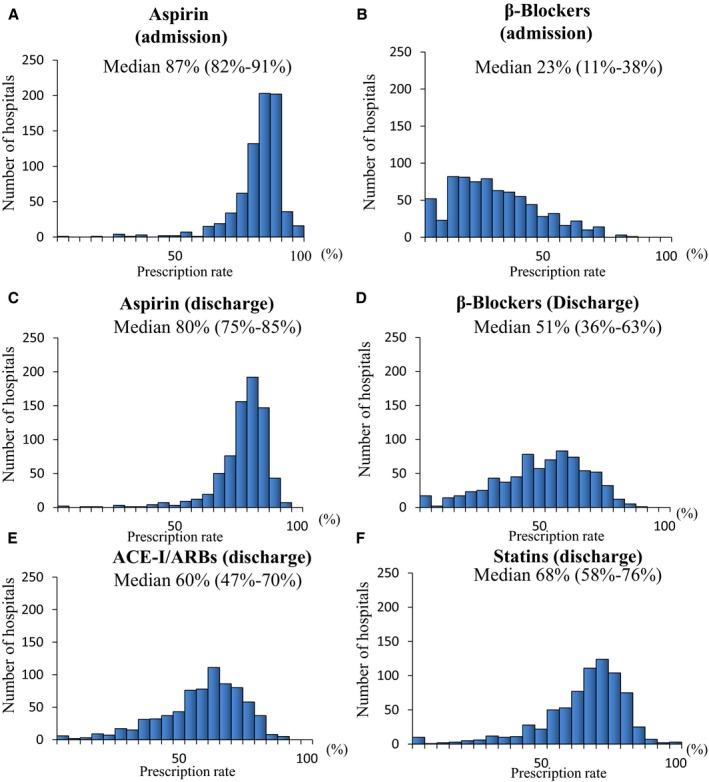
Distribution of prescription rates for aspirin (A) and β‐blockers (B) on admission, and aspirin (C), β‐blockers (D), ACEI/ARBs (E), and statins (F) at discharge among 741 hospitals in patients with AMI. ACEI indicates angiotensin‐converting enzyme inhibitor; AMI, acute myocardial infarction; ARB, angiotensin receptor blocker.
Correlation of Prescription Rate of Guideline‐Directed Drugs
Table S7 shows the correlation of each medication in 15 patterns overall; 7 of 15 combinations showed a weak correlation (Spearman correlation coefficient <0.3), and 6 were a combination of admission and discharge medications. Eight showed a moderate correlation (0.3‐0.7), and 6 were combinations of medications at discharge.
Relationships of Prescription Rates of Guideline‐Directed Medications and In‐Hospital Mortality
We categorized hospitals into quartiles according to the prescription rate of guideline‐directed medications for the following 4 regimens: aspirin at admission and discharge, β‐blockers at admission and discharge, ACEI/ARBs, and statins at discharge. Figure 2 shows that the in‐hospital mortality decreased from the lower to upper quartiles. The regression analysis indicated an inverse trend for the risk of death across quartiles (Table 2; all P‐values for the trend of in‐hospital mortality <0.001). The highest prescription rate quartile for each medication was associated with a significantly lower mortality than the lowest prescription rate quartile. For example, aspirin on admission and those at discharge were associated with 33% and 50% decrease of mortality (aspirin on admission, incidence rate ratio 0.67 [95% CI 0.61‐0.74], P<0.001; aspirin at discharge, incidence rate ratio 0.50 [95% CI 0.46‐0.55], P<0.001; β‐blocker on admission 0.83 [0.76‐0.92], P<0.001; β‐blocker at discharge 0.78 [0.71‐0.85], P<0.001; ACE/ARB 0.68 [0.62‐0.75], P<0.001; statin 0.63 [0.57‐0.70], P<0.001). After adjustment for age, sex, Charlson score, and Killip class and comparison with the Q1 group as a reference, a higher quantile was associated with a lower mortality rate. Further, to adjust for the differences among hospitals, we have examined using a Poisson mixed model and other hospital characteristics (hospital bed number, number of patients with AMI, and number of cardiologists) (Table S8).
Figure 2.
