Short abstract
See Article by Im et al
Keywords: Editorials, coronary artery disease, drug‐eluting stent, optical coherence tomography
Subject Categories: Optical Coherence Tomography (OCT), Percutaneous Coronary Intervention
Stent malposition, recognized as an entity with the advent of intravascular imaging, refers to the lack of full contact between stent struts and the vessel wall after percutaneous coronary intervention (Figure). Malapposition may be present immediately after placement of stents (acute stent malapposition), or it may develop later (late stent malapposition), which can, in turn, be categorized as late persistent malapposition (ongoing since the time of implantation) or late acquired malapposition (developing de novo during follow‐up).1
Figure 1.
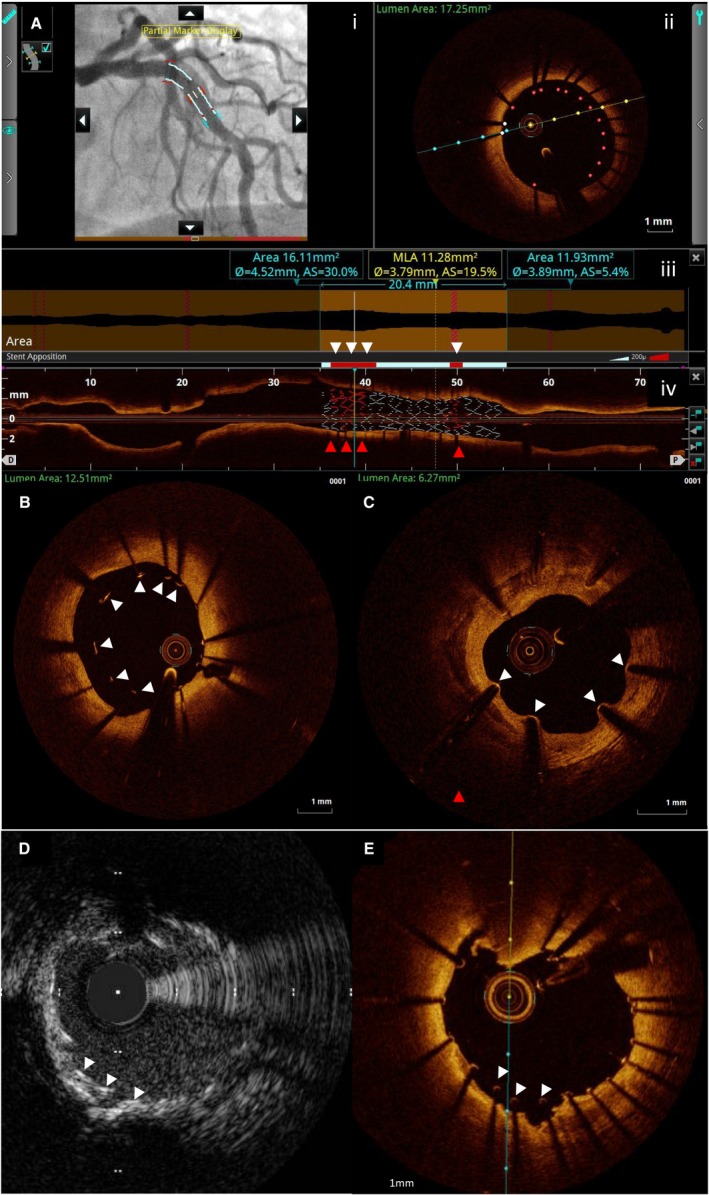
Intravascular imaging assessment of malapposition. A, Optical coherence tomography (OCT) automated detection of malapposition. The high resolution of OCT allows automatic detection of malapposition. Malapposed segments and stent struts are highlighted in red in the angiographic coregistration (i), OCT cross‐section (ii), automated measures apposition bar (white arrowheads: red segments of white bar denote malapposed segments) (iii), and rendered stent (red arrowheads: red stent struts of white rendered stent denote malapposed segments) in longitudinal OCT image (iv). B, OCT cross‐section showing acute malapposition (white arrowheads). C, OCT cross‐section showing neointimal growth toward malapposed struts 15 months after drug‐eluting stent implantation. Intravascular ultrasound (D) and OCT (E) coregistered cross‐sections from the same patient, showing malapposition (white arrowheads) much more easily visible by OCT because of its superior resolution. AS indicates area stenosis; MLA, minimum lumen area.
