Abstract
Background
Obesity is a precursor to heart failure with preserved ejection fraction. Biomarkers that identify preclinical metabolic heart disease (MHD) in young obese patients would help identify high‐risk individuals for heart failure prevention strategies. We assessed the predictive value of GAL3 (galectin–3), FSTL3 (follistatin‐like 3 peptide), and NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) to identify stage B MHD in young obese participants free of clinically evident cardiovascular disease.
Methods and Results
Asymptomatic obese patients (n=250) and non‐obese controls (n=21) underwent echocardiographic cardiac phenotyping. Obese patients were classified as MHD positive (MHD‐POS; n=94) if they had abnormal diastolic function or left ventricular hypertrophy and had estimated pulmonary artery systolic pressure ≥35 mm Hg. Obese patients without such abnormalities were classified as MHD negative (MHD‐NEG; n=52). Serum biomarkers timed with echocardiography. MHD‐POS and MHD‐NEG individuals were similarly obese, but MHD‐POS patients were older, with more diabetes mellitus and metabolic syndrome. Right ventricular coupling was worse in MHD‐POS patients (P<0.001). GAL3 levels were higher in MHD‐POS versus MHD‐NEG patients (7.7±2.3 versus 6.3±1.9 ng/mL, respectively; P<0.001). Both GAL3 and FSTL3 levels correlated with diastolic dysfunction and increased pulmonary artery systolic pressure but not with left ventricular mass. In multivariate models including all 3 biomarkers, only GAL3 remained associated with MHD (odds ratio: 1.30; 95% CI, 1.01–1.68; P=0.04).
Conclusions
In young obese individuals without known cardiovascular disease, GAL3 is associated with the presence of preclinical MHD. GAL3 may be useful in screening for preclinical MHD and identifying individuals with increased risk of progression to obesity‐related heart failure with preserved ejection fraction.
Keywords: echocardiography, obesity, prevention, remodeling heart failure
Subject Categories: Biomarkers, Pulmonary Hypertension, Heart Failure, Metabolic Syndrome, Echocardiography
Clinical Perspective
What Is New?
Obesity is a precursor to heart failure with preserved ejection fraction; metabolic heart disease, a cardiomyopathy characterized by left ventricular hypertrophy and/or diastolic dysfunction, is common in young obese individuals.
In this study, GAL3 (galectin–3) independently associated with metabolic heart disease, subclinical stage B heart failure enriched with pulmonary hypertension, in young obese individuals.
What Are the Clinical Implications?
This study suggests biomarkers may help us identify preclinical heart failure and asymptomatic pulmonary hypertension in high‐risk individuals with obesity and could serve as a screening biomarker to initiate preventative strategies for heart failure with preserved ejection fraction, including echocardiographic screening and therapy initiation.
Metabolic heart disease (MHD), a cardiomyopathy characterized by left ventricular hypertrophy (LVH) and/or diastolic dysfunction in the setting of preserved ejection fraction, is common in young obese individuals, with a prevalence ranging from 15% to 56%.1 The high frequency of MHD in this population is noteworthy for 2 main reasons. First, the prevalence of obesity is steadily rising in the United States, with current estimates at 40% in 2014.2 Second, obesity and metabolic syndrome are important precursors to heart failure with preserved ejection fraction (HFpEF), a major public health concern burdening our healthcare system with significant morbidity, mortality, and cost.3, 4
Heart failure (HF) is a progressive disorder, classified by the American College of Cardiology/American Heart Association (ACC/AHA) into 4 stages (A, B, C or D); stage B represents structural heart disease in the absence of clinical signs or symptoms of HF.5 Diastolic dysfunction, LVH, and pulmonary hypertension (PH) are preclinical echocardiographic abnormalities that precede clinical HF in individuals with stage B MHD.6, 7, 8 However, given the magnitude of the population at risk for MHD, the widespread use of echocardiography as a screening tool is neither practical nor cost effective. Inexpensive and convenient screening measures that can identify and mechanistically link to stage B MHD would help prioritize and monitor high‐risk individuals for further assessment and early intervention.9
BNP (B‐type natriuretic peptide) and NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) are biomarkers that are useful in the identification of stage B HF.10 However, both BNP and NT‐proBNP levels are depressed in obesity11 and tend to be lower in HFpEF12, 13 compared with HF with reduced ejection fraction, thus severely limiting their utility in this population. FSTL3 (follistatin‐like 3 peptide), similar to natriuretic peptides, is a cardiomyokine that is upregulated by hypertrophic stimuli14 with increased circulating levels in HF with reduced ejection fraction.15 Although FSTL3 has not been evaluated in HF and obesity, it may provide an alternative to natriuretic peptides in this setting. GAL3 (galectin–3) is elevated in symptomatic patients with HF with reduced ejection fraction or HFpEF,16 but little is known about its ability to identify preclinical stage B HF of any etiology or to identify MHD in the setting of obesity. Accordingly, our goal was to determine whether NT‐proBNP, FSTL3, or GAL3 was associated with preclinical MHD in obese participants. Stage B MHD was specifically defined as (1) the presence of increased left ventricular (LV) mass and/or diastolic dysfunction function that is (2) accompanied by PH, as elevated pulmonary pressure is a well‐established prognosticator and often portends hemodynamically important LV disease.6, 17, 18
Methods
The data that support the findings of this study are available from the corresponding author (D.M.G.) on reasonable request.
Study Population
Patients with obesity (n=250) were recruited from outpatient clinics at Boston Medical Center. Obesity was defined as a body mass index (BMI; calculated as kg/m2) ≥30. Nonobese volunteer controls with BMI <30 and no major comorbidities (n=44) were also recruited from Boston Medical Center. All participants with clinically recognized cardiovascular disease (HF, PH, coronary artery disease, valvular disease, angina, or atrial fibrillation), incidental asymptomatic LV systolic dysfunction (LV ejection fraction <50% on echocardiogram), or significant pulmonary disease were excluded. Of the 250 obese patients, 19 were excluded for missing or inadequate echocardiographic data (for the primary analysis), leaving 231 evaluable participants. Of the 44 control participants, 23 with evidence of asymptomatic echocardiographic abnormalities (incident valvular disease, diastolic abnormalities, LVH, or PH) or missing data were excluded from the analysis.
Categorization of MHD Disease
Primary analysis
For the primary analysis, MHD status classification of obese participants was based on the presence or absence of diastolic dysfunction, LVH, and PH. To be classified as MHD positive (MHD‐POS), a participant needed (1) to have diastolic dysfunction or LVH and (2) to have PH. Obese participants without diastolic dysfunction, LVH, or PH were classified as MHD negative (MHD‐NEG). Obese participants with PH, LVH, and/or diastolic dysfunction but not meeting criteria for MHD‐POS were classified as indeterminate. Of the 231 evaluable obese participants, 94 classified as MHD‐POS, 52 as MHD‐NEG, and 85 as indeterminate (Figure 1A).
Figure 1.
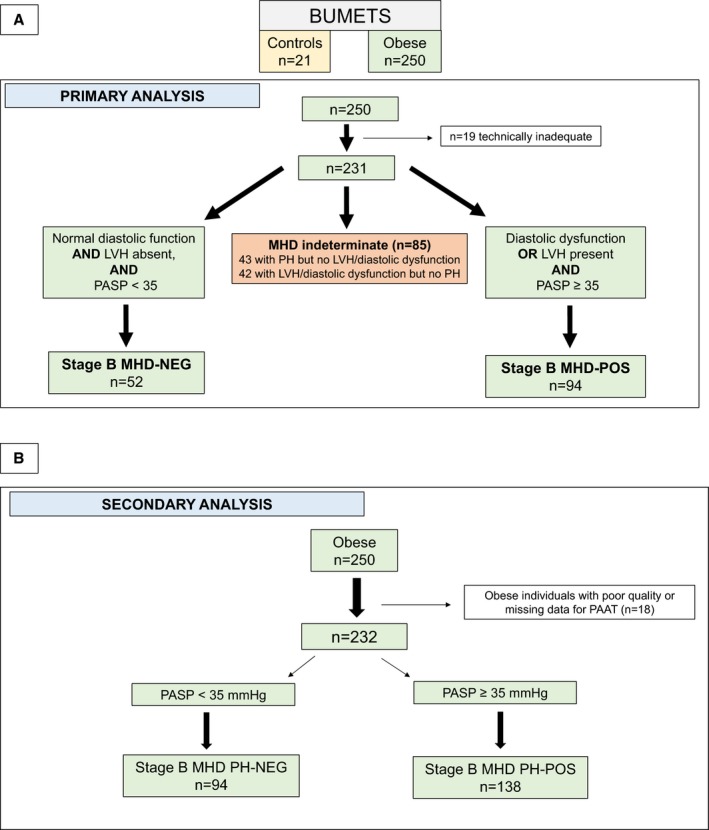
Flow diagram of the classification system of stage B MHD. A, Primary analysis used presence of diastolic dysfunction, LVH, and PH for classification of MHD. B, Secondary analysis used PH as the sole criterion for MHD presence. BUMETS indicates Boston University Metabolic Heart Disease Study; LVH, left ventricular hypertrophy; MHD, metabolic heart disease; MHD‐NEG, metabolic heart disease–negative; MHD‐POS, metabolic heart disease–positive; PAAT, pulmonary artery acceleration time; PASP, pulmonary artery systolic pressure; PH, pulmonary hypertension.