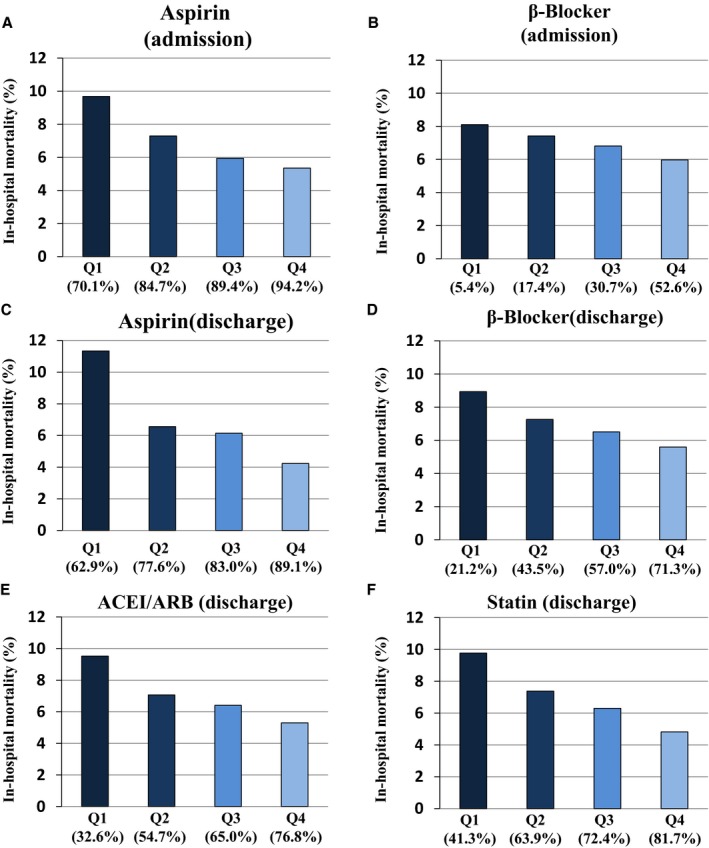
Relationship between in‐hospital mortality and prescription rates (quartiles) for aspirin (A) and β‐blockers (B) on admission, and aspirin (C), β‐blockers (D), ACEI/ARBs (E), and statins (F) at discharge among 741 hospitals in patients with AMI. ACEI indicates angiotensin‐converting enzyme inhibitor; AMI, acute myocardial infarction; ARB, angiotensin receptor blocker; Q1‐Q4, quartiles based on prescription rates.
Table 2.
Incidence Rate Ratio for In‐Hospital Mortality According to the Quartile of Each Guideline‐Directed Medication
| Q1 | Q2 | Q3 | Q4 | P for Trend | ||||
|---|---|---|---|---|---|---|---|---|
| IRR | IRR (95% CI) | P Value | IRR (95% CI) | P Value | IRR (95% CI) | P Value | ||
| Univariate analysis | ||||||||
| Aspirin (admission) | 1.00 | 0.83 (0.75‐0.91) | <0.001 | 0.70 (0.64‐0.77) | <0.001 | 0.67 (0.61‐0.74) | <0.001 | <0.001 |
| β‐Blocker (admission) | 1.00 | 0.98 (0.90‐1.08) | 0.699 | 0.90 (0.82‐0.99) | 0.035 | 0.83 (0.76‐0.92) | <0.001 | <0.001 |
| Aspirin (discharge) | 1.00 | 0.73 (0.67‐0.80) | <0.001 | 0.66 (0.61‐0.72) | <0.001 | 0.50 (0.46‐0.55) | <0.001 | <0.001 |
| β‐Blocker (discharge) | 1.00 | 0.88 (0.80‐0.97) | 0.009 | 0.79 (0.72‐0.87) | <0.001 | 0.78 (0.71‐0.85) | <0.001 | <0.001 |
| ACEI/ARB (discharge) | 1.00 | 0.86 (0.78, 0.94) | 0.001 | 0.78 (0.71‐0.86) | <0.001 | 0.68 (0.62‐0.75) | <0.001 | <0.001 |
| Statin (discharge) | 1.00 | 0.83 (0.76‐0.92) | <0.001 | 0.73 (0.66‐0.80) | <0.001 | 0.63 (0.57‐0.70) | <0.001 | <0.001 |
| Multivariate analysis* | ||||||||
| Aspirin (admission) | 1.00 | 0.88 (0.80‐0.97) | 0.008 | 0.76 (0.69‐0.84) | <0.001 | 0.74 (0.67‐0.82) | <0.001 | <0.001 |
| β‐Blocker (admission) | 1.00 | 1.00 (0.92‐1.10) | 0.960 | 0.93 (0.85‐1.02) | 0.139 | 0.86 (0.78‐0.95) | 0.002 | <0.001 |
| Aspirin (discharge) | 1.00 | 0.77 (0.70‐0.84) | <0.001 | 0.71 (0.65‐0.78) | <0.001 | 0.54 (0.49‐0.60) | <0.001 | <0.001 |
| β‐Blocker (discharge) | 1.00 | 0.89 (0.81‐0.97) | 0.011 | 0.82 (0.75‐0.90) | <0.001 | 0.82 (0.74‐0.90) | <0.001 | <0.001 |
| ACEI/ARB (discharge) | 1.00 | 0.89 (0.80‐0.98) | 0.014 | 0.82 (0.74‐0.91) | <0.001 | 0.74 (0.67‐0.81) | <0.001 | <0.001 |
| Statin (discharge) | 1.00 | 0.88 (0.80‐0.96) | 0.007 | 0.79 (0.72‐0.87) | <0.001 | 0.68 (0.62‐0.76) | <0.001 | <0.001 |
P<0.05 is statistically significant. ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; IRR, incidence rate ratio; Q1‐Q4, quartiles based on prescription rates.
Adjusted for age, sex, Charlson comorbidity index, and Killip class.