Acute stent malapposition occurs because of a mismatch between the stent and vessel lumen contours, and it can be the result of suboptimal stent deployment (stent undersizing or stent underexpansion) or secondary to vessel architecture/lesion characteristics (eg, occurring at bifurcation points, in large vessels, in long lesions requiring multiple overlapping stents, or secondary to stent struts interacting with an eccentric calcific plaque).1, 2 Design and alloy of the stent metal could also affect stent conformability to plaque and vessel irregularities and determine the degree of acute stent malapposition.3
Acute stent malapposition is a common finding after implantation of drug‐eluting stents (DESs), observed, on average, in 15% of stents by intravascular ultrasound (IVUS)4 and at a higher rate of 50% by optical coherence tomography (OCT),5 because of the higher resolution of OCT compared with IVUS and the ability for automatic detection of malapposed struts (Figure). As shown in serial OCT studies, acute stent malapposition may be corrected by vascular remodeling, resulting in complete neointimal integration during the follow‐up period (especially in malapposed struts with a distance to intimal surface of <0.35 mm).6 However, incomplete strut apposition may persist, leading to late persistent malapposition. In contrast, late acquired malapposition may result from positive (outward) remodeling causing an increase in vessel dimensions greater than abluminal tissue growth (responsible for approximately one third of late acquired malapposition7) or from abluminal thrombus dissolution after primary percutaneous coronary intervention or plaque regression without positive remodeling over time.8
Although intravascular imaging studies have established stent underexpansion as one of the most important independent predictors of stent‐related outcomes,9 the potential impact of acute stent malapposition on stent failure rates (ie, in‐stent restenosis and stent thrombosis) has been a matter of controversy.8 Bench‐top in vitro experiments,10 pathophysiological examinations,11 and small serial IVUS and OCT studies12 have supported the theoretical link between exposed, uncovered malapposed struts and increased propensity for local thrombus formation, possibly through causing local flow disturbances and delayed healing; however, several larger studies using IVUS4, 13 or OCT1, 9 have shown no relationship between acute stent malapposition and early, late, or very late stent thrombosis after DES implantation (a finding that is perhaps not surprising given the almost ubiquitous presence of a degree of malapposition on poststenting OCT). Moreover, no significant relationship has been established between the extent of acute stent malapposition and adverse clinical outcomes.2, 4 Indeed, in the IVUS substudy of the ADAPT‐DES (Assessment of Dual Antiplatelet Therapy With Drug‐Eluting Stents) study, the largest areas of stent malposition were observed in the group of patients with no major adverse cardiovascular events during the follow‐up.4
Late stent malapposition is a frequent finding in association with very late DES thrombosis,14 which may suggest a potential causal relationship between late malapposition and very late DES. However, this cause‐and‐effect relationship has been challenged8 by studies relating late stent malapposition, very late stent thrombosis, and inflammation15; studies reporting a high prevalence of strut fracture in very late stent thrombosis16; and OCT‐based studies indicating that neoatherosclerosis may be a more important cause of very late stent thrombosis than late stent malapposition.17 Nonetheless, the cumulative data on the safety of malapposition in metallic DES cannot be extrapolated to bioresorbable scaffolds because of differences in the design, composition, and biodegradation of the scaffolds.
One of the major limitations of the current data examining the clinical relevance and potential impact of different types of malapposition is the paucity of OCT‐based studies from large registries that do the following: (1) compare serial OCT images with paired OCT images that are acquired immediately after stent implantation; and (2) include careful, systematic, long‐term clinical follow‐up. In this issue of the Journal of the American Heart Association (JAHA), Im et al seek to address this inadequacy by reporting the long‐term clinical outcomes (>5 years compared with 2 years of follow‐up on the previous report from the same cohort) in 351 patients with 356 lesions.18 Postprocedural OCT images were analyzed in conjunction with subsequent OCT images that were acquired within 1 year (175±60 days) after stent implantation. Consistent with previous studies,1, 2, 3 late stent malapposition was detected in stents deployed in calcified lesions, in larger‐vessel diameters, or in more severely stenotic lesions compared with stents without malapposed struts.