Diastolic dysfunction was classified using the updated 2016 American Society of Echocardiography (ASE) guidelines on evaluation of LV diastolic dysfunction.19 Because of the high percentage of incomplete or absent tricuspid regurgitation jets in this cohort, tricuspid regurgitation velocity was replaced by pulmonary artery systolic pressure (PASP) calculated from pulmonary artery acceleration time (PAAT) using a value of ≥31 mm Hg—a value equivalent to the tricuspid regurgitation velocity requirement in the ASE criteria. LVH was classified as present if the LV mass (indexed to height2.7) was ≥45 g/m2.7 in women and ≥49 g/m2.7 for men.20 PH was classified as present if the estimated PASP derived from PAAT was ≥35 mm Hg.17
Secondary analysis
In a secondary analysis, obese participants were classified as PH‐POS if the estimated PASP derived from PAAT was ≥35 mm Hg and as PH‐NEG if PASP was <35 mm Hg. For this analysis, 18 patients were excluded for inadequate quality of the pulse‐wave Doppler signal from the right ventricular (RV) outflow tract, leaving 232 evaluable participants (93%) for analysis.
Clinical Assessment
A comprehensive medical history and physical examination were performed for all participants. Fasting laboratory values, resting heart rate, blood pressure (obtained after 10 minutes resting in a sitting position, averaged over 3 consecutive measurements), and anthropometrics were obtained for all patients. Diabetes mellitus was defined as fasting blood glucose level ≥126 mg/dL and/or active medical therapy with an oral hypoglycemic agent and/or insulin. Hypertension was defined as systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, and/or current antihypertensive therapy. Metabolic syndrome was defined as meeting ≥3 of the following 5 criteria: (1) increase waist circumference (≥102 cm for men or ≥88 cm for women), (2) increased fasting triglycerides (≥150 mg/dL), (3) high blood pressure (≥130/85 mm Hg or antihypertensive therapy), (4) decreased high‐density lipoprotein cholesterol (<40 mg/dL in men, <50 mg/dL in women), (5) impaired fasting glucose (≥100 mg/dL).21 Ideal body weight was calculated using the following equation: a+(2.3)([height in inches]−60), where a=50 kg for men and a=45.5 kg for women.22 The Boston University Medical Center institutional review board approved this study, and all participants provided informed consent before study enrollment.
Assessment of Cardiac Structure and Function
Two‐dimensional transthoracic echocardiograms using a 1‐ to 5‐MHz transducer and commercially available ultrasound machine (iE33; Phillips Medical Systems) was used by a single experienced sonographer (A.P.). Echocardiograms were interpreted offline in a blinded manner. Standard echocardiographic analysis was applied according to published recommendations.23 LV mass was calculated utilizing the cubed method with an index applying height to the power of 2.7 to account for body habitus in our obese cohort.24 LV diastolic function included pulse‐wave Doppler assessment of early (E) and late (A) transmitral inflow velocities, E/A ratio, E‐wave deceleration time, and tissue Doppler imaging of myocardial velocities averaging both the medial and lateral mitral annulus.19 LV filling pressure estimation, utilizing the mitral E wave and tissue Doppler mean e′ velocity as a ratio, was calculated as described previously.19
Right heart assessment was performed according to published recommendations for right atrial area and RV basal diameter.25 Tricuspid annular plane systolic excursion (TAPSE) was obtained by placing an M‐mode cursor on the lateral tricuspid annulus in an apical 4‐chamber view and capturing maximal annular movement during systole. Utilizing pulse‐wave Doppler interrogation of the pulmonary artery ejection, PAAT was measured as the time interval from the onset to peak flow velocity of pulmonary artery flow. RV ejection was the total time interval from onset to the cessation of pulmonary artery flow. PASPs were estimated from PAAT using a previously validated equation: 10([−0.004×PAAT]+2.1).26
All echocardiographic measurements were averaged over 3 consecutive cardiac cycles (when available). Echocardiographic measurements were performed by 2 trained cardiologists (D.M.G. and Y.W.) with repeated measurements of 10 scans showing an intraobserver coefficient of variation of 1.6% to 6.1% and an interobserver coefficient of variation of 1.8% to 7.0% for linear measurements. The intraobserver coefficient of variation was 7.3% with intraclass correlation coefficients ranging from 92% to 96%.
Biochemical Measurements
Following an overnight fast, blood samples were obtained from study participants the next morning. The citrated plasma samples were centrifuged immediately and stored at −80°C until assayed. No freeze–thaw cycles were performed before the assays described below. NT‐proBNP, GAL3, and FSTL3 levels were determined using ELISA kits. Assay calibration was performed according to the manufacturer's recommendations, with values normalized to a standard curve. Other biochemical testing including total cholesterol, HDL (high‐density lipoprotein), triglyceride, and creatinine concentrations were determined using standard clinical laboratory methods at Boston Medical Center. Estimated glomerular filtration rate (eGFR) was determined by the Chronic Kidney Disease Epidemiology Collaboration formula: eGFR (mL/min/1.73m2)=141×min (serum creatinine/κ,1)α×max (serum creatinine/κ,1)−1.209×0.993Age×1.018 (if female)×1.159 (if black); κ is 0.7 (females) and 0.9 (males) and α is −0.329 (females) and −0.411 (males).27
Statistical Analysis
Baseline characteristics and echocardiographic data were reported as mean±SD, median (interquartile range), or number (percentage) unless otherwise specified. Between‐group differences were compared using 1‐way ANOVA, Kruskal–Wallis, or χ2 analysis with Bonferroni correction for multiple‐comparison testing (P values considered statistically significant at <0.05/3=0.02) for between‐group comparisons. Natural logarithm transformations were applied to FSTL3 and NT‐proBNP levels for analyses to normalize right‐skewed distributions. Correlation testing for biomarkers and echocardiographic and clinical characteristics used pairwise Pearson correlation coefficients. Multivariate linear regression models were used to explore relationships between echocardiographic parameters and clinical characteristics. Univariate logistic regression models using MHD‐POS as the outcome of interest were applied to clinical and echocardiographic variables to determine associations. Variables that were significant (P<0.01) were subsequently applied in fully adjusted multivariate models to prevent model overfitting. All analyses were performed using SAS 9.3 (SAS Institute).
Results
Participant Characteristics
MHD‐POS and MHD‐NEG participants were similar in body weight, BMI, and body surface area, with 40% of all obese patients qualifying for class 3 obesity with a BMI ≥40 (Table 1). Control participants had a mean BMI of 25 with no diabetes mellitus, hypertension, or metabolic syndrome. MHD‐POS participants were older with a higher prevalence of comorbidities including diabetes mellitus, hypertension, hyperlipidemia, and metabolic syndrome than MHD‐NEG participants. Likewise, MHD‐POS (versus MHD‐NEG) participants were more likely to be receiving hypertensive therapy and hypoglycemic medications (oral or insulin therapy). MHD‐indeterminate participants, who were excluded from the primary analysis (n=85), were generally similar to MHD‐POS and MHD‐NEG participants regarding comorbidities and echocardiographic variables (Table S1).
Table 1.