Relationship of CPS and Outcome
The CPS for the 710 hospitals was widely distributed with a median score of 15, and CPS was inversely associated with in‐hospital mortality (Figure 3). The mortality rate declined as the score increased in the low‐score range. However, the score increased gradually and approached a plateau in the high‐score area (median mortality for CPS scores of 6, 15, and 24 were 10.6%, 6.8%, and 4.6%, respectively).
Figure 3.
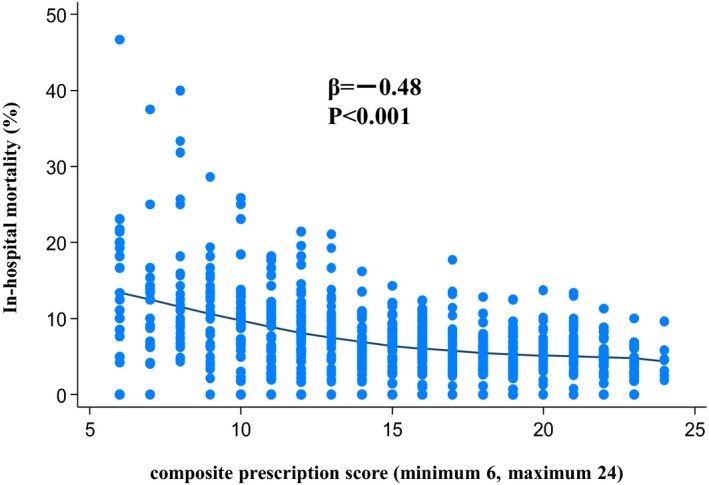
Association of composite prescription score of guideline‐directed medications and in‐hospital mortality in patients with AMI. AMI indicates acute myocardial infarction.
CPS and Hospital Characteristics
We examined the relationship between the highest CPS quartile and the factors representing hospital characteristics (Table 3 and Table S9). The number of hospital beds and the AMI case volume were positively correlated with CPS, as was the presence of a cardiac surgery division. However, the number of cardiologists per number of AMIs was not significantly correlated with CPS.
Table 3.
Relationship of the Highest Quartile of CPS and Hospital Characteristics
| Hospital Variables | OR | 95% CI | P Value |
|---|---|---|---|
| Hospital bed number per 10 beds | 1.02 | 1.01 to 1.03 | <0.001 |
| Number of patients with AMI per 10 | 1.12 | 1.10 to 1.15 | <0.001 |
| Cardiac surgery division | 1.64 | 1.23 to 2.17 | <0.001 |
| Number of cardiologists per number of AMIs | |||
| First quartile | Ref | Ref | |
| Second quartile | 1.10 | 0.77 to 1.57 | 0.6 |
| Third quartile | 0.91 | 0.63 to 1.32 | 0.62 |
| Fourth quartile | 0.71 | 0.48 to 1.04 | 0.075 |
P<0.05 is statistically significant. AMI indicates acute myocardial infarction; CPS, composite prescription score; OR, odds ratio.
Discussion
The major findings of the present study using a nationwide claim database containing more than 60 000 cases and over 700 hospitals between 2012 and 2013 are as follows: (1) there were wide variations in the prescription rates of guideline‐directed medications, (2) the prescription rates of these medications were inversely associated with in‐hospital mortality even after adjustment for variables, (3) the relationship between CPS and mortality appears to be closer to the plateau in the high‐score area, and (4) a high CPS score was associated with hospital performance (eg, the number of hospital beds, the AMI case volume, or involvement of a certified cardiologist).
Comparison of Guideline‐Directed Medication Prescription Rate With Studies in Other Countries
Among the guideline‐directed drugs, the prescription rate for aspirin on admission and discharge was high (87% and 80%, respectively). In contrast, the prescription rates for β‐blockers at admission and discharge and for ACEI/ARBs at discharge were low. Compared with the US study of the National Registry of Myocardial Infarction, the prescription rate for aspirin at admission was comparable between Japan (87%) and the United States (86%), whereas the prescription rate for β‐blockers at admission was lower in Japan than in the United States (23% versus 78%).17 The prescription rate for aspirin at discharge was comparable across different countries: United States 80%, the United Kingdom 98.1%, and Sweden (any antiplatelet therapy) 94.6%.17, 18, 19 However, the prescription rate of β‐blockers at discharge was lower in our study (51%) compared with the United States (75.8%), the United Kingdom (95.6%), and Sweden (88.7%).17, 18, 19 It was comparable to that in other Japanese registries (PACIFIC 49.5%, Credo Kyoto 43.7%, and Tokyo CCU 38.8%).20, 21, 22 In Japan the prescription rate for β‐blockers was relatively low in both PCI‐capable high‐quality hospitals and in the nationwide claims database. The prescription rate for ACEI/ARBs at discharge was also low in our population (52%) compared with that in other countries (United States 70.7%, the United Kingdom 93.9%, and Sweden, 56.2%).17, 18, 19 The prescription rate for statins was as follows: Japan 80%, the United Kingdom 96.5%, and Sweden 79.7%.18, 19 These findings indicate that the overall prescription rate for guideline‐recommended drugs, except for aspirin, was relatively low in Japan. It should be noted that the rate of PCI in Japan (85.7%) was higher than that of other nations: the United Kingdom 65.6% (2012‐2013) and the United States 64% (1994‐2006). The high rate of PCI may affect the medication strategy. In the 2013 ST‐elevation MI guidelines of the Japanese Circulation Society, the recommendation for β‐blocker use for low‐risk AMI (successful reperfusion, no left ventricular dysfunction, no severe arrhythmia) has been changed from Class 1 to Class 2A. Further investigation is needed for causes of relatively low prescription rate and secular change of the rate.