The most important finding of the study was that the cumulative 8‐year rate of composite cardiovascular events (cardiovascular death, target lesion‐ and target vessel‐related nonfatal myocardial infarction, target lesion and target vessel revascularization, and stent thrombosis) in patients with late stent malapposition was not significantly different from that in patients without late stent malapposition (7.3% versus 10.5%; P=0.822), a finding that is in agreement with accumulated evidence from multiple previous studies1, 2, 4, 5, 7, 9, 13, 19, 20, 21 (Table). Similarly, the clinical events were not different in subgroups on the basis of the type of late stent malapposition (ie, late persistent, late acquired, or a combination of the 2 versus no late stent malapposition) nor were they different between the first‐ and second‐generation DESs. Furthermore, there was no difference in clinical outcomes on the basis of cross‐sectional malapposition distance (≥400 versus <400 μm; P=0.87) or longitudinal length of strut malapposition (≥1 versus <1 mm; P=0.98).
Table 1.
IVUS and OCT Studies Correlating Stent Malapposition With Clinical Outcomes
| Authors | MISSION Intervention | Imaging Modality | Patients (Lesions) | Clinical Follow‐Up, mo | Outcome | P Value | |
|---|---|---|---|---|---|---|---|
| Malappositiona | No Malapposition | ||||||
| Van der Hoeven et al19 | MISSION Intervention | IVUS | 184 | 12 | 0% ST | 0% ST | NS |
| Guo et al7 | HORIZONS‐AMI | IVUS | 241 (263) | 12 | 0% Death or ST | 0% Death or ST | NS |
| Steinberg et al13 | TAXUS IV, V, and VI and ATLAS | IVUS | 1580 |
9 24 |
11.6% MACEs LASM: 8.3% MACEs |
8.8% MACEs 8.1% MACEs |
0.45 0.87 |
| Wang et al4 | ADAPT‐DES | IVUS | 2072 (2446) | 24 | 5.2% MACEs | 4.5% MACEs | 0.58 |
| Im et al1 | OCT | 351 (356) | 24 | LPSM vs LASM vs LPSM and LASM: 2.2%, 3.2%, and 0% MACEs, respectively | 3.2% MACEs | 1.0 | |
| Soeda et al20 | MGH OCT registry | OCT | 786 (900) | 12 | 1.7% DoCE | 2.9% DoCE | NS |
| Prati et al5 | CLI‐OPCI II | OCT | 832 (1002) | 12 | MACE HR: 1.15 (95% CI: 0.8–1.7) | 0.52 | |
| Prati et al21 | CLI‐OPCI ACS | OCT | 507 (588) | 12 | MACE HR: 0.84 (95% CI: 0.5–1.5) | 0.57 | |
| Romagnoli et al2 | CLI‐OPCI registry | OCT | 864 (1020) | 24 | MACE HR: 0.79 (95% CI: 0.5–1.2) | 0.26 | |
| Prati et al9 | CLI‐OPCI LATE | OCT | 1211 | 36 | DoCE HR: 0.92 (95% CI: 0.7–1.2) | 0.56 | |
| Im et al18 | OCT | 351 (356) | 96 |
LSM: 7.3% MACEs; LPSM vs LASM vs LPSM and LASM: 9.6%, 9.7%, and 0% MACEs, respectively |
10.5% MACEs 10.5% MACEs |
0.82 0.47 |
|
ADAPT‐DES indicates Assessment of Dual Antiplatelet Therapy With Drug‐Eluting Stents; CLI‐OPCI ACS, Centro per la Lotta contro l’Infarto‐Optimization of Percutaneous Coronary Intervention Acute coronary Syndrome; CLI‐OPCI LATE, Centro per la Lotta contro l’Infarto‐Optimization of Percutaneous Coronary Intervention Late; CLI‐OPCI, Centro per la Lotta contro l'Infarto–Optimization of Percutaneous Coronary Intervention II; DoCE, device‐oriented cardiovascular event; HORIZON S‐AMI, Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction; HR, hazard ratio; IVUS, intravascular ultrasound; LASM, late acquired stent malapposition; LPSM, late persistent stent malapposition; LSM, late stent malapposition; MACE, major adverse cardiovascular event; MGH OCT registry: Massachusetes General hospital Optical Coherence tomography registry; MISSION Intervention: A Prospective Randomised Controlled Trial to Evaluate the Efficacy of Drug‐Eluting Stents Versus Bare‐Metal Stents for the Treatment of Acute Myocardial Infarction; NS, nonsignificant; OCT, optical coherence tomography; ST, stent thrombosis; TAXUS ATLAS, Polymer‐Based, Paclitaxel‐Eluting TAXUS Liberté Stent in De Novo Lesions.
