Abstract
Background
Many older patients have a change in statin adherence—either an increase or a decrease—from before to after an acute myocardial infarction (AMI), but its association with mortality is unknown.
Methods and Results
Using Medicare administrative claims, a cohort of patients ≥66 years old with an AMI hospitalization from 2008 to 2010 was assembled. Statin adherence was measured for 180 days pre‐AMI and 180 days post‐AMI and categorized as severely nonadherent, moderately nonadherent, or adherent. Categorical change in statin adherence from pre‐ to post‐AMI was assessed. Patients were then followed for up to 18 months for all‐cause mortality. A Cox proportional hazards model was applied to estimate the effects of statin adherence change on all‐cause mortality, adjusted for patient baseline characteristics. Of 101 011 eligible patients, 20% had a categorical increase in adherence, 16% decreased, and 14% remained nonadherent both pre‐ and post‐AMI. Compared with patients who were always severely nonadherent (both pre‐ and post‐AMI), patients whose adherence increased from severely nonadherent to adherent (hazard ratio=0.83; 95% CI: 0.75–0.92) and patients who were always adherent (hazard ratio=0.88; 95% CI: 0.82–0.94) were less likely to die; patients whose adherence decreased from moderately nonadherent to severely nonadherent were more likely to die (hazard ratio=1.11; 95% CI: 1.01–1.22).
Conclusions
After an AMI, patients with decreased statin adherence had the worst mortality outcomes. However, patients with increased statin adherence had a similar risk of mortality compared with continuously adherent patients, suggesting that, even after an AMI, it is not too late to improve statin adherence.
Keywords: behavior change, medication adherence, myocardial infarction, older adults, secondary prevention
Subject Categories: Aging, Health Services, Mortality/Survival, Myocardial Infarction, Secondary Prevention
Clinical Perspective
What Is New?
This is the first study to investigate the association between changes in statin adherence from before to after an acute myocardial infarction (AMI) hospitalization and all‐cause mortality.
Patients who increased their statin adherence—even if they only increased from severely nonadherent to moderately nonadherent—had a similar risk of all‐cause mortality compared to patients who were always adherent.
Patients who decreased their statin adherence had a similar or an even higher risk of all‐cause mortality than patients who were always severely nonadherent.
What Are the Clinical Implications?
Many patients who increase their statin adherence after an AMI can experience similar benefits from statin therapy as patients who were always adherent, suggesting that, even after an AMI, it is not too late to improve statin adherence.
Among patients who were previously adherent to statin therapy, continued adherence is important after an AMI because decreasing statin adherence may lead to a higher risk of mortality compared to patients who were essentially never adherent to their statin.
After a patient experiences an AMI, counseling about the need for statin therapy that is tailored to the patient's previous experience with the medication may increase the likelihood of positive adherence changes and better clinical outcomes.
Statin therapy is a major component of guideline‐recommended secondary prevention after an acute myocardial infarction (AMI).1 Clinical benefit from statins after an AMI requires adherence to this medication therapy,2, 3 and statin adherence after an AMI was previously shown to have a dose–response‐type association with mortality.4 Clinical trials and many observational studies often assess the effectiveness of statins for secondary prevention among patients who are naïve to statin therapy; however, many patients are already taking a statin when they are hospitalized for an AMI, especially among older adults.5
Among US Medicare beneficiaries who were already taking a statin before being hospitalized for an AMI, 20% had increased statin adherence, 16% had decreased adherence, and 14% were consistently nonadherent both pre‐ and post‐AMI hospitalization.5 Other studies have found similar patterns of statin adherence changes after an AMI.6, 7 An important clinical question remains unknown among patients who were already taking a statin before experiencing an AMI: Does improving statin adherence matter, or is it too late to improve statin adherence after an AMI? No studies have evaluated whether and to what extent these changes in statin adherence may impact clinical outcomes. Therefore, the objective of this study was to investigate whether statin adherence change from before to after an AMI hospitalization is associated with all‐cause mortality in a large cohort of US Medicare beneficiaries.
Methods
The authors cannot make the Centers for Medicare and Medicaid Services (CMS) research data used for this study available to other researchers per the terms of the data use agreement. However, investigators can request access to Medicare administrative claims data through application with CMS.
Data Sources and Study Cohort
US Medicare 2007–2011 data including enrollment summaries, medical service claims (inpatient, outpatient, carrier, and skilled‐nursing facility), and prescription Part D claims from the CMS Chronic Conditions Data Warehouse were used to create a cohort of patients hospitalized for an AMI between 2008 and 2010. This cohort has previously been described,5 but this study additionally required patients to survive at least 180 days after hospital discharge. The eligibility criteria were (1) index AMI hospitalization between January 1, 2008, and December 31, 2010; (2) ≥66 years old; (3) continuous enrollment in Medicare Parts A, B, and D for ≥360 days prehospital admission, through the AMI hospitalization, and ≥180 days posthospital discharge; (4) discharged to home/self‐care and survived >180 days prehospital admission discharge; (5) filled ≥1 statin prescription between 360 and 14 days prehospital admission; and (6) no pre‐AMI end‐stage renal disease. Details on patient selection and attrition can be seen in Figure 1. AMI hospitalizations were identified using an International Classification of Diseases, Ninth Revision (ICD‐9) code of 410.x1 in the primary or secondary discharge field. If a patient had multiple AMIs during the index year, their index event was defined as their first observed AMI.
Figure 1.

Patient eligibility and attrition. AMI indicates acute myocardial infarction; US, United States.
The institutional review board of the University of North Carolina at Chapel Hill approved this study. The need for informed consent was waived because this was a secondary analysis of deidentified administrative claims data.
Assessment of Statin Adherence and Change in Statin Adherence
Our measurements of statin adherence and statin adherence change were previously described.5 Prescription claims for statins were identified in Part D event files. Adherence was measured using the proportion of days covered (PDC; 0–100%), adjusting for hospital stays and oversupply from previous prescription fills. Adherence was measured separately in both the 180 days pre‐ and 180 days post‐AMI hospitalization and then categorized as severely nonadherent (PDC <40%), moderately nonadherent (PDC 40–79.9%), or adherent (PDC ≥80%).4, 5
The primary exposure of interest was the categorical change in statin adherence from pre‐ to post‐AMI (9 categories for all combinations of pre‐ and post‐AMI adherence), with patients who were always severely nonadherent serving as the reference group. To simplify the display and description of results, statin adherence change was also categorized into the following groups (previously described as 5‐level adherence change5): a “major decrease” if patients were adherent pre‐AMI and severely nonadherent post‐AMI, a “moderate decrease” for all other adherence decreases, “no change,” a “major increase” if patients were severely nonadherent pre‐AMI and adherent post‐AMI, and a “moderate increase” for all other adherence increases.