Baseline Characteristics
| Nonobese Controls (n=21) | Obese MHD‐NEG (n=52) | Obese MHD‐POS (n=94) | P Value | |
|---|---|---|---|---|
| Age, y | 43±12 | 36±11 | 47±9b | <0.001 |
| Female, n (%) | 18 (86) | 47 (90) | 69 (73) | 0.04 |
| Black, n (%) | 9 (43) | 34 (65) | 61 (65) | 0.15 |
| Anthropometrics | ||||
| Height, m | 167±6 | 166±9 | 166±10 | 0.70 |
| Ideal body weight, kg | 60±6 | 58±8 | 59±9 | 0.68 |
| Actual body weight, kg | 69±8 | 103±22a | 109±22a | <0.001 |
| Body mass index, kg/m2 | 25±3 | 37±7a | 40±8a | <0.001 |
| Body surface area, m2 | 1.8±0.1 | 2.1±0.2a | 2.1±0.2a | <0.001 |
| Waist circumference, cm | 82±9 | 113±18a | 119±16a | <0.001 |
| Comorbidities, n (%) | ||||
| Diabetes mellitus | 0 (0) | 6 (12) | 37 (39) | <0.001 |
| Hypertension | 0 (0) | 16 (31) | 60 (64) | <0.001 |
| Current smoking | 1 (4) | 4 (8) | 13 (14) | 0.45 |
| Obstructive sleep apnea | 0 (0) | 6 (12) | 20 (21) | 0.02 |
| Hyperlipidemia | 1 (5) | 14 (27) | 50 (53) | <0.001 |
| Metabolic syndrome | 0 (0) | 28 (54) | 79 (84) | <0.001 |
| Medications, n (%) | ||||
| ACEI or ARB | 0 (0) | 8 (15) | 43 (46) | <0.001 |
| β‐Blocker | 0 (0) | 4 (8) | 23 (24) | 0.002 |
| Oral hypoglycemic agent | 0 (0) | 6 (12) | 26 (28) | 0.002 |
| Insulin | 0 (0) | 1 (2) | 15 (16) | 0.007 |
| Laboratory values | ||||
| Total cholesterol, mg/dL | 184 (178–217) | 182 (157–210) | 188 (165–206) | 0.85 |
| HDL, mg/dL | 61 (52–67) | 45 (39–53)a | 45 (39–53)a | <0.001 |
| Triglycerides, mg/dL | 60 (48–84) | 100 (70–151) | 117 (82–176)a | 0.008 |
| Triglyceride/HDL ratio | 1.1 (0.7–1.6) | 2.5 (1.3–3.3) | 2.7 (1.8–4.0)a | 0.001 |
| eGFR, mL/min/1.73 m2 | 99±16 | 128±25a | 122±28a | <0.001 |
| Biomarkers | ||||
| NT‐proBNP, pmol/L | 420 (279–736) | 554 (431–684) | 607 (485–773) | 0.36 |
| GAL3, ng/mL | 5.7±1.6 | 6.3±1.9 | 7.7±2.3a, b | <0.001 |
| FSTL3, pg/mL | 4665 (3976–5951) | 5335 (4414–6970) | 5768 (4477–7521) | 0.02 |
Data are mean±SD, median (interquartile range), or n (%). P value reflect overall group differences. P value listed reflects ANOVA comparison across all 3 groups; symbols denote between group comparisons with Bonferonni correction. ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; eGFR, estimated glomerular filtration rate; FSTL3, follistatin‐like 3 peptide; GAL3, galectin–3; HDL, high‐density lipoprotein; MHD‐NEG, metabolic heart disease–negative; MHD‐POS, metabolic heart disease–positive; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
P<0.02 vs obese MHD‐NEG participants.
P<0.02 vs controls.
Cardiac Structure and Function
As expected given the grouping criteria, MHD‐POS (versus MHD‐NEG) participants had higher LV mass and higher mean PASP (48±9 versus 27±4 mm Hg, respectively; Table 2). In addition, MHD‐POS individuals had more LV concentric remodeling. MHD‐POS participants had worse diastolic function, with increased A waves, lower E/A ratios, and increased left atrial size indexes.
Table 2.
Cardiac Structure and Function
| Nonobese Controls (n=21) | Obese MHD‐NEG (n=52) | Obese MHD‐POS (n=94) | P Value | |
|---|---|---|---|---|
| Left heart parameters | ||||
| LV diastolic dimension, mm | 45±4 | 46±4 | 46±5 | 0.54 |
| LVEDV, mL | 72 (61–80) | 79 (68–93) | 86 (71–104)a | 0.01 |
| LVEDV index, mL/m2 | 40±9 | 40±10 | 41±10 | 0.61 |
| LVM, g | 112±24 | 142±31a | 173±48a, b | <0.001 |
| LVM index, g/m2.7 | 28±6 | 36±6a | 44±12a, b | <0.001 |
| LVM/LVEDV, g/mL | 1.6±0.3 | 1.8±0.4 | 2.0±0.5a, b | <0.001 |
| Relative wall thickness | 0.4±0.1 | 0.4±0.1 | 0.5±0.1a, b | <0.001 |
| LVEF, % | 64±6 | 65±6 | 64±7 | 0.56 |
| Mitral E wave, cm/s | 70±13 | 83±17 | 78±19 | 0.03 |
| Mitral A wave, cm/s | 53±15 | 58±14 | 71±13a, b | <0.001 |
| Mitral E/A ratio | 1.4±0.4 | 1.5±0.4 | 1.1±0.3a, b | <0.001 |
| Mean e′ wave, cm/s | 12±2 | 11±2 | 9±2a, b | <0.001 |
| E/e′ ratio | 6±1 | 8±2 | 9±3a, b | <0.001 |
| Left atrial diameter, mm | 31±4 | 36±5a | 38±5a | <0.001 |
| LAV index (BSA), mL/m2 | 32±11 | 29±8 | 34±9a | 0.003 |
| LAV index (height), mL/height2.7 | 14±5 | 15±4 | 19±5a, b | <0.001 |
| Right heart parameters | ||||
| RV basal diameter, mm | 36±5 | 36±5 | 39±5 | 0.01 |
| TAPSE, mm | 23±4 | 23±3 | 23±4 | 0.97 |
| Right atrial area, cm2 | 14±3 | 14±3 | 17±4b | <0.001 |
| PAAT, ms | 181±29 | 168±14 | 106±19a, b | <0.001 |
| PASP, mm Hg | 25±6 | 27±4 | 48±9a, b | <0.001 |
| TAPSE/PASP (mm/mm Hg) | 1.0 (0.7–1.2) | 0.8 (0.8–1.0)a | 0.5 (0.4–0.6)a, b | <0.001 |
Data are mean±SD, median (interquartile range), or n (%). P values reflect overall group differences with ANOVA comparison across all 3 groups; symbol denotes between‐group comparisons with Bonferonni adjustment. BSA indicates body surface area; LAV, Left atrial volume; LV, left ventricle; LVEDV, left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction; LVM, left ventricular mass; MHD‐NEG, metabolic heart disease–negative; MHD‐POS, metabolic heart disease–positive; PAAT, pulmonary artery acceleration time; PASP, pulmonary artery systolic pressure; RV, right ventricle; TAPSE, tricuspid annular plane systolic excursion.
P<0.02 vs controls.
P<0.02 vs obese MHD‐NEG participants.
Biomarkers
Relationship to clinical characteristics
Whereas NT‐proBNP and FSTL3 were not different among the 3 groups, GAL3 was higher in MHD‐POS participants than in controls or MHD‐NEG individuals (Table 1). Among all MHD participants, GAL3 correlated with age (ρ=0.34, P<0.001), eGFR (ρ=−0.32, P<0.001), and diabetes mellitus (ρ=0.23, P=0.007) but not with height, weight, BMI, or hypertension (all P>0.05). FSTL3 correlated with age (ρ=0.35, P<0.001), weight (ρ=0.27, P=0.002), BMI (ρ=0.17, P=0.05), hypertension (ρ=0.29, P<0.001), diabetes mellitus (ρ=0.25, P=0.004), and eGFR (ρ=−0.24, P=0.006). In contrast, NT‐proBNP did not correlate with any clinical characteristic in the MHD‐POS or MHD‐NEG participants, including hypertension, diabetes mellitus, eGFR, and BMI.
Relationship to cardiac structure and function
Among the MHD participants, both GAL3 and FSTL3 correlated with measures of LV diastolic function (mean e′ velocity, E/A ratio, and E/e′ ratio), PASP, and the TAPSE/PASP ratio but did not correlate with LV volume or mass (Table 3 and Figure 2). By comparison, NT‐proBNP did not correlate with mean e′ velocity, E/e′ ratio, or LV mass or volume and correlated only with E/A.
Table 3.