Previous studies in other countries have revealed that there is often little or no correlation between each component of the quality indicators or PMs (performance measures).17, 18, 23 In our study all 15 prescription combinations demonstrate significant correlations. However, the relationship remained weak, especially between drugs prescribed on admission and at discharge. These data support the concept that a broad range of process metrics is needed to fully characterize hospital‐level care practices.17, 18, 23, 24
Prescription Rate and Short‐Term Mortality
To date, several reports have been published addressing whether prescription rates of guideline‐recommended medications are related to prognosis.17, 18, 23 A report from the United States revealed that quality indicators or PMs (performance measures), including prescription rates of guideline‐recommended drugs, are associated with short‐term mortality rates in patients with AMI and acute coronary syndrome.17, 23, 25 A recent study from the United Kingdom also showed that quality indicators, including prescription rates, are associated with mortality rates.18 In the present study we showed that the prescription rate of 4 medications and the CPS were associated negatively with in‐hospital mortality in patients with AMI. These findings show that even in a country in which the performance rate of PCI is as high as it is in Japan, the prescription rate of guideline‐recommended drugs during hospitalization is related to short‐term prognosis. A comparison study between Sweden and the United Kingdom26 showed that the potential for death prevention by optimal medical therapy was similar to or greater than that by reperfusion therapy. Especially in Japan, a relatively low prescription rate and large interhospital variation showed that there is much potential for improvement in short‐term mortality by increasing the rate of guideline‐directed medication prescriptions. Furthermore, according to the relationship of CPS and mortality rate, as the CPS increases, it appears to be closer to the plateau in the high‐score area. This result suggests that if prescription rates for guideline‐directed medications rise in hospitals with low prescription rates, the prognosis for patients with AMI may improve. To improve guideline‐directed medications in low‐CPS hospitals, intervention using a simple toolkit such as the ACS QUIK (Acute Coronary Syndrome Quality Improvement in Kerala) in India may be useful.27
Association of Guideline Adherence With Hospital Features
It has been revealed that hospital features are related to the guideline adherence of each hospital.17, 23 In the present analysis the highest quartile CPS was associated with the hospital case volume of AMIs, the number of hospital beds, and the parallel establishment of cardiovascular surgery. In contrast, there was no significant relationship between the prescription rates and the number of cardiovascular physicians per number of AMIs. These results suggest that factors of hospital performance are also related in part to prescription rates. Indeed, a previous study investigated multiple factors that included hospital structure and other factors contributing to the variation in mortality rates among hospitals beyond variations in hospital treatment.25, 28, 29, 30, 31, 32 Another study showed that hospitals with high performance were characterized by well‐organized features to improve AMI care across all departments.33 It is also possible that the low prescription rate not only contributes to the prognosis but also represents the difficulty in implementing high‐quality care for AMI. Because the severity of the patient's condition and comorbidities, difference in hospital levels, and regional factors including prehospital care may be involved, these factors that create a variation in prescription rates need to be improved.
Limitations
Our study has limitations. First, although DPC data must be confirmed by a doctor and are highly reliable, some of the data are based on medical claims. Therefore, there is a possibility that these data may contain certain errors, and some data may be underestimated (such as comorbidities) because they are not captured in claims. Second, although this research was conducted using nationwide databases and very closely represents the current situation in Japan, there is a possibility that actual conditions in nonspecialized facilities and small clinics are not reflected. Third, because this database does not contain data from before arrival at the hospital, there is a possibility that patients who die before hospital arrival may be missing. For the same reason, factors affecting the prognosis, such as time from onset to hospital arrival, cannot be included in the analysis. Fourth, missing data or unknown confounding factors may affect the analysis results. Fifth, although we have demonstrated that the prescription rate was inversely associated with in‐hospital mortality (Figure 2 and Table 2) and may reflect general activity in each hospital, further evaluation of quality as an indicator and variance of quality across institutes and settings is needed.
Conclusions
There were wide variations in the prescription rates of guideline‐directed medications for the treatment of Japanese AMI patients, and these rates were inversely associated with in‐hospital mortality. Therefore, there may be a necessity for interventions to improve short‐term mortality in hospitals with low prescription rates.