Assessment of Outcome
The study design was similar to our previous work,5 but after assessing statin adherence change, we followed patients up for mortality outcomes (Figure 2). All‐cause mortality was measured with the verified date of death found in the Medicare enrollment file. Patients were followed up from the end of the 180‐day post‐AMI adherence measurement period until whichever of the following occurred first: death, loss of continuous enrollment in Medicare Parts A/B/D, or end of available data for the patient (maximum of 18 months follow‐up). In other words, survival was measured starting at 181 days after index AMI hospital discharge up to a maximum of 24 months after the discharge date.
Figure 2.

Study timeline in months, relative to index hospital admission. A: Length of stay for the index AMI hospitalization goes from admission date (0i,adm) to discharge date (0i,dis). B: 12‐month period used to measure baseline comorbidities and identify prevalent statin users for study inclusion. C: 6‐month period used to measure pre‐AMI statin adherence. If a patient's first prescription claim for a statin was identified during this period (i.e. they were a “new user” according to our study definitions), pre‐AMI adherence was measured from that date until the index hospital admission date. Concurrent use of other cardiovascular medications also measured during this period. D: 3‐month period used to identify patients with dual Medicare and Medicaid eligibility. If a patient was dually enrolled during any of these 3 months, they were considered dual eligible for the entire study. E: 30‐day period after index hospital discharge date to measure whether patient followed up with a primary care provider and/or cardiologist. F: 6‐month period used to measure post‐AMI statin adherence. Concurrent use of other cardiovascular medications and hospitalizations for a stroke or a recurrent AMI were also measured during this period. Patients had to survive until the end of this period for study inclusion. G: Patients were followed up for all‐cause mortality from 6‐months after the index hospital discharge date until whichever occurred first: death, loss of continuous enrollment in Medicare Parts A/B/D, or end of available data for the patient (maximum of 18 months follow‐up). AMI indicates acute myocardial infarction.
Patient Characteristics
Other patient characteristics of interest that were measured fell into the following categories: (1) sociodemographic characteristics, (2) baseline clinical conditions and medication use, (3) characteristics of the index AMI hospitalization, and (4) postdischarge events and medication use.
Sociodemographic characteristics were measured from enrollment summary files and included age at index AMI admission, sex, race/ethnicity, dual eligibility in Medicare and Medicaid, and median household income. Dual eligibility was measured in the 3 months pre‐AMI. Median household income was calculated for individuals ≥65 years old at the US Census block group level.
Baseline comorbidities and recent cardiovascular procedures were measured in the 12 months before the index AMI admission from inpatient and outpatient medical claims. Comorbidities included previous AMI, dementia/Alzheimer's disease, depression, ischemic heart disease, unstable angina, lipid abnormalities, and rhabdomyolysis/myopathy. A modified Charlson Comorbidity Index was calculated that excluded AMI and dementia.5 Recent cardiovascular procedures included coronary artery bypass surgery and stent/percutaneous transluminal coronary angioplasty. Baseline use of secondary prevention medications was measured in the 6 months pre‐AMI from prescription claims (having at least 1 prescription claim for the medication class in the 180 days pre‐index AMI admission). Medications of interest included angiotensin‐converting enzyme (ACE) inhibitors/angiotensin II receptor blockers, β‐blockers, P2Y12 inhibitors, calcium channel blockers, and aldosterone receptor antagonists. Finally, we identified whether patients were “new users” of statins, defined as having their first prescription claim for a statin within 6 months before the index AMI.
Characteristics of the index AMI included type of AMI (subendocardial or transmural), procedures (coronary artery bypass surgery, percutaneous transluminal coronary angioplasty/stent, cardiac catheterization, angiocardiography, and infusion of platelet inhibitors), and complications (cardiogenic shock, cardiac dysrhythmias, hypotension, acute renal failure, and heart failure). Additionally, length of the index hospitalization, admission to an intensive care unit and/or coronary care unit, and consultation from a cardiologist were measured.
Outpatient follow‐up with a cardiologist and/or primary care provider was measured in the 30 days after hospital discharge.5 Hospitalizations for AMI and stroke were also measured from the index discharge date for 6 months. Use of secondary prevention medications during this same 6‐month period was also assessed, including measures for ACE inhibitors/angiotensin II receptor blockers, β‐blockers, P2Y12 inhibitors, calcium channel blockers, and aldosterone receptor antagonists (having at least 1 prescription claim for the medication class during the index AMI hospital stay or within 180 days after discharge). Finally, change in the simvastatin‐equivalent average daily dose was calculated (6‐month post‐AMI average daily dose minus 6‐month pre‐AMI average daily dose).5
Statistical Analyses
Distributions of patient characteristics were described. Additionally, the distribution of changes in statin adherence—stratified by pre‐AMI statin adherence—was described. All multivariable analyses (survival curves and Cox proportional hazards models) assessing the association between change in statin adherence and all‐cause mortality were adjusted for all variables described in the Patient Characteristics section above; multivariable analyses assessing the association between post‐AMI statin adherence and all‐cause mortality were adjusted for these same variables, as well as pre‐AMI statin adherence.
To visualize the association of statin adherence and all‐cause mortality after an AMI hospitalization in a familiar way, direct adjusted survival curves8 for the outcome of all‐cause mortality were plotted, stratified by post‐AMI statin adherence (3 curves). To show the importance of changes in statin adherence on this association, another set of direct adjusted survival curves was plotted, stratified by change in statin adherence from pre‐ to post‐AMI (9 curves). The adjusted survival probabilities with 95% CIs at 1‐year of follow‐up were also estimated for both sets of survival curves.
We then used a multivariable Cox proportional hazards model to estimate hazard ratios (HRs) and 95% CIs for the association between statin adherence and all‐cause mortality. The first model estimated the association between post‐AMI statin adherence and all‐cause mortality (patients who were severely nonadherent post‐AMI were the reference group). The final model, highlighting the importance of the change in statin adherence, estimated the association between changes in statin adherence and all‐cause mortality (patients who were severely nonadherent both pre‐ and post‐AMI were the reference group). Schoenfeld residuals for statin adherence change categories were plotted against survival time to assess the proportional hazards assumption.