Echocardiographic Correlates With Biomarkers in Obese Participants
| Biomarker | Variable | ρ | P Value |
|---|---|---|---|
| GAL3 | Diastolic function | ||
| Mean e′ | −0.30 | <0.001 | |
| Mitral E/A ratio | −0.27 | 0.002 | |
| E/e′ | 0.30 | <0.001 | |
| LAV indexed to BSA | −0.04 | 0.68 | |
| LV remodeling | |||
| LVM indexed to height2.7 | −0.05 | 0.56 | |
| LVM/LVEDV ratio, g/mL | 0.07 | 0.47 | |
| Right heart structure/function | |||
| TAPSE | 0.01 | 0.91 | |
| PASP | 0.33 | <0.001 | |
| TAPSE/PASP ratio | −0.30 | 0.002 | |
| FSTL3 | Diastolic function | ||
| Mean e′ | −0.35 | <0.001 | |
| Mitral E/A ratio | −0.34 | <0.001 | |
| E/e′ | 0.31 | <0.001 | |
| LAV indexed to BSA | −0.21 | 0.02 | |
| LV remodeling | |||
| LVM indexed to height2.7 | 0.04 | 0.67 | |
| LVM/LVEDV ratio, g/mL | 0.12 | 0.19 | |
| Right heart structure/function | |||
| TAPSE | −0.03 | 0.80 | |
| PASP | 0.21 | 0.02 | |
| TAPSE/PASP ratio | −0.24 | 0.02 | |
| NT‐proBNP | Diastolic function | ||
| Mean e′ | −0.08 | 0.40 | |
| Mitral E/A ratio | −0.20 | 0.03 | |
| E/e′ | 0.08 | 0.42 | |
| LAV indexed to BSA | −0.05 | 0.59 | |
| LV remodeling | |||
| LVM indexed to height2.7 | 0.05 | 0.59 | |
| LVM/LVEDV ratio, g/mL | −0.03 | 0.78 | |
| Right heart structure/function | |||
| TAPSE | 0.04 | 0.67 | |
| PASP | 0.11 | 0.25 | |
| TAPSE/PASP ratio | −0.18 | 0.08 | |
BSA indicates body surface area; FSTL3 indicates follistatin‐like 3 peptide; GAL3, galectin–3; LAV, left atrial volume; LV, left ventricular; LVEDV, left ventricular end‐diastolic volume; LVM, left ventricular mass; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; PASP, pulmonary artery systolic pressure; TAPSE, tricuspid annular plane systolic excursion.
Figure 2.
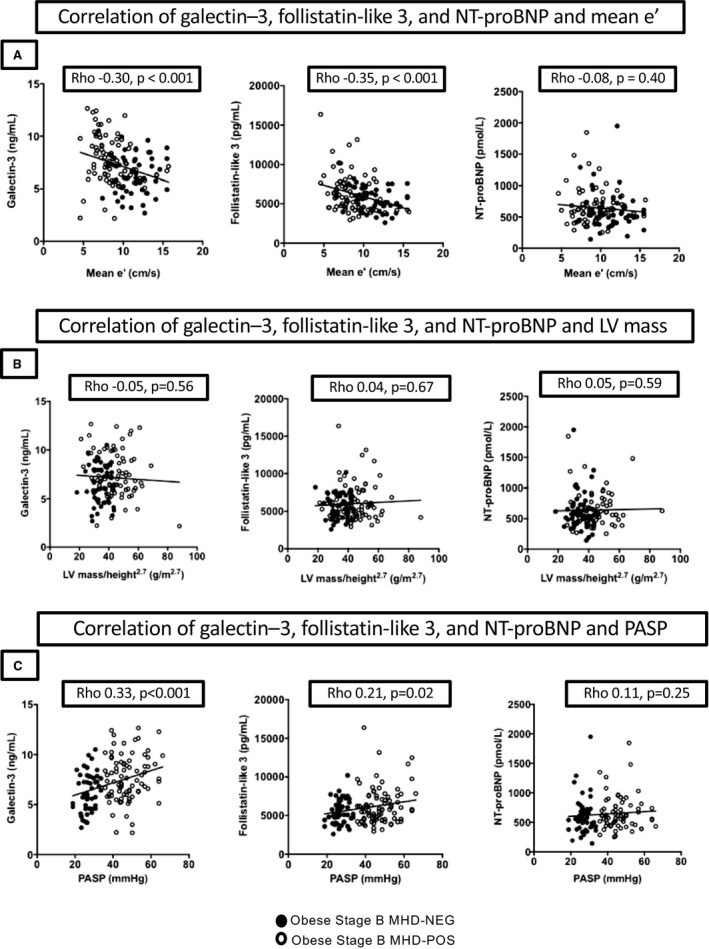
Correlations of biomarkers GAL3 (galectin–3), FSTL3 (follistatin‐like 3 peptide), and NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) with diastolic function, LV mass, and pulmonary pressures. A, Biomarker correlations with mean e′ velocity. B, Biomarker correlations with LV mass indexed to height.2.7 C, Biomarker correlations with PASP. LV indicates left ventricular; MHD‐NEG, metabolic heart disease–negative; MHD‐POS, metabolic heart disease–positive; PASP, pulmonary artery systolic pressure.
Cardiac Structure and Function and Pulmonary Hemodynamics in MHD
LVH and diastolic function
Of the MHD‐POS participants, 7% had isolated LVH, 59% had isolated abnormal diastolic function, and 34% had both LVH and diastolic dysfunction. Among these MHD‐POS participants, BMI was strongly correlated to LV mass indexed to height2.7 (ρ=0.52, P<0.001) but not with mean e′ (ρ=0.17, P=0.10). Among the MHD‐POS participants, 38% had concentric remodeling, 30% had concentric hypertrophy, and 12% had eccentric hypertrophy.
Pulmonary hypertension
Among all obese participants (both MHD‐POS and MHD‐NEG), PASP correlated with several measures of abnormal diastolic function including increased left atrial volume index (ρ=0.24, P=0.004), decreased mitral E/A ratio (ρ=−0.37, P<0.001), increased E/e′ (ρ=0.33, P<0.001), and decreased mean e′ (ρ=−0.46, P<0.001; Figure 3A). In addition, PASP correlated with increases in LV mass/volume ratio (ρ=0.27, P=0.001) and LV mass indexed to height2.7 (ρ=0.35, P<0.001; Figure 3B). In multivariate linear regression models with clinical variables, age (P=0.01), waist circumference (P=0.01), and diabetes mellitus (0.03) all associated with PASP. Mean e′ (P=0.002), E/A ratio (P=0.02), left atrial volume index (P=0.002), and LV mass indexed to height2.7 (P=0.009) were all independently predictive of PASP in echocardiographic multivariate models.
Figure 3.
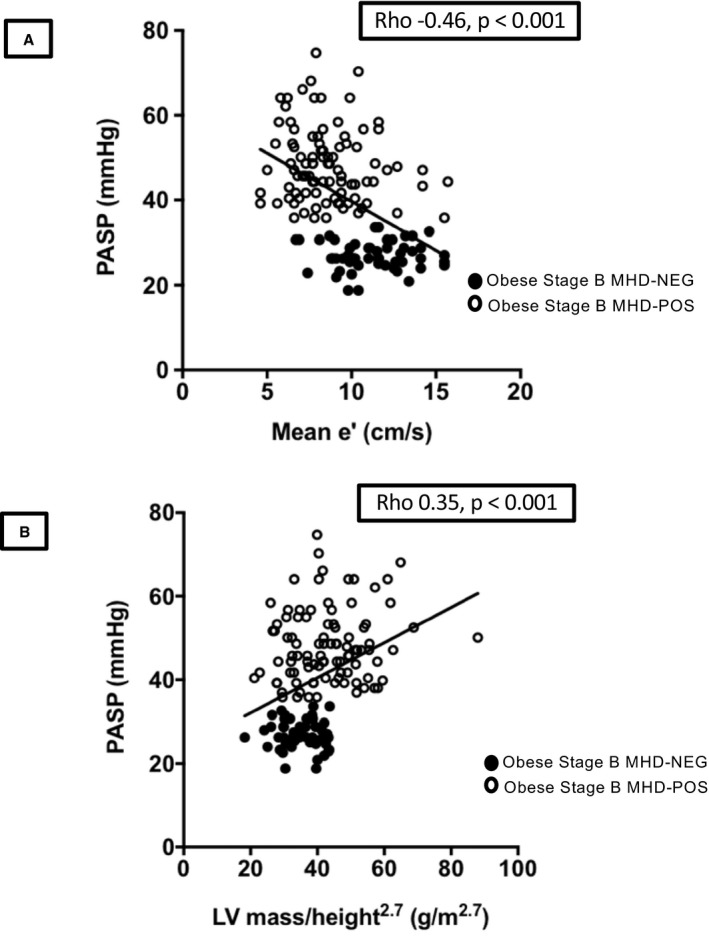
Relationship of left‐sided cardiac disease and pulmonary pressures in obese participants. A, Correlation of mean e′ velocity and PASP. B, Correlation of LV mass indexed to height2.7 and PASP. LV indicates left ventricular; MHD‐NEG, metabolic heart disease–negative; MHD‐POS, metabolic heart disease–positive; PASP, pulmonary artery systolic pressure.