Sources of Funding
The present work was supported in part by Health and Labor Sciences Research Grants from the Ministry of Health, Labor, and Welfare of Japan (H29‐Junkanki‐Ippan‐001) (Yasuda), (H29‐31‐Junkanki‐Ippan‐005) (Yasuda), and a grant from Japan Agency for Medical Research and Development (16ek02 10018h 00 03) and was supported by the Japanese Circulation Society. This work was also supported by JSPS KAKENHI grant number 17K09548 (K. Nakao). The funders had no role in the design and conduct of the study; in the collection, management, analysis, or interpretation of the data; in the preparation, review, or approval of the manuscript; or in the decision to submit this article for publication.
Disclosures
Dr Yasuda reports grants and personal fees from Takeda, grants and personal fees from Daiichi‐Sankyo, personal fees from Bristol‐Myers, grants and personal fees from Bristol‐Myers, and grants from Abbott unrelated to the submitted work. Dr Tsutsui reports grants from MSD, Daiichi‐Sankyo, Tanabe‐Mitsubishi, Teijin, Japan Tabaco, Nippon Boehringer, Actellion, and personal fees from MSD, Otsuka, Takeda, Tanabe‐Mitsubishi, Daiichi‐Sankyo, Nippon Boehringer, Novartis, Bayer, and Pfizer unrelated to the submitted work. Dr Saito has reported receiving personal fees from Mitsubishi Tanabe Pharma Corporation, Otsuka Pharmaceutical Co Ltd, Daiichi Sankyo Co Ltd, Novartis Pharma KK, Pfizer Japan Inc, and Nippon Boehringer Ingelheim Co Ltd. Dr Saito has received funding support from Mitsubishi Tanabe Pharma Corporation, Otsuka Pharmaceutical Co Ltd, Daiichi Sankyo Co Ltd, Novartis Pharma KK, Pfizer Japan Inc, Nippon Boehringer Ingelheim Co Ltd, Ono Pharmatical Co Ltd, St. Jude Medical Japan Co, Ltd, Bayer Holding Ltd, Terumo Corporation, Kyowa Hakko Kirin Co Ltd, Dainippon Sumitomo Pharma Co Ltd, Astellas Pharma Inc, Takeda Pharmaceutical Co Ltd, Teijin Pharma Ltd, Shionogi & Co Ltd, Kowa Pharmaceutical Co Ltd, and Actelion Pharmaceuticals Japan Ltd and also has a department endowed by MSD KK, all unrelated to the submitted work. Dr Komuro reports grants and personal fees from Takeda Pharmaceutical Company Limited, personal fees from Nippon Boehringer Ingelheim Co, Ltd, personal fees from MSD KK, grants from Astellas Pharma Inc, grants from Edwards Lifesciences Ltd, grants and personal fees from Mitsubishi Tanabe Pharma Corporation, personal fees from Actelion Pharmaceuticals Japan Ltd, grants and personal fees from Daiichi‐Sankyo, personal fees from Amgen Astellas BioPharma KK, grants from Otsuka Pharmaceutical Co Ltd, Kowa company Ltd, grants from Dainippon Sumitomo Pharma Co Ltd, grants from Teijin Pharma Ltd, grants from Toa Eiyo Ltd, grants from Nipro Corporation, grants from Terumo Corporation, and grants from Ono Pharmaceutical Co Ltd, unrelated to the submitted work.
Supporting information
Table S1. Baseline Hospital Characteristics Per Quantiles by Prescription Rate of Aspirin on Admission
Table S2. Baseline Hospital Characteristics Per Quantiles by Prescription Rate of β‐Blocker on Admission
Table S3. Baseline Hospital Characteristics Per Quantiles by Prescription Rate of Aspirin on Discharge
Table S4. Baseline Hospital Characteristics Per Quantiles by Prescription Rate of β‐Blocker on Discharge
Table S5. Baseline Hospital Characteristics Per Quantiles by Prescription Rate of ACEI/ARB on Discharge
Table S6. Baseline Hospital Characteristics Per Quantiles by Prescription Rate of Statin on Discharge
Table S7. Correlation of Hospital Prescription Rate of Guideline‐Directed Medications
Table S8. Incidence Rate Ratio for In‐Hospital Mortality According to the Quantile of Each Guideline‐Directed Medication by Mixed Poisson Regression Model
Table S9. Hospital Characteristics According to Composite Prescription Score
Figure S1. Study flow chart.
(J Am Heart Assoc. 2019;8:e009692 DOI: 10.1161/JAHA.118.009692.)