Several sensitivity analyses were conducted to assess the robustness and consistency of the estimates for the association between all‐cause mortality and statin adherence change. First, because some covariates were measured during the same period when the exposure of interest (statin adherence change) was measured, we iteratively added covariates to the Cox model based upon when they were measured to see how estimates were affected: crude estimates only followed by iterative adjustment for (1) sociodemographics, (2) pre‐AMI variables, (3) index AMI hospitalization variables, and (4) post‐AMI variables (this is the full model). Second, using the full study sample, 3 sensitivity analyses were conducted: (1) requiring a 10% absolute change in PDC from pre‐ to post‐AMI to officially be defined as an adherence change (to minimize the influence of small changes in adherence);5, 7 (2) adjusting for pre‐ and post‐AMI statin intensity1, 5 instead of change in simvastatin‐equivalent average daily dose; and (3) adding a liver disease variable to the model. Third, study eligibility was altered in 6 models by excluding (1) patients who were “new users,” (2) patients with pre‐AMI PDC of 0%, (3) “new users” and patients with pre‐AMI PDC of 0%, (4) patients with only 1 pre‐AMI statin prescription fill, (5) “new users” and patients with only 1 pre‐AMI statin fill, and (6) patients with an AMI or stroke hospitalization within 6 months after the index AMI discharge date. Finally, a sensitivity analysis was conducted among the subgroup of patients who were also taking an ACE inhibitor or an angiotensin II receptor blocker as well as a β‐blocker pre‐AMI; the association between statin adherence change and all‐cause mortality was adjusted for (1) changes in ACE inhibitor/angiotensin II receptor blocker and β‐blocker adherence, followed by iterative adjustment for (2) sociodemographics, (3) pre‐AMI variables, (4) index AMI hospitalization variables, and (5) post‐AMI variables.
All analyses were conducted with SAS 9.4 (SAS Institute Inc).
Results
Our final study sample included 101 011 Medicare beneficiaries who were already taking a statin before the index AMI hospitalization and survived at least 6 months after hospital discharge. Characteristics of the study sample can be seen in the Table. The distribution of changes in statin adherence from pre‐ to post‐AMI can be seen in Figure 3. Out of the entire study sample, the distribution of categorical changes in statin adherence was 3622 (3.6%) patients with a major decrease, 12 443 (12.3%) patients with a moderate decrease, 64 736 (64.1%) patients with no change (including 6892 [6.8%] patients who were always severely nonadherent and 6926 [6.9%] patients who were always moderately nonadherent), 15 022 (14.9%) patients with a moderate increase, and 5188 (5.1%) patients with a major increase. A total of 50 093 (49.6%) patients were nonadherent (PDC <80%) in the pre‐AMI and/or post‐AMI periods. The distribution of patient characteristics across statin adherence change groups has been previously described in this cohort5 (see Table S1 for results specific to this study).
Table 1.
Distribution of Patient Characteristics
| Patient Characteristics | Full Cohort: N=101 011, n (%) |
|---|---|
| Sociodemographics | |
| Age (y) | |
| 66–75 | 47 128 (46.7) |
| 76–85 | 39 698 (39.3) |
| 86+ | 14 185 (14.0) |
| Female | 54 886 (54.3) |
| Race/ethnicity | |
| White | 85 318 (84.5) |
| Black | 8495 (8.4) |
| Hispanic | 3001 (3.0) |
| Asian | 2227 (2.2) |
| Other | 1970 (2.0) |
| Pre‐AMI comorbidities and cardiovascular proceduresa | |
| Adjusted Charlson comorbidity indexb | |
| 0 | 21 946 (21.7) |
| 1–2 | 40 745 (40.3) |
| 3–5 | 29 876 (29.6) |
| 6–8 | 7102 (7.0) |
| 9+ | 1342 (1.3) |
| Baseline comorbidities and procedures | |
| Prior AMIc | 4288 (4.2) |
| Dementia/Alzheimer's diseased | 9396 (9.3) |
| Depression | 15 207 (15.1) |
| CABG | 1064 (1.1) |
| PTCA/stent | 6748 (6.7) |
| Pre‐AMI medications | |
| New user of statine | 10 220 (10.1) |
| Concurrent medicationsf | |
| ACE inhibitor/ARB | 65 524 (64.9) |
| β‐Blocker | 63 437 (62.8) |
| P2Y12 inhibitor | 31 546 (31.2) |
| Characteristics of index AMI hospitalization | |
| Procedures | |
| CABG | 6778 (6.7) |
| PTCA/stent | 39 479 (39.1) |
| Duration of hospitalization (d) | |
| 1–3 | 46 047 (45.6) |
| 4–6 | 30 966 (30.7) |
| 7–11 | 16 714 (16.5) |
| 12+ | 7284 (7.2) |
| 30‐d post‐AMI follow‐up | |
| None | 14 873 (14.7) |
| Primary care providerg only | 29 301 (29.0) |
| Cardiologist only | 19 161 (19.0) |
| Both | 37 676 (37.3) |
| Post‐AMI medications | |
| Concurrent medicationsh | |
| ACE inhibitor/ARB | 72 011 (71.3) |
| β‐Blocker | 87 887 (87.0) |
| P2Y12 inhibitor | 66 607 (65.9) |
| Post‐AMI clinical events | |
| Hospitalization for recurrent AMIi | 6386 (6.3) |
| Hospitalization for strokej | 972 (1.0) |
ACE indicates angiotensin‐converting enzyme; AMI, acute myocardial infarction; ARB, angiotensin II receptor blocker; CABG, coronary artery bypass surgery; ICD‐9, International Classification of Diseases, Ninth Revision; PTCA, percutaneous transluminal coronary angioplasty.
Measured in the 12 months before the index AMI hospital admission date.
Charlson comorbidity index does not include counts for AMI and dementia.
Charlson comorbidity index definition.
Medicare Chronic Conditions Data Warehouse definition.
First prescription claim for a statin was identified during the 6 months before the index AMI hospital admission date.
At least 1 prescription fill within medication class during the 6 months before the index AMI hospital admission date.
Primary care physician, physician assistant, or nurse practitioner.
At least 1 prescription fill within medication class during the index AMI hospitalization period or within 6 months after index AMI discharge date.
Inpatient ICD‐9 diagnosis code of 410.x1 in the primary or secondary discharge field within 6 months after index AMI discharge date.
Inpatient ICD‐9 diagnosis code of 430, 431, 433.x1, 434.x1, or 436 in the primary discharge field within 6 months after index AMI discharge date.
Figure 3.