RV uncoupling
Although TAPSE was similar across the 3 groups (Table 2), elevation of PASP in the MHD‐POS group was associated with a decrease in the TAPSE/PASP ratio suggestive of early right ventricle–pulmonary artery uncoupling (Figure 4A). Mean e′ was correlated with TAPSE/PASP (ρ=0.51, P<0.001; Figure 4B) and remained significant after adjustment for age, sex, LV ejection fraction, and LV mass indexed to height2.7 (P=0.001), suggesting a relationship between RV uncoupling and diastolic dysfunction.
Figure 4.
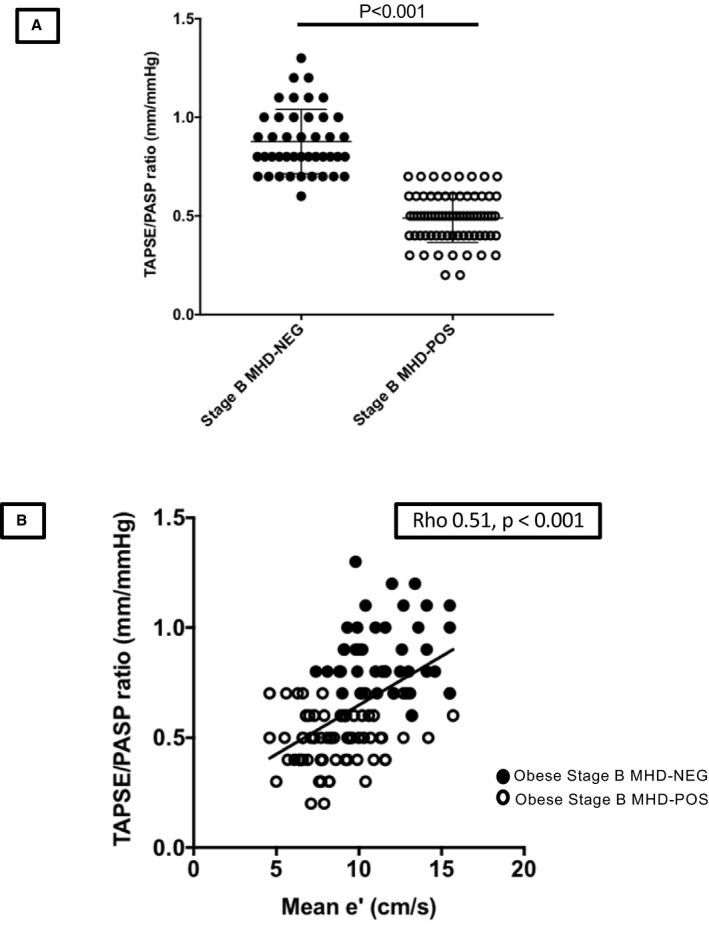
RV mechanics in stage B metabolic heart disease. A, Uncoupling of RV and pulmonary circulation in stage B metabolic heart disease. B, Relationship of LV diastolic function on RV mechanics in obese participants. LV indicates left ventricular; MHD, metabolic heart disease; MHD‐NEG, metabolic heart disease–negative; MHD‐POS, metabolic heart disease–positive; PASP, pulmonary artery systolic pressure; RV, right ventricular; TAPSE, tricuspid annular plane systolic excursion.
Primary Analysis: Clinical and Biomarker Correlates of MHD
Significant univariate clinical correlates of MHD‐POS individuals included age, female sex, hypertension, diabetes mellitus, waist circumference, and hyperlipidemia (Table S2). Among the 3 candidate biomarkers, GAL3 was associated with MHD‐POS individuals in univariate analysis, whereas FSTL3 and NT‐proBNP were not. In age‐ and sex‐adjusted multivariate logistic regression models (Table 4), GAL3 was the only biomarker that remained associated with MHD‐POS participants (odds ratio: 1.30; 95% CI, 1.01–1.68; P=0.04).
Table 4.
Logistic Regression Analysis for Presence of Obese Stage B MHD Phenotype
| OR (95% CI) | P Value | |
|---|---|---|
| GAL3 | ||
| Model 1: Age and sex | 1.28 (1.03–1.59) | 0.004 |
| Model 2: Clinical risk model | 1.24 (1.01–1.53) | 0.05 |
| Model 3: Model 1+FSTL3a+NT‐proBNPa | 1.30 (1.01–1.68) | 0.04 |
| FSTL3a | ||
| Model 1: Age and sex | 0.71 (0.57–1.36) | 0.56 |
| Model 2: Clinical risk model | 0.84 (0.53–1.35) | 0.47 |
| Model 3: Model 1+GAL3+NT‐proBNPa | 0.67 (0.41–1.10) | 0.11 |
| NT‐proBNPa | ||
| Model 1: Age and sex | 1.39 (0.85–2.28) | 0.19 |
| Model 2: Clinical risk model | 1.32 (0.83–2.11) | 0.24 |
| Model 3: Model 1+GAL3+FSTL3a | 1.49 (0.89–2.49) | 0.13 |
Clinical risk model: history of hypertension, diabetes mellitus, hyperlipidemia, waist circumference, and estimated glomerular filtration rate in addition to biomarker of interest. FSTL3 indicates follistatin‐like 3 peptide; GAL3, galectin–3; MHD, metabolic heart disease; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; OR, odds ratio.
FSTL3 and NT‐proBNP were log‐transformed in regression models for data normalization. OR for both biomarkers reflect 1‐SD increase in log‐transformed biomarker.
Secondary Analysis: Clinical and Biomarker Predictors of PH Presence
In a secondary analysis, obese participants were classified as PH‐POS solely on the basis of PASP ≥35 mm Hg or PH‐NEG if PASP was <35 mm Hg (Figure 1B). This criterion identified 138 obese PH‐POS participants and 94 PH‐NEG participants (Tables S3 and S4). In the PH‐POS individuals, (1) PASP correlated with mean e′ (ρ=−0.30, P<0.001; Figure S1A), (2) PASP correlated with LV mass indexed to height2.7 (ρ=−0.17, P=0.01), and (3) mean e′ correlated with the TAPSE/PASP ratio (ρ=0.33, P<0.001; Figure S1B). Similar univariate predictors of MHD PH‐POS individuals were noted, as seen in the former analysis (Table S5). As in the primary analysis, multivariate models with age and sex adjustment showed that only GAL3 was associated with PH‐POS participants (odds ratio: 1.33; 95% CI, 1.11–1.59; P=0.002; Table S6).
Discussion
The goal of this study was to determine whether biomarkers can be used for the early identification of preclinical (ie, ACC/AHA stage B) HF in a cohort of asymptomatic young obese participants. For the purpose of this analysis, stage B MHD was defined by the presence of increased LV mass and/or diastolic dysfunction that was accompanied by PH. There were 2 major findings. First, of the 3 biomarkers tested, we found that only GAL3 independently identified stage B MHD in otherwise asymptomatic young obese participants. Second, we found that in young obese participants, resting PH (1) is common, occurring in 59% participants; (2) correlates strongly with LV diastolic dysfunction and LVH; (3) correlates with RV uncoupling (lower TAPSE/PASP ratio); and (4) correlates with GAL3.
Classification of Stage B MHD Using LVH, Diastolic Dysfunction, and PH
Obesity is associated with an increased risk of incident HF,3, 4 and mounting evidence suggests that among patients with HFpEF, there is a distinct “metabolic” phenotype with obesity, type 2 diabetes mellitus, and/or metabolic syndrome.28 Obesity has been linked with subclinical alterations in cardiac structure (LVH) and function (diastolic dysfunction), which are generally viewed as cardiac hallmarks of MHD.29, 30 These structural and functional alterations are common in patients with obesity and may remain asymptomatic for extended periods. Importantly, LVH and diastolic dysfunction are important drivers in the progression to clinical HF, particularly HFpEF.31 A recent meta‐analysis that polled 7564 participants from 5 studies showed that asymptomatic diastolic dysfunction contributed to a 70% higher risk of progressing to clinical HF compared with participants without diastolic dysfunction.32
For this study, we defined MHD as the presence of diastolic dysfunction and/or LVH in the presence of PH. Diastolic dysfunction alone was prevalent in 59% of the MHD‐POS individuals in our study. As expected, in these participants, the A wave was increased, the E/A ratio was decreased, and the left atrium was larger. LVH was present in 7% of MHD‐POS participants; both entities, diastolic dysfunction and LVH, were present in 34%. The prevalence of LVH in our study (41%) is consistent with many prior observations, including a recent meta‐analysis of 22 studies in which the average prevalence of LVH in obese participants was 56% (range: 20–85%).1 As expected, LVH correlated with diastolic dysfunction, but as we33 and others34 have noted, there are many patients in whom diastolic dysfunction is not associated with LVH.