References
- 1. Newton JN, Briggs AD, Murray CJ, Dicker D, Foreman KJ, Wang H, Naghavi M, Forouzanfar MH, Ohno SL, Barber RM, Vos T, Stanaway JD, Schmidt JC, Hughes AJ, Fay DF, Ecob R, Gresser C, McKee M, Rutter H, Abubakar I, Ali R, Anderson HR, Banerjee A, Bennett DA, Bernabé E, Bhui KS, Biryukov SM, Bourne RR, Brayne CE, Bruce NG, Brugha TS, Burch M, Capewell S, Casey D, Chowdhury R, Coates MM, Cooper C, Critchley JA, Dargan PI, Dherani MK, Elliott P, Ezzati M, Fenton KA, Fraser MS, Fürst T, Greaves F, Green MA, Gunnell DJ, Hannigan BM, Hay RJ, Hay SI, Hemingway H, Larson HJ, Looker KJ, Lunevicius R, Lyons RA, Marcenes W, Mason‐Jones AJ, Matthews FE, Moller H, Murdoch ME, Newton CR, Pearce N, Piel FB, Pope D, Rahimi K, Rodriguez A, Scarborough P, Schumacher AE, Shiue I, Smeeth L, Tedstone A, Valabhji J, Williams HC, Wolfe CD, Woolf AD, Davis AC. Changes in health in England, with analysis by English regions and areas of deprivation, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:2257–2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ministry of Health, Labour and Welfare . Statistics. 2015;2015.
- 3. Krumholz HM, Wang Y, Chen J, Drye EE, Spertus JA, Ross JS, Curtis JP, Nallamothu BK, Lichtman JH, Havranek EP, Masoudi FA, Radford MJ, Han LF, Rapp MT, Straube BM, Normand SL. Reduction in acute myocardial infarction mortality in the United States: risk‐standardized mortality rates from 1995‐2006. JAMA. 2009;302:767–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE, Chavey WE, Fesmire FM, Hochman JS, Levin TN, Lincoff AM, Peterson ED, Theroux P, Wenger NK, Wright RS, Jneid H, Ettinger SM, Ganiats TG, Philippides GJ, Jacobs AK, Halperin JL, Albert NM, Creager MA, DeMets D, Guyton RA, Kushner FG, Ohman EM, Stevenson W, Yancy CW. 2012 ACCF/AHA focused update incorporated into the ACCF/AHA 2007 guidelines for the management of patients with unstable angina/non‐ST‐elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61:e179–e347. [DOI] [PubMed] [Google Scholar]
- 5. Amsterdam EA, Wenger NK, Brindis RG, Casey DE, Ganiats TG, Holmes DR, Jaffe AS, Jneid H, Kelly RF, Kontos MC, Levine GN, Liebson PR, Mukherjee D, Peterson ED, Sabatine MS, Smalling RW, Zieman SJ; ACC/AHA Task Force Members; Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons . 2014 AHA/ACC guideline for the management of patients with non‐ST‐elevation acute coronary syndromes: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130:2354–2394. [DOI] [PubMed] [Google Scholar]
- 6. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli‐Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimský P; ESC Scientific Document Group . 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST‐segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST‐segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119–177. [DOI] [PubMed] [Google Scholar]
- 7. Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, Bax JJ, Borger MA, Brotons C, Chew DP, Gencer B, Hasenfuss G, Kjeldsen K, Lancellotti P, Landmesser U, Mehilli J, Mukherjee D, Storey RF, Windecker S; ESC Scientific Document Group . 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation: Task Force for the management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2016;37:267–315. [DOI] [PubMed] [Google Scholar]
- 8. Krumholz HM, Anderson JL, Bachelder BL, Fesmire FM, Fihn SD, Foody JM, Ho PM, Kosiborod MN, Masoudi FA, Nallamothu BK; ACC/AHA Task Force on Performance Measures; American Academy of Family Physicians; American College of Emergency Physicians; American Academy of Cardiovascular and Pulmonary Rehabilitation; Society for Cardiovascular Angiography and Interventions; Society of Hospital Medicine . ACC/AHA 2008 performance measures for adults with ST‐elevation and non‐ST‐elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures (Writing Committee to Develop Performance Measures for ST‐Elevation and Non‐ST‐Elevation Myocardial Infarction): developed in collaboration with the American Academy of Family Physicians and the American College of Emergency Physicians: endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation, Society for Cardiovascular Angiography and Interventions, and Society of Hospital Medicine. Circulation. 2008;118:2596–2648. [DOI] [PubMed] [Google Scholar]
- 9. Schiele F, Gale CP, Bonnefoy E, Capuano F, Claeys MJ, Danchin N, Fox KA, Huber K, Iakobishvili Z, Lettino M, Quinn T, Rubini Gimenez M, Bøtker HE, Swahn E, Timmis A, Tubaro M, Vrints C, Walker D, Zahger D, Zeymer U, Bueno H. Quality indicators for acute myocardial infarction: a position paper of the Acute Cardiovascular Care Association. Eur Heart J Acute Cardiovasc Care. 2017;6:34–59. [DOI] [PubMed] [Google Scholar]