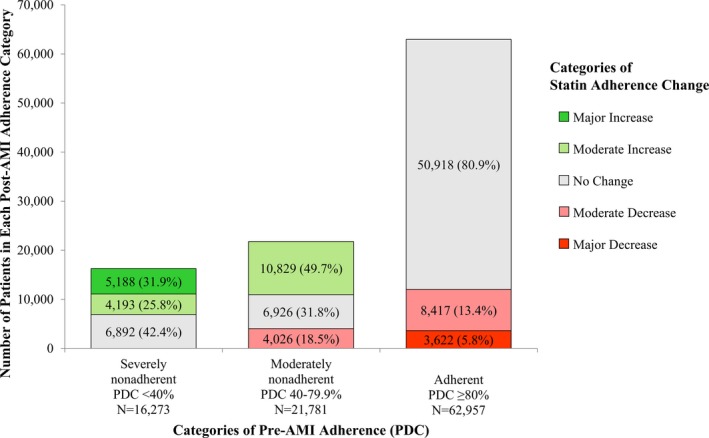
Distribution of categorical statin adherence change stratified by pre‐AMI statin adherence. Percentages were calculated for each pre‐AMI statin adherence category separately. AMI indicates acute myocardial infarction; PDC, proportion of days covered.
Starting at 181 days post‐AMI hospital discharge, patients were followed for a median of 346 days (interquartile range 244–447 days). During follow‐up, 13 274 (13.1%) patients died, 941 (0.9%) were censored from losing Part D continuous enrollment, and 27 (<0.1%) were censored from losing Part A/B continuous enrollment.
Survival Curves by Post‐AMI Statin Adherence
The direct adjusted survival curves for all‐cause mortality stratified by post‐AMI statin adherence (Figure 4A) showed a dose–response‐type relationship; patients who were severely nonadherent to statin therapy had the lowest probability of survival (12‐month survival 0.853 [95% CI: 0.847–0.859]), while patients who were adherent had the highest probability of survival (12‐month survival 0.879 [0.876–0.882]).
Figure 4.

Direct adjusted survival curves for all‐cause mortality after AMI, stratified by statin adherence. Adjusted for sociodemographics, baseline clinical conditions and medication use, whether the patient was a new user of statins (initiated statin within the 180 days pre‐index AMI), index hospitalization events, postdischarge clinical events and medication use, and changes in statin doses. Adjustment for medication use was accomplished by including a binary indicator variable for each of the following medication classes: (1) ACE inhibitor/ARB, (2) β‐blocker, (3) P2Y12 inhibitor, (4) calcium channel blocker, and (5) aldosterone receptor antagonist; a patient was classified as using a medication in the pre‐AMI period if they had at least 1 prescription claim in the 180 days before the index AMI and were classified as using a medication in the post‐AMI period if they had at least 1 prescription claim for the medication during the index AMI or within 180 days after discharge. Follow‐up begins 6 months after index AMI discharge (ie, Day 0 is 180 days after index AMI discharge). A, Stratified by post‐AMI statin adherence. B, Stratified by change in statin adherence from pre‐ to post‐AMI. *12‐month estimate calculated from 6 months post‐AMI discharge through 18 months post‐AMI discharge. ACE indicates angiotensin‐converting enzyme; AMI, acute myocardial infarction; ARB, angiotensin II receptor blocker.
Survival Curves by Statin Adherence Change
In Figure 4B, direct adjusted survival curves were further stratified by pre‐AMI adherence to give 9 strata representing all categorical changes in statin adherence. Compared with patients who were always severely nonadherent (dotted red line; 12‐month survival 0.864 [95% CI: 0.856–0.872]), patients with decreases in statin adherence (solid red, dashed red, and solid blue lines) tended to have similar or lower survival probabilities (12‐month estimates ranged from 0.852 [95% CI: 0.841–0.862] to 0.857 [95% CI: 0.849–0.864]). Compared with patients who were always adherent (solid green line; 12‐month survival 0.876 [95% CI: 0.873–0.879]), patients with increases in statin adherence (dotted green, dashed green, and dotted blue lines) tended to have similar or slightly higher survival probabilities (12‐month estimates ranged from 0.876 [95% CI: 0.865–0.872] to 0.884 [95% CI: 0.874–0.894]). See Figure S1 for the unadjusted survival curves.
Association Between Post‐AMI Statin Adherence and All‐Cause Mortality
When assessing the association between post‐AMI statin adherence and all‐cause mortality, patients who were adherent post‐AMI were less likely to die compared with patients who were severely nonadherent (hazard ratio [HR] 0.79; 95% CI: 0.74–0.83). Patients who were moderately nonadherent post‐AMI were also less likely to die compared with patients who were severely nonadherent but to a lesser degree (HR 0.89; 95% CI: 0.84–0.95), consistent with a dose–response‐type relationship. See Table S2 for full model results.
Association Between Statin Adherence Change and All‐Cause Mortality
In the final multivariable model, the association between statin adherence change (9 groups) and all‐cause mortality was estimated (Figure 5). Compared with patients who were severely nonadherent both pre‐ and post‐AMI, patients with decreases in statin adherence tended to have a higher risk of all‐cause mortality: a HR of 1.11 (95% CI: 1.01–1.22) for patients with a moderate decrease in statin adherence from moderately nonadherent to severely nonadherent and a HR of 1.09 (95% CI: 0.99–1.20) for patients with a major decrease in statin adherence. Patients with a moderate decrease from adherent to moderately nonadherent had a similar risk of all‐cause mortality compared with patients who were always severely nonadherent (HR 1.04; 95% CI: 0.96–1.13). Patients with moderate or major increases in statin adherence (HRs ranging from 0.83 [95% CI: 0.75–0.92] to 0.89 [95% CI: 0.80–0.99]) had similar risks of all‐cause mortality as patients who were consistently adherent both pre‐ and post‐AMI (HR 0.88; 95% CI: 0.82–0.94). See Table S3 for full model results. Schoenfeld residuals for statin adherence change were consistent with the proportional hazards assumption (Figure S2).
Figure 5.

All‐cause mortality adjusted hazard ratios (HRs) and 95% CIs by changes in statin adherence. Multivariable Cox proportional hazards model adjusted for sociodemographics, baseline clinical conditions and medication use, whether the patient was a new user of statins (initiated statin within the 180 days pre‐index AMI), index hospitalization events, postdischarge follow‐up, changes in statin doses, use of other secondary prevention medications, and AMI and stroke events that occurred within 180 days after index hospital discharge. Adjustment for medication use was accomplished by including a binary indicator variable for each of the following medication classes: (1) ACE inhibitor/ARB, (2) β‐blocker, (3) P2Y12 inhibitor, (4) calcium channel blocker, and (5) aldosterone receptor antagonist; a patient was classified as using a medication in the pre‐AMI period if they had at least 1 prescription claim in the 180 days before the index AMI and were classified as using a medication in the post‐AMI period if they had at least 1 prescription claim for the medication during the index AMI or within 180 days after discharge. The coloring scheme is only used to illustrate direction of adherence change, not for interpreting HRs. See Table S3 for a full list of and description of adjustment variables, as well as results from the full model. AMI indicates acute myocardial infarction; HR, hazard ratio; PDC, proportion of days covered.