A novel key aspect of this study is the inclusion of PH, in addition to LVH and/or diastolic dysfunction, in the identification of patients with MHD. The functionally important consequence of impaired LV relaxation and/or filling is an elevation in pulmonary artery pressures, which plays a pathophysiologic role in HFpEF patients.17 PH is a major determinant of clinical symptoms and outcomes in patients with symptomatic HFpEF.35 An inherent limitation of using diastolic relaxation and/or LVH to define MHD is that both measures are only indirectly related to PH. PASP was determined using PAAT, an approach that allowed pulmonary artery pressure to be estimated in >90% of patients in this study. Participants were deemed to have MHD only if diastolic dysfunction and/or LVH were associated with PH, which was defined as a resting PASP ≥35 mm Hg. Of the 250 obese participants in this study with analyzable echocardiographic data, 59% had LVH or diastolic dysfunction. With the additional requirement for PH, the number of obese participants classified as MHD‐POS decreased to 94 patients (38% of obese individuals included in the primary analysis). PASP correlated significantly with mean e′ (ρ=−0.46) and LV mass (ρ=0.35), indicating that LV disease was a major determinant of the elevated pulmonary pressures noted in the obese cohort. The goal of this added criterion was to increase the specificity of the definition of MHD. As discussed later, the use of PH alone as the definition of MHD for a secondary analysis was also robust, likely reflecting the ability of PH to provide an integrated measure of the hemodynamic severity of left‐sided dysfunction.
NT‐proBNP Fails to Associate With MHD
Natriuretic peptides are well‐established biomarkers for prognosis in HF patients.10 However, on the order of 30% of patients with established HFpEF—most notably, those who are younger and/or obese—have normal BNP levels,36 likely related to the observation that obesity and metabolic disease are associated with lower circulating natriuretic peptides.37 Conversely, natriuretic peptides are useful screening biomarkers for the detection of patients at risk for HF. In STOP‐HF (Natriuretic Peptide‐Based Screening and Collaborative Care for HF) and PONTIAC (NT‐proBNP Selected Prevention of Cardiac Events in a Population of Diabetic Patients Without a History of Cardiac Disease), natriuretic levels were used to randomize stage A patients to intensive therapy, which led to decreased rates of incident HF and hospitalization. Although the recruitment of these cohorts did not specifically target obesity or metabolic syndrome, the average BMIs suggested that enrollees, on average, were overweight (STOP‐HF) or had class 1 obesity (PONTIAC).38, 39 Therefore, it seemed possible that NT‐proBNP could be useful for the identification of stage B HF in our young obese population.
In our cohort, however, NT‐proBNP proved to be an ineffective biomarker for detection of stage B HF due to MHD. There were no differences in NT‐proBNP levels between MHD‐POS and MHD‐NEG individuals and no significant associations with clinical characteristics (eg, hypertension, diabetes mellitus, eGFR, and BMI) or measures of cardiac structure (ie, LVH, LV volume, RV parameters), most measures of diastolic function (mean e′, LA size, E/e′ ratio), or PH. The failure of NT‐proBNP to identify stage B MHD likely relates to confounding factors that suppress circulating natriuretic levels in the setting of obesity.37
FSTL3 Correlates to Metabolic Disease But Not MHD
FSTL3, like natriuretic peptides, is a cardiac myokine. FSTL3 appears to play a role in the regulation of myocyte growth and mediates paracrine activation of fibroblasts in the heart.40 FSTL gene expression increases in myocardium from patients with severe HF undergoing therapy with an LV assist device.15 FSTL3 also has a role in metabolic regulation; levels are increased in obesity,41 and deletion in mice leads to favorable visceral fat and glucose homeostasis.42 Because the levels of FSTL3 are increased in HF and there is no apparent suppression of levels in obesity, we reasoned that FSTL3 might be a useful biomarker for stage B MHD in obese individuals. Interestingly, in our population, plasma FSLT3 levels were higher in obese participants, particularly in those with MHD, and correlated significantly with diastolic indexes (mean e′, E/A ratio, E/e′ ratio) and higher PASP but not with LVH. However, in univariate and fully adjusted multivariate models, FSTL3 was not able to identify MHD among obese patients, suggesting that in these patients FSTL3 may reflect the abnormal metabolic milieu rather than the cardiac phenotype.
GAL3 Is Associated With MHD
A major finding of this study is that GAL3 is associated with stage B HF due to MHD in young obese individuals, and in particular, GAL3 links strongly to diastolic dysfunction and PH. In contrast to FSTL3, this association persisted with multivariable adjustments including age, sex, and clinical risk factors (hypertension, diabetes mellitus, hyperlipidemia, waist circumference, and eGFR). Prior studies have shown that plasma GAL3 levels are increased in both HFpEF patients and those with HF with reduced ejection fraction.43 In stage C HFpEF individuals, GAL3 is associated with poor outcomes.44 Likewise, GAL3 predicted incident HF in multivariate models in the Framingham Heart Study.45 Ours is the first study to test the role of GAL3 in predicting a preclinical HF phenotype in asymptomatic young obese individuals at high risk of HF development.
The mechanism responsible for elevated GAL3 in our participants likely relates to the underlying myocardial process. GAL3, a β‐galactoside binding lectin derived from macrophages, promotes cardiac fibroblast proliferation, collagen production, and inflammation and is associated with LV dysfunction.46 In mice, the inhibition of GAL3 blocked myocardial fibrosis, inflammation, and superoxide levels cause by a high‐fat diet, suggesting that GAL3 may play pathophysiologic role in cardiac dysfunction with obesity.47 Because LV diastolic dysfunction is a primary process in MHD, it is likely that GAL3 in some way reflects the biology of that process.
GAL3 Associates With PH
The measurement of PASP using PAAT allowed for several important findings. First, it provided the novel demonstration that GAL3 strongly associates with PH in these asymptomatic participants. PH was surprisingly common, occurring in 59% of the young asymptomatic obese participants in our study. Although prior studies have not assessed PH in asymptomatic young obese individuals, our findings are consistent with those of Brittain et al, who found that PH was associated with insulin resistance and diastolic dysfunction in a middle‐aged population.48 The implications of PH in our population are not clear; however, PH is a powerful predictor of mortality in patients with symptomatic HF35 and predicts HF admissions among black patients.3 It is also noteworthy that among our participants, PH was associated with early evidence of RV uncoupling, which portends a worse prognosis in patients with HFpEF.49
The source of the elevated GAL3 in our participants cannot be completely determined from this study. PH correlated with left heart disease in these individuals—in particular, diastolic dysfunction and LVH—suggesting that PH may be secondary to elevated left heart filling pressures. PH due to left heart disease (World Health Organization group 2) is the most frequent cause of PH worldwide. In addition, it is noteworthy that in patients with pulmonary arterial hypertension, GAL3 is associated with RV dysfunction,50 raising the possibility that among our participants, elevated GAL3 may in part reflect release from the lungs and/or the right ventricle in addition to the left heart.
Limitations
Because this study was noninvasive, we did not have invasive hemodynamics to exclude patients who may have had elevated pulmonary pressures not related to left‐sided disease (occult pulmonary embolism, significant obstructive sleep apnea, primary PH). However, with the mandatory inclusion of left‐sided cardiac disease, as reflected by LVH and/or diastolic dysfunction, we attempted to identify individuals who likely had PH due to left‐sided disease; individuals with any known pulmonary disease were excluded from the study. The sample size was diminished given exclusion of patients whose MHD status was indeterminate. However, the secondary analysis, which was based on the presence or absence of PH and included all 232 evaluable patients, confirmed the association of GAL3 with MHD.
Conclusions
We showed that stage B MHD and PH are common in asymptomatic young individuals who are obese. GAL3 is independently associated with MHD and PH in these high‐risk individuals, even among traditional risk factors, whereas NT‐proBNP and FSTL3 are not. The strong relationship between PH and GAL3 raises the possibility that GAL3 is involved in the pathophysiology of PH in metabolic disease and/or that PH itself may lead to an increase in GAL3 levels. GAL3 may be of value in identifying preclinical HF and/or PH in obese individuals, a targetable group for preventative HF interventions.