- 10. Ellrodt AG, Fonarow GC, Schwamm LH, Albert N, Bhatt DL, Cannon CP, Hernandez AF, Hlatky MA, Luepker RV, Peterson PN, Reeves M, Smith EE. Synthesizing lessons learned from Get With The Guidelines: the value of disease‐based registries in improving quality and outcomes. Circulation. 2013;128:2447–2460. [DOI] [PubMed] [Google Scholar]
- 11. Kumbhani DJ, Fonarow GC, Cannon CP, Hernandez AF, Peterson ED, Peacock WF, Laskey WK, Deedwania P, Grau‐Sepulveda M, Schwamm LH, Bhatt DL; Get With The Guidelines Steering Committee and Investigators . Temporal trends for secondary prevention measures among patients hospitalized with coronary artery disease. Am J Med. 2015;128:426. [DOI] [PubMed] [Google Scholar]
- 12. Yasuda S, Nakao K, Nishimura K, Miyamoto Y, Sumita Y, Shishido T, Anzai T, Tsutsui H, Ito H, Komuro I, Saito Y, Ogawa H; on the behalf of JROAD Investigators . The current status of cardiovascular medicine in Japan—analysis of a large number of health records from a nationwide claim‐based database, JROAD‐DPC. Circ J. 2016;80:2327–2335. [DOI] [PubMed] [Google Scholar]
- 13. Yasuda S, Miyamoto Y, Ogawa H. Current status of cardiovascular medicine in the aging society of Japan. Circulation. 2018;138:965–967. [DOI] [PubMed] [Google Scholar]
- 14. Tomoike H, Yokoyama H, Sumita Y, Hanai S, Kada A, Okamura T, Yoshikawa J, Doi Y, Hori M, Tei C; Scientific Committee of the JCS . Nationwide distribution of cardiovascular practice in Japan—results of Japanese Circulation Society 2010 annual survey. Circ J. 2015;79:1058–1067. [DOI] [PubMed] [Google Scholar]
- 15. Yasunaga H, Ide H, Imamura T, Ohe K. Impact of the Japanese diagnosis procedure combination‐based payment system on cardiovascular medicine‐related costs. Int Heart J. 2005;46:855–866. [DOI] [PubMed] [Google Scholar]
- 16. Wada T, Yasunaga H, Horiguchi H, Matsubara T, Fushimi K, Nakajima S, Yahagi N. Outcomes of argatroban treatment in patients with atherothrombotic stroke: observational nationwide study in Japan. Stroke. 2016;47:471–476. [DOI] [PubMed] [Google Scholar]
- 17. Bradley EH, Herrin J, Elbel B, McNamara RL, Magid DJ, Nallamothu BK, Wang Y, Normand SL, Spertus JA, Krumholz HM. Hospital quality for acute myocardial infarction: correlation among process measures and relationship with short‐term mortality. JAMA. 2006;296:72–78. [DOI] [PubMed] [Google Scholar]
- 18. Bebb O, Hall M, Fox KAA, Dondo TB, Timmis A, Bueno H, Schiele F, Gale CP. Performance of hospitals according to the ESC ACCA quality indicators and 30‐day mortality for acute myocardial infarction: national cohort study using the United Kingdom Myocardial Ischaemia National Audit Project (MINAP) register. Eur Heart J. 2017;38:974–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chung SC, Gedeborg R, Nicholas O, James S, Jeppsson A, Wolfe C, Heuschmann P, Wallentin L, Deanfield J, Timmis A, Jernberg T, Hemingway H. Acute myocardial infarction: a comparison of short‐term survival in national outcome registries in Sweden and the UK. Lancet. 2014;383:1305–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Daida H, Miyauchi K, Ogawa H, Yokoi H, Matsumoto M, Kitakaze M, Kimura T, Matsubara T, Ikari Y, Kimura K, Tsukahara K, Origasa H, Morino Y, Tsutsui H, Kobayashi M, Isshiki T; PACIFIC Investigators . Management and two‐year long‐term clinical outcome of acute coronary syndrome in Japan: prevention of atherothrombotic incidents following ischemic coronary attack (PACIFIC) registry. Circ J. 2013;77:934–943. [DOI] [PubMed] [Google Scholar]
- 21. Bao B, Ozasa N, Morimoto T, Furukawa Y, Nakagawa Y, Kadota K, Iwabuchi M, Shizuta S, Shiomi H, Tada T, Tazaki J, Kato Y, Hayano M, Natsuaki M, Fujiwara H, Mitsudo K, Nobuyoshi M, Kita T, Kimura T. β‐Blocker therapy and cardiovascular outcomes in patients who have undergone percutaneous coronary intervention after ST‐elevation myocardial infarction. Cardiovasc Interv Ther. 2013;28:139–147. [DOI] [PubMed] [Google Scholar]
- 22. Miyachi H, Takagi A, Miyauchi K, Yamasaki M, Tanaka H, Yoshikawa M, Saji M, Suzuki M, Yamamoto T, Shimizu W, Nagao K, Takayama M. Current characteristics and management of ST elevation and non‐ST elevation myocardial infarction in the Tokyo metropolitan area: from the Tokyo CCU network registered cohort. Heart Vessels. 2016;31:1740–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Peterson ED, Roe MT, Mulgund J, DeLong ER, Lytle BL, Brindis RG, Smith SC, Pollack CV, Newby LK, Harrington RA, Gibler WB, Ohman EM. Association between hospital process performance and outcomes among patients with acute coronary syndromes. JAMA. 2006;295:1912–1920. [DOI] [PubMed] [Google Scholar]