Sensitivity Analyses
When iteratively adding covariates to the model based on the timing of covariate measurement (Table S4), some estimates differed from the fully adjusted model but were still on the same side of the null; most crude estimates were further from the null than the fully adjusted model, and adjusting for additional variables brought estimates closer to the null. Most other sensitivity analyses (Tables S5 and S6) were consistent with findings from the final model (Figure 5). Among the subgroup of patients who were also taking an ACE inhibitor or angiotensin II receptor blocker and a β‐blocker pre‐AMI (N=48 580), the association between statin adherence change and all‐cause mortality, while adjusting for the change in adherence to these other medications, was mostly consistent with findings from the primary analysis (Table S7; the full model results for this sensitivity analysis can be seen in Table S8). The point estimate for statin adherence change from severely nonadherent to adherent was outside of the range from the primary analysis CIs; the 3 adherence change measures in this model were highly correlated and only 4% of the population was in this category of statin adherence change. All other findings from the “Fully adjusted model” sensitivity analysis in Table S7 were consistent with the final model (Figure 5).
Discussion
In our cohort of 101 011 older Medicare beneficiaries who were already taking a statin when hospitalized for an AMI, 20% had increased statin adherence, 16% had decreased adherence, and 14% were either severely or moderately nonadherent pre‐AMI and did not change their adherence category post‐AMI. Our study sheds light on the mortality risk associated with changes in statin adherence from before to after an AMI among older patients who were taking a statin before their AMI hospitalization. We found that patients with a categorical decrease in statin adherence had the greatest risk of all‐cause mortality between 6 and 24 months after hospital discharge. The results from our study also show that patients with increased statin adherence had a lower risk of all‐cause mortality compared with patients who were always severely nonadherent; indeed, the most intriguing and important finding from our study suggests the risk of death among patients with increased statin adherence was comparable to the risk among patients who were continuously adherent during the study period, even if they only increased from severely nonadherent to moderately nonadherent.
While statin adherence change after an AMI has been previously studied,5, 6, 7, 9, 10 to our best knowledge this is the first study that has investigated mortality outcomes associated with changes in statin adherence from pre‐ to post‐AMI among patients who were already taking a statin. Our findings for the association between post‐AMI adherence and mortality were consistent with a previous study, both in terms of magnitude and a dose–response‐type relationship;4 this 2007 study evaluating post‐AMI medication adherence found that—compared with patients who were adherent to statin therapy—patients who were severely nonadherent or moderately nonadherent had a 25% or 12% increased risk of mortality, respectively.4
A possible reason why patients may become less adherent to statin therapy post‐AMI aligns with the Sentinel Event Effect: a patient may believe that statin therapy is ineffective in preventing future events since an AMI occurred while taking a statin.5, 7, 11, 12 Also, many patients who were nonadherent to statins before their AMI did not improve their adherence after their AMI hospitalization; these patients may not understand the importance of improving their statin adherence. Contrary to these beliefs, our study found that patients with decreased statin adherence—even if they were adherent to statin therapy before their index AMI hospitalization—often had a higher risk of all‐cause mortality compared with patients who were always severely nonadherent. Additionally, while patients with decreased adherence from adherent to severely nonadherent had a similar risk of mortality compared with patients who were always severely nonadherent, their risk of mortality was considerably higher than that of patients who remained adherent both pre‐ and post‐AMI.
Therefore, our study provides evidence that (1) it is not too late to improve statin adherence after an AMI for patients who were taking a statin but were nonadherent to therapy before their AMI, and (2) continued adherence after an AMI is important among those patients who were previously adherent. Hospitalization for an AMI and clinician counseling about the need for adherence may act as a “wake‐up call” to improve statin adherence to prevent further coronary events.7, 11, 12, 13 Given the significant number of patients without an improvement in statin adherence after an AMI, cardiologists and other providers should emphasize that it is not too late to improve statin adherence among patients who were nonadherent to statin therapy pre‐AMI. Also, the consequence of decreasing statin adherence may be a clinically significant increase in the risk for post‐AMI mortality: an important message for patients who were adherent to statin therapy pre‐AMI. Statin therapy for both primary and secondary prevention has been shown to reduce the risk of future coronary events.14, 15, 16 Therefore, experiencing an AMI despite being adherent should not necessarily be viewed as a failure of statin therapy. If patients classified as adherent pre‐AMI had instead not taken their statin, they may have experienced an AMI even sooner. Our study also adjusted for within‐patient changes in simvastatin‐equivalent dosing, implying the importance of continued adherence or addressing nonadherence—independent of statin dose—even in patients who cannot tolerate high‐intensity statins. Importantly, clinicians may need to probe and address patient perceptions of statin effectiveness after an AMI, even among patients who were previously adherent to statin therapy.
Furthermore, our study results suggest that—among patients who were taking a statin before an AMI—measuring statin adherence as a change in adherence may give us a better understanding of the association between statin adherence and mortality after an AMI. Clinical interventions addressing the need for post‐AMI statin adherence among prevalent statin users that are tailored to patients after assessing their pre‐AMI statin adherence may lead to better clinical outcomes after AMI hospitalizations. While an AMI hospitalization may serve as a teachable moment regarding the need for statin therapy (and patients are already interacting with providers), such an intervention may be more impactful if delivered during follow‐up after hospital discharge.7, 17, 18 Future research could investigate how to best deliver these interventions.
Our observation that patients with decreased statin adherence had a higher risk of mortality compared with patients who were always severely nonadherent may reflect residual confounding. For example, sicker patients who have previously experienced a coronary event, or with high‐risk comorbidities such as diabetes mellitus, may have been more adherent to statins in the pre‐AMI period. Another potential reason that some patients experience an AMI despite being adherent is less responsiveness to the therapeutic effect of statins. A third potential reason is that statin adherence change may be a proxy for other health behavior changes (including changes in adherence to other secondary prevention medications, eg, ACE inhibitors/angiotensin II receptor blockers, and β‐blockers) that may also be associated with outcomes after an AMI. For example, factors associated with greater AMI severity (eg, requiring a coronary stent or coronary artery bypass surgery) have been previously associated with both increases in statin adherence and smoking cessation,5, 19 both of which may affect mortality outcomes. Future research could investigate these hypotheses further.