Sources of Funding
This study was supported by grants from the American Heart Association (FTF 17FTF33670369, Gopal), CJ Martin Fellowship from the National Health and Medical Research Council of Australia (APP1037603, Sverdlov), RACP Foundation/Servier Staff “Barry Young” Award and Heart Foundation of Australia Future Leader Fellowship (Award ID 101918, Sverdlov), National Institutes of Health (NO1‐00239, Colucci), and the National Center for Advancing Translational Sciences, National Institutes of Health (1UL1TR001430, Boston University CTSI).
Disclosures
None.
Supporting information
Table S1. Baseline Characteristics of Persons Removed From Stage B Metabolic Heart Disease Primary Analysis Due to Indeterminate Status
Table S2. Logistic Regression Analysis of Predictors of Obese Metabolic Heart Disease Phenotype
Table S3. Baseline Characteristics of Cohort With Metabolic Heart Disease Pulmonary Hypertension Phenotype
Table S4. Cardiac Structure and Function With Metabolic Heart Disease Pulmonary Hypertension Phenotype
Table S5. Logistic Regression Analysis of Predictors of Obese Stage B Metabolic Heart Disease Pulmonary Hypertension Phenotype
Table S6. Logistic Regression Analysis for Presence of Obese Stage B Metabolic Heart Disease Pulmonary Hypertension Phenotype
Figure S1. Correlations of diastolic function and right heart pressures and function in the stage B metabolic heart disease pulmonary hypertension phenotype.
(J Am Heart Assoc. 2019;8:e011100 DOI: 10.1161/JAHA.118.011100.)
References
- 1. Cuspidi C, Rescaldani M, Sala C, Grassi G. Left‐ventricular hypertrophy and obesity: a systematic review and meta‐analysis of echocardiographic studies. J Hypertens. 2014;32:16–25. [DOI] [PubMed] [Google Scholar]
- 2. Ogden CL, Carroll MD, Fryar CD, Flegal KM. Prevalence of obesity among adults and youth: United States, 2011–2014. NCHS Data Brief. 2015;288:1–8. [PubMed] [Google Scholar]
- 3. Caleyachetty R, Thomas GN, Toulis KA, Mohammed N, Gokhale KM, Balachandran K, Nirantharakumar K. Metabolically healthy obese and incident cardiovascular disease events among 3.5 million men and women. J Am Coll Cardiol. 2017;70:1429–1437. [DOI] [PubMed] [Google Scholar]
- 4. Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, Kannel WB, Vasan RS. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–313. [DOI] [PubMed] [Google Scholar]
- 5. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:1810–1852. [DOI] [PubMed] [Google Scholar]
- 6. Choudhary G, Jankowich M, Wu WC. Elevated pulmonary artery systolic pressure predicts heart failure admissions in African Americans: Jackson Heart Study. Circ Heart Fail. 2014;7:558–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Correa de Sa DD, Hodge DO, Slusser JP, Redfield MM, Simari RD, Burnett JC, Chen HH. Progression of preclinical diastolic dysfunction to the development of symptoms. Heart. 2010;96:528–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971;285:1441–1446. [DOI] [PubMed] [Google Scholar]
- 9. Jaffe AS, Januzzi JL Jr. Using biomarkers to guide heart failure therapy. Clin Chem. 2017;63:954–957. [DOI] [PubMed] [Google Scholar]
- 10. Chow SL, Maisel AS, Anand I, Bozkurt B, de Boer RA, Felker GM, Fonarow GC, Greenberg B, Januzzi JL Jr, Kiernan MS, Liu PP, Wang TJ, Yancy CW, Zile MR; American Heart Association Clinical Pharmacology Committee of the Council on Clinical Cardiology; Council on Basic Cardiovascular Sciences; Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; Council on Epidemiology and Prevention; Council on Functional Genomics and Translational Biology; Council on Quality of Care and Outcomes Research . Role of biomarkers for the prevention, assessment, and management of heart failure: a scientific statement from the American Heart Association. Circulation. 2017;135:e1054–e1091. [DOI] [PubMed] [Google Scholar]
- 11. McCord J, Mundy BJ, Hudson MP, Maisel AS, Hollander JE, Abraham WT, Steg PG, Omland T, Knudsen CW, Sandberg KR, McCullough PA; Breathing Not Properly Multinational Study Interventions . Relationship between obesity and B‐type natriuretic peptide levels. Arch Intern Med. 2004;164:2247–2252. [DOI] [PubMed] [Google Scholar]
- 12. Bursi F, Weston SA, Redfield MM, Jacobsen SJ, Pakhomov S, Nkomo VT, Meverden RA, Roger VL. Systolic and diastolic heart failure in the community. JAMA. 2006;296:2209–2216. [DOI] [PubMed] [Google Scholar]
- 13. Iwanaga Y, Nishi I, Furuichi S, Noguchi T, Sase K, Kihara Y, Goto Y, Nonogi H. B‐type natriuretic peptide strongly reflects diastolic wall stress in patients with chronic heart failure: comparison between systolic and diastolic heart failure. J Am Coll Cardiol. 2006;47:742–748. [DOI] [PubMed] [Google Scholar]
- 14. Shimano M, Ouchi N, Nakamura K, Oshima Y, Higuchi A, Pimentel DR, Panse KD, Lara‐Pezzi E, Lee SJ, Sam F, Walsh K. Cardiac myocyte‐specific ablation of follistatin‐like 3 attenuates stress‐induced myocardial hypertrophy. J Biol Chem. 2011;286:9840–9848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lara‐Pezzi E, Felkin LE, Birks EJ, Sarathchandra P, Panse KD, George R, Hall JL, Yacoub MH, Rosenthal N, Barton PJ. Expression of follistatin‐related genes is altered in heart failure. Endocrinology. 2008;149:5822–5827. [DOI] [PubMed] [Google Scholar]
- 16. Gopal DM, Kommineni M, Ayalon N, Koelbl C, Ayalon R, Biolo A, Dember LM, Downing J, Siwik DA, Liang CS, Colucci WS. Relationship of plasma galectin‐3 to renal function in patients with heart failure: effects of clinical status, pathophysiology of heart failure, and presence or absence of heart failure. J Am Heart Assoc. 2012;1:e000760 DOI: 10.1161/JAHA.112.000760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lam CS, Roger VL, Rodeheffer RJ, Borlaug BA, Enders FT, Redfield MM. Pulmonary hypertension in heart failure with preserved ejection fraction: a community‐based study. J Am Coll Cardiol. 2009;53:1119–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Maron BA, Hess E, Maddox TM, Opotowsky AR, Tedford RJ, Lahm T, Joynt KE, Kass DJ, Stephens T, Stanislawski MA, Swenson ER, Goldstein RH, Leopold JA, Zamanian RT, Elwing JM, Plomondon ME, Grunwald GK, Baron AE, Rumsfeld JS, Choudhary G. Association of borderline pulmonary hypertension with mortality and hospitalization in a large patient cohort: insights from the Veterans Affairs Clinical Assessment, Reporting, and Tracking Program. Circulation. 2016;133:1240–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF III, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Popescu BA, Waggoner AD. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29:277–314. [DOI] [PubMed] [Google Scholar]
- 20. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ; Chamber Quantification Writing Group; American Society of Echocardiography's Guidelines and Standards Committee; and European Association of Echocardiography. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. [DOI] [PubMed] [Google Scholar]
- 21. Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC Jr, Spertus JA, Costa F; American Heart Association; National Heart, Lung, and Blood Institute . Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. [DOI] [PubMed] [Google Scholar]
- 22. Pai MP, Paloucek FP. The origin of the “ideal” body weight equations. Ann Pharmacother. 2000;34:1066–1069. [DOI] [PubMed] [Google Scholar]
- 23. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. [DOI] [PubMed] [Google Scholar]
- 24. de Simone G, Kizer JR, Chinali M, Roman MJ, Bella JN, Best LG, Lee ET, Devereux RB; Strong Heart Study Investigators . Normalization for body size and population‐attributable risk of left ventricular hypertrophy: the Strong Heart Study. Am J Hypertens. 2005;18:191–196. [DOI] [PubMed] [Google Scholar]
- 25. Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713; quiz 786‐8. [DOI] [PubMed] [Google Scholar]