Indicates acute stent malapposition, unless otherwise specified.
Despite several limitations of the study by Im et al,18 including the single‐center, nonrandomized, cross‐sectional design, with patients arbitrarily included in the analysis on the basis of the performance of 2 serial OCTs, among others, it provides the longest follow‐up data to date correlating OCT‐detected stent malapposition with clinical outcomes. The results build on concordant findings at the 2‐year follow‐up point in the same cohort of patients1 and are in agreement with 2 recent analyses from the large, multicenter, OCT‐based registry, CLI‐OPCI (Centro per la Lotta contro l'Infarto–Optimization of Percutaneous Coronary Intervention),2, 9 including the CLI‐OPCI LATE (Centro per la Lotta contro l'Infarto‐Optimization of Percutaneous Coronary Intervention Late) study that enrolled ≈1200 patients with a median follow‐up of ≈3 years; in this study, acute stent malapposition (detected in ≈50% of stents) was not significantly related to the risk of long‐term stent failure (hazard ratio, 0.92).9
In conclusion, the study by Im et al18 adds long‐term data on the safety and lack of discernible clinical sequelae of different types of OCT‐detected stent malapposition. Until data are available to the contrary, the emphasis on correcting acute stent malapposition may not be based on current evidence and should be replaced with more attention paid to what is known to be important in optimizing stent‐related outcomes (ie, stent expansion and adequate lesion coverage).
Disclosures
Ali has served as a consultant to Abbott Vascular, Boston Scientific, Opsens Medical, Cardinal Health, and Canon; has equity/options in Shockwave Medical; and has received research grants from Abbott Vascular, the National Heart, Lung, and Blood Institute, and Cardiovascular Systems Inc. Mintz is a consultant to Boston Scientific and Philips Volcano. The remaining authors have no disclosures to report.
J Am Heart Assoc. 2019;8:e012262 DOI: 10.1161/JAHA.119.012262.
The opinions expressed in this article are not necessarily those of the editors or of the American Heart Association.
References
- 1. Im E, Kim BK, Ko YG, Shin DH, Kim JS, Choi D, Jang Y, Hong MK. Incidences, predictors, and clinical outcomes of acute and late stent malapposition detected by optical coherence tomography after drug‐eluting stent implantation. Circ Cardiovasc Interv. 2014;7:88–96. [DOI] [PubMed] [Google Scholar]
- 2. Romagnoli E, Gatto L, La Manna A, Burzotta F, Taglieri N, Saia F, Amico F, Marco V, Ramazzotti V, Di Giorgio A, Di Vito L, Boi A, Contarini M, Castriota F, Mintz GS, Prati F. Role of residual acute stent malapposition in percutaneous coronary interventions. Catheter Cardiovasc Interv. 2017;90:566–575. [DOI] [PubMed] [Google Scholar]
- 3. Tanigawa J, Barlis P, Dimopoulos K, Dalby M, Moore P, Di Mario C. The influence of strut thickness and cell design on immediate apposition of drug‐eluting stents assessed by optical coherence tomography. Int J Cardiol. 2009;134:180–188. [DOI] [PubMed] [Google Scholar]