Limitations
Our study has some limitations. First, residual confounding bias from unmeasured confounders and covariate misclassification error are possible in this quasi‐experimental observational study. We analyzed the change in medication adherence before and after an AMI, which may serve as a self‐control to mitigate this limitation. As previously mentioned, statin adherence change may also be a proxy for other health behavior changes that are unmeasured in administrative claims data; therefore, our results may be partially explained by other behavior changes, including adherence changes to other secondary prevention medications that were correlated with statin adherence change. This may bias the association between statin adherence change and mortality away from the null. However, patients who were always severely nonadherent or always adherent could not have decreases or increases in adherence, respectively, even though they may have changed other health behaviors. Additionally, the high correlation between statin adherence change and other health behavior changes increased the likelihood of multicollinearity; this would reduce our ability to interpret point estimates independent of these other behavior change variables if they were included in a regression model. The direction and magnitude of these biases is therefore difficult to assess, but our results were consistent across several sensitivity analyses.
Second, using administrative prescription claims data may lead to an overestimation of medication adherence because there is no information available about whether the patient actually took the medication. However, prescription claims records have good validity and correlation with other adherence measures20, 21, 22 and clinical outcomes.4 We applied standard algorithms in using prescription claims to measure adherence to mitigate this limitation. Prescription claims data may also underestimate adherence if prescriptions are paid for outside of a patient's Medicare Part D plan. However, this is not common for Medicare beneficiaries, and these claims are often adjudicated through Part D when they do occur.23, 24, 25 Medicare patients are also less likely than privately insured patients to use medication samples.26 Our results were consistent with a dose–response‐type association with post‐AMI statin adherence4 and robust to a more conservative measure of categorical adherence change.5, 7
Finally, our study includes both patients who were recently initiated on statin therapy before their index AMI and patients who had been taking statins for at least a year. Therefore, our study may be susceptible to a healthy user selection bias and time‐varying hazards.27, 28 However, in our sensitivity analyses, the findings were consistent when excluding “new users” and when excluding patients who may have discontinued statin therapy before their index AMI. Patients with primary nonadherence to statins29 and patients who discontinued statin therapy more than 1 year before experiencing an AMI were not included in this study. However, a body of work has already explored discontinuing statin therapy because of intolerance and re‐initiation of statin therapy.13, 30, 31, 32, 33
Conclusions
Even after an AMI, it is not too late to improve statin adherence. Patients who increased their statin adherence from before to after an AMI had a similar risk of all‐cause mortality as patients who were consistently adherent. However, continued adherence is also important because patients who decreased their statin adherence tended to have worse outcomes than patients who were always nonadherent. Among patients who were already taking a statin, counseling about the need for statin therapy after an AMI that is tailored to the patient's previous experience with the medication may increase the likelihood of positive adherence change and better clinical outcomes.
Sources of Funding
This research was supported in part by the US NIH National Institute on Aging grants 1R01AG046267‐01A1 & 1R21AG043668‐01A1 (principal investigator: Fang). Dr Hickson was supported by the US NIH National Heart, Lung, and Blood Institute through a National Research Service Award (NRSA) training grant (4T32HL007055‐41) as a postdoctoral research fellow with the Cardiovascular Disease Epidemiology Program at The University of North Carolina at Chapel Hill. Dr Hickson was also supported by the American Foundation for Pharmaceutical Education (AFPE) as the 2018 Pre‐Doctoral Fellow in Health Outcome Disparities.
Disclosures
Dr Robinson worked on grants from Acasti, Amarin, Amgen, Astra‐Zeneca, Esai, Esperion, Merck, Novo‐Nordisk, Regeneron, Sanofi, and Takeda. Dr Robinson was a consultant for Amgen, Merck, Novartis, Novo‐Nordisk, Pfizer, Regeneron, and Sanofi. The authors have no other disclosures.
Supporting information
Table S1. Patient Characteristics for the Full Cohort and Stratified by Change in Statin Adherence After AMI
Table S2. Full Model Results for the Association Between All‐Cause Mortality and Post‐AMI Statin Adherence, While Adjusting for pre‐AMI Statin Adherence
Table S3. Full Model Results for the Association Between All‐Cause Mortality and Change in Statin Adherence After Acute Myocardial Infarction (AMI)
Table S4. Crude Estimates and Models Adjusted Iteratively Based on the Timing of Covariate Measurement for the Association Between All‐Cause Mortality and Change in Statin Adherence After Acute Myocardial Infarction (AMI)
Table S5. Sensitivity Analyses Involving Changing Variable Definitions or Adding New Variables to the Model Estimating the Association Between All‐Cause Mortality and Change in Statin Adherence After Acute Myocardial Infarction (AMI)
Table S6. Sensitivity Analyses With Restricted Study Eligibility Estimating the Association Between All‐Cause Mortality and Change in Statin Adherence After Acute Myocardial Infarction (AMI)
Table S7. Association Between All‐Cause Mortality and Change in Statin Adherence, Adjusted for Changes in ACE/ARB and β‐Blocker Adherence, Among the Subgroup of Prevalent Users for all 3 Medication Classes: Models Adjusted Iteratively Based on the Timing of Covariate Measurement and Comparison to Final Model From Manuscript
Table S8. Full Model Results for the Association Between All‐Cause Mortality and Change in Statin Adherence After Acute Myocardial Infarction, Adjusted for Changes in ACE/ARB and β‐Blocker Adherence, Among the Subgroup of Prevalent Users for all 3 Medication Classes (N=48 580)
Figure S1. Detailed description of study timeline in months, relative to index hospital admission. A, Length of stay for the index AMI hospitalization goes from admission date (0i,adm) to discharge date (0i,dis). B, 12‐month period used to measure baseline comorbidities and identify prevalent statin users for study inclusion. C, 6‐month period used to measure pre‐AMI statin adherence. If a patient's first prescription claim for a statin was identified during this period (ie, they were a “new user” according to our study definitions), pre‐AMI adherence was measured from that date until the index hospital admission date. Concurrent use of other cardiovascular medications was also measured during this period. D, 3‐month period used to identify patients with dual Medicare and Medicaid eligibility. If a patient was dually enrolled during any of these 3 months, they were considered dual eligible for the entire study. E, 30‐day period after index hospital discharge date to measure whether patient followed up with a primary care provider and/or cardiologist. F, 6‐month period used to measure post‐AMI adherence. Concurrent use of other cardiovascular medications and hospitalizations for a stroke or a recurrent AMI were also measured during this period. Patients had to survive until the end of this period for study inclusion. G, Patients were followed up for all‐cause mortality from 6 months after the index hospital discharge date until the whichever occurred first: death, loss of continuous enrollment in Medicare Parts A/B/D, or end of available data for the patient (maximum of 18 months follow‐up). AMI indicates acute myocardial infarction.