- 26. Yared K, Noseworthy P, Weyman AE, McCabe E, Picard MH, Baggish AL. Pulmonary artery acceleration time provides an accurate estimate of systolic pulmonary arterial pressure during transthoracic echocardiography. J Am Soc Echocardiogr. 2011;24:687–692. [DOI] [PubMed] [Google Scholar]
- 27. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD‐EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Obokata M, Reddy YNV, Pislaru SV, Melenovsky V, Borlaug BA. Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation. 2017;136:6–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Messerli FH, Sundgaard‐Riise K, Reisin ED, Dreslinski GR, Ventura HO, Oigman W, Frohlich ED, Dunn FG. Dimorphic cardiac adaptation to obesity and arterial hypertension. Ann Intern Med. 1983;99:757–761. [DOI] [PubMed] [Google Scholar]
- 30. Wong CY, O'Moore‐Sullivan T, Leano R, Byrne N, Beller E, Marwick TH. Alterations of left ventricular myocardial characteristics associated with obesity. Circulation. 2004;110:3081–3087. [DOI] [PubMed] [Google Scholar]
- 31. Velagaleti RS, Gona P, Pencina MJ, Aragam J, Wang TJ, Levy D, D'Agostino RB, Lee DS, Kannel WB, Benjamin EJ, Vasan RS. Left ventricular hypertrophy patterns and incidence of heart failure with preserved versus reduced ejection fraction. Am J Cardiol. 2014;113:117–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Echouffo‐Tcheugui JB, Erqou S, Butler J, Yancy CW, Fonarow GC. Assessing the risk of progression from asymptomatic left ventricular dysfunction to overt heart failure: a systematic overview and meta‐analysis. JACC Heart Fail. 2016;4:237–248. [DOI] [PubMed] [Google Scholar]
- 33. Ayalon N, Gopal DM, Mooney DM, Simonetti JS, Grossman JR, Dwivedi A, Donohue C, Perez AJ, Downing J, Gokce N, Miller EJ, Liang CS, Apovian CM, Colucci WS, Ho JE. Preclinical left ventricular diastolic dysfunction in metabolic syndrome. Am J Cardiol. 2014;114:838–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yang H, Negishi K, Wang Y, Nolan M, Saito M, Marwick TH. Echocardiographic screening for non‐ischaemic stage B heart failure in the community. Eur J Heart Fail. 2016;18:1331–1339. [DOI] [PubMed] [Google Scholar]
- 35. Bursi F, McNallan SM, Redfield MM, Nkomo VT, Lam CS, Weston SA, Jiang R, Roger VL. Pulmonary pressures and death in heart failure: a community study. J Am Coll Cardiol. 2012;59:222–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Anjan VY, Loftus TM, Burke MA, Akhter N, Fonarow GC, Gheorghiade M, Shah SJ. Prevalence, clinical phenotype, and outcomes associated with normal B‐type natriuretic peptide levels in heart failure with preserved ejection fraction. Am J Cardiol. 2012;110:870–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Khan AM, Cheng S, Magnusson M, Larson MG, Newton‐Cheh C, McCabe EL, Coviello AD, Florez JC, Fox CS, Levy D, Robins SJ, Arora P, Bhasin S, Lam CS, Vasan RS, Melander O, Wang TJ. Cardiac natriuretic peptides, obesity, and insulin resistance: evidence from two community‐based studies. J Clin Endocrinol Metab. 2011;96:3242–3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Huelsmann M, Neuhold S, Resl M, Strunk G, Brath H, Francesconi C, Adlbrecht C, Prager R, Luger A, Pacher R, Clodi M. PONTIAC (NT‐proBNP selected prevention of cardiac events in a population of diabetic patients without a history of cardiac disease): a prospective randomized controlled trial. J Am Coll Cardiol. 2013;62:1365–1372. [DOI] [PubMed] [Google Scholar]
- 39. Ledwidge M, Gallagher J, Conlon C, Tallon E, O'Connell E, Dawkins I, Watson C, O'Hanlon R, Bermingham M, Patle A, Badabhagni MR, Murtagh G, Voon V, Tilson L, Barry M, McDonald L, Maurer B, McDonald K. Natriuretic peptide‐based screening and collaborative care for heart failure: the STOP‐HF randomized trial. JAMA. 2013;310:66–74. [DOI] [PubMed] [Google Scholar]
- 40. Panse KD, Felkin LE, Lopez‐Olaneta MM, Gomez‐Salinero J, Villalba M, Munoz L, Nakamura K, Shimano M, Walsh K, Barton PJ, Rosenthal N, Lara‐Pezzi E. Follistatin‐like 3 mediates paracrine fibroblast activation by cardiomyocytes. J Cardiovasc Transl Res. 2012;5:814–826. [DOI] [PubMed] [Google Scholar]
- 41. Brandt C, Pedersen M, Rinnov A, Andreasen AS, Moller K, Hojman P, Pedersen BK, Plomgaard P. Obesity and low‐grade inflammation increase plasma follistatin‐like 3 in humans. Mediators Inflamm. 2014;2014:364209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mukherjee A, Sidis Y, Mahan A, Raher MJ, Xia Y, Rosen ED, Bloch KD, Thomas MK, Schneyer AL. FSTL3 deletion reveals roles for TGF‐beta family ligands in glucose and fat homeostasis in adults. Proc Natl Acad Sci USA. 2007;104:1348–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. de Boer RA, Lok DJ, Jaarsma T, van der Meer P, Voors AA, Hillege HL, van Veldhuisen DJ. Predictive value of plasma galectin‐3 levels in heart failure with reduced and preserved ejection fraction. Ann Med. 2011;43:60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Edelmann F, Holzendorf V, Wachter R, Nolte K, Schmidt AG, Kraigher‐Krainer E, Duvinage A, Unkelbach I, Dungen HD, Tschope C, Herrmann‐Lingen C, Halle M, Hasenfuss G, Gelbrich G, Stough WG, Pieske BM. Galectin‐3 in patients with heart failure with preserved ejection fraction: results from the Aldo‐DHF trial. Eur J Heart Fail. 2015;17:214–223. [DOI] [PubMed] [Google Scholar]
- 45. Ho JE, Liu C, Lyass A, Courchesne P, Pencina MJ, Vasan RS, Larson MG, Levy D. Galectin‐3, a marker of cardiac fibrosis, predicts incident heart failure in the community. J Am Coll Cardiol. 2012;60:1249–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sharma UC, Pokharel S, van Brakel TJ, van Berlo JH, Cleutjens JP, Schroen B, Andre S, Crijns HJ, Gabius HJ, Maessen J, Pinto YM. Galectin‐3 marks activated macrophages in failure‐prone hypertrophied hearts and contributes to cardiac dysfunction. Circulation. 2004;110:3121–3128. [DOI] [PubMed] [Google Scholar]
- 47. Martinez‐Martinez E, Lopez‐Andres N, Jurado‐Lopez R, Rousseau E, Bartolome MV, Fernandez‐Celis A, Rossignol P, Islas F, Antequera A, Prieto S, Luaces M, Cachofeiro V. Galectin‐3 participates in cardiovascular remodeling associated with obesity. Hypertension. 2015;66:961–969. [DOI] [PubMed] [Google Scholar]
- 48. Brittain EL, Nwabuo C, Xu M, Gupta DK, Hemnes AR, Moreira HT, De Vasconcellos HD, Terry JG, Carr JJ, Lima JA. Echocardiographic pulmonary artery systolic pressure in the Coronary Artery Risk Development in Young Adults (CARDIA) study: associations with race and metabolic dysregulation. J Am Heart Assoc. 2017;6:e005111 DOI: 10.1161/JAHA.116.005111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Guazzi M, Dixon D, Labate V, Beussink‐Nelson L, Bandera F, Cuttica MJ, Shah SJ. RV contractile function and its coupling to pulmonary circulation in heart failure with preserved ejection fraction: stratification of clinical phenotypes and outcomes. JACC Cardiovasc Imaging. 2017;10:1211–1221. [DOI] [PubMed] [Google Scholar]
- 50. He J, Li X, Luo H, Li T, Zhao L, Qi Q, Liu Y, Yu Z. Galectin‐3 mediates the pulmonary arterial hypertension‐induced right ventricular remodeling through interacting with NADPH oxidase 4. J Am Soc Hypertens. 2017;11:275–289.e2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline Characteristics of Persons Removed From Stage B Metabolic Heart Disease Primary Analysis Due to Indeterminate Status
Table S2. Logistic Regression Analysis of Predictors of Obese Metabolic Heart Disease Phenotype
Table S3. Baseline Characteristics of Cohort With Metabolic Heart Disease Pulmonary Hypertension Phenotype
Table S4. Cardiac Structure and Function With Metabolic Heart Disease Pulmonary Hypertension Phenotype
Table S5. Logistic Regression Analysis of Predictors of Obese Stage B Metabolic Heart Disease Pulmonary Hypertension Phenotype
Table S6. Logistic Regression Analysis for Presence of Obese Stage B Metabolic Heart Disease Pulmonary Hypertension Phenotype
Figure S1. Correlations of diastolic function and right heart pressures and function in the stage B metabolic heart disease pulmonary hypertension phenotype.