- 4. Wang B, Mintz GS, Witzenbichler B, Souza CF, Metzger DC, Rinaldi MJ, Duffy PL, Weisz G, Stuckey TD, Brodie BR, Matsumura M, Yamamoto MH, Parvataneni R, Kirtane AJ, Stone GW, Maehara A. Predictors and long‐term clinical impact of acute stent malapposition: an assessment of Dual Antiplatelet Therapy With Drug‐Eluting Stents (ADAPT‐DES) intravascular ultrasound substudy. J Am Heart Assoc. 2016;5:e004438 DOI: 10.1161/JAHA.116.004438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Prati F, Romagnoli E, Burzotta F, Limbruno U, Gatto L, La Manna A, Versaci F, Marco V, Di Vito L, Imola F, Paoletti G, Trani C, Tamburino C, Tavazzi L, Mintz GS. Clinical impact of OCT findings during PCI: the CLI‐OPCI II study. JACC Cardiovasc Imaging. 2015;8:1297–1305. [DOI] [PubMed] [Google Scholar]
- 6. Gutierrez‐Chico JL, Wykrzykowska J, Nuesch E, van Geuns RJ, Koch KT, Koolen JJ, di Mario C, Windecker S, van Es GA, Gobbens P, Juni P, Regar E, Serruys PW. Vascular tissue reaction to acute malapposition in human coronary arteries: sequential assessment with optical coherence tomography. Circ Cardiovasc Interv. 2012;5:20–29, S1–S8. [DOI] [PubMed] [Google Scholar]
- 7. Guo N, Maehara A, Mintz GS, He Y, Xu K, Wu X, Lansky AJ, Witzenbichler B, Guagliumi G, Brodie B, Kellett MA Jr, Dressler O, Parise H, Mehran R, Stone GW. Incidence, mechanisms, predictors, and clinical impact of acute and late stent malapposition after primary intervention in patients with acute myocardial infarction: an intravascular ultrasound substudy of the Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction (HORIZONS‐AMI) trial. Circulation. 2010;122:1077–1084. [DOI] [PubMed] [Google Scholar]
- 8. Mintz GS. Why are we so concerned with acute incomplete stent apposition? Eur Heart J Cardiovasc Imaging. 2015;16:110–111. [DOI] [PubMed] [Google Scholar]
- 9. Prati F, Romagnoli E, La Manna A, Burzotta F, Gatto L, Marco V, Fineschi M, Fabbiocchi F, Versaci F, Trani C, Tamburino C, Alfonso F, Mintz GS. Long‐term consequences of optical coherence tomography findings during percutaneous coronary intervention: the Centro per la Lotta contro l'Infarto – Optimization of Percutaneous Coronary Intervention (CLI‐OPCI) LATE study. EuroIntervention. 2018;14:e443–e451. [DOI] [PubMed] [Google Scholar]
- 10. Foin N, Lu S, Ng J, Bulluck H, Hausenloy DJ, Wong PE, Virmani R, Joner M. Stent malapposition and the risk of stent thrombosis: mechanistic insights from an in vitro model. EuroIntervention. 2017;13:e1096–e1098. [DOI] [PubMed] [Google Scholar]
- 11. Joner M, Finn AV, Farb A, Mont EK, Kolodgie FD, Ladich E, Kutys R, Skorija K, Gold HK, Virmani R. Pathology of drug‐eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol. 2006;48:193–202. [DOI] [PubMed] [Google Scholar]
- 12. Ozaki Y, Okumura M, Ismail TF, Naruse H, Hattori K, Kan S, Ishikawa M, Kawai T, Takagi Y, Ishii J, Prati F, Serruys PW. The fate of incomplete stent apposition with drug‐eluting stents: an optical coherence tomography‐based natural history study. Eur Heart J. 2010;31:1470–1476. [DOI] [PubMed] [Google Scholar]
- 13. Steinberg DH, Mintz GS, Mandinov L, Yu A, Ellis SG, Grube E, Dawkins KD, Ormiston J, Turco MA, Stone GW, Weissman NJ. Long‐term impact of routinely detected early and late incomplete stent apposition: an integrated intravascular ultrasound analysis of the TAXUS IV, V, and VI and TAXUS ATLAS workhorse, long lesion, and direct stent studies. JACC Cardiovasc Interv. 2010;3:486–494. [DOI] [PubMed] [Google Scholar]