Figure S2. Unadjusted survival curves and 95% confidence bands for all‐cause mortality after acute myocardial infarction, stratified by statin adherence. Follow‐up begins 6 months after index AMI discharge (ie, Day 0 is 180 days after index AMI discharge). A, Stratified by post‐AMI statin adherence. B, Stratified by change in statin adherence from pre‐ to post‐AMI. *12‐month estimate calculated from 6 months post‐AMI discharge through 18 months post‐AMI discharge. AMI indicates acute myocardial infarction.
Figure S3. Schoenfeld residuals (dots) with smooth fitted spline (line) for statin adherence change.
(J Am Heart Assoc. 2019;8:e011378 DOI: 10.1161/JAHA.118.011378.)
This article was handled independently by Kerry‐Anne Rye, PhD, as a guest editor.
References
- 1. Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd‐Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC Jr, Watson K, Wilson PW, Eddleman KM, Jarrett NM, LaBresh K, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC Jr, Tomaselli GF. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S1–S45. [DOI] [PubMed] [Google Scholar]
- 2. Choudhry NK, Glynn RJ, Avorn J, Lee JL, Brennan TA, Reisman L, Toscano M, Levin R, Matlin OS, Antman EM, Shrank WH. Untangling the relationship between medication adherence and post‐myocardial infarction outcomes: medication adherence and clinical outcomes. Am Heart J. 2014;167:51–58.e5. [DOI] [PubMed] [Google Scholar]
- 3. Kuepper‐Nybelen J, Hellmich M, Abbas S, Ihle P, Griebenow R, Schubert I. Association of long‐term adherence to evidence‐based combination drug therapy after acute myocardial infarction with all‐cause mortality. A prospective cohort study based on claims data. Eur J Clin Pharmacol. 2012;68:1451–1460. [DOI] [PubMed] [Google Scholar]
- 4. Rasmussen JN, Chong A, Alter DA. Relationship between adherence to evidence‐based pharmacotherapy and long‐term mortality after acute myocardial infarction. JAMA. 2007;297:177–186. [DOI] [PubMed] [Google Scholar]
- 5. Hickson RP, Robinson JG, Annis IE, Killeya‐Jones LA, Korhonen MJ, Cole AL, Fang G. Changes in statin adherence following an acute myocardial infarction among older adults: patient predictors and the association with follow‐up with primary care providers and/or cardiologists. J Am Heart Assoc. 2017;6:e007106 DOI: 10.1161/JAHA.117.007106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. de Vries FM, Denig P, Vegter S, Bos HJ, Postma MJ, Hak E. Does a cardiovascular event change adherence to statin treatment in patients with type 2 diabetes? A matched cohort design. Curr Med Res Opin. 2015;31:595–602. [DOI] [PubMed] [Google Scholar]
- 7. Kronish IM, Ross JS, Zhao H, Muntner P. The impact of hospitalization for acute myocardial infarction on adherence to statins among older adults. Circ Cardiovasc Qual Outcomes. 2016;9:364–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang X, Loberiza FR, Klein JP, Zhang MJ. A SAS macro for estimation of direct adjusted survival curves based on a stratified Cox regression model. Comput Methods Programs Biomed. 2007;88:95–101. [DOI] [PubMed] [Google Scholar]
- 9. Beloin‐Jubinville B, Joly‐Mischlich T, Rouleau ED, Noiseux P, Blais L, Forget A, Beauchesne MF. Does hospitalization influence patients' medication adherence and community pharmacists' interventions? Ann Pharmacother. 2013;47:1143–1152. [DOI] [PubMed] [Google Scholar]
- 10. Cohen MJ, Shaykevich S, Cawthon C, Kripalani S, Paasche‐Orlow MK, Schnipper JL. Predictors of medication adherence postdischarge: the impact of patient age, insurance status, and prior adherence. J Hosp Med. 2012;7:470–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Boudreaux ED, Bock B, O'Hea E. When an event sparks behavior change: an introduction to the sentinel event method of dynamic model building and its application to emergency medicine. Acad Emerg Med. 2012;19:329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McBride CM, Emmons KM, Lipkus IM. Understanding the potential of teachable moments: the case of smoking cessation. Health Educ Res. 2003;18:156–170. [DOI] [PubMed] [Google Scholar]
- 13. Booth JN III, Colantonio LD, Rosenson RS, Safford MM, Chen L, Kilgore ML, Brown TM, Taylor B, Dent R, Monda KL, Muntner P, Levitan EB. Healthcare utilization and statin re‐initiation among Medicare beneficiaries with a history of myocardial infarction. J Am Heart Assoc. 2018;7:e008462 DOI: 10.1161/JAHA.117.008462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mills EJ, Rachlis B, Wu P, Devereaux PJ, Arora P, Perri D. Primary prevention of cardiovascular mortality and events with statin treatments: a network meta‐analysis involving more than 65,000 patients. J Am Coll Cardiol. 2008;52:1769–1781. [DOI] [PubMed] [Google Scholar]
- 15. Thavendiranathan P, Bagai A, Brookhart MA, Choudhry NK. Primary prevention of cardiovascular diseases with statin therapy: a meta‐analysis of randomized controlled trials. Arch Intern Med. 2006;166:2307–2313. [DOI] [PubMed] [Google Scholar]
- 16. Afilalo J, Duque G, Steele R, Jukema JW, de Craen AJ, Eisenberg MJ. Statins for secondary prevention in elderly patients: a hierarchical Bayesian meta‐analysis. J Am Coll Cardiol. 2008;51:37–45. [DOI] [PubMed] [Google Scholar]
- 17. Kripalani S, Jackson AT, Schnipper JL, Coleman EA. Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2:314–323. [DOI] [PubMed] [Google Scholar]
- 18. Colantonio LD, Huang L, Monda KL, Bittner V, Serban MC, Taylor B, Brown TM, Glasser SP, Muntner P, Rosenson RS. Adherence to high‐intensity statins following a myocardial infarction hospitalization among Medicare beneficiaries. JAMA Cardiol. 2017;2:890–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. O'Hea E, Abar B, Bock B, Chapman G, Boudreaux ED. Understanding smoking after acute illness: an application of the sentinel event method. Psychol Health. 2015;30:879–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hansen RA, Kim MM, Song L, Tu W, Wu J, Murray MD. Comparison of methods to assess medication adherence and classify nonadherence. Ann Pharmacother. 2009;43:413–422. [DOI] [PubMed] [Google Scholar]
- 21. Lau HS, de Boer A, Beuning KS, Porsius A. Validation of pharmacy records in drug exposure assessment. J Clin Epidemiol. 1997;50:619–625. [DOI] [PubMed] [Google Scholar]
- 22. Steiner JF, Prochazka AV. The assessment of refill compliance using pharmacy records: methods, validity, and applications. J Clin Epidemiol. 1997;50:105–116. [DOI] [PubMed] [Google Scholar]