- 24. Peterson ED, DeLong ER, Masoudi FA, O'Brien SM, Peterson PN, Rumsfeld JS, Shahian DM, Shaw RE. ACCF/AHA 2010 position statement on composite measures for healthcare performance assessment: a report of American College of Cardiology Foundation/American Heart Association Task Force on Performance Measures (Writing Committee to Develop a Position Statement on Composite Measures). J Am Coll Cardiol. 2010;55:1755–1766. [DOI] [PubMed] [Google Scholar]
- 25. Granger CB, Steg PG, Peterson E, López‐Sendón J, Van de Werf F, Kline‐Rogers E, Allegrone J, Dabbous OH, Klein W, Fox KA, Eagle KA; GRACE Investigators . Medication performance measures and mortality following acute coronary syndromes. Am J Med. 2005;118:858–865. [DOI] [PubMed] [Google Scholar]
- 26. Chung SC, Sundström J, Gale CP, James S, Deanfield J, Wallentin L, Timmis A, Jernberg T, Hemingway H; Swedish Web‐System for Enhancement and Development of Evidence‐Based Care in Heart Disease Evaluated According to Recommended Therapies/Register of Information and Knowledge About Swedish Heart Intensive Care Admissions; National Institute for Cardiovascular Outcomes Research/Myocardial Ischaemia National Audit Project; Cardiovascular Disease Research Using Linked Bespoke Studies and Electronic Health Records . Comparison of hospital variation in acute myocardial infarction care and outcome between Sweden and United Kingdom: population based cohort study using nationwide clinical registries. BMJ. 2015;351:h3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Huffman MD, Mohanan PP, Devarajan R, Baldridge AS, Kondal D, Zhao L, Ali M, Krishnan MN, Natesan S, Gopinath R, Viswanathan S, Stigi J, Joseph J, Chozhakkat S, Lloyd‐Jones DM, Prabhakaran D; Acute Coronary Syndrome Quality Improvement in Kerala (ACS QUIK) Investigators . Effect of a quality improvement intervention on clinical outcomes in patients in India with acute myocardial infarction: the ACS QUIK randomized clinical trial. JAMA. 2018;319:567–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Canto JG, Every NR, Magid DJ, Rogers WJ, Malmgren JA, Frederick PD, French WJ, Tiefenbrunn AJ, Misra VK, Kiefe CI, Barron HV. The volume of primary angioplasty procedures and survival after acute myocardial infarction. National Registry of Myocardial Infarction 2 Investigators. N Engl J Med. 2000;342:1573–1580. [DOI] [PubMed] [Google Scholar]
- 29. Ross JS, Normand SL, Wang Y, Ko DT, Chen J, Drye EE, Keenan PS, Lichtman JH, Bueno H, Schreiner GC, Krumholz HM. Hospital volume and 30‐day mortality for three common medical conditions. N Engl J Med. 2010;362:1110–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ko DT, Krumholz HM, Wang Y, Foody JM, Masoudi FA, Havranek EP, You JJ, Alter DA, Stukel TA, Newman AM, Tu JV. Regional differences in process of care and outcomes for older acute myocardial infarction patients in the United States and Ontario, Canada. Circulation. 2007;115:196–203. [DOI] [PubMed] [Google Scholar]
- 31. Werner RM, Bradlow ET. Relationship between Medicare's hospital compare performance measures and mortality rates. JAMA. 2006;296:2694–2702. [DOI] [PubMed] [Google Scholar]
- 32. McCrum ML, Joynt KE, Orav EJ, Gawande AA, Jha AK. Mortality for publicly reported conditions and overall hospital mortality rates. JAMA Intern Med. 2013;173:1351–1357. [DOI] [PubMed] [Google Scholar]
- 33. Curry LA, Spatz E, Cherlin E, Thompson JW, Berg D, Ting HH, Decker C, Krumholz HM, Bradley EH. What distinguishes top‐performing hospitals in acute myocardial infarction mortality rates? A qualitative study. Ann Intern Med. 2011;154:384–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline Hospital Characteristics Per Quantiles by Prescription Rate of Aspirin on Admission
Table S2. Baseline Hospital Characteristics Per Quantiles by Prescription Rate of β‐Blocker on Admission
Table S3. Baseline Hospital Characteristics Per Quantiles by Prescription Rate of Aspirin on Discharge
Table S4. Baseline Hospital Characteristics Per Quantiles by Prescription Rate of β‐Blocker on Discharge
Table S5. Baseline Hospital Characteristics Per Quantiles by Prescription Rate of ACEI/ARB on Discharge
Table S6. Baseline Hospital Characteristics Per Quantiles by Prescription Rate of Statin on Discharge
Table S7. Correlation of Hospital Prescription Rate of Guideline‐Directed Medications
Table S8. Incidence Rate Ratio for In‐Hospital Mortality According to the Quantile of Each Guideline‐Directed Medication by Mixed Poisson Regression Model
Table S9. Hospital Characteristics According to Composite Prescription Score
Figure S1. Study flow chart.