- 14. Adriaenssens T, Joner M, Godschalk TC, Malik N, Alfonso F, Xhepa E, De Cock D, Komukai K, Tada T, Cuesta J, Sirbu V, Feldman LJ, Neumann FJ, Goodall AH, Heestermans T, Buysschaert I, Hlinomaz O, Belmans A, Desmet W, Ten Berg JM, Gershlick AH, Massberg S, Kastrati A, Guagliumi G, Byrne RA; Prevention of Late Stent Thrombosis by an Interdisciplinary Global European Effort (PRESTIGE) Investigators . Optical coherence tomography findings in patients with coronary stent thrombosis: a report of the PRESTIGE Consortium (Prevention of Late Stent Thrombosis by an Interdisciplinary Global European Effort). Circulation. 2017;136:1007–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cook S, Eshtehardi P, Kalesan B, Raber L, Wenaweser P, Togni M, Moschovitis A, Vogel R, Seiler C, Eberli FR, Luscher T, Meier B, Juni P, Windecker S. Impact of incomplete stent apposition on long‐term clinical outcome after drug‐eluting stent implantation. Eur Heart J. 2012;33:1334–1343. [DOI] [PubMed] [Google Scholar]
- 16. Kosonen P, Vikman S, Jensen LO, Lassen JF, Harnek J, Olivecrona GK, Erglis A, Fossum E, Niemela M, Kervinen K, Ylitalo A, Pietila M, Aaroe J, Kellerth T, Saunamaki K, Thayssen P, Hellsten L, Thuesen L, Niemela K. Intravascular ultrasound assessed incomplete stent apposition and stent fracture in stent thrombosis after bare metal versus drug‐eluting stent treatment the Nordic Intravascular Ultrasound Study (NIVUS). Int J Cardiol. 2013;168:1010–1016. [DOI] [PubMed] [Google Scholar]
- 17. Kang SJ, Lee CW, Song H, Ahn JM, Kim WJ, Lee JY, Park DW, Lee SW, Kim YH, Mintz GS, Park SW, Park SJ. OCT analysis in patients with very late stent thrombosis. JACC Cardiovasc Imaging. 2013;6:695–703. [DOI] [PubMed] [Google Scholar]
- 18. Im E, Hong SJ, Ahn CM, Kim JS, Kim BK, Ko YG, Choi D, Jang Y, Hong MK. Long‐term clinical outcomes of late stent malapposition detected by optical coherence tomography after drug‐eluting stent implantation. J Am Heart Assoc. 2019;8:e011817 DOI: 10.1161/JAHA.118.011817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. van der Hoeven BL, Liem SS, Dijkstra J, Bergheanu SC, Putter H, Antoni ML, Atsma DE, Bootsma M, Zeppenfeld K, Jukema JW, Schalij MJ. Stent malapposition after sirolimus‐eluting and bare‐metal stent implantation in patients with ST‐segment elevation myocardial infarction: acute and 9‐month intravascular ultrasound results of the MISSION! intervention study. JACC Cardiovasc Interv. 2008;1:192–201. [DOI] [PubMed] [Google Scholar]
- 20. Soeda T, Uemura S, Park SJ, Jang Y, Lee S, Cho JM, Kim SJ, Vergallo R, Minami Y, Ong DS, Gao L, Lee H, Zhang S, Yu B, Saito Y, Jang IK. Incidence and clinical significance of poststent optical coherence tomography findings: one‐year follow‐up study from a multicenter registry. Circulation. 2015;132:1020–1029. [DOI] [PubMed] [Google Scholar]
- 21. Prati F, Romagnoli E, Gatto L, La Manna A, Burzotta F, Limbruno U, Versaci F, Fabbiocchi F, Di Giorgio A, Marco V, Ramazzotti V, Di Vito L, Trani C, Porto I, Boi A, Tavazzi L, Mintz GS. Clinical impact of suboptimal stenting and residual intrastent plaque/thrombus protrusion in patients with acute coronary syndrome: the CLI‐OPCI ACS Substudy (Centro per la Lotta Contro L'Infarto‐Optimization of Percutaneous Coronary Intervention in Acute Coronary Syndrome). Circ Cardiovasc Interv. 2016;9:e003726. [DOI] [PubMed] [Google Scholar]