- 23. Roberto PN, Stuart B. Out‐of‐plan medication in Medicare Part D. Am J Manag Care. 2014;20:743–748. [PubMed] [Google Scholar]
- 24. Stuart B, Loh FE. Medicare Part D enrollees' use of out‐of‐plan discounted generic drugs. J Am Geriatr Soc. 2012;60:387–388. [DOI] [PubMed] [Google Scholar]
- 25. Zhou L, Stearns SC, Thudium EM, Alburikan KA, Rodgers JE. Assessing Medicare Part D claim completeness using medication self‐reports: the role of veteran status and Generic Drug Discount Programs. Med Care. 2015;53:463–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brown JD, Doshi PA, Talbert JC. Utilization of free medication samples in the United States in a nationally representative sample: 2009–2013. Res Social Adm Pharm. 2016;13:193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lund JL, Richardson DB, Sturmer T. The active comparator, new user study design in pharmacoepidemiology: historical foundations and contemporary application. Curr Epidemiol Rep. 2015;2:221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ray WA. Evaluating medication effects outside of clinical trials: new‐user designs. Am J Epidemiol. 2003;158:915–920. [DOI] [PubMed] [Google Scholar]
- 29. Cheetham TC, Niu F, Green K, Scott RD, Derose SF, Vansomphone SS, Shin J , Tunceli K, Reynolds K. Primary nonadherence to statin medications in a managed care organization. J Manag Care Pharm. 2013;19:367–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Booth JN III, Colantonio LD, Chen L, Rosenson RS, Monda KL, Safford MM, Kilgore ML, Brown TM, Taylor B, Dent R, Muntner P, Levitan EB. Statin discontinuation, reinitiation, and persistence patterns among Medicare beneficiaries after myocardial infarction: a cohort study. Circ Cardiovasc Qual Outcomes. 2017;10:e003626. [DOI] [PubMed] [Google Scholar]
- 31. Serban MC, Colantonio LD, Manthripragada AD, Monda KL, Bittner VA, Banach M, Chen L, Huang L, Dent R, Kent ST, Muntner P, Rosenson RS. Statin intolerance and risk of coronary heart events and all‐cause mortality following myocardial infarction. J Am Coll Cardiol. 2017;69:1386–1395. [DOI] [PubMed] [Google Scholar]
- 32. Zhang H, Plutzky J, Shubina M, Turchin A. Continued statin prescriptions after adverse reactions and patient outcomes: a cohort study. Ann Intern Med. 2017;167:221–227. [DOI] [PubMed] [Google Scholar]
- 33. Zhang H, Plutzky J, Skentzos S, Morrison F, Mar P, Shubina M, Turchin A. Discontinuation of statins in routine care settings: a cohort study. Ann Intern Med. 2013;158:526–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Patient Characteristics for the Full Cohort and Stratified by Change in Statin Adherence After AMI
Table S2. Full Model Results for the Association Between All‐Cause Mortality and Post‐AMI Statin Adherence, While Adjusting for pre‐AMI Statin Adherence
Table S3. Full Model Results for the Association Between All‐Cause Mortality and Change in Statin Adherence After Acute Myocardial Infarction (AMI)
Table S4. Crude Estimates and Models Adjusted Iteratively Based on the Timing of Covariate Measurement for the Association Between All‐Cause Mortality and Change in Statin Adherence After Acute Myocardial Infarction (AMI)
Table S5. Sensitivity Analyses Involving Changing Variable Definitions or Adding New Variables to the Model Estimating the Association Between All‐Cause Mortality and Change in Statin Adherence After Acute Myocardial Infarction (AMI)
Table S6. Sensitivity Analyses With Restricted Study Eligibility Estimating the Association Between All‐Cause Mortality and Change in Statin Adherence After Acute Myocardial Infarction (AMI)
Table S7. Association Between All‐Cause Mortality and Change in Statin Adherence, Adjusted for Changes in ACE/ARB and β‐Blocker Adherence, Among the Subgroup of Prevalent Users for all 3 Medication Classes: Models Adjusted Iteratively Based on the Timing of Covariate Measurement and Comparison to Final Model From Manuscript
Table S8. Full Model Results for the Association Between All‐Cause Mortality and Change in Statin Adherence After Acute Myocardial Infarction, Adjusted for Changes in ACE/ARB and β‐Blocker Adherence, Among the Subgroup of Prevalent Users for all 3 Medication Classes (N=48 580)
Figure S1. Detailed description of study timeline in months, relative to index hospital admission. A, Length of stay for the index AMI hospitalization goes from admission date (0i,adm) to discharge date (0i,dis). B, 12‐month period used to measure baseline comorbidities and identify prevalent statin users for study inclusion. C, 6‐month period used to measure pre‐AMI statin adherence. If a patient's first prescription claim for a statin was identified during this period (ie, they were a “new user” according to our study definitions), pre‐AMI adherence was measured from that date until the index hospital admission date. Concurrent use of other cardiovascular medications was also measured during this period. D, 3‐month period used to identify patients with dual Medicare and Medicaid eligibility. If a patient was dually enrolled during any of these 3 months, they were considered dual eligible for the entire study. E, 30‐day period after index hospital discharge date to measure whether patient followed up with a primary care provider and/or cardiologist. F, 6‐month period used to measure post‐AMI adherence. Concurrent use of other cardiovascular medications and hospitalizations for a stroke or a recurrent AMI were also measured during this period. Patients had to survive until the end of this period for study inclusion. G, Patients were followed up for all‐cause mortality from 6 months after the index hospital discharge date until the whichever occurred first: death, loss of continuous enrollment in Medicare Parts A/B/D, or end of available data for the patient (maximum of 18 months follow‐up). AMI indicates acute myocardial infarction.
Figure S2. Unadjusted survival curves and 95% confidence bands for all‐cause mortality after acute myocardial infarction, stratified by statin adherence. Follow‐up begins 6 months after index AMI discharge (ie, Day 0 is 180 days after index AMI discharge). A, Stratified by post‐AMI statin adherence. B, Stratified by change in statin adherence from pre‐ to post‐AMI. *12‐month estimate calculated from 6 months post‐AMI discharge through 18 months post‐AMI discharge. AMI indicates acute myocardial infarction.
Figure S3. Schoenfeld residuals (dots) with smooth fitted spline (line) for statin adherence change.


